Abstract
A pressing need in neurobiology is the comprehensive identification and characterization of neuronal subclasses within the mammalian nervous system. To this end, we used constellation pharmacology as a method to interrogate the neuronal and glial subclasses of the mouse cerebellum individually and simultaneously. We then evaluated the data obtained from constellation-pharmacology experiments by cluster analysis to classify cells into neuronal and glial subclasses, based on their functional expression of glutamate, acetylcholine, and GABA receptors, among other ion channels. Conantokin peptides were used to identify N-methyl-d-aspartate (NMDA) receptor subtypes, which revealed that neurons of the young mouse cerebellum expressed NR2A and NR2B NMDA receptor subunits. Additional pharmacological tools disclosed differential expression of α-amino-3-hydroxy-5-methyl-4-isoxazloepropionic, nicotinic acetylcholine, and muscarinic acetylcholine receptors in different neuronal and glial subclasses. Certain cell subclasses correlated with known attributes of granule cells, and we combined constellation pharmacology with genetically labeled neurons to identify and characterize Purkinje cells. This study illustrates the utility of applying constellation pharmacology to classify neuronal and glial subclasses in specific anatomical regions of the brain.
Keywords: classifying neuronal subclasses, constellation pharmacology, calcium imaging, genetically labeled neurons, conantokin peptides
the comprehensive description of the neuronal subclasses in the mammalian nervous system is increasingly recognized as an essential goal for the progress of neuroscience. The achievement of this goal will require novel experimental techniques and methodologies (Bernard et al. 2009; Nelson et al. 2006; Wichterle et al. 2013). To this end, we have previously advanced an experimental approach that we call “constellation pharmacology.” In essence, constellation pharmacology uses calcium imaging with an array of subtype-selective pharmacological agents to interrogate the cell-specific combinations or constellations of receptors and ion channels expressed across a large population of cultured neuronal and glial cells, individually and simultaneously (Raghuraman et al. 2014; Smith et al. 2013; Teichert et al. 2012a, b, 2014). Cell subclasses are defined through constellation pharmacology by the specific combinations of receptors and ion channels that individual cells express. Two cells expressing the same combination of receptors and ion channels are considered members of the same cell subclass.
Many ligand- and voltage-gated ion channels expressed in neurons permit calcium flux (Dingledine et al. 1999; Hille 2001; Lipovsek et al. 2014; Pankratov and Lalo 2014), making constellation pharmacology amenable to probing a broad assortment of ion channels. Furthermore, some metabotropic receptors stimulate intracellular calcium release into the cytosol, and these can be examined through calcium imaging as well (Pin and Duvoisin 1995). Consequently, constellation pharmacology can address unique questions about neurons of the brain, including the following: how many neuronal subclasses are present in a given area of the brain? What cell-specific constellations of ion channels and receptors are expressed in those neuronal subclasses? How are those receptors and ion channels functionally linked to create neuronal subclasses with unique physiological properties and roles? How do the cell-specific constellations change as a function of development or disease?
Constellation pharmacology principally relies on two types of pharmacological challenges, known as receptor-agonist challenges and membrane-potential challenges (Teichert et al. 2012b). Receptor-agonist challenges use neurotransmitters, neurotransmitter analogs, and other subtype-selective agonists, in combination with subtype-selective antagonists, to reveal expression of ligand-gated ion channels and G protein-coupled receptors. By comparison, membrane-potential challenges use membrane depolarization in combination with subtype-selective antagonists of voltage-gated ion channels to reveal the specific voltage-gated ion channels expressed in individual cells.
In contrast to other techniques, constellation pharmacology uses several pharmacological challenges in combination, and hundreds of neurons can be interrogated simultaneously. Thus this technique can be used to classify and characterize many neuronal subclasses concurrently. Previous work in the ongoing development of constellation pharmacology has focused on neurons from the dorsal root ganglia and the ventral respiratory column, and these studies helped identify the constellations of receptors and ion channels expressed in cold-sensitive neurons and combinations of neuromodulators involved in breathing (Raghuraman et al. 2014; Teichert et al. 2012a, b, 2014).
In this paper, we extend the application of constellation pharmacology to the cerebellum. The classic work of Ramon y Cajal [see Sotelo (2003)] identified five primary neuronal subclasses within the cerebellum, which were defined by connectivity and morphology. The Purkinje, stellate, basket, Golgi, and granule cells have since become long-standing models for studying the architecture and neurophysiology of the cerebellum. However, with the complex role that the cerebellum plays in cognition and motor coordination, it may be the case that diverse neuronal subclasses exist among morphologically indistinguishable cells. This seems to be the case for the cerebral cortex, where hundreds of neuronal and glial subclasses are proposed (Molyneaux et al. 2007; Peters and Jones 1984; Ramon y Cajal 1995). The results presented here for the cerebellum highlight neuronal and glial diversity and demonstrate the ability to identify and examine cell subclasses in culture. Most importantly, we demonstrate for the first time the combination of constellation pharmacology and genetically labeled cells from reporter mice, which we advance as a powerful approach for the identification and classification of neuronal and glial subclasses throughout the nervous system.
METHODS
Culture of cerebellar neurons.
Methods for culturing neurons from mouse cerebellum were adapted from previously described methods for culturing neurons from the mouse ventral respiratory column (Raghuraman et al. 2014). For most experiments, wild-type C57BL/6 mice were used. For some experiments (as described in results), we used mice with genetically labeled Purkinje neurons, obtained by crossing a Pcp2-Cre driver mouse, B6.129-Tg(Pcp2-cre)2Mpin/J (stock number 004146; The Jackson Laboratory, Bar Harbor, ME) (Barski et al. 2000), with the Ai9 reporter mouse (stock number 007905; The Jackson Laboratory), which expresses tdTomato from the Rosa26 locus, after excision of a floxed stop cassette (Madisen et al. 2010). In all cases, mice, at postnatal days 7–8 (P7/8) were anesthetized on ice and decapitated per the University of Utah Institutional Animal Care and Use Committee-approved protocols. Mouse heads were pinned to a silicone-covered culture dish for dissection and removal of cerebellum. After removing skin and connective tissue to reveal the skull, skull bones were pierced at the connecting region between the interparietal and parietal bones. Upon opening the skull, the brain cavity was flushed immediately with ice-cold artificial cerebrospinal fluid (ACSF) at pH 7.6 that was bubbling with carbogen. For the remaining dissection, brain tissue was washed with ACSF every 1–3 min to keep tissue cold, moist, and oxygenated.
The hind skull was removed carefully so as not to disturb brain tissues; the exposed cerebellum was then removed from surrounding regions using a single cut with a fresh razor blade. The cut was initiated just above the cerebellum and continued at an ∼20° below-vertical angle to separate the cerebellum and brain stem from the remaining tissue. The separated piece was transferred to ice-cold ACSF, where the brain stem and other noncerebellar tissues were removed. The isolated cerebellum was chopped into approximately 1 mm × 1 mm pieces with a razor blade, and cerebellar pieces were transferred to 900 μl ice-cold HBSS. To prepare tissue for trituration and aid in isolation of individual cells, 100 μl 2.5% wt/vol trypsin was added (to produce a working concentration of 0.25% trypsin) and the mixture incubated for 5 min at 37°C. Trypsin was removed through dilution using several washes of ACSF. The tissue was then suspended in 4 ml MEM, supplemented with 10% vol/vol FBS (HyClone Laboratories, GE Healthcare Life Sciences, Logan, UT), 100 U/ml penicillin, 100 μg/ml streptomycin, 1× GlutaMAX (Thermo Fisher Scientific, Grand Island, NY), 10 mM HEPES, pH 7.4, and 0.4% wt/vol glucose.
Cerebellar tissue was dissociated in 2- to 4-ml supplemented MEM using a series of four fire-polished pasture pipettes with decreasing diameters. Trituration was performed gently by sucking tissue in and out of pasture pipettes, once every 4–6 s. Dissociation was considered complete once the solution appeared cloudy and had few visible pieces larger than 0.1 mm. The resulting cell suspension was diluted 1:5 with supplemented MEM, and 20 μl of the diluted cells was transferred to the center of a silicone donut affixed to a poly-d-lysine-coated, 24-well tissue-culture plate. The inner diameter of the silicone donut was 3 mm, and cells were plated to achieve a density of ∼100–200 cells/mm2. Cells were allowed to settle and adhere to the plate for 1 h in a 37°C incubator before flooding the well with 1 ml supplemented MEM or a 50:50 mixture of Neurobasal:MEM. Neurons were cultured overnight for 16–20 h at 37°C with 5% CO2 atmosphere.
