Abstract
When considering which components of the cell are the most critical to function and physiology, we naturally focus on the nucleus, the mitochondria that regulate energy and apoptotic signaling, or other organelles such as the endoplasmic reticulum, Golgi, ribosomes, etc. Few people will suggest that the membrane is the most critical element of a cell in terms of function and physiology. Those that consider the membrane critical will point to its obvious barrier function regulated by the lipid bilayer and numerous ion channels that regulate homeostatic gradients. What becomes evident upon closer inspection is that not all membranes are created equal and that there are lipid-rich microdomains that serve as platforms of signaling and a means of communication with the intracellular environment. In this review, we explore the evolution of membranes, focus on lipid-rich microdomains, and advance the novel concept that membranes serve as “capacitors for energy and metabolism.” Within this framework, the membrane then is the primary and critical regulator of stress and disease adaptation of the cell.
Keywords: membrane, cholesterol, metabolism, rafts, caveolae, energy
what is life? The answer can be literal, basic, and simple—the name of a breakfast cereal or a book written by Erwin Schrödinger—or more complex and abstract, invoking the philosophical and spiritual. The dictionary (thefreedictionary.com) definition states, “the property or quality that distinguishes living organisms from dead organisms and inanimate matter, manifested in functions such as metabolism, growth, reproduction, and response to stimuli or adaptation to the environment originating from within the organism.” In the most simplified and primary sense, life is manifest in a single cell and in a teleological view is something dependent on the plasma membrane. Since its observation in the seventeenth century with microscopy, the plasma membrane has been the focus of numerous investigations, and many advances in our understanding of the plasma membrane have been achieved thus far (24a). The plasma membrane is known to be a major coordinator between extracellular signals and intracellular responses, and it also plays a crucial role to link intracellular processes to cell-cell interactions and tissue organization (131). Therefore, the plasma membrane is involved in vast aspects of cellular function: from intracellular endosomes and extracellular vesicles to receptor signaling, presentation of surface proteins, and protein secretion (4).
The lipid bilayer plays an important role in compartmentalization and organization of the intracellular environment and various organelles necessary for homeostatic and specialized functions. The plasma membrane is a semipermeable barrier that provides protection from the extracellular environment and localizes membrane receptors and ion channels involved in signal transduction. Particular examples of membrane microdomains such as lipid rafts and caveolae have been studied in the context of localization of these signaling receptors and ion channels. However, very few researchers associate the plasma membrane, and its varied lipid-ordered domains, as a specialized cellular organelle important in regulating cellular metabolism and extracellular environment to cell/organelle communication.
Here we review 1) the evolution of membranes, considering lipid diversity, 2) the implication of membrane mechanics and elasticity for cell function, 3) the implication of cholesterol and other bioactive lipids as facilitators of metabolism by regulation of oxygen or providing direct raw materials to regulate cell function, and 4) the communication of the plasma membrane with intracellular components. Ultimately we link these observations to the role membranes play as capacitors for regulating energy, metabolism, and communication.
Evolution of Membranes
Lipids are amphipathic molecules—they have a hydrophilic head group and a hydrophobic tail group. In an aqueous environment, they spontaneously self-assemble such that the tails are protected from water. Lipids reorder the hydrogen bonds of water molecules forming a cagelike structure around the lipid, decreasing the entropy of water (22). This decrease in entropy is directly proportional to the surface area of the nonpolar regions of the lipids. The nonpolar regions of lipids in turn come together through hydrophobic interactions (22). These interactions become stronger while reaching thermodynamic stability by reducing the number of water molecules surrounding the hydrophobic tail region of the lipids (Fig. 1).
Fig. 1.

Self-assembly of biological membranes. Lipids are amphiphilic molecules. In aqueous environments, they self-assemble to form micelles or bilayers such that the hydrophilic head groups interface with water while the hydrophobic tails are protected. Left: free lipids in water have their hydrophobic tails exposed. The hydrogen bonds within water are broken or rearranged to account for the new hydrophobic entities. Center: the lipids can arrange themselves into a micelle, so that all the hydrophobic groups are protected from exposure to water. Right: the fatty acid chains in 2-tailed lipids are often too long to fit in micelles without exposure of the hydrophobic groups to water. Therefore, lipids often form bilayers and water forms a hydrogen bond cage along the surface of the head groups.
Modern cell membranes are made of glycerol phospholipids, although it is believed that early membranes self-assembled from monocarboxylate- and alcohol-derived one-chained amphiphilic molecules (79, 85, 161). Bacteria and eukaryotes have similar biochemistry and have ester-linked fatty acid phospholipids based on glycerol-3-phosphate (G3P) (49, 73, 79, 85). The discovery of glycerol-1-phosphate (G1P) ether linked to isoprenoid chains in Archaea and bacteria represents a major breakthrough in membrane evolution (49, 81, 117). The glycerol moieties for all the phospholipids in Archaea and bacteria are of opposite chiralities (80). The hydrophobic tail is fatty acid based in bacteria, while in archae it is isoprenoid based (49, 61, 85). This highlights the difference in chemical composition and biogenesis pathways (i.e., unique enzymes for lipid synthesis) in the archaeal and bacterial/eukaryotic membranes. This variation in lipids and their formation has given rise to the concept of the existence of a “last universal common ancestor” (LUCA), which was likely self-replicating genetic elements in hydrothermal vents living in bubbles made up of inorganic components and not bound by typical lipid membranes (61, 85, 161). The idea that early life did not have membrane structures was supported by studies suggesting that phospholipid biosynthesis appeared much later in evolution, independent of the lineage that gave rise to archae and bacteria (78, 80). The conundrum with this theory was evidence that the LUCA contained at least 100 genes (81). To provide for this level of complexity, compartmentalization likely had to exist early on to facilitate concentration rather than diffusion of components to allow for a critical mass to be achieved, initiating biochemical processes. This paradox was resolved by Martin and Russell, who suggested that the LUCA had minerals instead of phospholipids that served the role of providing a defined barrier to concentrate cell components in order for life to initiate and evolve (95).
The transition from inorganic to organic components defining the plasma membrane has also been controversial. At the heart of this controversy is the origin of life—a topic no less controversial or resolved. The “first life form” needed to balance existence, stability, growth, replication, and adaptation, and central to these pressures was a functional membrane. According to the early membrane hypothesis, cellularization occurred with simple lipids that were synthesized by inorganic transition metals or with enzymes that were nonstereospecific, eventually giving rise to precells (18, 71, 145), whereas some suggest that precells had ancient enzymes able to synthesize both G1P and G3P (155). The exchange of electrically charged compounds is shrouded in mystery, as ions penetrate hydrophobic membrane lipid through membrane proteins in the form of ion channels and translocases (Fig. 2). The chicken-and-egg question to resolve this problem is, “What came first, membranes or membrane proteins?” Two solutions emerged. One suggests that early cells likely had less restricted transport of materials, as resources for growth and adaptation were externally derived, suggesting that cells were heterotrophic. Support for such a concept was provided by studies showing that using simple fatty acids and their derivatives to model early protocells resulted in the generation of bilayer vesicles capable of internalizing nucleotides that could be used for replication reactions, suggesting that early, simple cells could possibly have acquired components for growth and replication from the external environment independent of specific transport machinery (31, 92). Such a model would allow cells and membranes to evolve and adapt as external pressures amassed components over a significant period of time to derive specific biochemical reactions, leading to the complexity we observe in modern cells to allow for specialization of function. The second solution suggested that the lipid bilayer evolved at the same time as membrane proteins along with the membrane bioenergetics (16, 59, 98). Given either of these paths, it is clear that the modern cell has evolved a highly functional lipid bilayer that contains a variety of components capable of facilitating growth and adaptation.
Fig. 2.
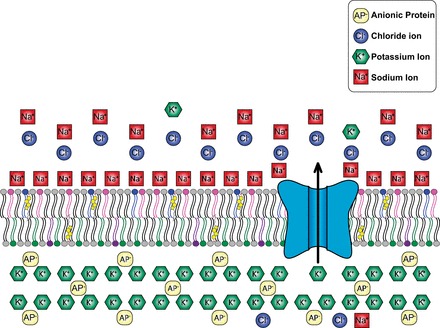
Ionic environment around the membrane. Membranes play an important role not only in compartmentalization of cellular compartments but also in maintaining an electrostatic potential across the membrane. In modern biological membranes, ion channels play an important role in regulating the homeostatic gradients of ions across the membrane and maintaining the membrane voltage potential. Figure illustrates the large variety of ions present in the cellular milieu.
