Abstract
Background.
Deficits in balance and muscle function are important risk factors for falls in older adults. Aging is associated with significant declines in muscle size and density, but associations of trunk muscle size and density with balance and falls in older adults have not been previously examined.
Methods.
Trunk muscle size (cross-sectional area) and attenuation (a measure of tissue density) were measured in computed tomography scans (at the L2 lumbar level) in a cohort of older adults (mean ± SD age of 81.9±6.4) residing in independent living communities. Outcome measures were postural sway measured during quiet standing and Short Physical Performance Battery (SPPB) at baseline, and falls reported by participants for up to 3 years after baseline measurements.
Results.
Higher muscle density was associated with reduced postural sway, particularly sway velocities, in both men and women, and better Short Physical Performance Battery score in women, but was not associated with falls. Larger muscle size was associated with increased postural sway in men and women and with increased likelihood of falling in men.
Conclusions.
The results suggest that balance depends more on muscle quality than on the size of the muscle. The unexpected finding that larger muscle size was associated with increased postural sway and increased fall risk requires further investigation, but highlights the importance of factors besides muscle size in muscle function in older adults.
Keywords: Balance-biomechanics, Sarcopenia, Incident falls, Muscle composition, Postural sway
Every year 30%–40% of adults aged 65 and older fall, and falls are a common cause of injury and death in older adults (1–3). Deficits in balance and muscle function are among the most important risk factors for falls (1,3), although studies measure balance in a wide variety of ways. A common approach to measuring balance is quantifying postural sway while standing on a force platform. Prospective studies indicate that postural sway increases with age (4,5), and that increased postural sway, particularly in mediolateral motion, is predictive of falls in older adults (6,7).
Muscles in the lumbar and abdominal region are thought to play an important role in trunk stability (8,9). Aging is associated with significant declines of muscle size and strength (10,11), and muscle size and tissue density can be measured on computed tomography (CT) scans. Low CT-based density indicates fat accumulation within the muscle tissue (12), and CT-based density is lower in older adults than younger adults (13). Low trunk muscle density is associated with lower functional capacity (14), faster declines in functional capacity (15), more low back pain (14), greater hyperkyphosis (16), and lower trunk extension strength (17) in older adults. In addition, a recent cross-sectional study found lower gluteal muscle density in fallers than nonfallers (18), and low thigh muscle density is predictive of fractures in older adults (19,20). However, despite the potential importance of trunk muscles to trunk stability, the associations of trunk muscle size and density with balance and falls in older adults have not been previously examined.
The aims of this study were to determine if CT-based trunk muscle size and density are cross-sectionally associated with postural sway and physical function, and predictive of incident falls in older adults. We hypothesized that higher trunk muscle size and density would be associated with lower postural sway and higher Short Physical Performance Battery (SPPB) score, and that higher trunk muscle size and density would be associated with reduced likelihood of falling.
Methods
Participants
This was a secondary analysis of data from a clinical trial testing low intensity whole body vibration to improve bone density (21). Detailed inclusion and exclusion criteria have been described previously (22). Briefly, participants were cognitively intact men (n = 57) and women (n = 117) over the age of 60 with osteopenia (sex-specific bone mineral density T-score < −1 and > −2.5) residing in independent living communities in the Boston metropolitan area. Exclusion criteria included known terminal disease, weight ≥ 250 pounds, nonambulatory, hip or total knee replacement, and history of recent fragility fracture. The initial trial period was 2 years, but 55 participants took part in an extension of the trial for a third year. For the primary study (21), participants were randomized to daily exposure to a vibrating platform (active), or to a nonvibrating platform (placebo), but groups were combined for this analysis. Protocols were approved by the Institutional Review Board of Hebrew SeniorLife and Beth Israel Deaconess Medical Center and participants supplied written informed consent.
