Abstract
Hepatitis B virus reactivation (HBVr) is an important complication of immunosuppressive drug therapy (ISDT). It can occur with active or resolved hepatitis B virus (HBV) infection with a clinical spectrum that ranges from mild elevations in liver tests to fulminant hepatic failure. The risk of it occurring is determined by the interplay between HBV serological status, level of viremia, and the immunosuppressive potency of the drug(s) used. Reactivation is most common during treatment of hematologic malignancies but also occurs with chemotherapy for breast cancer and numerous other solid organ malignancies, organ transplant, and immune suppression for nonmalignant conditions. The expansion of new biologic treatments for malignant and nonmalignant disorders has enlarged the population at risk. Increased awareness of HBVr among healthcare providers who prescribe ISDT, adoption of routine HBV screening, and linking the results of screening to antiviral prophylaxis are needed to reduce the incidence of this potentially fatal but preventable disorder.
Keywords: HBVr, chemotherapy, cancer, ISDT, liver
Hepatitis B virus reactivation (HBVr) was first described in the mid-1970s in the setting of cancer chemotherapy and kidney transplant. Since that time it has been shown to be a complication of many immunosuppressive drug therapies (ISDTs) that are routinely used across medical specialties for nonmalignant indications. Reactivation during ISDT is not rare, and hundreds of articles are published annually on this topic. It is a clinically important disorder because it can result in significant morbidity, liver failure, and even death. This review will describe how it can be prevented, and indicate those factors that continue to be impediments to a major reduction in incidence.
DEFINING HEPATITIS B REACTIVATION
A standardized definition of HBVr has not been established, but traditionally the diagnosis encompasses 2 key elements: an acute rise in serum hepatitis B virus (HBV) DNA and elevated serum aminotransferases. Virologic criteria have included (1) de novo detection of HBV DNA in patients with previously undetectable HBV DNA, (2) a rise in HBV DNA of at least 1 log (10-fold) IU/mL, or (3) HBV DNA levels rising above an arbitrary cutoff (for example, 20 000 IU) in patients with biochemical worsening [1]. Additional defining criteria include hepatitis B surface antigen (HBsAg) reverse seroconversion (seroreversion) in HBsAg-negative patients who are positive for antibody to hepatitis B core antigen (anti-HBc). Biochemical criteria for HBVr typically include a 2- or 3-fold increase in alanine aminotransferase above the upper limit of normal. Various methods of grading the severity of HBVr using virologic, biochemical, and clinical data have been proposed [2].
IMMUNOPATHOGENESIS AND PRESENTING FEATURES
The initiating events for HBVr have been described for cancer chemotherapy. During chemotherapy, an initial phase of enhanced HBV replication takes place several months before elevation of serum aminotransferases [3]. A second phase of immunologic restitution often follows withdrawal of chemotherapy, at which time overt hepatitis and hepatic failure may ensue [4]. As immunologic events begin weeks to months before the appearance of elevated aminotransferases, on-demand antiviral therapy may not protect against severe liver injury [5].
The clinical spectrum associated with HBVr can range from clinically inapparent to life-threatening liver injury. It is likely that many cases go unnoticed, as observational studies have demonstrated that a third or more of cases meeting virologic criteria for reactivation lack accompanying aminotransferase elevation [1]. Liver-related mortality is reported in most large clinical series. Some studies report a mortality rate of >50%, whereas others have described liver-related mortality in 0%–20% of cases [1, 6, 7]. Mortality is observed less frequently during ISDT treatment of nonmalignant conditions [1, 8]. Hospitalization may be required in >40% of cases occurring during cancer chemotherapy, and intensive care management is needed in approximately half of those hospitalized [7]. Delay in initiation or early discontinuation of cancer chemotherapy has been reported in as many as 40% of cases [7, 9].
Liver transplant may not be an option when liver failure occurs in a patient with underlying malignancy.
RISK ASSESSMENT: INTERPLAY OF FACTORS
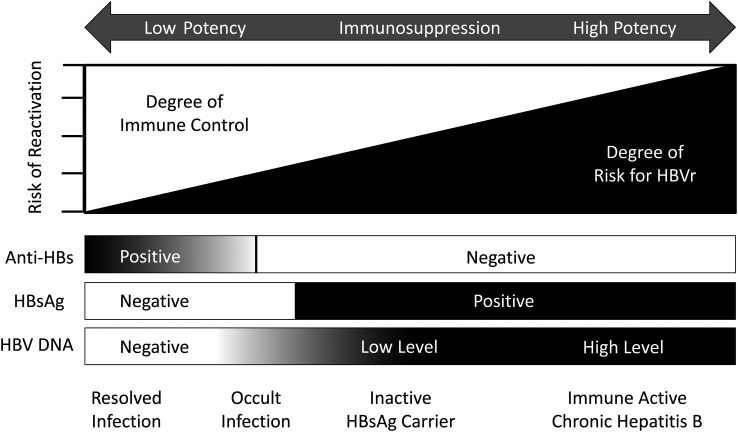
HBVr is precipitated by disruption of the host's immune control over viral replication. Clinical data support a complex interplay between the level of viral replication, host serologic status, and drug potency (Figure 1). Patients with active chronic hepatitis and elevated serum HBV DNA can reactivate with exposure to moderate or even minimally potent ISDT, whereas individuals with resolved infection (HBsAg negative, anti-HBc positive, with or without antibody to HBsAg [anti-HBs]) generally require intense immunosuppression. This can be explained by the biological gradient that exists for intrahepatic covalently closed circular HBV DNA (cccDNA), which is the genomic template for HBV replication. Patients with chronic hepatitis have the highest concentration, followed in order by inactive HBsAg carriers and those with past hepatitis B [10].
