Abstract
Increasing evidences have implicated somatic gain-of-function mutations at the telomerase reverse transcriptase (TERT) promoter as one of the major mechanisms that promote transcriptional activation of TERT and subsequently maintain telomere length in human cancers including glioma. To investigate the prognostic value of these mutations and telomere length, individually and their coexistence, in gliomas, we analyzed two somatic mutations C228T and C250T in the TERT promoter, relative telomere length (RTL), IDH1 mutation and MGMT methylation in 389 glioma patients, and explored their associations with patient characteristics and clinical outcomes. Our data showed that C228T and C250T mutations were found in 17.0% (66 of 389) and 11.8% (46 of 389) of gliomas, respectively, and these two mutations were mutually exclusive in this cancer. Moreover, they were significantly associated with WHO grade. We also found that the RTL was significant longer in gliomas than in meningiomas and normal brain tissues (Median, 0.89 vs. 0.44 and 0.50; P < 0.001), and demonstrated that the RTL was strongly correlated with tumor recurrence. Importantly, TERT promoter mutations or long RTL caused a significantly poorer survival than TERT wild-type or short RTL. Coexisting TERT promoter mutations and long RTL were more commonly associated with poor patient survival than they were individually. Notably, the patients with TERT promoter mutations particularly C228T or long RTL were resistant to radiotherapy. Collectively, TERT promoter mutations and long RTL are not only prognostic factors for poor clinical outcomes, but also the predictors of radiotherapy resistance in gliomas.
Keywords: glioma, TERT promoter mutations, relative telomere length, poor survival, radiotherapy resistance
INTRODUCTION
Glioma is the most common primary brain tumor in adults, and patients with malignant glioma always have a poor prognosis due to the progressive overgrowth and diffuse invasion [1]. Although recent advances in standard therapy including surgical resection followed by radiation and/or chemotherapy, the prognosis is still disappointing [2]. Thus, a better understanding of the mechanisms underlying glioma pathogenesis may lead to more precise prognostic prediction and more effective therapies for this disease.
Telomerase, a ribonucleoprotein that consists of an RNA subunit and a telomerase reverse transcriptase (TERT) catalytic subunit, maintains telomere length through adding telomeric repeats to chromosome ends [3]. Telomeres are shortened with each cell division in normal cells, whereas they are continuously elongated by telomerase in cancer cells [4]. Increased telomerase activity is perceived to be one of the hallmarks of human cancers including gliomas, overcoming replicative telomere shortening and conferring unrestricted growth of cancer cells [4, 5]. The TERT promoter has been well demonstrated to be the most important element of telomerase expression/activity through transcriptional regulation [6]. Recently, recurrent mutations at two hotspots termed C228T and C250T in the TERT promoter have been identified in diverse cancers including gliomas [7–12]. These mutations create new binding motifs for Ets/TCF transcription factors and cause two- to four-fold increase in transcriptional activity [7], and have been regarded as one of the major mechanisms of telomerase activation in gliomas [12]. In addition, a very recent study reveals that the transcription factor GABP selectively binds and activates the mutant TERT promoter, also contributing to aberrant expression of TERT gene in multiple cancers, including gliomas [13].
In this study, we sought to investigate TERT promoter mutations and relative telomere length (RTL) in a large cohort of Chinese patients with well-characterized gliomas, and explore their associations with clinical outcomes of these patients.
RESULTS
Patient characteristics
A total of 389 glioma specimens were analyzed in this study, including 247 diffuse astrocytomas (DA), 44 oligodendrogliomas (OL), 46 oligoastrocytomas (OA) and 52 glioblastomas (GBM). The patient group was composed of 216 men and 173 women with a mean age of 44.9 ± 14.1 years (range: 18–81). Median overall survival time for the total was 40.3 months. Malignant glioma patients were managed according to ESMO clinical recommendations as described previously [14]. Briefly, the patients were divided into four groups: i) subsequent to surgery, 94 patients received adjuvant radiotherapy alone (35–50 Gy of craniospinal radiotherapy); ii) 33 patients received adjuvant chemotherapy alone (temozolomide, 150 mg/m2 for 5 days for first cycle, 200 mg/m2 for 5 days every 28 days, ≥6 cycles); iii) 104 patients received the combined therapy; iv) 99 patients did not receive further therapy. Analysis of IDH1 mutations was performed in 330 tumor samples. One hundred and twenty patients (36.4%) harbored IDH1 mutations, with 118 displaying R132H (CGT → CAT) mutation and 2 displaying R132C (CGT → TGT) mutation (Supplementary Figure S1A). For MGMT promoter methylation analysis, DNA from 330 tumor samples was investigated by MSP assay. Methylated MGMT promoter sequences were detected in 190 cases (57.6%) (Supplementary Figure S1B). Other patient characteristics were summarized in Table 1.
Table 1. Association of TERT promoter mutations and the RTL with clinicopathological characteristics in gliomas (n = 389).
