Abstract
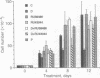
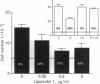
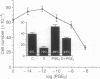
The synthetic glucocorticoid dexamethasone (1 microM to 1 pM) strongly (maximum greater than 80%) inhibits proliferation of the A549 human lung adenocarcinoma line (EC50 greater than 1 nM) and leads to the appearance, or a further increase (approximately 3-fold) in the expression on the cell surface, of the calcium and phospholipid binding protein lipocortin (annexin) 1. Both these effects, which are shared by hydrocortisone (1 microM) but not by progesterone or aldosterone (1 microM), are inhibited by the antiglucocorticoids RU38486 and RU43044 (1 microM). The nonsteroidal antiinflammatory drugs indomethacin (1 microM) and naproxen (10 microM) and human recombinant lipocortin 1 (0.05-5.0 micrograms/ml) also produce growth arrest in this cell line. During proliferation A549 cells spontaneously release prostaglandin E2 [10-20 ng (28-57 pmol) per ml per 5-day period] into the growth medium. In concentrations that cause growth-arrest, dexamethasone, indomethacin, and lipocortin 1 abolish the generation of this eicosanoid by A549 cells. Prostaglandin E2 itself (0.01-1 pM) stimulates cell growth and partially reverses (approximately 50%) the inhibition of growth caused by dexamethasone and indomethacin. Addition of the neutralizing anti-lipocortin 1 monoclonal antibody 1A (5 micrograms/ml), but not the nonneutralizing anti-lipocortin monoclonal antibody 1B, substantially reversed (greater than 80%) the inhibitory activity of dexamethasone on both growth and prostaglandin E2 synthesis. The generation of prostaglandin E2 by A549 cells seems to be an important regulator of cell proliferation in vitro and the dexamethasone-induced suppression of proliferation in this model is attributable to eicosanoid inhibition caused by lipocortin 1.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULLOUGH W. S. Hormones and mitotic activity. Vitam Horm. 1955;(13):261–292. doi: 10.1016/s0083-6729(08)61028-6. [DOI] [PubMed] [Google Scholar]
- Bailey J. M. New mechanisms for effects of anti-inflammatory glucocorticoids. Biofactors. 1991 Jun;3(2):97–102. [PubMed] [Google Scholar]
- Blackwell G. J., Carnuccio R., Di Rosa M., Flower R. J., Parente L., Persico P. Macrocortin: a polypeptide causing the anti-phospholipase effect of glucocorticoids. Nature. 1980 Sep 11;287(5778):147–149. doi: 10.1038/287147a0. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D. Calpactin in exocytosis. Nature. 1988 Jan 7;331(6151):20–20. doi: 10.1038/331020a0. [DOI] [PubMed] [Google Scholar]
- Carey F., Forder R., Edge M. D., Greene A. R., Horan M. A., Strijbos P. J., Rothwell N. J. Lipocortin 1 fragment modifies pyrogenic actions of cytokines in rats. Am J Physiol. 1990 Aug;259(2 Pt 2):R266–R269. doi: 10.1152/ajpregu.1990.259.2.R266. [DOI] [PubMed] [Google Scholar]
- Cirino G., Flower R. J. Human recombinant lipocortin 1 inhibits prostacyclin production by human umbilical artery in vitro. Prostaglandins. 1987 Jul;34(1):59–62. doi: 10.1016/0090-6980(87)90262-0. [DOI] [PubMed] [Google Scholar]
- Cirino G., Peers S. H., Flower R. J., Browning J. L., Pepinsky R. B. Human recombinant lipocortin 1 has acute local anti-inflammatory properties in the rat paw edema test. Proc Natl Acad Sci U S A. 1989 May;86(9):3428–3432. doi: 10.1073/pnas.86.9.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyran J. A., Lysz T. W., Lea M. A. Influence of inhibitors of eicosanoid metabolism on proliferation of rat hepatoma cells and on tumor-host interaction. Prostaglandins Leukot Essent Fatty Acids. 1990 Apr;39(4):311–317. doi: 10.1016/0952-3278(90)90011-9. [DOI] [PubMed] [Google Scholar]
