Abstract
Sucrose-specific regulation of gene expression is recognized as an important signaling response, distinct from glucose, which serves to modulate plant growth, metabolism, and physiology. The Arabidopsis MYB transcription factor Production of Anthocyanin Pigment-1 (PAP1) plays a key role in anthocyanin biosynthesis and expression of PAP1 is known to be regulated by sucrose. Sucrose treatment of Arabidopsis seedlings led to a 20-fold induction of PAP1 transcript, which represented a 6-fold increase over levels in glucose-treated seedlings. The PAP1 promoter was not sufficient for conferring a sucrose response to a reporter gene and did not correctly report expression of PAP1 in plants. Although we identified 3 putative sucrose response elements in the PAP1 gene, none were found to be necessary for this response. Using deletion analysis, we identified a 90 bp sequence within intron 1 of PAP1 that is necessary for the sucrose response. This sequence was sufficient for conferring a sucrose response to a minimal promoter: luciferase reporter when present in multiple copies upstream of the promoter. This work lays the foundation for dissecting the sucrose signaling pathway of PAP1 and contributes to understanding the interplay between sucrose signaling, anthocyanin biosynthesis, and stress responses.
Introduction
Regulation of genes by sugars such as glucose and sucrose has been well documented in plants and plays an important role in plant growth, development, and physiology [1–6]. Although it can be difficult to attribute signaling responses to a specific sugar molecule as opposed to one of its metabolites or catabolites, there is strong evidence for distinct signaling pathways for both glucose and sucrose [7–9]. Compared with glucose, sucrose-specific signaling pathways are not well characterized, and a sucrose-specific sensor functioning similar to that of hexokinase in glucose signaling has not been identified although several have been proposed [3, 7].
Genes regulated specifically by sucrose are generally defined as those that do not respond in a similar fashion to glucose or fructose. The best known examples of sucrose regulated genes are the Beta vulgaris sucrose transporter BvSUT1 from sugar beet [5, 9], patatin from potato [10, 11], and the Production of Anthocyanin Pigmentation-1 PAP1/MYB75 transcription factor that regulates anthocyanin biosynthesis in Arabidopsis thaliana [12, 13]. For these genes, there is minimal regulation in the presence of glucose or fructose. Other genes such as chalcone synthase [14], rolC [15], and beta amylase [16, 17] respond to glucose and other sugars yet show a stronger response to sucrose, while for others comparable responses have been observed for both sucrose and glucose [18–20]. It should also be noted that in many cases differences in gene responses to sugars have been reported. For example, glucose and fructose-induced expression of potato proteinase inhibitor II was reported to be equal to or half the response observed with sucrose [21, 22]. And higher expression of PAP1 in glucose versus sucrose-treated seedlings has also been reported [23]. This is most likely due to differences in how experiments were performed and/or underlying metabolic changes in sugar content and identity.
For some of these genes sugar response elements or sequences important for sugar regulation have been identified and nuclear binding proteins or activities characterized [4]. Genetic approaches have led to identification of genes that play important roles in regulating sugar expression of specific genes [24, 25]. However, overall the molecular pathways involved in sucrose-signaling are not well understood.
We are using Arabidopsis PAP1, a known sucrose-regulated gene, as a model to dissect sucrose signaling. Teng et al first identified PAP1 as important for sucrose-induced anthocyanin accumulation in Arabidopsis and showed that a PAP1 knock-out mutant lacked this response [12]. Solfanelli et al further showed that expression of PAP1 was specific to sucrose and not glucose or fructose [13], although a more recent paper reported higher PAP1 expression in glucose-treated seedlings compared to sucrose-treated seedlings [23]. Sucrose-induced expression of PAP1 has been shown to be modulated by hormones [23, 26–28], mutations in the AtSUC1 sucrose transporter [29], and alterations in calcium signaling [30]. In many cases it is not clear if these factors directly impact PAP1 expression or are secondary due to changes in endogenous sugar content. Although there have been several reports on the expression of PAP1 in response to sugars, sequences important for the sucrose-responsiveness of PAP1 have not been identified.
To begin to dissect the sucrose-regulated pathway of PAP1 we have localized sequences important for this response to an intron and further show that this intronic sequence can confer sucrose-responsiveness to a reporter gene. We also discuss the implications of PAP1 regulation by sucrose and how this might contribute to regulation of anthocyanin biosynthesis in plants.
Results
Sucrose specificity of anthocyanin biosynthesis
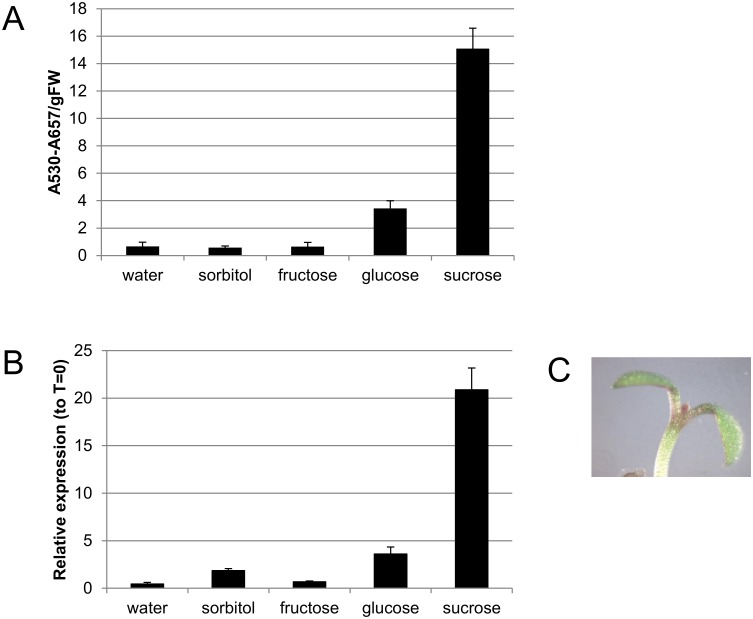
To confirm the sucrose-specific induction of anthocyanin biosynthesis, Arabidopsis Col-0 seeds were germinated in one-half-strength liquid Murashige and Skoog (MS) media and seedlings were treated with water or various sugars after 4 days of growth in continuous light. Seedlings were harvested 48 hours later and anthocyanins quantified (Fig 1A). Sucrose treated seedlings accumulated the most anthocyanin, although glucose treated seedlings also accumulated anthocyanin relative to the water treated control. These results are similar to those reported by Teng et al [12]. Expression of PAP1 was examined by RT-qPCR 24 hrs after addition of water or various sugars (Fig 1B). PAP1 transcript abundance paralleled anthocyanin accumulation; PAP1 levels were 20- and 3.6-fold greater after sucrose and glucose treatment, respectively.
Fig 1. Sucrose-specific anthocyanin biosynthesis in light grown seedlings.
A. Anthocyanin quantification in 4 day old seedlings treated with water or 90 mM sugar for 48 hours in continuous light. B. RT-qPCR of PAP1 transcript in 4 day old seedlings treated with water or 90 mM sugar for 24 hours in continuous light. C. Anthocyanin pigmentation in a seedling treated with 90 mM sucrose for 24 hours.
Identification of sequences important in conferring sucrose responsiveness of PAP1
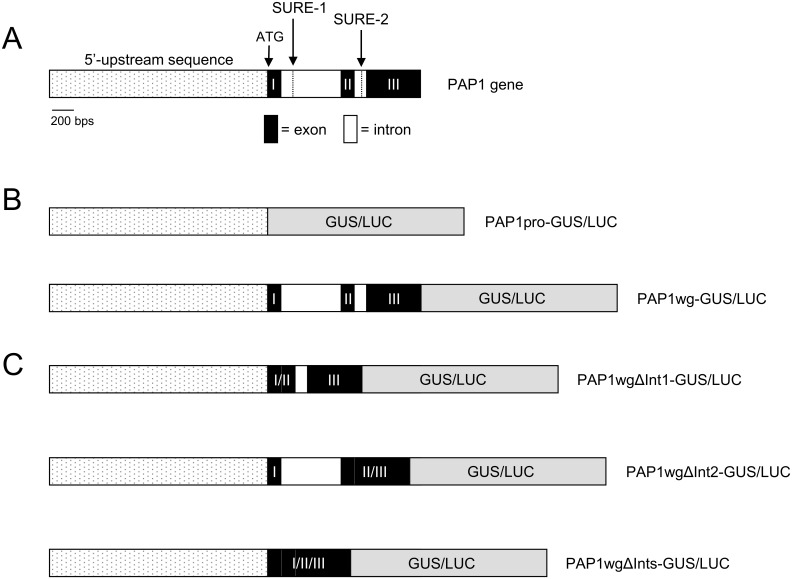
To define sequences important in governing the sucrose-induced expression of PAP1, we generated PAP1 promoter: reporter fusions with β-galactosidase and luciferase (PAP1pro:GUS or LUC). The promoter sequence was defined as 2006 bp upstream of the PAP1 start codon. A known sucrose response element (SRE), SURE-2, was identified in the promoter of PAP1 using the Plant cis-acting regulatory DNA element (PLACE) database [31]. SURE-1 (TTTTCTATT) and SURE-2 (AATACTAAT) elements were initially identified as important in conferring sucrose responsiveness of patatin, a lipolytic acyl hydrolase from potato [32]. SURE-1 and SURE-2 share sequence similarity with one another (5 out of 9 identical nucleotides) and with SP8 elements [32, 33]. The similarity between the SURE-2 elements in the PAP1 and patatin promoters extended beyond the 9 bp core element to 17 bp with only one discrepancy (patatin sequence: TATATAATACTAATAAA; PAP1 sequence has a T in place of the underlined A).
