Significance
Eukaryotic algae, which play a fundamental role in global CO2 fixation, enhance the performance of the carbon-fixing enzyme Rubisco by placing it into an organelle called the pyrenoid. Despite the ubiquitous presence and biogeochemical importance of this organelle, how Rubisco assembles to form the pyrenoid remains a long-standing mystery. Our discovery of an abundant repeat protein that binds Rubisco in the pyrenoid represents a critical advance in our understanding of pyrenoid biogenesis. The repeat sequence of this protein suggests elegant models to explain the structural arrangement of Rubisco enzymes in the pyrenoid. Beyond advances in basic understanding, our findings open doors to the engineering of algal pyrenoids into crops to enhance yields.
Keywords: pyrenoid, Rubisco, carbon fixation, Chlamydomonas reinhardtii, CO2-concentrating mechanism
Abstract
Biological carbon fixation is a key step in the global carbon cycle that regulates the atmosphere's composition while producing the food we eat and the fuels we burn. Approximately one-third of global carbon fixation occurs in an overlooked algal organelle called the pyrenoid. The pyrenoid contains the CO2-fixing enzyme Rubisco and enhances carbon fixation by supplying Rubisco with a high concentration of CO2. Since the discovery of the pyrenoid more that 130 y ago, the molecular structure and biogenesis of this ecologically fundamental organelle have remained enigmatic. Here we use the model green alga Chlamydomonas reinhardtii to discover that a low-complexity repeat protein, Essential Pyrenoid Component 1 (EPYC1), links Rubisco to form the pyrenoid. We find that EPYC1 is of comparable abundance to Rubisco and colocalizes with Rubisco throughout the pyrenoid. We show that EPYC1 is essential for normal pyrenoid size, number, morphology, Rubisco content, and efficient carbon fixation at low CO2. We explain the central role of EPYC1 in pyrenoid biogenesis by the finding that EPYC1 binds Rubisco to form the pyrenoid matrix. We propose two models in which EPYC1’s four repeats could produce the observed lattice arrangement of Rubisco in the Chlamydomonas pyrenoid. Our results suggest a surprisingly simple molecular mechanism for how Rubisco can be packaged to form the pyrenoid matrix, potentially explaining how Rubisco packaging into a pyrenoid could have evolved across a broad range of photosynthetic eukaryotes through convergent evolution. In addition, our findings represent a key step toward engineering a pyrenoid into crops to enhance their carbon fixation efficiency.
Rubisco, the most abundant enzyme in the biosphere (1), fixes CO2 into organic carbon that supports nearly all life on Earth (2, 3). Over the past 3 billion y, the enzyme became a victim of its own success as it drew down the atmospheric CO2 concentration to trace levels (4) and as the oxygen-producing reactions of photosynthesis filled our atmosphere with O2 (4). In today’s atmosphere, O2 competes with CO2 at Rubisco's catalytic site, producing the toxic compound phosphoglycolate (5). Phosphoglycolate must be metabolized at the expense of energy and loss of fixed carbon and nitrogen (6). To overcome Rubisco's limitations, many photosynthetic organisms have evolved carbon-concentrating mechanisms (CCMs) (7, 8). CCMs increase the CO2 concentration around Rubisco, decreasing O2 competition and enhancing carbon fixation.
At the heart of the CCM of eukaryotic algae is an organelle known as the pyrenoid (9). The pyrenoid is a spherical structure in the chloroplast stroma, discovered more than 130 y ago (10–12). Pyrenoids have been found in nearly all of the major oceanic eukaryotic primary producers and mediate ∼28–44% of global carbon fixation (SI Appendix, Table S1) (3, 13–17). A pyrenoid typically consists of a matrix surrounded by a starch sheath and traversed by membrane tubules continuous with the photosynthetic thylakoid membranes (18). This matrix is thought to consist primarily of tightly packed Rubisco and its chaperone, Rubisco activase (19). In higher plants and non–pyrenoid-containing photosynthetic eukaryotes, Rubisco is instead soluble throughout the chloroplast stroma. The molecular mechanism by which Rubisco aggregates to form the pyrenoid matrix remains enigmatic.
Two mechanisms for Rubisco accumulation in the pyrenoid have been proposed: (i) Rubisco holoenzymes could bind each other directly through hydrophobic residues (20), or (ii) a linker protein may link Rubisco holoenzymes together (18, 20). The second model is based on analogy to the well-characterized prokaryotic carbon concentrating organelle, the β-carboxysome, where Rubisco aggregation is mediated by a linker protein consisting of repeats of a domain resembling the Rubisco small subunit (21). Here we find that Rubisco accumulation in the pyrenoid of the model alga Chlamydomonas reinhardtii is mediated by a disordered repeat protein, which we term Essential Pyrenoid Component 1 (EPYC1). Our findings suggest a mechanism for aggregation of Rubisco in the pyrenoid matrix, and highlight similarities and differences between the mechanisms of assembly of the eukaryotic and prokaryotic organelles.
Results
EPYC1 Is an Abundant Pyrenoid Component.
