Abstract
Gram-positive bacteria are a common cause of urinary tract infection (UTI), particularly among individuals who are elderly, pregnant, or who have other risk factors for UTI. Here we review the epidemiology, virulence mechanisms, and host response to the most frequently isolated Gram-positive uropathogens: Staphylococcus saprophyticus, Enterococcus faecalis, and Streptococcus agalactiae. We also review several emerging, rare, misclassified, and otherwise underreported Gram-positive pathogens of the urinary tract including Aerococcus, Corynebacterium, Actinobaculum, and Gardnerella. The literature strongly suggests that urologic diseases involving Gram-positive bacteria may be easily overlooked due to limited culture-based assays typically utilized for urine in hospital microbiology laboratories. Some UTIs are polymicrobial in nature, often involving one or more Gram-positive bacteria. We herein review the risk factors and recent evidence for mechanisms of bacterial synergy in experimental models of polymicrobial UTI. Recent experimental data has demonstrated that, despite being cleared quickly from the bladder, some Gram-positive bacteria can impact pathogenic outcomes of co-infecting organisms. When taken together, the available evidence argues that Gram-positive bacteria are important uropathogens in their own right, but that some can be easily overlooked because they are missed by routine diagnostic methods. Finally, a growing body of evidence demonstrates that a surprising variety of fastidious Gram-positive bacteria may either reside in or be regularly exposed to the urinary tract and further suggests that their presence is widespread among women, as well as men. Experimental studies in this area are needed; however, there is a growing appreciation that the composition of bacteria found in the bladder could be a potentially important determinant in urologic disease, including susceptibility to UTI.
Gram-Positive and Polymicrobial UTI Epidemiology
Uncomplicated UTI
Uncomplicated urinary tract infection (UTI) is most common in young, sexually active, nonpregnant, premenopausal women. Gram-negative bacteria are isolated from 75 to 95% of these infections [1]. The remaining proportions of uncomplicated UTI are associated with a variety of organisms, including the Gram-positive bacteria Staphylococcus saprophyticus, Enterococcus faecalis, Streptococcus agalactiae (group B Streptococcus, GBS), and other less frequently isolated organisms. In demographic groups such as in pregnant women and the elderly, Gram-positive bacteria are found more often as etiologic agents of UTI. Symptoms associated with uncomplicated UTI caused by Gram-positive uropathogens are similar to those caused by Gram-negative organisms and usually include dysuria, urinary frequency, urinary urgency, and/or suprapubic pain. Fever, chills, costovertebral-angle tenderness, flank pain, and/or nausea are suggestive of upper urinary tract (kidney) involvement.
Point of care diagnosis of UTI
While the gold standard for UTI diagnosis is bacterial culture of the urine, dipstick urinalysis is commonly used in point-of-care diagnosis. In some clinical settings such as with infants, leukocyte esterase (LE) and pyuria (by dipstick analysis) have a very high sensitivity and specificity for UTI (>90% as defined by the culture of a uropathogen from urine with >100,000 colony forming units (CFU) per ml) [2, 3]. However, in contexts such as pregnancy, dipstick analysis using LE, pyuria, or presence of nitrites is less reliable as an indication of UTI per the microbiological definition of 105 CFU/ml cutoff [4, 5]. While dipstick urinalysis that is positive for LE and/or nitrites in a clean-catch urine sample is consistent with a UTI diagnosis, these tests can miss UTIs that meet the gold standard of bacteriuria diagnosis in relation to adverse outcomes in pregnancy (e.g. the microbiological 105 CFU/ml definition). One likely explanation is that nitrite tests are likely to be negative if the infecting organism does not reduce nitrate, as is the case for most Gram-positive uropathogens including S. saprophyticus, enterococci, and group B Streptococcus [6, 7]. Given the higher prevalence of Gram-positive bacteria as causes of UTI in certain populations such as the elderly, it is perhaps not surprising that some studies conclude that LE and nitrite are inadequate for UTI screening in this setting [8]. In short, while dipstick urinalyses can help to quickly identify UTI caused by Gram-negative bacteria, they are less useful for infections involving Gram-positive uropathogens and perform poorly in ruling out these infections with certainty.
Complicated UTI
Complicated UTI is defined as cystitis or pyelonephritis that occurs in individuals with predisposing anatomic, metabolic, or functional risk factors that make UTI more difficult to treat. Complicated UTIs often occur in nosocomial and/or institutional settings, particularly in individuals with structural or functional alterations of the urinary tract (often associated with urinary catheterization), or other underlying renal, metabolic, or immunological disorders [9]; these populations are at greater risk of Gram-positive and polymicrobial UTI [10, 11]. Another less frequently recognized anatomic risk factor for UTI is female genital cutting (FGM). A recent meta-analysis of five comparative studies showed that women who had experienced FGM were at 3-times higher risk of UTI compared to those who were uncut [12].
Catheter associated UTI
Catheter-associated UTI (CAUTI) account for 40% of all nosocomial infections [13] and are the most common complication of indwelling urinary catheters [14, 15]. Catheter-associated bacteria are thought to be derived largely from the patient’s own gut microbiota [16]. Bacteriuria occurs in 3–10% of patients following urinary catheterization [13, 17]. Catheter-associated bacteriuria is often asymptomatic [15] and there is no good way to distinguish between pathogenic CAUTI and asymptomatic bacteriuria (ASB); even the presence of neutrophils in the urine (pyuria), which are a strong identifier of uncomplicated UTI, is not a good diagnostic indicator of CAUTI [13]. Catheter-associated bacteria are largely in a biofilm state and are thus recalcitrant to antibiotic treatment [13, 18, 19]. However, if left untreated, these infections can lead to severe complications such as acute pyelonephritis, bacteremia, urosepsis, and death [13, 16]. While the well-adapted uropathogenic E. coli cause the majority of non-catheter-associated UTI in the community, the diversity of species associated with CAUTI is greater. For example, enterococci are rarely associated with community-acquired UTI but play a prominent role in the pathogenesis of CAUTI and are among the predominant pathogens isolated from polymicrobial communities on the surface of indwelling urinary catheters and biliary stents [20–22].
Laboratory models to study Gram-Positive UTI
Model systems to recapitulate and study infection by Gram-positive uropathogens have been adapted from those used to study UTI caused by Gram-negative bacteria. Transurethral inoculation with 1.5 ml of S. saprophtyicus at 1×109 colony forming units (CFU)/ml into the bladders of female albino WISTAR rats showed that at 7 days post infection, both bladders and kidneys were colonized at similar levels, leukocytes were present in the urine, and bladder inflammation and epithelial damage noted [23]. Subsequent studies in which 50µl of S. saprophyticus at 5×107 CFU/ml was transurethrally inoculated into 7–8 week old C3H/HeN female mice showed significantly higher CFU in the kidney compared to the bladder at 6 hours post infection and 2, 7, and 14 days post infection [24]. Bacterial persistence in the kidneys was observed in C3H/HeN mice but not in C57BL/6 mice, indicating that host factors contribute to the ability of S. saprophyticus to cause UTI. Under the same infection conditions, GBS showed similar kidney tropism at 1, 7, and 14 days post infection [25]. Similarly, E. faecalis preferentially infects the kidneys of C57Bl/6, outbred Harlan Sprague Dawley, and BALB/c female mice; however, these models require a 200µl inoculum volume to consistently establish infection [26–28]. Since E. faecalis is more commonly associated with CAUTI than ascending UTI, foreign body-associated UTI models have been developed in mice and rats to mimic the conditions of patients with indwelling urinary catheters [29, 30]. In the murine model, catheter material is inserted transurethrally into the murine bladder prior to bacterial inoculation, where it remains throughout the course of infection. E. faecalis establishes a robust infection in the catheter-containing bladder, in the kidneys, and on the catheter material itself where it forms a biofilm that facilitates persistent infection in the face of robust catheter-driven inflammation [30, 31]. The CAUTI murine model has recently been used to test the efficacy of novel UTI therapeutics and will continue to be useful in the search for antimicrobial agents aimed at preventing or dispersing Gram-positive biofilms that arise in catheterized individuals [32].
Epidemiology and animal models for polymicrobial UTI
The presence of multiple recognized uropathogens in midstream urine at titers >100,000 CFU/ml is consistent with a polymicrobial etiology of UTI. Polymicrobial infections occur most often among the elderly, immune compromised, and those with indwelling catheters, HIV, malignancy, and diabetes. Polymicrobial UTI is less common among young sexually active women. Since the highly polymicrobial microbiota of the GI and reproductive tracts are thought to be a major inoculation source leading to UTI, and since truly dual species or polymicrobial UTI do arise, several investigators have sought to examine the consequence of mixed microbial inoculation into the urinary tracts of model organisms. In a rat model, transurethral inoculation of Staphylococcus saprophyticus or Proteus mirabilis, resulted in ascending pyelonephritis significantly more often when the two organisms were inoculated together compared to single species infection, suggesting a synergistic virulence between the two species [33]. P. mirabilis also synergizes with UPEC in the murine urinary tract, such that co-infection gave rise to greater CFU for both P. mirabilis and UPEC, compared to either single species infection. The use of complementary, rather than competing, central metabolism pathways in the urinary tract by UPEC and P. mirabilis may limit competition and thus promote synergy between these two organisms [34]. Co-infection with the urease-positive Gram-negative organisms P. mirabilis and Providencia stuartii give rise to an increased incidence of urinary stones (urolithiasis) and bacteremia in a murine model of ascending UTI compared to monomicrobial infection [35], (ref), which may help explain why these organisms commonly co-occur in the urine of individuals with indwelling urinary catheters [20, 36, 37]. Similar studies in mice showed that Pseudomonas aeruuginosa and E. faecalis co-infection resulted in a more rapid development of pyelonephritis than observed when each species was inoculated alone [38]. Moreover, co-infection studies with group B Streptococcus and UPEC in a murine UTI model have demonstrated that the presence of GBS can modulate host immunity and alter host susceptibility to persistent high titer infection of the bladder and kidneys by UPEC [39]. Aged multiparous animals were particularly prone to UPEC infection in the context of GBS, demonstrating ~1000-fold higher titer UPEC infection in the presence of GBS compared to age-matched nulliparous controls [40]. Microscopic and microbial culture examinations, as well as culture-independent DNA sequence analysis of bacterial biofilms found on urinary catheters, show that CAUTI biofilms are often polymicrobial in nature [41–45]. By analogy to mixed species effects in ascending UTI, the nature of the mixed microbial community in CAUTI may also influence the spectrum or severity of sequelae.
In the next section of this chapter, we will examine the epidemiology, virulence mechanisms, and host response to the most frequently isolated Gram-positive uropathogens: Staphylococcus saprophyticus, Enterococcus faecalis, and Streptococcus agalactiae.
Urinary Tract Infection caused by Staphylococci
Epidemiology of S. saprophyticus UTI
Staphylococcus saprophyticus is a Gram-positive, coagulase negative, nonhemolytic coccus. Colonies of Staphylococcus saprophyticus are often yellow-pigmented [46]. S. saprophyticus causes 5–20% of community-acquired UTIs [47] and up to 42% of UTI among 16–25 year old women [48]. S. saprophyticus is second only to UPEC as the most common cause of uncomplicated UTI in this population [49, 50]. Similar to UPEC infection, recent sexual intercourse is also a risk factor for S. saprophyticus UTI [51, 52]. Over 40% of young, sexually active women are colonized with S. saprophyticus in the rectum, urethra, or cervix at any given time [52]. It is thought that a major source of urethral inoculation is the gastrointestinal (GI) microbiota. However, one study found no correlation between GI colonization and subsequent S. saprophyticus UTI [52]. S. saprophyticus colonization and UTI display an interesting seasonal variation, with the greatest prevalence in the late summer and early fall [48, 52–54]. The cause for this seasonality is not well understood. In the absence of complicating conditions, S. saprophyticus infection rarely causes UTI in males, but has been associated with urethritis and up to 17% of prostatitis [53, 55, 56]. One study of older men presenting with UTI in a veteran’s hospital found that the presence of S. saprophyticus was very rare (3 of 9314 urine samples were S. saprophyticus positive) [57]. When it is observed, male UTI caused by S. saprophyticus is found predominantly in elderly or institutionalized individuals [58]. UTI symptoms caused by S. saprophyticus are similar in spectrum to those caused by E. coli, but can be more severe than in patients with E. coli UTI [59, 60]; approximately 40% of patients with S. saprophyticus UTI present with acute pyelonephritis [58, 61].
Novobiocin resistance is a laboratory hallmark for identification of S. saprophyticus. However, antibiotic resistance in S. saprophyticus is uncommon [62]. As is the case for many coagulase negative staphylococcus species (CoNS) [63], methicillin-resistance can occur in S. saprophyticus and is found in ~1–8% of urine isolates [62, 64] via the acquisition of a penicillin binding protein (PBP) with low β-lactam affinity encoded by the mecA gene [65, 66]. mecA is carried on the staphylococcal cassette chromosome (SCC) mec (SCCmec) mobile genetic element (MGE) [50, 67, 68].
S. saprophyticus UTI virulence factors
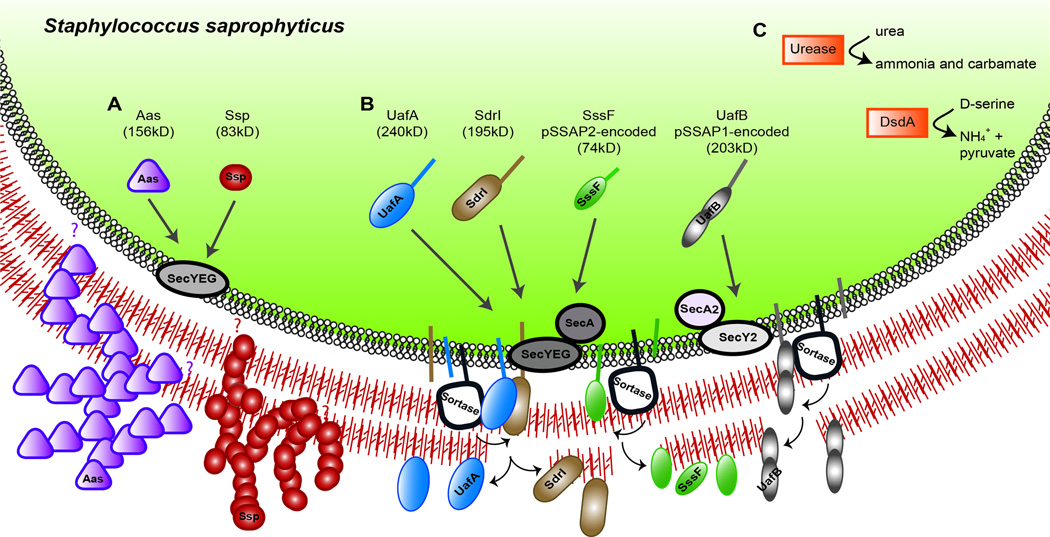
As the most frequent Gram-positive causative agent of UTI, S. saprophyticus is also the best studied with respect to virulence determinants necessary to cause infection. Scanning electron micrographs of murine bladders infected with wild type S. saprophyticus show the organism adhering over the entire surface of the bladder but with apparent selective adherence to the tight junctions between individual epithelial cells [69]. Several adhesins have been linked to S. saprophyticus colonization of the urinary tract (Figure 1A). Conserved among all S. saprophyticus strains, Aas is a hemagglutinin that has autolytic and adhesive properties, binds to fibronectin and human ureters in vitro, and has been implicated in colonization of rat kidneys in vivo [70–74]. Also widely conserved, the surface-associated lipase, Ssp, is found in >90% of S. saprophyticus strains, forms fimbria-like surface appendages, and is important for acute UTI as well as persistent kidney infection in a murine model [24, 74–76]. However, the mechanism by which Ssp contributes to in vivo infection is not well-understood.
Figure 1. S. saprophyticus virulence factors.
(A) Secreted surface proteins: Aas, possesses N-terminal signal sequence, but no motifs such as a transmembrane domain, LPXTG sortase recognition motif, or proline/glycine-rich cell wall-spanning domain to indicate the mode of attachment to the cell surface after translocation across the membrane (indicated by the purple question marks) [72]. Immuno-elctron microscopy shows Aas as part of a fuzzy surface layer that is absent when Aas is not expressed [75]. Ssp has a YSIRK-containing signal sequence but no sortase-recognition motif so its mode of attachment to the cell surface is uncertain (indicated by the red question mark); it is easily sheared from the cell surface. Electron microscopy and immuno-electron micrographs also show Ssp to exist as part of fuzzy surface layer, apparently consisting of 50–75nm fibrillar structures; the nature of these fibers in not known [75, 76]. (B) UafA, SdrI, SssF, and UafB contain an LPXTG motif and are predicted to be covalently attached to the cell wall [78, 79, 81]. The small arrows near the membrane-anchored sortase enzymes indicate the two-step transpeptidation reaction whereby sortase substrate are first cleaved within the LPXTG motif to create a sortase-substrate intermediate (and releasing the membrane domain and positively charged cytoplasmic tail, indicated by the straight line in the membrane) that is then resolved resulting in covalent linkage of the substrate to the cell wall [380]. UafB is genetically linked to accessory secretion genes secA2 and secY2 that are predicted to encode a dedicated accessory secretion system for UafB [79]. (C) Cytoplasmic enzymes that promote S saprophyticus survival in urine [381, 382].
Cell wall-attached surface proteins also mediate adherence in S. saprophyticus (Figure 1B). Sortase enzymes in Gram-positive bacteria covalently attach a subset of secreted proteins bearing sortase recognition motifs, or sorting signals, to the exterior cell wall [77]. Four sorting signal-containing proteins predicted to be cell wall-anchored via sortase enzymes have been described in S. saprophyticus: UafA, UafB, SdrI, and SssF. S. saprophyticus strains possess very few putative cell-wall associated sortase substrates compared to other staphylococci. The first sequenced S. saprophyticus strain ATCC 15305 encodes only one sortase substrate on its chromosome, UafA [78]. S. saprophyticus MS1146 encodes UafA, as well as UafB and SssF, on two different plasmids [79, 80]. SdrI was characterized on the unsequenced S. saprophyticus 7108 [81]. Uro-adherence factor A (UafA) is a hemagglutinin that mediates adhesion to bladder epithelial cells in vitro and is found in all S. saprophyticus strains examined to date [78]. Plasmid-encoded UafB, found in ~5% of strains examined, is a serine-rich glycoprotein that binds fibronectin, fibrinogen, and human bladder epithelial cells but does not promote bladder colonization in a murine UTI model [79]. Like the related serine-rich platelet binding proteins SraP in S. aureus and GspB in Streptococcus gordonii, which rely on an accessory SecA2 secretion system [82–84], UafB is encoded in a genetic locus that also contains gene for a putative accessory secretion apparatus [85]. In Gram-positive pathogens, these accessory Sec systems are associated with virulence [86]. SdrI is a cell wall-associated serine-aspartate-rich protein found in a minority of S. saprophyticus strains, that binds collagen, is associated with bacterial surface hydrophobicity, and plays a role in acute UTI and persistent kidney infections [24, 79, 81, 87]. SdrI shares sequence and structural homology with the adhesive Sdr proteins, including ClfA and ClfB, of Staphylococcus aureus and Staphylococcus epidermidis [81, 88, 89]. Plasmid-encoded SssF is highly conserved among S. saprophyticus strains, is involved in resistance to linoleic acid, but does not play a role in uropathogenesis in a murine model. Instead, SssF has been postulated to be important prior to urethral exposure, in biological niches such as the perineum or periurethral area where polyunsaturated fatty acids such as linoleic acid are particularly abundant [80].
In addition to cell-wall associated proteins, S. saprophyticus encodes urease that is important for efficient colonization of the bladder and kidneys, for inflammation in the bladder, and for dissemination to the spleen in a rat model of UTI [23]. The presence of urease-producing S. saprophyticus has been associated with the formation of urinary stones [90]. The ability of S. saprophyticus to tolerate high concentrations of D-serine that occur in the urine is conferred by D-serine deaminase, found in S. saprophyticus but in no other staphylococci [91] (Figure 1C). S. saprophyticus upregulates the virulence determinant Ssp in the presence of D-serine, and D-serine deaminase mutants are outcompeted by the isogenic wild type strain in the kidneys in a murine model of UTI [92]. S. saprophyticus express a capsule, whose encoding genetic locus in the sequenced strain is found on an SCC genetic element and whose genetic arrangement is similar to the S. aureus cap5 (cap8) locus [78]. The capsule of S. saprophyticus mediates resistance to complement-mediated opsonophagocytic killing by human neutrophils [93] but also prevents binding to urothelial cells, perhaps via masking and preventing interactions of adhesin(s) with the cells [78]. Given the contributions of capsule of other uropathogens, such as UPEC [94, 95], it will be of interest to determine at which stages of UTI this polysaccharide is acting.
Phenol soluble modulins (PSM) are staphylococcal pro-inflammatory cytolytic toxins characterized in S. aureus and S. epidermidis, are usually encoded within the core genomes, and are crucial in immune evasion as they are able to recruit, activate, and then lyse human neutrophils [96–99]. Many PSMs also have antibacterial activities alone or in cooperation with host antimicrobial peptides that can kill other microbes within a niche [100–102]. PSMs also mediate biofilm disassembly in their peptide form, whereas PSMs that assemble into amyloid-like extracellular fibrils under certain growth conditions promote biofilm stability [103–105]. One exceptional non-core genomic PSM has been found that is instead associated with the SCCmec MGE of S. aureus, termed PSMmec, genetically linking methicillin resistance and virulence [106]. PSMmec have been identified in other CoNS, including multiple human S. saprophyticus isolates [50, 106, 107]. A BLASTP search of the sequenced genome of methicillin-sensitive S. saprophyticus ATCC 15305 [78] for peptides homologous to PSMβ1 or PSMβ2 of S. aureus [96] revealed the presence of 3 putative PSM peptides with 55–80% identity to PSMβ1/2 that do not appear to be associated with MGEs (K.A. Kline, unpublished). Whether S. saprophyticus PSMs are expressed and functional in UTI remains to be experimentally determined.
Non-saprophyticus staphylococcal UTI
In contrast to S. saprophyticus which is a predominant cause of community acquired UTI, S. aureus UTI more often occurs in urinary-catheterized and pregnant individuals [108–110]. The majority of S. aureus UTI isolates are methicillin-resistant and S. aureus bacteriuria is associated with subsequent development of invasive infection [108]. Like S. saprophyticus, S. aureus also encodes an active urease enzyme. Two nickel ABC-transporters (Opp2 and Opp5a) have been identified as necessary for urease activity in vitro. These, along with a third ABC-transporter that imports nickel and cobalt when zinc is depleted, are both involved in UTI colonization and virulence in a mouse model [111, 112]. To our knowledge, no other S. aureus virulence factors have been examined during UTI.
Coagulase negative S. epidermidis is a member of the human skin microbiota and is an important opportunistic pathogen, especially in biofilm-associated infections associated with indwelling medical devices. Thus, the biofilm-forming properties of S. epidermidis are an area of active investigation and are summarized in a recent review [113]. CoNS, including S. epidermidis, are a lead cause of hospital-acquired infections where they are often methicillin resistant [114] and are associated with 2.5% of CAUTI [115]. In murine UTI studies in the absence of catheter, S. epidermidis is able to colonize the bladder at a similar frequency, but with significantly delayed kinetics, compared to E. coli or S. saprophyticus [69].
Host response to staphylococcal UTI
The immune response to S. saprophyticus UTI in humans is not markedly different from Gram-negative UTI and is characterized by symptoms such as lower urinary tract inflammation, pyuria, hematuria, and flank pain [49, 116]. In the mouse transurethral model of S. saprophyticus pathogenesis, inoculation of 107 bacteria into the murine bladder resulted in the recovery of 100-fold more CFU from the kidney than the bladder as early as 6 hours post infection and for as long as 2 weeks post infection [24]. The kidney tropism of S. saprophyticus in a murine model may reflect the propensity of this organism to cause pyelonephritis in humans [49, 61]. The innate inflammatory immune response to S. saprophyticus UTI reflected the higher kidney titers observed, with ~100-fold greater induction of numerous pro-inflammatory cytokines and significantly more neutrophils infiltrating in the kidney compared to the bladder, all of which peaked at 48 hours post infection. Enhanced macrophage recruitment to the bladder was also observed during acute stages of S. saprophyticus UTI [24]. Recently, a role for the mitochondrial respiratory chain has been implicated in the innate immune response to S. saprophyticus infection. A mouse strain carrying a heterogeneous knock-out of a subunit protein of the mitochondrial complex I (GRIM-19) is prone to spontaneous urinary tract infection by S. saprophyticus [117]. In response to infection, macrophages from GRIM-19 mice produce lower amounts of pro-inflammatory cytokines and are defective for intracellular killing of S. saprophyticus. [117]. This report reflects this first mechanistic study of how the host handles S. saprophyticus infection.
