Abstract
Background
Literature studies suggested a lower prevalence of coronary artery disease (CAD) in bicuspid aortic valve (BAV) than in tricuspid aortic valve (TAV) patients. However, this finding has been challenged. We performed a meta‐analysis to assess whether aortic valve morphology has a different association with CAD, concomitant coronary artery bypass grafting (CABG), and postoperative mortality.
Methods and Results
Detailed search was conducted according to the PRISMA (Preferred Reporting Items for Systematic reviews and Meta‐Analyses) guideline to identify all patients with BAV or TAV and presence of CAD, concomitant myocardial surgical revascularization, and the postoperative mortality. Thirty‐one studies on 3017 BAV and 4586 TAV patients undergoing aortic valve surgery were included. BAV patients showed a lower prevalence of CAD (odds ratio [OR]: 0.33; 95% CI: 0.17, 0.65), concomitant CABG (OR, 0.45; 95% CI: 0.35, 0.59), and postoperative mortality (OR, 0.62; 95% CI: 0.40, 0.97) than TAV. However, BAV subjects were significantly younger than TAV (mean difference: −7.29; 95% CI: −11.17, −3.41) were more frequently males (OR, 1.61; 95% CI: 1.33, 1.94) and exhibited a lower prevalence of hypertension (OR, 0.58; 95% CI: 0.39, 0.87) and diabetes (OR, 0.71; 95% CI: 0.54, 0.93). Interestingly, a metaregression analysis showed that younger age and lower prevalence of diabetes were associated with lower prevalence of CAD (Z value: −3.03; P=0.002 and Z value: −3.10; P=0.002, respectively) and CABG (Z value: −2.69; P=0.007 and Z value: −3.36; P=0.001, respectively) documented in BAV patients.
Conclusions
Analysis of raw data suggested an association of aortic valve morphology with prevalence of CAD, concomitant CABG, and postoperative mortality. Interestingly, the differences in age and diabetes have a profound impact on prevalence of CAD between BAV and TAV. In conclusion, our meta‐analysis suggests that the presence of CAD is independent of aortic valve morphology.
Keywords: aortic valve morphology, bicuspid aortic valve, coronary artery disease
Subject Categories: Meta Analysis, Valvular Heart Disease, Coronary Artery Disease
Introduction
Aortic valve stenosis (AVS) is considered the most prevalent form of valve disease.1, 2 The number of people affected by this progressive and debilitating pathology will increase because of the aging population, causing an ever‐increasing public health burden.3 Traditionally, it was thought that AVS was related to valve degeneration attributed to aging, caused by several years of mechanical stress and biological response to such injury. However, more recently, several risks factors have been linked to the development of AVS, including male sex, hypertension, hyperlipidemia, smoking, advanced age, and congenital bicuspid valve morphology.4, 5 Overall, AVS pathogenesis is a multifactorial process and seems to be related to coronary atherosclerosis. In detail, an important link between AVS and early stages of coronary atherosclerotic plaque has been hypothesized,6, 7, 8 and a multitude of studies have been published regarding the associations between AVS, coronary artery disease (CAD), and coronary artery bypass grafting (CABG).9, 10, 11, 12 In addition, a very recent study by Boudoulas et al.13 describes the association between aortic stenosis and CABG focusing on valve morphology and concluding that “the incidence of coronary artery disease is extremely high in patients with aortic stenosis and tricuspid aortic valve.” However, literature data are not consistent about this issue, and data about such an association have been challenged. In addition, the critical review of available studies highlights that, in most cases, only univariate unadjusted analyses were used, seldom taking into account for potential confounders. Thus, the aim of this study was to perform a meta‐analysis of literature studies enrolling patients undergoing aortic valve surgery to assess whether aortic valve morphology (tri‐ or bicuspid) impacts on prevalence of CAD, concomitant CABG, and postoperative mortality. In addition, to assess the presence of potential confounders, we evaluated the impact of distribution of major clinical and demographic variables between patients with bi‐ or tricuspid aortic valve.
Methods
A protocol for this review was prospectively developed, detailing the specific objectives, criteria for study selection, outcomes, and statistical methods.
Search Strategy
To identify all available studies pertaining to prevalence of CAD, defined by atherosclerosis of 1 or more arteries that supply blood to the heart causing oxygen deficiency in the myocardium, we included articles with anamnestic CAD also when it was not specified whether it was anatomical CAD (70% stenosis or greater in at least 1 major coronary artery) or clinical CAD (previous acute myocardial infarction or percutaneous coronary intervention). A detailed search was conducted according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta‐Analyses) guidelines14 to identify all concomitant myocardial surgical revascularization (CABG) and postoperative mortality in patients with bicuspid aortic valve (BAV) and tricuspid aortic valve (TAV) undergoing aortic valve surgery. A systematic search was performed in the electronic databases (PubMed, Web of Science, Scopus, and EMBASE), using the following search terms in all possible combinations: tricuspid aortic valve; bicuspid aortic valve; coronary artery disease; coronary artery bypass; myocardial infarction; mortality; and death. The last search was performed in November 2015. The search strategy was developed without any language or publication year restriction. In addition, the reference lists of all retrieved articles were manually reviewed. In case of missing data, the authors were contacted by e‐mail to try to retrieve original data. Two independent authors (P.P. and M.N.D.D.M.) analyzed each article and performed the data extraction independently. In case of disagreement, a third investigator was consulted (L.C.). Discrepancies were resolved by consensus. Selection results have been reported according to the PRISMA flow chart (Figure 1).
Figure 1.
