Significance
Transcriptional coactivator β-catenin promotes cell proliferation in development and tumorigenesis, via actions enhanced by PKA-catalyzed phosphorylation of its C-terminal region S675. Specific phosphorylation near the N terminus triggers ubiquitination and proteasomal degradation. Depletion of HeLa cell brefeldin A-inhibited guanine nucleotide-exchange factors (BIG)1 and/or BIG2 impaired β-catenin S675 phosphorylation and its transcriptional effects, as well as ADP-ribosylation factor activation-enhanced phospholipase D activity and vesicular trafficking, both required for restoration of transcriptional activation by appropriate BIG protein overexpression in depleted cells. We propose that interaction of β-catenin–bound BIG1 with BIG2 and its associated PKA complex, simultaneously or sequentially, are required for phosphorylation of S675, with nuclear entry of activated β-catenin followed by its acceleration of T-cell factor/lymphoid enhancer factor family gene transcription.
Keywords: ADP-ribosylation factor, cell migration, AKAP, phospholipase D
Abstract
Multifunctional β-catenin, with critical roles in both cell–cell adhesion and Wnt-signaling pathways, was among HeLa cell proteins coimmunoprecipitated by antibodies against brefeldin A-inhibited guanine nucleotide-exchange factors 1 and 2 (BIG1 or BIG2) that activate ADP-ribosylation factors (Arfs) by accelerating the replacement of bound GDP with GTP. BIG proteins also contain A-kinase anchoring protein (AKAP) sequences that can act as scaffolds for multimolecular assemblies that facilitate and limit cAMP signaling temporally and spatially. Direct interaction of BIG1 N-terminal sequence with β-catenin was confirmed using yeast two-hybrid assays and in vitro synthesized proteins. Depletion of BIG1 and/or BIG2 or overexpression of guanine nucleotide-exchange factor inactive mutant, but not wild-type, proteins interfered with β-catenin trafficking, leading to accumulation at perinuclear Golgi structures. Both phospholipase D activity and vesicular trafficking were required for effects of BIG1 and BIG2 on β-catenin activation. Levels of PKA-phosphorylated β-catenin S675 and β-catenin association with PKA, BIG1, and BIG2 were also diminished after BIG1/BIG2 depletion. Inferring a requirement for BIG1 and/or BIG2 AKAP sequence in PKA modification of β-catenin and its effect on transcription activation, we confirmed dependence of S675 phosphorylation and transcription coactivator function on BIG2 AKAP-C sequence.
Multifunctional β-catenin is an essential component of intercellular adherens junctions (AJs) that link cell surface cadherin and actin cytoskeleton via interactions with both. β-catenin is also a transcriptional coactivator in several pathways, including Wnt-initiated signaling (1, 2), which is critical in embryonic development and tissue homeostasis or pathology (2, 3). In the absence of “canonical” catenin-dependent Wnt signaling, cytoplasmic β-catenin is maintained at low levels via phosphorylations of S45 and S33, S37 and T41 by, respectively, casein kinase-1 and glycogen synthase kinase-3 (GSK-3) (1, 2). Subsequent degradation of β-catenin via an ubiquitin/proteasome pathway involves axin and adenomatous polyposis coli (APC) as scaffold proteins, plus β-transducin repeat-containing protein (β-TrCP), an E3 ubiquitin ligase. In one well-known model, binding of Wnt to Frizzled (Fz) and its coreceptor low-density lipoprotein receptor-like protein 5 or 6 (LRP5/6) resulted in GSK-3 sequestration in multivesicular bodies, thereby reducing its cytosolic level (4, 5). With lower GSK-3 activity, “stabilized” β-catenin (1, 2) entered the nucleus and, along with members of the T-cell factor (TCF)/lymphoid enhancer factor (LEF) family, activated transcription via Wnt-target gene promoters, including c-myc and cyclin D1 (6–8). In β-catenin knock-out mice, embryogenesis was arrested at gastrulation (9) and aberrant Wnt signaling led to tumor development (2, 3).
Although relationships between labile and stabilized β-catenin pools remain poorly understood, cadherins required for the AJ pool are generally considered to be negative regulators of Wnt signaling, because their presence in AJs means the presence also of β-catenin, preventing its nuclear accumulation (10). Initially, β-catenin was known as a component of intercellular adhesion complexes (11), which were responsible for cytoplasmic anchoring of cadherins. Nuclear levels of active β-catenin unreactive with antibodies to phosphorylated N terminus (12) correlated with Wnt transcriptional activity better than did total β-catenin (13). After exposure of cells to Wnt ligand, active β-catenin appeared at the plasma membrane before it was detected in nuclei (14), whereas S33-, S37-, and T41-phosphorylated β-catenin was more concentrated at cell–cell contacts than in cytoplasm (15). How events at the plasma membrane and cell–cell contacts influence β-catenin degradation coordinately with Wnt signaling is unclear. Evidence that β-catenin stability and transcriptional activity are influenced also by PKA- or Akt-catalyzed phosphorylation of S675 and S552 (16–18) reflects perhaps an intersection of canonical Wnt and cAMP signaling systems.
Brefeldin A (BFA)-inhibited guanine nucleotide-exchange factors (GEFs), BIG1 (∼200 kDa) and BIG2 (∼190 kDa), first purified together in ∼670-kDa multiprotein complexes from bovine brain cytosol (19–23), are 74% identical in amino acid sequences, with 90% identity in the Sec7 domains (Fig. 1A) responsible for ADP-ribosylation factor (Arf) activation. Microscopically, BIG1 was seen mainly at the trans-Golgi network (TGN), sometimes partially overlapping BIG2, which was associated also with recycling endosomes (24–26), e.g., in moving proteins and lipids among TGN, endosomes, and cell surface (24, 27–29). Although actions of BIG1 and BIG2 at the TGN were described as “redundant” (27), each protein clearly has specific roles in moving proteins and lipids that are not shared with the other (24, 30, 31). Overexpression in cultured cells of mutant BIG2 lacking Arf GEF activity markedly altered intracellular distributions of E-cadherin and β-catenin (32). GEF-inactivating BIG2 mutations were also found in patients with autosomal recessive periventricular heterotopia in whom severe malformations of the cerebral cortex result from impaired developmental neuron migration (32, 33). Along with Arf GEF activity of their Sec7 domains, BIG1 and BIG2 share identity in the much shorter amino acid sequence of an A kinase-anchoring protein (AKAP) element (Fig. 1A) that binds specific protein kinase A (PKA) regulatory (R) subunits. BIG1 lacks two other BIG2 AKAP sequences, but each BIG protein can act as scaffold or platform for the assembly and operation of multimolecular machines that limit cAMP signaling and effects of its other actions in space and time (34). Protein phosphatase 1γ (PP1γ) (35) and phosphodiesterase (PDE) 3A (36) that, respectively, dephosphorylate PKA-modified BIG1 or BIG2 and degrade cAMP, have also been identified in complexes with BIG1 and/or BIG2. More examples of important AKAP roles for the BIG proteins than the several already known will surely be found, as their participation in two seemingly major cellular vesicular trafficking and regulatory signaling systems is likely not fortuitous.
Fig. 1.
Interaction of β-catenin with BIG1 and/or BIG2. (A) Similar six-domain structures of BIG1 and BIG2 molecules include dimerization and cyclophilin binding (DCB, blue), homology upstream of Sec7 (HUS, green), Sec7 (BFA-inhibited Arf activation, yellow), and three homology downstream of Sec7 (HDS, pink). BIG1 contains one AKAP sequence and BIG2 three (AKAP-A, -B, -C, red). (B and C) Samples (5%, input) of endogenous HeLa cell proteins for IP (100 μg) and of proteins collected by IP (50%) with antibodies against BIG1, BIG2, or β-catenin (β-cat), or control IgG were analyzed by Western blotting with indicated antibodies. Exposure times differed in B and C to optimize images. (D) HA-BIG1 and FLAG–β-catenin, synthesized singly (wheat germ extract), were incubated together as indicated before IP with antitag antibodies. Samples of in vitro synthesized proteins before (5%, input) and from IP (100%) with antibodies against HA or FLAG were subjected to Western blotting, respectively, with the same antibodies. (E) Samples of in vitro synthesized BIG2 and FLAG–β-catenin were mixed before IP and analysis of collected proteins. (F) Yeast transformed with N terminus of Gal4p-activation domain fusion constructs of GSK-3β (3β) or indicated BIG1 amino acid sequences [1–1849 full length (F), 1–887 (N), or 888–1849 (C)], together with pGBKT7 vector with N terminus of full-length β-catenin fused to the DNA-binding domain of Gal4p were grown (3 d, 30 °C) on synthetic histidine-containing medium lacking leucine and tryptophan (−L−W); colonies from −L−W plates were grown (3 d, 30 °C) on selective medium lacking also histidine (−L−W−H) with or without adenine (−L−W−H−Ade).