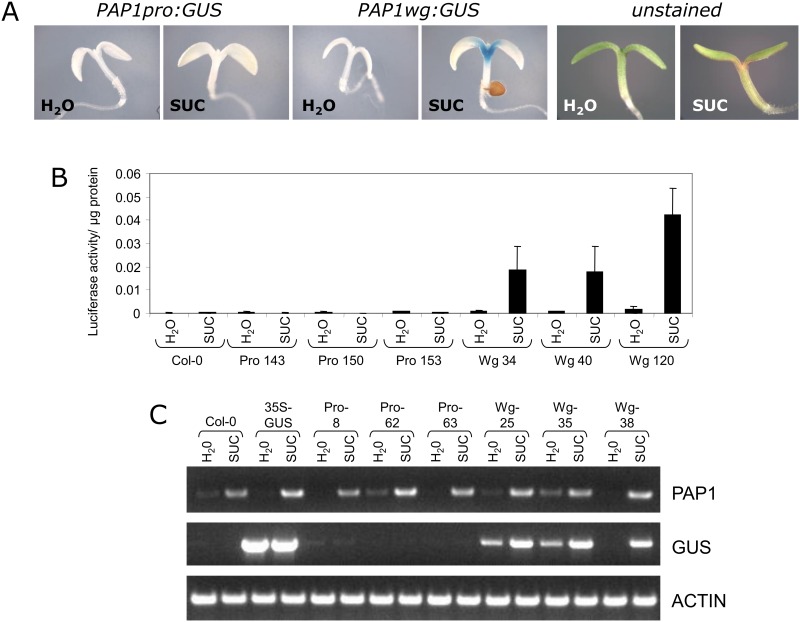
Constructs were transformed into Col-0 and transgenic plants were generated. For these and all subsequent experiments, 5–10 single copy homozygous lines were generated per construct and data is shown for 2–4 representative lines per construct. When plants were tested for sucrose-induced GUS or LUC activity as described above, no activity was observed (Fig 2A and 2B). RT-PCR of sucrose-treated PAP1pro:GUS plants showed that even though PAP1 transcript was present, the GUS transcript was not expressed in response to sucrose (Fig 2C).
Fig 2. Sucrose-responsiveness of PAP1 promoter and whole gene constructs.
A. Representative GUS staining of 4 day old seedlings treated with water or 90 mM sucrose for 24 hours. In sucrose-treated seedlings, anthocyanin pigmentation is observed at the base of the cotyledons (unstained seedlings). B. Luciferase activity of PAP1 promoter and whole gene constructs in 4 day old seedlings treated with water or 90 mM sucrose for 24 hours. C. RT-PCR of PAP1, GUS, and actin transcripts in 4 day old PAP1pro:GUS and PAP1wg:GUS seedlings treated with 90 mM sucrose for 4 hours. Numbers following Pro and Wg abbreviations refer to independent transgenic lines.
Several genes, including sucrose transporters, have been shown to require additional sequences contained within exons and introns for proper gene expression [34]. To test whether sequences in the exons and introns are important for sucrose-induced expression of PAP1, we fused the PAP1 promoter plus all exons and introns to GUS or LUC (PAP1wg:GUS or LUC) (Fig 3B). Transgenic plants harboring these constructs were generated and tested for GUS and LUC activity. Both reporter constructs showed sucrose-dependent reporter gene activity (Fig 2A and 2B). Furthermore, the PAP1wg:GUS lines had GUS staining at the base of the cotyledon and the upper part of the hypocotyl, reminiscent of anthocyanin pigmentation in seedlings (Figs 1C and 2A). RT-PCR of these plants showed that the GUS transcript was expressed in response to sucrose, similar to the expression of the endogenous PAP1 transcript (Fig 2C).
Fig 3. PAP1 gene organization and reporter gene constructs.
A. Schematic of PAP1 gene showing the location of the SURE-1 and SURE-2 elements. B. Schematic of PAP1 reporter gene constructs.
PAP1 gene expression during plant development
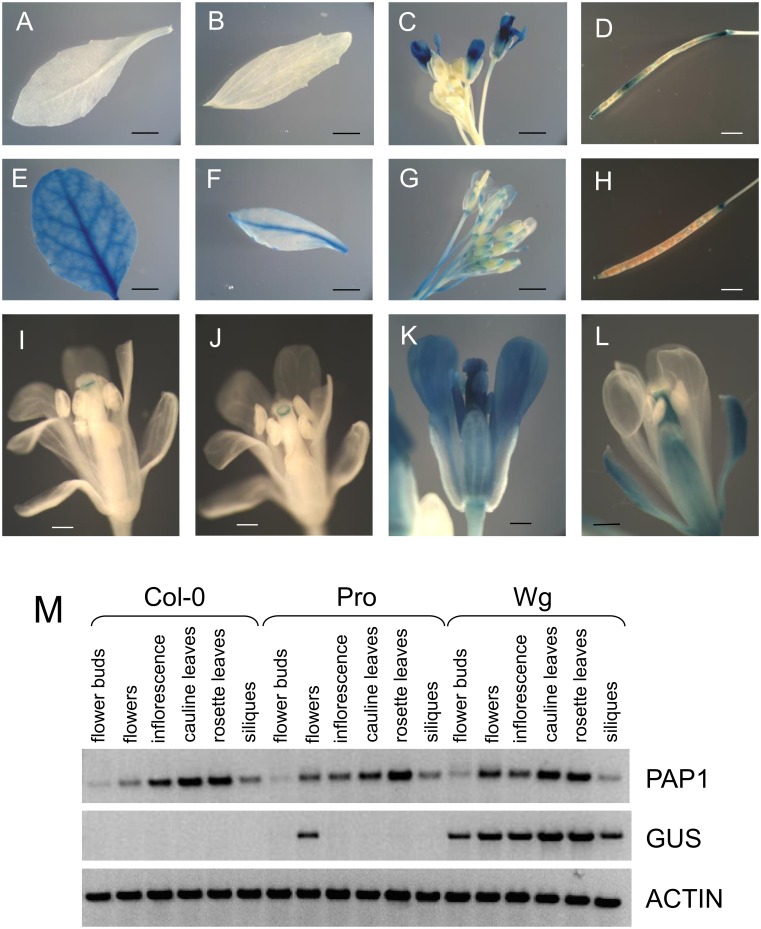
To determine whether PAP1pro:GUS plants correctly reported PAP1 expression at other developmental stages we compared the pattern of GUS expression of PAP1pro:GUS and PAP1wg:GUS lines. Seeds were germinated on MS media plus 2% sucrose and plants were harvested at various time points. In PAP1wg:GUS lines GUS staining was observed in the hypocotyl at 3, 5, and 7 days; no GUS staining was observed in PAP1pro:GUS lines (S1 Fig). Fourteen day old PAP1wg:GUS plants had strong GUS staining in leaves and cotyledons and faint patches of staining could also be observed in the roots (S2 Fig). No GUS staining was observed in PAP1pro:GUS lines. At 20 days PAP1wg:GUS lines had GUS staining in the leaves, mostly along the major veins and petioles (S3 Fig). PAP1pro:GUS lines did not show GUS staining except for a very small spot on the primary root several mm from the base of the rosette. This was distinct from the pattern of GUS staining observed in PAP1wg:GUS lines, in which GUS staining was limited to the top of the primary root near the base of the rosette. RT-PCR of 20 day old plants indicated PAP1 was expressed in both shoots and roots, while PAP1pro:GUS lines only showed GUS transcript in the roots (S3 Fig).
In flowering plants (28–42 days old) there was some variation in the pattern and intensity of GUS staining in different experiments that may be due to differences in plant development or environmental conditions. In all plants examined, PAP1pro:GUS lines showed GUS staining in petals, ovaries, and filaments of mature flowers and in some experiments staining was also observed in stigma and anthers (Fig 4C and 4K). Interestingly, in PAP1pro:GUS lines flower buds had little to no staining and immature flowers only showed a discrete band of staining just under the stigma, suggesting that the observed GUS expression pattern may be under developmental control (Fig 4C, 4I and 4J). Siliques were usually stained, but seeds were not (Fig 4D). In some experiments GUS staining could also be observed in cauline leaves (weak staining along the major vein), secondary inflorescences, and pedicels close to the junction with flowers and siliques. No staining was observed in rosette leaves (Fig 4A), stem, primary inflorescences, and sepals (Fig 4K).