CT-Based Muscle Measurements
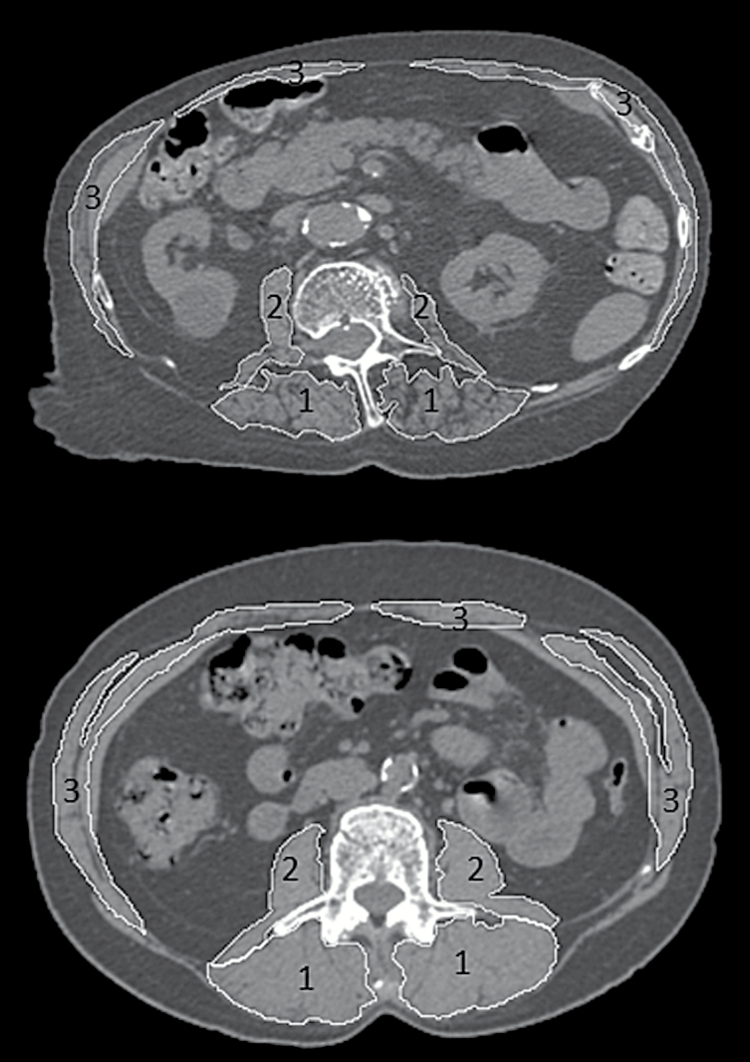
The independent variables were muscle cross-sectional area (CSA, cm2) and density (x-ray attenuation in Hounsfield Units, HU) as measured on CT scans for four muscle groups (all muscles, paraspinal, posterior abdominal, anterior abdominal), as shown in Figure 1. All participants underwent CT scanning of the lumbar spine (L1 and L2) at baseline on the same helical CT scanner operating at 120 kVp, 150 mAs, 48mm field of view, and 1mm slice thickness. Individual muscles were traced in the mid-vertebral slice at the L2 level, or the L1 level if L2 was not available (n = 4), using an image processing program (Analyze, Biomedical Imaging Resource, Mayo Clinic, Rochester, MN (23)). Muscle CSA was calculated as CSA within the muscle contour, and density as the mean of voxel attenuation within the muscle contour, averaging the right and left sides. Attenuation values were zeroed based on the attenuation of a nominally 0 HU chamber in a hydroxyapatite phantom (Image Analysis, Inc., Columbia, KY), and voxels outside the range of −50 to 150 HU were excluded to exclude pure fat or bone. This approach provides good inter- and intra-reader reliability (most intraclass correlation coefficients > .90) for muscle CSA and attenuation measurements (13), and all measurements in this study were performed by a single trained reader with similar reliability.
Figure 1.
Examples of muscle groups measured in computed tomography scans at the L2 level of the spine in a participant with low muscle density (top) and high muscle density (bottom). Muscle grouping examined included: 1) paraspinal muscles (erector spinae and transversospinalis), 2) posterior abdominal muscles (psoas major and quadratus lumborum), and 3) anterior abdominal muscles (rectus abdominis, external oblique, and internal oblique).
Postural Sway and Physical Function
Postural sway was measured at baseline with an eight-channel force plate (Kistler, Winterthur, Switzerland). As described previously (22,24), participants stood as still as possible with hands at sides, eyes open, and feet at shoulder width for 4 minutes while center of pressure (CoP) position data were sampled at 1000 Hz. Data were low-pass filtered at 50 Hz, and postural sway variables were calculated as previously described (24). Variables analyzed in this study were the root mean square of the anteroposterior (AP) CoP motion (mm), root mean square of the mediolateral (ML) CoP motion (mm), root mean square of the AP velocity of CoP motion (mm/s), and root mean square of the ML velocity of CoP motion (mm/s). Physical function was measured using the SPPB (25,26), which includes gait speed, sit to stand, and standing balance components.
