ABSTRACT
Never in Mitosis (NIMA) Related Kinase 2 (NEK2) plays a key role in regulating mitotic processes, including centrosome duplication and separation, microtubule stabilization, kinetochore attachment and spindle assembly checkpoint. NEK2 is aberrantly overexpressed in a wide variety of human cancers and has been implicated in various aspects of malignant transformation, including tumorigenesis, drug resistance and tumor progression. The close relationship between NEK2 and cancer has made it an attractive target for anticancer therapeutic development; however, the mechanisms of how NEK2 coordinates altered signaling to malignant transformation remains unclear. In this paper, we discuss the functional roles of NEK2 in cancer development; highlight some of the significant NEK2 signaling in cancer, and summarize recent advances in the development of NEK2 inhibitors.
KEYWORDS: Cancer, centrosome, inhibitor, mitosis, NEK2, oncogenic
Introduction
Never in Mitosis (NIMA) Related Kinases (NEKs) are a family of serine/threonine kinases that have a broadly structural similarity to the mitotic regulator NIMA of the filamentous fungus Aspergillus nidulans.1 The family is consists of 11 different members (NEK1-11), of which NEK2 exhibits the greatest sequence identity to NIMA, with 47% identical within the catalytic domains.2 The human NEK2 gene is located on the long arm of chromosome 1(1q32.2–1q41) and it is comprised of 8 exons.2,3 With the alternate splicing, NEK2 is expressed as 3 splice variants, namely NEK2A, NEK2B and NEK2C.3,4 NEK2A is the full length protein with 445 amino acids (48 KDa) and is the most studied variant. It is comprised of an N-terminal catalytic kinase domain and a C-terminal regulatory domain. The C-terminal domain possesses multiple regulatory motifs, including leucine zipper (LZ), coiled coil (CC), centrosome, and nucleolar localization and microtubule binding sites, PP1 binding site, APC binding site KEN-box and extended cyclin A-type destruction box (D-box) (Fig. 1).5 NEK2 is well recognized as a multifunctional protein with roles in cell cycle regulation, such as centrosome duplication and separation,6,7 microtubule stabilization,8,9 kinetochore attachment10,11 and spindle assembly checkpoint.12-14 In recent years, the oncogenic roles of NEK2 have attracted considerable attention. Plenty of studies have reported that NEK2 is highly expressed in various cancers and usually predicts poor overall survival. The essential roles of NEK2 as well as its important upstream and downstream proteins in drug resistance, tumor metastasis and progression have been gradually disclosed. Herein, we summarize current knowledge on the oncogenic NEK2 signaling in cancer and describe the mechanism-based development of therapeutic approaches targeting NEK2.
Figure 1.
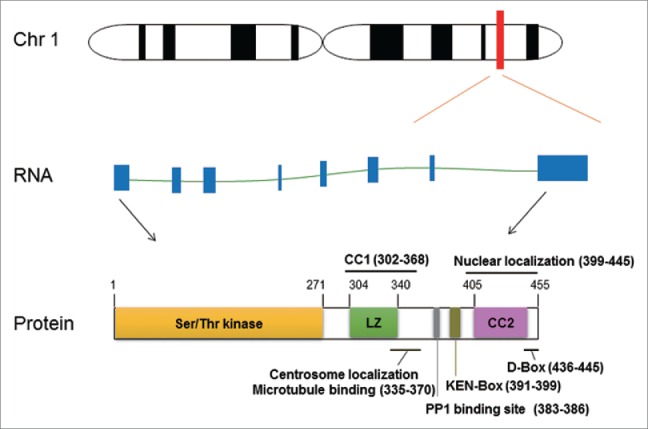
Genomic information of human NEK2 gene. The genomic locus of DJ-1 gene is located on the long arm q32 of chromosome 1 with 17,375 base pairs in length. NEK2 contains 8 exons (blue boxes), which currently has been transcribed with 5 transcript variants, and 3 of them coding proteins. The full-length transcript (NM_002497.3) and encoded protein structure is illustrated. The localization of the catalytic domain (serine/threonine kinase), leucine zipper (LZ), coiled coil (CC), PP1 binding site, KEN-box, D-box, centrosome localization microtubule binding site and nucleolar localization are indicated.
Regulation of NEK2 expression and activity
The expression of NEK2 exhibits a cell cycle-dependent pattern, which is low in G1 phase, peaking in S and G2 phase.15 Upon entry into mitosis, NEK2A undergoes a rapid disappearance whereas NEK2B persists until the subsequent G1 phase.3 Both the transcriptional and post-transcriptional regulation contributes to the dynamic protein level of NEK2s.
Several proteins have been demonstrated as transcriptional repressors of NEK2. Chromatin immunoprecipitation (ChIP) assay demonstrated that the E2F4, a member of the E2F transcription factor family, binds to the promoter of NEK2 in early G1. E2F4 functions as a transcriptional repressor in G0 and early G1 cells, which requires the binding to the pRB-related proteins p107 and p130. NEK2 mRNA is significantly derepressed in p107−/− and p130−/− mouse embryonic fibroblasts (MEFs).16 A direct and specific association of p53 with the distal NEK2 promoter has also been detected by ChIP assay. DNA methylation was restricted to the distal region of the NEK2 promoter. The DNA-demethylating agent 5aza-dC reduced the NEK2 transcript levels in HCT116 colon cancer cells but not in isogenic p53−/− cells. Stabilization of endogenous p53 by doxorubicin or ectopic expression of p53, but not a p53 DNA-binding mutant, decreased NEK2 expression.17 This study suggests that NEK2 is a novel p53-repressed gene and its binding region is protected by p53 from accumulating DNA methylation. Moreover, NEK2 is a direct functional target of MicroRNA-128, which suppressed NEK2 in colorectal cancer cells. Patients with high MicroRNA expression had significantly lower NEK2 expression and lower recurrence rates than those with low MicroRNA expression.18 In contrast to the above repressors, the expression of NEK2 is positively regulated by the forkhead transcription factor FoxM1. Overexpression of recombinant FoxM1 increases the mRNA level of NEK2, while FoxM1 depletion reduces NEK2 expression.19,20
Besides the transcriptional regulation, the cellular NEK2 abundance is also mediated by the ubiquitin-proteasome system (UPS). The sudden decreasing of NEK2A upon mitotic entry is resulted from proteasomal degradation, which depends on the binding of NEK2A to the anaphase promoting complex/cyclosome (APC/C) via 2 C-terminal motifs, the KEN-box and the D-box. Moreover, the proteasomal degradation of NEK2A may require its centrosomal localization.21-23 As to NEK2B, its abundance persists until the subsequent G1 phase may be explained by its absence of the binding site to APC/C. However, to our knowledge, the decrease of NEK2B levels in G1 phase remains unknown.
As a serine/threonine kinase, the phosphorylation of NEK2 is required for its activation. NEK2 dimerization via the LZ motif leads to trans-autophosphorylation.24 Mass spectrometric analysis identified 4 sites of autophosphorylation (Thr-170/ Ser-171, Thr-175, Thr-179, and Ser-241) within the catalytic domain. Autophosphorylation of Thr-170/ Ser-171 and Thr-175 increases NEK2 activity, while of Thr-179 and Ser-241 may negatively regulate NEK2. In the case of NEK2A, other autophosphorylation sites outside the kinase domain have been described; 4 around the KEN-box and 2 in the CC region may be involved in modulating dimerization and localization.25 Besides autophosphorylation, NEK2 activity is regulated by other kinases. Actually, the catalytic domain of NEK2 is phosphorylated and activated by p90RSK2, an effector of the MAPK pathway, which is important for the chromatin condensation in mouse spermatocytes.26 Moreover, the C-terminal of NEK2A, but not NEK2B, contains a binding site for protein phosphatase 1 (PP1) and the interaction of NEK2A with PP1 can lead to dephosphorylation and inhibition of NEK2A.27 Together, the controlled expression of NEK2 and its activity guarantee the performance of its biological function.
Biological function of NEK2
NEK2 plays an indispensable role in cell cycle progression, particularly with respect to the centrosome cycle (Fig. 2). NEK2 localizes to the centrosome and triggers centrosome separation by phosphorylating centrosome cohesion proteins C-Nap1, Rootletin and Cep68.28-30 NEK2 also regulates microtubule organization and stabilization. Through phosphorylation of ninein-like protein (Nlp) that is involved in microtubule anchoring at the centrosome during interphase, NEK2 promotes Nlp removal from the centrosome upon mitotic entry.31 NIP2/centrobin plays an essential role in stabilizing the microtubule structure, NEK2 antagonizes the microtubule stabilizing activity of centrobin by phosphorylates it.8,32 Moreover, NEK2 operates a faithful kinetochore microtubule attachments by phosphorylation of highly expressed in cancer 1 (Hec1).10,11 Through direct interaction with mitotic arrest deficient-like 1 (MAD1) or phosphorylation of Hec1 and Sgo1, NEK2 also modulates chromosome alignment and signaling of the spindle assembly checkpoint, thus regulating chromosome separation.12-14 In addition, NEK2 is involved in regulating chromatin condensation in meiosis. After phosphorylated and activated by the MAPK pathway, NEK2 phosphorylates the architectural chromatin protein high-mobility group protein A2 (HMGA2) and decreases the affinity of HMGA2 for DNA, thus might drive its release from the chromatin upon the G2/M progression, thereby promoting chromatin condensation in mouse spermatocytes.26,33
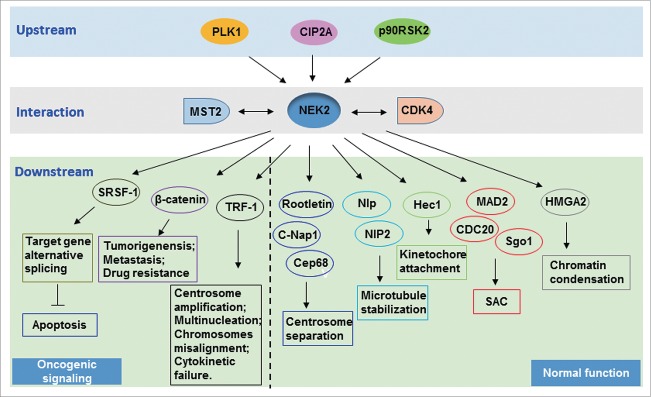
Figure 2.