Fig 4. GUS expression patterns (A-L) and RT-PCR (M) of PAP1pro:GUS (A-D, I-K) and PAP1wg:GUS (E-H, L) lines.
PAP1pro:GUS lines did not show expression in rosette (A) or cauline (B) leaves. Siliques usually showed patchy staining (D). No staining was observed in flower buds (C). Immature flowers (I, J) showed a band of staining just under the stigma, while mature flowers (C, K) showed expression in flower petals, ovaries, and filaments. PAP1wg:GUS lines showed staining in rosette (E) and cauline (F) leaves, flowers at all stages (G), and siliques (H). GUS staining was observed in sepals, ovaries, and filaments of flowers (L). Siliques of PAP1pro:GUS plants were not uniformly or consistently stained in this experiment. M. RT-PCR of 5 week old plants expressing PAP1 promoter and whole gene constructs. Scale bars are 3 mm in A, B, E, and F, 2 mm in C, D, G, and H, and 0.5 mm in I-L.
In comparison, GUS staining of PAP1wg:GUS lines was more uniformly distributed and present in most organs including rosette and cauline leaves (most notably along veins), flowers, and pedicels (Fig 4E–4G). In contrast to the staining observed in PAP1pro:GUS lines, PAP1wg:GUS lines showed GUS staining in sepals, but not petals (Fig 4L). The lack of GUS staining in petals is consistent with the lack of anthocyanins in Arabidopsis petals [35]. GUS staining was also observed in ovaries and filaments in flowers, but not stigma or anthers (Fig 4L). In some experiments staining was observed in stem and primary and secondary inflorescences, and siliques (Fig 4H). No GUS staining was observed in seeds.
GUS staining and GUS transcript observed in PAP1wg:GUS lines correlated well with native PAP1 transcript as detected by RT-PCR, which showed PAP1 transcript in rosette and cauline leaves, inflorescence, flower buds, flowers and siliques (Fig 4M). PAP1pro:GUS lines only showed GUS transcript in flowers, but not in any other tissues. These results suggest that the PAP1 promoter is not correctly reporting PAP1 expression in Arabidopsis. Our expression data for PAP1wg:GUS lines correlates well with expression data in the AtGenExpress atlas of Arabidopsis development [36]. In this dataset expression values of PAP1 are fairly low (with the exception of senescing leaves), but expression is detected in rosette and cauline leaves, 7 day old seedlings, and stage 15 flowers (including petals, stamen, and carpels). Taken together, the whole gene construct is not only necessary for the response to sucrose as it contains sequences necessary for controlling PAP1 expression as a function of tissue and development.
The expression pattern of a similar PAP1pro:GUS construct has been reported [37]. GUS expression was reported in hypocotyls and cotyledons of 2–3 day old seedlings and young emerging leaf tissue/primordia of 5–7 day old seedlings. As discussed above, we did not observe any GUS staining in seedlings of our PAP1pro:GUS lines when grown under our conditions or those reported by Gonzalez et al.
Importance of intronic sequences in conferring sucrose responsiveness of PAP1
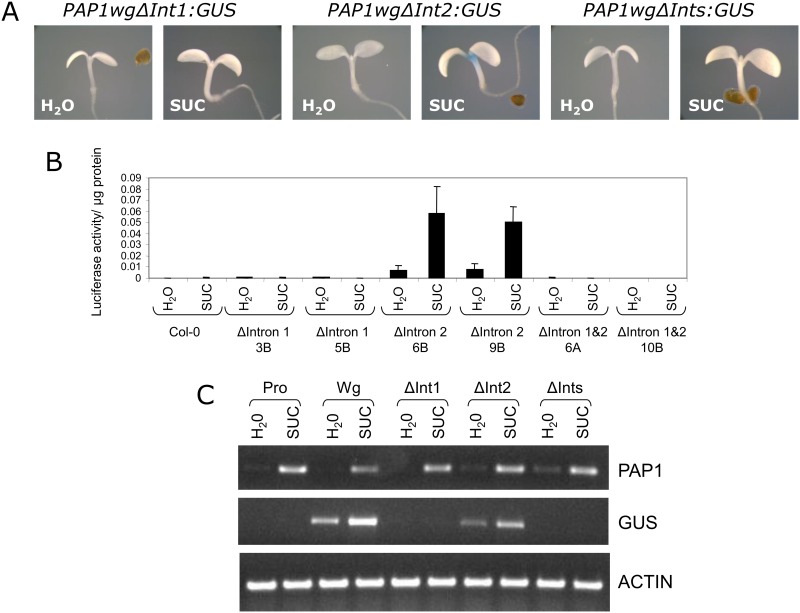
To further define sequences important in controlling sucrose-dependent expression of PAP1, we generated additional reporter gene constructs lacking one or both introns of PAP1 (PAP1wgΔInt1:GUS or LUC, PAP1wgΔInt2:GUS or LUC, PAP1wgΔInts:GUS or LUC) (Fig 3C). Transgenic plants were generated and tested for reporter gene activity. Constructs lacking intron 1 or both intron 1 and 2 (PAP1wgΔInt1:GUS or LUC and PAP1wgΔInts:GUS or LUC) did not have reporter gene activity in response to sucrose (Fig 5A and 5B). This was confirmed by RT-PCR, which showed no GUS transcript expressed in response to sucrose (Fig 5C). However, the construct lacking intron 2 (PAP1wgΔInt2:GUS) retained sucrose-induced reporter gene activity and GUS expression in response to sucrose. The pattern of GUS staining observed for PAP1wgΔInt2:GUS in seedlings and plants was identical to that observed for the construct containing the entire PAP1 gene (PAP1wg:GUS) (Figs 2A & 5A). However, the pattern of GUS staining in PAP1wgΔInt1:GUS and PAP1wgΔInts:GUS plants was much more limited. In these plants staining was observed in the major vein of rosette leaves with more intense staining near the base of the rosette and weaker staining towards the tip of the leaves (S4 Fig). Staining was also observed at the base of cauline leaves at the attachment point to the inflorescence. No other staining was observed in these lines.
Fig 5. Sucrose-responsiveness of PAP1 delta intron gene constructs.
A. Representative GUS staining of 4 day old seedlings treated with water or 90 mM sucrose for 24 hours. B. Luciferase activity of PAP1 delta intron constructs in 4 day old seedlings treated with water or 90 mM sucrose for 24 hours. C. RT-PCR of PAP1, GUS, and actin transcripts in 4 day old seedlings treated with 90 mM sucrose for 4 hours.
Role of SURE-1 in the sucrose-response
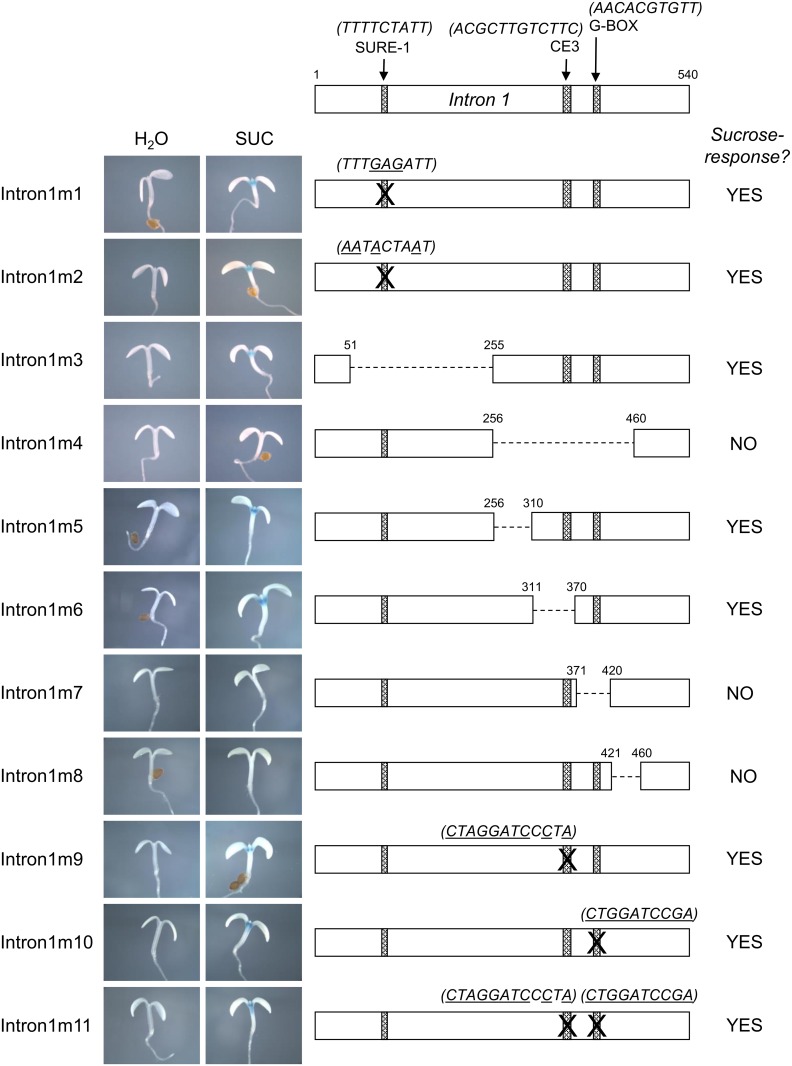
Having identified intron 1 as important for the sucrose response of PAP1, we next used PLACE to scan intron 1 for known cis-elements and identified the SURE-1 sucrose response element (TTTTCTATT) (Fig 3A). Interestingly, we also identified another SURE-2 element (AATACTAAT) within intron 2, which was shown to be dispensable for the sucrose response. To determine whether the SURE-1 element in intron 1 was necessary for the sucrose-responsiveness of PAP1, we generated 2 constructs in which the SURE-1 element was directly mutagenized. For intron1m1, the SURE-1 element (TTTTCTATT) was mutated to TTTGAGATT according to Grierson et al, who showed that this mutation abolished binding of a protein from nuclear extracts of potato tubers in electrophoresis mobility shift assays [32]. In the second construct, intron1m2, 4 nucleotides of the SURE-1 element were mutagenized to convert the SURE-1 element (TTTTCTATT) to the SURE-2 element (AATACTAAT). The SURE-2 element is present in the promoter and intron 2 of PAP1 and does not appear to be essential for the sucrose response as described above. The mutations were incorporated into PAP1wg:GUS constructs and neither mutation was observed to alter splicing. Both mutants retained sucrose-induced GUS activity and GUS expression in response to sucrose (Fig 6). This suggests that the SURE-1 element in intron 1 is not important in conferring sucrose responsiveness to PAP1.