Self-reported Falls
Falls history at baseline was self-reported as a fall within the previous 6 months. The primary falls outcome was the number of falls during the follow-up period of up to 3 years. Falls were self-reported every other month by a mail-in questionnaire asking participants if they had fallen to the ground or lower surface (22). If no questionnaire was received, study staff contacted participants by phone to ascertain fall status.
Statistical Analysis
Linear regression models were constructed to estimate the associations of muscle measurements with postural sway and with SPPB score. Associations between muscle measurements and incident fall rates were examined using Poisson regression, adjusting for the number of successfully completed fall questionnaires during follow-up. For each outcome measure, we conducted two analyses: unadjusted, including muscle CSA and density, and fully adjusted, which additionally controlled for age, weight, baseline fall history, and score on the Physical Activity Scale for the Elderly (27). Due to sex-specific differences in both postural sway and muscle, analyses were stratified by sex. Additional adjustment for treatment group (active vs placebo) was also examined but did not markedly alter the results. Similarly, baseline fall history was removed from adjusted models to check for potential bias, but results were similar and fall history was included in the reported analyses. Beta estimates and incidence rate ratios per 1-SD increase in muscle measurement are reported. A p value < .05 was considered to be statistically significant. All analyses were performed using SAS version 9.3 (SAS Institute, Inc., Cary, NC).
Results
Descriptive statistics for participant characteristics, postural sway, SPPB, and muscle measurements are reported in Table 1. At baseline, 17.9% of men and 13.7% of women reported having a fall during the previous 6 months. Follow-up time ranged from 0 to 36 months, with a median of 24 months, and mean ± SD of 21.4±11.4 months. During follow-up, a total of 57 falls were recorded in 19 men (33.3%), and a total of 119 falls were recorded in 57 women (48.7%).
Table 1.
Participant Characteristics, Postural Sway Measures, and Muscle Measurements at Baseline
| Men | Women | |
|---|---|---|
| N | 57 | 117 |
| Age at baseline (y) | 83.7 (5.9) | 81.1 (6.5) |
| Body weight (kg) | 79.7 (11.9) | 67.4 (12.2) |
| Physical Activity Scale for the Elderly | 72.4 (43.2) | 88.6 (51.7) |
| Fall in past 6 mo (%) | 17.9 | 13.7 |
| Postural sway and physical function | ||
| RMS of AP motion (mm) | 4.92 (1.60) | 4.00 (1.30) |
| RMS of AP velocity (mm/s) | 21.8 (11.2) | 14.9 (9.2) |
| RMS of ML motion (mm) | 3.28 (1.58) | 2.50 (1.27) |
| RMS of ML velocity (mm/s) | 10.1 (4.2) | 8.9 (2.8) |
| Short Physical Performance Battery | 8.9 (2.9) | 9.2 (2.6) |
| Muscle measurements | ||
| All muscles | ||
| CSA (cm2) | 53.9 (9.5) | 38.1 (6.1) |
| Density (HU) | 23.3 (8.6) | 17.4 (10.1) |
| Paraspinal | ||
| CSA (cm2) | 21.8 (4.3) | 15.7 (2.9) |
| Density (HU) | 27.2 (9.8) | 21.2 (12.1) |
| Posterior abdominal | ||
| CSA (cm2) | 8.2 (2.0) | 5.7 (1.3) |
| Density (HU) | 30.7 (7.2) | 29.7 (8.4) |
| Anterior abdominal | ||
| CSA (cm2) | 20.2 (4.7) | 14.4 (3.0) |
| Density (HU) | 18.4 (11.7) | 11.0 (12.0) |
Notes: AP = anteroposterior; CSA = cross-sectional area; ML = mediolateral; RMS = root mean square; HU = Hounsfield Units.
