Abstract
Anticancer potential of metformin has been extensively studied. However, its anticancer clinical use remains yet to be approved since sufficient concentration on target organs could not be achieved via conventional administration. To overcome this drawback, we aim to examine the efficiency of novel intravesical treatment of metformin on syngeneic orthotopic preclinical model. Three human and one murine bladder cancer cell lines were tested in vitro for inhibitory sensitivity by MTT and cologenic assays. AMPK pathway including AKT, Erk and S6K was examined by western blot and further explored by regulating activated levels using specific inhibitors. In vivo efficacy was determined by Kaplan-Meier survival curves and measurements of body and bladder weights plus tumor biomarkers. Lactic acid and metformin levels of plasma were measured by standard procedures. The results demonstrated that metformin activated AMPK and decreased phosphorylation of Akt and Erk. Furthermore, combinations of metformin with either Akt or Erk inhibitors synergistically diminished cancer proliferation, suggesting the involvement of Akt- and Erk- related pathways. Intravesical metformin 26 and 104 mg/kg, twice per week demonstrated a rapid elimination of the implanted tumor without any evidence of toxicity. In contrast, oral treatment at a dose of 800mg/kg/d exhibited little efficacy whereas severe toxicity existed if the dosage is higher. Collectively, intravesical metformin displays potent inhibition on bladder cancer in vitro and this preclinical study reveals the profound therapeutic application of metformin with durable tolerance via intravesical administration route.
Keywords: metformin, bladder cancer, localized administration, AMPK, preclinical model
INTRODUCTION
Bladder cancer is one of the most frequent cancers of the urinary tract, accounting for about 74,000 new cases and 16,000 deaths in the United States in 2015[1]. Even though transurethral resection has served as the standard treatment, recurrence and metastasis are often seen in clinic [2]. The commonest way to prevent recurrence and progression is supplemented with intravesical chemotherapy or immunosuppressive agents [3–4]. However, these methods are largely restricted with various degrees of side effects such as bone marrow suppression, allergic reactions and etc[5]. Thus, there is an unmet demand to discover safe and effective drugs for treating bladder cancer.
Abnormality of metabolism is one typical characteristic of tumors proposed in Warburg effect. There is renewed interest in developing novel anti-cancer breakthroughs modulating metabolism to limit neoplastic growth. Tumorigenesis is a multistep process and tumor cells often display alterations in their energy metabolism to support rapid growth [6]. Metabolic reprogramming might be a profound strategy for cancer treatment. Metformin, a widely prescribed drug for treating type II diabetes, is one of the most extensively recognized metabolism modulators. Epidemiological evidence indicates that metformin has a high potential efficacy as an anti-cancer drug [7–10]. Data collected from various xenograft cancer models suggest that metformin delays the progression and relapse of cancer but no much research in bladder cancer is available [11–12].
Moreover, over two hundreds of clinical trials on anticancer studies have either been completed or are on going. However, no positive clinical results have been reported yet. Concentration of metformin on target organs is probably the major obstacle for these unsatisfactory results [13]. We notice that oral administration is the general treatment route in these bench work studies and clinical trials. The maximum concentration of metformin in circulating system is less than 60 μM via oral administration, far less away from 2 mM, the minimum effective concentration in vitro [14]. Moreover, increase of oral dosage of metformin causes the risk of lactic acidosis, having a vicious circle with the lactic acidosis produced by tumors via anaerobic glycolysis. Therefore, alternative administration to achieve an effective dose is critical [13].
Thus, in this present study, we aim to explore the efficacy of metformin using intravesical administration to treat bladder cancer. To achieve this aim, MB49, the popular murine bladder cancer cell line, was applied to establish syngeneic orthotopic model. This study provides an effective strategy to eradicate bladder cancer.
RESULTS
Metformin inhibits bladder cancer cell proliferation
These cell lines were exposed to 0. 5∼64 mM metformin. Interestingly, metformin generally promoted cell growth at less than 0.5mM concentration, then turned to a dose-dependent inhibition of cell proliferation. The most sensitive cell line is UMUC3 with IC50 8.25mM while the most resistant one is J82 (IC5030.24mM) (Figure 1, Table 1).
Figure 1. Treatment with metformin on cell proliferation of various bladder cancer cell lines.
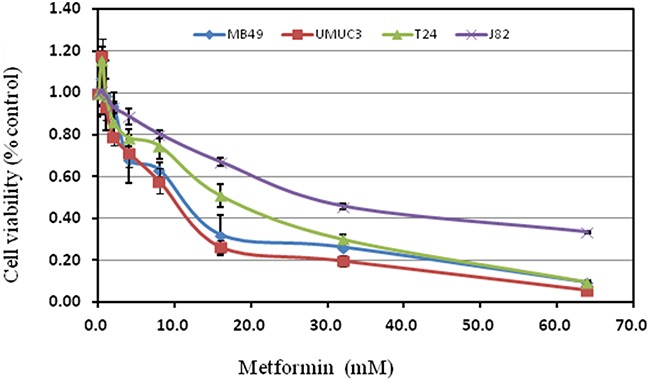
Cell viability was assessed with 48 hour metformin treatment at concentrations ranging from 0 to 64mM on human bladder cancer cell lines T24, UMUC3, J82 and murine bladder cancer cell line MB49 using a tetrazolium-based assay. Results are presented as the median of 5 independent experiments.