NEK2 interacting signaling. The figure is a schematic diagram depicting the interaction of NEK2 and its related proteins. This view represents frequently studied aspects of NEK2 activities along with several newly described interactions. Aberrant activation of NEK2 by its upstream proteins or overexpression of NEK2 results in the activation of its downstream proteins, not only leading to the malfunction of NEK2 in normal cell cycle regulation, but also interrupting apoptosis, metastasis and drug resistance.
Apart from its involvement in cell cycle regulation, novel functions of NEK2 have been disclosed recently. NEK2 has been reported as a novel regulator of B cell development and immunological response. Transgenic mice with conditional expression of NEK2 in the B cell lineage does not develop spontaneous tumor formation up to 24 month after induction, but develop spontaneous germinal centers and exhibit an enhanced T cell dependent immune response. Overexpression of NEK2 in the B cell lineage affects the development of B cells by increasing the proportion of immature B cells in the bone marrow and decreasing B-1 B cells in peritoneal cavity.34 In addition, NEK2 is involved in regulating cilia disassembly by phosphorylation and activation of Kif24, a microtubule depolymerizing kinesin. NEK2 and Kif24 are overexpressed in breast cancer cells, and depletion of either Nek2 or Kif24 restores ciliation and reduces proliferation of these cells.35 These novel biological functions significantly expand the traditional recognition of NEK2 as a centrosomal kinase.
Oncogenic role of NEK2 in human cancer
NEK2 is overexpressed in human cancers
NEK2 is frequently overexpressed in a wide variety of human cancers (Table 1). The aberrant expression of NEK2 was first reported in cell lines derived from Ewing's tumors through a microarray analysis of over 1700 cancer-associated genes.36 Accumulating evidences have shown that mRNA and/or protein level of NEK2 are up-regulated in primary tumor tissues or cancer cell lines listed as follows: diffuse large B-cell lymphoma (DLBCL),37,38 breast cancer,39-42 cervical cancer,39,43 ovarian cancer,39,44 prostate cancer,39,45 leukemia,39 cholangiocarcinoma,46 colorectal cancer,18,47,48 neuroblastoma,49 testicular seminomas,50 multiple myeloma,41 bladder cancer,41 glioblastoma,41 mantle cell lumphoma,41 mesothelioma,41 head and neck squamous cell carcinoma,41 melanmoma,41 peripheral nerve sheath tumor,51 pancreatic ductal adenocarcinoma,52 hepatocellular carcinoma,41,53 non-small cell lung cancer (NSCLC)54 and its subtype lung adenocarcinoma.41,55 Thus overexpression of NEK2 is a frequent event in cancer cells and indicated a potential role of NEK2 in cancer development.
Table 1.
Overview of overexpression of NEK2 in human primary tumors and cancer cell lines investigated.
| Human tumor type | Expression level | Test Technique | Clinical impact | Ref. |
|---|---|---|---|---|
| Ewing's tumors | Increased expression | Microarray | NA | 36 |
| Diffuse large B-cell lymphoma (DLBCL) | 3/5 patients >3 -fold; 4/7 patients 2.2-fold | Microarray; qRT-PCR | Tumor transformation | 37 |
| Higher | Microarray and immunohistochemistry | Tumor transformation | 38 | |
| Breast cancer | about 2-fold | Western blot and immunohistochemistry | Diseases progression | 39 |
| Higher; 2–13-folda | Western blot; qRT-PCR | Cell survival | 40 | |
| Over-expression | Microarray | Inferior survival | 41 | |
| Overexpression | Microarray, qPCR and immunohistochemistry | Greater risk of patient mortality and higher disease recurrence | 42 | |
| Cervical cancer | about 2-fold | Western blot | NA | 39 |
| Up-regulated | Microarray | NA | 43 | |
| Ovarian cancer | 2–5-fold | Western blot | NA | 39 |
| Upregulated | Microarray | Drug resistance | 44 | |
| Prostate cancer | about 2-fold | Western blot | NA | 39 |
| Up-regulated | qRT-PCR, western blot and Immunohistochemistry | Shorter recurrence-free time | 45 | |
| Leukemia | 1-5-fold | Western blot | NA | 39 |
| Over-expression | Microarray | Inferior survival | 41 | |
| Cholangiocarcinoma | Higher | RT-PCR and protein gel blot | Tumorigenic growth and survival | 46 |
| Colorectal cancer | Higher | Western blot | NA | 47 |
| Upregulated | Microarray | NA | 41 | |
| Higher | qRT-PCR | Poor prognoses | 18 | |
| 1.5–3.6-folda | Western blot, immunofluorescence microscopy and immunohistochemistry | Tumor progression and shortened overall survival | 48 | |
| Neuroblastoma | 2/20 patients' serum showed serological responses to NEK2 | Luciferase immunoprecipitation | Tumor antigen | 49 |
| Testicular seminomas | 5-folda | Western blot and immunohistochemistry | Progression of neoplastic transformation | 50 |
| Multiple Myeloma | Up-regulated | Microarray | Poor prognosis and drug resistance | 41 |
| Bladder cancer | Overexpression | Microarray | Inferior survival | 41 |
| Glioblastoma | Over-expression | Microarray | Inferior survival | 41 |
| Mantle cell lymphoma | Overexpression | Microarray | Inferior survival | 41 |
| Mesothelioma | Over-expression | Microarray | Inferior survival | 41 |
| Head and neck squamous cell carcinoma | Upregulated | Microarray | NA | 41 |
| Melanmoma | Up-regulated | Microarray | NA | 41 |
| Peripheral nerve sheath tumor | Upregulated and about 3-folda | Nanostring nCounter system and immunohistochemistry | Malignant transformation | 51 |
| Pancreatic ductal adenocarcinoma | 3-folda | Immunohistochemistry and qPCR | Poor prognosis | 52 |
| Hepatocellular carcinoma | Up-regulated | Microarray | NA | 41 |
| about 5-folda | Immunohistochemistry, western blot and qPCR | NA | 53 | |
| Non-small cell lung cancer (NSCLC) | Overexpression | Immunohistochemical and immunofluorescence techniques | Poorer overall survival rate and poor prognosis | 54 |
| Lung Adenocarcinoma | Over-expression | Microarray | Inferior survival | 41 |
| Overexpression | Microarray | Tumor progression | 55 |
These ‘x-fold’ were not directly presented by the authors of the articles and are calculated using the quantitative data in these articles.
NA: not answered
NEK2 overexpression leads to tumorigenensis
Given the important roles of NEK2 in the regulation of centrosome separation, microtubule organization, chromatin condensation, SAC, and chromosome congression during cell division, overexpression of NEK2 may perturb its normal biological function. Previous study has shown that overexpression of active NEK2 in osteosarcoma U2OS cells induces a premature separation of centrosomes, while overexpression of either active or inactive NEK2 leads to dispersal of centrosomal material and loss of a focused microtubules.6 Transfection of NEK2 in Her2+ breast cancer cells HCC1954 enhances centrosome amplification, leading to aneuploidy and chromosome instability (CIN).56 In consistence, overexpression of NEK2 in breast cancer cell lines, MDA-MB-231 and MCF-7, also leads to centrosome amplification and multinucleation.57 Moreover, in immortal HBL100 breast epithelial cells, ectopic expression of NEK2 induces an accumulation of multinucleated cells with supernumerary centrosomes.39 What's more, overexpression of NEK2 significantly increased cell proliferation in normal fibroblasts and in cell lines of multiple myeloma, lung cancer and breast cancer.41 These suggest that elevated NEK2 levels can lead to CIN and aneuploidy, which will ultimately trigger tumorigenesis.
NEK2 is required for the cancer phenotype
NEK2 is required for maintenance of the transformed phenotype of cancer cells, regulating transformed growth, survival, and chemo-resistance of several tumor types including cholangiocarcinoma,46 breast cancer,58 colorectal cancer,47 multiple myeloma,41 hepatoma53 and prostate cancer.45 Silencing NEK2 expression induces cell growth inhibition and enhances sensitivity of tumor cells to anticancer drugs such as cisplatin,47 paclitaxel,58 doxorubicin,58 and bortezomib.41 These findings demonstrate that NEK2 is functionally required for the transformed behavior of cancer cells and indicate the potential role of NEK2 in tumor progression and metastasis.
NEK2 contributes to drug resistance
Studies in multiple types of cancers have implicated the role of NEK2 in drug resistance. Multiple myeloma cells overexpressing NEK2 exhibited less sensitivity to anticancer drugs (bortezomib, doxorubicin and etopside)-induced apoptosis compared with control cells. Further study demonstrated that NEK2-induced drug resistance was mainly through activation of efflux drug pumps.41 NEK2 is also involved in aldehyde dehydrogenase 1-A1 (ALDH1A1)-induced drug resistance in multiple myeloma. Overexpression of ALDH1A1 in myeloma cells led to increases mRNA and protein levels of NEK2, whereas NEK2 depletion decreases drug efflux pump activity and drug resistance.59 In addition, NEK2 expression is upregulated in drug-resistant ovarian cancer cells compared with their parental counterparts, indicating NEK2 contributes to drug resistance in ovarian cancer.44 NEK2 is also highly expressed in breast cancer cell lines HCC1954 and JIMT-1 which are ER-PR-Her2+ and display primary resistance to trastuzumab, albeit the co-overexpression of PLK4 might be essential for drug resistance.60 Taken together, these findings indicate that NEK2 plays important role in drug resistance.
