Abstract
The brain is highly plastic and undergoes changes in response to many experiences. Learning especially can induce structural remodeling of dendritic spines, which is thought to relate to memory formation. Classical Pavlovian fear conditioning (FC) traditionally pairs an auditory cue with an aversive footshock, and has been widely used to study neural processes underlying associative learning and memory. Past research has found dendritic spine changes after FC in several structures. But, due to heterogeneity of cells within brain structures and limitations of traditional neuroanatomical techniques, it is unclear if all cells included in analyses were actually active during learning processes, even if known circuits are isolated. In this study, we employed a novel approach to analyze structural plasticity explicitly in neurons activated by exposure to either cued or uncued footshocks. We used male and female Arc-dVenus transgenic mice, which express the Venus fluorophore driven by the activity-related Arc promoter, to identify neurons that were active during either scenario. We then targeted fluorescent microinjections to Arc+ and neighboring Arc− neurons in the basolateral area of the amygdala (BLA) and auditory association cortex (TeA). In both BLA and TeA, Arc+ neurons had reduced thin and mushroom spine densities compared to Arc− neurons. This effect was present in males and females alike and also in both cued and uncued shock groups. Overall, this study adds to our understanding of how neuronal activity affects structural plasticity, and represents a methodological advance in the ways we can directly relate structural changes to experience-related neural activity.
Keywords: dendritic spines, amygdala, Arc, structural plasticity
INTRODUCTION
Dendritic spines are highly plastic and can change in number and shape after stimuli and experiences. Spine changes after learning paradigms are of particular interest because structural changes are thought to reflect underlying changes in synaptic strength (Trachtenberg et al., 2002; Matsuzaki et al., 2004; Zito et al., 2009; Kasai et al., 2010). Initial investigations into how dendritic spines change after learning employed Golgi staining techniques (Brandon and Coss, 1982; Patel and Stewart, 1988). Golgi-based approaches to learning-related structural plasticity are limited, however, because they traditionally rely on comparisons between behaviorally naïve animals and animals that underwent a learning experience, and thus observation of structural changes within an individual animal is impossible. One exception can be found in early studies of the motor cortex, in which unilateral reach training allows for elegant cross-hemisphere comparisons (Greenough et al., 1985). However, such an approach is difficult in the realm of classical Pavlovian fear conditioning (FC), which conventionally engages both hemispheres. More recently, in vivo two-photon imaging through a cranial window has allowed repeated imaging of discrete dendritic segments over the course of an experiment, making it possible to assess change in individual spines before and after a learning experience within the same animal. Using this technique, it has been shown that motor task learning can rapidly induce formation of new spines in motor cortex (Xu et al., 2009) and spine remodeling correlates with improved task performance after motor skill training (Yang et al., 2009). In contrast, FC leads to elimination of spines in frontal association cortex (Lai et al., 2012). However, due to the limitations of the technique it is not yet possible to image dendritic spines in deep structures further away from the cortical surface. Moreover, neither of these techniques allows exclusive analysis of the neurons that are recruited in the learning experience. Only a small subset of neurons in a given brain area are activated during learning (Han et al., 2009), and thus random sampling of spine segments likely includes neurons that were not activated. Therefore, any observed changes could represent global changes that are not specific to memory formation. To address this problem, Sanders et al. (2012) used GFP-GluR1cfos transgenic mice to tag hippocampal CA1 neurons activated by context FC and found reduced spine densities on activated neurons compared to non-activated neurons. It is unclear if this specificity is unique to CA1 or if it exists in other structures involved in FC.
Besides the hippocampus, the amygdala is an obvious area of interest when studying spine changes after FC. The basolateral complex, including the lateral amygdala, basolateral (BLA) and basomedial nuclei, has long been known to be important for fear expression and fear memory formation (Davis, 1992; Maren, 2001; Duvarci and Pare, 2014). The BLA is often seen as a relay station passing information from lateral amygdala on to medial central nucleus of the amygdala (Duvarci and Pare, 2014) and lesions of the BLA after cued FC block expression of conditioned fear, which suggest that it is an important site of learning-related plasticity (Anglada-Figueroa and Quirk, 2005). The BLA also maintains strong bidirectional connections to the hippocampus, and is thought to be critically involved in context FC (Maren, 2001; Pitkänen et al., 2006).
Here, we introduce a novel approach to assess dendritic spine morphology of activated vs. non-activated neurons in the BLA after cued FC or shock exposure alone. To identify activated neurons, we use Arc-dVenus transgenic mice, which express a destabilized Venus fluorophore driven by the activity-related Arc promoter (Eguchi and Yamaguchi, 2009). In order to eliminate background Venus expression or spine changes triggered by handling and exposure to a new environment, we used custom-made ‘home-cage’ conditioning boxes. We report that in male and female mice, Arc+ neurons exhibit reduced spine density compared to neighboring Arc− neurons after both cued and uncued shock. These findings lend insight into the neural processes that underlie experience- and activity-related structural plasticity.
EXPERIMENTAL PROCEDURES
Animals
Adult (10–14 week old) male (n = 15) and female (n = 17) Arc-dVenus transgenic mice (C57BL/6J background) were housed in the New Research Building Animal Facility at Harvard Medical School on a 12:12 light:dark schedule and given ad libitum access to food and water. These mice express a destabilized version of the fluorescent protein Venus, driven by the activity-related Arc promoter. Venus intensity in Arc-dVenus mice peaks at approximately 6 h post stimulation, and is fully decayed after 24 h (Eguchi and Yamaguchi, 2009; Gouty-Colomer et al., 2015). All procedures were approved by the Harvard Medical School Institutional Animal Care and Use Committee.
