Abstract
Voltage-gated sodium channels (VGSCs), composed of a pore-forming α subunit and up to two associated β subunits, are critical for the initiation of the action potential (AP) in excitable tissues. Building on the monumental discovery and description of sodium current in 1952, intrepid researchers described the voltage-dependent gating mechanism, selectivity of the channel, and general structure of the VGSC channel. Recently, crystal structures of bacterial VGSC α subunits have confirmed many of these studies and provided new insights into VGSC function. VGSC β subunits, first cloned in 1992, modulate sodium current but also have nonconducting roles as cell-adhesion molecules and function in neurite outgrowth and neuronal pathfinding. Mutations in VGSC α and β genes are associated with diseases caused by dysfunction of excitable tissues such as epilepsy. Because of the multigenic and drug-resistant nature of some of these diseases, induced pluripotent stem cells and other novel approaches are being used to screen for new drugs and further understand how mutations in VGSC genes contribute to pathophysiology.
The β subunits of voltage-gated sodium channels (VGSCs) modulate sodium current but also have nonconducting roles (e.g., in cell adhesion). Mutations in the VGSC genes are associated with various diseases (e.g., epilepsy).
Voltage-gated sodium channels (VGSCs) conduct inward current that depolarizes the plasma membrane and initiates the action potential (AP) in excitable cells, including neurons, cardiomyocytes, and skeletal muscle cells. Because of the intrinsic link between VGSCs and cellular excitability, it is not surprising that mutations in VGSC genes are linked with epilepsy, cardiac arrhythmia, neuropathic pain, migraine, and neuromuscular disorders. The goal of this review is to provide an overview of critical discoveries in VGSC physiology and discuss the challenges of studying VGSCs in disease, including some of the exciting techniques to address these challenges.
VGSC DISCOVERY AND STRUCTURE
In 1952, the Nobel Laureates Alan Lloyd Hodgkin and Andrew Fielding Huxley first recorded sodium current (INa) using their voltage-clamp technique on the squid giant axon. Their experiments showed three key features of INa—selective Na+ conductance, voltage-dependent activation, and rapid inactivation (Hodgkin and Huxley 1952). The Hodgkin–Huxley model mathematically described voltage-dependent initiation of the AP by inward INa, followed by fast inactivation of INa, and simultaneous activation of outward IK. Outward IK was postulated to reestablish the charge balance across the plasma membrane (Hodgkin and Huxley 1952). These results were supported by studies of peripheral motor neurons by Sir John Carew Eccles, who shared the Nobel Prize with Hodgkin and Huxley. Among other contributions, he identified deviations in membrane potential and APs because of injections of sodium into motor neurons (Coombs et al. 1955). In addition to this monumental discovery, refinement of the voltage-clamp technique opened the door for a plethora of research on membrane potential physiology.
In the 1960s, Bertil Hille and Clay Armstrong proposed the idea that INa and IK are conducted through specific ion channels. At that time, many studies centered on performing voltage-clamp recordings of squid axon with sodium-free solutions containing organic cations (e.g., ammonium) to determine the size of the ion-conducting pore. The axon membrane became transiently permeable to these ions following depolarization, and this permeability was abolished by tetrodotoxin (TTX) (Larramendi et al. 1956; Lorente De No et al. 1957; Tasaki et al. 1965, 1966; Tasaki and Singer 1966; Binstock and Lecar 1969). Hille added to this body of work by using additional metal and many organic cations to develop a model of the narrowest region of the ion-conducting pore of the channel (Hille 1971, 1972). Hille’s model proposed a partial dehydration of Na+ through interaction with a high-field-strength site at the extracellular end of the pore followed by rehydration in the lumen of the pore. This model is astonishingly close to what we now know to be true about VGSCs from crystal structure information (Payandeh et al. 2011; McCusker et al. 2012; Zhang et al. 2012). Armstrong and Bezanilla developed signal-averaging techniques to detect the movement of “gating charges” (Armstrong and Bezanilla 1973, 1974) corresponding to what we now understand to be the movement of voltage sensors, which respond to changes in membrane potential. Remarkably, without having any of the knowledge that we possess today about nucleotide/amino acid sequence and crystal structures, these early studies made many accurate predictions about VGSC structure and the functions of various channel domains.
Although early physiological studies provided evidence for the existence of a VGSC, the biochemical proof that tetrodotoxin/saxitoxin (STX) receptors were also ion-conducting did not yet exist. Membrane protein purification techniques were being established, but they required an extraordinarily large amount of time and biological starting material compared to today’s techniques. Photoaffinity labeling of STX receptors with a scorpion toxin derivative by William Catterall’s group identified a family of VGSC proteins that were designated α and β subunits (Beneski and Catterall 1980). Purification of VGSC protein to theoretical homogeneity was challenging because of the difficulty of solubilizing a high molecular weight, highly lipophilic membrane protein and the large amount of biological starting material required. Again, the Catterall laboratory was the first to overcome these challenges and purify VGSCs from rat brain (Hartshorne and Catterall 1981, 1984; Hartshorne et al. 1982). This work was closely followed by purification of VGSCs from rat and rabbit skeletal muscle (Barchi 1983; Kraner et al. 1985), and from chicken heart (Lombet and Lazdunski 1984). Reconstitution of purified VGSCs in a lipid bilayer membrane allowed observation of Na+ flux and confirmed that a functional VGSC protein had been purified (Hartshorne et al. 1985). The evolution of VGSC purification methods is discussed in detail in Catterall (1992). Purification from rat brain revealed that VGSCs are heterotrimers, composed of a single α subunit and two non-pore-forming β subunits, β1 and β2 (Hartshorne and Catterall 1981). We now know that there are more than two VGSC β subunits. Subsequent homology cloning and heterologous expression studies showed that each VGSC α is associated at the plasma membrane with a noncovalently linked β1 or β3 subunit and a covalently linked β2 or β4 subunit (Morgan et al. 2000; Yu et al. 2003).
To clone the first VGSC α subunit complementary DNA (cDNA), researchers from Shosaku Numa’s group took on the arduous task of purifying brain VGSCs and obtaining partial amino acid sequence by amino-terminal Edman degradation as well as through cleaved peptides from the α subunit. They then generated and screened multiple cDNA libraries based first on predicted degenerate cDNA sequences and then on cloned sequences. Assembly of the cloned VGSC cDNA fragments revealed what we now know to be Scn1a (Noda et al. 1984). Heterologous expression of the cloned cDNAs gave a functional channel, which we now recognize as the VGSC α subunit Nav1.1.
VGSC β SUBUNITS ARE REQUIRED TO RECAPITULATE PHYSIOLOGICAL EXPRESSION OF INa
Early cloning of Nav1.2 cDNA and its expression in oocytes resulted in INa that inactivated more slowly than INa recorded from neurons. Co-injection of low-molecular-weight rat brain messenger RNA (mRNA) was required for recapitulation of physiological INa in terms of rates of activation and inactivation, and voltage dependence (Auld et al. 1988; Krafte 1990). Cloning and expression of the VGSC β1 and β2 subunits showed that they mimicked the effects of low-molecular-weight mRNA on α subunit expression in oocytes (Isom et al. 1992, 1995b). Coexpression of Nav1.2 + β1 in oocytes resulted in a larger INa that activated and inactivated more rapidly and had a negative shift in the voltage-dependence of inactivation compared to α alone. Coexpression of Nav1.2 with both β1 and β2 further increased INa density (Isom et al. 1995b). As discussed by Calhoun and Isom, when expressed in heterologous systems, β subunits in general increase INa density, shift the voltage-dependence of current activation and inactivation, and accelerate the rates of activation and inactivation. However, the magnitude of current increase and direction of the shifts in activation and inactivation are dependent on the particular heterologous cell line and the identities of the β and α cDNAs expressed (Calhoun and Isom 2014). Importantly, heterologous expression systems cannot replicate the native cellular milieu, especially the multiprotein VGSC complexes that are known to form in specific subcellular domains of neurons and cardiac myocytes (Calhoun and Isom 2014). For example, in Scn1b null ventricular myocytes, peak and persistent sodium current is increased, mediated by increased Nav1.5 expression, with no effect on channel kinetics and voltage dependence (Lopez-Santiago et al. 2007). Furthermore, loss of Scn1b also results in increased TTX-S current and Scn3a expression in ventricular myocytes (Lin et al. 2015). However, in the dorsal root ganglia (DRG), critical neurons for peripheral pain sensation, Scn1b deletion causes a depolarizing shift in the voltage dependence of VGSC inactivation and decreased persistent INa (Lopez-Santiago et al. 2011). As heterologous expression systems cannot accurately replicate these cell types, in vivo and transgenic mouse models offer more appropriate methods for studying the functional roles of VGSC physiology.
MECHANISTIC INSIGHTS ON VGSC STRUCTURE AND FUNCTION
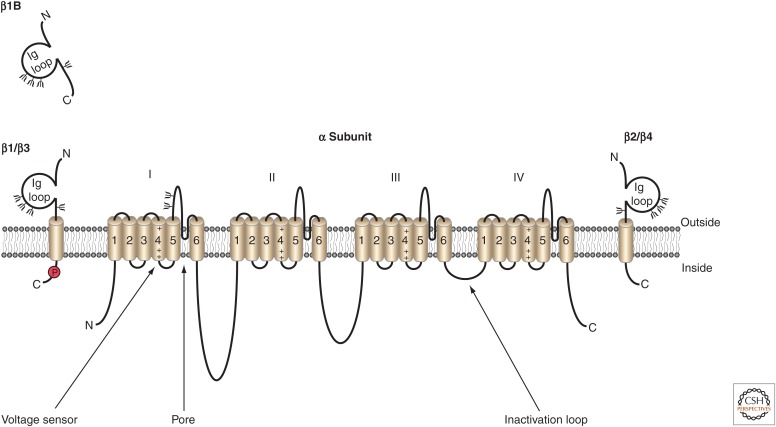
VGSC α subunits are highly evolutionarily conserved. Each α subunit is composed of four homologous domains, each containing six transmembrane helices (S1–S6), which come together in pseudotetrafold symmetry to form the ion-conducting pore (Fig. 1). As discussed earlier, long before the emergence of crystal structures, much of VGSC structure and function was inferred through studies modeling gating and using site-specific antibodies and toxins during electrophysiological recordings.