Figure 1.
Hepatitis B virus (HBV) reactivation (HBVr) risk based on potency of immunosuppressive drug therapy and degree of host immune control. The risk of HBVr occurs along a continuum influenced by host immune control of viral replication. This is reflected by serological status and/or the presence and level of HBV DNA. HBVr risk also increases in relation to the potency of the administered immunosuppressive drugs. In this graph, it is assumed that all patients with antibody to hepatitis B surface antigen are positive for antibody to hepatitis B core antigen. Abbreviations: anti-HBs, antibody to hepatitis B surface antigen; HBsAg, hepatitis B surface antigen.
PREDICTIVE VARIABLES
Increased age, male sex, extent of viral replication, and type of malignancy (probably related to intensity of the ISDT regimen) have been associated with HBVr during cancer chemotherapy. Multivariate analysis has demonstrated the presence of prechemotherapy HBV DNA detection in serum, use of anthracyclines or glucocorticoids, and diagnoses of breast cancer or lymphoma to be independent predictors of HBVr [11]. The most useful pretherapy predictor of HBVr is the serum HBV DNA level. Studies utilizing polymerase chain reaction testing have reported that HBVr occurs more frequently if baseline HBV DNA levels exceed 2000 IU/mL. The presence of hepatitis B e antigen (HBeAg) is also predictive of increased risk as it generally implies that serum HBV DNA exceeds 20 000 IU/mL. Debate exists about the protective role of anti-HBs in HBsAg-negative patients. Some studies have shown lower rates of HBVr, whereas others have not. The discordant observations have led to a recent recommendation by the American Gastroenterological Association that the presence of anti-HBs not be used to guide a need for antiviral prophylaxis [1].
REACTIVATION AND SPECIFIC IMMUNOSUPPRESSIVE DRUG THERAPY
B-Cell–Depleting Agents for Malignant Disorders
B-cell–depleting agents such as rituximab and ofatumumab are frequently used in the treatment of B-cell lymphoma and chronic lymphocytic leukemia. Both drugs target the B-cell surface antigen CD20 and inhibit B-cell activation. Rituximab increases the rate of HBVr during treatment of non-Hodgkin lymphoma. When combined with the traditional CHOP regimen (cyclophosphamide, doxorubicin, vincristine, and glucocorticoids), as many of 70% of HBsAg-positive patients and 10%–20% of HBsAg-negative patients with anti-HBc have been shown to reactivate [12, 13].
Rituximab-induced HBVr has a long-lived effect on B-cell depletion, which generally lasts for several months after therapy. One of the hallmarks of HBVr occurring with B-cell depletion is HBsAg seroreversion during treatment. This is a clinically important event because it has been associated with frequent hepatitis and severe liver injury. HBsAg seroreversion rarely occurs with other forms of ISDT other than traditional bone marrow or hematopoietic stem cell transplant.
Conventional Cancer Chemotherapy
The frequency of HBVr is highest with chemotherapy for leukemia or lymphoma. Rates of ≥50% have been routinely reported [1]. However, HBVr also occurs during treatment of solid organ malignancies including breast, colon, lung, stromal tumors of the gastrointestinal tract, head and neck cancer, retinoblastoma, sarcoma, and teratoma. Among solid organ malignancies, HBVr occurs most commonly with breast cancer where rates of 20%–40% are often reported [1]. This has been attributed to anthracyclines (doxorubicin or epirubicin), which are also used for ovarian, uterine, and lung cancer, and treatment of hepatocellular carcinoma (HCC) by transarterial chemoembolization (TACE).
The especially high rate of HBVr with hematologic malignancies is probably due to intense immunologic potency of the complex regimens used, and in particular, the incorporation of such drugs as rituximab and high-dose glucocorticoids. The high-dose glucocorticoids in CHOP regimens significantly increase the frequency of HBVr compared with glucocorticoid-free regimens [14]. The extensive immunologic conditioning in hematopoietic stem cell transplant is associated with reactivation in ≥50% of HBsAg-positive persons and 10%–20% of patients with resolved HBV infection [15].
Transarterial Chemoembolization for HCC
Reactivated hepatitis B is well documented in patients during TACE treatment of HCC. HBVr frequencies of 30%–40% have been reported by several investigative groups [1]. One study demonstrated that the risk increases with the degree of immunosuppression [16]. When compared with local ablation as the reference population, the adjusted hazard ratio for TACE with adriamycin was 2.5; for TACE with epirubicin and cisplatin, 4.2; and for TACE with the 2-drug regimen plus radiotherapy, 10.2.