Conditioning Home Cages (CHCs)
To reduce noise in Arc expression due to handling and novel environment exposure, animals were fear conditioned in custom “home cage” FC chambers. These chambers were designed and fabricated at the Harvard Medical School’s Research Instrumentation Core. Each cage consists of a Techniplast Safe Seal IVC Blue Line™ cage bottom with a custom acrylic lid. Each cage contains a clear, acrylic insert containing a small enclosure (13 cm × 5 cm × 5 cm) and a food hopper, to which the mice had free, continuous access. A water-delivery “nosepoke” is mounted to the front of each cage to provide water to housed animals. An IR-LED sensor mounted in each nosepoke triggers the release of a drop of water each time the nosepoke is entered by the mouse. Mice housed in the cages have access to a 13-cm × 24-cm × 24.5-cm region in front of the insert to freely move. The floor of each cage is made of fifteen 3-mm stainless steel dowels spaced by 8 mm oriented lengthwise along the cage. A custom-made connector connects these dowels to the output of a Med Associates Aversive Stimulator/Scrambler Module (env-414) which provides shocks during FC. The bar floor is elevated 3.5 cm from the bottom of the cage. The space beneath the bar floor is filled with mouse bedding to absorb mouse urine. Visaton BF-32-8 speakers mounted to the acrylic ceiling provided tones during conditioning. Shock delivery, water delivery and tone generation is regulated by a custom PCB containing a Teensy 3.0 microcontroller programed with custom firmware. Raspberry Pi model B computers mounted on each cage receive commands sent over a local network using Twisted-Python so that all four cages can be controlled in synchrony. Each Raspberry PI is equipped with a no-IR camera module that is mounted to the ceiling of each cage, allowing mice housed in the cages to be observed during and between behavioral sessions. Luxeon Rebel Deep Red (655 nm) LEDs mounted on the ceiling of each cage allow mice to be observed during the night.
FC procedure
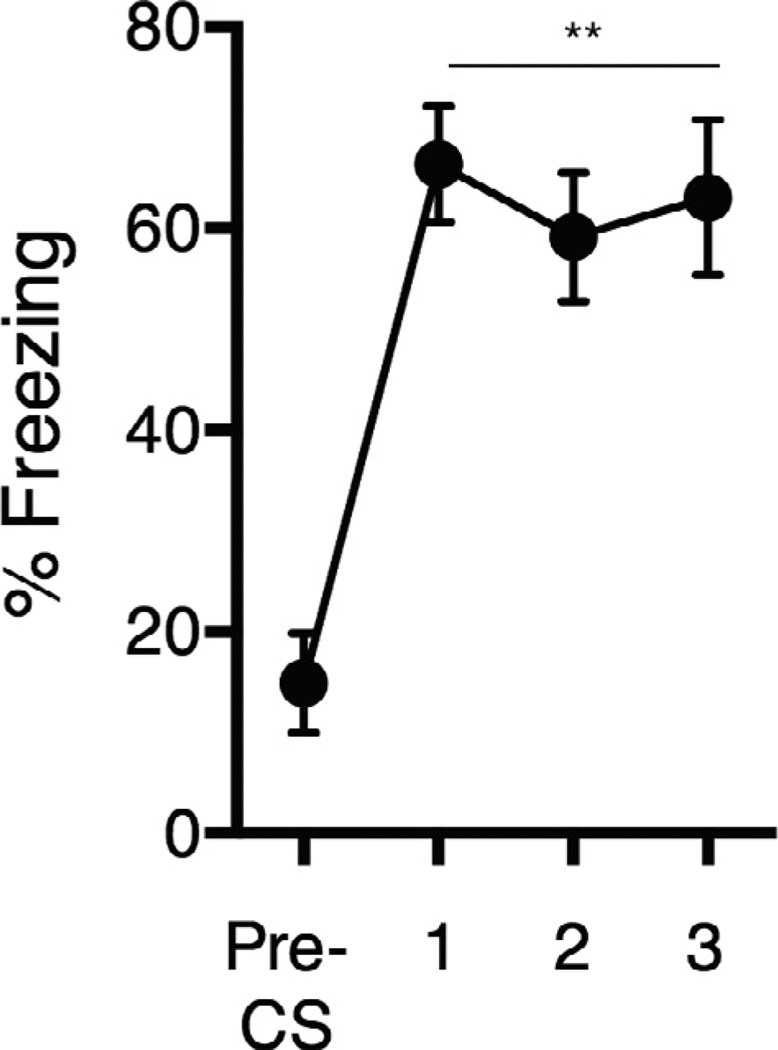
The cued shock protocol used in this study (described below) results in robust conditioned freezing to the auditory cue in mice up to a month after conditioning (Fig. 1), and therefore we believe that this protocol represents a traditional “cued FC” experience in which learning is induced. The experimental design for the current study is shown in Fig. 2. At 17:00 h on Day 1 animals were transferred to the custom conditioning cages and left undisturbed by experimenters until euthanasia at 15:00 h on Day 4 (Fig. 2A). Cued or uncued shocks began at 09:00 h on day 4. Animals in the cued shock group (males n = 8, females n = 10) were exposed to 7 CS-US pairings, consisting of a 10-s tone (80 db, 6 kHz) that co-terminated with a 1-s 0.5-mA foot shock. The mean inter-trial interval was 1 min 53 s and the duration of the session was 10 min. The uncued (“shock only”) group (males n = 7, females n = 7) received the same shock protocol but without tone presentations.
Fig. 1.
Cued fear conditioning in our Conditioning Home Cages (CHCs) induces robust fear memory. A separate cohort of mice underwent a cued conditioning protocol identical to that used here, and were then returned to standard housing. One month later, they were returned to the CHCs and exposed to the tone. Freezing significantly increased in response to the tone compared to pre-CS freezing levels (Wilcoxon matched-pairs t-test, **p < 0.01).