Urinary Tract Infection caused by Enterococci
Epidemiology of enterococcal UTI
Enterococci are a genus of Gram-positive lactic acid bacteria that typically occur as diplococci or in short chains. These facultative anaerobes are γ-hemolytic and can tolerate a diversity of environmental conditions including temperature ranges from 10–45°C, pH ranges from 4.6–9.9, sodium chloride concentrations up to 6.5%, bile salts up to 40%, and desiccation despite the fact that do not form spores [118]. Enterococcus species E. faecalis and E. faecium are responsible for a minority of community-acquired UTI, but together cause 15 to 30% of catheter-associated UTIs and are the third leading cause of hospital-acquired UTIs [13, 115, 119]. Diabetic individuals are also at increased risk for UTI [120, 121], which serves as a nidus for the higher incidence of bacteremia in this population [122, 123]. While some studies indicate no increase in the frequency of enterococcal UTI in diabetic women compared to non-diabetic women [124, 125], others report that Enterococcus spp. are more often associated with UTI among diabetics and cause 13% of ASB in diabetics compared to 4.9% in non-diabetics [121, 126]. These differential findings in humans have been reflected in animal studies. In a chemically-induced (streptozocin) model of murine diabetes, diabetic mice were more susceptible to E. faecalis ascending UTI compared to non-diabetic mice [127], whereas pyelonephritis after intravenous injection of E. faecalis was similar in chemically-induced (alloxan) diabetic rats and non-diabetic rats [128]. Diabetes is also a risk factor for prostatitis, for which enterococci are responsible for up to 10% of cases [129, 130]. The incidence of UTIs due to E. faecalis has risen steadily over the years and E. faecalis UTI now outnumbers E. faecium UTI 5:1 [131]. Infection due to multiple-drug-resistant enterococcal strains presents a significant medical problem, with vancomycin resistance increasingly prevalent among E. faecium isolates [131]. E. faecalis readily adheres to and develops biofilms on abiotic surfaces such as urinary catheters. Many enterococcal virulence factors involved in UTI described to date are also biofilm determinants. However, it is unclear whether these virulence factors function in a similar manner during biofilm formation and infection in the absence of abiotic devices.
E. faecalis UTI virulence factors
The E. faecalis surface protein Esp is a large ~200kD surface protein that is enriched among enterococcal bloodstream and endocarditis isolates compared to fecal isolates [132]. Esp is composed of multiple repetitive domains, sharing sequence similarity with Rib and C alpha virulence determinants in group B streptococci, which confer protective immunity and mediate immune evasion [132–135]. Esp promotes bladder colonization in a mouse model, as well as biofilm formation in vitro [136, 137].
Additional E. faecalis surface proteins that contribute to urovirulence in animal models include the collagen adhesin Ace and the enterococcal fibronectin binding protein EfbA [28, 138, 139]. Ace is regulated by the GrvRS two-component regulatory system and, accordingly, gvrR mutants are attenuated for biofilm formation in vitro and for virulence in a murine model of ascending UTI [140]. The ArgR family transcription factor AhrC is important for early biofilm formation in vitro, and ahrC mutans are significantly attenuated for biofilm growth on catheters as well as for bladder and kidney colonization during CAUTI [141]. Non-proteinacious E. faecalis factors monoglucosyl-diacylglycerol (MGlcDAG), diglucosyl-diacylglycerol (DGlcDAG), and D-alanylated lipotechoic acid (LTA) appear to limit UTI virulence because mutations in synthetic genes for each of these products increase colonization of urothelial cells in vitro and in vivo, suggesting that their expression interferes with the host-pathogen interaction in the urinary tract [142, 143].
Similar to other sortase-assembled pili in Gram-positive bacteria, biogenesis of the E. faecalis endocarditis and biofilm-associated pilus (Ebp) relies on a pilus-associated sortase (Sortase C in E. faecalis, SrtC) for pilus polymerization and the housekeeping Sortase A (SrtA) for cell wall anchoring [77, 144–147]. Temporal analysis of factors involved in biofilm formation in vitro showed that SrtA is important for the early attachment stage of biofilm formation, as is SrtC and the Ebp [144, 148]. Furthermore, SrtA and the Ebp are important for biofilm formation in vivo during CAUTI [30, 149]. However, since SrtA attaches multiple proteins to the cell wall, including aggregation substance [150] and Ebp, the contribution of SrtA may be attributable to multiple cell wall proteins in addition to the pilus. In contrast, E. faecalis SrtC and the Ebp, but not SrtA, are important for ascending UTI in the absence of catheter in an outbred mouse model [27, 151, 152] whereas Ebp is not required for ascending UTI in inbred mice [149]. An E. faecium ebp mutant is attenuated in the ascending UTI model [153]. Thus, it appears clear that E. faecalis Ebp is required for biofilm formation during infection, but its contribution to infection in the absence of foreign devices may be species dependent.
Ebp pili can bind extracellular matrix (ECM) proteins and human platelets in vitro [138, 154]. Recently, a predicted metal ion-dependent adhesion site (MIDAS) motif in the von Willebrand factor A (VWA) domain within the N-terminus of EbpA, the likely tip adhesin of the Ebp, was found to be essential for Ebp-dependent CAUTI, biofilm formation, and associated bladder colonization in vivo [155]. The VWA domain- and MIDAS motif-containing PilA of GBS, as well as the tip pilin RrgA of Streptococcus pneumoniae, bind extracellular matrix proteins [156, 157]. The VWA domain of the PilA tip pilin of GBS is also important for bacterial adhesion to human alveolar and intestinal epithelial cells in vitro. The MIDAS motif of E. faecalis EbpA mediates binding to host fibrinogen, which is released in the bladder in response to catheter implantation and subsequently coats the catheter surface, providing a possible explanation why E. faecalis is frequently associated with CAUTI [158].
Immune responses to enterococcal UTI
In a murine model, E. faecalis can colonize and persist in the kidneys, but is rapidly cleared from the bladder in the absence of apparent inflammation [26], suggesting lack of adherence by E. faecalis in the bladder. Nevertheless, urothelial cells containing intracellular E. faecalis have been observed in the urine of patients displaying lower urinary tract symptoms (LUTS) such as pain and issues associated with urine storage and voiding, and the same E. faecalis strains can invade human urothelial cells in vitro [159]. Kidney tropism is a common theme among Gram positive bacteria in the murine infection model [24, 25]. Mild inflammation observed in the infected kidneys at 2 days post infection, consisted primarily of monocytic cells and neutrophils, and was insufficient to clear the kidney infection. Aggregation substance promotes E. faecalis survival within human neutrophils [160]. E. faecalis can also survive longer within macrophages compared to non-pathogenic organisms such as E. coli DH5α and Lactococcus lactis [161] and it has been proposed that glycosaminoglycans on the macrophage surface are receptors for E. faecalis [162]. A number of enterococcal factors have been implicated in survival within macrophages, including extracellular polysaccharide, Ace, and oxidative stress responses [139, 162–166]. In addition, methionine sulfoxide reductases A and B, predicted to reverse protein oxidation, not only promote survival within activated murine peritoneal macrophages but also during ascending UTI [167]. Together these studies present a picture in which macrophages are a key responder to E. faecalis UTI and where survival within macrophages may be important for the ability of enterococci to persist within this niche.
The host signaling cascades leading to E. faecalis-mediated immune infiltration into the urinary tract is not yet well-delineated. In mice, toll-like receptor 2 (TLR2), which recognizes lipopeptides to initiate innate immune responses to Gram-positive bacteria, is not involved in the host response to E. faecalis either in TLR2-transiently transfected 293 cells in vitro or in the murine urinary tract [26, 168]. However, E. faecium-induced signaling in murine peritoneal macrophages requires both TLR2 and the intracellular adaptor protein MyD88 involved in TLR-mediated signaling, and these are thought to be important for signaling neutrophil recruitment during experimental peritonitis [169]. After E. faecalis uptake into macrophages, pro-inflammatory signaling cascades can also be induced via interaction between the intracellular macrophage nucleotide-binding oligimerization domain 2 (Nod2) protein and E. faecalis peptidoglycan fragments [85].
In contrast to ascending UTI, CAUTI is rarely symptomatic [15]. Moreover, urinary catherization alone, in the absence of bacterial infection, can give rise to dysuria, urinary urgency, urothelial damage, and bladder edema [15, 170–172]. Despite being commonly asymptomatic, CAUTI is accompanied by pyuria. However, Gram-negative associated CAUTI is more strongly associated with pyuria than those caused by Gram-positive organisms. CAUTI associated with Gram-positive organisms such as enterococci or CoNS are less inflammatory, as measured by leukocytes in the urine, compared to CAUTI associated with Gram-negative bacilli [173]. These features of Enterococcal CAUTI are also reflected in the murine CAUTI model where implantation of a foreign body (catheter) alone causes major histopathology including edema, urothelium damage, and proinflammatory cytokine expression. E. faecalis can overcome the robust catheter-mediated inflammatory response and replicate to high numbers as a biofilm on the catheter, as well as in the bladder and kidney [31]. Despite high E. faecalis CFU during CAUTI, markers of inflammation are not greatly enhanced in mice receiving the catheter implant together with E. faecalis compared to animals receiving catheter alone [30]. Infection of catheterized mice with E. faecalis results in a modest augmentation of inflammation in the bladder, characterized by a more than 2-fold increase of interleukin 1β (IL-1β) and macrophage inflammatory protein 1α (Mip-1α), despite bacterial loads in the bladder exceeding 106 CFU [30]. These, differential inflammatory responses to enterococcal CAUTI versus ascending UTI may be due, in part, to differing responses of macrophage to E. faecalis in a biofilm state compared to planktonic cells in vitro [174]. In addition, there is a significant decrease in the number of infiltrating activated macrophages during E. faecalis CAUTI compared to catheterization in the absence of bacteria suggesting an immunosuppressive capacity of E. faecalis [31]. The gene encoding a Toll/interleukin-1 receptor (TIR) domain containing protein (tcpF) is enriched among E. faecalis UTI isolates and can downregulate the host inflammatory response, presumably by interfering via molecular mimicry with the TIR-TIR interactions between TLRs required for TLR dimerization and subsequent signaling [175, 176]. Consistent with this finding, several studies of E. faecalis strains isolated from the gastrointestinal tract of healthy human infant guts have shown that a subset of strains were capable of suppressing inflammatory cytokine expression in human intestinal epithelial cells in vitro, as well as suppressing cytokine responses in a Dextran Sulphate Sodium salt (DSS) model of inflammatory intestinal colitis in vivo, although the presence of tcpF was not examined in these strains [177–179]. Together these studies indicate that high titer E. faecalis CAUTI is not highly inflammatory and can be immunosuppressive.
Urinary Tract Infection caused by Group B Streptococcus
Epidemiology of GBS UTI
Streptococcus agalactiae, otherwise known as group B Streptococcus (GBS) is a Gram-positive β-hemolytic chain-forming coccus that is a common asymptomatic inhabitant of the lower gastrointestinal and female reproductive tracts. GBS is estimated to cause approximately 1–2% of all monomicrobial UTIs [180]. Other studies of elderly populations with UTI show an involvement of GBS in as many as 39% of nursing home residents over 70 years of age [181]. Asymptomatic bacteriuria (ASB) and UTI caused by GBS are common not only among the elderly, but also in pregnant, diabetic, and immunocompromised individuals, as well as those with pre-existing urologic abnormalities, groups with higher risk of ascending pyelonephritis that can progress to bacteremia and/or urosepsis [10, 182–184]. While GBS may represent only a small fraction of total UTIs, the burden of GBS UTI is a major public health concern [10], with approximately 160,000 cases annually in the U.S.
Among nonpregnant adults, the incidence of systemic GBS infections is estimated at approximately 4.4 cases per 100,000 individuals; 14% of these are cases of urosepsis [185]. Common underlying conditions of individuals with GBS urosepsis include diabetes mellitus, malignancy, chronic kidney disease, recurrent urinary tract infections, obstructive neuropathy, and neurogenic bladder [185, 186]. In about 30% of cases, systemic infections caused by GBS in nonpregnant adults do not have an apparent focal origin such as cellulitis, pneumonia, or UTI. In contrast to the proportion of UTIs associated with GBS in young nonpregnant populations, estimated at 1–2% [180], it has been estimated that up to 7% of pregnant women have significant titers of GBS in urine [187, 188].
GBS bacteriuria in pregnancy
GBS is the leading cause of sepsis and meningitis in newborns and can be acquired by the newborn in utero or during passage through the colonized birth canal. In addition to colonizing the reproductive tract, GBS is often found to colonize the urinary tracts of pregnant women. Although GBS colonization of the urinary tract in pregnancy is often asymptomatic, GBS bacteriuria is an independent risk factor for maternal pyelonephritis and chorioamnionitis as well as neonatal GBS sepsis [187, 189–192]. The Centers for Disease Control recommend universal screening of GBS vaginal-rectal colonization at 35–37 weeks of gestation and antibiotic prophylaxis for culture-positive women during labor and delivery.
Screening and treatment for ASB is also recommended in pregnancy, since women with ASB are at higher risk of preterm delivery, have a 20–30-fold increased risk of pyelonephritis, and antibiotic treatment of ASB has been demonstrated to reduce these risks significantly [193–198]. The 2002 recommendations from the CDC stated that clinical microbiology labs should report any concentration of GBS detected in urine. The 2010 revised guidelines notes that this practice represents an
“increased workload for clinical microbiology laboratories, which do not generally report bacterial growth in urine of other pathogens at concentrations < 104 cfu/ml and rarely know whether urine samples are from pregnant women; as a result, some laboratories search for any GBS colonies in urine cultures from all women of reproductive age [199].”
Surprisingly, there is little published clinical data investigating whether low GBS titers (< 104 CFU/ml) in urine is associated with adverse maternal or neonatal outcomes. One study found that babies of women with low titer GBS bacteriuria were at higher risk of GBS disease compared to women without detectable GBS in urine [200]. Other studies have not been performed to confirm or refute this finding, but it has been argued that this study may have been biased because only a subset of the women underwent urine culture [199]. Clearly, additional studies are required to estimate the threat of low-level GBS in urine for neonatal GBS disease and determine the best course of action for screening and prophylactic measures. One study estimated that 4% of women who test negative for GBS rectal or vaginal colonization test positive for GBS in urine culture [201], suggesting that the urinary tract could be a distinct and independent niche for the bacterium that could be missed in routine third trimester screening procedures. Currently, the CDC recommends that women with GBS bacteriuria at any time in pregnancy should be given prophylactic antibiotics at the time of labor and delivery.
Invasive GBS disease in the elderly
UTI caused by GBS is approximately 10-times more frequent than GBS neonatal infections and is common among the elderly. Case fatality rates for invasive GBS infection are also higher among the elderly (~15%) compared to young infants with invasive infection (4–6%) [10]. However, in contrast to screening and prophylactic measures during pregnancy and post-partum periods, similar screening and prevention strategies are lacking in settings where elderly patients are at risk of invasive GBS disease. Attempts to develop an effective GBS vaccine have been successful in early trials in animals and humans [202–209], raising the possibility of future reductions in the incidence and severity of GBS disease in at-risk groups.
GBS virulence factors and induction of host immune responses
GBS can cause infections at a variety of body sites (skin, soft tissue, lung, peritoneum, urinary tract, etc.) and utilizes a wide range of virulence factors to injure and invade host tissues, resulting in disseminated infection (e.g. bacteremia, osteomyelitis, meningitis) [210]. Although much is known about the molecular epidemiology of GBS, the organism has not been studied extensively in animal models of UTI and thus, relatively little is known about which virulence factors may play important roles in this context.
Epidemiological analyses have shown that a variety of GBS serotypes are associated with UTI. However, serotype III GBS was found to cause a disproportionate number of acute symptomatic disease compared to other serotypes, which were more likely to be associated with asymptomatic bacteriuria [183]. These clinical studies have formed the basis for experimental studies of GBS urinary tract infections using serotype III GBS strains in mouse models of transurethral infection [25, 211, 212]. In the murine model, the presence of GBS robustly induces IL-1α [212], macrophage inflammatory protein-1α (MIP-1α), MIP-1β, IL-9, and IL-10 [25]. However, experimental studies have observed a striking lack of overall histological inflammation in the bladder during GBS cystitis [25] and marked differences in transcriptional responses compared to E. coli UTI [211]. A number of GBS virulence factors have been characterized in other models, but only a few studies have examined GBS virulence factors by inoculating wild type and mutant strains of bacteria into the mouse urinary tract [25, 39, 213]. These studies have demonstrated that sialic acid residues of the GBS capsular polysaccharide are necessary for optimal establishment of GBS in the urinary tract [25], whereas the β-hemolysin/cytolysin did not appear to have a significant effect on bacterial survival following transurethral infection [25, 213]. Additional studies are needed to identify GBS virulence factors of importance in the urinary tract and to understand the cellular, molecular, and biochemical details of host-microbe interactions in populations at-risk for GBS disease.
In the remainder of this chapter, we will 1) discuss several emerging, rare and/or underreported Gram-positive pathogens of the urinary tract, 2) introduce several recent studies that demonstrate some unexpected inhabitants of the urinary tract using culture-independent approaches for bacterial detection in urine, and 3) examine evidence that host urogenital colonization states may influence the risk of UTI.
Vaginal Microbiota, Bacterial Vaginosis (BV) and UTI
Mounting clinical evidence argues that the composition of a woman’s vaginal microbiota influences her risk of UTI. Women with a dominant population of vaginal lactobacilli are at a lower risk of UTI compared to women with more diverse microbiota, consisting of Gram-negative anaerobes, Actinobacteria, and other Firmicutes [214–216]. This vaginal condition is referred to by some as bacterial vaginosis (BV) and is often labeled as an ‘imbalance’ or dysbiosis of the vaginal microbiota because it has been associated with a wide variety of adverse health outcomes for women and their babies [217, 218]. Clinical features of BV (i.e. Amsel criteria) include vaginal pH >4.5, ‘thin’ grayish homogenous vaginal fluid, fishy odor upon KOH treatment of vaginal fluid and the presence of epithelial cells studded with bacteria in wet mount (i.e. ‘clue-cells’) [219–221]. Another method for BV diagnosis that has been used more extensively in recent years is the Nugent method [220], which is based on a morphotype scoring system using Gram-stained slides where a higher score indicates BV. Only recently have studies in experimental models demonstrated that a single bacterium, Gardnerella vaginalis, one of the common bacteria to overgrow in BV, is sufficient to yield clinical features and biochemical phenotypes of BV in a murine vaginal infection model [222, 223]. Studies examining the association between BV and UTI have found that women with BV have anywhere from a 2.2- to 13.7-fold increased risk of UTI depending on the population studied [214–216]. Further supporting these findings, clinical studies now provide compelling preliminary data that vaginal probiotic administration may be beneficial for women who are prone to recurrent UTI [224]. These studies are consistent with results of anaerobic culture studies presented above, showing that women with recurrent UTI were more likely to contain higher titers and a more diverse repertoire of mixed anaerobes in their urine than healthy (sexually active or inactive) women [225]. Taken together, these results suggest a possible linkage between dysbiosis of the vaginal microbiota and dysbiosis of the bladder microbiota.
Unfortunately, our understanding of why women with bacterial vaginosis are more prone to urinary tract infection is limited. It is thought that lactic acid, hydrogen peroxide, and other ‘defensive’ molecules produced by lactobacilli may create a hostile environment for potential pathogens, including uropathogens, in the vagina [226–228]. However, experimental studies are needed to more fully understand the causal relationships linking specific vaginal bacteria with UTI susceptibility in experimental models. While it remains a distinct possibility that lactobacilli act through specific mechanisms to discourage UTI [229], another possibility (though not a mutually exclusive one) is that one or more taxa of BV bacteria act through unknown mechanisms to enhance UTI susceptibility. As described below and illustrated in Table 1, and Figure 2 (with emphasis on Gram-positive bacteria) many of the bacterial genera that have been described in the BV-associated vaginal environment have also been observed in both the female and male urinary tracts using culture-independent approaches. These findings raise the possibility that fastidious organisms associated with BV could play a more direct role in the etiology of host-uropathogen interactions within the urinary tract.
Table 1. Genera of Gram-positive human urinary tract inhabitants and pathogens detected by culture-dependent and –independent techniques.
“culture negative” means specimens that did not reach the cutoff value, usually 105 CFU/ml urine in a clean catch specimen or 104 CFU/ml for collections by catheter or suprapubic aspiration.
|
Culture-Dependent Clinical Reports |
Culture-Independent Detection in Urinary Tract | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phylum | Genus | Bladder/ Kidney infection |
Bacteremia & Urosepsis |
Urethritis | Female, culture unknown |
Female, culture positive |
Female culture-negative, nitrite and leukocyte esterase negative or asymptomatic |
Male asymptomatic |
Male symptomatic |
|||
| TUC | CC,TUC | CC | TUC | SPA | urine | urethral swab |
urine | |||||
| Firmicutes | Staphylococcus | 5–20% of CAUTI [47] |
[328] | [53, 55, 56] |
[292, 329] | n/r | [318, 319] | [317, 329] | [317] | [319, 322– 324] |
[323] | [330] |
| Enterococcus | 15–30% of CAUTI [13, 115, 119] |
[328] | [331, 332] | [318] | [316, 333] | [319] | n/r | n/r | [322, 323] |
[323] | [330] | |
| Streptococcus | 1–2% of UTI [180] |
[180] | [334] | [292, 318, 329] |
n/r | [316, 318, 319] |
[317, 329] | [317] | [322– 324] |
[323] | [330] | |
| Aerococcus | [230, 232, 237, 239] |
[233–236, 278] |
n/r | [329, 335] | [317] | [318, 319] | [317] | [317] | [319, 322, 323] |
[323] | n/r | |
| Anaerococcus | n/r | n/r | n/r | [292, 329, 335] |
[333] | [316, 318, 319, 333] |
n/r | [317] | [319, 322– 324] |
[323] | n/r | |
| Peptostreptococcus | [321, 336]* | [337] | n/r | [335] | [333] | [318, 319] | n/r | n/r | [319, 322, 324] |
n/r | n/r | |
| Peptoniphilus | n/r | [338] | n/r | [335] | [333] | [317–319] | n/r | n/r | [319, 322] |
n/r | n/r | |
| Lactobacillus | [321, 339, 340] * |
n/r | n/r | [335] | [333] | [316–319, 333] |
[317, 329] | [317] | [319, 322– 324] |
[323] | [330] | |
| Finegoldia | n/r | n/r | n/r | [335] | [317, 318, 333] |
[319] | n/r | [317] | [319, 322– 324] |
[323] | [330] | |
| Veillonella | [341, 342] | [337, 341] |
n/r | [335] | [317] | n/r | [292, 329] | n/r | [322, 323] |
[324] | n/r | |
| Gemella | [343] | n/r | n/r | n/r | n/r | n/r | n/r | n/r | [319, 322, 323] |
[323] | n/r | |
| Actinobacteria | Corynebacterium | [246–249, 344– 349] |
[350] | [351–353] | [292, 329, 335] |
n/r | [316, 318, 319] |
[317, 329] | [317] | [319, 322, 323] |
[323] | n/r |
| Actinobaculum | [155, 261, 262, 266, 267, 269, 272, 274, 276, 277, 354] |
[270, 275, 278, 355] |
n/r | [335] | [317, 333] | [318, 319] | [292, 317, 329] |
[317] | [322] | n/r | n/r | |
| Gardnerella | [285, 300]* | [293, 300, 303, 356] |
[307–309, 357–359] |
[335] | [333] | [316, 318, 319, 333] |
[317, 322– 324, 329, 360] |
[317] | [319, 322] |
[323] | n/r | |
| Atopobium | n/r | n/r | n/r | [335] | [333] | [317, 319, 333] |
[292, 317, 329] |
[317] | [319, 322, 323, 361] |
[323] | [361] | |
| Actinomyces | [362–366] | [367] | n/r | [335] | n/r | [319] | [292] | n/r | [322] | n/r | n/r | |
| Bifidobacterium | n/r | n/r | n/r | [335] | [333] | n/r | [292, 329] | n/r | n/r | n/r | [330] | |
| Mycoplasma | [368–371] | n/r | [358, 372– 379] |
n/r | n/r | n/r | n/r | n/r | [319, 322, 323] |
[323] | n/r | |
n/r = not reported, CAUTI = community acquired UTI,
Questionable clinical significance, CC = clean catch, TUC = transurethral catheter, SPA = suprapubic aspiration.