Immunosuppressive Therapy for Inflammatory Disorders
Table 1 lists nonmalignant disorders and the ISDT commonly used for treatment. The most frequently reported settings for HBVr are during treatment of rheumatologic, dermatologic, and inflammatory bowel diseases. However, immunosuppressive therapy is also frequently used to treat renal conditions such as glomerulonephritis, neurologic disorders such as demyelinating diseases, or pulmonic conditions such as chronic asthma. The range of agents includes antimetabolites, glucocorticoids, biologic agents including tumor necrosis factor alpha (TNF-α) inhibitors, monoclonal antibodies, and calcineurin inhibitors. Newer agents that block costimulation of lymphocytes, tyrosine kinase inhibitors, and integrin inhibitors have been developed for multiple indications but have not had sufficient use to determine the magnitude of risk for HBVr.
Table 1.
Nonmalignant Diseases Commonly Treated With Immunosuppressive Drug Therapy
| Diagnosis | Immunosuppressive Drug Therapy |
|---|---|
| Rheumatoid arthritis | Rituximab, TNF-α inhibitors, abatacept, anakinra, tocilizumab, leflunomide, antimetabolites |
| Plaque psoriasis | TNF-α inhibitors, ustekinumab, cyclosporine, antimetabolites |
| Psoriatic arthritis | Glucocorticoids, ustekinumab, TNF-α inhibitors |
| Ankylosing spondylitis | Glucocorticoids, TNF-α inhibitors, rituximab |
| Crohn's disease | Glucocorticoids, calcineurin inhibitors, TNF-α inhibitors, antimetabolites, vedolizumab |
| Ulcerative colitis | Glucocorticoids, calcineurin inhibitors, TNF-α inhibitors, antimetabolites, vedolizumab |
| Granulomatosis with polyangiitis (Wegener granulomatosis) | Cyclophosphamide, high-dose glucocorticoids, rituximab |
| Microscopic polyangiitis | Rituximab, glucocorticoids, cyclophosphamide |
| Eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome) | High-dose glucocorticoids, antimetabolites, cyclophosphamide, leflunomide |
| Essential mixed cryoglobulinemia | Rituximab, cyclophosphamide |
| Systemic mastocytosis | High-dose glucocorticoids, imatinib |
| Myelodysplasia | Cytarabine-anthracycline therapy, azacitidine, imatinib |
| Multiple sclerosis | Glucocorticoids, interferon-β, natalizumab, mitoxantrone |
| Solid organ transplant | Calcineurin inhibitors, mycophenolate, rituximab, azathioprine |
| Severe asthma | Glucocorticoids |
| Nephrotic syndrome | Glucocorticoids, cyclophosphamide, rituximab |
Abbreviation: TNF, tumor necrosis factor.
Antimetabolites
The antimetabolites (azathioprine, 6-mercaptopurine [6-MP], and methotrexate) are traditionally used for rheumatoid arthritis, psoriasis, and inflammatory bowel disease. In conventional doses, these agents are associated with a low risk for HBVr (Table 1) [1].
Glucocorticoids
Glucocorticoids are more commonly associated with HBVr than are other traditional immunosuppressive agents. These agents have a direct suppressive effect on T-cell–mediated immunity and also stimulate a glucocorticoid responsive transcriptional element in the HBV genome [17]. Short-term exposure to moderate (10–20 mg of prednisone or equivalent) or high (>20 mg) doses enhances viral replication and lowers serum aminotransferase levels. Abrupt withdrawal often results in an immunologic rebound typified by an elevation of serum aminotransferases and a decline in serum HBV DNA [18]. Unless patients are rescued with antiviral therapy, the HBVr reactivation episodes may be protracted and occasionally severe. The dose and method of administration of glucocorticoid therapy affect the risk of HBVr. Treatment of asthma and emphysema for ≥3 months with moderate (20 mg) doses of glucocorticoids has caused HBVr [19]. Reactivation has not been described with inhaled or intra-articular glucocorticoids.
Biologics
TNF-α is an important proinflammatory cytokine that reduces HBV replication, and several of the available TNF-α inhibitors including infliximab, etanercept, and adalimumab have been shown to cause HBVr. These agents are commonly used in the management of rheumatoid arthritis, plaque psoriasis, and inflammatory bowel disease. Accurate assessment of the magnitude of risk posed by TNF-α inhibitor therapy is not available. A comprehensive review of HBVr attributed to these drugs revealed an overall frequency of 39% in HBsAg carriers and 5% in HBsAg-negative patients with anti-HBc [8]. Recent data specific to rheumatic diseases demonstrated HBVr in 12% of HBsAg carriers during TNF-α inhibitor therapy, while HBVr was reported in 2% of HBsAg-negative individuals positive for anti-HBc [20]. In an observational study of 146 patients with resolved HBV infection who had been given long-term TNF inhibitor therapy, none developed HBVr [21]. The majority of TNF inhibitor–treated patients with HBVr are administered additional immunosuppressive agents such as glucocorticoids, methotrexate, or calcineurin inhibitors. This practice may contribute to the varying rates of HBVr reported with TNF inhibition [22].
Other biologics such as tyrosine kinase inhibitors and monoclonal antibodies directed against various immune targets have been reported to precipitate HBVr. Agents associated with HBVr have included the tyrosine kinase inhibitors imatinib and nilotinib, which are used in the treatment of chronic myeloid leukemia. Reactivated hepatitis B has also been reported with abatacept, a T-cell costimulation modulator used in rheumatoid arthritis that inhibits CD80 and CD86 signaling; and ustekinumab, an interleukin (IL) 12/IL-23 inhibitor used in the treatment of psoriasis. The B-cell–depleting agents rituximab and ofatumumab have come under close scrutiny recently. The US Food and Drug Administration has recently required a box warning on these agents in which it recommends HBV screening and consideration for antiviral therapy by a specialist whenever HBsAg or anti-HBc is detected.