Here, we report coimmunoprecipitation (coIP) of endogenous HeLa cell β-catenin with BIG1 and BIG2, showing that Arf GEF functions of both were necessary and sufficient for changes in intracellular β-catenin distribution. Depletion of either BIG1 or BIG2 or overexpression of a GEF-inactive mutant led to β-catenin accumulation in the Golgi region. We found coIP of PKA catalytic subunit with β-catenin, BIG1, and/or BIG2, plus lower PKA-specific phosphorylation of β-catenin S675 after BIG1 or BIG2 knockdown. Mutation of the AKAP-C motif in BIG2 (37) decreased levels of S675 phosphorylation and products of β-catenin–target genes in cells depleted of BIG1 and/or BIG2, consistent with the view that BIG2 AKAP-C contributed to cAMP-regulation of β-catenin transcriptional activity.
Results
Interaction of β-Catenin with BIG1 and/or BIG2.
Analysis of proteins coimmunoprecipitated from HeLa cells, revealed that ∼1% of endogenous β-catenin coprecipitated with antibodies against BIG1 or BIG2, but not with control IgG (Fig. 1B). Immunoprecipitation (IP) of endogenous β-catenin collected also about 1% of BIG1 and 0.5% of BIG2 (Fig. 1C). To assess direct protein–protein interactions, human β-catenin, BIG1, and BIG2 were synthesized singly in vitro (wheat germ extract) and incubated together as indicated before IP. BIG1 was found with IP of β-catenin, and IP of BIG1 yielded also β-catenin (Fig. 1D), but no evidence for direct interaction of in vitro synthesized BIG2 and β-catenin was found (Fig. 1E). To identify BIG1 regions that interacted with β-catenin in yeast two-hybrid assays (Fig. 1F), Saccharomyces cerevisiae AH109 was cotransformed with constructs encoding BIG1 amino acids 1–1849 (F, full length), 1–885 (N), or 886–1849 (C) fused to the N-terminal Gal4p activation domain and full-length β-catenin fused to the Gal4p DNA-binding domain. Direct interaction of β-catenin with GSK-3β (38) was a positive control in these assays. BIG1-F and BIG1-N, but not BIG1-C, interacted with β-catenin (Fig. 1F), consistent with the conclusion that β-catenin associated with structural elements in the first 887 amino acids of BIG1.
Effects of BIG1 and/or BIG2 Depletion on Intracellular Distribution of β-Catenin.
Via specific molecular complexes, β-catenin performs diverse biological functions in different cellular locations (1, 3). Iodixanol density-gradient fractionation was used to assess intracellular distribution of endogenous proteins (Fig. S1A). BIG1 and BIG2 were most abundant in fractions 6–11, partially overlapping the lighter of two of β-catenin peaks. Endogenous β-catenin was seen microscopically (Fig. S1B) in irregular concentrations along the cell surface, seeming almost continuous in some regions. Perinuclear clusters were larger, corresponding perhaps to populations of transport vesicles. Large and small collections of BIG1 and BIG2 were scattered through the cytoplasm as earlier reported (23, 25, 26).
Fig. S1.
Density gradient distribution of endogenous HeLa cell proteins: BIG1, BIG2, and β-catenin. (A) Proteins in postnuclear supernatants of cells separated in iodixanol gradients as described in Materials and Methods. Lower gradient numbers correspond to lower and higher to greater densities. Amounts of protein are percentage in each fraction of the total amount of that protein recovered. (B) Cells were fixed and reacted with rabbit antibodies against BIG1 or BIG2 and mouse anti–β-catenin antibodies. (Scale bar, 10 μm.) (C) Quantification of Fig. 2A. β-Catenin in cells was quantified as the ratio of mean β-catenin staining intensity of Golgi to mean β-catenin staining intensity of cytosol (Golgi/cytosol). At least 20 cells were analyzed in each of three experiments. ***P < 0.005; *P < 0.05 vs. NT siRNA.
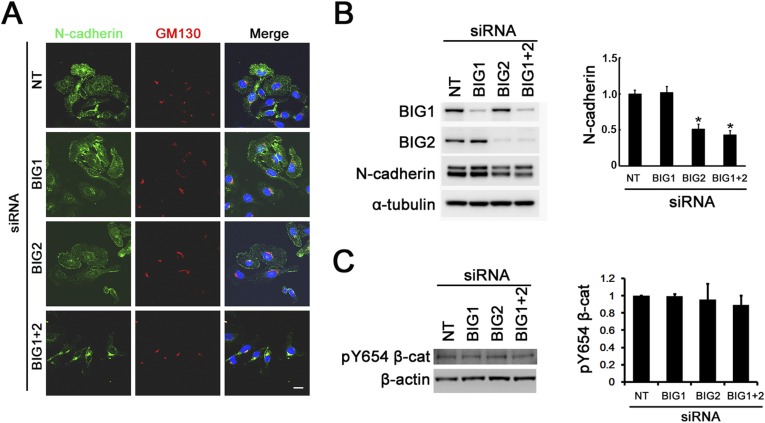
Overlap of β-catenin with both cis-Golgi GM130 (Fig. 2A and Fig. S1C) and trans-Golgi syntaxin-6 (Fig. 2B) in perinuclear regions seemed greater after BIG1 and/or BIG2 depletion. Unlike β-catenin, N-cadherin was seen associated with cis-Golgi GM130 only after BIG1 and BIG2 depletion (Fig. S2A), and amounts of total N-cadherin were significantly (>50%) lower after depletion of BIG2, not BIG1 (Fig. S2B). Phosphorylation of β-catenin Y654, reported to reduce its binding to cadherin (39, 40), was not evidently changed after BIG1 and/or BIG2 depletion (Fig. S2C).
Fig. 2.
Effects of BIG1 and/or BIG2 depletion on intracellular distribution of endogenous β-catenin. After a 72-h incubation with vehicle alone (mock) or siRNA, nontargeted (NT) or specific for BIG1 or BIG2 or both, cells were fixed, reacted with rabbit anti–β-catenin and mouse anti-GM130 antibodies (A), or mouse anti–syntaxin-6 antibodies (B), before preparation for confocal immunofluorescence microscopy. (Scale bar, 10 μm.)
Fig. S2.
Effects of BIG1 and/or BIG2 depletion on amounts of N-cadherin and Y654-phosphorylated (pY654) β-catenin. (A) After a 72-h incubation with siRNA, nontarget (NT) or specific for BIG1 or BIG2, or both, cells were fixed, reacted with antibodies against N-cadherin and GM130, and prepared for confocal immunofluorescence microscopy. (Scale bar, 10 μm.) (B) Samples (20 μg) of total lysate proteins were separated in 4–12% NuPAGE Bis⋅Tris gels, reacted with indicated antibodies, and quantified by densitometry; values were normalized to that of control (NT siRNA) cells = 1.0. Data are means ± SEM (n = 3), *P < 0.05 vs. NT siRNA. (C) Cells were incubated 72 h with NT or specific BIG1 and/or BIG2 siRNA before analyses of indicated proteins by Western blotting with antibodies against pY654 β-catenin (pY654–β-cat), densitometric quantification, and statistical analysis of data from three experiments as in B.
BIG1 and BIG2 are best known as Arf GEFs, the function for which they had been purified (19–22). Depletion of BIG1 or BIG2 using specific siRNAs or overexpression of a dominant-negative GEF-inactive Sec7 domain mutant interfered with vesicular transport of specific proteins from TGN or recycling endosomes to the plasma membrane (24, 27, 28, 41). Interference with β-catenin trafficking by expression of GEF-inactive BIG1 or BIG2, but not wild type (WT) or BIG1 AKAP mutant, led to its accumulation in perinuclear clusters (Fig. S3 A and B). GEF-inactive BIG1 (E793K) colocalized with GM130 (Fig. S3A), suggesting arrest of β-catenin at cis-Golgi, consistent with a report (32) that expression of BIG2 (E738K) in Madin-Darby canine kidney cells led to E-cadherin and β-catenin collection in Golgi structures. Absence of β-catenin in perinuclear regions of cells overexpressing WT BIG1 or BIG2 or BIG1 AKAP mutant is consistent with blockage of its translocation by inhibition of Arf activation, interfering with vesicular trafficking and/or phospholipase D (PLD) stimulation.
Fig. S3.
Effects of overexpressed GEF-inactive BIG1 or BIG2 mutants, but not BIG1 AKAP mutant, on cellular distribution of β-catenin. After a 24-h transfection with cDNA encoding HA-tagged BIG1 WT or mutants E793K, or AKAP (A), or GFP-tagged BIG2 WT or mutant E738K (B), cells were fixed, reacted with antibodies against β-catenin or GM130 (cis-Golgi marker), and HA or GFP, and prepared for confocal immunofluorescence microscopy. Perinuclear accumulation of β-catenin in cells expressing BIG1 (E793K) or BIG2 (E738K), but not WT BIG1 or BIG2 or BIG1 AKAP mutant, is consistent with blockage of Arf activation at membranes in a β-catenin translocation pathway. (Scale bar, 10 μm.)
Protein Kinase A Catalytic Subunit Interaction with BIG1, BIG2, and β-Catenin.
