Abstract
Background
Race and sex differences in silent myocardial infarction (SMI) are not well-established.
Methods and Results
The analysis included 9,498 participants from the ARIC study who were free of cardiovascular disease at baseline (visit-1; 1987–1989). Incident SMI was defined as ECG-evidence of MI without clinically documented MI (CMI) after the baseline until ARIC visit-4 (1996–1998). Coronary heart disease (CHD) and all-cause deaths were ascertained starting from ARIC visit-4 until 2010. During a median follow-up of 8.9 years, 317 (3.3%) participants developed SMI while 386 (4.1%) developed CMI. The incidence rates of both SMI and CMI were higher in men (5.08 and 7.96 per 1000-person years, respectively) than in women (2.93 and 2.25 per 1000-person years, respectively); p-value <.0001 for both. Blacks had non-significantly higher rate of SMI than whites (4.45 vs. 3.69 per 1000-person years; p-value=0.217) but whites had higher rate of CMI than blacks (5.04 vs. 3.24 per 1000-person years; p-value=0.002). SMI and CMI (vs. no MI) were associated with increased risk of CHD death (HR(95%CI): 3.06(1.88–4.99) and 4.74(3.26–6.90), respectively) and all-cause mortality (HR(95%CI):1.34(1.09–1.65) and 1.55(1.30–1.85), respectively). However, SMI and CMI were associated with increased mortality among both men and women, with potentially greater increased risk among women (interaction p-value= 0.089 and 0.051, respectively). No significant interactions by race were detected.
Conclusions
SMI represents over 45% of incident MIs and is associated with poor prognosis. Race and sex differences in the incidence and prognostic significance of SMI exist which may warrant considering SMI in personalized assessment of CHD risk.
Keywords: Silent Myocardial Infarction, Race, Sex, Coronary Heart Disease
INTRODUCTION
About 635,000 new cases of coronary heart disease (CHD) occur annually in the United States, with an additional 155,000 incidentally discovered asymptomatic silent MIs (SMI).1 SMI, defined as the presence of pathological Q waves in the absence of a history of typical cardiac symptoms, is one of the important cardiac abnormalities that can be reasonably detected through electrocardiogram (ECG) screening.2, 3
Given that SMI is characterized by no or mild symptoms, those patients are deprived from medical treatments that could prevent subsequent adverse outcomes, including a second MI or even death.4 This underscores the importance of detection of SMI in clinical practice. In clinical trials evaluating interventions to prevent or treat CHD, detection of unrecognized MI as a clinical end point has the potential to increase statistical power and allowing decreased sample sizes or reduced length of follow-up, cost, and potential harm from exposure.4
The reported incidence of SMI ranges from 22% to 60% of the total MI incidence, and the prognosis of these SMIs has been shown to be similar to, or worse than, clinically recognized MI 4–27. However, the current understanding of the epidemiology of SMI is based primarily on studies in white populations of European ancestry,8, 11–15, 18 or on studies with limited representation of both sexes.9, 10, 16, 18, 21, 23 The lack of race and sex diversity in these studies is occasionally complicated by small sample size as well.7, 28
The aim of this study was to examine the race and sex differences in the incidence and prognostic significance of SMI vs. MI with clinical manifestations (CMI) in the Atherosclerosis Risk in Communities (ARIC) study, a community-based predominantly biracial cohort study.
METHODS
Study population
The ARIC study was designed to investigate the causes of atherosclerosis and its clinical outcomes, as well as variation in cardiovascular risk factors, medical care, and disease by race and sex.29 From 1987 to 1989 (ARIC study baseline), 15 792 adults (55.2% women; age, 45–64 years) from 4 US communities (Washington County, Maryland; suburbs of Minneapolis, Minnesota; Jackson, Mississippi; and Forsyth County, North Carolina) were enrolled and underwent a phone interview and clinic visit. Additional examinations were conducted in 1990–1992 (visit 2), 1993–1995 (visit 3), 1996–1998 (visit 4), and 2011–2013 (visit 5). Participants were mostly white in the Washington County and Minneapolis sites, exclusively black in Jackson, and a mix of both in Forsyth County. The study was approved by each study site’s institutional review board. All participants provided written informed consent.
For the purpose of this analysis, we included all ARIC participants with good quality and complete ECG data at visits 1 to 4 as well as outcome events after visit 4. We excluded the follwing partcipants: 47 with reported race other than African-American or white, 136 with poor quality ECG, 3,775 with missing ECG in any of the ARIC first 4 visits (including 871 who died before visit 4), 429 with ECG diagnosis of bundle branch block, external pacemaker or Wolff-Parkinson-White pattern, and 201 with missing one or more of baseline cardiovascular disease (CVD) risk factors. We also excluded 1,706 participants with history of CVD at baseline which was defined as the presence of ECG evidence of MI, or a self-reported history of physician-diagnosed MI, coronary artery bypass surgery, coronary angioplasty, heart failure, or stroke. After all exclusions (n=6,294), 9,498 remained and were included in the analysis.
