Significance
Human activities have greatly increased emissions of reactive forms of nitrogen to the atmosphere. This perturbation to the nitrogen cycle has produced large increases of nitrogen deposition to sensitive ecosystems. Over recent decades, attention has focused on wet and dry deposition of nitrate stemming from fossil fuel combustion emissions of nitrogen oxides. Successful decreases in nitrogen oxides emissions in the United States have substantially decreased nitrate deposition. By contrast, emissions of ammonia, an unregulated air pollutant, and resulting deposition of ammonium have grown. Expanded observations demonstrate that deposition of reactive nitrogen in the United States has shifted from a nitrate-dominated to an ammonium-dominated condition. Recognition of this shift is critical to formulating effective future policies to protect ecosystems from excess nitrogen deposition.
Keywords: ammonia, dry deposition, wet deposition, nitrogen oxides, agriculture
Abstract
Rapid development of agriculture and fossil fuel combustion greatly increased US reactive nitrogen emissions to the atmosphere in the second half of the 20th century, resulting in excess nitrogen deposition to natural ecosystems. Recent efforts to lower nitrogen oxides emissions have substantially decreased nitrate wet deposition. Levels of wet ammonium deposition, by contrast, have increased in many regions. Together these changes have altered the balance between oxidized and reduced nitrogen deposition. Across most of the United States, wet deposition has transitioned from being nitrate-dominated in the 1980s to ammonium-dominated in recent years. Ammonia has historically not been routinely measured because there are no specific regulatory requirements for its measurement. Recent expansion in ammonia observations, however, along with ongoing measurements of nitric acid and fine particle ammonium and nitrate, permit new insight into the balance of oxidized and reduced nitrogen in the total (wet + dry) US nitrogen deposition budget. Observations from 37 sites reveal that reduced nitrogen contributes, on average, ∼65% of the total inorganic nitrogen deposition budget. Dry deposition of ammonia plays an especially key role in nitrogen deposition, contributing from 19% to 65% in different regions. Future progress toward reducing US nitrogen deposition will be increasingly difficult without a reduction in ammonia emissions.
Beginning in the mid-20th century, emissions of anthropogenic reactive nitrogen (Nr) to the atmosphere accelerated rapidly due to increased fossil fuel combustion and intensive agricultural activities (1–4). Once emitted to the atmosphere, Nr compounds are deposited to terrestrial and aquatic ecosystems through dry and wet processes. Although nitrogen is an essential and often limiting element for ecosystems, increases in Nr deposition resulting from increased emissions have raised concerns around the world due to its adverse environmental impacts, including decreased biological diversity, increased soil acidification, and lake eutrophication (5–9). Critical loads (CLs) have been widely used to quantify levels of Nr deposition that ecosystems can sustain without significant harmful effects (10, 11). Twenty-four of the 45 national parks in the contiguous United States were estimated to receive Nr deposition in 2013 exceeding the local CL (12). Atmospheric Nr (an important ingredient of ozone and fine particle formation) has also been linked with climate change and human health degradation (8, 13, 14).
Atmospheric Nr sources are dominated by emissions of nitrogen oxides (NOx = NO + NO2) and ammonia (NH3) (8). NOx is produced by a wide range of high temperature processes including lightning and the combustion of fossil fuels by vehicles, electric power generating units, and other industrial and natural combustion sources. NOx is oxidized in the atmosphere and converted to a variety of forms, including nitric acid, with a short timescale (typically 1 d or less). Reis et al. (15) attributed more than 80% of the NH3 emissions in the United States to the agricultural sector, including emissions from livestock waste and volatilization of N-based fertilizer.
During the last two decades, US NOx emissions have steadily declined due to effective regulations designed to decrease NOx contributions to ozone, fine particles, and acid deposition (16). Data from the National Emissions Inventory (NEI) Air Pollutant Emissions Trends (https://www.epa.gov/air-emissions-inventories/air-pollutant-emissions-trends-data) indicate that NOx emissions decreased by nearly 41% from 1990 to 2010. Further reductions are expected in coming years due to additional policy actions (e.g., the Cross-State Air Pollution Rule, reductions in mobile source emissions, and 5-y reviews of the National Ambient Air Quality Standards). Meanwhile, NH3 emissions have been reported to increase by 11% between 1990 and 2010 (17), with contributing factors including regional growth in livestock numbers and increased application of NOx controls such as selective catalytic reduction. Unlike NOx, NH3 emissions in the United States are not regulated. In 2006, US anthropogenic NH3 emissions were estimated at 2.8 Tg N/y; they are projected to increase to between 3.3 and 4.2 Tg N/y by 2050, mainly due to increases in N fertilizer application and livestock growth (12).
Through dry deposition processes, gaseous HNO3 and NH3 are rapidly removed from the atmosphere and deposited to surface ecosystems. HNO3 and NH3 can both be incorporated into atmospheric particles; this includes their reaction with each other to form fine particle ammonium nitrate, reaction of ammonia with sulfuric acid to form fine particle ammonium sulfate, and reactions of nitric acid with soil dust or sea salt to form coarse particle nitrate (18), among other species. These aerosol particles can also deposit nitrate and/or ammonium via dry deposition. Nitric acid, ammonia, and particulate nitrate and ammonium are scavenged by clouds and precipitation, producing wet deposition of ammonium and nitrate. HNO3 and NO3− are generally referred to as oxidized N, whereas NH3 and NH4+ are termed reduced N; both oxidized and reduced N represent significant Nr inputs in unmanaged ecosystems (19, 20).
Publications from the 1990s and early 2000s recognized that deposition of oxidized N dominated the US atmospheric reactive N deposition budget (21, 22). With the ensuing emissions reductions of NOx and emissions increases of NH3, relative contributions of oxidized and reduced N have likely changed in recent years. Modeling studies (12, 23) have suggested this change and point to its likely continuation. A recent analysis of US National Atmospheric Deposition Program (NADP) observations (24) illustrates decreases in US wet nitrate deposition and increases in wet ammonium deposition in many regions. Focusing on the Midwest and Eastern United States, Sickles and Shadwick (25) reported that reductions in NOx emissions have decreased oxidized nitrogen dry and wet deposition and that particulate ammonium concentrations now exceed concentrations of particulate nitrate plus gaseous nitric acid in the region. Ammonia gas was not considered in their study.
From an observational perspective, the absence of gaseous ammonia observations has prevented a broad, national understanding of the overall contributions of oxidized and reduced forms of inorganic N to the total Nr deposition budget. Making use of longer-term wet and dry deposition records and newly available NH3 measurements from regions across the country, we examine the overall contributions of oxidized and reduced forms of inorganic nitrogen to wet, dry, and total deposition budgets to better inform discussions of strategies to decrease N deposition to sensitive ecosystems.
Results and Discussion
Analyses of wet deposition records provide important insight into the shift from oxidized to reduced nitrogen deposition across the contiguous United States. Recently expanded measurements of gas and particle phase reactive nitrogen species permit an assessment of current contributions of oxidized and reduced nitrogen to the US Nr dry deposition budget. By combining these analyses, we gain a better understanding of the importance of both oxidized and reduced nitrogen to the total (wet + dry) Nr deposition budget across much of the United States.
Oxidized vs. Reduced N in Wet N Deposition.
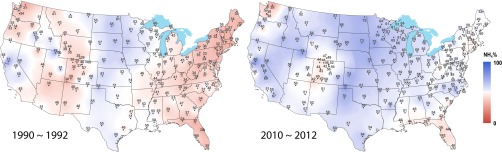
Although wet N deposition was dominated by oxidized N (NO3−) across much of the country in the early 1990s, most locations now receive a majority of their wet N deposition as reduced N (NH4+) (Fig. 1), a trend also recently reported by Du et al. (24). During the period 1990–1992, 69% of the observation sites were subjected to oxidized N contributions in excess of 50%; 20 y later, 69% of the sites instead received wet deposition of reduced N greater than 50%.
