Abstract
Background
Concussion remains a symptom-based diagnosis clinically, yet preclinical studies investigating traumatic brain injury, of which concussion is believed to represent a ‘mild’ form, emphasize histological endpoints with functional assessments often minimized or ignored all together. Recently, clinical studies have identified the importance of cognitive and neuropsychiatric symptoms, in addition to somatic complaints, following concussion. How these findings may translate to preclinical studies is unclear at present.
Objective
To address the contrasting endpoints utilized clinically compared to those in preclinical studies and the potential role of functional assessments in a commonly used model of diffuse axonal injury (DAI)..
Methods
Animals were subjected to DAI using the impact-acceleration model. Functional and behavioral assessments were conducted over 1 week following DAI prior to completion of histological assessment at 1-week post-DAI.
Results
We show, despite the suggestion that this model represents concussive injury, no functional impairments as determined using common measures of motor, sensorimotor, cognitive, and neuropsychiatric function following injury over the course of 1 week. The lack of functional deficits is in sharp contrast to neuropathologic findings indicating neural degeneration, astrocyte reactivity, and microglial activation.
Conclusion
Future studies are needed to identify functional assessments, neurophysiologic techniques, and imaging assessments more apt to distinguish differences following so-called ‘mild’ traumatic brain injury (TBI) in preclinical models and determine whether these models are truly studying concussive or subconcussive injury. These studies are needed to not only understand mechanism of injury and production of subsequent deficits, but also for rigorous evaluation of potential therapeutic agents.
Keywords: concussion, subconcussive injury, traumatic brain injury, functional assessment, diffuse axonal injury
INTRODUCTION
The diagnosis of concussion, believed to represent a form of mild traumatic brain injury, remains challenging due to the lack of definitive diagnostic tests such as imaging, neuropsychological testing, accelerometer recordings 1, or the identification of biomarkers. While advances have been made in the research environment in many of these areas as evidenced by the advent of diffusion tensor imaging (DTI) for imaging axonal injury 2, 3 and the identification of biomarkers such as neuron-specific enolase, S100B, and glial fibrillary acidic protein, clinical diagnosis of concussion continues to rely largely on the self-reported symptoms of the patient 1, 4. Despite this reliance on clinically observed and measured, or self-reported, symptoms for the diagnosis of concussion in humans, preclinical studies modeling ‘mild’ TBI or concussion continue to emphasize histologic measures of injury for characterizing model severity, revealing a clear disconnect between preclinical and clinical studies. Consequently, it remains unclear the precise severity of preclinical models and how accurately these models replicate concussive injury in humans. While variations of the fluid percussion injury model, a commonly used preclinical model of TBI suspected to potentially replicate concussion with the use of ‘mild’ settings (i.e. less than 1.5 atmospheres), have been characterized to varying degrees using both behavioral and histological endpoints, the same cannot be said of the impact-acceleration model, another widely used preclinical model of TBI and one that has been described as potentially more relevant based on the ability to model closed head injury and acceleration-deceleration biomechanics associated with concussion 5, 6. Furthermore, this model produces significant diffuse axonal injury, a common pathological feature of concussion and one that may correlate to cognitive performance 7. As the diagnosis of concussion clinically represents a symptom-based diagnosis without the presence of gross anatomical changes, it remains unclear how adequate current preclinical models are at replicating a concussive-like state and associated pathophysiology. This deficiency will likely limit meaningful translation of therapeutics and preventative measures from bench-to-bedside and for these reasons, the suggestion has been made that studies should clearly characterize how concussion is defined within the context of a preclinical model with regards to behavioral deficits produced 5.
Efforts have been made in numerous preclinical studies, particularly those utilizing the fluid percussion model of brain injury, to incorporate functional assessments in an attempt to relate histological and/or biochemical assessments with functional impairment. Notably, while proposed diagnostic tools in the clinic for concussive injury have grown increasingly technically advanced, particularly when considering neuroimaging and neuropsychological testing, preclinical assessments used in TBI models have historically been largely simplistic in nature. Frequently measured modalities include sensorimotor assessments, learning and memory, and less frequently, anxiety-like or depressive-like behaviors. Increasing emphasis has been placed in recent years on the incorporation of more advanced tests and those addressing neurobehavioral disorders, as these are one of the most prominent long-term consequences of TBI exposure clinically 8. Motor function can be assessed with activity measurements and open field exploration, while balance/coordination has been assayed using the rotarod, inclined plane, beam walk, and balance beam 9–19. Learning and memory have been evaluated following TBI using various iterations of the Morris water maze 7, 10, 13, 19–33, radial arm maze 10, 15, 18, T-maze 33, 34, and avoidance chambers 19, 33, 35, 36. Anxiety-like measures utilized previously include the elevated-plus maze and measures of peripheral versus central exploratory activity within the open field test 7, 16, 18, 32, 36–40. Depressive-like activity has been assessed in the forced swim test and tail suspension test 19, 32, 33, 35, 36, 39, 41. How these various behavioral measures are affected following TBI is dependent on numerous factors including but not limited to the severity of injury, location of injury, type of injury (global versus focal, open versus closed), age at time of injury, and the length of recovery period prior to assessment. Characterization of these deficits or changes following injury may allow for better understanding of model severity and relationship to clinical practice rather than the more commonly utilized histological measures that are limited to controlled preclinical studies.
In addition to being a symptom-based diagnosis lacking clear diagnostic tests, concussion occurs clinically in a varied population ranging from the pediatric to geriatric patient. Preclinical studies have largely neglected to consider the role of the aging process in the severity of injury sustained and subsequent recovery characteristics. While TBI is generally thought to be a condition associated with the adolescent and young-adult population, the prolonged careers of athletes and military personnel necessitates the consideration of age as an experimental variable or paradigm. Previous preclinical work assessing the effect of aging on outcome from TBI, as well as other forms of neurologic injury, has generally revealed a greater initial injury severity and prolonged deficits in comparison to young-adult animals. These studies largely utilized models requiring craniotomy 13, 42, 43 rather than simulating true closed-head injury more analogous to that sustained on the athletic or battlefield in the form of a concussion. Consequently, it remains unclear how age alters functional and histological outcome following diffuse axonal injury.
In this work we address the discrepancy between clinical diagnosis of concussion and the potential role of functional and behavioral measures in the preclinical study of TBI and, more specifically, diffuse axonal injury (DAI) produced using the impact-acceleration model. We also investigate the effect of aging on the injury process and how this may influence behavioral/functional deficits. We perform a basic characterization of neural injury following impact-acceleration injury and relate observed changes, or lack thereof, to those observed with behavioral/functional studies.
METHODS
Animals
Two age groups, one 3–4 months (n=16) and the other 9–12 months (n=15), of male Sprague Dawley rats were utilized in this work. All animals were acquired from Hilltop at 3–4 months of age and housed in the West Virginia University vivarium for aging when required. Animals were given standard rat chow and water ad libitum. All work involving live animals was approved by the Institutional Animal Care and Use Committee of West Virginia University and was performed according to the principles of the Guide for the Care and Use of Laboratory Animals, published by the Institute of Laboratory Resources, National Research Council (National Institutes of Health publication 85-23-2985).
Induction of Traumatic Brain Injury
Each age cohort of animals was divided into two groups, with animals receiving either a sham surgery or impact-acceleration injury. Anesthesia was induced and maintained using isoflurane at a 4% and 2% concentration, respectively. Adequate anesthesia was confirmed by evaluating the response to heel tendon pinch. Body temperature was controlled during the approximately 10-minute procedure with a homeothermic heating blanket and rectal probe. Animals were prepared in sterile fashion and received an impact-acceleration injury as described previously 44–46. Briefly, a 10 mm diameter and 3 mm thick stainless steel disk was affixed to the skull between bregma and lambda with cyanoacrylate. The animal was placed in a prone position on a foam bed with the metal disk directly beneath a 2 m tall plexiglass tube. A 450-gram weight was dropped from the top of the tube, striking the metal disk. The disk was then removed, the skull inspected, and the wound sutured. The animal was then returned to its cage and monitored post-operatively.
Functional Characterization
Functional testing was performed beginning 24 hours post-injury and continued over a subsequent one-week period. Assays were included to assess overall activity (locomotor activity), coordination/balance (rotarod), cognition (Morris water maze), anxiety-like activity (elevated plus maze), and depression-like activity (forced swim test). Testing was spread over various days to minimize the level of exertion required by the animal in an effort to reduce the potential for fatigue, consistent with the work of Shultz and colleagues 7. Assays requiring more extensive exertion or those of longer duration were the only task completed on a given day. At 24 hours post-injury, animals underwent activity monitoring and a single trial in the Morris Water Maze. At 48 hours, the elevated-plus maze and the first rotarod fixed speed test were conducted. At 72 hours, the accelerating rotarod test was conducted. At 96 hours the forced swim test was performed, and at 120 hours activity monitoring was conducted. The final day prior to sacrifice, day 6 (144 hours), the probe trial in the water maze was performed along with the second fixed speed rotarod test.
Locomotor Activity
Total activity was assessed using an automated activity monitoring system (San Diego Instruments) that records beam breaks in the x, y, and z planes. Animals were acclimated to the room for 1 hour prior to initiation of testing. Testing chambers consist of a square Plexiglass housing and 16 × 16 photobeam array to detect lateral movements. An 8 × 8 array, located above the 16 × 16 array, detects rearing-associated movements. Activity was quantified over 30 minutes with fine, ambulatory, and rearing beam breaks summed to produce the total beam breaks.