Lipid Diversity, Lipid-Sterol Asymmetry, and Lipochaperone Function
As the cell membrane evolved, there was a concerted refinement of the lipid composition but also an increase in complexity driven by not only the asymmetric distribution of these lipids but also the incorporation of membrane-localized proteins that allowed for more complex cellular functions. The plasma membranes of modern animal cells contain four major phospholipids [i.e., phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), and sphingomyelin (SM)] (24a) (Fig. 3). The outer leaflet of the plasma membrane consists mainly of PC and SM, whereas PE and PS are the predominant phospholipids of the inner leaflet (24a). A fifth phospholipid, phosphatidylinositol, is also localized to the inner leaflet (24a). In addition to the phospholipids, the plasma membranes contain glycolipids and cholesterol (24a). How plasma membranes are compartmentalized and organized and how lipids and proteins contribute to this is currently being intensively investigated. There is overwhelming evidence that the plasma membrane is not a homogeneous or random structure (88) but rather highly asymmetric, with different proteins and lipid compositions in the two leaflets organized to facilitate specific cellular functions (28, 133).
Fig. 3.
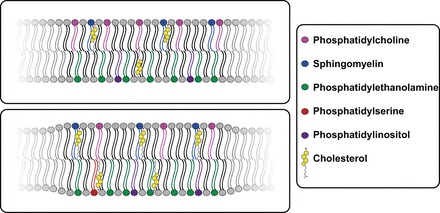
Diversity of membrane lipids. Modern biological membranes are made of different types of lipids including phospholipids, sphingomyelin, and cholesterol. Changes in local concentration of these lipids can lead to microdomains in the membrane and potentially lipid rafts. This complexity and diversity is a large evolutionary advance over early membranes that were thought to self-assemble from monocarboxylate- and alcohol-derived 1-chained amphiphilic molecules.
Lipid rafts are specific domains of the plasma membrane rich in cholesterol and sphingolipids (140, 152). Lipid rafts are liquid ordered as they are enriched in cholesterol, whereas the remainder of the bilayer is in a liquid-disordered phase (14). Furthermore, these cholesterol-enriched domains are more rigid than the surrounding membrane and are detergent resistant (120), and they form distinct sites in the membrane for organizing signaling proteins that drive cellular processes (111). Caveolae are a subset of lipid rafts that form distinct flasklike, invaginated subcellular structures on the plasma membrane resistant to detergent solubilization (103, 123, 141). Caveolae are enriched in cholesterol, glycosphingolipids, SM, and caveolin protein in addition to other signaling proteins (15, 82, 90, 121, 132, 153, 154). In many cell types, caveolae can be arranged singly or in chains or grapelike clusters (103, 104, 108, 109). Caveolae may function in protein trafficking, signal transduction, and endocytosis (115, 116). Although studies have suggested indirect involvement in metabolism through enrichment of signaling molecules that regulate processes such as biochemical and redox signaling (110, 111), assessment of their lipid and protein compositions suggests a potential for direct contribution to cellular energy and metabolism.
Differences exist in the two leaflets of the plasma membrane, leading to lipid asymmetry. Synaptic plasma membranes (SPMs) have been studied in much detail to shed light on this aspect. Like most plasma membranes, the inner leaflet (i.e., cytofacial leaflet) is rich in PE and PS; however, the outer leaflet (i.e., exofacial leaflet) is rich in SM and PC (34, 75). In mouse SPM, cholesterol asymmetry is observed, with more cholesterol on the inner leaflet than on the outer leaflet (63–66). This distribution can change with stress. Mice fed ethanol have a twofold increase in outer leaflet cholesterol (although the total cholesterol from the both leaflets remains the same) (1, 138, 162), and aging also leads to increased outer leaflet cholesterol compared with young mice (64). Previous evidence suggests that depletion of SM by sphingomyelinase altered the distribution of cholesterol and increased the pool of intracellular cholesterol (125) and also made the cholesterol more susceptible to oxidation (142). Cholesterol has highest affinity for SM and less for phospholipids such as PC and PS. It has been shown that affinity for phospholipids such as PC and PS is curvature dependent (163). P-glycoprotein (P-gp), which belongs to the family of multiple drug resistance ABC transporters, has been shown to stimulate cholesterol distribution from the inner to the outer leaflet of the plasma membrane (48, 67). Whether such asymmetry in cholesterol affects lipid raft formation is unclear, but studies suggest alteration in membrane lipid rafts and caveolae with age leading to neurological and cardiac dysfunction (45, 55, 114). Recent evidence has indicated the ability of caveolin expression to rescue neurocognitive decline with age (91). Such findings suggest the critical role of ordered membrane domains in regulating cell physiology.
Although folding of proteins is largely directed by their amino acid sequence, the surrounding environment, especially lipids, is important in protein folding, misfolding, and unfolding (6, 137). This is true for soluble, peripheral, and integral membrane proteins. Studies in mutant Escherichia coli have shown that absence of PE caused misfolding of the membrane protein lactose permease although insertion into the membrane was unaffected (7, 8). For heme, even substituting PE with PS, phosphatidylglycerol, or cardiolipin did not lead to refolding into the correct conformation (32), suggesting a highly ordered and specific role for lipids in protein folding. In recent experiments, it was shown that plant-derived ricin toxin A subunit, which belongs to the A-B family of toxins, unfolded in the presence of anionic lipids (128). Ricin A chain has a very stable secondary structure even after detaching from its B subunit. In the presence of anionic lipids present in specialized cellular membrane such as the endoplasmic reticulum (ER), an unfolding of the toxin was induced, resulting in ER exit to the cytosol where the toxin can refold to induce toxicity. In contrast to ricin toxin, cholera toxin, which belongs to the A-B5 family of toxins derived from bacteria Vibrio cholerae, spontaneously unfolds after dissociating from B subunit and is extruded out of the ER for degradation. Because of lysine over arginine bias, it escapes ubiquitination and proteasomal degradation. In the cytosol, the A subunit refolds and interacts with ADP ribosylation factor-6 and ADP ribosylates GTP-bound Gsα, locking it in the active state (3, 69, 147). Recently, it has been shown that the inner leaflet of plasma membrane may facilitate refolding of the unfolded cholera toxin A subunit, likely in a lipid raft-specific mechanism (127).
Mechanics of Biological Membranes and How Their Elasticity Affects Function and Interaction with Proteins
Unique lipids within membranes create diversity at multiple levels, one such being unique mechanical properties that have important implications for function. In an aqueous medium, lipids aggregate into quasi-two-dimensional bilayer sheets and adopt a configuration that minimizes the exposure of their hydrophobic parts. In the plane of the membrane the membrane resembles a nearly incompressible viscous fluid, while in bending it behaves somewhat like an elastic solid. In-plane lipid flow has been observed in experimental systems such as tether formation and micropipette aspiration of membranes (40). In cellular processes, the plasma membrane is thought to have a membrane “reservoir” that can act as a source of lipids (126). This reservoir allows for lipid flow during dynamic events such as spreading, motility, and endocytosis (126) and may be critical to stress adaptation and metabolism.
Continuum models of bilayer membranes have been used to study the deformation of membranes and to explain many biological phenomena. The most popular model of lipid bilayers is the Helfrich model (56), in which the energy per unit area of the membrane depends only on the mean and Gaussian curvatures of the membrane. This model has been widely used to study the shape of red blood cells (37, 68) and to explain biological phenomena such as the formation of membrane tubes (35) and the shapes of lipid vesicles (70, 101, 139).
The basis for this modeling effort is the same principle that is the basis for the definition of equilibrium: equilibrium is attained when the free energy of the system is minimum. In this case, the membrane is assumed to be at mechanical equilibrium and the modeling effort is based on the principle of virtual work. This principle states that when the system energy is minimized, the resulting state is the same as one of mechanical equilibrium. By using the principle of virtual work, we are assuming that mechanical equilibrium occurs more rapidly than the other biochemical processes (this is a reasonable assumption in the cellular environment). It is important to recognize here that mechanical equilibrium does not mean thermodynamic or chemical equilibrium.
One of the fascinating observations about lipid membranes is that they can be described with a Hamiltonian function, which corresponds to the total energy of the system, using just the geometry of the membrane through curvatures. Ignoring fluctuations of the membrane, the elastic energy per unit area, W, is given by the Helfrich Hamiltonian (56),
Here, H is the mean curvature, given by (κ1 + κ2)/2 (see Fig. 4 for how the curvatures κ1 and κ2 are defined), and K is the Gaussian curvature, given by κ1κ2. C is the spontaneous curvature of the membrane—it results from a break in symmetry in individual monolayers (“up-down”)—such as due to differential partitioning of membrane molecules with noncylindrical shapes. kB is the bending modulus, and k̄ is the Gaussian modulus. The first term in the above equation explicitly accounts for the local elastic energy penalty due to any deviation of the local mean curvature of the membrane, H, from the preferred, so-called spontaneous curvature, C. The second term penalizes the existence of a Gaussian curvature. Thus the elastic energy of the membrane can be written solely in terms of its geometric properties. This is a remarkable aspect of lipid membranes because it indicates that for length scales only a few times bigger than the thickness of the membrane (the membrane is roughly 5 nm thick, and this theory applies above the thermal wavelength of the membranes, which is roughly 20 nm), the behavior of the membrane can be described solely in terms of its geometric properties. This feature has been proven experimentally and theoretically many times but was first recognized in independent works by Helfrich (56), Canham (17), and Evans (39).
Fig. 4.