NEK2 expression is associated with tumor progression and predicts poor prognosis
Elevated NEK2 expression is usually correlated with malignant transformation and tumor progression. DLBCL is a general transformed type of follicular lymphoma (FL), the expression of NEK2 is much higher in DLBCL than in FL, thus identifying the transformed DLBCL from FL.37,38 In contrast to the restricted NEK2 staining only in tumor cells of ductal carcinoma in situ (DCIS), an early step in breast cancer, the NEK2 positive cells are present throughout the stroma and are no longer confined within ducts or lobules, indicating NEK2 might precede metastasis in breast cancer.39 Actually, NEK2 is one of these 5 genes that build molecular grade index (MGI) to provide prognostic information for breast cancer.61 In consistence, the high NEK2 mRNA expression patients of colorectal cancer showed greater tumor depth, lymphatic invasion and peritoneal dissemination than the low NEK2 expression patients.18 Another group also showed that NEK2 was not only over-expressed in tumors, but also in metastatic sites, including lymph node and liver metastases.48 Moreover, the enhanced NEK2 overexpression with advanced tumor stage is also observed in patients with prostate cancer,45 testicular seminomas,50 peripheral nerve sheath tumor,51 and lung adenocarcinoma,55 which indicating an contributory role of NEK2 in cancer progression. With regard to overall survival, patients with high NEK2 expression have poorer prognosis than those with low NEK2 expression in breast cancer,41,60 leukemia,41 colorectal cancer,18,48 multiple myeloma,41 bladder cancer,41 glioblastoma,41 mantle cell lymphoma,41 mesothelioma,41 pancreatic ductal adenocarcinoma,52 NSCLC54 and its subtype lung adenocarcinoma.41 Thus, NEK2 expression may be useful for indicating tumor progression and diseases prognosis.
Oncogenic NEK2 signaling
Despite the relationship between NEK2 and cancer has been frequently reported, and CIN is considered as an important link between malfunction of NEK2 and tumor formation, the detailed mechanisms of how NEK2 coordinates altered signaling with tumorigenesis remains elusive. Here we discuss the oncogenic NEK2 signaling in cancer, including its key upstream regulators and downstream effectors, which lead to tumorigenensis, tumor progression and drug resistance (Fig. 2).
Upstream regulators of NEK2
PLK1
Polo-like kinase 1 (PLK1), a serine-threonine kinase, plays a pivotal role in regulating many key stages of the cell cycle, including centrosomes maturation, bipolar spindle assembly, M phase entry, sister chromatid cohesion, formation of kinetochore-microtubule attachments and finally mitotic exit and cytokinesis.62 A previous report demonstrated that PLK1 controls the NEK2-PP1γ antagonism in centrosome disjunction by phosphorylating the mammalian sterile 20-like kinase 2 (MST2).63 MST2 is a component of Hippo pathway and directly interact with NEK2A, and regulates its ability to localize to centrosomes, and phosphorylate C-Nap1 and rootletin.64 The absence phosphorylation of MST2 by PLK1 promotes assembly of NEK2A-PP1γ-MST2 complexes, in which PP1γ counteracts NEK2A kinase activity. In contrast, PLK1 phosphorylation of MST2 prevents PP1γ binding to MST2-NEK2A, allowing NEK2A to promote centrosome disjunction.63 In addition, PLK1 was shown to regulate NEK2 phosphorylation and stabilization of β-catenin at mitotic centrosomes.65 Thus NEK2 might work as an executor for the PLK1-associated development of human cancers.
CIP2A
Cancerous inhibitor of protein phosphatase 2A (CIP2A) is correlated with the aberrant proliferation of human cancer cells and with cancer progression in a large variety of human malignancies.66 A recent study has demonstrated that CIP2A is involved in regulation of centrosome separation and mitotic spindle dynamics, which is through the regulation of NEK2. The authors first showed that NEK2 is a binding partner for CIP2A by a yeast 2-hybrid assay and coimmunoprecipitation assays. Then they showed silencing of CIP2A reduced NEK2 activity while overexpression of CIP2A enhanced NEK2 activity. The activating effect of CIP2A on NEK2 depends on direct interaction, which is independent on PP1 or PP2A.67 This suggests that NEK2 is an essential target for CIP2A-induced tumorigenesis.
CDK4
Cyclin-dependent kinase 4 (CDK4) is a key regulator of the G1/S transition. It forms a complex with Cyclin D1, phosphorylates and inactivates the retinoblastoma (Rb) protein, drives progression through the cell cycle.68 CDK4 has also been reported to regulate centrosome duplication. Abolishing the expression of CDK4 abrogated both centrosome amplification and binucleation in p53-null MEFs and in Her2+ breast cancer cells.56,69 Silencing of CDK4 decreased the protein levels of NEK2, indicating a molecular connection between CDK4 and NEK2. Overexpression of NEK2 restored CDK4 knockdown-induced reduction of centrosome amplification. Interestingly, expression of CDK4 protein was also restored in cells overexpressing NEK2. Moreover, NEK2 knockdown led to a marked reduction in CDK4 protein expression, suggesting there is a potential signaling loop between CDK4 and NEK2.56 Therefore, abnormal expression of either CDK4 or NEK2 will alter its normal function, leading to CIN and aneuploidy that finally cause cancer.
Downstream effectors of NEK2
The mitotic proteins
As described above, NEK2 regulates chromosome separation by modulating the SAC through direct interaction or phosphorylation Hec1, MAD1, MAD2 and CDC20. Of note, the interaction of NEK2 and Hec1 has attracted great attention. NEK2 phosphorylates Hec1 at Ser165, which is essential for faithful kinetochore microtubule attachments and successful chromosome alignment in mitosis.10,11,13 It is found that following the phosphorylation of S165 during earlier stages of mitosis, the kinetochore pS165 signal diminishes after metaphase alignment and prior to anaphase onset, correlating with the timing of proper attachment of microtubules to kinetochores. Expressing phosphor-mimicking Hec1 (Hec1S165E) hampers chromosome alignment and triggers severe mitotic arrest associated with increased Mad1/Mad2 signals at prometaphase kinetochores. Thus it is reasonable to presume that overexpression of NEK2 may interrupt the dynamic changes of Hec1 phosphorylation during mitosis, thus leading to tumorigenensis.13 Meaningfully, disruption of Hec1/Nek2 protein-protein interaction by small molecules has shown impressive anticancer activities (see below), suggesting a potential treatment for cancer therapy.
In addition, NEK2 physically binds to and phosphorylates both MAD2 and CDC20. Overexpression of NEK2 enhances the ability of MAD2 to induce a delay in mitosis, in which kinase activity of NEK2 is essential.70 In contrast, elimination of NEK2 by siRNA decreases the assembly of MAD2 to kinetochores and causes aberrant premature chromosome segregation.12 Thus aberrant expression of NEK2 may promote aneuploidy by disrupting the mitotic checkpoint signaling, leading to malignant transformation.
TRF-1
Telomeric repeat binding factor 1 (TRF1) is a double-stranded telomere DNA-binding protein that negatively regulates telomere elongation by telomerase and promotes efficient DNA replication at telomeres.71 TRF1 also plays important roles in cell cycle regulation. TRF1 depletion disrupts proper sister chromatid cohesion and induces merotelic kinetochore attachment.72 It is found that NEK2 directly binds and phosphorylates TRF1 through multiple sites on TRF1.57,73 Moreover, mitotic aberrations through NEK2 overexpression are likely to require TRF1. NEK2 overexpression in breast cancer cells, MDA-MB-231 and MCF7, results in centrosome amplification, multinucleation, chromosomes misalignment and cytokinetic failure, which leads to aneuploidy. Interestingly, silencing of TRF1 prevents all these phenomena. Moreover, adding exogenous TRF1 back in NEK2-overexpressed cells with endogenous TRF1 depletion, cells had re-induced cytokinetic failure. Together, all these results suggests that TRF1 is indispensable for overexpressed NEK2 to trigger abnormal mitosis and chromosomal instability.57
β-catenin
β-catenin is a multifunctional protein that plays important roles in Wnt signaling pathway, cell-cell adhesion, centrosome disjunction and bipolar spindle formation.74,75 Previous studies have demonstrated that NEK2 binds to and phosphorylates β-catenin, prevents β-catenin ubiquitination and degradation, leading to the stabilization and accumulation of β-catenin at centrosomes in mitosis.65,76 The clinicopathological correlation of NEK2 and β-catenin has been disclosed in breast carcinoma. The increased NEK2 cytoplasmic expression was correlated with the increased β-catenin cytoplasmic expression in pure DCIS, concomitant DCIS and IDC.77 In addition, NEK2 also influence extra-centrosomal β-catenin localization. Overexpression of NEK2 in NSCLC A549 cells led to a severe reduction of β-catenin signal at the cell-cell adherens junction complexes and increase of β-catenin signal in and around the nucleus, an invasive state of cells.78 In consistence, overexpression of NEK2 in multiple myeloma ARP1 cells and lung cancer H1299 cells resulted in nuclear accumulation of β-catenin, while NEK2 knockdown decreased the expression of nuclear β-catenin.41 Moreover, overexpression of NEK2 in resected colorectal cancer tissues was associated with lower tumor membranous β-catenin expression and higher cytoplasmic and nuclear β-catenin accumulation.48 Together, these studies indicated NEK2 upregulation may influence localization of extra-centrosomal β-catenin, thus triggering tumorigenensis, metastasis and drug resistance.
