Abstract
Circulating tumor cells (CTCs) are a hallmark of invasive behavior of cancer, responsible for the development of metastasis. Their detection and analysis have significant impacts in cancer biology and clinical practice. However, CTCs are rare events and contain heterogeneous subpopulations, requiring highly sensitive and specific techniques to identify and capture CTCs with high efficiency. Nanotechnology shows strong promises for CTC enrichment and detection owning to the unique structural and functional properties of nanoscale materials. In this review, we discuss the CTC enrichment and detection technologies based on a variety of functional nanosystems and nanostructured substrates, with the goal to highlight the role of nanotechnology in the advancement of basic and clinical CTC research.
Keywords: : circulating tumor cells, detection, enrichment, functional nanoparticles, microfluidics, nanostructured substrates, nanotechnology
Metastasis is the major cause of death in cancer patients, accounting for about 90% of the mortality. Although the mechanism of metastasis is not fully understood, it is known that a mandatory step of the metastatic cascade is the transport of tumor cells that are shed from the primary tumor site throughout the bloodstream of cancer patients [1]. During transport, a small population (<0.01%) of these circulating tumor cells (CTCs) arrests in a capillary bed at a distant site where they extravagate and seed the growth of a secondary tumor. The clinical value of CTC detection remains to be learned, but many studies have shown their great potential [2]. It has been realized that detection and characterization of CTCs may provide a noninvasive liquid biopsy for characterizing and monitoring cancer [3]. The prognostic significance of CTC detection has been demonstrated in several types of cancers including breast, prostate, colon, melanoma and lung cancer [4–8]. CTCs are also useful in monitoring and predicting the response to ongoing therapy [9–11]. In addition, detection of CTCs shows strong promise for early cancer detection since they have been found in blood during early stages of tumorigenesis [12]. Furthermore, molecular profiling of CTCs may offer insights into mechanisms of cancer progression and provide new therapeutic targets [13].
CTC detection and analysis, however, are very challenging [14]. The major challenge is that CTCs are rare events, as few as one CTC mixed with about 10 million white blood cells (WBCs) and 5 billion red blood cells (RBCs) in 1 ml of blood of metastatic patients [15]. Another key challenge is that they are heterogeneous in population due to tumor heterogeneity and potential changes of molecular characteristics during the epithelial-to-mesenchymal transition (EMT) [16]. As a result, significant advancement in this area has only been made in the last two decades, even though CTCs were first discovered in 1869 [17]. To date, a vast number of isolation and detection techniques have been developed, with about 100 companies offering CTC-related products and devices and over 400 clinical trials ongoing [18]. However, there is only one technique that has been approved by The US FDA for clinical utilization, the CellSearch system (Veridex, LLC) [15]. This technique is used to enumerate CTCs in patients with metastatic breast, prostate and colorectal cancer to help inform clinical decision making [6].
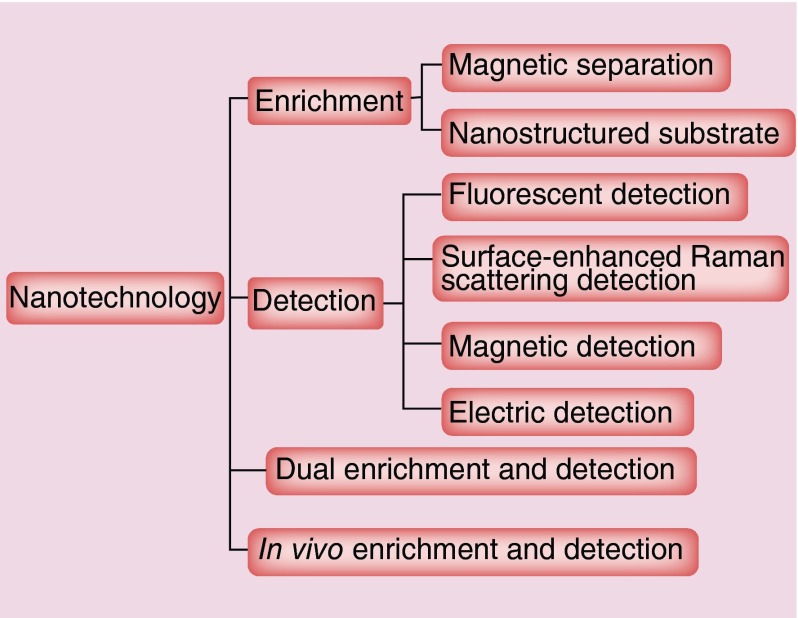
During the last two decades, the tremendous development of nanotechnology has led to the generation of a variety of advanced nanoscale materials, including metal, metal oxide, semiconductor and polymeric nanomaterials with a vast range of applications, such as in medicine [19], energy conversion and storage [20], electronics [21] and catalysis [22]. The fantasy of nanomaterials is driven by their exceptional structural and functional properties that are often not available from either bulk materials or discrete molecules due to the nanoscale effects. For example, gold nanoparticles (Au NPs) with near infrared absorption such as Au nanoshells, nanorods, nanocages and hollow nanospheres show about million times higher absorption coefficient than organic light-absorbing dye molecules, making them a new generation of photothermal agents to ablate tumor [23–27]. For CTC enrichment and detection, nanomaterials have been playing an important role, with boosted interests in recent years. The rationale is that when nanomaterials are linked with targeting ligands, they can recognize CTCs with high specificity, allowing for isolation, detection and characterization using the functional properties of the nanomaterials. In addition, nanomaterials have large surface-to-volume ratio, which enables highly efficient cellular binding in the complex blood matrix. Furthermore, nanomaterials can be readily manipulated to allow multiplexed detection and analysis, which are very important to address the heterogeneous problem of CTCs. In this review, we discuss recent progress on CTC enrichment and detection approaches using various nanoplatforms, with the goal to highlight the role of nanotechnology in the advancement of basic and clinical CTC research. These techniques are classified as ex vivo and in vivo methods, with the former category composed of enrichment, detection and emerging dual enrichment and detection methods (Figure 1).
Figure 1. . Nanotechnology applications in circulating tumor cells enrichment and detection.
CTC enrichment
Due to the rarity of CTCs, an initial step is often needed to isolate and enrich the tumor cells from blood cells. Current enrichment methods can be divided into two categories: those based on physical properties such as size, density and deformability, and those based on biological properties such as protein expressions [28]. Classic approaches in the former category are density gradient centrifugation, membrane filtration and microchip-based capture platforms. Approaches in the latter category include magnetic separation, substrate- and microchip-based capture platforms. The most commonly used marker for CTC recognition is EpCAM. Since epithelial cells are not usually found in circulation, the findings of EpCAM-positive cells indicate the presence of CTCs.
Magnetic nanoparticles
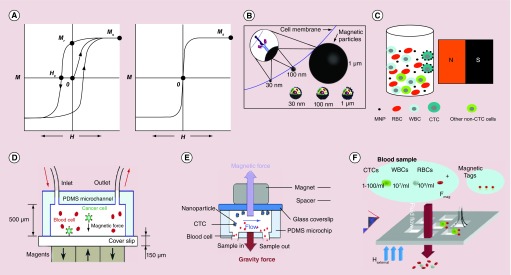
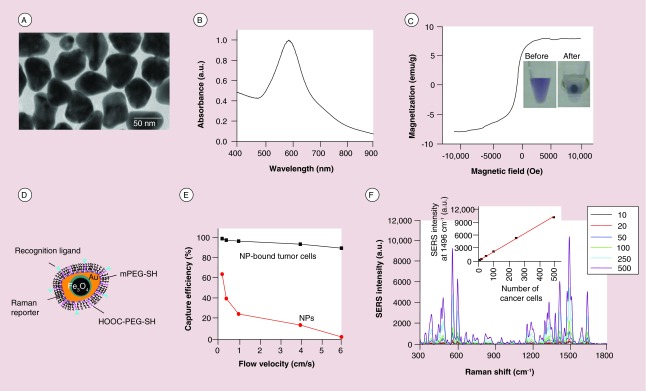
Magnetic nanoparticles (MNPs), commonly composed of magnetic elements such as iron (Fe) and cobalt (Co), show alignment of their magnetic moment in the presence of magnetic field. This magnetic alignment eventually pins down in the same direction of the external magnetic field under saturation [29]. Depending on the particle size, shape and composition, the magnetic response can be ferromagnetic or superparamagnetic (Figure 2A) [30]. Ferromagnetic NPs show a remnant magnetization after removal of the field, while superparamagnetic NPs do not have a remnant magnetization due to thermal fluctuations. The magnetic response causes the movement of the NPs in the direction of applied magnetic gradient and thus the MNPs can be separated from the resting solution.
Figure 2. . Magnetic nanoparticles for magnetic enrichment of circulating tumor cells.
(A) Ferromagnetic and superparamagnetic properties of MNPs. (B) Schematic of immunomagnetically labeled cell with particles of different diameters. (C) Schematic of bulk magnetic separation under a stationary condition. (D) The principle of microchip-based immunomagnetic enrichment in an upright mode. (E) The principle of microchip-based immunomagnetic enrichment in an inverted mode. (F) Magnetic shifter device comprising an array of magnetic pores for magnetic CTC filtration.
CTC: Circulating tumor cell; MNP: Magnetic nanoparticle; PDMS: Polydimethylsiloxane; RBC: Red blood cell; WBC: White blood cell.
(A) Reproduced with permission [30] © The Royal Society of Chemistry (2009); (B) Reprinted with permission from [32] © Elsevier; (D) Reproduced with permission from [55] © The Royal Society of Chemistry (2011); (E) Reproduced with permission from [56] © Springer (2013); (F) Reproduced with permission from [62] © The Royal Society of Chemistry (2014).
Magnetic separation using magnetic particles is one of the leading CTC enrichment methods [31]. This method is easy to manipulate and exhibits high capture efficiency and specificity. Captured cells can be easily recovered by removing the magnetic field. The particles can be either microbeads (>0.5 μm) that are generally made of polymeric matrix with embedded magnetic materials, or MNPs (5–200 nm). MNPs have several distinct advantages over microbeads. They have higher cellular binding capability and excellent stability in whole blood. The small size of NPs allows the ability to attach many NPs to a cell without cell aggregation resulting in higher magnetic susceptibility (Figure 2B) [32]. Furthermore, the NPs allow for multiplexed detection by using different sized NPs or NPs labeled with different detection tags.