Calcium imaging and cluster analysis.
Calcium imaging was performed with protocols similar to those described previously for peripheral and central nervous system neurons from the mouse (Raghuraman et al. 2014; Teichert et al. 2012a, b, 2014). Briefly, cultured cerebellar neurons were incubated with 2.5 μM Fura-2-acetoxymethyl ester dye in MEM culture media for 1 h at 37°C and an additional 30 min at room temperature before experimentation. At initiation of imaging, the dye was removed with multiple washes of ACSF, pH 7.4, at room temperature. The fluorescence emission intensity was measured at 510 nm, with alternating excitation wavelengths of 340 and 380 nm. Data were recorded as the ratio of emission intensity measured for each excitation wavelength (340:380 ratio) using standard calcium imaging techniques. Image recording and experiments generally lasted ~1 h, with antagonist incubations and agonist pulses spaced throughout the experiment. All agonists and antagonist compounds were dissolved at desired concentrations in ACSF buffer. Magnesium-free (Mg2+-Free) ACSF was used in instances where N-methyl-d-aspartate (NMDA) receptors were to be activated to prevent NMDA receptor blockade by magnesium ions.
Ligand concentrations for figures showing calcium-imaging traces were as described. All ligands were prepared in ACSF, with the exception of 200 μM NMDA with 20 μM d-serine in Mg2+-Free buffer (NMDA/d-serine), which was prepared in Mg2+-Free ACSF. Ligands (see Fig. 1) included NMDA/d-serine, 300 μM glutamate, 200 μM glycine, 1 mM acetylcholine, 200 μM nicotine, 1 mM carbamylcholine, 30 mM potassium (K+), and 30 mM potassium with 1 mM GABA (K+/GABA). Ligands (see Fig. 2) were the same as seen in Fig. 1, with the addition of α-amino-3-hydroxy-5-methyl-4-isoxazloepropionic (AMPA), which was prepared at 300 μM. Ligands (see Fig. 3) were the same as seen in Fig. 1, with the addition of atropine at 10 μM. Ligands (see Fig. 4) were the same as seen in Fig. 1, with the addition of N-(5-chloro-2,4-dimethoxyphenyl)-N′-(5-methyl-3-isoxazolyl)-urea [PNU-120596 (PNU)] at 10 μM. In Fig. 6, NMDA/d-serine was used in conjunction with conantokin peptides, which were each applied at a working concentration of 3.3 μM.
Fig. 1.
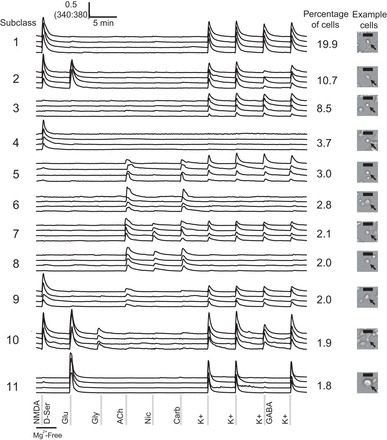
Cellular subclasses of the cerebellum classified by receptor-agonist and membrane-potential challenges. The 11 most frequently observed subclasses are shown. Four traces, each from an individual cell, are depicted as representative members of each subclass. The percentages of cerebellar cells clustered into each subclass are provided, and an example of cell morphology and size of cells in each subclass is provided on the right. Black scale bars represent 20 μm. Cells that responded to potassium depolarization are neurons, and those that did not respond to depolarization are presumed to be glia. Ligand concentrations of the pharmacological agents are the following: NMDA/d-serine, 200 μM N-methyl-d-aspartate (NMDA) with 20 μM d-serine in magnesium-free (Mg2+-Free) buffer; Glu, 300 μM glutamate; Gly, 200 μM glycine; ACh, 1 mM acetylcholine; Nic, 200 μM nicotine; Carb, 1 mM carbamylcholine; K+, 30 mM potassium; and K+/GABA, 30 mM potassium with 1 mM GABA. Data were generated from 4 mice, 7 experimental wells, and 5,825 cells.
Fig. 2.
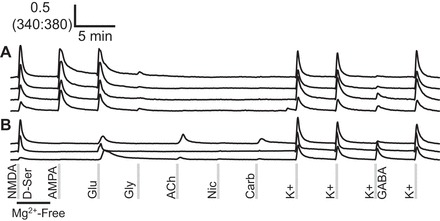
The majority of glutamate-responsive neurons in the cerebellum expresses α-amino-3-hydroxy-5-methyl-4-isoxazloepropionic (AMPA)-type glutamate receptors. More than 95% of neurons that responded to glutamate also responded to AMPA with a similar magnitude, indicating that they express AMPA-type glutamate receptors (A). A few cells responded to glutamate and not AMPA; these presumably express kainate or metabotropic glutamate receptors (B). Ligand concentrations are listed in Fig. 1.
Fig. 3.
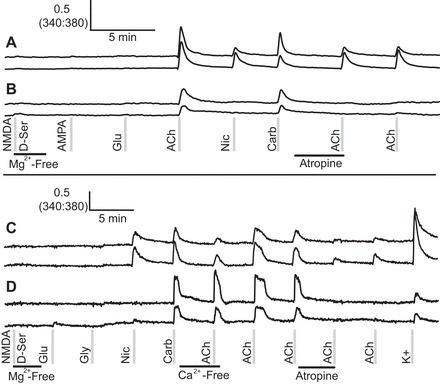
Some cerebellar neurons express a combination of nicotinic and muscarinic acetylcholine receptors, whereas other cerebellar neurons only express muscarinic acetylcholine receptors. A: calcium-imaging traces from cells that expressed both muscarinic and nicotinic acetylcholine receptors, indicated by a partial block of an acetylcholine response in the presence of atropine and by a weaker response to nicotine than to acetylcholine. B: calcium-imaging traces from cells that only express muscarinic acetylcholine receptors. These cells responded to acetylcholine but not to nicotine, and their responses to acetylcholine were completely blocked by atropine. C and D: similar neurons to those shown in A and B, respectively, in an experiment where extracellular calcium was removed from the bath solution. C: the response to acetylcholine was partially blocked in the absence of extracellular calcium or by the application of atropine, suggesting that these neurons express both nicotinic and muscarinic receptors. D: neurons responded fully to acetylcholine in the absence of extracellular calcium, but their responses were completely blocked by atropine, indicating that these neurons only expressed muscarinic receptors. Ca2+-Free, calcium free.
Fig. 4.
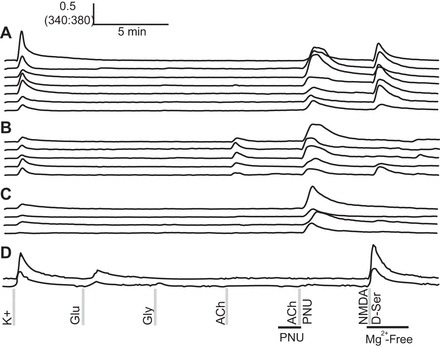
N-(5-chloro-2,4-dimethoxyphenyl)-N′-(5-methyl-3-isoxazolyl)-urea [PNU-120596 (PNU)] revealed cells expressing α7 nicotinic acetylcholine receptors. Functional expression of α7 nicotinic acetylcholine receptors was found in a subset of cells from subclass 1 of Fig. 1 (A), a subset of acetylcholine-responsive cells (B), and a subset of cells that did not respond to other agonists tested (C). D: representative traces from cells that did not respond to acetylcholine under any condition tested in this experiment.
Fig. 6.
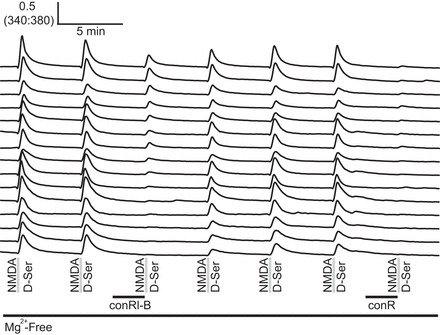
Cerebellar neurons expressed NR2A and NR2B NMDA receptor subtypes in a spectrum of ratios. The combined use of conRl-B and conantokin R (conR) revealed different expression levels for NR2A and NR2B in cerebellar neurons. Cells that were weakly affected by 3.3 μM conRl-B, yet completely inhibited by 3.3 μM conR, are shown at the top and represent cells that express a mixture of NR2B and NR2A NMDA receptors. Cells strongly inhibited by 3.3 μM conRl-B and 3.3 μM conR are shown at the bottom, representing cells that predominantly express NR2B.