| Characteristics | TERT promoter mutations (n = 389) | RTL (n = 389) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | C228T | C250T | ||||||||||
| Yes (%) | No (%) | P | Yes (%) | No (%) | P | Yes (%) | No (%) | P | ≤0.62 (%) | >0.62 (%) | P | |
| No. of Patients | 112 (28.8) | 277 (71.2) | 66 (17.0) | 323 (83.0) | 46 (11.8) | 343 (88.2) | 143 (36.8) | 246 (63.2) | ||||
| Gender | ||||||||||||
| Male | 51 (23.6) | 165 (76.4) | 0.013 | 34 (15.7) | 182 (84.3) | 0.499 | 17 (7.9) | 199 (92.1) | 0.007 | 84 (38.9) | 132 (61.1) | 0.343 |
| Female | 61(35.3) | 112(64.7) | 32 (18.5) | 141 (81.5) | 29 (16.8) | 144 (83.2) | 59 (34.1) | 114 (65.9) | ||||
| Age, years | ||||||||||||
| Mean | 48 | 44 | 0.005 | 47 | 44 | 0.112 | 49 | 44 | 0.037 | 45 | 45 | 0.833 |
| SD | 13.7 | 14.1 | 14.4 | 14.1 | 12.8 | 14.2 | 13.1 | 14.8 | ||||
| WHO grade | ||||||||||||
| I | 5 (15.2) | 28 (84.8) | 0.039 | 1 (3.0) | 32 (97.0) | 0.072 | 4 (12.1) | 29 (87.9) | 0.858 | 11 (33.3) | 22 (66.7) | 0.685 |
| II | 40 (24.7) | 122 (75.3) | 22 (13.6) | 140 (86.4) | 18 (11.1) | 144 (88.9) | 65 (40.1) | 97 (59.9) | ||||
| III | 41 (32.3) | 86 (67.7) | 27 (21.3) | 100 (78.7) | 14 (11.0) | 113 (89.0) | 45 (35.4) | 82 (64.6) | ||||
| IV | 26 (38.8) | 41 (61.2) | 16 (23.9) | 51 (76.1) | 10 (14.9) | 57 (85.1) | 22 (32.8) | 45 (67.2) | ||||
| Localization | ||||||||||||
| Frontal lobe | 58 (33.9) | 113 (66.1) | 0.069 | 36 (21.1) | 135 (78.9) | 0.020 | 22 (12.9) | 149 (87.1) | 0.010 | 59 (34.5) | 112 (65.5) | 0.083 |
| Temporal lobe | 41 (29.1) | 100 (70.9) | 22 (15.6) | 119 (84.4) | 19 (13.5) | 122 (86.5) | 50 (35.5) | 91 (64.5) | ||||
| Parietal lobe | 19 (28.4) | 48 (71.6) | 12 (17.9) | 55 (82.1) | 7 (10.4) | 60 (89.6) | 22 (32.8) | 45 (67.2) | ||||
| Occipital lobe | 9 (39.1) | 14 (60.9) | 7 (30.4) | 16 (69.6) | 2 (8.7) | 21 (91.3) | 7 (30.4) | 16 (69.6) | ||||
| Cerebellum | 2 (11.8) | 15 (88.2) | 2 (11.8) | 15 (88.2) | 0 (0.0) | 17 (100.0) | 12 (70.6) | 5 (29.4) | ||||
| Others | 6 (14.3) | 36 (85.7) | 3 (7.1) | 39 (92.8) | 3 (7.1) | 39 (92.8) | 16 (38.1) | 26 (61.9) | ||||
| Pathological diagnosis | ||||||||||||
| DA | 66 (26.7) | 181 (73.3) | 0.157 | 37 (15.0) | 210 (85.0) | 0.006 | 29 (11.7) | 218 (88.3) | 0.767 | 91 (36.8) | 156 (63.2) | 0.496 |
| OL | 10 (22.7) | 34 (77.3) | 3 (6.8) | 41 (93.2) | 7 (15.9) | 37 (84.1) | 14 (31.8) | 30 (68.2) | ||||
| OA | 19 (41.3) | 27 (58.7) | 15 (32.6) | 31 (67.4) | 4 (8.7) | 42 (91.3) | 21 (45.6) | 25 (54.3) | ||||
| GBM | 17 (32.7) | 35 (67.3) | 11 (21.2) | 41 (78.8) | 6 (11.5) | 46 (88.5) | 17 (32.7) | 35 (67.3) | ||||
| Recurrence▼ | ||||||||||||
| Yes | 61 (30.7) | 138 (69.3) | 0.171 | 34 (17.1) | 165 (82.9) | 0.225 | 27 (13.6) | 172 (86.4) | 0.498 | 57 (28.6) | 142 (71.4) | 0.005 |
| No | 31 (23.7) | 100 (76.3) | 17 (13.0) | 114 (87.0) | 14 (10.7) | 117 (89.3) | 58 (44.3) | 73 (55.7) | ||||
| KPS▼ | ||||||||||||
| ≥ 80 | 41 (23.3) | 135 (76.7) | 0.050 | 22 (14.3) | 132 (85.7) | 0.648 | 29 (18.8) | 125 (81.2) | 0.001 | 61 (39.6) | 93 (60.4) | 0.105 |
| < 80 | 51 (33.1) | 103 (66.9) | 29 (16.5) | 147 (83.5) | 12 (6.8) | 164 (93.1) | 54 (30.7) | 122 (69.3) | ||||
| Seizures▼ | ||||||||||||
| Yes | 42 (30.2) | 97 (69.8) | 0.457 | 20 (14.4) | 119 (85.6) | 0.758 | 22 (15.8) | 117 (84.2) | 0.129 | 44 (31.7) | 95 (68.3) | 0.349 |
| No | 50 (26.2) | 141 (73.8) | 31 (16.2) | 160 (83.8) | 19 (9.9) | 172 (90.1) | 71 (37.2) | 120 (62.8) | ||||
| Radiotherapy▼ | ||||||||||||
| Yes | 54 (27.3) | 144 (72.7) | 32 (16.2) | 166 (83.8) | 22 (11.1) | 176 (88.9) | 64 (32.3) | 143 (67.7) | ||||
| No | 38 (28.8) | 94 (71.2) | 19 (14.4) | 113 (85.6) | 19 (14.4) | 113 (85.6) | 51 (38.6) | 81 (61.4) | ||||
| Chemotherapy▼ | ||||||||||||
| Yes | 37 (27.0) | 100 (73.0) | 21 (15.3) | 116 (84.7) | 16 (11.7) | 121 (88.3) | 50 (36.5) | 87 (63.5) | ||||
| No | 55 (28.5) | 138 (71.5) | 30 (15.5) | 163 (84.5) | 25 (13.0) | 168 (87.0) | 65 (33.7) | 128 (66.3) | ||||
| IDH1 mutation▼ | ||||||||||||
| Yes | 34 (28.3) | 86 (71.7) | 0.899 | 21 (17.5) | 99 (82.5) | 0.527 | 13 (10.8) | 107 (89.2) | 0.604 | 37 (30.8) | 83 (69.2) | 0.280 |
| No | 58 (27.6) | 152 (72.4) | 30 (14.3) | 180 (85.7) | 28 (13.3) | 182 (86.7) | 78 (37.1) | 132 (62.9) | ||||
| MGMT methylation▼ | ||||||||||||
| Yes | 57 (30.0) | 133 (70.0) | 0.324 | 35 (18.4) | 155 (81.6) | 0.091 | 22 (11.6) | 168 (88.4) | 0.615 | 66 (34.7) | 124 (65.3) | 1.000 |
| No | 35 (25.0) | 105 (75.0) | 16 (11.4) | 124 (88.6) | 19 (13.6) | 121 (86.4) | 49 (35.0) | 91 (65.0) | ||||
Abbreviations: Relative telomere length (RTL); Diffuse astrocytoma (DA); Oligodendroglioma (OL); Oligoastrocytoma (OA); Glioblastoma (GBM); Karnofsky performance status (KPS);
Only 330 patients have complete survival information.
TERT promoter mutations and the RTL in gliomas
Two hot-spot mutations (C228T and C250T) in the TERT promoter were investigated in a total of 389 glioma samples by Pyrosequencing and Sanger sequencing in this study. In Figure 1A, representative pyrograms and electropherograms of the two TERT promoter mutations in gliomas were shown. Figure 1B and 1C summarize TERT promoter mutations found in different subtypes of gliomas. These two mutations were collectively found in 28.8% (112 of 389) of gliomas. C228T and C250T mutations were found in 17.0% (66 of 389) and 11.8% (46 of 389) of gliomas, respectively. All mutations found in this study were heterozygous, and these two mutations were mutually exclusive in this cancer.
Figure 1. TERT promoter mutations in gliomas.
