Abstract
Background
Trauma exposure and posttraumatic stress disorder (PTSD) have been linked to myocardial infarction and stroke in women, with biological and behavioral mechanisms implicated in underlying risk. The third most common cardiovascular illness, venous thromboembolism (VTE), is a specific health risk for women. Given previous associations with other cardiovascular diseases, we hypothesized that high levels of trauma and PTSD symptoms would be associated with higher risk of incident VTE in younger and middle‐aged women.
Methods and Results
We used proportional hazards models to estimate hazard ratios (HRs) and 95% CIs for new‐onset VTE (960 events) over 22 years in 49 296 women in the Nurses’ Health Study II. Compared to no trauma exposure, both trauma exposure and PTSD symptoms were significantly associated with increased risk of developing VTE, adjusting for demographics, family history, and childhood adiposity. Women with the most PTSD symptoms exhibited the greatest risk elevation: trauma/6 to 7 symptoms: HR=2.42 (95% CI, 1.83–3.20); trauma/4 to 5 symptoms: HR=2.00 (95% CI, 1.55–2.59); trauma/1 to 3 symptoms: HR=1.44 (95% CI, 1.12–1.84); trauma/no symptoms: HR=1.72 (95% CI, 1.43–2.08). Results were similar, although attenuated, when adjusting for VTE‐relevant medications, medical conditions, and health behaviors.
Conclusions
Women with the highest PTSD symptom levels had nearly a 2‐fold increased risk of VTE compared to women without trauma exposure in fully adjusted models. Trauma exposure alone was also associated with elevated VTE risk. Trauma and PTSD symptoms may be associated with a hypercoagulable state. Treatment providers should be aware that women with trauma exposure and PTSD symptoms may be vulnerable to VTE.
Keywords: deep vein thrombosis, posttraumatic stress disorder, pulmonary embolism, trauma, venous thromboembolism
Subject Categories: Epidemiology, Mental Health, Women
Introduction
Venous thromboembolism (VTE), encompassing deep vein thrombosis (DVT) and pulmonary embolism (PE), is the third most common cause of cardiovascular death and the leading preventable cause of hospital‐based death.1, 2 Although VTE has been identified as a major public health concern for men and women,2 certain factors make it particularly relevant to women's health.3 Female‐specific risk factors for VTE related to reproductive hormone exposure (eg, due to contraceptive therapy, pregnancy, and hormone therapy [HT]) have been documented,3 and VTE is a leading cause of mortality among pregnant and recently postpartum women in the United States.2 Additionally, VTE incidence rates are higher in women of childbearing age than in men of the same age,4, 5 likely reflecting VTE induced by pregnancy and/or oral contraceptive use during childbearing years. Given the disease burden and highly recurrent nature of VTE—a largely preventable condition—greater insight into factors that may increase risk or promote prevention, particularly in women, is a significant public health priority.2, 3 Furthermore, given that 26% to 47% of VTE cases are classified as occurring in the absence of major established risk factors (eg, cancer, surgery, and pregnancy),6 better understanding of VTE risk has important clinical relevance.
VTE shares risk factors (eg, hypertension, obesity, cigarette smoking) and pathophysiology (eg, inflammation, hypercoagulability, endothelial damage) with atherothrombosis,7 the primary cause of cardiovascular disease.8 Consequently, in recent years, VTE has been conceptualized as part of a pancardiovascular syndrome that includes coronary artery disease, peripheral artery disease, and cerebrovascular disease.7 Growing evidence suggests that psychosocial factors, such as depression, which have been linked to cardiovascular risk more broadly, are also associated with VTE risk in particular.9, 10 The majority of individuals in the general population (at least 60% according to most studies11, 12) are exposed to 1 or more traumatic events (eg, sexual assault, physical assault, combat, natural disasters) during their lifetime. Posttraumatic stress disorder (PTSD) is a psychological consequence of exposure to such traumatic events, and is characterized by symptoms of re‐experiencing of the trauma, avoidance of trauma reminders, negative alterations in mood and thinking, and hyperarousal. PTSD is twice as common in women than in men; ≈1 in 10 women are estimated to develop PTSD in their lifetime.11 PTSD has been increasingly identified as a cardiovascular risk factor,13 with effects particularly striking in women.14 Furthermore, PTSD has been linked to pathophysiological processes implicated in VTE, including inflammation,15 hypercoagulability,16 and endothelial damage.17 One study suggested that PE incidence was higher after compared with before exposure to a natural disaster, a type of traumatic event commonly associated with psychological distress.18 However, research examining a range of traumatic events commonly associated with psychological distress, PTSD, and risk for developing VTE among initially healthy individuals is lacking.
In this study, we investigated associations of trauma exposure and PTSD symptoms with risk of incident VTE over a 22‐year period using data from the Nurses’ Health Study II (NHS II), an ongoing cohort study of younger and middle‐aged women. Whereas research on PTSD and risk for chronic disease often conflates trauma exposure and PTSD symptoms, we were able to examine the effects of trauma and PTSD symptoms separately. Consistent with prior findings related to trauma exposure, PTSD symptoms, and incident myocardial infarction (MI) and stroke in this sample,14 we hypothesized that, compared to no trauma exposure, trauma exposure and PTSD symptoms would be associated with increased risk of incident VTE. Models adjusted for a range of covariates determined from previous research,19, 20 including demographic factors, VTE‐relevant medical risk factors, medications, and adult health conditions and behaviors. Although some of these covariates are likely confounders (eg, socioeconomic status, parity) of the associations of interest, others could lie on the pathway between trauma/PTSD symptoms and VTE (eg, adult body mass index, cigarette smoking).
Methods
Sample
The NHS II sample comprises 116 430 US female registered nurses who were enrolled in 1989 when they were 25 to 42 years of age and followed with biennial questionnaires. Women who reported a history of VTE (“DVT/PE”) at baseline in 1989 were excluded to examine incident VTE. In 2008, a subset of 60 804 women (aged 44–62 years) were sent a supplemental questionnaire querying trauma exposure and PTSD symptoms,21 and 54 224 women returned the questionnaire (89% response rate). The sample is predominantly white (94%), and most women (78%) were married at baseline. This study was approved by the Partners Healthcare Human Research Committee. Returning questionnaires via mail represented implied consent.