Organ Transplantation
The calcineurin inhibitors cyclosporine and tacrolimus are commonly used in solid organ transplants. These agents inhibit T-cell activation and transcription of IL-2. The current standard of care for HBsAg-positive patients undergoing liver transplant is to provide combined prophylaxis with hepatitis B immunoglobulin (HBIG) and nucleoside analogue therapy. Using this approach, recurrent hepatitis B (defined as HBsAg and HBV DNA positive) occurs in no more than 5% of patients [23]. Many of the failed cases have occurred due to lamivudine-resistant HBV; as a result, many centers now use entecavir or tenofovir. The high potency and low potential for resistance with these agents have led some centers to investigate HBIG-free regimens in carefully selected patients [23].
There is a well-recognized risk of HBV transmission from anti-HBc–positive donor organs. The rate of HBV transmission (and subsequent HBVr) appears to vary by organ type, with the greatest risk in liver transplant recipients [24]. Antiviral prophylaxis is recommended for recipients of anti-HBc positive liver transplant, particularly if recipients are both anti-HBs and anti-HBc negative, in which case patients are usually treated with long-term antiviral prophylaxis [25].
PREVENTION AND MANAGEMENT
Recognition and Screening
Recognition of the HBV status of persons requiring ISDT is essential to preventing HBVr because it allows determination of the need for antiviral prophylaxis. Table 2 illustrates available guidelines that advocate routine screening for all individuals exposed to ISDT. These guidelines vary slightly, but each endorses screening for HBsAg in all patients and anti-HBc when high-risk ISDT is given. Unfortunately, HBV screening and antiviral prophylaxis are currently underutilized by oncologists, rheumatologists, dermatologists, and other prescribers of these drugs.
Table 2.
Guidelines in the Prevention of Hepatitis B Reactivation Associated With Immunosuppressive Drug Therapy
| Guideline | Recommended Screening Test |
Nucleoside Antiviral Prophylaxis |
||||
|---|---|---|---|---|---|---|
| HBsAg | anti-HBc | HBV DNA | Candidates | Timinga | Durationa | |
| US Centers for Disease Control (2008) | All | All | … | HBsAg+ | … | … |
| American Association for the Study of Liver Diseases (2009) | High-risk patients | High-risk patients | … | HBsAg+ | At onset | 6 mo if HBV DNA <2000 IU/mL vs >6 mo if HBV DNA >2000 IU/mL |
| Asian Pacific Association for the Study of the Liver (2012) | All | Biologic agent used | … | HBsAg+ | Prior to onset | 6 mo |
| anti-HBc+ and biologic agent | Defer and monitor HBV DNA | |||||
| European Association for the Study of the Liver (2012) | All | All | HBsAg– and anti-HBc+ patients | HBsAg+ or HBV DNA+ | Prior to onset | 12 mo |
| anti-HBc+ | If high-risk agent; otherwise defer and monitor HBV DNA | |||||
| American Gastroenterological Association Institute (2015) | Moderate- to high-risk patients | Moderate- to high-risk patients | HBsAg+ or anti-HBc+ patients | anti-HBc+ | Prior to onset if high/intermediate-risk agent | 6 mo vs 12 mo if B-cell–depleting agent |
Abbreviations: anti-HBc, antibody to hepatitis B core antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus.
a Timing in relation to initiation of immunosuppressive drug therapy and duration after completion.
Screening in Cancer Care
Two large retrospective reviews of screening practices in US cancer centers determined that <20% of patients underwent HBV screening before cancer chemotherapy [26, 27]. Similarly, data reported from surveys of oncologists in the United States, Canada, and Australia have noted universal screening practices in only 13%–22% of respondents [28–31]. In a recent international survey of the American Association for the Study of Liver Diseases, only 40% of 188 HBVr cases were screened for HBsAg and anti-HBc before initiation of cancer chemotherapy, and an additional 13% had HBsAg screening alone. Only 10% of these cases received prophylactic antiviral therapy [7]. In the latest iteration of the guidelines of the American Society of Clinical Oncology, HBV screening (HBsAg and anti-HBc) was only recommended for patients about to undergo highly aggressive chemotherapy such as rituximab-CHOP or in the setting of hematopoietic stem cell transplant [32].
Other Medical Specialties
In general, rheumatologists, dermatologists, and gastroenterologists have more readily adopted HBV screening. However, physician awareness of the risk for HBVr with biologic agents remains less than optimal. In a national survey of American rheumatologists, 69% reported routine screening for HBV before treatment with biologic agents, including TNF-α inhibitor therapy and monoclonal antibody therapy [33]. A national survey of dermatologists found only 52% were aware that HBVr may result from TNF-α inhibitor therapy [34]. Ambiguous or weakly worded practice guidelines of their specialty organizations further contribute to low rates of screening. In none of the existing guidelines are recommendations made about referring HBV-infected patients to specialists for consideration of antiviral prophylaxis.