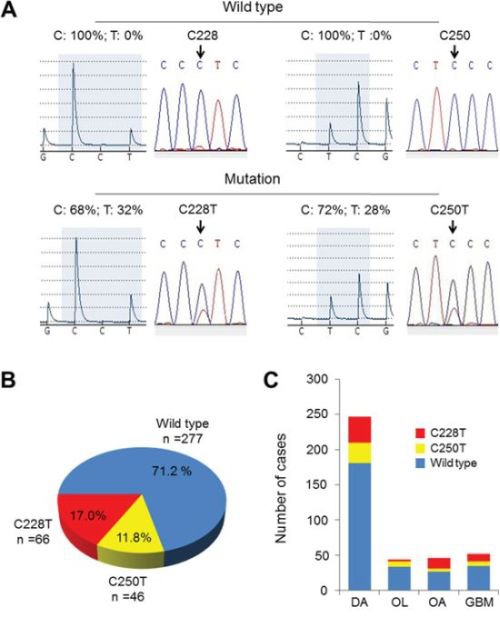
A. Shown are representative pyrograms (left) and electropherograms (right) of the TERT wild-type and two TERT promoter mutations as indicated for gliomas. B. Pie chart of C228T and C250T somatic mutation status in 389 primary gliomas. C. Frequency of TERT promoter mutations (C228T, red; C250T, yellow) in each tumor subtype. DA, diffuse astrocytoma; OL, oligodendroglioma; OA, oligoastrocytoma; GBM, glioblastoma.
Comparing with previous reports [12], the prevalence of TERT promoter mutations is relatively lower in the present study. To support this finding, ATRX activation was detected by IHC assay in 60 glioma tissues, including 18 cases with TERT promoter mutations and 42 TERT wild-type cases. As shown in Figure 2, positive immunostaining of ATRX in cases with TERT promoter mutations and TERT wild-type was 72.2% (13/18) and 7.1% (3/42), respectively. There was a significantly positive relationship between TERT promoter mutations and ATRX activation (Χ2 = 27.289, P < 0.001) in gliomas, as supported by the previous studies that TERT promoter and ATRX mutations (usually caused ATRX inactivation) were mutually exclusive [9, 15].
Figure 2. Differential expression of ATRX in gliomas.
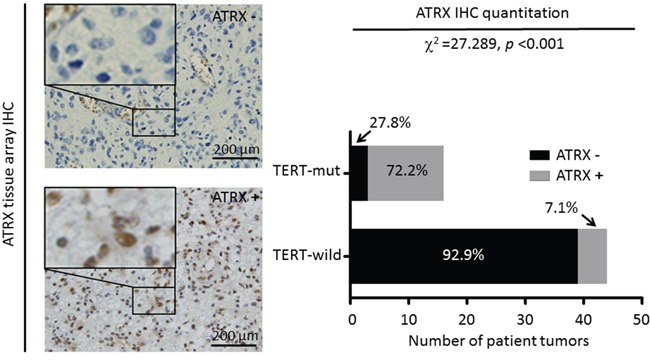
Immunohistochemical (IHC) assays were performed to detect ATRX activation using anti-ATRX antibody. Shown are representative IHC images of glioma tissues with or without ATRX staining (left panel) (magnification, ×200). A comparison of the proportion of patients with TERT promoter mutations or TERT wild-type associated with positive immunostaining of ATRX (right panel). The relationship between them was evaluated using Fisher's exact test.
Next, the RTL was measured in 389 gliomas, 50 meningiomas and 8 normal brain tissues by using real-time quantitative PCR. As shown in Supplementary Figure S2A, the RTL was significantly longer in gliomas than that in meningiomas and normal brain tissues (Median, 0.89 vs. 0.44 and 0.50; P < 0.001). In addition, we also investigated the relationship between TERT promoter mutations and the RTL in gliomas. The results showed that the RTL was significantly shorter in the cases with TERT promoter mutations than in the cases with TERT wild-type (P = 0.017) (Supplementary Figure S2B), indicating that other molecular mechanisms may be involved in regulating telomere length in the latter. It has been reported that many TERT wild-type gliomas harbor highly frequent ATRX mutations, which induce the Alternative Lengthening of Telomeres (ALT) phenotype [15, 16]. As expected, we found highly frequent ATRX inactivation (∼92.9%) in TERT wild-type gliomas in the present study (Figure 2), and demonstrated that the RTL was significantly longer in ATRX-negative tumors than that in ATRX-positive tumors (Median, 0.90 vs. 0.49; P = 0.002) (Supplementary Figure S2C). These findings suggest that telomere length may also be regulated by ALT phenotype such as ATRX inactivation in TERT wild-type tumors, as supported by the previous studies [16]. A recent study showed that gliomas with TERT promoter mutations had shorter telomere length compared to TERT wild-type gliomas [17], which was consistent with our findings. In addition, coexisting TERT promoter mutations and long RTL were also found in 55 of 330 (16.7%) gliomas.
Association of TERT promoter mutations and variable RTL with clinicopathological characteristics of glioma patients
The relationship between TERT promoter mutations and patient characteristics was summarized in Table 1. The results showed that there was a significantly positive association of TERT promoter mutations with WHO grade (P = 0.039). The prevalence of TERT promoter mutations was only 15.2% (5 of 33) in patients with grade I gliomas, whereas these mutations were found in 24.7% (40 of 162), 32.3% (41 of 127) and 38.8% (26 of 67) in patients with grade II-IV gliomas, respectively. These results suggest that TERT promoter mutations were more frequent with high-grade gliomas. To investigate the relationship between the RTL with patient characteristics, we defined the upper limit of the overall 95% confidence interval (CI, 0.45–0.62) for 50 meningioma samples as cut-off value. Glioma patients were then categorized into two groups by use of this cut-off point: short telomere (≤0.62) and long telomere (>0.62). Also shown in Table 1, the RTL was only significantly associated with tumor recurrence (P < 0.001).
To assess the association of TERT promoter mutations and the RTL with gender, age, WHO grade, pathological diagnosis, recurrence, seizures, KPS score, IDH1 mutation, and MGMT methylation, we conducted multiple multivariable logistic regressions. As shown in Table 2, TERT promoter mutations remained closely associated with WHO grade (OR = 1.81, 95% CI = 1.23–2.66; P = 0.003). The RTL was still significantly associated with tumor recurrence (OR = 1.70, 95% CI = 1.00–2.89; P = 0.049) after adjustment. In addition, the RTL was significantly associated with age (OR = 2.24, 95% CI = 1.09–4.60; P = 0.029).