Bulk magnetic separation
Classical magnetic separation is done with an external permanent magnet, usually neodymium-iron-boron (NdFeB) magnet, to separate MNP-bound CTCs in a bulk solution under a stationary condition (Figure 2C). Since the magnetic force is proportional to the number of bound NPs [33], the NP-bound cells are isolated much faster than free NPs in a solution under the same magnetic field and thereby selectively enriched. The FDA-approved CellSearch system uses this approach to enrich CTCs by using 120–200 nm Fe NPs (ferrofluid) linked with anti-EpCAM antibodies [34]. In combination with immunofluroescence detection targeting cytokeratin, the system reaches over 80 recovery rate of spiked breast cancer cells [35]. Although the CellSearch system is currently the gold standard to detect CTCs, a major limitation is that it only captures and detects EpCAM-positive cells. EpCAM expression is often heterogeneous in cancer cells and its downregulation has also been correlated with CTCs in peripheral blood [16]. This may explain why up to 70% patients known to have metastatic disease failed to exhibit detectable CTCs using the CellSearch system [36]. Another commercialized technique that uses magnetic enrichment is AdnaTest (AdnaGen AG, Germany) [37]. This technique uses a dual-capture assay, in which CTCs are captured with a mixture of large microbeads (4.5 μm superparamagnetic Dynabeads®) linked with one of two different antibodies: one against EpCAM, and the other against tumor marker such as MUC-1 or HER2 depending on the type of cancer. Subsequent detection is done with multiplexed reverse transcriptase polymerase chain reaction (RT-PCR) to recognize tumor associated mRNAs. Compared with the CellSearch system, this method improves the enrichment step by capturing CTCs with expression of any one of the two antigens. But, it is not clear whether it outperforms the CellSearch system, due to the difference in the size of the magnetic particles and the detection methods [38,39]. Different from CellSearch and AdnaTest, the commercialized magnetic activated cell sorting (MACS) technique traps CTCs labeled with superparamagnetic Fe NPs (˜30 nm) within a magnetized steelwool column [40,41]. When the column is removed from the external magnetic field, the trapped cells are no longer bound to the steelwool and eluted from the column with a buffered solution. A new magnetic separation strategy that can get rid of nonspecifically labeled cells was developed by Talasaz et al. in 2009, which is named MagSweeper [42]. The MagSweeper uses magnetic neodymium rods to capture tumor cells labeled with anti-EpCAM linked Dynabeads in a circular loop motion. Due to the use of multiple magnetic rods and the enrichment in a motion mode that avoids nonspecifically bound species, the separator can enrich CTCs by 108-fold with 100% purity.
While the positive selection is appealing, a drawback of this method is that CTCs with low or no expression of the targeted makers can not be captured. This problem can be avoided by using negative depletion with immunomagnetic beads [43]. A general approach for negative selection with magnetic separation is to firstly lyse RBCs and then use MNPs coated with anti-CD45 antibodies to separate WBCs [44–48]. As demonstrated by Yang et al., this method can reduce the number of normal blood cells by 106-fold [44]. It gives a recovery of approximately 83% on spiked head and neck cancer cells. However, due to the huge amount of normal blood cells, it is very challenging to deplete all of these background cells. In addition, lysis of RBCs may also cause loss and damage of the rare tumor cells
Microchip-based magnetic separation
Microfluidic devices have become one of the mainstream platforms for CTC enrichment and detection due to many advantages including miniaturization, portability, cost–effectiveness and the abilities of online isolation/detection and single cell analysis [49]. Numerous microchip platforms have been developed based on affinity, size or other physical properties [50]. A new direction in immunomagnetic separation is to perform the separation on a microfluidic device, due to the benefits of both immunomagnetic separation and microfluidic device. Under the flow condition, the capture efficiency depends on the ratio of magnetic force and drag force [51]. A cell with many bound NPs has a large ratio of the two forces than free NPs and can thus be selectively captured.
Cell isolation in the microfluidic channels is often simply performed with permanents magnets under the chip (Figure 2D) [51–57]. The capture efficiency and sample throughput can be precisely controlled through the design of the fluidic channels, the control of the flow rate, and the magnetic field strength. Zhang and co-authors have extensively studied both experimentally and theoretically the effects of these parameters on cell capturing efficiency [51,55,56]. They showed that over 85% spiked cancer cells in blood can be captured with EpCAM targeted Fe3O4 NPs at a speed of 10 ml/h using NdFeB block magnets with a maximum energy product of 42 MGOe [55]. They also showed that the performance of the separation can be improved by inverting the microchannel (magnet placed on top of the channel) (Figure 2E) [56]. In this mode, the direction of the gravity is opposite to that of the magnetic field force. Thus, the effects of RBC sedimentation on CTC capture is greatly reduced. Using the inverted microchip-based immunomagnetic separation with Fe3O4-Au core-shell NPs, Sokolov and co-authors demonstrated that CTC capture efficiency can be markedly improved by duplex targeting [57].
In some devices, the microfluidic channels are structurally designed to facilitate or enhance cell capture [58–61]. For example, the device reported by Chen et al. contains an array of magnetic microposts fabricated inside the channel to generate a strong magnetic force when magnetized by the external permanent magnet [59]. Validation studies showed that the device can capture 90% spiked tumor cells labeled with anti-EpCAM conjugated ferrofluid NPs at a flow rate of 6 ml/h with anti-EpCAM conjugated Fe NPs. To improve sample throughput and capture efficiency, Earhart et al. recently developed a magnetic shifter device, composed of an array of 40 µm holes in a silicon nitride membrane and a 12 µm thick coating of a magnetically soft permalloy (Figure 2F) [62]. Due to the extremely high field gradients at the pore edges and high density of pores (approximately 200 pores/mm2), the magnetic microfilter combined with anti-EpCAM conjugated MNPs, can capture 96% spiked cancer cells in blood at a flow rate of 10 ml/h.
Nanostructured substrates
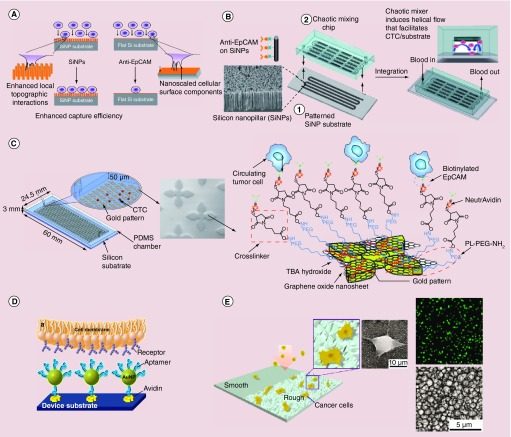
The past few years have witnessed the emergence of nanostructured substrates as a new platform for capturing and enriching CTCs [63,64]. When the substrates are functionalized with targeting ligands, CTCs are captured on the substrate through ligand-antigen binding. Compared to flat substrates, the major advantage of the nanostructured ones is the enhanced local topographic interactions between the substrates and targeting cell surface, which results in vastly enhanced cell capture affinity (Figure 3A) [65]. In addition, nanostructures can be coated with ligands with much higher densities than flat surfaces and thus can introduce multivalent effects to improve binding affinity. Furthermore, when the nanostructures are embedded into a microfluidic device, they lower the rolling velocity of cells in microfluidic channels and thus further enhance cellular binding.
Figure 3. . Nanostructured substrates for circulating tumor cells enrichment.
(A) 3D silicon nanopillar substrates showing enhanced cell binding affinity as compared with the flat substrate. (B) Silicon nanopillar-embedded chaotic mixing microfluidic device. (C) Silicon substrates coated with graphene oxide nanosheet targeting CTCs through EpCAM recognition. (D) Gold nanoparticles-coated substrate to capture CTCs with aptamer ligands. (E) Roughened glass substrate showing capture of tumor cells without using CTC biomarkers.
CTC: Circulating tumor cell; PDMS: Polydimethylsiloxane; SiNP: Silicon nanopillar; TBA: Tetrabutylammonium.
(A) Reproduced with permission from [65] © John Wiley and Sons (2009); (B) Reproduced with permission from [66] © John Wiley and Sons (2011); (C) Reprinted with permission from [74] © Macmillan Publishers Ltd: (Nature Nanotechnology; 2013); (D) Adapted with permission from [78] © American Chemical Society (2013); (E) Reprinted with permission from [80] © American Chemical Society (2013).
Different types of nanostructured substrates have been reported for CTC enrichment, including nanoarray [65–71], nanofiber [72,73], nanosheet [74,75], deposited NP substrates [76–78] roughened surface [79,80] and nanoporous substrates [81,82]. The integrated NanoVelcro device, which has been developed by Tseng and co-authors through a combination of anti-EpCAM coated vertically oriented silicon nanopillars with vastly enhanced CTC-capture affinity and an overlaid microfluidic chaotic mixing chip capable of promoting cell-substrate contact frequency, is very promising (Figure 3B) [66]. The spiking experiments demonstrated that it can capture over 95% tumor cells. Studies on patient samples showed that the device captured significantly greater number of CTCs compared with the CellSearch system. A similar device was later developed by the same group, but with horizontally distributed poly(lactic-co-glycolic acid) (PLGA)-nanofiber as the embedded materials [73]. To facilitate the release of the tumor cells after capture, they later coated the nanoarray surface with thermal responsive polymer, poly (N-isopropylacrylamide) (PIPAAm) to manipulate cell release by changing temperature [69]. The new generation NanoVelcro devices can both capture (at 37°C) and release (at 4°C) over 90% tumor cells, with over 90% cells remaining viable.
Recently, Yoon et al. reported the use of graphene oxide (GO) to capture CTCs [74]. In their work, GO nanosheets were adsorbed onto a flower-shaped gold surface on a silicon substrate and chemically functionalized with EpCAM antibodies (Figure 3C). The recovery rates of 3–5 and 10–20 spiked breast cancer cells reached 73% and 94.2%, respectively, at flow rate of 1–3 ml/h. Without the GO sheet, the chip captures 13.3 and 48% for 3–5 and 10–20 cells, respectively. This demonstrated highly efficient CTC enrichment for low concentration CTCs.
