Abstract
Somatic mutation theory of cancer has directed cancer research during the last century. A deluge of information on cellular, molecular, and genetic behavior was uncovered, but so was a mind-numbing complexity that still challenges research and concepts, and expectations in the war on cancer have by and large not been fulfilled. A change of paradigm beyond reductionism has been called for, especially as research ubiquitously points at the importance of tissue, microenvironment, extracellular matrix, embryonic and morphogenetic fields, and fields of tissue maintenance and organization in the processes of carcinogenesis, cancer control, and cancer progression, as well as in the control of cellular and genetic behavior. Holistic, organismic systems concepts open new perspectives for cancer research and treatment, as well as general biological understanding.
Key Words: Cancer, reductionism, holism, biology, lymphoma, leukemia, organism, systems medicine
摘要
上个世纪,癌症体细胞突变理论 主导着癌症研究。大量有关细 胞、分子和基因行为的信息涌现 出来,但却极为复杂,令人费 解,这也给研究和理念带来挑 战,总的来说,并未符合对癌症 之战的预期。人们呼吁改变超越 简化论的模式,特别是处处指向 组织、微环境、细胞外基质、胚 胎和形态发生场所、维护组织领 域和组织癌变过程、癌症控制和 癌症演化以及细胞和基因行为控 制组织的重要性的相关研究。整 体的有机系统概念可为癌症研究 和治疗以及常规的生物学理解开 启全新视角。
SINOPSIS
La teoría del cáncer por mutación somática ha dirigido la investigación sobre el cáncer durante el último siglo. Se ha descubierto un aluvión de información sobre las conductas celular, molecular y genética, pero también su abrumadora complejidad, que continúa poniendo a prueba la investigación y los conceptos y, en general, no se han cumplido las expectativas en la guerra contra el cáncer. Se ha pedido un cambio de paradigma más allá del reduccionismo, especialmente porque la investigación señala la importancia indudable de los campos tisulares, microambientales, de la matriz extracelular, embriónicos y morfogenéticos, y de los campos de mantenimiento y organización tisular en los procesos de carcinogenia, control canceroso y progresión cancerosa, así como en el control de la conducta celular y genética. Los conceptos de los sistemas holísticos y orgánicos abren nuevas perspectivas para la investigación y el tratamiento del cáncer, además de impulsar la comprensión biológica en general.
“Brücke and I, we have sworn to assert the truth that no forces are effective in the organism other than the strictly physico-chemical.”
—Emil du Bois-Reymond, physiologist (1818-1896)
REDUCTIONISTIC AND HOLISTIC PARADIGMS
Cancer research—like all life sciences—has developed in the tension between nothing-buttery and morethanism1–3: The reductionistic paradigm rests on the hypothesis that every living organism is “nothing but” physical and chemical reactions and can be fully explained by interactions of its parts, while the holistic paradigm implies that the whole of a living organism is “more than” the sum of its constituent parts (Box 1).
Box 1.
A Comparison of the Reductionistic and Holistic Paradigms3
| Reductionistic Paradigm | Holistic Paradigm |
|---|---|
| Particularism and Reductionism: Everything in nature can be reduced to and explained by its parts—cells, molecules, and atoms—and their interaction. | Holism: There is a supramolecular, holistic order in nature; holistic, hierarchical higher natural laws can be found and applied. |
| Materialism, mechanicism: Every living organism and process can be explained in total by laws of physics and chemistry, equivalent to a machine. | Living nature is a quality of its own: There are special natural laws in living nature, above and in addition to physics and chemistry that can be found and applied. |
| Darwinism: The only cause of evolutionary change is chance and selection by survival advantage. | Evolutionary holism: There are specific laws of nature that guide evolution. |
Both paradigms date back to the ancient Greeks but have intensively directed research during the last centuries. The reductionistic paradigm was dominant during the last century. It was tremendously successful in guiding experimental science in generating an enormous mass of information on cellular and molecular structures, characteristics, behavior, and interactions. This wealth of information, however, also revealed the immense complexity that currently confronts modern biology.
Multiparticularism: In human or animal organisms, tens of thousands of relevant functionally diverse biological molecules have been analyzed; hundreds or thousands of them are involved in the same process—such as tumor growth, tumor immunology, or gene function.
Multirelationism: These molecules interact with dozens or hundreds of other molecules, selectively and nonlinearly, meaning that the net effect in a biological process can differ substantially from its effect under isolated experimental conditions (“a small change in initial conditions can be vastly amplified to produce chaotic behavior”4).
Pleiotropism, pluripotence, or multifunctionality: Each cell or molecule, like cytokines, can have multiple functions and effects.
Redundancy: Different molecules and cells have the same effect.
Context-dependency of the effects: The effect of a certain molecule or cell on another cell or process is dependent on the context and can be the opposite. “In networks, context is everything.”5
These different dimensions of “mind-numbing”6 complexity that crushed basic concepts of cellular and molecular interactions7 preclude the precise and clear-cut understanding of how these molecules are integrated in cell, tissue, organ function, or organism as well as a reliable explanation and a precise prediction of biological processes by its constituent parts: “When we get to a certain network complexity, we completely fail to understand how it works”6 or “Even if you construct a complete list of all the processes known to occur within a cell, that won't tell you how it works.”8 Faced with this challenge—to calculate coherent behavior out of more than a universe full of informational data—biology needs an approach “beyond reductionism,”9 a “whole-istic biology”10 approach to understand these complex systems. Systems biology became one of the emerging fields in an attempt to understand complex networks with methods like computational biology, simulations, and mathematical modeling. Still, living organisms have essential characteristics that reach beyond the complex interrelationship of thousands of molecules in networks: multilevel systems; an ability to react, adapt, and restore functions; the regulation and harmonization of all functions on different levels. To understand living organisms, a paradigm shift—a conceptual breakthrough—is needed.11,12
REDUCTIONISTIC CANCER CONCEPT: ARE WE READY TO GO BEYOND?
In cancer research and concepts, “reductionism has been the driving obsession.”13 Cancer is understood as a cellular disease caused by cancer cells. Normal cells transform into cancer cells through random mutations in certain genes. (“Cancer is caused by alterations in oncogenes, tumor-suppressor genes, and microRNA genes.”14) These mutations can be caused by chemicals, ionizing radiation, or viruses or during normal replication of chromosomes. This leads to increased and uncontrolled cell proliferation through gain-of-function mutations in oncogenes (they stimulate proliferation, survival, metastases) and through loss-of-function mutations in tumor-suppressor genes (they regulate and inhibit cell proliferation, promote DNA repair, induce apoptosis). These mutations are passed on to the daughter cells. Several mutations are needed to initiate cancer (multi-step development). Further mutations accumulate and lead to cancer progression: increased genetic instability generating genetic diversity; growth advantage through self-sufficiency in growth signals and insensitivity to anti-growth signals; aggressiveness; cell survival, evasion of natural cell death (apoptosis); sustained angiogenesis; tissue invasion; metastatic spread, etc. Cancer cells are distinct from normal cells; they are autonomous, uncontrolled by the microenvironment, tissue, organism, and the malignant process is irreversible, progressive, and cumulative, finally leading to the death of the host.14–17 The adequate therapy according to this paradigm is to completely and aggressively eradicate all cancer cells, accepting mutilation of the patient; accordingly, wording and metaphors in oncology—research, treatment, patient communication, advertisement—are predominantly military.