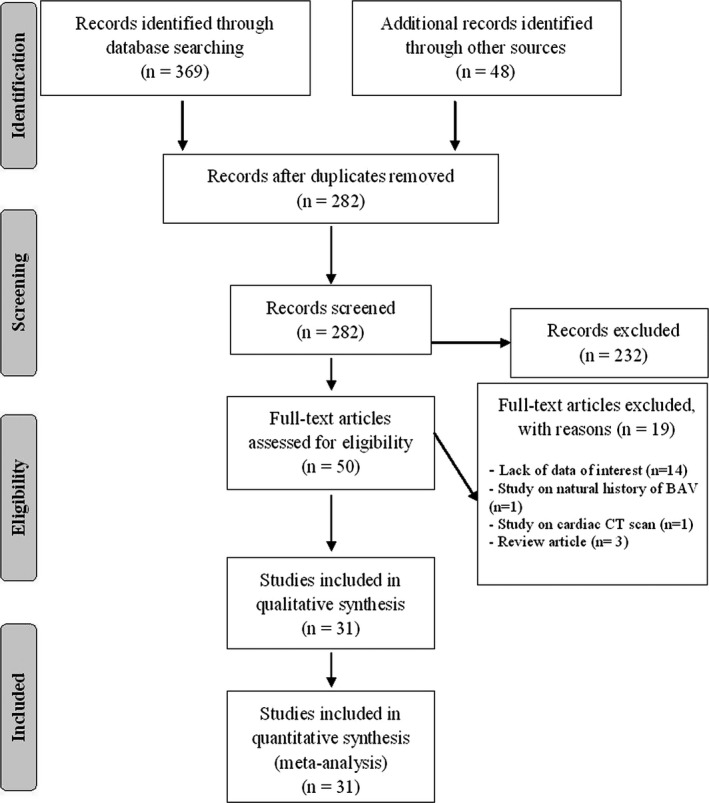
PRISMA flow diagram. BAV indicates bicuspid aortic valve; CT, computed tomography.
Data Extraction and Quality Assessment
According to the prespecified protocol, all studies evaluating prevalence of CAD or use of CABG or postoperative mortality in patients with BAV and in those with TAV undergoing aortic valve replacement were included in the analysis. Case reports, reviews, animal studies, and studies on natural history of patients with aortic valve disease (not undergoing surgery) were excluded. In each study, data regarding sample size, major clinical and demographic variables, number of patients with CAD, and number of those undergoing CABG and those dying postoperatively were extracted. Formal quality score adjudication was not used, because previous investigations failed to demonstrate its usefulness.15
Statistical Analysis and Risk of Bias Assessment
Statistical analysis was carried out using Comprehensive Meta‐analysis (version 2 [2005]; Biostat, Englewood, NJ). Differences among BAV and TAV subjects were expressed as mean difference (MD) with pertinent 95% CIs for continuous variables, and as odds ratio (OR) with pertinent 95% CI for dichotomous variables. Overall effect was tested using Z scores, and significance was set at P<0.05. Statistical heterogeneity among studies was assessed with the chi‐square Cochran's Q test and with the I2 statistic, which measures the inconsistency across study results and describes the proportion of total variation in study estimates, that is, attributed to heterogeneity rather than sampling error. In detail, I2 values of 0% indicate no heterogeneity, 25% low, 25% to 50% moderate, and 50% high heterogeneity.16
Publication bias was assessed by the Egger test and represented graphically by funnel plots of the standard difference in means versus SE. Visual inspection of funnel plot asymmetry was performed to address for possible small‐study effect, as well as the Egger test to address publication bias, over and above any subjective evaluation. P<0.10 was considered statistically significant.17 In order to be as conservative as possible, the random‐effect method was used to take into account the variability among included studies.
Metaregression Analyses
We hypothesized that differences in prevalence of CAD, use of CABG, or postoperative mortality between BAV and TAV patients may be affected by differences in clinical and demographic characteristics of patients included in different studies (mean age, sex, body mass index [BMI], hypertension, diabetes mellitus, hyperlipidemia, and smoking habit). To assess the possible effect of such variables in explaining the different results observed across studies, we planned to perform metaregression analyses after implementing a regression model with prevalence of CAD, use of CABG, or postoperative mortality between BAV and TAV patients as dependent variables (y) and the variables mentioned above as independent variables (x).
Results
After excluding duplicate results, the search retrieved 282 articles. Of these studies, 232 were excluded because they were off the topic after scanning the title and/or the abstract and 19 because they were reviews/comments/case reports or they lacked data of interest.
Thus, 31 articles (3017 BAV patients and 4586 TAV patients) were included in the final analysis (Figure 1). In detail, 15 studies with data on prevalence of CAD (1163 BAV and 2234 TAV patients), 16 reporting on use of CABG (1782 BAV and 1886 TAV patients), and 16 on postoperative mortality rate (1067 BAV and 2399 TAV patients) were included.
Study Characteristics
Major characteristics of the 31 studies included in the meta‐analysis are shown in Table 1.
Table 1.
Demographic and Clinical Data of BAV and TAV
| Author (Year) | Reported Outcomes | Observation Time | Subjects | Age, y | Males | Hypertension | Hyperlipidemia | Diabetes | BMI | Smoking | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdulkareem 201318 | CABG | BAV | 192 | 58 | 71.3 | ||||||
| TAV | 203 | 65 | 62.5 | ||||||||
| Ali 201019 | CABG, mortality | 7 years | BAV | 90 | 63 | 78.9 | 31.1 | 12.2 | 5.6 | ||
| TAV | 125 | 70 | 58.4 | 32.0 | 16.8 | 11.2 | |||||
| Asano 201220 | CABG, mortality | 5 years | BAV | 86 | 46.3 | ||||||
| TAV | 58 | 70 | |||||||||
| Badiu 201021 | CAD, CABG, mortality | 5 years | BAV | 11 | 37 | 100.0 | 45.5 | 9.1 | |||
| TAV | 91 | 48 | 63.7 | 69.2 | 36.3 | 4.4 | |||||
| Boudoulas 201513 | CABG | BAV | 95 | 62 | 71.6 | 70 | 61 | 31 | 53 | ||