Universal Versus Targeted Screening
Strategies for HBV screening in patients undergoing ISDT may involve a universal approach, in which all patients are screened, or a targeted approach, in which only patients perceived to be at increased risk undergo screening. Most practice guidelines have recommended a universal approach to HBsAg and anti-HBc screening for individuals with a moderate to high risk of HBVr as determined by the potency of the immunosuppressive agent or regimen (Table 2). Targeted screening, however, may be an alternative when individuals in populations with a low (<2%) prevalence of infection are given drugs that are not considered high risk for the induction of HBVr. In such instances, screening with HBsAg alone may suffice. Limited cost-efficacy data are available in regard to universal vs targeted screening. However, universal screening for HBsAg has been reported to be a cost-effective practice in patients with hematologic malignancies [35, 36].
Management Strategies
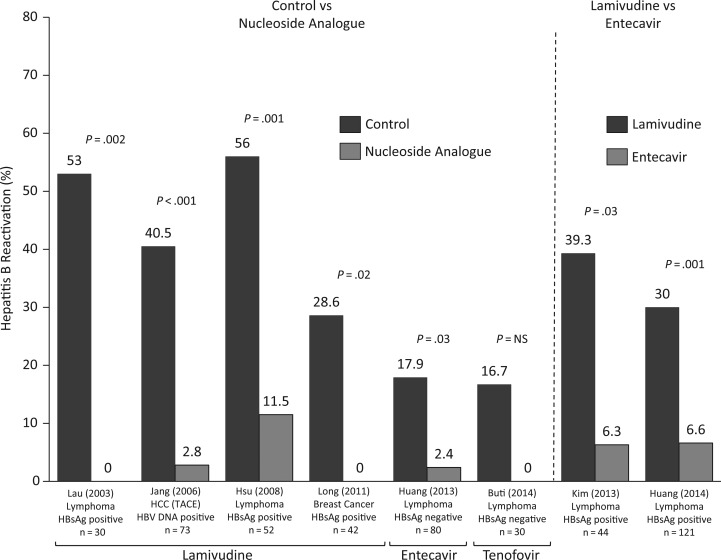
Different management strategies have been considered for patients at risk of HBVr. Strategies include prophylactic antiviral therapy initiated before or immediately after starting ISDT, preemptive antiviral therapy when a 1-log increase or de novo detection of HBV DNA occurs prior to onset of an overt hepatitis flare, and deferred therapy initiated at the time serum aminotransferase levels increase. Of these, prophylactic antiviral therapy has been shown to be significantly more effective in reducing the incidence of HBVr (>80%) and liver injury [1, 37]. Several prospective randomized controlled trials (RCTs) and 2 systematic analyses, mostly involving the use of lamivudine and more recently entecavir or tenofovir, have demonstrated a significant decrease in HBVr with prophylactic antiviral therapy (Figure 2) [5, 13, 37–44]. The RCTs encompass a range of malignancies, including breast cancer, HCC, and non-Hodgkin lymphoma. Most of these studies were done in HBsAg-positive patients, but RCTs demonstrate similar benefits of prophylaxis in patients with resolved infection who have hematological malignancies [41, 42].
Figure 2.
Prospective randomized controlled trials evaluating antiviral prophylaxis for hepatitis B virus (HBV) reactivation are shown involving a range of malignancies and mostly individuals positive for hepatitis B surface antigen [5, 13, 38–43]. Studies compared nucleoside analogue prophylaxis vs deferred approach (controls) or lamivudine vs entecavir. The higher frequency of HBV reactivation in lamivudine-treated patients on the right side of the figure most likely reflects the use of more aggressive immunosuppression in the studies by Kim [13] and Huang [43] . Rituximab was a component of chemotherapy in these 2 studies but was not incorporated into the treatment regimens on the left side of the graph. Abbreviations: HBsAg, hepatitis B surface antigen; HCC, hepatocellular carcinoma; NS, not significant; TACE, transarterial chemoembolization.
Lamivudine has been more widely used for prophylaxis, but recent data using entecavir have shown this to be more effective in the prevention of HBVr due to greater potency and lower level of drug resistance (Figure 2) [13, 43, 45]. Tenofovir has also been shown to be effective in patients with hematologic malignancies [42]. Most patients with severe reactivation are likely to be continued on antiviral therapy for an indefinite period. In such patients, the use of entecavir or tenofovir is preferred.
Prophylactic antiviral therapy should continue for 6 months after cancer chemotherapy, provided this does not incorporate B-cell depletion therapy. A comprehensive review and meta-analysis of 183 cases of rituximab-associated HBVr found that the majority of events appeared within the first 3 months after discontinuation of rituximab; however, one-third occurred >6 months after discontinuation [46]. Therefore, most experts agree that antiviral therapy should be maintained for 12 months after discontinuation of rituximab. At the current time, evidence-based guidelines are lacking in recommendations on how long to continue antiviral prophylaxis when TNF inhibitors and other biologic agents are discontinued. Due to the intermediate risk for HBV reactivation when these agents are used in HBsAg-positive patients, the authors recommend that the duration of antiviral therapy should be guided by the baseline HBV DNA status. For example, antiviral therapy can be discontinued when TNF inhibitors are no longer required in HBsAg-positive individuals with undetectable HBV DNA before antiviral therapy, continued for 1–3 months in those who had <2000 IU of HBV DNA, and continued for 6 months in the less common situation where levels of HBV DNA exceeded 2000 IU.