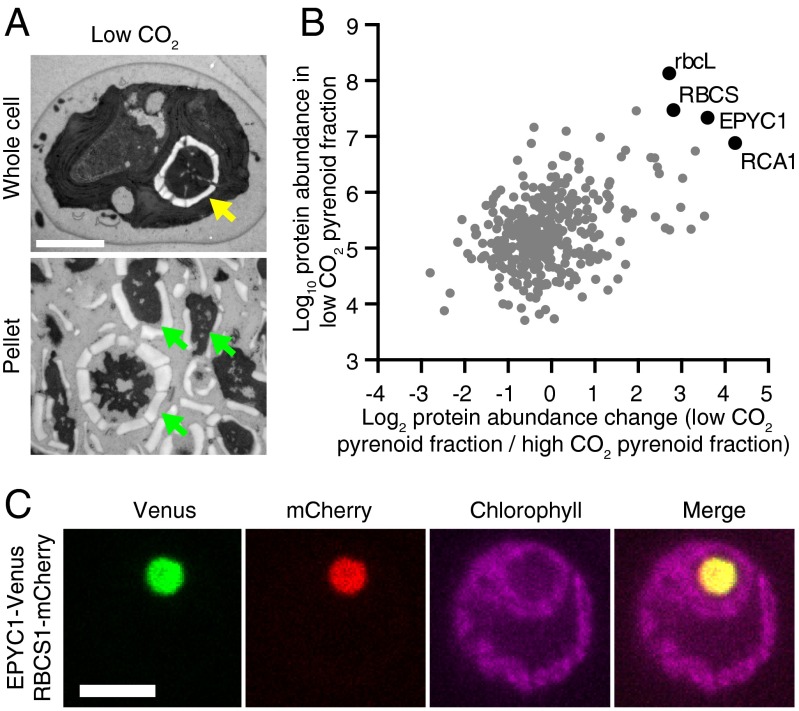
We hypothesized that the pyrenoid contains unidentified components that are important for its biogenesis. Therefore, we used mass spectrometry to analyze the protein composition of the pyrenoid of Chlamydomonas, before and after applying a stimulus that induces pyrenoid growth. When cells are transferred from high CO2 (2–5% CO2 in air) to low CO2 (∼0.04% CO2 in air), the CCM is induced (22) and the pyrenoid increases in size (23). We developed a protocol for isolating largely intact pyrenoids by cell lysis and centrifugation, and applied this protocol to cells before and after a shift from high to low CO2 (Fig. 1A and SI Appendix, Fig. S1 A–C). Mass spectrometry indicated that the most abundant proteins in the low-CO2 pyrenoid fraction included the Rubisco large (rbcL) and small (RBCS) subunits, as well as Rubisco activase (RCA1) (Fig. 1B, SI Appendix, Fig. S1D, and Dataset S1).
Fig. 1.
EPYC1 is an abundant pyrenoid protein. (A) TEM images of Chlamydomonas whole cells and pyrenoid-enriched pellet fraction from cells grown at low CO2. The yellow arrow indicates the pyrenoid, and green arrows indicate pyrenoid-like structures. (Scale bar: 2 µm.) (B) Mass spectrometry analysis of 366 proteins in pyrenoid-enriched pellet fractions from low- and high-CO2–grown cells (mean of four biological replicates; raw data are provided in SI Appendix and Dataset S1). RbcL, RBCS, EPYC1, and RCA1 (black) are abundant in low-CO2 pellets, as determined by iBAQ (y-axis). In addition, these proteins showed increased abundance in low-CO2 pellets compared with high-CO2 pellets, as determined by label-free quantification (LFQ; x-axis). (C) Confocal microscopy of EPYC1-Venus and RBCS1-mCherry coexpressed in WT cells. (Scale bar: 5 µm.)
Strikingly, a fourth protein, previously identified as a low-CO2–induced nuclear-encoded protein (LCI5; Cre10.g436550) (24), was found in the low-CO2 pyrenoid fraction with comparable abundance to Rubisco (Fig. 1B). Based on the data presented herein, we propose naming this protein Essential Pyrenoid Component 1 (EPYC1). Under low CO2, the stoichiometry of EPYC1, estimated by intensity-based absolute quantification (iBAQ), was ∼1:6 with rbcL and ∼1:1 with RBCS (25). Consistent with EPYC1 being a component of the pyrenoid, the abundance of EPYC1 in the pyrenoid fraction was increased by ∼12-fold after the shift from high to low CO2 (Fig. 1B and SI Appendix, Fig. S1D and Dataset S1), an increase similar to that of rbcL (7-fold), RBCS (7-fold), and RCA1 (19-fold). To confirm the pyrenoid localization of EPYC1, we coexpressed fluorescently tagged EPYC1 and RBCS. Venus-tagged EPYC1 showed clear colocalization with mCherry-tagged RBCS in the pyrenoid (Fig. 1C and SI Appendix, Fig. S1E).
EPYC1 Is Essential for a Functional CCM.
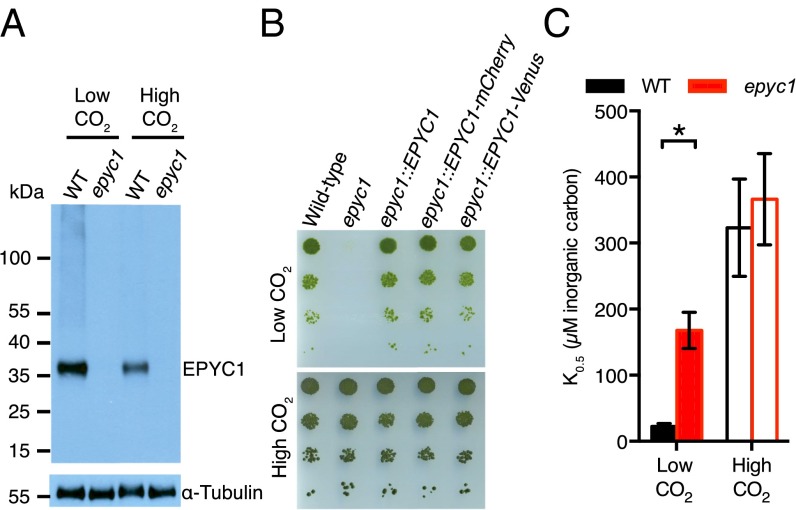
The high abundance of EPYC1 in the pyrenoid led us to ask whether EPYC1 is required for the CCM. We isolated a mutant in the 5′ UTR of the EPYC1 gene (SI Appendix, Fig. S2A and Table S2), which contains markedly reduced levels of EPYC1 mRNA (SI Appendix, Fig. S2B and Table S3) and EPYC1 protein (Fig. 2A), and lacks transcriptional regulation in response to CO2 (SI Appendix, Fig. S2B). Similar to previously described mutants in other components of the CCM, the epyc1 mutant showed defective photoautotrophic growth in low CO2, which was rescued by high CO2 and by reintroducing the EPYC1 gene (Fig. 2B and SI Appendix, Fig. S2 C–E).
Fig. 2.