NIS-Elements (Nikon Instruments, Melville, NY) was used to define regions of interest (ROIs) corresponding to each cell in a field of view and to capture calcium-imaging data for each cell individually and simultaneously. Cell-culture images were produced in NIS-Elements. Dose-response curves were plotted in Prism 6 (GraphPad Software, La Jolla, CA). The time course of the 340:380 ratio for each ROI (trace) was analyzed using a set of R functions (R Core Team 2013) for quantitative analysis of peak heights, clustering, and determination of cell-phenotype percentages. Traces with obvious abnormal response patterns were removed before the analysis. The MALDIquant package (Gibb and Strimmer 2012) was used to correct baselines, smooth traces, and detect peaks. All traces were baseline corrected using the estimateBaseline function with the “TopHat” method and smoothed using the smoothIntensity function with the “SavitzkyGolay” method and a halfWindowSize of 3. Peaks were detected using the detectPeaks function with the “MAD” method, a halfWindowSize of 30, and a signal-to-noise ratio limit (SNR.lim) setting of 4. Peak heights were calculated through subtraction of the baseline from maximum deflection of the 340:380 ratio within a defined window for each receptor-agonist or membrane-potential challenge. All ROIs were scored as a yes/no response to each input, based on the presence of a peak in the response window region. We used a SNR threshold of 4 and a value above calcium baseline of 0.05 for the 340:380-nm ratio, as thresholds for response to each stimulus. These threshold values gave a false-positive rate < 0.001 (e.g., peaks during times of no input). A “no” response was defined as not meeting or exceeding the thresholds defined above. The resulting binary response profiles were clustered using the “cluster” library in R. The “dist” function was used to calculate the Manhattan distances between binary profiles from each ROI. The “hclust” function was used to cluster based on this distance and “cutree” used to define groups of identical binary response profiles. We used permutation to estimate the significance of clustering in the following iterative steps: 1) cluster the binary profiles and record the size of the largest group; 2) permute the response profiles for each input within each experimental well (this makes the null assumption that response at one input is random with respect to response at any other input); we permute within each well to account for well-to-well differences in frequency; 3) cluster based on the permuted response profiles, and record the size of the largest group; 4) repeat steps 2 and 3 10,000 times to obtain a null distribution of maximum group size; and 5) compare the group size obtained in step 1 with the null distribution to estimate the probability of getting a group that is as large or larger by chance; remove all response profiles corresponding to the group identified in step 1, and repeat steps 1–5 with the remaining ROIs.
With seven different challenges (variables), there were 128 (27) possible response profiles, where each cell was scored as a responder or nonresponder to each challenge, given a threshold response for scoring. Of the 128 groups possible with 7 unique inputs, 75 were observed. Of these, 35 were significant, according to the permutation test at a threshold <0.01. The amount of response for the application of K+/GABA was estimated as the ratio of peak height at that application to the average of the two previous peak heights in response to K+ without GABA.
Peptide synthesis.
Peptide sequences for conantokins from Conus rolani cDNA were created using a standard, solid-phase N-(9-fluorenyl) methoxycarbonyl (FMOC) protocol on an Apex 396 automated peptide synthesizer (AAPPTec, Louisville, KY). Methods for peptide synthesis have been described previously (Gowd et al. 2012). Briefly, peptides were constructed on a commercial FMOC-l-Asn (Trt)-RinkAmide 4-methylbenzhydrylamine resin from Peptides International (Louisville, KY), using side-chain protection and coupling activation steps. Coupling reactions were performed for 60 min with 10-fold excess amino acid, except for the nonstandard amino acid γ-carboxyglutamate (Gla), for which coupling reactions were 90 min with threefold excess Gla. Decoupling was performed for 20 min with 20% piperidine in dimethylformamide, and cleavage from resin was performed with a 3-h treatment with Reagent K. Peptide products were filtered and precipitated in ice-cold methyl tert-butyl ether. Crude peptides were ultimately purified by reverse-phase HPLC using a preparative C18 Vydac column and eluted with a linear gradient from 0.1% trifluoroacetic acid (TFA) in water to 0.1% TFA in 90% acetonitrile. Purity of peptides was assessed with an analytical C18 Vydac column. The molecular masses of purified peptides were determined by electrospray ionization mass spectrometry at the University of Utah Mass Spectrometry Core Facility.
RESULTS
Cluster analysis to define neuronal subclasses with receptor-agonist and membrane-potential challenges.
Receptor-agonist and membrane-potential challenges were used to investigate the combinations of glutamate, glycine, acetylcholine, and GABA receptors and voltage-gated ion channels expressed in cultured cerebellar neurons from 7- to 8-day-old mice. Glutamate receptor agonists (glutamate, NMDA/d-serine, and glycine) and acetylcholine receptor agonists (nicotine, acetylcholine, and carbamylcholine) were used to examine expression of a subset of the ligand-gated ion channels. In cells expressing receptors for these ligands, agonists elicited an increase in intracellular calcium that was indicated by a positive deflection of the 340:380 nm ratio. Neurons were additionally stimulated with membrane depolarization or membrane depolarization in the presence of GABA. Cells that expressed voltage-gated calcium channels responded with calcium influx. In most cells, GABA inhibited the calcium influx elicited by depolarization. GABAA receptors have an inhibitory influence on neurons by opening a chloride-permeable channel, which hyperpolarizes neurons and would be expected to counteract a membrane depolarization.
The seven different receptor-agonist and membrane-potential challenges shown in Fig. 1 were used for a cluster analysis to estimate the number of unique cell subclasses in the cerebellum, as described in methods (the response to K+/GABA was not used as a variable for cluster analysis). With seven different challenges (variables), there were 128 (27) possible response profiles, where each cell was scored as a responder or nonresponder to each challenge, given a threshold response for scoring. Of 128 possible response profiles, 75 unique response profiles were actually observed. Of these, 35 were significant, according to a permutation test at a threshold <0.01, as described in methods. These 35 unique response profiles may be considered an estimate of the lower bound for the number of discrete cell subclasses present in the cerebellum.
Figure 1 shows the 11 most frequently encountered response profiles from experiments that used the 7 receptor-agonist and membrane-potential challenges described above. These represent the majority of cells found in the cerebellum of a 7-day-old mouse. In Fig. 1, four calcium-imaging traces are shown as representative responses for cells in each subclass; each trace represents a recording from an individual cell. Figure 1 additionally shows the percentage of the total cerebellar cell population assigned to each subclass.
Differences among cellular subclasses.
Subclass 1, representing the most frequently observed subclass, comprises cells that responded to NMDA and depolarization, and the cells in this subclass accounted for ∼20% of all cells. By comparison, neurons assigned to the second most frequent subclass responded to NMDA, glutamate, and depolarization and accounted for ∼11% of all cells. Interestingly, subclasses 1 and 2 exhibited characteristics consistent with granule cells. These neurons were detected at a high frequency, expressed NMDA receptors, and were predominantly small cells. These results are consistent with previous findings that cerebellar granule cells express NMDA receptors, are the most abundant neurons of the cerebellum, and are the smallest neurons of the cerebellum (Farrant et al. 1994; Goldowitz and Hamre 1998).
Subclass 3 cells responded only to depolarization and comprised ∼9% of the cell population. Presumably, these cells express other ligand-gated receptors for agonists not tested in these experiments. Subclass 4 cells responded only to NMDA and encompassed ∼4% of the cells. Cells that did not respond to depolarization are likely to be glia. Subclass 5 cells responded to acetylcholine, carbamylcholine, and depolarization, whereas subclass 6 cells responded to the same cholinergic agonists but did not respond to depolarization. These subclasses each encompassed ∼3% of the cell population.
Subclass 7 cells responded to all acetylcholine receptor agonists and to depolarization and accounted for ∼2% of cells. Subclass 8, ∼2% of all cells, responded to all acetylcholine receptor agonists but not to depolarization and may also be glia. Subclass 9 responded to NMDA, acetylcholine, and depolarization, accounting for ∼2% of cells.
Subclass 10 cells responded to NMDA, glutamate, glycine, and depolarization, also accounting for ∼2% of cells. Intriguingly, subclass 10 responded directly to glycine. Glycine receptors generally hyperpolarize neurons (Dutertre et al. 2012). Because glycine-responsive cells always responded to NMDA as well, we presume that these signals are derived from NMDA receptor subtypes that are sensitive to glycine stimulation. NMDA receptors built from NR12/NR32 subunits are activated by glycine and are resistant to blockade by magnesium ions (Henson et al. 2010; Madry et al. 2007; Smothers and Woodward 2007; Yao et al. 2008). Subclass 11 was unique by responding to glutamate and to depolarization but not to NMDA.