Phosphorylation of S552 or S675 in the C-terminal armadillo-repeat region of β-catenin by PKA had been associated with increased β-catenin transcriptional activity (16, 17, 42), but mechanisms were unclear. Levels of phosphorylated S675 and S552 were decreased similarly by depletion of BIG1 or BIG2 (Fig. 3A and Fig. S4A). Endogenous PKA catalytic subunit α (Cα) was coprecipitated from cells with BIG1 or BIG2 antibodies, but not control IgG (Fig. 3B) and IP of Cα collected BIG1 and BIG2, as well as β-catenin (Fig. 3C). Both BIGs were required for Cα-catalyzed phosphorylation of β-catenin (Fig. 3D). Depletion of either decreased phosphorylation of Akt S473, consistent with effects of the GEFs on β-catenin also via Akt (Fig. S4B). Generation and nuclear accumulation of active β-catenin, newly translated, with no phosphorylation of S37 or T41, are important consequences of Wnt signaling (14). Levels of active β-catenin, like those of β-catenin pS675, were lower in cells after BIG1 or BIG2 depletion, although total amounts of β-catenin protein were unchanged (Fig. 3A).
Fig. 3.
Effects of BIG1 and/or BIG2 depletion on levels of pS675 β-catenin, active β-catenin (unreactive with antibodies to phosphorylated N terminus), total β-catenin, and interaction between β-catenin and PKA Cα. (A) Cells were incubated 72 h with vehicle alone (mock) or with nontarget (NT) or specific BIG1 and/or BIG2 siRNA before analysis of indicated proteins by Western blotting and densitometric quantification. **P < 0.005 vs. NT siRNA. (B and C) CoIP of endogenous β-catenin with PKA catalytic subunit Cα and BIG1 or BIG2. Samples (5%, input) of total cell proteins used for IP (200 μg) and 50% of proteins collected by IP with antibodies against BIG1, BIG2, or control IgG (B) or Cα antibodies (C) were analyzed. (D) Proteins from Cα IP of extracts of cells incubated 72 h without (none) or with indicated siRNA, were reacted with antibodies against Cα, β-catenin, BIG1, BIG2, and β-actin. *P < 0.05 vs. NT siRNA.
Fig. S4.
Effects of BIG1 and/or BIG2 depletion on levels of specifically serine-phosphorylated and total β-catenin or Akt. (A and B) Cells were incubated 72 h with vehicle alone (mock) or with NT or specific BIG1 and/or BIG2 siRNA before analyses of indicated proteins by Western blotting and densitometric quantification. *P < 0.05; **P < 0.01 vs. NT siRNA.
Phosphorylation of β-catenin S45 by casein kinase-1 and by GSK-3 at Thr-41, Ser-37, and Ser-33 has been reported (1, 2). There were, however, no significant effects of BIG1 or BIG2 depletion on levels of those specific modifications (Fig. S4A), suggesting they were not involved in β-catenin regulation by BIG1 or BIG2.
Overexpression of BIG1-F or -N, or Mutants of BIG1 AKAP or BIG2 AKAP-A or -B Reversed Effects of BIG1 or BIG2 Depletion on pS675 β-Catenin.
Reversal of the effect of BIG1 depletion on β-catenin phosphorylation in HeLa cells by its overexpression (Fig. 4A) confirmed specificity, implicating BIG1 in regulation of pS675 β-catenin. Consistent with the necessity for Arf activation (Fig. S5A), overexpression of GEF-inactive BIG1 (E793K) did not reverse effects of BIG1 depletion on pS675 β-catenin. Failure of overexpression of BIG1-C and BIG1-S (BIG1-Sec7, 698–887) fragments to reverse the effects of BIG1 depletion, although both BIG1-N and -F were effective (Fig. S5B), excluded a role for the BIG1 C terminus in S675 phosphorylation (43). We confirmed (Fig. 4 B and C) that overexpression of BIG1 AKAP mutant or BIG2 WT, or AKAP-A, or -B, but not AKAP-C, mutants (34, 37) reversed the effects of BIG2 depletion, consistent with critical action of the BIG2, but not BIG1, AKAP-C sequence in this regulation.
Fig. 4.
Overexpression of BIG1 WT, BIG1 AKAP, or BIG2 AKAP-A or -B mutants, but not BIG2 AKAP-C mutant, reversed effects of BIG1 or BIG2 depletion on levels of pS675 β-catenin. (A) After a 72-h depletion of BIG1, cells were incubated 24 h with 4 μg of empty vector (EV) or 4 or 2 μg of HA-BIG1 WT before analysis of proteins. *P < 0.01 vs. NT siRNA, **P < 0.05 vs. BIG1 siRNA + EV. (B and C) After 72 h with BIG1 (B) or BIG2 (C) siRNA, cells were incubated for 48 h with 4 or 2 μg of WT or HA-tagged BIG1, or 4 μg of BIG2 WT or AKAP-A (A), -B (B), -C (C) mutants before analysis of proteins. *P < 0.01 vs. NT siRNA; **P < 0.01 vs. BIG1 or BIG2 siRNA.
Fig. S5.
Overexpression of BIG1-F, BIG1-N, but not BIG1 (E793K) mutant, or BIG1-S, or -C, reversed effects of BIG1 or BIG2 depletion on levels of pS675 β-catenin. (A) After a 72-h depletion of BIG1, cells were incubated 24 h with 4 μg of empty vector (EV) or 4 or 2 μg of HA-BIG1 E793K mutant before analysis of proteins. *P < 0.01 vs. NT siRNA. (B) Western blots of proteins from cells after 72 h with BIG1 siRNA followed by 24 h with plasmids (4 μg) for expression of, respectively, BIG1-full length (F), -N terminus (N), -Sec7 domain (S), or -C terminus. *P < 0.05 vs. NT siRNA; **P < 0.05 vs. BIG1 siRNA.
Major effects of Arf activation often include enhanced vesicular transport and/or increased PLD activities at specific intracellular sites (44). We found that 5-fluoro-2-indolyl des-chlorohalopemide (FIPI), an inhibitor of both PLD1 and PLD2 (45), or PKA inhibitor H-89 prevented restoration of β-catenin S675 phosphorylation in cells depleted of BIG1 (Fig. 5 A and B), suggesting that both vesicular trafficking and PLD action, as well as PKA, were important in BIG1 regulation of β-catenin S675 phosphorylation.
Fig. 5.
Effects of FIPI or PKA (H-89) on levels of pS675 β-catenin. (A and B) After a 72-h depletion of BIG1, cells were incubated 24 h with 4 μg of EV or WT HA-BIG1 plus 1 h without or with vehicle, 750 nM FIPI, or 10 μM H-89 before analyses of proteins. *P < 0.01 vs. NT siRNA; **P < 0.05 vs. BIG1 siRNA + EV.
Effects of BIG1 or BIG2 Depletion on Nuclear-Activated β-Catenin and Its Transcriptional Activity.
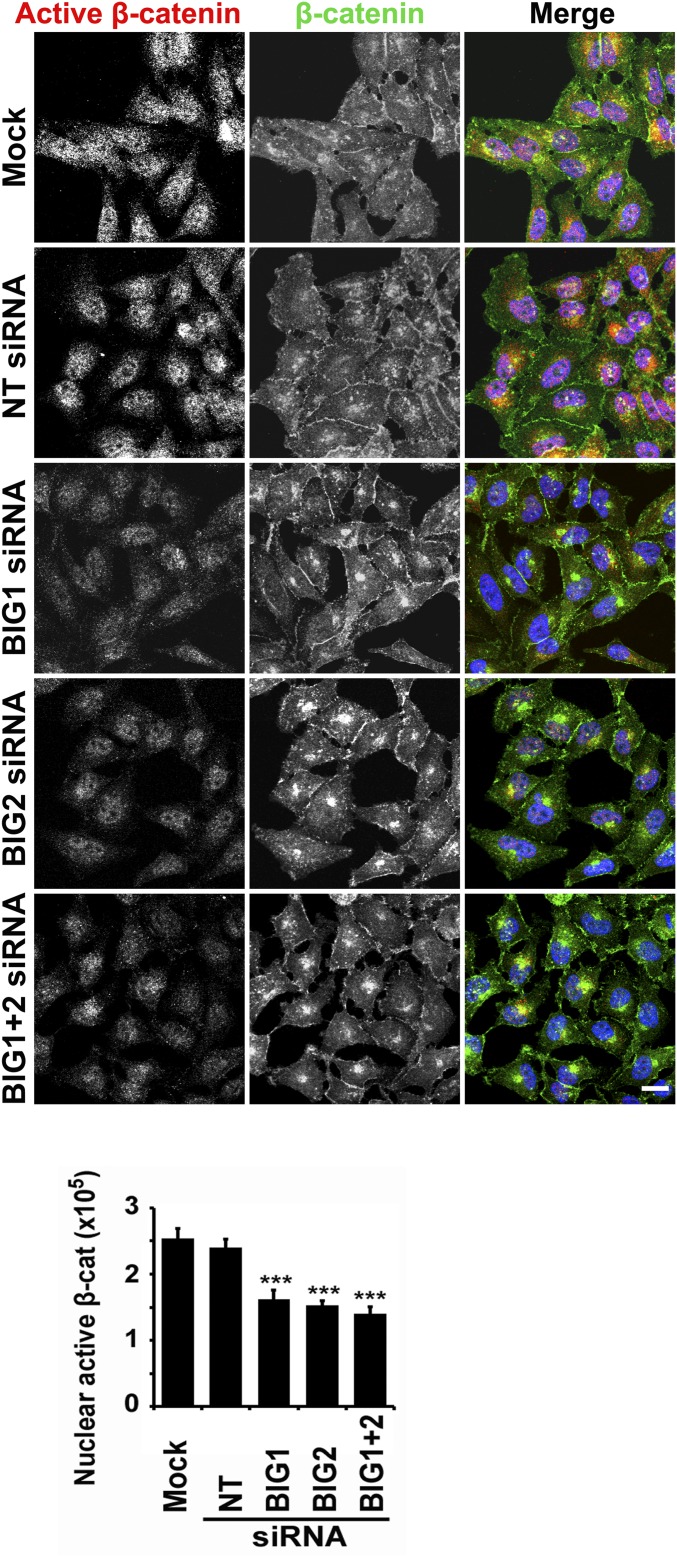
Association of PKA-catalyzed phosphorylation of β-catenin S552 and S675 with its increased transcriptional activity has been reported (16, 17, 42). Because subcellular fractionation may not accurately reflect intracellular localization of proteins in living cells (e.g., redistribution after cell breakage of molecules not tightly bound to nuclear or cytoplasmic structures), immunofluorescence microscopy was used to assess effects of BIG1 and/or BIG2 depletion on intracellular localization of activated β-catenin. In control [nontargeted (NT) siRNA] cells, activated β-catenin was completely nuclear, and declined notably after depletion of BIG1 and/or BIG2 (Fig. S6). As only a small fraction of total β-catenin (i.e., appropriately unphosphorylated and active), was acted upon by BIG1 and BIG2, it is perhaps no surprise that total β-catenin was not significantly altered (Fig. S6).