Table 2. Multivariable analysis of characteristics of glioma patients divided according to TERT promoter mutations and the RTL (n = 330).
| Characteristics | TERT promoter mutations | RTL | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall | C228T | C250T | ||||||
| OR* (95% CI) | P | OR* (95% CI) | P | OR* (95% CI) | P | OR* (95% CI) | P | |
| Gender | 2.12 (1.26–3.56) | 0.005 | 1.26 (0.67–2.36) | 0.478 | 3.19 (1.51–6.75) | 0.002 | 1.33 (0.83–2.14) | 0.243 |
| Age1 | 1.94 (1.00–3.76) | 0.052 | 2.11 (0.96–4.64) | 0.062 | 1.37 (0.55–3.42) | 0.503 | 2.24 (1.09–4.60) | 0.029 |
| WHO grade2 | 1.81 (1.23–2.66) | 0.003 | 1.52 (0.95–2.44) | 0.083 | 1.80 (1.08–3.01) | 0.025 | 0.95 (0.67–1.33) | 0.751 |
| Pathological diagnosis3 | 0.94 (0.72–1.22) | 0.643 | 1.08 (0.79–1.47) | 0.628 | 0.80 (0.55–1.17) | 0.248 | 0.95 (0.74–1.22) | 0.680 |
| Recurrence | 1.46 (0.79–2.70) | 0.225 | 1.19 (0.56–2.54) | 0.646 | 1.54 (0.67–3.57) | 0.311 | 1.70 (1.00–2.89) | 0.049 |
| Seizures | 1.52 (0.88–2.63) | 0.133 | 0.99 (0.51–1.94) | 0.980 | 2.13 (0.99–4.56) | 0.052 | 1.23 (0.75–2.01) | 0.417 |
| KPS4 | 2.68 (1.50–4.80) | 0.001 | 1.02 (0.51–2.04) | 0.964 | 6.33 (2.73–14.71) | <0.001 | 1.32 (0.79–2.20) | 0.289 |
| IDH1 mutation | 0.96 (0.55–1.71) | 0.900 | 1.50 (0.76–2.98) | 0.246 | 0.53 (0.24–1.20) | 0.131 | 1.45 (0.86–2.45) | 0.168 |
| MGMT methylation | 1.27 (0.74–2.18) | 0.391 | 1.67 (0.84–3.30) | 0.142 | 0.81 (0.39–1.70) | 0.577 | 0.99 (0.60–1.61) | 0.951 |
| RTL | 0.58 (0.34–1.01) | 0.053 | 0.43 (0.22–0.82) | 0.011 | 1.07 (0.49–2.32) | 0.871 | — | — |
OR: odds ratio with 95% confidence interval;
Age (≥60; <60);
WHO grade (I; II; III; IV);
Pathological diagnosis (Diffuse astrocytoma; Oligodendroglioma; Oligoastrocytoma; Glioblastoma);
KPS (≥80; <80).
Effect of TERT promoter mutations and variable RTL on poor patient survival
Whether TERT promoter mutations and variable RTL are indeed associated with poor overall survival (OS), as suggested by their association with clinicopathologic features of glioma patients, was subsequently investigated by univariate survival analysis. As shown in Table 3, TERT promoter mutations (HR = 1.49, 95% CI, 1.05–2.10; P = 0.026) and long RTL (HR = 1.50, 95% CI, 1.04–2.17; P = 0.030) were significantly associated with poor survival in glioma patients. Next, we used Kaplan-Meier survival analysis to further determine the effect of TERT promoter mutations and variable RTL on patient survival. As expected, the patients with TERT promoter mutation (P = 0.023) or long RTL (P = 0.027) had significantly poorer survival than those without mutations or with short RTL (Figure 3A and 3B). Accordingly, 1-, 2- and 3-year OS rates were worse in the former that in the latter (Table 4). Although the number was small, our data showed that the RTL significantly affected the survival in the patients with grade I gliomas. The patients with long RTL had worse survival than those with short RTL (P = 0.044) (Figure 3C). In addition, we demonstrated that the patients with coexisting TERT promoter mutations and long RTL had much worse survival than others (Table 4 and Figure 3D). Given that the majority of gliomas in the present study are astrocytomas, similar analysis has been done only in astrocytomas. Similar to the findings in Figure 3, TERT promoter mutations and longer RTL caused a significantly poorer survival of astrocytoma patients than TERT wild-type or short RTL (Supplementary Figures S3A and S3B). In addition, we also analyzed the relationship between TERT promoter status and patient survival for other histological types including oligodendroglioma, oligoastrocytoma and glioblastoma. Similar results were also found, except for oligoastrocytoma because of the limited number (Supplementary Figure S4).
Table 3. Prognostic value of TERT promoter mutations and the RTL in univariate and multivariate cox regression analysis in glimoas (n = 330).
| Characteristics | Univariateanalysis | Multivariate analysis forTERT | Multivariate analysis for RTL | |||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P | Hazard Ratio (95% CI) | P | Hazard Ratio (95% CI) | P | |
| Gender | 1.15 (0.84–1.59) | 0.385 | 1.19 (0.85–1.64) | 0.310 | 1.17 (0.85–1.63) | 0.338 |
| Age | 3.24 (2.26–4.64) | <0.001 | 1.04 (1.02–1.04) | <0.001 | 1.03 (1.02–1.04) | <0.001 |
| Pathological diagnosis1 | 1.09 (0.94–1.27) | 0.271 | 1.13 (0.97–1.32) | 0.122 | 1.14 (0.98–1.33) | 0.091 |
| IDH1 mutations | 0.59 (0.41–0.84) | 0.004 | 0.48 (0.40–0.89) | 0.018 | 0.68 (0.47–0.99) | 0.022 |
| MGMT methylation | 0.60 (0.44–0.83) | 0.002 | 0.51 (0.36–0.71) | <0.001 | 0.51 (0.36–0.72) | <0.001 |
| Radiotherapy | 0.89 (0.64–1.23) | 0.467 | 0.74 (0.53–1.04) | 0.084 | 0.66 (0.46–0.94) | 0.020 |
| Chemotherapy | 1.75 (1.27–2.42) | 0.001 | 2.09 (1.49–2.94) | <0.001 | 2.23 (1.58–3.16) | <0.001 |
| TERT promoter mutations | 1.49 (1.05–2.10) | 0.026 | 1.39 (1.13–2.17) | 0.020 | - | - |
| RTL | 1.50 (1.04–2.17) | 0.030 | - | - | 1.72 (1.18–2.50) | 0.005 |
Pathological diagnosis (Diffuse astrocytoma; Oligodendroglioma; Oligoastrocytoma; Glioblastoma)
Figure 3. Kaplan-Meier analysis of overall survival for glioma patients assessed for the mutational status of TERT promoter and the RTL individually or in combination.
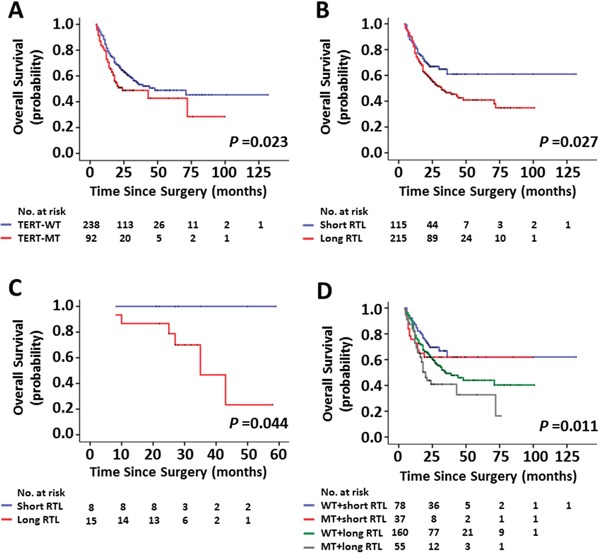
A. The patients with TERT promoter mutations had a worse survival than those without mutations. Long RTL caused a poorer overall survival than short RTL in all patients B. and in the patients with grade I gliomas C. respectively. D. Overall survival in glioma patients where patient groups were defined by the mutational status of TERT promoter and the RTL. The patients with coexisting TERT promoter mutations and long RTL had much worse survival than others. WT and MT represent the TERT promoter wild-type and -mutated tumors, respectively.