In addition to the cell subclasses presented in Fig. 1, we observed cells that did not respond to any compound or to depolarization. The nonresponding cells were 28% of all cells and may represent glia or dead neurons. Additional, unique response patterns were observed at lower frequencies; these were excluded from Fig. 1 but may still contain additional subclasses of interest for future studies.
A summary of the binary scoring for the 11 most frequent cell subclasses observed in the cerebellum is shown in Table 1. Interestingly, all cell subclasses that responded to depolarizing stimuli were sensitive to inhibition by GABA. Subclass 11 was the most sensitive to GABA, showing a 60% average inhibition of response to depolarization in the presence of GABA. Subclass 7 was the least sensitive to GABA, with an average inhibition of 18%. Several cell subclasses also had different average cell sizes (cross-sectional area of cell soma). The most significant difference was observed in subclass 11, which had a larger average size (106 μm2) than any of the other cell subclasses. Modest size differences were observed in other subclasses, with average sizes ranging from 55 to 68 μm2.
Table 1.
Summary of binary scoring for subclasses of cerebellar neurons and glia (corresponding to Fig. 1)
| Subclass | Count | NMDA | Glu | Gly | ACh | Nic | Carb | K+ | GABA Block [SE] | Size, μm2 [SE] |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1161 | + | + | 0.33 [0.01] | 59 [0.76] | |||||
| 2 | 623 | + | + | + | 0.44 [0.01] | 59 [1.1] | ||||
| 3 | 498 | + | 0.25 [0.01] | 60 [1.2] | ||||||
| 4 | 217 | + | NA | 60 [1.7] | ||||||
| 5 | 177 | + | + | + | 0.23 [0.02] | 56 [2.0] | ||||
| 6 | 166 | + | + | NA | 63 [2.4] | |||||
| 7 | 120 | + | + | + | + | 0.18 [0.03] | 55 [2.4] | |||
| 8 | 116 | + | + | + | NA | 62 [2.9] | ||||
| 9 | 113 | + | + | + | 0.27 [0.03] | 57 [2.7] | ||||
| 10 | 110 | + | + | + | + | 0.20 [0.03] | 68 [2.1] | |||
| 11 | 106 | + | + | 0.60 [0.03] | 106 [7.8] | |||||
| NR | 1615 | |||||||||
| MISC | 711 | |||||||||
| Total | 5825 |
Cellular subclasses that exhibited a response above the threshold to each of the pharmacological challenges are marked with a plus sign. The average fractional block caused by GABA (compared with the response to high K+ in the absence of GABA) and the mean cell size (cross-sectional cell area) in each cellular subclass are shown with SE values in brackets. Ligand concentrations of the pharmacological agents are the following: NMDA, 200 μM N-methyl-d-aspartate (NMDA) with 20 μM d-serine in magnesium-free buffer; Glu, 300 μM glutamate; Gly, 200 μM glycine; ACh, 1 mM acetylcholine; Nic, 200 μM nicotine; Carb, 1 mM carbamylcholine; K+, 30 mM potassium. NA, not applicable, because these cells did not respond to depolarization with high K+ in the absence of GABA; NR, cells nonresponsive to any of the pharmacological challenges; MISC, miscellaneous.
Identifying glutamate receptor subtypes.
To define the molecular source of the glutamate response in cells belonging to subclasses 2, 10, and 11, we used the selective glutamate receptor agonist AMPA, which activates AMPA-type glutamate receptors but not kainate nor NMDA-type glutamate receptors (Keinanen et al. 1990; Lees 2000). The application of AMPA revealed that most glutamate responses occurred through AMPA receptors in our cerebellar cell cultures.
Figure 2 shows that AMPA activated most cells that responded to glutamate and caused a similar magnitude response as that elicited by glutamate. Figure 2A shows traces from individual neurons that illustrate a diversity of AMPA-responsive cells that belong to subclass 2 of Fig. 1. Furthermore, some cells responded to glutamate and not AMPA (Fig. 2B); these cells were very low frequency. The glutamate-responsive cells that were not stimulated by AMPA presumably express kainate-type glutamate receptors or metabotropic glutamate receptors that stimulate intracellular calcium release.
Identifying acetylcholine receptor subtypes.
Some acetylcholine-responsive cells responded to all three cholinergic agonists (see subclasses 7 and 8 of Fig. 1), whereas others responded to acetylcholine and carbamylcholine only (see subclasses 5 and 6 of Fig. 1). Because carbamylcholine has mixed agonist activity on nicotinic and muscarinic acetylcholine receptors (Jensen et al. 2003), we hypothesized that cells responding to acetylcholine and carbamylcholine, but not to nicotine, were expressing muscarinic receptors and not expressing nicotinic receptors. To test this hypothesis, we used atropine, a selective antagonist of muscarinic acetylcholine receptors (Giachetti 1986), and calcium-free (Ca2+-Free) bath solution.
Figure 3 depicts the results of these experiments. Figure 3A shows cells that responded to acetylcholine, nicotine, and carbamylcholine and had diminished response to acetylcholine in the presence of atropine. Figure 3B shows cells that responded to acetylcholine and carbamylcholine, and the acetylcholine response was completely blocked by atropine. Figure 3C shows traces from cells that were acetylcholine, nicotine, and carbamylcholine responsive that had a diminished response to acetylcholine in Ca2+-Free media. Figure 3D shows traces from cells that responded to acetylcholine and carbamylcholine and responded fully to the application of acetylcholine in Ca2+-Free solution. From these results, we conclude that cells in Fig. 3, A and C, representing cells in subclasses 7 and 8 of Fig. 1, express a mixture of nicotinic and muscarinic acetylcholine receptors, and cells in Fig. 3, B and D, representing cells in subclasses 5 and 6 of Fig. 1, predominantly express muscarinic acetylcholine receptors.
PNU reveals expression of α7 nicotinic acetylcholine receptors.
The α7 nicotinic acetylcholine receptors exhibit fast desensitization to acetylcholine (Wang and Sun 2005), and calcium signals from α7 receptors are not expected to be seen in standard calcium imaging protocols, as reported previously (Smith et al. 2013). However, calcium signals from α7 receptors can be observed upon application of acetylcholine in the presence of PNU, which positively modulates α7 receptors (Barron et al. 2009; Hurst et al. 2005; Smith et al. 2013). We applied PNU to reveal expression of α7 nicotinic acetylcholine receptors in cerebellar neurons, and the results revealed expression of α7 acetylcholine receptors in neurons that express other acetylcholine receptor subtypes and in neurons that previously exhibited no response to acetylcholine.
Foremost, PNU revealed acetylcholine responses in a subset of cells from subclass 1 (Fig. 4A). Subclass 1 cells previously responded to NMDA and depolarization but did not show any response to acetylcholine. Consistent with the working hypothesis that subclass 1 cells are granule cells, α7 receptors have been shown to be present at low levels in the granule cell layer, and nicotine alleviates ethanol-induced toxicity in cultured granule cells (Caruncho et al. 1997; Fucile et al. 2004; Tizabi et al. 2003).
PNU also amplified acetylcholine responses in cells that responded to acetylcholine in the absence of PNU (Fig. 4B). This indicated the coexpression of two or more acetylcholine receptors in a subset of acetylcholine-responsive cells. Finally, PNU revealed α7 expression in cells that did not respond to any of the other agonists tested (Fig. 4C), indicating a cell subclass that expresses α7 nicotinic receptors but lacks other subtypes of acetylcholine or glutamate receptors. Some of these cells would comprise a subset of subclass 3 cells in Fig. 1.
Conantokin peptides elucidate NMDA receptor subtypes.
Peptide toxins from venomous cone snails are often highly selective pharmacological tools that can be used to identify specific receptor subtypes. One family of peptides found in the venoms of cone snails is conantokins, which inhibit NMDA receptors and exhibit subtype selectivity (Castellino and Prorok 2000; Layer et al. 2004; Lewis et al. 2012). We used conantokins to identify the NMDA receptor composition in cultured cerebellar neurons that responded to NMDA. The NR2B-selective conRl-B and the NR2A- and NR2B-inhibiting conantokin R (conR) were incorporated for this purpose (Blandl et al. 2001; Gowd et al. 2012; White et al. 2000).