Methods of cancer research and the concepts of the cancer cell were inspired and guided by two tremendously successful disciplines: parasitology, whose dramatic successes at the end of the 19th and beginning of the 20th centuries inspired all areas of medical research,18 particularly with the successful discovery of antibiotics, and by the leading discipline of the 20th century, genetics. Seminal steps in the evolution of the reductionistic cancer paradigm are shown in Box 2.
Box 2.
Seminal Steps in the Evolution of the Reductionistic Cancer Concept3
|
In 1971, US President Richard Nixon declared the “war on cancer” (National Cancer Act of 1971) and announced the goal to cure cancer in the bicentennial year 1976.19 With a national commitment and a similar concentrated effort that split the atom and took man to the moon, this dreaded disease should be conquered.20 Although, unquestionably, the treatment and survival rates have substantially improved in some types of cancer such as lymphomas, leukemia, childhood malignancies, and testicular cancer, the overall goal is far from reached despite almost unlimited research funding and the transformation of cancer-drug development into a multibillion dollar industry. Up to 1990, death rates continuously increased, and since then, they slowly decreased, mainly due to cancer prevention through tobacco control and other endeavors.21 Most patients with solid tumors do not respond to any given drug.22 Altogether, death rates have changed little, and cancer has remained a major fatal disease in the industrialized world.
Reasons are manifold: inadequate tumor models used in the many cancer-drug screening programs; significant acute and long-term toxicity of anticancer drugs; low response rates in patients due to low drug sensitivity (currently, expectations go to select responders and individually target the treatment to molecular characteristics of cancer cells); and rapid evolution of aggressive drug-resistant cells due to high mutation rates and selective pressure, resulting in transitory treatment responses.22,23 But basically, the empirical observations revealed high complexity that thwarted the presumption of cancer as a “simple story.”24 The somatic mutation theory was challenged by the multiple discoveries that genes were not the clear-cut “coded instructions”25 for life, the “command center,”26 or “hidden ruler of life”25 as presupposed, where the function and structure clearly and unambiguously follows out of the DNA sequence. Instead, it was revealed that genes themselves are de facto regulated on many levels: by histones; methylation; splicing (DNA sequences have to be cut out and put together to generate a “gene”) and alternative splicing (DNA sequences may produce different RNAs and hence different proteins);27–29 RNA-editing;30 RNA-transport from nucleus to cytoplasm;31 modification of translation;32,33 stabilization or degradation of mRNA; posttranslational modifications;34 acetylation; methylation, etc. So, a certain DNA sequence can participate in the synthesis of many different proteins and many different functions. It is a passive source of material upon which a cell has to draw to synthesize proteins and tissues with certain functions35 depending on its regulation. Besides, the DNA itself also turned out to be less stable than anticipated, but rather fluid, flexible, and able to reorganize rapidly.36–38
In the face of the disappointing results—“The war against cancer is far from over”39—the need for a turning point in cancer research and cancer paradigm is called for40–44: “The time has come, to shift the cancer paradigm.”45 This is especially so, as mounting evidence points to the importance of the microenvironment, the tissue and organism in regulating genetic function and cellular behavior, in cancer development.
MICROENVIRONMENT REGULATES PHENOTYPE AND GENOTYPE OF CANCER CELLS
Why do we harbor so many potentially malignant tumors without getting cancer? In autopsies, cancer is found quite frequently, in many more instances than it appears during life. For instance, in the prostate glands from deceased young male patients, small foci of histological cancer were found in 27% and 34% of the men in their 30s and 40s, respectively.46 Autopsies of 110 young and middle-aged Danish women (20-54 years) found malignancy in the breast of 22 women (20%, mostly carcinoma in situ), with multicentric and bilateral lesions in nearly half of them. Of the women in their 40s, 39% had malignant lesions in their breasts. The life-long cumulated frequency of clinical, invasive breast cancer in the Danish population, however, is just 6.5%.47 In 101 consecutive autopsies of thyroids, occult papillary carcinoma was found in 36%. Ten glands even contained two to five tumor foci. Only a minimal proportion will ever become a clinical carcinoma. These lesions were even regarded as a normal finding not to be treated when incidentally found.48 There are many other examples.42
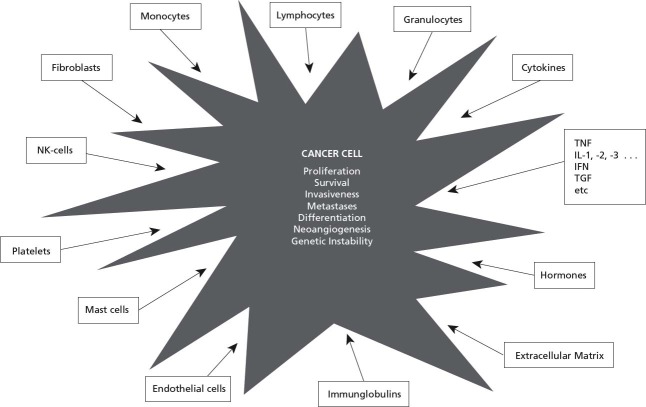
Cancer cells are less autonomous than assumed: There is deluge of data showing that cell proliferation, survival, apoptosis, differentiation, polarity of cells, gene function, invasiveness, ability to metastasize, angiogenesis, and drug resistance are enhanced, inhibited, or otherwise modified by the microenvironment, the surrounding cells or molecules (Figure 1), including cytokines, lymphocytes, granulocytes, macrophages, fibroblasts, and many others. Interestingly, identical cytokines and identical cells quite commonly have different and often opposite effects on tumor cells' behavior, depending again on the context.3,7,49,50
Figure 1.
The behavior of cancer cells is influenced by various other cells and molecules.