Trauma and PTSD Symptom Assessment
Lifetime exposure to 15 traumatic events (eg, physical assault, sudden death of a loved one), in addition to “a seriously traumatic event not already covered,” was assessed with a modified version of the Brief Trauma Questionnaire.21, 22 The Brief Trauma Questionnaire is considered a reliable and valid measure of trauma exposure that parallels interview measures of trauma exposure.21, 22, 23 Kappa coefficients for the presence of trauma have been found to range from 0.60 to 1.00, with most kappas above 0.74.23 Women reported which event occurred first (if reporting multiple events) and which was their worst experience, along with their ages at the first and worst events. Women also reported whether they ever experienced PTSD symptoms in relation to their worst trauma. Seven PTSD symptoms occurring subsequent to the worst trauma were queried using the Short Screening Scale for Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV) PTSD.24 The trauma exposure and PTSD symptom data were used to derive information on PTSD symptom severity and age of onset. Self‐reported age of PTSD symptom onset has had excellent test–retest reliability in this sample (intraclass correlation coefficient=0.95 based on comparisons of reported age of PTSD symptom onset on the questionnaire and reported age of onset in a subset of women who completed a “gold standard” highly structured diagnostic interview for PTSD25).
For each year of the study, participants were classified into 5 groups according to dates of trauma exposure and severity of PTSD symptoms: (1) no trauma exposure, (2) trauma‐exposed and reported no PTSD symptoms, (3) trauma‐exposed and reported 1 to 3 symptoms, (4) trauma‐exposed and reported 4 to 5 symptoms, and (5) trauma‐exposed and reported 6 to 7 symptoms. Women who reported never having experienced trauma were classified as having no trauma exposure. PTSD symptoms were only assessed in relation to women's self‐defined worst trauma. Therefore, age of PTSD symptom onset was established according to the age of worst trauma. Thus, following their first trauma, women were categorized as being trauma‐exposed with no PTSD symptoms. After their worst trauma, women were categorized as having the number of PTSD symptoms reported with respect to the worst event. If women reported only 1 trauma, the dates of the first and worst traumas were the same. The trauma/PTSD symptom groups were based on previous research with the Short Screening Scale for DSM‐IV PTSD recommending scores of 4+24 and 6+26 as clinical cutoffs for PTSD. Thus, we had 1 group of women with trauma and subclinical PTSD symptoms and 2 groups of women with trauma and PTSD symptom numbers above the recommended clinical cutoff on this screening questionnaire, with the latter 2 grouped with respect to increasing symptom severity. Furthermore, whereas most research on PTSD and chronic disease has often conflated trauma and PTSD symptoms, we were able to examine VTE onset in women with no trauma exposure, trauma exposure and no symptoms, and trauma exposure and varying symptom severity.
VTE Assessment
At each biennial follow‐up, women reported whether they had a physician‐diagnosed VTE (“DVT/PE”) in the past 2 years. In the current study, we considered cases of incident VTE as reported on the 1991–2011 questionnaires (n=960). As in prior research in the NHS II using self‐reported medical conditions,27, 28 we used the date of the first questionnaire on which VTE was reported for time to event. The severity of diagnosis, in conjunction with the extensive treatment and therapeutic monitoring and follow‐up that patients with VTE undergo, make it likely that VTE events are reported with high accuracy, particularly in this cohort of nurses with high health literacy. Validation of DVT and review of medical records for PE cases in the NHS II supports that nurse VTE reports are highly accurate when compared to medical record review.20, 29 In a validation study of 101 self‐reported cases of DVT for which medical records were available, 95 (94%) cases were confirmed, 2 (2%) cases were probable, and only 4 (4%) cases were not confirmed.29 Additionally, per protocols in the NHS II cohort, medical records are routinely requested from nurses who have no history of malignancy and who report a PE; record review for this subset of cases is conducted by physicians blind to the trauma/PTSD symptom primary exposure. Imaging was considered diagnostic of PE if a ventilation/perfusion lung scan was read by a radiologist as high probability for PE, or if there was a filling defect on contrast‐enhanced computed tomography scan of the pulmonary vasculature or on catheter‐based pulmonary angiography. Idiopathic PE, the focus for the current study, was defined by the absence of recent surgery, major physical trauma (eg, fracture), or active malignancy in the medical record. Cases confirmed by record review were considered “definite cases.” We classified PE events as “probable cases” if a participant or relative verbally confirmed the report when it was followed up but access to medical records was denied or unable to be attained.14 We considered the subset of probable and definite idiopathic PE cases (n=157) as our outcome in a sensitivity analysis.
Covariates
The following demographics and early childhood factors were examined as potential confounders: age, race/ethnicity (African American, Latina, Asian, white, other), maximum parental education at the participant's birth (high school or less, some college, 4+ years of college), parental history of MI,30 and somatotype at age 5 (to estimate childhood adiposity).
Consistent with prior research in the NHS cohorts,19, 31 time‐varying indicators for VTE‐relevant medical risk factors, medications, and adult health behaviors and conditions were also included as covariates. Parity (nulliparous, 1, 2–3, 4+ children), oral contraceptive use (never used, current user, former user; oral contraceptives were predominantly estrogen and progesterone‐containing: less than 2.26% of women used progestin‐only oral contraceptives), and menopausal status and HT use (premenopausal, postmenopausal/never HT, postmenopausal/past HT, postmenopausal/current HT, postmenopausal/missing HT, unknown menopausal status) were assessed biennially. Current nonaspirin nonsteroidal anti‐inflammatory drug use (yes/no) was assessed with each biennial questionnaire except for the 1991 assessment. Physician‐diagnosed hypertension, hypercholesterolemia, coronary heart disease (MI or angina), cancer (excluding nonmelanoma skin cancer), type 2 diabetes mellitus, and rheumatologic disease (rheumatoid arthritis or systemic lupus erythematosus) were also reported at each biennial assessment with the exception of rheumatologic disease, which was not queried on the 1989 or 1995 questionnaires. Adult health behaviors were assessed at baseline via self‐report and updated biennially, unless otherwise noted. Adult body mass index in kg/m2 was computed from self‐reported height and weight (validated in prior NHS research32), and categorized as <22.5, 22.5 to <25, 25 to <27.5, 27.5 to <30, 30 to <35, and 35+.19 Women were classified as nonsmokers, former smokers, or current smokers of 1 to 14, 15 to 24, or 25+ cigarettes/day. Alcohol consumption (0, 1–<5, 5–<10, 10–<20, or 20+ g/day) was assessed in 1989, 1991, 1995, 1999, 2003, and 2007. Physical activity (<3, 3–<9, 9–<18, 18–<27, or 27+ metabolic equivalent hours/week) was measured in 1989, 1991, 1997, 2001, 2005, and 2009. Diet was assessed every 4 years beginning in 1991. Diet quality was quantified based on the Alternative Healthy Eating Index33 and divided into quintiles, with the highest quintile representing the healthiest diet. Antidepressant use is frequently prescribed for PTSD34 and is associated with increased risk for VTE.35 Lifetime antidepressant use was assessed in 1993, and regular past 2‐year antidepressant use was assessed in 1997, 2001, 2003, 2005, and 2007. Women who endorsed lifetime antidepressant use in 1993 were coded as having used antidepressants from 1989 to 1993; use was updated as available.