Figure 5 shows the results of a titration with conRl-B that revealed NR2B-mediated NMDA signaling in neurons of the cerebellum. In this experiment, all NMDA-responsive neurons were sensitive to inhibition by conRl-B. Figure 5A shows example traces from single cells in this titration experiment. Normalized dose-inhibition curves were plotted for NMDA-responsive cells and are shown in Fig. 5B. Figure 5C shows the histogram of calculated IC50 values for all NMDA-responsive cells.
Fig. 5.
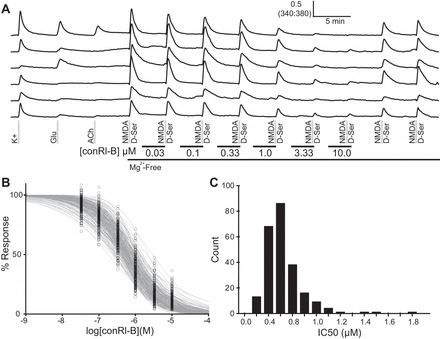
Inhibition of NMDA response with conantokin Rl-B (conRl-B) indicates functional expression of NR2B-containing NMDA receptors in cerebellar neurons. A: representative traces illustrating the inhibition of NMDA-stimulated calcium signals by conRl-B at various concentrations. B: dose-inhibition curves plotted for individual neurons illustrate the effect of conRl-B on NMDA receptors expressed in live neurons. C: the distribution of IC50 values for cerebellar neurons calculated from a curve fit using the Hill equation for normalized data.
The mean IC50 value for inhibition by conRl-B was 0.64 ± 0.31 μM. This value is higher than the 0.1 μM IC50 value determined for conRl-B, acting on heterologously expressed NR1/NR2B NMDA receptors in oocytes (Gowd et al. 2012). One explanation for the higher IC50 value observed in cultured cerebellar neurons is that other NMDA receptor subtypes are also expressed. In situ hybridization studies of the mouse cerebellum have shown expression of NR2B and NR2A type NMDA receptor subunits in the cerebellum at this stage (P7) of development (Farrant et al. 1994; Takahashi et al. 1996; Watanabe et al. 1994).
To determine whether NR2A subunits were also expressed in cultured cerebellar neurons, we combined two conantokin peptides with differing subtype selectivities. The NR2B-selective conRl-B was used in combination with the NR2A- and NR2B-selective conR; these peptides have similar IC50 values on NR2B receptor subtypes (Gowd et al. 2012; White et al. 2000). Figure 6 depicts representative traces from cells in this experiment that illustrate a spectrum of NMDA receptor expression characteristics. A majority of cells was equally inhibited by conRl-B and conR, indicating predominant expression of NR2B NMDA receptor subtypes. Traces representative of these cells are shown (Fig. 6, bottom). However, a smaller subset of cells showed stronger inhibition with conR than with conRl-B, indicating that these cells express a mixture of NR2A and NR2B NMDA receptor subtypes; these cells are also depicted (Fig. 6, top).
Reporter mice enable identification of Purkinje neurons.
A drawback of constellation pharmacology is that identification of morphologically distinct cell subclasses (within intact tissues) is difficult in cell culture. For this reason, we explored the use of genetically labeled neuronal cell subclasses from reporter mice. The Cre-driver mouse, B6.129-Tg(Pcp2-cre)2Mpin/J, expresses Cre recombinase under the control of the Pcp2 promoter, which was originally reported to drive gene expression exclusively in Purkinje neurons (Barski et al. 2000) but was later reported to drive gene expression in a somewhat broader set of cells throughout the brain (Madisen et al. 2010). We crossed this mouse with the Ai9 reporter mouse, which expresses tdTomato from the Rosa26 locus after excision of a floxed stop cassette by Cre recombinase (Madisen et al. 2010). This mating resulted in progeny that expressed tdTomato in large-diameter Purkinje neurons and a few other morphologically distinct cells, as demonstrated previously (Madisen et al. 2010).
Figure 7 shows the results of a constellation pharmacology experiment with genetically labeled Purkinje neurons that expressed tdTomato. Figure 7, A and B, depicts a cerebellar slice from a 12-day-old mouse, illustrating expression of tdTomato in the Purkinje neuron monolayer. Figure 7C depicts neurons expressing tdTomato in a culture prepared from the cerebellum of a 7-day-old mouse for constellation pharmacology. Figure 7D illustrates two large fluorescent neurons that responded to glutamate and depolarization. These are the Purkinje neurons that have been shown to express AMPA receptors but not NMDA receptors (Farrant and Cull-Candy 1991; Hartmann and Konnerth 2005; Lambolez et al. 1992). Figure 7D additionally highlights a large neuron that was not fluorescent but exhibited the same pharmacological profile. We presume this is also a Purkinje neuron that had not started expressing Cre recombinase or tdTomato. [The expression of Cre recombinase from the Pcp2 promoter begins at P6 and is not fully developed until P21 (Barski et al. 2000).] Figure 7E depicts a small, morphologically distinct fluorescent neuron that exhibited a different pharmacological profile from the cells in Fig. 7D. Some fluorescent cells were observed in the granule cell layer, and this cell exhibits a pharmacological profile consistent with granule cells. Some Cre-recombinase activity has been observed in non-Purkinje cells previously (Barski et al. 2000; Madisen et al. 2010). Because gene expression in cultured neurons may be different from intact brain, we compared the response profiles of genetically labeled Purkinje neurons after 4 and 24 h in culture. At these two time points, Purkinje neurons shared a common response profile, suggesting that any potential drift in gene expression in culture is likely to be gradual.
Fig. 7.
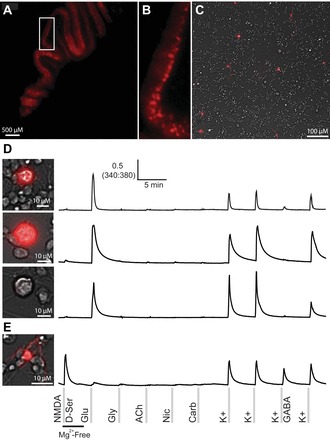
Reporter mice, with genetically labeled neurons expressing tdTomato, facilitated identification of Purkinje neurons in culture. Red fluorescence of the Purkinje cell monolayer from a postnatal day 12 (P12) mouse is shown (A and B). Fluorescent neurons in a culture of cerebellar cells (overlaid on a brightfield image) from a P7 mouse are shown (C). D: three different Purkinje neurons and their response profiles. Purkinje neurons were large and round, brightly fluorescent, and responded to glutamate and depolarization. However, not all Purkinje neurons were labeled at P7. The genetic labeling of Purkinje neurons has been reported to be complete by P21. This also shows a large and round nonfluorescent cell with the same profile as the fluorescent cells. E: a small fluorescent cell with a different morphology in culture and a different response profile that was consistent with granule cells.
Ultimately, we were able to identify Purkinje neurons using a combination of tdTomato expression, relatively large cell size with round soma, and a distinctive functional profile (i.e., expression of AMPA but not NMDA receptors). The Purkinje cells are found in subclass 11 of Fig. 1. Multiple pieces of evidence corroborate our identification of subclass 11 as Purkinje neurons. For example, these neurons were of relatively low frequency, as expected, and were significantly larger than cells in any other cell subclasses, as shown in Fig. 1 and Table 1. Additionally, GABA significantly inhibited these neurons, and our results indicate the expression of AMPA receptors, GABA receptors, and voltage-gated ion channels in Purkinje neurons.
We demonstrated the relatedness of cell subclasses using a heat map that grouped subclasses with common response profiles. The cellular subclasses depicted in the heat map of Fig. 8 correspond to the same subclasses in Fig. 1 and Table 1. In Fig. 8, subclasses 1, 2, 9, and 10 were grouped; cells in these subclasses shared common properties of NMDA response, K+ response, and small cell areas. We propose that subclasses 1 and 2 are granule cells, and this analysis suggests that subclasses 9 and 10 may also be granule cells. Subclasses 3–8 were also grouped, including four subclasses (5–8) that responded to acetylcholine receptor agonists. Subclasses 4, 6, and 8 are likely to be glial cells, because they did not respond to depolarization. The categorization of subclasses 3, 5, and 7 as neuronal or glial cells is presently ambiguous, because they responded relatively weakly to depolarization. Notably, the Purkinje cells of subclass 11 were found to be distinct from other subclasses as large cells that responded to glutamate but not NMDA. These results demonstrate that not only can we identify distinct neuronal subclasses in culture, but also, we can investigate how those subclasses are related to each other.