Muscle CSA and density were associated with the outcome measures in fully adjusted models as reported in Table 2 (unadjusted model results are available in Supplementary Table 1). Larger CSA of paraspinal and posterior abdominal muscles in men and larger CSA of anterior abdominal muscles in women were associated with increased AP motion. Larger CSA of anterior abdominal and posterior abdominal muscles was associated with increased AP velocity in women, but not men. Muscle CSA was not associated with ML motion, whereas larger CSA of anterior abdominal muscles was associated with increased ML velocity in women only. Higher density of the paraspinal and posterior abdominal muscles was associated with reduced AP motion in men (unadjusted and adjusted models) and women (unadjusted models only), with reduced ML motion in men (unadjusted models only) and women (unadjusted and fully adjusted models), and with reduced AP velocity and ML velocity in men and women (unadjusted and adjusted models). Higher density of the anterior abdominal muscles was associated with reduced AP velocity in women (unadjusted model only). Higher muscle density was associated with better SPPB performance in women, but this association was only significant for the posterior abdominal muscles in men (unadjusted model only).
Table 2.
Associations of Postural Sway With Muscle Size and Density in Men and Women
| Men | Women | |
|---|---|---|
| RMS of AP motion | ||
| All muscles | ||
| CSA | 0.78 (0.16, 1.39) | 0.24 (−0.03, 0.52) |
| Density | −0.70(−1.19, −0.22) | −0.11 (−0.40, 0.19) |
| Paraspinal | ||
| CSA | 0.67 (0.10, 1.23) | 0.03 (−0.24, 0.30) |
| Density | −0.79 (−1.26, −0.31) | −0.23 (−0.51, 0.04) |
| Posterior abdominal | ||
| CSA | 0.49 (0.06, 0.91) | 0.06 (−0.19, 0.32) |
| Density | −0.91 (−1.36, −0.45) | −0.14 (−0.42, 0.14) |
| Anterior abdominal | ||
| CSA | 0.56 (−0.04, 1.16) | 0.39 (0.14, 0.64) |
| Density | −0.39 (−0.91, 0.12) | 0.05 (−0.22, 0.32) |
| RMS of AP velocity | ||
| All muscles | ||
| CSA | −1.63 (−5.76, 2.51) | 2.84 (1.10, 4.58) |
| Density | −4.62 (−7.89, −1.35) | −2.49 (−4.21, −0.63) |
| Paraspinal | ||
| CSA | −1.36 (−5.27, 2.55) | 1.52 (−0.24, 3.27) |
| Density | −4.23 (−7.49, −0.97) | −2.20 (−3.99, −0.41) |
| Posterior abdominal | ||
| CSA | 0.52 (−2.23, 3.27) | 2.24 (0.59, 3.89) |
| Density | −6.76 (−9.72, −3.81) | −2.22 (−4.04, −0.40) |
| Anterior abdominal | ||
| CSA | −0.26 (−4.41, 3.88) | 2.69 (1.01, 4.36) |
| Density | −2.89 (−6.41, 0.64) | −1.57 (−3.33, 0.19) |
| RMS of ML motion | ||
| All muscles | ||
| CSA | 0.25 (−0.41, 0.90) | 0.04 (−0.23, 0.30) |
| Density | −0.38 (−0.90, 0.14) | −0.19 (−0.47, 0.09) |
| Paraspinal | ||
| CSA | 0.15 (−0.44, 0.75) | −0.13 (−0.39, 0.12) |
| Density | −0.42 (−0.92, 0.02) | −0.36 (−0.63, −0.10) |
| Posterior abdominal | ||
| CSA | 0.35 (−0.11, 0.80) | 0.06 (−0.18, 0.31) |
| Density | −0.47 (−0.96, 0.02) | −0.30 (−0.57, −0.02) |
| Anterior abdominal | ||
| CSA | 0.15 (−0.47, 0.77) | 0.21 (−0.04, 0.46) |
| Density | −0.16 (−0.68, 0.37) | −0.01 (−0.27, 0.26) |
| RMS of ML velocity | ||
| All muscles | ||
| CSA | 0.26 (−1.33, 1.85) | 0.67 (0.12, 1.21) |
| Density | −2.14 (−3.40, −0.50) | −0.56 (−1.15, 0.02) |
| Paraspinal | ||
| CSA | −0.17 (−1.65, 1.30) | 0.09 (−0.45, 0.63) |
| Density | −2.00 (−3.23, −0.77) | −0.80 (−1.35, −0.25) |
| Posterior abdominal | ||
| CSA | 0.61 (−0.47, 1.68) | 0.48 (−0.03, 1.00) |
| Density | −2.60 (−3.76, −1.44) | −0.68 (−1.24, −0.11) |
| Anterior abdominal | ||