Figure 1.
Voltage-gated sodium channel (VGSC) α and β subunit topology. The pore-forming α subunit is composed of four homologous domains (I–IV), each with six transmembrane segments. The fourth segment in each domain contains the voltage-sensing domain of the channel. The inactivation loop between domains III and IV contains the isoleucine, phenylalanine, and methionine (IFM) domain required for channel inactivation. Up to two β subunits may be associated with VGSCs. β1 and β3 subunits associate noncovalently, whereas β2 and β4 subunits associate via a disulfide link. (From Brackenbury and Isom 2011; reprinted, with permission, © Frontiers Media SD.)
The sliding-helix model of VGSC voltage-dependent activation was proposed in the 1980s by Catterall (1986a,b). The S4 segments of each domain that serve as voltage sensors contain arginine residues at every third position, which pair with negatively charged residues in nearby transmembrane segments. On membrane depolarization, these residues change ion-pair partners, causing each S4 helix to rotate or slide outward, pushing a single arginine residue in each of the four segments out, and generating gating current that activates, or opens, the channel pore (Payandeh et al. 2011). Data from potassium channel structures prompted the paddle model as an alternate mechanism of voltage-dependent activation (Jiang et al. 2003); however, the solving of multiple prokaryotic α subunit crystal structures showed the validity of the sliding-helix model for VGSCs (Payandeh et al. 2011; Vargas et al. 2012).
Another critical property of VGSCs is voltage-dependent inactivation, which occurs despite an ongoing depolarizing pulse. Early studies identified the intracellular loop between domains III and IV as the inactivation gate (Fig. 1). This was proposed to “swing” into the pore in a voltage-dependent manner, physically blocking ion conduction, and effectively inactivating the pore (Vassilev et al. 1988, 1989). A short amino acid sequence in the inactivation gate, isoleucine, phenylalanine, and methionine (IFM), was identified as critical to the process of inactivation. Mutation of these three residues to glutamine resulted in a noninactivating channel (West et al. 1992; Kellenberger 1997). These studies, combined with a three-dimensional nuclear magnetic resonance (NMR) structure of the inactivation gate (Rohl et al. 1999), helped form the current understanding of the molecular basis for fast inactivation of VGSCs.
Recent crystal structures of bacterial VGSCs have led to a more detailed understanding of the pore and selectivity filter. The membrane reentrant P-loop, located between transmembrane helices S5 and S6 of each domain that line the pore, was identified as the target for the pore-blocking TTX, suggesting its close location to the pore (Noda et al. 1989; Terlau et al. 1991). The crystal structure of a bacterial VGSC showed that the four P-loops together form a ring of glutamates, located near the extracellular end of the pore (Payandeh et al. 2011). As predicted in 1992, these highly conserved glutamates form a high-field-strength site critical in determining ion selectivity of the channel (Heinemann et al. 1992). These residues stabilize multiple ionic occupancy states, preferentially conducting hydrated Na+ through the pore (Payandeh et al. 2011). In contrast, potassium channels conduct dehydrated K+ (Doyle et al. 1998).
Capturing crystal structures of bacterial VGSCs in different activation states has provided new insights and biochemical modeling tools. A closed-pore conformation of the Arcobacter channel (NavAb), crystallized within a lipid-based bicelle, offered the first VGSC 3D structure (Payandeh et al. 2011). A putatively inactive NavRh channel from the marine bacterium Rickettsiales showed significant differences from NavAb and supplied new information about conformational rearrangements required for inactivation (Zhang et al. 2012). Finally, the apparently open conformation of the NavM structure from the marine bacterium Magnetococcus presented further insights into channel gating and selectivity (McCusker et al. 2012).
Despite the wealth of information provided by these prokaryotic VGSC structures, crystallization of a eukaryotic VGSC is the essential next step. Although prokaryotic VGSCs are homotetramers, mammalian VGSC α subunits are single polypeptides containing four nonidentical domains. Further, the extensive extracellular and intracellular loops of mammalian channels are not present in their prokaryotic orthologs. Thus, major gaps in the field include the crystal structure of a mammalian α subunit and the co-crystal structure of an α subunit associated with β subunits. This information will be critical in fully understanding mammalian VGSC structure.
VGSC Diversity
Originally, VGSC α subunit proteins were named according to the tissue from which they were purified. For example, brain type II, rat II, and R-II are all outdated terms for Nav1.2 (Catterall et al. 2005). To date, nine VGSC α proteins (Nav1.1–1.9) and five β proteins (β1–4 and β1B), encoded by SCN(X)A and SCN1B-SCN4B genes, respectively, have been identified in mammalian genomes.
A BLAST search of the cDNAs encoding β1 and β2 in 1995 revealed important, and unexpected, new information. Both proteins contained areas of homology with known CAMs of the immunoglobulin (Ig) superfamily, for example, contactin and myelin P0. This led to the hypothesis that β1 and β2 function as CAMs in addition to channel modulation (Isom et al. 1995b). We now know that all five β subunit proteins contain an extracellular Ig domain and have CAM function, as discussed later in this review. β1–4 (encoded by SCN1B-SCN4B) are type 1 transmembrane proteins with an extracellular amino terminus (containing the Ig domain) and an intracellular carboxyl terminus (Isom et al. 1992, 1995b; Morgan et al. 2000). In contrast, β1B, a splice variant of SCN1B, is soluble and, thus, a portion is secreted. Retention of, intron 3 results in generation of an alternate carboxy-terminal domain that does not contain a transmembrane region (Patino et al. 2011). Recently, the three-dimensional crystal structures of the human Ig domains of β3 and β4 were solved. β3 formed a trimer when crystalized (Namadurai et al. 2014). In contrast, the β4 Ig domain was monomeric in crystal form (Gilchrist et al. 2013). Although this could represent a physiological difference in homophilic interactions, it could also be because of the removal of 31 amino acids at the β4 amino terminus to facilitate crystallization (Gilchrist et al. 2013). Comparisons of these structures will give new insights into the function of β subunits, but the co-crystal structure of α and β subunits remains the next frontier.
VGSC α and β subunits are highly expressed in central and peripheral neurons, cardiac myocytes, skeletal muscle, and some nonexcitable cells (Maier et al. 2004; Patino and Isom 2010; Brunklaus et al. 2014), including breast cancer cells (Fraser et al. 2005), astrocytes, and oligodendrocytes. Table 1 shows the gene name, known tissue expression, and diseases associated with mutations for each VGSC subunit (adapted from Patino and Isom 2010; Catterall 2012; Brunklaus et al. 2014). VGSC expression is developmentally regulated. For example, Nav1.3, β3, and β1B are most prevalent in embryonic and neonatal rodent brain. This expression profile then changes such that Nav1.1, Nav1.2, Nav1.6, β1, β2, and β4 are predominant in the adult brain, albeit distribution is not equivalent across brain regions (Kazen-Gillespie et al. 2000; Catterall et al. 2005; Patino et al. 2011).
Table 1.
VGSC genes, expression patterns, and disease associations
| Gene symbol | Type | Tissue distribution | Consequence of mutations | TTX sensitivity |
|---|---|---|---|---|
| SCN1A | Nav1.1 | CNS, PNS, heart, DRG | DS, familial autism, FHM3, FS+, GEFS+, SUDEP | + |
| SCN2A | Nav1.2 | CNS, PNS, DRG | BFNIS, DS, EOEE, familial autism, GEFS, OS | + |
| SCN3A | Nav1.3 | CNS, PNS, heart, DRG | Unclear | + |
| SCN4A | Nav1.4 | Skeletal muscle, heart, DRG | PAM, PMC, HyperPP, HypoPP, SNEL | + |
| SCN5A | Nav1.5 | Skeletal muscle, heart, CNS, DRG | AF, AS, BS, DCM, LQTS, PCCD, SIDS, SSS, SUDEP | - |
| SCN8A | Nav1.6 | CNS, PNS, heart, DRG | EOEE; cognitive impairment, paralysis, ataxia, dystonia | + |
| SCN9A | Nav1.7 | DRG | CIP, IEM, PEPD, PPN | + |
| SCN10A | Nav1.8 | DRG | PPN | - |
| SCN11A | Nav1.9 | DRG | PPN | - |
| SCN1B | β1 | CNS, PNS, heart, DRG, glia (oligodendrocytes, Schwann cells, astrocytes, radial glia) | AF, BS, DS, GEFS+, LQTS, PCCD, TLE | N/A |
| SCN2B | β2 | CNS, PNS, heart, DRG | AF, BS | N/A |
| SCN3B | β3 | CNS, PNS, heart, DRG | AF, BS, PCCD, SIDS, ventricular fibrillation | N/A |
| SCN4B | β4 | CNS, PNS, heart, DRG | LQTS, SIDS | N/A |
| SCN1B | β1B | Fetal CNS, PNS, heart, DRG | BS, PCCD, epilepsy | N/A |
Modified, with permission, from Patino and Isom 2010, © Elsevier Ireland; Catterall 2012, © Wiley; Brunklaus et al. 2014, © BMJ.
AF, Atrial fibrillation; AS, atrial standstill; BFNIS, benign familial neonatal-infantile seizures; BS, Brugada syndrome; CIP, channelopathy-associated insensitivity to pain; CNS, central nervous system; DCM, dilated cardiomyopathy; DRG, dorsal root ganglia; DS, Dravet syndrome; EOEE, early-onset epileptic encephalopathy; FHM3, familial hemiplegic migraine type 3; FS+, febrile seizures plus; GEFS+, genetic epilepsy with febrile seizures plus; HyperPP, hyperkalemic periodic paralysis, HypoPP, hypokalemic periodic paralysis; IEM, inherited erythromelaglia; LQTS, long QT syndrome; OS, Ohtahara syndrome; PAM, potassium-aggravated myotonia; PCCD, progressive cardiac conduction disease; PEPD, paroxysmal extreme pain disorder formally known as familial rectal pain syndrome; PMC, paramyotonia congenital; PNS, peripheral nervous system; PPN, painful peripheral neuropathies; SIDS, sudden infant death syndrome; SNEL, severe neonatal episodic laryngospasm; SSS, sick sinus syndrome; SUDEP, sudden unexplained death in epilepsy; TLE, temporal lobe epilepsy.