Fig. 1.
Comparisons of the 3-y average NH4+ percentage of wet inorganic nitrogen deposition across the United States in 1990–1992 (Left) and 2010–2012 (Right). To help visualize spatial patterns, isopleths were produced by interpolating NH4+ mole percentages at individual monitoring sites using a cubic inverse-distance weighting of sites within 500 km of each observation station. The black dots on the map represent locations of sites with 3-y data available for each time period. The NH4+ percentage on a molar basis [(NH4+%) = (NH4+)/(NO3− + NH4+) × 100%] is noted at each site.
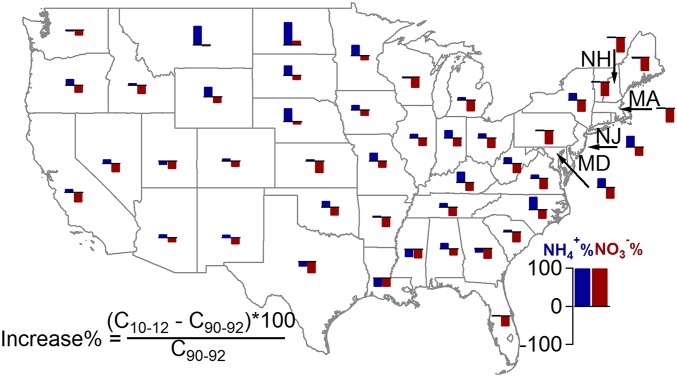
Changes in fractional contributions of oxidized and reduced N depend on the combined changes in wet deposition fluxes of NH4+ and NO3−. Fig. 2 examines these changes for 45 of the 48 contiguous United States with available data. In every state but North Dakota, nitrate wet deposition fluxes decreased, with an average decrease of 29%. The nationwide decrease of oxidized N in wet deposition is consistent with the downward trend of US NOx emissions. With the successful implementation of the Clean Air Act (CAA) and the 1990 Amendments, NOx emissions were estimated to decline by 36% between 1990 and 2008 (13). Nitrate wet deposition decreases were largest in the Northeastern United States, an area where large NOx emissions reductions were implemented. Lehmann and Gay (26) examined trends in nitrate concentrations in US wet deposition in detail for a period ending in 2009 and also highlight large reductions in the Northeastern United States.
Fig. 2.
Absolute percentage change of NH4+ and NO3− in wet deposition across the country. C10–12 is the average NH4+ or NO3− flux (kg N/ha per year) in each state between 2010 and 2012 and C90–92 is the average NH4+ or NO3− flux (kg N/ha per year) between 1990 and 1992. Only sites in Fig. 1 with both 1990–1992 and 2010–2012 data available are used to calculate the average flux for each state.
Thirty-seven of 45 states experienced increased ammonium wet deposition over the last two decades; for these states, the average increase was 22% (Fig. 2). Increases in ammonium wet deposition were especially common in the northern plains states; relatively large increases were also seen in North Carolina, Kentucky, Maryland, and New Jersey. Substantial increases in ammonium ion concentrations in precipitation in the Central and Western United States were previously reported through 2004 by Lehmann et al. (27). The increasing NH4+ wet deposition is broadly consistent with the estimates of increasing NH3 emissions since the 1990s (17).
Oxidized vs. Reduced Dry Inorganic N Deposition.
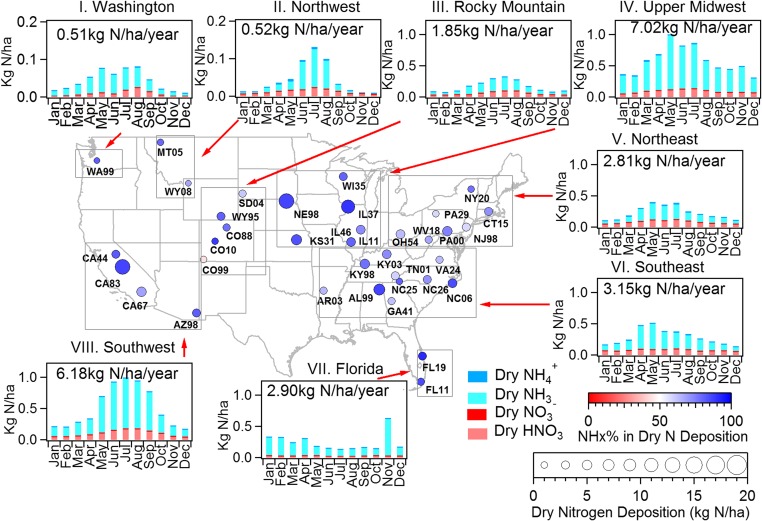
Gas phase nitric acid and ammonia and particulate ammonium and nitrate are potentially important contributors to dry inorganic N deposition. Limited historical measurements, especially for ammonia, prevent an analysis of long-term trends of oxidized vs. reduced dry inorganic nitrogen deposition like those presented above for wet deposition. Recent efforts to measure gas phase ammonia concentrations more routinely by the NADP Ammonia Monitoring Network (AMoN) and Interagency Monitoring of Protected Visual Environments (IMPROVE) NHx networks, however, allow comparison of the current balance between oxidized and reduced inorganic N dry deposition. We focus here on characterizing spatial patterns for the period 2011–2013. Fig. 3 illustrates (by circle size) the current magnitude of dry inorganic N deposition across the United States. Significant spatial variability is seen from site to site, reflecting difference in species concentrations. Estimated annual sums of dry deposition by gaseous ammonia and nitric acid and particulate ammonium and nitrate range from 0.49 (WY08) to 13.4 kg N/ha per year (NE98). Reduced N contributes more than 50% of the total calculated dry inorganic N deposition at all sites except Mesa Verde National Park (CO99; 44%) in southwest Colorado. This remote arid site is expected to have relatively small agricultural impacts (28) but greater influence of NOx emitted from nearby oil and gas development (29) and the large, coal-fired Four Corners and San Juan power plants. The highest fractional and absolute reduced N contributions are seen, not surprisingly, in areas with substantial agricultural activity, including sites in Illinois (IL37 exhibits the highest reduced N fraction at 90%), Nebraska, and the Central Valley of California.
Fig. 3.
Spatial and temporal trends in dry inorganic N deposition at 37 locations across the United States. Included are deposition of gaseous nitric acid and ammonia and PM2.5 ammonium and nitrate. Fractional reduced N contributions are represented by circle color. The total deposition from these four species is indicated by circle size. The bar charts depict monthly average contributions of individual dry reduced and oxidized N deposition pathways for eight selected regions. The average total dry inorganic N deposition fluxes in different regions are shown by the number in each figure.