Rotarod
Two versions of rotarod testing were conducted, a fixed speed and an accelerating variant. All studies were conducted using the OMNI-ROTOR (Omnitech, Inc.). Rats were trained over a period of five consecutive days with 5 trials per day. Animals were placed on the rotarod and the rotarod was accelerated to a maximum of 24 RPM over a period of 20 seconds. Once reaching 24 RPM, the maximum time allowed per trial was 60 seconds.
For the fixed speed testing, animals were tested at speeds of 12, 16, 20, 24, 28, 32, 36, and 40 RPM for up to 60 seconds. Three trials were conducted at each speed with a rest period of 30 minutes between each trial. For accelerated testing, the rotarod was set to reach a maximum speed of 50 RPM over a period of 4 minutes with a total test duration of 5 minutes per trial. The accelerated test was repeated twice for each animal. For both fixed and accelerating tests, the latency to fall was recorded.
Elevated Plus Maze
The elevated plus maze, a measure of anxiety-related activity, was conducted as previously described 47, 48. Animals were placed one at a time in the center of the maze, facing an open arm. Data were recorded over a period of five minutes using ANY-Maze Version 4.63 video tracking software (Stoelting Co.). Specifically, time spent in open versus closed arms, number of entries into each arm type, speed within given arms, and distance traveled were recorded. A body percentage of 90% was required to be in a given arm to register a change in arm presence.
Forced Swim Test
The forced swim test, described at length by Porsolt et al, has been used as a measure of depressive-like activity 49, 50. Animals were acclimated to the testing room for 60 minutes prior to initiation of testing. On the day before testing, animals were habituated to the water-filled testing cylinder for 7 minutes, a length of time consistent with the testing period. During the testing period, animals were placed in the water for a total of 7 minutes, the first 2 of which served as an acclimation period. Data related to immobility time, speed, distance traveled, and number of immobile episodes was recorded over the subsequent 5 minutes.
Morris Water Maze
The Morris Water Maze was used to assess memory as previously described. Animals were trained prior to induction of injury and tested post-injury for the ability to recall the platform location. All trials were conducted in a 182-cm diameter pool using Videomex 4.50 tracking software (Columbus Instruments) for data acquisition. The water temperature was maintained at 19–22 °C and a platform (12 cm x 12 cm) made of plexiglass was used. The platform was hidden from the rat by using a black pool and black platform that was submerged a distance of 1.5 inches. Animals were trained on Days 1–6 via a series of 4 trials per day, with each trial starting from a different location around the pool, and each with a maximum time of 2 minutes. Once animals found the platform, 15 seconds was allowed to elapse for spatial acquisition to occur. If animals were unsuccessful in finding the platform after two minutes, they were placed on the platform for 15 seconds. After induction of injury, on Day 1, a one trial test with the platform in place was utilized to assess memory. Once the platform was discovered, animals were removed immediately to prevent additional learning. On Day 6, a traditional probe trial was performed in which the platform was removed and time spent in each quadrant and the counter zone recorded.
Tissue Preparation
At the conclusion of functional testing, 7 days after injury, all surviving animals were anesthetized and immediately perfused transcardially with physiologic saline followed by 4% paraformaldehyde. The entire brain, brainstem, and rostral spinal cord were extracted and placed in 4% paraformaldehyde for 24 hours. Following fixation, the brain was blocked into 2 mm thick sections and processed using the Tissue-Tek VIP 5 Automatic Tissue Processor (Sakura Finetek, Torrence, CA). Processed tissues were paraffin-embedded with the Tissue-Tek Tec 5 embedding system (Sakura Finetek, Torrence, CA) and sliced (6 µm) using a Leica RM2235 microtome (Leica Microscopes, Buffalo Grove, IL). Sections were mounted on glass slides and heat-fixed. Immediately prior to staining, tissues were de-paraffinized using a series of xylene and alcohol washes as described previously 51.
Fluoro-Jade B (FJB) Staining
FJB staining was used to identify neural degeneration. For FJB labeling, slides were incubated in 0.06% potassium permanganate for 10 minutes following rehydration through a series of alcohol and deionized (dH2O) water rinses. After incubation in potassium permanganate, slides were rinsed for 2 minutes in dH2O water and then incubated for 20 minutes with FJB in 0.1% acetic acid. Slides were washed three times in dH2O.
Glial Fibrillary Acidic Protein (GFAP) Staining