VGSC subcellular localization is critical for normal physiological functions. In neurons, VGSCs are concentrated at the axon initial segment (AIS), where the AP is initiated, and nodes of Ranvier in myelinated axons, which are critical for saltatory conduction. In cardiomyocytes, VGSCs are differentially localized at intercalated discs and transverse tubules (see Bao and Isom 2014; Calhoun and Isom 2014 for reviews that discuss this in more detail).
Despite their high sequence similarity, each VGSC α subunit has subtle differences in biophysical properties and pharmacological sensitivities (as summarized in Catterall et al. 2005; Kwong and Carr 2015). Many of the agents that target α subunits are toxins, including TTX, STX, μ-conotoxins, and their derivatives, such as 4,9-anhydro-TTX, which preferentially blocks Nav1.6 (Rosker et al. 2007; Kwong and Carr 2015). VGSCs are canonically separated into TTX-sensitive (TTX-S) and TTX-resistant categories (TTX-R), which are blocked by nanomolar or micromolar concentrations of TTX, respectively (Catterall et al. 2005). Interestingly, mutation of a single residue changes TTX sensitivity (Noda et al. 1989). Modulation of INa by these toxins (reviewed in Gilchrist et al. 2014) can be affected by β subunit expression. For example, expression of β1 and β3, but not β2 or β4, tend to increase the rate of association and binding affinity of μ-conotoxins for VGSCs. However, modulation of INa by STX or TTX was unaffected by β subunit expression (Isom et al. 1995a; Zhang et al. 2013). In vivo, a combination of electrophysiology and toxicology are used to infer which VGSCs contribute to aspects of endogenous INa. Although challenging, in vivo studies are crucial, as heterologous results can be difficult to translate to in vivo physiological and pathophysiological mechanisms.
Posttranslational Modifications of α and β Subunits
VGSC α subunits are heavily glycosylated, although the extent of glycosylation varies among subunits (Bennett 2002). 40–50% of these added carbohydrate residues are sialic acid moieties such that each channel contains ∼100 sialic acid residues (Miller et al. 1983; Roberts and Barchi 1987). Differential glycosylation of VGSC subunits can affect channel gating, putatively because of the local negative charge of sialic acid residues. In general, when α subunits are less glycosylated, channel gating occurs at more depolarized voltages (Recio-Pinto et al. 1990; Bennett et al. 1997; Zhang et al. 1999; Tyrrell et al. 2001). For example, Nav1.9 expressed in neonatal DRG neurons is more glycosylated than in adult DRG, suggesting developmental regulation of glycosylation. This may account for developmental differences in gating of persistent INa attributed to Nav1.9 (Tyrrell et al. 2001). Voltage-dependent activation and inactivation of Nav1.4, but not Nav1.5, shifts when expressed in cells incapable of sialylation, indicating that not all VGSCs are affected equally (Bennett 2002). Glycosylation accounts for about one-third of the molecular weights of β subunits. Mature β subunits contain three to four N-linked glycosylation sites in the Ig domain (Isom et al. 1992; McCormick et al. 1998) all of which are exposed in the β3 crystal structure (Namadurai et al. 2014). Sialic acid residues in these sites affect β1- and β2-mediated modulation of INa and channel surface expression (Johnson et al. 2004; Johnson and Bennett 2006). Critically, VGSC glycosylation varies developmentally and among cell types; for example, neonatal and adult myocytes have distinct differences in glycogen expression, as do atrial and ventricular myocytes (Stocker and Bennett 2006). Therefore, tissue-specific variations in β subunit processing likely include altered sialylation/glycosylation and may contribute to differential modulation of INa by β subunits among cell types (Zhang et al. 1999; Bennett 2002).
β1 can be phosphorylated on intracellular tyrosine residue Y-181 (Malhotra et al. 2002, 2004). Tyrosine phosphorylation regulates β1-mediated recruitment of ankyrin to points of cell–cell contact (Malhotra et al. 2002) and likely occurs via fyn kinase, an src family kinase (Brackenbury et al. 2008; Nelson et al. 2014). Receptor tyrosine phosphatase β interacts with the intracellular domain of β1 and may modulate β1 phosphorylation (Ratcliffe et al. 2000). In cardiac myocytes, nonphosphorylated β1 localizes at the T-tubules with ankyrinB, whereas phosphorylated β1 localizes at intercalated discs with connexin-43, N-cadherin, and Nav1.5 (Malhotra et al. 2004). Therefore, the phosphorylation state Y-181 regulates β1 association with cytoskeletal proteins, as well as its subcellular localization.
β subunits are substrates for sequential proteolytic cleavage by BACE1 (β-secretase) and γ-secretase, similar to amyloid precursor protein. BACE1 cleavage releases the extracellular domain (ECD), followed by a γ-secretase-mediated cleavage event to release the carboxy-terminal fragment (CTF) (Wong et al. 2005). These cleaved peptides may have important physiological functions. For example, the β2-CTF translocates to the nucleus and promotes Scn1a mRNA and protein expression when heterologously expressed in cultured neurons (Kim et al. 2005, 2007). Consistent with this proposed mechanism, total and surface expression of Nav1.1 in acutely dissociated hippocampal slices is significantly reduced in BACE1 null mice compared to wild-type (Kim et al. 2011). The physiological importance of BACE1 and γ-secretase cleavage of the other β subunits is not understood. As β1B can act as a CAM, it is possible that the β1-ECD, which contains the identical Ig loop domain, functions as a ligand for cell adhesion (Patino et al. 2011). γ-Secretase activity and, thus, formation of the β1-CTF is critical for β1-mediated neurite outgrowth in vitro (Brackenbury and Isom 2011). Determining whether these functions occur in vivo represents a critical next step in our understanding of posttranslational processing of VGSCs.
β Subunits Are Multifunctional
Effects on Surface Expression
Coexpression of β subunits with α results in increased INa density, in part, as a result of promotion of α cell-surface expression. Following posttranslational processing, α subunits are stored in an intracellular pool associated with the plasma membrane (Schmidt et al. 1985; Schmidt and Catterall 1986). Concomitant with plasma membrane insertion, α and β2 covalently associate via a disulfide bond (Chen et al. 2012). Scn2b null mice on the C57BL/6×129SV background have a 50% reduction in hippocampal INa density and level of surface α subunits (Chen et al. 2002). However, a second study of Scn2b null mice congenic on the C57BL/6 background saw no change in hippocampal INa (Uebachs et al. 2010), suggesting genetic background influences on β2 function. The effect of β2 on channel surface expression may also depend on the particular α subunit. Scn2b null small-fast DRG neurons have reduced TTX-S INa, but left unchanged TTX-R INa (Lopez-Santiago et al. 2006). An Scn2b-linked Brugada syndrome mutation results in decreased surface Nav1.5 protein compared to wild-type (Riuró et al. 2013). Thus, modulation of α subunit surface expression by β2 may contribute to disease mechanisms. This is not unique to β2; β1 and β1B also promote VGSC surface expression (reviewed in Calhoun and Isom 2014).
Effects of β4 on Resurgent INa
Resurgent INa is caused by the transient opening of VGSCs during recovery from inactivation following the AP. This specialized current is an important adaptation for high-frequency firing neurons, such as cerebellar Purkinje neurons (Raman and Bean 1997). For this to occur, a “blocking protein,” putatively the ICD of β4, must bind to open channels during depolarization and unbind on repolarization, shortening the typical refractory period and producing a resurgent INa. Without β4 expression, resurgent current is lost from cerebellar Purkinje neurons, but is rescued by a β4 ICD peptide (Grieco et al. 2005). This same β4 peptide is sufficient to invoke resurgent INa in Nav1.7-expressing HEK cells (Theile and Cummins 2011). With β4 knockdown in cerebellar granule neurons, resurgent INa is decreased, but is rescued by the β4 peptide (Bant and Raman 2010). Other β subunits may also contribute to modulation of resurgent INa, as Scn1b null cerebellar granule neurons have reduced resurgent INa (Brackenbury et al. 2010).
Cell Adhesion and Neurite Outgrowth
β Subunits are multifunctional. They play critical roles in neurite outgrowth, migration, and maintenance of nodes of Ranvier. β Subunits are CAMs and, as such, recruit other signaling proteins to the VGSC complex. Most is known about the CAM interactions of β1 and β2, which participate in trans-homophilic adhesion, via the Ig loop, with β1 or β2 molecules on adjacent cells (Malhotra et al. 2000). β1 or β2 trans-homophilic adhesion recruits the cytoskeletal protein ankyrin to points of cell–cell contact (Malhotra et al. 2000). Ankyrin recruitment is abolished by phosphorylation of β1-Y181 (Malhotra et al. 2002). β1–β1 trans-homophilic adhesion promotes neurite outgrowth via a pathway requiring fyn kinase, the CAM contactin, γ-secretase activity, and localized INa (Brackenbury et al. 2008, 2010; Brackenbury and Isom 2011). Because β1B contains the identical Ig loop, it similarly promotes neurite outgrowth of neurons expressing β1 (Patino et al. 2011). Together, these properties may contribute to neuronal development in vivo; Scn1b null mice have neuronal pathfinding dysfunction and differences in subcellular localization of α subunits at AIS (Chen et al. 2004; Brackenbury et al. 2008).
β1, and likely β1B, interacts heterophilically with other CAMs and extracellular matrix proteins, including contactin, neurofascin-186, NrCAM, N-cadherin, and tenascin-R (Kazarinova-Noyes et al. 2001; Ratcliffe et al. 2001; Malhotra et al. 2004; McEwen and Isom 2004; Patino et al. 2011). Some of these interactions affect β1-modulation of α surface expression. For instance, coexpression of NF186 or contactin with Nav1.2 and β1 increases Nav1.2 surface expression compared with Nav1.2 and β1 alone, although NF186 and contactin have no impact on Nav1.2 expression in the absence of β1 (Kazarinova-Noyes et al. 2001; McEwen and Isom 2004). Heterophilic interactions with tenascin-R, an extracellular matrix protein secreted by oligodendrocytes during myelination, repels cells expressing β1 or β2 and, therefore, may play a role in restricting VGSCs to nodes of Ranvier in myelinated axons (Xiao et al. 1999). The cell-adhesive properties of β1 and β1B have likely clinical relevance, as most known SCN1B epilepsy mutations are located in or near the Ig loop domain (reviewed in O’Malley and Isom 2015).