For the substrates deposited with NPs (either by physical adsorption or by covalent binding), the multivalent effects play an important role in cell attachment. As demonstrated by Sheng et al. (Figure 3D), each gold (Au) NP (approximately 14 nm) is coated with 95 aptamer ligands [78]. When the Au NPs are coated on the channel of a microfluidic device, they enhanced cellular binding by 39-fold as compared with flat surface coated with aptamer alone. This increased the cell capture efficiency from 49 to 92%, indicating the strong promise of such nanostructured device for CTC capture and enrichment.
A marker- and size-independent methodology was lately reported by Chen et al. using nanoroughened substrates (Figure 3E) [80]. This method utilizes differential adhesion preference of cancer cells to nanorough surfaces when compared with normal blood cells. Using reactive ion etching, they made roughened glass surfaces with precisely controlled root-mean-square roughness from 1 to 150 nm. They found that as the roughness root-mean-square increases, capture yield of spiked cancer cells increases, reaching 80% for the 150-nm substrates. The device was tested with multiple breast cancer cell lines and compared with the flat surface that captures less than 20% cells in all tested cell lines.
CTC detection
Following isolation and enrichment, CTCs are detected and analyzed using either cytometric or nucleic acid-based approaches [83]. While cytometric methods analyze the cells based on protein expressions, the nucleic acid methods detect genetic alterations specific to tumor cells. Cytometric methods include immunohistochemistry imaging, spectroscopic detection and flow cytrometry. The advantage of cytometric methods over nucleic-acid based methods is the possibility to further characterize the cells since cell lysis is not required in the former procedures. When CTCs are examined microscopically, cell morphology can also been examined. Nucleic acid-based methods can analyze genetic information on whole cell or extracted RNA or DNA using PCR, RT-PCR, quantitative real-time RT-PCR, whole-genome amplification and FISH [84]. In general, nucleic acid methods have high sensitivity but low specificity due to interference from the expression of markers in normal cells. Nanomaterials are used in a variety of detection methods by taking advantages of their unique functional properties. Based on the mechanism of signal readout, the types of nanomaterials used in this area are classified as optical, magnetic and conductive NPs.
Optical nanoparticles
Fluorescent nanoparticles
Fluorescence is a leading technique for CTC detection and analysis. Generally, it is done with organic dyes as the imaging agents. However, it has been widely realized that the use of organic dyes is limited by photobleaching, low signal intensity, spectral overlapping and the need for multiple light sources to excited different fluorophores in multiplexed detection. Alternatively, quantum dots (QDs) have large absorption coefficient, narrow emission, high photostability and superior brightness [85]. Their emission can be precisely tuned by changing the size and composition of the NPs, which results in multicolor NPs with a single excitation laser source [86]. Due to these excellent properties, they have been widely used in biomedical imaging during the last decade [87]. However, they have not attracted much attention in CTC imaging [88–90]. The major concern is their cytotoxicity that may cause cell molecular changes and damage [91]. To avoid the toxicity issue, QDs can be used to detect CTCs by monitoring extracted nucleic acids. An example is the microfluidic bead-based nucleic acid sensor developed by Zhang et al. using multienzyme-nanoparticle amplification and QD labels [92]. By measuring the fluorescence signal intensity from QDs, the amount of targeted DNA can be quantified. The method can detect 1 spiked colorectal cancer cell in the blood.
Another class of fluorescent NPs is upconversion NPs (UCNPs) that contain lanthanide ions and show strong emission under NIR excitations [93]. Due to the NIR excitation, cellular autofluorescence is minimized and thus UCNPs enable imaging in biological samples with high sensitivity. In 2014, Fang et al. demonstrated the first application of UCNPs for CTC detection [94]. Using aptamer-linked NaYF4 (Yb:Er) UCNPs targeting PTK-7 on cancer cells, in combination with magnetic enrichment with superparamagnetic Av-conjguated Fe3O4 NPs, they showed linear correlation between the fluorescence intensity with the number of PTK-7 positive CCRF-CEM cells spiked in whole blood. As few as 10 cells spiked in 10 ml of whole blood were detected. The captured cells were further examined with a confocal microscope, which indicated a purity of 70–90% depending on the concentration of spiked tumor cells.
Surface-enhanced Raman scattering nanoparticles
When organic dye with highly delocalized pi electrons is adsorbed onto metal NPs, the fluorescence signal of the dye is quenched and the Raman signals are strongly enhanced, as high as 1012–14 times leading to detection sensitivity down to single molecule and single particle level [95,96]. The dye-adsorbed metal NPs, termed surface-enhanced Raman scattering nanoparticles (SERS NPs), have emerged as a new generation of optical labels for biomedical imaging and diagnosis [97,98]. Different from the fluorescence technique, SERS gives sharp fingerprint-like signals (10–100-times narrower than fluorescence signals), distinct from biological autofluorescence/scattering background. This allows ultrasensitive detection without the need for tedious signal separation. The fingerprinting signals offer excellent multiplexity capability for multicolor imaging and detection [99,100]. The capability of using a single excitation source for multicolor probes and minimal photobleaching are additional advantages.
In 2008, Sha et al. demonstrated for the first time the potential of SERS NPs for CTC detection using spiked breast cancer cells [101]. 50 nm Au SERS NPs (Nanoplex Biotags) are linked with HER2 antibodies to target SK-BR-3 cells. By coupling with immunomagnetic enrichment with anti-EpCAM conjugated magnetic beads, the method can detect 10 tumor cells/ml blood. The studies used a sample holder with a magnetic assembly that focuses the tumor cells on a precise location on the wall of the tube for detection. A recent modified strategy was to magnetically enrich the labeled cells in a tube under a flow condition, followed by the detection by SERS technology [102]. The flow condition can be facilely translated into microfluidic modality for single cell analysis.
In 2011, Wang et al. developed a SERS-based assay using Au NPs covalently linked with epidermal growth factor peptide in combination with density gradient centrifugation enrichment and tested the assay with patient blood samples [103]. QSY21 quencher was used as the Raman reporter. The method can detect five to 50 spiked head and neck cancer cells in blood. Studies on clinic blood samples showed that CTCs in 17 out of 19 patients with head and neck cancer were detected, with CTC number ranging 1–720. A major advantage of this methodology is the specific detection of CTCs in the presence of WBCs, due to the use of small epidermal growth factor peptide rather than whole antibody ligands.
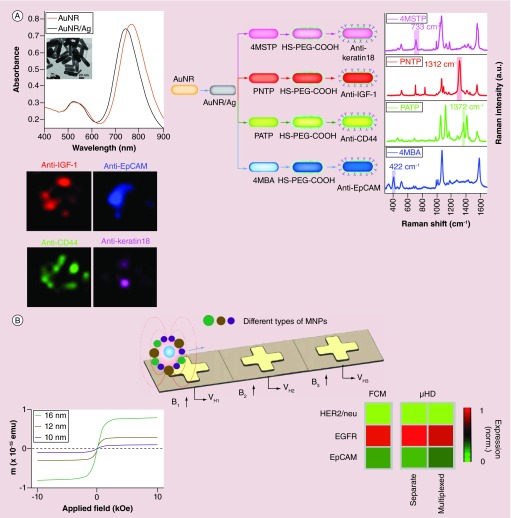
Recently, Zhang demonstrated the use of SERS NPs for CTC detection and enumeration at single cell level by combining a membrane substrate-based enrichment [104]. Nitrocellulose membrane substrate was functionalzed with anti-EpCAM antibodies to capture CTCs. The captured CTCs were labeled with Au SERS NPs linked with EpCAM antibodies. Microscopic SERS imaging was performed to detect and count CTCs. A significant advancement was made in the same year by Nima et al. who applied SERS for multicolor CTC imaging [105]. In their studies, four color SERS NPs were formed using Ag-coated Au nanorods (NRs) and four different Raman reporters (Figure 4A). Each color NP was linked with specific antibodies to target one of the four markers on breast cancer cells, IGF-1, anti-EpCAM, anti-CD 44 and anti-keratin 18. Using the SERS probe cocktail and the signature SERS peak from each reporter, the four markers on the same cell were imaged with a confocal Raman microscope. This represents a significant advancement in developing SERS-based technologies for CTC detection and analysis.
Figure 4. . Nanoparticles for multiplexed detection, imaging and profiling of circulating tumor cells.
(A) Multiplexed imaging of circulating tumor cells using four color Au-Ag core-shell SERS nanorods. Top Left: Absorption and TEM image of Ag-Au core-shell nanorods. Red: Au nanorods. Black: Au-Ag core-shell nanorods. Right: Preparation and SERS spectra of 4 color Au-Au SERS nanoprobes. Down left: Raman imaging of tumor cells with four color Au-Au SERS nanoprobes. (B) Multiplexed detection and profiling of CTCs with MnFe2O4 MNPs in combination with a micro-Hall detector. Top: Schematic showing the principle of multiplexed profiling of CTC marker expressions using MNPs of different sizes in conjunction with a micro-Hall detector. Down left: Magnetic properties of MNPs with different sizes. Down right: The heat map showing comparison of the relative expression levels of three different tumor makers measured using the micro-Hall detection method and flow cytometry.
MNP: Magnetic nanoparticle; MSTP: 4-(methylsulfanyl) thiophenol; PNTP: P-nitrobenzoic acid; PATP: P-aminobenzoic acid; 4MBA: 4-mercaptobenzoic acid.
(A) Reprinted with permission from [105] © Macmillan Publishers Ltd:(Science Reports; 2014); (B) Adapted with permission from [106] and reprinted with permission from © AAAS (2012).
For color images please see online at: www.futuremedicine.com/doi/full/10.2217/NNM.15.32
Magnetic nanoparticles
While MNPs have been typically utilized for CTC enrichment, they can be used as detection agents as well. In 2012, Weissleder, Lee and co-authors demonstrated an innovative technology using MNPs that can directly detect CTCs in whole blood and quantitatively measure specific biomarkers in a multiplexed fashion (Figure 4B) [106]. They developed a microfluidic chip-based micro-Hall detector (μHD) to detect induced magnetic moments by magnetically labeled cells in the presence of an external magnetic field based on the Hall effect. As the signal intensity is proportional to the number of bound MNPs and thus level of biomarkers, this method is able to detect and profile the targeted biomarkers on single cells in the presence of vast numbers of blood cells. Using MNPs of different sizes, they can profile multiple markers. The method has been tested with three different cancer cell lines and three different markers. The molecular profiles of these markers agreed well with those from flow cytometry. Studies on blood samples from 20 ovarian cancer patients showed that CTCs were detected in 100% patients in contrast to 20% patients with the CellSearch system. The method detected a higher number of CTCs in patients of ovarian cancer than CellSearch across all the patient samples due to the use of a multiplexed targeting strategy. A potential drawback with this technology is that CTCs with low level of protein expressions may not be detected because the tumor cells require over 106 MNPs in order to be detected.