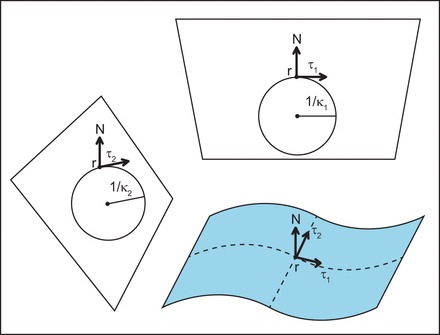
Continuum modeling of the membrane. Theory can help us understand how membranes behave at the continuum level. The membrane can be represented as a 2-dimensional surface; this representation allows us to think about the mechanical properties of the membrane. The 2 principal curvatures κ1 and κ2 are calculated with methods of differential geometry, and represent the local change in the shape of the surface. The 2 principal curvatures of the membrane have been used to describe the curvature-dependent elastic energy of the membrane. In mechanical equilibrium, all forces acting on the membrane must be balanced, and therefore the energy of the membrane must be minimized. This approach relates the energetics of the membrane to the shape of the membrane at mechanical equilibrium.
Then, the total energy of the membrane is just the integral of W over the membrane area, A. Since the membrane has a high stretch modulus (it is easier to bend the membrane than to stretch it), it is often treated as incompressible. To represent this incompressibility, a Lagrange multiplier, λ, often interpreted as membrane tension, is added to augment the Hamiltonian. The total energy is written as
The minimum energy state associated with mechanical equilibrium of the membrane results from minimizing the total membrane energy E. The mathematics behind this operation is quite complex; the interested reader is referred to References 36 and 118. However, the result is a partial differential equation, called the “shape equation,” that gives the relationship between membrane shape through H and K and externally applied forces and/or protein-induced spontaneous curvature (35, 89).
The elegance of this approach lies in the ability to predict the shape of the membrane (cellular and vesicular) under different conditions. Perhaps the most famous example of the use of the Helfrich Hamiltonian was to describe how the biconcave shape of the red blood cell could be interpreted as one of the solutions to the shape equation (17, 56, 139). While many cellular features such as the cytoskeleton and membrane protein composition were ignored in this model, it is nonetheless fascinating that the shape of a red blood cell could be described with an equation for mechanical equilibrium by minimizing the associated elastic energy. This was the advent of mechanobiology at the cellular level and ushered in the use of energy to describe not just metabolism but also mechanical forces and membrane shapes (further discussed below).
Another seminal work that highlights the application of this model is the formation of membrane tubes (35). Tubes and membrane tethers are oft-observed features in cells. By modeling the force acting on the membrane as a point load (an idealization) and the resulting tether as a cylinder, Derenyi and coworkers were able to describe the relationship between the radius of the tube, membrane tension, and the bending modulus. Furthermore, they were able to predict the force-length relationship for membrane tethers and the force required to form the cylindrical tube. These examples highlight how energy considerations can give insight into the response of lipid membranes from a mechanical standpoint.
Recently, there have been further developments in the field of membrane mechanics. The elastic models used thus far to study lipid membranes focus mainly on length scales that are much larger than the thickness of the bilayer. The Helfrich model assumes that the lipids are aligned normal to the membrane surface at all times and that curvatures are of the order of the bilayer thermal wavelength (∼20 nm). This approach captures the changes in membrane shape that occur at large length scales larger than the thickness of the bilayer (∼5 nm). There are, however, circumstances under which lipids are not aligned normal with the surface. The lipids are then said to tilt relative to the membrane surface; this in turn induces a change in membrane thickness, which is simply the projection of the lipid length onto the surface normal. Lipid molecules are chiral: they possess a directionality and generally need not be aligned normal to the membrane surface. For example, the tilt angle of gel-phase dipalmitoylphosphatidylcholine (DPPC) was found to be ∼32° (150). Even in the liquid phase, lipids can tilt in regions adjacent to protein inclusions (158). Membrane fission and fusion are critical cellular processes that take place at lengths comparable to the membrane thickness (∼5 nm). Lipids may also exhibit tilt in the neighborhood of an inserted protein and manifest associated defect structures. We have recently developed a continuum framework to study this process (124). Experimentally observed ripple phases are characterized by oscillatory thickness variations induced by spatially nonuniform tilt (86). Membrane fusion has been studied with dissipative particle dynamics (DPD) approaches (51, 52). These studies have shown that when two fusing membranes are in close proximity the lipids tilt, splay, and flip from one monolayer to another (51, 52, 93). These models are beginning to shed light on how the energetics of the membrane at different levels can be harnessed into mechanical forces and the role they play in governing membrane morphology and topology.
The evolutionary diversity that has emerged in the plasma membrane is quite astounding. The early role of the membrane was to serve as a passive barrier to concentrate chemical components in a cell to provide for a critical mass to initiate life. Modern membranes have evolved to the point of highly organized shape and structure facilitated by ordered and disordered lipids, encrusted with a complement of proteins that allow for further complexity of function. What is curious is how the pressure to evolve emerged.
Cholesterol and Oxygen: Coevolution of Membranes and Metabolism
Once the existence and replication of unicellular organisms was established, the evolutionary pressure was to build organisms of increasing complexity that ultimately led to the generation of eukaryotic cells capable of multicellular organization, function, and growth and enhanced survival while facing mounting environmental stressors. Biochemical processes that utilize biological fuel to synthesize energy were necessary to facilitate organization, function, growth, and survival. The first of many natural resources that cells needed to adapt for use was oxygen. The early atmosphere lacked oxygen, but a sharp rise was seen around 3 billion years ago with cyanobacteria generating oxygen via photosynthesis (113). It is curious that major events in maximum organismal size and “growth spurts” in evolution are marked by spikes in atmospheric oxygen (112, 113). Interestingly, atmospheric oxygen levels were the highest (somewhere near 30%) when dinosaurs roamed the Earth, with a sharp decline around the same time as mass extinction events, and then stabilized to modern-day levels over the past 100–200 million years (113).
The oxygen paradox highlights the mystery that oxygen is so critical a fuel for the metabolic machinery of life yet so toxic to all forms of life inhabiting this planet (130). The key to this paradox are the two simple, yet biologically fundamental, redox reactions: the first reaction, which is essentially the reverse of photosynthesis in which mitochondria utilize oxygen to enzymatically generate a proton gradient fueling oxidative phosphorylation, and the second reaction, where oxygen in excess is consumed to generate reactive species that induce cellular damage (84, 160). To maximize the first reaction and minimize the second, oxygen levels needed to remain under tight control (105). It has been suggested that the evolutionary response to this paradox was the creation of cholesterol within membranes to tame oxygen for biological use, a primary feature that linked membranes and metabolism (13, 74, 97).
Cholesterol is a critical component of the plasma membrane. It is necessary for the generation of lipid rafts, which provide a liquid-ordered domain in membranes that create platforms for signaling (120). There is debate regarding the coevolution of cholesterol with oxygen, where some suggest that cholesterol came first and others suggest that oxygen had to be present initially (13). The latter theory is more largely accepted, as numerous steps in the synthesis of cholesterol require molecular oxygen (97), suggesting that it may have served as an adaptation in membranes to accommodate for rising levels of atmospheric oxygen to allow organisms to survive on land. The link between oxygen, cholesterol, and the drive to eukaryotic evolution is further supported by the fact that cholesterol is abundant in eukaryotic membranes but almost completely absent in prokaryotes and in intracellular organelles thought to have derived from symbiotic relationships (i.e., mitochondria) (97, 135). In addition, plants that generate oxygen through photosynthesis have membrane compositions in which sterols represent a third to half of total lipids (47). As a component of the membrane that allowed for adaptation, cholesterol serves a number of functions for eukaryotes. Given the oxygen paradox described above, the initial function of cholesterol was likely to act as a barrier to limit the exposure of the intracellular environment to oxygen and its radicals that could contribute to injury of cellular components, and such a concept is supported by studies in CHO cells showing variances in membrane oxygen gradients relative to cholesterol content (74). Other examples include the mammalian lens of the eye, which is a highly structured compartment made up of fiber cells that have significantly enriched levels of cholesterol. In oxygen permeability studies, it has been shown that at near-physiological temperatures lipid extracts from calf lenses with high cholesterol content had the most limited oxygen diffusion relative to other artificial mixtures with lower cholesterol content (159). In such scenarios, the barrier function, to bind up and limit oxygen exposure of the internal cellular environment, is an important feature of cholesterol. The effect was to limit cellular oxygen concentration to curb the toxic generation of free radicals to protect from injury.