Much less is known about the CAM functions of β3 and β4. Studies of β3 homophilic adhesion give conflicting results: the β3 Ig domain appears to interact with full-length β3 when expressed heterologously (Yereddi et al. 2013), yet another group reported that β3 expression could not induce aggregation of Drosophila S2 cells, unlike β1 and β2 (McEwen et al. 2009). β3 and β1 likely do not associate, as β3 does not interact with a peptide containing the β1-ECD (McEwen and Isom 2004). β4 did not promote neurite outgrowth of cerebellar granule neurons, but this observation does not preclude other CAM interactions (Davis et al. 2004).
PATHOPHYSIOLOGICAL ROLES OF VGSCs
Mutations in VGSC genes are associated with many types of genetic epilepsy, peripheral neuropathy, long QT syndrome, neuromuscular disorders, and other diseases associated with dysfunction of excitable tissues. Recent reviews discuss the complex pathophysiological mechanisms of sodium channelopathies associated with pain and cardiac disease (Dib-Hajj et al. 2010; Adsit et al. 2013; Bao and Isom 2014; O’Malley and Isom 2015). Here, we will focus on the role of VGSCs in the genetic epilepsies and cancer as examples of disease resulting from aberrant or loss of VGSC function in mammals.
Epilepsy
Epilepsy results from an imbalance between excitation and inhibition in the brain. More than two million Americans have epilepsy, which can be caused by genetic mutations or brain injury (Hirtz et al. 2007). Epilepsy significantly impacts the quality of life for both patients and caregivers because of the unpredictability of seizures and the risk of comorbidities including cognitive decline, intellectual disability, developmental delay, and sudden unexpected death in epilepsy (SUDEP).
Mutations in genes encoding VGSC α and β subunits are associated with epilepsies with a wide range of phenotypic severities, including genetic epilepsy with febrile seizures plus (GEFS+), an inherited epilepsy with a wide range of phenotypes, and Dravet syndrome (DS), one of the most devastating pediatric epileptic encephalopathies. An intriguing discussion of the complex mechanisms underlying DS was published recently (Chopra and Isom 2014). In 1998, the first VGSC mutation associated with epilepsy was identified in SCN1B in a patient with GEFS+. However, GEFS+ is incompletely penetrant and family members with the same SCN1B mutation present with a wide range of epilepsy severities (Wallace et al. 1998), a now familiar characteristic of the genetic epilepsies. Since this initial discovery, a large number of mutations in SCN1A, SCN2A, SCN8A, and SCN1B (Escayg et al. 2000; O’Malley and Isom 2015) have been implicated in multiple types of epilepsy (see Table 1) (reviewed in part by Shi et al. 2012; Steinlein 2014; Wagnon and Meisler 2015). More than 1200 mutations have been identified in SCN1A alone (Meng et al. 2015). Identifying the pathophysiological mechanisms underlying DS is of particular importance, as traditional antiepileptic drugs often aggravate seizures in DS patients and development of new therapeutics to help these patients is especially critical. Although 70%–80% of DS patients have an identified heterozygous de novo SCN1A mutation (Marini et al. 2011), homozygous mutations in SCN1B have also been reported (Patino et al. 2009; Ogiwara et al. 2012).
Studies of DS SCN1A mutations have shown that a majority result in loss-of-function (Meng et al. 2015). Studies of Scn1a+/− null mice and knockin mice expressing human mutations have led to the “interneuron hypothesis,” suggesting that selective INa loss in GABAergic interneurons causes epilepsy via loss of inhibitory tone (Yu et al. 2006). However, this has become controversial (Chopra and Isom 2014; Isom 2014). More recent studies of DS patient-derived induced pluripotent stem cell (iPSC) neurons showed either that both inhibitory and excitatory (Liu et al. 2013) or excitatory neurons (Jiao et al. 2013) are hyperexcitable compared to nonepileptic controls (Mistry et al. 2014). These studies suggest that SCN1A haploinsufficiency may result in compensatory up-regulation of other VGSC α subunits. Hyperexcitability of both inhibitory and excitatory neurons may lead to seizures in DS via increased network excitability or synchronization of firing.
Scn1b null mice also model DS; they experience spontaneous seizures and early lethality. At least one DS SCN1B mutation causes β1 to be retained intracellularly, preventing surface expression and, thus, function (Patino et al. 2009). Scn1b null mice have differences in neuronal pathfinding that are observed before seizure onset (Brackenbury et al. 2013). Thus, SCN1B-linked DS mutations may alter neuronal excitability by affecting α subunit surface expression/function as well as by altering neuronal development via changes in cell adhesion.
A major concern for epilepsy patients and their families is the risk of SUDEP. Research suggests that a “perfect storm” of seizures, cardiac arrhythmias, respiratory dysfunction, and autonomic and/or parasympathetic dysfunction contributes to SUDEP (Auerbach et al. 2013; Kalume, 2013; Massey et al. 2014). Heterozygous Scn1a-R1407X knockin GEFS+/DS mice, as well as Scn1b null DS mice, have increased transient and persistent INa in cardiac myocytes, which may provide substrates for cardiac arrhythmias and contribute to SUDEP (Lopez-Santiago et al. 2007; Auerbach et al. 2013). Fully understanding the mechanisms underlying SUDEP will be essential to epilepsy treatment.
One of the greatest challenges in epilepsy research is the need for novel antiepileptic drugs. Even with optimal treatment, 20–30% of all epilepsy patients are pharmacoresistant, defined as being unresponsive to at least two different, tolerated antiepileptic drugs (Kwan et al. 2010, 2011). Even in nonrefractory patients, antiepileptic drugs treat disease symptoms rather than preventing disease progression and often have intolerable side effects. A major goal of studying epilepsy-associated VGSC mutations is to further understand epileptogenesis (how a brain becomes epileptic) to inform new antiepileptic drug discovery.
Emerging Roles for VGSCs in Cancer
An exciting area of research is the role of VGSCs in nonexcitable cells, including multiple cancer cell types. Almost two decades ago, researchers observed that cancer cell lines with higher VGSC expression have increased cell motility and metastatic potential. Furthermore, the invasive capacity of these cells could be reduced by incubation with TTX (Grimes et al. 1995; Laniado et al. 1997). Since then, both α and β subunits have been detected in many cancer cell lines and in some patient biopsies. VGSC α subunits are expressed in some cervical, ovarian, breast, and colon tumors in vivo (Fraser et al. 2005; Gao et al. 2010; House et al. 2010; Hernandez-Plata et al. 2012), although some prostate, breast, cervical, and lung cancers express β subunits (reviewed in Patel and Brackenbury, 2015). Studies have identified both positive and negative associations between VGSC expression and metastatic potential. Nav1.5 expression in breast cancer cells correlates positively with increased risk of recurrence and metastasis (Fraser et al. 2005; Yang et al. 2012), and a similar trend has been described for colon, prostate, and ovarian cancers. However, there is an inverse correlation between α expression and clinical grade in glioma and no correlation in lung cancer cell lines (Schrey et al. 2002; Onganer and Djamgoz 2005; Roger et al. 2007). Expression of α subunits can potentiate lateral motility, adhesion, process extension, and other cellular behaviors associated with metastasis (Brackenbury 2012). It is not clear how increased expression of the pore-forming α subunits potentiates invasive changes in the tumor cells. Three potential models have been proposed: increased Na+ influx enhances H+ efflux, thus activating pH-dependent extracellular matrix degradation and invasion; regulation of an “invasion gene network,” particularly by SCN5A, and increased intracellular Ca2+, which enhances formation of invasive projections (reviewed in Brackenbury 2012).
SCN1B and SCN2B expression are associated with metastatic potential in prostate cancer cells (Diss et al. 2008; Jansson et al. 2012). However, studies of the invasive potential of SCN1B-expressing breast cancer in vitro have been contradicted by in vivo studies. β1 expression is associated with less invasive breast cancer cell lines (Chioni et al. 2009); however, in a mouse model of breast cancer, overexpression of β1 increased tumor growth, metastasis, and angiogenesis (Nelson et al. 2014). β1 trans-homophilic adhesion mediates process outgrowth from breast cancer cells (Nelson et al. 2014), thus it is proposed that β1 promotes metastasis by a mechanism similar to β1-mediated neurite outgrowth.
NEW FRONTIERS AND NOVEL TECHNIQUES
The list of pathogenic VGSC gene mutations is growing rapidly. The explosion of whole-genome sequencing has led to multi-institution efforts, such as the NINDS-funded Epi4K Project (www.ninds.nih.gov/news_and_events/news_articles/pressrelease_childhood_epilepsy_genes_08112013.htm), with the goal of sequencing the genomes of 4000 patients with epilepsy, and the England Department of Health funded 100,000 Genomes Project (genomicsengland.co.uk) (Kearney 2014; Siva 2015). However, even with familial genetic information in hand, determining whether mutations are causative and understanding their pathophysiology are not simple tasks. A given patient can have mutations in several genes, which may be causative, benign, or modify another mutation. A single causative mutation may result in a wide range of phenotypes among individuals, for example, DS and GEFS+ patients may express the same SCN1A or SCN1B mutation. Genetic background is critical. Scn1a+/− mouse models of DS have phenotypes of varying severity depending on genetic background (Yu et al. 2006; Miller et al. 2014). This issue can now be addressed directly with the induced-pluripotent stem cell (iPSC) technique. Patient iPSCs, usually derived from a skin cell biopsy, can theoretically be differentiated into any cell type, including cardiomyocytes, and specific neuronal subtypes (Parent and Anderson 2015). With this remarkable technique, we now have the opportunity to study disease-causing mutations in human cells within the context of the patient’s unique genomic background (Takahashi et al. 2007). Furthermore, endogenous genes that may contribute to pathology remain present. Brain organoids made from human iPSC-derived cells with a mutation in a gene involved in pluripotent cell maintenance were used to model microcephaly, providing proof-of-concept for use of organoids to model other neurological diseases (Lancaster et al. 2013). Another advantage of iPSCs is the opportunity to perform drug screening on differentiated human cells, alongside animal models, thus providing insight into toxicity and species-specific effects before clinical trials (reviewed in Ko and Gelb 2014). Patient-derived iPSC cells are critical for precision medicine approaches. For instance, antiepileptic drug effectiveness or toxicity may be optimized on patient-derived iPSC-neurons before patient treatment. As the iPSC technique is further refined and developed, it will provide additional, critical tools in therapeutic development.