Fig. S6.
Effects of BIG1 and/or BIG2 depletion on cellular localization of active β-catenin. After a 72-h incubation with vehicle (mock) or siRNA, NT, or specific for BIG1 or BIG2, or both, HeLa cells were fixed, reacted with antibodies against β-catenin (total) or active β-catenin (without phosphorylation of Ser37 and Thr41), and prepared for confocal immunofluorescence microscopy. (Scale bar, 10 μm.) To quantify active β-catenin in nuclei of HeLa cells, nuclear perimeter was demarcated and fluorescence intensity quantified in pixels using ImageJ software. In each group, >50 transfected cells were counted. ***P < 0.005 vs. NT siRNA.
In nuclei, β-catenin interacts with transcription factors of the TCF/LEF-1 family, to accomplish transcriptional activation of target genes, such as MYC (6) and CCND1, which encodes cyclin D (7, 8). To explore mechanisms of BIG1 and/or BIG2 action on β-catenin phosphorylation by PKA, we estimated levels of MYC and CCND1 mRNA using RT-PCR. As amounts of both MYC and CCND1 mRNA in HeLa cells were significantly lower after depletion of BIG1 or BIG2 (Fig. 6A), TCF/LEF-1 transcriptional activities were compared in control or BIG1- or BIG2-depleted cells transfected with TCF/LEF-1 luciferase reporter TOP-Flash or a negative control vector FOP-Flash. Treatment of cells with BIG1- and/or BIG2-specific siRNA decreased TCF/LEF-1 transcriptional activity significantly (Fig. 6B). Consistent with the lower level of nuclear activated β-catenin after depletion of BIG1 and/or BIG2, TCF/LEF-1 transcriptional activity was ∼50% that of control cells.
Fig. 6.
Effects of BIG1 and/or BIG2 depletion on β-catenin–induced transcriptional activity. (A) HeLa cells were incubated with vehicle (mock) or siRNA, NT or specific for BIG1 or BIG2 or both for 72 h before mRNA was extracted, cDNA synthesized, and level of cyclin D1 or c-myc mRNA quantified by RT-PCR. *P < 0.05 vs. NT siRNA. (B) After a 48-h depletion of BIG1 and/or BIG2, cells were assayed for reporter activity. *P < 0.05 vs. NT siRNA. (C) Proposed model for enhancement of β-catenin S675 phosphorylation and transcriptional activity that requires presence of β-catenin bound to BIG1 that is interacting with BIG2. AKAP-C sequence of BIG2 supports a PKA assembly that phosphorylates β-catenin S675. Such dynamic macromolecular machines would be situated appropriately for nuclear translocation of pS675 β-catenin to promote transcriptional activity of TCF/LEF-1 family genes. Identification of other molecules, which may accompany β-catenin into nuclei, could also be important.
Wnt, by binding to its receptor Frizzled and coreceptor LRP5/6, inhibited the activity of GSK-3 and stimulated β-catenin transcriptional activity (4, 5). As transcriptional activity of β-catenin induced by Wnt3a was decreased by BIG1 or BIG2 depletion (Fig. S7A), effects of BIG1 and/or BIG2 depletion on amounts of plasma membrane Wnt receptors were assessed by reaction of cell-surface proteins with reversible biotinylating agent sulfo-NHS-SS-biotin before quantification of total and cell-surface proteins by Western blotting (Fig. S7B). Lack of effects of GEF depletion on levels of cell-surface or total coreceptors LRP6 and Frizzled 6 suggested they did not explain BIG protein depletion effects on β-catenin. Major differences, however, in effects of BIG1 and BIG2 depletion on both amounts and distribution of integrin β1 (Fig. S7 C and D), clearly need further investigation.
Fig. S7.
Effects of BIG1 and/or BIG2 depletion on Wnt3a-induced transcriptional activity and amounts of cell-surface receptor proteins. (A) After a 48-h depletion of BIG1 and/or BIG2, cells were incubated for 24 h with TOP-Flash or FOP-Flash, together with pRL-TK reporter plasmids followed by a 1-h incubation with 100 ng/mL Wnt3a, before TCF/LEF reporter activity was measured as described in Materials and Methods. *P < 0.01 vs. NT siRNA, **P < 0.05 vs. NT siRNA + Wnt3a. (B) Cells were incubated (72 h) with vehicle (mock) or siRNA, NT, or specific for BIG1 or BIG2 or both. Samples (20 μg) of total cell lysate or biotinylated cell-surface proteins from replicate samples were separated in 4–12% Bolt Bis⋅Tris Plus gels (Invitrogen), followed by reaction with indicated antibodies. (C and D) Amounts of LRP6, Frizzled 6, and integrin β1 in total cell lysate (C) or at cell surface (D) from three experiments like that in B were quantified by densitometry and values expressed relative to that of control (mock) cells = 1.0. Data are means ± SEM, *P < 0.05, **P < 0.01 vs. NT siRNA.
To explore BIG1 and BIG2 regulation of the transcriptional activity of β-catenin via S675 phosphorylation, effects of S675 mutants were assessed. Overexpression of β-catenin S675D, potential mimic of pS675, reversed effects of BIG1 or BIG2 depletion, whereas S675A did not, indicating that BIG1 and BIG2 regulated nuclear action via S675 that had been phosphorylated by PKA (Fig. S8 A–D). Data (Fig. S8 E and F) were consistent with a model (Fig. 6C) in which simultaneous interaction of BIG2 with PKA and BIG1 with β-catenin bound, is required for regulating both β-catenin phosphorylation and transcriptional activities.
Fig. S8.
Effects of BIG1 and/or BIG2 depletion on β-catenin–induced transcriptional activity and BIG2 AKAP-C mutant on phosphorylation of β-catenin S675. After a 48-h depletion of BIG1 or BIG2, cells were transfected (24 h) without or with 4 μg of EV or β-catenin (S675D) phosphomimetic mutant before measurement of TCF/LEF reporter activity (A). *P < 0.05 vs. NT siRNA, **P < 0.05 vs. BIG1 siRNA + EV or BIG2 siRNA + EV. Expression of β-catenin mutant (S675A) that cannot be phosphorylated was, however, ineffective (B). *P < 0.05 vs. EV. (C and D) After a 48-h depletion of BIG1 or BIG2, cells were transfected (24 h) with EV or appropriate WT BIG1 or BIG2 followed by 24-h incubation with TOP-Flash or FOP-Flash together with pRL-TK reporter plasmids. During a final 1-h incubation, 10 μM H-89 protein kinase inhibitor or DMSO (vehicle) was present, as indicated, before measurement of TCF/LEF reporter activity as in A. *P < 0.05 vs. NT siRNA, **P < 0.05 vs. BIG1 siRNA + EV or BIG2 siRNA + EV. (E and F) After a 48-h depletion of BIG1 or BIG2, cells were transfected (24 h) with 4 μg of EV, BIG1 AKAP mutant (E), or indicated BIG2 AKAP mutant (F), and TCF/LEF reporter activity was measured as in A. *P < 0.05 vs. NT siRNA, **P < 0.05 vs. BIG1 siRNA + EV or BIG2 siRNA + EV.