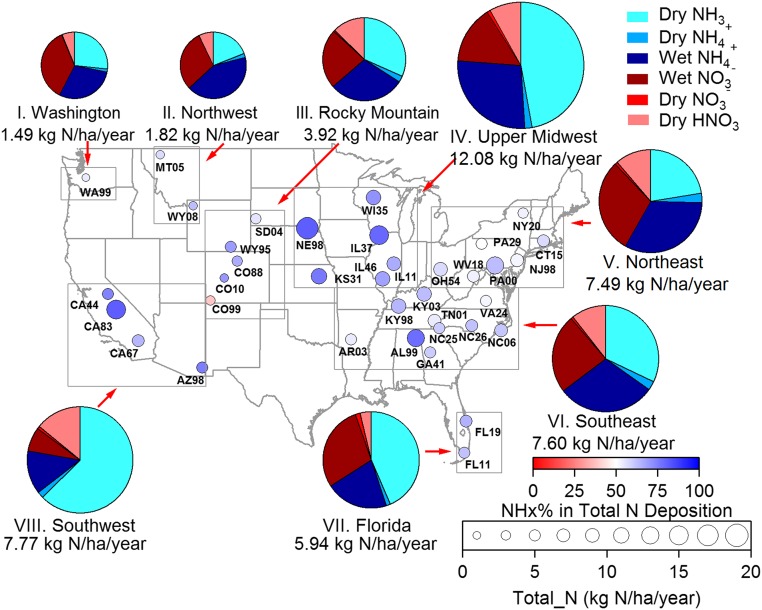
To examine overall dry deposition patterns, sites were grouped into eight regions (by proximity and similar trends) as follows (Table S1 and Fig. 3): Washington (I), Northwest (II; Montana and northern Wyoming), Rocky Mountain (III; western South Dakota and southern Wyoming, CO), Upper Midwest (IV; Wisconsin, Illinois, eastern Kansas, and eastern Nebraska), Northeast (V; New York, Connecticut, New Jersey, Pennsylvania, Ohio, and West Virginia), Southeast (VI; Kentucky, Virginia, Tennessee, North Carolina, Georgia, Alabama, and Arkansas), Florida (VII), and Southwest (VIII; California and southern Arizona). The lowest regional average dry N deposition flux was found in the Washington region (0.51 kg N/ha per year) and the highest in the Upper Midwest (7.0 kg N/ha per year), one of the nation’s primary food production areas with large NH3 emissions from livestock and fertilizer use.
Table S1.
| Region | Site_ID | Site name | State | Latitude (°N) | Longitude (°W) | Period of record | AMoN site | CASTNET site | NTN site | IMPROVE NHx site |
| Washington (I) | WA99 | Mount Rainier National Park | WA | 46.7582 | −122.124 | 07/11∼06/13 | WA99 | MOR409 | WA99 | |
| Northwest (II) | MT05 | Glacier National Park | MT | 48.5105 | −113.997 | 07/11∼06/12 | GLR468 | MT05 | GLACS | |
| WY08 | Yellowstone National Park | WY | 44.5653 | −110.4 | 07/11∼06/12 | YELL408 | WY08 | YELLS | ||
| Rocky Mountain (III) | CO10 | Gothic | CO | 38.9561 | −106.986 | 09/12∼06/13 | CO10 | GTH161 | CO10 | |
| CO88 | Rocky Mountain National Park | CO | 40.2778 | −105.545 | 07/11∼06/13 | CO88 | ROM406 | CO19 | ||
| CO99 | Mesa Verde National Park | CO | 37.1984 | −108.491 | 07/11∼06/12 | MEV405 | CO99 | MEVES | ||
| SD04 | Wind Cave | SD | 43.5576 | −103.484 | 07/11∼06/12 | WNC429 | SD04 | WICAS | ||
| WY95 | Brooklyn Lake | WY | 41.3647 | −106.241 | 07/12∼06/13 | WY95 | CNT169 | WY95 | ||
| Upper Midwest (IV) | IL11 | Bondville | IL | 40.0528 | −88.3719 | 07/11∼06/13 | IL11 | BVL130 | IL11 | |
| IL37 | Stockton | IL | 42.2869 | −89.9997 | 07/11∼06/13 | IL37 | STK138 | IL18 | ||
| IL46 | Alhambra | IL | 38.8689 | −89.6219 | 07/11∼06/13 | IL46 | ALH157 | IL46 | ||
| KS31 | Konza Prairie | KS | 39.1022 | −96.6092 | 07/11∼06/13 | KS31 | KNZ184 | KS31 | ||
| NE98 | Santee | NE | 42.8292 | −97.8541 | 07/11∼06/13 | NE98 | SAN189 | SD99 | ||
| WI35 | Perkinstown | WI | 45.2064 | −90.5978 | 07/11∼06/13 | WI35 | PRK134 | WI35 | ||
| Northeast (V) | CT15 | Abington | CT | 41.84 | −72.0101 | 07/11∼06/13 | CT15 | ABT147 | CT15 | |
| NJ98 | Washington Crossing | NJ | 40.3125 | −74.8729 | 07/11∼06/13 | NJ98 | WSP144 | NJ99 | ||
| NY20 | Huntington Wildlife | NY | 43.9731 | −74.2231 | 07/12∼06/13 | NY20 | HWF187 | NY20 | ||
| OH54 | Deer Creek State Park | OH | 39.6359 | −83.2606 | 07/11∼06/13 | OH54 | DCP114 | OH54 | ||
| PA00 | Arendtsville | PA | 39.9231 | −77.3078 | 07/11∼06/13 | PA00 | ARE128 | PA00 | ||
| PA29 | Kane Experimental Forest | PA | 41.5978 | −78.7675 | 07/11∼06/13 | PA29 | KEF112 | PA29 | ||
| WV18 | Parsons | WV | 39.0897 | −79.6622 | 07/11∼06/13 | WV18 | PAR107 | WV18 | ||
| Southeast (VI) | AL99 | Sand Mountain Research & Extension Center | AL | 34.2886 | −85.9699 | 07/11∼06/13 | AL99 | SND152 | AL99 | |
| AR03 | Caddo Valley | AR | 34.1795 | −93.0992 | 07/11∼06/13 | AR03 | CAD150 | AR03 | ||
| GA41 | Georgia Station | GA | 33.1805 | −84.4103 | 07/11∼06/13 | GA41 | GAS153 | GA41 | ||
| KY03 | Mackville | KY | 37.7047 | −85.0489 | 07/11∼06/13 | KY03 | MCK131 | KY03 | ||
| KY98 | Cadiz | KY | 36.7841 | −87.8499 | 07/11∼06/13 | KY98 | CDZ171 | KY99 | ||
| NC06 | Beaufort | NC | 34.8846 | −76.6207 | 07/11∼06/13 | NC06 | BFT142 | NC06 | ||
| NC25 | Coweeta | NC | 35.0605 | −83.4305 | 07/11∼06/13 | NC25 | COW137 | NC25 | ||
| NC26 | Candor | NC | 35.2632 | −79.8365 | 07/11∼06/13 | NC26 | CND125 | NC36 | ||
| TN01 | Great Smoky Mountains National Park | TN | 35.6331 | −83.9422 | 07/11∼06/13 | TN01 | GRS420 | TN01 | ||
| VA24 | Prince Edward | VA | 37.1652 | −78.3073 | 07/11∼06/13 | VA24 | PED108 | VA24 | ||
| Florida (VII) | FL11 | Everglades National Park | FL | 25.39 | −80.68 | 07/11∼06/13 | FL11 | EVE419 | FL11 | |
| FL19 | Indian River | FL | 27.8492 | −80.4554 | 07/11∼06/13 | FL19 | IRL141 | FL99 | ||
| Southwest (VIII) | AZ98 | Chiricahua National Monument | AZ | 32.0097 | −109.389 | 07/11∼06/13 | AZ98 | CHA467 | AZ98 | |
| CA44 | Yosemite National Park | CA | 37.7133 | −119.706 | 07/11∼06/13 | CA44 | YOS404 | CA99 | ||
| CA67 | Joshua Tree National Park | CA | 34.0695 | −116.389 | 07/11∼06/13 | CA67 | JOT403 | CA67 | ||
| CA83 | Sequoia National Park | CA | 36.4894 | −118.823 | 07/11∼06/13 | CA83 | SEK430 | CA75 |
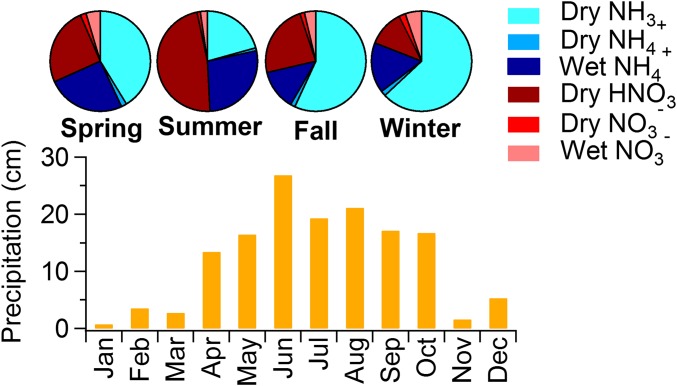
In most regions, dry ammonia and nitric acid deposition display strong seasonal patterns, with higher values in summer and lower values in winter. These seasonal patterns are driven mostly by seasonal concentration patterns rather than changes in deposition velocity. Ammonia emissions increase with warmer summertime temperatures due to enhanced volatilization (30, 31). Active summertime photochemistry speeds conversion of NOx to nitric acid, whereas warmer summertime temperatures reduce formation of particulate ammonium nitrate, leaving more nitric acid and ammonia in the gas phase (32). Interestingly, dry NH3 deposition is elevated during the winter in the Upper Midwest compared with other regions. Higher winter ammonia concentrations in this region might reflect trapping of cold season ammonia emissions (from livestock and/or winter fertilizer application) near the surface by a shallow boundary layer (28). Dry N deposition exhibits a winter seasonal maximum in Florida. Increased summertime precipitation here suppresses summertime atmospheric concentrations and therefore dry deposition of reduced and oxidized N species. In Florida wet N deposition contributed more than 75% of total (wet + dry) inorganic N deposition during summer when there was more precipitation (Fig. S1); dry deposition of reduced N was the dominant input during the drier winter season.