Fig. 8.
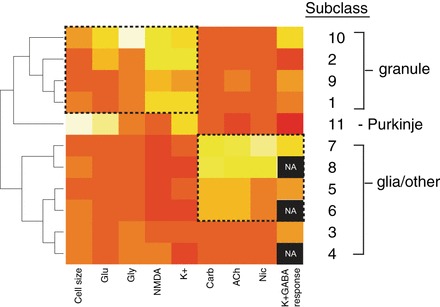
Heat map of mean values for 11 cerebellar cellular subclasses presented in Table 1. The color scale varies from red (low) to yellow (high). For cell size, yellow represents an average large cell area and red, a small cell area. For each pharmacological challenge, yellow represents an average large response and red, a small response. Black represents data that are not available (NA), because the corresponding cells did not respond to K+ depolarization. Hence, their K+ response could not be blocked by GABA. Cellular subclasses have been clustered and ordered according to the similarities in mean values for each variable. Subclasses 1 and 2, proposed to be granule cells, cluster with subclasses 9 and 10, which may represent additional granule cell subclasses. Subclass 11 is comprised of the Purkinje neurons that were identified by genetic labeling from reporter mice, cell morphology, and a distinctive functional profile. Additional cellular subclasses that did not respond to depolarization (4, 6, and 8) are likely to be glial cells and have clustered with other acetylcholine-responsive cellular subclasses at the bottom of the heat map. The categorization of subclasses 3, 5, and 7 as neuronal or glial cells is ambiguous, because they responded relatively weakly to depolarization.
DISCUSSION
The cerebellum is responsible for the coordination and timing of muscle contractions and is known to play various roles in higher-order cognitive functions (Akshoomoff and Courchesne 1992; Del Olmo et al. 2007; Deluca et al. 2014; Stein 1986). Neuronal subclasses in the cerebellum were originally defined by connectivity and morphology (Addison 1911; Braitenberg and Atwood 1958; Snider 1940), and more recently, these subclasses have been studied with electrophysiology (Hockberger et al. 1987; Llinas and Sugimori 1980; Uusisaari et al. 2007) and immunohistochemistry (Ashton et al. 2006; Baimbridge and Miller 1982; Saito et al. 1974). These studies have led to the identification of several cellular subclasses, including granule, Purkinje, basket, stellate, and Golgi cells, among others. Two of the most thoroughly studied subclasses are the granule and the Purkinje cells. Granule cells are small and numerous and comprise a majority of cells in the cerebellum (Goldowitz and Hamre 1998). In contrast, Purkinje cells are large, and relatively few yet have critical roles in cerebellar development and physiology (Goldowitz and Hamre 1998).
Here, we used constellation pharmacology to survey neuronal diversity in the cerebellum. In Fig. 1 and Table 1, we highlighted 11 unique cell subclasses that were defined by functional expression of glutamate, acetylcholine, and GABA receptors and voltage-gated ion channels. Additional experiments revealed that many of these cell subclasses could be subdivided with additional pharmacology. In addition to the 11 cell subclasses highlighted in Fig. 1 and Table 1, cluster analysis suggested that there may be >35 unique cellular subclasses in the cerebellum.
Certain cell subclasses defined in this work show consistencies with known cell subclasses in the cerebellum. For example, granule cells are known to be the most abundant cells of the cerebellum and the smallest cells of the cerebellum and express NMDA receptors (Akazawa et al. 1994; Burgoyne and Cambray-Deakin 1988; Cathala et al. 2000; Gray 1961; Komuro and Yacubova 2003). Consistent with granule cells, subclasses 1 and 2 were abundant in cerebellar cell culture, were some of the smallest cells, and expressed NMDA receptors. Therefore, we infer that these cell subclasses are granule cell neurons.
Additional cellular subclasses from our experiments showed correlational evidence of being granule cells. For example, cell subclasses 9 and 10 were grouped with subclasses 1 and 2 during the heat map analysis. All of these subclasses have relatively small cell areas and expressed NMDA receptors, in addition to other receptors. The results suggest that at least four granule cell subclasses may be present in the cerebellum. However, it could also be the case that these cells represent different maturational stages of the granule cells. Nonetheless, it was shown previously that some granule cells in cerebellar lobules 9 and 10 express muscarinic acetylcholine receptors (Takayasu et al. 2003; Vilaró et al. 1992); consistent with those studies, our results show that subclass 9 is a lower-frequency cell that was responsive to NMDA and acetylcholine and was found to group with other putative granule cells by cluster analysis (see Figs. 1 and 8).
Synapse formation or gene-expression changes during cell culture could confound classification of neuronal cells through constellation pharmacology. Although we cannot rule out the potential for synapse formation and gene-expression changes, several lines of evidence suggest that synapses are not distorting the classification scheme. First, when cerebellar neurons are plated at lower densities, where synapse formation is less probable, cell subclasses and their frequencies are not altered significantly. Second, the fluorescent Purkinje neurons in our experiments shared a common functional profile, after 4 and 24 h in culture, indicating that the observed response profiles were not altered by synaptic input or by obvious changes in gene expression. Third, we believe that the short culture times (<24 h) make functional synapse formation unlikely. These observations suggest that our results reflect the diversity of individual cells in the intact cerebellum. Nonetheless, we will continue to investigate the potential influence of synapse formation and receptor expression changes in short-term cultures.
We believe that cell bodies in culture accurately reflect the transmembrane proteins expressed at synapses in the brain. Previous studies have shown that receptors and ion channels expressed in synapses and on axons are also expressed on cell bodies (Black et al. 2012; Jeffry et al. 2009). Additionally, our results suggest that cell bodies studied in culture truthfully represent neurons of the brain. For example, Purkinje neurons are known to express AMPA and GABA receptors on dendrites (Farrant and Cull-Candy 1991; Fritschy et al. 1992; Hartmann and Konnerth 2005; He et al. 2015; Lambolez et al. 1992; Pirker et al. 2000), and we have identified subclass 11 (see Fig. 1) as Purkinje neurons in culture, which lacked dendrites and were shown to express AMPA and GABA receptors on the cell body. Therefore, we believe that results obtained through constellation pharmacology provide a realistic reflection of the cellular subclasses found in the intact cerebellum, but these results will ultimately need to be verified in the intact brain.
Selective pharmacology demonstrated that a subset of subclass 1 cells expresses α7-type nicotinic acetylcholine receptors (see Fig. 4), which indicates that cell subclasses can be subdivided further. Continued studies with constellation pharmacology will enable a comprehensive characterization of agonist receptors and voltage-gated channels expressed in neuronal subclasses of the cerebellum. This study focused on glutamate, acetylcholine, and GABA, because these are well-documented neurotransmitters in the cerebellum (Ottersen and Walberg 2002). However, serotonin, epinephrine, homocysteic acid, and several peptides are also shown to contribute to cerebellar neurotransmission (Ottersen and Walberg 2002). Experiments that examine glutamatergic, cholinergic, GABAergic, serotonergic, histaminergic, and peptidergic receptor expression should reveal additional neuronal subclasses and disclose the constellations of ion channels expressed in those subclasses.
Perhaps the most important advance presented here is the combination of constellation pharmacology with genetically labeled neuronal subclasses. Although we have previously published several research articles that describe constellation pharmacology (Raghuraman et al. 2014; Smith et al. 2013; Teichert et al. 2012a, b, 2014, 2015), we demonstrate in this study the combination of constellation pharmacology and genetically labeled cells for the first time. We believe that this combination of methods is a powerful approach for the identification and classification of neuronal and glial cell subclasses. In this particular case, we identified Purkinje neurons by their fluorescent label (expression of tdTomato), large size relative to other cells in the cerebellum, and functional profile that was consistent with previous reports (i.e., expression of AMPA and GABA receptors but not NMDA receptors). Although we have not yet identified all neuronal subclasses in the cerebellum, including the interneurons, we anticipate that the extension of genetic labeling and profiling techniques to focus on these cells will facilitate their characterization. Our current results thus enable us to continue to investigate ion channel expression in individual Purkinje cells using constellation pharmacology and to track gene expression in Purkinje neurons through development or in disease models. We believe this general experimental approach—the combination of genetically labeled cells and constellation pharmacology—can and should be applied broadly to identify and classify neuronal and glial cell subclasses throughout the nervous system.