| CSA | 0.73 (−0.88, 2.33) | 0.92 (0.41, 1.43) |
| Density | −1.35 (−2.71, 0.01) | −0.15 (−0.69, 0.39) |
| SPPB | ||
| All muscles | ||
| CSA | 0.47 (−0.54, 1.48) | 0.14 (−0.33, 0.62) |
| Density | 0.34 (−0.45, 1.14) | 0.75 (0.24, 1.26) |
| Paraspinal | ||
| CSA | 0.31 (−0.60, 1.22) | 0.22 (−0.25, 0.68) |
| Density | 0.48 (−0.27, 1.22) | 0.50 (0.01, 0.98) |
| Posterior abdominal | ||
| CSA | 0.08 (−0.64, 0.80) | −0.22 (−0.65, 0.21) |
| Density | 0.67 (−0.09, 1.44) | 1.03 (0.56, 1.50) |
| Anterior abdominal | ||
| CSA | 0.10 (−0.86, 1.07) | −0.08 (−0.53, 0.37) |
| Density | 0.15 (−0.66, 0.97) | 0.72 (0.24, 1.19) |
Notes: Beta coefficients per SD increase in muscle measure (95% confidence interval) for fully adjusted models. Fully adjusted models include age, weight, Physical Activity Scale for the Elderly, and baseline falls history as covariates. Associations in bold are significant (p < .05). AP = anteroposterior; CSA = cross-sectional area; ML = mediolateral; RMS = root mean square; SPPB = Short Physical Performance Battery.
Larger muscle CSA was associated with increased incidence of falls in men for all muscle groups in adjusted models (Table 3). In women, higher posterior abdominal muscle density was associated with reduced incidence of falls (unadjusted model only, see Supplementary Table 2).
Table 3.
Incidence Rate Ratios (IRRs) per SD Increase in Muscle Measure (95% confidence interval) for Falls During Follow-up in Men and Women, Fully Adjusted Models
| Men | Women | |
|---|---|---|
| All muscles | ||
| CSA | 2.35 (1.24, 4.45) | 1.14 (0.84, 1.55) |
| Density | 1.44 (0.69, 2.97) | 1.05 (0.74, 1.48) |
| Paraspinal | ||
| CSA | 2.04 (1.12, 3.70) | 0.86 (0.63, 1.17) |
| Density | 2.11 (0.91, 4.89) | 1.05 (0.77, 1.44) |
| Posterior abdominal | ||
| CSA | 2.12 (1.13, 3.97) | 1.21 (0.94, 1.56) |
| Density | 0.81 (0.41, 1.60) | 0.83 (0.61, 1.12) |
| Anterior abdominal | ||
| CSA | 2.20 (1.12, 4.34) | 1.23 (0.92, 1.64) |
| Density | 0.87 (0.43, 1.80) | 1.16 (0.84, 1.60) |
Notes: Models adjust for follow-up time and include age, weight, Physical Activity Scale for the Elderly, and baseline falls history as covariates. IRRs in bold are significant (p < .05). CSA = cross-sectional area.
Discussion
The results support the hypothesis that high trunk muscle density is indicative of better balance, but this did not translate into reduced likelihood of falling. However, larger trunk muscle size was indicative of worse balance and increased risk for falls. The finding that larger muscle size indicates more postural sway suggests that muscular strength alone is not sufficient for good balance. Strength is likely important, as strength training programs generally improve measures of balance in older adults (28). However, current evidence does not indicate that strength training alone reduces falls in older adults, although exercise interventions that include multiple types of exercise can reduce falls, as can Tai Chi movement training (29). Balance likely depends on other components of muscular function such as neuromuscular control and fatigue resistance. Muscle fatigue reduces postural stability (30), as does prolonged standing (31), and the 4-minute testing period in this study might have induced fatigue and increased postural sway in some older participants. The finding that lower muscle density was associated with more postural sway suggests that low density may be a marker of declines in these other areas of muscle function.