EPYC1 is an essential component of the carbon-concentrating mechanism. (A) EPYC1 protein levels in WT and epyc1 mutant cells grown at low and high CO2 were probed by Western blot analysis with anti-EPYC1 antibodies. Anti-tubulin is shown as a loading control. (B) Growth phenotypes of WT, epyc1, and three lines complemented with EPYC1. Serial 1:10 dilutions of WT, epyc1, epyc1::EPYC1, epyc1::EPYC1-mCherry, and epyc1::EPYC1-Venus lines were spotted on TP minimal medium and grown at low and high CO2 under 500 µmol photons m−2 s−1 illumination. (C) Inorganic carbon affinity of WT and epyc1 cells. Cells were pregrown at low and high CO2, and whole-cell inorganic carbon affinity was measured as the concentration of inorganic carbon at half-maximal O2 evolution. Data are a mean of five low-CO2 or three high-CO2 biological replicates. Error bars represent SEM. *P = 0.0055, Student’s t test.
We further tested the CCM activity in the epyc1 mutant by measuring whole-cell affinity for inorganic carbon, inferred from photosynthetic O2 evolution. When grown under low CO2, the epyc1 mutant showed a reduced affinity for inorganic carbon (increased K0.5) relative to WT (P = 0.0055, Student’s t test; n = 5) (Fig. 2C and SI Appendix, Fig. S2F and Table S4). The affinity of the epyc1 mutant under low CO2 was slightly greater than that of WT at high CO2, indicating a residual level of CCM activity. This activity may be due to trace levels of EPYC1 in the epyc1 mutant (SI Appendix, Fig. S2 A and B), or a normal CO2 concentration followed by inefficient capture by Rubisco.
EPYC1 Is Required for Normal Pyrenoid Size, Number, and Matrix Density.
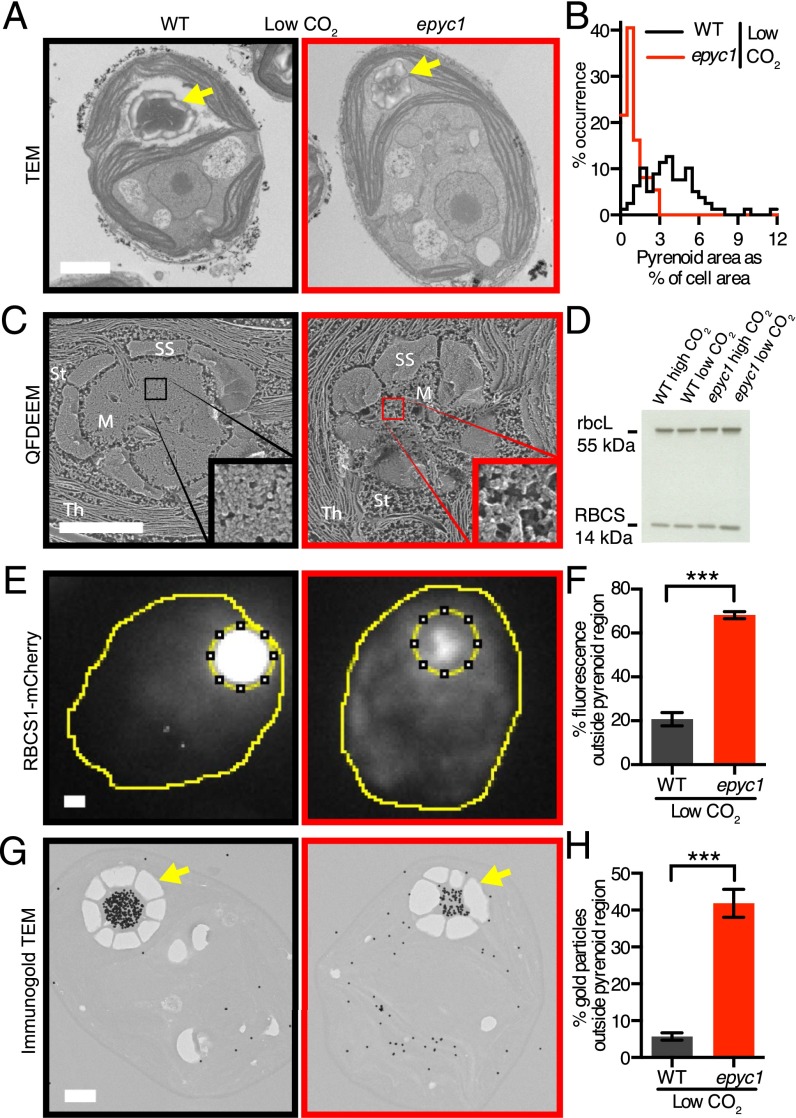
Given that EPYC1 is in the pyrenoid and is required for the CCM, we explored whether the epyc1 mutant shows any visible defects in pyrenoid structure. Thin-section transmission electron microscopy (TEM) revealed that the epyc1 mutant had smaller pyrenoids than WT at both low and high CO2 (low CO2: n = 37–79, P < 10−19, Welch’s t test; high CO2: n = 18–22, P < 10−5, Welch’s t test) (Fig. 3 A and B and SI Appendix, Fig. S3 A and B and Fig. S4). Chlamydomonas typically has one pyrenoid per cell (17). The epyc1 mutant showed a higher frequency of multiple pyrenoids; 13% of nondividing epyc1 cells (n = 231) showed multiple pyrenoids, compared with 3% of WT cells (n = 252; P = 0.00048, Fisher’s exact test of independence) (SI Appendix, Table S5). Higher-resolution quick-freeze deep-etch electron microscopy (QFDEEM) indicated a lower packing density of granular material in the pyrenoid matrix of the epyc1 mutant (Fig. 3C and SI Appendix, Figs. S3C and S5). This defect was most noticeable when cells were grown in low CO2, but was also visible at high CO2.
Fig. 3.