The following variables were used in sensitivity analyses adjusting for type of worst traumatic event, depression, and engagement with the medical system, respectively. We classified women's worst traumatic events (ie, the traumas to which PTSD symptoms were anchored) as (1) interpersonal violence (childhood physical abuse, physical assault, unwanted sexual contact), (2) other traumatic event to self (eg, car accident, natural disaster exposure), or (3) traumatic event occurring to others (eg, sudden death of a loved one, witnessing violence). Lifetime depression history (experiencing depressive symptoms for 2+ weeks) was first assessed in 2001, and women reported whether they experienced depressive symptoms for 2+ weeks or received a diagnosis of depression in the past 2 years at the 2003, 2005, 2007, and 2009 biennial assessments. Women who endorsed lifetime depression in 2001 were coded as having depression from 1989 to 2001, and depression history was updated as available based on the 2003, 2005, 2007, and 2009 questionnaires. Women also reported whether they had a physical exam in the past 2 years on the 1989, 2001, 2003, 2005, 2007, and 2009 biennial questionnaires. Women who reported a physical exam in 1989 were coded as having had a physical from 1989 to 1999; physical exam status was updated as available.
Exclusions
A flowchart of exclusions for deriving the analytic sample (n=49 296) is shown in Figure. Compared to women in the analytic sample, participants who provided trauma/PTSD symptom data but who were excluded from analyses were similar on demographics, family history, and childhood adiposity. Cumulative VTE incidence rates from 1989 to 2011 were comparable for those included (1.9%) and excluded (2.1%), as well as for those who did (2.0%) and did not (1.8%) receive the trauma and PTSD screening supplemental questionnaire.
Figure 1.
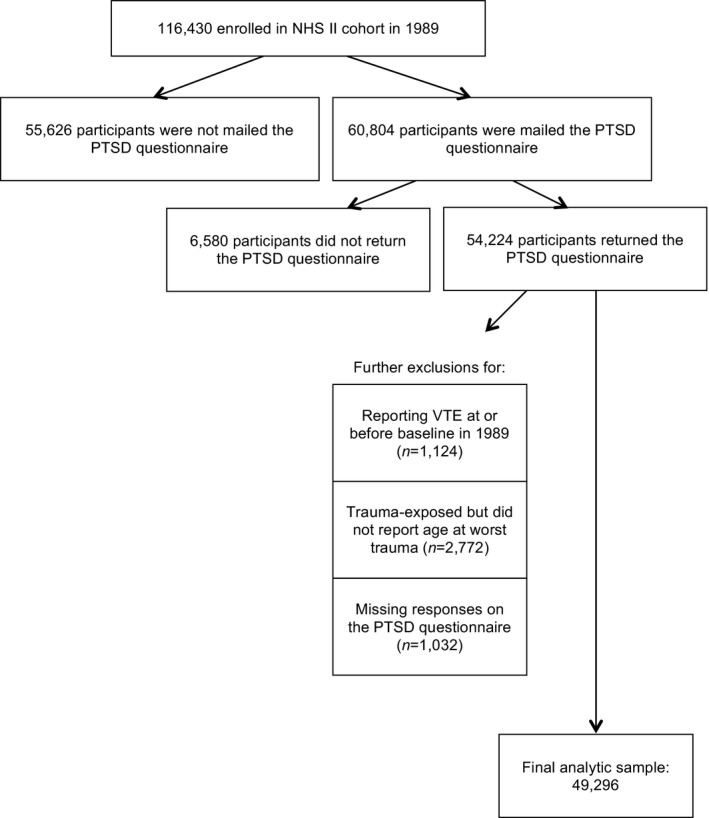
Exclusions for deriving the analytic sample. NHS II indicates Nurses’ Health Study II; PTSD, posttraumatic stress disorder; VTE, venous thromboembolism.
Statistical Analysis
We investigated whether trauma exposure and PTSD symptoms were associated with incident VTE with Cox proportional hazards models to calculate hazard ratios (HRs) and 95% CIs. Participants contributed person‐time from baseline in 1989 until VTE onset, death, the end of follow‐up in 2011, or their last returned questionnaire (ie, if they did not remain in the study to complete the 2011 assessment).
We tested a series of increasingly adjusted models. Model 1 adjusted for age. Model 2 adjusted for true potential confounders including demographics, family history, and childhood adiposity. Model 3 adjusted for VTE‐relevant medical risk factors, medications, and adult health behaviors and conditions. These covariates included parity, oral contraceptive use, nonaspirin nonsteroidal anti‐inflammatory drug use, menopausal status and HT use, antidepressant use, hypertension, hypercholesterolemia, coronary heart disease, cancer, type 2 diabetes mellitus, rheumatologic disease, body mass index, physical activity, diet quality, cigarette smoking, and alcohol consumption. Missing data in covariates were handled by treating missing as a separate category. Variables added in Model 3 were time‐varying covariates and were updated every 2, 4, or 6 years as available. Models were lagged such that for each period of prediction (eg, VTE status on the 1995 questionnaire), covariates added in Model 3 were lagged by 1 period (eg, data from the 1993 questionnaire) and trauma/PTSD symptom status was lagged to represent the year prior to the period for these covariates (eg, trauma/PTSD symptoms in 1992). We lagged models in this way to reduce the likelihood of prodromal disease influencing PTSD symptoms and the health behaviors and conditions assessed close in time to PTSD symptoms yet preceding VTE.