With a different detection mechanism, the same groups developed a micronuclear magnetic resonance (μNMR)-based method for detecting and counting CTCs in whole blood [107]. The μNMR measures the transverse relaxation time (T2) of water proton in a solution sample. When the sample contains CTCs labeled with MNPs, the MNPs produce local dipole fields with strong spatial dependence, which accelerates the transverse relaxation of water protons and thus shorter T2 than nontargeted objects [108]. The method involves RBC lysis after MNP labeling. Experiments with spiked cancer cells showed recovery rates of 30–45% depending on the concentration of the tumor cells. The method detected CTCs in 87% of patients with ovarian cancer, with higher CTC number than the CellSearch system in advanced cases.
Conductive nanoparticles
Carbon nanotubes (CNTs) have remarkable electronic properties, behaving as a metal or semiconductor depending on their diameter and helicity [109]. Mechanical deformations or chemical binding can induce strong variations of its conductance, which can be easily detected by electron current signals. Such unique properties make CNTs excellent chemical and biological sensors. In 2008, Shao et al. demonstrated the first application of CNTs for electric detection of cancer cells in blood. They made single wall CNT field effect transistor array device containing 20 pairs of electrodes with a single CNT between each pair. The CNTs were functionalized with antibodies to recognize breast cancer cells. The binding of cancer cells to the CNTs induced 60% decrease in conductivity whereas control experiments produced less than 5% decrease in conductivity. The key advantage of this assay is that the sensing area is limited to few receptors in cells and thus may potentially detecting CTCs with low protein expressions. It also directly detects cancer cells in blood, without the need of preenrichment. However, it has difficulty to count CTCs because the signal is only determined by a single cell reaching the spacing between the electrodes. The volume of analyzed blood is also very small (<10 μl), which may miss rare CTCs in patient blood.
A recent study by Liu et al. demonstrated a quantitative CNT-based sensor for direct detection of cancer cells in whole blood using real time electrical impedance sensing [110]. Multilayer CNTs were assembled on an indium tin oxide electrode surface and modified with EpCAM antibodies. Cell binding induced increase of the electron-transfer resistance. Using liver cancer as a model, they demonstrated that this electrical response was linearly proportional to the concentration of the cancer cells in whole blood, with a detection of limit of five cells per ml of blood. Rather than whole cell detection, Kwon described a method to detect proteins collected from lysed cancer cells using CNT patterned surface coupled with scanning probe microscopy imaging [111]. CNTs served as a substrate to recognize carcinoembryonic antigens (CEAs) expressed on CTCs and scanning probe microscopy was used to image CEAs bound on the CNTs. This quantitative assay can detect not only a single CTC but also single CEA molecule, indicating its superior sensitivity.
Dual enrichment & detection with hybrid nanoparticles
Due to their scarcity of CTCs in blood, materials that allow dual enrichment/detection modalities are highly desirable for developing simple, rapid and efficient detection methods. The dual functional materials bridge the gap between the enrichment and detection technologies, simplifying sample processes and minimizing CTC loss and damage. Thus, dual functional nanomaterials are very promising for developing new generation of CTC detection technologies.
An earlier strategy was developed by Maeda et al. who made magnetic-fluorescent nanocomposites with bacterial MNPs (BacMPs) and commercial QDs for dual magnetic isolation and fluorescence detection [112]. The BacMPs were functionalized with anti-EpCAM using gene fusion techniques. In their proof-of-concept studies, the nanocomposite, under optimized conditions, led to a 90% recovery rate for NCI-H358 lung cancer cells from 1 ml of PBS suspension whereas less than 10% for JM non-epithelial model cells. Following magnetic separation, the cancer cells were detected by fluorescence imaging because of the QD moieties in the composites. Magnetic-fluorescent composites using MNPs and organic dyes have also been reported [113,114]. Recently, Ray and co-authors used Fe3O4-Au core-shell NPs and organic dye to form the hybrid nanosystem [115]. The use of magnetic-plasmonic core-shell NPs allows subsequent destruction of captured tumor cells with photothermal therapy.
We recently developed a dual capture and detection assay using highly integrated magnetic-plasmonic core-shell SERS NPs (Figure 5) [116]. We synthesized Fe3O4-Au core-shell NPs in different shapes based on a seed-mediated growth method [116,117]. These anisotropic core-shell NPs are 30–40-times higher in SERS activities than traditional spherical counterparts. The integrated nanoprobes were made from oval-shaped Fe3O4-Au core-shell NPs, which were adsorbed with QSY21 Raman reporters and linked with one of two different antibodies, EpCAM or HER2. To further minimize cell loss, we constructed an online capture and detection system, in which tumor cells were firstly captured online with a macromagnet and then immobilized by a micromagnet in the absence of the macromagnet for SERS detection. Using the duplex targeted nanoprobes in combination with the online flow system, we demonstrated that over 90% SK-BR-3 breast cancer cells can be captured at a flow velocity of 6 cm/s without significant interference from free NPs. Spiking experiments with SK-BR-3 cells showed that the intensity of the SERS signals from the Raman reporter was linearly correlated to the number of cells in the whole blood. Our method showed a limit of detection of 1–2 cells per milliliter of human whole blood. With further development using microfluidic device, multiplexed targeting and microscopic Raman detection, the technique will lead to a new generation of versatile system for highly sensitive and specific detection and profiling of CTCs in whole blood.
Figure 5. . Magnetic-plasmonic hybrid nanoparticles for dual enrichment and detection of circulating tumor cells.
(A) TEM image, (B) absorption spectrum and (C) M-H curve and magnetic separation micrograph of oval shape IO-Au core-shell NPs. (D) Structure of oval shape IO-Au SERS nanoprobes. (E) Capture efficiencies of nanoparticle-bound SK-BR-3 tumor cells (black) and free oval shale IO-Au core-shell nanoparticles (red). (F) Detection of spiked SK-BR-3 cells in whole blood with oval shape IO-Au SERS nanoprobes in combination with an on-line magnetic enrichment and SERS detection system. The limit of detection is calculated to be 1-2 cells/ml blood.
NP: Nanoparticle.
Reprinted with permission from [116] © Future Science Group (2014).
In vivo enrichment & detection with nanomaterials
Techniques capable of detecting and quantifying CTCs in vivo are valuable because they are noninvasive and can monitor CTC level in real-time. They detect CTCs in the entire blood volume of the body, which can enhance sensitivity up to 102–103 times as compared with ex vivo methods [118]. In vivo CTC detection has been done on superficial vessels with a mouse model by in vivo flow cytometry using traditional fluorescence [119–126] or newly developed photothermal (PT) and photoacoustic (PA) [127–134] detection methods, or a combination of these methods [135,136].
An example of the use of nanotechnology in CTC detection with in vivo fluorescence flow cytometry was the use of two color QDs (Qdot 585 and Qdot655) to track the circulation of breast cancer cells of two different cell lines [122]. This work, reported by Tkaczyk et al., was the first study showing that two different populations of circulating tumor cells can be quantified simultaneously in the blood circulation in a mouse model. The technique has the potential to track different CTC subtypes in vivo. However, limitations of the fluorescence-based methods exist, including potential cytotoxiciy of QDs and interference from light scattering and autofluorescence. These drawbacks can be avoided in PT/PA detection methods, which are based on the absorption properties of light from pulsed laser by endogenous biomolecules or exogenous contrast agents and subsequent nonradiative relaxation of absorbed laser energy into heat. In PT imaging, the heat induces the variations of the refractive index in the cells, which is detected with phase-contrast imaging technique with a second, collinear probe laser pulse [137]. In PA flow cytometry the temperature fluctuations resulting from the pulsed heating by the laser generates pressure waves that is detected with an ultrasound transducer [138]. PAFC combines high sensitivity and spectral specificity of optical methods with high spatial resolution and tissue penetration of ultrasounds methods, and thus very promising for in vivo detection.
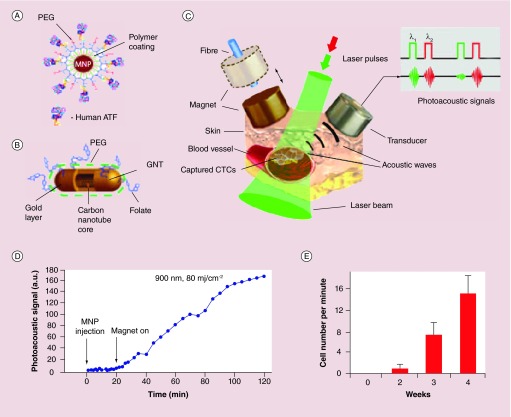
Using low toxicity metal-based NPs, Zharov and co-authors have conducted extensive studies on CTC detection with in vivo PT/PA flow cytometry from single color to two color detection [130–133]. In their studies, a groundbreaking approach is the use of MNPs and gold coated carbon nanotube for magnetic enrichment and PA detection of CTCs in vivo (Figure 6) [130]. In this method, 10 nm Fe2O3 NPs were functionalized with the aminoterminal fragment (ATF) of the urokinase plasminogen activator to target urokinase plasminogen activator receptor on breast cancer cells. The MNPs served as dual magnetic and PA contrast agents. GNTs have a higher PA contrast than MNPs in the NIR region and thus were used as a second PA agent to increase detection sensitivity. GNTs were also used as a second targeting agent through linked folate ligands to increase detection specificity. Results showed that the sensitivity limit is 35 GNTs and 720 MNPs. Selective capture (over 90%) of MNP-labeled cancer cells over free MNPs were achieved at a flow velocity of 2–8 cm/s. The technique was examined by detecting CTCs in the blood circulation of a nude mouse with breast cancer xenografts through the ear vein. After injection of a cocktail of the two nanoprobes, they observed photoacoustic signals when the magnet is placed near the ear vein. The signals further increased with time, indicating successful enrichment of CTCs. By converting the signals into cell number, they were able to monitor CTC level during tumor development. They observed that the CTC rate increased dramatically with time, correlating to different stages of tumor. Zharov and co-authors further demonstrated the ability to kill CTCs in vivo following detection using photothermal melanin NPs [129] or cancer stem cells (CSCs) using GNTs [131]. Incorporation the killing modality with the detection methods is beneficial because it directly reduces the risk of metastasis by eradicating these diseased cells.