EPYC1 is essential for Rubisco aggregation in the pyrenoid. (A) Representative TEMs of WT and epyc1 cells grown at low CO2. (B) Quantification of the pyrenoid area as a percentage of cell area of WT and epyc1 cells grown at low CO2. Data are from TEM images as represented in A. epyc1: n = 37; WT: n = 79. P < 10−19, Welch’s t test. (C) QFDEEM of the pyrenoid of WT and epyc1 cells grown at low CO2. M, pyrenoid matrix; St, stroma; Th, thylakoids; SS, starch sheath. (Inset) Four hundred percent zoom view of the pyrenoid matrix. (D) Rubisco protein levels in WT and epyc1 cells grown at low and high CO2 were probed by Western blot analysis. (E) Localization of Rubisco was determined by microscopy of WT and the epyc1 mutant containing RBCS1-mCherry. The sum of the fluorescence signals from Z stacks was used for quantitation. (F) The fraction of RBCS1-mCherry signal from outside the pyrenoid region (inner dotted line in E) was quantified in WT and epyc1 cells at low CO2. epyc1: n = 27; WT: n = 27. ***P < 10−15, Student’s t test. (G) Representative images of anti-Rubisco immunogold labeling of WT and epyc1 cells grown at low CO2. Gold particles were enlarged 10× for visibility. (H) The fraction of immunogold particles outside the pyrenoid was quantified. WT: n = 26 cells, 8,123 gold particles; epyc1: n = 27 cells, 2,708 gold particles. ***P < 10−15, Student’s t test. In F and H, data are mean values, with error bars indicating SEM. Yellow arrows indicate pyrenoids. (Scale bars: 1 µm.)
Interestingly, the epyc1 mutant retains a number of canonical pyrenoid characteristics (17), including correct localization in the chloroplast, the presence of a starch sheath under low CO2, and traversing membrane tubules, suggesting that normal levels of EPYC1 are not required for these characteristics. In addition, the epyc1 mutant showed normal levels of the carbonic anhydrase CAH3, which is thought to be central in delivering CO2 to Rubisco in the pyrenoid (SI Appendix, Fig. S2G).
EPYC1 Is Required for Rubisco Assembly into the Pyrenoid.
Our observations of decreased pyrenoid size and apparent matrix density in the epyc1 mutant could be explained by decreased whole-cell levels of Rubisco. However, Western blot analysis revealed no detectable difference in rbcL and RBCS abundance in epyc1 relative to WT cells or between cells grown at low and high CO2 levels (Fig. 3D and SI Appendix, Fig. S3D). This result led us to hypothesize that the localization of Rubisco was perturbed in the epyc1 mutant. To test this hypothesis, we generated WT and epyc1 cell lines expressing Rubisco tagged with mCherry, and determined the distribution of fluorescence signal by microscopy. Remarkably, a large fraction of Rubisco was found outside the pyrenoid in the epyc1 mutant. In epyc1 cells grown in low CO2, 68% of fluorescence from Rubisco tagged with mCherry was found outside the pyrenoid region, compared with 21% in WT cells (n = 27; P < 10−15, Student’s t test) (Fig. 3 E and F and SI Appendix, Fig. S6). Immunogold-EM confirmed the mislocalization of Rubisco in epyc1. In pyrenoid-containing sections of low-CO2–grown epyc1 cells, 42% of anti-Rubisco immunogold particles were found outside the pyrenoid, whereas only 6% were found outside the pyrenoid in WT (WT: n = 26 cells, 8,123 gold particles; epyc1: n = 27 cells, 2,708 gold particles; P < 10−15, Student’s t test) (Fig. 3 G and H and SI Appendix, Fig. S7).
If EPYC1 functions in the recruitment of Rubisco to the pyrenoid solely at low CO2 (23), then the epyc1 mutant could be trapped in a “high-CO2” state of Rubisco localization (23). However, the epyc1 mutant showed a defect in Rubisco localization even under high CO2 (SI Appendix, Fig. S3 E and F and Fig. S6), where the fraction of Rubisco-mCherry fluorescence outside the pyrenoid region increased to 80% in epyc1, compared with 68% in the WT (WT: n = 20; epyc1: n = 20; P = 10−6, Student’s t test). We conclude that EPYC1 is required for Rubisco localization to the pyrenoid not only at low CO2, but also at high CO2.
EPYC1 and Rubisco Are Part of the Same Complex.
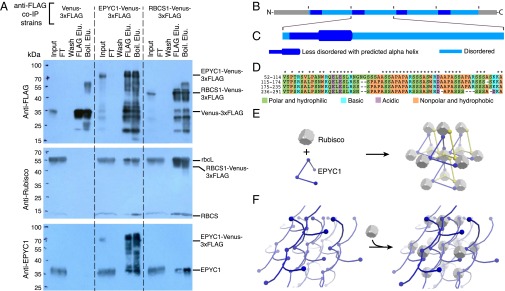
EPYC1 could promote the localization of Rubisco to the pyrenoid by a physical interaction. Thus, we immunoprecipitated EPYC1 and Rubisco, and probed the eluates by Western blot analysis (Fig. 4A and SI Appendix, Fig. S8A). Immunoprecipitation of tagged EPYC1 pulled down the Rubisco holoenzyme, and, reciprocally, tagged RBCS1 pulled down EPYC1. We conclude that EPYC1 and Rubisco are part of the same supramolecular complex in the pyrenoid. The high abundance of EPYC1 in the pyrenoid, EPYC1’s physical interaction with Rubisco, and the dependence of Rubisco on EPYC1 for localization to the pyrenoid all suggest that EPYC1 plays a structural role in pyrenoid biogenesis.
Fig. 4.
EPYC1 forms a complex with Rubisco. (A) Anti-FLAG coimmunoprecipitation (co-IP) of WT cells expressing Venus-3×FLAG, EPYC1-Venus-3×FLAG, and RBCS1-Venus-3×FLAG. For each co-IP, the input, flow-through (FT), fourth wash (wash), 3×FLAG elution (FLAG Elu.), and boiling elution (Boil. Elu.) were probed with anti-FLAG, anti-Rubisco, or anti-EPYC1. Labels on the right show the expected sizes of proteins. (B) Analysis of the EPYC1 protein sequence showing that EPYC1 consists of four nearly identical repeats. (C) Each repeat has a highly disordered domain (light blue) and a less-disordered domain (dark blue) containing a predicted alpha-helix (thicker line) rich in charged residues. (D) Amino acid alignments of the four repeats. Asterisks indicate residues that are identical in all four repeats. (E and F) Two models illustrate how EPYC1 could bind the Rubisco holoenzyme in a manner compatible with the observed packing of Rubisco in the pyrenoid. (E) EPYC1 and Rubisco could form a codependent network. If each EPYC1 can bind four Rubisco holoenzymes, and each Rubisco holoenzyme can bind eight EPYC1s, eight EPYC1 proteins could connect each Rubisco to twelve neighboring Rubiscos. (F) EPYC1 could form a scaffold onto which Rubisco binds. Both arrangements could expand indefinitely in every direction. For clarity, the spacing between Rubisco holoenzymes was increased and EPYC1 is depicted in both yellow and blue.