The most consistent result was a negative association between muscle density and postural sway, particularly sway velocity. For example, 1 SD (about 10–12 HU) higher paraspinal muscle density translated to about 10%–20% lower sway velocity. This is the first study to directly associate muscle density with a quantitative measure like postural sway, providing novel evidence that low trunk muscle density is associated with poor balance in older adults. Similarly, positive associations were found between muscle density and SPPB score in women, consistent with reported associations with the Health ABC Physical Performance Battery, which also incorporates tests that challenge balance (14,15). The lack of such associations in men may be due to limited statistical power and/or smaller effects. However, no consistent association was found between muscle density and fall incidence. Thus, although low density muscle may indicate impaired balance control, it is not a strong predictor of fall risk.
Unexpectedly, the results indicate that larger trunk muscle size is associated with worse balance and more falls. For example, 1 SD larger muscle CSA indicates about 15% larger AP motion in men, and about 19% larger AP velocity in women. Similarly, men (but not women) with 1 SD larger muscle CSA were more than twice as likely to fall during follow-up. These findings are counter to our hypotheses, necessitating considerations of possible explanations. First, we questioned whether larger muscle might be of lower quality, although in post hoc analysis, CSA was positively correlated with muscle density (r = .275, p < .001 for all muscles), which does not support this idea. Second, one might speculate that greater strength without proper neuromuscular control could impair balance. Antagonistic co-contraction is increased in older adults at the ankle, possibly in an attempt to increase stability and counteract declines in postural control, but this co-contraction is associated with increased postural sway (32). Increased age-related co-contraction in the trunk muscles might negatively affect postural sway more in individuals with larger or stronger muscles. Third, muscle CSA may not be representative of muscle strength and function as it relates to balance and fall risk in older adults. The association of trunk strength with muscle CSA in older adults is moderate (eg, r = .61 with total L4-level muscle CSA (17)), leaving a large amount of variance unexplained by muscle size. Furthermore, muscle strength declines more rapidly with age than muscle mass or CSA (10,11), suggesting that other factors besides size are important for strength in older adults. Fourth, greater weight is associated with increased postural sway (33), as well as greater muscle CSA (34), although including weight as a covariate did not change the association of CSA with balance or falls. Fifth, individuals with larger muscles may be more confident or allow greater postural sway, as strength training and agility training both decrease fear of falling in older adults while having little effect on postural stability or fall risk score (35). Finally, individuals with larger muscles may be more physically active, and very active older adults are more likely to fall (36,37), although we adjusted for self-reported physical activity. Overall, the results suggest that larger muscle size in older adults worsens balance and likelihood of falls, a finding that should be confirmed and explained in future research.
This study has several important limitations. The study cohort of osteopenic older adults from independent living communities may limit the applicability of the findings in other populations, and the sample size, particularly in men, limited statistical power. Falls were self-reported, raising the possibility for omissions or reporting errors. Only four commonly reported measures of postural sway were analyzed, although other measures may be calculated from force platform data, and such measures do not fully represent “balance,” which is multifactorial in nature. Similarly, falls have numerous risk factors besides musculoskeletal or postural measures, including cognitive, visual, vestibular, and environmental factors. Finally, CT-based measures of muscle size and density remain primarily of investigative interest and their clinical utility remains uncertain.
The unique aspects of this study include the comparison of trunk muscle size and density with quantitative measures of balance and with incident falls. Higher muscle density, reflecting lower fat content in the muscle, is indicative of less postural sway in older men and women, whereas larger muscle size is indicative of increased postural sway in men and women and of increased likelihood of falling in men. These apparently opposite effects are the most intriguing result, and future research should clarify how muscle size and density are related to muscle strength and function, and how age-related changes might affect these relationships. This could help explain our findings and would have implications for assessing muscle properties when predicting functional outcomes.
Supplementary Material
Please visit the article online at http://gerontologist.oxfordjournals.org/ to view supplementary material.
Funding
This work was supported by the National Institutes of Health (R01AG025489, K99AG042458, R01AR053986, and P30AG028747).
Conflict of Interest
Dr. Kiel has received grant funding from Eli Lilly, received royalty payments from Wolters Kluwer for authoring an UpToDate chapter on falls, and served on scientific advisory boards for Novartis. Dr. Rubin is a founder of Marodyne Medical. All other authors state they have no disclosures.