SRSF1
Alternative splicing is a major resource contributing to structural transcript variation and proteome diversity. Aberrant splicing is frequently happened in cancer cells, thus enhancing their ability to adapt to the adverse growth conditions of the tumor microenvironment.79 A novel function for NEK2 in splicing regulation has been demonstrated recently. NEK2 is found to be localized in the nucleus of cancer cells derived from tissues of patients with testicular seminoma, breast, lung, colon, prostate and cervix cancer. Moreover, NEK2 co-localizes in splicing speckles with SRSF1 and SRSF2, two splicing factor. NEK2 phosphorylates SRSF1 and affects the splicing activity of SRSF1 toward reporter minigenes and endogenous targets. Knockdown of NEK2 induces expression of pro-apoptotic variants from SRSF1-target genes and sensitizes cells to apoptosis, which were recapitulated by knockdown of SRSF1.80 This study demonstrates a novel mechanism that helps explain the malfunction of NEK2 and cancer development.
Therapeutic targeting of NEK2
Given the compelling evidence mentioned above, there is no doubt that NEK2 is an ideal target for intervention in cancer. Generally speaking, targeting NEK2 could be executed by: i) modulating the protein level by using RNA interference; ii) blocking of ATP binding site; and iii) interfering protein interactions. We will discuss the progression of these interventions in detail as follows.
RNA interference
As the essential roles of NEK2 in tumorigenesis, progression and drug resistance, RNAi-based down-regulation of NEK2 can be a viable therapeutic strategy. Several preclinical studies using RNA interference targeted at NEK2 have shown the anti-tumor effect against different type of cancers. Suppression of the NEK2 expression with siRNA inhibited cell proliferation and induced cell death of breast cancer, cholangiocarcinoma, colorectal cancer, multiple myeloma, hepatoma and prostate cancer cells in vitro, and led to a reduction of tumor size in xenograft-nude mouse model.40-42,45-47,53 Moreover, silencing of NEK2 expression in breast cancer and colorectal cancer cells dramatically increased the susceptibility to chemotherapeutic drugs, such as paclitaxel, doxorubicin and cisplatin.47,58 Despite the success in preclinical reports, the RNAi-based NEK2 intervention therapeutics for clinical application still require plenty of work, including the problems related with the RNA interference technology itself and the potential side effect that might be resulted from NEK2 silencing in patients.
Blocking of ATP-binding site
The clinical successes of a number of kinase-targeted drugs have attracted an enormous amount of attention during the past decades of years. NEK2 is one of serine/threonine kinases that are ideal for drug development. The first compound reported to exhibit an inhibitory effect on NEK2 was SU11652 (compound 1), which was observed during the structural analysis of the NEK2 kinase domain. As a cell-permeable compound, SU11652 inhibited a number of tyrosine kinases and serine/threonine kinases, including platelet-derived growth factor receptor β, vascular endothelial growth factor receptor 2, fibroblast growth factor receptor, EGFR, and Kit family members.25 During the development of thiophene-based inhibitors of PLK1, 2 compounds (compound 2 and 3) were found to have activity against NEK2, even though the inhibition on PLK1 (IC50=2 nM) were greater than on NEK2 (IC50=21/25 nM).81
Then a series of viridin/wortmannin-like compounds (compound 4–6) were identified to inhibit the NEK2 activity by a high-throughput screening, however, these compounds generally exhibited similar levels of activity against Aurora A, Cdk1 and PLK1.82 Similarly, a series of aminopyrazine inhibitors were developed based on the initial hit compound (compound 7) identified by HTS. These compounds inhibited the NEK2 kinase in both the phosphorylated and unphosphorylated states, thus inhibiting its entire lifespan. However, none of them were active in cells because of insufficient membrane permeability. Moreover, as PLK1 possess similar ATP binding site of NEK2, some aminopyrazines inhibitors exhibited poor selectivity against NEK2.83 To identify selective NEK2 inhibitors with low activities on PLK1, series of compounds derived from the thiophene-based PLK1 inhibitor (compound 2) were synthesized and evaluated. A potent NEK2 inhibitor (compound 8) characterized by more than hundred-fold selectivity against PLK1 was identified and it showed an unusual binding mode to the DFG-out conformation of NEK2. However, this compound was lack of cellular activity.84 By combining key scaffold of the above series of compounds, aminopyrazines and benzimidazoles, the hybrids finally led to a potent compound (aminopyridine, compound 9) with the ability to reversibly inhibit activity of NEK2, thus modulate the phosphorylation of NEK2 substrates in cells, and sufficient selectivity against the most relevant cell cycle kinases, including PLK1, Mps1, Aurora A and CDK2.85
Compared with noncovalent inhibitors, irreversible inhibitors have plenty of advantages including prolonged pharmacodynamics, high potency and selectivity. Irreversible inhibition of NEK2 kinase can be achieved by targeting the enzyme's cysteine 22 (Cys-22) residue lying near the catalytic site. Up to now, 2 irreversible NEK2 inhibitors, oxindole propynamide (JH295, compound 10)86 and 2-arylamino-6-ethynylpurines (NCL-00017509, compound 11)87 have been reported. JH295 used an N-arylpropiolamide as Michael acceptor for attack by the Cys-22. Meaningfully, JH295 did not affect the activities of other mitotic kinases, CDK1, PLK1, Aurora B, or Mps1. Given this selective profile, JH295 is useful for determining the biological roles of NEK2 beyond RNAi knockdown method. Two-arylamino-6-ethynylpurines binds to NEK2 by conjugate addition of the Cys-22 to the terminus of the electrophilic alkyne to afford a (Z)-thioalkenyl-purine. A model study further indicated heterocyclic scaffolds are likely to be more promising for inhibition of NEK2.88
Interruption of protein interaction
Previous studies have reported that NEK2 plays important roles in cell division by interacting with a series of proteins, including Hec1, c-Nap1, Rootletin, MAD1 and MAD2, thus interrupting these interactions would affect the biologic function of NEK2. Of note, preclinical experiments has demonstrated the disrupting of NEK2 and Hec1 binding was effective for cancer intervention. A chemical genetic screening has identified a small molecular (INH1, compound 12), which specifically disrupt the Hec1/NEK2 interaction via direct Hec1 binding, consequently leading to metaphase chromosome misalignment, spindle aberrancy, and eventual cell death. INH1 effectively inhibited the proliferation of multiple human breast cancer cells in vitro and retarded tumor growth in nude mice bearing xenografts derived from the human breast cancer MDA-MB-468.89 To further improve the efficacy of INH1, a series of INH analogs were designed, synthesized and evaluated.90 Of note, 2 INH derivatives INH41 (compound 13) and INH154 (compound 14) have recently been reported to binding to Hec1, thus blocking Hec1 phosphorylation by NEK2, and kill cancer cells at the nanomolar range. Moreover, the binding of INHs to Hec1 formed a virtual death-trap to trigger NEK2 degradation through proteasome pathway.91 Another series of INH derivatives named TAIs were also synthesized to improve the anti-cancer activity of INH1. TAI-1 (compound 15) was highly potent with a wide anti-cancer spectrum in vitro and was effective orally in in vivo xenografted mouse models of triple negative breast cancer, colon cancer and liver cancer.92 To optimize the oral bioavailability, solubility, and pharmacokinetic parameters, the further development led to compound TAI-95 (compound 16). TAI-95 was active on a number of breast cancer and liver cancer cell lines at nM levels and demonstrated strong inhibition of tumor growth of breast cancer and liver cancer models without inducing weight loss or other obvious toxicity.93,94 In addition, another series of Hec1/NEK2 inhibitors, 4-aryl-N-arylcarbonyl-2-aminothiazoles were synthesized. Compound 17 bearing C-4′ 4-methoxyphenoxy and 4-(o-fluoropyridyl)carbonyl groups showed low nanomolar in vitro antiproliferative activity and significant in vivo antitumor activity in mice bearing human MDA-MB-231 xenografts.95
Concluding remarks
As an important mitotic kinase, NEK2 plays key role in regulating cell cycle progression. However, overexpression of NEK2 is frequently observed in a variety of human cancers. Elevated expression of NEK2 appears to participate in the initiation, maintenance, progression, metastasis of cancer and is positively associated with poor prognosis. In addition, NEK2 has been shown to promote chemoresistance in a number of cancer types and silencing of NEK2 sensitizes these tumor cells to chemotherapeutics. These facts suggest that NEK2 is a promising therapeutic target for cancer treatment. Recently, great efforts have been made to develop the NEK2 inhibitors (Table 2). As reviewed above, some NEK2 inhibitors have shown low nanomolar in vitro anti-proliferative activity and significant in vivo antitumor activity in xenograft nude mouse model. However, up to now, there is no NEK2 inhibitor that has been reported to undergo clinical trial, thus the development of NEK2 inhibitors is in the very early stage. There are still many questions that need to be addressed to maximize the effect of anti-NEK2 therapy. First, the specificity of currently available ATP-competitive inhibitors should be improved without losing their biological activities. Second, the molecular basis of how NEK2 interacts with its binding targets remains unknown. A better understanding of this will be fundamentally important for providing new insights into the development of novel NEK2 inhibitors. Third, although many novel interaction partners have been reported, the oncogenic NEK2 signaling deserves to be further defined for future drug discovery against NEK2. Fourth, identifying robust and sensitive biomarkers which may lead to more successful clinical trials of NEK2 inhibitors is greatly encouraged. In conclusion, current knowledge demonstrates that NEK2 is oncogenic in a variety of human cancers; novel strategies targeting NEK2 will bring convincing outcomes for cancer therapy.
Table 2.