The EPYC1 Protein Consists of Four Nearly Identical Repeats.
To gain insight into how EPYC1 might contribute to pyrenoid biogenesis, we performed a detailed analysis of the EPYC1 protein sequence. This analysis indicated that EPYC1 consists of four nearly identical ∼60-aa repeats (Fig. 4 B–D), flanked by short N and C termini, in contrast to a previous study suggesting only three repeats (26). We found that each repeat consists of a predicted disordered domain and a shorter, less disordered domain containing a predicted alpha helix (Fig. 4C and SI Appendix, Fig. S8 B and C). Given that these repeats cover >80% of the EPYC1 protein, it is likely that the Rubisco binding sites are contained within the repeats.
We Propose Two Models for Rubisco Assembly into the Pyrenoid Matrix by EPYC1.
If each repeat of EPYC1 binds Rubisco, then EPYC1 could link multiple Rubisco holoenzymes together to form the pyrenoid matrix. Multiple Rubisco binding sites on EPYC1 could arrange Rubisco into the hexagonal closely packed or cubic closely packed arrangement observed in recent cryoelectron tomography studies of the Chlamydomonas pyrenoid (18). EPYC1 and Rubisco could interact in one of two fundamental ways: (i) EPYC1 and Rubisco could form a codependent network (Fig. 4E), or (ii) EPYC1 could form a scaffold onto which Rubisco binds (Fig. 4F). Importantly, the 60-aa repeat length of EPYC1 is sufficient to span the observed 2- to 4.5-nm gap between Rubisco holoenzymes in the pyrenoid (18), and a stretched-out repeat could potentially span the observed 15-nm Rubisco center-to-center distance. A promising candidate for an EPYC1-binding site on Rubisco would be the two alpha-helices of the small Rubisco subunit. When these helices are exchanged for higher-plant alpha-helices, pyrenoids fail to form and the CCM does not function, but holoenzyme assembly is normal (20).
Proteins with Similar Physicochemical Properties to EPYC1 Are Present in a Diverse Range of Eukaryotic Algae.
The primary sequences of disordered proteins like EPYC1 are known to evolve rapidly compared with those of structured proteins, but their physicochemical properties are under selective pressure and are evolutionarily maintained (27). Therefore, we searched for proteins with similar physicochemical properties (i.e., repeat number, length, high isoelectric point, disorder profile, and absence of transmembrane domains) across a broad range of algae (SI Appendix, Table S6). Excitingly, proteins with similar properties are found in most pyrenoid-containing algae and appear to be absent from pyrenoid-less algae, suggesting that EPYC1-like proteins may play similar roles in pyrenoids across eukaryotic algae. A thorough assessment of the generality of linker proteins will be enabled by future proteomic analyses of pyrenoids from a diverse range of algae.
Discussion
Our data provide strong support for the concept that Rubisco clustering into the pyrenoid is required for an efficient CCM in eukaryotic algae (9). Current models of the CCM (17, 28) suggest that CO2 is released at a high concentration from the thylakoid tubules traversing the pyrenoid matrix. The mislocalization of Rubisco to the stroma of the epyc1 mutant could decrease the efficiency of CO2 capture by Rubisco, explaining the severe CCM defect observed in this mutant.
The observations presented here suggest that Rubisco packaging to form the matrix of the eukaryotic pyrenoid is achieved by a different mechanism than that used in the well-characterized prokaryotic β-carboxysome. In the β-carboxysome, aggregation of Rubisco is mediated by the protein CcmM (CO2 concentrating mechanism protein M). CcmM contains multiple repeats of a domain resembling the Rubisco small subunit, and incorporation of these domains into separate Rubisco holoenzymes is thought to produce a link between Rubisco holoenzymes (21). Given that the EPYC1 repeats show no homology to Rubisco and are highly disordered, it is likely that they bind to the surface of Rubisco holoenzymes rather than becoming incorporated in the place of small subunits. The simplicity of such a surface-binding mechanism potentially explains how Rubisco packaging into a pyrenoid could have evolved across a broad range of photosynthetic eukaryotes through convergent evolution (17, 29), leading to the dominant role of pyrenoids in aquatic CO2 fixation. Such a surface-binding mechanism may even organize Rubisco in prokaryotic α-carboxysomes, where the intrinsically disordered Rubisco-binding repeat protein CsoS2 plays a poorly understood role in assembly (30).
In addition to being a key structural component, EPYC1 could regulate Rubisco partitioning to the pyrenoid or Rubisco kinetic properties. The Rubisco content of the pyrenoid changes in response to CO2 (23 and our data), whereas total cellular Rubisco remains constant (Fig. 3D). Given that EPYC1 is required for Rubisco localization to the pyrenoid, changes in EPYC1 abundance and/or Rubisco-binding affinity could affect Rubisco partitioning to the pyrenoid. Consistent with this hypothesis, EPYC1 was previously found to be up-regulated at both the transcript and protein levels in response to light and low CO2 (26), and our data further support this finding (Fig. 2A and SI Appendix, Fig. S2A). Moreover, previous studies have shown that EPYC1 becomes phosphorylated at multiple sites in response to low CO2 (26, 31), potentially affecting its binding affinity for Rubisco.
Another mode of regulation of EPYC1–Rubisco binding could be through the methylation of Rubisco. Rubisco is methylated in multiple residues (32), and in Chlamydomonas, the predicted methyltransferase CIA6 is required for Rubisco localization to the pyrenoid (33). It is also possible that EPYC1 binding to Rubisco alters the kinetic properties of Rubisco to fine-tune its performance in the pyrenoid.