Supplementary Material
References
- 1. Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75:51–61. doi:10.1016/j.maturitas.2013.02.009 [DOI] [PubMed] [Google Scholar]
- 2. Deandrea S, Lucenteforte E, Bravi F, Foschi R, La Vecchia C, Negri E. Risk factors for falls in community-dwelling older people: a systematic review and meta-analysis. Epidemiology. 2010;21:658–668. doi:10.1097/EDE.0b013e3181e89905 [DOI] [PubMed] [Google Scholar]
- 3. Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing. 2006;35(suppl 2):ii37–ii41. [DOI] [PubMed] [Google Scholar]
- 4. Baloh RW, Corona S, Jacobson KM, Enrietto JA, Bell T. A prospective study of posturography in normal older people. J Am Geriatr Soc. 1998;46:438–443. [DOI] [PubMed] [Google Scholar]
- 5. Du Pasquier RA, Blanc Y, Sinnreich M, Landis T, Burkhard P, Vingerhoets FJ. The effect of aging on postural stability: a cross sectional and longitudinal study. Neurophysiol Clin. 2003;33:213–218. [DOI] [PubMed] [Google Scholar]
- 6. Piirtola M, Era P. Force platform measurements as predictors of falls among older people - a review. Gerontology. 2006;52:1–16. [DOI] [PubMed] [Google Scholar]
- 7. Swanenburg J, de Bruin ED, Uebelhart D, Mulder T. Falls prediction in elderly people: a 1-year prospective study. Gait Posture. 2010;31:317–321. doi:10.1016/j.gaitpost.2009.11.013 [DOI] [PubMed] [Google Scholar]
- 8. Panjabi MM. The stabilizing system of the spine. Part I. Function, dysfunction, adaptation, and enhancement. J Spinal Disord. 1992;5:383–389; discussion 397. [DOI] [PubMed] [Google Scholar]
- 9. Stokes IA, Gardner-Morse MG, Henry SM. Abdominal muscle activation increases lumbar spinal stability: analysis of contributions of different muscle groups. Clin Biomech (Bristol, Avon). 2011;26:797–803. doi:10.1016/j.clinbiomech.2011.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Delmonico MJ, Harris TB, Visser M, et al. ; Health, Aging, and Body. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–1585. doi:10.3945/ajcn.2009.28047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. [DOI] [PubMed] [Google Scholar]
- 12. Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol (1985). 2000;89:104–110. [DOI] [PubMed] [Google Scholar]
- 13. Anderson DE, D’Agostino JM, Bruno AG, Demissie S, Kiel DP, Bouxsein ML. Variations of CT-based trunk muscle attenuation by age, sex, and specific muscle. J Gerontol A Biol Sci Med Sci. 2013;68:317–323. doi:10.1093/gerona/gls168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hicks GE, Simonsick EM, Harris TB, et al. Cross-sectional associations between trunk muscle composition, back pain, and physical function in the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2005;60:882–887. [DOI] [PubMed] [Google Scholar]
- 15. Hicks GE, Simonsick EM, Harris TB, et al. Trunk muscle composition as a predictor of reduced functional capacity in the health, aging and body composition study: the moderating role of back pain. J Gerontol A Biol Sci Med Sci. 2005;60:1420–1424. [DOI] [PubMed] [Google Scholar]
- 16. Katzman W, Cawthon P, Hicks GE, et al. Association of spinal muscle composition and prevalence of hyperkyphosis in healthy community-dwelling older men and women. J Gerontol A Biol Sci Med Sci. 2012;67:191–195. doi:10.1093/gerona/glr160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anderson DE, Bean JF, Holt NE, Keel JC, Bouxsein ML. Computed tomography-based muscle attenuation and electrical impedance myography as indicators of trunk muscle strength independent of muscle size in older adults. Am J Phys Med Rehabil. 2014;93:553–561. doi:10.1097/PHM.0000000000000059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Inacio M, Ryan AS, Bair WN, Prettyman M, Beamer BA, Rogers MW. Gluteal muscle composition differentiates fallers from non-fallers in community dwelling older adults. BMC Geriatr. 2014;14:37. doi:10.1186/1471-2318-14-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schafer AL, Vittinghoff E, Lang TF, et al. ; Health, Aging, and Body Composition (Health ABC) Study. Fat infiltration of muscle, diabetes, and clinical fracture risk in older adults. J Clin Endocrinol Metab. 