Table 4. Overall survival by grouping with TERT promoter mutations and the RTL (n = 330).
| Characteristics | Overall survival rate (%) | Overall survival time (month) | |||
|---|---|---|---|---|---|
| 1 year (95% CI) | 2 years (95% CI) | 3 years (95% CI) | Median | 95% CI | |
| TERT promoter mutations | |||||
| No (WT) | 81.5 (76.6–86.4) | 64.3 (58.0–70.6) | 53.3 (45.9–60.7) | 48.2 | 15.6–68.9 |
| Yes (MT) | 72.8 (63.8–81.8) | 48.8 (38.0–59.6) | 42.7 (28.0–57.4) | 23.5 | 3.7–44.3 |
| RTL | |||||
| Short (≤0.62) | 82.6 (75.7–89.5) | 66.9 (58.1–75.7) | 61.0 (49.6–72.4) | Not reached | — |
| Long (>0.62) | 77.2 (71.5–82.9) | 56.6 (49.7–63.5) | 46.7 (39.1–54.3) | 32.0 | 20.2–43.8 |
| Combinations | |||||
| WT and Short RTL | 87.2 (79.8–94.6) | 69.6 (59.2–80.0) | 62.0 (48.1–75.9) | Not reached | — |
| MT and Short RTL | 73.0 (58.7–87.3) | 61.9 (46.2–77.6) | 61.9 (46.2–77.6) | Not reached | — |
| WT and Long RTL | 78.8 (72.5–85.1) | 61.7 (54.1–69.3) | 49.3 (40.5–58.1) | 35.0 | 20.2–49.8 |
| MT and Long RTL | 72.7 (60.9–84.5) | 40.9 (27.2–54.6) | 32.7 (14.7–50.7) | 20.0 | 16.2–23.8 |
Abbreviations: WT, TERT promoter wild type; MT, TERT promoter mutations; RTL, relative telomere length
Next, we attempted to explore whether the prognostic value of TERT promoter mutations and variable RTL as found in univariate analysis is attributable to their association with other factors or whether they contribute independently to survival. Thus, Cox multivariate regression analysis was performed in this study. Also shown in Table 3, TERT promoter mutations (HR = 1.39; 95% CI, 1.13–2.17; P = 0.020) and long RTL (HR = 1.72; 95% CI, 1.18–2.50; P = 0.005) are predictors of poor OS for glioma patients as independently variable with respect to gender, age, pathological diagnosis, IDH1 mutations, MGMT methylation, radiotherapy and chemotherapy.
Notably, we also demonstrated that the patients with symptoms of seizure had better survival than patients without the symptoms (P = 0.012) (Supplementary Figure S5). There is a strong possibility that the majority of the patients with symptoms of seizure are diagnosed as low-grade tumors in this study, accounting for 59.7% of all cases with symptoms of seizure, as supported by a previous study [18].
Impact of TERT promoter mutations and the RTL on outcomes of radiotherapy and chemotherapy in glioma patients
As adjuvant therapy after surgical resection for glioma, radiotherapy and chemotherapy can significantly improve the patient OS [19]. In this study, we tested the effect of TERT promoter mutations and the RTL on outcomes of radiotherapy and chemotherapy in a cohort of gliomas. As shown in Figure 4A, although the difference did not reach statistical significance, TERT promoter mutations were associated with lower 2-year OS rate in the patients receiving both radiotherapy and chemotherapy compared with TERT wild-type (34.3% ± 9.8% vs. 52.6% ± 5.9%, P = 0.092). Further analysis revealed that these mutations significantly affected 2-year OS rate in the patients only receiving radiotherapy (58.3% ± 9.6% vs. 81.8% ± 4.8%, P = 0.041) (Figure 4B), whereas they did not affect 2-year OS rate in the cases receiving chemotherapy compared with TERT wild-type (P = 0.304, figure not shown). Next, to divide the patients into two groups, we chose median RTL for all cases receiving radiotherapy and/or chemotherapy as cut-off values. As shown in Figure 4C, long RTL did not significantly affected 2-year OS rate in the patients receiving both radiotherapy and chemotherapy than short RTL (P = 0.350). Similar to TERT promoter mutations, long RTL caused a significantly poorer radiotherapy outcome (2-year OS rate: 65.6% ± 7.0% vs. 84.7% ± 5.4%, P = 0.025) in the patients only receiving radiotherapy than short RTL (Figure 4D). Moreover, we similarly did not find significant effect of long RTL on chemotherapy outcome in these patients (P = 0.824, figure not shown). Similarly, the astrocytoma patients with TERT promoter mutations or long RTL were also resistant to radiotherapy (Supplementary Figures S3C and S3D). To further clarify the role of TERT promoter mutations in radiotherapy outcome, C250T and C228T mutations were separately analyzed for their correlation with patients' response to radiotherapy. The results showed that C228T mutation, but not C250T mutation, significantly affected patients' response to radiotherapy (Figure 5).
Figure 4. Kaplan-Meier estimates of overall survival by the mutational status of TERT promoter and the RTL.
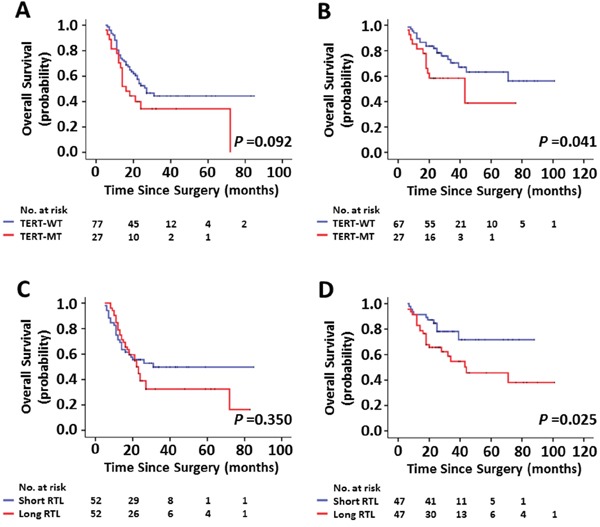
A, C. Represent Kaplan-Meier analysis of 104 patients receiving both radiotherapy and chemotherapy after surgery. B, D. Represent Kaplan-Meier analysis of 94 patients only receiving postoperative radiotherapy.
Figure 5. Effect of C228T and C250T mutations individually on radiotherapy response.

Represent Kaplan-Meier analysis of 94 patients only receiving postoperative radiotherapy grouped by C228T (left) and C250T (right) mutations, respectively.