Slides were incubated in rabbit anti-cow GFAP antibody (Dako, Glostrup, Denmark) at a dilution of 1:500 in 4% horse serum in DPBS overnight. Tissues were then washed 3 times in DPBS and incubated in biotinylated anti-rabbit IgG antibody (Vector Laboratories, Burlingame, CA) diluted at 1:10,000 in 4% horse serum in DPBS for 4 hours. Following secondary antibody incubation, Avidin D-HRP (Vector Laboratories, Burlingame, CA) diluted at 1:1,000 in DPBS for 1 hour was applied. DAB chromagen solution (Vector Laboratories, Burlingame, CA) was applied for 5 minutes. Tissues were then rinsed 3 times in DPBS and dried overnight.
Ionized Calcium Binding Adapter Molecule 1 (IBA-1) Staining
IBA-1 labeling of activated microglia utilized an identical protocol as that used for GFAP staining with the following exceptions: the primary antibody was rabbit anti-Iba 1 (Wako Chemicals USA, Richmond, VA) at a dilution of 1:500 and the chromagen solution was Nova Red (Vector Laboratories, Burlingame, CA).
Statistical Analysis
Data were analyzed using GraphPad Prism version 4.0. One-way ANOVA with Tukey’s post-test was performed to determine differences between experimental groups on the accelerating rotarod, elevated plus maze, forced swim test, and Morris Water Maze. Two-way ANOVA with repeated measures was utilized for fixed speed rotarod testing analysis. A p-value of <0.05 was considered statistically significant.
RESULTS
Diffuse axonal injury induced using the impact-acceleration model produces neural degeneration, reactive astrocytes, and microglial activation in both age groups
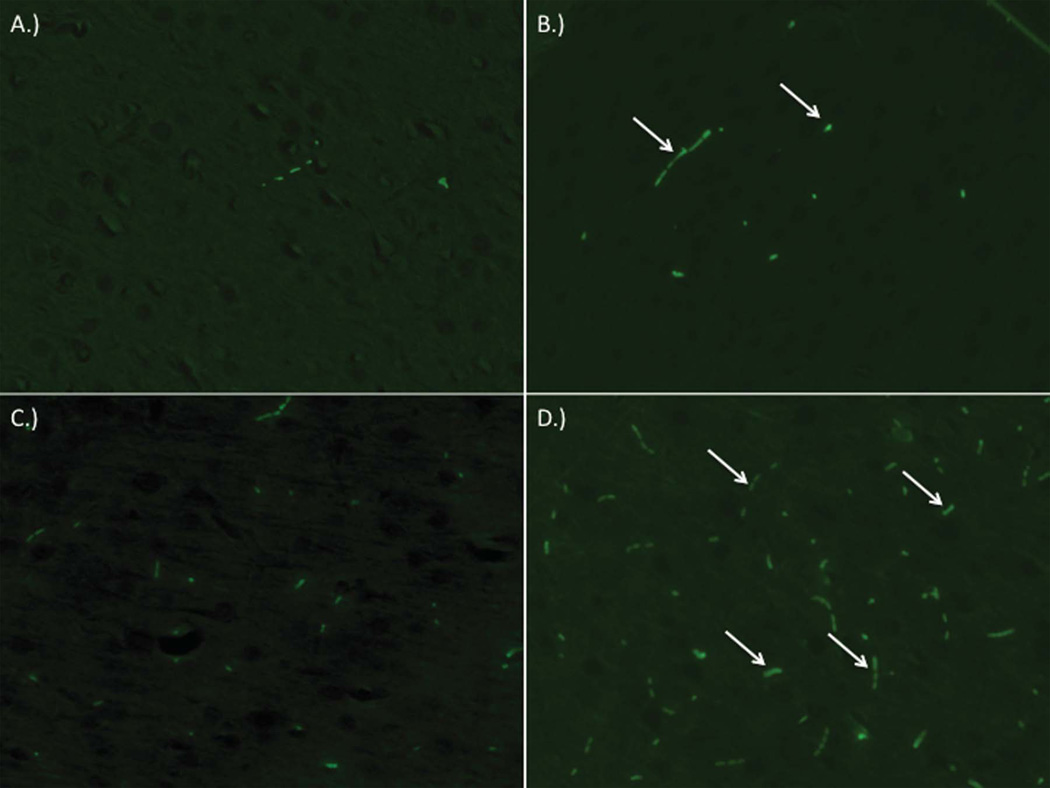
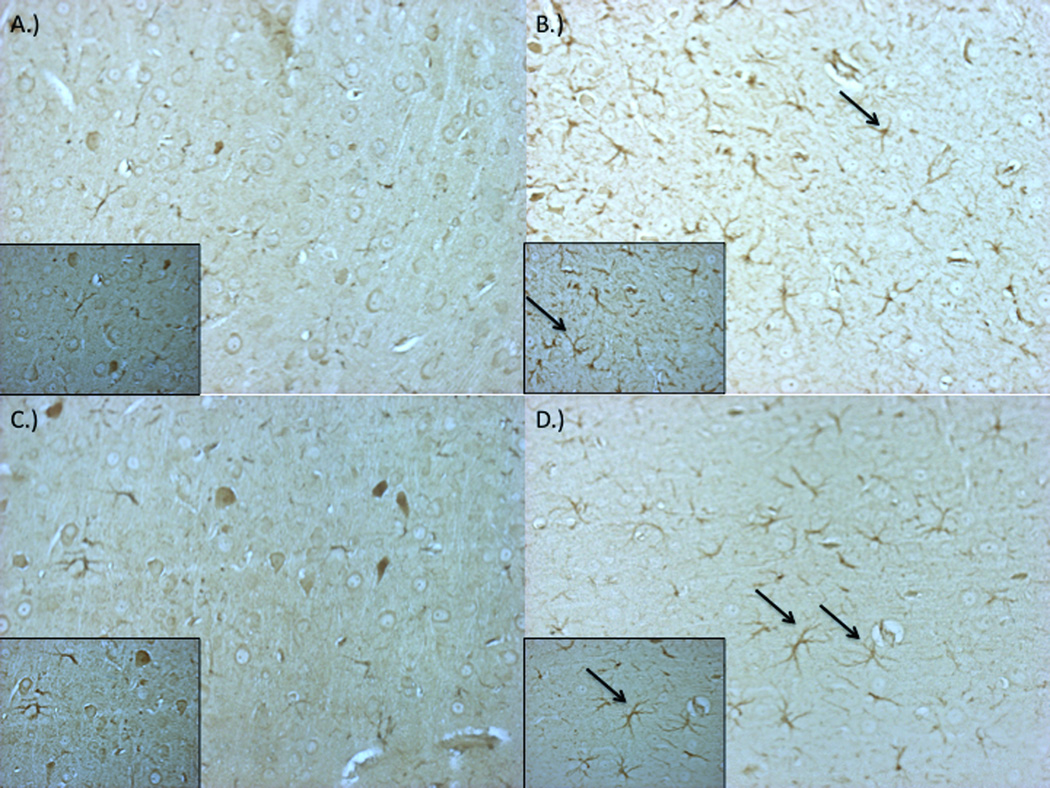
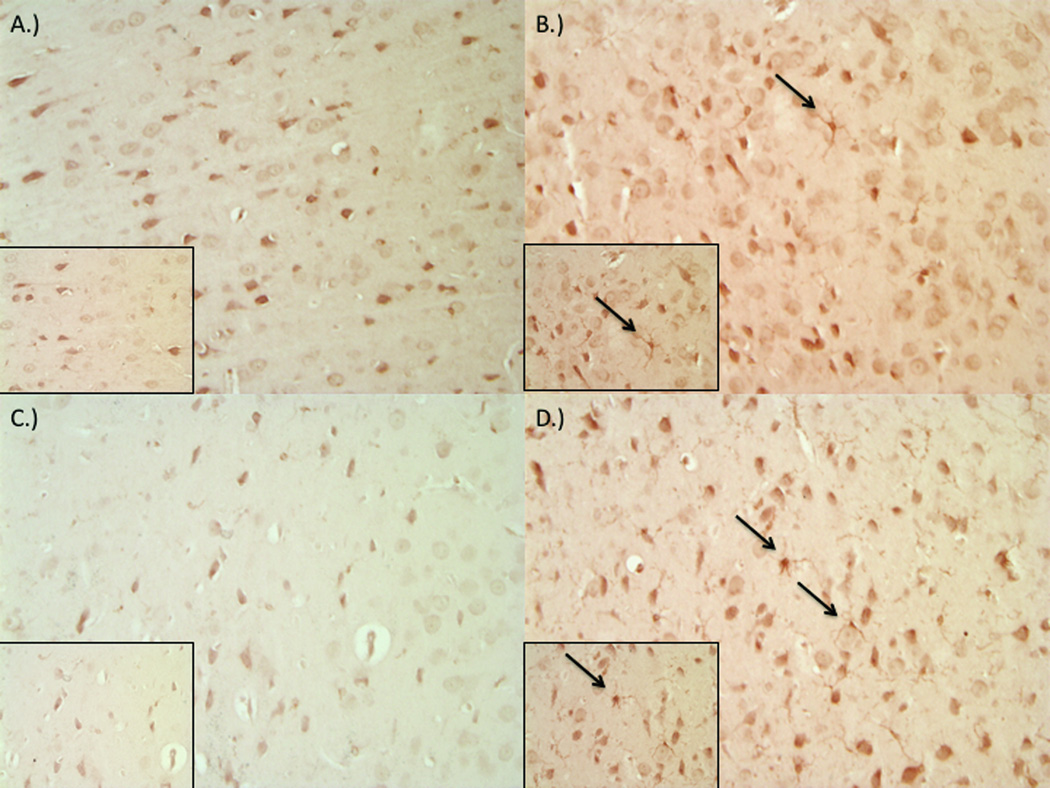
Fluoro-Jade B staining of brain sections revealed diffuse degeneration of neurons throughout the cortex and striatum as indicated by increased fluorescent labeling (Figure 1). While injury induced clear degeneration in both age groups examined in comparison to control (Figure 1a,c), young-adult specimens (Figure 1b) exhibited diminished damage, whereas older animals exhibited a more severe response and level of damage (Figure 1d). A diffuse proliferation and hypertrophy of astrocytes, visualized using GFAP staining, was also seen across tissue regions in young and old groups relative to control (Figure 2). Microglial activation was enhanced post-injury in both age groups as indicated by Iba-1 staining (Figure 3). Microglia in the injured brain exhibit increased ramification of processes as well as overall hypertrophy.
Figure 1.
Impact-acceleration injury results in neural degeneration as evident by Fluoro-Jade B staining in both 3–4 month and 9–12 month-old male rats. Representative sections from sham 3–4 month-old (A), injured 3–4 month-old (B), sham 9–12 month-old (C), and injured 9–12 month-old (D) rats.
Figure 2.
Impact-acceleration injury results in reactive astrocytes as evident by glial fibrillary acidic protein (GFAP) staining in both 3–4 month and 9–12 month-old male rats. Representative sections from sham 3–4 month-old (A), injured 3–4 month-old (B), sham 9–12 month-old (C), and injured 9–12 month-old (D) rats.
Figure 3.
Impact-acceleration injury results in microglial activation as evident by ionized calcium binding adapter molecule 1 (Iba-1) staining in both 3–4 month and 9–12 month-old male rats. Representative sections from sham 3–4 month-old (A), injured 3–4 month-old (B), sham 9–12 month-old (C), and injured 9–12 month-old (D) rats.
Impact-Acceleration injury fails to produce deficits in total activity and motor coordination across multiple time points in either age group up to one-week post-injury
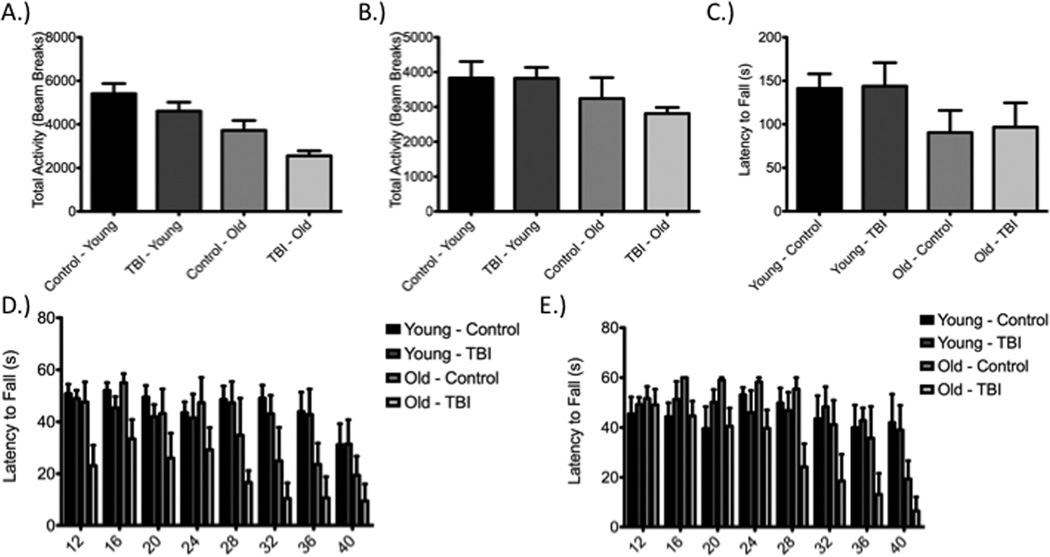
Analysis using a one-way ANOVA and Tukey’s post-hoc of total activity indicated by beam breaks revealed no significant difference between injury and control groups within respective age groups, at both Day 1 (Figure 4a) and Day 5 (Figure 4b) post-injury, although differences were observed across age groups on Day 1 [Day 1: F(3,29) = 10.26, P < 0.05; Young: q=2.07, P>0.05; Old: q=2.78, P>0.05 and Day 5: F(3,29) = 2.12, P > 0.05; Young: q=0.02, P>0.05; Old: q=1.118, P>0.05]. More complex motor function or coordination was assessed using two variations of the rotarod – accelerating (Figure 4c) and fixed speed (Figure 4d,e). The fixed speed test conducted on Day 2 post-injury demonstrated no significant interaction between speed and experimental group using two-way ANOVA and a Bonferroni post-hoc test when comparing within a respective age group (Table 1). When repeated on Day 6, the fixed speed test again failed to demonstrate significant deficits between respective age groups (Table 2). The accelerating rotarod test, conducted on day 3 post-injury, did not reveal any deficits across groups, regardless of age [F(3,23)=1.165, P>0.05; Young: q=0.11, P>0.05; Old: q=0.22, P>0.05].
Figure 4.
Diffuse axonal injury (DAI), produced through impact-acceleration injury, does not result in sensorimotor deficits. Total activity was not altered within respective age groups on Day 1 (A) or Day 5 (B) post-injury. Similar findings were seen in the accelerated rotarod test on Day 3 post-injury (C) and the fixed speed rotarod test conducted on Day 2 (D) and Day 6 (E) post-injury.
Table 1.