Another challenge to the field is the non-specificity of clinically available VGSC-targeted drugs. There are six different potential sites for small molecule targeting on VGSCs, as shown by the wide variety of toxin binding sites. However, most of the current antiarrhythmic, antiepileptic, and analgesic VGSC drugs on the market, for example, lamotrigine, phenytoin, and lidocaine, target the “local anesthetic” site located in domain IVS6 (Kwong and Carr 2015). This site is highly conserved among α subunits; thus, these drugs have little selectivity for specific VGSCs and often cause adverse effects. Development of VGSC agents that target other binding sites remains an important task. Although, in most cases, toxins cannot be used directly for therapeutics, they can greatly inform drug design; small molecules can be developed that preserve beneficial effects while reducing harm. Drugs targeting specific toxin binding sites may allow more selective blockade of specific α subunits to treat disease, such as neuropathic pain, cardiac arrhythmias, epilepsies, and perhaps even cancer (Stevens et al. 2011; Xiao et al. 2014).
Transgenic zebrafish are an economical and rapid vertebrate system that can be used to screen small molecule libraries and FDA-approved drugs for novel applications. Zebrafish with mutations in scn1Lab, the gene orthologous to SCN1A, have spontaneous seizures that are resistant to many antiepileptic drugs and appear to model DS. Peter de Witte’s group showed that DS zebrafish responded to fenfluramine (Zhang et al. 2015). Scott Baraban used locomotion tracking in mutant zebrafish to monitor behavioral seizures in a drug screen, which was validated using diazepam, valproate, and stiripentol, antiepileptic drugs effective in some DS patients. Out of 320 compounds tested, clemizole, an FDA-approved antihistamine, suppressed spontaneous seizures in vivo (Baraban et al. 2013). This approach can be adapted for screening potential therapeutics for any monogenic epilepsy, especially if combined with the CRISPR/Cas9 technique. Discovered in 2010 as a bacterial defense mechanism against phages (Deveau et al. 2010), the CRISPR/Cas9 system has since been adapted to easily and quickly introduce heritable genetic mutations in mice, rats, zebrafish, and rabbits (Chang et al. 2013; Li et al. 2013; Yang et al. 2014a). Compared to zinc-finger nucleases and transcription activator-like effector nucleases (TALENs), CRISPR/Cas creates double-stranded DNA breaks in a sequence-specific manner at sites complementary to a “single-guide RNA.” Because these are easy to design, the CRISPR/Cas9 system vastly improves the precision, speed, and accuracy of creating transgenic cell lines (including iPSCs) and animal models for single gene mutations (Yang et al. 2014b). These and other cutting edge techniques will allow us to discover novel disease mechanisms and therapeutics for diseases linked to VGSC mutations for another six decades (Catterall 2012).
Footnotes
Editors: Paul J. Kammermeier, Ian Duguid, and Stephan Brenowitz
REFERENCES
- Adsit GS, Vaidyanathan R, Galler CM, Kyle JW, Makielski JC. 2013. Channelopathies from mutations in the cardiac sodium channel protein complex. J Mol Cell Cardiol 61: 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong CM, Bezanilla F. 1973. Currents related to movement of the gating particles of the sodium channels. Nature 242: 459–461. [DOI] [PubMed] [Google Scholar]
- Armstrong CM, Bezanilla F. 1974. Charge movement associated with the opening and closing of the activation gates of the Na channels. J Gen Physiol 63: 533–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach DS, Jones J, Clawson BC, Offord J, Lenk GM, Ogiwara I, Yamakawa K, Meisler MH, Parent JM, Isom LL. 2013. Altered cardiac electrophysiology and SUDEP in a model of Dravet syndrome. PLoS ONE 8: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auld VJ, Goldin AL, Krafte DS, Marshall J, Dunn JM, Catterall WA, Lester HA, Davidson N, Dunn RJ. 1988. A rat brain Na+ channel α subunit with novel gating properties. Neuron 1: 449–461. [DOI] [PubMed] [Google Scholar]
- Bant JS, Raman IM. 2010. Control of transient, resurgent, and persistent current by open-channel block by Na channel β4 in cultured cerebellar granule neurons. Proc Natl Acad Sci 107: 12357–12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y, Isom LL. 2014. Nav1.5 and regulatory β subunits in cardiac sodium channelopathies. Card Electrophysiol Clin 6: 679–694. [Google Scholar]
- Baraban SC, Dinday MT, Hortopan GA. 2013. Drug screening in Scn1a zebrafish mutant identifies clemizole as a potential Dravet syndrome treatment. Nat Commun 4: 2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchi RL. 1983. Protein components of the purified sodium channel from rat skeletal muscle sarcolemma. J Neurochem 40: 1377–1385. [DOI] [PubMed] [Google Scholar]
- Beneski DA, Catterall WA. 1980. Covalent labeling of protein components of the sodium channel with a photoactivable derivative of scorpion toxin. Proc Natl Acad Sci 77: 639–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett ES. 2002. Isoform-specific effects of sialic acid on voltage-dependent Na+ channel gating: Functional sialic acids are localized to the S5-S6 loop of domain I. J Physiol 538: 675–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett E, Urcan MS, Tinkle SS, Koszowski AG, Levinson SR. 1997. Contribution of sialic acid to the voltage dependence of sodium channel gating. A possible electrostatic mechanism. J Gen Physiol 109: 327–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binstock L, Lecar H. 1969. Ammonium ion currents in the squid giant axon. J Gen Physiol 53: 342–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury WJ. 2012. Voltage-gated sodium channels and metastatic disease. Channels (Austin) 6: 352–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury WJ, Isom LL. 2011. Na Channel β subunits: Overachievers of the ion channel family. Front Pharmacol 2: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury WJ, Davis TH, Chen C, Slat EA, Detrow MJ, Dickendesher TL, Ranscht B, Isom LL. 2008. Voltage-gated Na+ channel β1 subunit-mediated neurite outgrowth requires Fyn kinase and contributes to postnatal CNS development in vivo. J Neurosci 28: 3246–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury WJ, Calhoun JD, Chen C, Miyazaki H, Nukina N, Oyama F, Ranscht B, Isom LL. 2010. Functional reciprocity between Na+ channel Nav1.6 and β1 subunits in the coordinated regulation of excitability and neurite outgrowth. Proc Natl Acad Sci 107: 2283–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury WJ, Yuan Y, O’Malley HA, Parent JM, Isom LL. 2013. Abnormal neuronal patterning occurs during early postnatal brain development of Scn1b-null mice and precedes hyperexcitability. Proc Natl Acad Sci 110: 1089–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunklaus A, Ellis R, Reavey E, Semsarian C, Zuberi SM. 2014. Genotype phenotype associations across the voltage-gated sodium channel family. J Med Genet 51: 650–658. [DOI] [PubMed] [Google Scholar]
- Calhoun JD, Isom LL. 2014. The role of non-pore-forming β subunits in physiology and pathophysiology of voltage-gated sodium channels. In Voltage gated sodium channels (ed. Ruben PC), pp. 51–89. Springer, Berlin. [DOI] [PubMed] [Google Scholar]
- Catterall WA. 1986a. Molecular properties of voltage-sensitive sodium channels. Annu Rev Biochem 55: 953–985. [DOI] [PubMed] [Google Scholar]
- Catterall WA. 1986b. Voltage-dependent gating of sodium channels: Correlating structure and function. Trends Neurosci 9: 7–10. [Google Scholar]
- Catterall WA. 1992. Cellular and molecular biology of voltage-gated sodium channels. Physiol Rev 72: S15–S48. [DOI] [PubMed] [Google Scholar]
- Catterall WA. 2012. Voltage-gated sodium channels at 60: Structure, function and pathophysiology. J Physiol 590: 2577–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Goldin AL, Waxman SG. 2005. International Union of Pharmacology. XLVII: Nomenclature and structure–function relationships of voltage-gated sodium channels. Pharmacol Rev 57: 397–409. [DOI] [PubMed] [Google Scholar]
- Chang N, Sun C, Gao L, Zhu D, Xu X, Zhu X, Xiong J-W, Xi JJ. 2013. Genome editing with RNA-guided Cas9 nuclease in zebrafish embryos. Cell Res 23: 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Bharucha V, Chen Y, Westenbroek RE, Brown A, Malhotra JD, Jones D, Avery C, Gillespie PJ, Kazen-Gillespie KA, et al. 2002. Reduced sodium channel density, altered voltage dependence of inactivation, and increased susceptibility to seizures in mice lacking sodium channel β2-subunits. Proc Natl Acad Sci 99: 17072–17077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Westenbroek RE, Xu X, Edwards CA, Sorenson DR, Chen Y, McEwen DP, O’Malley Ha, Bharucha V, Meadows LS, et al. 2004. Mice lacking sodium channel β1 subunits display defects in neuronal excitability, sodium channel expression, and nodal architecture. J Neurosci 24: 4030–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Calhoun JD, Zhang Y, Lopez-Santiago L, Zhou N, Davis TH, Salzer JL, Isom LL. 2012. Identification of the cysteine residue responsible for disulfide linkage of Na+ channel α and β2 subunits. J Biol Chem 287: 39061–39069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chioni A-MM, Brackenbury WJ, Calhoun JD, Isom LL, Djamgoz MBA. 2009. A novel adhesion molecule in human breast cancer cells: Voltage-gated Na+ channel β1 subunit. Int J Biochem Cell Biol 41: 1216–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra R, Isom LL. 2014. Untangling the dravet syndrome seizure network: The changing face of a rare genetic epilepsy. Epilepsy Curr 14: 86–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs J, Eccles J, Fatt P. 1955. The electrical properties of the motoneurone membrane. J Physiol 130: 291–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TH, Chen C, Isom LL. 2004. Sodium channel β1 subunits promote neurite outgrowth in cerebellar granule neurons. J Biol Chem 279: 51424–51432. [DOI] [PubMed] [Google Scholar]