Silent MI
Incident SMI was defined as ECG evidence of new MI at ARIC visit 2, visit 3 or visit 4 that was not present at the baseline visit (visit 1) in the absence of documented CMI. Participants with both SMI and CMI between ARIC visit 1 and visit 4 were considered as having CMI. Identical electrocardiographs (MAC PC, Marquette Electronics Inc., Milwaukee, Wisconsin) were used at all clinical sites, and resting 10-second standard simultaneous 12-lead ECGs were recorded in all participants using strictly standardized procedures. All ECGs were processed in a central ECG laboratory (initially at Dalhousie University, Halifax, Nova Scotia, Canada and later at the EPICARE Center, Wake Forest School of Medicine, Winston-Salem, North Carolina, USA), where all ECGs were visually inspected for technical errors and quality. ECG evidence of MI was defined by new appearance of Minnesota Code ECG Classifications as a major Q/QS wave abnormality (MC 1.1 or MC 1.2), or minor Q/QS waves abnormality (MC 1.3) plus major ST-T abnormality (MC 4.1, MC 4.2, MC 5.1 or MC 5.2).30, 31 Traditional serial change comparisons30 were not used.
CHD death and all-cause mortality
CHD death and all-cause mortality were ascertained after ARIC visit 4 (1996–1998) through December 31, 2010 from death certificates. Deaths and hospitalization events were ascertained in each clinical center during an annual follow-up phone interview or through review of community hospital discharge indexes. Incident CHD events included definite or probable hospitalized MI (clinical myocardial infarction, i.e. CMI in this analysis) or definite CHD death. All CHD events classification and specific criteria including the adjudication process have been previously described.32–34 CMI was based on physician review and adjudication of chest pain, cardiac biomarkers/enzymes from hospitalizations, ECG evidence including a new pathological Q wave, CHD history, the underlying cause of death from death certificates, and other associated information. All eligible hospitalized events were classified as either definite, probable, suspect, or no MI. Definite or probable MI was combined to define a clinically manifest MI (CMI) in this analysis. The definite hospitalized CMI met one or more of the following criteria: 1) Evolving diagnostic ECG pattern; 2) Diagnostic ECG pattern and abnormal enzymes; 3) Cardiac pain and abnormal enzymes plus evolving ST-T pattern or equivocal ECG pattern. The probable hospitalized MI met one or more of the following criteria in the absence of sufficient evidence for definite hospitalized MI: 1) Cardiac pain and abnormal enzymes; 2) Cardiac pain and equivocal enzymes and either evolving ST-T pattern or diagnostic ECG pattern. 3) Abnormal enzymes and evolving ST-T pattern. Criteria for each of these diagnostic elements in the algorithm remained constant over the study period and are described in detail in the ARIC Study Surveillance Manual.31, 33, 34
Covariates
Baseline age, sex, race, education level, income, and smoking status were determined by self-report. Body mass index at baseline was calculated as weight in kilograms divided by height in meters squared. Blood samples were obtained after an 8-hour fasting period. Baseline diabetes mellitus was defined as a fasting glucose level ≥126 mg/dL (or nonfasting glucose ≥200 mg/dL), a self-reported physician diagnosis of diabetes mellitus, or the use of diabetes medications. Baseline hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or the use of blood pressure–lowering medications. At each study visit, medication history was obtained by self-report of medication intake during last 2 weeks and by reviewing medications brought by the participants to their visits. Each medication was coded by trained and certified interviewers with the use of a computerized medication classification system. Prevalent stroke and peripheral arterial disease were identified by self-reported history of a previous physician diagnosis. Prevalent heart failure was identified by the Gothenburg criteria or self-reported history of heart failure medication use in the past 2 weeks.
Statistical methods
Frequency distributions of the variables used in analyses were first inspected to rule out anomalies and outliers. Descriptive statistics were used to determine mean values, standard deviations, and percentile distributions for continuous variables, and frequencies and percentages for categorical variables.
During the period from visit 1 to visit 4, incidence rates of SMI and CMI were calculated per 1000 person-years and compared in all ARIC participants as well as stratified by age, sex and race/ethnicity.
Cox proportional hazards analysis was used to examine the associations of SMI and CMI (vs. no MI) occurring from visit 1 to visit 4 with CHD death and all-cause mortality occurring after visit 4. The follow up time included the time elapsed between the identification of SMI or CMI plus the time from visit 4 to the event. Non-CHD deaths were treated as censored. Models were incrementally adjusted as follows: Model 1 adjusted for baseline demographics (age, sex and race), and Model 2 adjusted for variables in Model 1 plus study field center, body mass index, income, education, smoking status, systolic blood pressure, blood pressure lowering medications, diabetes mellitus, ratio of total cholesterol/high density lipoprotein cholesterol, use of cholesterol lowering medications, use of aspirin, family history of CHD and serum creatinine (all variables measured at baseline). Interactions by sex and race were examined in Model 2. We examined the assumption of proportional hazards by computation of Schoenfeld residuals and inspection of log (−log [survival function]) curves, and they were met.