A strong influence on tumor cell behavior is particularly exerted by the extracellular matrix.42,51–53 Extracellular matrix also regulates normal development and function of an organism: embryogenesis, growth and differentiation of cells, gene function, apoptosis, tissue specificity, tissue structure, and normal homeostasis of the adult organism.54–58 The classic feature of cancer tissue is the disturbed communication between cells and extracellular matrix.56,59,60 Breaking cell-cell bonds and intercellular communication is known to induce neoplastic behavior.61 Experimentally, the interaction between tumor cells and extracellular matrix could be improved by correcting the extracellular matrix receptor integrin (treating with inhibitory β1-integrin antibodies in a 3-dimensional basement membrane culture). This led to a striking morphological and functional reversion of the breast cancer cells to a normal phenotype. Also, their malignancy in vivo was substantially reduced despite persisting prominent mutations, amplifications, and deletions on the genetic level.62
Cells can have a multitude of chromosomal mutations, but as long as the cells are in an appropriate cellular microenvironment that allows a cell to adopt a normal structure, the cell will display a normal phenotype.56 Normal breast tissue adjacent to breast cancer tissue, for instance, displayed a normal morphological appearance in situ, although the cells contained chromosomal aberrations (loss of heterozygosity) identical to mutations that characterize invasive breast cancer.51,63 Cancer researcher Bissell concluded,
The structure of the tissue is dominant over the genome, and that we may need a new paradigm for how epithelia-specific genes are regulated in vivo. We also argue that unless the structure of the tissue is critically altered, malignancy will not progress, even in the presence of multiple chromosomal mutations.56
The normal microenvironment and architecture of the tissue can constrain tumor development but, conversely, can also promote and induce cancer.42,53
TUMOR TISSUE AS A POPULATION
Not only do the microenvironment, surrounding cells, molecules, and extracellular matrix influence the behavior of the tumor, tumor cells themselves also influence each other. Tumor tissue consists of a heterogeneous and dynamic cell population. The multiple subclones in a tumor tissue differ in important behavioral properties, such as growth rate, ability to metastasize, and sensitivity to treatment. When different tumor subpopulations with different characteristics are mixed together to mimic tumor heterogeneity, they influence each other's growth, metastatic behavior, and response to chemotherapeutics or hormones in a way that cannot be predicted from the behavior of the individual subclones. Growth can be induced, enhanced, reduced, or inhibited; insensitive clones can become sensitive and vice versa: Cell clones interact “any way they can.”64–68 The growth of multiple tumors in the same experimental host often results in a mutual decrease in growth rate. After removal of a tumor, the growth of the remaining tumors or metastases is accelerated. When a second tumor was implanted, the growth of the others decreased.65,69,70 Individual cell types do not function in isolation in a complex system. Cancer should therefore be dealt with and investigated as an integrated organ, a cancer cell society, with its own characteristics, rather than a collection of independently growing cells.68,71
MALIGNANT TRANSFORMATION AND REVERSION OF MALIGNANCY BY THE ENVIRONMENT
Not only the phenotype but also the malignancy itself, the malignant transformation and its reversal, can be induced by the environment. For instance, cancer cells can induce a malignant transformation in neighboring normal cells. This was shown in athymic nude mice transplanted with human tumor cells—a classic experimental model in cancer research. When cells had grown to tumors, these were harvested and investigated: They consisted of either only human cancer cells (as expected), a mixture of both human cancer cells and mouse (host) transformed cells with abnormal karyotypes or chromosomal constitutions, or only transformed mouse cells with specific chromosomal abnormalities and malignant phenotypes.72–76
The initial dogma “once a cancer cell, always a cancer cell” was disproven by Barry Pierce and his colleagues, who demonstrated the differentiation of malignant neoplastic cells to benign cell types.77–79 Back in the 1950s, his observations first of all led to a rejection by Cancer's editor, because “everybody knows that cancer cells cannot differentiate.”80 Today, this phenomenon—cancer cells can differentiate to normal cells with normal behavior when exposed to certain environments or certain substances—is well known.81
Illmensee and Mintz were the first to demonstrate that teratocarcinoma cells, when transplanted into normal early embryos (embryoblasts) can reverse to normal cells (Figure 2). These embryos develop into normal healthy mice, which are in fact chimeras, a mosaic of tissues from normal embryonic cells derived from their “natural” parents and of cells derived from the teratocarcinoma that reversed to normality. The ex-carcinoma cells were found in all tissues—skin, eye, blood, bowel, heart, kidney, muscle, reproductive glands, and others. Subsequent breeding of a male showed that it produced viable sperm derived from the original teratocarcinoma cells. The resultant offspring did not develop tumors.82–85 Similar experiments were conducted in other laboratories with other carcinoma cells such as myeloic leukemic cells,86,87 neuroblastoma cells,88,89 and melanoma cells 90,91 and showed that the normal embryonic milieu can reprogram meta-static tumor cells toward benign ones with normal behavior that display cell morphologies resembling the host cells.
Figure 2.
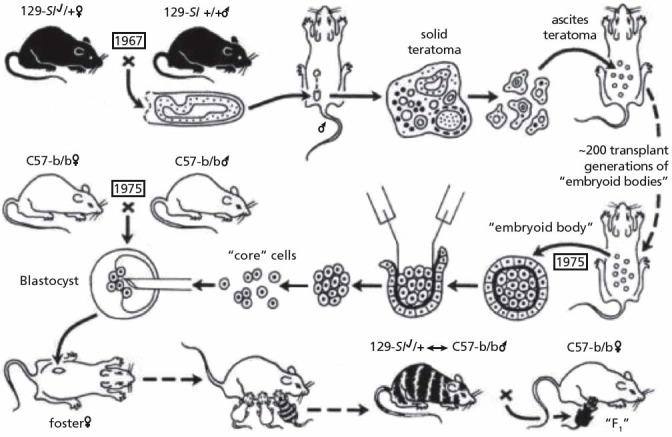
Experiments by Mintz and Illmensee: Metastatic melanoma was produced by placing a 6-day male 129 embryo under a testis capsule. Ascites tumor of embryoid bodies was generated and was maintained in 200 transplant generations. The malignant core cells were injected into blastocysts from parents of C57BL/6 mice, which were then transferred to the uterus of pseudopregnant mothers. Normal mice, which were chimeras, were born with coal-colored mosaicism or internal tissue contribution of the 129 tumor cells. A mosaic male produced viable sperm of the 129 genotype. Despite originating from malignant cells, the progeny did not produce tumors. Figure source: Mintz B, Illmensee K, 1975.81 Reprinted with permission from the author.
The importance of cytoplasmatic, epigenetic regulation for gene (and oncogene) function and for the malignant or benign phenotype of the cell was shown by transplantation of nuclei from frog renal adenocarcinoma into activated but enucleated normal frog ova: no growth of cancer cells was observed, but a blastulae development up to early stage tadpoles with normal appearance occurred.92–94 Transplantation of tissue of these tadpoles into normal hosts generated tissue indistinguishable from normal tissue.95 Similar results also were observed after transplantation of the nucleus of other cancer types, such as medulloblastomas, melanomas, and some breast cancer and lymphoma cell lines, into enucleated oocytes.96,97
Adult tissue can also lead to reconversion of malignant to benign growth. For instance, the transplantation of basal cell carcinomas into the uterus of rats first led to tumor formation, which then regressed. After 4 to 6 weeks, the tumors had disappeared, and progressively larger stretches of the uterine lumen were covered with stratified epithelium, which resembled epidermis.98 Highly aggressive cancer cells derived from liver epithelial cells produced aggressively growing tumors when transplanted subcutaneously; however, when transplanted into the liver, they lost aggressiveness and neo-plastic behavior, either partly or completely with a fully differentiated morphology and integration into hepatic plates. This regulatory capacity of the liver microenvironment declined with advanced age. Although transplanted tumor cells partly responded to epigenetic differentiation signals of the hepatic tissue of older rats or when younger rats grew older, their progeny proliferated again, formed foci, and when removed from the liver, reverted to an aggressively tumorigenic phenotype.99,100
Cancer development as a physiological response to environmental perturbations or altered tissue environment was also confirmed by Farber et al, who treated rats with carcinogens, leading to a multitude of small nodules in the liver. These were resistant to a variety of agents, including carcinogens, and showed a whole array of differences from the normal liver tissue. They were regarded as a physiological pattern of adaption to survive in a hostile environment. Only 2% to 5% of the nodules persisted and became the origin of slow evolution to cancer; the majority (95%-98%) of the nodules disappeared again by differentiation, remodeling, reorganization, and rearrangement of their component hepatocytes and blood vessels to form normal liver tissue.101,102
CANCER AND EMBRYOLOGY AND TISSUE RENEWAL
Cancer cells and tissue closely resemble embryonic and regenerating cells and tissue in their proliferation, migration, invasiveness, neoangiogenesis, undifferentiation, synthesis of fetal proteins, potential immortality, induction of immunological tolerance in the host, etc. However, there is one key difference: In embryonic and regenerating tissues, these processes are highly organized, whereas in cancer they are displaced and unregulated. Cancer, therefore, is seen as “a problem of developmental biology”103,104 and as a caricature of the normal process of tissue renewal, where carcinoma cells grossly exaggerate normal characteristics,104 or as “wounds that do not heal.”105
Interestingly, the ability to develop cancer is inversely correlated to the propensity for regeneration: Cancer has been found only in animals with a low or absent regenerative capacity of complete limbs or head, while animals with a strong ability to rebuild lost limbs hardly ever develop cancer.106–110 This is especially noticeable in hydra, which can replace all limbs without any difficulties. It can be split in many small particles which assemble again to a new organism. It is potentially immortal. Never has any cancer or other tumor formation been found in hydras.