PERSPECTIVES
As HBVr from ISDT is a potentially serious health issue that is nearly totally preventable, identifying patients at risk by HBV screening followed by antiviral prophylaxis is essential. Unfortunately, these 2 management tools are greatly underutilized. There are several factors that contribute to this, including (1) poor recognition that HBVr is a common complication of ISDT; (2) lack of strong guidance in oncology and medical subspecialty practice guidelines; and (3) failure to recognize the far greater therapeutic benefit of antiviral prophylaxis over on-demand treatment. Clinicians need to consider that HBVr is not only a cause of significant morbidity and some mortality, but also frequently leads to interruption of ISDT. An expanded array of biologic agents has become available over the past decade and this trend will continue. Thus, the incidence of HBVr will almost certainly continue to increase in the future if current screening and antiviral treatment practices do not change.
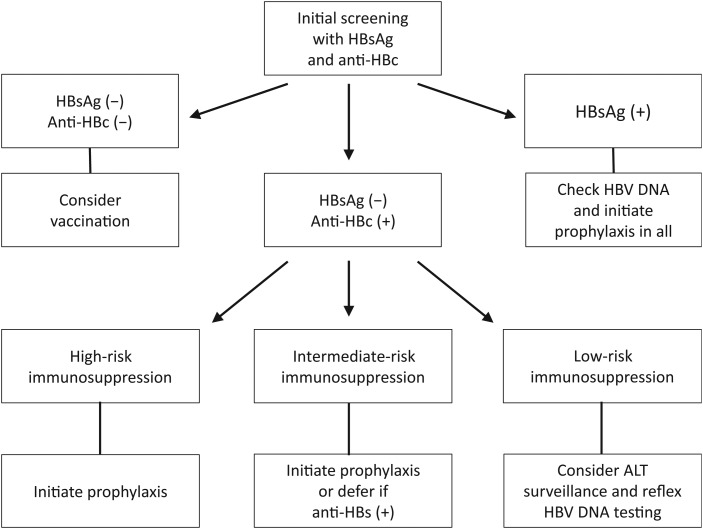
The authors’ recommendations for HBV screening and antiviral prophylaxis are presented in Figure 3. For the time being, the authors recommend that HBsAg-positive patients who are in need of ISDT and whose care is not managed by infectious disease specialists should be referred to liver disease specialists wherever possible. The appropriateness of antiviral therapy in HBsAg negative, anti-HBc–positive patients often involves more complex decision making and is likely to evolve further as new information becomes available on the risk of HBVr with prolonged cytokine inhibitor therapy and newer biologic agents. It is important that the language of revised management recommendations for hepatitis B as proposed by the practice guideline committees of the relevant medical organizations state these points clearly.
Figure 3.
Management algorithm for patients requiring immunosuppressive drug therapy. The initial screening with hepatitis B surface antigen (HBsAg) and antibody to hepatitis B core antigen (anti-HBc) leads to identification of 3 groups of patients. All susceptible individuals should be considered for vaccination. Those with resolved hepatitis B (HBsAg negative, anti-HBc positive) should be checked for hepatitis B virus (HBV) DNA if given intermediate- to high-risk immunosuppressive drug therapy (ISDT) (see text and [1] for drug classification according to degree of risk). All HBsAg-positive patients should have HBV DNA testing and antiviral prophylaxis. Patients positive for anti-HBc for whom deferred therapy is chosen should undergo regular HBV DNA and alanine aminotransferase (ALT) testing if given treatment with intermediate-risk ISDT. Such patients can undergo regular ALT monitoring with reflex HBV DNA testing if given low-risk ISDT. Low-resistance antivirals such as entecavir or tenofovir are preferred for prophylaxis and in the event that HBV reactivation occurs.