All analyses were performed with SAS version 9.3 (SAS Institute Inc., Cary, North Carolina). A 2-sided p <0.05 was considered significant. However, because the interactions tests were used for only screening for effect modification (interactions) by sex and race and not testing a hypothesized effect modification, we used a more relaxed p-value of 0.10 to define significance to detect interaction.35
RESULTS
This analysis included 9,498 participants (age at baseline 54.0 (± 5.7) years, 56.9% women, and 20.3% African American). From baseline through the 4th ARIC visit, 317 participants developed SMI while 386 developed CMI. Table 1 shows the baseline characteristics of the study participants stratified by MI status.
Table 1.
Baseline (1987–89) Participants Characteristic Stratified by Incident Myocardial Infarction during follow up (1996–1998).
| Mean± SD or n (%) | No MI (n=8,795) | SMI (n=317) | CMI (n=386) | p-value* | p-value† |
|---|---|---|---|---|---|
| Age (years) | 54 ±5.6 | 55 ±5.9 | 55 ±5.6 | 0.289 | <.001 |
| Women | 5154 (59) | 139 (44) | 107 (28) | <.001 | <.001 |
| African-American | 1802 (20) | 74 (23) | 54 (14) | 0.001 | 0.003 |
| Education ≤ high school | 4483 (51) | 161 (51) | 227 (59) | 0.033 | 0.011 |
| Current smoker | 1814 (21) | 80 (25) | 120 (31) | 0.004 | <.001 |
| Body mass index (kg/m2) | 27 ±5.0 | 29 ±5.7 | 28 ±4.3 | 0.063 | <.001 |
| Systolic blood pressure (mmHg) | 118 ±17 | 125 ±19 | 125 ±19 | 0.783 | <.001 |
| Hypertension | 2347 (27) | 128 (41) | 152 (39) | 0.783 | <.001 |
| Antihypertensive medication | 1966 (22) | 109 (34) | 116 (30) | 0.221 | <.001 |
| Diabetes | 644 (7.4) | 53 (17) | 64 (17) | 0.970 | <.001 |
| Ratio of total /HDL cholesterol | 4.4 ±1.6 | 4.8 ±1.7 | 5.7 ±1.6 | <.001 | <.001 |
| Cholesterol lowering medication | 202 (2.3) | 7 (2.2) | 12 (3.1) | 0.463 | 0.578 |
| Aspirin use | 4016 (46) | 144 (46) | 166 (43) | 0.531 | 0.579 |
| Family history of coronary heart disease | 3462 (39) | 138 (44) | 199 (52) | 0.034 | <.001 |
| Serum creatinine (mg/dL) | 1.1 ±0.3 | 1.1 ±0.2 | 1.2 ±0.2 | 0.130 | <.001 |
p-value for comparison between silent and clinical MI using unpaired student T test and Chi2 for continuous and categorical variables, respectively
p-value for comparison among the three groups using analysis of variance and Chi2 for continuous and categorical variables, respectively.
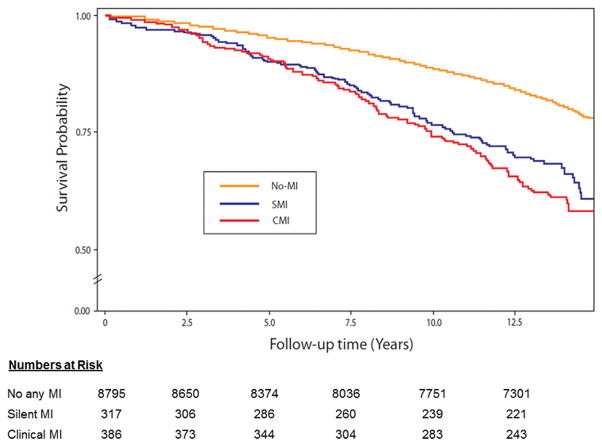
Table 2 shows the incidence rate (per 1000 person-years) of SMI and CMI, overall and stratified by sex and race. Overall, the incidence rate of CMI was slightly higher than SMI. However, sex and race differences in the incidence of SMI and CMI were observed. The incidence rates of both SMI and CMI were higher in men compared to women (p-value <.0001). On the other hand, blacks had non-significantly higher rate of SMI than whites (p-value= 0.217) but white had higher rate of CMI than blacks (p-value=0.002). Figure 1 shows the incidence rates of SMI and CMI in white men, black men, white women and black women. As shown, the incidence rate of SMI was higher than CMI in black women, which is the opposite of what is observed in white men in whom CMI was more common than SMI. On the other hand, the incidence rates of SMI were comparable to those of CMI in white women and black men. During a median follow up of 13.2 years follow-up, 1,833 all-cause mortality cases were detected of which 189 were CHD deaths. Figure 2 and Figure 3 show the event (CHD death and all-cause mortality, respectively) -free survival curves by MI status (no MI, SMI and CMI). In multivariable adjusted Cox proportional hazards analysis, both SMI and CMI (compared to no MI) were associated with increased risk of CHD death (Table 3) and all-cause mortality (Table 4). However, SMI and CMI were associated with increased risk of mortality among both men and women, with potentially greater increased risk among women (interaction p-value=0.089 and 0.051, respectively). No significant interaction by race was detected.
Table 2.