| TAV | 175 | 71 | 55.4 | 87 | 60 | 47 | 48 | ||||
| Branchetti 201422 | CAD | BAV | 74 | 55.5 | 64.9 | 31.1 | 27.0 | 5.4 | 43.2 | ||
| TAV | 61 | 64.4 | 70.5 | 42.6 | 42.6 | 13.1 | 19.7 | ||||
| Costopoulos 201423 | Mortality | 1 year | BAV | 21 | 76.7 | 57.1 | 66.7 | 28.6 | 26.6 | ||
| TAV | 447 | 79.8 | 47.4 | 77.2 | 30.2 | 26.1 | |||||
| Davies 199624 | CABG | BAV | 296 | ||||||||
| TAV | 125 | ||||||||||
| Delius 199825 | Mortality | 10 years | BAV | 16 | |||||||
| TAV | 31 | ||||||||||
| Eleid 201326 | CAD, CABG | BAV | 47 | 58 | 76.6 | 68.1 | 48.9 | 8.5 | 23.4 | ||
| TAV | 53 | 66 | 75.5 | 86.8 | 49.1 | 11.3 | 13.2 | ||||
| Etz 201527 | CAD, mortality | Inhospital | BAV | 32 | 46.7 | 71.9 | 46.9 | 15.6 | |||
| TAV | 347 | 61.6 | 63.7 | 72.0 | 9.2 | 10.7 | |||||
| Girdauskas 201428 | Mortality | 10 years | BAV | 153 | 54 | 73.2 | 48.4 | 11.1 | 35.9 | ||
| TAV | 172 | 64 | 47.7 | 57.0 | 15.7 | 40.1 | |||||
| Holubec 201429 | CAD, CABG, mortality | 2 years | BAV | 60 | 45a | 81.7 | 45.0 | ||||
| TAV | 40 | 59a | 67.5 | 75.0 | |||||||
| Hwang 201130 | CAD, mortality | 10 years | BAV | 45 | 59.6 | 60.0 | 44.4 | 2.2 | 6.7 | 26.7 | |
| TAV | 43 | 58.3 | 48.8 | 23.3 | 2.3 | 9.3 | 9.3 | ||||
| Jackson 20149 | CAD | BAV | 292 | 61.1 | 73.6 | 51.0 | 11.3 | ||||
| TAV | 355 | 717 | 69.9 | 74.6 | 14.9 | ||||||
| Kochman 201431 | CAD, mortality | 1 year | BAV | 28 | 77.6 | 46.4 | 60.7 | 39.3 | |||
| TAV | 84 | 79.1 | 47.6 | 65.5 | 34.5 | ||||||
| Kvitting 201332 | CABG | BAV | 63 | 43 | 79.4 | 36.5 | 4.8 | 26 | |||
| TAV | 170 | 36 | 67.6 | 25.9 | 2.4 | 24 | |||||
| Liu 201533 | CAD, mortality | 30 days | BAV | 15 | 75.4 | 60.0 | 33.3 | 23.6 | |||
| TAV | 25 | 75.8 | 68.0 | 56.0 | 12.0 | 21.7 | |||||
| Mosalanezhad 201434 | CABG, mortality | 8 years | BAV | 30 | 42 | 93.3 | |||||
| TAV | 20 | 59 | 75.0 | ||||||||
| Nakamura 201435 | CAD | BAV | 17 | 70 | 76.5 | 58.8 | 35.3 | 17.6 | 35.3 | ||
| TAV | 59 | 77 | 52.5 | 79.7 | 33.9 | 20.3 | 40.7 | ||||
| Philip 201510 | CAD | BAV | 200 | 57 | 23.5 | 21.5 | 10.5 | ||||
| TAV | 200 | 78 | 76.5 | 78.5 | 31.5 | ||||||
| Roberts 200311 | CABG | BAV | 232 | 64.7 | 72.0 | ||||||
| TAV | 267 | 74 | 51.3 | ||||||||
| Roberts 2007a36 | CABG, mortality | 5 years | BAV | 102 | |||||||
| TAV | 18 | ||||||||||
| Roberts 2007b37 | CABG, mortality | 4 years | BAV | 187 | |||||||
| TAV | 235 | ||||||||||
| Roberts 2007c38 | CABG | BAV | 54 | ||||||||
| TAV | 142 | ||||||||||
| Roberts 2007d39 | CABG, mortality | 13 years | BAV | 180 | |||||||
| TAV | 107 | ||||||||||
| Rylski 201412 | CAD, mortality | Inhospital | BAV | 41 | 55a | 63.4 | 56.1 | 9.8 | |||
| TAV | 588 | 61a | 64.1 | 81.1 | 9.2 | ||||||
| Shim 201140 | CAD | BAV | 50 | 52 | 78.0 | 40.0 | 20.0 | 12.0 | 24.7 | 32.0 | |
| TAV | 50 | 52 | 78.0 | 50.0 | 28.0 | 8.0 | 25.2 | 42.0 | |||
| Stephan 199741 | CABG | BAV | 57 | 67 | 57.9 | ||||||
| TAV | 57 | 73 | 54.4 | ||||||||
| Warner 201342 | CAD | BAV | 10 | 46.5 | 60.0 | 50.0 | 40.0 | 10.0 | 30.1 | 10.0 | |
| TAV | 13 | 46.3 | 76.9 | 38.5 | 46.2 | 23.1 | |||||
| Yuan 201043 | CAD | BAV | 241 | 56.1 | 77.2 | 30.7 | 24.1 | 10.8 | |||
| TAV | 225 | 62.8 | 64.0 | 41.8 | 24.9 |
Data reported as median value. BAV indicates bicuspid aortic valve; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; TAV, tricuspid aortic valve.
The number of patients varied from 23 to 647, mean age from 36 to 79.8 years, and prevalence of male sex from 47.4% to 100%. Presence of hypertension was reported by 23.3% to 87.0% of patients, smoking habit by 9.3% to 53.0%, diabetes mellitus by 2.4% to 47.0%, and hyperlipidemia by 2.2% to 78.5%. Mean BMI varied from 21.7 to 30.1 kg/m2. Length of follow‐up for mortality assessment ranged from in‐hospital stay period to 13 years with a median of 5 years.
Coronary Artery Disease
Fifteen studies,1 for a total of 1163 BAV and 2234 TAV patients, showed that CAD was reported by 13.5% of BAV and 30.3% of TAV patients (OR, 0.33; 95% CI: 0.17, 0.65; P=0.001; Figure 2). Heterogeneity among studies was significant (I2=86%; P<0.001), and it was not reduced by the exclusion of one study at a time. In addition, after excluding 2 studies by Kochman et al.31 and Liu et al.,33 including patients undergoing transcatheter aortic valve implantation (TAVI), the results were entirely confirmed (OR, 0.31; 95% CI: 0.14, 0.66; P=0.002; I2=88%; P<0.001).
Figure 2.
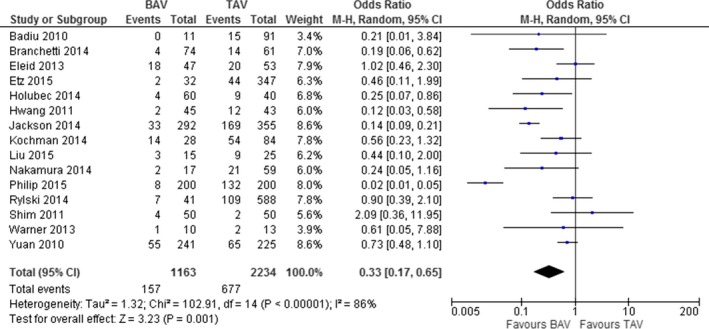
Prevalence of coronary artery disease in patients with bicuspid (BAV) and tricuspid aortic valve (TAV).