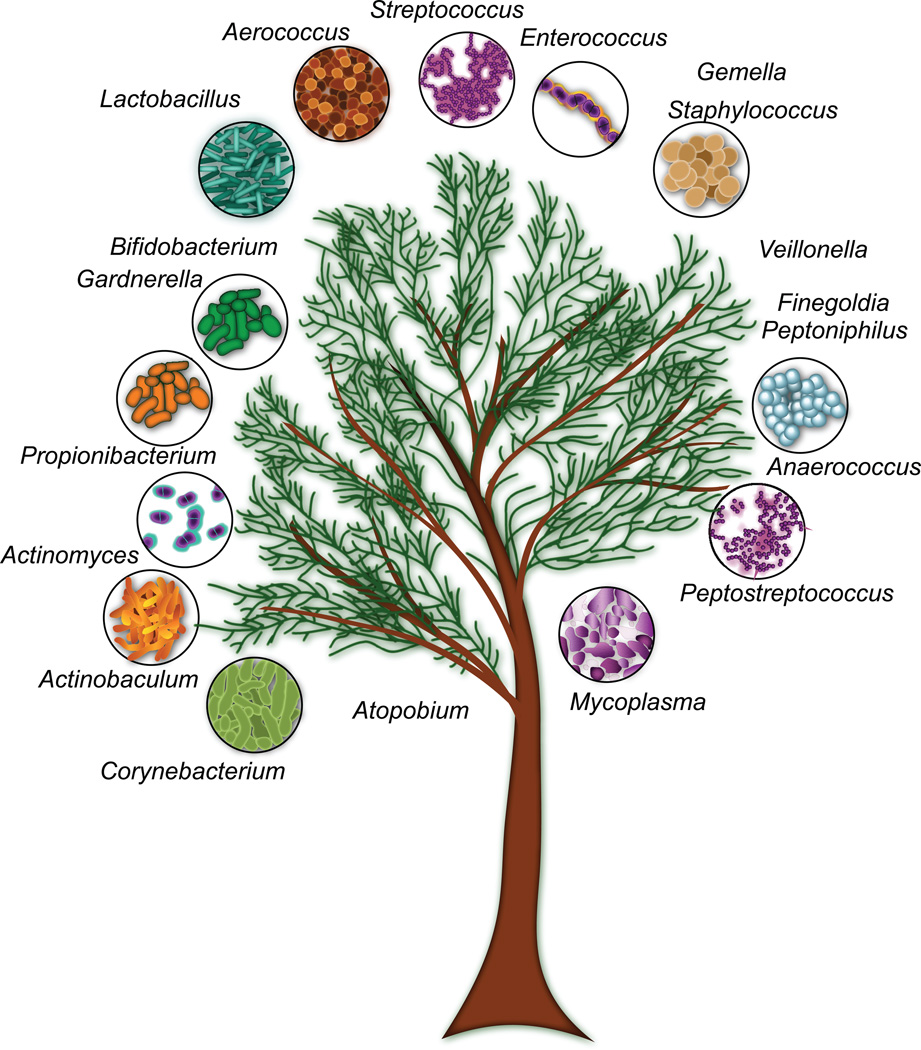
Figure 2. Gram-positive inhabitants and pathogens of the human urinary tract.
Approximate phylogenetic relationships between Gram-positive bacteria are illustrated in this schematic representation. Please refer to the text and Table 1 for additional information and references describing these genera as uropathogens or inhabitants of the human urinary tract. On the left, Bifidobacterium and Gardnerella belong to the order Bifidobacteriales, which together with the orders Priopionibacteriales, Actinomycetales, and Corynebacteriales belong to the class Actinobacteria (a.k.a. “high GC Gram positive bacteria”). Atopobium belongs to the order Coriobacteriales and the class Coriobacteriia. The classes Coriobacteriia and Actinobacteria both belong to the phylum Actinobacteria. The remaining genera with the exception of Mycoplasma belong to the phylum Firmicutes. Whereas Peptostreptococcus, Anerococcus, Finegoldia and Peptoniphilus belong to the order Clostridiales and the class Clostridia, Staphylococcus and Gemella belong to the order Bacilliales and the class Bacilli. Members of the order Lactobacilliales (Lactobacillus, Aerococcus, Enterococcus, and Streptococcus), are also classified as Bacilli. The genus Veillonella is also a member of the Firmicutes, but belongs to the class and order Negativicutes and Selenomonodales respectively. On the other hand, Mycoplasma belongs to the phylum Tenericutes, the class Mollicutes and the order Mycoplasmatales.
Rare, Emerging, and Under-reported Gram-Positive and Polymicrobial Etiologies of UTI
In the next section of this chapter, we present several specific examples of Gram-positive bacteria that are rare, emerging, or underreported, including species of Aerococcus, Corynebacterium, Actinobaculum, and the potential uropathogen Gardnerella vaginalis. These organisms may be missed as causes of UTI due to 1) misclassification due to lack of distinguishing phenotypic criteria, 2) dismissal of significant growth as ‘microbiota contamination’, or 3) lack of detection by standard approaches.
Aerococcus as a cause of UTI
Aerococcus is a genus of microaerophilic, facultatively anaerobic, α-hemolytic, Gram-positive cocci that are catalase- and oxidase-negative and leucine aminopeptidase positive. One unique characteristic of aerococci is that they divide on 2 planes at right angles, resulting in tetrads and irregular clusters. Species of Aerococcus are commonly isolated from air, dust, and vegetation, and are also common isolates from the human vagina and urinary tract. Several species of Aerococcus can cause urinary tract infections and urosepsis, including A. urinae, A. viridans, and A. sanguinicola [230–236]. One study reported that 0.8% of all urine specimens cultured during a 4-month period in a Denmark hospital yielded growth of “Aerococcus-like” organisms [237]. Patients with UTI caused by Aerococcus are most often elderly and many have urological abnormalities or other risk factors for UTI [230, 233, 237]. Many of the cases described are invasive systemic infections in which Aerococcus is isolated from blood along with significant Aerococcus titers in urine.
Aerococci have a number of biochemical and physiological similarities with lactococci, pediococci, enterococci, and streptococci [238]. In particular, phenotypic similarities between Aerococcus and viridans group streptococci have made it difficult for clinical labs to distinguish between them using routine phenotypic tests. For example, one study examined the ability of three commonly used bacterial identification systems (API 20 STREP, ID 32 STREP, and VITEK 2 ID-GPC card, bioMérieux) to correctly identify 30 urinary tract isolates representing different species of Aerococcus. This study revealed that Aerococcus isolates (with the exception of A. viridans) were commonly misidentified or identified with low discrimination [239]. Molecular tools such as amplification and sequencing of 16S rRNA, which are not commonly employed in clinical microbiology labs, are needed for accurate identification of Aerococcus species. The result is that aerococci are often misclassified and/or discarded as likely contaminants.
Prompt and accurate identification of Aerococcus is necessary to avoid life-threatening systemic infection by this potential pathogen in susceptible individuals that present with uncomplicated UTI. Most isolates of Aerococcus urinae have been characterized as resistant to sulfonamides [240, 241]. Case reports of A. urinae UTI demonstrate that when patients are treated with antibiotics effective against the organism, bacteriuria cleared and the patient recovered fully; however, in other situations when A. urinae was not recognized or effective antibiotic treatment was not promptly provided, patients often progressed from simple UTI to invasive systemic infections [234, 235, 240, 242, 243].
Corynebacterium urealyticum
Corynebacteria are Gram-positive, non-motile, non-spore forming, facultatively anaerobic Actinobacteria that are common components of the skin microbiota and increasingly recognized as opportunistic pathogens [244, 245]. Corynebacterium urealyticum has been associated with asymptomatic bacteriuria, and rarely, with acute and chronic infections of the urinary tract. C. urealyticum (previously known as Corynebacterium group D2) is the most common cause of alkaline encrusting cystitis and pyelitis, a chronic inflammatory condition of the urinary tract in which the bacterium causes painful localized ulcerations with deposits of ammonium magnesium phosphate (a.k.a. struvite) that can be visualized on plain radiography [246–248]. Alkaline urine and the presence of struvite crystals in urine sediments are characteristic features of C. urealyticum encrusting cystitis and occur due to bacterial expression of urease activity, which catalyzes the conversion of urea to ammonia and carbon dioxide. The presence of a previous urological procedure and/or mucosal lesion appears to be necessary for urea-splitting bacteria such as C. urealyticum to cause encrusting UTI. Immune compromised patients with underlying urologic disease, those that have undergone long-term hospitalization and/or previous treatment with broad-spectrum antibiotics are also at greater risk for developing alkaline encrusted UTI. While this type of UTI is considered rare, one prospective study showed that 9.8% of renal transplant recipients (n=163) had C. urealyticum in urine, with nearly half of positive patients experiencing no symptoms until at least 1 month after first detection of the organism [249].
C. urealyticum infection can often be missed in routine urine culture because the organism is slow growing and requires enriched media. Moreover, similar to other Actinobacteria, C. urealyticum requires at least 48 hours of growth on blood agar plates to produce pinpoint colonies, which may be dismissed as “contaminating microbiota,” since it is generally thought that species of Corynebacteria other than C. diptheriae are nonpathogenic. The presence of alkaline urine or struvite crystals in urine sediment should prompt cultures of longer duration. A number of cases of C. urealyticum UTI also report that Gram-staining of urine specimens reveals Gram-positive bacilli and polymorphonuclear cells, despite routine urine cultures that come back negative. Molecular detection techniques can also be employed when tests are negative, but there is strong suspicion of encrusting cystitis or pyelitis with C. urealyticum. Encrusting cystitis caused by C. urealyticum is treated by endoscopic removal of encrustations, acidification of the urinary tract, and treatment with appropriate antibiotics. A number of reports document resistance of C. urealyticum isolates to ampicillin, cephalothin, gentamicin, imipenem, tetracycline, ciprofloxacin, ofloxacin and sometimes to tetracycline, erythromycin, and rifampicin [250, 251]; however, most isolates were susceptible to synercid and linezolid [250].
Actinobaculum schaalii
The genus Actinobaculum and the species Actinobaculum schaalii were first described in 1997 following the isolation of organisms described as Actinomyces-like or Corynebacterium-, like from human blood and urine that displayed >6% sequence divergence from the nearest relative, Actinobaculum suis [252], a swine uropathogen also previously known as Actinomyces suis, Corynebacterium suis or Eubacterium suis [253–255]. Species of Actinobaculum are members of the family Actinomycetacea, which also includes the genera Actinomyces, Aracanobacterium, and Mobiluncus [256]. These bacteria are facultative or obligate anaerobes and their detection in clinical specimens often requires a full 48 hours of growth on blood agar plates in the presence of a 5% carbon dioxide atmosphere. Phylogenetically, these bacteria belong to the high G content Gram-positives, but phenotypically, they can appear ‘Gram-variable’ since their cell walls are relatively thin compared to other Gram-positive bacteria and thus more easily decolorized during the Gram-staining procedure. Currently, the genus Actinobaculum contains four species: A. suis, A. schaalii, A. massiliense, and A. urinale. To date, A. urinale and A. massiliense have only been described in a few cases of human pathology, including urinary tract infections. In contrast, A. suis is a well-documented uropathogen of female pigs [254, 257–259] that appears to colonize the prepuce in a large proportion of wild and domesticated adult males [255, 260]. Here we focus on A. schaalii, which appears to be an emerging human uropathogen, particularly among the elderly and those with underlying urologic abnormalities [261].
A. schaalii is a small rod-shaped (straight or slightly curved and sometimes branching), nonmotile, nonsporulating, weakly β-hemolytic, facultative anaerobe requiring CO2 for growth and testing negative for catalase, oxidase, urease, and nitrite production. A. schaalii is resistant to trimethoprim and ciprofloxacin, antibiotics used as first-line treatments for urinary tract infection. Although the natural history surrounding this species is still unclear, culture-dependent and –independent studies published to date suggest that this bacterium may be a commensal inhabitant of the human urinary tract. To date there have been approximately 130 reported cases of human infection with A. schaalii, mostly as small retrospective studies and case reports [252, 261–279]. Approximately 85% of A. schaalii infections reported so far occur in the urinary tract (i.e. single organism cystitis, pyelonephritis, or urosepsis) or have disseminated (e.g. bacteremia, endocarditis) from an unknown primary source. These infections most often occur in elderly individuals and those with underlying urologic conditions such as chronic renal failure or urologic obstruction [261]. In clinical cases of A. schaalii UTI, Gram-positive bacteria are often evident by direct microscopy, and yet, typical aerobic urine culture often produces a negative result [266, 268]. Cases of apparent urosepsis where A. schallii was isolated using anaerobic blood culture techniques were documented only after culture conditions were altered, eventually leading to the identification of A. schaalii in urine.
A. schaalii has been considered an extremely rare uropathogen. However, recent literature suggests that A. schaalii may be underreported due to inadequate culture and identification techniques. Due to fastidious growth conditions required for A. schaalii culture, and the similar appearance of the colonies to resident microbiota of the skin and genitourinary tract, it is likely this organism has been overlooked or dismissed as “contamination” when it may be clinically significant in certain settings [252]. Traditional phenotypic tests are still inadequate for identification of A. schaalii. Instead, studies have successfully used the API Coryne and Rapid ID 32A strip test systems (bioMérieux) together with molecular methodologies (sequencing of 16S ribosomal DNA and/or species-specific primers for quantitative PCR) for identification and/or enumeration of A. schaalii.
A recent study examined 252 randomly selected urines from individuals in 3 hospitals in Viborg County, Denmark using a validated quantitative polymerase chain reaction that specifically analyzed the presence and titers of A. schaalii. The authors of this study found that 22% of persons >60 years of age harbored at least 104 CFU/ml of urine [268]. Additional studies are needed to estimate the prevalence of A. schaalii in the urinary tract in other populations and to define the possible clinical significance of this organism.
Gardnerella vaginalis
Gardnerella vaginalis belongs to the class Actinobacteria (also frequently called ‘high GC Gram-positives’) and the order Bifidobacteriales. G. vaginalis is best known for its connection to bacterial vaginosis (BV), a condition marked by G. vaginalis overgrowth in the vagina. BV has been linked to higher risks of a wide variety of adverse women’s health outcomes, including UTI [214–216, 280, 281]. In addition to the ever more apparent role of G. vaginalis in BV, a growing body of evidence implicates the organism as a potential uropathogen. G. vaginalis (see Figure 3 for images of this organism) is a fastidious bacterium that cannot be recovered under the same conditions as typical uropathogens. It requires incubation in the presence of 5% CO2 or better yet, under anaerobic conditions. Although it can be recovered on sheep blood agar under these conditions, the organism cannot survive in acidic urine or in urine stored at room temperature or at 37°C (rather than refrigerated) prior to recovery. G. vaginalis prevalence is significantly underestimated if plates are incubated for only 24 hours, but instead requires extended incubation (48–72 hrs) [282].
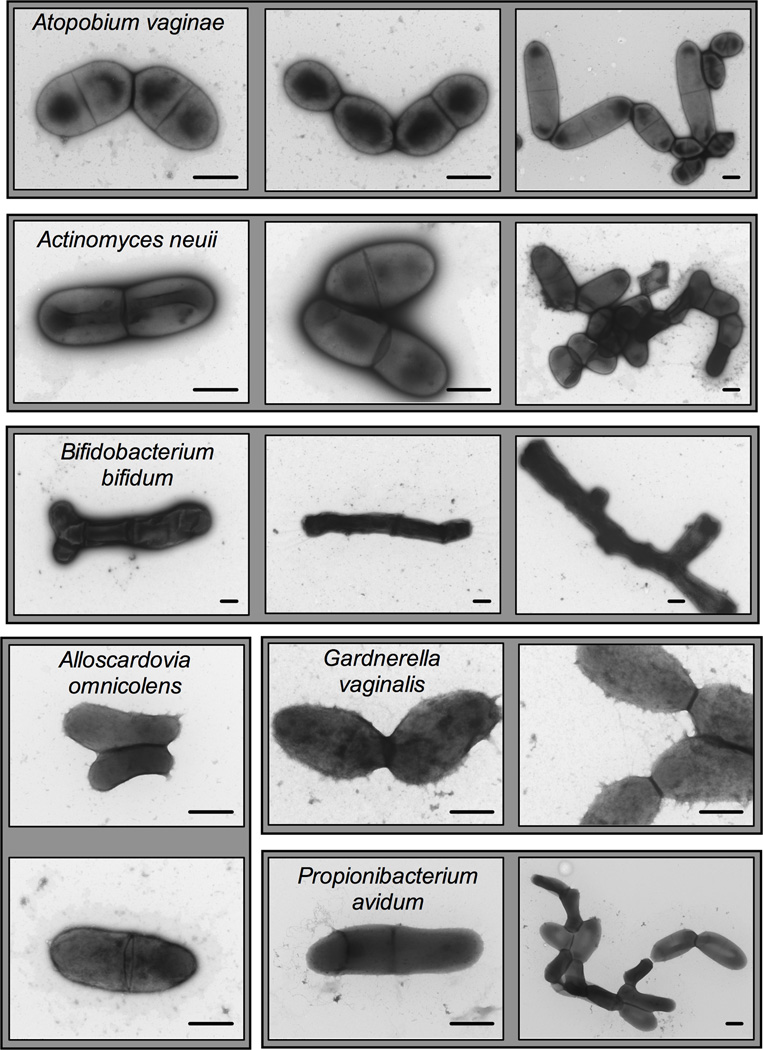
Figure 3. Transmission electron micrographs of several urogenital isolates from the phylum Actinobacteria.
Strains grown for 24–48 hours underwent negative staining with uranyl acetate and were examined by TEM. These strains were isolated from the urine or vaginas of pregnant or nonpregnant women and are available through BEI resources. Strain names are as follows: Actinomyces neuii, MJR8396A; Alloscardovia omnicolens, CMW7705A; Atopobium vaginae, CMW7778A; Bifidobacterium bifidum, MJR8628B; Gardnerella vaginalis, PSS7772B; Propionibacterium avidum, MJR7694. Scale bars are 500nm. Shaded backgrounds contain images of the same strain.
In two large studies encompassing culture analysis of a total of ~33,000 urine specimens, G. vaginalis was reported in 0.6–2.3% of all samples at titers >104 CFU/ml of urine. G. vaginalis was often identified in pure culture and in a context of patient reported symptoms and/or pyuria [283, 284]. One of these studies (reporting G. vaginalis in 0.6% of urines) only examined plates at the 24 hour time point [284], suggesting that 0.6% may be an underestimation. The larger of these two studies examined hospital inpatients and reported that among the patients with G. vaginalis bacteriuria, 58% had evident pyuria and 10% had pyelonephritis [285]. These patients were also more likely to have a history of recurrent UTI. Other studies provide further support that G. vaginalis presence in urine is not simply the result of periurethral or vaginal contamination and further suggest a possible role of G. vaginalis in renal disease. Several studies in the 1960’s-80’s identified G. vaginalis identified in urine isolated by suprapubic aspiration, thus bypassing possible urogenital contaminants [286–290]. These studies suggest that pregnancy renders women more likely to harbor G. vaginalis in their bladders and that recovery of the organism was especially common in women with underlying renal disease. Another study described Gardnerella as common isolate from aspirates of individuals with reflux scarring and so-called “sterile pyelonephritis” [291]. In addition to these culture-based studies, more recent studies using culture independent approaches have echoed these early studies. In fact, one study in particular concludes that G. vaginalis is found at high levels in urines collected by suprapubic (needle) aspiration from many older adult women [292].
In addition to its apparent ability to infect the bladder and kidneys, G. vaginalis has also been described in bloodstream infections. One study reported thirty cases of bacteremia caused by G. vaginalis among obstetric patients over a 4-year period and suggests that bacteremia caused by this organism may be significantly underreported [293]. Although cases of G. vaginalis bacteremia are enriched in the gynecologic setting, occurring after birth [294–296] or following procedures such as endometrial ablation [297] or vaginal myomectomy [298], G. vaginalis bloodstream infection is not limited to women [299–304]. Indeed, the evidence suggests that female to male transmission of G. vaginalis can occur during sexual encounters and that regular condom use reduces the likelihood that G. vaginalis can be isolated from male urine [305, 306]. In one example of bloodstream infection in a male, a previously healthy individual with flank pain was found to have urolithiasis (kidney stones) with fever, elevated peripheral neutrophils, and elevated creatinine (indicating damage to the kidneys) [301]. Blood cultures revealed the man had urosepsis caused by a coccobacilli that produced pinpoint grey colonies on chocolate agar. In this case the hospital microbiology lab was not equipped to identify the organism. Instead, a national microbiology laboratory performed an extensive workup, identifying the organism as G. vaginalis. Another example involved an uncircumcised man with a previous history of diabetes mellitus and an ongoing sexual partner with recurrent BV. The man was shown to have bloodstream G. vaginalis, infective endocarditis, along with apparent septic emboli on one kidney and in the brain [300]. In this case, routine culture of the urine was also unable to identify G. vaginalis as the culprit.
G. vaginalis has also been described in polymicrobial infections in individuals with underlying urologic abnormalities or following urologic procedures. One case series describes G. vaginalis as commonly co-occurring with members of the genus Bacteroides in abscesses following urological procedures [285]. Sturm concluded this case series by recommending that microbiology labs should perform Gram-staining of urine specimens and should follow up results of pyuria in the context of visible coccobacilli by performing culture under conditions capable of G. vaginalis recovery. Given the apparently high risk of G. vaginalis infection following urological instrumentation, Sturm suggests that routine culture for this organism may be warranted during preoperative testing.
The role of G. vaginalis as a genitourinary pathogen is still controversial. Recent work in an experimental model of vaginal infection with G. vaginalis demonstrated that strain JCP8151B, isolated from a woman with BV, was sufficient to induce features of BV in mice [110]. This is the first animal model in which any BV bacterium has been shown to induce the formation of “clue” cells – exfoliated vaginal epithelial cells coated in bacteria – one of the hallmark microscopic features of BV. Interestingly, multiple studies of urinary tract infections involving G. vaginalis report the presence of clue-like cells in urine, both from women [291] and from men [307–309]. Interestingly, a wide variety of genera from the phylum Actinobacteria have been implicated in UTI or identified as part of the urinary microbiome, including not only Gardnerella, but also Actinobaculum, Corynebacterium, Actinomyces, Atopobium, Alloscardovia, and Bifidobacterium (see Figure 3 for images of these bacteria). Few of these genera are accepted as true uropathogens. Thus, experimental studies of infection in animal models are needed to help settle the debate about their potential roles as uropathogens, either in the classical sense or by predisposing the urinary tract to infection or other urologic diseases.
Urine “contamination” with normal microbiota or polymicrobial UTI?
The potential biological and clinical significance of polymicrobial growth in urine depends on many factors. On one hand, the finding of multiple colony types after urine culture is a valid and justifiable concern to suspect contamination of the urine specimen with periurethral and/or vaginal microbiota. For this reason, most clinical microbiology labs will not evaluate plates with polymicrobial growth, but rather dismiss them as “contamination” and request another specimen. For instance, in suspected cases of uncomplicated cystitis, E. faecalis and GBS are often assumed to represent contamination of the urine specimen originating from the periuethral area during collection [1]. In fact, one recent study evaluated titers of these Gram-positives in midstream urine and compared to titers obtained when the same women were subjected to catheterization for urine collection. This study concludes that"enterococci (in 10% of cultures) and group B streptococci (in 12% of cultures) were not predictive of bladder bacteriuria at any colony count (Spearman's r=0.322 for enterococci and 0.272 for group B streptococci).” However, only a few patients in this study had Enterococcus or GBS at levels considered significant for a UTI diagnosis (>100,000cfu/ml). Further studies are needed to define 1) the relationship between Gram-positive bacteria in midstream and catheter-collected urine when patients meet the threshold for UTI (105 CFU/ml), 2) the likelihood of cystitis when these organisms are detected in pure culture versus in the context of multiple other colony types, and 3) whether these relationships are similar across all patient groups. We caution against dismissing Gram-positive pathogens as unimportant. For example, while S. saprophyticus is now established as the predominant Gram-positive uropathogen, it was originally considered to be a urinary contaminant [310, 311].
There are multiple cited cases of polymicrobial bloodstream infection with identical organisms present in both urine and blood cultures [for examples see [312–314]]. In these cases, polymicrobial bloodstream infection supports the interpretation of polymicrobial UTI, especially when multiple of the organisms identified in the blood are also identified in urine. Moreover, since cases of disseminated polymicrobial infection occur most often in compromised persons with underlying risk factors for UTI, the organisms involved are more likely to be members of the ‘normal’ microbiota that may be overlooked in urine specimens as nonpathogens. In another related case, a woman received a suprapubic catheter following urogynecological surgery and later developed an abscess near the location of bladder catheter insertion. Although hospital microbiology lab returned the result of this urine culture as “contaminated,” identification of bacteria from the anaerobically cultured surgically drained pus demonstrated a polymicrobial infection with E. coli, G. vaginalis, and Peptostreptococcus productus [283].