Notes
Supplement sponsorship. This article appears as part of the supplement “Hepatitis B,” sponsored by the CDC Foundation and Gilead.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 2015; 148:221–44. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez SA, Perrillo RP. Reactivation of hepatitis B virus due to chemotherapy or immunosuppressive drug therapy. In: Liaw YF, Zoulim F. Hepatitis B virus in human diseases. 1st ed New York: Springer, 2015. [Google Scholar]
- 3.Hui CK, Cheung WW, Zhang HY et al. Kinetics and risk of de novo hepatitis B infection in HBsAg-negative patients undergoing cytotoxic chemotherapy. Gastroenterology 2006; 131:59–68. [DOI] [PubMed] [Google Scholar]
- 4.Perrillo RP. Acute flares in chronic hepatitis B: the natural and unnatural history of an immunologically mediated liver disease. Gastroenterology 2001; 120:1009–22. [DOI] [PubMed] [Google Scholar]
- 5.Lau GK, Yiu HH, Fong DY et al. Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology 2003; 125:1742–9. [DOI] [PubMed] [Google Scholar]
- 6.Kawsar HI, Shahnewaz J, Gopalakrishna KV, Spiro TP, Daw HA. Hepatitis B reactivation in cancer patients: role of prechemotherapy screening and antiviral prophylaxis. Clin Adv Hematol Oncol 2012; 10:370–8. [PubMed] [Google Scholar]
- 7.Hwang JP, Barbo AG, Perrillo RP. Hepatitis B reactivation during cancer chemotherapy: an international survey of the membership of the American Association for the Study of Liver Diseases. J Viral Hepat 2015; 22:346–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez-Alvarez R, Diaz-Lagares C, Garcia-Hernandez F et al. Hepatitis B virus (HBV) reactivation in patients receiving tumor necrosis factor (TNF)-targeted therapy: analysis of 257 cases. Medicine 2011; 90:359–71. [DOI] [PubMed] [Google Scholar]
- 9.Yeo W, Ho WM, Hui P et al. Use of lamivudine to prevent hepatitis B virus reactivation during chemotherapy in breast cancer patients. Breast Cancer Res Treat 2004; 88:209–15. [DOI] [PubMed] [Google Scholar]
- 10.Werle-Lapostolle B, Bowden S, Locarnini S et al. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology 2004; 126:1750–8. [DOI] [PubMed] [Google Scholar]
- 11.Yeo W, Zee B, Zhong S et al. Comprehensive analysis of risk factors associating with hepatitis B virus (HBV) reactivation in cancer patients undergoing cytotoxic chemotherapy. Br J Cancer 2004; 90:1306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong HJ, Ni LN, Sheng GF, Song HL, Xu JZ, Ling Y. Risk of hepatitis B virus (HBV) reactivation in non-Hodgkin lymphoma patients receiving rituximab-chemotherapy: a meta-analysis. J Clin Virol 2013; 57:209–14. [DOI] [PubMed] [Google Scholar]
- 13.Kim SJ, Hsu C, Song YQ et al. Hepatitis B virus reactivation in B-cell lymphoma patients treated with rituximab: analysis from the Asia Lymphoma Study Group. Eur J Cancer 2013; 49:3486–96. [DOI] [PubMed] [Google Scholar]
- 14.Cheng AL, Hsiung CA, Su IJ et al. Steroid-free chemotherapy decreases risk of hepatitis B virus (HBV) reactivation in HBV-carriers with lymphoma. Hepatology 2003; 37:1320–8. [DOI] [PubMed] [Google Scholar]
- 15.Hammond SP, Borchelt AM, Ukomadu C, Ho VT, Baden LR, Marty FM. Hepatitis B virus reactivation following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2009; 15:1049–59. [DOI] [PubMed] [Google Scholar]
- 16.Jang JW, Kwon JH, You CR et al. Risk of HBV reactivation according to viral status and treatment intensity in patients with hepatocellular carcinoma. Antivir Ther 2011; 16:969–77. [DOI] [PubMed] [Google Scholar]
- 17.Tur-Kaspa R, Burk RD, Shaul Y, Shafritz DA. Hepatitis B virus DNA contains a glucocorticoid-responsive element. Proc Natl Acad Sci U S A 1986; 83:1627–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perrillo RP, Regenstein FG, Peters MG et al. Prednisone withdrawal followed by recombinant alpha interferon in the treatment of chronic type B hepatitis. A randomized, controlled trial. Ann Intern Med 1988; 109:95–100. [DOI] [PubMed] [Google Scholar]
- 19.Kim TW, Kim MN, Kwon JW et al. Risk of hepatitis B virus reactivation in patients with asthma or chronic obstructive pulmonary disease treated with corticosteroids. Respirology 2010; 15:1092–7. [DOI] [PubMed] [Google Scholar]
- 20.Lee YH, Bae SC, Song GG. Hepatitis B virus (HBV) reactivation in rheumatic patients with hepatitis core antigen (HBV occult carriers) undergoing anti-tumor necrosis factor therapy. Clin Exp Rheumatol 2013; 31:118–21. [PubMed] [Google Scholar]
- 21.Barone M, Notarnicola A, Lopalco G et al. Safety of long-term biologic therapy in rheumtaologic patients with a previously resolved hepatitis B viral infection. Hepatology 2015; 62:40–6. [DOI] [PubMed] [Google Scholar]
- 22.Perrillo RP. Tumor necrosis factor inhibitor therapy for HBV infected individuals: how loud is the alarm bell? Hepatology 2015; 62:16–8. [DOI] [PubMed] [Google Scholar]
- 23.Cholongitas E, Papatheodoridis GV. High genetic barrier nucleos(t)ide analogue(s) for prophylaxis from hepatitis B virus recurrence after liver transplantation: a systematic review. Am J Transplant 2013; 13:353–62. [DOI] [PubMed] [Google Scholar]
- 24.Wachs ME, Amend WJ, Ascher NL et al. The risk of transmission of hepatitis B from HBsAg(-), HBcAb(+), HBIgM(-) organ donors. Transplantation 1995; 59:230–4. [PubMed] [Google Scholar]