Figure 6. . Nanoparticles for in vivo magnetic enrichment and photoacoustic detection of circulating tumor cells.
(A) MNP probes targeting urokinase plasminogen activator receptors on tumor cells. (B) GNT probes targeting folate receptors on tumor cells. (C) Enrichment and detection setup. (D) Photoacoustic signals from CTCs in abdominal vessels at week 1 of tumor development with and without magnetic enrichment. (E) The average CTC rate in mouse ear vein over a period of 4 weeks after tumor development.
ATF: Aminoterminal fragment; CTC: Circulating tumor cell; GNT: Gold nanotube; MNP: Magnetic nanoparticle; PEG: Polyethylene glycol.
Reprinted with permission from [130] © Macmillan Publishers Ltd (2009).
Conclusion
Nanotechnology receives intense attention for the enrichment and detection of CTCs during the last decade owing to the unique structural and functional properties of nanoscale materials. Magnetic NPs are used for the enrichment of CTCs in blood samples in the FDA-approved CellSearch system. Many new nanotechnology-based techniques that improve upon the CellSearch system have been developed and showed strong potential for clinical applications. Most techniques use NPs, such as magnetic, fluorescent, metallic, conductive NPs or nanostructured substrates, to enrich or detect CTCs in blood samples. Hybrid NPs have been recently developed to bridge current enrichment and detect methodologies. Nanotechnology has also been used to detect CTCs in vivo. The use of photothermal NPs in the in vivo technologies allows subsequent eradication of CTCs, which is promising for prevention of cancer metastasis.
Future perspective
In the next 5–10 years, sensitivity and specificity remain the key issues to be addressed in future technologies. The dual functional nanomaterials such as magnetic-optical core-shell nanoparticles are very promising to reduce false negatives by bridging current enrichment and detection methods. Multiplexed targeting is another way to improve detection sensitivity by capturing and analyzing CTC subpopulations. Nanomaterials are ideal for developing multiplexed assay as each particle can be linked with different targeting ligands. In addition, incorporation of nanomaterials with microfluidic devices will continue to be an optimistic strategy in CTC enrichment and detection because of the combined benefits of nanotechnology and microchip technology.
Another future direction of the field will be molecular characterization of CTCs that may better inform clinical decision making. The ability to reliably and efficiently characterize CTC gene and protein expression profiles remains limited. The SERS NPs may revolutionize protein analysis at the single cell level. SERS NPs can profile CTC protein markers in a high throughput fashion. Multicolor SERS NPs (>10 colors) can be formulated without changing the size and shape of the nanostructures. Signals from each color NPs can be facilely obtained by deconvolution with classic least square regression. Thus, the multicolor SERS NPs will lead to novel characterization methods in a high throughput fashion.
Executive summary.
Background
Circulating tumor cells (CTCs) provide a potentially noninvasive liquid biopsy for characterizing and monitoring cancer, helping inform clinical decision making.
A variety of techniques have been developed to enrich and detect CTCs during the last two decades, with the CellSearch system being approved by FDA for utilization in the clinic to count CTCs in blood of patients with metastatic breast, prostate and colon cancer.
Nanotechnology plays an important role in CTC enrichment and detection with increasing interests in recent years owing to the unique structural and functional properties of nanoscale materials.
Nanotechnology in enrichment & detection of CTCs
Magnetic nanoparticles have been widely used for CTC enrichment based on classic bulk immunomagnetic separation or microchip-based immunomagnetic separation.
A variety of nanostructured substrates have been developed to capture and enrich CTCs.
Magnetic, fluorescent, plasmonic and conductive nanoparticles have been recently used to detect CTCs either directly in whole blood or in combination with enrichment methods.
Hybrid functional nanoparticles have emerged as new contrast agents for dual enrichment and detection of CTCs.
In vivo enrichment and detection technologies have been developed based on magnetic and plasmonic nanoparticles.
Conclusion
Nanomaterials are used in the US FDA-approved CellSearch system.
Many new technologies with significant improvement and great potential for clinical applications have been developed using various nanoplatforms, mainly magnetic, fluorescent, metallic and conductive NPs as well as nanostructured substrates.
Nanotechnology has been proven promising for in vivo detection of CTCs.
Future perspective
Sensitivity and specificity remain the key issues to be addressed in future technologies.
Dual functional nanoparticles with multiplexing capability are very promising in developing new generation of CTC enrichment and detection technologies.
Nanomaterials are very promising for molecular characterization of CTCs, especially for profiling of CTC protein expressions at the single cell level.
Acknowledgements
The authors thank the financial support by NIH (Grant No: 1R15 CA 195509-01) and the University of Memphis FedEx Institute of Technology Innovation Fund.
Footnotes
Disclaimer
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Financial & competing interest disclosure
The authors acknowledge the NIH funding ageny (Grant No 1R15CA195509-01). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as either: • of interest; •• of considerable interest
- 1.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liberko M, Kolostova K, Bobek V. Essentials of circulating tumor cells for clinical research and practice. Crit. Rev. Oncol. Hematol. 2013;88:338–356. doi: 10.1016/j.critrevonc.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Alix-Panabières CKP. Circulating tumor cells: liquid biopsy of cancer. Clin. Chem. 2013;59(1):110–118. doi: 10.1373/clinchem.2012.194258. [DOI] [PubMed] [Google Scholar]
- 4.Mocellin S, Hoon D, Ambrosi A, Nitti D, Rossi CR. The prognostic value of circulating tumor cells in patients with melanoma: a systematic review and meta-analysis. Clin. Cancer Res. 2006;12:4605–4613. doi: 10.1158/1078-0432.CCR-06-0823. [DOI] [PubMed] [Google Scholar]
- 5.De Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2008;14(19):6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 6.Miller MC, Doyle GV, Terstappen LWMM. Significance of circulating tumor cells detected by the CellSearch systemin patients with metastatic breast colorectal and prostate cancer. J. Oncol. 2010;2010:1–8. doi: 10.1155/2010/617421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krebs MG, Sloane R, Priest L, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J. Clin. Oncol. 2011;29(12):1556–1563. doi: 10.1200/JCO.2010.28.7045. [DOI] [PubMed] [Google Scholar]
- 8.Hiltermann TJN, Pore MM, van den Berg A, et al. Circulating tumor cells in small-cell lung cancer: A predictive and prognostic factor. Ann. Oncol. 2012;23(11):2937–2942. doi: 10.1093/annonc/mds138. [DOI] [PubMed] [Google Scholar]
- 9.Hartkopf AD, Wagner P, Wallwiener D, Fehm T, Rothmund R. Changing levels of circulating tumor cells in monitoring chemotherapy response in patients with metastatic breast cancer. Anticancer Res. 2011;31(3):979–984. [PubMed] [Google Scholar]
- 10.Miyamoto DT, Sequist LV, Lee RJ. Circulating tumour cells—monitoring treatment response in prostate cancer. Nat. Rev. Clin. Oncol. 2014;11(7):401–412. doi: 10.1038/nrclinonc.2014.82. [DOI] [PubMed] [Google Scholar]
- 11.Smerage JB, Barlow WE, Hortobagyi GN, et al. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J. Clin. Oncol. 2014;32:3483–3489. doi: 10.1200/JCO.2014.56.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hüsemann Y, Geigl JB, Schubert F, et al. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13(1):58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Danila DC, Fleisher M, Scher HI. Circulating tumor cells as biomarkers in prostate cancer. Clin. Cancer Res. 2011;17(12):3903–3912. doi: 10.1158/1078-0432.CCR-10-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.den Toonder J. Circulating tumor cells: the grand challenge. Lab. Chip. 2011;11(3):375–377. doi: 10.1039/c0lc90100h. [DOI] [PubMed] [Google Scholar]
- 15.Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 2004;10(20):6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]; • This paper determined the performance of the US FDA-approved CellSearch system for circulating tumor cell detection.
- 16.Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashworth TR. A case of cancer in which cells similar to those in the tumors were seen in the blood after death. Aust. Med. J. 1869;14:146–147. [Google Scholar]
- 18.Arya SK, Lim B, Rahman ARA. Enrichment, detection and clinical significance of circulating tumor cells. Lab. Chip. 2013;13(11):1995–2027. doi: 10.1039/c3lc00009e. [DOI] [PubMed] [Google Scholar]; •• A very good review of various techniques for circulating tumor cell enrichment and detection.