It has been estimated that up to 20% of women presenting with classic symptoms of UTI have culture-negative urine [315]. In the cited example “culture-negative” included samples without a single dominant species (i.e. polymicrobial growth), samples with “secondary pathogens” at titers <104/ml urine, or “doubtful pathogens” at titers <105 CFU/ml urine. Unfortunately, the term sterile is often applied incorrectly to these “culture-negative” contexts. For example, Domann et al. found that 9.2% of urine specimens collected from renal transplant recipients did not produce significant growth under aerobic conditions, but had evidence of intact bacterial rods and/or cocci that were identified as fastidious anaerobes using culture-independent molecular approaches [316]. Alternatively, what might appear to be a monomicrobial infection when observed by aerobic culture may actually be a polymicrobial infection when characterized by culture-independent techniques. For example, one recent study detailed a case study where a woman had what appeared to be a typical culture-positive UTI with >105 CFU/ml of monomicrobial E. coli according to aerobic clinical lab results. The culture-independent approach performed in parallel revealed that urine specimens collected by catheterization and suprapubic aspiration from this patient contained Actinobaculum and Aerococcus at levels that far exceeded E. coli [317]. Other studies that have examined the incidence and significance of Actinobaculum and Aerococcus in urine specimens have shown that up to 90% and 69% of the time, respectively, significant titers of these organisms were found alongside significant titers of typical uropathogens such as E. coli [230, 268].
Another recent study performed anaerobic culture for fastidious anaerobes in urine of women who suffer from recurrent chronic episodes of cystitis and compared these results to a similarly aged groups of young women without UTI [225]. The study reports that women with recurrent cystitis were more likely to contain higher titers and a more diverse repertoire of mixed anaerobes in their urine than healthy women without UTI. In fact, this study had two healthy control groups: one with women who reported regular sexual activity and another with women who reported no history of sexual intercourse. Results of the study strongly suggest that sexual activity does not provide a simple explanation for why women with recurrent chronic cystitis have fastidious bacteria in their urine. Taken together, these findings suggest that fastidious organisms and species assumed to represent contamination of urine specimens in urine cultures are routinely overlooked. We argue that additional studies specifically evaluating contested potential uropathogens in specific patient settings such as those who suffer from recurrent UTI, those at risk for complicated UTI, and those with other urologic problems of uncertain etiology (e.g. interstitial cystitis, urinary urgency incontinence, preeclampsia etc) are still needed.
“Uncultivated” Bacterial Inhabitants of the Urinary Tract
Clinical microbiology labs employ various combinations of culture-dependent approaches in the analysis of urine specimens. These approaches include the use of selective and nonselective agar media for enumeration and identification of bacteria under aerobic conditions. Incubation of agar plates is typically performed at 35–37°C for 18–24 hours. Some labs use additional approaches to cast a slightly wider net to avoid false-negative results, often depending on clinical findings or other factors, extending aerobic incubation to 2–3 days, incubating plates in the presence of 5% CO2, or performing Giemsa- or Gram-staining of urine specimens. In the last few years, culture-independent approaches have evaluated whether the urinary tract contains microbes that are not cultivated under typical aerobic conditions used for urine culture. Table 1 summarizes the results of the urinary tract microbiome studies that have been performed to date with an emphasis on Gram-positive organisms observed using culture-independent approaches (see right side of Table, ‘culture-independent’ detection).
In one study, Domann et al. compared the use of traditional urine culture to the use of a culture-independent denaturing high performance liquid chromatography (DHPLC) to separate and identify PCR-amplified 16S rRNA sequences from genomic DNA purified from midstream urine specimens [316]. In this study of renal transplant recipients, a group at high risk for UTI. Interestingly, while the authors report 100% correspondence between culture-based method and detection of the corresponding aerobic bacteria in DHPLC, they also demonstrate that 9.2% of all patients examined were ‘culture-negative’ using standard approaches, yet appeared to contain significant numbers of fastidious bacteria detected using the DHPLC approach. Most of the culture-negative, DHPLC-positive specimens were polymicrobial, and many of the genera detected in these urines have been previously associated with BV (see above). Specifically, Gardnerella vaginalis, Anaerococcus lactolyticus, and Lactobacillus iners were among the Gram-positive bacteria detected in urines of renal transplant recipients and Prevotella, Bacteroides, Dialister, Leptotrichia, and Fusobacteria were among the Gram-negative bacteria detected. Many of these urines were positive for leukocyte esterase activity, strongly suggesting that the organisms present were the cause of symptomatic UTI as opposed to the possibility of urine contamination by vaginal bacteria. The authors state that standard curves of bacteria in pure culture were used to estimate CFU in urine using the DHPLC method and that in many cases bacteria were in clinically-relevant ranges of >5×104 CFU/ml. Similar studies using midstream urine to assess bacterial diversity using 16S RNA sequencing have also implied that a variety of vaginal bacteria may inhabit the female urinary tract [318] [319]. One limitation of these studies is that the collection method (midstream “clean catch”) does not adequately address the possibility of urine contamination with vaginal or periurethral microbes.
One line of evidence that microbes detected by culture-independent approaches are not simply a product of urine contamination by vaginal bacteria comes from a study in which bacteria recovered from catheters were identified by culture-dependent and -independent approaches [320]. As with previous studies, the authors demonstrate the presence of many bacteria that are currently regarded as vaginal bacteria (see Table 1). As with previous studies, examples of patients were provided in whom culture independent approaches detect high levels of bacteria such as Actinobacculum that were not amenable to culture using routine methods. The authors concluded that catheterized patients who had a UTI were more likely to have lower diversity of organisms recovered from catheters compared to patients who did not have a UTI. However, it is not clear from the methods what time point the 16S data contributing to this interpretation were collected (e.g. during UTI or prior to UTI). Thus, it is not clear if higher diversity of the urinary tract microbiota may protect against UTI or if the presence of a UTI drowns out the signal of other bacteria.
In an attempt to demonstrate beyond reasonable doubt that ‘vaginal bacteria’ in the urinary tract were in fact located in the bladder, a recent study by Wolfe et al., compared the bacterial composition of urine from asymptomatic adult women collected by various methods including midstream ‘clean catch,’ transurethral catheterization, and direct suprapubic aspiration from the bladder [317]. Suprapubic aspiration eliminates concerns of vaginal or periurethral contamination. All urines used in the study were culture-negative using the clinical microbiology laboratory-recommended cutoff of 104 CFU/ml. The authors showed that a subpopulation of urines collected by transurethral catheterization did not yield significant growth under typical clinical microbiology laboratory conditions (for urine), but contained Gram-positive and Gram-negative bacteria that were evident by direct microscopy and 16S rRNA amplification and sequencing. Surprisingly, bacterial genomic DNA could be amplified from 21/23 urine samples collected by direct needle aspiration from the bladder. Organisms present in these specimens could often be observed in corresponding catheter and midstream specimens collected at the same time, but rarely from ‘mock needle stick’ samples. Bacteria detected in urine specimens obtained by catheterization or aspiration included the anaerobic genera Actinobaculum, Anaerococcus, Atopobium, Gardnerella, Prevotella, Sneathia, and Veillonella among others (see Table 1). These organisms are all fastidious anaerobes that would not be detected using typical aerobic urine culture and were not detected in mock needlesticks that did not enter the bladder. In addition to these somewhat unexpected organisms, more typical potential uropathogens were also revealed by this study, including species of Streptococcus and Staphylococcus.
These findings are supported by a few much earlier studies. For example, in a 1979 study. 185 pregnant women (admitted for pregnancy complications) underwent suprapubic bladder aspiration and their urine was subjected to anaerobic culture. 6.4% of these women had anaerobes in their urine including members of the genera Peptostreptococcus, Veillonella, Clostridium, and Bifidobacterium [321]. These findings provide clear evidence that urine taken directly from the bladder, and thus not exposed to potential contaminants of the vagina, periurethral area or distal urethra, often contains organisms that are undetectable using routine clinical microbiology procedures.
Independent investigations have also evaluated the microbial content of male urine or urethral swabs using culture-independent approaches. These studies reveal strikingly similar patterns of fastidious and anaerobic bacteria seen in female urogenital specimens, including more typical Gram-positive uropathogens (e.g. Staphylococcus, Enterococcus, Streptococcus), as well as other bacteria previously considered to be specific to the female urogenital tract (e.g. Gardnerella, Atopobium, Actinobaculum, Anaerococcus, Lactobacillus, etc, see Table 1) [322, 323]. Another recent study investigated the microbiota of urine and coronal sulcus specimens from adolescent males, showing that bacteria associated with BV are present in young men that report having no history of partnered sexual activity [324]. These data provide further evidence that specific bacterial species not cultivated using routine approaches are in fact present in the genitourinary tracts of both men and women and are not necessarily acquired through sexual contact. Note that some of these species have also been reported in case studies of urinary tract infection, most often in populations with underlying risk factors for UTI (see Table 1 references).
One of the major limitations of culture-independent urinary microbiome studies is that it is not possible to know whether the bacteria are alive or dead. A skeptic might ask"is the presence of microbial DNA in urine, even if taken by needle aspiration from the bladder, indicative of a live microbial community?” Teams led by Schreckenberger, Wolfe, and Brubaker recently used an expanded quantitative urine culture (EQUC) method to address this important question [292, 325]. These authors demonstrated previously that urine collected from women by transurethral catheterization and by suprapubic aspiration had very similar microbiome profiles. Thus, subsequent studies used catheter-based collection of urine because this was the least invasive methods that still provided confidence that the urine was not contaminated with vaginal bacteria. The authors performed 16S rDNA-based community profiling in parallel with EQUC and demonstrated that many of the fastidious bacteria detected in catheterized urine by culture-independent approaches can in fact be recovered in laboratory culture [292, 325]. Whether these bacteria represent stable communities living in the bladder still remains to be fully characterized.
This same group most recently performed community profiling and EQUC on catheter-collected urine samples to test the hypothesis that the urinary microbiome may contribute to urinary urgency incontinence (UUI) [292]. Overall, this paper sets an impressive standard for the field, providing a truly multidisciplinary approach to provide evidence that challenges previous assumptions regarding a condition (UUI) of uncertain etiology that has nevertheless been defined as noninfectious. The authors conclude that the urinary microbiomes of women with UUI were in fact different from women in the control group, having lesser proportions of Lactobacilli and greater proportions of Gardnerella, Actinobacculum, Actinomyces, Aerococcus, Arthrobacter, Corynebacterium, Oligella, Staphylococcus, and Streptococcus. This paper also reports that women in the UUI group were more likely to harbor Lactobacillus gasseri in their bladders compared to women in the control group, which were more likely to harbor Lactobacillus crispatus. In addition to the culture independent component of this study, the authors also performed EQUC to demonstrate that many of the bacteria detected using molecular approaches could also be cultured in the laboratory. One potential limitation of this study is that the UUI group was found to be ~2-fold less likely to be using estrogen, despite being on average 14 years older than the control group (63 [±12] versus 49 [±14]; P < 0.05). Differences in age and hormonal status have a wide variety of physiological implications and have been correlated with specific phenotypic features of the vaginal microbiota (e.g. presence or amounts of various bacteria) [326, 327]. These factors could therefore be important confounders that may contribute to the differences observed between the cases and controls.
Another study from the same groups used the same population of women suffering from UUI who were randomized to two treatment arms of a large clinical trial, but instead employed qPCR targeting the 16S ribosomal DNA (rDNA) to assign women simply as positive or negative for bacteria presence. Only women who were negative for UTI by the clinical (culture-based) definition at the time of urine sample retrieval, prior to randomization were included in the qPCR study. The authors report no statistically significant differences in median qPCR levels depending on whether women went on to have a post procedure UTI (UTI 2.58 ×105 vs no UTI 1.35 × 105 copies/ml) or whether treatment for UUI was successful. Rather, using a categorical variable (presence/absence), the authors demonstrate that women positive for bacterial DNA in catheterized urine suffered a higher number of UUI episodes (5.71[±2.60] vs 4.72 [±2.86]). However, qPCR positive women were less likely to suffer from post-procedure UTI compared to qPCR negative women (10% vs 24% respectively).
One of the limitations of the urinary tract microbiome studies performed to date is that the abundance and composition of microbes in the urinary tract is relative and based on the total number of sequence reads, which can vary widely from individual to individual. Going forward, one of the challenges will be to measure absolute abundances of specific uncultivated species in larger epidemiological studies, and to evaluate the potential risks posed by urinary tract colonization with different bacterial species or subsets. Experimental models in animals should also be employed to help to directly examine the hypothesis that some of these Gram-positive anaerobes are pathogenic in the urinary tract. Such models will also be required to test specific hypotheses related to the potential roles of urogenital bacteria in determining host susceptibility to UTI caused by common uropathogens.
Many of the fastidious anaerobes described in urine microbiome studies (see Table 1) are taxa that have also been associated with bacterial vaginosis. Clinical studies demonstrating a higher risk of UTI in women with BV further suggest that some of these bacteria may simultaneously cause dysbiosis in the vagina and the bladder. However, contamination of urine with vaginal bacteria is not a viable explanation for these findings given that the bacteria were also detected in urines collected by catheterization and suprapubic aspiration (see Table 1). As described above, the presence of clue cells (i.e. squamous epithelial cells covered in G. vaginalis and possibly other bacteria) is characteristic of women with BV [222]. However, consistent with the idea of urinary tract dysbiosis, women with “sterile pyelonephritis” who were identified as having a high infectious bladder burden of G. vaginalis also had clue cells in urine directly aspirated from the bladder [291]. In fact, clue cells have also been reported in urethral and semen specimens from men who were identified as having G. vaginalis-associated infections [307–309]. These findings support the interpretation that G. vaginalis may attach itself to epithelial cells of the urethra and bladder and induce exfoliation, thus resulting in urinary clue cells that much resemble the vaginal clue cells seen in women with BV.
Experimental studies are needed to determine whether these ‘BV bacteria’ have a direct causal role in determining host susceptibility to acute or recurrent UTI. Findings that women suffering from recurrent UTI have fewer recurrences following vaginal probiotic L. crispatus administration suggest that vaginal lactobacilli may have a protective role in UTI, or that they may displace other organisms that have a detrimental role. This interpretation is also indirectly supported by observations that Lactobacillus is the most common dominant organism in the urinary tract (among older women) and that having qPCR-positive urine was associated with a reduced likelihood of UTI (see above). Finally, the fact that these bacteria can also be found in the male urinary tract, even among adolescent males with no sexual history, strongly suggests that these ‘BV bacteria’ are not simply vaginal bacteria transiently exposed to the urinary tract during sexual activities, but rather that these organisms may have a wider tissue tropism that includes the urinary tract.
In summary, we have 1) reviewed the epidemiology and virulence characteristics of the most common Gram-positive uropathogens, including Staphylococcus saprophyticus, Enterococcus faecalis, and group B Streptococcus, 2) reviewed the natural history of several less common uropathogens and the reasons why these bacteria are systematically overlooked, 3) summarized recent literature suggesting that a wide variety of Gram-positive bacteria are common inhabitants of the human urinary tract, and 4) presented evidence that polymicrobial infections involving Gram-positive bacteria may be more prevalent than previously appreciated and also may impact pathologic outcomes in the urinary tract.
Fifty years ago it was thought that the major uropathogenic organisms did not include Gram-positive bacteria. Ten years ago it was assumed that the uninfected urinary tract was sterile. Currently, it is widely held that Gram-positive bacteria either alone or alongside Gram-negative uropathogens in the urine are likely to be contaminants of no consequence. The first two assumptions have been disproved as technology has advanced. The literature summarized in this chapter calls into question the last assumption. We propose that future studies will illuminate a previously unappreciated role for members of the polymicrobial microbiota in the urinary tract, vagina, and gut in UTI susceptibility and disease progression.
Describes Staphylococcus, Enterococcus, and Streptococcus as uropathogens, discusses polymicrobial urinary tract infection, the significance of polymicrobial growth upon urine culture, and the potential under-reporting of fastidious organisms and atypical Gram-positives as causes of urinary tract infection. Finally, this chapter summarizes current literature regarding the microbiota of the urinary tract and discusses the potential role of urogenital microbiota in UTI susceptibility.
Contributor Information
Kimberly A. Kline, Singapore Centre on Environmental Life Sciences Engineering, School of Biological Sciences, Nanyang Technological University, Singapore, Phone: +65 9755-3601, Fax: +65 6316-7349, kkline@ntu.edu.sg
Amanda L. Lewis, Department of Molecular Microbiology, Campus Box 8230, Washington University School of Medicine, 660 S. Euclid Avenue, St. Louis, MO 63110, USA, Phone: 314-286-0016, Fax: 314-747-0264, allewis@wustl.edu
References
- 1.Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028–1037. doi: 10.1056/NEJMcp1104429. [DOI] [PubMed] [Google Scholar]
- 2.Schroeder AR, Chang PW, Shen MW, Biondi EA, Greenhow TL. Diagnostic accuracy of the urinalysis for urinary tract infection in infants<3 months of age. Pediatrics. 2015;135:965–971. doi: 10.1542/peds.2015-0012. [DOI] [PubMed] [Google Scholar]
- 3.Clyne M. Paediatrics: dipstick adequate for febrile UTI test. Nat Rev Urol. 2014;11:304. doi: 10.1038/nrurol.2014.116. [DOI] [PubMed] [Google Scholar]
- 4.Demilie T, Beyene G, Melaku S, Tsegaye W. Diagnostic accuracy of rapid urine dipstick test to predict urinary tract infection among pregnant women in Felege Hiwot Referral Hospital, Bahir Dar, North West Ethiopia. BMC Res Notes. 2014;7:481. doi: 10.1186/1756-0500-7-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jido TA. Urinary tract infections in pregnancy: evaluation of diagnostic framework. Saudi J Kidney Dis Transpl. 2014;25:85–90. doi: 10.4103/1319-2442.124496. [DOI] [PubMed] [Google Scholar]
- 6.Mehnert-Kay SA. Diagnosis and management of uncomplicated urinary tract infections. Am Fam Physician. 2005;72:451–456. [PubMed] [Google Scholar]
- 7.Al Majid F, Buba F. The Predictive and Discriminant Values of Urine Nitrites in Urinary Tract Infection. Biomed Res. 2010;21:297–299. [Google Scholar]
- 8.Arinzon Z, Peisakh A, Shuval I, Shabat S, Berner YN. Detection of urinary tract infection (UTI) in long-term care setting: is the multireagent strip an adequate diagnostic tool? Arch Gerontol Geriatr. 2009;48:227–231. doi: 10.1016/j.archger.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Wagenlehner FM, Naber KG. Current challenges in the treatment of complicated urinary tract infections and prostatitis. Clin Microbiol Infect. 12(Suppl 3):67–80. doi: 10.1111/j.1469-0691.2006.01398.x. [DOI] [PubMed] [Google Scholar]
- 10.Edwards MS, Baker CJ. Group B streptococcal infections in elderly adults. Clin Infect Dis. 2005;41:839–847. doi: 10.1086/432804. [DOI] [PubMed] [Google Scholar]
- 11.Matthews SJ, Lancaster JW. Urinary tract infections in the elderly population. Am J Geriatr Pharmacother. 2011;9:286–309. doi: 10.1016/j.amjopharm.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Berg RC, Underland V, Odgaard-Jensen J, Fretheim A, Vist GE. Effects of female genital cutting on physical health outcomes: a systematic review and meta-analysis. BMJ Open. 2014;4:e006316. doi: 10.1136/bmjopen-2014-006316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maki DG, Tambyah PA. Engineering out the risk for infection with urinary catheters. Emerg Infect Dis. 2001;7:342–347. doi: 10.3201/eid0702.010240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain P, Parada JP, David A, Smith LG. Overuse of the indwelling urinary tract catheter in hospitalized medical patients. Arch Intern Med. 1995;155:1425–1429. [PubMed] [Google Scholar]