Overview of NEK2 inhibitors.
| Action Mode | Compound | Name | Structure | NEK2IC50 (μM) | Ref. |
|---|---|---|---|---|---|
| Blocking ATP-binding site | 1 | SU11652 | 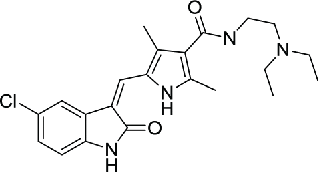 |
8.0 | 25 |
| 2–3 | Thiophene-based compounds |
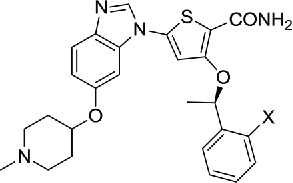 2 X=CF3 2 X=CF3
|
0.025 | 81 | |
| 3 X=Cl | 0.021 | ||||
| 4–6 | Viridin/wortmannin-like compounds | 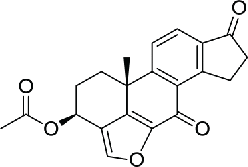 |
11.9 | 82 | |
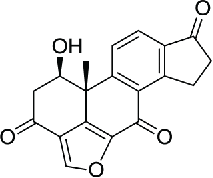 |
1.4 | ||||
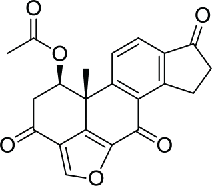 |
4.4 | ||||
| 7 | Aminopyrazine hit compound | 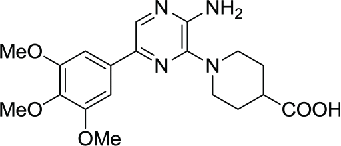 |
0.870 | 83 | |
| 8 | Benzimidazole compound | 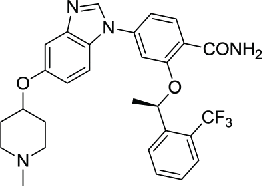 |
0.360 | 84 | |
| 9 | Aminopyridine compound | 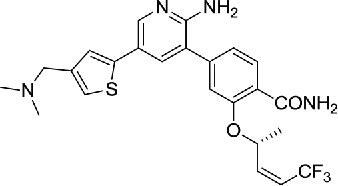 |
0.022 | 85 | |
| 10 | Oxindole propynamide (JH295) | 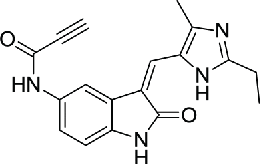 |
0.770 | 86 | |
| 11 | 2-arylamino-6-ethynylpurine (NCL-00017509) | 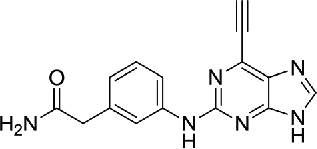 |
0.056 | 87 | |
| Interruption of protein interaction | 12 | INH1 | 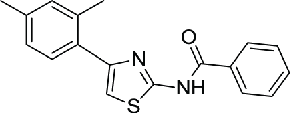 |
ND | 89 |
| 13 | INH41 | 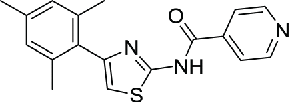 |
ND | 91 | |
| 14 | INH154 | 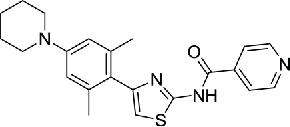 |
ND | 91 | |
| 15 | TAI-1 | 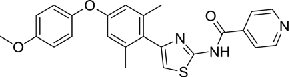 |
ND | 92 | |
| 16 | TAI-95 | 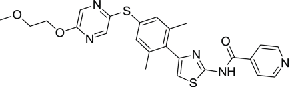 |
ND | 93,94 | |
| 17 | 4-aryl-N-arylcarbonyl-2-aminothiazole | 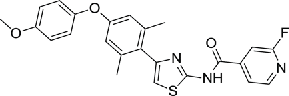 |
ND | 95 |
ND: not determined
Abbreviations
- ALDH1A1
aldehyde dehydrogenase 1-A1
- APC/C
anaphase promoting complex/cyclosome
- CC
coiled coil
- CDK4
cyclin-dependent kinase 4
- ChIP
Chromatin immunoprecipitation
- CIN
chromosome instability
- CIP2A
cancerous inhibitor of protein phosphatase 2A
- DCIS
ductal carcinoma in situ
- DLBCL
diffuse large B-cell lymphoma
- FL
follicular lymphoma
- Hec1
highly expressed in cancer 1
- HMGA2
high-mobility group protein A2
- LZ
leucine zipper
- MAD1
mitotic arrest deficient-like 1
- MEFs
mouse embryonic fibroblasts
- MGI
molecular grade index
- MST2
mammalian sterile 20-like kinase 2
- NEK2
Never in Mitosis (NIMA) Related Kinase 2
- NEKs
Never in Mitosis (NIMA) Related Kinases
- Nlp
ninein-like protein
- NSCLC
non-small cell lung cancer
- PLK1
polo-like kinase 1
- PP1
protein phosphatase 1
- Rb:
retinoblastoma
- TRF1
telomeric repeat binding factor 1
- UPS
ubiquitin-proteasome system.
Acknowledgments
We thank all members of our laboratory who critically commented on this manuscript.
Funding
This work was funded by grants from the National Natural Science Foundation of China (No. 81402953), The Science and Technology Commission of Shanghai Municipality (No. 14431902700) and China Postdoctoral Science Foundation (No. 2014M551362).
References
- [1].Fry AM, O'Regan L, Sabir SR, Bayliss R. Cell cycle regulation by the NEK family of protein kinases. J Cell Sci 2012; 125:4423-33; PMID:23132929; http://dx.doi.org/ 10.1242/jcs.111195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Schultz SJ, Fry AM, Sutterlin C, Ried T, Nigg EA. Cell cycle-dependent expression of Nek2, a novel human protein kinase related to the NIMA mitotic regulator of Aspergillus nidulans. Cell Growth Differ 1994; 5:625-35; PMID:7522034 [PubMed] [Google Scholar]
- [3].Hames RS, Fry AM. Alternative splice variants of the human centrosome kinase Nek2 exhibit distinct patterns of expression in mitosis. Biochem J 2002; 361:77-85; PMID:11742531; http://dx.doi.org/ 10.1042/bj3610077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wu W, Baxter JE, Wattam SL, Hayward DG, Fardilha M, Knebel A, Ford EM, da Cruz e Silva EF, Fry AM. Alternative splicing controls nuclear translocation of the cell cycle-regulated Nek2 kinase. J Biol Chem 2007; 282:26431-40; PMID:17626005; http://dx.doi.org/ 10.1074/jbc.M704969200 [DOI] [PubMed] [Google Scholar]
- [5].Hayward DG, Fry AM. Nek2 kinase in chromosome instability and cancer. Cancer Lett 2006; 237:155-66; PMID:16084011; http://dx.doi.org/ 10.1016/j.canlet.2005.06.017 [DOI] [PubMed] [Google Scholar]
- [6].Fry AM, Meraldi P, Nigg EA. A centrosomal function for the human Nek2 protein kinase, a member of the NIMA family of cell cycle regulators. EMBO J 1998; 17:470-81; PMID:9430639; http://dx.doi.org/ 10.1093/emboj/17.2.470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Faragher AJ, Fry AM. Nek2A kinase stimulates centrosome disjunction and is required for formation of bipolar mitotic spindles. Mol Biol Cell 2003; 14:2876-89; PMID:12857871; http://dx.doi.org/ 10.1091/mbc.E03-02-0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jeong Y, Lee J, Kim K, Yoo JC, Rhee K. Characterization of NIP2/centrobin, a novel substrate of Nek2, and its potential role in microtubule stabilization. J Cell Sci 2007; 120:2106-16; PMID:17535851; http://dx.doi.org/ 10.1242/jcs.03458 [DOI] [PubMed] [Google Scholar]
- [9].Sonn S, Jeong Y, Rhee K. Nip2/centrobin may be a substrate of Nek2 that is required for proper spindle assembly during mitosis in early mouse embryos. Mol Reprod Dev 2009; 76:587-92; PMID:19117032; http://dx.doi.org/ 10.1002/mrd.20990 [DOI] [PubMed] [Google Scholar]
- [10].Chen Y, Riley DJ, Zheng L, Chen PL, Lee WH. Phosphorylation of the mitotic regulator protein Hec1 by Nek2 kinase is essential for faithful chromosome segregation. J Biol Chem 2002; 277:49408-16; PMID:12386167; http://dx.doi.org/ 10.1074/jbc.M207069200 [DOI] [PubMed] [Google Scholar]
- [11].Du J, Cai X, Yao J, Ding X, Wu Q, Pei S, Jiang K, Zhang Y, Wang W, Shi Y, et al.. The mitotic checkpoint kinase NEK2A regulates kinetochore microtubule attachment stability. Oncogene 2008; 27:4107-14; PMID:18297113; http://dx.doi.org/ 10.1038/onc.2008.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lou Y, Yao J, Zereshki A, Dou Z, Ahmed K, Wang H, Hu J, Wang Y, Yao X. NEK2A interacts with MAD1 and possibly functions as a novel integrator of the spindle checkpoint signaling. J Biol Chem 2004; 279:20049-57; PMID:14978040; http://dx.doi.org/ 10.1074/jbc.M314205200 [DOI] [PubMed] [Google Scholar]
- [13].Wei R, Ngo B, Wu G, Lee WH. Phosphorylation of the Ndc80 complex protein, HEC1, by Nek2 kinase modulates chromosome alignment and signaling of the spindle assembly checkpoint. Mol Biol Cell 2011; 22:3584-94; PMID:21832156; http://dx.doi.org/ 10.1091/mbc.E11-01-0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fu G, Ding X, Yuan K, Aikhionbare F, Yao J, Cai X, Jiang K, Yao X. Phosphorylation of human Sgo1 by NEK2A is essential for chromosome congression in mitosis. Cell Res 2007; 17:608-18; PMID:17621308; http://dx.doi.org/ 10.1038/cr.2007.55 [DOI] [PubMed] [Google Scholar]
- [15].Fry AM, Schultz SJ, Bartek J, Nigg EA. Substrate specificity and cell cycle regulation of the Nek2 protein kinase, a potential human homolog of the mitotic regulator NIMA of Aspergillus nidulans. J Biol Chem 1995; 270:12899-905; PMID:7759549; http://dx.doi.org/ 10.1074/jbc.270.47.28357 [DOI] [PubMed] [Google Scholar]
- [16].Ren B, Cam H, Takahashi Y, Volkert T, Terragni J, Young RA, Dynlacht BD. E2F integrates cell cycle progression with DNA repair, replication, and G(2)/M checkpoints. Genes Dev 2002; 16:245-56; PMID:11799067; http://dx.doi.org/ 10.1101/gad.949802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nabilsi NH, Ryder DJ, Peraza-Penton AC, Poudyal R, Loose DS, Kladde MP. Local depletion of DNA methylation identifies a repressive p53 regulatory region in the NEK2 promoter. J Biol Chem 2013; 288:35940-51; PMID:24163369; http://dx.doi.org/ 10.1074/jbc.M113.523837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Takahashi Y, Iwaya T, Sawada G, Kurashige J, Matsumura T, Uchi R, Ueo H, Takano Y, Eguchi H, Sudo T, et al.. Up-regulation of NEK2 by microRNA-128 methylation is associated with poor prognosis in colorectal cancer. Ann Surg Oncol 2014; 21:205-12; PMID:24046120; http://dx.doi.org/ 10.1245/s10434-013-3264-3 [DOI] [PubMed] [Google Scholar]