We conducted 5 sensitivity analyses. First, given the broad range of trauma exposure in our sample and the fact that conditional probability of developing PTSD varies for different traumas (eg, interpersonal violence has a higher conditional probability of PTSD compared to natural disaster exposure),11 we re‐estimated Model 2 adjusting for worst trauma type (interpersonal violence, other traumatic event to self, traumatic event occurring to others; women not exposed to trauma were coded as “none” and were included as the reference group) as a categorical covariate. Second, we examined the association between trauma/PTSD symptom status with incidence of the subset of 157 PE events confirmed by additional information or record review over the 22‐year study period. Third, given that depression is often comorbid with PTSD and has been associated with VTE risk,9, 10 we re‐estimated Model 2 adjusting for lifetime history of depression. Fourth, we addressed possible ascertainment bias (ie, that women with PTSD symptoms might be more likely to present to treatment providers with symptoms that would also prompt assessment for VTE) by covarying whether nurses had physical exams in addition to Model 2 covariates as a way to account for engagement with the medical system. Fifth, we considered the potential for recall bias by analyzing prospectively only new‐onset VTE events reported after assessment of trauma/PTSD symptoms (2008) on the 2009 and 2011 questionnaires (n=48 525; 189 new VTE events).
Results
Table 1 presents participant characteristics by trauma exposure and PTSD symptoms at baseline. Over two thirds of women (69%, n=34 118) reported being exposed to a traumatic event by the 1989 baseline assessment. Of those reporting trauma exposure at baseline, most (72%; n=24 714) reported no PTSD symptoms, 14% reported 1 to 3 symptoms (n=4706), 8% reported 4 to 5 symptoms (n=2817), and 6% reported 6 to 7 symptoms (n=1881). Over the course of the study period, an additional 5326 women reported trauma exposure.
Table 1.
Baseline Participant Characteristics as a Function of Trauma Exposure and PTSD Symptoms at the NHS II 1989 Assessment (N=49 296)
| No Trauma (n=15 178) | Trauma‐Exposed (n=34 118) | ||||
|---|---|---|---|---|---|
| No Symptoms (n=24 714) | 1 to 3 Symptoms (n=4706) | 4 to 5 Symptoms (n=2817) | 6 to 7 Symptoms (n=1881) | ||
| Mean (SD) or % (n) | Mean (SD) or % (n) | Mean (SD) or % (n) | Mean (SD) or % (n) | Mean (SD) or % (n) | |
| Age, y | 34 (5) | 35 (5) | 35 (4) | 35 (4) | 35 (4) |
| Parents’ education at birth, ≥college, % | 23.6 (3584) | 22.3 (5503) | 22.6 (1065) | 22.2 (625) | 24.9 (468) |
| Parental history of myocardial infarction, % | 33.6 (5097) | 37.0 (9137) | 37.6 (1771) | 39.7 (1118) | 37.2 (699) |
| Highest somatotype, age 5, % | 6.2 (939) | 7.0 (1722) | 6.8 (318) | 7.6 (214) | 8.4 (158) |
| White race, % | 93.5 (14 186) | 93.8 (23 179) | 94.2 (4431) | 94.7 (2668) | 94.6 (1779) |
| Parity, % | |||||
| Nulliparous | 33.1 (5029) | 26.3 (6491) | 25.9 (1221) | 26.4 (745) | 30.2 (568) |
| 1 child | 17.5 (2650) | 18.7 (4614) | 18.3 (862) | 19.9 (561) | 19.8 (372) |
| 2 to 3 children | 46.5 (7056) | 50.4 (12 455) | 50.4 (2373) | 48.3 (1361) | 44.6 (838) |
| 4+ children | 2.9 (443) | 4.7 (1154) | 5.3 (250) | 5.3 (150) | 5.5 (103) |
| Oral contraceptive use, % | |||||
| Never | 18.7 (2845) | 15.3 (3771) | 14.2 (667) | 13.2 (373) | 13.1 (246) |
| Former user | 66.0 (10 021) | 73.1 (18 059) | 75.2 (3541) | 76.6 (2159) | 77.5 (1458) |
| Current user | 15.1 (2295) | 11.6 (2860) | 10.5 (493) | 10.0 (281) | 9.4 (177) |
| Premenopausal status, % | 97.8 (14 837) | 97.1 (23 997) | 96.8 (4555) | 96.0 (2705) | 95.3 (1792) |
| Nonaspirin nonsteroidal anti‐inflammatory drug user, % | 16.1 (2443) | 19.2 (4742) | 20.2 (950) | 23.7 (668) | 27.0 (507) |
| Hypercholesterolemia, % | 9.4 (1419) | 10.7 (2640) | 10.5 (494) | 13.0 (365) | 13.2 (249) |
| Hypertension, % | 4.1 (618) | 5.1 (1259) | 5.8 (271) | 7.2 (202) | 6.9 (129) |
| Coronary heart disease, %a | 0.1 (22) | 0.3 (67) | 0.7 (33) | 1.0 (27) | 1.0 (19) |
| Cancer, %b | 0.5 (81) | 1.0 (244) | 1.1 (51) | 1.0 (27) | 1.1 (21) |
| Type 2 diabetes mellitus, % | 0.5 (75) | 0.7 (168) | 0.6 (26) | 0.7 (19) | 0.9 (17) |
| Rheumatologic disease, %c | 0.5 (73) | 0.8 (202) | 1.1 (53) | 1.3 (37) | 1.4 (26) |
| Body mass index, kg/m2 | 23.5 (4.6) | 23.9 (4.8) | 24.0 (4.9) | 24.2 (5.1) | 24.2 (5.3) |
| Cigarette smoking, % | |||||
| Never | 72.3 (10 978) | 65.9 (16 297) | 62.3 (2933) | 60.8 (1712) | 55.7 (1047) |
| Former smoker | 18.2 (2755) | 22.3 (5518) | 24.8 (1167) | 26.8 (756) | 27.6 (519) |
| Current smoker | 9.2 (1399) | 11.4 (2821) | 12.5 (587) | 11.9 (335) | 16.4 (308) |
| Alcohol intake, g/day | 2.9 (5.0) | 3.0 (5.6) | 3.2 (6.0) | 2.9 (5.5) | 3.1 (6.0) |
| Physical activity, MET h/weekd | 24.2 (35.7) | 24.7 (35.9) | 23.4 (33.2) | 24.0 (34.3) | 24.3 (31.4) |
| Worst diet (1st quintile) on the alternative healthy eating index, %e | 20.3 (3074) | 18.1 (4481) | 16.8 (791) | 16.8 (472) | 15.3 (288) |
| Antidepressant use, %f | 6.4 (970) | 10.2 (2533) | 13.9 (655) | 22.1 (622) | 32.7 (616) |
NHS II indicates Nurses’ Health Study II; PTSD, posttraumatic stress disorder.