Fig. S1.
Pie charts of seasonal N deposition species pathways (Upper) and total monthly measured precipitation (Lower) in Florida area (FL11 and FL19).
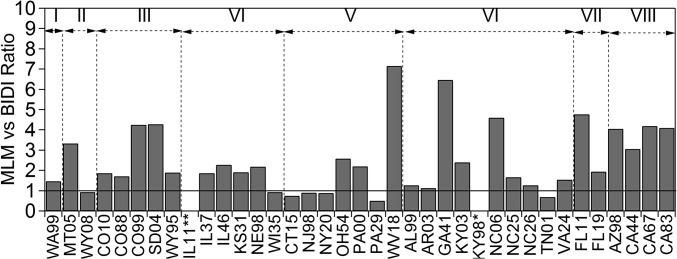
At the annual scale, ammonia dry deposition rates estimated using the multilayer model (MLM) approach are larger than those derived from the bidirectional model by a factor of 1.90 (median MLM/bidirectional flux ratio of 35 sites listed in Fig. 4). A reduction in NH3 dry deposition rates, relative to the unidirectional flux framework, was also observed on implementation of NH3 bidirectionality in the Community Multiscale Air Quality Model (33). MLM vs. bidirectional model differences vary across regions but generally result from stomatal and ground compensation points, as well as the effects of surface acidity, represented in the bidirectional framework. The net result of these processes is to reduce the atmosphere-surface NH3 concentration gradient, and therefore the flux, relative to the unidirectional MLM deposition velocity approach, which assumes a zero surface concentration. Model differences are generally greatest in summer (Fig. S2) when temperature driven stomatal and soil compensation points are at a maximum. On average, the bidirectional and MLM approaches yield comparable net fluxes during winter when compensation points are lowest and surfaces are more acidic. Further discussion of the MLM vs. the bidirectional model is included in SI Methods.
Fig. 4.
Ratio of annual NH3 dry deposition rates estimated using the MLM vs. bidirectional approaches. Regions are indicated at top of graph. *Due to a lack of meteorological data, the bidirectional flux model is not parameterized appropriately for site KY98. **Due to vegetation type, the bidirectional flux model is not parameterized appropriately for site IL11.
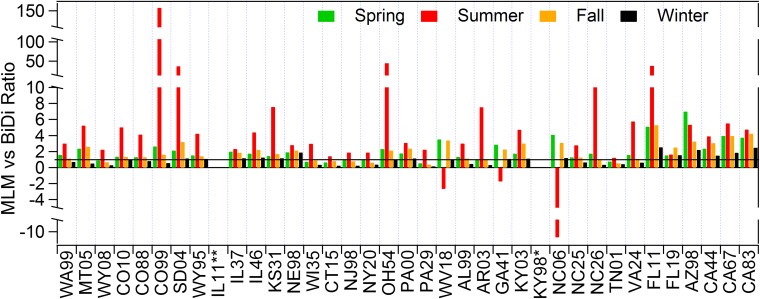
Fig. S2.
Seasonal ratios of MLM vs. bidirectional NH3 dry deposition estimates. *Due to lack of meteorological data, bidirectional flux model is not parameterized appropriately for the KY98 site. **Due to feature of surface plants, bidirectional flux model is not parameterized appropriately for the IL11 site.
The relatively large overall differences between the MLM and bidirectional NH3 flux estimates warrant brief discussion of the significant uncertainties that persist in modeling dry deposition of reactive nitrogen. MLM HNO3 deposition velocities, on which the NH3 deposition velocity used here is based, may contain up to ±25% uncertainty related to error in the measurements that drive the model and underlying process parameterizations (34). Models of HNO3 deposition velocity also differ substantially themselves. For example, a multisite evaluation of the MLM and Big Leaf Model used in the Canadian Air and Precipitation Monitoring Network (CAPMoN) showed a median bias in hourly HNO3 deposition velocities of −35%, with MLM yielding lower values (35). Regarding bidirectional NH3 models, comparisons to average measured NH3 fluxes over nonagricultural ecosystems generally demonstrate agreement within ±30% using site-specific process parameterizations (36–39), although differences can be much larger under specific meteorological and surface conditions. Uncertainty may also be significantly larger when applying generalized parameterizations as done here. In that regard, recent versions of bidirectional models (40–42) have not yet been rigorously compared with each other or against flux measurements for natural ecosystems in North America. Although our analyses use commonly used approaches for both HNO3 deposition velocity and bidirectional NH3 flux, the abovementioned uncertainties are included to emphasize that the dry deposition component of the N deposition budget is significantly more uncertain than the wet fraction, a point that should be considered in the interpretation of our results.
Fractional Reduced N Contributions to the Total Inorganic N Deposition Budget.
With wet and dry deposition estimates available for 37 locations, the total wet plus dry nitrogen deposition budgets can be estimated across the United States (Fig. 5). Fractional deposition contributions by each wet and dry deposition pathway for each of the eight regions are also illustrated in Fig. 5. Reduced N deposition fractions in the eight regions range from 58% (Washington, I) to 78% (Southwest, VIII), with dry NH3 deposition alone contributing between 19% (Northwest, II) and 63% (Southwest, VIII). Fractional reduced N contributions at individual sites range from 42% at CO99 (Mesa Verde National Park) to 84% at CA83 in California’s Central Valley. Ammonia dry deposition fractions ranged from 11% (PA27) to 74% (CA83). The spatial patterns of reduced N deposition fraction generally reflect spatial variations in agricultural activity including animal husbandry. Assuming that the biases between the MLM deposition velocity and bidirectional flux approaches shown in Fig. 4 are generally representative, a full assessment using the bidirectional approach would, at many sites, reduce overall deposition rates and the relative fraction of NH3 dry deposition. However, the general pattern observed in Fig. 5 remains consistent; NHx still contributes the majority of inorganic N deposition at the national scale.