Concomitant CABG
Sixteen studies,2 reporting on 1782 BAV and 1886 TAV patients, showed that the number of patients undergoing concomitant CABG was significantly lower between BAV than TAV patients (31.0% vs 45.7%; OR, 0.45; 95% CI: 0.35, 0.59; P<0.001; Figure 3). Heterogeneity among studies was significant (I2=56%; P=0.003). However, after excluding the study by Asano et al.,20 all results were confirmed without heterogeneity (OR, 0.49; 95% CI: 0.39, 0.61; P<0.001; I2=37%; P=0.07).
Figure 3.
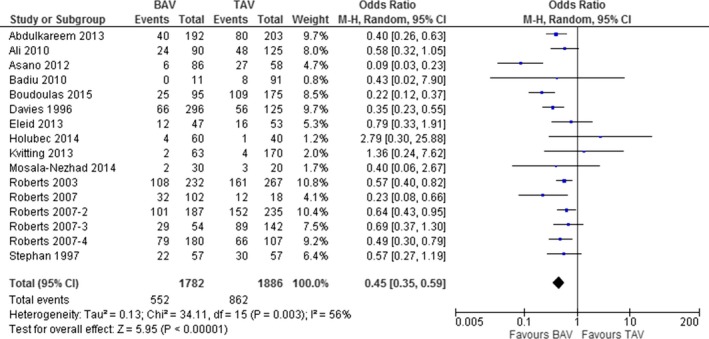
Prevalence of coronary artery bypass grafting (CABG) in patients with bicuspid (BAV) and tricuspid aortic valve (TAV).
Postoperative Mortality
The 16 studies3 evaluating postoperative mortality showed a slightly significant difference in rate of mortality between BAV and TAV patients (16.2% vs 18.8%; OR, 0.62; 95% CI: 0.40, 0.97; P=0.04; I2=65%; P<0.001; Figure 4). Interestingly, a metaregression analysis showed that the mortality rate was independent by the length of the observation (Z value: −1.17; P=0.240). In addition, we conducted a subanalysis removing the 3 studies23, 31, 33 that analyzed patients undergoing TAVI; the results did not differ from the previous ones (OR, 0.51; 95% CI: 0.32, 0.84; P=0.006; I2=63%; P=0.001).
Figure 4.
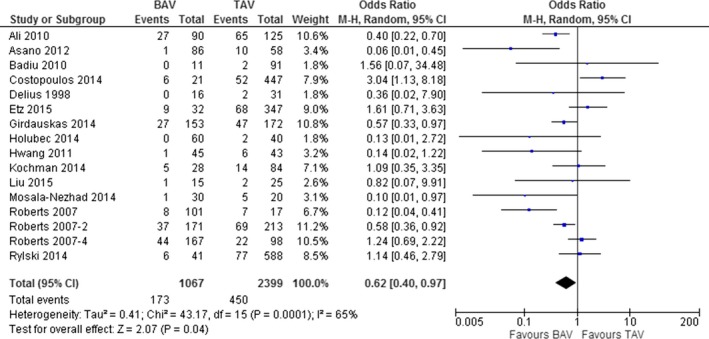
Post‐operative mortality in patients with bicuspid (BAV) and tricuspid aortic valve (TAV).
Clinical and Demographic Variables
As showed in Figure S1, when major clinical and demographic characteristics have been compared between BAV and TAV patients, we found that BAV subjects were significantly younger than TAV (MD, −7.29; 95% CI: −11.17, −3.41; P<0.001). In addition, BAV subjects were more frequently males (72.7% vs 60.1%; OR, 1.61; 95% CI: 1.33, 1.94; P<0.001) and exhibited a lower prevalence of hypertension (43.0% vs 65.4%; OR, 0.58; 95% CI: 0.39, 0.87; P<0.001) and diabetes (11.6% vs 16.3%; OR, 0.71; 95% CI: 0.54, 0.93; P=0.01) than TAV. In contrast, no significant differences were found in prevalence of hyperlipidemia and smoking habit as well as in mean BMI between BAV and TAV patients.
Interestingly, a metaregression analysis showed that younger age and lower prevalence of diabetes were associated with lower prevalence of CAD (Z value: −3.03; P=0.002 and Z value: −3.10; P=0.002, respectively; Figure 5) and CABG (Z value: −2.69; P=0.007 and Z value: −3.36; P=0.001, respectively; Figure 6) documented in BAV patients as compared to TAV patients.
Figure 5.
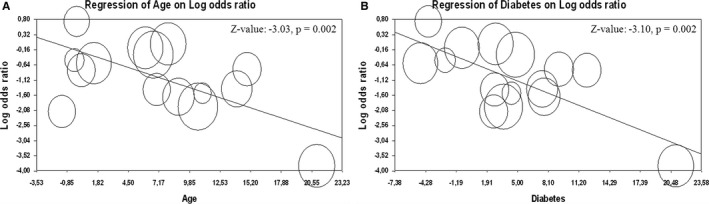
Meta‐regression analysis. Effect of the difference in mean age (A) and in prevalence of diabetes (B) on prevalence of coronary artery disease in patients with bicuspid (BAV) and tricuspid aortic valve (TAV).
Figure 6.
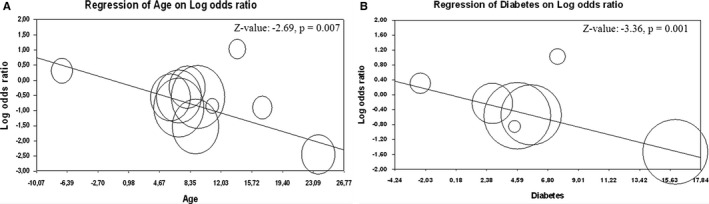
Meta‐regression analysis. Effect of the difference in mean age (A) and in prevalence of diabetes (B) on prevalence of concomitant coronary artery bypass grafting in patients with bicuspid (BAV) and tricuspid aortic valve (TAV).