Along with advancing our understanding of the molecular mechanisms underlying global carbon fixation, our findings may contribute to the future engineering of crops with enhanced photosynthesis. There is great interest in introducing a CCM into C3 plants, given that this enhancement is predicted to increase yields by up to 60% and to improve the efficiency of nitrogen and water use (34). Although much remains to be done to improve our understanding of the algal CCM, recent work suggests that algal components may be relatively easy to engineer into higher plants (35). Our discovery of a possible mechanism for Rubisco assembly to form the pyrenoid is a key step toward engineering an algal CCM into crops.
Materials and Methods
Strains and Culture Conditions.
WT Chlamydomonas CC-1690 (36) was used for pyrenoid enrichment and proteomics. WT Chlamydomonas cMJ030 (CC-4533) (37) was used for all other experiments. The epyc1 mutant was isolated from a collection of high-CO2–requiring mutants generated by transformation of the pMJ016c mutagenesis cassette into cMJ030 (37). All experiments were performed under photoautotrophic conditions supplemented with high CO2 (3% or 5% vol/vol CO2-enriched air) or low CO2 (air, ∼0.04% vol/vol CO2).
Proteomics.
Pyrenoid enrichment was performed using a modified protocol based on previous studies (38, 39). In brief, cells were harvested by centrifugation (3,220 × g for 2 min at 4 °C), lysed by sonication, and then centrifuged again at 500 × g for 3 min at 4 °C to obtain a soluble fraction and a pellet fraction. Shotgun proteomics on the soluble and pellet fractions was performed as described by Mühlhaus et al. (40). Raw MS data files were processed with MaxQuant version 1.5.2.8 (41).
Cloning.
EPYC1 (Cre10.g436550) and RBCS1 (Cre02.g120100) ORFs were amplified from gDNA and cloned into pLM005 (Venus) or pLM006 (mCherry) by Gibson assembly (42).
Transformation of Chlamydomonas.
Constructs were transformed into the nuclear genome of WT and epyc1 strains by electroporation as described by Zhang et al. (37). To screen for Venus- and mCherry-expressing colonies, transformation plates were imaged with a Typhoon Trio fluorescence scanner (GE Healthcare).
Microscopy.
TEM images of the enriched pyrenoid fraction and whole cells before pyrenoid enrichment were prepared and taken according to Nordhues et al. (43). TEM imaging for pyrenoid area analysis and immunogold localization of Rubisco was based on methods described by Meyer et al. (20). QFDEEM was performed as described by Heuser (44). Fluorescence microscopy was performed using a spinning-disk confocal microscope (Leica DMI6000) with the following settings: Venus, 514 nm excitation with 543/22 nm emission; mCherry, 561 nm excitation with 590/20 nm emission; and chlorophyll, 561 nm excitation with 685/40 nm emission.
Quantitative Real-Time PCR.
EPYC1 gene transcript levels were determined by qRT-PCR. CDNA was synthesized from total RNA, and relative gene expression was measured in real time using SYBR Green. Gene expression was calculated according to the method of Livak and Schmittgen (45), relative to RCK1 (Cre06.g278222) (46). The primers used are listed in SI Appendix, Table S2.
Western Blot Analysis.
Protein levels of EPYC1 and CAH3 in WT and the epyc1 mutant were measured according to Heinnickel et al. (47). Rubisco levels were measured as described by Meyer et al. (20).
O2 Evolution and Spot Tests.
Apparent affinity for inorganic carbon was determined using the oxygen evolution method described by Badger et al. (48). Spot tests were performed by spotting serially diluted WT, epyc1, and complemented cell lines onto Tris-phosphate (TP) plates. Plates were incubated in low or high CO2 under 100 or 500 µmol photons m−2 s−1 of light for 7 d.
Coimmunoprecipitation.
Cell lysate from WT cells expressing the bait proteins (Venus-3×FLAG, EPYC1-Venus-3×FLAG, or RbcS1-Venus-3×FLAG) was incubated with anti-FLAG M2 antibody (Sigma-Aldrich) bound to protein G Dynabeads (Life Technologies). Bait proteins with interaction partners were eluted by 3×FLAG competition, followed by boiling in 1× Laemmli buffer.
EPYC1 Sequence Analysis.
To understand the intrinsic disorder of EPYC1, the full-length amino acid sequence was run through several structural disorder prediction programs, including VL3, VLTX (49), and GlobPlot 2 (50). To look for regions of secondary structure, the full-length and repeat regions of the EPYC1 amino acid sequence were analyzed by PSIPRED v3.3 (51) and Phyre2 (52).
Proteins with EPYC1-Like Physicochemical Properties in Other Algae.
Complete translated genomic sequences from pyrenoid and non-pyrenoid algae were analyzed for tandem repeats using Xstream (53). The isoelectric point, disorder profile (54), and presence of transmembrane domains (55) of Xstream hits were calculated. Proteins with three or more repeats, a pI >8, an oscillating disorder profile with a frequency between 40 and 80 amino acids, and no transmembrane domains were classified as potential EPYC1-like Rubisco linker proteins.
More detailed information on the materials and methods used in this study is provided in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank J. N. Skepper, L. Carter, and M. Rütgers for TEM support, discussions on immunogold optimization, and ultramicrotomy; H. Cartwright for confocal microscopy support; S. Ramundo for technical advice with coimmunoprecipitation; W. Patena for help with data analysis; and W. Frommer, V. Walbot, P. Walter, and T. Cuellar for comments on the manuscript. The project was funded by National Science Foundation Grants EF-1105617 and IOS-1359682 (to L.C.M.M., L.P., G.R., and M.C.J.); the Carnegie Institution for Science (L.C.M.M. and M.C.J.); National Institutes of Health Grant T32GM007276 (to E.S.F.R., V.K.C., and A.I.); Biotechnology and Biological Sciences Research Council Grant BB/M007693/1 (to M.T.M. and H.G.); the Federal Ministry of Education and Research, Germany, within the frame of the GoFORSYS Research Unit for Systems Biology (Grant FKZ 0313924, to T.M.-A., F.S., M. Schroda, and M. Stitt); and the International Max Planck Research School of the Max Planck Society (T.M.-A. and T.M.).
Footnotes
Conflict of interest statement: The authors wish to note that the Carnegie Institution for Science has submitted a provisional patent application on aspects of the findings.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1522866113/-/DCSupplemental.