- 19.Aguilar ZP, editor. Elsevier. 2012. Nanomaterials For Medical Applications; p. 544. [Google Scholar]
- 20.Zhang Q, Uchaker E, Candelaria SL, Cao G. Nanomaterials for energy conversion and storage. Chem. Soc. Rev. 2013;42(7):3127–3171. doi: 10.1039/c3cs00009e. [DOI] [PubMed] [Google Scholar]
- 21.Wang ZL, Zhu G, Yang Y, Wang S, Pan C. Progress in nanogenerators for portable electronics. Materials Today. 2012;15(12):532–543. [Google Scholar]
- 22.Zhou B, Hermans S, Somorjai GA. Nanotechnology in Catalysis. In: Lockwood DJ, editor. Nanostructure Science and Technology (Volume 3) Springer Science and Businees Media, LLC; NY, USA: 2007. p. p333. [Google Scholar]
- 23.Jain PK, Lee KS, El-Sayed IH, El-Sayed MA. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: applications in biological imaging and biomedicine. J. Phys. Chem. B. 2006;110(14):7238–7248. doi: 10.1021/jp057170o. [DOI] [PubMed] [Google Scholar]
- 24.Lal S, Clare SE, Halas NJ. Nanoshell-enabled photothermal cancer therapy: impending clinical impact. Acc. Chem. Res. 2008;41(12):1842–1851. doi: 10.1021/ar800150g. [DOI] [PubMed] [Google Scholar]
- 25.Huang X, Neretin S, El-Sayed MA. Gold nanorods: from synthesis and properties to biological and biomedical applications. Adv. Mater. 2009;21(48):4880–4910. doi: 10.1002/adma.200802789. [DOI] [PubMed] [Google Scholar]
- 26.Chen J, Wiley B, Li ZY, et al. Gold nanocages: engineering their structure for biomedical applications. Adv. Mater. 2005;17(18):2255–2261. [Google Scholar]
- 27.Melancon MP, Lu W, Yang Z, et al. In vitro and in vivo targeting of hollow gold nanoshells directed at epidermal growth factor receptor for photothermal ablation therapy. Mol. Cancer Ther. 2008;7(6):1730–1739. doi: 10.1158/1535-7163.MCT-08-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alix-Panabières C, Pantel K. Technologies for detection of circulating tumor cells: facts and vision. Lab. Chip. 2014;14(1):57–62. doi: 10.1039/c3lc50644d. [DOI] [PubMed] [Google Scholar]
- 29.Kodama RH. Magnetic nanoparticles. J. Magn. Magn. Mater. 1999;200(1–3):359–372. [Google Scholar]
- 30.Frey NA, Peng S, Cheng K, Sun S. Magnetic nanoparticles: synthesis, functionalization, and applications in bioimaging and magnetic energy storage. Chem. Soc. Rev. 2009;38(9):2532–2542. doi: 10.1039/b815548h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoeppener AELM, Swennenhuis JF, Terstappen LWMM. Immunomagnetic separation technologies, in minimal residual disease and circulating tumor cells in breast cancer. In: Ignatiadis M, Sotiriou C, Pantel K, editors. Springer-Verlag, Berlin Heidelberg; Germany: 2012. pp. 43–58. [Google Scholar]
- 32.Xu H, Aguilar ZP, Yang L, et al. Antibody conjguated magnetic iron oxide nanoparticles for cancer cell separation in fresh whole blood. Biomaterials. 2011;32:9758–9765. doi: 10.1016/j.biomaterials.2011.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pamme N. Magnetism and microfluidics. Lab. chip. 2006;6(1):24–38. doi: 10.1039/b513005k. [DOI] [PubMed] [Google Scholar]
- 34.Liberti PA, Rao CG, Terstappen LWMM. Optimization of ferrofluids and protocols for the enrichment of breast tumor cells in blood. J. Magn. Magn. Mater. 2001;225(1–2):301–307. [Google Scholar]
- 35.Riethdorf S, Fritsche H, Muller V, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin. Cancer Res. 2007;13(3):920–928. doi: 10.1158/1078-0432.CCR-06-1695. [DOI] [PubMed] [Google Scholar]
- 36.van der Auwera I, Peeters D, Benoy IH, et al. Circulating tumour cell detection: a direct comparison between the CellSearch System, the AdnaTest and CK-19/mammaglobin RT-PCR in patients with metastatic breast cancer. Br. J. Cancer. 2010;102(2):276–284. doi: 10.1038/sj.bjc.6605472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lankiewicz S, Rivero BG, Böcher O. Quantitative real-time RT-PCR of disseminated tumor cells in combination with immunomagnetic cell enrichment. Mol. Biotechnol. 2006;34(1):15–26. doi: 10.1385/MB:34:1:15. [DOI] [PubMed] [Google Scholar]
- 38.Andreopoulou E, Yang LY, Rangel KM, et al. Comparison of assay methods for detection of circulating tumor cells in metastatic breast cancer: AdnaGen AdnaTest BreastCancer Select/Detect™ versus Veridex CellSearch™ system. Int. J. Cancer. 2012;130(7):1590–1597. doi: 10.1002/ijc.26111. [DOI] [PubMed] [Google Scholar]
- 39.Muller V, Riethdorf S, Rack B, et al. Prognostic impact of circulating tumor cells assessed with the CellSearch System™ and AdnaTest Breast™ in metastatic breast cancer patients: the DETECT study. Breast Cancer Res. 2012;14(4):R118. doi: 10.1186/bcr3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miltenyi S, Muller W, Weichel W, Radbruch A. High gradient magnetic cell separation with MACS. Cytometry. 1990;11:231–238. doi: 10.1002/cyto.990110203. [DOI] [PubMed] [Google Scholar]
- 41.Martin VM, Siewert C, Scharl A, et al. Immunomagnetic enrichment of disseminated epithelial tumor cells from peripheral blood by MACS. Exp. Hematol. 1998;26(3):252–264. [PubMed] [Google Scholar]
- 42.Talasaz AH, Powell AA, Huber DE, et al. Isolating highly enriched populations of circulating epithelial cells and other rare cells from blood using a magnetic sweeper device. Proc. Natl Acad. Sci. USA. 2009;106(10):3970–3975. doi: 10.1073/pnas.0813188106. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper reported a novel immunomagnetic cell separator to process circulating tumor cells with high purity.
- 43.Diamond E, Lee GY, Akhtar NH, et al. Isolation and characterization of circulating tumor cells in prostate cancer. Front. Oncol. 2012;2(131):1–11. doi: 10.3389/fonc.2012.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang L, Lang JC, Balasubramanian P, et al. Optimization of an enrichment process for circulating tumor cells from the blood of head and neck cancer patients through depletion of normal cells. Biotechnol. Bioeng. 2009;102(2):521–534. doi: 10.1002/bit.22066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin HC, Hsu HC, Hsieh CH, et al. A negative selection system PowerMag for effective leukocyte depletion and enhanced detection of EpCAM positive and negative circulating tumor cells. Clin. Chim. Acta. 2013;419:77–84. doi: 10.1016/j.cca.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 46.Wu Y, Deighan CJ, Miller BL, et al. Isolation and analysis of rare cells in the blood of cancer patients using a negative depletion methodology. Method. 2013;64(2):169–182. doi: 10.1016/j.ymeth.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joshi P, Jacobs B, Derakhshan A, et al. Enrichment of circulating melanoma cells (CMCs) using negative selection from patients with metastatic melanoma. Oncotarget. 2014;5(9):2450–2461. doi: 10.18632/oncotarget.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lara O, Tong X, Zborowski M, Chalmers JJ. Enrichment of rare cancer cells through depletion of normal cells using density and flow-through, immunomagnetic cell separation. Exp. Hematol. 2004;32(10):891–904. doi: 10.1016/j.exphem.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 49.Hajba L, Guttman A. Circulating tumor-cell detection and capture using microfluidic devices. Trends Analyt. Chem. 2014;59:9–16. [Google Scholar]
- 50.Balic M, Lin H, Williams A, Datar RH, Cote RJ. Progress in circulating tumor cell capture and analysis: implications for cancer management. Expert Rev. Mol. Diagn. 2012;12(3):303–312. doi: 10.1586/erm.12.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoshino K, Chen P, Huang YY, Zhang X. Computational analysis of microfluidic immunomagnetic rare cell separation from a particulate blood flow. Anal. Chem. 2012;84(10):4292–4299. doi: 10.1021/ac2032386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xia N, Hunt TP, Mayers BT, et al. Combined microfluidic-micromagnetic separation of living cells in continuous flow. Biomed. Microdevices. 2006;8(4):299–308. doi: 10.1007/s10544-006-0033-0. [DOI] [PubMed] [Google Scholar]
- 53.Adams JD, Thevoz P, Bruus H, Soh HT. Integrated acoustic and magnetic separation in microfluidic channels. Appl. Phys. Lett. 2009;95(25):254103. doi: 10.1063/1.3275577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Modak N, Datta A, Ganguly R. Cell separation in a microfluidic channel using magnetic microspheres. Microfluid. Nanofluidics. 2009;6:647–660. [Google Scholar]
- 55.Hoshino K, Huang YY, Lane N, et al. Microchip-based immunomagnetic detection of circulating tumor cells. Lab. Chip. 2011;11(20):3449–3457. doi: 10.1039/c1lc20270g. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This paper report a new method of microchip-based immunomagnetic detection of circulating tumor cells that combines the benefits of both immunomagnetic assay and microflyidic device.