- Deveau H, Garneau JE, Moineau S. 2010. CRISPR/Cas system and its role in phage-bacteria interactions. Annu Rev Microbiol 64: 475–493. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Cummins TR, Black JA, Waxman SG. 2010. Sodium channels in normal and pathological pain. Annu Rev Neurosci 33: 325–347. [DOI] [PubMed] [Google Scholar]
- Diss JKJ, Fraser SP, Walker MM, Patel A, Latchman DS, Djamgoz MBA. 2008. β-subunits of voltage-gated sodium channels in human prostate cancer: Quantitative in vitro and in vivo analyses of mRNA expression. Prostate Cancer Prostatic Dis 11: 325–333. [DOI] [PubMed] [Google Scholar]
- Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. 1998. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science 280: 69–77. [DOI] [PubMed] [Google Scholar]
- Escayg A, MacDonald BT, Meisler MH, Baulac S, Huberfeld G, An-Gourfinkel I, Brice A, LeGuern E, Moulard B, Chaigne D, et al. 2000. Mutations of SCN1A, encoding a neuronal sodium channel, in two families with GEFS+2. Nat Genet 24: 343–345. [DOI] [PubMed] [Google Scholar]
- Fraser S, Diss J, Chioni A, Mycielska M, Pan H, Yamaci R, Pani F, Siwy Z, Krasowska M, Grzywna Z, et al. 2005. Voltage-gated sodium channel expression and potentiation of human breast cancer metastasis. Clin Cancer Res 11: 5381–5389. [DOI] [PubMed] [Google Scholar]
- Gao R, Shen Y, Cai J, Lei M, Wang Z. 2010. Expression of voltage-gated sodium channel α subunit in human ovarian cancer. Oncol Rep 23: 1293–1299. [DOI] [PubMed] [Google Scholar]
- Gilchrist J, Das S, Van Petegem F, Bosmans F. 2013. Crystallographic insights into sodium-channel modulation by the β4 subunit. Proc Natl Acad Sci 110: E5016–E5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist J, Olivera BM, Bosmans F. 2014. Animal toxins influence voltage-gated sodium channel function. Handb Exp Pharmacol 221: 203–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco TM, Malhotra JD, Chen C, Isom LL, Raman IM. 2005. Open-channel block by the cytoplasmic tail of sodium channel β4 as a mechanism for resurgent sodium current. Neuron 45: 233–244. [DOI] [PubMed] [Google Scholar]
- Grimes JA, Fraser SP, Stephens GJ, Downing JE, Laniado ME, Foster CS, Abel PD, Djamgoz MB. 1995. Differential expression of voltage-activated Na+ currents in two prostatic tumour cell lines: Contribution to invasiveness in vitro. FEBS Lett 369: 290–294. [DOI] [PubMed] [Google Scholar]
- Hartshorne RP, Catterall WA. 1981. Purification of the saxitoxin receptor of the sodium channel from rat brain. Proc Natl Acad Sci 78: 4620–4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne RP, Catterall WA. 1984. The sodium channel from rat brain. Purification and subunit composition. J Biol Chem 259: 1667–1675. [PubMed] [Google Scholar]
- Hartshorne RP, Messner DJ, Coppersmith JC, Catterall WA. 1982. The saxitoxin receptor of the sodium channel from rat brain. Evidence for two nonidentical β subunits. J Biol Chem 257: 13888–13891. [PubMed] [Google Scholar]
- Hartshorne RP, Keller BU, Talvenheimo JA, Catterall WA, Montal M. 1985. Functional reconstitution of the purified brain sodium channel in planar lipid bilayers. Proc Natl Acad Sci 82: 240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann SH, Terlau H, Stühmer W, Imoto K, Numa S. 1992. Calcium channel characteristics conferred on the sodium channel by single mutations. Nature 356: 441–443. [DOI] [PubMed] [Google Scholar]
- Hernandez-Plata E, Ortiz CS, Marquina-Castillo B, Medina-Martinez I, Alfaro A, Berumen J, Rivera M, Gomora JC. 2012. Overexpression of Nav1.6 channels is associated with the invasion capacity of human cervical cancer. Int J Cancer 130: 2013–2023. [DOI] [PubMed] [Google Scholar]
- Hille B. 1971. The permeability of the sodium channel to organic cations in myelinated nerve. J Gen Physiol 58: 599–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. 1972. The permeability of the sodium channel to metal cations in myelinated nerve. J Gen Physiol 59: 637–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. 2007. How common are the “common” neurologic disorders? Neurology 68: 326–337. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. 1952. A quantitative description of membrane current and its application to conduction and excitation in nerves. J Physiol 117: 500–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House CD, Vaske CJ, Schwartz AM, Obias V, Frank B, Luu T, Sarvazyan N, Irby R, Strausberg RL, Hales TG, et al. 2010. Voltage-gated Na+ channel SCN5A is a key regulator of a gene transcriptional network that controls colon cancer invasion. Cancer Res 70: 6957–6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom LL. 2014. “It was the interneuron with the parvalbumin in the hippocampus!” “No, it was the pyramidal cell with the glutamate in the cortex!” searching for clues to the mechanism of dravet syndrome—The plot thickens. Epilepsy Curr 14: 350–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom LL, De Jongh KS, Patton DE, Reber BF, Offord J, Charbonneau H, Walsh K, Goldin AL, Catterall WA. 1992. Primary structure and functional expression of the β1 subunit of the rat brain sodium channel. Science 256: 839–842. [DOI] [PubMed] [Google Scholar]
- Isom LL, Scheuer T, Brownstein AB, Ragsdale DS, Murphy BJ, Catterall WA. 1995a. Functional co-expression of the β1 and type IIA α subunits of sodium channels in a mammalian cell line. J Biol Chem 270: 3306–3312. [DOI] [PubMed] [Google Scholar]
- Isom LL, Ragsdale DS, De Jongh KS, Westenbroek RE, Reber BF, Scheuer T, Catterall WA. 1995b. Structure and function of the β2 subunit of brain sodium channels, a transmembrane glycoprotein with a CAM motif. Cell 83: 433–442. [DOI] [PubMed] [Google Scholar]
- Jansson KH, Lynch JE, Lepori-Bui N, Czymmek KJ, Duncan RL, Sikes RA. 2012. Overexpression of the VSSC-associated CAM, β-2, enhances LNCaP cell metastasis associated behavior. Prostate 72: 1080–1092. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Lee A, Chen J, Ruta V, Cadene M, Chait BT, MacKinnon R. 2003. X-ray structure of a voltage-dependent K+ channel. Nature 423: 33–41. [DOI] [PubMed] [Google Scholar]
- Jiao J, Yang Y, Shi Y, Chen J, Gao R, Fan Y, Yao H, Liao W, Sun X-F, Gao S. 2013. Modeling Dravet syndrome using induced pluripotent stem cells (iPSCs) and directly converted neurons. Hum Mol Genet 22: 1–12. [DOI] [PubMed] [Google Scholar]
- Johnson D, Bennett ES. 2006. Isoform-specific effects of the β2 subunit on voltage-gated sodium channel gating. J Biol Chem 281: 25875–25881. [DOI] [PubMed] [Google Scholar]
- Johnson D, Montpetit ML, Stocker PJ, Bennett ES. 2004. The sialic acid component of the β1 subunit modulates voltage-gated sodium channel function. J Biol Chem 279: 44303–44310. [DOI] [PubMed] [Google Scholar]
- Kalume F. 2013. Sudden unexpected death in Dravet syndrome: Respiratory and other physiological dysfunctions. Respir Physiol Neurobiol 189: 324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazarinova-Noyes K, Malhotra JD, McEwen DP, Mattei LN, Berglund EO, Ranscht B, Levinson SR, Schachner M, Shrager P, Isom LL, et al. 2001. Contactin associates with Na+ channels and increases their functional expression. J Neurosci 21: 7517–7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazen-Gillespie KA, Ragsdale DS, D’Andrea MR, Mattei LN, Rogers KE, Isom LL. 2000. Cloning, localization, and functional expression of sodium channel β1A subunits. J Biol Chem 275: 1079–1088. [DOI] [PubMed] [Google Scholar]
- Kearney JA. 2014. Epi4K phase I: Gene discovery in epileptic encephalopathies by exome sequencing. Epilepsy Curr 14: 208–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger S. 1997. Molecular analysis of the putative inactivation particle in the inactivation gate of brain type IIA Na+ channels. J Gen Physiol 109: 589–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Ingano LAM, Carey BW, Pettingell WH, Kovacs DM. 2005. Presenilin/γ-secretase-mediated cleavage of the voltage-gated sodium channel β2-subunit regulates cell adhesion and migration. J Biol Chem 280: 23251–23261. [DOI] [PubMed] [Google Scholar]
- Kim DY, Carey BW, Wang H, Ingano LAM, Binshtok AM, Wertz MH, Pettingell WH, He P, Lee VM-Y, Woolf CJ, et al. 2007. BACE1 regulates voltage-gated sodium channels and neuronal activity. Nat Cell Biol 9: 755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Gersbacher MT, Inquimbert P, Kovacs DM. 2011. Reduced sodium channel Nav1.1 levels in BACE1-null mice. J Biol Chem 286: 8106–8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H, Gelb BD. 2014. Concise review: Drug discovery in the age of the induced pluripotent stem cell. Stem Cells Transl Med 3: 396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krafte DS. 1990. Inactivation of cloned Na channels expressed in Xenopus oocytes. J Gen Physiol 96: 689–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraner SD, Tanaka JC, Barchi RL. 1985. Purification and functional reconstitution of the voltage-sensitive sodium channel from rabbit T-tubular membranes. J Biol Chem 260: 6341–6347. [PubMed] [Google Scholar]
- Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Hauser WA, Mathern G, Moshé SL, Perucca E, Wiebe S, French J. 2010. Definition of drug resistant epilepsy: Consensus proposal by the Ad Hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 51: 1069–1077. [DOI] [PubMed] [Google Scholar]