Fig. 5.
Spatial trends in total reactive inorganic N deposition across the United States from July 2011 to June 2013. Fractional reduced N contributions to total N deposition (dry + wet) at the 37 sites are represented by circle color. The total inorganic nitrogen deposition is indicated by circle size. The pie charts show average fractional contributions of individual reduced and oxidized N deposition pathways for the eight regions, with each pie area proportional to the average total inorganic nitrogen deposition (also listed under each pie).
The site-specific circle sizes in Fig. 5 indicate the combined wet plus dry inorganic N deposition fluxes. Some regions exhibit majority dry deposition [e.g., dry deposition contributions of 58% and 79% in the Upper Midwest (IV) and Southwest (VIII), respectively], whereas others are more strongly influenced by wet deposition [e.g., wet deposition contributions of 66% and 72% in the Washington (I) and Southeast (VI) regions, respectively]. The largest deposition fluxes at individual sites tend to be observed at locations where fractional reduced N contributions are large. The maximum regional average inorganic N deposition flux (12.1 kg N/ha per year) was observed in the Upper Midwest region (IV); relatively large deposition fluxes were also observed for California and the eastern United States. These spatial patterns are similar to those identified in recent model simulations (43).
Implications and Summary.
Increases in agricultural emissions of ammonia and the success of regulatory policies in decreasing NOx emissions over the last two decades are changing the face of US reactive nitrogen deposition. Although US wet inorganic N deposition was once dominated by nitrate, wet inorganic N deposition now comes mostly from ammonium at nearly 70% of US monitoring sites. Although estimates of dry deposition fluxes of inorganic N inherently contain more uncertainty, dry and total (wet plus dry) inorganic N deposition fluxes also appear to be dominated by reduced N in most parts of the country. Decreases in wet and dry deposition fluxes of oxidized inorganic N species are expected to continue into the future as the United States continues to lower NOx emissions. Current projections of ammonia emissions growth, meanwhile, suggest that reduced N deposition levels will grow in the future. In addition to the adverse impacts of reduced N deposition on ecosystem health, ammonia is an important precursor to fine particle formation. Fine particles decrease visibility (44) and negatively impact human health and increase health care costs (45, 46). Reductions in US ammonia emissions from agricultural and nonagricultural sources, whether by regulation or voluntary actions (e.g., agricultural producer adoption of best management practices), would yield a variety of positive benefits for ecosystems and society. Increased study of atmospheric ammonia concentrations and improved measures of ammonia dry deposition fluxes are needed to design optimal strategies for achieving such benefits.
SI Methods
Bidirectional Ammonia Flux Model.
The net air-surface flux of NH3 above natural terrestrial ecosystems is governed by the competing processes of emission and deposition within the underlying vegetation and soil. Vegetation (i.e., apoplast) and soil pore water contain dissolved NH4+ and a corresponding NH3 gas phase equilibrium, therefore exhibiting a “compensation point” relative to the surrounding atmosphere (54, 55). The NH3 compensation point is the atmospheric concentration at which the net surface exchange is zero, i.e., the NH3 concentration at which the atmosphere is at equilibrium with the underlying surface. The surface is a sink for atmospheric NH3 when the atmospheric concentration exceeds the compensation point and emits NH3 to the atmosphere under the opposite condition. Although there are many instances where NH3 is only deposits to or emits from the surface (i.e., unidirectional flux), from a mechanistic standpoint, NH3 air-surface exchange is considered “bidirectional.”
Bidirectional NH3 flux is calculated using the two-layer canopy compensation point model developed by Nemitz et al. (53), which relates the net canopy-scale NH3 flux (Ft) to the net emission potential of the canopy (i.e., foliage and soil), or surface concentration [χ(zo)], which is in turn related to the canopy compensation point (χc). The system of equations describing the net canopy flux (Ft), as well as component vegetation [i.e., stomatal (Fs), cuticular (Fw)] and ground (Fg) fluxes, is given by Nemitz et al. (53). The model requires inputs of atmospheric NH3 concentration along with parameterizations for atmospheric [aerodynamic resistance (Ra) and boundary layer (Rb)], in-canopy [aerodynamic (Rac), boundary-layer resistances (Rbg), and ground (Rg = Rac + Rbg)], and leaf-level [stomatal (Rs) and cuticular (Rw)] resistances, as well as stomatal (Γs) and ground (Γg) emission potentials. Here, the ground emission potential does not distinguish between soil and litter layers but is rather a bulk property of the surface.
The aerodynamic resistance (Ra) is calculated as a function of the standard deviation (SD) of the measured wind direction (σθ), and wind speed (u) according to Hicks et al. (56), assuming that the atmosphere is considered unstable when global radiation (G) exceeds 100 W/m2 (47)
| [S1a] |
and
| [S1b] |
Under low wind speed (<2 m/s), Eq. S1a is modified to increase the proportionality constant as
| [S1c] |
It should be noted that the upper limits on the SD of wind direction (σθ) for stable and unstable periods used by the CASTNET MLM were not used in the bidirectional model. Friction velocity (u*) is calculated from the near-neutral approximation as a function of Ra and u (47). The boundary-layer resistance is calculated according to Hicks et al. (56). The in-canopy aerodynamic (turbulent) resistance (Rac) is calculated according to Massad et al. (40) as a function of u* and canopy height. The additional boundary layer resistance (Rbg) at the ground is calculated according to Schuepp (57), where the ground friction velocity (u*g) assumes the form of Pleim et al. (42). The sum of Rac and Rbg establishes the total ground resistance (Rg). Canopy height, leaf area index, zero-plane displacement, and surface roughness length follow the MLM approach (47).
The bulk stomatal resistance to NH3 transfer (Rs) is assumed equal to that of water vapor (H2O) corrected for differences in molecular diffusivity. Stomatal resistance to H2O is calculated as a function of G, air temperature (T), and the vegetation-specific minimum resistance (Rsmin) according to the rather simple parameterization of Wesely (58). Minimum stomatal resistances assume the same values specified for the MLM model (47). Parameterization of the cuticular resistance (Rw) is calculated according to Massad et al. (40) as a function of relative humidity, surface type (forest, seminatural, grassland), and the atmospheric acid ratio (AR). Site-specific values of AR were calculated seasonally using CASTNET-measured concentrations of SO2 and HNO3, AMoN-measured NH3 concentrations, and HCl concentrations generated by the Community Multiscale Air Quality Model (CMAQ Version 5.0.2, https://www.cmascenter.org/cmaq/).
The stomatal (i.e., vegetation) emission potential Γs was parameterized according to Massad et al. (40) as a function of total nitrogen deposition assuming the “unmanaged” case, and a value of Γg = 20 was assigned for the ground emission potential. Emission potentials are calculated for each AMoN site using collocated NADP/NTN wet deposition and CASTNET dry deposition data averaged over a 5-y period between 2006 and 2010. Vegetation and soil emission potentials are then used to calculate vegetation (χs) and ground (χg) compensation points (in units of µg/m3 to represent air concentration) as a function of temperature following ref. 59. Note that N deposition derived from NADP/NTN wet deposition and CASTNET dry deposition does not include dry deposition of NH3 or deposition of organic N compounds and therefore represents a lower limit for total N deposition and thus the vegetation emission potential.
Implementation of Bidirectional NH3 Model.