Turbellaria, a flatworm, has a strong regenerative ability, which differs within the body parts. When the turbellaria was treated with carcinogens, tumors could only be induced in nonregenerative parts of the worm but not in parts with a high regenerative capacity. Still, these tumors did not lead to the death of the worm and healed spontaneously with interesting features. After some growth, an open wound appeared at the tumor site, followed by change of the histological appearance: the tissue became increasingly structured, cells moved to the wound, tumor cells successively differentiated and showed the morphology of the normal local tissue until the tumor had completely disappeared. This process resembled normal regeneration. Sometimes super-numerary organs like little tails developed.110 Similar observations were made in other powerful regeneration models, such as the lenses and iris or limbs from newts, showing that the autonomous growth of malignant cells can be brought under control or reintegrated into the biological system by the regenerative process.111–113 Correspondingly, an impaired, incomplete, or uncoordinated regenerative process after chronic injury or inflammation can give rise to a malignant tumor.112 This resembles acute feverish infectious diseases in humans that are associated with a reduced cancer incidence and even with cancer remission, while chronic inflammation—which seems to be the result of an individual's inability to eliminate infection and restore immune homeostasis—increases cancer risk.114
PHYSICAL ISOLATION AND CANCER
Physical isolation from the biological system alone can induce malignant tumor growth, as was discovered accidentally when rats' kidneys were wrapped in cellophane film to produce experimental hypertension, but instead unexpectedly induced sarcomas that hardly ever develop spontaneously in these rats. Further investigations revealed that sarcomas could be induced just by embedding plastic films subcutaneously. The sarcomas were induced regardless of the chemical structure of the plastic film. The physical form, the physical separation, was critical. When the plastic had other physical forms, such as perforated film, fibers, textiles, powders, or granules, the sarcoma-inducing ability disappeared and few or no sarcomas were induced any more.115–118 Thus, cancer can be induced by disturbed tissue topography—by disturbing the normal homeostatic relations and interaction between cells, tissues, and organs, their morphogenetic field.119
TOWARDS AN SYSTEMS-ORIENTED (ORGANISMIC) CONCEPT IN CANCER RESEARCH
Michael Sporn, one of the leading figures in oncogene research (Box 2) stated, “Carcinoma is a disease of the whole organism. Although molecular and cell biology have immense power as analytical tools, the ultimate understanding and control of the process of carcinogenesis will require a new synthesis at the levels of tissue, organ, and organism.”44 But what is an organism? The postgenomic era overcomes its crisis by making networks to the centerpiece of the new paradigm, of the systems biology's endeavor to understand biological processes, to find the coordinating principles. While the notion of “system” has been known in biology for a century—with its eminent thinkers Paul Weiss, Ludwig von Bertalanffy, Walter M. Elsasser, and others—its meaning varied over time. Today, systems biology is based on the premise that a living organism consists of an interacting and dynamic network of genes, proteins, and other molecules that cause the function and behavior of the whole macroscopic organism. It uses bioinformatics and embraces a variety of experimental and computational approaches, including data mining to find hidden patterns (automated discovery) and simulation-based analysis to test hypotheses. This is facilitated by the rapid progress in molecular biology generating large quantitative datasets on the one side and the substantial advances in software and computational power on the other side.120,121
Creating these network models of biological process, however, still remains a “flat Earth systems biology.”11 They do not address a whole host of considerations, including the fundamental characteristics of the living organism; the hierarchically structured different levels; emergence (“the behavior of the overall system typically manifests itself in totally new phenomena, called emergent behavior”4); cross-level interaction; vertical causality; harmonization; the development of the supracellular form or Gestalt of organs or body parts; the positional information within the whole despite parts being displaced; regeneration; robustness; and the reliable functioning of tissues, organs, and organism over decades or even a century. “For a conceptual breakthrough a new paradigm is needed … beyond the current parameters of networks and of systems.”11
Holistic thinking in biology can be traced back to ancient Greece and had its zeniths during German idealism with one of its outstanding figures, Johann Wolfgang von Goethe.1,2,122 The concept of “system” was introduced into modern biology by Paul Weiss. He pointed out that the essence of a living system is its hierarchical order, with the macrodeterminism as its most decisive element: the macroscopic level has a high determinism, a high grade of order and predictability, and can maintain its order while the constituent parts are highly variable.123,124
The property of the system as a whole to build, operate, and maintain itself in a state of desirable orderliness, predictable from its repetitive and systematic recurrence and endurance, in spite of the continued flux and infinitely greater range of variation, hence, unpredictability, of the behavior of its constituent units, which carry out the building, operation, and maintenance…. In a system, the structure of the whole coordinates the play of the parts.”123
The living system, ie, organism, is hierarchically ordered and consists of several interacting subordinate sub-systems. Each sub-system regulates its own subordinate smaller parts, restraining their degree of freedom (Figure 3). The system is lawful, and its laws can be found without knowing all of the details of the involved elementary processes.123 In this sense, Weiss also derived a field theory for developmental biology. The field is structured and ascribes certain qualities, direction, intensities, and functions to every point of the field. It organizes the material. The field concept is similar to the ones in physics.125 Weiss was also inspired by the Gestalt theory of Christian von Ehrenfels, Wertheimer, Köhler, Koffka, Lewin, and others, who made seminal discoveries that fundamentally influenced the theories of perception and Gestalt psychology.125–129 A Gestalt is by definition a wholeness. It is more than the sum of its parts, and it is perceived independent of its parts. Gestalt became a model notion for systems biology. In general, scientific objectivity and scientific reasoning, as well as clinical judgment, often rely on Gestalt cognition.130,131
Figure 3.