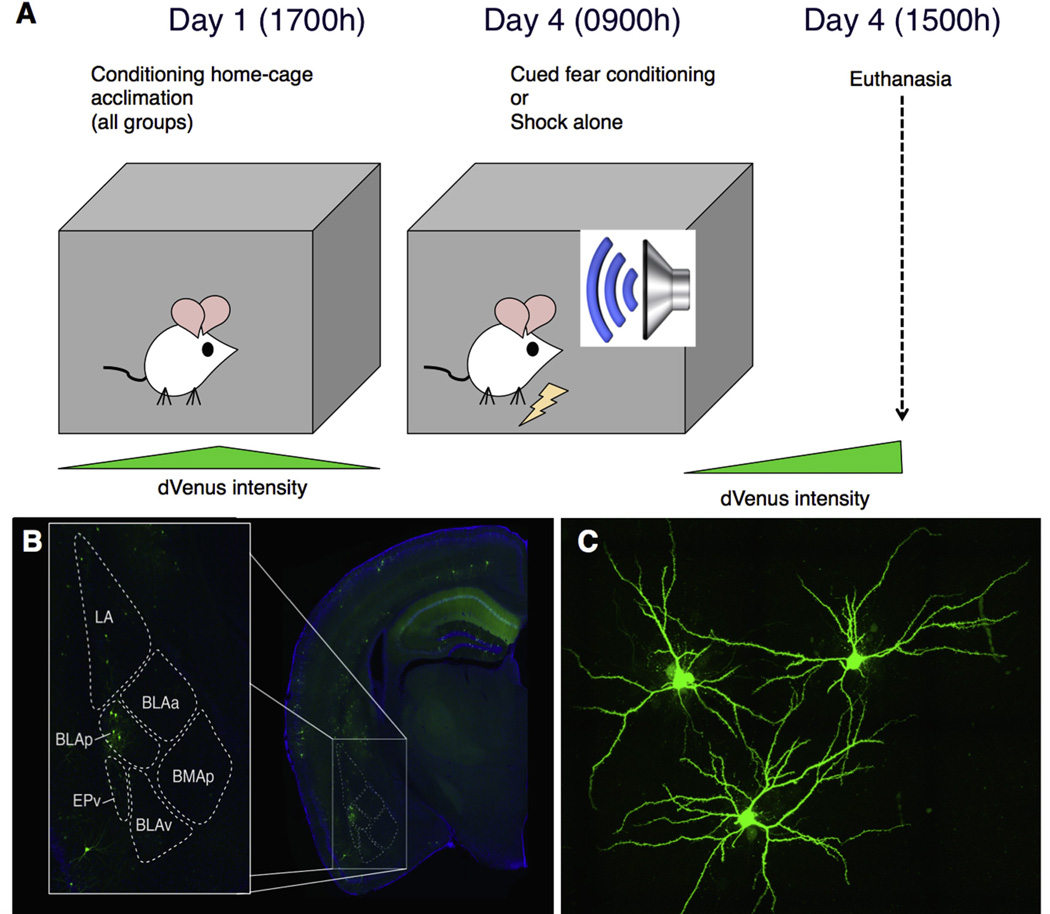
Fig. 2.
Experimental design and approach. (A) Animals were transferred to the custom fear conditioning home cages 3 days prior to fear conditioning, to allow Venus decay from handling and novel environment. On day 3, animals were exposed to either 9 tone-shock pairings or 9 shocks, and then euthanized 6 h later. (B) Arc+ neurons in the BLAp were identified, and both Arc+ and neighboring Arc− neurons were iontophoretically filled with Lucifer Yellow for imaging and spine morphology analysis (C).
Euthanasia and tissue preparation
Six hours after the last shock presentation, animals were deeply anesthetized with isoflurane and sacrificed by transcardial perfusion of 4% paraformaldehyde in .1 mol/L phosphate buffer (phosphate-buffered saline (PBS), pH 7.4). Brains were extracted and post-fixed in paraformaldehyde over night and then placed in .1% sodium azide in PBS at 4 °C for storage.
Morphology analysis
BLA and temporal association area (TeA) containing coronal sections of 240-µm thickness were collected using a vibrating microtome (Leica Microsystems, Inc, Buffalo Grove, Illinois, USA). To identify Arc+ neurons, Venus-expressing neurons were visualized through a GFP filter (Zeiss Microscopy, Thornwood, New York, USA) on a Zeiss Axio Examiner A.1 microscope (Zeiss Microscopy). Iontophoretic microinjections of Lucifer Yellow dye were performed into Arc+ and neighboring Arc neurons. BLA neurons were targeted within the “BLAp” region (defined by Allen Brain Atlas; Fig. 2B) in coronal sections ranging from Bregma −1.755 mm to −2.255 mm. TeA neurons were sampled from layer 2 of the “TeA” region from the same Bregma range. For each brain region of interest (BA and TeA) four Arc+/Arc− neuron pairs, for a total of eight neurons per animal were selected for spine analysis. From each neuron four dendritic segments were imaged, for a total of 32 segments per animal (16 segments per Arc+ and 16 segments per Arc− group). Z-stacks were acquired using an Olympus FV1000 confocal microscope (Optical Analysis Corporation, Nashua, New Hampshire, USA) with 100× lens, zoom of 3.7, NA 1.4, and step size of 0.33 µm. In TeA, only basal dendrites were sampled. Raw Z-stacks were deconvolved with AutoQuant (Media Cybernetics, Rockville, Maryland, USA) and analyzed for spine number and shape (thin, stubby, or mushroom) using NeuronStudio software (Computational Neurobiology and Imaging Center, New York, New York, USA) (classification criteria described in detail by Rodriguez et al. (2008)).