Discussion
After confirming the association of in vitro synthesized BIG1 with β-catenin or BIG2 in vitro and coIP of the three endogenous proteins from HeLa cells, we showed that depletion of BIG1 and/or BIG2 altered the intracellular distribution of β-catenin that had accumulated at perinuclear Golgi structures dependent on Arf-activating GEF functions of both BIG proteins. Additionally, coIP of endogenous PKA catalytic Cα subunit with β-catenin, BIG1, and/or BIG2 likely reflected AKAP roles of these Arf GEFs (34), consistent with the decreased PKA-catalyzed phosphorylation of β-catenin S675 that followed depletion of BIG1 and/or BIG2, and the failure of BIG2 with mutated AKAP-C to replace wild-type in BIG2-depleted cells. These data describe a mechanism through which BIG2, acting as an AKAP, modulated β-catenin function.
Phosphorylation of β-catenin S552 and S675 by PKA was reported more than 5 y ago (16, 17). Tetrameric PKA comprises two C subunits maintained inactive by interaction with a regulatory R subunit dimer. Active catalytic subunits released by cAMP binding to the R subunits phosphorylate substrates determined by intracellular site and molecular composition of the AKAP-based complexes in which they function. Intracellular localization of PKA action is achieved via specific interaction of R subunits with an AKAP domain (46) that maintains necessary spatial and temporal control of cAMP concentration and action via scaffolding and functional contributions to multimolecular complexes that include PKA itself, its substrate(s), enzymes for cAMP synthesis and degradation, as well as additional enzymes and other molecules that participate in pathway regulation or cross-talk (46). A decade ago, three functional AKAP sequences in BIG2 were reported (34), demonstrating PKA–RIα interaction with BIG2 and BIG1 in HepG2 cells. Here, we show coIP of BIG1 and BIG2 from HeLa cells with antibodies against PKA-Cα, and decreased levels of β-catenin S675 phosphorylation after depletion of BIG1 or BIG2, consistent with BIG1 and/or BIG2 regulation of β-catenin action via PKA. Phosphorylation of S675 by PKA in HEK293 and L cells had been shown to stabilize β-catenin and alter its intracellular localization (16), but was later found to promote β-catenin interaction with transcriptional coactivator cAMP-response element binding (CREB) protein in nuclei and did not affect β-catenin stability or intracellular localization (17). Whether such differences reflect complexity of regulation of Wnt/β-catenin signaling and/or relate to specific experimental conditions or biological systems is unclear.
PKA-catalyzed phosphorylation of GSK-3 might also lead to nuclear accumulation of β-catenin (47, 48). We found no changes in total HeLa cell β-catenin after BIG1 and/or BIG2 depletion, although amounts of both pS675 and nonphosphorylated S33, 37/T41 β-catenin were significantly lower. Our observation of diminished β-catenin transcriptional activity after BIG1 and/or BIG2 depletion extends earlier reports of the altered activity of β-catenin without significant changes in total amounts (17, 18, 49). Although we saw interactions of BIG1 and BIG2 with β-catenin, whether or not such physical association is required for effects on β-catenin activation remains to be determined.
Interference with the activation of Arf, essential for several vesicular trafficking pathways (50), is potentially a mechanism for effects of BIG1 and/or BIG2 depletion (21–23) on β-catenin signaling. Involvement of receptor internalization and endosomal trafficking in Wnt receptor signaling was also suggested (2). Generation of PtdIns(4,5)P2 by PIP5K, another possible action of Arf–GTP, is required for activation of Wnt coreceptor LRP6 (4, 5) that promotes oligomerization and phosphorylation of LRP6 (51, 52). Participation of both Arf1 and Arf6 in PIP5K recruitment and activation at trans-Golgi and plasma membranes has been reported (53, 54). Inhibition of an Arf GTPase-activating protein (GAP), by the small molecule QS11, increased cell content of both Arf1–GTP and Arf6–GTP, with synergistic activation of Wnt/β-catenin signaling (55). Incubation of HEK293 cells with Wnt3A-conditioned medium transiently increased amounts of Arf1–GTP and Arf6–GTP, whereas interference with Wnt-stimulated PtdIns(4,5)P2 synthesis and LRP6 phosphorylation after depletion of Arf1 or Arf6 were also reported (56).
PLD activities increased by Arf activation generated phosphatidic acid at specific intracellular sites where enhanced PIP5K action serves in regulation of membrane trafficking (44, 57). Inhibition of PLD activity by FIPI prevented restoration of β-catenin S675 phosphorylation by BIG1 overexpression in cells previously depleted of BIG1. Failure of BIG1 (E793K) overexpression to reverse effects of BIG1 depletion on β-catenin phosphorylation was consistent with Arf activation-dependent β-catenin translocation or delivery of other essential components to the proposed regulatory complex (Fig. 6C).
Routes for internalization of Wnt receptor complexes, via caveolin- or clathrin-mediated routes in different studies (58, 59), were not explored. We did find, however, no changes in levels of total or cell-surface Frizzled 6 or LRP6, excluding this explanation for BIG1/2 effects. Activation of Arf6 by Wnt5A stimulation reportedly disrupted N-cadherin–β-catenin complexes and blocked elevation of cytoplasmic and nuclear β-catenin, enhancement of β-catenin–induced transcription, and tumor cell invasion (60). Robust responses to Wnt3a signaling were demonstrated in HeLa cells (61), despite very low levels of HeLa cell N-cadherin (62), and the absence of E-cadherin (63), which can associate with β-catenin to form cell surface junctional complexes. We found N-cadherin content of HeLa cells decreased after BIG2 depletion, but cell-surface N-cadherin appeared unaffected (Fig. S2 A and B). Phosphorylation of β-catenin Y654 dramatically reduced its binding to cadherin, enhanced β-catenin–driven transcription (39, 40), and increased PKA phosphorylation of β-catenin S675 (39). Phosphorylation of β-catenin Y654 was not affected by BIG1 and/or BIG2 depletion (Fig. S2C). Whether the correlation of Y654 and S675 phosphorylations by two different enzymes at perhaps different sites is obligatory, or the two reactions occur also independently, is unknown.
Localization of β-catenin in nuclei has been related to cell proliferation, migration, and invasion (1, 3, 60). Phosphorylation of β-catenin clearly influenced its intracellular distribution (1, 3), and reversible O-glycosylation may affect its nuclear accumulation and transcriptional action (64–66). Correct N-glycosylation and trafficking of integrin β1 required BIG1 (30) and BIG2 (28), respectively, but their roles in O-glycosylation are unknown. We speculate that accumulation of β-catenin at Golgi structures after BIG1 or BIG2 depletion contributes to inhibition of Wnt signaling by delaying β-catenin transit to and action in nuclei.
The importance of β-catenin transcriptional activity in tumorigenesis and developmental events that depend on β-catenin–enhanced transcription (1, 3) can lead to uncontrolled cell proliferation, with altered cell migration and invasion (1). In addition to roles for BIG1 in control of cell polarity during directed migration (31), and appropriate integrin β1 N-glycosylation (30) as well as for BIG2 in its intracellular trafficking (28), we show here that both BIG1 and BIG2, via multiple, different actions, contributed to PKA-catalyzed phosphorylation of β-catenin with resulting enhancement of cyclin D and c-myc transcription, whether or not initiated by Wnt signaling.
Materials and Methods
Sources of antibodies, reagents, and plasmids are in SI Materials and Methods. HeLa cells were grown, and confocal immunofluorescence microscopy, as well as luciferase assays, were performed as described in SI Materials and Methods. Procedures for RT-PCR, IP, and in vitro assays (yeast two-hybrid analysis, wheat germ extract system experiments) are also described in SI Materials and Methods.
SI Materials and Methods
Antibodies and Reagents.
Affinity-purified rabbit antibodies against human BIG1 and BIG2 have been described (23). Antibodies against β-catenin (610154), α-catenin (610194), syntaxin-6 (610635), calreticulin (612137), and GM130 (610823) were purchased from BD Biosciences; against HA (3724), phospho-(S675) β-catenin (4176), nonphosphorylated (S33/37/T41) β-catenin (8814), LRP6 (2560), and Frizzled 6 (5158) from Cell Signaling; against active β-catenin (05-665), β-catenin (06-734), and GAPDH (AB2302) from Millipore; against phospho-(Y654) β-catenin (CP4021) from ECM Biosciences; against PKA catalytic subunit α (sc-28315) and N-cadherin (sc-7939) from Santa Cruz Biotechnology; against FLAG (F1804), c-myc (C3956), α-tubulin (T5168), β-actin (A5441), and βCOP (G6160) from Sigma-Aldrich; and against GFP (632592) from Clontech. Horseradish peroxidase-conjugated sheep anti-rabbit and anti-mouse Ig (IgG) and TnT T7 coupled wheat germ extract system were purchased from Promega and Alexa Fluor 594- or 488-conjugated goat anti-rabbit or anti-mouse IgG (H+L) from Invitrogen. Protease inhibitor mixture tablets were purchased from Roche, Wnt3a from R&D Systems, and inhibitors of PLD1 and PLD2 (5-fluoro-2-indolyl des-chlorohalopemide, FIPI) and of PKA (H89) from Sigma-Aldrich.