Fixed speed rotarod testing on Day 2 post-DAI does not reveal deficits between injured and sham animals within respective age groups. Deficits are seen when comparing injured aged animals to younger animals (both control and injured) at higher speeds.
| Experimental Group | Fixed Speed on Rotarod (RPM) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group #1 | Group #2 | 12 | 16 | 20 | 24 | 28 | 32 | 36 | 40 |
| Y – I | Y – S | t=0.19 P>0.05 |
t=0.66 P>0.05 |
t=0.74 P>0.05 |
t=0.20 P>0.05 |
t=0.13 P>0.05 |
t=0.59 P>0.05 |
t=0.11 P>0.05 |
t=0.02 P>0.05 |
| Y – I | O – S | t=0.12 P>0.05 |
t=0.85 P>0.05 |
t=0.11 P>0.05 |
t=0.51 P>0.05 |
t=1.09 P>0.05 |
t=1.59 P>0.05 |
t=1.68 P>0.05 |
t=1.05 P>0.05 |
| Y – I | O – I | t=2.65 P>0.05 |
t=1.22 P>0.05 |
t=1.63 P>0.05 |
t=1.26 P>0.05 |
t=3.14 P<0.05 |
t=3.34 P<0.01 |
t=3.29 P<0.01 |
t=2.25 P>0.05 |
| Y – S |
O – S |
t=0.28 P>0.05 |
t=0.25 P>0.05 |
t=0.54 P>0.05 |
t=0.33 P>0.05 |
t=1.17 P>0.05 |
t=2.05 P>0.05 |
t=1.72 P>0.05 |
t=1.00 P>0.05 |
| Y – S | O – I | t=2.73 P>0.05 |
t=1.83 P>0.05 |
t=2.31 P>0.05 |
t=1.41 P>0.05 |
t=3.15 P<0.05 |
t=3.80 P<0.01 |
t=3.27 P<0.05 |
t=2.14 P>0.05 |
| O – S | O – I | t=2.14 P>0.05 |
t=1.88 P>0.05 |
t=1.50 P>0.05 |
t=1.58 P>0.05 |
t=1.59 P>0.05 |
t=1.26 P>0.05 |
t=1.13 P>0.05 |
t=0.87 P>0.05 |
Table 2.
Fixed speed rotarod testing on Day 6 post-DAI does not reveal deficits between injured and sham animals within respective age groups. Deficits are seen when comparing injured aged animals to younger animals (both control and injured) at higher speeds.
| Experimental Group | Fixed Speed on Rotarod (RPM) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group #1 | Group #2 | 12 | 16 | 20 | 24 | 28 | 32 | 36 | 40 |
| Y – I | Y – S | t=0.36 P>0.05 |
t=0.67 P>0.05 |
t=1.01 P>0.05 |
t=0.68 P>0.05 |
t=0.29 P>0.05 |
t=0.46 P>0.05 |
t=0.28 P>0.05 |
t=0.27 P>0.05 |
| Y – I | O – S | t=0.20 P>0.05 |
t=0.74 P>0.05 |
t=0.76 P>0.05 |
t=1.05 P>0.05 |
t=0.73 P>0.05 |
t=0.61 P>0.05 |
t=0.60 P>0.05 |
t=1.63 P>0.05 |
| Y – I | O – I | t=0.12 P>0.05 |
t=0.67 P>0.05 |
t=0.96 P>0.05 |
t=0.63 P>0.05 |
t=2.26 P>0.05 |
t=2.97 P<0.05 |
t=2.97 P<0.05 |
t=3.12 P<0.05 |
| Y – S |
O – S |
t=0.50 P>0.05 |
t=1.30 P>0.05 |
t=1.61 P>0.05 |
t=0.43 P>0.05 |
t=0.46 P>0.05 |
t=0.19 P>0.05 |
t=0.35 P>0.05 |
t=1.88 P>0.05 |
| Y – S | O – I | t=0.34 P>0.05 |
t=0.03 P>0.05 |
t=0.09 P>0.05 |
t=1.29 P>0.05 |
t=2.47 P>0.05 |
t=2.40 P>0.05 |
t=2.58 P>0.05 |
t=3.41 P>0.05 |
| O – S | O – I | t=0.22 P>0.05 |
t=1.31 P>0.05 |
t=1.57 P>0.05 |
t=1.58 P>0.05 |
t=2.67 P>0.05 |
t=1.93 P>0.05 |
t=1.93 P>0.05 |
t=1.09 P>0.05 |
Diffuse axonal injury does not alter reference memory in both acute and subacute periods as determined using the Morris Water Maze
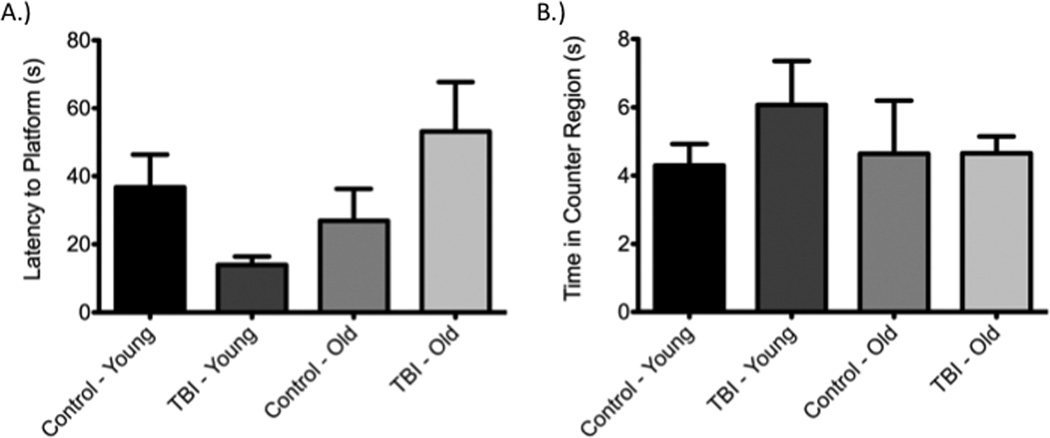
Acute analysis of reference memory using the Morris Water Maze at 24 hours post-injury (Figure 5) showed no deficits as a consequence of injury in either the young or aged group [F(3,27)=3.78, P>0.05; Young: q=2.58, P>0.05; Old: q=2.62, P>0.05]. Similar findings to those at 24 hours were observed in a probe trial conducted on day 6 post-injury. Specifically, no difference between the injured and non-injured groups within respective age groups was seen with regards to time spent in the counter region, the region in which the platform previously was placed [F(3,27)=0.58, P>0.05; Young: q=1.612, P>0.05; Old: q=0.01, P>0.05]. Importantly, prior to injury and subsequent testing, no difference was observed on the final day of training between age-matched experimental groups based on latency to find the platform [F(3,27) = 0.29, P > 0.05; Young: q=0.48, P>0.05; Old: q=0.11, P>0.05].
Figure 5. Diffuse axonal injury did not produce retrograde amnesia on Day 1 (A) or Day 6 (B) post-injury as determined using the Morris water maze.
Measures of anxiety-like and depressive-like behaviors are unaltered following injury
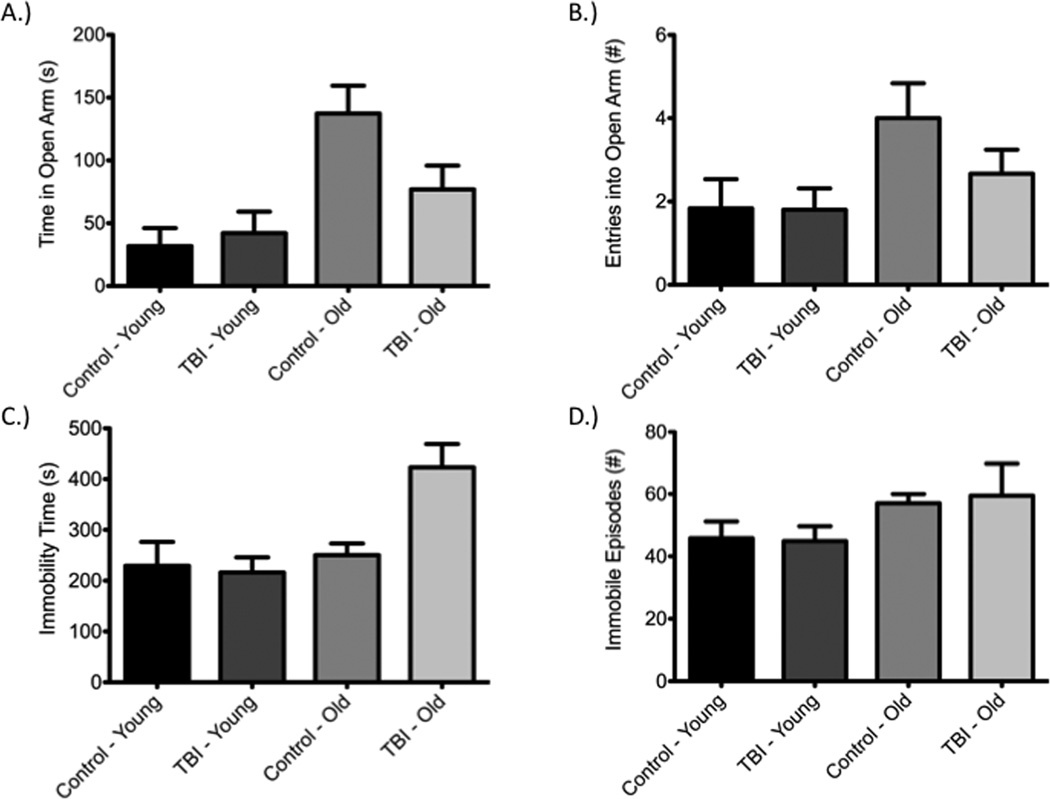
The elevated-plus maze, a commonly utilized measure of anxiety-like behavior, failed to show differences between control and injured animals within respective age groups on day 2 post-injury (Figure 6a,b). Time spent in the open arms [F(3,29)=5.00, P<0.05; Young: q=0.55, P>0.05; Old: q=3.00, P>0.05] and number of entries into the open arms [F(3,29)=2.147, P>0.05; Young: q=0.05, P>0.05; Old: q=1.97, P>0.05] were specifically analyzed. The forced swim test, a measure of depressive-like activity, was conducted on day 4 post-injury. Again, no significant differences were observed between injured and control groups within respective age groups (Figure 6c,d). Both total immobility time [F(3,28)=6.67, P<0.05; Young: q=0.33, P>0.05; Old: q=3.70, P>0.05] and total number of immobile episodes [F(3,28)=1.02, P>0.05; Young: q=0.12, P>0.05; Old: q=0.28; P>0.05] were assessed.