Statistical analysis
All statistical analyses were conducted using GraphPad Prism software (GraphPad Software, Inc, La Jolla, California, USA). Spine densities from segments sampled from Arc+ and Arc− neurons were first averaged per animal. Sampling from Arc− and Arc+ neurons within animals served as an internal control, and allowed for within-animal evaluation of Arc effects on dendritic morphology. Thus, all comparisons were made within animals between average spine densities for Arc− and Arc+ neurons. Comparisons were made by using mixed-design ANOVA with Arc+/Arc− as within subject factor and Cued/Shock only as between subject factor. Spine densities were averaged per animal to make animals the unit of analysis. There is evidence that structural dynamics and activity-dependent plasticity of mushroom and thin spines are different (Matsuzaki et al., 2004; Bourne and Harris, 2007; Matsuo et al., 2008), thus we analyzed each spine type separately. Significance was set at p < 0.05.
RESULTS
To assess potential sex differences in Arc+ vs. Arc− neurons, we first looked at the magnitude of difference in thin and mushroom spine density in Arc+ compared to Arc− neurons by calculating a ratio of Arc+ vs. Arc− spine density and compared the ratio index between males and females for each experimental group. No overall sex differences were found in any condition (data not shown; cued: BLA thin spines: t = 0.5322, p = 0.6019; BLA mushroom spines: t = 0.08976, p = 0.9296; TeA thin spines: t = 0.3901, p = 0.7024; TeA mushroom spines: t = 1.848, p = 0.0858. shock only: BLA thin spines: t = 0.1812, p = 0.8593; BLA mushroom spines: t = 0.3624, p = 0.7234; TeA thin spines: t = 0.2549, p = 0.8031; TeA mushroom spines: t = 0.5379, p = 0.6005). Additionally, we compared overall mushroom and thin spine densities between males and females for each experimental group and again found no sex-differences in either cued (BLA thin spines: t = 0.1104, p = 0.9134; BLA mushroom spines: t = 1.195, p = 0.2496; TeA thin spines: t = 0.07126, p = 0.9442; TeA mushroom spines: t = 0.1408, p = 0.8900) or shock-only group (BLA thin spines: t = 0.6156, p = 0.5496; BLA mushroom spines: t = 0.6280, p = 0.5418; TeA thin spines: t = 0.9541, p = 0.3588; TeA mushroom spines: t = 0.7682, p = 0.4572). Consequently, male and female animals were combined for all subsequent analyses. Moreover, lack of sex differences shows that inclusion of female animals does not lead to increased variability in this particular paradigm and that our findings extend to both sexes.
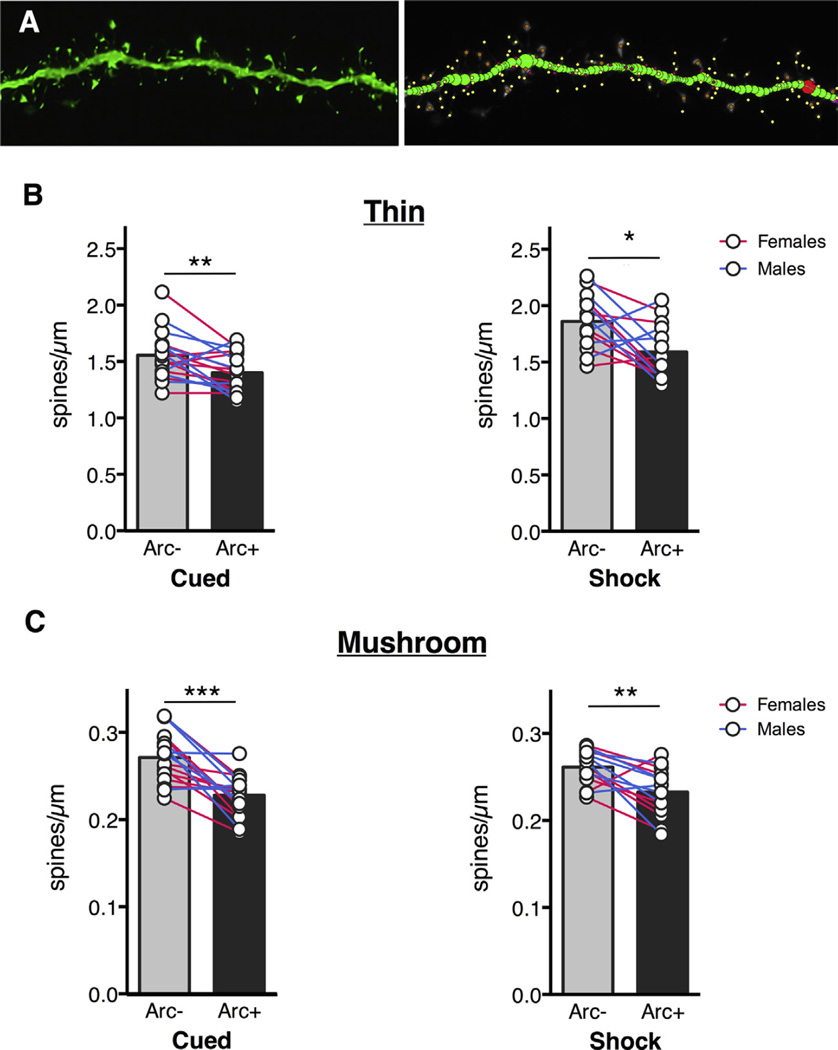
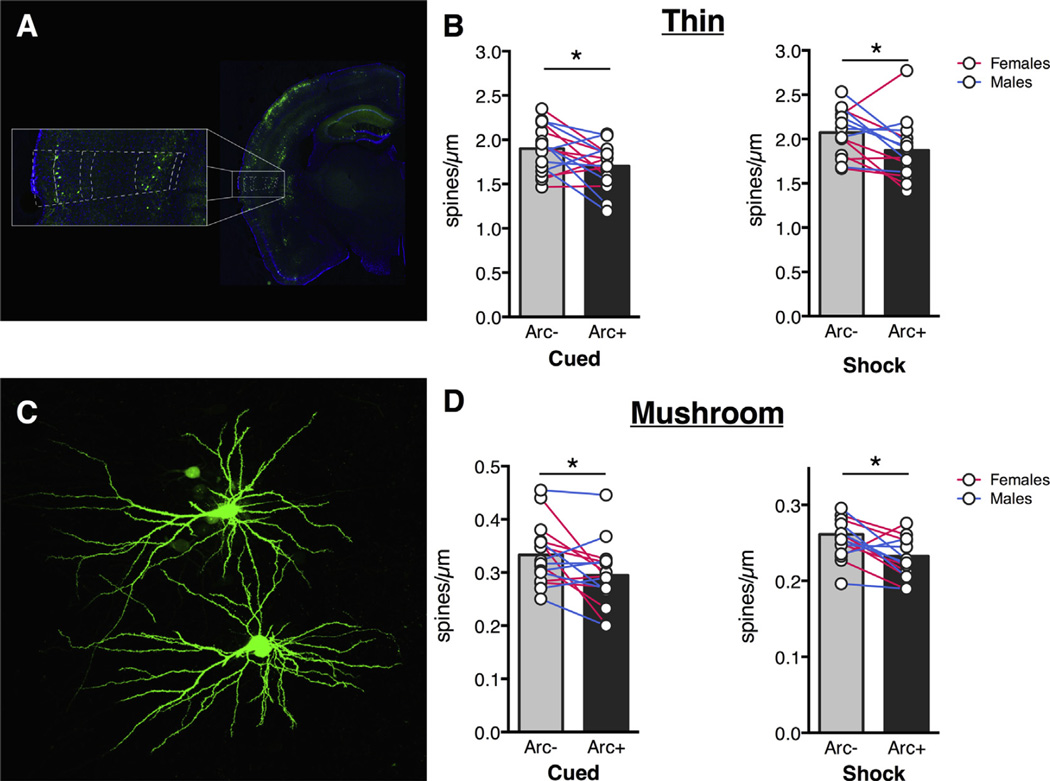
In the BLA, Arc+ neurons had fewer thin spines compared to Arc− neurons in both the cued group the shock-only group (Fig. 3B; main effect of Arc: F(1,30) = 19.36, p = 0.0001). A Sidak’s multiple comparisons test revealed that the effect was present in both groups (Cued: p = 0.0424; Shock only: p = 0.0016). Surprisingly, there was also a main effect of experimental group, with Shock-only animals having higher thin spine densities than animals from the cued condition (main effect of experimental group: F(1,30) = 16.62, p = 