- Kwan P, Schacter SC, Brodie MJ. 2011. Drug-resistant epilepsy. N Engl J Med 365: 919–926. [DOI] [PubMed] [Google Scholar]
- Kwong K, Carr MJ. 2015. Voltage-gated sodium channels. Curr Opin Pharmacol 22: 131–139. [DOI] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin C-A, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. 2013. Cerebral organoids model human brain development and microcephaly. Nature 501: 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laniado ME, Lalani EN, Fraser SP, Grimes JA, Bhangal G, Djamgoz MB, Abel PD. 1997. Expression and functional analysis of voltage-activated Na+ channels in human prostate cancer cell lines and their contribution to invasion in vitro. Am J Pathol 150: 1213–1221. [PMC free article] [PubMed] [Google Scholar]
- Larramendi L, Lorente De No R, Vidal F. 1956. Restoration of sodium-deficient frog nerve fibres by an isotonic solution of guanidinium chloride. Nature 178: 316–317. [DOI] [PubMed] [Google Scholar]
- Li D, Qiu Z, Shao Y, Chen Y, Guan Y, Liu M, Li Y, Gao N, Wang L, Lu X, et al. 2013. Heritable gene targeting in the mouse and rat using a CRISPR-Cas system. Nat Biotechnol 31: 681–683. [DOI] [PubMed] [Google Scholar]
- Lin X, O’Malley H, Chen C, Auerbach D, Foster M, Shekhar A, Zhang M, Coetzee W, Jalife J, Fishman GI, et al. 2015. Scn1b deletion leads to increased tetrodotoxin-sensitive sodium current, altered intracellular calcium homeostasis and arrhythmias in murine hearts. J Physiol 593: 1389–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lopez-Santiago LF, Yuan Y, Jones JM, Zhang H, O’Malley HA, Patino GA, O’Brien JE, Rusconi R, Gupta A, et al. 2013. Dravet syndrome patient-derived neurons suggest a novel epilepsy mechanism. Ann Neurol 74: 128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombet A, Lazdunski M. 1984. Characterization, solubilization, affinity labeling and purification of the cardiac Na+ channel using Tityus toxin γ. Eur J Biochem 141: 651–660. [DOI] [PubMed] [Google Scholar]
- Lopez-Santiago LF, Pertin M, Morisod X, Chen C, Hong S, Wiley J, Decosterd I, Isom LL. 2006. Sodium channel β2 subunits regulate tetrodotoxin-sensitive sodium channels in small dorsal root ganglion neurons and modulate the response to pain. J Neurosci 26: 7984–7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Santiago LF, Meadows LS, Ernst SJ, Chen C, Malhotra JD, McEwen DP, Speelman A, Noebels JL, Maier SKG, Lopatin AN, et al. 2007. Sodium channel Scn1b null mice exhibit prolonged QT and RR intervals. J Mol Cell Cardiol 43: 636–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Santiago LF, Brackenbury WJ, Chen C, Isom LL. 2011. Na+ channel Scn1b gene regulates dorsal root ganglion nociceptor excitability in vivo. J Biol Chem 286: 22913–22923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente De No R, Vidal F, Larramendi L. 1957. Restoration of sodium-deficient frog nerve fibres by onium ions. Nature 179: 737–738. [DOI] [PubMed] [Google Scholar]
- Maier SKG, Westenbroek RE, McCormick KA, Curtis R, Scheuer T, Catterall WA. 2004. Distinct subcellular localization of different sodium channel α and β subunits in single ventricular myocytes from mouse heart. Circulation 109: 1421–1427. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Kazen-Gillespie K, Hortsch M, Isom LL. 2000. Sodium channel β subunits mediate homophilic cell adhesion and recruit ankyrin to points of cell–cell contact. J Biol Chem 275: 11383–11388. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Koopmann MC, Kazen-Gillespie KA, Fettman N, Hortsch M, Isom LL. 2002. Structural requirements for interaction of sodium channel β1 subunits with ankyrin. J Biol Chem 277: 26681–26688. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Thyagarajan V, Chen C, Isom LL. 2004. Tyrosine-phosphorylated and nonphosphorylated sodium channel β1 subunits are differentially localized in cardiac myocytes. J Biol Chem 279: 40748–40754. [DOI] [PubMed] [Google Scholar]
- Marini C, Scheffer IE, Nabbout R, Suls A, De Jonghe P, Zara F, Guerrini R. 2011. The genetics of Dravet syndrome. Epilepsia 52: 24–29. [DOI] [PubMed] [Google Scholar]
- Massey CA, Sowers LP, Dlouhy BJ, Richerson GB. 2014. Mechanisms of sudden unexpected death in epilepsy: The pathway to prevention. Nat Rev Neurol 10: 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick KA, Isom LL, Ragsdale D, Smith D, Scheuer T, Catterall WA. 1998. Molecular determinants of Na+ channel function in the extracellular domain of the β1 subunit. J Biol Chem 273: 3954–3962. [DOI] [PubMed] [Google Scholar]
- McCusker EC, Bagnéris C, Naylor CE, Cole AR, D’Avanzo N, Nichols CG, Wallace BA. 2012. Structure of a bacterial voltage-gated sodium channel pore reveals mechanisms of opening and closing. Nat Commun 3: 1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen DP, Isom LL. 2004. Heterophilic interactions of sodium channel β1 subunits with axonal and glial cell adhesion molecules. J Biol Chem 279: 52744–52752. [DOI] [PubMed] [Google Scholar]
- McEwen DP, Chen C, Meadows LS, Lopez-Santiago LF, Isom LL. 2009. The voltage-gated Na+ channel β3 subunit does not mediate trans homophilic cell adhesion or associate with the cell adhesion molecule contactin. Neurosci Lett 462: 272–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, Xu H-Q, Yu L, Lin G-W, He N, Su T, Shi Y-W, Li B, Wang J, Liu X-R, et al. 2015. The SCN1A mutation database: Updating information and analysis of the relationships among genotype, functional alteration, and phenotype. Hum Mutat 36: 573–580. [DOI] [PubMed] [Google Scholar]
- Miller JA, Agnew WS, Levinson SR. 1983. Principal glycopeptide of the tetrodotoxin/saxitoxin binding protein from Electrophorus electricus: Isolation and partial chemical and physical characterization. Biochemistry 22: 462–470. [DOI] [PubMed] [Google Scholar]
- Miller AR, Hawkins NA, McCollom CE, Kearney JA. 2014. Mapping genetic modifiers of survival in a mouse model of Dravet syndrome. Genes Brain Behav 13: 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry AM, Thompson CH, Miller AR, Vanoye CG, George AL, Kearney JA. 2014. Strain- and age-dependent hippocampal neuron sodium currents correlate with epilepsy severity in Dravet syndrome mice. Neurobiol Dis 65: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan K, Stevens EB, Shah B, Cox PJ, Dixon AK, Lee K, Pinnock RD, Hughes J, Richardson PJ, Mizuguchi K, et al. 2000. β3: An additional auxiliary subunit of the voltage-sensitive sodium channel that modulates channel gating with distinct kinetics. Proc Natl Acad Sci 97: 2308–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namadurai S, Balasuriya D, Rajappa R, Wiemhöfer M, Stott K, Klingauf J, Edwardson JM, Chirgadze DY, Jackson AP. 2014. Crystal structure and molecular imaging of the Nav channel β3 subunit indicates a trimeric assembly. J Biol Chem 289: 10797–10811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M, Millican-Slater R, Forrest LC, Brackenbury WJ. 2014. The sodium channel β1 subunit mediates outgrowth of neurite-like processes on breast cancer cells and promotes tumour growth and metastasis. Int J Cancer 135: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M, Shimizu S, Tanabe T, Takai T, Kayano T, Ikeda T, Takahashi H, Nakayama H, Kanaoka Y, Minamino N, et al. 1984. Primary structure of Electrophorus electricus sodium channel deduced from cDNA sequence. Nature 312: 121–127. [DOI] [PubMed] [Google Scholar]
- Noda M, Suzuki H, Numa S, Stühmer W. 1989. A single point mutation confers tetrodotoxin and saxitoxin insensitivity on the sodium channel II. FEBS Lett 259: 213–216. [DOI] [PubMed] [Google Scholar]
- Ogiwara I, Nakayama T, Yamagata T, Ohtani H, Mazaki E, Tsuchiya S, Inoue Y, Yamakawa K. 2012. A homozygous mutation of voltage-gated sodium channel βI gene SCN1B in a patient with Dravet syndrome. Epilepsia 53: e200–e203. [DOI] [PubMed] [Google Scholar]
- O’Malley HA, Isom LL. 2015. Sodium channel β subunits: Emerging targets in channelopathies. Annu Rev Physiol 77: 481–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onganer PU, Djamgoz MBA. 2005. Small-cell lung cancer (human): Potentiation of endocytic membrane activity by voltage-gated Na+ channel expression in vitro. J Membr Biol 75: 67–75. [DOI] [PubMed] [Google Scholar]
- Parent JM, Anderson SA. 2015. Reprogramming patient-derived cells to study the epilepsies. Nat Neurosci 18: 360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel F, Brackenbury WJ. 2015. Dual roles of voltage-gated sodium channels in development and cancer. Int J Dev Biol 59: 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino GA, Isom LL. 2010. Electrophysiology and beyond: Multiple roles of Na+ channel β subunits in development and disease. Neurosci Lett 486: 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino GA, Claes LRF, Lopez-Santiago LF, Slat EA, Dondeti RSR, Chen C, O’Malley HA, Gray CBB, Miyazaki H, Nukina N, et al. 2009. A functional null mutation of SCN1B in a patient with Dravet syndrome. J Neurosci 29: 10764–10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino GA, Brackenbury WJ, Bao Y, Lopez-Santiago LF, O'Malley HA, Chen C, Calhoun JD, Lafrenière RG, Cossette P, Rouleau GA, et al. 2011. Voltage-gated Na+ channel β1B: A secreted cell adhesion molecule involved in human epilepsy. J Neurosci 31: 14577–14591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payandeh J, Scheuer T, Zheng N, Catterall WA. 2011. The crystal structure of a voltage-gated sodium channel. Nature 475: 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Bean BP. 1997. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. J Neurosci 17: 4517–4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe CF, Qu Y, McCormick KA, Tibbs VC, Dixon JE, Scheuer T, Catterall WA. 2000. A sodium channel signaling complex: modulation by associated receptor protein tyrosine phosphatase β. Nat Neurosci 3: 437–444. [DOI] [PubMed] [Google Scholar]