An intriguing alternative thesis our group and others have proposed advances the concept of the membrane and cholesterol within it acting as a capacitor for energy and metabolism by acting as a storage molecule for cellular substrates that can be utilized under stress conditions. One of the richest sources of organ-level caveolae and cholesterol is the lung, an organ primarily involved in oxygen uptake and exchange, where in response to hypoxia there is an upregulation of membrane cholesterol (10). At the cellular level, the highest density of membrane caveolae is in endothelial cells, cells that line luminal surfaces of vessels (96) that are critical for delivering oxygen. Early evidence in myocardial ischemia-reperfusion injury showed that rampant ultrastructural injury to the plasma membrane (100) was a likely trigger and interventions such as preconditioning in which sublethal stresses protect from subsequent lethal injury resulted from preservation of the membrane ultrastructure (99, 100). Our group has shown that preconditioning results in increased membrane order, cholesterol content, and formation of caveolar microdomains (151). In fact, mice genetically engineered to overexpress caveolin in the cardiac myocyte show increased adaptation to stress including ischemia-reperfusion injury and pressure overload, two processes that have clearly been linked to a mismatch in oxygen availability and proper utilization (60, 151). Studies also show that mice lacking caveolin are more sensitive to cardiovascular stress and disease (110, 111). Recent evidence from our group suggests that caveolae, which are heavily enriched in cholesterol, may form nanocontacts with mitochondria (discussed below) close to the plasma membrane to more efficiently manage metabolism and energy especially in stress adaptation, and this general principle may be conserved evolutionarily and broadly applicable to cancer, neurodegeneration, and many other diseases that are metabolically dependent (44). Studies have shown that in response to stress cholesterol content of the mitochondrion (a compartment that typically has no cholesterol) rises, leading to mitochondrial dysfunction (2, 107, 134), and this is further confirmed in caveolin-knockout animals (9). Therefore, it appears that if injury damages membrane structures or membranes have limited caveolin content leading to an inability to concentrate cholesterol to temper and regulate metabolism, the end result is uncontrolled delivery and accumulation of cholesterol and presumably oxygen to mitochondria, leading to cellular injury. Hughes et al. have shown in yeast that sterols can act as oxygen sensors, an observation also confirmed in mammalian cells (33, 62), suggesting that membrane cholesterol content may be critical not only to limiting the amount of oxygen delivered to the cell but, more importantly, to how a cell physiologically responds and adapts to perturbations in oxygen availability for metabolic processes.
With regard to the earlier discussion of membrane mechanics and elasticity, how does energy minimization relate to oxygen transport and cholesterol in red blood cells? First, the membrane bending modulus is a function of membrane composition, and an increase in membrane cholesterol is known to increase the membrane bending modulus (25). This means that it is going to cost more energy to deform a membrane with higher cholesterol content. An effective readout of the membrane properties is that the shape of the red blood cell and different values of bending modulus (depending on the membrane composition and cholesterol content) are indicative of different shapes. A mathematical modeling study related the shape of red blood cells to oxygen transport and showed that shape changes can explain the loss in oxygen flux across the membrane (156). Thus relatively simple mechanical models can provide insight into the relationship between cell shape and transport properties.
Bioactive Lipids
In applying the idea of a capacitor to membrane lipids themselves, a more recent concept has emerged where lipids have been shown to be biologically active. That is, they can be stored in the membrane as dormant, inert structural components that have the potential to be released and activated upon a specific cellular cue. We will not discuss in detail the emerging field of bioactive lipids; however, the reader is encouraged to refer to excellent topical reviews in this area (see Refs. 11, 29, 53, 54, 106, although many more examples exist in the literature). We will highlight some background in this area and point to selected examples that link to energy and metabolism.
The first description of “bioactive lipids” can be drawn from the classic description of the cleavage of phosphatidylinositol 4,5-bisphosphate (PIP2), a phospholipid localized to the plasma membrane, by phospholipase A to form diacylglycerol and inositol 1,4,5-trisphosphate, which act as second messengers to activate protein kinase C (102). Many more lipid-derived signaling molecules including arachidonic acid (which can give rise to a multitude of additional lipid mediators through specific processing by enzymes such as cyclooxygenase, lipoxygenase, and cytochrome P-450), ceramide, sphingosine-1-phosphate, and other lysophospholipids have been identified (11, 53, 54). There is some evidence suggesting that lipid rafts may contain and enrich biologically active lipids and precursors such as arachidonic acid (120, 122) providing a conduit to modulate cell metabolism. These activated metabolites then regulate cellular function under various physiological and pathophysiological settings. It is important to note that oxygen is a key cofactor in many enzymes used to further functionalize arachidonic acid and lipid peroxidation has also been shown to activate many lipids including cholesterol (29).
Membrane Communication with Intracellular Components
There is direct correlation between membrane complexity and variety of lipids as is evident by comparison of the prokaryotic and eukaryotic membranes. The diversity and complexity of eukaryotes are such that, whereas hundreds of lipid species can be attributed to prokaryotes, thousands are associated with the eukaryotes. This enhanced diversity of lipids entails higher membrane complexity and function in the eukaryotes. The rise of double membrane-bound intracellular organelles is one such example. Each organelle such as the ER, endosomes, the Golgi, etc., is rich in one or more types of unique lipids. The lipid variety dictates association of particular types of proteins and compartmentalizing enzymes and metabolism. This also provides a unique identity to each of the organelles and prevents them from coalescing into one another. The lipid diversity guarantees a much more stable, robust membrane that can withstand changes in the surrounding pH, temperature, and osmolarity (148). The remainder of this review focuses on the role of membrane lipid domain communication of plasma membrane with intracellular components; many of these features are critical for energy and metabolism.
Evolution of endomembranes and membrane-endoplasmic and membrane-mitochondrial interactions.
It has been widely believed that the endomembrane originated from de novo vesicle formation. The biggest proponents of this theory, Matin and Muller, proposed that eukaryotes evolved because of the symbiosis of archaebacterium and α-proteobacterium that are considered ancestors of mitochondria (94). This symbiosis was largely driven by hydrogen exchange. According to this hypothesis, it was assumed that the evolution of endomembranes took place inside the cytoplasm of archaebacteria after symbiosis with α-proteobacterium that had the genes for fatty acid ester lipid biosynthesis after assimilation into the archae genome. Since α-proteobacterium is a eubacterium, it slowly replaced the archae ether-linked lipids with ester-linked lipids. Alternative theories suggest that the endomembranes evolved from the plasma membrane by folding inward, which could have been in the form of invagination, tabulation, or vesiculation (5, 20, 21, 30, 38, 72). In either condition, the plasma membrane subsequently had to be involved in regulating the intracellular environment. Independent of signaling cascades utilizing second messengers and regulator proteins, this was achieved by physical association and communication between the plasma membrane and intracellular compartments.
Plasma membrane and endoplasmic reticulum connection.
Plasma membranes have been identified to interact at ubiquitous sites with ER in almost all eukaryotes (19). These contact sites are believed to have multiple functions (87, 143, 149), including an evolutionarily conserved role in the regulation of lipid composition and metabolism at the plasma membrane (143, 149). This connection involves mainly calcium signaling processes (57) regulating fundamental biological processes such as memory, vision, fertilization, muscle contraction, proliferation, cell migration, immune response, and transcription. A classical well-known contact site is the spatial arrangement between the ER and the plasma membrane known as transverse tubules (136). To trigger muscle contraction, the massive influx of Ca2+ into the cytosol activates myosin movement along actin filaments in the sarcomeres. This Ca2+ influx is achieved by the opening of voltage-gated plasma membrane Ca2+ channels, such as the dihydropyridine receptor, and the synchronized opening of the main sarcoplasmic reticulum Ca2+ channel, the ryanodine receptor (12, 57). A physical coupling at the contact sites between the plasma membrane and the sarcoplasmic reticulum ensures this synchronized coordination. ER has one of the most elaborate network of membranes inside the cells. This network is important for regulating cross talk between the ER and other organelles and the plasma membrane. The ER-plasma membrane junction, apart from undergoing mutual exchange of lipids and ions, can also regulate signaling, cellular architecture, plasma membrane domain organization, and ER shape. This is predominantly found in yeast but has also been described in immune cells, insect photoreceptors, plant cells, and neurons (58). At the contact site, ER adjacent to the plasma membrane has been shown to be devoid of ribosomes (41, 43). The distance between ER and plasma membrane has been measured as ∼30 nm in yeast, but in mammalian cells it has been shown to be as close as 10 nm (119). This denotes that the spacing between the two organelles is regulated.
Plasma membrane and mitochondrial connection.
The plasma membrane has been reported to be in close contact with mitochondria in several mammalian cell types. For instance, in HeLa cells, ∼10% of the plasma membrane is covered with mitochondria (46). It is not clear whether plasma membrane directly comes in contact with mitochondria, and this area is still under intensive investigation. Mitochondria appear to be connected indirectly to the plasma membrane in rat leukemia cells and cardiomyocytes through an ER stack, a connection important for calcium signaling (26). A recent proteomic study suggests that the plasma membrane connexin Cx32, a structural subunit of gap junctions, interacts with mitochondrial proteins in murine hepatocytes (42). However, in an interesting recent finding, the first molecular and functional characterization of a mitochondrion-plasma membrane contact site was reported in yeast (77, 83). Electron tomography revealed direct contacts of mitochondria and invaginations of the plasma membrane without participation of the ER (77). Stomatin-like protein 2 (SLP-2), which is expressed predominantly in mitochondria, belongs to the stomatin-prohibitin-flotillin-HflC/K superfamily (23, 27, 76, 146). These proteins are involved in organizing the cardiolipin-rich microdomains and regulate mitochondrial function as well as biogenesis. Studies show that there are two populations of SLP-2, one over the mitochondria and the other on the plasma membrane. During T-cell activation, both populations coalesce together at the immunological synapse. SLP-2 was shown to compartmentalize mitochondria as well as plasma membrane into functional domains. Furthermore, it has also been shown that there is an exchange of membrane between plasma membrane and mitochondria in T cells (24, 129). Our group and others have recently shown in heart and cardiac myocytes that caveolae and mitochondria are in close proximity and upon stress these microdomains form a physical interaction that may be dependent on G protein activation (44, 157) and may specifically target such mitochondria for posttranslational modification and regulation (144). Such data suggest that membrane associations with intracellular compartments may involve both physical and signal-regulated processes.