Similar to the MLM, the bidirectional NH3 model was run at an hourly time step using hourly CASTNET meteorology and assuming an hourly NH3 air concentration equivalent to the corresponding 2-wk integrated AMoN concentration. Note that for this analysis, AMoN and CASTNET are collocated. Hourly fluxes are summed over time to produce seasonal and annual total (net, Ft) and component (Fs, Fw, and Fg) fluxes.
Comparison of Bidirectional and Unidirectional NH3 Dry Deposition Models.
The net flux estimated from the bidirectional NH3 model was directly compared with the unidirectional (deposition) flux estimated by multiplying the AMoN NH3 concentration by 0.7 × the MLM (CASTNET)-derived deposition velocity for HNO3. Common inputs of hourly CASTNET meteorology, AMoN NH3 concentrations, and canopy physical characteristics were used to compare seasonal and annual fluxes for the dominant vegetation type at each collocated CASTNET/AMoN site. The objective of this exercise was to examine the relative differences in fluxes to inform the potential uncertainty of the 0.7 × MLM approach. The comparison is only valid for the natural surfaces for which the bidirectional NH3 model has been parameterized, which excludes fertilized and nitrogen fixing crops and other surfaces specified by CASTNET including, water, sand, and rock.
Meteorological data were discontinued at CASTNET sites in 2010 at all but five sites. For this reason, the meteorological dataset for each site was chosen based on 90% completeness for the primary variables starting at 2009 and working backward until the 90% annual completeness criterion was met. This meteorological dataset was then matched with AMoN NH3 concentration data for the year of 2012 (NH3 data in 2013 were used for sites GTH161 and CNT169). Because the objective was to compare the models using common inputs, it was not necessary to match the year chosen for meteorology with that of the chemical inputs (AMoN, NADP). At each site, the models assume the dominant vegetation type as specified by the CASTNET site characteristics for MLM.
Differences in MLM vs. Bidirectional NH3 Dry Deposition Estimates.
Across regions (Fig. 4), there is considerable variability in the difference between MLM and bidirectional estimates among sites. In some cases, bidirectional flux estimates exceed MLM estimates. MLM estimates consistently exceed bidirectional flux estimates in the Rocky Mountain (III), Florida (VII), and Southwest (VIII) regions. In the Southwest (VIII), low surface acid ratios produce lower rates of NH3 deposition to leaf cuticles, and lower net fluxes, under the bidirectional framework than would occur on more acidic surfaces. In the Rocky Mountain (III), model differences arise from a combination of processes. For example, site CO99 experiences very low NH3 air concentrations. During warm conditions, the stomatal compensation point exceeds the atmospheric NH3 concentration, resulting in a net stomatal emission that offsets deposition to the leaf cuticle and ground, thereby producing a low net annual deposition flux. Similar competing flux processes and corresponding low annual net fluxes are observed at sites WV18 and GA41 (Fig. 4).
Differences in MLM vs. bidirectional model estimates are generally greatest in summer (Fig. S2). Although highest atmospheric NH3 concentrations typically occur during the hottest months, the temperature-driven stomatal and soil compensation points are also at a maximum. At several sites, stomatal emissions during the summer are substantial enough to largely offset (e.g., sites CO99, SD04, OH54, and FL11 in Fig. S2) or even exceed (e.g., sites WV18, GA41, and NC06 in Fig. S2) deposition to the cuticle and ground. Furthermore, atmospheric acidity generally reaches a minimum during summer owing to seasonally lower concentrations of SO2 and higher concentrations of NH3. Leaf surfaces are therefore less acidic, resulting in lower cuticular deposition rates relative to what would be observed for that same atmospheric NH3 concentration in other seasons. On average, bidirectional and MLM estimates are most comparable in winter when compensation points are lowest and surfaces are more acidic (i.e., larger acid ratios).
Methods
Weekly precipitation concentrations of NH4+ and NO3− were obtained from the NADP National Trends Network (NTN; nadp.isws.illinois.edu/ntn/). Weekly gaseous HNO3 concentrations and particulate NH4+ and NO3− concentrations were obtained from the Clean Air Status and Trends Network (CASTNET; https://www.epa.gov/castnet). Biweekly concentrations of gaseous NH3 were taken from the NADP AMoN (nadp.isws.illinois.edu/AMoN/). To gain greater spatial coverage of airborne NH3 concentrations, especially in the western United States, NHx (NH3 + NH4+) measurements from a pilot IMPROVE NHx monitoring network (28) were also used. More detailed information about these observation networks can be found in Table S2.
Table S2.
Summary of data from US national networks used in the study
| Network | Deposition species | Data period | Source |
| AMoN* | Dry deposition: gaseous NH3 | 2011–2013 | nadp.isws.illinois.edu/AMoN |
| CASTNET† | Dry deposition: gaseous HNO3, particulate NH4+, NO3− | 2011–2013 | https://www.epa.gov/castnet |
| NTN‡ | Wet deposition: NH4+, NO3− | 1990–2013 | nadp.isws.illinois.edu/NTN |
| IMPROVE NHx§ | Dry deposition: gaseous NH3, particulate NH4+, NO3− | 2011–2012 | (28) |
The AMoN is operated by the NADP, which measures biweekly NH3 concentrations using passive diffusion (Radiello) samplers.
The CASTNET is funded by the Environmental Protection Agency (EPA) and NPS, which measures weekly HNO3 and particulate NH4+ and NO3− concentrations using three-stage filter pack samplers.
The NTN is operated by the NADP, which measures weekly NH4+, NO3− concentrations in precipitation samples.
The IMPROVE NHx study was conducted from April 2011 to August 2012, which measured the sum of gaseous NH3 and fine particle NH4+ concentrations using a single, acid-coated filter with 1-in-3 d sampling period. Colocated measurements of NH4+, NO3− and sulfate (SO42−) collected on nylon filters provide two methods to determine the split of measured NHx between gaseous NH3 and fine particle NH4+; here we assume that fine particle NO3− and SO42− are fully neutralized by NH4+ to estimate the NH4+ concentration, which was then subtracted from the NHx concentration to obtain a lower bound estimate of the NH3 concentration (28).
Wet deposition data were obtained from NTN sites for the periods 1990–1992 and 2010–2012. The number of sites analyzed changed due to network development over this period. From 1990 to 1992, there were 195 sites; 238 sites were available for the 2010–2012 period. Sites were not included if data were unavailable for ≥1 y in either period examined.
Oxidized and reduced N gas and particle concentrations were obtained for 37 sites (Table S1) where NTN and CASTNET monitoring stations were collocated with AMoN and/or IMPROVE NHx sites. At 30 of these locations, 2 y of measurements (July 2011 to June 2013) were available. The remaining sites had data availability of at least 1 y. Concentrations of all species that contribute to the Nr deposition budget are not measured at these sites. Important missing compounds include inorganic (e.g., NO2) and organic N (e.g., alkyl nitrates, peroxyacetyl nitrate, and amines) species. Wet deposition of organic N is also not routinely measured and therefore not considered in this analysis.
Wet N deposition was determined from the amount of total precipitation and the aqueous concentrations of NH4+ and NO3−. Dry N deposition was calculated for each species as the product of the N species concentration and a deposition velocity. Deposition velocities of gaseous HNO3 and particulate NH4+ and NO3− were provided by CASTNET for each of its measurement sites based on the MLM (47), with input of on-site meteorology and local site characteristics. Gaps in the meteorological data were addressed by using the CASTNET substitution method (48). The deposition velocity of NH3 is difficult to determine due to the bidirectional nature of the dry NH3 flux that depends strongly on local conditions (40). To estimate dry NH3 deposition here, its deposition velocity was calculated as 70% of the HNO3 deposition velocity following previous estimates (49–51). A review of field observations suggests that the NH3 deposition velocity is at least half and perhaps as high as the HNO3 deposition velocity. Our choice of 70% agrees well with the findings of Neirynck et al. (52) and Nemitz et al. (39).