Sensitivity Analysis
Given the statistically significant difference in distribution of age, sex, hypertension, and diabetes between BAV and TAV patients, all the analyses have been repeated after including only studies enrolling patients comparable for all these variables.30, 33, 40, 41, 42 Interestingly, the difference in prevalence of CAD (OR, 0.65; 95% CI, 0.33, 1.26; P=0.20) and mortality rate (OR, 1.77; 95% CI, 0.81, 3.87; P=0.15) were no longer significant. None of the 5 studies enrolling patients comparable for age, sex, hypertension, and diabetes provided information about concomitant CABG in BAV and TAV subjects. Thus, the sensitivity analysis was not performed for this outcome. When stratifying results according to the study design (retrospective or prospective), we found that the difference in CAD, concomitant CABG, and postoperative mortality between BAV and TAV patients were consistently confirmed only by retrospective studies. Interestingly, all the differences between the 2 groups were no longer significant in prospective studies (Table 2).
Table 2.
Stratification of the Studies: Retrospective and Prospective
| No. of Studies | OR (95% CI) | Heterogeneity | |
|---|---|---|---|
| CAD | |||
| Retrospective studies | 8 | 0.37 (0.18, 0.76; P=0.007) | I2: 84%; P<0.001 |
| Prospective studies | 7 | 0.30 (0.07, 1.32; P=0.11) | I2: 87%; P<0.001 |
| CABG | |||
| Retrospective studies | 15 | 0.45 (0.35, 0.59; P<0.001) | I2: 59%; P=0.002 |
| Prospective studies | 1 | 0.43 (0.02, 7.90; P=0.57) | Not evaluable |
| Mortality | |||
| Retrospective studies | 13 | 0.57 (0.34, 0.94; P=0.03) | I2: 72%; P<0.001 |
| Prospective studies | 3 | 1.08 (0.41, 2.86; P=0.88) | I2: 0%; P=0.95 |
CABG indicates coronary artery bypass grafting; CAD, coronary artery disease; OR, odds ratio.
Publication Bias
Because it is recognized that publication bias can affect results of meta‐analyses, we attempted to assess this potential bias using funnel plot visual analysis (Figure S2).
Visual inspection of funnel plots of effect size versus SE for studies evaluating CAD, CABG, and mortality in BAV and in TAV patients suggested a symmetric distribution of studies around the effect size, and the Egger test confirmed the lack of publication bias for all these outcomes (CAD, P=0.868; CABG, P=0.914; mortality, P=0.403).
Discussion
This meta‐analysis, which includes more than 7500 patients undergoing aortic valve surgery, shows, in agreement with previously published data, a lower prevalence of CAD (13.5% vs 30.3%) and concomitant CABG (31% vs 45.7%) in BAV than in TAV patients, accompanied by a marginally lower rate of postoperative mortality (16.2% vs 18.8%). We also show that BAV patients enrolled in the included studies were ≈7 years younger, more often males (72.7% vs 60.1%), and exhibited lower prevalence of hypertension (43.0% vs 65.4%) and diabetes (11.6% vs 16.3%) compared to TAV patients (Table S1).
Altogether, these data suggest that the lower cardiovascular risk reported in BAV patients may be partly explained by younger age and lower prevalence of some cardiovascular risk factors in this clinical setting. Interestingly, a meta‐regression analysis confirmed and extended this hypothesis, showing that age and diabetes have a profound impact on the difference in prevalence of CAD and CABG between BAV and TAV patients. Moreover, when the analyses have been repeated after including only those studies enrolling patients matched for age, sex, hypertension, and diabetes, the difference in prevalence of CAD and postoperative mortality were no longer significant between the 2 groups of patients. Thus, patients with BAV typically develop aortic stenosis at a younger age, usually before 65 years, compared to aortic stenosis in patients with TAV, which more often develops after age 70.44
Several studies analyzed prevalence of CAD in BAV and TAV patients, concluding that this atherosclerotic disease is uncommon in BAV, but is associated with TAV disease.9, 24 However, recent developments suggest that “incidence of CAD is high in patients with aortic valve degeneration, both in those with tricuspid and bicuspid aortic valve.”13
Our analysis shows that although the incidence of all the considered variables is lower in BAV compared to TAV patients, a higher mean age and a higher prevalence of hypertension and diabetes is found in the TAV group. The potential impact of these confounding covariates should be taken into account. Indeed, our sensitivity analysis shows that once hypertension, sex, diabetes, and age are taken into account and only studies with patients comparable for these variables are included, the differences in prevalence of CAD between BAV and TAV patients is no longer significant.
The finding that, in the TAV group, higher mean age and increased prevalence of diabetes and hypertension are paralleled by a higher prevalence of CAD, concomitant CABG, and postoperative mortality somehow supports the hypothesis that the etiopathogenetic mechanism underling aortic valve degeneration is similar or complementary to coronary atherosclerosis. Indeed, recent evidence suggested that risk factors responsible for pathogenesis of atherosclerosis are also related to development of aortic calcification and stenosis.13, 45, 46 However, the reasons why many patients with CAD do not develop aortic stenosis are still under debate. The major explanation may reside in the anatomic variation in size and diameter in normal tricuspid aortic valve47 and the genetic predisposition to aortic calcification, such as lipoprotein(a) expression levels encoded by the lipoprotein(a) gene.48
Another finding of our analysis is the marginally lower postoperative mortality in BAV than in TAV patients. Evaluating the risk of post operative mortality in BAV and TAV patients, it is interesting to highlight that the only study19 providing an adjusted multivariate analysis showed that valve morphology did not impact on mortality rate. Moreover, similar findings have been confirmed by Roberts et al.37, 39 by means of an unadjusted analysis. On the other hand, it is noteworthy to stress that 4 studies20, 27, 28, 32 consistently highlighted that an increasing age was the main predictor of postoperative mortality. Overall, these evidences confirm and extend our data suggesting that the difference in mean age between BAV and TAV might, at least in part, explain the difference in postoperative mortality documented in these 2 groups. Indeed, when we have analyzed only studies on patients comparable for age, sex, hypertension, and diabetes, the differences in postoperative mortality rate between BAV and TAV patients were no longer significant. In addition, after stratifying results according to study design, we found that differences between BAV and TAV patients were confirmed only by retrospective studies. Interestingly, all the differences between the 2 groups were no longer significant in prospective studies.
We recognize that our study has several potential limitations. The studies included in our meta‐analysis have different inclusion and exclusion criteria with no clear definition of CAD. In addition, most of the patients included in the analysis had concomitant cardiovascular risk factors. Given that meta‐analysis is performed on aggregate data and some missing information is present in each study, the multivariate approach allowed for the adjustment for some (but not all) potential confounders. Thus, although results of metaregression analyses were able to refine analyses by assessing the influence of most clinical and demographic variables on the observed results, caution is necessary in overall results interpretation. Moreover, heterogeneity among the studies was generally significant. Although it was not possible to conclusively ascertain sources of heterogeneity, publication bias did not affect results of our meta‐analysis.