- 15.Tambyah PA, Maki DG. Catheter-associated urinary tract infection is rarely symptomatic: a prospective study of 1,497 catheterized patients. Arch Intern Med. 2000;160:678–682. doi: 10.1001/archinte.160.5.678. [DOI] [PubMed] [Google Scholar]
- 16.Warren JW. Catheter-associated urinary tract infections. Infect Dis Clin North Am. 1997;11:609–622. doi: 10.1016/s0891-5520(05)70376-7. [DOI] [PubMed] [Google Scholar]
- 17.Stensballe J, Tvede M, Looms D, Lippert FK, Dahl B, Tønnesen E, Rasmussen LS. Infection risk with nitrofurazone-impregnated urinary catheters in trauma patients: a randomized trial. Ann Intern Med. 2007;147:285–293. doi: 10.7326/0003-4819-147-5-200709040-00002. [DOI] [PubMed] [Google Scholar]
- 18.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis K. Multidrug tolerance of biofilms and persister cells. Curr Top Microbiol Immunol. 2008;322:107–131. doi: 10.1007/978-3-540-75418-3_6. [DOI] [PubMed] [Google Scholar]
- 20.Dedeić-Ljubović A, Hukić M. Catheter-related urinary tract infection in patients suffering from spinal cord injuries. Bosn J Basic Med Sci. 2009;9:2–9. doi: 10.17305/bjbms.2009.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desai PJ, Pandit D, Mathur M, Gogate A. Prevalence, identification and distribution of various species of enterococci isolated from clinical specimens with special reference to urinary tract infection in catheterized patients. Indian J Med Microbiol. 2001;19:132–137. [PubMed] [Google Scholar]
- 22.Johnson AP, Warner M, Speller DC. In-vitro activity of quinupristin/dalfopristin (Synercid) against isolates of Streptococcus pneumoniae, Staphylococcus aureus and enterococcus spp. J Antimicrob Chemother. 1997;40:604–605. doi: 10.1093/jac/40.4.604. [DOI] [PubMed] [Google Scholar]
- 23.Gatermann S, John J, Marre R. Staphylococcus saprophyticus urease: characterization and contribution to uropathogenicity in unobstructed urinary tract infection of rats. Infect Immun. 1989;57:110–116. doi: 10.1128/iai.57.1.110-116.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kline KA, Ingersoll MA, Nielsen HV, Sakinc T, Henriques-Normark B, Gatermann S, Caparon MG, Hultgren SJ. Characterization of a novel murine model of Staphylococcus saprophyticus urinary tract infection reveals roles for Ssp and SdrI in virulence. Infect Immun. 2010;78:1943–1951. doi: 10.1128/IAI.01235-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kline KA, Schwartz DJ, Lewis WG, Hultgren SJ, Lewis AL. Immune activation and suppression by group B streptococcus in a murine model of urinary tract infection. Infect Immun. 2011;79:3588–3595. doi: 10.1128/IAI.00122-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kau AL, Martin SM, Lyon W, Hayes E, Caparon MG, Hultgren SJ. Enterococcus faecalis tropism for the kidneys in the urinary tract of C57BL/6J mice. Infect Immun. 2005;73:2461–2468. doi: 10.1128/IAI.73.4.2461-2468.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh KV, Nallapareddy SR, Murray BE. Importance of the ebp (endocarditis- and biofilm-associated pilus) locus in the pathogenesis of Enterococcus faecalis ascending urinary tract infection. J Infect Dis. 2007;195:1671–1677. doi: 10.1086/517524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torelli R, Serror P, Bugli F, Paroni Sterbini F, Florio AR, Stringaro A, Colone M, De Carolis E, Martini C, Giard JC, Sanguinetti M, Posteraro B. The PavA-like fibronectin-binding protein of Enterococcus faecalis, EfbA, is important for virulence in a mouse model of ascending urinary tract infection. J Infect Dis. 2012;206:952–960. doi: 10.1093/infdis/jis440. [DOI] [PubMed] [Google Scholar]
- 29.Kim HY, Choe HS, Lee DS, Yoo JM, Lee SJ. A novel rat model of catheter-associated urinary tract infection. Int Urol Nephrol. 2015;47:1259–1263. doi: 10.1007/s11255-015-1038-5. [DOI] [PubMed] [Google Scholar]
- 30.Guiton PS, Hung CS, Hancock LE, Caparon MG, Hultgren SJ. Enterococcal biofilm formation and virulence in an optimized murine model of foreign body-associated urinary tract infections. Infect Immun. 2010;78:4166–4175. doi: 10.1128/IAI.00711-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guiton PS, Hannan TJ, Ford B, Caparon MG, Hultgren SJ. Enterococcus faecalis overcomes foreign body-mediated inflammation to establish urinary tract infections. Infect Immun. 2013;81:329–339. doi: 10.1128/IAI.00856-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guiton PS, Cusumano CK, Kline KA, Dodson KW, Han Z, Janetka JW, Henderson JP, Caparon MG, Hultgren SJ. Combinatorial small-molecule therapy prevents uropathogenic Escherichia coli catheter-associated urinary tract infections in mice. Antimicrob Agents Chemother. 2012;56:4738–4745. doi: 10.1128/AAC.00447-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hjelm E, Lundell-Etherden I, Mårdh PA. Ascending urinary tract infections in rats induced by Staphylococcus saprophyticus and Proteus mirabilis. Acta Pathol Microbiol Immunol Scand [B] 1987;95:347–350. doi: 10.1111/j.1699-0463.1987.tb03136.x. [DOI] [PubMed] [Google Scholar]
- 34.Alteri CJ, Himpsl SD, Mobley HL. Preferential use of central metabolism in vivo reveals a nutritional basis for polymicrobial infection. PLoS Pathog. 2015;11:e1004601. doi: 10.1371/journal.ppat.1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Armbruster CE, Smith SN, Yep A, Mobley HL. Increased incidence of urolithiasis and bacteremia during Proteus mirabilis and Providencia stuartii coinfection due to synergistic induction of urease activity. J Infect Dis. 2014;209:1524–1532. doi: 10.1093/infdis/jit663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kunin CM. Blockage of urinary catheters: role of microorganisms and constituents of the urine on formation of encrustations. J Clin Epidemiol. 1989;42:835–842. doi: 10.1016/0895-4356(89)90096-6. [DOI] [PubMed] [Google Scholar]
- 37.Mobley HL, Warren JW. Urease-positive bacteriuria and obstruction of long-term urinary catheters. J Clin Microbiol. 1987;25:2216–2217. doi: 10.1128/jcm.25.11.2216-2217.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsuchimori N, Hayashi R, Shino A, Yamazaki T, Okonogi K. Enterococcus faecalis aggravates pyelonephritis caused by Pseudomonas aeruginosa in experimental ascending mixed urinary tract infection in mice. Infect Immun. 1994;62:4534–4541. doi: 10.1128/iai.62.10.4534-4541.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kline KA, Schwartz DJ, Gilbert NM, Hultgren SJ, Lewis AL. Immune modulation by group B Streptococcus influences host susceptibility to urinary tract infection by uropathogenic Escherichia coli. Infect Immun. 2012;80:4186–4194. doi: 10.1128/IAI.00684-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kline KA, Schwartz DJ, Gilbert NM, Lewis AL. Impact of host age and parity on susceptibility to severe urinary tract infection in a murine model. PLoS One. 2014;9:e97798. doi: 10.1371/journal.pone.0097798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barford JM, Anson K, Hu Y, Coates AR. A model of catheter-associated urinary tract infection initiated by bacterial contamination of the catheter tip. BJU Int. 2008;102:67–74. doi: 10.1111/j.1464-410X.2008.07465.x. [DOI] [PubMed] [Google Scholar]
- 42.Matsukawa M, Kunishima Y, Takahashi S, Takeyama K, Tsukamoto T. Bacterial colonization on intraluminal surface of urethral catheter. Urology. 2005;65:440–444. doi: 10.1016/j.urology.2004.10.065. [DOI] [PubMed] [Google Scholar]
- 43.Nickel JC, Downey JA, Costerton JW. Ultrastructural study of microbiologic colonization of urinary catheters. Urology. 1989;34:284–291. doi: 10.1016/0090-4295(89)90327-0. [DOI] [PubMed] [Google Scholar]
- 44.Macleod SM, Stickler DJ. Species interactions in mixed-community crystalline biofilms on urinary catheters. J Med Microbiol. 2007;56:1549–1557. doi: 10.1099/jmm.0.47395-0. [DOI] [PubMed] [Google Scholar]
- 45.Frank DN, Wilson SS, St Amand AL, Pace NR. Culture-independent microbiological analysis of foley urinary catheter biofilms. PLoS One. 2009;4:e7811. doi: 10.1371/journal.pone.0007811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nicolle LE, Hoban SA, Harding GK. Characterization of coagulase-negative staphylococci from urinary tract specimens. J Clin Microbiol. 1983;17:267–271. doi: 10.1128/jcm.17.2.267-271.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hooton TM, Stamm WE. Diagnosis and treatment of uncomplicated urinary tract infection. Infect Dis Clin North Am. 1997;11:551–581. doi: 10.1016/s0891-5520(05)70373-1. [DOI] [PubMed] [Google Scholar]
- 48.Wallmark G, Arremark I, Telander B. Staphylococcus saprophyticus: a frequent cause of acute urinary tract infection among female outpatients. J Infect Dis. 1978;138:791–797. doi: 10.1093/infdis/138.6.791. [DOI] [PubMed] [Google Scholar]
- 49.Hovelius B, Mårdh PA. Staphylococcus saprophyticus as a common cause of urinary tract infections. Rev Infect Dis. 1984;6:328–337. doi: 10.1093/clinids/6.3.328. [DOI] [PubMed] [Google Scholar]
- 50.Zong Z, Peng C, Lü X. Diversity of SCCmec elements in methicillin-resistant coagulase-negative staphylococci clinical isolates. PLoS One. 2011;6:e20191. doi: 10.1371/journal.pone.0020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gillespie WA, Sellin MA, Gill P, Stephens M, Tuckwell LA, Hilton AL. Urinary tract infection in young women, with special reference to Staphylococcus saprophyticus. J Clin Pathol. 1978;31:348–350. doi: 10.1136/jcp.31.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rupp ME, Soper DE, Archer GL. Colonization of the female genital tract with Staphylococcus saprophyticus. J Clin Microbiol. 1992;30:2975–2979. doi: 10.1128/jcm.30.11.2975-2979.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Colodner R, Ken-Dror S, Kavenshtock B, Chazan B, Raz R. Epidemiology and clinical characteristics of patients with Staphylococcus saprophyticus bacteriuria in Israel. Infection. 2006;34:278–281. doi: 10.1007/s15010-006-5655-x. [DOI] [PubMed] [Google Scholar]
- 54.Ferry S, Burman LG, Mattsson B. Urinary tract infection in primary health care in northern SwedenIEpidemiology. Scand J Prim Health Care. 1987;5:123–128. doi: 10.3109/02813438709013988. [DOI] [PubMed] [Google Scholar]
- 55.Hovelius B, Thelin I, Mårdh PA. Staphylococcus saprophyticus in the aetiology of nongonococcal urethritis. Br J Vener Dis. 1979;55:369–374. doi: 10.1136/sti.55.5.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carson CC, McGraw VD, Zwadyk P. Bacterial prostatitis caused by Staphylococcus saprophyticus. Urology. 1982;19:576–578. doi: 10.1016/0090-4295(82)90002-4. [DOI] [PubMed] [Google Scholar]
- 57.Kauffman CA, Hertz CS, Sheagren JN. Staphylococcus saprophyticus: role in urinary tract infections in men. J Urol. 1983;130:493–494. doi: 10.1016/s0022-5347(17)51268-9. [DOI] [PubMed] [Google Scholar]
- 58.Hovelius B, Colleen S, Mårdh PA. Urinary tract infections in men caused by Staphylococcus saprophyticus. Scand J Infect Dis. 1984;16:37–41. doi: 10.3109/00365548409068407. [DOI] [PubMed] [Google Scholar]
- 59.Jellheden B, Norrby RS, Sandberg T. Symptomatic urinary tract infection in women in primary health care. Bacteriological, clinical and diagnostic aspects in relation to host response to infection. Scand J Prim Health Care. 1996;14:122–128. doi: 10.3109/02813439608997082. [DOI] [PubMed] [Google Scholar]
- 60.Rupp ME, Archer GL. Coagulase-negative staphylococci: pathogens associated with medical progress. Clin Infect Dis. 1994;19:231–243. doi: 10.1093/clinids/19.2.231. quiz 244–245. [DOI] [PubMed] [Google Scholar]
- 61.Latham RH, Running K, Stamm WE. Urinary tract infections in young adult women caused by Staphylococcus saprophyticus. JAMA. 1983;250:3063–3066. [PubMed] [Google Scholar]
- 62.Kahlmeter G ECO.SENS. An international survey of the antimicrobial susceptibility of pathogens from uncomplicated urinary tract infections: the ECO.SENS Project. J Antimicrob Chemother. 2003;51:69–76. doi: 10.1093/jac/dkg028. [DOI] [PubMed] [Google Scholar]
- 63.Diekema DJ, Pfaller MA, Schmitz FJ, Smayevsky J, Bell J, Jones RN, Beach M SENTRY Partcipants Group. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin Infect Dis. 2001;32(Suppl 2):S114–S132. doi: 10.1086/320184. [DOI] [PubMed] [Google Scholar]
- 64.Higashide M, Kuroda M, Ohkawa S, Ohta T. Evaluation of a cefoxitin disk diffusion test for the detection of mecA-positive methicillin-resistant Staphylococcus saprophyticus. Int J Antimicrob Agents. 2006;27:500–504. doi: 10.1016/j.ijantimicag.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 65.Hartman BJ, Tomasz A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984;158:513–516. doi: 10.1128/jb.158.2.513-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matsuhashi M, Song MD, Ishino F, Wachi M, Doi M, Inoue M, Ubukata K, Yamashita N, Konno M. Molecular cloning of the gene of a penicillin-binding protein supposed to cause high resistance to beta-lactam antibiotics in Staphylococcus aureus. J Bacteriol. 1986;167:975–980. doi: 10.1128/jb.167.3.975-980.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Katayama Y, Ito T, Hiramatsu K. A new class of genetic element, staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 2000;44:1549–1555. doi: 10.1128/aac.44.6.1549-1555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Higashide M, Kuroda M, Omura CT, Kumano M, Ohkawa S, Ichimura S, Ohta T. Methicillin-resistant Staphylococcus saprophyticus isolates carrying staphylococcal cassette chromosome mec have emerged in urogenital tract infections. Antimicrob Agents Chemother. 2008;52:2061–2068. doi: 10.1128/AAC.01150-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McTaggart LA, Rigby RC, Elliott TS. The pathogenesis of urinary tract infections associated with Escherichia coli, Staphylococcus saprophyticus and S. epidermidis. J Med Microbiol. 1990;32:135–141. doi: 10.1099/00222615-32-2-135. [DOI] [PubMed] [Google Scholar]
- 70.Meyer HG, Wengler-Becker U, Gatermann SG. The hemagglutinin of Staphylococcus saprophyticus is a major adhesin for uroepithelial cells. Infect Immun. 1996;64:3893–3896. doi: 10.1128/iai.64.9.3893-3896.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gatermann S, Meyer HG. Staphylococcus saprophyticus hemagglutinin binds fibronectin. Infect Immun. 1994;62:4556–4563. doi: 10.1128/iai.62.10.4556-4563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hell W, Meyer HG, Gatermann SG. Cloning of aas, a gene encoding a Staphylococcus saprophyticus surface protein with adhesive and autolytic properties. Mol Microbiol. 1998;29:871–881. doi: 10.1046/j.1365-2958.1998.00983.x. [DOI] [PubMed] [Google Scholar]
- 73.Gatermann S, Marre R, Heesemann J, Henkel W. Hemagglutinating and adherence properties of Staphylococcus saprophyticus: epidemiology and virulence in experimental urinary tract infection of rats. FEMS Microbiol Immunol. 1988;1:179–185. doi: 10.1111/j.1574-6968.1988.tb02372.x. [DOI] [PubMed] [Google Scholar]
- 74.Kleine B, Gatermann S, Sakinc T. Genotypic and phenotypic variation among Staphylococcus saprophyticus from human and animal isolates. BMC Res Notes. 2010;3:163. doi: 10.1186/1756-0500-3-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gatermann S, Kreft B, Marre R, Wanner G. Identification and characterization of a surface-associated protein (Ssp) of Staphylococcus saprophyticus. Infect Immun. 1992;60:1055–1060. doi: 10.1128/iai.60.3.1055-1060.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sakinc T, Woznowski M, Ebsen M, Gatermann SG. The surface-associated protein of Staphylococcus saprophyticus is a lipase. Infect Immun. 2005;73:6419–6428. doi: 10.1128/IAI.73.10.6419-6428.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schneewind O, Missiakas DM. Protein secretion and surface display in Gram-positive bacteria. Philos Trans R Soc Lond B Biol Sci. 2012;367:1123–1139. doi: 10.1098/rstb.2011.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuroda M, Yamashita A, Hirakawa H, Kumano M, Morikawa K, Higashide M, Maruyama A, Inose Y, Matoba K, Toh H, Kuhara S, Hattori M, Ohta T. Whole genome sequence of Staphylococcus saprophyticus reveals the pathogenesis of uncomplicated urinary tract infection. Proc Natl Acad Sci USA. 2005;102:13272–13277. doi: 10.1073/pnas.0502950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.King NP, Beatson SA, Totsika M, Ulett GC, Alm RA, Manning PA, Schembri MA. UafB is a serine-rich repeat adhesin of Staphylococcus saprophyticus that mediates binding to fibronectin, fibrinogen and human uroepithelial cells. Microbiology. 2011;157:1161–1175. doi: 10.1099/mic.0.047639-0. [DOI] [PubMed] [Google Scholar]
- 80.King NP, Sakinç T, Ben Zakour NL, Totsika M, Heras B, Simerska P, Shepherd M, Gatermann SG, Beatson SA, Schembri MA. Characterisation of a cell wall-anchored protein of Staphylococcus saprophyticus associated with linoleic acid resistance. BMC Microbiol. 2012;12:8. doi: 10.1186/1471-2180-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sakinc T, Kleine B, Gatermann SG. SdrI, a serine-aspartate repeat protein identified in Staphylococcus saprophyticus strain 7108, is a collagen-binding protein. Infect Immun. 2006;74:4615–4623. doi: 10.1128/IAI.01885-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bensing BA, Gibson BW, Sullam PM. The Streptococcus gordonii platelet binding protein GspB undergoes glycosylation independently of export. J Bacteriol. 2004;186:638–645. doi: 10.1128/JB.186.3.638-645.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bensing BA, López JA, Sullam PM. The Streptococcus gordonii surface proteins GspB and Hsa mediate binding to sialylated carbohydrate epitopes on the platelet membrane glycoprotein Ibalpha. Infect Immun. 2004;72:6528–6537. doi: 10.1128/IAI.72.11.6528-6537.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Siboo IR, Chambers HF, Sullam PM. Role of SraP, a Serine-Rich Surface Protein of Staphylococcus aureus, in binding to human platelets. Infect Immun. 2005;73:2273–2280. doi: 10.1128/IAI.73.4.2273-2280.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim YG, Shaw MH, Warner N, Park JH, Chen F, Ogura Y, Núñez G. Cutting edge: crohn’s disease-associated Nod2 mutation limits production of proinflammatory cytokines to protect the host from Enterococcus faecalis-induced lethality. J Immunol. 2011;187:2849–2852. doi: 10.4049/jimmunol.1001854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rigel NW, Braunstein M. A new twist on an old pathway--accessory Sec [corrected] systems. Mol Microbiol. 2008;69:291–302. doi: 10.1111/j.1365-2958.2008.06294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kleine B, Ali L, Wobser D, Sakιnç T. The N-terminal repeat and the ligand binding domain A of SdrI protein is involved in hydrophobicity of S. saprophyticus. Microbiol Res. 2015;172:88–94. doi: 10.1016/j.micres.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 88.von Eiff C, Peters G, Heilmann C. Pathogenesis of infections due to coagulase-negative staphylococci. Lancet Infect Dis. 2002;2:677–685. doi: 10.1016/s1473-3099(02)00438-3. [DOI] [PubMed] [Google Scholar]
- 89.McCrea KW, Hartford O, Davis S, Eidhin DN, Lina G, Speziale P, Foster TJ, Höök M. The serine-aspartate repeat (Sdr) protein family in Staphylococcus epidermidis. Microbiology. 2000;146:1535–1546. doi: 10.1099/00221287-146-7-1535. [DOI] [PubMed] [Google Scholar]
- 90.Fowler JE., Jr Staphylococcus saprophyticus as the cause of infected urinary calculus. Ann Intern Med. 1985;102:342–343. doi: 10.7326/0003-4819-102-3-342. [DOI] [PubMed] [Google Scholar]
- 91.Sakinç T, Michalski N, Kleine B, Gatermann SG. The uropathogenic species Staphylococcus saprophyticus tolerates a high concentration of D-serine. FEMS Microbiol Lett. 2009;299:60–64. doi: 10.1111/j.1574-6968.2009.01731.x. [DOI] [PubMed] [Google Scholar]
- 92.Korte-Berwanger M, Sakinc T, Kline K, Nielsen HV, Hultgren S, Gatermann SG. Significance of the D-serine-deaminase and D-serine metabolism of Staphylococcus saprophyticus for virulence. Infect Immun. 2013;81:4525–4533. doi: 10.1128/IAI.00599-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Park S, Kelley KA, Vinogradov E, Solinga R, Weidenmaier C, Misawa Y, Lee JC. Characterization of the structure and biological functions of a capsular polysaccharide produced by Staphylococcus saprophyticus. J Bacteriol. 2010;192:4618–4626. doi: 10.1128/JB.00104-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bahrani-Mougeot FK, Buckles EL, Lockatell CV, Hebel JR, Johnson DE, Tang CM, Donnenberg MS. Type 1 fimbriae and extracellular polysaccharides are preeminent uropathogenic Escherichia coli virulence determinants in the murine urinary tract. Mol Microbiol. 2002;45:1079–1093. doi: 10.1046/j.1365-2958.2002.03078.x. [DOI] [PubMed] [Google Scholar]
- 95.Anderson GG, Goller CC, Justice S, Hultgren SJ, Seed PC. Polysaccharide capsule and sialic acid-mediated regulation promote biofilm-like intracellular bacterial communities during cystitis. Infect Immun. 2010;78:963–975. doi: 10.1128/IAI.00925-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, DeLeo FR, Otto M. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 2007;13:1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 97.Kaito C, Saito Y, Nagano G, Ikuo M, Omae Y, Hanada Y, Han X, Kuwahara-Arai K, Hishinuma T, Baba T, Ito T, Hiramatsu K, Sekimizu K. Transcription and translation products of the cytolysin gene psm-mec on the mobile genetic element SCCmec regulate Staphylococcus aureus virulence. PLoS Pathog. 2011;7:e1001267. doi: 10.1371/journal.ppat.1001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Diep BA, Otto M. The role of virulence determinants in community-associated MRSA pathogenesis. Trends Microbiol. 2008;16:361–369. doi: 10.1016/j.tim.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mehlin C, Headley CM, Klebanoff SJ. An inflammatory polypeptide complex from Staphylococcus epidermidis: isolation and characterization. J Exp Med. 1999;189:907–918. doi: 10.1084/jem.189.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cogen AL, Yamasaki K, Muto J, Sanchez KM, Crotty Alexander L, Tanios J, Lai Y, Kim JE, Nizet V, Gallo RL. Staphylococcus epidermidis antimicrobial delta-toxin (phenol-soluble modulin-gamma) cooperates with host antimicrobial peptides to kill group A Streptococcus. PLoS One. 2010;5:e8557. doi: 10.1371/journal.pone.0008557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cogen AL, Yamasaki K, Sanchez KM, Dorschner RA, Lai Y, MacLeod DT, Torpey JW, Otto M, Nizet V, Kim JE, Gallo RL. Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J Invest Dermatol. 2010;130:192–200. doi: 10.1038/jid.2009.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Marchand A, Verdon J, Lacombe C, Crapart S, Héchard Y, Berjeaud JM. Anti-Legionella activity of staphylococcal hemolytic peptides. Peptides. 2011;32:845–851. doi: 10.1016/j.peptides.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 103.Tsompanidou E, Sibbald MJ, Chlebowicz MA, Dreisbach A, Back JW, van Dijl JM, Buist G, Denham EL. Requirement of the agr locus for colony spreading of Staphylococcus aureus. J Bacteriol. 2011;193:1267–1272. doi: 10.1128/JB.01276-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Periasamy S, Joo HS, Duong AC, Bach TH, Tan VY, Chatterjee SS, Cheung GY, Otto M. How Staphylococcus aureus biofilms develop their characteristic structure. Proc Natl Acad Sci USA. 2012;109:1281–1286. doi: 10.1073/pnas.1115006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schwartz K, Syed AK, Stephenson RE, Rickard AH, Boles BR. Functional amyloids composed of phenol soluble modulins stabilize Staphylococcus aureus biofilms. PLoS Pathog. 2012;8:e1002744. doi: 10.1371/journal.ppat.1002744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Queck SY, Khan BA, Wang R, Bach TH, Kretschmer D, Chen L, Kreiswirth BN, Peschel A, Deleo FR, Otto M. Mobile genetic element-encoded cytolysin connects virulence to methicillin resistance in MRSA. PLoS Pathog. 2009;5:e1000533. doi: 10.1371/journal.ppat.1000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Monecke S, Engelmann I, Archambault M, Coleman DC, Coombs GW, Cortez de Jäckel S, Pelletier-Jacques G, Schwarz S, Shore AC, Slickers P, Ehricht R. Distribution of SCCmec-associated phenol-soluble modulin in staphylococci. Mol Cell Probes. 2012;26:99–103. doi: 10.1016/j.mcp.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 108.Muder RR, Brennen C, Rihs JD, Wagener MM, Obman A, Stout JE, Yu VL. Isolation of Staphylococcus aureus from the urinary tract: association of isolation with symptomatic urinary tract infection and subsequent staphylococcal bacteremia. Clin Infect Dis. 2006;42:46–50. doi: 10.1086/498518. [DOI] [PubMed] [Google Scholar]