Expression Plasmids.
Plasmids β-catenin-pcDNA3 (67), GSK3β-pCMV-Tag 5A (68), M50 Super 8× TOP-Flash, and M51 Super 8× FOP-Flash (69) were purchased from Addgene and those encoding Renilla (pRL-TK) from Promega. For yeast two-hybrid analyses, GSK3β and fragments of BIG1 and BIG2 were subcloned into pGADT7, and β-catenin into pGBKT7 vectors. Plasmids pCMV6-XL4-BIG2 WT and inactive mutants AKAP-A, -B, -C have been described (37).
BIG1 AKAP domain mutation was introduced using QuikChange II XL site-directed mutagenesis kit (Agilent Technologies) with HA-BIG1(V583W) primer 5′-ATATTTGAAAGATGGAATGATCTATCAAAA-3′. β-catenin S675 mutations were similarly introduced using QuikChange II site-directed mutagenesis kit (Agilent Technologies) with primers 5′-TACAAGAAACGGCTTGACGTTGAGCTGACCAGC-3′ (S675D) and 5′-TACAAGAAACGGCTTGCAGTTGAGCTGACCAGC-3′ (S675A).
Cell Culture and Transfection.
HeLa cells used in all experiments, grown in DMEM (Invitrogen) with 10% (vol/vol) FBS (Invitrogen) in a humidified 5% (vol/vol) CO2 atmosphere at 37 °C, were transfected with 100 nM BIG1- or BIG2-specific siRNA. Sense sequences were GUCCAAAUGUCCUCGCAUA (BIG1) and CCAAAAGAUUGACCGAUUA (BIG2), with Dharmacon siCONTROL nontargeting siRNA as negative control. DharmaFECT1 transfection reagent was used according to manufacturer’s instructions with a 72-h incubation. HilyMax DNA transfection reagent (Dojindo Molecular Technologies) was used 24 h before cell analysis for transient protein expression according to the manufacturer’s protocol.
Western Blotting and Immunoprecipitation.
To quantify protein (BCA kit, Pierce), collected cells were broken in radio-immunoprecipitation assay (RIPA) buffer (50 mM Tris·HCl, pH 7.4, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, and EDTA-free protease inhibitor mixture). Samples of proteins (15 μg) were separated by SDS/PAGE, identified by Western blotting, and quantified by densitometry.
To cross-link interacting proteins before IP, cells were incubated (30 min, room temperature) with 2 mM dithiobis-succinimidylpropionate (Pierce), a thiol-cleavable cross-linker dissolved in dry DMSO, which was quenched with 20 mM Tris buffer, pH 7.5, before cells were mechanically broken in ice-cold Nonidet P-40 buffer (50 mM Tris·HCl, pH 7.4, 50 mM NaCl, 1% Nonidet P-40 and EDTA-free protease inhibitor mixture). Samples of proteins (100 μg) were incubated overnight (4 °C with mixing) with 1 μg of specific antibodies or control IgG, and 3 h more after adding 15 μL of Dynabeads Protein G (Invitrogen). Beads were washed five times with Nonidet P-40 buffer before proteins were eluted, separated by SDS/PAGE in 4–12% NuPAGE gel (Invitrogen), transferred to nitrocellulose membranes (Invitrogen) that were blocked with 5% (wt/vol) nonfat milk (Bio-Rad), reacted with primary and secondary (horseradish peroxidase-coupled) antibodies, developed using Super Signal Chemiluminescent substrate (Pierce), and exposed in the LAS-4000 system (Fujifilm). In most experiments, samples of input (5%, before IP) and of proteins from IP (50%) were analyzed.
Yeast Two-Hybrid Analyses.
Yeast two-hybrid analyses were performed in Saccharomyces cerevisiae strain AH109 (Clontech) according to the manufacturer’s instructions. Briefly, after transformation with pGADT7 and pGBKT7 fusion constructs plus carrier DNA using lithium acetate, yeast was grown on nonselective plates (lacking leucine and tryptophan) and colonies were transferred to selective plates (lacking histidine and/or adenine).
In Vitro Protein Synthesis and Binding Assays.
TNT coupled wheat germ extract system (Promega) was used for transcription according to the manufacturer’ s recommendations. Reactions containing template DNA 1 μg, wheat germ extract 25 μL, reaction buffer 2 μL, RNA polymerase (SP6 or T7) 1 μL, 1 μL of 1 mM amino acid mixture minus methionine, 1 mM amino acid mixture minus leucine 1 μL, and nuclease-free water to a total volume of 50 μL, were incubated (30 °C, 2 h). For in vitro binding reactions, 20-μL samples of protein products were incubated (4 °C, 4 h) in Nonidet P-40 buffer before IP and analysis by Western blotting.
Subcellular Fractionation.
Fractionation of postnuclear supernatant (PNS) by density-gradient centrifugation has been described (31). Briefly, cells (90% confluent, 10-cm plates) were washed with PBS, harvested by scraping in ice-cold homogenization buffer (0.25 M sucrose, 10 mM Tris-Cl, pH 7.4, 1 mM magnesium acetate), incubated on ice (15 min), and broken by passage ∼15 times through a 30-gauge needle to ∼90% complete breakage. Homogenates were centrifuged (800 × g, 6 min, 4 °C) to remove nuclei and unbroken cells; samples (0.6 mL) of PNS were transferred to step gradients containing 2%, 6%, 10%, 14%, 18%, 22%, and 26% (wt/vol) iodixanol (0.6 mL each, total volume 4.2 mL). After centrifugation (100,000 × g, 4 °C, 3 h), 15 fractions were collected from the top and 15-μL samples of each were subjected to SDS/PAGE for analysis by Western blotting and densitometric quantification.
Confocal Immunofluorescence Microscopy.
Cells (7 × 104 per well) were grown on Lab-Tek four-well chamber slides (Nunc) 24 h before fixation [4% (wt/vol) paraformaldehyde in PBS, 15 min, 25 °C] and permeabilization (0.01% Triton X-100 and 0.05% SDS in PBS, 5 min). After 30 min in blocking buffer (0.1% saponin, 0.2% BSA in PBS), cells were incubated with primary antibodies in the same buffer (1 h), washed with PBS, incubated with Alexa Fluor-conjugated secondary antibodies in blocking buffer (1 h), and mounted in VECTASHIELD mounting medium with DAPI (Vector Laboratories). Images were acquired using LSM 510 META laser confocal microscope (Carl Zeiss) with 40×/1.3 N.A. oil objective lens and 488- or 543-nm lasers. Pinholes were set to scan ∼1-μm layers (resolution 512 × 512 pixels). Projection view and optical sections were processed digitally using CLSM5 Zeiss Browse Image software. Figures were assembled and labeled using Adobe Photoshop (Adobe Systems).
RNA Extraction, Reverse Transcription, and PCR.
Total RNA and first-strand cDNA were prepared using RNeasy Plus mini kit and QuantiTect reverse transcription kit (Qiagen) according to manufacturer’s instructions. Semiquantitative reverse transcription-PCR was performed to estimate β-catenin/TCF target gene (c-myc, and cyclin D1) mRNA levels. Primer sequences (forward and reverse) were: β-actin, 5′-CTGGGACGACATGGAGAAAA-3′ and 5′-AAGGAAGGCTGGAAGAGTGC-3′; c-myc, 5′-ATTTCTACTGCGACGAGGAGG-3′ and 5′-TGCTGTCGTTGAGAGGGTAGG-3′; and cyclin D1, 5′-CTGGAGCCCGTGAAAAAGAGC-3′ and 5′-CTGGAGAGGAAGCGTGTGAGG-3′. For PCR (total volume 50 μL), we used ∼1 μg of cDNA, 5 pmol of each primer, 200 μm of each dNTP, and 1 unit of Taq polymerase (TaKaRa). After denaturation (95 °C, 3 min), 25 PCR cycles of 95 °C/40 s, 53 °C/40 s, and 72 °C/40 s ended with 10 min at 72 °C. PCR products were subjected to electrophoresis in 2% agarose gel in TBE buffer (89 mm Tris-base, pH 7.6, 89 mm boric acid, 2 mm EDTA), before staining with ethidium bromide and photography on a UV light box.
Luciferase Assays of β-Catenin Transcriptional Activity.
Cells growing in 12-well plates were transiently transfected with TOP-Flash or FOP-Flash reporter plasmid (1 µg), containing eight copies of optimal or mutant TCF/LEF consensus-binding sites upstream of firefly luciferase, together with Renilla luciferase reporter plasmid (pRL-TK, 0.1 µg), using dual luciferase assay kit (Promega), according to the manufacturer’s instructions. After 24 h, cells were collected and lysed in passive lysis buffer (Promega). Firefly luciferase activity (TCF-responsive reporter TOP-Flash) of each sample was normalized for transfection efficiency to its Renilla luciferase activity driven by TK promoter.