- Ratcliffe CF, Westenbroek RE, Curtis R, Catterall WA. 2001. Sodium channel β1 and β3 subunits associate with neurofascin through their extracellular immunoglobulin-like domain. J Cell Biol 154: 427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recio-Pinto E, Thornhill WB, Duch DS, Levinson SR, Urban BW. 1990. Neuraminidase treatment modifies the function of electroplax sodium channels in planar lipid bilayers. Neuron 5: 675–684. [DOI] [PubMed] [Google Scholar]
- Riuró H, Beltran-Alvarez P, Tarradas A, Selga E, Campuzano O, Vergés M, Pagans S, Iglesias A, Brugada J, Brugada P, et al. 2013. A missense mutation in the sodium channel β2 subunit reveals SCN2B as a new candidate gene for Brugada syndrome. Hum Mutat 34: 961–966. [DOI] [PubMed] [Google Scholar]
- Roberts RH, Barchi RL. 1987. The voltage-sensitive sodium channel from rabbit skeletal muscle. Chemical characterization of subunits. J Biol Chem 262: 2298–2303. [PubMed] [Google Scholar]
- Roger S, Rollin J, Barascu A, Besson P, Raynal P-I, Iochmann S, Lei M, Bougnoux P, Gruel Y, Le Guennec J-Y. 2007. Voltage-gated sodium channels potentiate the invasive capacities of human non-small-cell lung cancer cell lines. Int J Biochem Cell Biol 39: 774–786. [DOI] [PubMed] [Google Scholar]
- Rohl CA, Boeckman FA, Baker C, Scheuer T, Catterall WA, Klevit RE. 1999. Solution structure of the sodium channel inactivation gate. Biochemistry 38: 855–861. [DOI] [PubMed] [Google Scholar]
- Rosker C, Lohberger B, Hofer D, Steinecker B, Quasthoff S, Schreibmayer W. 2007. The TTX metabolite 4, 9-anhydro-TTX is a highly specific blocker of the Nav1. 6 voltage-dependent sodium channel. Am J Physiol Cell Physiol 293: 783–789. [DOI] [PubMed] [Google Scholar]
- Schmidt JW, Catterall WA. 1986. Biosynthesis and processing of the α subunit of the voltage-sensitive sodium channel in rat brain neurons. Cell 46: 437–444. [DOI] [PubMed] [Google Scholar]
- Schmidt J, Rossie S, Catterall WA. 1985. A large intracellular pool of inactive Na channel α subunits in developing rat brain. Proc Natl Acad Sci 82: 4847–4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrey M, Codina C, Kraft R, Beetz C, Kal R, Stefan W, Patt S. 2002. Molecular characterization of voltage-gated sodium channels in human gliomas. 13: 20–25. [DOI] [PubMed] [Google Scholar]
- Shi X, Yasumoto S, Kurahashi H, Nakagawa E, Fukasawa T, Uchiya S, Hirose S. 2012. Clinical spectrum of SCN2A mutations. Brain Dev 34: 541–545. [DOI] [PubMed] [Google Scholar]
- Siva N. 2015. UK gears up to decode 100,000 genomes from NHS patients. Lancet 385: 103–104. [DOI] [PubMed] [Google Scholar]
- Steinlein OK. 2014. Mechanisms underlying epilepsies associated with sodium channel mutations. Prog Brain Res 213: 97–111. [DOI] [PubMed] [Google Scholar]
- Stevens M, Peigneur S, Tytgat J. 2011. Neurotoxins and their binding areas on voltage-gated sodium channels. Front Pharmacol 2: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker PJ, Bennett ES. 2006. Differential sialylation modulates voltage-gated Na+ channel gating throughout the developing myocardium. J Gen Physiol 127: 253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. 2007. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872. [DOI] [PubMed] [Google Scholar]
- Tasaki I, Singer I. 1966. Membrane macromolecules and nerve excitability: A physico-chemical interpretation of excitation in squid giant axons. Ann NY Acad Sci 137: 792–806. [DOI] [PubMed] [Google Scholar]
- Tasaki I, Singer I, Watanabe A. 1965. Excitation of internally perfused squid giant axons in sodium-free media. Proc Natl Acad Sci 54: 763–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki I, Singer I, Watanabe A. 1966. Excitation of squid giant axons in sodium-free external media. Am J Physiol 211: 746–754. [DOI] [PubMed] [Google Scholar]
- Terlau H, Heinemann SH, Stühmer W, Pusch M, Conti F, Imoto K, Numa S. 1991. Mapping the site of block by tetrodotoxin and saxitoxin of sodium channel II. FEBS Lett 293: 93–96. [DOI] [PubMed] [Google Scholar]
- Theile JW, Cummins TR. 2011. Recent developments regarding voltage-gated sodium channel blockers for the treatment of inherited and acquired neuropathic pain syndromes. Front Pharmacol 2: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell L, Renganathan M, Dib-Hajj SD, Waxman SG. 2001. Glycosylation alters steady-state inactivation of sodium channel Nav1.9/NaN in dorsal root ganglion neurons and is developmentally regulated. J Neurosci 21: 9629–9637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebachs M, Opitz T, Royeck M, Dickhof G, Horstmann M-T, Isom LL, Beck H. 2010. Efficacy loss of the anticonvulsant carbamazepine in mice lacking sodium channel β subunits via paradoxical effects on persistent sodium currents. J Neurosci 30: 8489–8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas E, Yarov-Yarovoy V, Khalili-Araghi F, Catterall WA, Klein ML, Tarek M, Lindahl E, Schulten K, Perozo E, Bezanilla F, et al. 2012. An emerging consensus on voltage-dependent gating from computational modeling and molecular dynamics simulations. J Gen Physiol 140: 587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev PM, Scheuer T, Catterall WA. 1988. Identification of an intracellular peptide segment involved in sodium channel inactivation. Science 241: 1658–1661. [DOI] [PubMed] [Google Scholar]
- Vassilev P, Scheuer T, Catterall WA. 1989. Inhibition of inactivation of single sodium channels by a site-directed antibody. Proc Natl Acad Sci 86: 8147–8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagnon JL, Meisler MH. 2015. Recurrent and non-recurrent mutations of SCN8A in epileptic encephalopathy. Front Neurol 6: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace RH, Wang DW, Singh R, Scheffer IE, George AL, Phillips HA, Saar K, Reis A, Johnson EW, Sutherland GR, et al. 1998. Febrile seizures and generalized epilepsy associated with a mutation in the Na+-channel α1 subunit gene SCN1B. Nat Genet 19: 366–370. [DOI] [PubMed] [Google Scholar]
- West JW, Patton DE, Scheuer T, Wang Y, Goldin AL, Catterall WA. 1992. A cluster of hydrophobic amino acid residues required for fast Na+-channel inactivation. Proc Natl Acad Sci 89: 10910–10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H-K, Sakurai T, Oyama F, Kaneko K, Wada K, Miyazaki H, Kurosawa M, De Strooper B, Saftig P, Nukina N. 2005. β subunits of voltage-gated sodium channels are novel substrates of β-site amyloid precursor protein-cleaving enzyme (BACE1) and γ-secretase. J Biol Chem 280: 23009–23017. [DOI] [PubMed] [Google Scholar]
- Xiao ZC, Ragsdale DS, Malhotra JD, Mattei LN, Braun PE, Schachner M, Isom LL. 1999. Tenascin-R is a functional modulator of sodium channel β subunits. J Biol Chem 274: 26511–26517. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Blumenthal K, Cummins TR, Sciences B. 2014. Gating pore currents demonstrate selective and specific modulation of individual sodium channel voltage sensors by biological toxins. Mol Pharmacol 46202: 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Kozminski DJ, Wold LA, Modak R, Calhoun JD, Isom LL, Brackenbury WJ. 2012. Therapeutic potential for phenytoin: Targeting Nav1.5 sodium channels to reduce migration and invasion in metastatic breast cancer. Breast Cancer Res Treat 134: 603–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Xu J, Zhu T, Fan J, Lai L, Zhang J, Chen YE. 2014a. Effective gene targeting in rabbits using RNA-guided Cas9 nucleases. J Mol Cell Biol 6: 97–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Wang H, Jaenisch R. 2014b. Generating genetically modified mice using CRISPR/Cas-mediated genome engineering. Nat Protoc 9: 1956–1968. [DOI] [PubMed] [Google Scholar]
- Yereddi NR, Cusdin FS, Namadurai S, Packman LC, Monie TP, Slavny P, Clare JJ, Powell AJ, Jackson AP. 2013. The immunoglobulin domain of the sodium channel β3 subunit contains a surface-localized disulfide bond that is required for homophilic binding. FASEB J 27: 568–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FH, Westenbroek RE, Silos-Santiago I, McCormick KA, Lawson D, Ge P, Ferriera H, Lilly J, DiStefano PS, Catterall WA, et al. 2003. Sodium channel β4, a new disulfide-linked auxiliary subunit with similarity to β2. J Neurosci 23: 7577–7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FH, Mantegazza M, Westenbroek RE, Robbins CA, Kalume F, Burton KA, Spain WJ, McKnight GS, Scheuer T, Catterall WA. 2006. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat Neurosci 9: 1142–1149. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hartmann HA, Satin J. 1999. Glycosylation influences voltage-dependent gating of cardiac and skeletal muscle sodium channels. J Membr Biol 171: 195–207. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ren W, DeCaen P, Yan C, Tao X, Tang L, Wang J, Hasegawa K, Kumasaka T, He J, et al. 2012. Crystal structure of an orthologue of the NaChBac voltage-gated sodium channel. Nature 486: 130–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M-M, Wilson MJ, Azam L, Gajewiak J, Rivier JE, Bulaj G, Olivera BM, Yoshikami D. 2013. Co-expression of NaVβ subunits alters the kinetics of inhibition of voltage-gated sodium channels by pore-blocking μ-conotoxins. Br J Pharmacol 168: 1597–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kecskés A, Copmans D, Langlois M, Crawford AD, Ceulemans B, Lagae L, de Witte PAM, Esguerra CV. 2015. Pharmacological characterization of an antisense knockdown zebrafish model of Dravet syndrome: Inhibition of epileptic seizures by the serotonin agonist fenfluramine. PLoS ONE 10: e0125898. [DOI] [PMC free article] [PubMed] [Google Scholar]