References
- 1.Ellis RJ. The most abundant protein in the world. Trends Biochem Sci. 1979;4(11):241–244. [Google Scholar]
- 2.Falkowski PG, Barber RT, Smetacek V. Biogeochemical controls and feedbacks on ocean primary production. Science. 1998;281(5374):200–207. doi: 10.1126/science.281.5374.200. [DOI] [PubMed] [Google Scholar]
- 3.Field CB, Behrenfeld MJ, Randerson JT, Falkowski P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science. 1998;281(5374):237–240. doi: 10.1126/science.281.5374.237. [DOI] [PubMed] [Google Scholar]
- 4.Dismukes GC, et al. The origin of atmospheric oxygen on Earth: The innovation of oxygenic photosynthesis. Proc Natl Acad Sci USA. 2001;98(5):2170–2175. doi: 10.1073/pnas.061514798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Somerville CR, Ogren WL. A phosphoglycolate phosphatase-deficient mutant of Arabidopsis. Nature. 1979;280(5725):833–836. [Google Scholar]
- 6.Bauwe H, Hagemann M, Fernie AR. Photorespiration: Players, partners and origin. Trends Plant Sci. 2010;15(6):330–336. doi: 10.1016/j.tplants.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Sage RF, Sage TL, Kocacinar F. Photorespiration and the evolution of C4 photosynthesis. Annu Rev Plant Biol. 2012;63:19–47. doi: 10.1146/annurev-arplant-042811-105511. [DOI] [PubMed] [Google Scholar]
- 8.Giordano M, Beardall J, Raven JA. CO2 concentrating mechanisms in algae: Mechanisms, environmental modulation, and evolution. Annu Rev Plant Biol. 2005;56:99–131. doi: 10.1146/annurev.arplant.56.032604.144052. [DOI] [PubMed] [Google Scholar]
- 9.Badger MR, et al. The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast-based CO2-concentrating mechanisms in algae. Can J Bot. 1998;76(6):1052–1071. [Google Scholar]
- 10.Schmitz F. Die Chromatophoren der Algen: Vergleichende Untersuchungen über Bau und Entwicklung der Chlorophyllkörper und der analogen Farbstoffkörper der Algen. M. Cohen & Sohn; Bonn, Germany: 1882. [Google Scholar]
- 11.Vaucher J-P. Histoire des Conferves D’eau Douce: Contenant Leurs Différents Modes De Reproduction, Et La Description De Leurs Principales Espèces, Suivie De L’histoire Des Trémelles Et Des Ulves D’eau Douce. JJ Paschoud; Geneva, Switzerland: 1803. [Google Scholar]
- 12.Brown R. Pyrenoid: Its structure distribution and function. J Phycol. 1967;3(Suppl 1):5–7. [Google Scholar]
- 13.Behrenfeld MJ, et al. Biospheric primary production during an ENSO transition. Science. 2001;291(5513):2594–2597. doi: 10.1126/science.1055071. [DOI] [PubMed] [Google Scholar]
- 14.Rousseaux CS, Gregg WW. Interannual variation in phytoplankton primary production at a global scale. Remote Sens. 2013;6(1):1–19. [Google Scholar]
- 15.Mann GD. Chloroplast morphology, movements and inheritance in diatoms. In: Chaudhary BR, Agrawal SB, editors. Cytology, Genetics and Molecular Biology of Algae. SPB Academic Publishing; Amsterdam: 1996. pp. 249–274. [Google Scholar]
- 16.Thierstein HR, Young JR, editors. Coccolithophores: From Molecular Processes to Global Impact. Springer; Heidelberg, Germany: 2004. [Google Scholar]
- 17.Meyer M, Griffiths H. Origins and diversity of eukaryotic CO2-concentrating mechanisms: Lessons for the future. J Exp Bot. 2013;64(3):769–786. doi: 10.1093/jxb/ers390. [DOI] [PubMed] [Google Scholar]
- 18.Engel BD, et al. Native architecture of the Chlamydomonas chloroplast revealed by in situ cryo-electron tomography. eLife. 2015;4:e04889. doi: 10.7554/eLife.04889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKay RML, Gibbs SP. Composition and function of pyrenoids: Cytochemical and immunocytochemical approaches. Can J Bot. 1991;69(5):1040–1052. [Google Scholar]
- 20.Meyer MT, et al. Rubisco small-subunit α-helices control pyrenoid formation in Chlamydomonas. Proc Natl Acad Sci USA. 2012;109(47):19474–19479. doi: 10.1073/pnas.1210993109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long BM, Badger MR, Whitney SM, Price GD. Analysis of carboxysomes from Synechococcus PCC7942 reveals multiple Rubisco complexes with carboxysomal proteins CcmM and CcaA. J Biol Chem. 2007;282(40):29323–29335. doi: 10.1074/jbc.M703896200. [DOI] [PubMed] [Google Scholar]
- 22.Berry J, Boynton J, Kaplan A, Badger M. Carnegie Institution of Washington Year Book. Vol 75. Carnegie Institution of Washington; Washington, DC: 1976. Growth and photosynthesis of Chlamydomonas reinhardtii as a function of CO2 concentration; pp. 423–432. [Google Scholar]
- 23.Borkhsenious ON, Mason CB, Moroney JV. The intracellular localization of ribulose-1,5-bisphosphate carboxylase/oxygenase in Chlamydomonas reinhardtii. Plant Physiol. 1998;116(4):1585–1591. doi: 10.1104/pp.116.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miura K, et al. Expression profiling-based identification of CO2-responsive genes regulated by CCM1 controlling a carbon-concentrating mechanism in Chlamydomonas reinhardtii. Plant Physiol. 2004;135(3):1595–1607. doi: 10.1104/pp.104.041400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwanhäusser B, et al. Global quantification of mammalian gene expression control. Nature. 2011;473(7347):337–342. doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- 26.Turkina MV, Blanco-Rivero A, Vainonen JP, Vener AV, Villarejo A. CO2 limitation induces specific redox-dependent protein phosphorylation in Chlamydomonas reinhardtii. Proteomics. 2006;6(9):2693–2704. doi: 10.1002/pmic.200500461. [DOI] [PubMed] [Google Scholar]
- 27.Dunker AK, Silman I, Uversky VN, Sussman JL. Function and structure of inherently disordered proteins. Curr Opin Struct Biol. 2008;18(6):756–764. doi: 10.1016/j.sbi.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Stessman DJ, Spalding MH. The CO2-concentrating mechanism and photosynthetic carbon assimilation in limiting CO2: How Chlamydomonas works against the gradient. Plant J. 2015;82(3):429–448. doi: 10.1111/tpj.12829. [DOI] [PubMed] [Google Scholar]