Myocardial infarction or angina.
Excluding nonmelanoma skin cancer.
Rheumatoid arthritis or systemic lupus erythematosus.
MET hours/week=metabolic equivalent hours/week.
First assessed in 1991.
First assessed in 1993.
There were 960 self‐reported VTE events over the 22‐year follow‐up. Compared to no trauma exposure, trauma alone and trauma with PTSD symptoms were each associated with heightened risk of VTE, with the greatest elevation in risk observed with the highest PTSD symptom levels (Table 2). For example, the risk of VTE was 2.42‐fold higher among women with trauma/6 to 7 symptoms than in women with no trauma exposure after adjusting for demographics, family history, and childhood adiposity (Table 2, Model 2). Most trauma and PTSD symptom groups demonstrated statistically significantly elevated risk in the fully adjusted model (excepting trauma/1–3 symptoms), despite attenuation in the HRs (Table 2, Model 3).
Table 2.
Adjusted Hazard Ratios (95% CI) for the Association of Trauma Exposure and PTSD Symptoms With Risk of Incident Venous Thromboembolism (960 Self‐Reported Events), 1989–2011
| No Trauma | Trauma‐Exposed | ||||
|---|---|---|---|---|---|
| No Symptoms | 1 to 3 Symptoms | 4 to 5 Symptoms | 6 to 7 Symptoms | ||
| Cases, n (person‐y) | 144 (280 907) | 520 (567 661) | 117 (148 868) | 102 (92 408) | 77 (59 083) |
| Hazard Ratio (95% CI) | |||||
| Model 1: Age‐adjusted model | 1 (ref) | 1.73 (1.44–2.08) | 1.44 (1.13–1.85) | 2.04 (1.58–2.63) | 2.44 (1.85–3.23) |
| Model 2: Minimally adjusted modela | 1 (ref) | 1.72 (1.43–2.08) | 1.44 (1.12–1.84) | 2.00 (1.55–2.59) | 2.42 (1.83–3.20) |
| Model 3: Fully adjusted modelb | 1 (ref) | 1.60 (1.33–1.93) | 1.26 (0.98–1.62) | 1.65 (1.27–2.15) | 1.90 (1.43–2.54) |
PTSD indicates posttraumatic stress disorder.
Adjusted for age, race/ethnicity, parental education, parental history of myocardial infarction, and age 5 somatotype.
Additionally adjusted for parity, oral contraceptive use, nonaspirin nonsteroidal anti‐inflammatory drug use, menopausal status and hormone therapy use, antidepressant use, hypercholesterolemia, hypertension, coronary heart disease (myocardial infarction or angina), cancer (excluding nonmelanoma skin cancer), type 2 diabetes mellitus, rheumatologic disease (rheumatoid arthritis or systematic lupus erythematosus), body mass index, physical activity, diet quality, cigarette smoking, and alcohol consumption.
To address whether the type of worst traumatic event accounted for associations between PTSD symptoms and incident VTE, we included worst trauma type as a categorical covariate along with Model 2 covariates. Although the HRs were attenuated in this model, all the trauma/PTSD symptom groups were significantly associated with increased risk of developing VTE except for the trauma/1 to 3 symptoms group (notably, this group was not characterized by significantly elevated risk of incident VTE in the fully adjusted model): trauma/no symptoms: HR=1.39 (95% CI, 1.03–1.87); trauma/1 to 3 symptoms: HR=1.18 (95% CI, 0.84–1.65); trauma/4 to 5 symptoms: HR=1.66 (95% CI, 1.17–2.35); trauma/6 to 7 symptoms: HR=2.02 (95% CI, 1.40–2.91).
Risk estimates for trauma exposure and the highest level of PTSD symptoms were also elevated when we restricted analyses to the 157 probable and definite cases of idiopathic PE that were confirmed by additional information or record review. However, in this sensitivity analysis, HRs in the minimally adjusted model were substantially attenuated and not statistically significant, and confidence intervals were wide (trauma/no symptoms: HR=1.26 [95% CI, 0.84–1.91]; trauma/1–3 symptoms: HR=1.14 [95% CI, 0.65–2.00]; trauma/4–5 symptoms: HR=0.87 [95% CI, 0.42–1.78]; trauma/6–7 symptoms: HR=1.53 [95% CI, 0.77–3.05]).
When covarying lifetime depression along with Model 2 covariates, all trauma and PTSD symptom groups remained significantly associated with increased VTE risk, although HRs were attenuated, especially for those with more PTSD symptoms (trauma/no symptoms: HR=1.68 [95% CI, 1.39–2.02]; trauma/1–3 symptoms: HR=1.34 [95% CI, 1.05–1.72]; trauma/4–5 symptoms: HR=1.77 [95% CI, 1.36–2.29]; trauma/6–7 symptoms: HR=2.06 [95% CI, 1.55–2.74]). Trauma exposure and PTSD symptoms continued to be characterized by heightened risk for developing first VTE during follow‐up when we accounted for physical exam attendance in addition to Model 2 covariates (trauma/no symptoms: HR=1.72 [95% CI, 1.42–2.06]; trauma/1–3 symptoms: HR=1.43 [95% CI, 1.12–1.83]; trauma/4–5 symptoms: HR=1.99 [95% CI, 1.54–2.57]; trauma/6–7 symptoms: HR=2.38 [95% CI, 1.80–3.15]).
In the analysis of 189 prospectively detected new‐onset VTE events, trauma exposure and PTSD symptoms were associated with nominally increased risk of incident VTE compared to no trauma exposure when adjusting for Model 2 covariates (trauma/no symptoms: HR=1.55 [95% CI, 1.01–2.38]; trauma/1–3 symptoms: HR=1.55 [95% CI, 0.93–2.57]; trauma/4–5 symptoms: HR=1.22 [95% CI, 0.66–2.25]; trauma/6–7 symptoms: HR=1.82 [95% CI, 0.96–3.43]). However, aside from the HR for trauma/no symptoms, results did not reach statistical significance when analyses were restricted to the smaller number of cases reported in this brief follow‐up period.