- Davidson J., Flower R. J., Milton A. S., Peers S. H., Rotondo D. Antipyretic actions of human recombinant lipocortin-1. Br J Pharmacol. 1991 Jan;102(1):7–9. doi: 10.1111/j.1476-5381.1991.tb12122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errasfa M., Russo-Marie F. A purified lipocortin shares the anti-inflammatory effect of glucocorticosteroids in vivo in mice. Br J Pharmacol. 1989 Aug;97(4):1051–1058. doi: 10.1111/j.1476-5381.1989.tb12561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower R. J. Eleventh Gaddum memorial lecture. Lipocortin and the mechanism of action of the glucocorticoids. Br J Pharmacol. 1988 Aug;94(4):987–1015. doi: 10.1111/j.1476-5381.1988.tb11614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller P. J., Verity K. Somatostatin gene expression in the thymus gland. J Immunol. 1989 Aug 1;143(3):1015–1017. [PubMed] [Google Scholar]
- Goulding N. J., Godolphin J. L., Sharland P. R., Peers S. H., Sampson M., Maddison P. J., Flower R. J. Anti-inflammatory lipocortin 1 production by peripheral blood leucocytes in response to hydrocortisone. Lancet. 1990 Jun 16;335(8703):1416–1418. doi: 10.1016/0140-6736(90)91445-g. [DOI] [PubMed] [Google Scholar]
- Goulding N. J., Luying P., Guyre P. M. Characteristics of lipocortin 1 binding to the surface of human peripheral blood leucocytes. Biochem Soc Trans. 1990 Dec;18(6):1237–1238. doi: 10.1042/bst0181237. [DOI] [PubMed] [Google Scholar]
- Haigler H. T., Christmas P. Annexin 1 is secreted by the human prostate. Biochem Soc Trans. 1990 Dec;18(6):1104–1106. doi: 10.1042/bst0181104. [DOI] [PubMed] [Google Scholar]
- Hofert J. F., White A. Effect of a single injection of cortisol on the incorporation of 3H-thymidine and 3H-deoxycytidine into lymphatic tissue DNA of adrenalectomized rats. Endocrinology. 1968 Apr;82(4):767–776. doi: 10.1210/endo-82-4-767. [DOI] [PubMed] [Google Scholar]
- Hofert J. F., White A. Inhibitory effect of a liver extract on the incorporation of 3H-deoxycytidine into thymus DNA of adrenalectomized and adrenalectomized-hepatectomized rats. Endocrinology. 1968 Apr;82(4):777–785. doi: 10.1210/endo-82-4-777. [DOI] [PubMed] [Google Scholar]
- Isacke C. M., Lindberg R. A., Hunter T. Synthesis of p36 and p35 is increased when U-937 cells differentiate in culture but expression is not inducible by glucocorticoids. Mol Cell Biol. 1989 Jan;9(1):232–240. doi: 10.1128/mcb.9.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivarie R. D., Baxter J. D., Morris J. A. Interaction of thyroid and glucocorticoid hormones in rat pituitary tumor cells. Specificity and diversity of the responses analyzed by two-dimensional gel electrophoresis. J Biol Chem. 1981 May 10;256(9):4520–4528. [PubMed] [Google Scholar]
- Ivarie R. D., O'Farrell P. H. The glucocorticoid domain: steroid-mediated changes in the rate of synthesis of rat hepatoma proteins. Cell. 1978 Jan;13(1):41–55. doi: 10.1016/0092-8674(78)90136-8. [DOI] [PubMed] [Google Scholar]
- Karasik A., Pepinsky R. B., Shoelson S. E., Kahn C. R. Lipocortins 1 and 2 as substrates for the insulin receptor kinase in rat liver. J Biol Chem. 1988 Aug 25;263(24):11862–11867. [PubMed] [Google Scholar]
- Loeb J. N., Borek C., Yeung L. L. Suppression of DNA synthesis in hepatoma cells exposed to glucocorticoid hormone in vitro. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3852–3856. doi: 10.1073/pnas.70.12.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makman M. H., Dvorkin B., White A. Influence of cortisol on the utilization of precursors of nucleic acids and protein by lymphoid cells in vitro. J Biol Chem. 1968 Apr 10;243(7):1485–1497. [PubMed] [Google Scholar]