- 29.Villarreal JC, Renner SS. Hornwort pyrenoids, carbon-concentrating structures, evolved and were lost at least five times during the last 100 million years. Proc Natl Acad Sci USA. 2012;109(46):18873–18878. doi: 10.1073/pnas.1213498109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai F, et al. Advances in understanding carboxysome assembly in Prochlorococcus and Synechococcus implicate CsoS2 as a critical component. Life (Basel) 2015;5(2):1141–1171. doi: 10.3390/life5021141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H, et al. The global phosphoproteome of Chlamydomonas reinhardtii reveals complex organellar phosphorylation in the flagella and thylakoid membrane. Mol Cell Proteomics. 2014;13(9):2337–2353. doi: 10.1074/mcp.M114.038281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor TC, Backlund A, Bjorhall K, Spreitzer RJ, Andersson I. First crystal structure of Rubisco from a green alga, Chlamydomonas reinhardtii. J Biol Chem. 2001;276(51):48159–48164. doi: 10.1074/jbc.M107765200. [DOI] [PubMed] [Google Scholar]
- 33.Ma Y, Pollock SV, Xiao Y, Cunnusamy K, Moroney JV. Identification of a novel gene, CIA6, required for normal pyrenoid formation in Chlamydomonas reinhardtii. Plant Physiol. 2011;156(2):884–896. doi: 10.1104/pp.111.173922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Long SP, Marshall-Colon A, Zhu XG. Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell. 2015;161(1):56–66. doi: 10.1016/j.cell.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 35.Atkinson N, et al. Introducing an algal carbon-concentrating mechanism into higher plants: Location and incorporation of key components. Plant Biotechnol J. 2015 doi: 10.1111/pbi.12497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sager R. Inheritance in the green alga Chlamydomonas reinhardi. Genetics. 1955;40(4):476–489. doi: 10.1093/genetics/40.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang R, et al. High-throughput genotyping of green algal mutants reveals random distribution of mutagenic insertion sites and endonucleolytic cleavage of transforming DNA. Plant Cell. 2014;26(4):1398–1409. doi: 10.1105/tpc.114.124099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuchitsu K, Tsuzuki M, Miyachi S. Changes of starch localization within the chloroplast induced by changes in CO2 concentration during growth of Chlamydomonas reinhardtii: Independent regulation of pyrenoid starch and stroma starch. Plant Cell Physiol. 1988;29(8):1269–1278. [Google Scholar]
- 39.Kuchitsu K, Tsuzuki M, Miyachi S. Polypeptide composition and enzyme activities of the pyrenoid and its regulation by CO2 concentration in unicellular green algae. Can J Bot. 1991;69(5):1062–1069. [Google Scholar]
- 40.Mühlhaus T, Weiss J, Hemme D, Sommer F, Schroda M. Quantitative shotgun proteomics using a uniform 15N-labeled standard to monitor proteome dynamics in time course experiments reveals new insights into the heat stress response of Chlamydomonas reinhardtii. Mol Cell Proteom. 2011;10(9):M110.004739. doi: 10.1074/mcp.M110.004739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies, and proteome-wide protein quantification. Nat Biotechnol. 2008;26(12):1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 42.Gibson DG, et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods. 2009;6(5):343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 43.Nordhues A, et al. Evidence for a role of VIPP1 in the structural organization of the photosynthetic apparatus in Chlamydomonas. Plant Cell. 2012;24(2):637–659. doi: 10.1105/tpc.111.092692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heuser JE. The origins and evolution of freeze-etch electron microscopy. J Electron Microsc (Tokyo) 2011;60(Suppl 1):S3–S29. doi: 10.1093/jmicro/dfr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 46.Schloss JA. A Chlamydomonas gene encodes a G protein β subunit-like polypeptide. Mol Gen Genet. 1990;221(3):443–452. doi: 10.1007/BF00259410. [DOI] [PubMed] [Google Scholar]
- 47.Heinnickel ML, et al. Novel thylakoid membrane GreenCut protein CPLD38 impacts accumulation of the cytochrome b6f complex and associated regulatory processes. J Biol Chem. 2013;288(10):7024–7036. doi: 10.1074/jbc.M112.427476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Badger MR, Kaplan A, Berry JA. Internal inorganic carbon pool of Chlamydomonas reinhardtii: Evidence for a carbon dioxide-concentrating mechanism. Plant Physiol. 1980;66(3):407–413. doi: 10.1104/pp.66.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Obradovic Z, et al. Predicting intrinsic disorder from amino acid sequence. Proteins. 2003;53(Suppl 6):566–572. doi: 10.1002/prot.10532. [DOI] [PubMed] [Google Scholar]
- 50.Linding R, Russell RB, Neduva V, Gibson TJ. GlobPlot: Exploring protein sequences for globularity and disorder. Nucleic Acids Res. 2003;31(13):3701–3708. doi: 10.1093/nar/gkg519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buchan DW, Minneci F, Nugent TC, Bryson K, Jones DT. Scalable web services for the PSIPRED Protein Analysis Workbench. Nucleic Acids Res. 2013;41(Web Server issue) W1:W349–W357. doi: 10.1093/nar/gkt381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10(6):845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Newman AM, Cooper JB. XSTREAM: A practical algorithm for identification and architecture modeling of tandem repeats in protein sequences. BMC Bioinformatics. 2007;8(1):382. doi: 10.1186/1471-2105-8-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Romero P, et al. Sequence complexity of disordered protein. Proteins. 2001;42(1):38–48. doi: 10.1002/1097-0134(20010101)42:1<38::aid-prot50>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 55.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J Mol Biol. 2001;305(3):567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.