Figure 6.
Impact-acceleration injury was not associated with changes in anxiety- or depressive-like behavior as determined using the elevated-plus maze and forced swim test, respectively. Both the time spent in open arms (A) and number of entries into open arms (B) were not significantly altered within respective age groups when comparing injured and sham animals. Similar findings were observed on the forced swim test with regards to immobility time (C) and number of immobile episodes (D).
Aging alters select behavioral measures at baseline and post-injury in male animals
While no differences were observed in total activity within respective age groups at both 24 hours and day 4, comparison across age groups showed reduced activity in the older cohort of animals subjected to injury when compared to both younger control animals [F(3,29)=10.26, P<0.05; q=7.21, P<0.0001] and younger injured animals [F(3,29)=10.26, P<0.05; q=5.95, P<0.05] at 24 hours post-injury (Figure 4a). At day 4 post-injury, no difference in total activity was observed between any groups (Figure 4b). Evaluation of fixed-speed rotarod performance revealed a similar phenomena in that at day 2 post-injury (Figure 4c), no difference was observed within respective age groups or between control groups, but older injured animals performed worse than both younger controls and younger injured animals at higher speeds (Table 1). On day 6, deficits were still observed when contrasting older injured animals with both younger controls and younger injured animals (Table 2). On the accelerating version of rotarod testing, neither aging nor injury affected the latency to fall [F(3,23)=1.17, P>0.05; younger control-younger injury: q=0.11, P>0.05; younger control-older control: q=1.76, P>0.05; younger control-older injury: q=1.79, P>0.05; younger injury-older control: q=1.91, P>0.05; younger injury-older injury: q=1.97, P>0.05; older control-older injury: q=0.22, P>0.05].
Reference memory at 24 hours was reduced in the older injured group in comparison to the younger injured group as determined by latency to platform measurements using the Morris Water Maze [F(3,27)=3.78, P<0.05; q=4.65; P<0.05]. On day 6 post-injury, no differences were seen between any of the control or treatment groups with regard to time spent in the counter region, irrespective of age [F(3,27)=0.58, P>0.05; younger control-younger injury: q=1.61, P<0.05; younger control-older control: q=0.27, P>0.05; younger control-older injured: q=0.30, P>0.05; younger injured-older control: q=1.23, P>0.05; younger injured-older injured: q=1.35, P>0.05; older control-older injured: q=0.01, P>0.05].
Aging appears to alter anxiety-like and depressive-like behaviors as indicated by the elevated-plus maze and forced swim test, respectively. Older control animals spent more time in the open arm of the elevated-plus maze than both younger controls (q=4.82, P<0.05) and younger injured animals (q=4.81, P<0.05). No difference was observed when comparing younger and older injured groups (q=2.09, P>0.05). In the forced swim test, older injured animals spent more time immobile than both younger controls (q=4.72, P<0.05) and younger injured animals (q=5.79, P<0.05).
DISCUSSION
We have demonstrated a lack of functional and behavioral deficits despite evidence of histologic changes utilizing the impact-acceleration model of TBI, and more specifically, diffuse axonal injury (DAI). Preclinical studies of TBI and DAI have consistently emphasized histologic measures for assessing the severity of injury despite the fact that concussion is a clinical diagnosis based on the observation or reporting of somatic as well as cognitive or neuropsychologic events. It would seem logical then that preclinical studies need to incorporate functional assays as not only an experimental endpoint but also to reconcile preclinical models with the severity of injury being studied. Others have explored this concept, primarily using the fluid percussion model of TBI, and have shown impaired hippocampal spatiotemporal representation in the absence of histological changes 34. Similarly, a study using low-level fluid percussion injury has identified the presence of neuroinflammation after injury despite reporting no difference in axonal injury and functional impairments 52.
By observing no effect of injury on an array of behavioral and functional measures following impact-acceleration injury, even though changes in injury markers were observed on post-mortem histopathology, additional questions are raised. One, is the impact-acceleration (Marmarou) model utilized herein truly a model of concussive injury or rather, would it be more appropriately termed a model of subconcussive injury? Second, what preclinical behavioral measures are most appropriate for detecting the subtleties of injury progression and how do these emulate and approximate those used clinically? Third, how does symptom expression relate to neurologic health or neurodegeneration? Four, how are neuropathological findings and functional analysis altered by aging?
Subconcussive Injury: An Emerging Phenomenon
Emerging evidence indicates the presence of long-term neurodegeneration upon neuropathological examination of persons with no history of concussion 53, 54. Importantly, Talavage and colleagues have identified for the first time persons failing to exhibit symptoms associated with concussion but clear deficits on neurocognitive (ImPACT) and neuropsychologic (fMRI) measures 55. This work, completed in high school football players, indicates changes in neurologic function and health without evidence of concussive injury but rather repetitive subconcussive blows 55. As these players did not exhibit obvious injury, participation and exposure to further injury continued, a potentially problematic scenario considering some biomechanical analyses suggest a potential accumulation of injury and altered neurophysiology consistent with the number of subconcussive impacts rather than the shear magnitude 55, 56. The notion of pathological changes occurring in the brain in the absence of clinical presentation of concussion is particularly alarming and indicates the need for further research as does identification of persistent deficits in working memory months after initial TBI 57, 58.
As clinically measured subconcussive injury likely requires the utilization of increasingly advanced testing modalities such as ImPACT, fMRI, or force accelerometers, translation of this work to preclinical models based on functional assessments will likely necessitate an advanced array of assessment modalities, specifically those assessing reaction time and processing or attention deficits, as these may more closely measure, in preclinical models, the deficits observed in clinic in patients following concussion. While the methodology of these techniques is beyond the scope of this work, various tests assessing these parameters have been extensively reviewed elsewhere 59, 60. The same can be said of more advanced measures of learning and memory such as shuttle avoidance and operant conditioning.
Symptom Expression as a Correlate of Neuropatholgy
While the acute effects of clinically defined concussive injury are apparent based upon a combination of self-reported symptoms by the patient and a thorough neurologic assessment by a trained clinician, diagnosis of concussion has historically been viewed as a transient neurologic disturbance with no long-term effects. This view has radically changed in recent years with the emergence of chronic traumatic encephalopathy (CTE), a neurodegenerative condition associated with repetitive head injury – both of the concussive and subconcussive types. CTE is notable in that a history of repetitive head trauma proceeds by years, often decades, development of disease-related symptoms. While the disease pathophysiology remains to be elucidated, it has been hypothesized that the initial brain trauma initiates degenerative processes that emerge slowly over time, hence the existence of a latent period between trauma and symptom emergence. The role the quantity, severity, type, and location of impacts plays in symptom development and severity remain unknown, but it is apparent that more sensitive measures to detect neural damage may be required. In this work we’ve shown the lack of acute deficits across a spectrum of functional assays despite the presence of neuropathological findings. The existence of neural degeneration and glial response without corresponding symptom expression, albeit using relatively simplistic rodent behavioral tests, indicates the potential for induction of neural damage without clear deficits clinically, at least in the acute period. How deficits develop over time, as well as how neuropathological findings progress and develop with time, remains to be seen but may provide further insight into the development of long-term neurodegenerative diseases associated with neurotrauma, such as CTE, and warrants future study.
Prior work from preclinical studies has revealed differences in deficit development that are largely dependent upon injury mechanism (focal versus global), injury severity, and time of measurement post-injury. This concept is most well described using the Morris Water Maze in which all three factors (mechanism, severity, and time) have been explored to varying degrees 7, 10, 13, 19–25, 29, 30, 32, 33, 61. Other assessments of learning and memory such as radial arm maze 10, 15, 18, novel object recognition 19, 62, avoidance tasks (active and passive) 19, 33, 35, 36, fear conditioning 16, 63, swim T-maze 33, 34, and the dig task 33 have revealed similar findings. The same is true of assessments focusing on anxiety- and depressive-like behaviors, with injury mechanism, severity, and recovery time all likely influencing outcomes. More simplistic functional tests, mainly those assessing motor and/or balance, have also been used following injury in preclinical models of TBI. These tests display a range of findings with many failing to report deficits with more mild variations of injury or deficits that resolve within a short period of time post-injury 9–13, 15–18. More severe injury models, such as CCI, have been reported as producing lasting deficits 19.
The frequent lack of functional deficits observed following injury in preclinical models is perhaps most alarming considering the frequently reported histological damage indicating lasting effects of brain injury, even when injury is below the threshold of detection based on commonly used functional and behavioral assays 52. Clinical studies have identified similar discrepancies between symptom resolution and metabolic disturbances. Specifically, Vagnozzi and colleagues showed a resolution of symptoms by day 15 post-concussion in all athletes studied, yet metabolite ratios did not normalize until day 30 64.