0.0003). No significant interaction was found (F (1,30) = 1.414, p = 0.2437). The same effect of Arc was found for mushroom spines in both groups (Fig. 3C; main effect of Arc: F(1,30) = 38.20, p < 0.0001). A Sidak’s multiple comparisons test revealed that the effect was present in both groups (Cued: p < 0.0001, Shock only: p = 0.0054). There was no main effect of experimental condition (F(1,30) = 0.1299, p = 0.7211) or significant interaction (F(1,30) = 1.621, p = 0.2128). We also looked at dendritic spine differences in the temporal association area (TeA) and found similar trends. For both groups we found fewer thin spines on Arc+ neurons (Fig. 4B; main effect of Arc: F(1,28) = 12.24, p = 0.0016) and Sidak’s multiple comparisons test showed that the effect was present in both groups (Cued: p = 0.0332, Shock only: p = 0.0449; Fig. 3A). There was no significant interaction (F(1,28) = 0.0005, p = 0.9823) and a near-significant effect of experimental condition (F(1,28), p = 0.0559). Additionally, mushroom spine density was also lower in Arc+ neurons (Fig. 4C; main effect of Arc: F(1,28) = 14.88, p = 0.0006) and Sidak’s multiple comparisons test showed that both groups showed this effect (Cued: p = 0.0215, Shock only: p = 0.0217). There was no significant interaction (F (1,28) = 0.01569, p = 0.9012) and no main effect of experimental group (F(1,28) = 2.258, p = 0.1441).
Fig. 3.
BLA Arc+ neurons have fewer spines compared to Arc− neurons after cued or uncued shock presentations. (A) Representative BLA dendritic segment and neuronstudio rendering. Thin spines: yellow, mushroom spines: orange. Thin spine density (B) and mushroom spine density (C) is lower in Arc+ neurons in cued and shock-only conditions. Circles represent averages for individual animals, bars represent group averages. Stars represent significance level of Sidak’s multiple comparison test after ANOVA. *p < 0.05, **p < 0.01, ***p < 0.001. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 4.
TeA Arc+ neurons have fewer spines compared to Arc− neurons after cued or uncued shock presentations. (A) Dashed lines show TeA location. Neurons were filled in layer 2. Thin spine density (B) and mushroom spine density (D) is lower in Arc+ neurons in cued and shock-only conditions. Circles represent averages for individual animals, bars represent group averages. Stars represent significance level of Sidak’s multiple comparison test after ANOVA. (C) Filled layer 2 TeA neurons. *p < 0.05.
DISCUSSION
In this study, we introduce a novel approach to assess activity-dependent dendritic spine plasticity in a within-subject design. We used Arc-dVenus mice to identify neurons activated by cued or uncued shock and used targeted microinjections of fluorescent dye to visualize dendritic spines for morphology analysis. We found fewer spines on activated neurons in both the cued and shock-only conditions. These results are in line with a previous study in which fewer spines where found on CA1 neurons activated by context FC compared to non-activated neurons (Sanders et al., 2012). Our results indicate that these effects are not specific to CA1, but extend to the BLA and TeA.