Increasing evidences have demonstrated that IDH1 mutations and MGMT methylation are associated with survival benefit in gliomas [20, 21]. This is as supported by our data that the cases with IDH1-mutated or MGMT-methylated tumors had better survival than those with IDH1-wild type or MGMT-unmethylated tumors (Supplementary Figure S6). Thus, we performed a Cox multivariate regression analysis to determine whether these two genetic events contribute independently to radiotherapy outcome of glioma patients. As shown in Figure 6, TERT promoter mutations (HR = 2.30; 95% CI, 1.09–4.86; P = 0.029) and long RTL (HR = 2.55; 95% CI, 1.18–5.52; P = 0.018) were two predictors of poor survival in glioma patients receiving radiotherapy as an independently variable with respect to WHO grade, KPS score, IDH1 mutations and MGMT methylation, respectively. Collectively, our data suggest that glioma patients with TERT promoter mutation and long RTL are resistant to postoperative radiotherapy.
Figure 6. Multivariate Cox regression analysis.
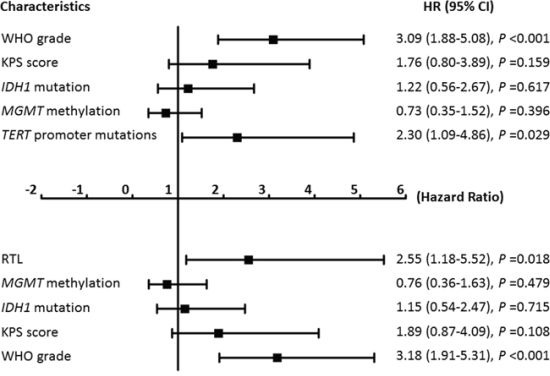
Multivariate Cox regression analysis of tumor characteristics TERT promoter mutations and the RTL on overall survival in the patients (n = 94) only receiving postoperative radiotherapy.
DISCUSSION
In this study, we found 28.8% (112 of 389) glioma patients harbored TERT promoter mutations, which was relatively lower than previously reported [12]. However, we found highly frequent ATRX inactivation (∼92.9%) in TERT wild-type gliomas, and there was a significantly inverse relationship between TERT promoter mutations and ATRX inactivation. These observations suggest that each of these two molecular events plays a relatively independent role in maintaining the immortalization of glioma cells, ultimately contributing to glioma tumorigenesis. Given that TERT promoter mutations have been widely found in diverse cancers [7–12], these mutations are likely to be one of the mechanisms of telomerase activation in this cancer. As described in other cancers such as melanomas, thyroid cancer, bladder cancer and laryngeal cancer [7, 22–24], the present study and previous study [12] confirmed that C228T and C250T mutations were mutually exclusive in gliomas, suggesting that each of them is individually sufficient to play a significant oncogenic role in glioma pathogenesis. Importantly, these mutations have been demonstrated to be absent in benign tumors and normal subjects [7, 8, 24], implicating that they may be potentially valuable diagnostic and prognostic markers in human cancers including gliomas.
Considering the association of TERT promoter mutations with poor survival of primary gliomas [25–27], we investigated the effect of TERT promoter mutations on the prognosis of a cohort of 330 glioma patients in this study. As expected, TERT promoter mutations were closely associated with WHO grade, suggesting that these mutations may contribute to clinical outcomes of glioma patients. It is more noteworthy that these mutations predict poor patient survival, which was consistent with a previous study [11, 25–27]. These observations suggest that TERT promoter mutations play a critical role in glioma pathogenesis and are believed to be linked with the progression of this cancer.
As “mitotic clock”, telomere length reflects the life of differentiated somatic cells [28]. Over the years, telomere length measurement has been widely used as a marker for cell proliferation [3, 29]. Although the current findings are still in debate, telomere length variation is strongly implicated in the process of tumorigenesis [30]. In recent years, although there is still controversy, variable telomere length in peripheral blood leukocytes has been demonstrated to be associated with an increased risk of various cancers [31, 32], including gliomas [33]. However, only one study has addressed the link of telomere length and histopathologic parameters and patient survival in a limited number of glioblastomas, revealing that telomere length is associated with age and KPS score, as well as weakly contributes to patient survival [34]. In the present study, we measured telomere length in a cohort of 330 gliomas and investigated its association with clinopathologic characteristics and prognosis of glioma patients. Our data showed that telomere length was significantly correlated with age and tumor recurrence. Importantly, long telomere length is an independent factor for predicting poor patient survival. It is well known that patients with lower-grade gliomas always have longer survival times than patients with high-grade gliomas. However, in fact, a small number of patients with lower-grade gliomas have also very poor survival. In this study, we measured the RTL in 23 grade I tumors, which are considered to be “borderline tumor” and majority of scholars believe that it can be cured after surgery [1], and investigated the association of the RTL with patient survival. Our data demonstrated that the RTL significantly affected patient survival. None of patients with short RTL were dead, whereas 6 of 15 (40%) patients with long RTL were dead. These results suggest that telomere length may be an important predictor for clinical outcomes of the patients with low-grade gliomas.
Meanwhile, our data also showed that coexisting TERT promoter mutations and long RTL were significantly associated with poor patient survival than they were individually. Importantly, we observed that TERT promoter mutations particularly C228T and long telomere length significantly impacted radiotherapy outcome in glioma patients, which appears independent of WHO grade, KPS score, IDH1 mutations and MGMT methylation. Although the exact mechanisms remain unclear, we have sound reasons to believe that these two genetic alterations can be used as useful biomarkers for predicting outcome of postoperative radiotherapy in gliomas.
In summary, we investigated TERT promoter mutations and alteration of telomere length in a large cohort of primary gliomas in Northwest China, and demonstrated that these mutations and long telomere length were closely associated with aggressive tumor behaviors and poor patient survival. Moreover, to our knowledge, the present study is the first to show that TERT promoter mutations and long telomere length affect radiotherapy outcome in glioma patients. Collectively, these observations raise the possibility that these two molecular events may be one of major driving forces for the progression of glioma, and may be potentially valuable prognostic markers for glioma patients.
MATERIALS AND METHODS
Patients and tissue samples
Gliomas (n = 389), benign meningiomas (n = 50) and normal brain tissues (n = 8) (form cerebral contusion and laceration patients) were randomly obtained at the First Affiliated Hospital of Xi'an Jiaotong University and the Tangdu Hospital of The Fourth Military Medical University between January 2004 and September 2013. Histopathological diagnosis was confirmed by at least two senior neuropathologists according to the World Health Organization (WHO) classification [1]. This study was approved by the local ethics committee, and written informed consent was obtained from all patients.
DNA extraction
All tissues sections were reviewed by board-certified pathologists to ensure that ≥50% of the cells used for DNA purification were neoplastic. Genomic DNA was extracted from fresh or frozen tissues by using standard procedures of protease K digestion, phenol/chloroform extraction and ethanol precipitation. Genomic DNA was extracted from paraffin-embedded tissues as previously described [35]. Briefly, after a treatment for 8 to 10 h at room temperature with xylene to remove paraffin, tissues were digested with 1% sodium dodecyl sulfate (SDS) and 0.5 mg/ml proteinase K at 48°C for 48 h, with addition of several spiking aliquots of concentrated proteinase K to facilitate digestion. DNA was then isolated after standard phenol/chloroform extraction and ethanol precipitation protocols.