Incidence of Silent and Clinically Manifest Myocardial Infarction by Sex and Race: ARIC 1987–89 to 1996–98.
| SMI | CMI | |||
|---|---|---|---|---|
|
| ||||
| Events N (%) |
Incidence per 1000 person-years | Events N (%) |
Incidence per 1000 person-years | |
| All population (n=9,498) | 317 (3.3) | 3.84 (2.84–4.84) | 386 (4.1) | 4.68 (3.51–5.84) |
| Men (n=4,098) | 178 (4.3) | 5.08 (3.34–6.82) | 279 (6.8) | 7.96 (5.64–10.3) |
| Women (n=5,400) | 139 (2.6) | 2.93 (1.77–4.09) | 107 (2.0) | 2.25 (1.18–3.33) |
| White (n=7,568) | 243 (3.2) | 3.69 (2.59–4.79) | 332 (4.4) | 5.04 (3.69–6.39) |
| Black (n=1,930) | 74 (3.8) | 4.45 (2.05–6.84) | 54 (2.8) | 3.24 (1.13–5.36) |
MI= myocardial infarction; SMI= Silent MI; CMI= MI with clinical manifestations.
Figure 1.
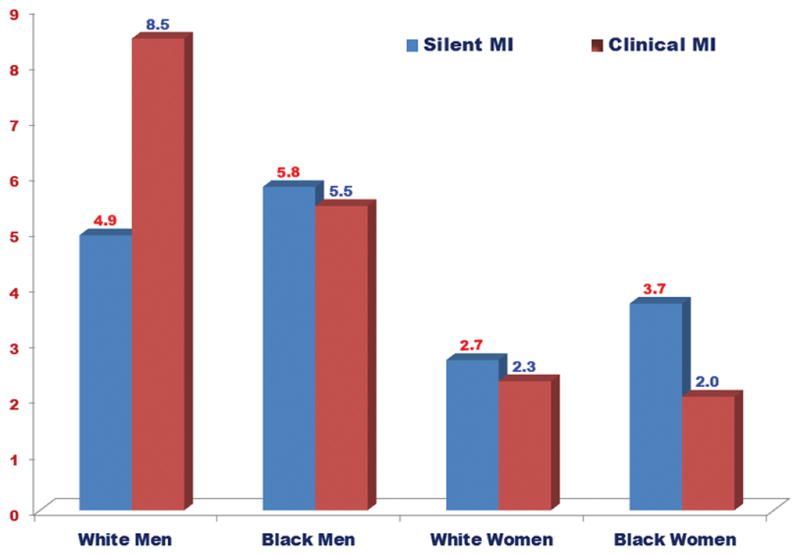
Sex-race specific incidence rates (per 1000 person-years) of silent and clinical myocardial infarctions.
Figure 2.
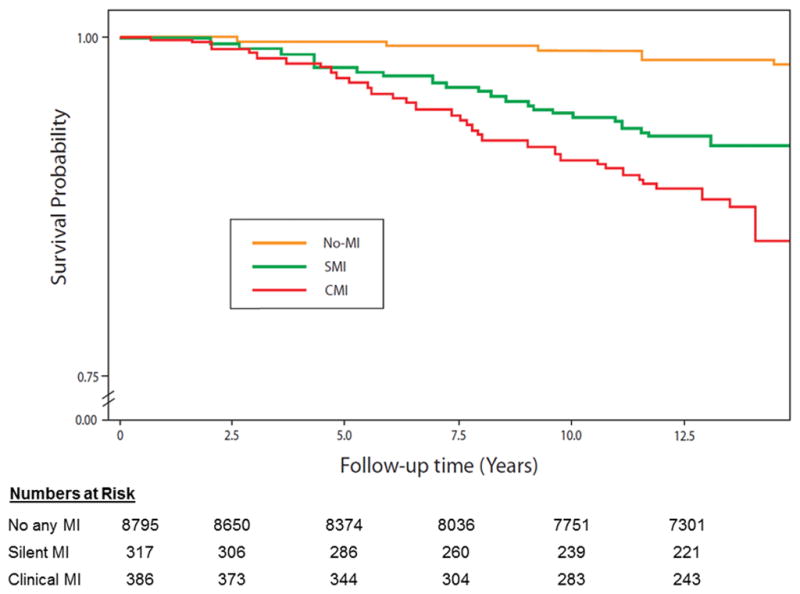
Coronary heart disease survival probability curves by myocardial infarction status.
Figure 3.
All-cause mortality survival probability curves by myocardial infarction status.
Table 3.
Risk of Coronary Heart Disease Death Associated with Silent and Clinically Manifest Myocardial Infarction by Sex and Race.