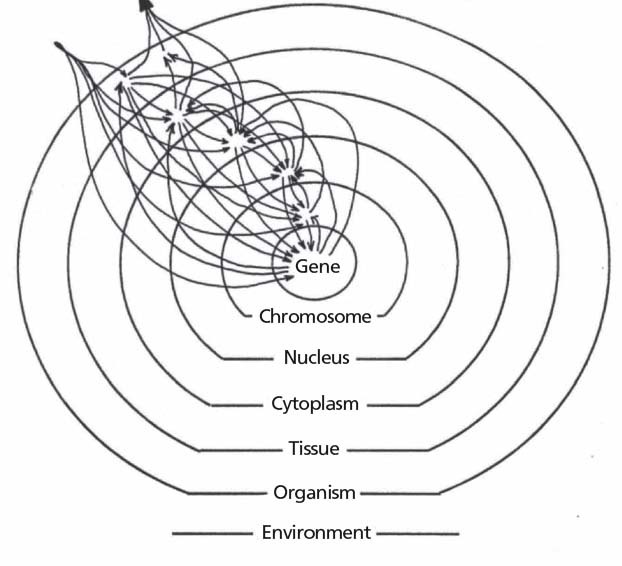
Concept of living organism by Paul Weiss: Interactive relations between hierarchically ordered subsystems. Adapted from Weiss PA, 1967.123
These strong concepts influenced many fields of modern science, of holistic and organismic concepts of biology and medicine. One of the most outstanding and well-elaborated organismic concepts of cancer was framed by the molecular and developmental biologists Sonnenschein and Soto. These distinguished cell researchers from Boston start with the question, “Why do cells proliferate?” The dominant premise is that quiescence, G0, is the default state of a cell in Metazoa and that cells divide only when they receive a positive signal, ie, are stimulated by growth factors. This notion has acquired an axiomatic quality although it is seldom discussed and has never been proven. Sonnenschein and Soto now propose the contrary: proliferation is the default state of cells, with the consequence that their control is mediated negatively by inhibitory signals. This is based on an evolutionary perspective and on their long experience working with cell cultures. Among microbiologists, it is generally accepted that proliferation is the default state of prokaryotes and unicellular eukaryotes; the ability to proliferate is constitutive to these organisms and largely depends on the availability of nutrition. As multicellular organisms evolved from unicellular organisms, a more complex control and restraint of cell proliferation evolved as well. Rate of proliferation depends on cell type, tissue, organ development, and physiological stage. Since cell cycle markers have been highly conserved from yeast to humans, is it implausible that the default state of proliferation inherent to unicellular organisms would have switched so fundamentally to quiescence. Instead, one can presume that proliferation is a constitutive property of all cells—unicellular and multicellular—meaning that cells will exercise their constitutive, built-in capacity to proliferate when adequately nourished and when extracellular or intracellular inhibitors are absent or inactive. This complies with experimental observations on cell proliferation.41,132
When proliferation is the default state of a cell, the control of proliferation has to be negative. Growth factors then block this inhibition of cell proliferation. The major difference between unicellular and multicellular systems is the coordinated control of cell regulation. And therein lies the new perspective on organisms and cancer, evolved from the concept of the morphogenetic field, which is a basic paradigm of embryology and developmental biology:
We posit that in normal, adult, multicellular organisms there are discrete units of tissue maintenance and/or organization; histologically, they comprise the parenchyma and the stroma of an organ. During embryogenesis, adjacent stroma and epithelia exert instructive influences on each other resulting in organ formation. This complex web of interactive signaling continues throughout the lifespan of the individual. We further postulate that these units of tissue maintenance and/or organization are tridimensional and carry positional and historical information. They maintain the normal architecture of all organs and guide tissue turnover, remodeling and healing through a dynamic process.41,132
These units of tissue organization field regulate cellular behavior, proliferation, mobility, differentiation, survival, invasiveness, etc. They are the primary locus of cancer development and the primary target of carcinogenic agents. Cancerogenesis begins with a disruption of normal interaction between cells and the subadjacent stroma of an organ. This leads to structural and functional changes in the affected tissue. Individual cells within these lesions recognize misinformation, which may lead to increased proliferation and disorders in organization or appearance such as dysplasia or metaplasia. Further deterioration of the tissue organization and/or maintenance field leads to carcinoma in situ. If the disturbance ceases, the process can still be restored. If the disturbance persists, cancer develops with increasing proliferation, invasiveness, metastatic ability, autonomy, survival, genetic instability, etc, due to diminished regulation by the disturbed morphogenetic field.41,132
This tissue organization field theory of cancer implies consequences for carcinogenesis research. Investigation of isolated cancer cells is of little relevance. Important, however, are investigations in the complete tissue, where the core changes are represented, such as in hyperplasia, dysplasia, metaplasia, and carcinoma in situ. Placing cells in a culture dish already disrupts the tissue field, potentially producing malignant cellular behavior because
The exploration of the tissue organization field theory of carcinogenesis requires the use of tissue recombination and transplantation experiments, comparable to the ones that informed embryologists about the inductive and permissive influences in organogenesis.41,132
The experimental observations described above are compatible with the tissue organization field theory of neoplasia. This theory is also consistent with the conclusion of many researchers who have characterized cancer as
a disease of organization133;
a breakdown of the hierarchical organization65;
an ongoing systemic failure in growth control134; homoeostase,44 or communication between epithelium and stroma44;
a chronic, maladaptive tissue and organismic response to injury44;
a dynamic developmental disorder119;
a disturbance of the morphogenetic field, specifically the field for tissue organization and/or maintenance132;
a change in the growth-restraining forces134;
a failure of host's organs and tissues to exercise growth control134;
a multicellular life that has partially escaped from the hierarchical controls of tissue, organ, and organismal life136;
an embryonic growth potential released from restraint134; and
a disease of the whole organism.44
Further research within this organismic, holistic paradigm that understands cancer as deranged organs acting in the context of an organism has to concentrate on the tissue, organ, and organism.3 Key requirements are new and elaborate paradigms and conceptual breakthroughs as well as the investigation of microenvironmental regulation, normal development, tissue architecture, homeostasis, hygiogenesis, repair, and regeneration. Rather than “Why do smokers get cancer?,” the question that is relevant is, “Why don't smokers get cancer?” Meaning: How do some organisms manage to restore health despite strong carcinogenic influences? Additionally, the restorative function of high feverous infections and the historic success of fever therapy with bacterial vaccines will be of renewed interest.114 Salutogenetic metaphors may replace the current military ones.
Disclosure The authors declare no competing interests.
Contributor Information
Gunver Kienle, Gunver S. Kienle, Dr med, is senior research scientists at the Institute for Applied Epistemology and Medical Methodology at the University of Witten/Herdecke in Freiburg, Germany..