Fig 6. Summary of sucrose responsiveness of PAP1 Intron 1 mutants.
Detailed schematic depicting mutations of PAP1 intron 1 and representative GUS staining of 4 day old seedlings treated with water or 90 mM sucrose for each mutation.
Deletion analysis defines a 90 bp region in intron 1 as important for the sucrose-response of PAP1
In order to identify sequences in intron 1 that are important for the sucrose-response of PAP1 we deleted regions of intron 1 within the context of the PAP1wg:GUS construct. Intron 1 is 540 bp and initially we tested two constructs with non-overlapping 205 bp deletions (intron1m3 and intron1m4, Fig 6). These deletions did not include the first 50 or last 80 nucleotides of the intron so as not to disrupt splicing, which was verified by RT-PCR for all constructs. Nucleotides 51–255 of intron 1 (including the SURE-1 element) were deleted in intron1m3 and nucleotides 256–460 were deleted in intron1m4 (Fig 6). The intron1m3 construct retained sucrose-induced GUS activity and expression, while no GUS expression or activity was observed in sucrose-treated seedlings of intron1m4 (Fig 6). These results further verify that the SURE-1 element is not important for the sucrose-response of PAP1 because removal of the SURE-1 element (in intron1m3) did not affect the sucrose-response.
Smaller (40–60 bp) deletions were made within the 205 bp region deleted in intron1m4 and these constructs were tested for sucrose responsiveness (intron1m5- 8, Fig 6). In addition, two potential cis-elements within this 205 bp region were also mutagenized (intron1m9-11, Fig 6). These potential cis-elements included a 10 nt palindromic G-box sequence with similarity to an abscisic acid response element (G/ABRE consensus: (T/C)ACGTG(T/G)C, PAP1 sequence: AACACGTGTT) [38] and a sequence resembling Coupling Element 3 (ACGCGTGTCCTC) that has been found to be associated with ABREs (PAP1 sequence: ACGCTTGTCTTC) [39, 40] (Fig 6). Both of these elements were mutated individually (intron1m9 and 10) as well as together (intron1m11). All three of these constructs retained a sucrose-response, indicating these putative elements are not important for sucrose-dependent expression (Fig 6).
Deletion constructs intron1m5 and 6 also retained sucrose-responsiveness, but sucrose-induced GUS activity was absent in constructs intron1m7 and intron1m8. RT-PCR and sequencing confirmed that splicing was not affected in these mutants. This identified a 90 bp region of intron 1, that when deleted (in constructs intron1m7&8), abolished the sucrose-response.
A 90 bp region is sufficient for conferring a sucrose response
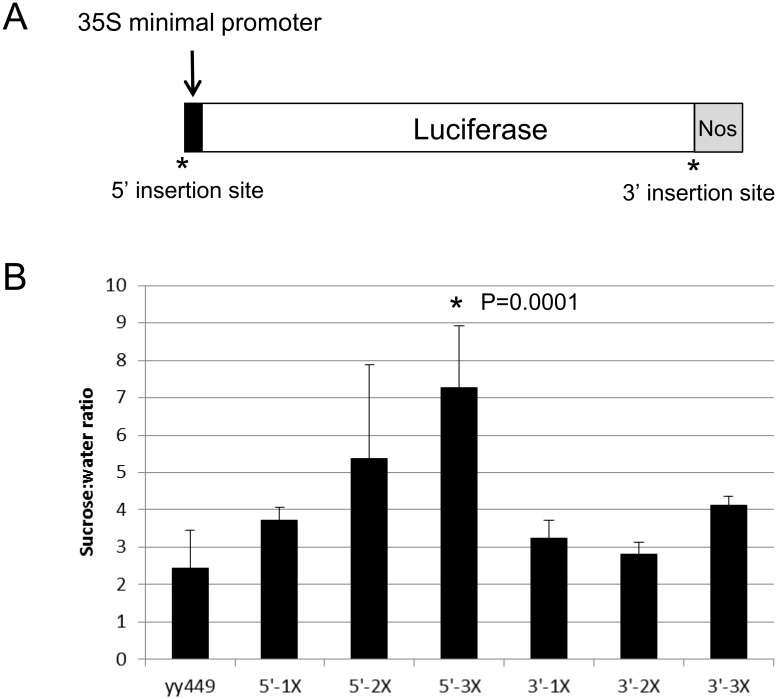
To determine if this 90 bp region in the intron of PAP1 is sufficient to confer a sucrose response, we cloned 1–3 copies of this region upstream of the 35S minimal promoter and LUC reporter gene (5’-1X, 5’-2X, 5’-3X; Fig 7). We also cloned the same fragments downstream of LUC (3’-1X, 3’-2X, 3’-3X; Fig 7) to test whether the spatial context of the element was important. Relative to the empty yy449 vector, only the 5’-3X construct had a significantly higher sucrose response although there was a trend of increased sucrose response as the number of 90 bp elements increased. Constructs with the 90 bp element 3’ of LUC did not show a significant sucrose response.
Fig 7. Sucrose-responsiveness of 90 bp sucrose response element: minimal promoter constructs.
A. One to 3 copies of the 90 bp SRE was inserted upstream (5’) of the 35S minimal promoter or downstream of the LUC coding sequence (3’) in the vector yy449. B. Sucrose response of transgenic seedlings harboring the empty vector (yy449) or 1–3 copies of the 90 bp SRE in either the 5’ or 3’ insertion site. Error bars represent standard deviation of 2–4 independent single-copy homozygous transgenic lines. Significance was calculated using ANOVA to examine the influence of construct on the sucrose to water ratio. After determining there was a significant influence of construct, a post-hoc Tukey’s HSD was performed using a confidence level of 0.05.
Discussion
Intronic sequences are important in regulating the sucrose responsiveness of PAP1
Given the fundamental roles that sugars play in plant metabolism, understanding how sugars also serve as signaling molecules is vital towards gaining a comprehensive understanding of plant growth and development. Sugars such as glucose and sucrose are known to regulate the expression of large numbers of genes as evidenced by reporter gene studies [11, 14–16, 18, 19, 21, 22, 41–44] and large scale gene expression analyses [4, 45–50]. We used reporter gene assays in combination with mutant approaches to identify sequences important for regulating the sucrose response of PAP1. Two notable findings have come from this work. First, the sequence shown to be necessary and sufficient for the sucrose response of PAP1 was located in an intron. Although there are many genes that are known to be regulated by intronic sequences, including the floral Arabidopsis MADS box gene AGAMOUS [51, 52], and several sucrose transporters including AtSUC1, AtSUC9, and LeSUT1 [34, 53], there are no reports of sugar response elements in an intron. Furthermore, bioinformatic approaches to identify cis elements typically limit searches to upstream regions [54, 55]. Second, despite the presence of 3 known SUREs in the promoter and first and second introns of PAP1, none were found to be important. The promoter of PAP1, which contains a SURE-2 element with extended identity to the SURE-2 element of patatin was not sufficient for conferring sucrose responsiveness. And removal of the SURE-2 in intron 2 (by deletion of the entire intron) did not abolish the sucrose response of PAP1. In addition, mutation of SURE-1 did not abolish the sucrose response. Although it is possible that our two mutations of SURE-1 failed to alter the element sufficiently for complete loss of function, complete removal of SURE-1 (in construct intron1m3) does not affect the sucrose response thus demonstrating this element is dispensable for sucrose regulation. Although SURE-1 has been implicated in regulating sucrose responsiveness in numerous genes [19, 32, 43, 44, 56–59], in at least one other case SURE elements were not important [60]. Furthermore, the presence of SURE elements in promoter sequences of Arabidopsis genes was not or only weakly correlated with the regulation of these genes by sucrose [54].