- 109.Baraboutis IG, Tsagalou EP, Lepinski JL, Papakonstantinou I, Papastamopoulos V, Skoutelis AT, Johnson S. Primary Staphylococcus aureus urinary tract infection: the role of undetected hematogenous seeding of the urinary tract. Eur J Clin Microbiol Infect Dis. 2010;29 doi: 10.1007/s10096-010-0967-2. 1095-101. [DOI] [PubMed] [Google Scholar]
- 110.Gilbert NM, O’Brien VP, Hultgren S, Macones G, Lewis WG, Lewis AL. Urinary tract infection as a preventable cause of pregnancy complications: opportunities, challenges, and a global call to action. Glob Adv Health Med. 2013;2:59–69. doi: 10.7453/gahmj.2013.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hiron A, Posteraro B, Carrière M, Remy L, Delporte C, La Sorda M, Sanguinetti M, Juillard V, Borezée-Durant E. A nickel ABC-transporter of Staphylococcus aureus is involved in urinary tract infection. Mol Microbiol. 2010;77:1246–1260. doi: 10.1111/j.1365-2958.2010.07287.x. [DOI] [PubMed] [Google Scholar]
- 112.Remy L, Carrière M, Derré-Bobillot A, Martini C, Sanguinetti M, Borezée-Durant E. The Staphylococcus aureus Opp1 ABC transporter imports nickel and cobalt in zinc-depleted conditions and contributes to virulence. Mol Microbiol. 2013;87:730–743. doi: 10.1111/mmi.12126. [DOI] [PubMed] [Google Scholar]
- 113.Otto M. Molecular basis of Staphylococcus epidermidis infections. Semin Immunopathol. 2012;34:201–214. doi: 10.1007/s00281-011-0296-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Widerström M, Wiström J, Sjöstedt A, Monsen T. Coagulase-negative staphylococci: update on the molecular epidemiology and clinical presentation, with a focus on Staphylococcus epidermidis and Staphylococcus saprophyticus. Eur J Clin Microbiol Infect Dis. 2012;31:7–20. doi: 10.1007/s10096-011-1270-6. [DOI] [PubMed] [Google Scholar]
- 115.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK National Healthcare Safety Network Team, Participating National Healthcare Safety Network Facilities. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 116.Hedman P, Ringertz O. Urinary tract infections caused by Staphylococcus saprophyticus. A matched case control study. J Infect. 1991;23:145–153. doi: 10.1016/0163-4453(91)92045-7. [DOI] [PubMed] [Google Scholar]
- 117.Chen Y, Lu H, Liu Q, Huang G, Lim CP, Zhang L, Hao A, Cao X. Function of GRIM-19, a mitochondrial respiratory chain complex I protein, in innate immunity. J Biol Chem. 2012;287:27227–27235. doi: 10.1074/jbc.M112.340315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fisher K, Phillips C. The ecology, epidemiology and virulence of Enterococcus. Microbiology. 2009;155:1749–1757. doi: 10.1099/mic.0.026385-0. [DOI] [PubMed] [Google Scholar]
- 119.Richards MJ, Edwards JR, Culver DH, Gaynes RP National Nosocomial Infections Surveillance System. Nosocomial infections in medical intensive care units in the United States. Crit Care Med. 1999;27:887–892. doi: 10.1097/00003246-199905000-00020. [DOI] [PubMed] [Google Scholar]
- 120.Nicolle LE, Friesen D, Harding GK, Roos LL. Hospitalization for acute pyelonephritis in Manitoba, Canada, during the period from 1989 to 1992; impact of diabetes, pregnancy, and aboriginal origin. Clin Infect Dis. 1996;22:1051–1056. doi: 10.1093/clinids/22.6.1051. [DOI] [PubMed] [Google Scholar]
- 121.Boyko EJ, Fihn SD, Scholes D, Abraham L, Monsey B. Risk of urinary tract infection and asymptomatic bacteriuria among diabetic and nondiabetic postmenopausal women. Am J Epidemiol. 2005;161:557–564. doi: 10.1093/aje/kwi078. [DOI] [PubMed] [Google Scholar]
- 122.Carton JA, Maradona JA, Nuño FJ, Fernandez-Alvarez R, Pérez-Gonzalez F, Asensi V. Diabetes mellitus and bacteraemia: a comparative study between diabetic and non-diabetic patients. Eur J Med. 1992;1:281–287. [PubMed] [Google Scholar]
- 123.MacFarlane IA, Brown RM, Smyth RW, Burdon DW, FitzGerald MG. Bacteraemia in diabetics. J Infect. 1986;12:213–219. doi: 10.1016/s0163-4453(86)94112-5. [DOI] [PubMed] [Google Scholar]
- 124.Bonadio M, Meini M, Gigli C, Longo B, Vigna A. Urinary tract infection in diabetic patients. Urol Int. 1999;63:215–219. doi: 10.1159/000030453. [DOI] [PubMed] [Google Scholar]
- 125.Bonadio M, Costarelli S, Morelli G, Tartaglia T. The influence of diabetes mellitus on the spectrum of uropathogens and the antimicrobial resistance in elderly adult patients with urinary tract infection. BMC Infect Dis. 2006;6:54. doi: 10.1186/1471-2334-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ronald A. The etiology of urinary tract infection: traditional and emerging pathogens. Am J Med. 2002;113(Suppl 1A):14S–19S. doi: 10.1016/s0002-9343(02)01055-0. [DOI] [PubMed] [Google Scholar]
- 127.Rosen DA, Hung CS, Kline KA, Hultgren SJ. Streptozocin-induced diabetic mouse model of urinary tract infection. Infect Immun. 2008;76:4290–4298. doi: 10.1128/IAI.00255-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Raffel L, Pitsakis P, Levison SP, Levison ME. Experimental Candida albicans, Staphylococcus aureus, and Streptococcus faecalis pyelonephritis in diabetic rats. Infect Immun. 1981;34:773–779. doi: 10.1128/iai.34.3.773-779.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Millán-Rodríguez F, Palou J, Bujons-Tur A, Musquera-Felip M, Sevilla-Cecilia C, Serrallach-Orejas M, Baez-Angles C, Villavicencio-Mavrich H. Acute bacterial prostatitis: two different sub-categories according to a previous manipulation of the lower urinary tract. World J Urol. 2006;24:45–50. doi: 10.1007/s00345-005-0040-4. [DOI] [PubMed] [Google Scholar]
- 130.Brede CM, Shoskes DA. The etiology and management of acute prostatitis. Nat Rev Urol. 2011;8:207–212. doi: 10.1038/nrurol.2011.22. [DOI] [PubMed] [Google Scholar]
- 131.Huycke MM, Sahm DF, Gilmore MS. Multiple-drug resistant enterococci: the nature of the problem and an agenda for the future. Emerg Infect Dis. 1998;4:239–249. doi: 10.3201/eid0402.980211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shankar V, Baghdayan AS, Huycke MM, Lindahl G, Gilmore MS. Infection-derived Enterococcus faecalis strains are enriched in esp, a gene encoding a novel surface protein. Infect Immun. 1999;67:193–200. doi: 10.1128/iai.67.1.193-200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li J, Kasper DL, Ausubel FM, Rosner B, Michel JL. Inactivation of the alpha C protein antigen gene, bca, by a novel shuttle/suicide vector results in attenuation of virulence and immunity in group B Streptococcus. Proc Natl Acad Sci USA. 1997;94:13251–13256. doi: 10.1073/pnas.94.24.13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Larsson C, Stålhammar-Carlemalm M, Lindahl G. Experimental vaccination against group B streptococcus, an encapsulated bacterium, with highly purified preparations of cell surface proteins Rib and alpha. Infect Immun. 1996;64:3518–3523. doi: 10.1128/iai.64.9.3518-3523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Madoff LC, Michel JL, Gong EW, Kling DE, Kasper DL. Group B streptococci escape host immunity by deletion of tandem repeat elements of the alpha C protein. Proc Natl Acad Sci USA. 1996;93:4131–4136. doi: 10.1073/pnas.93.9.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shankar N, Lockatell CV, Baghdayan AS, Drachenberg C, Gilmore MS, Johnson DE. Role of Enterococcus faecalis surface protein Esp in the pathogenesis of ascending urinary tract infection. Infect Immun. 2001;69:4366–4372. doi: 10.1128/IAI.69.7.4366-4372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tendolkar PM, Baghdayan AS, Gilmore MS, Shankar N. Enterococcal surface protein, Esp, enhances biofilm formation by Enterococcus faecalis. Infect Immun. 2004;72:6032–6039. doi: 10.1128/IAI.72.10.6032-6039.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nallapareddy SR, Singh KV, Sillanpää J, Zhao M, Murray BE. Relative contributions of Ebp Pili and the collagen adhesin ace to host extracellular matrix protein adherence and experimental urinary tract infection by Enterococcus faecalis OG1RF. Infect Immun. 2011;79:2901–2910. doi: 10.1128/IAI.00038-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lebreton F, Riboulet-Bisson E, Serror P, Sanguinetti M, Posteraro B, Torelli R, Hartke A, Auffray Y, Giard JC. ace, Which encodes an adhesin in Enterococcus faecalis, is regulated by Ers and is involved in virulence. Infect Immun. 2009;77:2832–2839. doi: 10.1128/IAI.01218-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Roh JH, Singh KV, La Rosa SL, Cohen AL, Murray BE. The two-component system GrvRS (EtaRS) regulates ace expression in Enterococcus faecalis OG1RF. Infect Immun. 2015;83:389–395. doi: 10.1128/IAI.02587-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Frank KL, Guiton PS, Barnes AM, Manias DA, Chuang-Smith ON, Kohler PL, Spaulding AR, Hultgren SJ, Schlievert PM, Dunny GM. AhrC and Eep are biofilm infection-associated virulence factors in Enterococcus faecalis. Infect Immun. 2013;81:1696–1708. doi: 10.1128/IAI.01210-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Diederich AK, Wobser D, Spiess M, Sava IG, Huebner J, Sakιnç T. Role of glycolipids in the pathogenesis of Enterococcus faecalis urinary tract infection. PLoS One. 2014;9:e96295. doi: 10.1371/journal.pone.0096295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wobser D, Ali L, Grohmann E, Huebner J, Sakinc T. A novel role for D-alanylation of lipoteichoic acid of enterococcus faecalis in urinary tract infection. PLoS One. 2014;9:e107827. doi: 10.1371/journal.pone.0107827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nallapareddy SR, Singh KV, Sillanpää J, Garsin DA, Höök M, Erlandsen SL, Murray BE. Endocarditis and biofilm-associated pili of Enterococcus faecalis. J Clin Invest. 2006;116:2799–2807. doi: 10.1172/JCI29021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schlüter S, Franz CM, Gesellchen F, Bertinetti O, Herberg FW, Schmidt FR. The high biofilm-encoding Bee locus: a second pilus gene cluster in Enterococcus faecalis? Curr Microbiol. 2009;59:206–211. doi: 10.1007/s00284-009-9422-y. [DOI] [PubMed] [Google Scholar]
- 146.Nielsen HV, Flores-Mireles AL, Kau AL, Kline KA, Pinkner JS, Neiers F, Normark S, Henriques-Normark B, Caparon MG, Hultgren SJ. Pilin and sortase residues critical for endocarditis- and biofilm-associated pilus biogenesis in Enterococcus faecalis. J Bacteriol. 2013;195:4484–4495. doi: 10.1128/JB.00451-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kline KA, Kau AL, Chen SL, Lim A, Pinkner JS, Rosch J, Nallapareddy SR, Murray BE, Henriques-Normark B, Beatty W, Caparon MG, Hultgren SJ. Mechanism for sortase localization and the role of sortase localization in efficient pilus assembly in Enterococcus faecalis. J Bacteriol. 2009;191:3237–3247. doi: 10.1128/JB.01837-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Guiton PS, Hung CS, Kline KA, Roth R, Kau AL, Hayes E, Heuser J, Dodson KW, Caparon MG, Hultgren SJ. Contribution of autolysin and Sortase a during Enterococcus faecalis DNA-dependent biofilm development. Infect Immun. 2009;77:3626–3638. doi: 10.1128/IAI.00219-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nielsen HV, Guiton PS, Kline KA, Port GC, Pinkner JS, Neiers F, Normark S, Henriques-Normark B, Caparon MG, Hultgren SJ. The metal ion-dependent adhesion site motif of the Enterococcus faecalis EbpA pilin mediates pilus function in catheter-associated urinary tract infection. MBio. 2012;3 doi: 10.1128/mBio.00177-12. e00177–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kristich CJ, Manias DA, Dunny GM. Development of a method for markerless genetic exchange in Enterococcus faecalis and its use in construction of a srtA mutant. Appl Environ Microbiol. 2005;71:5837–5849. doi: 10.1128/AEM.71.10.5837-5849.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kemp KD, Singh KV, Nallapareddy SR, Murray BE. Relative contributions of Enterococcus faecalis OG1RF sortase-encoding genes, srtA and bps (srtC), to biofilm formation and a murine model of urinary tract infection. Infect Immun. 2007;75:5399–5404. doi: 10.1128/IAI.00663-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sillanpää J, Chang C, Singh KV, Montealegre MC, Nallapareddy SR, Harvey BR, Ton-That H, Murray BE. Contribution of individual Ebp Pilus subunits of Enterococcus faecalis OG1RF to pilus biogenesis, biofilm formation and urinary tract infection. PLoS One. 2013;8:e68813. doi: 10.1371/journal.pone.0068813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sillanpää J, Nallapareddy SR, Singh KV, Prakash VP, Fothergill T, Ton-That H, Murray BE. Characterization of the ebp(fm) pilus-encoding operon of Enterococcus faecium and its role in biofilm formation and virulence in a murine model of urinary tract infection. Virulence. 2010;1:236–246. doi: 10.4161/viru.1.4.11966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nallapareddy SR, Sillanpää J, Mitchell J, Singh KV, Chowdhury SA, Weinstock GM, Sullam PM, Murray BE. Conservation of Ebp-type pilus genes among Enterococci and demonstration of their role in adherence of Enterococcus faecalis to human platelets. Infect Immun. 2011;79:2911–2920. doi: 10.1128/IAI.00039-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Branci S, Ewertsen C, Thybo S, Nielsen HV, Jensen F, Wettergren A, Larsen PN, Bygbjerg IC. Cystic echinococcosis of the liver: experience from a Danish tertiary reference center (2002–2010) J Travel Med. 2012;19:28–34. doi: 10.1111/j.1708-8305.2011.00577.x. [DOI] [PubMed] [Google Scholar]
- 156.Banerjee A, Kim BJ, Carmona EM, Cutting AS, Gurney MA, Carlos C, Feuer R, Prasadarao NV, Doran KS. Bacterial Pili exploit integrin machinery to promote immune activation and efficient blood-brain barrier penetration. Nat Commun. 2011;2:462. doi: 10.1038/ncomms1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hilleringmann M, Giusti F, Baudner BC, Masignani V, Covacci A, Rappuoli R, Barocchi MA, Ferlenghi I. Pneumococcal pili are composed of protofilaments exposing adhesive clusters of Rrg A. PLoS Pathog. 2008;4:e1000026. doi: 10.1371/journal.ppat.1000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Flores-Mireles AL, Pinkner JS, Caparon MG, Hultgren SJ. EbpA vaccine antibodies block binding of Enterococcus faecalis to fibrinogen to prevent catheter-associated bladder infection in mice. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3009384. 254ra127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Horsley H, Malone-Lee J, Holland D, Tuz M, Hibbert A, Kelsey M, Kupelian A, Rohn JL. Enterococcus faecalis subverts and invades the host urothelium in patients with chronic urinary tract infection. PLoS One. 2013;8:e83637. doi: 10.1371/journal.pone.0083637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rakita RM, Vanek NN, Jacques-Palaz K, Mee M, Mariscalco MM, Dunny GM, Snuggs M, Van Winkle WB, Simon SI. Enterococcus faecalis bearing aggregation substance is resistant to killing by human neutrophils despite phagocytosis and neutrophil activation. Infect Immun. 1999;67:6067–6075. doi: 10.1128/iai.67.11.6067-6075.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gentry-Weeks CR, Karkhoff-Schweizer R, Pikis A, Estay M, Keith JM. Survival of Enterococcus faecalis in mouse peritoneal macrophages. Infect Immun. 1999;67:2160–2165. doi: 10.1128/iai.67.5.2160-2165.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Baldassarri L, Bertuccini L, Creti R, Filippini P, Ammendolia MG, Koch S, Huebner J, Orefici G. Glycosaminoglycans mediate invasion and survival of Enterococcus faecalis into macrophages. J Infect Dis. 2005;191:1253–1262. doi: 10.1086/428778. [DOI] [PubMed] [Google Scholar]
- 163.La Carbona S, Sauvageot N, Giard JC, Benachour A, Posteraro B, Auffray Y, Sanguinetti M, Hartke A. Comparative study of the physiological roles of three peroxidases (NADH peroxidase, Alkyl hydroperoxide reductase and Thiol peroxidase) in oxidative stress response, survival inside macrophages and virulence of Enterococcus faecalis. Mol Microbiol. 2007;66:1148–1163. doi: 10.1111/j.1365-2958.2007.05987.x. [DOI] [PubMed] [Google Scholar]
- 164.Lebreton F, van Schaik W, Sanguinetti M, Posteraro B, Torelli R, Le Bras F, Verneuil N, Zhang X, Giard JC, Dhalluin A, Willems RJ, Leclercq R, Cattoir V. AsrR is an oxidative stress sensing regulator modulating Enterococcus faecium opportunistic traits, antimicrobial resistance, and pathogenicity. PLoS Pathog. 2012;8:e1002834. doi: 10.1371/journal.ppat.1002834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Giard JC, Riboulet E, Verneuil N, Sanguinetti M, Auffray Y, Hartke A. Characterization of Ers, a PrfA-like regulator of Enterococcus faecalis. FEMS Immunol Med Microbiol. 2006;46:410–418. doi: 10.1111/j.1574-695X.2005.00049.x. [DOI] [PubMed] [Google Scholar]
- 166.Verneuil N, Rincé A, Sanguinetti M, Auffray Y, Hartke A, Giard JC. Implication of hypR in the virulence and oxidative stress response of Enterococcus faecalis. FEMS Microbiol Lett. 2005;252:137–141. doi: 10.1016/j.femsle.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 167.Zhao C, Hartke A, La Sorda M, Posteraro B, Laplace JM, Auffray Y, Sanguinetti M. Role of methionine sulfoxide reductases A and B of Enterococcus faecalis in oxidative stress and virulence. Infect Immun. 2010;78:3889–3897. doi: 10.1128/IAI.00165-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303:1522–1526. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]
- 169.Leendertse M, Willems RJ, Giebelen IA, van den Pangaart PS, Wiersinga WJ, de Vos AF, Florquin S, Bonten MJ, van der Poll T. TLR2-dependent MyD88 signaling contributes to early host defense in murine Enterococcus faecium peritonitis. J Immunol. 2008;180:4865–4874. doi: 10.4049/jimmunol.180.7.4865. [DOI] [PubMed] [Google Scholar]
- 170.Goble NM, Clarke T, Hammonds JC. Histological changes in the urinary bladder secondary to urethral catheterisation. Br J Urol. 1989;63:354–357. doi: 10.1111/j.1464-410x.1989.tb05216.x. [DOI] [PubMed] [Google Scholar]
- 171.Peychl L, Zalud R. Changes in the urinary bladder caused by short-term permanent catheter insertion. Cas Lek Cesk. 2008;147:325–329. [PubMed] [Google Scholar]
- 172.Delnay KM, Stonehill WH, Goldman H, Jukkola AF, Dmochowski RR. Bladder histological changes associated with chronic indwelling urinary catheter. J Urol. 1999;161:1106–1108. discussion 1108–1109. [PubMed] [Google Scholar]
- 173.Tambyah PA, Maki DG. The relationship between pyuria and infection in patients with indwelling urinary catheters: a prospective study of 761 patients. Arch Intern Med. 2000;160:673–677. doi: 10.1001/archinte.160.5.673. [DOI] [PubMed] [Google Scholar]
- 174.Daw K, Baghdayan AS, Awasthi S, Shankar N. Biofilm and planktonic Enterococcus faecalis elicit different responses from host phagocytes in vitro. FEMS Immunol Med Microbiol. 2012;65:270–282. doi: 10.1111/j.1574-695X.2012.00944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kraemer TD, Quintanar Haro OD, Domann E, Chakraborty T, Tchatalbachev S. The TIR Domain Containing Locus of Enterococcus faecalis Is Predominant among Urinary Tract Infection Isolates and Downregulates Host Inflammatory Response. Int J Microbiol. 2014;2014:918143. doi: 10.1155/2014/918143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chan SL, Low LY, Hsu S, Li S, Liu T, Santelli E, Le Negrate G, Reed JC, Woods VL, Jr, Pascual J. Molecular mimicry in innate immunity: crystal structure of a bacterial TIR domain. J Biol Chem. 2009;284:21386–21392. doi: 10.1074/jbc.C109.007591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ng LHM, Chow WL, Lee YK. Infant intestinal Enterococcus faecalis down-regulates inflammatory responses in human intestinal cell lines. World Journal of Gastroenterology. 2008;7:017. doi: 10.3748/wjg.14.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang S, Ng LH, Chow WL, Lee YK. Infant intestinal Enterococcus faecalis down-regulates inflammatory responses in human intestinal cell lines. World J Gastroenterol. 2008;14:1067–1076. doi: 10.3748/wjg.14.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang S, Hibberd ML, Pettersson S, Lee YK. Enterococcus faecalis from healthy infants modulates inflammation through MAPK signaling pathways. PLoS One. 2014;9:e97523. doi: 10.1371/journal.pone.0097523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Dis Mon. 2003;49:53–70. doi: 10.1067/mda.2003.7. [DOI] [PubMed] [Google Scholar]
- 181.Beyer I, Mergam A, Benoit F, Theunissen C, Pepersack T. Management of urinary tract infections in the elderly. Z Gerontol Geriatr. 2001;34:153–157. doi: 10.1007/s003910170080. [DOI] [PubMed] [Google Scholar]
- 182.Haft RF, Kasper DL. Group B streptococcus infection in mother and child. Hosp Pract (Off Ed) 1991;26:111–122. 125–128, 133–134. doi: 10.1080/21548331.1991.11704238. [DOI] [PubMed] [Google Scholar]
- 183.Ulett KB, Benjamin WH, Jr, Zhuo F, Xiao M, Kong F, Gilbert GL, Schembri MA, Ulett GC. Diversity of group B streptococcus serotypes causing urinary tract infection in adults. J Clin Microbiol. 2009;47:2055–2060. doi: 10.1128/JCM.00154-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Trivalle C, Martin E, Martel P, Jacque B, Menard JF, Lemeland JF. Group B streptococcal bacteraemia in the elderly. J Med Microbiol. 1998;47:649–652. doi: 10.1099/00222615-47-7-649. [DOI] [PubMed] [Google Scholar]
- 185.Farley MM, Harvey RC, Stull T, Smith JD, Schuchat A, Wenger JD, Stephens DS. A population-based assessment of invasive disease due to group B Streptococcus in nonpregnant adults. N Engl J Med. 1993;328:1807–1811. doi: 10.1056/NEJM199306243282503. [DOI] [PubMed] [Google Scholar]
- 186.Chaiwarith R, Jullaket W, Bunchoo M, Nuntachit N, Sirisanthana T, Supparatpinyo K. Streptococcus agalactiae in adults at Chiang Mai University Hospital: a retrospective study. BMC Infect Dis. 2011;11:149. doi: 10.1186/1471-2334-11-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Muller AE, Oostvogel PM, Steegers EA, Dörr PJ. Morbidity related to maternal group B streptococcal infections. Acta Obstet Gynecol Scand. 2006;85:1027–1037. doi: 10.1080/00016340600780508. [DOI] [PubMed] [Google Scholar]
- 188.Persson K, Bjerre B, Elfström L, Polberger S, Forsgren A. Group B streptococci at delivery: high count in urine increases risk for neonatal colonization. Scand J Infect Dis. 1986;18:525–531. doi: 10.3109/00365548609021657. [DOI] [PubMed] [Google Scholar]
- 189.Nicolle LE. Uncomplicated urinary tract infection in adults including uncomplicated pyelonephritis. Urol Clin North Am. 2008;35:1–12. doi: 10.1016/j.ucl.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 190.Nicolle LE. Complicated pyelonephritis: unresolved issues. Curr Infect Dis Rep. 2007;9:501–507. doi: 10.1007/s11908-007-0075-3. [DOI] [PubMed] [Google Scholar]