- Mukherjee A. B., Waite R. G., Cohen M. M., Bernstein R. Incorporation of uridine- 3 H and sodium acetate- 14 C in lymphocytes derived from normal and leukemic individuals. Cancer Res. 1972 Sep;32(9):1833–1836. [PubMed] [Google Scholar]
- Munck A. Glucocorticoid inhibition of glucose uptake by peripheral tissues: old and new evidence, molecular mechanisms, and physiological significance. Perspect Biol Med. 1971 Winter;14(2):265–269. doi: 10.1353/pbm.1971.0002. [DOI] [PubMed] [Google Scholar]
- Pepinsky R. B., Sinclair L. K., Dougas I., Liang C. M., Lawton P., Browning J. L. Monoclonal antibodies to lipocortin-1 as probes for biological function. FEBS Lett. 1990 Feb 26;261(2):247–252. doi: 10.1016/0014-5793(90)80564-y. [DOI] [PubMed] [Google Scholar]
- Pepinsky R. B., Sinclair L. K. Epidermal growth factor-dependent phosphorylation of lipocortin. Nature. 1986 May 1;321(6065):81–84. doi: 10.1038/321081a0. [DOI] [PubMed] [Google Scholar]
- Pratt W. B., Aronow L. The effect of glucocorticoids on protein and nucleic acid synthesis in mouse fibroblasts growing in vitro. J Biol Chem. 1966 Nov 25;241(22):5244–5250. [PubMed] [Google Scholar]
- Relton J. K., Strijbos P. J., O'Shaughnessy C. T., Carey F., Forder R. A., Tilders F. J., Rothwell N. J. Lipocortin-1 is an endogenous inhibitor of ischemic damage in the rat brain. J Exp Med. 1991 Aug 1;174(2):305–310. doi: 10.1084/jem.174.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen J. M., Fina J. R., Milholland J., Rosen F. Inhibition of glucose uptake in lymphosarcoma P1798 by cortisol and its relationship to the biosynthesis of deoxyribonucleic acid. J Biol Chem. 1970 Apr 25;245(8):2074–2080. [PubMed] [Google Scholar]
- Römisch J., Schorlemmer U., Fickenscher K., Pâques E. P., Heimburger N. Anticoagulant properties of placenta protein 4 (annexin V). Thromb Res. 1990 Dec 1;60(5):355–366. doi: 10.1016/0049-3848(90)90218-2. [DOI] [PubMed] [Google Scholar]
- Schlaepfer D. D., Haigler H. T. Expression of annexins as a function of cellular growth state. J Cell Biol. 1990 Jul;111(1):229–238. doi: 10.1083/jcb.111.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. F., Tetley T. D., Guz A., Flower R. J. Detection of lipocortin 1 in human lung lavage fluid: lipocortin degradation as a possible proteolytic mechanism in the control of inflammatory mediators and inflammation. Environ Health Perspect. 1990 Apr;85:135–144. doi: 10.1289/ehp.85-1568329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland R. L., Reddel R. R., Green M. D. Effects of oestrogens on cell proliferation and cell cycle kinetics. A hypothesis on the cell cycle effects of antioestrogens. Eur J Cancer Clin Oncol. 1983 Mar;19(3):307–318. doi: 10.1016/0277-5379(83)90127-x. [DOI] [PubMed] [Google Scholar]
- Violette S. M., King I., Browning J. L., Pepinsky R. B., Wallner B. P., Sartorelli A. C. Role of lipocortin I in the glucocorticoid induction of the terminal differentiation of a human squamous carcinoma. J Cell Physiol. 1990 Jan;142(1):70–77. doi: 10.1002/jcp.1041420110. [DOI] [PubMed] [Google Scholar]
- Wallner B. P., Mattaliano R. J., Hession C., Cate R. L., Tizard R., Sinclair L. K., Foeller C., Chow E. P., Browing J. L., Ramachandran K. L. Cloning and expression of human lipocortin, a phospholipase A2 inhibitor with potential anti-inflammatory activity. Nature. 1986 Mar 6;320(6057):77–81. doi: 10.1038/320077a0. [DOI] [PubMed] [Google Scholar]
- William F., Mroczkowski B., Cohen S., Kraft A. S. Differentiation of HL-60 cells is associated with an increase in the 35-kDa protein lipocortin I. J Cell Physiol. 1988 Dec;137(3):402–410. doi: 10.1002/jcp.1041370303. [DOI] [PubMed] [Google Scholar]