A 90 bp intronic sequence functions as a sucrose response element
In addition to SURE-1 and SURE-2, other SREs that have been identified include the B-box [32, 61], SP8 elements [33], TGGACGG element [17], ATCATT element [62], site II elements (TGGGCY) [62], SUC-6 element (GAANGAGANGA) [54], CMSRE-1 and -2 [20], as well as several elements that respond to both sucrose and reactive oxygen species [54]. None of these motifs are present within the 90 bp region we identified, although this sequence shares similarity to elements such as SURE-1, SURE-2, SP8, B-box site M1, and TTACTA in being AT-rich (67% AT content for 90 bp region).
When placed outside the context of an intron upstream of the 35S minimal promoter, the 90 bp sequence was able to function as a SRE, although it only had a robust sucrose response when present in 3 tandem copies. The sucrose response of constructs with one copy of the 90 bp region was not significantly different from the empty vector unlike what was observed in PAP1wg lines, which contain one copy of the 90 bp region in the context of intron 1. This indicates that other sequences may be required for a robust sucrose-response and/or that the spatial context of the 90 bp sequence is important. Evidence for the latter is suggested by the fact that the 90 bp sequence did not function when placed downstream of LUC (in 3’ constructs). In these constructs the element was located 1700 bp downstream of the start codon, while in the context of the PAP1 gene the element is 491 bp downstream of the start codon. The difference in distance of the element relative to the transcriptional start may be one reason that the element did not function effectively.
Contribution of sucrose and other regulatory elements in the expression of PAP1
The correlation between sucrose responsiveness and pattern of GUS staining in our transgenic plants suggests that sequences conferring sucrose responsiveness are also responsible for the observed expression pattern of PAP1 in plants. Removal of intron 1 (in constructs PAP1wgΔInt1:GUS and PAP1wgΔInts:GUS) severely limits GUS expression quantitatively and qualitatively as compared to PAP1wg:GUS lines. Furthermore, PAP1wgΔInt2:GUS and both SURE-1 mutants (intron1m1 and intron1m2) retained sucrose responsiveness and both had GUS staining patterns nearly identical to PAP1wg:GUS lines. The ability of sucrose to govern the expression of PAP1 may be related to the protective role of anthocyanins during stress responses. For instance, stress conditions such as high light and cold are known to increase sucrose content and accumulation of anthocyanins in these conditions may serve to protect cells from damage by virtue of their ability to absorb light and serve as antioxidants and osmoprotectants [63–66].
Differences in the expression pattern between PAP1pro:GUS and PAP1wg:GUS lines further underscores the complexity of PAP1 regulation. PAP1wg:GUS plants showed GUS staining that was reminiscent of anthocyanin pigmentation in seedlings and plants (hypocotyls in young seedlings and leaves with noticeable staining along major veins) and in several other tissues as well (cauline leaves, inflorescences, immature and mature flowers, siliques). RT-PCR confirmed that PAP1 transcript was present in all of these organs and tissues in wild-type Col-0 plants. The pattern of GUS staining in PAP1pro:GUS was remarkably different and nearly opposite that of the PAP1wg:GUS plants. We first observed staining in the roots of 20 day old PAP1pro:GUS plants, but this was at a different location than the root staining observed in PAP1wg:GUS plants. No rosette staining was observed in PAP1pro:GUS lines while strong rosette expression was observed in PAP1wg:GUS lines. Both lines had GUS expression in mature flowers but PAP1pro:GUS lines had strong petal and no sepal staining while PAP1wg:GUS lines had strong sepal and no petal staining. Because the PAP1 promoter sequence is present in the PAP1wg:GUS construct, the discrepancy in staining patterns may be explained by the presence of sequences within the introns and/or exons of PAP1 that repress petal staining. Repressor elements are most likely within the exons of PAP1 because petal staining is not observed in the PAP1wgΔInts:GUS line, which contains the PAP1 promoter plus exons, but no introns. Furthermore, because the PAP1wgΔInts:GUS line had very limited GUS staining that was nothing like the staining observed in PAP1pro:GUS lines, PAP1 exons must repress all promoter expression elements and contain sequences conferring the weak expression observed in rosette and cauline leaves.
Conclusions
A 90 bp intronic sequence was identified as important for regulating the sucrose response of PAP1 and was sufficient for conferring a sucrose response to a reporter gene when present in multiple copies. Intronic sequence was also shown to be important for the proper pattern of PAP1 gene expression. Pigmentation of flowers, fruits, and vegetables remains a target for crop improvement [67] and understanding the many layers of PAP1 regulation may lead to new approaches for modifying anthocyanin content of these plants.
Materials and Methods
Sucrose treatment
Surface-sterilized seeds were put into a 250 ml flask containing 50 ml of sterilized ½-strength MS basal salts pH 5.7 (Phytotechnology Laboratories, Shawnee Mission, KS). Seeds were kept at 4°C for 2 days then transferred to a continuous light or dark chamber with shaking at ~100 rpm. Four days after transfer to the chamber, sucrose (or an equivalent volume of water) was added to 90 mM from a 1.8 M stock. Seedlings were harvested at the indicated timepoints following addition of either sucrose or water.
RT-PCR
Seedlings were harvested and frozen immediately in liquid N2. Total RNA was extracted using the RNeasy Plant mini kit (Qiagen, Valencia, CA) and treated with DNase I (Fermentas, Glen Burnie, MD). Two μg of RNA was reverse transcribed using MMLV Reverse Transcriptase (Ambion, Austin, TX) according to the manufacturer’s protocol. Two to 5 ul of cDNA were used in 20 μl PCR reactions. PAP1 and actin were amplified using primers from Teng et al (MYB75F/R and ACT8F/R) [12]. GUS transcript was amplified using the following primers: GusF: 5’-ACCGTTTGTGTGAACAACGA-3’ and GusR: 5’-GGCACAGCACATCAAAGAGA-3’. In some experiments PAP1:GUS chimera transcript was amplified using the following primers: PAP1_2031F: 5’-CGAAAAGGTGCTTGGACTACT-3’ and GUS_424R: 5’-TCTGCCAGTTCAGTTCGTTG-3’.
RT-qPCR
Total RNA was extracted using the RNeasy Plant mini kit (Qiagen, Valencia, CA) and treated with TURBO DNA-free kit (Ambion, Austin, TX). One μg of RNA was reverse transcribed using iScript Reverse Transcription Supermix (BioRad, Hercules, CA) according to the manufacturer’s protocol. Twenty μl reactions were prepared using 1 ul of cDNA, 0.5 to 0.8 uM primer, and SYBR Premix Ex Taq II (Perfect Real Time) reagent (Takara Bio Inc, Otsu, Shiga Japan). All reactions were performed in triplicate. PAP1 and ubiquitin primer sequences are from Solfanelli et al [13]. Amplification was carried out on a Roche 480 Light Cycler (Roche, Madison, WI) with the following cycling parameters: One cycle of 95°C for 5 min followed by 45 cycles of amplification (95°C for 10 s: 60°C for 10 s; 72°C for 10 s). Cq values were calculated using the Light Cycler 480 SW 1.5 software and the Absolute quantification/2nd derivative method. Primer efficiencies were calculated from the slope of Cq values plotted as a concentration of cDNA (ranging from 0.01–1 μg) and were 102 and104% for PAP1 and ubiquitin, respectively. Cq values and primer efficiencies were input into the Relative Expression Software tool (REST) 2009 version [68] and fold changes were determined relative to ubiquitin.
Anthocyanin quantification
Anthocyanin quantification was modified from Solfanelli et al [13]. Anthocyanins were extracted in 1 ml 1% HCl in MeOH for 16–24 hours at 4°C. 0.9 ml of the extract was combined with 0.9 ml water and 1 ml chloroform. Extracts were vortexed and clarified by low speed centrifugation. 0.9 ml of the aqueous phase was removed and Abs 530 and 657 were determined. Anthocyanin content is reported as Abs 530-657/mg fresh weight.
PAP1 promoter, whole gene, and delta intron-reporter constructs
The 2 kb 5’-upstream region of the PAP1 gene (At1g56650) was amplified from A. thaliana genomic DNA (Col-0) using the Expand High Fidelity PCR system (Roche Applied Science, Indianapolis, IN) and the following primers, which contain restriction enzyme sites (underlined): PAP1HindIIIF: 5’-AACACTACAAAAAAGCTTAACTGCATTTAG-3’ and PAP1proSmaIR: 5’-TGGACGAACCCTCCCGGGAACAAAGATAG-3’ or PAP1HindIIIF and PAP1HindIIIR: 5’-TGGACGAACCCTAAGCTTAACAAAGATAG-3’. A 3379 bp fragment containing the 2 kb 5’-upstream region and all exons and introns of PAP1 gene was amplified using PAP1HindIIIF and PAP1wgSmaIR: 5’-CAAATGTTCGAACCCGGGATCAAATTTCAC-3’ or PAP1wgHindIIIR: 5’-CAAATGTTCGAAAAAGCTTTCAAATTTCAC-3’. The whole gene constructs were designed to be translationally fused to either the GUS or LUC ORF and the insertion of a HindIII site for cloning the whole gene construct into pLPTV-BAR resulted in a change of the last amino acid of PAP1 from Asp to Glu.