Discussion
This is the first study to demonstrate that trauma and PTSD symptoms are associated with increased risk of incident VTE in a large cohort of younger and middle‐aged women over a 22‐year period. Trauma exposure and elevated PTSD symptoms have been identified as risk factors for other cardiovascular diseases in this cohort of women,14 and our work extends these findings to the third most common cause of cardiovascular death: VTE. Women with the highest PTSD symptom levels had the greatest elevation in risk of developing VTE, even when adjusting for a variety of potential confounders and mediators that were lagged to reflect women's health status prior to VTE onset. Women with trauma exposure who did not report PTSD symptoms also exhibited increased risk of VTE incidence compared to women with no trauma exposure, although the magnitude of elevated risk was not as substantial as for women with high PTSD symptom levels. Initial research has linked psychosocial factors, including chronic stress36 and depression,9, 10 to VTE risk. Our study further underscores the role that psychosocial variables may play in contributing to the development of VTE, and suggests that the experience of extremely stressful, traumatic events and related posttraumatic stress reactions may have even more widespread cardiotoxic effects than has previously been considered.
VTE is characterized by high mortality rates and a high rate of recurrence among survivors,2 and it has been identified as an important health concern for women in particular.3 Furthermore, studying the link between PTSD symptoms and VTE specifically in a sample of younger and middle‐aged women has particular relevance for understanding VTE risk in women. Even though the overall rate of first VTE is similar in men and women,4 incidence rates are higher in women of childbearing age than in men of the same age.4, 5 This difference in incidence rate likely reflects, in part, VTE due to pregnancy and/or oral contraceptive use during childbearing years. However, roughly a quarter to a half of VTE cases, overall, are classified as occurring in the absence of major established risk factors,6 thus emphasizing the need for greater understanding of susceptibility to VTE. PTSD is twice as common in women as in men,11 and our findings suggest that elevated PTSD symptoms (over and above the effects of trauma type and depression, a psychological disorder that is frequently comorbid with PTSD and has also been linked with risk of VTE9, 10) may be an important vulnerability factor for developing VTE in women. Given that VTE is a leading cause of mortality among pregnant and recently postpartum women,3 and in light of female‐specific risk factors for VTE related to reproductive hormone exposure,3 pregnant women with PTSD symptoms or women with PTSD symptoms who are using hormone therapy may represent especially vulnerable groups with respect to VTE risk. Additional research in these particular populations is needed, but our findings highlight certain individuals who might benefit from close monitoring by healthcare providers in order to prevent possible VTE.
Both biological and behavioral mechanisms likely underlie the associations between trauma, PTSD symptoms, and VTE risk. Acute (eg, mental stress) and chronic stress (eg, job strain, low socioeconomic status) have been associated with a hypercoagulable state as indicated by increased blood levels of circulating procoagulant markers (eg, fibrinogen, clotting factor VII) that are known to counteract anticoagulant processes.37 Furthermore, there is cross‐sectional evidence suggesting that PTSD is characterized by elevated levels of clotting markers.16 Hypercoagulable states may trigger VTE by increasing the likelihood of clot formation, and longitudinal research that examines the effects of trauma exposure and PTSD on coagulation and fibrinolysis is needed. Increased inflammation and endothelial damage—both of which have been observed in those with elevated PTSD symptoms15, 17—are thought to heighten susceptibility to developing VTE as well.7 Thus, several of the covariates included in the fully adjusted model likely represent additional underlying processes linking trauma/PTSD symptoms to VTE; this is consistent with the attenuation in HRs in the fully adjusted model relative to the minimally adjusted model. Elevated PTSD symptoms are associated with increased risk for numerous chronic medical conditions, including MI and stroke,14 type 2 diabetes mellitus,38 and rheumatologic disease,39 which in turn have also been linked to VTE risk7, 40 and could be markers for underlying inflammation or hypercoagulability.
Trauma and PTSD symptoms have also been associated with health risk behaviors that have been tied to increased risk of developing VTE in women. For example, women with elevated PTSD symptoms in the NHS II have been found to be at increased risk of becoming overweight or obese over time,41 and body mass index has been identified as a major risk factor for PE in NHS participants.19 Furthermore, compared to women with no history of trauma, women with elevated PTSD symptoms in our sample were more likely to smoke cigarettes and use antidepressants—2 behavioral factors that have also been linked to vulnerability to developing VTE.7, 35 Although preliminary, findings from our fully adjusted model highlight some candidate behavioral mechanisms underlying associations between trauma, PTSD symptoms, and incident VTE. Moreover, studying these processes separately in women and in men holds promise for best understanding underlying mechanisms of risk given sex differences in health behaviors (eg, men are more likely to smoke than women but women have been found to be less likely to meet current aerobic physical activity guidelines than men5). Ultimately, it is likely that trauma exposure and PTSD symptoms are associated with various forms of dysregulation at the biological and behavioral level that contribute to risk for numerous chronic medical conditions, including VTE. Further work is needed to delineate the relative contributions of biological and behavioral factors that underlie the associations between trauma exposure, PTSD symptoms, and VTE onset in women. Such work has the potential to not only further understanding of mechanistic processes but also identify more specific targets for prevention and intervention.
These novel findings contribute importantly to a growing literature suggesting that trauma and PTSD symptoms may truly inflict cardiovascular damage, harming health beyond the significant mental health burden imposed. However, several limitations are worth noting. Trauma and PTSD symptoms were assessed retrospectively, although the reliability of dating of PTSD symptom onset has previously been demonstrated in this sample.25 Additionally, because PTSD symptoms were assessed with a screening measure, some misclassification is possible, and further research with more comprehensive diagnostic interviews may not only add more precision but also address the extent to which diagnoses of PTSD are related to VTE. Furthermore, we assessed PTSD symptoms based on DSM‐IV PTSD criteria. Even though most of the DSM‐5 and DSM‐IV PTSD criteria are highly similar, research using DSM‐5 PTSD criteria is needed. Other limitations are that our primary outcome comprised VTE events based on self‐report, and we lacked information on the exact date of VTE (as in prior NHS II research with self‐reported outcomes,27, 28 we used the date of the first questionnaire on which VTE was reported for time to event). However, being nurses, NHS II participants have high health literacy, and reporting of VTE events has been validated in this cohort.29 Furthermore, when we restricted analyses to the small subset of PE events confirmed by additional information or record review, although not statistically significant, effect sizes were consistent with the notion that trauma exposure and elevated PTSD symptoms are associated with increased VTE risk. In fact, the PE cases considered here likely represent only a subset of possible VTE events since medical records were requested for PE events solely in women without a history of malignancy. Although additional research with confirmed PE and DVT cases would be useful, we suspect results would be similar.