Conclusion
Was life spontaneous or predesigned? This is a debate that has burned for eons and is likely never to be fully extinguished. A key aspect of the cell that propelled life to evolve and grow into more complex cells and ultimately multicellular organisms was the plasma membrane. It evolved from serving a basic function as a barrier to a cellular component critical to maintaining and regulating cellular physiology and metabolism. A unique feature of the eukaryotic membrane, which includes lipids such as cholesterol, allowed for this unique transition. Thus, the plasma membrane becomes a critical feature of this most basic definition of what it means to be alive. Given the diversity of membrane lipids, their protein composition, and their primacy in exposure and response to the external environment and stressors, it is likely that modulators of membrane biology may be a key and largely overlooked target in developing novel therapeutics for a variety of diseases.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-091071 (H. H. Patel), HL-107200 (H. H. Patel), and HL-066941 (H. H. Patel), and VA Merit BX001963 (H. H. Patel).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.R., A.K., P.R., and H.H.P. drafted manuscript; S.R., A.K., A.R.B., P.R., and H.H.P. edited and revised manuscript; S.R., A.K., A.R.B., P.R., and H.H.P. approved final version of manuscript; A.R.B., P.R., and H.H.P. prepared figures.
REFERENCES
- 1.Bae MK, Jeong DK, Park NS, Lee CH, Cho BH, Jang HO, Yun I. The effect of ethanol on the physical properties of neuronal membranes. Mol Cells 19: 356–364, 2005. [PubMed] [Google Scholar]
- 2.Baggetto LG, Clottes E, Vial C. Low mitochondrial proton leak due to high membrane cholesterol content and cytosolic creatine kinase as two features of the deviant bioenergetics of Ehrlich and AS30-D tumor cells. Cancer Res 52: 4935–4941, 1992. [PubMed] [Google Scholar]
- 3.Banerjee T, Taylor M, Jobling MG, Burress H, Yang Z, Serrano A, Holmes RK, Tatulian SA, Teter K. ADP-ribosylation factor 6 acts as an allosteric activator for the folded but not disordered cholera toxin A1 polypeptide. Mol Microbiol 94: 898–912, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bissig C, Gruenberg J. Lipid sorting and multivesicular endosome biogenesis. Cold Spring Harb Perspect Biol 5: a016816, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blobel G. Intracellular protein topogenesis. Proc Natl Acad Sci USA 77: 1496–1500, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogdanov M, Dowhan W. Lipid-assisted protein folding. J Biol Chem 274: 36827–36830, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Bogdanov M, Dowhan W. Phospholipid-assisted protein folding: phosphatidylethanolamine is required at a late step of the conformational maturation of the polytopic membrane protein lactose permease. EMBO J 17: 5255–5264, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogdanov M, Umeda M, Dowhan W. Phospholipid-assisted refolding of an integral membrane protein. Minimum structural features for phosphatidylethanolamine to act as a molecular chaperone. J Biol Chem 274: 12339–12345, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Bosch M, Mari M, Herms A, Fernandez A, Fajardo A, Kassan A, Giralt A, Colell A, Balgoma D, Barbero E, Gonzalez-Moreno E, Matias N, Tebar F, Balsinde J, Camps M, Enrich C, Gross SP, Garcia-Ruiz C, Perez-Navarro E, Fernandez-Checa JC, Pol A. Caveolin-1 deficiency causes cholesterol-dependent mitochondrial dysfunction and apoptotic susceptibility. Curr Biol 21: 681–686, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Botto L, Beretta E, Daffara R, Miserocchi G, Palestini P. Biochemical and morphological changes in endothelial cells in response to hypoxic interstitial edema. Respir Res 7: 7, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brash AR. Arachidonic acid as a bioactive molecule. J Clin Invest 107: 1339–1345, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brette F, Orchard C. Resurgence of cardiac t-tubule research. Physiology (Bethesda) 22: 167–173, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Brown AJ, Galea AM. Cholesterol as an evolutionary response to living with oxygen. Evolution 64: 2179–2183, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Brown DA, London E. Structure of detergent-resistant membrane domains: does phase separation occur in biological membranes? Biochem Biophys Res Commun 240: 1–7, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68: 533–544, 1992. [DOI] [PubMed] [Google Scholar]
- 16.Brown JR, Doolittle WF. Root of the universal tree of life based on ancient aminoacyl-tRNA synthetase gene duplications. Proc Natl Acad Sci USA 92: 2441–2445, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canham PB. The minimum energy of bending as a possible explanation of the biconcave shape of the human red blood cell. J Theor Biol 26: 61–81, 1970. [DOI] [PubMed] [Google Scholar]
- 18.Cao TB, Saier MH Jr. The general protein secretory pathway: phylogenetic analyses leading to evolutionary conclusions. Biochim Biophys Acta 1609: 115–125, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Carrasco S, Meyer T. STIM proteins and the endoplasmic reticulum-plasma membrane junctions. Annu Rev Biochem 80: 973–1000, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cavalier-Smith T. The origin of cells: a symbiosis between genes, catalysts, and membranes. Cold Spring Harb Symp Quant Biol 52: 805–824, 1987. [DOI] [PubMed] [Google Scholar]
- 21.Cavalier-Smith T. The phagotrophic origin of eukaryotes and phylogenetic classification of Protozoa. Int J Syst Evol Microbiol 52: 297–354, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Chandler D. Interfaces and the driving force of hydrophobic assembly. Nature 437: 640–647, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Chevallet M, Lescuyer P, Diemer H, van Dorsselaer A, Leize-Wagner E, Rabilloud T. Alterations of the mitochondrial proteome caused by the absence of mitochondrial DNA: a proteomic view. Electrophoresis 27: 1574–1583, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christie DA, Kirchhof MG, Vardhana S, Dustin ML, Madrenas J. Mitochondrial and plasma membrane pools of stomatin-like protein 2 coalesce at the immunological synapse during T cell activation. PLoS One 7: e37144, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24a.Cooper GM. The Cell: A Molecular Approach. Sunderland, MA: Sinauer, 2000. [Google Scholar]
- 25.Cooper RA. Influence of increased membrane cholesterol on membrane fluidity and cell function in human red blood cells. J Supramol Struct 8: 413–430, 1978. [DOI] [PubMed] [Google Scholar]
- 26.Csordas G, Varnai P, Golenar T, Roy S, Purkins G, Schneider TG, Balla T, Hajnoczky G. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol Cell 39: 121–132, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Da Cruz S, Xenarios I, Langridge J, Vilbois F, Parone PA, Martinou JC. Proteomic analysis of the mouse liver mitochondrial inner membrane. J Biol Chem 278: 41566–41571, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Daleke DL. Regulation of transbilayer plasma membrane phospholipid asymmetry. J Lipid Res 44: 233–242, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Davies SS, Guo L. Lipid peroxidation generates biologically active phospholipids including oxidatively N-modified phospholipids. Chem Phys Lipids 181: 1–33, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Duve C. Evolution of the peroxisome. Ann NY Acad Sci 168: 369–381, 1969. [DOI] [PubMed] [Google Scholar]
- 31.Deamer DW. Origins of life: how leaky were primitive cells? Nature 454: 37–38, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Debnath D, Bhattacharya S, Chakrabarti A. Phospholipid assisted folding of a denatured heme protein: effect of phosphatidylethanolamine. Biochem Biophys Res Commun 301: 979–984, 2003. [DOI] [PubMed] [Google Scholar]
- 33.DeBose-Boyd RA. Feedback regulation of cholesterol synthesis: sterol-accelerated ubiquitination and degradation of HMG CoA reductase. Cell Res 18: 609–621, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demchenko AP, Yesylevskyy SO. Nanoscopic description of biomembrane electrostatics: results of molecular dynamics simulations and fluorescence probing. Chem Phys Lipids 160: 63–84, 2009. [DOI] [PubMed] [Google Scholar]