PCR products were cloned into the pDrive vector using the Qiagen PCR Cloning Kit (Qiagen, Valencia, CA) and sequenced. pDrive containing the 2 kb PAP1 promoter amplified with PAP1HindIIIF and PAP1proSmaIR was first digested with Sma I and the resulting 3676 bp fragment (containing the 2 kb PAP1 promoter plus vector sequence) was isolated. This fragment was partially digested with Hind III and a 2005 bp fragment was isolated and cloned into the Sma I and Hind III sites of pBI101.3. pDrive containing the 2 kb PAP1 promoter amplified with PAP1HindIIIF and PAP1proHindIIIR was partially digested with Hind III and the resulting 2005 kb fragment was isolated and cloned into the Hind III site of the pLPTV-BAR vector. pDrive containing the whole gene PAP1 sequence amplified with PAP1HindIIIF and PAP1wgSmaIR was first digested with Sma I and the resulting 5543 bp fragment was isolated. This fragment was partially digested with Hind III and a 3379 bp fragment was isolated and cloned into the Sma I and Hind III sites of pBI101.3. pDrive containing the whole gene PAP1 promoter sequence amplified with PAP1HindIIIF and PAP1wgHindIIIR was partially digested with Hind III and the resulting 3379 kb fragment was isolated and cloned into the Hind III site of the pLPTV-BAR vector. Sequence identity and orientation were confirmed by sequencing.
To generate PAP1wg constructs lacking one or both introns, a full length PAP1 cDNA was amplified from Arabidopsis seedlings. To create PAP1wgΔIntron2, a 531 bp Age I-Spe I fragment (containing Intron 2) was removed and replaced with a 442 bp Age I-Spe I fragment from PAPI cDNA. To create PAP1wgΔIntron1, a synthetic DNA fragment was constructed from the Sca I site in the 5’-upstream region of the PAP1 gene to the Age I site in Exon 2 and omitted Exon 1 (GENEART, Regensburg, Germany). This 430 bp Sca I- Age I fragment was used to replace the corresponding 969 bp fragment from PAP1wg and PAP1wgΔIntron2 to create PAP1wgΔIntron1 and PAP1wgΔIntrons1&2, respectively.
Intron1m1-8 reporter constructs
Mutations were introduced by PCR [69]. For the intron1m1 mutation, a 50 μl reaction was performed with 50 ng pBI101.3-PAP1wg plasmid, 200 μM dNTPs, 6% DMSO, 2.5 U PFU Ultra HF polymerase (Stratagene, La Jolla, CA) and 150 ng of the following primers: mut1F: 5’-TAATCACTACCAATAGTCTTCGTTCTCTCTATTTGAGATTCAGAAAATTGATTAATACCCGG-3’
mut1R: 5’-CCGGGTATTAATCAATTTTCTGAATCTCAAATAGAGAGAACGAAGACTATTGGTAGTGATTA-3’. Cycling conditions were 95°C for 1 minute followed by 16 cycles of 95°C for 50s, 60°C for 1 min 68°C for 32 min, and a final extension time of 68°C for 7 min. Reactions were digested with 20 U of DpnI (New England Biolabs, Ipswich, MA) for 2 hrs and 5 μl was used to transform DH5α cells. For the intron1m2 mutation, a 50 μl reaction was performed with 50 ng pDrive-PAP1wg plasmid and the following primers according to Zheng et al: mut2F: 5’- gtcttcgttctctctaaatactaatcagaaaattgattaatacccggtattaaaaaaaaaaaaaaaaatttgtttaaatgagtac-3’ and mut2R: 5’- ttaatcaattttctgattagtatttagagagaacgaagactattggtagtgattatagatattcatatttgtgtgtgtgt-3’ [70]. Plasmids with the intron1m2 mutation were digested with Sca I and Age I and the resulting 970 bp fragment (containing intron 1) was used to replace the corresponding fragment in pBI101.3-PAP1wg.
For the intron1m3 and intron1m4 deletion mutations, single primers were used to generate mutations in pDrive-PAP1wg according to Makarova et al [71]. The primers used were: mut3R: 5’- GTAAAAATCTTCGTTTTTTGTGTGTGTGTGTGTCGGTTAGTGTGT-3’ and mut4F: 5’-TTTCTTTTGCTGTTCGTATTTGTTTTACACCTATAAAATATATAGAAGGAG-3’. Mutations were incorporated into pBI101.3-PAP1wg as described above.
For the intron1m5-8 deletions, synthetic DNA fragments were constructed in the context of the Sca I-Age I fragment that encompasses intron 1, and incorporating the specific deletions shown in Fig 5 (GENEART, Regensburg, Germany). These Sca I- Age I fragments were used to replace the corresponding 969 bp fragment from PAP1wg.
Minimal promoter constructs
For the 5’ constructs, a synthetic DNA fragment was constructed with the 90 bp region of interest flanked by HindIII and BamHI (5’) and BglII (3’) restriction sites (GeneArt® Gene Synthesis by Life Technologies): AAGCTTAAAGGGATCCtaaatgaattcgtgggaaaattttgtatgaacacgtgtttctgtgttggaacagttctttatttttattggtgtgcatagattcttcctgAGATCT. This fragment was digested with HindIII and BglII and ligated into HindIII and BamHI-digested yy449 vector (Genbank accession AB638628.1). This process was repeated once or twice more to obtain plasmids with two (192 bp) or 3 (288 bp) copies of the 90 bp region. For the 3’ constructs, 1–3 copies of the 90 bp fragment were synthesized with flanking XbaI sites. Multiple copy elements were separated with the spacer AGATCC. These fragments were cloned into the XbaI site of yy449.
Generation of transgenic plants
Plasmids were transformed into Agrobacterium tumefaciens strain GV3101 by electroporation. A. thaliana ecotype Col-0 was transformed by the floral dip method as described [72]. Seeds were surface-sterilized by treating with 95% ethanol for 10 min followed by 20% bleach supplemented with 0.1% Tween-20 for 5 min, and rinsed several times in sterile water. Seeds were suspended in 0.1% agargel and plated on MS plates supplemented with 50 μg ml-1 kanamycin (pBI101.3) or sowed directly on soil and sprayed with Liberty herbicide (pLPTV). Resistant plants were checked by PCR for the presence of the transgene.
Gus staining
Seedlings were stained in 100 mM sodium phosphate buffer, pH 7, 10 mM EDTA, 0.1% Triton X-100, 2 mM K4Fe(CN)6, 2 mM K3Fe(CN)6, and 1 mg ml-1 5-bromo-4-chloro-3-indolyl ß-D glucuronide for 24–48 hours at 37°C. Seedlings were then repeatedly destained using 70% ethanol and photographed.
Luciferase assay
Seedlings ground to a fine powder and luciferase activity was measured using the Luciferase Assay System (Promega, Madison, WI) according to the manufacturer’s protocol. Protein content in cell extracts was measured by Bradford assay using BSA as standard.
Supporting Information
(PPTX)
(PPTX)
(PPTX)
(PPT)
Acknowledgments
We thank Yoshi Yamamoto for the gift of the yy449 vector, Alyssa McKenzie for research help, and Cris Argueso and Corey Broeckling for helpful comments on the manuscript.