| Events/1000 person years | Model 1* HR (95% CI) |
Model 2† HR (95% CI) |
Interaction p-value†† | |
|---|---|---|---|---|
| All Population | N/A | |||
| No MI (n=8,795) | 0.7 | 1 (ref.) | 1 (ref.) | |
| Silent MI (n=317) | 3.2 | 4.10 (2.57–6.53) | 3.06 (1.88–4.99) | |
| Clinical MI (n=386) | 5.5 | 6.85 (4.78–9.79) | 4.74 (3.26–6.90) | |
|
| ||||
| Men | 0.089 | |||
| No MI (n=3,641) | 1.0 | 1 (ref.) | 1 (ref.) | |
| Silent MI (n=178) | 3.6 | 3.23 (1.79–5.81) | 2.77 (1.51–5.10) | |
| Clinical MI (n=279) | 5.5 | 5.49 (3.61–8.34) | 4.39 (2.83–6.63) | |
| Women | ||||
| No MI (n=5,154) | 0.4 | 1 (ref.) | 1 (ref.) | |
| Silent MI (n=139) | 2.8 | 6.92 (3.26–14.7) | 3.79 (1.65–8.73) | |
| Clinical MI (n=107) | 5.5 | 12.7 (6.66–24.0) | 5.67 (2.78–11.6) | |
|
| ||||
| White | 0.204 | |||
| No MI (n=6,993) | 0.5 | 1 (ref.) | 1 (ref.) | |
| Silent MI (n=243) | 2.6 | 4.01 (2.23–7.24) | 3.30 (1.82–6.01) | |
| Clinical MI (n=332) | 4.6 | 6.60 (4.31–10.1) | 4.52 (2.92–6.99) | |
| Black | ||||
| No MI (n=1,802) | 1.1 | 1 (ref.) | 1 (ref.) | |
| Silent MI (n=74) | 5.4 | 4.15 (1.93–8.89) | 2.62 (1.06–6.48) | |
| Clinical MI (n=54) | 11.5 | 7.22 (3.75–13.9) | 5.57 (2.60–11.9) | |
HR=hazard ratio; CI=confidence interval; MI= myocardial infarction
Model 1 adjusted for age, sex, and race.
Model 2 adjusted for variables in model 1 plus study field center, body mass index, education, smoking status, systolic blood pressure, blood pressure lowering medications, diabetes mellitus, ratio of total cholesterol/high density lipoprotein, use of cholesterol lowering medications, use of aspirin, family history of coronary heart disease and serum creatinine (all at baseline).
Interactions tested in Model 2.
Table 4.
Risk of All-cause Mortality Associated with Different Patterns of Myocardial Infarction.
| Events/1000 person years | Model 1* HR (95% CI) |
Model 2 † HR (95% CI) |
Interaction P-value†† | |
|---|---|---|---|---|
| All participants | N/A | |||
| No MI (n=8,795) | 8.4 | 1 (ref.) | 1 (ref.) | |
| Silent MI (n=317) | 15.9 | 1.63 (1.33–1.99) | 1.34 (1.09–1.65) | |
| Clinical MI (n=386) | 18.7 | 1.85 (1.56–2.20) | 1.55 (1.30–1.85) | |
|
| ||||
| Men | 0.051 | |||
| No MI (n=3,641) | 11.0 | 1 (ref.) | 1 (ref.) | |
| Silent MI (n=178) | 17.3 | 1.43 (1.11–1.85) | 1.23 (0.94–1.60) | |
| Clinical MI (n=279) | 18.7 | 1.65 (1.34–2.02) | 1.45 (1.18–1.78) | |
| Women | ||||
| No MI (n=5,154) | 6.6 | 1 (ref.) | 1 (ref.) | |
| Silent MI (n=139) | 14.0 | 2.05 (1.49–2.81) | 1.58 (1.13–2.20) | |
| Clinical MI (n=107) | 18.9 | 2.59 (1.89–3.56) | 1.83 (1.32–2.54) | |
|
| ||||
| White | 0.178 | |||
| No MI (n=6,993) | 8.0 | 1 (ref.) | 1 (ref.) | |
| Silent MI (n=243) | 14.6 | 1.50 (1.18–1.90) | 1.31 (1.03–1.67) | |
| Clinical MI (n=332) | 18.1 | 1.80 (1.49–2.17) | 1.48 (1.22–1.79) | |
| Black | ||||
| No MI (n=1,802) | 9.8 | 1 (ref.) | 1 (Ref.) | |
| Silent MI (n=74) | 20.1 | 2.03 (1.40–2.96) | 1.45 (0.96–2.21) | |
| Clinical MI (n=54) | 23.0 | 2.14 (1.41–3.26) | 1.97 (1.27–3.05) | |
HR=hazard ratio; CI=confidence interval; MI= myocardial infarction
Model 1 adjusted for age, sex, and race.
Model 2 adjusted for variables in model 1 plus study field center, body mass index, education, smoking status, systolic blood pressure, blood pressure lowering medications, diabetes mellitus, ratio of total cholesterol/high density lipoprotein, use of cholesterol lowering medications, use of aspirin, family history of coronary heart disease and serum creatinine (all at baseline).
Interactions tested in Model 2.
DISCUSSION
In this analysis from the ARIC study, one of the largest community-based biracial cohort studies in the US, we examined the sex and racial differences in the incidence and prognostic significance of silent vs. clinically-manifested MI. The three key findings are: 1) SMI is common; about 45% of the MIs are silent; 2) Both SMI and CMI are associated with poor prognosis with CMI showing slightly stronger association with risk of death than SMI; 3) There are race and sex differences in the incidence and prognostic significance of SMI. These findings highlights the importance of detection of SMI, and the potential impact of such detection on personalized prevention of CHD that takes into account race and sex. This is further underscored by the known sex and race disparity in CHD incidence and prognosis,36 and the fact that those with SMI are deprived from medical attention compared to those with CMI.