- [19].Laoukili J, Kooistra MR, Bras A, Kauw J, Kerkhoven RM, Morrison A, Clevers H, Medema RH. FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat Cell Biol 2005; 7:126-36; PMID:15654331; http://dx.doi.org/ 10.1038/ncb1217 [DOI] [PubMed] [Google Scholar]
- [20].Wonsey DR, Follettie MT. Loss of the forkhead transcription factor FoxM1 causes centrosome amplification and mitotic catastrophe. Cancer Res 2005; 65:5181-9; PMID:15958562; http://dx.doi.org/ 10.1158/0008-5472.CAN-04-4059 [DOI] [PubMed] [Google Scholar]
- [21].Hames RS, Wattam SL, Yamano H, Bacchieri R, Fry AM. APC/C-mediated destruction of the centrosomal kinase Nek2A occurs in early mitosis and depends upon a cyclin A-type D-box. EMBO J 2001; 20:7117-27; PMID:11742988; http://dx.doi.org/ 10.1093/emboj/20.24.7117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hames RS, Crookes RE, Straatman KR, Merdes A, Hayes MJ, Faragher AJ, Fry AM. Dynamic recruitment of Nek2 kinase to the centrosome involves microtubules, PCM-1, and localized proteasomal degradation. Mol Biol Cell 2005; 16:1711-24; PMID:15659651; http://dx.doi.org/ 10.1091/mbc.E04-08-0688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sedgwick GG, Hayward DG, Di Fiore B, Pardo M, Yu L, Pines J, Nilsson J. Mechanisms controlling the temporal degradation of Nek2A and Kif18A by the APC/C-Cdc20 complex. EMBO J 2013; 32:303-14; PMID:23288039; http://dx.doi.org/ 10.1038/emboj.2012.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fry AM, Arnaud L, Nigg EA. Activity of the human centrosomal kinase, Nek2, depends on an unusual leucine zipper dimerization motif. J Biol Chem 1999; 274:16304-10; PMID:10347187; http://dx.doi.org/ 10.1074/jbc.274.23.16304 [DOI] [PubMed] [Google Scholar]
- [25].Rellos P, Ivins FJ, Baxter JE, Pike A, Nott TJ, Parkinson DM, Das S, Howell S, Fedorov O, Shen QY, et al.. Structure and regulation of the human Nek2 centrosomal kinase. J Biol Chem 2007; 282:6833-42; PMID:17197699; http://dx.doi.org/ 10.1074/jbc.M609721200 [DOI] [PubMed] [Google Scholar]
- [26].Di Agostino S, Rossi P, Geremia R, Sette C. The MAPK pathway triggers activation of Nek2 during chromosome condensation in mouse spermatocytes. Development 2002; 129:1715-27; PMID:11923207 [DOI] [PubMed] [Google Scholar]
- [27].Helps NR, Luo X, Barker HM, Cohen PT. NIMA-related kinase 2 (Nek2), a cell-cycle-regulated protein kinase localized to centrosomes, is complexed to protein phosphatase 1. Biochem J 2000; 349:509-18; PMID:10880350; http://dx.doi.org/ 10.1042/bj3490509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fry AM, Mayor T, Meraldi P, Stierhof YD, Tanaka K, Nigg EA. C-Nap1, a novel centrosomal coiled-coil protein and candidate substrate of the cell cycle-regulated protein kinase Nek2. J Cell Biol 1998; 141:1563-74; PMID:9647649; http://dx.doi.org/ 10.1083/jcb.141.7.1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bahe S, Stierhof YD, Wilkinson CJ, Leiss F, Nigg EA. Rootletin forms centriole-associated filaments and functions in centrosome cohesion. J Cell Biol 2005; 171:27-33; PMID:16203858; http://dx.doi.org/ 10.1083/jcb.200504107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Man X, Megraw TL, Lim YP. Cep68 can be regulated by Nek2 and SCF complex. Eur J Cell Biol 2015; 94:162-72; PMID:25704143; http://dx.doi.org/ 10.1016/j.ejcb.2015.01.004 [DOI] [PubMed] [Google Scholar]
- [31].Rapley J, Baxter JE, Blot J, Wattam SL, Casenghi M, Meraldi P, Nigg EA, Fry AM. Coordinate regulation of the mother centriole component nlp by nek2 and plk1 protein kinases. Mol Cell Biol 2005; 25:1309-24; PMID:15684383; http://dx.doi.org/ 10.1128/MCB.25.4.1309-1324.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Park J, Rhee K. NEK2 phosphorylation antagonizes the microtubule stabilizing activity of centrobin. Biochem Biophys Res Commun 2013; 431:302-8; PMID:23291182; http://dx.doi.org/ 10.1016/j.bbrc.2012.12.106 [DOI] [PubMed] [Google Scholar]
- [33].Di Agostino S, Fedele M, Chieffi P, Fusco A, Rossi P, Geremia R, Sette C. Phosphorylation of high-mobility group protein A2 by Nek2 kinase during the first meiotic division in mouse spermatocytes. Mol Biol Cell 2004; 15:1224-32; PMID:14668482; http://dx.doi.org/ 10.1091/mbc.E03-09-0638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gu Z, Zhou W, Huang J, Yang Y, Wendlandt E, Xu H, He X, Tricot G, Zhan F. Nek2 is a novel regulator of B cell development and immunological response. Biomed Res Int 2014; 2014:621082; PMID:25485281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kim S, Lee K, Choi JH, Ringstad N, Dynlacht BD. Nek2 activation of Kif24 ensures cilium disassembly during the cell cycle. Nat Commun 2015; 6:8087; PMID:26290419; http://dx.doi.org/ 10.1038/ncomms9087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wai DH, Schaefer KL, Schramm A, Korsching E, Van Valen F, Ozaki T, Boecker W, Schweigerer L, Dockhorn-Dworniczak B, Poremba C. Expression analysis of pediatric solid tumor cell lines using oligonucleotide microarrays. Int J Oncol 2002; 20:441-51; PMID:11836553 [PubMed] [Google Scholar]
- [37].de Vos S, Hofmann WK, Grogan TM, Krug U, Schrage M, Miller TP, Braun JG, Wachsman W, Koeffler HP, Said JW. Gene expression profile of serial samples of transformed B-cell lymphomas. Lab Invest 2003; 83:271-85; PMID:12594241; http://dx.doi.org/ 10.1097/01.LAB.0000053913.85892.E9 [DOI] [PubMed] [Google Scholar]
- [38].Andreasson U, Dictor M, Jerkeman M, Berglund M, Sundstrom C, Linderoth J, Rosenquist R, Borrebaeck CA, Ek S. Identification of molecular targets associated with transformed diffuse large B cell lymphoma using highly purified tumor cells. Am J Hematol 2009; 84:803-8; PMID:19844990; http://dx.doi.org/ 10.1002/ajh.21549 [DOI] [PubMed] [Google Scholar]
- [39].Hayward DG, Clarke RB, Faragher AJ, Pillai MR, Hagan IM, Fry AM. The centrosomal kinase Nek2 displays elevated levels of protein expression in human breast cancer. Cancer Res 2004; 64:7370-6; PMID:15492258; http://dx.doi.org/ 10.1158/0008-5472.CAN-04-0960 [DOI] [PubMed] [Google Scholar]
- [40].Tsunoda N, Kokuryo T, Oda K, Senga T, Yokoyama Y, Nagino M, Nimura Y, Hamaguchi M. Nek2 as a novel molecular target for the treatment of breast carcinoma. Cancer Sci 2009; 100:111-6; PMID:19038001; http://dx.doi.org/ 10.1111/j.1349-7006.2008.01007.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhou W, Yang Y, Xia J, Wang H, Salama ME, Xiong W, Xu H, Shetty S, Chen T, Zeng Z, et al.. NEK2 induces drug resistance mainly through activation of efflux drug pumps and is associated with poor prognosis in myeloma and other cancers. Cancer Cell 2013; 23:48-62; PMID:23328480; http://dx.doi.org/ 10.1016/j.ccr.2012.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cappello P, Blaser H, Gorrini C, Lin DC, Elia AJ, Wakeham A, Haider S, Boutros PC, Mason JM, Miller NA, et al.. Role of Nek2 on centrosome duplication and aneuploidy in breast cancer cells. Oncogene 2014; 33:2375-84; PMID:23708664; http://dx.doi.org/ 10.1038/onc.2013.183 [DOI] [PubMed] [Google Scholar]
- [43].Koch M, Wiese M. Gene expression signatures of angiocidin and darapladib treatment connect to therapy options in cervical cancer. J Cancer Res Clin Oncol 2013; 139:259-67; PMID:23052694; http://dx.doi.org/ 10.1007/s00432-012-1317-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Liu X, Gao Y, Lu Y, Zhang J, Li L, Yin F. Upregulation of NEK2 is associated with drug resistance in ovarian cancer. Oncol Rep 2014; 31:745-54; PMID:24337664 [DOI] [PubMed] [Google Scholar]