Helmut Kiene, Helmut Kiene, Dr med, is senior research scientists at the Institute for Applied Epistemology and Medical Methodology at the University of Witten/Herdecke in Freiburg, Germany..
REFERENCES
- 1.Dijksterhuis EJ. Die Mechanisierung des Weltbildes. Berlin, Göttingen, Heidelberg: Springer; 1956. [Google Scholar]
- 2.Gloy K. Das Verständnis der Natur. 2. Die Geschichte des ganzheitlichen Denkens. Beck; 1996. [Google Scholar]
- 3.Kienle GS, Kiene H. Beyond reductionism: zur Notwendigkeit komplexer, organismischer Ansätze in der Tumorimmunologie und Onkologie. In: Kienle GS, Kiene H: Die Mistel in der Onkologie. Stuttgart, New York: Schattauer Verlag; 2003:333–432. [Google Scholar]
- 4.Bonn D. Biocomplexity: look at the whole, not the parts. Lancet. 2001;357:288. [DOI] [PubMed] [Google Scholar]
- 5.Orosz CG. An introduction to immuno-ecology and immuno-informatics. In: Segel LA, Cohen IR, editors. Design principles for the immune system and other distributed autonomous systems. Oxford, New York: Oxford University Press; 2001:125–49. [Google Scholar]
- 6.Service RF. Exploring the systems of life. Science. 1999;284:80–3. [DOI] [PubMed] [Google Scholar]
- 7.Nathan C, Sporn MB. Cytokines in context. J Cell Biol. 1991;113(5):981–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Normile D. Building working cells “in silico.” Science. 1999;284:80–1. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher R, Appenzeller T. Beyond reductionism. Science. 1999;284:79. [Google Scholar]
- 10.Chong L, Ray B. Whole-istic biology. Science. 2002;295:1661. [Google Scholar]
- 11.Mesarovic M, Sreenath SN. Beyond the flat earth perspective in systems biology. Biol Theory. 2006;1(1):33–4. [Google Scholar]
- 12.Mesarovic MD, Sreenath SN, Keene JD. Search for organising principles: understanding in systems biology. Systems Biology. 2004;1(1):19–27. [DOI] [PubMed] [Google Scholar]
- 13.Weinberg RA. The genetic origins of human cancer. Cancer. 1988;61:1963–8. [DOI] [PubMed] [Google Scholar]
- 14.Croce CM. Oncogenes and cancer. N Engl J Med. 2008;358(5):502–11. [DOI] [PubMed] [Google Scholar]
- 15.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. [DOI] [PubMed] [Google Scholar]
- 16.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. [DOI] [PubMed] [Google Scholar]
- 17.Muzny DM, Bainbridge MN, Chang K, Dinh HH, Drummond JA, Fowler G, et al. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silverstein AM. A history of immunology. San Diego, New York, Boston, London, Sydney, Tokyo, Toronto: Academic Press Inc; 1989. [Google Scholar]
- 19.Brennan R, Federico S, Dyer MA. The war on cancer: have we won the battle but lost the war? Oncotarget. 2010;1(2):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nixon R. State of the Union Address. 1971. January 22. [Google Scholar]
- 21.Jemal A, Ward E, Thun M. Declining death rates reflect progress against cancer. PLoS ONE. 2010;5(3):e9584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chabner BA, Roberts TG. Chemotherapy and the war on cancer. Nature Rev Cancer. 2005;5(1):65–72. [DOI] [PubMed] [Google Scholar]
- 23.Gura T. Systems for identifying new drugs are often faulty. Science. 1997;278:1041–2. [DOI] [PubMed] [Google Scholar]
- 24.Weinberg RA. Racing to the beginning of the road. The search for the origin of cancer. London, New York, Toronto, Sydney, Auckland: Bantam Press; 1997. [Google Scholar]
- 25.Dulbecco R. The design of life. New Haven, CT: Yale University Press; 1990. [Google Scholar]
- 26.Vinson V, Purnell BA, Chin GJ, Marx J. Macromolecular ballet. Science. 2000;288(5470):1369. [Google Scholar]
- 27.Sharp PA. Split genes and RNA splicing. Cell. 1994;77:805–15. [DOI] [PubMed] [Google Scholar]
- 28.Tamkun JW, Schwarzbauer JE, Hynes RO. A single rat fibronectin gene generates three different mRNAs by alternative splicing of a complex exon. Proc Natl Acad Sci. 1984;81:5140–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez AJ. Alternative splicing of pre-mRNA: developmental consequences and mechanisms of regulation. Annu Rev Genet. 1998;32(2):279–305. [DOI] [PubMed] [Google Scholar]
- 30.Brennicke A, Marchfelder A, Binder S. RNA editing. FEMS Microbiol Rev. 1999;23(3):297–316. [DOI] [PubMed] [Google Scholar]
- 31.Terry LJ, Shows EB, Wente SR. Crossing the nuclear envelope: hierarchical regulation of nucleocytoplasmic transport. Science STKE. 2007;318(5855):1412. [DOI] [PubMed] [Google Scholar]
- 32.Farabaugh PJ. Programmed translational frameshifting. Microbiol Rev. 1996;60(1):103–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Groisman I, Engelberg-Kulka H. Translational bypassing: a new reading alternative of the genetic code. Biochem Cell Biol. 1995;73(11-12):1055–9. [DOI] [PubMed] [Google Scholar]
- 34.Walsh CT, Garneau-Tsodikova S, Gatto GJ., Jr Protein posttranslational modifications: the chemistry of proteome diversifications. Angewandte Chemie Intl Ed. 2005;44(45):7342–72. [DOI] [PubMed] [Google Scholar]
- 35.Nijhout HF. Metaphors and the role of genes in development. Bioessays. 1990;12(9):441–6. [DOI] [PubMed] [Google Scholar]
- 36.Walbot V, Cullis CA. Rapid genomic change in higher plants. Ann Rev Plant Physiol. 1985;36:367–96. [Google Scholar]
- 37.Bennetzen JL. Transposable element contributions to plant gene and genome evolution. Plant Mol Biol. 2000;42(1):251–69. [PubMed] [Google Scholar]
- 38.Deragon JM, Capy P. Impact of transposable elements on the human genome. Ann Med. 2000;32(4):264–73. [DOI] [PubMed] [Google Scholar]
- 39.Bailar JC, III, Gornik HL. Cancer undefeated. N Engl J Med. 1997. May 29;336(22):1569–74. [DOI] [PubMed] [Google Scholar]
- 40.Goldstein I, Madar S, Rotter V. Cancer research, a field on the verge of a paradigm shift? Trends Mol Med. 2012;18(6):299–303. [DOI] [PubMed] [Google Scholar]
- 41.Sonnenschein C, Soto AM. Theories of carcinogenesis: an emerging perspective. Elsevier; 2008:372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bissell MJ, Hines WC. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nature Medicine. 2011;17(3):320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Platoni K. Thinking outside the cell. Oakland, CA: East Bay Express; 2007. [Google Scholar]
- 44.Sporn MB. The war on cancer. Lancet. 1996;347:1377–81. [DOI] [PubMed] [Google Scholar]
- 45.Schipper H, Goh CR, Wang T-UL. Shifting the cancer paradigm: Must we kill to cure? J Clin Oncol. 1995;13(4):801–7. [DOI] [PubMed] [Google Scholar]
- 46.Sakr WA, Haas GP, Cassin BF, Pontes JE, Crissman JD. The frequency of carcinoma and intraepithelial neoplasia of the prostate in young male patients. J Urol. 1993;150(2 Pt 1):379. [DOI] [PubMed] [Google Scholar]
- 47.Nielsen M, Thomsen JL, Primdahl S, Dyreborg U, Andersen JA. Breast cancer and atypia among young and middle-aged women: a study of 110 medicolegal autopsies. Br J Cancer 1987;56:814–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harach HR, Franssila KO, Wasenius VM. Occult papillary carcinoma of the thyroid. A “normal” finding in Finland. A systematic autopsy study. Cancer. 1985;56(3):531–8. [DOI] [PubMed] [Google Scholar]
- 49.Balkwill FR. Cytokine therapy of cancer. The importance of knowing the context. Eur Cytokine Netw. 1994;5(4):379–85. [PubMed] [Google Scholar]
- 50.Sporn MB, Roberts AB. The multifunctional nature of peptide growth factors. In: Sporn MB, Roberts AB, editors. Peptide growth factors and their receptors. New York: Springer-Verlag; 1990. p. 3–15. [Google Scholar]