In conclusion, patients undergoing aortic valve replacement might represent a potential bias. Patients undergoing surgery are a subset of patients with BAV or TAV, and this could influence the results. This might limit the reproducibility of reported results. However, a large study49 reporting on natural history of BAV and TAV patients not undergoing surgery confirmed the lack of difference in cardiovascular mortality between the 2 groups. In addition, the studies included analyzed not only aortic valve stenosis pathology, but also aortic valve regurgitation. However, in the studies analyzing both aortic valve pathologies,18, 22, 26, 27, 35 there are no significative differences between BAV and TAV groups.
A further potential confounder is represented by inclusion of studies analyzing patients’ candidate for TAVI, who, besides the older age, have a greater prevalence of comorbidities. However, a subanalysis excluding these 3 studies23, 31, 33 confirmed all our findings.
In conclusion, the results of the meta‐analysis here presented shows that analysis of raw data clearly suggests an association of aortic valve morphology with prevalence of CAD, concomitant CABG, and postoperative mortality. Interestingly, differences in age and diabetes have a profound impact on prevalence of CAD and concomitant CABG between BAV and TAV patients. Thus, based on our analysis, BAV patients do not exhibit a lower risk of CAD compared to TAV patients. However, further studies or patient‐level meta‐analysis are needed to adjust results for potential confounders and definitely address this issue.
Author Contributions
Paolo Poggio and Matteo Nicola Dario Di Minno conceived and designed the study, performed statistical analysis, interpreted results, and drafted the manuscript; Laura Cavallotti, Paola Songia, Alessandro Di Minno, Pasquale Ambrosino, and Liborio Mammana acquired clinical data and drafted the manuscript; Alessandro Parolari and Elena Tremoli interpreted results and performed critical revisions. All authors read and approved the final version of the manuscript. Paolo Poggio and Matteo Nicola Dario Di Minno had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of data analysis.
Sources of Funding
This work was supported by the Fondazione Gigi e Pupa Ferrari ONLUS, the Italian Ministry of Health (RC2014 BIO61 and RC2015 BIO30), and the Fondazione Umberto Veronesi.
Disclosures
None.
Supporting information
Figure S1. Distribution of major clinical and demographic characteristics in patients with bicuspid (BAV) and tricuspid aortic valve (TAV). A, Sex, (B) age, (C) hypertension, (D) diabetes, (E) hyperlipedimia, (F) BMI, and (G) smoking. BMI indicates body mass index.
Figure S2. Funnel plots of effect size vs standard error for studies evaluating the prevalence of coronary artery disease (A), coronary artery bypass grafting (B), and postoperative mortality (C) in patients with bicuspid (BAV) and tricuspid aortic valve (TAV).
Table S1. Summary Table of Meta‐Analysis Results
(J Am Heart Assoc. 2016;5:e003200 doi: 10.1161/JAHA.116.003200)
Notes
References
- 1. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez‐Sarano M. Burden of valvular heart diseases: a population‐based study. Lancet. 2006;368:1005–1011. [DOI] [PubMed] [Google Scholar]
- 2. Osnabrugge RL, Mylotte D, Head SJ, Van Mieghem NM, Nkomo VT, LeReun CM, Bogers AJ, Piazza N, Kappetein AP. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta‐analysis and modeling study. J Am Coll Cardiol. 2013;62:1002–1012. [DOI] [PubMed] [Google Scholar]
- 3. Pawade TA, Newby DE, Dweck MR. Calcification in aortic stenosis: the skeleton key. J Am Coll Cardiol. 2015;66:561–577. [DOI] [PubMed] [Google Scholar]
- 4. Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634. [DOI] [PubMed] [Google Scholar]
- 5. Patel DK, Green KD, Fudim M, Harrell FE, Wang TJ, Robbins MA. Racial differences in the prevalence of severe aortic stenosis. J Am Heart Assoc. 2014;3:e000879 doi: 10.1161/JAHA.114.000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chandra HR, Goldstein JA, Choudhary N, O'Neill CS, George PB, Gangasani SR, Cronin L, Marcovitz PA, Hauser AM, O'Neill WW. Adverse outcome in aortic sclerosis is associated with coronary artery disease and inflammation. J Am Coll Cardiol. 2004;43:169–175. [DOI] [PubMed] [Google Scholar]
- 7. Kizer JR, Wiebers DO, Whisnant JP, Galloway JM, Welty TK, Lee ET, Best LG, Resnick HE, Roman MJ, Devereux RB. Mitral annular calcification, aortic valve sclerosis, and incident stroke in adults free of clinical cardiovascular disease: the Strong Heart Study. Stroke. 2005;36:2533–2537. [DOI] [PubMed] [Google Scholar]
- 8. Olsen MH, Wachtell K, Bella JN, Gerdts E, Palmieri V, Nieminen MS, Smith G, Ibsen H, Devereux RB; substudy L . Aortic valve sclerosis relates to cardiovascular events in patients with hypertension (a LIFE substudy). Am J Cardiol. 2005;95:132–136. [DOI] [PubMed] [Google Scholar]
- 9. Jackson V, Eriksson MJ, Caidahl K, Eriksson P, Franco‐Cereceda A. Ascending aortic dilatation is rarely associated with coronary artery disease regardless of aortic valve morphology. J Thorac Cardiovasc Surg. 2014;148:2973–2980.e2971. [DOI] [PubMed] [Google Scholar]
- 10. Philip F, Faza NN, Schoenhagen P, Desai MY, Tuzcu EM, Svensson LG, Kapadia SR. Aortic annulus and root characteristics in severe aortic stenosis due to bicuspid aortic valve and tricuspid aortic valves: implications for transcatheter aortic valve therapies. Catheter Cardiovasc Interv. 2015;86:E88–E98. [DOI] [PubMed] [Google Scholar]