Detection of TERT promoter and IDH1 mutations
Two hot-spot somatic mutations (C228T and C250T) in the TERT core promoter were examined by pyrosequencing assay using the primers and conditions previously described [36]. Sanger sequencing was subsequently used to confirm the results of pyrosequencing using the same primer pair as for pyrosequencing assay. A fragment of 129 bp length spanning the catalytic domain of IDH1 including codon 132 was amplified by PCR with the following primers: 5′-CGG TCT TCA GAG AAG CCA TT-3′ (forward) and 5′-GCA AAA TCA CAT TAT TGC CAA C-3′ (reverse) as previously described [37]. PCR products were analyzed by Sanger sequencing.
Relative telomere length (RTL) measurement
Relative telomere length (RTL) was determined by real-time quantitative PCR as previously described [38]. Theoretically, the RTL can be measured using the primers that hybridize the telomeric hexamer repeats, because the number of binding sites for the primers increases as average telomere length increases. Two telomere primer sequences were as follows: tel-1,5′-CGG TTT GTT TGG GTT TGG GTT TGG GTT TGG GTT TGG GTT-3′, and tel-2,5′-GGC TTG CCT TAC CCT TAC CCT TAC CCT TAC CCT TAC CCT-3′. A single copy gene, 36B4, was used as a reference. The primer sequences were 5′-CAG CAA GTG GGA AGG TGT AAT CC-3′ (36B4u) and 5′-CCC ATT CTA TCA TCA ACG GGT ACA A-3′ (36B4d). The reactions were carried out in a total of volume of 20 μl containing 25 ng of genome DNA, 1 × Maxima SYBR Green (SYBR Premix Ex TaqTM II, TAKARA), 270 nM tel-1 and 900 nM tel-2 for telomere or 300 nM 36B4u and 500 nM 36B4d for reference gene 36B4. The standard curve was established using serial dilutions of normal leukocyte DNA with a quantity range of 3.12 to 50 ng. Amplification conditions and RTL measure were described previously [38].
Analysis of MGMT methylation
Genomic DNA was subjected to bisulfite treatment as described previously [35]. Next, Methylation-Specific PCR (MSP) was performed to evaluate methylation status of MGMT promoter with primers specific for either methylated or unmethylated DNA, as previously described [19]. The primer sequences for the methylated reaction were 5′-TTT CGA CGT TCG TAG GTT TTC GC-3′ (forward) and 5′-GCA CTC TTC CGA AAA CGA AAC G-3′ (reverse), and for the unmethylated reaction they were 5′-TTT GTG TTT TGA TGT TTG TAG GTT TTT GT-3′ (forward) and 5′-AAC TCC ACA CTC TTC CAA AAA CAA AAC A-3′ (reverse). The annealing temperature was 59°C. Normal leukocyte DNA treated in vitro with SssI methyltransferase (New England Biolabs, Beverly, MA) was used as a positive control for methylated alleles of MGMT, and untreated normal leukocyte DNA was used as a negative control. The PCR products were electrophoresed on a 1.2% agarose gel and visualized under UV illumination using an ethidium bromide stain.
Immunohistochemical (IHC) analysis of ATRX
IHC assays were performed as previously described [39]. In brief, paraffin-embedded sections (5 μm) were deparaffinized and rehydrated in a graded series of ethanol, and washed in distilled water. After antigen retrieval and blocking, the sections were incubated with anti-ATRX antibody (Abcam, ab97508; 1:300 dilution) overnight at 4°C. Slides were then counterstained with hematoxylin. The mutational status of TERT promoter was scored in a double-blinded manner.
Statistical analysis
The Mann-Whitney U test was used to compare the RTL between glioma tissues and control subjects. The correlation between TERT promoter mutations and the RTL with clinicpathological characteristics was analyzed by Fisher's exact probability test (two-sided) or Chi-square test. Multivariate models were developed that adjusted for the most important covariates, including gender, age, WHO grade, pathological, disease recurrence, seizures, KPS score, IDH1 mutation and MGMT methylation. Survival length was determined from the day of primary tumor surgery to the day of death or last clinical follow-up. Kaplan–Meier method was used for survival analysis grouping with TERT promoter mutations or appropriate cut-off value of RTL. Differences between curves were analyzed using the log-rank test. Multivariate Cox regression analysis was used to evaluate the effect of TERT promoter mutations and the RTL on survival and resistance to radiotherapy. All statistical analyses were performed using the SPSS statistical package (11.5, Chicago, IL, USA). P values < 0.05 were considered significant.
SUPPLEMENTARY FIGURES
ACKNOWLEDGMENTS AND FUNDING
This work was supported by the National Natural Science Foundation of China (No. 81272933, 81372217and 81472622), the Fundamental Research Funds for the Central Universities.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn EH. Structure and function of telomeres. Nature. 1991;350:569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 4.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 5.Hiraga S, Ohnishi T, Izumoto S, Miyahara E, Kanemura Y, Matsumura H, Arita N. Telomerase activity and alterations in telomere length in human brain tumors. Cancer Res. 1998;58:2117–2125. [PubMed] [Google Scholar]
- 6.Kyo S, Takakura M, Fujiwara T, Inoue M. Understanding and exploiting hTERT promoter regulation for diagnosis and treatment of human cancers. Cancer Sci. 2008;99:1528–1538. doi: 10.1111/j.1349-7006.2008.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957–959. doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, Kadel S, Moll I, Nagore E, Hemminki K, Schadendorf D, Kumar R. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339:959–961. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 9.Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, Diaz LA, Jr, Friedman AH, Friedman H, Gallia GL, Giovanella BC, Grollman AP, He TC, He Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110:6021–6026. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vinagre J, Almeida A, Populo H, Batista R, Lyra J, Pinto V, Coelho R, Celestino R, Prazeres H, Lima L, Melo M, da Rocha AG, Preto A, et al. Frequency of TERT promoter mutations in human cancers. Nat Commun. 2013;4:2185. doi: 10.1038/ncomms3185. [DOI] [PubMed] [Google Scholar]
- 11.Nonoguchi N, Ohta T, Oh JE, Kim YH, Kleihues P, Ohgaki H. TERT promoter mutations in primary and secondary glioblastomas. Acta Neuropathol. 2013;126:931–937. doi: 10.1007/s00401-013-1163-0. [DOI] [PubMed] [Google Scholar]
- 12.Arita H, Narita Y, Fukushima S, Tateishi K, Matsushita Y, Yoshida A, Miyakita Y, Ohno M, Collins VP, Kawahara N, Shibui S, Ichimura K. Upregulating mutations in the TERT promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol. 2013;126:267–276. doi: 10.1007/s00401-013-1141-6. [DOI] [PubMed] [Google Scholar]