Role of Aging in Neuropathology & Functional Deficits Post-TBI
Aging has been attributed, via clinical experience, with poorer outcomes post-TBI 29, 65, 66. Consequently, there has been an increased emphasis on understanding the effects of aging post-TBI in recent years in an effort to improve the translation of therapeutics from bench-to-bedside. In preclinical studies, aging has been associated with an increase in mortality rate as well as prolonged suppression of reflexes 42, 67. Importantly, aging has also been associated with diminished functional performance both before 29, 47, and after, injury 27, 29, 42, 47. While the injury models and functional/behavioral measures utilized across studies vary, it is clear that older animals exhibit diminished learning and memory, and often complex sensorimotor abilities, in comparison to young-adult animals. In work by Mehan and colleagues, 20 month-old animals performed worse in the Morris water maze and beam walking tasks compared to young-adult animals following laterally administered controlled cortical impact injury 29, 47. Use of the impact-acceleration model has produced similar findings in that aged animals, despite being subjected to a less severe injury in terms of energy transferred, exhibited a markedly worse impairment in the water maze at 1 week, 3 weeks, and 5 weeks post-injury in comparison to young-adult animals 27. Others have identified similar deficits in middle-aged animals compared to young-adult animals with cortical contusion producing more severe deficits in the bilateral tactile adhesive removal test and acquisition, as well as memory, of a reference-based task 68. Similarly, aging has been associated with larger lesion cavities following CCI 68 and an altered biochemical response as demonstrated by age-dependent changes in protein expression post-injury 29, 67. Why the aged brain appears more susceptible to brain injury remains unclear and necessitates further investigation. Future studies also need to address differences in histopathology across age in relation to functional deficits, allowing for a more direct comparison of the effects of neural injury on functional deficits. Of particular interest may be changes, often degenerative or inflammatory, across neural cell types (neurons, astrocytes, and microglia).
Future Directions
Future studies need to elucidate the effect of TBI across a spectrum of time points to address the possibility of delayed effects and the development of deficits over time. While this work demonstrates the lack of detectable functional deficits following a single injury using an array of behavioral measures during the acute and subacute period, chronic deficits that may take longer to develop were not studied. Clinically, changes in affect and cognitive abilities generally arise years after sustaining concussive injury, at least in those cases associated with a diagnosis of CTE 53, 69–72. This reality further indicates the need for long-term preclinical studies to assess not only behavioral changes but also pathological changes.
In addition to the need for longer-term single impact studies, perhaps more importantly, long-term studies incorporating repetitive injury are warranted based on clinical indications and injury paradigms potentially experienced on the athletic or battlefields, frequent locations of concussive injury. Unfortunately, at this point in time preclinical models of disease processes associated with a history of repetitive-concussive injury, such as CTE, have not been established. The lack of representative animal models for these increasingly diagnosed conditions, both in terms of functional/behavioral changes as well as histolgic changes observed on post-mortem analysis, has limited elucidation of disease pathophysiology and therapeutic development. Prior work using models of repeat injury have contributed significantly to understanding disease pathophysiology and characterization of deficits 14, 17, 25, 62, 73–82, but how to best correlate the findings of these works with clinical conditions such as CTE remains unclear at present.
While numerous models have been utilized to study TBI in rodent models, how these models replicate the clinical scenario, particularly of such a heterogeneous injury as TBI, is unclear. Emerging evidence in preclinical as well as clinical literature indicates a potential role for magnetic resonance spectroscopy (MRS) to gain insight into the biochemistry and pathophysiology of the injury process. Similarly, diffusion tensor imaging (DTI) has been utilized for identification of axonal injury post-TBI. Application of technologies such as these to preclinical models may allow for an enhanced understanding of injury pathophysiology and the severity of injury induced using various models.
Limitations
Animals were not assessed until 24 hours post-injury to minimize the potential for anesthetic influences on behavior. By waiting until one day post-injury this issue was avoided. Future studies are likely needed to assess the effect of TBI in this immediate post-injury period. Despite the delay in initial assessments, one would have expected if this was truly a model of concussive injury that some deficits may be detected based on the persistence of symptoms clinically beyond one day in many cases, even though 80–90% of those afflicted with concussion report resolution of symptoms within 7 days. Additional studies using an array of assessments for each aspect of behavior may be worthwhile, as some assessments may prove more sensitive than those utilized in the current work. How the recovery period and resolution of symptoms correlates between clinical and preclinical studies remains to be seen. Furthermore, animals were sacrificed at one week post-injury for neuropathological analysis, preventing the study of potentially late developing deficits weeks or months post-injury. Finally, while we investigate a single injury paradigm in this work, repeat injury models are of significant interest for modeling the clinical scenario (athletes or soldiers exposed to repetitive injuries). Numerous repeat injury paradigms using a variety of models have made significant contributions to the understanding of TBI pathophysiology and development of histological and behavioral deficits 14, 17, 25, 62, 73–82. Using a model such as that used within this work but for repeat injuries may demonstrate a behavioral phenotype more closely resembling concussion. Other modifications of the model, such as a higher release point or additional weight, may yield similar findings with regard to a more severe concussion-like phenotype.
CONCLUSION
Future studies are needed to identify functional assessments, neurophysiologic techniques, and imaging assessments more apt to distinguish differences following so-called ‘mild’ TBI in preclinical models and determine whether these models are truly studying concussive or subconcussive injury. These studies are needed to not only understand mechanism of injury and production of subsequent deficits, but also for rigorous evaluation of potential therapeutic agents.
Acknowledgments
The authors would like to thank Dakota Jackson, Ahmad Hanif, and Emily Reilly for assistance with experimental protocols and the West Virginia University Microscopy Imaging Facility for assistance with microscopy. The authors would also like to thank Ms. Diana Richardson of CDC NIOSH for assistance in tissue preparation. This work was supported by grant funding to Ryan Turner (5T32GM08174) and Jason D. Huber (5RO1-NS061954) from the National Institutes of Health.
Footnotes
Disclosure: The authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
REFERENCES
- 1.Duhaime AC, Beckwith JG, Maerlender AC, et al. Spectrum of acute clinical characteristics of diagnosed concussions in college athletes wearing instrumented helmets: clinical article. J Neurosurg. 2012 Dec;117(6):1092–1099. doi: 10.3171/2012.8.JNS112298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mac Donald CL, Dikranian K, Song SK, Bayly PV, Holtzman DM, Brody DL. Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury. Exp Neurol. 2007 May;205(1):116–131. doi: 10.1016/j.expneurol.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mac Donald CL, Johnson AM, Cooper D, et al. Detection of blast-related traumatic brain injury in U.S. military personnel. N Engl J Med. 2011 Jun 2;364(22):2091–2100. doi: 10.1056/NEJMoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finnoff JT, Jelsing EJ, Smith J. Biomarkers, genetics, and risk factors for concussion. PM R. 2011 Oct;3(10 Suppl 2):S452–S459. doi: 10.1016/j.pmrj.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Dashnaw ML, Petraglia AL, Bailes JE. An overview of the basic science of concussion and subconcussion: where we are and where we are going. Neurosurg Focus. 2012 Dec;33(6) doi: 10.3171/2012.10.FOCUS12284. E5: 1–9. [DOI] [PubMed] [Google Scholar]
- 6.Levin H, Robertson CS. Mild TBI in Translation. J Neurotrauma. 2012 Oct 9; doi: 10.1089/neu.2012.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shultz SR, MacFabe DF, Foley KA, Taylor R, Cain DP. A single mild fluid percussion injury induces short-term behavioral and neuropathological changes in the Long-Evans rat: support for an animal model of concussion. Behav Brain Res. 2011 Oct 31;224(2):326–335. doi: 10.1016/j.bbr.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Fromm L, Heath DL, Vink R, Nimmo AJ. Magnesium attenuates post-traumatic depression/anxiety following diffuse traumatic brain injury in rats. J Am Coll Nutr. 2004 Oct;23(5):529S–533S. doi: 10.1080/07315724.2004.10719396. [DOI] [PubMed] [Google Scholar]