GRANTS
Support for this work was provided by the National Institute of General Medical Science (Grant GM48677).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.J.C., B.M.O., and R.W.T. conception and design of research; K.J.C., L.S.L., and S.R. performed experiments; K.J.C., L.S.L., and K.C. analyzed data; K.J.C., L.S.L., B.M.O., and R.W.T. interpreted results of experiments; K.J.C. and L.S.L. prepared figures; K.J.C. drafted manuscript; K.J.C., K.C., S.R., M.P.H., B.M.O., and R.W.T. edited and revised manuscript; K.J.C., L.S.L., M.P.H., B.M.O., and R.W.T. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge Sam Espino and Tosifa Memon for assistance with mouse cell preparations and technical aspects of constellation pharmacology. The authors thank Tyler Harris for assistance in performing experiments, Peter Huynh for reading and providing comments on early drafts of the manuscript, and Dr. John White for providing the Ai9 mice used in these experiments and for technical help with breeding. The authors also thank Joanna Gajewiak for synthesizing the peptides used in this study.
REFERENCES
- Addison WH. The development of the Purkinje cells and of the cortical layers in the cerebellum of the albino rat. J Comp Neurol 21: 459–487, 1911. [Google Scholar]
- Akazawa C, Shigemoto R, Bessho Y, Nakanishi S, Mizuno N. Differential expression of five N-methyl-d-aspartate receptor subunit mRNAs in the cerebellum of developing and adult rats. J Comp Neurol 347: 150–160, 1994. [DOI] [PubMed] [Google Scholar]
- Akshoomoff NA, Courchesne E. A new role for the cerebellum in cognitive operations. Behav Neurosci 106: 731–738, 1992. [DOI] [PubMed] [Google Scholar]
- Ashton JC, Friberg D, Darlington CL, Smith PF. Expression of the cannabinoid CB2 receptor in the rat cerebellum: an immunohistochemical study. Neurosci Lett 396: 112–116, 2006. [DOI] [PubMed] [Google Scholar]
- Baimbridge KG, Miller JJ. Immunohistochemical localization of calcium-binding protein in the cerebellum, hippocampal formation and olfactory bulb of the rat. Brain Res 245: 223–229, 1982. [DOI] [PubMed] [Google Scholar]
- Barron SC, McLaughlin JT, See JA, Richards VL, Rosenberg RL. An allosteric modulator of alpha7 nicotinic receptors, N-(5-chloro-2, 4-dimethoxyphenyl)-N′-(5-methyl-3-isoxazolyl)-urea (PNU-120596), causes conformational changes in the extracellular ligand binding domain similar to those caused by acetylcholine. Mol Pharmacol 76: 253–263, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barski JJ, Dethleffsen K, Meyer M. Cre recombinase expression in cerebellar Purkinje cells. Genesis 28: 93–98, 2000. [PubMed] [Google Scholar]
- Bernard A, Sorensen SA, Lein ES. Shifting the paradigm: new approaches for characterizing and classifying neurons. Curr Opin Neurobiol 19: 530–536, 2009. [DOI] [PubMed] [Google Scholar]
- Black JA, Frézel N, Dib-Hajj SD, Waxman SG. Expression of Nav1.7 in DRG neurons extends from peripheral terminals in the skin to central preterminal branches and terminals in the dorsal horn. Mol Pain 8: 82, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandl T, Zajicek J, Prorok M, Castellino FJ. Sequence requirements for the N-methyl-d-aspartate receptor antagonist activity of conantokin-R. J Biol Chem 276: 7391–7396, 2001. [DOI] [PubMed] [Google Scholar]
- Braitenberg V, Atwood RP. Morphological observations on the cerebellar cortex. J Comp Neurol 109: 1–33, 1958. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, Cambray-Deakin MA. The cellular neurobiology of neuronal development: the cerebellar granule cell. Brain Res Rev 13: 77–101, 1988. [DOI] [PubMed] [Google Scholar]
- Caruncho HJ, Guidotti A, Lindstrom J, Costa E, Pesold C. Subcellular localization of the α7 nicotinic receptor in rat cerebellar granule cell layer. Neuroreport 8: 1431, 1997. [DOI] [PubMed] [Google Scholar]
- Castellino FJ, Prorok M. Conantokins: inhibitors of ion flow through the N-methyl-d-aspartate receptor channels. Curr Drug Targets 1: 219–235, 2000. [DOI] [PubMed] [Google Scholar]
- Cathala L, Misra C, Cull-Candy S. Developmental profile of the changing properties of NMDA receptors at cerebellar mossy fiber-granule cell synapses. J Neurosci 20: 5899–5905, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Olmo MF, Cheeran B, Koch G, Rothwell JC. Role of the cerebellum in externally paced rhythmic finger movements. J Neurophysiol 98: 145–152, 2007. [DOI] [PubMed] [Google Scholar]
- Deluca C, Golzar A, Santandrea E, Lo Gerfo E, Eštočinová J, Moretto G, Fiaschi A, Panzeri M, Mariotti C, Tinazzi M, Chelazzi L. The cerebellum and visual perceptual learning: evidence from a motion extrapolation task. Cortex 58: 52–71, 2014. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev 51: 7–62, 1999. [PubMed] [Google Scholar]
- Dutertre S, Becker CM, Betz H. Inhibitory glycine receptors: an update. J Biol Chem 287: 40216–40223, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Cull-Candy SG. Excitatory amino acid receptor-channels in Purkinje cells in thin cerebellar slices. Proc Biol Sci 244: 179–184, 1991. [DOI] [PubMed] [Google Scholar]
- Farrant M, Feldmeyer D, Takahashi T, Cull-Candy SG. NMDA-receptor channel diversity in the developing cerebellum. Nature 368: 335–339, 1994. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Benke D, Mertens S, Oertel WH, Bachi T, Möhler H. Five subtypes of type A gamma-aminobutyric acid receptors identified in neurons by double and triple immunofluorescence staining with subunit-specific antibodies. Proc Natl Acad Sci USA 89: 6726–6730, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucile S, Renzi M, Lauro C, Limatola C, Ciotti T, Eusebi F. Nicotinic cholinergic stimulation promotes survival and reduces motility of cultured rat cerebellar granule cells. Neuroscience 127: 53–61, 2004. [DOI] [PubMed] [Google Scholar]
- Giachetti A, Giraldo E, Ladinsky H, Montagna E. Binding and functional profiles of the selective M1 muscarinic receptor antagonists trihexyphenidyl and dicyclomine. Br J Pharmacol 89: 83–90, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb S, Strimmer K. MALDIquant: a versatile R package for the analysis of mass spectrometry data. Bioinformatics 28: 2270–2271, 2012. [DOI] [PubMed] [Google Scholar]
- Goldowitz D, Hamre K. The cells and molecules that make a cerebellum. Trends Neurosci 21: 375–382, 1998. [DOI] [PubMed] [Google Scholar]
- Gowd KH, Han TS, Twede V, Gajewiak J, Smith MD, Watkins M, Platt RJ, Toledo G, White HS, Olivera BM, Bulaj G. Conantokins derived from the Asprella clade impart conRl-B, an N-methyl d-aspartate receptor antagonist with a unique selectivity profile for NR2B subunits. Biochemistry 51: 4685–4692, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EG. The granule cells, mossy synapses and Purkinje spine synapses of the cerebellum: light and electron microscope observations. J Anat 95: 345, 1961. [PMC free article] [PubMed] [Google Scholar]
- Hartmann J, Konnerth A. Determinants of postsynaptic Ca2+ signaling in Purkinje neurons. Cell Calcium 37: 459–466, 2005. [DOI] [PubMed] [Google Scholar]
- He Q, Duguid I, Clark B, Panzanelli P, Patel B, Thomas P, Fritschy JM, Smart TG. Interneuron- and GABAA receptor-specific inhibitory synaptic plasticity in cerebellar Purkinje cells. Nat Commun 6: 7364, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson MA, Roberts AC, Pérez-Otaño I, Philpot BD. Influence of the NR3A subunit on NMDA receptor functions. Prog Neurobiol 91: 23–37, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer Associates, 2001. [Google Scholar]