Although the general direction of activity-related spine changes was the same in Sanders et al and the current work, there are several experimental design differences between the two studies that are worth noting. First, we analyzed spine densities 6 h after conditioning, which is much earlier than the 24-h time point used by Sanders et al. We do not know whether our observations would continue to match those of Sanders et al had we also waited 24 h before sacrifice, but we look forward to delineating the timeline of structural changes in our model in the future. Second, we used different immediate early genes as markers for neuronal activity. While it is not clear how much c-fos and Arc overlap after a given experience (for review: Minatohara et al., 2015), there is evidence for differential expression in the BLA during opiate withdrawal (Lucas et al., 2008), in the hippocampus after exposure to a novel environment (Christensen et al., 2013), and in the nucleus accumbens after immobilization stress (Ons et al., 2004). Finally, there may be functional differences in recruitment of BLA and TeA neurons vs. those in CA1 after aversive associative learning and/or shock exposure. Given our observation that both cued and uncued shock conditions resulted in fewer spines on activated BLA and TeA neurons, these differences may not be due to learning-related changes per se, but rather to the experience of aversive foot shocks regardless of associated auditory stimuli.
It is not yet possible to determine whether the reduced spine density in Arc+ neurons reflects a selection effect (i.e., neurons with fewer spines are more likely to be activated) or active removal of spines (i.e., “pruning”) in response to the aversive experience. We were especially surprised to observe fewer mushroom spines in activated neurons at this time point, since mushroom spines are generally thought to be more stable than thin spines. However, this notion is mainly based on examination of basal turnover rate of spines over time (Trachtenberg et al., 2002; Holtmaat et al., 2005), and it is less clear if mushroom spines are less likely than thin spines to shrink or be eliminated in response to neuronal activity. Spines on activated neurons could be reduced to strengthen only activated synapses to increase specificity of synaptic contacts on the neurons. Decreases in spine density after a putative learning event are seemingly counter-intuitive, considering reports of increased spine density in CA1 after a spatial learning task (Moser et al., 1994) or trace eyeblink conditioning (Leuner et al., 2003), and increased number of synapses in motor cortex after motor skill learning (Kleim et al., 2002). However, these studies did not distinguish between activated and non-activated neurons. Weakening of inactive synapses within strongly activated neurons could be mediated by Arc, the activity marker used in this study. Arc plays a role in homeostatic synaptic scaling by accelerating AMPA receptor endocytosis (Chowdhury et al., 2006) and reducing AMPAR-mediated synaptic currents (Rial Verde et al., 2006), specifically targeting inactive synapses through an “inverse” synaptic tagging mechanism (Okuno et al., 2012).
In this study, we analyzed dendritic spines in neurons that were activated by cued or uncued shock exposure and found fewer spines on activated neurons. The finding that Arc+ neurons had fewer spines regardless of experimental condition suggests that at this time point, activated BLA and TeA neurons may primarily reflect processing of the shock. In support of this, previous work has shown that shock presentation alone is sufficient to disinhibit layer 2/3 pyramidal neurons in auditory cortex (including TeA region (Letzkus et al., 2011)). Additionally, the BLA is involved in both auditory and contextual conditioning (Maren, 2001) so comparable morphological profiles in Arc+ neurons across cued and uncued shock conditions would be expected. Finally, it is also possible that activated neurons represent distinct stimuli processing in cued and uncued shock, but either undergo similar patterns of remodeling, and/or are recruited according to similar mechanistic “rules.” We look forward to further dissecting these possibilities in the future.
To our knowledge, this is the first within-animal report of spine profiles in active vs. inactive neurons after either cued or uncued shock exposure, demonstrating reliably lower spine density in active neurons in both males and females. Although (as noted above) we cannot attribute our effects to learning per se, it will be interesting to see if spine differences change at different time points after FC vs. after memory recall or extinction testing. More work is also needed in order to determine whether these findings extend to activated neurons in other brain regions (e.g., prefrontal cortex) and in other common behavioral paradigms.
Despite years of research on dendritic morphology, the exact relationship between structural changes and functional outcomes is still unclear. As techniques advance, however, future research will get closer to answering these questions. For example, Hayashi-Takagi et al. (2015) developed a technique to label and shrink spines in the motor cortex that formed after motor learning tasks and showed that erasure of newly formed spines resulted in loss of learned motor skills. It will be interesting to see this groundbreaking technique applied to other established learning and memory processes such as FC.
Acknowledgments
The authors thank Alexis Stefano for help with spine analysis.
Abbreviations
- BLA
basolateral amygdala
- FC
fear conditioning
- PBS
phosphate-buffered saline
- TeA
temporal association area.