This NH3 deposition velocity approach is a simple approximation of unidirectional air-surface exchange, ignoring important bidirectional exchange processes that influence the magnitude and direction of the flux. To assess the potential importance of these bidirectional exchange processes and their impact on annual reactive N deposition budgets, NH3 fluxes derived from the unidirectional approach were compared with fluxes estimated using a two-layer bidirectional flux model (53). The bidirectional model uses hourly CASTNET meteorology and 2-wk integrated AMoN NH3 concentrations to estimate NH3 exchange with soil and vegetation, as well as net fluxes above the vegetation. Ammonia compensation points and leaf surface resistances were parameterized following the recommendations of Massad et al. (40) for natural vegetation. Development of this modeling framework is ongoing. Thus, the comparison is constrained to the dominant natural vegetation type at each site for which the Massad et al. (40) parameterizations are applicable, which excludes fertilized and nitrogen fixing crops and some other surfaces specified by CASTNET, including water, sand, and rock. Details of the bidirectional model and comparison are included in SI Methods.
Acknowledgments
We thank Amy Sullivan, Katie Benedict, Aohan Tang, Qijing Bian, Arsineh Hecobian, and Yixing Shao (Colorado State University); Brian Michael (University of Illinois); and Nate Hyde (WaveMetrics) for helpful suggestions and support. Funding for this work was provided by the National Park Service.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: All datasets related to this manuscript have been deposited in a repository operated by the Colorado State University, https://dspace.library.colostate.edu/handle/10217/171382.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1525736113/-/DCSupplemental.
References
- 1.Galloway JN, et al. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science. 2008;320(5878):889–892. doi: 10.1126/science.1136674. [DOI] [PubMed] [Google Scholar]
- 2.Erisman JW, Galloway J, Seitzinger S, Bleeker A, Butterbach-Bahl K. Reactive nitrogen in the environment and its effect on climate change. Curr Opin Environ Sustain. 2011;3(5):281–290. [Google Scholar]
- 3.Galloway JN, Cowling EB. Reactive nitrogen and the world: 200 years of change. AMBIO. 2002;31(2):64–71. doi: 10.1579/0044-7447-31.2.64. [DOI] [PubMed] [Google Scholar]
- 4.Liu X, et al. Enhanced nitrogen deposition over China. Nature. 2013;494(7438):459–462. doi: 10.1038/nature11917. [DOI] [PubMed] [Google Scholar]
- 5.Clark CM, Tilman D. Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands. Nature. 2008;451(7179):712–715. doi: 10.1038/nature06503. [DOI] [PubMed] [Google Scholar]
- 6.Phoenix GK, et al. Impacts of atmospheric nitrogen deposition: Responses of multiple plant and soil parameters across contrasting ecosystems in long-term field experiments. Glob Change Biol. 2012;18(4):1197–1215. [Google Scholar]
- 7.Holtgrieve GW, et al. A coherent signature of anthropogenic nitrogen deposition to remote watersheds of the Northern Hemisphere. Science. 2011;334(6062):1545–1548. doi: 10.1126/science.1212267. [DOI] [PubMed] [Google Scholar]
- 8.Galloway JN, et al. Nitrogen cycles: Past, present, and future. Biogeochemistry. 2004;70(2):153–226. [Google Scholar]
- 9.Janssens IA, et al. Reduction of forest soil respiration in response to nitrogen deposition. Nat Geosci. 2010;3(5):315–322. [Google Scholar]
- 10.Nilsson J. Critical Loads for Sulphur and Nitrogen. Air Pollution and Ecosystems. Springer; New York: 1988. pp. 85–91. [Google Scholar]
- 11.Porter E, Blett T, Potter DU, Huber C. Protecting resources on federal lands: Implications of critical loads for atmospheric deposition of nitrogen and sulfur. Bioscience. 2005;55(7):603–612. [Google Scholar]
- 12.Ellis RA, et al. Present and future nitrogen deposition to national parks in the United States: Critical load exceedances. Atmos Chem Phys. 2013;13(17):9083–9095. [Google Scholar]
- 13.Davidson E, et al. Excess nitrogen in the U.S. environment: Trends, risks, and solutions. Issues Ecol. 2012;15(Winter 2012):1–16. [Google Scholar]
- 14.Gruber N, Galloway JN. An Earth-system perspective of the global nitrogen cycle. Nature. 2008;451(7176):293–296. doi: 10.1038/nature06592. [DOI] [PubMed] [Google Scholar]
- 15.Reis S, Pinder R, Zhang M, Lijie G, Sutton M. Reactive nitrogen in atmospheric emission inventories. Atmos Chem Phys. 2009;9(19):7657–7677. [Google Scholar]
- 16.Hand JL, et al. Widespread reductions in haze across the United States from the early 1990s through 2011. Atmos Environ. 2014;94:671–679. [Google Scholar]
- 17.Xing J, et al. Historical gaseous and primary aerosol emissions in the United States from 1990 to 2010. Atmos Chem Phys. 2013;13(15):7531–7549. [Google Scholar]
- 18.Lee T, et al. Observations of fine and coarse particle nitrate at several rural locations in the United States. Atmos Environ. 2008;42(11):2720–2732. [Google Scholar]
- 19.Galloway JN, Cowling EB, Seitzinger SP, Socolow RH. Reactive nitrogen: Too much of a good thing? AMBIO. 2002;31(2):60–63. doi: 10.1579/0044-7447-31.2.60. [DOI] [PubMed] [Google Scholar]
- 20.Fowler D, et al. Regional mass budgets of oxidized and reduced nitrogen and their relative contribution to the nitrogen inputs of sensitive ecosystems. Environ Pollut. 1998;102(1 suppl):337–342. [Google Scholar]
- 21.Bytnerowicz A, Fenn ME. Nitrogen deposition in California forests: A review. Environ Pollut. 1996;92(2):127–146. doi: 10.1016/0269-7491(95)00106-9. [DOI] [PubMed] [Google Scholar]
- 22.Holland EA, Braswell BH, Sulzman J, Lamarque J-F. Nitrogen deposition onto the United States and Western Europe: Aynthesis of observations and models. Ecol Appl. 2005;15(1):38–57. [Google Scholar]
- 23.Paulot F, Jacob DJ, Henze DK. Sources and processes contributing to nitrogen deposition: An adjoint model analysis applied to biodiversity hotspots worldwide. Environ Sci Technol. 2013;47(7):3226–3233. doi: 10.1021/es3027727. [DOI] [PubMed] [Google Scholar]
- 24.Du E, de Vries W, Galloway JN, Hu X, Fang J. Changes in wet nitrogen deposition in the United States between 1985 and 2012. Environ Res Lett. 2014;9(9):095004. [Google Scholar]
- 25.Sickles II, Shadwick D. Air quality and atmospheric deposition in the eastern US: 20 years of change. Atmos Chem Phys. 2015;15(1):173–197. [Google Scholar]
- 26.Lehmann CM, Gay DA. Monitoring long-term trends of acidic wet deposition in US precipitation: Results from the National Atmospheric Deposition Program. Powerpl Chem. 2011;13(7):386–393. [Google Scholar]
- 27.Lehmann CM, Bowersox VC, Larson RS, Larson SM. Monitoring Long-Term Trends in Sulfate and Ammonium in US Precipitation: Results from the National Atmospheric Deposition Program/National Trends Network. Acid Rain-Deposition to Recovery. Springer; New York: 2007. pp. 59–66. [Google Scholar]