- 11. Roberts WC, Ko JM. Weights of operatively‐excised stenotic unicuspid, bicuspid, and tricuspid aortic valves and their relation to age, sex, body mass index, and presence or absence of concomitant coronary artery bypass grafting. Am J Cardiol. 2003;92:1057–1065. [DOI] [PubMed] [Google Scholar]
- 12. Rylski B, Desai ND, Bavaria JE, Vallabhajosyula P, Moser W, Pochettino A, Szeto WY, Milewski RK. Aortic valve morphology determines the presentation and surgical approach to acute type A aortic dissection. Ann Thorac Surg. 2014;97:1991–1996; discussion 1996‐1997. [DOI] [PubMed] [Google Scholar]
- 13. Boudoulas KD, Wolfe B, Ravi Y, Lilly S, Nagaraja HN, Sai‐Sudhakar CB. The aortic stenosis complex: aortic valve, atherosclerosis, aortopathy. J Cardiol. 2015;65:377–382. [DOI] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG; Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Open Med. 2009;3:e123–e130. [PMC free article] [PubMed] [Google Scholar]
- 15. Juni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta‐analysis. JAMA. 1999;282:1054–1060. [DOI] [PubMed] [Google Scholar]
- 16. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta‐analysis. BMJ. 2001;323:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abdulkareem N, Soppa G, Jones S, Valencia O, Smelt J, Jahangiri M. Dilatation of the remaining aorta after aortic valve or aortic root replacement in patients with bicuspid aortic valve: a 5‐year follow‐up. Ann Thorac Surg. 2013;96:43–49. [DOI] [PubMed] [Google Scholar]
- 19. Ali A, Patel A, Ali ZA, Abu‐Omar Y, Freed D, Sheikh AY, Athanasiou T, Pepper J. Stentless aortic valve replacement in patients with bicuspid aortic valve disease: clinical outcome and aortic diameter changes during follow‐up. Eur J Cardiothorac Surg. 2010;38:134–140. [DOI] [PubMed] [Google Scholar]
- 20. Asano M, Kunihara T, Aicher D, El Beyrouti H, Rodionycheva S, Schafers HJ. Mid‐term results after sinutubular junction remodelling with aortic cusp repair. Eur J Cardiothorac Surg. 2012;42:1010–1015. [DOI] [PubMed] [Google Scholar]
- 21. Badiu CC, Eichinger W, Bleiziffer S, Hermes G, Hettich I, Krane M, Bauernschmitt R, Lange R. Should root replacement with aortic valve‐sparing be offered to patients with bicuspid valves or severe aortic regurgitation? Eur J Cardiothorac Surg. 2010;38:515–522. [DOI] [PubMed] [Google Scholar]
- 22. Branchetti E, Bavaria JE, Grau JB, Shaw RE, Poggio P, Lai EK, Desai ND, Gorman JH, Gorman RC, Ferrari G. Circulating soluble receptor for advanced glycation end product identifies patients with bicuspid aortic valve and associated aortopathies. Arterioscler Thromb Vasc Biol. 2014;34:2349–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Costopoulos C, Latib A, Maisano F, Testa L, Bedogni F, Buchanan L, Naganuma T, Sticchi A, Sato K, Miyazaki T, Figini F, Giannini F, Taramasso M, Naim C, Carlino M, Chieffo A, Montorfano M, Alfieri O, Colombo A. Comparison of results of transcatheter aortic valve implantation in patients with severely stenotic bicuspid versus tricuspid or nonbicuspid valves. Am J Cardiol. 2014;113:1390–1393. [DOI] [PubMed] [Google Scholar]
- 24. Davies MJ, Treasure T, Parker DJ. Demographic characteristics of patients undergoing aortic valve replacement for stenosis: relation to valve morphology. Heart. 1996;75:174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Delius RE, Samyn MM, Behrendt DM. Should a bicuspid aortic valve be replaced in the presence of subvalvar or supravalvar aortic stenosis? Ann Thorac Surg. 1998;66:1337–1342. [DOI] [PubMed] [Google Scholar]
- 26. Eleid MF, Forde I, Edwards WD, Maleszewski JJ, Suri RM, Schaff HV, Enriquez‐Sarano M, Michelena HI. Type A aortic dissection in patients with bicuspid aortic valves: clinical and pathological comparison with tricuspid aortic valves. Heart. 2013;99:1668–1674. [DOI] [PubMed] [Google Scholar]
- 27. Etz CD, von Aspern K, Hoyer A, Girrbach FF, Leontyev S, Bakhtiary F, Misfeld M, Mohr FW. Acute type A aortic dissection: characteristics and outcomes comparing patients with bicuspid versus tricuspid aortic valve. Eur J Cardiothorac Surg. 2015;48:142–150. [DOI] [PubMed] [Google Scholar]
- 28. Girdauskas E, Disha K, Borger MA, Kuntze T. Long‐term prognosis of ascending aortic aneurysm after aortic valve replacement for bicuspid versus tricuspid aortic valve stenosis. J Thorac Cardiovasc Surg. 2014;147:276–282. [DOI] [PubMed] [Google Scholar]
- 29. Holubec T, Zacek P, Jamaliramin M, Emmert MY, Tuna M, Nedbal P, Dominik J, Harrer J, Falk V, Vojacek J. Valve cuspidity: a risk factor for aortic valve repair? J Card Surg. 2014;29:585–592. [DOI] [PubMed] [Google Scholar]
- 30. Hwang HY, Shim MS, Park EA, Ahn H. Reduction aortoplasty for the ascending aortic aneurysm with aortic valve disease. Does bicuspid valve matter? Circ J. 2011;75:322–328. [DOI] [PubMed] [Google Scholar]
- 31. Kochman J, Huczek Z, Scislo P, Dabrowski M, Chmielak Z, Szymanski P, Witkowski A, Parma R, Ochala A, Chodor P, Wilczek K, Reczuch KW, Kubler P, Rymuza B, Koltowski L, Scibisz A, Wilimski R, Grube E, Opolski G. Comparison of one‐ and 12‐month outcomes of transcatheter aortic valve replacement in patients with severely stenotic bicuspid versus tricuspid aortic valves (results from a multicenter registry). Am J Cardiol. 2014;114:757–762. [DOI] [PubMed] [Google Scholar]
- 32. Kvitting JP, Kari FA, Fischbein MP, Liang DH, Beraud AS, Stephens EH, Mitchell RS, Miller DC. David valve‐sparing aortic root replacement: equivalent mid‐term outcome for different valve types with or without connective tissue disorder. J Thorac Cardiovasc Surg. 2013;145:117–126, 127.e111‐115; discussion 126‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu XB, Jiang JB, Zhou QJ, Pu ZX, He W, Dong AQ, Feng Y, Jiang J, Sun Y, Xiang MX, He YX, Fan YQ, Dong L, Wang JA. Evaluation of the safety and efficacy of transcatheter aortic valve implantation in patients with a severe stenotic bicuspid aortic valve in a Chinese population. J Zhejiang Univ Sci B. 2015;16:208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mosala Nezhad Z, de Kerchove L, Hechadi J, Tamer S, Boodhwani M, Poncelet A, Noirhomme P, Rubay J, El Khoury G. Aortic valve repair with patch in non‐rheumatic disease: indication, techniques and durability†. Eur J Cardiothorac Surg. 2014;46:997–1005; discussion 1005. [DOI] [PubMed] [Google Scholar]