- [45].Zeng YR, Han ZD, Wang C, Cai C, Huang YQ, Luo HW, Liu ZZ, Zhuo YJ, Dai QS, Zhao HB, et al.. Overexpression of NIMA-related kinase 2 is associated with progression and poor prognosis of prostate cancer. BMC Urol 2015; 15:90; PMID:26320076; http://dx.doi.org/ 10.1186/s12894-015-0085-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kokuryo T, Senga T, Yokoyama Y, Nagino M, Nimura Y, Hamaguchi M. Nek2 as an effective target for inhibition of tumorigenic growth and peritoneal dissemination of cholangiocarcinoma. Cancer Res 2007; 67:9637-42; PMID:17942892; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-1489 [DOI] [PubMed] [Google Scholar]
- [47].Suzuki K, Kokuryo T, Senga T, Yokoyama Y, Nagino M, Hamaguchi M. Novel combination treatment for colorectal cancer using Nek2 siRNA and cisplatin. Cancer Sci 2010; 101:1163-9; PMID:20345485; http://dx.doi.org/ 10.1111/j.1349-7006.2010.01504.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Neal CP, Fry AM, Moreman C, McGregor A, Garcea G, Berry DP, Manson MM. Overexpression of the Nek2 kinase in colorectal cancer correlates with beta-catenin relocalization and shortened cancer-specific survival. J Surg Oncol 2014; 110:828-38; PMID:25043295; http://dx.doi.org/ 10.1002/jso.23717 [DOI] [PubMed] [Google Scholar]
- [49].Kohler ME, Johnson BD, Palen K, Chen QR, Khan J, Orentas RJ. Tumor antigen analysis in neuroblastoma by serological interrogation of bioinformatic data. Cancer Sci 2010; 101:2316-24; PMID:20718755; http://dx.doi.org/ 10.1111/j.1349-7006.2010.01694.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Barbagallo F, Paronetto MP, Franco R, Chieffi P, Dolci S, Fry AM, Geremia R, Sette C. Increased expression and nuclear localization of the centrosomal kinase Nek2 in human testicular seminomas. J Pathol 2009; 217:431-41; PMID:19023884; http://dx.doi.org/ 10.1002/path.2471 [DOI] [PubMed] [Google Scholar]
- [51].Stricker TP, Henriksen KJ, Tonsgard JH, Montag AG, Krausz TN, Pytel P. Expression profiling of 519 kinase genes in matched malignant peripheral nerve sheath tumor/plexiform neurofibroma samples is discriminatory and identifies mitotic regulators BUB1B, PBK and NEK2 as overexpressed with transformation. Mod Pathol 2013; 26:930-43; PMID:23370767; http://dx.doi.org/ 10.1038/modpathol.2012.242 [DOI] [PubMed] [Google Scholar]
- [52].Ning Z, Wang A, Liang J, Liu J, Zhou T, Yan Q, Wang Z. Abnormal expression of Nek2 in pancreatic ductal adenocarcinoma: a novel marker for prognosis. Int J Clin Exp Pathol 2014; 7:2462-9; PMID:24966957 [PMC free article] [PubMed] [Google Scholar]
- [53].Zhang MX, Xu XM, Zhang P, Han NN, Deng JJ, Yu TT, Gan YY, He XQ, Long ZX. Effect of silencing NEK2 on biological behaviors of HepG2 in human hepatoma cells and MAPK signal pathway. Tumour Biol 2015 [DOI] [PubMed] [Google Scholar]
- [54].Zhong X, Guan X, Dong Q, Yang S, Liu W, Zhang L. Examining Nek2 as a better proliferation marker in non-small cell lung cancer prognosis. Tumour Biol 2014; 35:7155-62; PMID:24763826; http://dx.doi.org/ 10.1007/s13277-014-1935-8 [DOI] [PubMed] [Google Scholar]
- [55].Bidkhori G, Narimani Z, Hosseini Ashtiani S, Moeini A, Nowzari-Dalini A, Masoudi-Nejad A. Reconstruction of an integrated genome-scale co-expression network reveals key modules involved in lung adenocarcinoma. PLoS One 2013; 8:e67552; PMID:23874428; http://dx.doi.org/ 10.1371/journal.pone.0067552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Harrison Pitner MK, Saavedra HI. Cdk4 and nek2 signal binucleation and centrosome amplification in a her2+ breast cancer model. PLoS One 2013; 8:e65971; PMID:23776583; http://dx.doi.org/ 10.1371/journal.pone.0065971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lee J, Gollahon L. Mitotic perturbations induced by Nek2 overexpression require interaction with TRF1 in breast cancer cells. Cell Cycle 2013; 12:3599-614; PMID:24091727; http://dx.doi.org/ 10.4161/cc.26589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lee J, Gollahon L. Nek2-targeted ASO or siRNA pretreatment enhances anticancer drug sensitivity in triplenegative breast cancer cells. Int J Oncol 2013; 42:839-47; PMID:23340795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Yang Y, Zhou W, Xia J, Gu Z, Wendlandt E, Zhan X, Janz S, Tricot G, Zhan F. NEK2 mediates ALDH1A1-dependent drug resistance in multiple myeloma. Oncotarget 2014; 5:11986-97; PMID:25230277; http://dx.doi.org/ 10.18632/oncotarget.2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Marina M, Saavedra HI. Nek2 and Plk4: prognostic markers, drivers of breast tumorigenesis and drug resistance. Front Biosci (Landmark Ed) 2014; 19:352-65; PMID:24389189; http://dx.doi.org/ 10.2741/4212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ma XJ, Salunga R, Dahiya S, Wang W, Carney E, Durbecq V, Harris A, Goss P, Sotiriou C, Erlander M, et al.. A five-gene molecular grade index and HOXB13:IL17BR are complementary prognostic factors in early stage breast cancer. Clin Cancer Res 2008; 14:2601-8; PMID:18451222; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-5026 [DOI] [PubMed] [Google Scholar]
- [62].Liu X. Targeting Polo-Like Kinases: A Promising Therapeutic Approach for Cancer Treatment. Transl Oncol 2015; 8:185-95; PMID:26055176; http://dx.doi.org/ 10.1016/j.tranon.2015.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Mardin BR, Agircan FG, Lange C, Schiebel E. Plk1 controls the Nek2A-PP1gamma antagonism in centrosome disjunction. Curr Biol 2011; 21:1145-51; PMID:21723128; http://dx.doi.org/ 10.1016/j.cub.2011.05.047 [DOI] [PubMed] [Google Scholar]
- [64].Mardin BR, Lange C, Baxter JE, Hardy T, Scholz SR, Fry AM, Schiebel E. Components of the Hippo pathway cooperate with Nek2 kinase to regulate centrosome disjunction. Nat Cell Biol 2010; 12:1166-76; PMID:21076410; http://dx.doi.org/ 10.1038/ncb2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Mbom BC, Siemers KA, Ostrowski MA, Nelson WJ, Barth AI. Nek2 phosphorylates and stabilizes beta-catenin at mitotic centrosomes downstream of Plk1. Mol Biol Cell 2014; 25:977-91; PMID:24501426; http://dx.doi.org/ 10.1091/mbc.E13-06-0349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Khanna A, Pimanda JE, Westermarck J. Cancerous inhibitor of protein phosphatase 2A, an emerging human oncoprotein and a potential cancer therapy target. Cancer Res 2013; 73:6548-53; PMID:24204027; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-1994 [DOI] [PubMed] [Google Scholar]
- [67].Jeong AL, Lee S, Park JS, Han S, Jang CY, Lim JS, Lee MS, Yang Y. Cancerous inhibitor of protein phosphatase 2A (CIP2A) protein is involved in centrosome separation through the regulation of NIMA (never in mitosis gene A)-related kinase 2 (NEK2) protein activity. J Biol Chem 2014; 289:28-40; PMID:24214971; http://dx.doi.org/ 10.1074/jbc.M113.507954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Dickson MA. Molecular pathways: CDK4 inhibitors for cancer therapy. Clin Cancer Res 2014; 20:3379-83; PMID:24795392; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-1551 [DOI] [PubMed] [Google Scholar]
- [69].Adon AM, Zeng X, Harrison MK, Sannem S, Kiyokawa H, Kaldis P, Saavedra HI. Cdk2 and Cdk4 regulate the centrosome cycle and are critical mediators of centrosome amplification in p53-null cells. Mol Cell Biol 2010; 30:694-710; PMID:19933848; http://dx.doi.org/ 10.1128/MCB.00253-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Liu Q, Hirohashi Y, Du X, Greene MI, Wang Q. Nek2 targets the mitotic checkpoint proteins Mad2 and Cdc20: a mechanism for aneuploidy in cancer. Exp Mol Pathol 2010; 88:225-33; PMID:20034488; http://dx.doi.org/ 10.1016/j.yexmp.2009.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Walker JR, Zhu XD. Post-translational modifications of TRF1 and TRF2 and their roles in telomere maintenance. Mech Ageing Dev 2012; 133:421-34; PMID:22634377; http://dx.doi.org/ 10.1016/j.mad.2012.05.002 [DOI] [PubMed] [Google Scholar]