- 9.Ding Y, Yao B, Lai Q, McAllister JP. Impaired motor learning and diffuse axonal damage in motor and visual systems of the rat following traumatic brain injury. Neurol Res. 2001 Mar-Apr;23(2–3):193–202. doi: 10.1179/016164101101198334. [DOI] [PubMed] [Google Scholar]
- 10.Hallam TM, Floyd CL, Folkerts MM, et al. Comparison of behavioral deficits and acute neuronal degeneration in rat lateral fluid percussion and weight-drop brain injury models. J Neurotrauma. 2004 May;21(5):521–539. doi: 10.1089/089771504774129865. [DOI] [PubMed] [Google Scholar]
- 11.Heath DL, Vink R. Magnesium sulphate improves neurologic outcome following severe closed head injury in rats. Neurosci Lett. 1997 Jun 13;228(3):175–178. doi: 10.1016/s0304-3940(97)00394-7. [DOI] [PubMed] [Google Scholar]
- 12.Heath DL, Vink R. Improved motor outcome in response to magnesium therapy received up to 24 hours after traumatic diffuse axonal brain injury in rats. J Neurosurg. 1999 Mar;90(3):504–509. doi: 10.3171/jns.1999.90.3.0504. [DOI] [PubMed] [Google Scholar]
- 13.Hylin MJ, Orsi SA, Zhao J, et al. Behavioral and histopathological alterations resulting from mild fluid percussion injury. J Neurotrauma. 2013 Jan 9; doi: 10.1089/neu.2012.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kane MJ, Angoa-Perez M, Briggs DI, Viano DC, Kreipke CW, Kuhn DM. A mouse model of human repetitive mild traumatic brain injury. J Neurosci Methods. 2012 Jan 15;203(1):41–49. doi: 10.1016/j.jneumeth.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyeth BG, Jenkins LW, Hamm RJ, et al. Prolonged memory impairment in the absence of hippocampal cell death following traumatic brain injury in the rat. Brain Res. 1990 Sep 3;526(2):249–258. doi: 10.1016/0006-8993(90)91229-a. [DOI] [PubMed] [Google Scholar]
- 16.Meyer DL, Davies DR, Barr JL, Manzerra P, Forster GL. Mild traumatic brain injury in the rat alters neuronal number in the limbic system and increases conditioned fear and anxiety-like behaviors. Exp Neurol. 2012 Apr 1; doi: 10.1016/j.expneurol.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Mouzon B, Chaytow H, Crynen G, et al. Repetitive mild traumatic brain injury in a mouse model produces learning and memory deficits accompanied by histological changes. J Neurotrauma. 2012 Dec 10;29(18):2761–2773. doi: 10.1089/neu.2012.2498. [DOI] [PubMed] [Google Scholar]
- 18.Rostami E, Davidsson J, Ng KC, et al. A Model for Mild Traumatic Brain Injury that Induces Limited Transient Memory Impairment and Increased Levels of Axon Related Serum Biomarkers. Front Neurol. 2012;3:115. doi: 10.3389/fneur.2012.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Z, Loane DJ, Murray MG, 2nd, Stoica BA, Faden AI. Comparing the predictive value of multiple cognitive, affective, and motor tasks after rodent traumatic brain injury. J Neurotrauma. 2012 Oct 10;29(15):2475–2489. doi: 10.1089/neu.2012.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adelson PD, Fellows-Mayle W, Kochanek PM, Dixon CE. Morris water maze function and histologic characterization of two age-at-injury experimental models of controlled cortical impact in the immature rat. Childs Nerv Syst. 2013 Jan;29(1):43–53. doi: 10.1007/s00381-012-1932-4. [DOI] [PubMed] [Google Scholar]
- 21.Beaumont A, Marmarou A, Czigner A, et al. The impact-acceleration model of head injury: injury severity predicts motor and cognitive performance after trauma. Neurol Res. 1999 Dec;21(8):742–754. doi: 10.1080/01616412.1999.11741008. [DOI] [PubMed] [Google Scholar]
- 22.Beaumont A, Marmarou C, Marmarou A. The effects of human corticotrophin releasing factor on motor and cognitive deficits after impact acceleration injury. Neurol Res. 2000 Oct;22(7):665–673. doi: 10.1080/01616412.2000.11740737. [DOI] [PubMed] [Google Scholar]
- 23.Berman RF, Verweij BH, Muizelaar JP. Neurobehavioral protection by the neuronal calcium channel blocker ziconotide in a model of traumatic diffuse brain injury in rats. J Neurosurg. 2000 Nov;93(5):821–828. doi: 10.3171/jns.2000.93.5.0821. [DOI] [PubMed] [Google Scholar]
- 24.Dapul HR, Park J, Zhang J, et al. Concussive Injury before or after Controlled Cortical Impact Exacerbates Histopathology and Functional Outcome in a Mixed Traumatic Brain Injury Model in Mice. J Neurotrauma. 2013 Feb 20; doi: 10.1089/neu.2012.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeFord SM, Wilson MS, Rice AC, et al. Repeated mild brain injuries result in cognitive impairment in B6C3F1 mice. J Neurotrauma. 2002 Apr;19(4):427–438. doi: 10.1089/08977150252932389. [DOI] [PubMed] [Google Scholar]
- 26.Gurkoff GG, Giza CC, Hovda DA. Lateral fluid percussion injury in the developing rat causes an acute, mild behavioral dysfunction in the absence of significant cell death. Brain Res. 2006 Mar 10;1077(1):24–36. doi: 10.1016/j.brainres.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Maughan PH, Scholten KJ, Schmidt RH. Recovery of water maze performance in aged versus young rats after brain injury with the impact acceleration model. J Neurotrauma. 2000 Dec;17(12):1141–1153. doi: 10.1089/neu.2000.17.1141. [DOI] [PubMed] [Google Scholar]
- 28.Meehan WP, 3rd, Zhang J, Mannix R, Whalen MJ. Increasing Recovery Time Between Injuries Improves Cognitive Outcome After Repetitive Mild Concussive Brain Injuries in Mice. Neurosurgery. 2012 Jun 27; doi: 10.1227/NEU.0b013e318265a439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehan ND, Strauss KI. Combined age- and trauma-related proteomic changes in rat neocortex: a basis for brain vulnerability. Neurobiol Aging. 2012 Sep;33(9):1857–1873. doi: 10.1016/j.neurobiolaging.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt RH, Scholten KJ, Maughan PH. Time course for recovery of water maze performance and central cholinergic innervation after fluid percussion injury. J Neurotrauma. 1999 Dec;16(12):1139–1147. doi: 10.1089/neu.1999.16.1139. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt RH, Scholten KJ, Maughan PH. Cognitive impairment and synaptosomal choline uptake in rats following impact acceleration injury. J Neurotrauma. 2000 Dec;17(12):1129–1139. doi: 10.1089/neu.2000.17.1129. [DOI] [PubMed] [Google Scholar]
- 32.Washington PM, Forcelli PA, Wilkins T, Zapple DN, Parsadanian M, Burns MP. The Effect of Injury Severity on Behavior: A Phenotypic Study of Cognitive and Emotional Deficits after Mild, Moderate, and Severe Controlled Cortical Impact Injury in Mice. J Neurotrauma. 2012 Aug 3; doi: 10.1089/neu.2012.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zohar O, Rubovitch V, Milman A, Schreiber S, Pick CG. Behavioral consequences of minimal traumatic brain injury in mice. Acta Neurobiol Exp (Wars) 2011;71(1):36–45. doi: 10.55782/ane-2011-1821. [DOI] [PubMed] [Google Scholar]
- 34.Eakin K, Miller JP. Mild traumatic brain injury is associated with impaired hippocampal spatiotemporal representation in the absence of histological changes. J Neurotrauma. 2012 Apr 10;29(6):1180–1187. doi: 10.1089/neu.2011.2192. [DOI] [PubMed] [Google Scholar]
- 35.Milman A, Zohar O, Maayan R, Weizman R, Pick CG. DHEAS repeated treatment improves cognitive and behavioral deficits after mild traumatic brain injury. Eur Neuropsychopharmacol. 2008 Mar;18(3):181–187. doi: 10.1016/j.euroneuro.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Schwarzbold ML, Rial D, De Bem T, et al. Effects of traumatic brain injury of different severities on emotional, cognitive, and oxidative stress-related parameters in mice. J Neurotrauma. 2010 Oct;27(10):1883–1893. doi: 10.1089/neu.2010.1318. [DOI] [PubMed] [Google Scholar]
- 37.Cutler SM, VanLandingham JW, Murphy AZ, Stein DG. Slow-release and injected progesterone treatments enhance acute recovery after traumatic brain injury. Pharmacol Biochem Behav. 2006 Jul;84(3):420–428. doi: 10.1016/j.pbb.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 38.Hogg S, Moser PC, Sanger DJ. Mild traumatic lesion of the right parietal cortex of the rat: selective behavioural deficits in the absence of neurological impairment. Behav Brain Res. 1998 Jun;93(1–2):143–155. doi: 10.1016/s0166-4328(97)00146-0. [DOI] [PubMed] [Google Scholar]
- 39.Jones NC, Cardamone L, Williams JP, Salzberg MR, Myers D, O'Brien TJ. Experimental traumatic brain injury induces a pervasive hyperanxious phenotype in rats. J Neurotrauma. 2008 Nov;25(11):1367–1374. doi: 10.1089/neu.2008.0641. [DOI] [PubMed] [Google Scholar]
- 40.Liu YR, Cardamone L, Hogan RE, et al. Progressive metabolic and structural cerebral perturbations after traumatic brain injury: an in vivo imaging study in the rat. J Nucl Med. 2010 Nov;51(11):1788–1795. doi: 10.2967/jnumed.110.078626. [DOI] [PubMed] [Google Scholar]
- 41.Shapira M, Licht A, Milman A, Pick CG, Shohami E, Eldar-Finkelman H. Role of glycogen synthase kinase-3beta in early depressive behavior induced by mild traumatic brain injury. Mol Cell Neurosci. 2007 Apr;34(4):571–577. doi: 10.1016/j.mcn.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Hamm RJ, Jenkins LW, Lyeth BG, White-Gbadebo DM, Hayes RL. The effect of age on outcome following traumatic brain injury in rats. J Neurosurg. 1991 Dec;75(6):916–921. doi: 10.3171/jns.1991.75.6.0916. [DOI] [PubMed] [Google Scholar]
- 43.Harmon KG, Drezner JA, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Br J Sports Med. 2013 Jan;47(1):15–26. doi: 10.1136/bjsports-2012-091941. [DOI] [PubMed] [Google Scholar]