Several previous studies have examined the prevalence, incidence and prognostic significance of SMI.4–26 In literature reviews by Pride et al,4 and by Sheifer et al,37 SMI constituted up to 44% of the total MIs, and carried a prognosis that was as poor as that for CMIs. The prevalence and incidence of SMI differed, however, from one study to another. In the Cardiovascular Health Study (CHS), which is a predominantly white population of elderly aged 65 years and older, SMI accounted for 22% of the prevalent MIs.19 In a similar cohort of elderly patients aged >75 years, the Bronx Aging Study, SMIs represented 44% of the total MIs.17 On the other hand, in the Heart and Estrogen/progestin Replacement Study Trial, which included only women, SMI constituted only 4% of the total MI,38 which is much lower than the Reykjavik Study in Women in which SMIs represented 33% of the total MIs.10 Similarly, different studies showed different prognosis of SMI, with some reporting similar or poorer prognosis17, 26 and others showing better prognosis with SMI compared to CMI.7, 39
Differences in the incidence and prognostic significance among different studies could be explained by differences in the population studied (e.g. distribution of age, race and sex) and the method by which SMI is detected (e.g. Q wave in the ECG, myocardial scar in the cardiac magnetic resonance imaging, or areas of akinesia in the echocardiography). Even within studies that used ECG to define silent MI there are differences, some used serial Q/ST/T changes26 and others used MI at each point of time as our study. Regardless of these differences, the overall incidence and prognostic significance of SMI in these studies generally accord with our results. However, none of these studies had the large sample size or the ethnically diverse community-based population with good representation of both sexes as our study. Hence, the race and sex differences in the incidence and prognostic significance of SMI were not appropriately examined in previous studies. Therefore, our results expand upon the previous studies and also extend our previous ARIC report on SMI that examined the incidence but not the prognostic significance of these MIs.40
Our observations of the race and sex differences in the incidence and prognostic significance of SMI adds to the accumulating evidence of the sex and racial differences in CVD outcomes and the potential differences in the impact of risk factors among sexes and races. Because we adjusted for several potential confounders, it is less likely that our observed sex and racial differences were confounded by differences in MI-associated morbidities. Future investigation should assess whether genetic background, emerging risk factors, access to health care, awareness, and adherence to medications contribute to sex and racial differences.
Our results should be read in the context of certain limitations. Our analyses included only whites and blacks, and hence our results may not be generalizable to other races/ethnicities. Although we adjusted for several potential confounders in the models examining the association between SMI and CMI with outcomes, residual confounding remains a possibility as all similar studies. Q-waves often disappear after MI, and hence the SMI incidence in our study might be underestimated given the time between visits. Also, the increasing sensitivity of troponin in the past decade probably have yielded more CMI, and subsequently less SMI in the later stages of ARIC compared to before. Although this should not impact the race and sex differences, it may impact the trend of SMI over time. Also, there were no significant changes in the sensitivity of troponin before 1998, the date our ascertainment of silent MI ended. Despite these limitations, our study was able to document the race and sex differences in the incidence and prognostic significance of SMI and compare the results to CMI in a large, well-designed prospective cohort study with long term follow-up; the ARIC study. Other strengths include standardized ECG procedures and carefully documented outcomes events ascertained by an independent adjudication committee.
In conclusion, in the ARIC study we showed that SMI is as common as CMI; 45% of the MIs are silent, and that both SMI and CMI are associated with poor outcomes. However, there are race and sex differences in the incidence and prognostic significance of SMI. Thus, accidental ECG finding of MI in persons without a history of MI may warrant enhanced CHD prevention efforts that take into account sex and race differences.
Clinical Perspectives.