- 191.Anderson BL, Simhan HN, Simons KM, Wiesengeld HC. Untreated asymptomatic group B streptococcal bacteriuria early in pregnancy and chorioamnionitis at delivery. Am J Obstet Gynecol. 2007;196:524, e1–e5. doi: 10.1016/j.ajog.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 192.Kessous R, Weintraub AY, Sergienko R, Lazer T, Press F, Wiznitzer A, Sheiner E. Bacteruria with group-B streptococcus: is it a risk factor for adverse pregnancy outcomes? J Matern Fetal Neonatal Med. 2012;25:1983–1986. doi: 10.3109/14767058.2012.671872. [DOI] [PubMed] [Google Scholar]
- 193.Colgan R, Nicolle LE, McGlone A, Hooton TM. Asymptomatic bacteriuria in adults. Am Fam Physician. 2006;74:985–990. [PubMed] [Google Scholar]
- 194.Kincaid-Smith P, Bullen M. Bacteriuria in Pregnancy. Lancet. 1965;1:395–399. doi: 10.1016/s0140-6736(65)90001-2. [DOI] [PubMed] [Google Scholar]
- 195.Smaill F. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev. 2001;2:CD000490. doi: 10.1002/14651858.CD000490. [DOI] [PubMed] [Google Scholar]
- 196.Smaill F. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev. 2000;2:CD000490. doi: 10.1002/14651858.CD000490. [DOI] [PubMed] [Google Scholar]
- 197.Mittendorf R, Williams MA, Kass EH. Prevention of preterm delivery and low birth weight associated with asymptomatic bacteriuria. Clin Infect Dis. 1992;14:927–932. doi: 10.1093/clinids/14.4.927. [DOI] [PubMed] [Google Scholar]
- 198.Romero R, Oyarzun E, Mazor M, Sirtori M, Hobbins JC, Bracken M. Meta-analysis of the relationship between asymptomatic bacteriuria and preterm delivery/low birth weight. Obstet Gynecol. 1989;73:576–582. [PubMed] [Google Scholar]
- 199.Verani JR, McGee L, Schrag SJ Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC) Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59(RR-10):1–36. [PubMed] [Google Scholar]
- 200.Weng CKK, Sheng X, Byington C. Pregnancy outcomes in women with Group B Streptococcal bacteriuria. Annual Meeting of the Pediatric Academic Societies; Vancouver, Canada. 2010. [Google Scholar]
- 201.Hammoud MS, Al-Shemmari M, Thalib L, Al-Sweih N, Rashwan N, Devarajan LV, Elsori H. Comparison between different types of surveillance samples for the detection of GBS colonization in both parturient mothers and their infants. Gynecol Obstet Invest. 2003;56:225–230. doi: 10.1159/000074825. [DOI] [PubMed] [Google Scholar]
- 202.Margarit I, Rinaudo CD, Galeotti CL, Maione D, Ghezzo C, Buttazzoni E, Rosini R, Runci Y, Mora M, Buccato S, Pagani M, Tresoldi E, Berardi A, Creti R, Baker CJ, Telford JL, Grandi G. Preventing bacterial infections with pilus-based vaccines: the group B streptococcus paradigm. J Infect Dis. 2009;199:108–115. doi: 10.1086/595564. [DOI] [PubMed] [Google Scholar]
- 203.Baker CJ, Rench MA, Paoletti LC, Edwards MS. Dose-response to type V group B streptococcal polysaccharide-tetanus toxoid conjugate vaccine in healthy adults. Vaccine. 2007;25:55–63. doi: 10.1016/j.vaccine.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 204.Palazzi DL, Rench MA, Edwards MS, Baker CJ. Use of type V group B streptococcal conjugate vaccine in adults 65–85 years old. J Infect Dis. 2004;190:558–564. doi: 10.1086/422010. [DOI] [PubMed] [Google Scholar]
- 205.Baker CJ, Paoletti LC, Rench MA, Guttormsen HK, Edwards MS, Kasper DL. Immune response of healthy women to 2 different group B streptococcal type V capsular polysaccharide-protein conjugate vaccines. J Infect Dis. 2004;189:1103–1112. doi: 10.1086/382193. [DOI] [PubMed] [Google Scholar]
- 206.Baker CJ, Rench MA, Fernandez M, Paoletti LC, Kasper DL, Edwards MS. Safety and immunogenicity of a bivalent group B streptococcal conjugate vaccine for serotypes II and III. J Infect Dis. 2003;188:66–73. doi: 10.1086/375536. [DOI] [PubMed] [Google Scholar]
- 207.Baker CJ, Rench MA, McInnes P. Immunization of pregnant women with group B streptococcal type III capsular polysaccharide-tetanus toxoid conjugate vaccine. Vaccine. 2003;21:3468–3472. doi: 10.1016/s0264-410x(03)00353-0. [DOI] [PubMed] [Google Scholar]
- 208.Shen X, Lagergård T, Yang Y, Lindblad M, Fredriksson M, Wallerström G, Holmgren J. Effect of pre-existing immunity for systemic and mucosal immune responses to intranasal immunization with group B Streptococcus type III capsular polysaccharide-cholera toxin B subunit conjugate. Vaccine. 2001;19:3360–3368. doi: 10.1016/s0264-410x(00)00532-6. [DOI] [PubMed] [Google Scholar]
- 209.Xue G, Yu L, Li S, Shen X. Intranasal immunization with GBS surface protein Sip and ScpB induces specific mucosal and systemic immune responses in mice. FEMS Immunol Med Microbiol. 2010;58:202–210. doi: 10.1111/j.1574-695X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 210.Maisey HC, Doran KS, Nizet V. Recent advances in understanding the molecular basis of group B Streptococcus virulence. Expert Rev Mol Med. 2008;10:e27. doi: 10.1017/S1462399408000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tan CK, Carey AJ, Cui X, Webb RI, Ipe D, Crowley M, Cripps AW, Benjamin WH, Jr, Ulett KB, Schembri MA, Ulett GC. Genome-wide mapping of cystitis due to Streptococcus agalactiae and Escherichia coli in mice identifies a unique bladder transcriptome that signifies pathogen-specific antimicrobial defense against urinary tract infection. Infect Immun. 2012;80:3145–3160. doi: 10.1128/IAI.00023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ulett GC, Webb RI, Ulett KB, Cui X, Benjamin WH, Crowley M, Schembri MA. Group B Streptococcus (GBS) urinary tract infection involves binding of GBS to bladder uroepithelium and potent but GBS-specific induction of interleukin 1alpha. J Infect Dis. 2010;201:866–870. doi: 10.1086/650696. [DOI] [PubMed] [Google Scholar]
- 213.Kulkarni R, Randis TM, Antala S, Wang A, Amaral FE, Ratner AJ. β-Hemolysin/cytolysin of Group B Streptococcus enhances host inflammation but is dispensable for establishment of urinary tract infection. PLoS One. 2013;8:e59091. doi: 10.1371/journal.pone.0059091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sumati AH, Saritha NK. Association of urinary tract infection in women with bacterial vaginosis. J Glob Infect Dis. 2009;1:151–152. doi: 10.4103/0974-777X.56254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sharami SH, Afrakhteh M, Shakiba M. Urinary tract infections in pregnant women with bacterial vaginosis. J Obstet Gynaecol. 2007;27:252–254. doi: 10.1080/01443610701194846. [DOI] [PubMed] [Google Scholar]
- 216.Hillebrand L, Harmanli OH, Whiteman V, Khandelwal M. Urinary tract infections in pregnant women with bacterial vaginosis. Am J Obstet Gynecol. 2002;186:916–917. doi: 10.1067/mob.2002.123987. [DOI] [PubMed] [Google Scholar]
- 217.Huang B, Fettweis JM, Brooks JP, Jefferson KK, Buck GA. The changing landscape of the vaginal microbiome. Clin Lab Med. 2014;34:747–761. doi: 10.1016/j.cll.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Cone RA. Vaginal microbiota and sexually transmitted infections that may influence transmission of cell-associated HIV. J Infect Dis. 2014;210(Suppl 3):S616–S621. doi: 10.1093/infdis/jiu459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Joesoef MR, Hillier SL, Josodiwondo S, Linnan M. Reproducibility of a scoring system for gram stain diagnosis of bacterial vaginosis. J Clin Microbiol. 1991;29:1730–1731. doi: 10.1128/jcm.29.8.1730-1731.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Schwebke JR, Hillier SL, Sobel JD, McGregor JA, Sweet RL. Validity of the vaginal gram stain for the diagnosis of bacterial vaginosis. Obstet Gynecol. 1996;88:573–576. doi: 10.1016/0029-7844(96)00233-5. [DOI] [PubMed] [Google Scholar]
- 222.Gilbert NM, Lewis WG, Lewis AL. Clinical features of bacterial vaginosis in a murine model of vaginal infection with Gardnerella vaginalis. PLoS One. 2013;8:e59539. doi: 10.1371/journal.pone.0059539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lewis WG, Robinson LS, Gilbert NM, Perry JC, Lewis AL. Degradation, foraging, and depletion of mucus sialoglycans by the vagina-adapted Actinobacterium Gardnerella vaginalis. J Biol Chem. 2013;288:12067–12079. doi: 10.1074/jbc.M113.453654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Stapleton AE, Au-Yeung M, Hooton TM, Fredricks DN, Roberts PL, Czaja CA, Yarova-Yarovaya Y, Fiedler T, Cox M, Stamm WE. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52:1212–1217. doi: 10.1093/cid/cir183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Naboka IuL, Kogan MI, Vasileva LI, Gudima IA, Miroshnichenki EA, Ibishev KhS. Bacterial mixed infection in women with chronic recurrent cystitis. Zh Mikrobiol Epidemiol Immunobiol. 2011;1:8–12. [PubMed] [Google Scholar]
- 226.Eschenbach DA, Davick PR, Williams BL, Klebanoff SJ, Young-Smith K, Critchlow CM, Holmes KK. Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J Clin Microbiol. 1989;27:251–256. doi: 10.1128/jcm.27.2.251-256.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Klebanoff SJ, Coombs RW. Viricidal effect of Lactobacillus acidophilus on human immunodeficiency virus type 1: possible role in heterosexual transmission. J Exp Med. 1991;174:289–292. doi: 10.1084/jem.174.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Mirmonsef P, Gilbert D, Zariffard MR, Hamaker BR, Kaur A, Landay AL, Spear GT. The effects of commensal bacteria on innate immune responses in the female genital tract. Am J Reprod Immunol. 2011;65:190–195. doi: 10.1111/j.1600-0897.2010.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Reid G, Burton J. Use of Lactobacillus to prevent infection by pathogenic bacteria. Microbes Infect. 2002;4:319–324. doi: 10.1016/s1286-4579(02)01544-7. [DOI] [PubMed] [Google Scholar]
- 230.Sierra-Hoffman M, Watkins K, Jinadatha C, Fader R, Carpenter JL. Clinical significance of Aerococcus urinae: a retrospective review. Diagn Microbiol Infect Dis. 2005;53:289–292. doi: 10.1016/j.diagmicrobio.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 231.Senneby E, Petersson AC, Rasmussen M. Clinical and microbiological features of bacteraemia with Aerococcus urinae. Clin Microbiol Infect. 2012;18:546–550. doi: 10.1111/j.1469-0691.2011.03609.x. [DOI] [PubMed] [Google Scholar]
- 232.Murray TS, Muldrew KL, Finkelstein R, Hampton L, Edberg SC, Cappello M. Acute pyelonephritis caused by Aerococcus urinae in a 12-year-old boy. Pediatr Infect Dis J. 2008;27:760–762. doi: 10.1097/INF.0b013e318170af46. [DOI] [PubMed] [Google Scholar]
- 233.Ibler K, Truberg Jensen K, Ostergaard C, Sönksen UW, Bruun B, Schønheyder HC, Kemp M, Dargis R, Andresen K, Christensen JJ. Six cases of Aerococcus sanguinicola infection: clinical relevance and bacterial identification. Scand J Infect Dis. 2008;40:761–765. doi: 10.1080/00365540802078059. [DOI] [PubMed] [Google Scholar]
- 234.de Jong MF, Soetekouw R, ten Kate RW, Veenendaal D. Aerococcus urinae: severe and fatal bloodstream infections and endocarditis. J Clin Microbiol. 2010;48:3445–3447. doi: 10.1128/JCM.00835-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Christensen JJ, Jensen IP, Faerk J, Kristensen B, Skov R, Korner B Danish ALO Study Group. Bacteremia/septicemia due to Aerococcus-like organisms: report of seventeen cases. Clin Infect Dis. 1995;21:943–947. doi: 10.1093/clinids/21.4.943. [DOI] [PubMed] [Google Scholar]
- 236.Christensen JJ, Gutschik E, Friis-Møller A, Korner B. Urosepticemia and fatal endocarditis caused by aerococcus-like organisms. Scand J Infect Dis. 1991;23:717–721. doi: 10.3109/00365549109024299. [DOI] [PubMed] [Google Scholar]
- 237.Christensen JJ, Korner B, Kjaergaard H. Aerococcus-like organism--an unnoticed urinary tract pathogen. APMIS. 1989;97:539–546. doi: 10.1111/j.1699-0463.1989.tb00828.x. [DOI] [PubMed] [Google Scholar]
- 238.Facklam R, Hollis D, Collins MD. Identification of gram-positive coccal and coccobacillary vancomycin-resistant bacteria. J Clin Microbiol. 1989;27:724–730. doi: 10.1128/jcm.27.4.724-730.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Cattoir V, Kobal A, Legrand P. Aerococcus urinae and Aerococcus sanguinicola, two frequently misidentified uropathogens. Scand J Infect Dis. 2010;42:775–780. doi: 10.3109/00365548.2010.485576. [DOI] [PubMed] [Google Scholar]
- 240.Zhang Q, Kwoh C, Attorri S, Clarridge JE., III Aerococcus urinae in urinary tract infections. J Clin Microbiol. 2000;38:1703–1705. doi: 10.1128/jcm.38.4.1703-1705.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Schuur PM, Kasteren ME, Sabbe L, Vos MC, Janssens MM, Buiting AG. Urinary tract infections with Aerococcus urinae in the south of The Netherlands. Eur J Clin Microbiol Infect Dis. 1997;16:871–875. doi: 10.1007/BF01700552. [DOI] [PubMed] [Google Scholar]
- 242.Heilesen AM. Septicaemia due to Aerococcus urinae. Scand J Infect Dis. 1994;26:759–760. doi: 10.3109/00365549409008648. [DOI] [PubMed] [Google Scholar]
- 243.Gritsch W, Nagl M, Hausdorfer J, Gschwendtner A, Pechlaner C, Wiedermann CJ. Septicaemia and endomyocarditis caused by Aerococcus urinae. Wien Klin Wochenschr. 1999;111:446–447. [PubMed] [Google Scholar]
- 244.Soriano F, Rauch A. Microbiological and clinical features of Corynebacterium urealyticum: urinary tract stones and genomics as the Rosetta Stone. Clin Microbiol Infect. 2008;14:632–643. doi: 10.1111/j.1469-0691.2008.02023.x. [DOI] [PubMed] [Google Scholar]
- 245.Cogen AL, Nizet V, Gallo RL. Skin microbiota: a source of disease or defence? Br J Dermatol. 2008;158:442–455. doi: 10.1111/j.1365-2133.2008.08437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Chung SY, Davies BJ, O’Donnell WF. Mortality from grossly encrusted bilateral pyelitis, ureteritis, and cystitis by Corynebacterium group D2. Urology. 2003;61:463. doi: 10.1016/s0090-4295(02)02283-5. [DOI] [PubMed] [Google Scholar]
- 247.Meria P, Margaryan M, Haddad E, Dore B, Lottmann HB. Encrusted cystitis and pyelitis in children: an unusual condition with potentially severe consequences. Urology. 2004;64:569–573. doi: 10.1016/j.urology.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 248.Johnson MH, Strope SA. Encrusted cystitis. Urology. 2012;79:e31–e32. doi: 10.1016/j.urology.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.López-Medrano F, García-Bravo M, Morales JM, Andrés A, San Juan R, Lizasoain M, Aguado JM. Urinary tract infection due to Corynebacterium urealyticum in kidney transplant recipients: an underdiagnosed etiology for obstructive uropathy and graft dysfunction-results of a prospective cohort study. Clin Infect Dis. 2008;46:825–830. doi: 10.1086/528713. [DOI] [PubMed] [Google Scholar]
- 250.Sánchez Hernández J, Mora Peris B, Yagüe Guirao G, Gutiérrez Zufiaurre N, Muñoz Bellido JL, Segovia Hernández M, García Rodríguez JA. In vitro activity of newer antibiotics against Corynebacterium jeikeium, Corynebacterium amycolatum and Corynebacterium urealyticum. Int J Antimicrob Agents. 2003;22:492–496. doi: 10.1016/s0924-8579(03)00121-3. [DOI] [PubMed] [Google Scholar]
- 251.Fernández-Natal I, Guerra J, Alcoba M, Cachón F, Soriano F. Bacteremia caused by multiply resistant corynebacterium urealyticum: six case reports and review. Eur J Clin Microbiol Infect Dis. 2001;20:514–517. doi: 10.1007/pl00011297. [DOI] [PubMed] [Google Scholar]
- 252.Lawson PA, Falsen E, Akervall E, Vandamme P, Collins MD. Characterization of some Actinomyces-like isolates from human clinical specimens: reclassification of Actinomyces suis (Soltys and Spratling) as Actinobaculum suis comb. nov. and description of Actinobaculum schaalii sp. nov. Int J Syst Bacteriol. 1997;47:899–903. doi: 10.1099/00207713-47-3-899. [DOI] [PubMed] [Google Scholar]
- 253.Wegienek J, Reddy CA. Nutritional and metabolic features of Eubacterium suis. J Clin Microbiol. 1982;15:895–901. doi: 10.1128/jcm.15.5.895-901.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Wendt M, Liebhold M, Kaup F, Amtsberg G, Bollwahn W. Corynebacterium suis infection in swine. 1. Clinical diagnosis with special consideration of urine studies and cystoscopy. Tierarztl Prax. 1990;18:353–357. [PubMed] [Google Scholar]
- 255.Jones JE, Dagnall GJ. The carriage of Corynebacterium suis in male pigs. J Hyg (Lond) 1984;93:381–388. doi: 10.1017/s0022172400064949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Schaal KP, Yassin AF, Stackebrandt E. The Family Actinomycetaceae: The Genera Actinomyces, Actinobaculum, Arcanobacterium, Varibaculum, and Mobiluncus. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E, editors. The Prokaryotes. New York, NY: Springer; 2006. pp. 430–537. [Google Scholar]
- 257.Waldmann KH. Pyelocystitis in breeding sows. Tierarztl Prax. 1987;15:263–267. [PubMed] [Google Scholar]
- 258.Percy DH, Ruhnke HL, Soltys MA. A case of infectious cystitis and pyelonephritis of swine caused by Corynebacterium suis. Can Vet J. 1966;7:291–292. [PMC free article] [PubMed] [Google Scholar]
- 259.Kaup FJ, Liebhold M, Wendt M, Drommer W. Corynebacterium suis infections in swine. 2. Morphological findings in the urinary tract with special reference to the bladder. Tierarztl Prax. 1990;18:595–599. [PubMed] [Google Scholar]
- 260.Pleschakowa V, Leibold W, Amtsberg G, Konine D, Wendt M. The prevalence of Actinobaculum suis in boars of breeding herds in the Omsk region (Russian Federation) by indirect immunofluorescence technique. Dtsch Tierarztl Wochenschr. 2004;111:67–69. [PubMed] [Google Scholar]
- 261.Cattoir V. Actinobaculum schaalii: review of an emerging uropathogen. J Infect. 2012;64:260–267. doi: 10.1016/j.jinf.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 262.Tschudin-Sutter S, Frei R, Weisser M, Goldenberger D, Widmer AF. Actinobaculum schaalii - invasive pathogen or innocent bystander? A retrospective observational study. BMC Infect Dis. 2011;11:289. doi: 10.1186/1471-2334-11-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Vanden Bempt I, Van Trappen S, Cleenwerck I, De Vos P, Camps K, Celens A, Van De Vyvere M. Actinobaculum schaalii causing Fournier’s gangrene. J Clin Microbiol. 2011;49:2369–2371. doi: 10.1128/JCM.00272-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Bank S, Hansen TM, Søby KM, Lund L, Prag J. Actinobaculum schaalii in urological patients, screened with real-time polymerase chain reaction. Scand J Urol Nephrol. 2011;45:406–410. doi: 10.3109/00365599.2011.599333. [DOI] [PubMed] [Google Scholar]
- 265.Alvarez-Paredes L, López-García P, Ruiz-García M, Royo-García G. Actinobaculum schaalii infection. Enferm Infecc Microbiol Clin. 2012;30:505–506. doi: 10.1016/j.eimc.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 266.Andersen LB, Bank S, Hertz B, Søby KM, Prag J. Actinobaculum schaalii, a cause of urinary tract infections in children? Acta Paediatr. 2012;101:e232–e234. doi: 10.1111/j.1651-2227.2011.02586.x. [DOI] [PubMed] [Google Scholar]
- 267.Bank S, Jensen A, Hansen TM, Søby KM, Prag J. Actinobaculum schaalii, a common uropathogen in elderly patients, Denmark. Emerg Infect Dis. 2010;16:76–80. doi: 10.3201/eid1601.090761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Nielsen HL, Søby KM, Christensen JJ, Prag J. Actinobaculum schaalii: a common cause of urinary tract infection in the elderly population. Bacteriological and clinical characteristics. Scand J Infect Dis. 2010;42:43–47. doi: 10.3109/00365540903289662. [DOI] [PubMed] [Google Scholar]
- 269.Tavassoli P, Paterson R, Grant J. Actinobaculum schaalii: An Emerging Uropathogen? Case Rep Urol. 2012;2012:468516. doi: 10.1155/2012/468516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Beguelin C, Genne D, Varca A, Tritten ML, Siegrist HH, Jaton K, Lienhard R. Actinobaculum schaalii: clinical observation of 20 cases. Clin Microbiol Infect. 2011;17:1027–1031. doi: 10.1111/j.1469-0691.2010.03370.x. [DOI] [PubMed] [Google Scholar]
- 271.Hoenigl M, Leitner E, Valentin T, Zarfel G, Salzer HJ, Krause R, Grisold AJ. Endocarditis caused by Actinobaculum schaalii, Austria. Emerg Infect Dis. 2010;16:1171–1173. doi: 10.3201/eid1607.100349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Larios OE, Bernard KA, Manickam K, Ng B, Alfa M, Ronald A. First report of Actinobaculum schaalii urinary tract infection in North America. Diagn Microbiol Infect Dis. 2010;67:282–285. doi: 10.1016/j.diagmicrobio.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 273.Fendukly F, Osterman B. Isolation of Actinobaculum schaalii and Actinobaculum urinale from a patient with chronic renal failure. J Clin Microbiol. 2005;43:3567–3569. doi: 10.1128/JCM.43.7.3567-3569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Pajkrt D, Simoons-Smit AM, Savelkoul PH, van den Hoek J, Hack WW, van Furth AM. Pyelonephritis caused by Actinobaculum schaalii in a child with pyeloureteral junction obstruction. Eur J Clin Microbiol Infect Dis. 2003;22:438–440. doi: 10.1007/s10096-003-0933-3. [DOI] [PubMed] [Google Scholar]
- 275.Jöhnk ML, Olsen AB, Prag JB, Søby KM. Severe phimosis as cause of urosepsis with Actinobaculum schaalii. Ugeskr Laeger. 2012;174:1539–1540. [PubMed] [Google Scholar]
- 276.Reinhard M, Prag J, Kemp M, Andresen K, Klemmensen B, Højlyng N, Sørensen SH, Christensen JJ. Ten cases of Actinobaculum schaalii infection: clinical relevance, bacterial identification, and antibiotic susceptibility. J Clin Microbiol. 2005;43:5305–5308. doi: 10.1128/JCM.43.10.5305-5308.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.García-Bravo M, González-Fernández MB, García-Castro MA, Jaime-Muniesa ML. Urinary tract infection caused by Actinobaculum schaalii in an elderly patient. Rev Esp Quimioter. 2011;24:52–53. [PubMed] [Google Scholar]
- 278.Sturm PD, Van Eijk J, Veltman S, Meuleman E, Schülin T. Urosepsis with Actinobaculum schaalii and Aerococcus urinae. J Clin Microbiol. 2006;44:652–654. doi: 10.1128/JCM.44.2.652-654.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Haller P, Bruderer T, Schaeren S, Laifer G, Frei R, Battegay M, Flückiger U, Bassetti S. Vertebral osteomyelitis caused by Actinobaculum schaalii: a difficult-to-diagnose and potentially invasive uropathogen. Eur J Clin Microbiol Infect Dis. 2007;26:667–670. doi: 10.1007/s10096-007-0345-x. [DOI] [PubMed] [Google Scholar]
- 280.Amatya R, Bhattarai S, Mandal PK, Tuladhar H, Karki BM. Urinary tract infection in vaginitis: a condition often overlooked. Nepal Med Coll J. 2013;15:65–67. [PubMed] [Google Scholar]
- 281.Harmanli OH, Cheng GY, Nyirjesy P, Chatwani A, Gaughan JP. Urinary tract infections in women with bacterial vaginosis. Obstet Gynecol. 2000;95:710–712. doi: 10.1016/s0029-7844(99)00632-8. [DOI] [PubMed] [Google Scholar]
- 282.Lam MH, Birch DF. Survival of Gardnerella vaginalis in human urine. Am J Clin Pathol. 1991;95:234–239. doi: 10.1093/ajcp/95.2.234. [DOI] [PubMed] [Google Scholar]
- 283.Josephson S, Thomason J, Sturino K, Zabransky R, Williams J. Gardnerella vaginalis in the urinary tract: incidence and significance in a hospital population. Obstet Gynecol. 1988;71:245–250. [PubMed] [Google Scholar]
- 284.Clarke RW, Collins LE, Maskell R. Gardnerella vaginalis as a urinary pathogen. J Infect. 1989;19:191–193. doi: 10.1016/s0163-4453(89)92189-0. [DOI] [PubMed] [Google Scholar]
- 285.Sturm AW. Gardnerella vaginalis in infections of the urinary tract. J Infect. 1989;18:45–49. doi: 10.1016/s0163-4453(89)93642-6. [DOI] [PubMed] [Google Scholar]
- 286.Lam MH, Birch DF, Fairley KF. Prevalence of Gardnerella vaginalis in the urinary tract. J Clin Microbiol. 1988;26:1130–1133. doi: 10.1128/jcm.26.6.1130-1133.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Gilbert GL, Garland SM, Fairley KF, McDowall DM. Bacteriuria due to ureaplasmas and other fastidious organisms during pregnancy: prevalence and significance. Pediatr Infect Dis. 1986;5(Suppl):S239–S243. doi: 10.1097/00006454-198611010-00007. [DOI] [PubMed] [Google Scholar]