2010;95:E368–E372. doi:10.1210/jc.2010-0780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lang T, Cauley JA, Tylavsky F, Bauer D, Cummings S, Harris TB; Health ABC Study. Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J Bone Miner Res. 2010;25:513–519. doi:10.1359/jbmr.090807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kiel DP, Hannan MT, Barton BA, et al. Low-magnitude mechanical stimulation to improve bone density in persons of advanced age: a randomized, placebo-controlled trial. J Bone Miner Res. 2015;30:1319–1328. doi:10.1002/jbmr.2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kiel DP, Hannan MT, Barton BA, et al. Insights from the conduct of a device trial in older persons: low magnitude mechanical stimulation for musculoskeletal health. Clin Trials. 2010;7:354–367. doi:10.1177/1740774510371014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Robb RA. The biomedical imaging resource at Mayo Clinic. IEEE Trans Med Imaging. 2001;20:854–867. doi:10.1109/42.952724 [DOI] [PubMed] [Google Scholar]
- 24. Muir JW, Kiel DP, Hannan M, Magaziner J, Rubin CT. Dynamic parameters of balance which correlate to elderly persons with a history of falls. PLoS One. 2013;8:e705–e766. doi:10.1371/journal.pone.0070566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. [DOI] [PubMed] [Google Scholar]
- 26. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 27. Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The physical activity scale for the elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52:643–651. [DOI] [PubMed] [Google Scholar]
- 28. Granacher U, Gollhofer A, Hortobágyi T, Kressig RW, Muehlbauer T. The importance of trunk muscle strength for balance, functional performance, and fall prevention in seniors: a systematic review. Sports Med. 2013;43:627–641. doi:10.1007/s40279-013-0041-1 [DOI] [PubMed] [Google Scholar]
- 29. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;9:CD007146. doi:10.1002/14651858.CD007146.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Helbostad JL, Sturnieks DL, Menant J, Delbaere K, Lord SR, Pijnappels M. Consequences of lower extremity and trunk muscle fatigue on balance and functional tasks in older people: a systematic literature review. BMC Geriatr. 2010;10:56. doi:10.1186/1471-2318-10-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Freitas SM, Wieczorek SA, Marchetti PH, Duarte M. Age-related changes in human postural control of prolonged standing. Gait Posture. 2005;22:322–330. [DOI] [PubMed] [Google Scholar]
- 32. Nagai K, Yamada M, Uemura K, Yamada Y, Ichihashi N, Tsuboyama T. Differences in muscle coactivation during postural control between healthy older and young adults. Arch Gerontol Geriatr. 2011;53:338–343. doi:10.1016/j.archger.2011.01.003 [DOI] [PubMed] [Google Scholar]
- 33. Hue O, Simoneau M, Marcotte J, et al. Body weight is a strong predictor of postural stability. Gait Posture. 2007;26:32–38. doi:10.1016/j.gaitpost.2006.07.005 [DOI] [PubMed] [Google Scholar]
- 34. Anderson DE, D’Agostino JM, Bruno AG, Manoharan RK, Bouxsein ML. Regressions for estimating muscle parameters in the thoracic and lumbar trunk for use in musculoskeletal modeling. J Biomech. 2012;45:66–75. doi:10.1016/j.jbiomech.2011.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu-Ambrose T, Khan KM, Eng JJ, Lord SR, McKay HA. Balance confidence improves with resistance or agility training. Increase is not correlated with objective changes in fall risk and physical abilities. Gerontology. 2004;50:373–382. doi:10.1159/000080175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chan BK, Marshall LM, Winters KM, Faulkner KA, Schwartz AV, Orwoll ES. Incident fall risk and physical activity and physical performance among older men: the Osteoporotic Fractures in Men Study. Am J Epidemiol. 2007;165:696–703. doi:10.1093/aje/kwk050 [DOI] [PubMed] [Google Scholar]
- 37. Peeters GM, Verweij LM, van Schoor NM, et al. Which types of activities are associated with risk of recurrent falling in older persons? J Gerontol A Biol Sci Med Sci. 2010;65:743–750. doi:10.1093/gerona/glq013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.