Cell-Surface Protein Isolation.
To isolate biotinylated cell-surface proteins, Pierce Cell Surface Protein Isolation Kit (89881, Thermo Scientific) was used according to the manufacturer’s protocol. For labeling surface proteins, cells on 100-mm plates were washed three times with ice-cold PBS and incubated (30 min, 4 °C) in 10 mL of PBS containing 0.24 mg/mL EZ-link sulfo-NHS-SS-biotin before reaction-quenching solution (Thermo Scientific) was added. Cells were then washed twice with TBS, collected by scraping gently, and homogenized in 400 μL of lysis buffer (Thermo Scientific). Cell lysates (300 μg of protein in 300 μL) were mixed end over end (1 h, room temperature) with 300 μL of immobilized NeutrAvidin agarose beads (Thermo Scientific), which were then washed and biotinylated proteins eluted in 300 μL of SDS sample buffer containing 50 mM DTT. Samples (20 μL) were subjected to SDS/PAGE and proteins analyzed by immunoblotting.
Statistical Analysis.
Data are means ± SEM of values from at least three experiments with significance defined as P < 0.05 using Student’s t test.
Acknowledgments
We thank Drs. Christian Combs and Daniela Malide [Light Microscopy Core Facility, National Heart, Lung, and Blood Institute (NHLBI)] for their much appreciated assistance with confocal microscopy and Dr. Michael A. Frohman for expert advice on PLD inhibitors. This work was supported by the Intramural Research Program, NIH, NHLBI, and in part by the Taiwan Ministry of Science and Technology (Grants MOST 103-2320-B-006-039-MY3 and MOST 104-2320-B-006-032-MY3) (to C.-C.L.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1601918113/-/DCSupplemental.
References
- 1.Valenta T, Hausmann G, Basler K. The many faces and functions of β-catenin. EMBO J. 2012;31(12):2714–2736. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kikuchi A, Yamamoto H, Sato A, Matsumoto S. New insights into the mechanism of Wnt signaling pathway activation. Int Rev Cell Mol Biol. 2011;291:21–71. doi: 10.1016/B978-0-12-386035-4.00002-1. [DOI] [PubMed] [Google Scholar]
- 3.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Taelman VF, et al. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell. 2010;143(7):1136–1148. doi: 10.1016/j.cell.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng X, et al. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438(7069):873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He TC, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281(5382):1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 7.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398(6726):422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 8.Shtutman M, et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA. 1999;96(10):5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haegel H, et al. Lack of beta-catenin affects mouse development at gastrulation. Development. 1995;121(11):3529–3537. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- 10.Howard S, Deroo T, Fujita Y, Itasaki N. A positive role of cadherin in Wnt/β-catenin signalling during epithelial-mesenchymal transition. PLoS One. 2011;6(8):e23899. doi: 10.1371/journal.pone.0023899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCrea PD, Turck CW, Gumbiner B. A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science. 1991;254(5036):1359–1361. doi: 10.1126/science.1962194. [DOI] [PubMed] [Google Scholar]
- 12.van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H. Wnt signaling controls the phosphorylation status of beta-catenin. J Biol Chem. 2002;277(20):17901–17905. doi: 10.1074/jbc.M111635200. [DOI] [PubMed] [Google Scholar]
- 13.Staal FJ, van Noort M, Strous GJ, Clevers HC. Wnt signals are transmitted through N-terminally dephosphorylated beta-catenin. EMBO Rep. 2002;3(1):63–68. doi: 10.1093/embo-reports/kvf002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hendriksen J, et al. Plasma membrane recruitment of dephosphorylated beta-catenin upon activation of the Wnt pathway. J Cell Sci. 2008;121(11):1793–1802. doi: 10.1242/jcs.025536. [DOI] [PubMed] [Google Scholar]
- 15.Maher MT, Flozak AS, Stocker AM, Chenn A, Gottardi CJ. Activity of the beta-catenin phosphodestruction complex at cell-cell contacts is enhanced by cadherin-based adhesion. J Cell Biol. 2009;186(2):219–228. doi: 10.1083/jcb.200811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hino S, Tanji C, Nakayama KI, Kikuchi A. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase stabilizes beta-catenin through inhibition of its ubiquitination. Mol Cell Biol. 2005;25(20):9063–9072. doi: 10.1128/MCB.25.20.9063-9072.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taurin S, Sandbo N, Qin Y, Browning D, Dulin NO. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase. J Biol Chem. 2006;281(15):9971–9976. doi: 10.1074/jbc.M508778200. [DOI] [PubMed] [Google Scholar]
- 18.Fang D, et al. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282(15):11221–11229. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morinaga N, Tsai SC, Moss J, Vaughan M. Isolation of a brefeldin A-inhibited guanine nucleotide-exchange protein for ADP ribosylation factor (ARF) 1 and ARF3 that contains a Sec7-like domain. Proc Natl Acad Sci USA. 1996;93(23):12856–12860. doi: 10.1073/pnas.93.23.12856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morinaga N, Moss J, Vaughan M. Cloning and expression of a cDNA encoding a bovine brain brefeldin A-sensitive guanine nucleotide-exchange protein for ADP-ribosylation factor. Proc Natl Acad Sci USA. 1997;94(24):12926–12931. doi: 10.1073/pnas.94.24.12926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Togawa A, Morinaga N, Ogasawara M, Moss J, Vaughan M. Purification and cloning of a brefeldin A-inhibited guanine nucleotide-exchange protein for ADP-ribosylation factors. J Biol Chem. 1999;274(18):12308–12315. doi: 10.1074/jbc.274.18.12308. [DOI] [PubMed] [Google Scholar]
- 22.Morinaga N, Adamik R, Moss J, Vaughan M. Brefeldin A inhibited activity of the sec7 domain of p200, a mammalian guanine nucleotide-exchange protein for ADP-ribosylation factors. J Biol Chem. 1999;274(25):17417–17423. doi: 10.1074/jbc.274.25.17417. [DOI] [PubMed] [Google Scholar]
- 23.Yamaji R, et al. Identification and localization of two brefeldin A-inhibited guanine nucleotide-exchange proteins for ADP-ribosylation factors in a macromolecular complex. Proc Natl Acad Sci USA. 2000;97(6):2567–2572. doi: 10.1073/pnas.97.6.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen X, et al. Association of brefeldin A-inhibited guanine nucleotide-exchange protein 2 (BIG2) with recycling endosomes during transferrin uptake. Proc Natl Acad Sci USA. 2006;103(8):2635–2640. doi: 10.1073/pnas.0510599103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin HW, Morinaga N, Noda M, Nakayama K. BIG2, a guanine nucleotide exchange factor for ADP-ribosylation factors: Its localization to recycling endosomes and implication in the endosome integrity. Mol Biol Cell. 2004;15(12):5283–5294. doi: 10.1091/mbc.E04-05-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao X, Lasell TK, Melançon P. Localization of large ADP-ribosylation factor-guanine nucleotide exchange factors to different Golgi compartments: Evidence for distinct functions in protein traffic. Mol Biol Cell. 2002;13(1):119–133. doi: 10.1091/mbc.01-08-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishizaki R, Shin HW, Mitsuhashi H, Nakayama K. Redundant roles of BIG2 and BIG1, guanine-nucleotide exchange factors for ADP-ribosylation factors in membrane traffic between the trans-Golgi network and endosomes. Mol Biol Cell. 2008;19(6):2650–2660. doi: 10.1091/mbc.E07-10-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen X, et al. Brefeldin A-inhibited ADP-ribosylation factor activator BIG2 regulates cell migration via integrin β1 cycling and actin remodeling. Proc Natl Acad Sci USA. 2012;109(36):14464–14469. doi: 10.1073/pnas.1211877109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kondo Y, et al. ARF1 and ARF3 are required for the integrity of recycling endosomes and the recycling pathway. Cell Struct Funct. 2012;37(2):141–154. doi: 10.1247/csf.12015. [DOI] [PubMed] [Google Scholar]