- 35. Nakamura Y, Ryugo M, Shikata F, Okura M, Okamura T, Yasugi T, Izutani H. The analysis of ascending aortic dilatation in patients with a bicuspid aortic valve using the ratio of the diameters of the ascending and descending aorta. J Cardiothorac Surg. 2014;9:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roberts WC, Ko JM, Filardo G, Henry AC, Hebeler RF Jr, Cheung EH, Matter GJ, Hamman BL. Valve structure and survival in quinquagenarians having aortic valve replacement for aortic stenosis (+/‐aortic regurgitation) with versus without coronary artery bypass grafting at a single US medical center (1993 to 2005). Am J Cardiol. 2007;100:1584–1591. [DOI] [PubMed] [Google Scholar]
- 37. Roberts WC, Ko JM, Filardo G, Henry AC, Hebeler RF Jr, Cheung EH, Matter GJ, Hamman BL. Valve structure and survival in septuagenarians having aortic valve replacement for aortic stenosis (+/‐aortic regurgitation) with versus without coronary artery bypass grafting at a single US medical center (1993 to 2005). Am J Cardiol. 2007;100:1157–1165. [DOI] [PubMed] [Google Scholar]
- 38. Roberts WC, Ko JM, Garner WL, Filardo G, Henry AC, Hebeler RF Jr, Matter GJ, Hamman BL. Valve structure and survival in octogenarians having aortic valve replacement for aortic stenosis (+/‐ aortic regurgitation) with versus without coronary artery bypass grafting at a single US medical center (1993 to 2005). Am J Cardiol. 2007;100:489–495. [DOI] [PubMed] [Google Scholar]
- 39. Roberts WC, Ko JM, Filardo G, Henry AC, Hebeler RF Jr, Cheung EH, Matter GJ, Hamman BL. Valve structure and survival in sexagenarians having aortic valve replacement for aortic stenosis (+/‐aortic regurgitation) with versus without coronary artery bypass grafting at a single US medical center (1993 to 2005). Am J Cardiol. 2007;100:1286–1292. [DOI] [PubMed] [Google Scholar]
- 40. Shim CY, Cho IJ, Yang WI, Kang MK, Park S, Ha JW, Jang Y, Chung N. Central aortic stiffness and its association with ascending aorta dilation in subjects with a bicuspid aortic valve. J Am Soc Echocardiogr. 2011;24:847–852. [DOI] [PubMed] [Google Scholar]
- 41. Stephan PJ, Henry AC III, Hebeler RF Jr, Whiddon L, Roberts WC. Comparison of age, gender, number of aortic valve cusps, concomitant coronary artery bypass grafting, and magnitude of left ventricular‐systemic arterial peak systolic gradient in adults having aortic valve replacement for isolated aortic valve stenosis. Am J Cardiol. 1997;79:166–172. [DOI] [PubMed] [Google Scholar]
- 42. Warner PJ, Al‐Quthami A, Brooks EL, Kelley‐Hedgepeth A, Patvardhan E, Kuvin JT, Heffernan KS, Huggins GS. Augmentation index and aortic stiffness in bicuspid aortic valve patients with non‐dilated proximal aortas. BMC Cardiovasc Disord. 2013;13:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yuan SM, Jing H, Lavee J. The bicuspid aortic valve and its relation to aortic dilation. Clinics (Sao Paulo). 2010;65:497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Otto CM. Calcification of bicuspid aortic valves. Heart. 2002;88:321–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O'Brien KD. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–853. [DOI] [PubMed] [Google Scholar]
- 46. Capoulade R, Clavel MA, Dumesnil JG, Chan KL, Teo KK, Tam JW, Cote N, Mathieu P, Despres JP, Pibarot P. Impact of metabolic syndrome on progression of aortic stenosis: influence of age and statin therapy. J Am Coll Cardiol. 2012;60:216–223. [DOI] [PubMed] [Google Scholar]
- 47. Shibayama K, Harada K, Berdejo J, Tanaka J, Mihara H, Itabashi Y, Shiota T. Comparison of aortic root geometry with bicuspid versus tricuspid aortic valve: Real‐time three‐dimensional transesophageal echocardiographic study. J Am Soc Echocardiogr. 2014;27:1143–1152. [DOI] [PubMed] [Google Scholar]
- 48. Thanassoulis G, Campbell CY, Owens DS, Smith JG, Smith AV, Peloso GM, Kerr KF, Pechlivanis S, Budoff MJ, Harris TB, Malhotra R, O'Brien KD, Kamstrup PR, Nordestgaard BG, Tybjaerg‐Hansen A, Allison MA, Aspelund T, Criqui MH, Heckbert SR, Hwang SJ, Liu Y, Sjogren M, van der Pals J, Kalsch H, Muhleisen TW, Nothen MM, Cupples LA, Caslake M, Di Angelantonio E, Danesh J, Rotter JI, Sigurdsson S, Wong Q, Erbel R, Kathiresan S, Melander O, Gudnason V, O'Donnell CJ, Post WS. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368:503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Roberts WC, Vowels TJ, Filardo G, Ko JM, Mathur RP, Shirani J. Natural history of unoperated aortic stenosis during a 50‐year period of cardiac valve replacement. Am J Cardiol. 2013;112:541–553. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Distribution of major clinical and demographic characteristics in patients with bicuspid (BAV) and tricuspid aortic valve (TAV). A, Sex, (B) age, (C) hypertension, (D) diabetes, (E) hyperlipedimia, (F) BMI, and (G) smoking. BMI indicates body mass index.
Figure S2. Funnel plots of effect size vs standard error for studies evaluating the prevalence of coronary artery disease (A), coronary artery bypass grafting (B), and postoperative mortality (C) in patients with bicuspid (BAV) and tricuspid aortic valve (TAV).
Table S1. Summary Table of Meta‐Analysis Results