Data Availability
All relevant data are within the paper and Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1.Sheen J, Zhou L, Jang JC. Sugars as signaling molecules. Curr Opin Plant Biol. 1999;2(5):410–8. Epub 1999/10/06. . [DOI] [PubMed] [Google Scholar]
- 2.Smeekens S. Sugar-induced signal transduction in plants. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:49–81. Epub 2004/03/12. 10.1146/annurev.arplant.51.1.49 . [DOI] [PubMed] [Google Scholar]
- 3.Tognetti JA, Pontis HG, Martinez-Noel GM. Sucrose signaling in plants: a world yet to be explored. Plant Signal Behav. 2013;8(3):e23316 Epub 2013/01/22. 10.4161/psb.23316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rolland F, Baena-Gonzalez E, Sheen J. Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol. 2006;57:675–709. Epub 2006/05/04. 10.1146/annurev.arplant.57.032905.105441 . [DOI] [PubMed] [Google Scholar]
- 5.Chiou TJ, Bush DR. Sucrose is a signal molecule in assimilate partitioning. Proc Natl Acad Sci U S A. 1998;95(8):4784–8. Epub 1998/04/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ainsworth EA, Bush D. Carbohydrate export from the leaf—A highly regulated process and target to enhance photosynthesis and productivity. Plant Physiol. 2011;155:157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheen J. Master regulators in plant glucose signaling networks. 2014;57(2):67–79. Epub 2014/12/23. 10.1007/s12374-014-0902-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wind J, Smeekens S, Hanson J. Sucrose: Metabolite and signaling molecule. Phytochemistry. 2010;71:1610–4. 10.1016/j.phytochem.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 9.Vaughn MW, Harrington GN, Bush DR. Sucrose-mediated transcriptional regulation of sucrose symporter activity in the phloem. Proc Natl Acad Sci U S A. 2002;99(16):10876–80. Epub 2002/08/01. 10.1073/pnas.172198599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wenzler H, Mignery G, Fisher L, Park W. Sucrose-regulated expression of a chimeric potato tuber gene in leaves of transgenic tobacco plants. Plant Mol Biol. 1989;13(4):347–54. Epub 1989/10/01. . [DOI] [PubMed] [Google Scholar]
- 11.Jefferson R, Goldsbrough A, Bevan M. Transcriptional regulation of a patatin-1 gene in potato. Plant Mol Biol. 1990;14(6):995–1006. Epub 1990/06/01. . [DOI] [PubMed] [Google Scholar]
- 12.Teng S, Keurentjes J, Bentsink L, Koornneef M, Smeekens S. Sucrose-specific induction of anthocyanin biosynthesis in Arabidopsis requires the MYB75/PAP1 gene. Plant Physiol. 2005;139(4):1840–52. Epub 2005/11/22. 10.1104/pp.105.066688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P. Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol. 2006;140(2):637–46. Epub 2005/12/31. 10.1104/pp.105.072579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsukaya H, Ohshima T, Naito S, Chino M, Komeda Y. Sugar-dependent expression of the CHS-A gene for chalcone synthase from petunia in transgenic Arabidopsis. Plant Physiol. 1991;97(4):1414–21. Epub 1991/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yokoyama R, Hirose T, Fujii N, Aspuria ET, Kato A, Uchimiya H. The rolC promoter of Agrobacterium rhizogenes Ri plasmid is activated by sucrose in transgenic tobacco plants. Mol Gen Genet. 1994;244(1):15–22. Epub 1994/07/08. . [DOI] [PubMed] [Google Scholar]
- 16.Mita S, Suzuki-Fujii K, Nakamura K. Sugar-inducible expression of a gene for beta-amylase in Arabidopsis thaliana. Plant Physiol. 1995;107(3):895–904. Epub 1995/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maeo K, Tomiya T, Hayashi K, Akaike M, Morikami A, Ishiguro S, et al. Sugar-responsible elements in the promoter of a gene for beta-amylase of sweet potato. Plant Mol Biol. 2001;46(5):627–37. Epub 2001/08/23. . [DOI] [PubMed] [Google Scholar]
- 18.Visser RG, Stolte A, Jacobsen E. Expression of a chimaeric granule-bound starch synthase-GUS gene in transgenic potato plants. Plant Mol Biol. 1991;17(4):691–9. Epub 1991/10/01. . [DOI] [PubMed] [Google Scholar]
- 19.Kim KN, Guiltinan MJ. Identification of cis-acting elements important for expression of the starch-branching enzyme I gene in maize endosperm. Plant Physiol. 1999;121(1):225–36. Epub 1999/09/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morikami A, Matsunaga R, Tanaka Y, Suzuki S, Mano S, Nakamura K. Two cis-acting regulatory elements are involved in the sucrose-inducible expression of the sporamin gene promoter from sweet potato in transgenic tobacco. Mol Genet Genomics. 2005;272(6):690–9. Epub 2005/01/18. 10.1007/s00438-004-1100-y . [DOI] [PubMed] [Google Scholar]
- 21.Kim SR, Costa MA, An GH. Sugar response element enhances wound response of potato proteinase inhibitor II promoter in transgenic tobacco. Plant Mol Biol. 1991;17(5):973–83. Epub 1991/11/01. . [DOI] [PubMed] [Google Scholar]
- 22.Johnson R, Ryan CA. Wound-inducible potato inhibitor II genes: enhancement of expression by sucrose. Plant Molecular Biology. 1990;14:527–36. [DOI] [PubMed] [Google Scholar]
- 23.Luo QJ, Mittal A, Jia F, Rock CD. An autoregulatory feedback loop involving PAP1 and TAS4 in response to sugars in Arabidopsis. Plant Mol Biol. 2012;80(1):117–29. Epub 2011/05/03. 10.1007/s11103-011-9778-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rolland F, Moore B, Sheen J. Sugar sensing and signaling in plants. Plant Cell. 2002;14 Suppl:S185–205. Epub 2002/06/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rook F, Bevan MW. Genetic approaches to understanding sugar-response pathways. Journal of Experimental Botany. 2003;54:495–501. [DOI] [PubMed] [Google Scholar]
- 26.Loreti E, Povero G, Novi G, Solfanelli C, Alpi A, Perata P. Gibberellins, jasmonate and abscisic acid modulate the sucrose-induced expression of anthocyanin biosynthetic genes in Arabidopsis. New Phytol. 2008;179(4):1004–16. Epub 2008/06/10. 10.1111/j.1469-8137.2008.02511.x . [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Van den Ende W, Rolland F. Sucrose induction of anthocyanin biosynthesis is mediated by DELLA. Mol Plant. 2014;7(3):570–2. Epub 2013/11/19. 10.1093/mp/sst161 . [DOI] [PubMed] [Google Scholar]
- 28.Jeong SW, Das PK, Jeoung SC, Song JY, Lee HK, Kim YK, et al. Ethylene suppression of sugar-induced anthocyanin pigmentation in Arabidopsis. Plant Physiol. 2010;154(3):1514–31. Epub 2010/09/30. 10.1104/pp.110.161869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sivitz AB, Reinders A, Ward JM. Arabidopsis sucrose transporter AtSUC1 is important for pollen germination and sucrose-induced anthocyanin accumulation. Plant Physiology. 2008;147:92–100. 10.1104/pp.108.118992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin DH, Choi MG, Lee HK, Cho M, Choi SB, Choi G, et al. Calcium dependent sucrose uptake links sugar signaling to anthocyanin biosynthesis in Arabidopsis. Biochem Biophys Res Commun. 2013;430(2):634–9. Epub 2012/12/12. 10.1016/j.bbrc.2012.11.100 . [DOI] [PubMed] [Google Scholar]
- 31.Higo K, Ugawa Y, Iwamoto M, Korenaga T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999;27(1):297–300. Epub 1998/12/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grierson C, Du JS, de Torres Zabala M, Beggs K, Smith C, Holdsworth M, et al. Separate cis sequences and trans factors direct metabolic and developmental regulation of a potato tuber storage protein gene. Plant J. 1994;5(6):815–26. Epub 1994/06/01. . [DOI] [PubMed] [Google Scholar]
- 33.Ishiguro S, Nakamura K. The nuclear factor SP8BF binds to the 5'-upstream regions of three different genes coding for major proteins of sweet potato tuberous roots. Plant Mol Biol. 1992;18(1):97–108. Epub 1992/01/01. . [DOI] [PubMed] [Google Scholar]
- 34.Sivitz AB, Reinders A, Johnson ME, Krentz AD, Grof CP, Perroux JM, et al. Arabidopsis sucrose transporter AtSUC9. High-affinity transport activity, intragenic control of expression, and early flowering mutant phenotype. Plant Physiol. 2007;143(1):188–98. Epub 2006/11/14. 10.1104/pp.106.089003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shirley BW, Kubasek WL, Storz G, Bruggemann E, Koornneef M, Ausubel FM, et al. Analysis of Arabidopsis mutants deficient in flavonoid biosynthesis. Plant J. 1995;8(5):659–71. Epub 1995/11/01. . [DOI] [PubMed] [Google Scholar]
- 36.Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, et al. A gene expression map of Arabidopsis thaliana development. Nat Genet. 2005;37(5):501–6. Epub 2005/04/05. 10.1038/ng1543 . [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez A, Zhao M, Leavitt JM, Lloyd AM. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J. 2008;53(5):814–27. Epub 2007/11/27. 10.1111/j.1365-313X.2007.03373.x . [DOI] [PubMed] [Google Scholar]
- 38.Hattori T, Totsuka M, Hobo T, Kagaya Y, Yamamoto-Toyoda A. Experimentally determined sequence requirement of ACGT-containing abscisic acid response element. Plant Cell Physiol. 2002;43(1):136–40. Epub 2002/02/06. . [DOI] [PubMed] [Google Scholar]