We also lacked information on several established VTE risk factors, including thrombophilia and genetic risk for clotting disorders. Furthermore, even though warfarin/Coumadin use is unlikely to confound associations between trauma, PTSD symptoms, and VTE, we were not able to adjust sufficiently for these medications due to limited information in the NHS II. In addition, women needed to remain in the study until 2008 in order to provide data on trauma and PTSD symptoms, and survivor bias is thus a possible concern. However, study retention is high (>90% biennial response rate), and the mortality rate is low (only 1.6% of the NHS II cohort was deceased by 2008). Finally, because the NHS II sample was representative of the nursing profession at the time of recruitment in 1989, the cohort is predominantly white and highly educated.
Despite these limitations, the current study has several important and unique strengths. The NHS II cohort is a large sample of civilian women exposed to a wide range of traumatic experiences. The sample is richly characterized with respect to risk for chronic disease, and the women have repeatedly provided information on health behaviors, medication use, and medical conditions for over 20 years. Thus, not only is this the first study to our knowledge to investigate associations between trauma exposure, PTSD symptoms, and incident VTE specifically in women, but the unique characteristics of the NHS II cohort facilitate a highly detailed examination of these issues, going beyond tests of the primary association to take account of a broad array of potential confounders and speculating possible underlying mechanisms, as well as considering effects of trauma and PTSD symptoms separately from one another.
Conclusions
Within the past decade, the National Institutes of Health, Center for Disease Control, and Surgeon General have made concerted efforts directed at VTE prevention.2 Given that VTE is associated with long‐term complications and high mortality rates, it is critical to diagnose cases early and accurately. However, this effort is frequently complicated by lack of VTE awareness on the part of patients and healthcare providers and by the often‐silent presentation of VTE.2 Our findings indicate that women with trauma exposure and elevated PTSD symptoms may be especially susceptible to developing VTE compared to women without trauma exposure. A growing body of evidence consistently suggests that elevated PTSD symptoms are a risk factor for cardiovascular diseases such as MI and stroke,14 and our results suggest that PTSD symptoms are associated with even more widespread cardiovascular effects than perhaps initially recognized. Screening for VTE risk in women with trauma and PTSD symptoms may help to offset this vulnerability and reduce the disease burden of VTE in women. Additional research is needed to ascertain whether women with trauma exposure and PTSD symptoms may particularly benefit from VTE prevention measures, including weight loss and smoking cessation. Ultimately, it is critical to test whether mitigating symptoms of PTSD and trauma‐related distress can offset the risk of developing VTE in these vulnerable individuals.
Sources of Funding
This study was supported by the National Institutes of Health grants R01MH101269‐01A1, K01HL130650, T32MH017119, and UM1CA176726, and the Yerby Postdoctoral Fellowship.
Disclosures
None.
Acknowledgments
We acknowledge the Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital, and Harvard Medical School for managing the NHS II.
(J Am Heart Assoc. 2016;5:e003197 doi: 10.1161/JAHA.116.003197)
References
- 1. Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet. 2012;379:1835–1846. [DOI] [PubMed] [Google Scholar]
- 2. Beckman MG, Hooper WC, Critchley SE, Ortel TL. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38:S495–S501. [DOI] [PubMed] [Google Scholar]
- 3. Eichinger S, Evers J, Glasier A, La Vecchia C, Martinelli I, Skouby S, Somigliana E, Baird D, Benagiano G, Crosignani P. Venous thromboembolism in women: a specific reproductive health risk. Hum Reprod Update. 2013;19:471–482. [DOI] [PubMed] [Google Scholar]
- 4. Nordström M, Lindblad B, Bergqvist D, Kjellström T. A prospective study of the incidence of deep‐vein thrombosis within a defined urban population. J Intern Med. 1992;232:155–160. [DOI] [PubMed] [Google Scholar]
- 5. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. [DOI] [PubMed] [Google Scholar]
- 6. White RH. Four topics in venous thromboembolism: the epidemiology of venous thromboembolism. Circulation. 2003;107:I4–I8. [DOI] [PubMed] [Google Scholar]
- 7. Piazza G, Goldhaber SZ. Venous thromboembolism and atherothrombosis: an integrated approach. Circulation. 2010;121:2146–2150. [DOI] [PubMed] [Google Scholar]
- 8. Viles‐Gonzalez JF, Fuster V, Badimon JJ. Atherothrombosis: a widespread disease with unpredictable and life‐threatening consequences. Eur Heart J. 2004;25:1197–1207. [DOI] [PubMed] [Google Scholar]
- 9. Lee CW, Liao CH, Lin CL, Liang JA, Sung FC, Kao CH. Depression and risk of venous thromboembolism: a population‐based retrospective cohort study. Psychosom Med. 2015;77:591–598. [DOI] [PubMed] [Google Scholar]
- 10. von Känel R, Margani A, Stauber S, Meyer FA, Biasiutti FD, Vökt F, Wissmann T, Lämmle B, Lukas PS. Depressive symptoms as a novel risk factor for recurrent venous thromboembolism: a longitudinal observational study in patients referred for thrombophilia investigation. PLoS One. 2015;10:e0125858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. [DOI] [PubMed] [Google Scholar]
- 12. Kilpatrick D, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM‐IV and DSM‐5 criteria. J Trauma Stress. 2013;26:537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wentworth BA, Stein MB, Redwine LS, Xue Y, Taub PR, Clopton P, Nayak KR, Maisel AS. Post‐traumatic stress disorder: a fast track to premature cardiovascular disease? Cardiol Rev. 2013;21:16–22. [DOI] [PubMed] [Google Scholar]