- Hockberger PE, Tseng HY, Connor JA. Immunocytochemical and electrophysiological cerebellar granule cells in explant cultures. J Neurosci 7: 1370–1383, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst RS, Hajós M, Raggenbass M, Wall TM, Higdon NR, Lawson JA, Rutherford-Root KL, Berkenpas MB, Hoffmann WE, Piotrowski DW, Groppi VE, Allaman G, Ogier R, Bertrand S, Bertrand D, Arneric SP. A novel positive allosteric modulator of the α7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci 25: 4396–4405, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffry JA, Yu SQ, Sikand P, Parihar A, Evans MS, Premkumar LS. Selective targeting of TRPV1 expressing sensory nerve terminals in the spinal cord for long lasting analgesia. PloS One 4: e7021, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen AA, Mikkelsen I, Frølund B, Bräuner-Osborne H, Falch E, Krogsgaard-Larsen P. Carbamoylcholine homologs: novel and potent agonists at neuronal nicotinic acetylcholine receptors. Mol Pharmacol 64: 865–875, 2003. [DOI] [PubMed] [Google Scholar]
- Keinanen K, Wisden W, Sommer B, Werner P, Herb A, Verdoorn TA, Sakmann B, Seeburg PH. A family of AMPA-selective glutamate receptors. Science 249: 556–560, 1990. [DOI] [PubMed] [Google Scholar]
- Komuro H, Yacubova E. Recent advances in cerebellar granule cell migration. Cell Mol Life Sci 60: 1084–1098, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambolez B, Audinat E, Bochet P, Crépel F, Rossier J. AMPA receptor subunits expressed by single Purkinje cells. Neuron 9: 247–258, 1992. [DOI] [PubMed] [Google Scholar]
- Layer RT, Wagstaff JD, White HS. Conantokins: peptide antagonists of NMDA receptors. Curr Med Chem 11: 3073–3084, 2004. [DOI] [PubMed] [Google Scholar]
- Lees GJ. Pharmacology of AMPA/kainate receptor ligands and their therapeutic potential in neurological and psychiatric disorders. Drugs 59: 33–78, 2000. [DOI] [PubMed] [Google Scholar]
- Lewis RJ, Dutertre S, Vetter I, Christie MJ. Conus venom peptide pharmacology. Pharmacol Rev 64: 259–298, 2012. [DOI] [PubMed] [Google Scholar]
- Lipovsek M, Fierro A, Pérez EG, Boffi JC, Millar NS, Fuchs PA, Katz E, Elgoyhen AB. Tracking the molecular evolution of calcium permeability in a nicotinic acetylcholine receptor. Mol Biol Evol 31: 3250–3265, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol 305: 171–195, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13: 133–140, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madry C, Mesic I, Bartholomäus I, Nicke A, Betz H, Laube B. Principal role of NR3 subunits in NR1/NR3 excitatory glycine receptor function. Biochem Biophys Res Commun 354: 102–108, 2007. [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JRL, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci 8: 427–437, 2007. [DOI] [PubMed] [Google Scholar]
- Nelson SB, Sugino K, Hempel CM. The problem of neuronal cell types: a physiological genomics approach. Trends Neurosci 29: 339–345, 2006. [DOI] [PubMed] [Google Scholar]
- Ottersen OP, Walberg F. Neurotransmitters in the cerebellum. In: The Cerebellum and Its Disorders, edited by Manto MU, Pandolfo M. Cambridge, UK: Cambridge University Press, 2002, p. 38–48. [Google Scholar]
- Pankratov Y, Lalo U. Calcium permeability of ligand-gated Ca2+ channels. Eur J Pharmacol 739: 60–73, 2014. [DOI] [PubMed] [Google Scholar]
- Peters AJ, Jones EG. Classification of cortical neurons. In: Cerebral Cortex, Vol. 1. Cellular Components of the Cerebral Cortex. New York: Plenum, 1984. [Google Scholar]
- Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology 34: 1–26, 1995. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 101: 815–850, 2000. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2013. [Google Scholar]
- Raghuraman S, Garcia AJ, Anderson TM, Twede VD, Curtice KJ, Chase K, Ramirez JM, Olivera BM, Teichert RW. Defining modulatory inputs into CNS neuronal subclasses by functional pharmacological profiling. Proc Natl Acad Sci USA 111: 6449–6454, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon y Cajal S. Histology of the Nervous System of Man and Vertebrates. New York: Oxford University Press, 1995. [Google Scholar]
- Saito K, Barber R, Wu JY, Matsuda T, Roberts E, Vaughn JE. Immunohistochemical localization of glutamate decarboxylase in rat cerebellum. Proc Natl Acad Sci USA 71: 269–273, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NJ, Hone AJ, Memon T, Bossi S, Smith TE, McIntosh JM, Olivera BM, Teichert RW. Comparative functional expression of nAChR subtypes in rodent DRG neurons. Front Cell Neurosci 7: 225, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smothers CT, Woodward JJ. Pharmacological characterization of glycine-activated currents in HEK 293 cells expressing N-methyl-d-aspartate NR1 and NR3 subunits. J Pharmacol Exp Ther 322: 739–748, 2007. [DOI] [PubMed] [Google Scholar]
- Snider RS. Morphology of the cerebellar nuclei in the rabbit and cat. J Comp Neurol 72: 399–413, 1940. [Google Scholar]
- Sotelo C. Viewing the brain through the master hand of Ramon y Cajal. Nat Rev Neurosci 4: 71–77, 2003. [DOI] [PubMed] [Google Scholar]
- Stein JF. Role of the cerebellum in the visual guidance of movement. Nature 323: 217–221, 1986. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Feldmeyer D, Suzuki N, Onodera K, Cull-Candy SG, Sakimura K, Mishina M. Functional correlation of NMDA receptor epsilon subunits expression with the properties of single-channel and synaptic currents in the developing cerebellum. J Neurosci 16: 4376–4382, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayasu Y, Iino M, Furuya N, Ozawa S. Muscarine-induced increase in frequency of spontaneous EPSCs in Purkinje cells in the vestibulo-cerebellum of the rat. J Neurosci 23: 6200–6208, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichert RW, Memon T, Aman JW, Olivera BM. Using constellation pharmacology to define comprehensively a somatosensory neuronal subclass. Proc Natl Acad Sci USA 111: 2319–2324, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichert RW, Raghuraman S, Memon T, Cox JL, Foulkes T, Rivier JE, Olivera BM. Characterization of two neuronal subclasses through constellation pharmacology. Proc Natl Acad Sci USA 109: 12758–12763, 2012a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichert RW, Schmidt EW, Olivera BM. Constellation pharmacology: a new paradigm for drug discovery. Annu Rev Pharmacol Toxicol 55: 573–589, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichert RW, Smith NJ, Raghuraman S, Yoshikami D, Light AR, Olivera BM. Functional profiling of neurons through cellular neuropharmacology. Proc Natl Acad Sci USA 109: 1388–1395, 2012b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y, Al-Namaeh M, Manaye KF, Taylor RE. Protective effects of nicotine on ethanol-induced toxicity in cultured cerebellar granule cells. Neurotox Res 5: 315–321, 2003. [DOI] [PubMed] [Google Scholar]
- Uusisaari M, Obata K, Knöpfel T. Morphological and electrophysiological properties of GABAergic and non-GABAergic cells in the deep cerebellar nuclei. J Neurophysiol 97: 901–911, 2007. [DOI] [PubMed] [Google Scholar]
- Vilaró MT, Wiederhold KH, Palacios JM, Mengod G. Muscarinic M2 receptor mRNA expression and receptor binding in cholinergic and non-cholinergic cells in the rat brain: a correlative study using in situ hybridization histochemistry and receptor autoradiography. Neuroscience 47: 367–393, 1992. [DOI] [PubMed] [Google Scholar]
- Wang H, Sun X. Desensitized nicotinic receptors in brain. Brain Res Rev 48: 420–437, 2005. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Mishina M, Inoue Y. Distinct spatiotemporal expressions of five NMDA receptor channel subunit mRNAs in the cerebellum. J Comp Neurol 343: 513–519, 1994. [DOI] [PubMed] [Google Scholar]
- White HS, McCabe RT, Armstrong H, Donevan SD, Cruz LJ, Abogadie FC, Torres J, Rivier JE, Paarmann I, Hollmann M, Olivera BM. In vitro and in vivo characterization of conantokin-R, a selective NMDA receptor antagonist isolated from the venom of the fish-hunting snail Conus radiatus. J Pharmacol Exp Ther 292: 425–432, 2000. [PubMed] [Google Scholar]
- Wichterle H, Gifford D, Mazzoni E. Neuroscience. Mapping neuronal diversity one cell at a time. Science 341: 726–727, 2013. [DOI] [PubMed] [Google Scholar]
- Yao Y, Harrison CB, Freddolino PL, Schulten K, Mayer ML. Molecular mechanism of ligand recognition by NR3 subtype glutamate receptors. EMBO J 27: 2158–2170, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