- 13.Bell RJ, Rube HT, Kreig A, Mancini A, Fouse SF, Nagarajan RP, Choi S, Hong C, He D, Pekmezci M, Wiencke JK, Wrensch MR, Chang SM, et al. The transcription factor GABP selectively binds and activates the mutant TERT promoter in cancer. Science. 2015;348:1036–1039. doi: 10.1126/science.aab0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stupp R, Roila F, Group EGW. Malignant glioma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;4:126–128. doi: 10.1093/annonc/mdp151. [DOI] [PubMed] [Google Scholar]
- 15.Cancer Genome Atlas Research N. Brat DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR, Cooper LA, Rheinbay E, Miller CR, Vitucci M, Morozova O, Robertson AG, Noushmehr H, et al. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N Engl J Med. 2015;372:2481–2498. doi: 10.1056/NEJMoa1402121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heaphy CM, de Wilde RF, Jiao Y, Klein AP, Edil BH, Shi C, Bettegowda C, Rodriguez FJ, Eberhart CG, Hebbar S, Offerhaus GJ, McLendon R, Rasheed BA, et al. Altered telomeres in tumors with ATRX and DAXX mutations. Science. 2011;333:425. doi: 10.1126/science.1207313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heidenreich B, Rachakonda PS, Hosen I, Volz F, Hemminki K, Weyerbrock A, Kumar R. TERT promoter mutations and telomere length in adult malignant gliomas and recurrences. Oncotarget. 2015;6:10617–10633. doi: 10.18632/oncotarget.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smits A, Duffau H. Seizures and the natural history of World Health Organization Grade II gliomas: a review. Neurosurgery. 2011;68:1326–1333. doi: 10.1227/NEU.0b013e31820c3419. [DOI] [PubMed] [Google Scholar]
- 19.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 20.Esteller M, Garcia-Foncillas J, Andion E, Goodman SN, Hidalgo OF, Vanaclocha V, Baylin SB, Herman JG. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343:1350–1354. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- 21.Bleeker FE, Atai NA, Lamba S, Jonker A, Rijkeboer D, Bosch KS, Tigchelaar W, Troost D, Vandertop WP, Bardelli A, Van Noorden CJ. The prognostic IDH1(R132) mutation is associated with reduced NADP+-dependent IDH activity in glioblastoma. Acta Neuropathol. 2010;119:487–494. doi: 10.1007/s00401-010-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Bishop J, Shan Y, Pai S, Liu D, Murugan AK, Sun H, El-Naggar AK, Xing M. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr Relat Cancer. 2013;20:603–610. doi: 10.1530/ERC-13-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rachakonda PS, Hosen I, de Verdier PJ, Fallah M, Heidenreich B, Ryk C, Wiklund NP, Steineck G, Schadendorf D, Hemminki K, Kumar R. TERT promoter mutations in bladder cancer affect patient survival and disease recurrence through modification by a common polymorphism. Proc Natl Acad Sci U S A. 2013;110:17426–17431. doi: 10.1073/pnas.1310522110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qu Y, Dang S, Wu K, Shao Y, Yang Q, Ji M, Shi B, Hou P. TERT promoter mutations predict worse survival in laryngeal cancer patients. Int J Cancer. 2014;135:1008–1010. doi: 10.1002/ijc.28728. [DOI] [PubMed] [Google Scholar]
- 25.Eckel-Passow JE, Lachance DH, Molinaro AM, Walsh KM, Decker PA, Sicotte H, Pekmezci M, Rice T, Kosel ML, Smirnov IV, Sarkar G, Caron AA, Kollmeyer TM, et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. N Engl J Med. 2015;372:2499–2508. doi: 10.1056/NEJMoa1407279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simon M, Hosen I, Gousias K, Rachakonda S, Heidenreich B, Gessi M, Schramm J, Hemminki K, Waha A, Kumar R. TERT promoter mutations: a novel independent prognostic factor in primary glioblastomas. Neuro Oncol. 2015;17:45–52. doi: 10.1093/neuonc/nou158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mosrati MA, Malmstrom A, Lysiak M, Krysztofiak A, Hallbeck M, Milos P, Hallbeck AL, Bratthall C, Strandeus M, Stenmark-Askmalm M, Soderkvist P. TERT promoter mutations and polymorphisms as prognostic factors in primary glioblastoma. Oncotarget. 2015;6:16663–16673. doi: 10.18632/oncotarget.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanza RP, Cibelli JB, Blackwell C, Cristofalo VJ, Francis MK, Baerlocher GM, Mak J, Schertzer M, Chavez EA, Sawyer N, Lansdorp PM, West MD. Extension of cell life-span and telomere length in animals cloned from senescent somatic cells. Science. 2000;288:665–669. doi: 10.1126/science.288.5466.665. [DOI] [PubMed] [Google Scholar]
- 29.Counter CM, Avilion AA, LeFeuvre CE, Stewart NG, Greider CW, Harley CB, Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992;11:1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu L, Li S, Stohr BA. The role of telomere biology in cancer. Annu Rev Pathol. 2013;8:49–78. doi: 10.1146/annurev-pathol-020712-164030. [DOI] [PubMed] [Google Scholar]
- 31.Wentzensen IM, Mirabello L, Pfeiffer RM, Savage SA. The association of telomere length and cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20:1238–1250. doi: 10.1158/1055-9965.EPI-11-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hou L, Zhang X, Gawron AJ, Liu J. Surrogate tissue telomere length and cancer risk: shorter or longer? Cancer Lett. 2012;319:130–135. doi: 10.1016/j.canlet.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 33.Wang S, Chen Y, Qu F, He S, Huang X, Jiang H, Jin T, Wan S, Xing J. Association between leukocyte telomere length and glioma risk: a case-control study. Neuro Oncol. 2014;16:505–512. doi: 10.1093/neuonc/not240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lotsch D, Ghanim B, Laaber M, Wurm G, Weis S, Lenz S, Webersinke G, Pichler J, Berger W, Spiegl-Kreinecker S. Prognostic significance of telomerase-associated parameters in glioblastoma: effect of patient age. Neuro Oncol. 2013;15:423–432. doi: 10.1093/neuonc/nos329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi J, Zhang G, Yao D, Liu W, Wang N, Ji M, He N, Shi B, Hou P. Prognostic significance of aberrant gene methylation in gastric cancer. Am J Cancer Res. 2012;2:116–129. [PMC free article] [PubMed] [Google Scholar]
- 36.Qu Y, Shi L, Wang D, Zhang B, Yang Q, Ji M, Shi B, Hou P. Low frequency of TERT promoter mutations in a large cohort of gallbladder and gastric cancers. Int J Cancer. 2014;134:2993–2994. doi: 10.1002/ijc.28633. [DOI] [PubMed] [Google Scholar]
- 37.Balss J, Meyer J, Mueller W, Korshunov A, Hartmann C, von Deimling A. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008;116:597–602. doi: 10.1007/s00401-008-0455-2. [DOI] [PubMed] [Google Scholar]
- 38.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi J, Liu W, Sui F, Lu R, He Q, Yang Q, Lv H, Shi B, Hou P. Frequent amplification of AIB1, a critical oncogene modulating major signaling pathways, is associated with poor survival in gastric cancer. Oncotarget. 2015;6:14344–14359. doi: 10.18632/oncotarget.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