- 56.Huang Y, Hoshino K, Chen P, et al. Immunomagnetic nanoscreeing of circulating tumor cells with a motion controlled microfluidic system. Biomed. Microdevices. 2013;15(4):673–681. doi: 10.1007/s10544-012-9718-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu CH, Huang YY, Chen P, et al. Versatile immunomagnetic nanocarrier platform for capturing cancer cells. ACS Nano. 2013;7(10):8816–8823. doi: 10.1021/nn403281e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kang JH, Krause S, Tobin H, Mammoto A, Kanapathipillai M, Ingber DE. A combined micromagnetic-microfluidic device for rapid capture and culture of rare circulating tumor cells. Lab. Chip. 2012;12(12):2175–2181. doi: 10.1039/c2lc40072c. [DOI] [PubMed] [Google Scholar]
- 59.Chen X, Liu J, Zhu J, Sun Y, Fan J. Optimization of microfluidic immunomagnetic chip for circulting tumor cell capture. Sensor Mater. 2013;25(9):667–671. [Google Scholar]
- 60.Yu X, He R, Li S, et al. Magneto-controllable capture and release of cancer cells by using a micropillar device decorated with graphite oxide-coated magnetic nanoparticles. Small. 2013;9(22):3895–3901. doi: 10.1002/smll.201300169. [DOI] [PubMed] [Google Scholar]
- 61.Kim S, Han SI, Park MJ, et al. Circulating tumor cell microseparator based on lateral magnetophoresis and immunomagnetic nanobeads. Anal. Chem. 2013;85(5):2779–2786. doi: 10.1021/ac303284u. [DOI] [PubMed] [Google Scholar]
- 62.Earharta CM, Hughes CE, Gaster RS, et al. Isolation and mutational analysis of circulating tumor cells from lung cancer patients with magnetic sifters and biochips. Lab. Chip. 2014;14(1):78–88. doi: 10.1039/c3lc50580d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang L, Asghar W, Demirci U, Wan Y. Nanostructured substrates for isolation of circulating tumor cells. Nano Today. 2013;8:374–387. doi: 10.1016/j.nantod.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoon HJ, Kozminsky M, Nagrath S. Emerging role of nanomaterials in circulating tumor cell isolation and analysis. ACS Nano. 2014;8(3):1995–2017. doi: 10.1021/nn5004277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang S, Wang H, Jiao J, Chen KJ, et al. Three-dimensional nanostructured substrates toward efficient capture of circulating tumor cells. Angew. Chem. Int. Ed. 2009;48(47):8970–8973. doi: 10.1002/anie.200901668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang S, Liu K, Liu J, et al. Highly efficient capture of circulating tumor cells by using nanostructured silicon substrates with integrated chaotic micromixers. Angew. Chem. Int. Ed. 2011;50(13):3084–3088. doi: 10.1002/anie.201005853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee SK, Kim GS, Wu Y, et al. Nanowire substrate-based laser scanning cytometry for quantitation of circulating tumor cells. Nano Lett. 2012;12(6):2697–2704. doi: 10.1021/nl2041707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brökera P, Lücke K, Perpeet M, Gronewold TMA. A nanostructured SAW chip-based biosensor detecting cancer cells. Sensor Actuat. B. 2012;165(1):1–6. [Google Scholar]
- 69.Hou S, Zhao H, Zhao L, et al. Capture and stimulated release of circulating tumor cells on polymer-grafted silicon nanostructures. Adv. Mater. 2013;25(11):1547–1551. doi: 10.1002/adma.201203185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shen Q, Xu L, Zhao L, et al. Specific capture and release of circulating tumor cells using aptamer-modifi ed nanosubstrates. Adv. Mater. 2013;25(16):2368–2373. doi: 10.1002/adma.201300082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hong WY, Jeon SH, Lee ES, Cho Y. An integrated multifunctional platform based on biotin-doped conducting polymer nanowires for cell capture, release, and electrochemical sensing. Biomaterials. 2014;35(36):9573–9580. doi: 10.1016/j.biomaterials.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 72.Zhang N, Deng Y, Tai Q, et al. Electrospun TiO2 nanofi ber-based cell capture assay for detecting circulating tumor cells from colorectal and gastric cancer patients. Adv. Mater. 2012;24(20):2756–2760. doi: 10.1002/adma.201200155. [DOI] [PubMed] [Google Scholar]
- 73.Hou S, Zhao L, Shen Q, Yu J, et al. Polymer nanofiber-embedded microchips for detection, isolation, and molecular analysis of single circulating melanoma cells. Angew. Chem. Int. Ed. 2013;52(12):3379–3383. doi: 10.1002/anie.201208452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yoon HJ, Kim TH, Zhang Z, et al. Sensitive capture of circulating tumour cells by functionalized graphene oxide nanosheets. Nat. Nanotechnol. 2013;8:735–881. doi: 10.1038/nnano.2013.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gu Y, Cheng J, Li Y, et al. Detection of circulating tumor cells in prostate cancer based on carboxylated graphene oxide modified light addressable potentiometric sensor. Biosens. Bioelectron. 2015;66:24–31. doi: 10.1016/j.bios.2014.10.070. [DOI] [PubMed] [Google Scholar]
- 76.Sekine J, Luo SC, Wang S, Zhu B, Tseng HR, Yu H. Functionalized conducting polymer nanodots for enhanced cell capturing: the synergistic effect of capture agents and nanostructures. Adv. Mater. 2011;23(41):4788–4792. doi: 10.1002/adma.201102151. [DOI] [PubMed] [Google Scholar]
- 77.Han W, Allio BA, Foster DG, King MR. Nanoparticle coatings for enhanced capture of flowing cells in microtubes. ACS Nano. 2010;4(1):174–180. doi: 10.1021/nn900442c. [DOI] [PubMed] [Google Scholar]
- 78.Sheng W, Chen T, Tan W, Fan ZH. Multivalent DNA nanospheres for enhanced capture of cancer cells in microfluidic devices. ACS Nano. 2013;7(8):7067–7076. doi: 10.1021/nn4023747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wan Y, Mahmood MAI, Li N, et al. Nanotextured substrates with immobilized aptamers for cancer cell isolation and cytology. Cancer. 2011;118(4):1145–1154. doi: 10.1002/cncr.26349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen W, Weng S, Zhang F, et al. Nanoroughened surfaces for efficient capture of circulating tumor cells without using capture antibodies. ACS Nano. 2013;7(1):566–575. doi: 10.1021/nn304719q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen GD, Fachin F, Fernandez-Suarez M, Wardle BL, Toner M. Nanoporous elements in microfl uidics for multiscale manipulation of bioparticles. Small. 2011;7(8):1061–1067. doi: 10.1002/smll.201002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ng E, Hoshino K, Zhang X. Microfluidic immunodetection of cancer cells via site-specific microcontact printing of antibodies on nanoporous surface. Method. 2013;63(3):266–275. doi: 10.1016/j.ymeth.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 83.Mostert B, Sleijfer S, Foekens JA, Gratama JW. Circulating tumor cells (CTCs): Detection methods and their clinical relevance in breast cancer. Cancer Treat. Rev. 2009;35(5):463–467. doi: 10.1016/j.ctrv.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 84.Yu M, Stott S, Toner M, Maheswaran S, Haber DA. Circulating tumor cells: approaches to isolation and characterization. J. Cell Biol. 2011;192(3):373–382. doi: 10.1083/jcb.201010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Michalet X, Pinaud F, Lacoste TD, et al. Properties of fluorescent semiconductor nanocrystals and their application to biological labeling. Single Mol. 2001;2(4):261–276. [Google Scholar]
- 86.Bruchez M, Jr, Moronne M, Gin P, Weiss S, Alivisatos AP. Semiconductor nanocrystals as fluorescent biological labels. Science. 1998;281(5385):2013–2016. doi: 10.1126/science.281.5385.2013. [DOI] [PubMed] [Google Scholar]
- 87.Kairdolf BA, Smith AM, Stokes TH, Wang MD, Young AN, Nie S. Semiconductor quantum dots for bioimaging and biodiagnostic applications. Ann. Rev. Anal. Chem. 2013;6:143–162. doi: 10.1146/annurev-anchem-060908-155136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hsieh YH, Liu SJ, Chen HW, Lin YK, Liang KS, Lai LJ. Highly sensitive rare cell detection based on quantum dot probe fluorescence analysis. Anal. Bioanal. Chem. 2010;396(3):1135–1141. doi: 10.1007/s00216-009-3323-6. [DOI] [PubMed] [Google Scholar]
- 89.Hsieh YH, Lai LJ, Liu SJ, Liang KS. Rapid and sensitive detection of cancer cells by coupling with quantum dots and immunomagnetic separation at low concentrations. Biosens. Bioelectron. 2011;26(10):4249–4252. doi: 10.1016/j.bios.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 90.Lee HJ, Cho HY, Oh JH, et al. Simultaneous captureand in situ analysis of circulating tumor cells using multiple hybrid nanoparticles. Biosens. Bioelectron. 2013;47:508–514. doi: 10.1016/j.bios.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 91.Winnik FM, Maysinger D. Quantum dot cytotoxicity and ways to reduce it. Acc. Chem. Res. 2013;46(3):672–680. doi: 10.1021/ar3000585. [DOI] [PubMed] [Google Scholar]
- 92.Zhang H, Fu X, Hu J, Zhu Z. Microfluidic bead-based multienzyme-nanoparticle amplification for detection of circulating tumor cells in the blood using quantum dots labels. Anal. Chim. Acta. 2013;779:64–71. doi: 10.1016/j.aca.2013.03.060. [DOI] [PubMed] [Google Scholar]
- 93.DaCosta MV, Doughan S, Han Y, Krul UJ. Lanthanide upconversion nanoparticles and applications in bioassays and bioimaging: a review. Anal. Chim. Acta. 2014;832:1–33. doi: 10.1016/j.aca.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 94.Fang S, Wan C, Xiang J, et al. Aptamer-conjugated upconversion nanoprobes assisted by magnetic separation for effective isolation and sensitive detection of circulating tumor cells. Nano Res. 2014;7(9):1327–1336. [Google Scholar]
- 95.Nie S, Emory SR. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science. 1997;275(5303):1102–1106. doi: 10.1126/science.275.5303.1102. [DOI] [PubMed] [Google Scholar]
- 96.Kneipp K, Wang Y, Kneipp H, et al. Single molecule detection using surface-enhanced Raman scattering (SERS) Phys. Rev. Lett. 1997;78:1667–1670. [Google Scholar]
- 97.Doering WE, Piotti ME, Natan MJ, Freeman RG. SERS as a foundation for nanoscale, optically detected biological labels. Adv. Mater. 2007;19(20):3100–3108. [Google Scholar]
- 98.Sha MY, Xu H, Penn SG, Cromer R. SERS nanoparticles: a new optical detection modality for cancer diagnosis. Nanomedicine. 2007;2(5):725–734. doi: 10.2217/17435889.2.5.725. [DOI] [PubMed] [Google Scholar]
- 99.Zavaleta CL, Smith BR, Walton I, et al. Multiplexed imaging of surface enhanced Raman scattering nanotags in living mice using noninvasive Raman spectroscopy. Proc. Natl Acad. Sci. USA. 2009;106(32):13511–13566. doi: 10.1073/pnas.0813327106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.von Maltzahn G, Centrone A, Park JH, et al. SERS-coded gold nanorods as a multifunctional platform for densely multiplexed near-infrared imaging and photothermal heating. Adv. Mater. 2009;21(31):1–6. doi: 10.1002/adma.200803464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sha MY, Xu HX, Natan MJ, Cromer R. Surface-enhanced Raman scattering tags for rapid and homogeneous detection of circulating tumor cells in the presence of human whole blood. J. Am. Chem. Soc. 2008;130(51):17214–17215. doi: 10.1021/ja804494m. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper reports the first application of surface-enhanced Raman scattering spectroscopy for circulating tumor cell detection.