- 288.Savige JA, Gilbert GL, Fairley KF, McDowall DR. Bacteriuria due to Ureaplasma urealyticum and Gardnerella vaginalis in women with preeclampsia. J Infect Dis. 1983;148:605. doi: 10.1093/infdis/148.3.605. [DOI] [PubMed] [Google Scholar]
- 289.McDowall DR, Buchanan JD, Fairley KF, Gilbert GL. Anaerobic and other fastidious microorganisms in asymptomatic bacteriuria in pregnant women. J Infect Dis. 1981;144:114–122. doi: 10.1093/infdis/144.2.114. [DOI] [PubMed] [Google Scholar]
- 290.McFadyen IR, Eykyn SJ. Suprapubic aspiration of urine in pregnancy. Lancet. 1968;1:1112–1114. doi: 10.1016/s0140-6736(68)90185-2. [DOI] [PubMed] [Google Scholar]
- 291.Fairley KF, Birch DF. Unconventional bacteria in urinary tract disease: gardnerella vaginalis. Kidney Int. 1983;23:862–865. doi: 10.1038/ki.1983.107. [DOI] [PubMed] [Google Scholar]
- 292.Pearce MM, Hilt EE, Rosenfeld AB, Zilliox MJ, Thomas-White K, Fok C, Kliethermes S, Schreckenberger PC, Brubaker L, Gai X, Wolfe AJ. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. MBio. 2014;5 doi: 10.1128/mBio.01283-14. e01283–e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Reimer LG, Reller LB. Gardnerella vaginalis bacteremia: a review of thirty cases. Obstet Gynecol. 1984;64:170–172. [PubMed] [Google Scholar]
- 294.Datcu R, Charib K, Kjaeldgaard P. Septic shock caused by Gardnerella vaginalis and Peptostreptococcus species after Cesarean section. Ugeskr Laeger. 2009;171:1012. [PubMed] [Google Scholar]
- 295.Amaya RA, Al-Dossary F, Demmler GJ. Gardnerella vaginalis bacteremia in a premature neonate. J Perinatol. 2002;22:585–587. doi: 10.1038/sj.jp.7210757. [DOI] [PubMed] [Google Scholar]
- 296.La Scolea LJ, Jr, Dryja DM, Dillon WP. Recovery of Gardnerella vaginalis from blood by the quantitative direct plating method. J Clin Microbiol. 1984;20:568–569. doi: 10.1128/jcm.20.3.568-569.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.McCool RA, DeDonato DM. Bacteremia of Gardnerella vaginalis after endometrial ablation. Arch Gynecol Obstet. 2012;286:1337–1338. doi: 10.1007/s00404-012-2447-7. [DOI] [PubMed] [Google Scholar]
- 298.Agostini A, Beerli M, Franchi F, Bretelle F, Blanc B. Garnerella vaginalis bacteremia after vaginal myomectomy. Eur J Obstet Gynecol Reprod Biol. 2003;108:229. doi: 10.1016/s0301-2115(02)00432-3. [DOI] [PubMed] [Google Scholar]
- 299.Alidjinou EK, Bonnet I, Canis F, Dewulf G, Mazars E, Cattoen C. Gardnerella vaginalis bacteremia in a male patient. Med Mal Infect. 2013;43:434–435. doi: 10.1016/j.medmal.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 300.Yoon HJ, Chun J, Kim JH, Kang SS, Na DJ. Gardnerella vaginalis septicaemia with pyelonephritis, infective endocarditis and septic emboli in the kidney and brain of an adult male. Int J STD AIDS. 2010;21:653–657. doi: 10.1258/ijsa.2010.009574. [DOI] [PubMed] [Google Scholar]
- 301.Lagacé-Wiens PR, Ng B, Reimer A, Burdz T, Wiebe D, Bernard K. Gardnerella vaginalis bacteremia in a previously healthy man: case report and characterization of the isolate. J Clin Microbiol. 2008;46:804–806. doi: 10.1128/JCM.01545-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Bastida Vilá MT, López Onrubia P, Rovira Lledos J, Martinez Martinez JA, Expósito Aguilera M. Garderella vaginalis bacteremia in an adult male. Eur J Clin Microbiol Infect Dis. 1997;16:400–401. doi: 10.1007/BF01726374. [DOI] [PubMed] [Google Scholar]
- 303.Denoyel GA, Drouet EB, De Montclos HP, Schanen A, Michel S. Gardnerella vaginalis bacteremia in a man with prostatic adenoma. J Infect Dis. 1990;161:367–368. doi: 10.1093/infdis/161.2.367. [DOI] [PubMed] [Google Scholar]
- 304.Legrand JC, Alewaeters A, Leenaerts L, Gilbert P, Labbe M, Glupczynski Y. Gardnerella vaginalis bacteremia from pulmonary abscess in a male alcohol abuser. J Clin Microbiol. 1989;27:1132–1134. doi: 10.1128/jcm.27.5.1132-1134.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Swidsinski A, Doerffel Y, Loening-Baucke V, Swidsinski S, Verstraelen H, Vaneechoutte M, Lemm V, Schilling J, Mendling W. Gardnerella biofilm involves females and males and is transmitted sexually. Gynecol Obstet Invest. 2010;70:256–263. doi: 10.1159/000314015. [DOI] [PubMed] [Google Scholar]
- 306.Verstraelen H, Verhelst R, Vaneechoutte M, Temmerman M. The epidemiology of bacterial vaginosis in relation to sexual behaviour. BMC Infect Dis. 2010;10:81. doi: 10.1186/1471-2334-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Chowdhury MN. Gardnerella vaginalis carriage in male patients. Trop Geogr Med. 1986;38:137–140. [PubMed] [Google Scholar]
- 308.Burdge DR, Bowie WR, Chow AW. Gardnerella vaginalis-associated balanoposthitis. Sex Transm Dis. 1986;13:159–162. doi: 10.1097/00007435-198607000-00009. [DOI] [PubMed] [Google Scholar]
- 309.Lefevre JC, Lepargneur JP, Bauriaud R, Bertrand MA, Blanc C. Clinical and microbiologic features of urethritis in men in Toulouse, France. Sex Transm Dis. 1991;18:76–79. doi: 10.1097/00007435-199118020-00004. [DOI] [PubMed] [Google Scholar]
- 310.Raz R, Colodner R, Kunin CM. Who are you--Staphylococcus saprophyticus? Clin Infect Dis. 2005;40:896–898. doi: 10.1086/428353. [DOI] [PubMed] [Google Scholar]
- 311.Torres Pereira A. Coagulase-negative strains of staphylococcus possessing antigen 51 as agents of urinary infection. J Clin Pathol. 1962;15:252–253. doi: 10.1136/jcp.15.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Siegman-Igra Y, Kulka T, Schwartz D, Konforti N. The significance of polymicrobial growth in urine: contamination or true infection. Scand J Infect Dis. 1993;25:85–91. [PubMed] [Google Scholar]
- 313.Bishara J, Leibovici L, Huminer D, Drucker M, Samra Z, Konisberger H, Pitlik S. Five-year prospective study of bacteraemic urinary tract infection in a single institution. Eur J Clin Microbiol Infect Dis. 1997;16:563–567. doi: 10.1007/BF02447917. [DOI] [PubMed] [Google Scholar]
- 314.Woods TD, Watanakunakorn C. Bacteremia due to Providencia stuartii: review of 49 episodes. South Med J. 1996;89:221–224. doi: 10.1097/00007611-199602000-00013. [DOI] [PubMed] [Google Scholar]
- 315.Ferry SA, Holm SE, Stenlund H, Lundholm R, Monsen TJ. Clinical and bacteriological outcome of different doses and duration of pivmecillinam compared with placebo therapy of uncomplicated lower urinary tract infection in women: the LUTIW project. Scand J Prim Health Care. 2007;25:49–57. doi: 10.1080/02813430601183074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Domann E, Hong G, Imirzalioglu C, Turschner S, Kühle J, Watzel C, Hain T, Hossain H, Chakraborty T. Culture-independent identification of pathogenic bacteria and polymicrobial infections in the genitourinary tract of renal transplant recipients. J Clin Microbiol. 2003;41:5500–5510. doi: 10.1128/JCM.41.12.5500-5510.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Wolfe AJ, Toh E, Shibata N, Rong R, Kenton K, Fitzgerald M, Mueller ER, Schreckenberger P, Dong Q, Nelson DE, Brubaker L. Evidence of uncultivated bacteria in the adult female bladder. J Clin Microbiol. 2012;50:1376–1383. doi: 10.1128/JCM.05852-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Siddiqui H, Nederbragt AJ, Lagesen K, Jeansson SL, Jakobsen KS. Assessing diversity of the female urine microbiota by high throughput sequencing of 16S rDNA amplicons. BMC Microbiol. 2011;11:244. doi: 10.1186/1471-2180-11-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Lewis DA, Brown R, Williams J, White P, Jacobson SK, Marchesi JR, Drake MJ. The human urinary microbiome; bacterial DNA in voided urine of asymptomatic adults. Front Cell Infect Microbiol. 2013;3:41. doi: 10.3389/fcimb.2013.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Horwitz D, McCue T, Mapes AC, Ajami NJ, Petrosino JF, Ramig RF, Trautner BW. Decreased microbiota diversity associated with urinary tract infection in a trial of bacterial interference. J Infect. 2015;71:358–367. doi: 10.1016/j.jinf.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Dankert J, Mensink WF, Aarnoudse JG, Meijer-Severs GJ, Huisjes HJ. The prevalence of anaerobic bacteria in suprapubic bladder aspirates obtained from pregnant women. Zentralbl Bakteriol [Orig A] 1979;244:260–267. [PubMed] [Google Scholar]
- 322.Nelson DE, Van Der Pol B, Dong Q, Revanna KV, Fan B, Easwaran S, Sodergren E, Weinstock GM, Diao L, Fortenberry JD. Characteristic male urine microbiomes associate with asymptomatic sexually transmitted infection. PLoS One. 2010;5:e14116. doi: 10.1371/journal.pone.0014116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Dong Q, Nelson DE, Toh E, Diao L, Gao X, Fortenberry JD, Van der Pol B. The microbial communities in male first catch urine are highly similar to those in paired urethral swab specimens. PLoS One. 2011;6:e19709. doi: 10.1371/journal.pone.0019709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Nelson DE, Dong Q, Van der Pol B, Toh E, Fan B, Katz BP, Mi D, Rong R, Weinstock GM, Sodergren E, Fortenberry JD. Bacterial communities of the coronal sulcus and distal urethra of adolescent males. PLoS One. 2012;7:e36298. doi: 10.1371/journal.pone.0036298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Hilt EE, McKinley K, Pearce MM, Rosenfeld AB, Zilliox MJ, Mueller ER, Brubaker L, Gai X, Wolfe AJ, Schreckenberger PC. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol. 2014;52:871–876. doi: 10.1128/JCM.02876-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Borgdorff H, Verwijs MC, Wit FW, Tsivtsivadze E, Ndayisaba GF, Verhelst R, Schuren FH, van de Wijgert JH. The impact of hormonal contraception and pregnancy on sexually transmitted infections and on cervicovaginal microbiota in african sex workers. Sex Transm Dis. 2015;42:143–152. doi: 10.1097/OLQ.0000000000000245. [DOI] [PubMed] [Google Scholar]
- 327.Lüthje P, Hirschberg AL, Brauner A. Estrogenic action on innate defense mechanisms in the urinary tract. Maturitas. 2014;77:32–36. doi: 10.1016/j.maturitas.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 328.Ackermann RJ, Monroe PW. Bacteremic urinary tract infection in older people. J Am Geriatr Soc. 1996;44:927–933. doi: 10.1111/j.1532-5415.1996.tb01862.x. [DOI] [PubMed] [Google Scholar]
- 329.Pearce MM, Zilliox MJ, Rosenfeld AB, Thomas-White KJ, Richter HE, Nager CW, Visco AG, Nygaard IE, Barber MD, Schaffer J, Moalli P, Sung VW, Smith AL, Rogers R, Nolen TL, Wallace D, Meikle SF, Gai X, Wolfe AJ, Brubaker L Pelvic Floor Disorders Network. The female urinary microbiome in urgency urinary incontinence. Am J Obstet Gynecol. 2015;213:347.e1–347.e11. doi: 10.1016/j.ajog.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Nickel JC, Stephens A, Landis JR, Chen J, Mullins C, van Bokhoven A, Lucia MS, Melton-Kreft R, Ehrlich GD MAPP Research Network. Search for Microorganisms in Men with Urologic Chronic Pelvic Pain Syndrome: A Culture-Independent Analysis in the MAPP Research Network. J Urol. 2015;194:127–135. doi: 10.1016/j.juro.2015.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Marconi M, Pilatz A, Wagenlehner F, Diemer T, Weidner W. Impact of infection on the secretory capacity of the male accessory glands. Int Braz J Urol. 2009;35:299–308. doi: 10.1590/s1677-55382009000300006. discussion 308–309. [DOI] [PubMed] [Google Scholar]
- 332.Ivanov YB. Microbiological features of persistent nonspecific urethritis in men. J Microbiol Immunol Infect. 2007;40:157–161. [PubMed] [Google Scholar]
- 333.Imirzalioglu C, Hain T, Chakraborty T, Domann E. Hidden pathogens uncovered: metagenomic analysis of urinary tract infections. Andrologia. 2008;40:66–71. doi: 10.1111/j.1439-0272.2007.00830.x. [DOI] [PubMed] [Google Scholar]
- 334.Pillai A, Deodhar L, Gogate A. Microbiological study of urethritis in men attending a STD clinic. Indian J Med Res. 1990;91:443–447. [PubMed] [Google Scholar]
- 335.Siddiqui H, Lagesen K, Nederbragt AJ, Jeansson SL, Jakobsen KS. Alterations of microbiota in urine from women with interstitial cystitis. BMC Microbiol. 2012;12:205. doi: 10.1186/1471-2180-12-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Casullo VA, Bottone E, Herold BC. Peptostreptococcus asaccharolyticus renal abscess: a rare cause of fever of unknown origin. Pediatrics. 2001;107:E11. doi: 10.1542/peds.107.1.e11. [DOI] [PubMed] [Google Scholar]
- 337.Teo KP, Jacob SC, Lim SH. Post-caesarean septicaemia in Kandang Kerbau Hospital, Singapore, 1993–1995. Med J Malaysia. 1997;52:325–330. [PubMed] [Google Scholar]
- 338.Brown K, Church D, Lynch T, Gregson D. Bloodstream infections due to Peptoniphilus spp.: report of 15 cases. Clin Microbiol Infect. 2014;20:O857–O860. doi: 10.1111/1469-0691.12657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Bernier M, Njomnang Soh P, Lochet A, Prots L, Félice R, Senescau A, Fabre R, Philippon A. Lactobacillus delbrueckii: probable agent of urinary tract infections in very old women. Pathol Biol (Paris) 2012;60:140–142. doi: 10.1016/j.patbio.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 340.Darbro BW, Petroelje BK, Doern GV. Lactobacillus delbrueckii as the cause of urinary tract infection. J Clin Microbiol. 2009;47:275–277. doi: 10.1128/JCM.01630-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Yagihashi Y, Arakaki Y. Acute pyelonephritis and secondary bacteraemia caused by Veillonella during pregnancy. BMJ Case Rep. 2012 doi: 10.1136/bcr-2012-007364. pii:bcr-2012-007364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Meijer-Severs GJ, Aarnoudse JG, Mensink WF, Dankert J. The presence of antibody-coated anaerobic bacteria in asymptomatic bacteriuria during pregnancy. J Infect Dis. 1979;140:653–658. doi: 10.1093/infdis/140.5.653. [DOI] [PubMed] [Google Scholar]
- 343.García-Lechuz JM, Cuevas-Lobato O, Hernángomez S, Hermida A, Guinea J, Marín M, Peláez T, Bouza E. Extra-abdominal infections due to Gemella species. Int J Infect Dis. 2002;6:78–82. doi: 10.1016/s1201-9712(02)90142-6. [DOI] [PubMed] [Google Scholar]
- 344.Ahmad NM, Ahmad KM. Corynebacterium minutissimum pyelonephritis with associated bacteraemia: a case report and review of literature. J Infect. 2005;51:e299–e303. doi: 10.1016/j.jinf.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 345.Audard V, Garrouste-Orgeas M, Misset B, Ali AB, Gattolliat O, Meria P, Carlet J. Fatal septic shock caused by Corynebacterium D2. Intensive Care Med. 2003;29:1376–1379. doi: 10.1007/s00134-003-1865-1. [DOI] [PubMed] [Google Scholar]
- 346.Beteta López A, Gil Ruiz MT, Vega Prado L, Fajardo Olivares M. Cystitis and haematuria due to Corynebacterium striatum. A case report and review. Actas Urol Esp. 2009;33:909–912. doi: 10.1016/s0210-4806(09)72880-3. [DOI] [PubMed] [Google Scholar]
- 347.Craig J, Grigor W, Doyle B, Arnold D. Pyelonephritis caused by Corynebacterium minutissimum. Pediatr Infect Dis J. 1994;13:1151–1152. doi: 10.1097/00006454-199412000-00018. [DOI] [PubMed] [Google Scholar]
- 348.Fontana I, Bertocchi M, Rossi AM, Gasloli G, Santori G, Ferro C, Patti V, Rossini A, Valente U. Corynebacterium urealyticum infection in a pediatric kidney transplant recipient: case report. Transplant Proc. 2010;42:1367–1368. doi: 10.1016/j.transproceed.2010.03.054. [DOI] [PubMed] [Google Scholar]
- 349.Pagnoux C, Bérezné A, Damade R, Paillot J, Aouizerate J, Le Guern V, Salmon D, Guillevin L. Encrusting cystitis due to Corynebacterium urealyticum in a patient with ANCA-associated vasculitis: case report and review of the literature. Semin Arthritis Rheum. 2011;41:297–300. doi: 10.1016/j.semarthrit.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 350.Matusnami M, Otsuka T, Ohkusu K, Sogi M, Kitazono H, Hosokawa N. Urosepsis caused by Globicatella sanguinis and Corynebacterium riegelii in an adult: case report and literature review. J Infect Chemother. 2012;18:552–554. doi: 10.1007/s10156-011-0335-x. [DOI] [PubMed] [Google Scholar]
- 351.Galan-Sanchez F, Aznar-Marin P, Marin-Casanova P, Garcia-Martos P, Rodriguez-Iglesias M. Urethritis due to Corynebacterium glucuronolyticum. J Infect Chemother. 2011;17:720–721. doi: 10.1007/s10156-011-0237-y. [DOI] [PubMed] [Google Scholar]
- 352.Abdolrasouli A, Roushan A. Corynebacterium propinquum associated with acute, nongonococcal urethritis. Sex Transm Dis. 2013;40:829–831. doi: 10.1097/OLQ.0000000000000027. [DOI] [PubMed] [Google Scholar]
- 353.Swartz SL, Kraus SJ, Herrmann KL, Stargel MD, Brown WJ, Allen SD. Diagnosis and etiology of nongonococcal urethritis. J Infect Dis. 1978;138:445–454. doi: 10.1093/infdis/138.4.445. [DOI] [PubMed] [Google Scholar]
- 354.Greub G, Raoult D. “Actinobaculum massiliae,” a new species causing chronic urinary tract infection. J Clin Microbiol. 2002;40:3938–3941. doi: 10.1128/JCM.40.11.3938-3941.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Gomez E, Gustafson DR, Rosenblatt JE, Patel R. Actinobaculum bacteremia: a report of 12 cases. J Clin Microbiol. 2011;49:4311–4313. doi: 10.1128/JCM.00798-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Lagacé-Wiens PR, Ng B, Reimer A, Burdz T, Wiebe D, Bernard K. Gardnerella vaginalis bacteremia in a previously healthy man: case report and characterization of the isolate. J Clin Microbiol. 2008;46:804–806. doi: 10.1128/JCM.01545-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Aydin MD, Agaçfidan A, Güvener Z, Kadioglu A, Ang O. Bacterial pathogens in male patients with urethritis in Istanbul. Sex Transm Dis. 1998;25:448–449. doi: 10.1097/00007435-199809000-00013. [DOI] [PubMed] [Google Scholar]
- 358.Iser P, Read TH, Tabrizi S, Bradshaw C, Lee D, Horvarth L, Garland S, Denham I, Fairley CK. Symptoms of non-gonococcal urethritis in heterosexual men: a case control study. Sex Transm Infect. 2005;81:163–165. doi: 10.1136/sti.2004.010751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Kumar B, Sharma M. Carriage of Gardnerella vaginalis in the urethra of Indian men. Indian J Med Res. 1994;99:252–254. [PubMed] [Google Scholar]
- 360.McKechnie ML, Hillman R, Couldwell D, Kong F, Freedman E, Wang H, Gilbert GL. Simultaneous identification of 14 genital microorganisms in urine by use of a multiplex PCR-based reverse line blot assay. J Clin Microbiol. 2009;47:1871–1877. doi: 10.1128/JCM.00120-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Manhart LE, Khosropour CM, Liu C, Gillespie CW, Depner K, Fiedler T, Marrazzo JM, Fredricks DN. Bacterial vaginosis-associated bacteria in men: association of Leptotrichia/Sneathia spp. with nongonococcal urethritis. Sex Transm Dis. 2013;40:944–949. doi: 10.1097/OLQ.0000000000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Agrawal P, Vaiphei K. Renal actinomycosis. BMJ Case Rep. 2014 doi: 10.1136/bcr-2014-205892. pii:bcr2014205892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Juhász J, Galambos J, Surján L Jr. Renal actinomycosis associated with bilateral necrosing renal papillitis. Int Urol Nephrol. 1980;12:199–203. doi: 10.1007/BF02217136. [DOI] [PubMed] [Google Scholar]
- 364.Herbland A, Leloup M, Levrat Q, Guillaume F, Verrier V, Bouillard P, Landois T, Ouaki CF, Lesieur O. Fulminant course of unilateral emphysematous pyelonephritis revealing a renal actinomycosis caused by Actinomyces meyeri, an unknown cause of septic shock. J Intensive Care. 2014;2:42. doi: 10.1186/2052-0492-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Jang SM, Na W, Jun YJ, Paik SS. Primary vesical actinomycosis diagnosed by routine urine cytology. Acta Cytol. 2010;54:658–659. doi: 10.1159/000325198. [DOI] [PubMed] [Google Scholar]
- 366.Ieven M, Verhoeven J, Gentens P, Goossens H. Severe infection due to Actinomyces bernardiae: case report. Clin Infect Dis. 1996;22:157–158. doi: 10.1093/clinids/22.1.157. [DOI] [PubMed] [Google Scholar]
- 367.Horliana AC, Chambrone L, Foz AM, Artese HP, Rabelo MS, Pannuti CM, Romito GA. Dissemination of periodontal pathogens in the bloodstream after periodontal procedures: a systematic review. PLoS One. 2014;9:e98271. doi: 10.1371/journal.pone.0098271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Angelova I, Talakova C, Belovezhdov N. Mycoplasma infections of the kidneys. Vutr Boles. 1988;27:110–113. [PubMed] [Google Scholar]
- 369.Küchle C, Abele-Horn M, Menninger M, Held E, Heesemann J. Mycoplasma hominis. A rare causative agent of acute pyelonephritis. Dtsch Med Wochenschr. 1997;122:542–544. doi: 10.1055/s-2008-1047651. [DOI] [PubMed] [Google Scholar]
- 370.Thomsen AC. Occurrence and pathogenicity of Mycoplasma hominis in the upper urinary tract: a review. Sex Transm Dis. 1983;10(Suppl):323–326. [PubMed] [Google Scholar]
- 371.Wong SS, Yuen KY. Acute pyelonephritis caused by Mycoplasma hominis. Pathology. 1995;27:61–63. doi: 10.1080/00313029500169482. [DOI] [PubMed] [Google Scholar]
- 372.Anagrius C, Loré B, Jensen JS. Mycoplasma genitalium: prevalence, clinical significance, and transmission. Sex Transm Infect. 2005;81:458–462. doi: 10.1136/sti.2004.012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Elsner P, Hartmann AA, Wecker I. Gardnerella vaginalis is associated with other sexually transmittable microorganisms in the male urethra. Zentralbl Bakteriol Mikrobiol Hyg [A] 1988;269:56–63. doi: 10.1016/s0176-6724(88)80084-1. [DOI] [PubMed] [Google Scholar]
- 374.Falk L, Fredlund H, Jensen JS. Signs and symptoms of urethritis and cervicitis among women with or without Mycoplasma genitalium or Chlamydia trachomatis infection. Sex Transm Infect. 2005;81:73–78. doi: 10.1136/sti.2004.010439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Högdahl M, Kihlström E. Leucocyte esterase testing of first-voided urine and urethral and cervical smears to identify Mycoplasma genitalium-infected men and women. Int J STD AIDS. 2007;18:835–838. doi: 10.1258/095646207782716983. [DOI] [PubMed] [Google Scholar]
- 376.Ishihara S, Yasuda M, Ito S, Maeda S, Deguchi T. Mycoplasma genitalium urethritis in men. Int J Antimicrob Agents. 2004;24(Suppl 1):S23–S27. doi: 10.1016/j.ijantimicag.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 377.Moi H, Reinton N, Moghaddam A. Mycoplasma genitalium is associated with symptomatic and asymptomatic non-gonococcal urethritis in men. Sex Transm Infect. 2009;85:15–18. doi: 10.1136/sti.2008.032730. [DOI] [PubMed] [Google Scholar]
- 378.Shigehara K, Kawaguchi S, Sasagawa T, Furubayashi K, Shimamura M, Maeda Y, Konaka H, Mizokami A, Koh E, Namiki M. Prevalence of genital Mycoplasma, Ureaplasma, Gardnerella, and human papillomavirus in Japanese men with urethritis, and risk factors for detection of urethral human papillomavirus infection. J Infect Chemother. 2011;17:487–492. doi: 10.1007/s10156-010-0203-0. [DOI] [PubMed] [Google Scholar]
- 379.Wong ES, Hooton TM, Hill CC, McKevitt M, Stamm WE. Clinical and microbiological features of persistent or recurrent nongonococcal urethritis in men. J Infect Dis. 1988;158:1098–1101. doi: 10.1093/infdis/158.5.1098. [DOI] [PubMed] [Google Scholar]
- 380.Marraffini LA, Dedent AC, Schneewind O. Sortases and the art of anchoring proteins to the envelopes of gram-positive bacteria. Microbiol Mol Biol Rev. 2006;70:192–221. doi: 10.1128/MMBR.70.1.192-221.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Mobley HLT. Urease. In: Mobley HLT, Mendz GL, Hazell SL, editors. Helicobacter pylori: Physiology and Genetics. Washington, DC: ASM Press; 2001. pp. 179–191. [PubMed] [Google Scholar]
- 382.Roesch PL, Redford P, Batchelet S, Moritz RL, Pellett S, Haugen BJ, Blattner FR, Welch RA. Uropathogenic Escherichia coli use d-serine deaminase to modulate infection of the murine urinary tract. Mol Microbiol. 2003;49:55–67. doi: 10.1046/j.1365-2958.2003.03543.x. [DOI] [PubMed] [Google Scholar]