- [72].Ohishi T, Muramatsu Y, Yoshida H, Seimiya H. TRF1 ensures the centromeric function of Aurora-B and proper chromosome segregation. Mol Cell Biol 2014; 34:2464-78; PMID:24752893; http://dx.doi.org/ 10.1128/MCB.00161-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Prime G, Markie D. The telomere repeat binding protein Trf1 interacts with the spindle checkpoint protein Mad1 and Nek2 mitotic kinase. Cell Cycle 2005; 4:121-4; PMID:15611654; http://dx.doi.org/ 10.4161/cc.4.1.1351 [DOI] [PubMed] [Google Scholar]
- [74].Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science 2004; 303:1483-7; PMID:15001769; http://dx.doi.org/ 10.1126/science.1094291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Kaplan DD, Meigs TE, Kelly P, Casey PJ. Identification of a role for beta-catenin in the establishment of a bipolar mitotic spindle. J Biol Chem 2004; 279:10829-32; PMID:14744872; http://dx.doi.org/ 10.1074/jbc.C400035200 [DOI] [PubMed] [Google Scholar]
- [76].Bahmanyar S, Kaplan DD, Deluca JG, Giddings TH Jr., O'Toole ET, Winey M, Salmon ED, Casey PJ, Nelson WJ, Barth AI. beta-Catenin is a Nek2 substrate involved in centrosome separation. Genes Dev 2008; 22:91-105; PMID:18086858; http://dx.doi.org/ 10.1101/gad.1596308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Wang S, Li W, Lv S, Wang Y, Liu Z, Zhang J, Liu T, Niu Y. Abnormal expression of Nek2 and beta-catenin in breast carcinoma: clinicopathological correlations. Histopathology 2011; 59:631-42; PMID:22014044; http://dx.doi.org/ 10.1111/j.1365-2559.2011.03941.x [DOI] [PubMed] [Google Scholar]
- [78].Das TK, Dana D, Paroly SS, Perumal SK, Singh S, Jhun H, Pendse J, Cagan RL, Talele TT, Kumar S. Centrosomal kinase Nek2 cooperates with oncogenic pathways to promote metastasis. Oncogenesis 2013; 2:e69; PMID:24018644; http://dx.doi.org/ 10.1038/oncsis.2013.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Sveen A, Kilpinen S, Ruusulehto A, Lothe RA, Skotheim RI. Aberrant RNA splicing in cancer; expression changes and driver mutations of splicing factor genes. Oncogene 2015; PMID:26300000 [DOI] [PubMed] [Google Scholar]
- [80].Naro C, Barbagallo F, Chieffi P, Bourgeois CF, Paronetto MP, Sette C. The centrosomal kinase NEK2 is a novel splicing factor kinase involved in cell survival. Nucleic Acids Res 2014; 42:3218-27; PMID:24369428; http://dx.doi.org/ 10.1093/nar/gkt1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Emmitte KA, Adjabeng GM, Andrews CW, Alberti JG, Bambal R, Chamberlain SD, Davis-Ward RG, Dickson HD, Hassler DF, Hornberger KR, et al.. Design of potent thiophene inhibitors of polo-like kinase 1 with improved solubility and reduced protein binding. Bioorg Med Chem Lett 2009; 19:1694-7; PMID:19237286; http://dx.doi.org/ 10.1016/j.bmcl.2009.01.094 [DOI] [PubMed] [Google Scholar]
- [82].Hayward DG, Newbatt Y, Pickard L, Byrne E, Mao G, Burns S, Sahota NK, Workman P, Collins I, Aherne W, et al.. Identification by high-throughput screening of viridin analogs as biochemical and cell-based inhibitors of the cell cycle-regulated nek2 kinase. J Biomol Screen 2010; 15:918-27; PMID:20664067; http://dx.doi.org/ 10.1177/1087057110376537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Whelligan DK, Solanki S, Taylor D, Thomson DW, Cheung KM, Boxall K, Mas-Droux C, Barillari C, Burns S, Grummitt CG, et al.. Aminopyrazine inhibitors binding to an unusual inactive conformation of the mitotic kinase Nek2: SAR and structural characterization. J Med Chem 2010; 53:7682-98; PMID:20936789; http://dx.doi.org/ 10.1021/jm1008727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Solanki S, Innocenti P, Mas-Droux C, Boxall K, Barillari C, van Montfort RL, Aherne GW, Bayliss R, Hoelder S. Benzimidazole inhibitors induce a DFG-out conformation of never in mitosis gene A-related kinase 2 (Nek2) without binding to the back pocket and reveal a nonlinear structure-activity relationship. J Med Chem 2011; 54:1626-39; PMID:21366329; http://dx.doi.org/ 10.1021/jm1011726 [DOI] [PubMed] [Google Scholar]
- [85].Innocenti P, Cheung KM, Solanki S, Mas-Droux C, Rowan F, Yeoh S, Boxall K, Westlake M, Pickard L, Hardy T, et al.. Design of potent and selective hybrid inhibitors of the mitotic kinase Nek2: structure-activity relationship, structural biology, and cellular activity. J Med Chem 2012; 55:3228-41; PMID:22404346; http://dx.doi.org/ 10.1021/jm201683b [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Henise JC, Taunton J. Irreversible Nek2 kinase inhibitors with cellular activity. J Med Chem 2011; 54:4133-46; PMID:21627121; http://dx.doi.org/ 10.1021/jm200222m [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Carbain B, Bayliss R, Boxall K, Coxon C, Lebraud H, Matheson C, Turner D, Zhen-Wang L, Griffin RJ. 118 2-arylamino-6-ethynylpurines as Potent Irreversible Inhibitors of the Mitotic Kinase Nek2. Eur J Cancer 2012; 48, Supplement 6:37; PMID:21664123; http://dx.doi.org/ 10.1016/S0959-8049(12)71916-021664123 [DOI] [Google Scholar]
- [88].Lebraud H, Coxon CR, Archard VS, Bawn CM, Carbain B, Matheson CJ, Turner DM, Cano C, Griffin RJ, Hardcastle IR, et al.. Model system for irreversible inhibition of Nek2: thiol addition to ethynylpurines and related substituted heterocycles. Org Biomol Chem 2014; 12:141-8; PMID:24213855; http://dx.doi.org/ 10.1039/C3OB41806E [DOI] [PubMed] [Google Scholar]
- [89].Wu G, Qiu XL, Zhou L, Zhu J, Chamberlin R, Lau J, Chen PL, Lee WH. Small molecule targeting the Hec1/Nek2 mitotic pathway suppresses tumor cell growth in culture and in animal. Cancer Res 2008; 68:8393-9; PMID:18922912; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Qiu XL, Li G, Wu G, Zhu J, Zhou L, Chen PL, Chamberlin AR, Lee WH. Synthesis and biological evaluation of a series of novel inhibitor of Nek2/Hec1 analogues. J Med Chem 2009; 52:1757-67; PMID:19243176; http://dx.doi.org/ 10.1021/jm8015969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Hu CM, Zhu J, Guo XE, Chen W, Qiu XL, Ngo B, Chien R, Wang YV, Tsai CY, Wu G, et al.. Novel small molecules disrupting Hec1/Nek2 interaction ablate tumor progression by triggering Nek2 degradation through a death-trap mechanism. Oncogene 2015; 34:1220-30; PMID:24662830; http://dx.doi.org/ 10.1038/onc.2014.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Huang LY, Lee YS, Huang JJ, Chang CC, Chang JM, Chuang SH, Kao KJ, Tsai YJ, Tsai PY, Liu CW, et al.. Characterization of the biological activity of a potent small molecule Hec1 inhibitor TAI-1. J Exp Clin Cancer Res 2014; 33:6; PMID:24401611; http://dx.doi.org/ 10.1186/1756-9966-33-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Huang LY, Chang CC, Lee YS, Chang JM, Huang JJ, Chuang SH, Kao KJ, Lau GM, Tsai PY, Liu CW, et al.. Activity of a novel Hec1-targeted anticancer compound against breast cancer cell lines in vitro and in vivo. Mol Cancer Ther 2014; 13:1419-30; PMID:24694948; http://dx.doi.org/ 10.1158/1535-7163.MCT-13-0700 [DOI] [PubMed] [Google Scholar]
- [94].Huang LY, Chang CC, Lee YS, Huang JJ, Chuang SH, Chang JM, Kao KJ, Lau GM, Tsai PY, Liu CW, et al.. Inhibition of Hec1 as a novel approach for treatment of primary liver cancer. Cancer Chemother Pharmacol 2014; 74:511-20; PMID:25038613; http://dx.doi.org/ 10.1007/s00280-014-2540-7 [DOI] [PubMed] [Google Scholar]
- [95].Lee YS, Chuang SH, Huang LY, Lai CL, Lin YH, Yang JY, Liu CW, Yang SC, Lin HS, Chang CC, et al.. Discovery of 4-aryl-N-arylcarbonyl-2-aminothiazoles as Hec1/Nek2 inhibitors. Part I: optimization of in vitro potencies and pharmacokinetic properties. J Med Chem 2014; 57:4098-110; PMID:24773549; http://dx.doi.org/ 10.1021/jm401990s [DOI] [PubMed] [Google Scholar]