- 39.Shen Q, Zhang P, Ho T. Modular nature of abscisic acid (ABA) response complexes: composite promoter units that are necessary and sufficient for ABA induction of gene expression in barley. Plant Cell. 1996;8:1107–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi H, Hong J, Ha J, Kang J, Kim S. ABFs, a family of ABA-responsive element binding factors. J Biol Chem. 2000;275:1723–30. [DOI] [PubMed] [Google Scholar]
- 41.Wenzler HC, Mignery GA, Fisher LM, Park WD. Analysis of a chimeric class-I patatin-GUS gene in transgenic potato plants: High-level expression in tubers and sucrose-inducible expression in cultured leaf and stem explants. Plant Mol Biol. 1989;12(1):41–50. Epub 1989/01/01. 10.1007/bf00017446 . [DOI] [PubMed] [Google Scholar]
- 42.Tang Z, Sadka A, Morishige DT, Mullet JE. Homeodomain leucine zipper proteins bind to the phosphate response domain of the soybean VspB tripartite promoter. Plant Physiol. 2001;125(2):797–809. Epub 2001/02/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu H, Kim SY, Park WD. High-level tuber expression and sucrose inducibility of a potato Sus4 sucrose synthase gene require 5' and 3' flanking sequences and the leader intron. Plant Cell. 1995;7(9):1387–94. Epub 1995/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X, Xing J, Gianfagna TJ, Janes HW. Sucrose regulation of ADP-glucose pyrophosphorylase subunit genes transcript levels in leaves and fruits. Plant Sci. 2002;162(2):239–44. Epub 2002/05/07. . [DOI] [PubMed] [Google Scholar]
- 45.Han L, Li JL, Jin M, Su YH. Transcriptome analysis of Arabidopsis seedlings responses to high concentrations of glucose. Genet Mol Res. 2015;14(2):4784–801. Epub 2015/05/13. 10.4238/2015.May.11.11 . [DOI] [PubMed] [Google Scholar]
- 46.Kunz S, Pesquet E, Kleczkowski LA. Functional dissection of sugar signals affecting gene expression in Arabidopsis thaliana. PLoS One. 2014;9(6):e100312 Epub 2014/06/21. 10.1371/journal.pone.0100312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Osuna D, Usadel B, Morcuende R, Gibon Y, Blasing OE, Hohne M, et al. Temporal responses of transcripts, enzyme activities and metabolites after adding sucrose to carbon-deprived Arabidopsis seedlings. Plant J. 2007;49(3):463–91. Epub 2007/01/16. 10.1111/j.1365-313X.2006.02979.x . [DOI] [PubMed] [Google Scholar]
- 48.Gonzali S, Loreti E, Solfanelli C, Novi G, Alpi A, Perata P. Identification of sugar-modulated genes and evidence for in vivo sugar sensing in Arabidopsis. J Plant Res. 2006;119(2):115–23. Epub 2006/02/08. 10.1007/s10265-005-0251-1 . [DOI] [PubMed] [Google Scholar]
- 49.Usadel B, Blasing OE, Gibon Y, Retzlaff K, Hohne M, Gunther M, et al. Global transcript levels respond to small changes of the carbon status during progressive exhaustion of carbohydrates in Arabidopsis rosettes. Plant Physiol. 2008;146(4):1834–61. Epub 2008/02/29. 10.1104/pp.107.115592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blasing OE, Gibon Y, Gunther M, Hohne M, Morcuende R, Osuna D, et al. Sugars and circadian regulation make major contributions to the global regulation of diurnal gene expression in Arabidopsis. Plant Cell. 2005;17(12):3257–81. Epub 2005/11/22. 10.1105/tpc.105.035261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sieburth LE, Meyerowitz EM. Molecular dissection of the AGAMOUS control region shows that cis elements for spatial regulation are located intragenically. Plant Cell. 1997;9(3):355–65. Epub 1997/03/01. 10.1105/tpc.9.3.355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deyholos MK, Sieburth LE. Separable whorl-specific expression and negative regulation by enhancer elements within the AGAMOUS second intron. Plant Cell. 2000;12(10):1799–810. Epub 2000/10/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weise A, Lalonde S, Kuhn C, Frommer WB, Ward JM. Introns control expression of sucrose transporter LeSUT1 in trichomes, companion cells and in guard cells. Plant Mol Biol. 2008;68(3):251–62. Epub 2008/07/04. 10.1007/s11103-008-9366-9 . [DOI] [PubMed] [Google Scholar]
- 54.Geisler M, Kleczkowski LA, Karpinski S. A universal algorithm for genome-wide in silicio identification of biologically significant gene promoter putative cis-regulatory-elements; identification of new elements for reactive oxygen species and sucrose signaling in Arabidopsis. Plant J. 2006;45(3):384–98. Epub 2006/01/18. 10.1111/j.1365-313X.2005.02634.x . [DOI] [PubMed] [Google Scholar]
- 55.Rombauts S, Florquin K, Lescot M, Marchal K, Rouze P, van de Peer Y. Computational approaches to identify promoters and cis-regulatory elements in plant genomes. Plant Physiol. 2003;132(3):1162–76. Epub 2003/07/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scarpella E, Simons EJ, Meijer AH. Multiple regulatory elements contribute to the vascular-specific expression of the rice HD-Zip gene Oshox1 in Arabidopsis. Plant Cell Physiol. 2005;46(8):1400–10. Epub 2005/06/21. 10.1093/pcp/pci153 . [DOI] [PubMed] [Google Scholar]
- 57.Kwak MS, Noh SA, Oh MJ, Huh GH, Kim KN, Lee SW, et al. Two sweetpotato ADP-glucose pyrophosphorylase isoforms are regulated antagonistically in response to sucrose content in storage roots. Gene. 2006;366(1):87–96. Epub 2005/12/13. 10.1016/j.gene.2005.09.021 . [DOI] [PubMed] [Google Scholar]
- 58.Sun C, Palmqvist S, Olsson H, Boren M, Ahlandsberg S, Jansson C. A novel WRKY transcription factor, SUSIBA2, participates in sugar signaling in barley by binding to the sugar-responsive elements of the iso1 promoter. Plant Cell. 2003;15(9):2076–92. Epub 2003/09/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.K J, L CT, Larsson H, Rask L. Differential accumulation of Arabidopsis thaliana Sbe2.1 and Sbe2.2 transcripts in response to light. Plant Sci. 1998;135:183–93. [Google Scholar]
- 60.Mutisya J, Sun C, Palmqvist S, Baguma Y, Odhiambo B, Jansson C. Transcriptional regulation of the sbeIIb genes in sorghum (Sorghum bicolor) and barley (Hordeum vulgare): importance of the barley sbeIIb second intron. J Plant Physiol. 2006;163(7):770–80. Epub 2006/04/18. 10.1016/j.jplph.2005.04.038 . [DOI] [PubMed] [Google Scholar]
- 61.Zourelidou M, de Torres-Zabala M, Smith C, Bevan MW. Storekeeper defines a new class of plant-specific DNA-binding proteins and is a putative regulator of patatin expression. Plant J. 2002;30(4):489–97. Epub 2002/05/25. . [DOI] [PubMed] [Google Scholar]
- 62.Comelli RN, Gonzalez DH. Identification of regulatory elements involved in expression and induction by sucrose and UV-B light of the Arabidopsis thaliana COX5b-2 gene, encoding an isoform of cytochrome c oxidase subunit 5b. Physiol Plant. 2009;137(3):213–24. Epub 2009/09/29. 10.1111/j.1399-3054.2009.01285.x . [DOI] [PubMed] [Google Scholar]
- 63.Couee I, Sulmon C, Gouesbet G, El Amrani A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J Exp Bot. 2006;57(3):449–59. Epub 2006/01/07. 10.1093/jxb/erj027 . [DOI] [PubMed] [Google Scholar]
- 64.Gould KS. Nature's swiss army knife: The diverse protective roles of anthocyanins in leaves. J Biomed Biotechnol. 2004;2004(5):314–20. Epub 2004/12/04. 10.1155/s1110724304406147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hatier JH, Gould KS. Foliar anthocyanins as modulators of stress signals. J Theor Biol. 253 Netherlands2008. p. 625–7. 10.1016/j.jtbi.2008.04.018 [DOI] [PubMed] [Google Scholar]
- 66.Chalker-Scott L. Environmental significance of anthocyanins in plant stress responses. Photochemistry and Photobiology. 1999;70(1):1–9. [Google Scholar]
- 67.Zhang Y, Butelli E, Martin C. Engineering anthocyanin biosynthesis in plants. Curr Opin Plant Biol. 2014;19:81–90. Epub 2014/06/08. 10.1016/j.pbi.2014.05.011. 24907528. 10.1016/j.pbi.2014.05.011 [DOI] [PubMed] [Google Scholar]
- 68.Pfaffl MW, Horgan GW, Dempfle L. Relative Expression Software Tool (REST©) for group wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acid Research. 2002;30(9):E36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weiner M, Costa G. Rapid PCR site-directed mutagenesis In: Dieffenback C, Dvelsler G, editors. PCR Primer: A Laboratory Manual. Cold Spring Harbor, NY: CSH Laboratory Press; 1995. [Google Scholar]
- 70.Zheng L, Baumann U, Reymond JL. An efficient one-step site-directed and site-saturation mutagenesis protocol. Nucleic Acids Res. 2004;32(14):e115 Epub 2004/08/12. 10.1093/nar/gnh110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Makarova O, Kamberov E, Margolis B. Generation of deletion and point mutations with one primer in a single cloning step. Biotechniques. 2000;29(5):970–2. Epub 2000/11/21. . [DOI] [PubMed] [Google Scholar]
- 72.Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16(6):735–43. Epub 1999/03/09. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PPTX)
(PPTX)
(PPTX)
(PPT)
Data Availability Statement
All relevant data are within the paper and Supporting Information files.