- 44.Mills JD, Bailes JE, Sedney CL, Hutchins H, Sears B. Omega-3 fatty acid supplementation and reduction of traumatic axonal injury in a rodent head injury model. J Neurosurg. 2011 Jan;114(1):77–84. doi: 10.3171/2010.5.JNS08914. [DOI] [PubMed] [Google Scholar]
- 45.Mills JD, Bailes JE, Turner RC, Dodson SC, Sakai J, Maroon JC. Anabolic steroids and head injury. Neurosurgery. 2012 Jan;70(1):205–209. doi: 10.1227/NEU.0b013e3182250918. discussion 209–210. [DOI] [PubMed] [Google Scholar]
- 46.Mills JD, Hadley K, Bailes JE. Dietary supplementation with the omega-3 fatty acid docosahexaenoic acid in traumatic brain injury. Neurosurgery. 2011 Feb;68(2):481–474. doi: 10.1227/NEU.0b013e3181ff692b. discussion 481. [DOI] [PubMed] [Google Scholar]
- 47.Turner RC, Seminerio MJ, Naser ZJ, et al. Effects of aging on behavioral assessment performance: implications for clinically relevant models of neurological disease. J Neurosurg. 2012 Jun 29; doi: 10.3171/2012.5.JNS112224. [DOI] [PubMed] [Google Scholar]
- 48.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2(2):322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978 Feb 15;47(4):379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 50.Porsolt RD, Brossard G, Hautbois C, Roux S. Rodent models of depression: forced swimming and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci. 2001 May; doi: 10.1002/0471142301.ns0810as14. Chapter 8:Unit 8 10A. [DOI] [PubMed] [Google Scholar]
- 51.Turner RC, Naser ZJ, Bailes JE, Smith DW, Fisher JA, Rosen CL. Effect of slosh mitigation on histologic markers of traumatic brain injury: laboratory investigation. J Neurosurg. 2012 Dec;117(6):1110–1118. doi: 10.3171/2012.8.JNS12358. [DOI] [PubMed] [Google Scholar]
- 52.Shultz SR, MacFabe DF, Foley KA, Taylor R, Cain DP. Sub-concussive brain injury in the Long-Evans rat induces acute neuroinflammation in the absence of behavioral impairments. Behav Brain Res. 2012 Apr 1;229(1):145–152. doi: 10.1016/j.bbr.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 53.Baugh CM, Stamm JM, Riley DO, et al. Chronic traumatic encephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging Behav. 2012 Jun;6(2):244–254. doi: 10.1007/s11682-012-9164-5. [DOI] [PubMed] [Google Scholar]
- 54.Stern RA, Riley DO, Daneshvar DH, Nowinski CJ, Cantu RC, McKee AC. Long-term consequences of repetitive brain trauma: chronic traumatic encephalopathy. PM R. 2011 Oct;3(10 Suppl 2):S460–S467. doi: 10.1016/j.pmrj.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 55.Talavage TM, Nauman E, Breedlove EL, et al. Functionally-Detected Cognitive Impairment in High School Football Players Without Clinically-Diagnosed Concussion. J Neurotrauma. 2010 Oct 1; doi: 10.1089/neu.2010.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Breedlove EL, Robinson M, Talavage TM, et al. Biomechanical correlates of symptomatic and asymptomatic neurophysiological impairment in high school football. J Biomech. 2012 Apr 30;45(7):1265–1272. doi: 10.1016/j.jbiomech.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 57.Broglio SP, Eckner JT, Paulson HL, Kutcher JS. Cognitive decline and aging: the role of concussive and subconcussive impacts. Exerc Sport Sci Rev. 2012 Jul;40(3):138–144. doi: 10.1097/JES.0b013e3182524273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gosselin N, Bottari C, Chen JK, et al. Evaluating the cognitive consequences of mild traumatic brain injury and concussion by using electrophysiology. Neurosurg Focus. 2012 Dec;33(6) doi: 10.3171/2012.10.FOCUS12253. E7: 1–7. [DOI] [PubMed] [Google Scholar]
- 59.Blokland A. Reaction time responding in rats. Neurosci Biobehav Rev. 1998 Oct;22(6):847–864. doi: 10.1016/s0149-7634(98)00013-x. [DOI] [PubMed] [Google Scholar]
- 60.Bushnell PJ, Strupp BJ. Assessing Attention in Rodents. 2009 [PubMed] [Google Scholar]
- 61.Zhou Y, Riccio DC. Concussion-induced retrograde amnesia in rats. Physiol Behav. 1995 Jun;57(6):1107–1115. doi: 10.1016/0031-9384(95)00019-f. [DOI] [PubMed] [Google Scholar]
- 62.Prins ML, Hales A, Reger M, Giza CC, Hovda DA. Repeat traumatic brain injury in the juvenile rat is associated with increased axonal injury and cognitive impairments. Dev Neurosci. 2010;32(5–6):510–518. doi: 10.1159/000316800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reger ML, Poulos AM, Buen F, Giza CC, Hovda DA, Fanselow MS. Concussive brain injury enhances fear learning and excitatory processes in the amygdala. Biol Psychiatry. 2012 Feb 15;71(4):335–343. doi: 10.1016/j.biopsych.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vagnozzi R, Signoretti S, Cristofori L, et al. Assessment of metabolic brain damage and recovery following mild traumatic brain injury: a multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain. 2010 Nov;133(11):3232–3242. doi: 10.1093/brain/awq200. [DOI] [PubMed] [Google Scholar]
- 65.Anderson J, Sandhir R, Hamilton ES, Berman NE. Impaired expression of neuroprotective molecules in the HIF-1alpha pathway following traumatic brain injury in aged mice. J Neurotrauma. 2009 Sep;26(9):1557–1566. doi: 10.1089/neu.2008.0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wali B, Sayeed I, Stein DG. Improved behavioral outcomes after progesterone administration in aged male rats with traumatic brain injury. Restor Neurol Neurosci. 2011;29(1):61–71. doi: 10.3233/RNN-2011-0579. [DOI] [PubMed] [Google Scholar]
- 67.Timaru-Kast R, Luh C, Gotthardt P, et al. Influence of age on brain edema formation, secondary brain damage and inflammatory response after brain trauma in mice. PLoS One. 2012;7(8):e43829. doi: 10.1371/journal.pone.0043829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoane MR, Lasley LA, Akstulewicz SL. Middle age increases tissue vulnerability and impairs sensorimotor and cognitive recovery following traumatic brain injury in the rat. Behav Brain Res. 2004 Aug 12;153(1):189–197. doi: 10.1016/j.bbr.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 69.Omalu BI, Fitzsimmons RP, Hammers J, Bailes J. Chronic traumatic encephalopathy in a professional American wrestler. J Forensic Nurs. 2010 Fall;6(3):130–136. doi: 10.1111/j.1939-3938.2010.01078.x. [DOI] [PubMed] [Google Scholar]
- 70.Omalu BI, DeKosky ST, Hamilton RL, et al. Chronic traumatic encephalopathy in a national football league player: part II. Neurosurgery. 2006 Nov;59(5):1086–1092. doi: 10.1227/01.NEU.0000245601.69451.27. discussion 1092–1083. [DOI] [PubMed] [Google Scholar]
- 71.Omalu BI, DeKosky ST, Minster RL, Kamboh MI, Hamilton RL, Wecht CH. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery. 2005 Jul;57(1):128–134. doi: 10.1227/01.neu.0000163407.92769.ed. discussion 128–134. [DOI] [PubMed] [Google Scholar]
- 72.McKee AC, Stein TD, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013 Jan;136(Pt 1):43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bennett RE, Mac Donald CL, Brody DL. Diffusion tensor imaging detects axonal injury in a mouse model of repetitive closed-skull traumatic brain injury. Neurosci Lett. 2012 Apr 4;513(2):160–165. doi: 10.1016/j.neulet.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dapul HR, Park J, Zhang J, et al. Concussive injury before or after controlled cortical impact exacerbates histopathology and functional outcome in a mixed traumatic brain injury model in mice. J Neurotrauma. 2013 Mar 1;30(5):382–391. doi: 10.1089/neu.2012.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fujita M, Wei EP, Povlishock JT. Intensity- and interval-specific repetitive traumatic brain injury can evoke both axonal and microvascular damage. J Neurotrauma. 2012 Aug 10;29(12):2172–2180. doi: 10.1089/neu.2012.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Klemenhagen KC, O'Brien SP, Brody DL. Repetitive concussive traumatic brain injury interacts with post-injury foot shock stress to worsen social and depression-like behavior in mice. PLoS One. 2013;8(9):e74510. doi: 10.1371/journal.pone.0074510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shitaka Y, Tran HT, Bennett RE, et al. Repetitive closed-skull traumatic brain injury in mice causes persistent multifocal axonal injury and microglial reactivity. J Neuropathol Exp Neurol. 2011 Jul;70(7):551–567. doi: 10.1097/NEN.0b013e31821f891f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Y, Arun P, Wei Y, et al. Repeated Blast Exposures cause DNA Fragmentation in Mice. J Neurotrauma. 2013 Sep 27; doi: 10.1089/neu.2013.3074. [DOI] [PubMed] [Google Scholar]
- 79.Genovese RF, Simmons LP, Ahlers ST, Maudlin-Jeronimo E, Dave JR, Boutte AM. Effects of mild TBI from repeated blast overpressure on the expression and extinction of conditioned fear in rats. Neuroscience. 2013 Dec 19;254:120–129. doi: 10.1016/j.neuroscience.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 80.Valiyaveettil M, Alamneh Y, Wang Y, et al. Contribution of systemic factors in the pathophysiology of repeated blast-induced neurotrauma. Neurosci Lett. 2013 Feb 28;539:1–6. doi: 10.1016/j.neulet.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 81.Mannix R, Meehan WP, Mandeville J, et al. Clinical correlates in an experimental model of repetitive mild brain injury. Ann Neurol. 2013 Jul;74(1):65–75. doi: 10.1002/ana.23858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Prins ML, Alexander D, Giza CC, Hovda DA. Repeated mild traumatic brain injury: mechanisms of cerebral vulnerability. J Neurotrauma. 2013 Jan 1;30(1):30–38. doi: 10.1089/neu.2012.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]