This report from the Atherosclerosis Risk in Communities (ARIC) study, one of the largest community-based biracial cohort studies in the US, shows that presence of asymptomatic or silent myocardial infarction on screening electrocardiograms is a common finding; about 45% of the total number of myocardial infarctions in the study were silent. These silent myocardial infarctions were associated with increased risk of death in a magnitude that is relatively comparable to myocardial infarctions with clinical manifestations. However, race and sex differences in the incidence and prognostic significance of silent myocardial infarction were observed in this study. These findings highlights the importance of detection of silent myocardial infarctions, and the potential impact of such detection on personalized prevention of coronary heart disease that takes into account race and sex.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Funding Sources: The ARIC Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute (NHLBI) contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
Footnotes
Disclosures: None.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics – 2015 Update. A report from the American Heart Association. Circulation. 2015;131:e226–e240. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Thygesen K, Alpert JS, White HD on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal Definition of Myocardial Infarction. Circulation. 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 3.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD the writing group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. [Google Scholar]
- 4.Pride YB, Piccirillo BJ, Gibson CM. Prevalence, Consequences, and Implications for Clinical Trials of Unrecognized Myocardial Infarction. Am J Cardiol. 2013;111:914–918. doi: 10.1016/j.amjcard.2012.11.042. [DOI] [PubMed] [Google Scholar]
- 5.Aguilar D, Goldhaber SZ, Gans DJ, Levey AS, Porush JG, Lewis JB, Rouleau JL, Berl T, Lewis EJ, Pfeffer MA. Clinically unrecognized Q-wave myocardial infarction in patients with diabetes mellitus, systemic hypertension, and nephropathy. Am J Cardiol. 2004;94:337–339. doi: 10.1016/j.amjcard.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 6.Boland A, Gerardy J, Mossay D, Delapierre D, Seutin V. Pirlindole and dehydropirlindole protect rat cultured neuronal cells against oxidative stress-induced cell death through a mechanism unrelated to MAO-A inhibition. Br J Pharmacol. 2002;135:713–720. doi: 10.1038/sj.bjp.0704519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis TM, Fortun P, Mulder J, Davis WA, Bruce DG. Silent myocardial infarction and its prognosis in a community-based cohort of type 2 diabetic patients: the Fremantle Diabetes Study. Diabetologia. 2004;47:395–399. doi: 10.1007/s00125-004-1344-4. [DOI] [PubMed] [Google Scholar]
- 8.de Torbal A, Boersma E, Kors JA, van Herpen G, Deckers JW, van der Kuip DA, Stricker BH, Hofman A, Witteman JC. Incidence of recognized and unrecognized myocardial infarction in men and women aged 55 and older: the Rotterdam Study. Eur Heart J. 2006;27:729–736. doi: 10.1093/eurheartj/ehi707. [DOI] [PubMed] [Google Scholar]
- 9.Grimm RH, Jr, Tillinghast S, Daniels K, Neaton JD, Mascioli S, Crow R, Pritzker M, Prineas RJ. Unrecognized myocardial infarction: experience in the Multiple Risk Factor Intervention Trial (MRFIT) Circulation. 1987;75:II6–II8. [PubMed] [Google Scholar]
- 10.Jonsdottir LS, Sigfusson N, Sigvaldason H, Thorgeirsson G. Incidence and prevalence of recognised and unrecognised myocardial infarction in women: the Reykjavik Study. Eur Heart J. 1998;19:1011–1018. doi: 10.1053/euhj.1998.0980. [DOI] [PubMed] [Google Scholar]
- 11.Kannel WB. Prevalence and clinical aspects of unrecognized myocardial infarction and sudden unexpected death. Circulation. 1987;75:II4–II5. [PubMed] [Google Scholar]
- 12.Kannel WB, Abbott RD. Incidence and prognosis of unrecognized myocardial infarction: an update on the Framingham study. N Engl J Med. 1984;311:1144–1147. doi: 10.1056/NEJM198411013111802. [DOI] [PubMed] [Google Scholar]
- 13.Kannel WB, Cupples LA, Gagnon DR. Incidence, precursors and prognosis of unrecognized myocardial infarction. Adv Cardiol. 1990;37:202–214. doi: 10.1159/000418828. [DOI] [PubMed] [Google Scholar]
- 14.Kannel WB, McNamara PM, Feinleib M, Dawber TR. The unrecognized myocardial infarction: fourteen-year follow-up experience in the Framingham study. Geriatrics. 1970;25:75–87. [PubMed] [Google Scholar]
- 15.Margolis JR, Kannel WS, Feinleib M, Dawber TR, McNamara PM. Clinical features of unrecognized myocardial infarction—silent and symptomatic: eighteen year follow-up: the Framingham study. Am J Cardiol. 1973;32:1–7. doi: 10.1016/s0002-9149(73)80079-7. [DOI] [PubMed] [Google Scholar]
- 16.Medalie JH, Goldbourt U. Unrecognized myocardial infarction: five-year incidence, mortality, and risk factors. Ann Intern Med. 1976;84:526–531. doi: 10.7326/0003-4819-84-5-526. [DOI] [PubMed] [Google Scholar]
- 17.Nadelmann J, Frishman WH, Ooi WL, Tepper D, Greenberg S, Guzik H, Lazar EJ, Heiman M, Aronson M. Prevalence, incidence and prognosis of recognized and unrecognized myocardial infarction in persons aged 75 years or older: the Bronx Aging Study. Am J Cardiol. 1990;66:533–537. doi: 10.1016/0002-9149(90)90477-i. [DOI] [PubMed] [Google Scholar]