Footnotes
UNCITED REFERENCE
REFERENCES
- Anglada-Figueroa D, Quirk GJ. Lesions of the basal amygdala block expression of conditioned fear but not extinction. [Accessed November 6, 2015];J Neurosci. 2005 25:9680–9685. doi: 10.1523/JNEUROSCI.2600-05.2005. Available at: < http://www.jneurosci.org/content/25/42/9680.short>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? [Accessed July 21, 2014];Curr Opin Neurobiol. 2007 17:381–386. doi: 10.1016/j.conb.2007.04.009. Available at: < http://www.ncbi.nlm.nih.gov/pubmed/17498943>. [DOI] [PubMed] [Google Scholar]
- Brandon JG, Coss RG. Rapid dendritic spine stem shortening during one-trial learning: the honeybee’s first orientation flight. [Accessed March 25, 2016];Brain Res. 1982 252:51–61. doi: 10.1016/0006-8993(82)90977-5. Available at: < http://www.sciencedirect.com/science/article/pii/0006899382909775>. [DOI] [PubMed] [Google Scholar]
- Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. [Accessed September 3, 2015];Neuron. 2006 52:445–459. doi: 10.1016/j.neuron.2006.08.033. Available at: < http://www.sciencedirect.com/science/article/pii/S0896627306006829>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DZ, Thomsen MS, Mikkelsen JD. Reduced basal and novelty-induced levels of activity-regulated cytoskeleton associated protein (Arc) and c-Fos mRNA in the cerebral cortex and hippocampus of APPswe/PS1ΔE9 transgenic mice. Neurochem Int. 2013;63:54–60. doi: 10.1016/j.neuint.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. Available at: < http://neuro.annualreviews.org/cgi/doi/10.1146/annurev.neuro.15.1.353>. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Pare D. Amygdala microcircuits controlling learned fear. [Accessed June 30, 2015];Neuron. 2014 82:966–980. doi: 10.1016/j.neuron.2014.04.042. Available at: < http://www.sciencedirect.com/science/article/pii/S08966273140035 72>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi M, Yamaguchi S. In vivo and in vitro visualization of gene expression dynamics over extensive areas of the brain. [Accessed December 28, 2015];Neuroimage. 2009 44:1274–1283. doi: 10.1016/j.neuroimage.2008.10.046. Available at: < http://www.sciencedirect.com/science/article/pii/S1053811908011713>. [DOI] [PubMed] [Google Scholar]
- Gouty-Colomer LA, Hosseini B, Marcelo IM, Schreiber J, Slump DE, Yamaguchi S, Houweling AR, Jaarsma D, Elgersma Y, Kushner SA. Arc expression identifies the lateral amygdala fear memory trace. [Accessed November 17, 2015];Mol Psychiatry 2015. 2015 doi: 10.1038/mp.2015.18. Available at: < http://dx.doi.org/10.1038/mp.2015.18>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, Larson JR, Withers GS. Effects of unilateral and bilateral training in a reaching task on dendritic branching of neurons in the rat motor-sensory forelimb cortex. [Accessed March 24, 2016];Behav Neural Biol. 1985 44:301–314. doi: 10.1016/s0163-1047(85)90310-3. Available at: < http://www.sciencedirect.com/science/article/pii/S0163104785903103>. [DOI] [PubMed] [Google Scholar]
- Han J-H, Kushner SA, Yiu AP, Hsiang H-LL, Buch T, Waisman A, Bontempi B, Neve RL, Frankland PW, Josselyn SA. Selective erasure of a fear memory. Science. 2009;323:1492–1496. doi: 10.1126/science.1164139. [DOI] [PubMed] [Google Scholar]
- Hayashi-Takagi A, Yagishita S, Nakamura M, Shirai F, Wu YI, Loshbaugh AL, Kuhlman B, Hahn KM, Kasai H. Labelling and optical erasure of synaptic memory traces in the motor cortex. [Accessed September 10, 2015];Nature. 2015 525:333–338. doi: 10.1038/nature15257. Available at: < http://dx.doi.org/10.1038/nature15257>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Müller C, Lüthi A. Switching on and off fear by distinct neuronal circuits. [Accessed July 9, 2014];Nature. 2008 454:600–606. doi: 10.1038/nature07166. Available at: < http://dx.doi.org/10.1038/nature07166>. [DOI] [PubMed] [Google Scholar]
- Holtmaat AJGD, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, Knott GW, Svoboda K. Transient and persistent dendritic spines in the neocortex in vivo. [Accessed February 8, 2016];Neuron. 2005 45:279–291. doi: 10.1016/j.neuron.2005.01.003. Available at: < http://www.cell.com/article/S0896627305000048/fulltext>. [DOI] [PubMed] [Google Scholar]
- Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J. Structural dynamics of dendritic spines in memory and cognition. [Accessed September 19, 2015];Trends Neurosci. 2010 33:121–129. doi: 10.1016/j.tins.2010.01.001. Available at: < http://www.sciencedirect.com/science/article/pii/S0166223610000020>. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Barbay S, Cooper NR, Hogg TM, Reidel CN, Remple MS, Nudo RJ. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. [Accessed September 17, 2015];Neurobiol Learn Mem. 2002 77:63–77. doi: 10.1006/nlme.2000.4004. Available at: < http://www.sciencedirect.com/science/article/pii/S1074742700940048>. [DOI] [PubMed] [Google Scholar]
- Lai CSW, Franke TF, Gan W-B. Opposite effects of fear conditioning and extinction on dendritic spine remodelling. [Accessed October 6, 2015];Nature. 2012 483:87–91. doi: 10.1038/nature10792. Available at: < http://dx.doi.org/10.1038/nature10792>. [DOI] [PubMed] [Google Scholar]
- Letzkus JJ, Wolff SBE, Meyer EMM, Tovote P, Courtin J, Herry C, Lüthi A. A disinhibitory microcircuit for associative fear learning in the auditory cortex. [Accessed October 29, 2015];Nature. 2011 480:331–335. doi: 10.1038/nature10674. Available at: < http://dx.doi.org/10.1038/nature10674>. [DOI] [PubMed] [Google Scholar]