- 25.Perrillo R. Hepatitis B virus prevention strategies for antibody to hepatitis B core antigen-positive liver donation: a survey of North American, European, and Asian-Pacific transplant programs. Liver Transpl 2009; 15:223–32. [DOI] [PubMed] [Google Scholar]
- 26.Hwang JP, Fisch MJ, Zhang H et al. Low rates of hepatitis B virus screening at the onset of chemotherapy. J Oncol Pract 2012; 8:e32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wi CI, Loo NM, Larson JJ et al. Low level of hepatitis B virus screening among patients receiving chemotherapy. Clin Gastroenterol Hepatol 2015; 13:970–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khokhar OS, Farhadi A, McGrail L, Lewis JH. Oncologists and hepatitis B: a survey to determine current level of awareness and practice of antiviral prophylaxis to prevent reactivation. Chemotherapy 2009; 55:69–75. [DOI] [PubMed] [Google Scholar]
- 29.Tran TT, Rakoski MO, Martin P, Poordad F. Screening for hepatitis B in chemotherapy patients: survey of current oncology practices. Aliment Pharmacol Ther 2010; 31:240–6. [DOI] [PubMed] [Google Scholar]
- 30.Lee RS, Bell CM, Singh JM, Hicks LK. Hepatitis B screening before chemotherapy: a survey of practitioners' knowledge, beliefs, and screening practices. J Oncol Pract 2012; 8:325–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Day FL, Link E, Thursky K, Rischin D. Current hepatitis B screening practices and clinical experience of reactivation in patients undergoing chemotherapy for solid tumors: a nationwide survey of medical oncologists. J Oncol Pract 2011; 7:141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Artz AS, Somerfield MR, Feld JJ et al. American Society of Clinical Oncology provisional clinical opinion: chronic hepatitis B virus infection screening in patients receiving cytotoxic chemotherapy for treatment of malignant diseases. J Clin Oncol 2010; 28:3199–202. [DOI] [PubMed] [Google Scholar]
- 33.Stine JG, Khokhar OS, Charalambopoulos J, Shanmugam VK, Lewis JH. Rheumatologists' awareness of and screening practices for hepatitis B virus infection prior to initiating immunomodulatory therapy. Arthritis Care Res 2010; 62:704–11. [DOI] [PubMed] [Google Scholar]
- 34.Stine JG, Bass M, Ibrahim D, Khokhar OS, Lewis JH. Dermatologists' awareness of and screening practices for hepatitis B virus infection before initiating tumor necrosis factor-alpha inhibitor therapy. South Med J 2011; 104:781–8. [DOI] [PubMed] [Google Scholar]
- 35.Zurawska U, Hicks LK, Woo G et al. Hepatitis B virus screening before chemotherapy for lymphoma: a cost-effectiveness analysis. J Clin Oncol 2012; 30:3167–73. [DOI] [PubMed] [Google Scholar]
- 36.Hay AE, Meyer RM. Hepatitis B, rituximab, screening, and prophylaxis: effectiveness and cost effectiveness. J Clin Oncol 2012; 30:3155–7. [DOI] [PubMed] [Google Scholar]
- 37.Katz LH, Fraser A, Gafter-Gvili A, Leibovici L, Tur-Kaspa R. Lamivudine prevents reactivation of hepatitis B and reduces mortality in immunosuppressed patients: systematic review and meta-analysis. J Viral Hepat 2008; 15:89–102. [DOI] [PubMed] [Google Scholar]
- 38.Jang JW, Choi JY, Bae SH et al. A randomized controlled study of preemptive lamivudine in patients receiving transarterial chemo-lipiodolization. Hepatology 2006; 43:233–40. [DOI] [PubMed] [Google Scholar]
- 39.Hsu C, Hsiung CA, Su IJ et al. A revisit of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in non-Hodgkin's lymphoma: a randomized trial. Hepatology 2008; 47:844–53. [DOI] [PubMed] [Google Scholar]
- 40.Long M, Jia W, Li S et al. A single-center, prospective and randomized controlled study: Can the prophylactic use of lamivudine prevent hepatitis B virus reactivation in hepatitis B s-antigen seropositive breast cancer patients during chemotherapy? Breast Cancer Res Treat 2011; 127:705–12. [DOI] [PubMed] [Google Scholar]
- 41.Huang YH, Hsiao LT, Hong YC et al. Randomized controlled trial of entecavir prophylaxis for rituximab-associated hepatitis B virus reactivation in patients with lymphoma and resolved hepatitis B. J Clin Oncol 2013; 31:2765–72. [DOI] [PubMed] [Google Scholar]
- 42.Buti M, Manzano ML, Morillas RM et al. Tenofovir DF prevents HBV reactivation in anti-HBc positive patients with hematologic malignancies treated with rituximab: 12-months results of a randomized study (PREBLIN study) [Abstract 1661]. Hepatology 2014; 60(4 suppl):997A. [Google Scholar]
- 43.Huang H, Li X, Zhu J et al. Entecavir vs lamivudine for prevention of hepatitis B virus reactivation among patients with untreated diffuse large B-cell lymphoma receiving R-CHOP chemotherapy: a randomized clinical trial. JAMA 2014; 312:2521–30. [DOI] [PubMed] [Google Scholar]
- 44.Loomba R, Rowley A, Wesley R et al. Systematic review: the effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med 2008; 148:519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li HR, Huang JJ, Guo HQ et al. Comparison of entecavir and lamivudine in preventing hepatitis B reactivation in lymphoma patients during chemotherapy. J Viral Hepat 2011; 18:877–83. [DOI] [PubMed] [Google Scholar]
- 46.Evens AM, Jovanovic BD, Su YC et al. Rituximab-associated hepatitis B virus (HBV) reactivation in lymphoproliferative diseases: meta-analysis and examination of FDA safety reports. Ann Oncol 2011; 22:1170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]