- 51.Bissell MJ, LaBarge MA. Context, tissue plasticity, and cancer: are tumor stem cells also regulated by the microenvironment? Cancer Cell. 2005;7(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kenny PA, Bissell MJ. Tumor reversion: correction of malignant behavior by microenvironmental cues. Intl J Cancer. 2003;107(5):688–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Devel Cell. 2010;18(6):884–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hagios C, Lochter A, Bissell MJ. Tissue architecture: the ultimate regulator of epithelial function? Phil Trans R Soc Lond B. 1998;353(1370):857–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J Theoret Biol. 1982;99:31–68. [DOI] [PubMed] [Google Scholar]
- 56.Bissell MJ, Weaver VM, Lelièvre SA, Wang F, Petersen OW, Schmeichel KL. Tissue structure, nuclear organization, and gene expression in normal and malignant breast. Cancer Res. 1999;59(7 Suppl):1757s–64s. [PubMed] [Google Scholar]
- 57.Adams JC, Watt FM. Regulation of development and differentiation by the extracellular matrix. Development. 1993;117:1183–98. [DOI] [PubMed] [Google Scholar]
- 58.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–8. [DOI] [PubMed] [Google Scholar]
- 59.Cunha GR. Role of mesenchymal-epithelial interactions in normal and abnormal development of the mammary gland and prostate. Cancer. 1994;74:1030–44. [DOI] [PubMed] [Google Scholar]
- 60.Kaufman DG, Arnold JT. Stromal-epithelial interactions in normal and neoplastic development. In: Sirica AE, ed. Cellular and molecular pathogenesis. Philadelphia: Lippincott-Raven Publishers; 1996:403–31. [Google Scholar]
- 61.Rubin H. Epigenetic nature of neoplastic transformation. In: Hodges GM, Rowlatt C, eds. Developmental biology and cancer. Boca Raton: CRC-Press; 1994:61–84. [Google Scholar]
- 62.Weaver VM, Petersen OW, Wang F, et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137(1):231–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deng G, Lu Y, Zlotnikov G, Thor AD, Smith HS. Loss of heterozygosity in normal tissue adjacent to breast carcinomas. Science. 1996;274:2057–9. [DOI] [PubMed] [Google Scholar]
- 64.Heppner GH. Tumor cell societies. J Natl Cancer Inst. 1989;81(9):648–9. [DOI] [PubMed] [Google Scholar]
- 65.Heppner GH, Miller FR. The cellular basis of tumor progression. Intl Rev Cytol. 1998;177:1–56. [DOI] [PubMed] [Google Scholar]
- 66.Poste G, Greig R. On the genesis and regulation of cellular heterogeneity in malignant tumors. Invasion Metastasis. 1982;2:137–76. [PubMed] [Google Scholar]
- 67.Heppner G. Cell-to-cell interaction in regulating diversity of neoplasms. Semin Cancer Biol. 1991;2:97–103. [PubMed] [Google Scholar]
- 68.Heppner G. Cancer cell societies and tumor progression. Stem Cells. 1993;11:199–203. [DOI] [PubMed] [Google Scholar]
- 69.Prehn RT. The inhibition of tumor growth by tumor mass. Cancer Res. 1991;51:2–4. [PubMed] [Google Scholar]
- 70.Demicheli R, Retsky MW, Hrushesky WJ, Baum M, Gukas ID. The effects of surgery on tumor growth: a century of investigations. Ann Oncol. 2008;19(11):1821–8. [DOI] [PubMed] [Google Scholar]
- 71.Heppner GH. Summation and synthesis: from the immunology point of view. In: Yang SS, Warner HR, editors. The underlying molecular, cellular, and immunological factors in cancer. New York: Plenum Press; 1993:315–20. [DOI] [PubMed] [Google Scholar]
- 72.Ozen M, Multani AS, Kuniyasu H, Chung LW, von Eschenbach AC, Pathak S. Specific histologic and cytogenetic evidence for in vitro malignant transformation of murine host cells by three human prostate cancer cell lines. Oncol Res. 1997;9(8):433–8. [PubMed] [Google Scholar]
- 73.Pathak S, Nemeth MA, Multan AS, Thalmann GN, von Eschenbach AC, Chung LW. Can cancer cells transform normal host cells into malignant cells? Br J Cancer. 1997;76(9):1134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pathak S, Nemeth MA, Multani AS. Human tumor xenografts in nude mice are not always of human origin. A warning signal. Cancer. 1998;83(9):1891–2. [DOI] [PubMed] [Google Scholar]
- 75.Goldenberg DM, Pavia RA. In vivo horizontal oncogenesis by a human tumor in nude mice. Proc Natl Acad Sci. 1982;79:2389–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goldenberg DM, Pavia RA. Malignant potential of murine stromal cells after transplantation of human tumors into nude mice. Science. 1981;212:65–7. [DOI] [PubMed] [Google Scholar]
- 77.Pierce GB, Dixon FJ. Testicular teratomas. I. Demonstration of teratogenesis by metamorphosis of multipotential cells. Cancer. 1959;12(3):573–83. [DOI] [PubMed] [Google Scholar]
- 78.Pierce GB, Dixon FJ, Verney EL. Teratocarcinogenic and tissue-forming potentials of the cell types comprising neoplastic embryoid bodies. Lab Invest. 1960;9(6):583–602. [PubMed] [Google Scholar]
- 79.Pierce GB, Verney EL. An in vitro and in vivo study of differentiation in teratocarcinomas. Cancer. 1961;14:1017–29. [DOI] [PubMed] [Google Scholar]
- 80.Pierce GB. On the boundary between development and neoplasia. An interview with Professor G. Barry Pierce [interview by Juan Arechaga]. Int J Dev Biol. 1993;37(1):5–16. [PubMed] [Google Scholar]
- 81.Nowak D, Stewart D, Koeffler HP. Differentiation therapy of leukemia: 3 decades of development. Blood. 2009;113(16):3655–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mintz B, Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc Natl Acad Sci. 1975;72(9):3585–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brinster RL. The effect of cells transferred into the mouse blastocyst on subsequent development. J Exp Med. 1974;140:1049–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Illmensee K, Mintz B. Totipotency and normal differentiation of single tetratocarcinoma cells cloned by injection into blastocysts. Proc Natl Acad Sci USA. 1976;73(2):549–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Papaioannou VE, McBurney MW, Gardner RL, Evans MJ. Fate of tetratocarcinoma cells injected into early mouse embryos. Nature. 1975;258:70–3. [DOI] [PubMed] [Google Scholar]
- 86.Gootwine E, Webb CG, Sachs L. Participation of myeloid lekaemic cells injected into embryos in haematopoietic differentiation in adult mice. Nature. 1982;299:63–5. [DOI] [PubMed] [Google Scholar]
- 87.Webb CG, Gootwine E, Sachs L. Developmental potential of myeloid leukemia cells injected into midgestation embryos. Devel Biol. 1984;101:221–4. [DOI] [PubMed] [Google Scholar]
- 88.Wells RS, Miotto KA. Widespread inhibition of neuroplastoma cells in the 13-to 17-day-old mouse embryo. Cancer Res. 1986;46:1659–62. [PubMed] [Google Scholar]
- 89.Podesta AH, Mullins J, Pierce GB, Wells RS. The neurula stage mouse embryo in control of neuroplastoma. Proc Natl Acad Sci. 1984;81:7608–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gerschenson M, Graves K, Carson SD, Wells RS, Pierce GB. Regulation of melanoma by the embryonic skin. Proc Natl Acad Sci. 1986;83:7307–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kulesa PM, Kasemeier-Kulesa JC, Teddy JM, et al. Reprogramming metastatic melanoma cells to assume a neural crest cell-like phenotype in an embryonic microenvironment. Proc Natl Acad Sci USA. 2006;103(10):3752–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McKinnell RG, Deggins BA, Labat DD. Transplantation of pluripotential nuclei from triploid frog tumors. Science. 1969;165:394–6. [DOI] [PubMed] [Google Scholar]