- 14. Sumner JA, Kubzansky LD, Elkind MS, Roberts AL, Agnew‐Blais J, Chen Q, Cerdá M, Rexrode KM, Rich‐Edwards JW, Spiegelman D, Suglia SF, Rimm EB, Koenen KC. Trauma exposure and posttraumatic stress disorder symptoms predict onset of cardiovascular events in women. Circulation. 2015;132:251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baker DG, Nievergelt CM, O'Connor DT. Biomarkers of PTSD: neuropeptides and immune signaling. Neuropharmacology. 2012;62:663–673. [DOI] [PubMed] [Google Scholar]
- 16. von Känel R, Hepp U, Buddeberg C, Keel M, Mica L, Aschbacher K, Schnyder U. Altered blood coagulation in patients with posttraumatic stress disorder. Psychosom Med. 2006;68:598–604. [DOI] [PubMed] [Google Scholar]
- 17. von Känel R, Hepp U, Traber R, Kraemer B, Mica L, Keel M, Mausbach BT, Schnyder U. Measures of endothelial dysfunction in plasma of patients with posttraumatic stress disorder. Psychiatry Res. 2008;158:363–373. [DOI] [PubMed] [Google Scholar]
- 18. Watanabe H, Kodama M, Tanabe N, Nakamura Y, Nagai T, Sato M, Okabe M, Aizawa Y. Impact of earthquakes on risk for pulmonary embolism. Int J Cardiol. 2008;129:152–154. [DOI] [PubMed] [Google Scholar]
- 19. Kabrhel C, Varraso R, Goldhaber SZ, Rimm EB, Camargo CA. Prospective study of BMI and the risk of pulmonary embolism in women. Obesity. 2009;17:2040–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kabrhel C, Varraso R, Goldhaber SZ, Rimm E, Camargo CA Jr. Physical inactivity and idiopathic pulmonary embolism in women: prospective study. BMJ. 2011;343:d3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morgan CA III, Hazlett G, Wang S, Richardson EG Jr, Schnurr P, Southwick SM. Symptoms of dissociation in humans experiencing acute, uncontrollable stress: a prospective investigation. Am J Psychiatry. 2001;158:1239–1247. [DOI] [PubMed] [Google Scholar]
- 22. Schnurr PP, Vieilhauer MJ, Weathers F, Findler M. The Brief Trauma Questionnaire. White River Junction, VT: National Center for PTSD; 1999. [Google Scholar]
- 23. Schnurr PP, Spiro A III, Vielhauer MJ, Findler MN, Hamblen JL. Trauma in the lives of older men: findings from the Normative Aging Study. J Clin Geropsychol. 2002;8:175–187. [Google Scholar]
- 24. Breslau N, Peterson EL, Kessler RC, Schultz LR. Short screening scale for DSM‐IV posttraumatic stress disorder. Am J Psychiatry. 1999;156:908–911. [DOI] [PubMed] [Google Scholar]
- 25. Koenen KC, De Vivo I, Rich‐Edwards J, Smoller JW, Wright RJ, Purcell SM. Protocol for investigating genetic determinants of posttraumatic stress disorder in women from the Nurses’ Health Study II. BMC Psychiatry. 2009;9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kimerling R, Ouimette P, Prins A, Nisco P, Lawler C, Cronkite R, Moos RH. Brief report: utility of a short screening scale for DSM‐IV PTSD in primary care. J Gen Intern Med. 2006;21:65–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Forman JP, Stampfer MJ, Curhan GC. Non‐narcotic analgesic dose and risk of incident hypertension in US women. Hypertension. 2005;46:500–507. [DOI] [PubMed] [Google Scholar]
- 28. Riley EH, Wright RJ, Jun HJ, Hibert EN, Rich‐Edwards JW. Hypertension in adult survivors of child abuse: observations from the Nurses’ Health Study II. J Epidemiol Community Health. 2010;64:413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pun VC, Hart JE, Kabrhel C, Camargo CA Jr, Baccarelli AA, Laden F. Prospective study of ambient particulate matter exposure and risk of pulmonary embolism in the Nurses’ Health Study cohort. Environ Health Perspect. 2015;123:1265–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lind C, Enga KF, Mathiesen EB, Njølstad I, Brækkan SK, Hansen JB. Family history of myocardial infarction and cause‐specific risk of myocardial infarction and venous thromboembolism: the Tromsø Study. Circ Cardiovasc Genet. 2014;7:684–691. [DOI] [PubMed] [Google Scholar]
- 31. Varraso R, Kabrhel C, Goldhaber SZ, Rimm EB, Camargo CA Jr. Prospective study of diet and venous thromboembolism in US women and men. Am J Epidemiol. 2012;175:114–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self‐reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–473. [DOI] [PubMed] [Google Scholar]
- 33. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Friedman MJ, Davidson JRT. Pharmacotherapy for PTSD In: Friedman MJ, Keane TM, Resisk PA, eds. Handbook of PTSD: Science and Practice. New York, NY: Guilford Press; 2007:376–405. [Google Scholar]
- 35. Jick SS, Li L. Antidepressant drug use and risk of venous thromboembolism. Pharmacotherapy. 2008;28:144–150. [DOI] [PubMed] [Google Scholar]
- 36. Rosengren A, Fredén M, Hansson PO, Wilhelmsen L, Wedel H, Eriksson H. Psychosocial factors and venous thromboembolism: a long‐term follow‐up study of Swedish men. J Thromb Haemost. 2008;6:558–564. [DOI] [PubMed] [Google Scholar]
- 37. von Känel R, Mills PJ, Fainman C, Dimsdale JE. Effects of psychological stress and psychiatric disorders on blood coagulation and fibrinolysis: a biobehavioral pathway to coronary artery disease? Psychosom Med. 2001;63:531–544. [DOI] [PubMed] [Google Scholar]
- 38. Roberts AL, Agnew‐Blais JC, Spiegelman D, Kubzansky LD, Mason SM, Galea S, Hu FB, Rich‐Edwards JW, Koenen KC. Posttraumatic stress disorder and incidence of type 2 diabetes mellitus in a sample of women: a 22‐year longitudinal study. JAMA Psychiatry. 2015;72:203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee YC, Agnew‐Blais J, Malspeis S, Keyes K, Costenbader K, Kubzansky LD, Roberts AL, Koenen KC, Karlson EW. Posttraumatic stress disorder and risk for incident rheumatoid arthritis. Arthritis Care Res. 2016;68:292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee JJ, Pope JE. A meta‐analysis of the risk of venous thromboembolism in inflammatory rheumatic diseases. Arthritis Res Ther. 2014;16:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kubzansky LD, Bordelois P, Jun HJ, Roberts AL, Cerda M, Bluestone N, Koenen KC. The weight of traumatic stress: a prospective study of posttraumatic stress disorder symptoms and weight status in women. JAMA Psychiatry. 2014;71:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]