- 35.Derenyi I, Julicher F, Prost J. Formation and interaction of membrane tubes. Phys Rev Lett 88: 238101, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Deserno M. Fluid lipid membranes: from differential geometry to curvature stresses. Chem Phys Lipids 185: 11–45, 2015. [DOI] [PubMed] [Google Scholar]
- 37.Deuling HJ, Helfrich W. Red blood cell shapes as explained on the basis of curvature elasticity. Biophys J 16: 861–868, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Devos D, Dokudovskaya S, Alber F, Williams R, Chait BT, Sali A, Rout MP. Components of coated vesicles and nuclear pore complexes share a common molecular architecture. PLoS Biol 2: e380, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Evans EA. Bending resistance and chemically induced moments in membrane bilayers. Biophys J 14: 923–931, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischer TM. Is the surface area of the red cell membrane skeleton locally conserved? Biophys J 61: 298–305, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flucher BE. Structural analysis of muscle development: transverse tubules, sarcoplasmic reticulum, and the triad. Dev Biol 154: 245–260, 1992. [DOI] [PubMed] [Google Scholar]
- 42.Fowler SL, Akins M, Zhou H, Figeys D, Bennett SA. The liver connexin32 interactome is a novel plasma membrane-mitochondrial signaling nexus. J Proteome Res 12: 2597–2610, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franzini-Armstrong C, Jorgensen AO. Structure and development of E-C coupling units in skeletal muscle. Annu Rev Physiol 56: 509–534, 1994. [DOI] [PubMed] [Google Scholar]
- 44.Fridolfsson HN, Kawaraguchi Y, Ali SS, Panneerselvam M, Niesman IR, Finley JC, Kellerhals SE, Migita MY, Okada H, Moreno AL, Jennings M, Kidd MW, Bonds JA, Balijepalli RC, Ross RS, Patel PM, Miyanohara A, Chen Q, Lesnefsky EJ, Head BP, Roth DM, Insel PA, Patel HH. Mitochondria-localized caveolin in adaptation to cellular stress and injury. FASEB J 26: 4637–4649, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fridolfsson HN, Patel HH. Caveolin and caveolae in age associated cardiovascular disease. J Geriatr Cardiol 10: 66–74, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frieden M, Arnaudeau S, Castelbou C, Demaurex N. Subplasmalemmal mitochondria modulate the activity of plasma membrane Ca2+-ATPases. J Biol Chem 280: 43198–43208, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Furt F, Simon-Plas F, Mongrand S. Lipids of the plant plasma membrane. Plant Plasma Membr 19: 3–30, 2011. [Google Scholar]
- 48.Garrigues A, Escargueil AE, Orlowski S. The multidrug transporter, P-glycoprotein, actively mediates cholesterol redistribution in the cell membrane. Proc Natl Acad Sci USA 99: 10347–10352, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gattinger A, Schloter M, Munch JC. Phospholipid etherlipid and phospholipid fatty acid fingerprints in selected euryarchaeotal monocultures for taxonomic profiling. FEMS Microbiol Lett 213: 133–139, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Grafmuller A, Shillcock J, Lipowsky R. The fusion of membranes and vesicles: pathway and energy barriers from dissipative particle dynamics. Biophys J 96: 2658–2675, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grafmuller A, Shillcock J, Lipowsky R. Pathway of membrane fusion with two tension-dependent energy barriers. Phys Rev Lett 98: 218101, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Grzelczyk A, Gendaszewska-Darmach E. Novel bioactive glycerol-based lysophospholipids: new data—new insight into their function. Biochimie 95: 667–679, 2013. [DOI] [PubMed] [Google Scholar]
- 54.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9: 139–150, 2008. [DOI] [PubMed] [Google Scholar]
- 55.Head BP, Peart JN, Panneerselvam M, Yokoyama T, Pearn ML, Niesman IR, Bonds JA, Schilling JM, Miyanohara A, Headrick J, Ali SS, Roth DM, Patel PM, Patel HH. Loss of caveolin-1 accelerates neurodegeneration and aging. PLoS One 5: e15697, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Helfrich W. Elastic properties of lipid bilayers: theory and possible experiments. Z Naturforsch C 28: 693–703, 1973. [DOI] [PubMed] [Google Scholar]
- 57.Helle SC, Kanfer G, Kolar K, Lang A, Michel AH, Kornmann B. Organization and function of membrane contact sites. Biochim Biophys Acta 1833: 2526–2541, 2013. [DOI] [PubMed] [Google Scholar]
- 58.Henkart M, Landis DM, Reese TS. Similarity of junctions between plasma membranes and endoplasmic reticulum in muscle and neurons. J Cell Biol 70: 338–347, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hilario E, Gogarten JP. The prokaryote-to-eukaryote transition reflected in the evolution of the V/F/A-ATPase catalytic and proteolipid subunits. J Mol Evol 46: 703–715, 1998. [DOI] [PubMed] [Google Scholar]
- 60.Horikawa YT, Panneerselvam M, Kawaraguchi Y, Tsutsumi YM, Ali SS, Balijepalli RC, Murray F, Head BP, Niesman IR, Rieg T, Vallon V, Insel PA, Patel HH, Roth DM. Cardiac-specific overexpression of caveolin-3 attenuates cardiac hypertrophy and increases natriuretic peptide expression and signaling. J Am Coll Cardiol 57: 2273–2283, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huber R, Eder W, Heldwein S, Wanner G, Huber H, Rachel R, Stetter KO. Thermocrinis ruber gen. nov., sp. nov., a pink-filament-forming hyperthermophilic bacterium isolated from Yellowstone National Park. Appl Environ Microbiol 64: 3576–3583, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hughes AL, Todd BL, Espenshade PJ. SREBP pathway responds to sterols and functions as an oxygen sensor in fission yeast. Cell 120: 831–842, 2005. [DOI] [PubMed] [Google Scholar]
- 63.Igbavboa U, Avdulov NA, Chochina SV, Wood WG. Transbilayer distribution of cholesterol is modified in brain synaptic plasma membranes of knockout mice deficient in the low-density lipoprotein receptor, apolipoprotein E, or both proteins. J Neurochem 69: 1661–1667, 1997. [DOI] [PubMed] [Google Scholar]
- 64.Igbavboa U, Avdulov NA, Schroeder F, Wood WG. Increasing age alters transbilayer fluidity and cholesterol asymmetry in synaptic plasma membranes of mice. J Neurochem 66: 1717–1725, 1996. [DOI] [PubMed] [Google Scholar]
- 65.Igbavboa U, Eckert GP, Malo TM, Studniski AE, Johnson LN, Yamamoto N, Kobayashi M, Fujita SC, Appel TR, Muller WE, Wood WG, Yanagisawa K. Murine synaptosomal lipid raft protein and lipid composition are altered by expression of human apoE 3 and 4 and by increasing age. J Neurol Sci 229–230: 225–232, 2005. [DOI] [PubMed] [Google Scholar]
- 66.Igbavboa U, Hamilton J, Kim HY, Sun GY, Wood WG. A new role for apolipoprotein E: modulating transport of polyunsaturated phospholipid molecular species in synaptic plasma membranes. J Neurochem 80: 255–261, 2002. [DOI] [PubMed] [Google Scholar]
- 67.Igbavboa U, Sun GY, Weisman GA, He Y, Wood WG. Amyloid beta-protein stimulates trafficking of cholesterol and caveolin-1 from the plasma membrane to the Golgi complex in mouse primary astrocytes. Neuroscience 162: 328–338, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iglic A. A possible mechanism determining the stability of spiculated red blood cells. J Biomech 30: 35–40, 1997. [DOI] [PubMed] [Google Scholar]
- 69.Inoue T, Tsai B. How viruses use the endoplasmic reticulum for entry, replication, and assembly. Cold Spring Harb Perspect Biol 5: a013250, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jaric M, Seifert U, Wintz W, Wortis M. Vesicular instabilities: the prolate-to-oblate transition and other shape instabilities of fluid bilayer membranes. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics 52: 6623–6634, 1995. [DOI] [PubMed] [Google Scholar]
- 71.Jekely G. Did the last common ancestor have a biological membrane? Biol Direct 1: 35, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jekely G. Small GTPases and the evolution of the eukaryotic cell. Bioessays 25: 1129–1138, 2003. [DOI] [PubMed] [Google Scholar]
- 73.Kates M, Yengoyan LS, Sastry PS. A diether analog of phosphatidyl glycerophosphate in Halobacterium cutirubrum. Biochim Biophys Acta 98: 252–268, 1965. [DOI] [PubMed] [Google Scholar]
- 74.Khan N, Shen J, Chang TY, Chang CC, Fung PC, Grinberg O, Demidenko E, Swartz H. Plasma membrane cholesterol: a possible barrier to intracellular oxygen in normal and mutant CHO cells defective in cholesterol metabolism. Biochemistry 42: 23–29, 2003. [DOI] [PubMed] [Google Scholar]