- 102.Shi W, Paproski RJ, Moore R, Zemp R. Detection of circulating tumor cells using targeted surface-enhanced Raman scattering nanoparticles and magnetic enrichment. J. Biomed. Optics. 2014;19(5):056014. doi: 10.1117/1.JBO.19.5.056014. [DOI] [PubMed] [Google Scholar]
- 103.Wang X, Qian XM, Beitler JJ, et al. Detection of circulating tumor cells in human peripheral blood using surface-enhanced Raman scattering nanoparticles. Cancer Res. 2011;71(5):1526–1532. doi: 10.1158/0008-5472.CAN-10-3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang P, Zhang R, Gao M, Zhang X. Novel nitrocellulose membrane substrate for efficient analysis of circulating tumor cells coupled with surface-enhanced Raman scattering imaging. ACS Appl. Mater. Interfaces. 2014;6(1):370–376. doi: 10.1021/am404406c. [DOI] [PubMed] [Google Scholar]
- 105.Nima ZA, Mahmood M, Xu Y, et al. Circulating tumor cell identification by functionalized silver-gold nanorods with multicolor, super-enhanced SERS and photothermal resonances. Sci. Rep. 2014;4:4752/1–8. doi: 10.1038/srep04752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Issadore D, Chung J, Shao H, et al. Ultrasensitive clinical enumeration of rare cells ex vivo using a micro-hall detector. Sci. Transl. Med. 2012;4(141):141ra92/1–10. doi: 10.1126/scitranslmed.3003747. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This paper reports an innovative application of magnetic nanoparticles for circulating tumor cell detection rather than conventional usage for magnetic separation.
- 107.Ghazani AA, Pectasides M, Sharma A, et al. Molecular characterization of scant lung tumor cells using iron-oxide nanoparticles and micro-nuclear magnetic resonance. Nanomed: Nanotechnol. Biol. Med. 2014;10(3):661–668. doi: 10.1016/j.nano.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee H, Yoon TJ, Figueiredo JL, Swirski FK, Weissleder R. Rapid detection and profiling of cancer cells in fine-needle aspirates. Proc. Natl Acad. Sci. USA. 2009;106(30):12459–12464. doi: 10.1073/pnas.0902365106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bernholc J, Brenner D, Nardelli MB, Meunier V, Roland C. Mechanical and electric properties of nanotubes. Annu. Rev. Mater. Res. 2002;32:347–375. [Google Scholar]
- 110.Liu Y, Zhu F, Dan W, Fu Y, Liu S. Construction of carbon nanotube based nanoarchitectures for selective impedimetric detection of cancer cells in whole blood. Analyst. 2014;139:5086–5092. doi: 10.1039/c4an00758a. [DOI] [PubMed] [Google Scholar]
- 111.Kwon T, Park J, Lee G, et al. Carbon nanotube-patterned surface-based recognition of carcinoembryonic antigens in tumor cells for cancer diagnosis. J. Phys. Chem. Lett. 2013;4(7):1126–1130. doi: 10.1021/jz400087m. [DOI] [PubMed] [Google Scholar]
- 112.Maeda Y, Yoshino T, Matsunaga T. Novel nanocomposites consisting of in vivo-biotinylated bacterial magnetic particles and quantum dots for magnetic separation and fluorescent labeling of cancer cells. J. Mater. Chem. 2009;19:6361–6366. [Google Scholar]
- 113.Mi Y, Li K, Liu Y, Pu KY, Liu B, Feng SS. Herceptin functionalized polyhedral oligomeric silsesquioxane-conjugated oligomers-silica/iron oxide nanoparticles for tumor cell sorting and detection. Biomaterials. 2011;32(32):8226–8233. doi: 10.1016/j.biomaterials.2011.07.040. [DOI] [PubMed] [Google Scholar]
- 114.Kim JH, Chung HH, Jeong MS, Song MR, Kang KW, Kim JS. One-step detection of circulating tumor cells in ovarian cancer using enhanced fluorescent silica nanoparticles. Int. J. Nanomed. 2013;8:2247–2257. doi: 10.2147/IJN.S45059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fan Z, Shelton M, Singh AK, Senapati D, Khan SA, Ray PC. Multifunctional plasmonic shell magnetic core nanoparticles for targeted diagnostics, isolation, and photothermal destruction of tumor cells. ACS Nano. 2012;6(2):1065–1073. doi: 10.1021/nn2045246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bhana S, Chaffin E, Wang Y, Mishra SR, Huang X. Capture and detection of cancer cells in whole blood with magnetic-optical nanoovals. Nanomedicine. 2014;9(5):593–606. doi: 10.2217/nnm.13.77. [DOI] [PubMed] [Google Scholar]; •• This paper reports a novel method that is capable of capturing and detecting circulating tumor cells in whole blood with a single nanostructure agent.
- 117.Bhana S, Rai BK, Mishra SR, Wang Y, Huang X. Synthesis and properties of near infrared-absorbing magnetic-optical nanopins. Nanoscale. 2012;4(16):4939–4942. doi: 10.1039/c2nr31291c. [DOI] [PubMed] [Google Scholar]
- 118.Galanzha EI, Zharov VP. Circulating tumor cell detection and capture by photoacoustic flow cytometry in vivo and ex vivo . Cancer. 2013;5(4):1691–1738. doi: 10.3390/cancers5041691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Georgakoudi I, Solban N, Novak J, et al. In vivo flow cytometry: a new method for enumerating circulating cancer cells. Cancer Res. 2004;64(15):5044–5047. doi: 10.1158/0008-5472.CAN-04-1058. [DOI] [PubMed] [Google Scholar]
- 120.He W, Wang H, Hartmann LC, Cheng JX, Low PS. In vivo quantification of rare circulating tumor cells by multiphoton intravital flow cytometry. Proc. Natl Acad. Sci. USA. 2007;104(28):11760–11765. doi: 10.1073/pnas.0703875104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Boutrus S, Greiner C, Hwu D, et al. Portable two-color in vivo flow cytometer for real-time detection of fluorescently-labeled circulating cells. J. Biomed. Opt. 2007;12(2):020507. doi: 10.1117/1.2722733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tkaczyk ER, Zhong CF, Ye JY, et al. In vivo monitoring of multiple circulating cell populations using two-photon flow cytometry. Opt. Commun. 2008;281:888–894. doi: 10.1016/j.optcom.2007.10.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tkaczyk ER, Tkaczyk AH, Katnik S, et al. Extended cavity laser enhanced two-photon flow cytometry. J. Biomed. Opt. 2008;13(4):041319/1–12. doi: 10.1117/1.2967983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chang YC, Ye JY, Thomas TP, Cao ZY, et al. Fiber-optic multiphoton flow cytometry in whole blood and in vivo . J. Biomed. Opt. 2010;15(4):047004/1–9. doi: 10.1117/1.3463481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hristozova T, Konschak R, Budach V, Tinhofer I. A simple multicolor flow cytometry protocol for detection and molecular characterization of circulating tumor cells in epithelial cancers. Cytometry A. 2012;81A:489–495. doi: 10.1002/cyto.a.22041. [DOI] [PubMed] [Google Scholar]
- 126.Nedosekin DA, Verkhusha VV, Melerzanov AV, Zharov VP, Galanzha EI. In vivo photoswitchable flow cytometry for direct tracking of single circulating tumor cells. Chem. Biol. 2014;21(6):792–801. doi: 10.1016/j.chembiol.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zharov V, Galanzha E, Shashkov E, Khlebtsov N, Tuchin V. In vivo photoacoustic flow cytometry for monitoring circulating singe cancer cells and contrast agents. Opt. Lett. 2006;31(24):3623–3625. doi: 10.1364/ol.31.003623. [DOI] [PubMed] [Google Scholar]
- 128.Holan SH, Viator JA. Automated wavelet denoising of photoacoustic signals for circulating melanoma cell detection and burn image reconstruction. Phys. Med. Biol. 2008;53(12):N227–N236. doi: 10.1088/0031-9155/53/12/N01. [DOI] [PubMed] [Google Scholar]
- 129.Galanzha EI, Shashkov EV, Spring PM, Suen JY, Zharov VP. In vivo, noninvasive, label-free detection and eradication of circulating metastatic melanoma cells using two-color photoacoustic flow cytometry with a diode laser. Cancer Res. 2009;69(20):7926–7934. doi: 10.1158/0008-5472.CAN-08-4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Galanzha EI, Shashkov EV, Kelly T, Kim JW, Yang L, Zharov VP. In vivo magnetic enrichment and multiplex photoacoustic detection of circulating tumour cells. Nat. Nanotechnol. 2009;4:855–860. doi: 10.1038/nnano.2009.333. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This paper described an innovative approach that enrichs and detects circulating tumor cells in vivo by integrating multiplexed targeting, magnetic separation and photoacoustic detection.
- 131.Galanzha EI, Kim JM, Zharov VP. Nanotechnology-based molecular photoacoustic and photothermal flow cytometry platform for in-vivo detection and killing of circulating cancer stem cells. J. Biophoton. 2009;2(12):725–735. doi: 10.1002/jbio.200910078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zharov VP. Ultrasharp nonlinear photothermal and photoacoustic resonances and holes beyond the spectral limit. Nat. Photon. 2011;5:110–116. doi: 10.1038/nphoton.2010.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nedosekin DA, Juratli MA, Sarimollaoglu M, et al. Photoacoustic and photothermal detection of circulating tumor cells, bacteria and nanoparticles in cerebrospinal fluid in vivo and ex vivo . J. Biophoton. 2013;6(6–7):523–533. doi: 10.1002/jbio.201200242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Galanzha E, Nedosekin DA, Sarimollaoglu M, et al. Photoacoustic and photothermal cytometry using photoswitchable proteins and nanoparticles with ultrasharp resonances. J. Biophoton. 2015;8(1–2):81–93. doi: 10.1002/jbio.201300140. [DOI] [PubMed] [Google Scholar]
- 135.Juratli M, Sarimollaoglu M, Siegel E, et al. Real-time monitoring of circulating tumor cell release during tumor manipulation using in vivo photoacoustic and fluorescent flow cytometry. Head Neck. 2014;36(8):1207–1215. doi: 10.1002/hed.23439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nedosekin DA, Sarimollaoglu M, Galanzha EI, et al. Synergy of photoacoustic and fluorescence flow cytometry of circulating cells with negative and positive contrasts. J. Biophoton. 2013;6:425–434. doi: 10.1002/jbio.201200047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zharov VP, Galanzha EI, Tuchin VV. Photothermal image flow cytometry in vivo . Opt. Lett. 2005;30(6):628–630. doi: 10.1364/ol.30.000628. [DOI] [PubMed] [Google Scholar]
- 138.Galanzha EI, Zharov VP. Photoacoustic flow cytometry. Method. 2012;57:280–296. doi: 10.1016/j.ymeth.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]