- 28.Chen X, et al. Seasonal ambient ammonia and ammonium concentrations in a pilot IMPROVE NHx monitoring network in the western United States. Atmos Environ. 2014;91:118–126. [Google Scholar]
- 29.Rodriguez MA, Barna MG, Moore T. Regional impacts of oil and gas development on ozone formation in the western United States. J Air Waste Manag Assoc. 2009;59(9):1111–1118. doi: 10.3155/1047-3289.59.9.1111. [DOI] [PubMed] [Google Scholar]
- 30.Brunke R, Alvo P, Schuepp P, Gordon R. Effect of meteorological parameters on ammonia loss from manure in the field. J Environ Qual. 1988;17(3):431–436. [Google Scholar]
- 31.Sommer SG, Olesen JE, Christensen BT. Effects of temperature, wind speed and air humidity on ammonia volatilization from surface applied cattle slurry. J Agric Sci. 1991;117(01):91–100. [Google Scholar]
- 32.Li Y, et al. Observations of ammonia, nitric acid, and fine particles in a rural gas production region. Atmos Environ. 2014;83(0):80–89. [Google Scholar]
- 33.Bash JO, Cooter EJ, Dennis RL, Walker JT, Pleim JE. Evaluation of a regional air-quality model with bidirectional NH3 exchange coupled to an agroecosystem model. Biogeosciences. 2013;10(3):1635–1645. [Google Scholar]
- 34.Cooter EJ, Schwede DB. Sensitivity of the National Oceanic and Atmospheric Administration multilayer model to instrument error and parameterization uncertainty. J Geophys Res, D, Atmospheres. 2000;105(D5):6695–6707. [Google Scholar]
- 35.Schwede D, Zhang L, Vet R, Lear G. An intercomparison of the deposition models used in the CASTNET and CAPMoN networks. Atmos Environ. 2011;45(6):1337–1346. [Google Scholar]
- 36.Flechard CR, Fowler D, Sutton MA, Cape JN. A dynamic chemical model of bi-directional ammonia exchange between semi-natural vegetation and the atmosphere. Q J R Meteorol Soc. 1999;125(559):2611–2641. [Google Scholar]
- 37.Neirynck J, Ceulemans R. Bidirectional ammonia exchange above a mixed coniferous forest. Environ Pollut. 2008;154(3):424–438. doi: 10.1016/j.envpol.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 38.Kruit RJW, et al. Modeling the surface–atmosphere exchange of ammonia. Atmos Environ. 2010;44(7):945–957. [Google Scholar]
- 39.Nemitz E, Sutton M, Wyers G, Jongejan P. Gas-particle interactions above a Dutch heathland: I. Surface exchange fluxes of NH3, SO2, HNO3 and HCl. Atmos Chem Phys. 2004;4(4):989–1005. [Google Scholar]
- 40.Massad R-S, Nemitz E, Sutton M. Review and parameterisation of bi-directional ammonia exchange between vegetation and the atmosphere. Atmos Chem Phys. 2010;10(21):10359–10386. [Google Scholar]
- 41.Zhang L, Wright L, Asman W. Bi‐directional air‐surface exchange of atmospheric ammonia: A review of measurements and a development of a big‐leaf model for applications in regional‐scale air‐quality models. J Geophys Res, D, Atmospheres. 2010;115:D20310. [Google Scholar]
- 42.Pleim JE, Bash JO, Walker JT, Cooter EJ. Development and evaluation of an ammonia bidirectional flux parameterization for air quality models. J Geophys Res, D, Atmospheres. 2013;118(9):3794–3806. [Google Scholar]
- 43.Schwede DB, Lear GG. A novel hybrid approach for estimating total deposition in the United States. Atmos Environ. 2014;92(0):207–220. [Google Scholar]
- 44.Malm WC. Introduction to Visibility. Cooperative Institute for Research in the Atmosphere, NPS Visibility Program, Colorado State University; Fort Collins, CO: 1999. [Google Scholar]
- 45.Paulot F, Jacob DJ. Hidden cost of U.S. agricultural exports: Particulate matter from ammonia emissions. Environ Sci Technol. 2014;48(2):903–908. doi: 10.1021/es4034793. [DOI] [PubMed] [Google Scholar]
- 46.Stokstad E. Air pollution. Ammonia pollution from farming may exact hefty health costs. Science. 2014;343(6168):238. doi: 10.1126/science.343.6168.238. [DOI] [PubMed] [Google Scholar]
- 47.Meyers TP, Finkelstein P, Clarke J, Ellestad TG, Sims PF. A multilayer model for inferring dry deposition using standard meteorological measurements. J Geophys Res, D, Atmospheres. 1998;103(D17):22645–22661. [Google Scholar]
- 48.Bowker GE, Schwede DB, Lear GG, Warren-Hicks WJ, Finkelstein PL. Quality assurance decisions with air models: A case study of imputation of missing input data using EPA’s multi-layer model. Water Air Soil Pollut. 2011;222(1-4):391–402. [Google Scholar]
- 49.Benedict KB, et al. A seasonal nitrogen deposition budget for Rocky Mountain National Park. Ecol Appl. 2013;23(5):1156–1169. doi: 10.1890/12-1624.1. [DOI] [PubMed] [Google Scholar]
- 50.Beem KB, et al. Deposition of reactive nitrogen during the Rocky Mountain Airborne Nitrogen and Sulfur (RoMANS) study. Environ Pollut. 2010;158(3):862–872. doi: 10.1016/j.envpol.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 51.Benedict KB, et al. Observations of atmospheric reactive nitrogen species in Rocky Mountain National Park and across northern Colorado. Atmos Environ. 2013;64:66–76. [Google Scholar]
- 52.Neirynck J, et al. Fluxes of oxidised and reduced nitrogen above a mixed coniferous forest exposed to various nitrogen emission sources. Environ Pollut. 2007;149(1):31–43. doi: 10.1016/j.envpol.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 53.Nemitz E, Milford C, Sutton MA. A two-layer canopy compensation point model for describing bi-directional biosphere-atmosphere exchange of ammonia. Q J R Meteorol Soc. 2001;127(573):815–833. [Google Scholar]
- 54.Langford A, Fehsenfeld F, Zachariassen J, Schimel D. Gaseous ammonia fluxes and background concentrations in terrestrial ecosystems of the United States. Global Biogeochem Cycles. 1992;6(4):459–483. [Google Scholar]
- 55.Farquhar GD, Firth PM, Wetselaar R, Weir B. On the gaseous exchange of ammonia between leaves and the environment: Determination of the ammonia compensation point. Plant Physiol. 1980;66(4):710–714. doi: 10.1104/pp.66.4.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hicks B, Baldocchi D, Meyers T, Hosker R, Jr, Matt D. A preliminary multiple resistance routine for deriving dry deposition velocities from measured quantities. Water Air Soil Pollut. 1987;36(3-4):311–330. [Google Scholar]
- 57.Schuepp PH. Turbulent transfer at the ground: On verification of a simple predictive model. Boundary-Layer Meteorol. 1977;12(2):171–186. [Google Scholar]
- 58.Wesely M. Parameterization of surface resistances to gaseous dry deposition in regional-scale numerical models. Atmospheric Environ (1967) 1989;23(6):1293–1304. [Google Scholar]
- 59.Hill PW, Raven JA, Loubet B, Fowler D, Sutton MA. Comparison of gas exchange and bioassay determinations of the ammonia compensation point in Luzula sylvatica (Huds.) Gaud. Plant Physiol. 2001;125(1):476–487. doi: 10.1104/pp.125.1.476. [DOI] [PMC free article] [PubMed] [Google Scholar]