- 75.Kiessling V, Wan C, Tamm LK. Domain coupling in asymmetric lipid bilayers. Biochim Biophys Acta 1788: 64–71, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kirchhof MG, Chau LA, Lemke CD, Vardhana S, Darlington PJ, Marquez ME, Taylor R, Rizkalla K, Blanca I, Dustin ML, Madrenas J. Modulation of T cell activation by stomatin-like protein 2. J Immunol 181: 1927–1936, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klecker T, Scholz D, Fortsch J, Westermann B. The yeast cell cortical protein Num1 integrates mitochondrial dynamics into cellular architecture. J Cell Sci 126: 2924–2930, 2013. [DOI] [PubMed] [Google Scholar]
- 78.Koga Y. Early evolution of membrane lipids: how did the lipid divide occur? J Mol Evol 72: 274–282, 2011. [DOI] [PubMed] [Google Scholar]
- 79.Koga Y, Kyuragi T, Nishihara M, Sone N. Did archaeal and bacterial cells arise independently from noncellular precursors? A hypothesis stating that the advent of membrane phospholipid with enantiomeric glycerophosphate backbones caused the separation of the two lines of descent. J Mol Evol 46: 54–63, 1998. [DOI] [PubMed] [Google Scholar]
- 80.Koga Y, Morii H. Biosynthesis of ether-type polar lipids in archaea and evolutionary considerations. Microbiol Mol Biol Rev 71: 97–120, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koonin EV. Comparative genomics, minimal gene-sets and the last universal common ancestor. Nat Rev Microbiol 1: 127–136, 2003. [DOI] [PubMed] [Google Scholar]
- 82.Kurzchalia TV, Dupree P, Parton RG, Kellner R, Virta H, Lehnert M, Simons K. VIP21, a 21-kD membrane protein is an integral component of trans-Golgi-network-derived transport vesicles. J Cell Biol 118: 1003–1014, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lackner LL, Ping H, Graef M, Murley A, Nunnari J. Endoplasmic reticulum-associated mitochondria-cortex tether functions in the distribution and inheritance of mitochondria. Proc Natl Acad Sci USA 110: E458–E467, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lane N. Oxygen: The Molecule That Made the World. Oxford, UK: Oxford Univ. Press, 2002. [Google Scholar]
- 85.Langworthy TA, Smith PF, Mayberry WR. Lipids of Thermoplasma acidophilum. J Bacteriol 112: 1193–1200, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lenz O, Schmid F. Structure of symmetric and asymmetric “ripple” phases in lipid bilayers. Phys Rev Lett 98: 058104, 2007. [DOI] [PubMed] [Google Scholar]
- 87.Levine T, Loewen C. Inter-organelle membrane contact sites: through a glass, darkly. Curr Opin Cell Biol 18: 371–378, 2006. [DOI] [PubMed] [Google Scholar]
- 88.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science 327: 46–50, 2010. [DOI] [PubMed] [Google Scholar]
- 89.Lipowsky R. Spontaneous tubulation of membranes and vesicles reveals membrane tension generated by spontaneous curvature. Faraday Discuss 161: 305–331, 2013. [DOI] [PubMed] [Google Scholar]
- 90.Liu P, Anderson RG. Compartmentalized production of ceramide at the cell surface. J Biol Chem 270: 27179–27185, 1995. [DOI] [PubMed] [Google Scholar]
- 91.Mandyam CD, Schilling JM, Cui W, Egawa J, Niesman IR, Kellerhals SE, Staples MC, Busija AR, Risbrough VB, Posadas E, Grogman GC, Chang JW, Roth DM, Patel PM, Patel HH, Head BP. Neuron-targeted caveolin-1 improves molecular signaling, plasticity, and behavior dependent on the hippocampus in adult and aged mice. Biol Psychiatry (October 7, 2015). doi: 10.1016/j.biopsych.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mansy SS, Schrum JP, Krishnamurthy M, Tobe S, Treco DA, Szostak JW. Template-directed synthesis of a genetic polymer in a model protocell. Nature 454: 122–125, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marrink SJ, Mark AE. The mechanism of vesicle fusion as revealed by molecular dynamics simulations. J Am Chem Soc 125: 11144–11145, 2003. [DOI] [PubMed] [Google Scholar]
- 94.Martin W, Muller M. The hydrogen hypothesis for the first eukaryote. Nature 392: 37–41, 1998. [DOI] [PubMed] [Google Scholar]
- 95.Martin W, Russell MJ. On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Philos Trans R Soc Lond B Biol Sci 358: 59–83, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Massey KA, Schnitzer JE. Caveolae and cancer. Recent Results Cancer Res 180: 217–231, 2010. [DOI] [PubMed] [Google Scholar]
- 97.Mouritsen OG, Zuckermann MJ. What's so special about cholesterol? Lipids 39: 1101–1113, 2004. [DOI] [PubMed] [Google Scholar]
- 98.Mulkidjanian AY, Galperin MY, Koonin EV. Co-evolution of primordial membranes and membrane proteins. Trends Biochem Sci 34: 206–215, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74: 1124–1136, 1986. [DOI] [PubMed] [Google Scholar]
- 100.Murry CE, Richard VJ, Reimer KA, Jennings RB. Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circ Res 66: 913–931, 1990. [DOI] [PubMed] [Google Scholar]
- 101.Nelson P, Powers T, Seifert U. Dynamical theory of the Pearling instability in cylindrical vesicles. Phys Rev Lett 74: 3384–3387, 1995. [DOI] [PubMed] [Google Scholar]
- 102.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science 258: 607–614, 1992. [DOI] [PubMed] [Google Scholar]
- 103.Palade GE. Fine structure of blood capillaries. J Appl Phys 24: 1424, 1953. [Google Scholar]
- 104.Palade GE, Bruns RR. Structural modulations of plasmalemmal vesicles. J Cell Biol 37: 633–649, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Papa S, Skulachev VP. Reactive oxygen species, mitochondria, apoptosis and aging. Mol Cell Biochem 174: 305–319, 1997. [PubMed] [Google Scholar]
- 106.Papackova Z, Cahova M. Fatty acid signaling: the new function of intracellular lipases. Int J Mol Sci 16: 3831–3855, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Parlo RA, Coleman PS. Enhanced rate of citrate export from cholesterol-rich hepatoma mitochondria. The truncated Krebs cycle and other metabolic ramifications of mitochondrial membrane cholesterol. J Biol Chem 259: 9997–10003, 1984. [PubMed] [Google Scholar]
- 108.Parton RG, Molero JC, Floetenmeyer M, Green KM, James DE. Characterization of a distinct plasma membrane macrodomain in differentiated adipocytes. J Biol Chem 277: 46769–46778, 2002. [DOI] [PubMed] [Google Scholar]
- 109.Parton RG, Way M, Zorzi N, Stang E. Caveolin-3 associates with developing T-tubules during muscle differentiation. J Cell Biol 136: 137–154, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Patel HH, Insel PA. Lipid rafts and caveolae and their role in compartmentation of redox signaling. Antioxid Redox Signal 11: 1357–1372, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Patel HH, Murray F, Insel PA. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol 48: 359–391, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Payne JL, Boyer AG, Brown JH, Finnegan S, Kowalewski M, Krause RA Jr, Lyons SK, McClain CR, McShea DW, Novack-Gottshall PM, Smith FA, Stempien JA, Wang SC. Two-phase increase in the maximum size of life over 35 billion years reflects biological innovation and environmental opportunity. Proc Natl Acad Sci USA 106: 24–27, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Payne JL, McClain CR, Boyer AG, Brown JH, Finnegan S, Kowalewski M, Krause RA Jr, Lyons SK, McShea DW, Novack-Gottshall PM, Smith FA, Spaeth P, Stempien JA, Wang SC. The evolutionary consequences of oxygenic photosynthesis: a body size perspective. Photosynth Res 107: 37–57, 2011. [DOI] [PubMed] [Google Scholar]
- 114.Peart JN, Pepe S, Reichelt ME, Beckett N, See Hoe L, Ozberk V, Niesman IR, Patel HH, Headrick JP. Dysfunctional survival-signaling and stress-intolerance in aged murine and human myocardium. Exp Gerontol 50: 72–81, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pelkmans L, Burli T, Zerial M, Helenius A. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell 118: 767–780, 2004. [DOI] [PubMed] [Google Scholar]
- 116.Pelkmans L, Helenius A. Endocytosis via caveolae. Traffic 3: 311–320, 2002. [DOI] [PubMed] [Google Scholar]
- 117.Pereto J, Lopez-Garcia P, Moreira D. Ancestral lipid biosynthesis and early membrane evolution. Trends Biochem Sci 29: 469–477, 2004. [DOI] [PubMed] [Google Scholar]
- 118.Philips R, Kondev J, Theriot J, Orme N, Garcia H. Physical Biology of the Cell. New York: Garland Science, 2009. [Google Scholar]
- 119.Pichler H, Gaigg B, Hrastnik C, Achleitner G, Kohlwein SD, Zellnig G, Perktold A, Daum G. A subfraction of the yeast endoplasmic reticulum associates with the plasma membrane and has a high capacity to synthesize lipids. Eur J Biochem 268: 2351–2361, 2001. [DOI] [PubMed] [Google Scholar]
- 120.Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res 44: 655–667, 2003. [DOI] [PubMed] [Google Scholar]