- 30.Shen X, Hong MS, Moss J, Vaughan M. BIG1, a brefeldin A-inhibited guanine nucleotide-exchange protein, is required for correct glycosylation and function of integrin beta1. Proc Natl Acad Sci USA. 2007;104(4):1230–1235. doi: 10.1073/pnas.0610535104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li CC, et al. Effects of brefeldin A-inhibited guanine nucleotide-exchange (BIG) 1 and KANK1 proteins on cell polarity and directed migration during wound healing. Proc Natl Acad Sci USA. 2011;108(48):19228–19233. doi: 10.1073/pnas.1117011108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheen VL, et al. Mutations in ARFGEF2 implicate vesicle trafficking in neural progenitor proliferation and migration in the human cerebral cortex. Nat Genet. 2004;36(1):69–76. doi: 10.1038/ng1276. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, et al. Brefeldin A-inhibited guanine exchange factor 2 regulates filamin A phosphorylation and neuronal migration. J Neurosci. 2012;32(36):12619–12629. doi: 10.1523/JNEUROSCI.1063-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H, Adamik R, Pacheco-Rodriguez G, Moss J, Vaughan M. Protein kinase A-anchoring (AKAP) domains in brefeldin A-inhibited guanine nucleotide-exchange protein 2 (BIG2) Proc Natl Acad Sci USA. 2003;100(4):1627–1632. doi: 10.1073/pnas.0337678100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuroda F, Moss J, Vaughan M. Regulation of brefeldin A-inhibited guanine nucleotide-exchange protein 1 (BIG1) and BIG2 activity via PKA and protein phosphatase 1gamma. Proc Natl Acad Sci USA. 2007;104(9):3201–3206. doi: 10.1073/pnas.0611696104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puxeddu E, et al. Interaction of phosphodiesterase 3A with brefeldin A-inhibited guanine nucleotide-exchange proteins BIG1 and BIG2 and effect on ARF1 activity. Proc Natl Acad Sci USA. 2009;106(15):6158–6163. doi: 10.1073/pnas.0901558106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Islam A, et al. cAMP-dependent protein kinase A (PKA) signaling induces TNFR1 exosome-like vesicle release via anchoring of PKA regulatory subunit RIIbeta to BIG2. J Biol Chem. 2008;283(37):25364–25371. doi: 10.1074/jbc.M804966200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hart MJ, de los Santos R, Albert IN, Rubinfeld B, Polakis P. Downregulation of beta-catenin by human Axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta. Curr Biol. 1998;8(10):573–581. doi: 10.1016/s0960-9822(98)70226-x. [DOI] [PubMed] [Google Scholar]
- 39.van Veelen W, et al. β-catenin tyrosine 654 phosphorylation increases Wnt signalling and intestinal tumorigenesis. Gut. 2011;60(9):1204–1212. doi: 10.1136/gut.2010.233460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piedra J, et al. Regulation of beta-catenin structure and activity by tyrosine phosphorylation. J Biol Chem. 2001;276(23):20436–20443. doi: 10.1074/jbc.M100194200. [DOI] [PubMed] [Google Scholar]
- 41.Shinotsuka C, Waguri S, Wakasugi M, Uchiyama Y, Nakayama K. Dominant-negative mutant of BIG2, an ARF-guanine nucleotide exchange factor, specifically affects membrane trafficking from the trans-Golgi network through inhibiting membrane association of AP-1 and GGA coat proteins. Biochem Biophys Res Commun. 2002;294(2):254–260. doi: 10.1016/S0006-291X(02)00456-4. [DOI] [PubMed] [Google Scholar]
- 42.Taurin S, Sandbo N, Yau DM, Sethakorn N, Dulin NO. Phosphorylation of beta-catenin by PKA promotes ATP-induced proliferation of vascular smooth muscle cells. Am J Physiol Cell Physiol. 2008;294(5):C1169–C1174. doi: 10.1152/ajpcell.00096.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le K, Li CC, Ye G, Moss J, Vaughan M. Arf guanine nucleotide-exchange factors BIG1 and BIG2 regulate nonmuscle myosin IIA activity by anchoring myosin phosphatase complex. Proc Natl Acad Sci USA. 2013;110(34):E3162–E3170. doi: 10.1073/pnas.1312531110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riebeling C, Morris AJ, Shields D. Phospholipase D in the Golgi apparatus. Biochim Biophys Acta. 2009;1791(9):876–880. doi: 10.1016/j.bbalip.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su W, et al. 5-Fluoro-2-indolyl des-chlorohalopemide (FIPI), a phospholipase D pharmacological inhibitor that alters cell spreading and inhibits chemotaxis. Mol Pharmacol. 2009;75(3):437–446. doi: 10.1124/mol.108.053298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welch EJ, Jones BW, Scott JD. Networking with AKAPs: Context-dependent regulation of anchored enzymes. Mol Interv. 2010;10(2):86–97. doi: 10.1124/mi.10.2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujino H, West KA, Regan JW. Phosphorylation of glycogen synthase kinase-3 and stimulation of T-cell factor signaling following activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. J Biol Chem. 2002;277(4):2614–2619. doi: 10.1074/jbc.M109440200. [DOI] [PubMed] [Google Scholar]
- 48.Fang X, et al. Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc Natl Acad Sci USA. 2000;97(22):11960–11965. doi: 10.1073/pnas.220413597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ji H, et al. EGF-induced ERK activation promotes CK2-mediated disassociation of alpha-Catenin from beta-Catenin and transactivation of beta-Catenin. Mol Cell. 2009;36(4):547–559. doi: 10.1016/j.molcel.2009.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donaldson JG, Jackson CL. ARF family G proteins and their regulators: Roles in membrane transport, development and disease. Nat Rev Mol Cell Biol. 2011;12(6):362–375. doi: 10.1038/nrm3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schramp M, Thapa N, Heck J, Anderson R. PIPKIγ regulates β-catenin transcriptional activity downstream of growth factor receptor signaling. Cancer Res. 2011;71(4):1282–1291. doi: 10.1158/0008-5472.CAN-10-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan W, et al. Wnt3a-mediated formation of phosphatidylinositol 4,5-bisphosphate regulates LRP6 phosphorylation. Science. 2008;321(5894):1350–1353. doi: 10.1126/science.1160741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Godi A, et al. ARF mediates recruitment of PtdIns-4-OH kinase-beta and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat Cell Biol. 1999;1(5):280–287. doi: 10.1038/12993. [DOI] [PubMed] [Google Scholar]
- 54.Honda A, et al. Phosphatidylinositol 4-phosphate 5-kinase alpha is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell. 1999;99(5):521–532. doi: 10.1016/s0092-8674(00)81540-8. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Q, et al. Small-molecule synergist of the Wnt/beta-catenin signaling pathway. Proc Natl Acad Sci USA. 2007;104(18):7444–7448. doi: 10.1073/pnas.0702136104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim W, et al. ADP-ribosylation factors 1 and 6 regulate Wnt/β-catenin signaling via control of LRP6 phosphorylation. Oncogene. 2013;32(28):3390–3396. doi: 10.1038/onc.2012.373. [DOI] [PubMed] [Google Scholar]
- 57.Roth MG. Molecular mechanisms of PLD function in membrane traffic. Traffic. 2008;9(8):1233–1239. doi: 10.1111/j.1600-0854.2008.00742.x. [DOI] [PubMed] [Google Scholar]
- 58.Jiang Y, He X, Howe PH. Disabled-2 (Dab2) inhibits Wnt/β-catenin signalling by binding LRP6 and promoting its internalization through clathrin. EMBO J. 2012;31(10):2336–2349. doi: 10.1038/emboj.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamamoto H, Sakane H, Yamamoto H, Michiue T, Kikuchi A. Wnt3a and Dkk1 regulate distinct internalization pathways of LRP6 to tune the activation of beta-catenin signaling. Dev Cell. 2008;15(1):37–48. doi: 10.1016/j.devcel.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 60.Grossmann AH, et al. The small GTPase ARF6 stimulates β-catenin transcriptional activity during WNT5A-mediated melanoma invasion and metastasis. Sci Signal. 2013;6(265):ra14. doi: 10.1126/scisignal.2003398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang W, et al. A genome-wide RNAi screen for Wnt/beta-catenin pathway components identifies unexpected roles for TCF transcription factors in cancer. Proc Natl Acad Sci USA. 2008;105(28):9697–9702. doi: 10.1073/pnas.0804709105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herrenknecht K, et al. The uvomorulin-anchorage protein alpha catenin is a vinculin homologue. Proc Natl Acad Sci USA. 1991;88(20):9156–9160. doi: 10.1073/pnas.88.20.9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vessey CJ, et al. Altered expression and function of E-cadherin in cervical intraepithelial neoplasia and invasive squamous cell carcinoma. J Pathol. 1995;176(2):151–159. doi: 10.1002/path.1711760208. [DOI] [PubMed] [Google Scholar]
- 64.Olivier-Van Stichelen S, et al. O-GlcNAcylation stabilizes β-catenin through direct competition with phosphorylation at threonine 41. FASEB J. 2014;28(8):3325–3338. doi: 10.1096/fj.13-243535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hart GW. Minireview series on the thirtieth anniversary of research on O-GlcNAcylation of nuclear and cytoplasmic proteins: Nutrient regulation of cellular metabolism and physiology by O-GlcNAcylation. J Biol Chem. 2014;289(50):34422–34423. doi: 10.1074/jbc.R114.609776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bond MR, Hanover JA. A little sugar goes a long way: The cell biology of O-GlcNAc. J Cell Biol. 2015;208(7):869–880. doi: 10.1083/jcb.201501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kolligs FT, Hu G, Dang CV, Fearon ER. Neoplastic transformation of RK3E by mutant beta-catenin requires deregulation of Tcf/Lef transcription but not activation of c-myc expression. Mol Cell Biol. 1999;19(8):5696–5706. doi: 10.1128/mcb.19.8.5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou BP, et al. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6(10):931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 69.Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol. 2003;13(8):680–685. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]