- Leuner B, Falduto J, Shors TJ. Associative memory formation increases the observation of dendritic spines in the hippocampus. [Accessed January 19, 2016];J Neurosci. 2003 23:659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. Available at: < http://www.jneurosci.org/content/23/2/659.short>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M, Frenois F, Vouillac C, Stinus L, Cador M, Le Moine C. Reactivity and plasticity in the amygdala nuclei during opiate withdrawal conditioning: differential expression of c-fos and arc immediate early genes. [Accessed January 28, 2016];Neuroscience. 2008 154:1021–1033. doi: 10.1016/j.neuroscience.2008.04.006. Available at: < http://www.sciencedirect.com/science/article/pii/S0306452208005666>. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. [Accessed November 11, 2015];Annu Rev Neurosci. 2001 24:897–931. doi: 10.1146/annurev.neuro.24.1.897. Available at: < http://www.annualreviews.org/doi/full/10.1146/annurev.neuro.24.1.897>. [DOI] [PubMed] [Google Scholar]
- Matsuo N, Reijmers L, Mayford M. Spine-type-specific recruitment of newly synthesized AMPA receptors with learning. [Accessed December 3, 2015];Science. 2008 319:1104–1107. doi: 10.1126/science.1149967. Available at: < http://www.sciencemag.org/content/319/5866/1104.full>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GCR, Kasai H. Structural basis of long-term potentiation in single dendritic spines. [Accessed December 16, 2014];Nature. 2004 429:761–766. doi: 10.1038/nature02617. Available at: < http://dx.doi.org/10.1038/nature02617>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minatohara K, Akiyoshi M, Okuno H. Role of immediate-early genes in synaptic plasticity and neuronal ensembles underlying the memory trace. [Accessed January 21, 2016];Front Mol Neurosci. 2015 8:78. doi: 10.3389/fnmol.2015.00078. Available at: < http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4700275&tool=pmcentrez&rendertype=abstract>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Trommald M, Andersen P. An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial learning in adult rats suggests the formation of new synapses. [Accessed January 6, 2016];Proc Natl Acad Sci U S A. 1994 91:12673–12675. doi: 10.1073/pnas.91.26.12673. Available at: < http://www.pnas.org/content/91/26/12673.short>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno H, Akashi K, Ishii Y, Yagishita-Kyo N, Suzuki K, Nonaka M, Kawashima T, Fujii H, Takemoto-Kimura S, Abe M, Natsume R, Chowdhury S, Sakimura K, Worley PF, Bito H. Inverse synaptic tagging of inactive synapses via dynamic interaction of Arc/Arg3.1 with CaMKIIβ. [Accessed May 4, 2015];Cell. 2012 149:886–898. doi: 10.1016/j.cell.2012.02.062. Available at: < http://www.sciencedirect.com/science/article/pii/S0092867412004151>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ons S, Martí O, Armario A. Stress-induced activation of the immediate early gene Arc (activity-regulated cytoskeleton-associated protein) is restricted to telencephalic areas in the rat brain: relationship to c-fos mRNA. [Accessed January 28, 2016];J Neurochem. 2004 89:1111–1118. doi: 10.1111/j.1471-4159.2004.02396.x. Available at: < http://www.ncbi.nlm.nih.gov/pubmed/15147503>. [DOI] [PubMed] [Google Scholar]
- Patel SN, Stewart MG. Changes in the number and structure of dendritic spines 25 hours after passive avoidance training in the domestic chick, Gallus domesticus. [Accessed March 25, 2016];Brain Res. 1988 449:34–46. doi: 10.1016/0006-8993(88)91021-9. Available at: < http://www.sciencedirect.com/science/article/pii/0006899388910219>. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat: a review. [Accessed December 3, 2015];Ann N Y Acad Sci. 2006 911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. Available at: < http://doi.wiley.com/10.1111/j.1749-6632.2000.tb06738.x>. [DOI] [PubMed] [Google Scholar]
- Rial Verde EM, Lee-Osbourne J, Worley PF, Malinow R, Cline HT. Increased expression of the immediate-early gene arc/arg3.1 reduces AMPA receptor-mediated synaptic transmission. [Accessed January 29, 2016];Neuron. 2006 52:461–474. doi: 10.1016/j.neuron.2006.09.031. Available at: http://www.sciencedirect.com/science/article/pii/S0896627306007380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, Ehlenberger DB, Dickstein DL, Hof PR, Wearne SL. Automated three-dimensional detection and shape classification of dendritic spines from fluorescence microscopy images. [Accessed January 4, 2016];PLoS One. 2008 3:e1997. doi: 10.1371/journal.pone.0001997. Available at: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0001997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J, Cowansage K, Baumgärtel K, Mayford M. Elimination of dendritic spines with long-term memory is specific to active circuits. [Accessed November 16, 2015];J Neurosci. 2012 32:12570–12578. doi: 10.1523/JNEUROSCI.1131-12.2012. Available at: < http://www.jneurosci.org/content/32/36/12570.short>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K. Long-term in vivo imaging of experience- dependent synaptic plasticity in adult cortex. [Accessed August 18, 2015];Nature. 2002 420:788–794. doi: 10.1038/nature01273. Available at: < http://dx.doi.org/10.1038/nature01273>. [DOI] [PubMed] [Google Scholar]
- Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y. Rapid formation and selective stabilization of synapses for enduring motor memories. [Accessed December 23, 2015];Nature. 2009 462:915–919. doi: 10.1038/nature08389. Available at: < http://dx.doi.org/10.1038/nature08389>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan W-B. Stably maintained dendritic spines are associated with lifelong memories. [Accessed January 14, 2016];Nature. 2009 462:920–924. doi: 10.1038/nature08577. Available at: < http://dx.doi.org/10.1038/nature08577>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zito K, Scheuss V, Knott G, Hill T, Svoboda K. Rapid functional maturation of nascent dendritic spines. [Accessed January 14, 2016];Neuron. 2009 61:247–258. doi: 10.1016/j.neuron.2008.10.054. Available at: http://www.sciencedirect.com/science/article/pii/S0896627308009653. [DOI] [PMC free article] [PubMed] [Google Scholar]