- 18.Rosenman RH, Friedman M, Jenkins CD, Straus R, Wurm M, Kositchek R. Clinically unrecognized myocardial infarction in the Western Collaborative Group Study. Am J Cardiol. 1967;19:776–782. doi: 10.1016/0002-9149(67)90498-5. [DOI] [PubMed] [Google Scholar]
- 19.Sheifer SE, Gersh BJ, Yanez ND, III, Ades PA, Burke GL, Manolio TA. Prevalence, predisposing factors, and prognosis of clinically unrecognized myocardial infarction in the elderly. J Am Coll Cardiol. 2000;35:119–126. doi: 10.1016/s0735-1097(99)00524-0. [DOI] [PubMed] [Google Scholar]
- 20.Shlipak MG, Elmouchi DA, Herrington DM, Lin F, Grady D, Hlatky MA. The incidence of unrecognized myocardial infarction in women with coronary heart disease. Ann Intern Med. 2001;134:1043–1047. doi: 10.7326/0003-4819-134-11-200106050-00010. [DOI] [PubMed] [Google Scholar]
- 21.Sigurdsson E, Thorgeirsson G, Sigvaldason H, Sigfusson N. Unrecognized myocardial infarction: epidemiology, clinical characteristics, and the prognostic role of angina pectoris: the Reykjavik Study. Ann Intern Med. 1995;122:96–102. doi: 10.7326/0003-4819-122-2-199501150-00003. [DOI] [PubMed] [Google Scholar]
- 22.Stokes J, III, Dawber TR. The silent coronary: the frequency and clinical characteristics of unrecognized myocardial infarction in the Framingham study. Ann Intern Med. 1959;50:1359–1369. doi: 10.7326/0003-4819-50-6-1359. [DOI] [PubMed] [Google Scholar]
- 23.Yano K, MacLean CJ. The incidence and prognosis of unrecognized myocardial infarction in the Honolulu, Hawaii, Heart Program. Arch Intern Med. 1989;149:1528–1532. [PubMed] [Google Scholar]
- 24.Burgess DC, Hunt D, Li L, Zannino D, Williamson E, Davis TM, Laakso M, Kesaniemi YA, Zhang J, Sy RW, Lehto S, Mann S, Keech AC. Incidence and predictors of silent myocardial infarction in type 2 diabetes and the effect of fenofibrate: an analysis from the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Eur Heart J. 2010;31:92–99. doi: 10.1093/eurheartj/ehp377. [DOI] [PubMed] [Google Scholar]
- 25.Kannel WB, Sorlie P, McNamara PM. Prognosis after initial myocardial infarction: the Framingham study. Am J Cardiol. 1979;44:53–59. doi: 10.1016/0002-9149(79)90250-9. [DOI] [PubMed] [Google Scholar]
- 26.Crow RS1, Prineas RJ, Hannan PJ, Grandits G, Blackburn H. Prognostic associations of Minnesota Code serial electrocardiographic change classification with coronary heart disease mortality in the Multiple Risk Factor Intervention Trial. Am J Cardiol. 1997;80:138–144. doi: 10.1016/s0002-9149(97)00307-x. [DOI] [PubMed] [Google Scholar]
- 27.Ammar KA, Samee S, Makwana R, Urban L, Mahoney DW, Kors JA, Redfield MM, Jacobsen S, Rodeheffer RJ. Echocardiographic characteristics of electrocardiographically unrecognized myocardial infarctions in a community population. Am J Cardiol. 2005;96:1069. doi: 10.1016/j.amjcard.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 28.Kehl DW, Farzaneh-Far R, Na B, Whooley MA. Prognostic value of electrocardiographic detection of unrecognized myocardial infarction in persons with stable coronary artery disease: data from the Heart and Soul Study. Clin Res Cardiol. 2011;100:359–366. doi: 10.1007/s00392-010-0255-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 30.Prineas RJ, Crow RS, Blackburn H. The Minnesota Code Manual of Electrocardiographic Findings. Boston: John Wright PSB; 1982. p. 203. [Google Scholar]
- 31.Prineas RJ, Crow RS, Zhang ZM. The Minnesota Code Manual of Electrocardiographic Findings. 2. Published by Springer; London: 2010. [Google Scholar]
- 32.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 33.Rosamond W, Chambless L, Heiss G, Mosley T, Coresh J, Whitsel E, Wagenknecht L, Ni H, Folsom A. Twenty-two year trends in incidence of myocardial infarction, CHD mortality, and case-fatality in four US communities, 1987 to 2008. Circulation. 2012;125:1848–1857. doi: 10.1161/CIRCULATIONAHA.111.047480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.ARIC Investigators. Manual 3: Surveillance Component Procedures Manual of Operations. 2015 Version 6.4. http://www2.cscc.unc.edu/aric/surveillance-manuals.
- 35.Frongillo E. [Accessed July 29, 2015];Evaluating Statistical Interactions. https://www.cscu.cornell.edu/news/statnews/stnews64.pdf.
- 36.Safford MM, Brown TM, Muntner PM, Durant RW, Glasser S, Halanych JH, Shikany JM, Prineas RJ, Samdarshi T, Bittner VA, Lewis CE, Gamboa C, Cushman M, Howard V, Howard G REGARDS Investigators. Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308:1768–74. doi: 10.1001/jama.2012.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheifer SE, Manolio TA, Gersh BJ. Unrecognized myocardial infarction. Ann Intern Med. 2001;135:801–811. doi: 10.7326/0003-4819-135-9-200111060-00010. [DOI] [PubMed] [Google Scholar]
- 38.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 39.Kwong RY, Chan AK, Brown KA, Chan CW, Reynolds HG, Tsang S, Davis RB. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113:2733–2743. doi: 10.1161/CIRCULATIONAHA.105.570648. [DOI] [PubMed] [Google Scholar]
- 40.Boland LL, Folsom AR, Sorlie PD, Taylor HA, Rosamond WD, Chambless LE, Cooper LS. Occurrence of unrecognized myocardial infarction in subjects aged 45 to 65 years (the ARIC study) Am J Cardiol. 2002;90:927–931. doi: 10.1016/s0002-9149(02)02655-3. [DOI] [PubMed] [Google Scholar]