- 93.King TJ, DiBerardino MA. Transplantation of nuclei from the frog renal adenocarcinoma. I. Development of tumor nuclear-transplant embryos. Ann N Y Acad Sci. 1965;126:115–26. [DOI] [PubMed] [Google Scholar]
- 94.DiBerardino MA, Mizell M, Hoffner NJ, Friesendorf DG. Frog larvae cloned from nuclei of pronephric adenocarcinoma. Differentiation. 1983;23:213–7. [DOI] [PubMed] [Google Scholar]
- 95.McKinnell RG, Lust JM, Sauerbier W, et al. Genomic plasticity of the Lucke renal carcinoma: a review. Int J Dev Biol. 1993;37:213–9. [PubMed] [Google Scholar]
- 96.Li L, Connelly MC, Wetmore C, Curran T, Morgan JI. Mouse embryos cloned from brain tumors. Cancer Res. 2003;63(11):2733. [PubMed] [Google Scholar]
- 97.Hochedlinger K, Blelloch R, Brennan C, Y, et al. Reprogramming of a melanoma genome by nuclear transplantation. Genes Devel. 2004;18(15):1875–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cooper M, Pinkus H. Intrauterine transplantation of rat basal cell carcinoma as a model for reconversion of malignant to benign growth. Cancer Res. 1977;37:2544–7. [PubMed] [Google Scholar]
- 99.Coleman WB, Wennerberg AE, Smith GJ, Grisham JW. Regulation of the differentiation of diploid and some aneuploid rat liver epithelial (stemlike) cells by the hepatic microenvironment. Am J Pathol. 1993;142:1373–82. [PMC free article] [PubMed] [Google Scholar]
- 100.McCullough KD, Coleman WB, Ricketts SL, Wilson JW, Smith GJ, Grisham JW. Plasticity of the neoplastic phenotype in vino is regulated by epigenetic factors. Proc Natl Acad Sci USA. 1998;95(26):15333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Farber E, Sarma DS. Hepatocarcinogenesis: A dynamic cellular perspective. Lab Invest. 1987;56(1):4–22. [PubMed] [Google Scholar]
- 102.Farber E, Rubin H. Cellular adaptation in the origin and development of cancer. Cancer Res. 1991;51:2751–61. [PubMed] [Google Scholar]
- 103.Pierce GB, Shikes R, Fink LM. Cancer. A problem of developmental biology. Englewood Cliffs, NJ: Prentice-Hall; 1978. [Google Scholar]
- 104.Pierce GB, Speers WC. Tumors as caricatures of the process of tissue renewal: Prospects for therapy by directing differentiation. Cancer Res. 1988;48:1996–2004. [PubMed] [Google Scholar]
- 105.Dvorak HF. Tumours: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–9. [DOI] [PubMed] [Google Scholar]
- 106.Domagk G. Die experimentelle Geschwulstforschung. Handbuch der allgemeinen Pathologie. 1956;6(3. Teil):242–367. [Google Scholar]
- 107.Tsonis PA. Regeneration in vertebrates. Devel Biol. 2000;221(2):273–84. [DOI] [PubMed] [Google Scholar]
- 108.Gersch M. Zellentartung und Zellwucherung bei Regenwürmern nach Behandlung mit cancerogenen Kohlenwasserstoffen. Archiv der Geschwulstforschung. 1957;10(2):101–18. [PubMed] [Google Scholar]
- 109.Gersch M. Zellentartung und Zellwucherung bei wirbellosen Tieren. Archiv der Geschwulstforschung. 1951;3(1):1–18. [PubMed] [Google Scholar]
- 110.Seilern-Aspang F. Experimentelle Beiträge zur Frage der Zusammenhänge: Regenerationsfähigkeit—Geschwulstbildung. Roux' Archiv für Entwicklungsmechanik. 1960;152:491–516. [DOI] [PubMed] [Google Scholar]
- 111.Okamoto M. Simultaneous demonstration of lens regeneration from dorsal iris and tumour production from ventral iris in the same newt eye after carcinogen administration. Differentiation. 1997;61(5):285–92. [DOI] [PubMed] [Google Scholar]
- 112.Oviedo NJ, Beane WS. Regeneration: The origin of cancer or a possible cure? Amsterdam: Elsevier; 2009:557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Seilern-Aspang F, Kratochwil K. Induction and differentiation of an epithelial tumour in the newt (Triturus cristatus). J Embryol Exp Morphol. 1962;10(3):337–56. [PubMed] [Google Scholar]
- 114.Kienle GS. Fever in cancer treatment: Coley's therapy and epidemiologic observations. Glob Adv Health Med. 2012;1(1):90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bischoff F, Bryson G. Carcinogenesis through solid state surfaces. Prog Exp Tumor Res. 1964;5:85–133. [DOI] [PubMed] [Google Scholar]
- 116.Oppenheimer BS, Oppenheimer ET, Stout AP. Sarcomas induced in rats by implanting cellophane. Proc Soc Exp Biol Med. 1948;67:33–4. [DOI] [PubMed] [Google Scholar]
- 117.Brand KG, Buoen LC, Johnson KH, Brand I. Etiological factors, stages, and the role of the foreign body in foreign body tumorigenesis: a review. Cancer Res. 1975;35:279–86. [PubMed] [Google Scholar]
- 118.Oppenheimer BS, Oppenheimer ET, Stout AP. Sarcomas induced in rodents by imbedding various plastic films. Proc Soc Exp Biol & Med. 1952;79:366–9. [DOI] [PubMed] [Google Scholar]
- 119.Rubin H. Cancer as a dynamic developmental disorder. Cancer Res. 1985;45:2935–42. [PubMed] [Google Scholar]
- 120.Kitano H. Systems biology: a brief overview. Science. 2002;295(5560):1662–4. [DOI] [PubMed] [Google Scholar]
- 121.Kitano H. Computational systems biology. Nature. 2002;420(6912):206–10. [DOI] [PubMed] [Google Scholar]
- 122.Steiner R. Goethes Weltanschauung. 8 ed. Dornach: Rudolf Steiner Verlag; 1990. [Google Scholar]
- 123.Weiss PA. The science of life. Mount Kisco, New York: Futura Company; 1973. [Google Scholar]
- 124.Weiss PA. Das lebende System: Ein Beispiel für den Schichtendeterminismus. In: Koestler A, Smythies JR, editors. Das neue Menschenbild. Die Revolutionierung der Wissenschaften vom Leben. Ein internationales Symposion. Wien: Molden; 1967:13–70. [Google Scholar]
- 125.Drack M, Apfalter W, Pouvreau D. On the making of a system theory of life: Paul A Weiss and Ludwig von Bertalanffy's conceptual connection. The Q Rev Biol. 2007;82(4):349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ash MG, Woodward WR. Gestalt psychology in German culture, 1890-1967: holism and the quest for objectivity. Cambridge: Cambridge University Press; 1998. [DOI] [PubMed] [Google Scholar]
- 127.Köhler W. Die Aufgabe der Gestaltpsychologie. Berlin, New York: Walter de Gruyter; 1971. [Google Scholar]
- 128.von Ehrenfels C. Über Gestaltqualitäten. VjschrwissPh. 1890;14. [Google Scholar]
- 129.Wertheimer M. Untersuchungen zur Lehre der Gestalt. Teil 1. Psychol Forsch. 1922;1:47–58. [Google Scholar]
- 130.Daston L, Galison P. Objectivity. New York: Zone Books; 2007. [Google Scholar]
- 131.Kienle GS, Kiene H. Clinical judgement and the medical profession. J Eval Clin Pract. 2011;17(4):621–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sonnenschein C, Soto AM. The society of cells. Cancer and control of cell proliferation. 1 ed. New York: Springer-Verlag; 1999. [Google Scholar]
- 133.Smithers DW. On the nature of neoplasia in man. Edinburgh and London: E. & S. Livingstone Ltd; 1964. [Google Scholar]
- 134.Devitt JE. Breast cancer: Have we missed the forest because of the tree? Lancet. 1994;344:734–5. [DOI] [PubMed] [Google Scholar]
- 135.Pierce GB, Cox WF., Jr Neoplasms as caricatures of tissue renewal. In: Saunders GF, editor. Cell differentiation and neoplasia. New York: Raven Press; 1978. p. 57–66. [Google Scholar]
- 136.Clark WH. The nature of cancer: Morphogenesis and progressive (self)-disorganization in neoplastic development. Acta Oncol. 1995;34(1):3–21. [DOI] [PubMed] [Google Scholar]