- 121.Pike LJ, Casey L. Localization and turnover of phosphatidylinositol 4,5-bisphosphate in caveolin-enriched membrane domains. J Biol Chem 271: 26453–26456, 1996. [DOI] [PubMed] [Google Scholar]
- 122.Pike LJ, Han X, Chung KN, Gross RW. Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: a quantitative electrospray ionization/mass spectrometric analysis. Biochemistry 41: 2075–2088, 2002. [DOI] [PubMed] [Google Scholar]
- 123.Rajendran L, Simons K. Lipid rafts and membrane dynamics. J Cell Sci 118: 1099–1102, 2005. [DOI] [PubMed] [Google Scholar]
- 124.Rangamani P, Benjamini A, Agrawal A, Smit B, Steigmann DJ, Oster G. Small scale membrane mechanics. Biomech Model Mechanobiol 13: 697–711, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rao AM, Igbavboa U, Semotuk M, Schroeder F, Wood WG. Kinetics and size of cholesterol lateral domains in synaptosomal membranes: modification by sphingomyelinase and effects on membrane enzyme activity. Neurochem Int 23: 45–52, 1993. [DOI] [PubMed] [Google Scholar]
- 126.Raucher D, Sheetz MP. Characteristics of a membrane reservoir buffering membrane tension. Biophys J 77: 1992–2002, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ray S, Taylor M, Banerjee T, Tatulian SA, Teter K. Lipid rafts alter the stability and activity of the cholera toxin A1 subunit. J Biol Chem 287: 30395–30405, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ray S, Taylor M, Burlingame M, Tatulian SA, Teter K. Modulation of toxin stability by 4-phenylbutyric acid and negatively charged phospholipids. PLoS One 6: e23692, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rezaul K, Wu L, Mayya V, Hwang SI, Han D. A systematic characterization of mitochondrial proteome from human T leukemia cells. Mol Cell Proteomics 4: 169–181, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rosen GM. Free Radicals: Biology and Detection by Spin Trapping. New York: Oxford Univ. Press, 1999. [Google Scholar]
- 131.Rossy J, Ma Y, Gaus K. The organisation of the cell membrane: do proteins rule lipids? Curr Opin Chem Biol 20C: 54–59, 2014. [DOI] [PubMed] [Google Scholar]
- 132.Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell 68: 673–682, 1992. [DOI] [PubMed] [Google Scholar]
- 133.Rothman JE, Lenard J. Membrane asymmetry. Science 195: 743–753, 1977. [DOI] [PubMed] [Google Scholar]
- 134.Rouslin W, MacGee J, Wesselman AR, Adams RJ, Gupte S. Canine myocardial ischemia: increased mitochondrial cholesterol, a marker of mitochondrial membrane injury. J Mol Cell Cardiol 12: 1475–1482, 1980. [DOI] [PubMed] [Google Scholar]
- 135.Saenz JP, Sezgin E, Schwille P, Simons K. Functional convergence of hopanoids and sterols in membrane ordering. Proc Natl Acad Sci USA 109: 14236–14240, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sandow A. Excitation-contraction coupling in muscular response. Yale J Biol Med 25: 176–201, 1952. [PMC free article] [PubMed] [Google Scholar]
- 137.Sarnataro D, Campana V, Paladino S, Stornaiuolo M, Nitsch L, Zurzolo C. PrPC association with lipid rafts in the early secretory pathway stabilizes its cellular conformation. Mol Biol Cell 15: 4031–4042, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schroeder F, Morrison WJ, Gorka C, Wood WG. Transbilayer effects of ethanol on fluidity of brain membrane leaflets. Biochim Biophys Acta 946: 85–94, 1988. [DOI] [PubMed] [Google Scholar]
- 139.Seifert U, Berndl K, Lipowsky R. Shape transformations of vesicles: phase diagram for spontaneous-curvature and bilayer-coupling models. Phys Rev A 44: 1182–1202, 1991. [DOI] [PubMed] [Google Scholar]
- 140.Simons K, Ikonen E. Functional rafts in cell membranes. Nature 387: 569–572, 1997. [DOI] [PubMed] [Google Scholar]
- 141.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 1: 31–39, 2000. [DOI] [PubMed] [Google Scholar]
- 142.Slotte JP, Hedstrom G, Rannstrom S, Ekman S. Effects of sphingomyelin degradation on cell cholesterol oxidizability and steady-state distribution between the cell surface and the cell interior. Biochim Biophys Acta 985: 90–96, 1989. [DOI] [PubMed] [Google Scholar]
- 143.Stefan CJ, Manford AG, Emr SD. ER-PM connections: sites of information transfer and inter-organelle communication. Curr Opin Cell Biol 25: 434–442, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sun J, Nguyen T, Aponte AM, Menazza S, Kohr MJ, Roth DM, Patel HH, Murphy E, Steenbergen C. Ischaemic preconditioning preferentially increases protein S-nitrosylation in subsarcolemmal mitochondria. Cardiovasc Res 106: 227–236, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Szostak JW, Bartel DP, Luisi PL. Synthesizing life. Nature 409: 387–390, 2001. [DOI] [PubMed] [Google Scholar]
- 146.Taylor SW, Fahy E, Zhang B, Glenn GM, Warnock DE, Wiley S, Murphy AN, Gaucher SP, Capaldi RA, Gibson BW, Ghosh SS. Characterization of the human heart mitochondrial proteome. Nat Biotechnol 21: 281–286, 2003. [DOI] [PubMed] [Google Scholar]
- 147.Teter K, Jobling MG, Sentz D, Holmes RK. The cholera toxin A13 subdomain is essential for interaction with ADP-ribosylation factor 6 and full toxic activity but is not required for translocation from the endoplasmic reticulum to the cytosol. Infect Immun 74: 2259–2267, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Thomas JA, Rana FR. The influence of environmental conditions, lipid composition, and phase behavior on the origin of cell membranes. Orig Life Evol Biosph 37: 267–285, 2007. [DOI] [PubMed] [Google Scholar]
- 149.Toulmay A, Prinz WA. Lipid transfer and signaling at organelle contact sites: the tip of the iceberg. Curr Opin Cell Biol 23: 458–463, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tristram-Nagle S, Zhang R, Suter RM, Worthington CR, Sun WJ, Nagle JF. Measurement of chain tilt angle in fully hydrated bilayers of gel phase lecithins. Biophys J 64: 1097–1109, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tsutsumi YM, Horikawa YT, Jennings MM, Kidd MW, Niesman IR, Yokoyama U, Head BP, Hagiwara Y, Ishikawa Y, Miyanohara A, Patel PM, Insel PA, Patel HH, Roth DM. Cardiac-specific overexpression of caveolin-3 induces endogenous cardiac protection by mimicking ischemic preconditioning. Circulation 118: 1979–1988, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.van Meer G, Simons K. Lipid polarity and sorting in epithelial cells. J Cell Biochem 36: 51–58, 1988. [DOI] [PubMed] [Google Scholar]
- 153.Vinten J, Johnsen AH, Roepstorff P, Harpoth J, Tranum-Jensen J. Identification of a major protein on the cytosolic face of caveolae. Biochim Biophys Acta 1717: 34–40, 2005. [DOI] [PubMed] [Google Scholar]
- 154.Vinten J, Voldstedlund M, Clausen H, Christiansen K, Carlsen J, Tranum-Jensen J. A 60-kDa protein abundant in adipocyte caveolae. Cell Tissue Res 305: 99–106, 2001. [DOI] [PubMed] [Google Scholar]
- 155.Wachtershauser G. From pre-cells to Eukarya—a tale of two lipids. Mol Microbiol 47: 13–22, 2003. [DOI] [PubMed] [Google Scholar]
- 156.Wang CH, Popel AS. Effect of red blood cell shape on oxygen transport in capillaries. Math Biosci 116: 89–110, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang J, Schilling JM, Niesman IR, Headrick JP, Finley JC, Kwan E, Patel PM, Head BP, Roth DM, Yue Y, Patel HH. Cardioprotective trafficking of caveolin to mitochondria is Gi-protein dependent. Anesthesiology 121: 538–548, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Watkins EB, Miller CE, Majewski J, Kuhl TL. Membrane texture induced by specific protein binding and receptor clustering: active roles for lipids in cellular function. Proc Natl Acad Sci USA 108: 6975–6980, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Widomska J, Raguz M, Subczynski WK. Oxygen permeability of the lipid bilayer membrane made of calf lens lipids. Biochim Biophys Acta 1768: 2635–2645, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Woese CR, Kandler O, Wheelis ML. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci USA 87: 4576–4579, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Woese CR, Magrum LJ, Fox GE. Archaebacteria. J Mol Evol 11: 245–251, 1978. [DOI] [PubMed] [Google Scholar]
- 162.Wood WG, Gorka C, Schroeder F. Acute and chronic effects of ethanol on transbilayer membrane domains. J Neurochem 52: 1925–1930, 1989. [DOI] [PubMed] [Google Scholar]
- 163.Yesylevskyy SO, Demchenko AP, Kraszewski S, Ramseyer C. Cholesterol induces uneven curvature of asymmetric lipid bilayers. ScientificWorldJournal 2013: 965230, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