Table 1. Inhibitory concentration 50%(IC50) for metformin.
| Metformin(mM) | Bladder cancer cell lines | ||||
| MB49 | UMUC3 | T24 | J82 | ||
| 10.41 | 8.25 | 14.25 | 30.24 | ||
Metformin suppresses colony formation
Colony formation was examined in the presence of metformin with either regular continuous or intermittent fashion. In regular continuous fashion, it was found that 0.5mM metformin favored the colony formation as shown in Figure 2A, Supplementary Figure S1. However, suppressive effect increased when the concentration of metformin was higher than 2mM in T24 and UMUC3 cell lines. Inhibitory effect in J82 was not significant, showing similar pattern observed in proliferation assay described above.
Figure 2. Evaluation of colony suppression of metformin on bladder cancer cell lines.
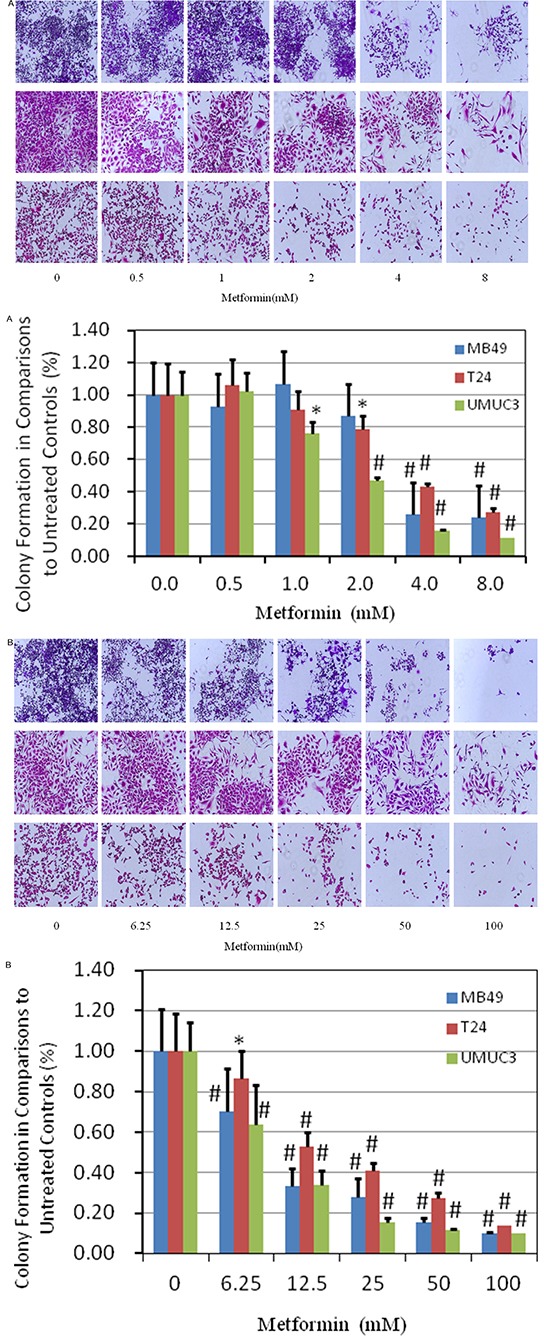
A. clonogenic assay was assessed after 7 day metformin treatment at various concentrations and stained with crystal violet at the end of the experiment. Above: images were taken through an inverted microscope with ×10 magnification. Below: the quantification of colony was determined by microplate area scan at OD 550nm, Results are presented as the median of 5 independent experiments (*P<0.05, #P<0.01). B. colony formation assay was carried out with two hour treatment at labeled concentrations, twice per week for two weeks and stained with crystal violet at the end of the experiment. Above: images were taken through an inverted microscope with ×10 magnification. Below: the quantification of colony was determined by microplate area scan at OD 550nm, Results are presented as the median of 5 independent experiments (*P<0.05, #P<0.01).
To mimic intravesical treatment, we designed an intermittent treatment protocol with 2 hour metformin incubation twice per week for two weeks (Figure 2B, Supplementary Figure S2). Understandably, much higher concentration of metformin is needed for inhibiting the colony formation, compared with that in continuous fashion in general (Figure 2B, Supplementary Figure S2). MB49 and UMUC3 were more sensitive than T24, same trend observed in continuous fashion. The dramatic suppression on colony formation of intermittent treatment imply that intravesical treatment is applicable to cause significant cell killing effects.
Metformin activates AMPK
AMPK, a central energy sensor, is known as a crucial factor in the interaction between metabolism and cancer [15]. As the activation of AMPK correlates tightly with phosphorylation at Thr-172 (p-AMPKα), we assessed activation of AMPK by determining phosphorylation AMPKα and its primary downstream targeting enzyme such as p-p70s6k, p-4EBP1 and p-ACC. As shown in Figure 3A–3C, metformin activated AMPK markedly and decreased the phosphorylation of downstream proteins including 4EBP1 and P70S6K. In the meantime, metformin increases the protein level of p-ACC then inhibits FASN, its downstream of AMPK-ACC, consistent with previous reports [16–17].
Figure 3. Effects of metformin on AMPK intracellular signaling pathways in three bladder cancer cell lines MB49, T24, and UMUC3.
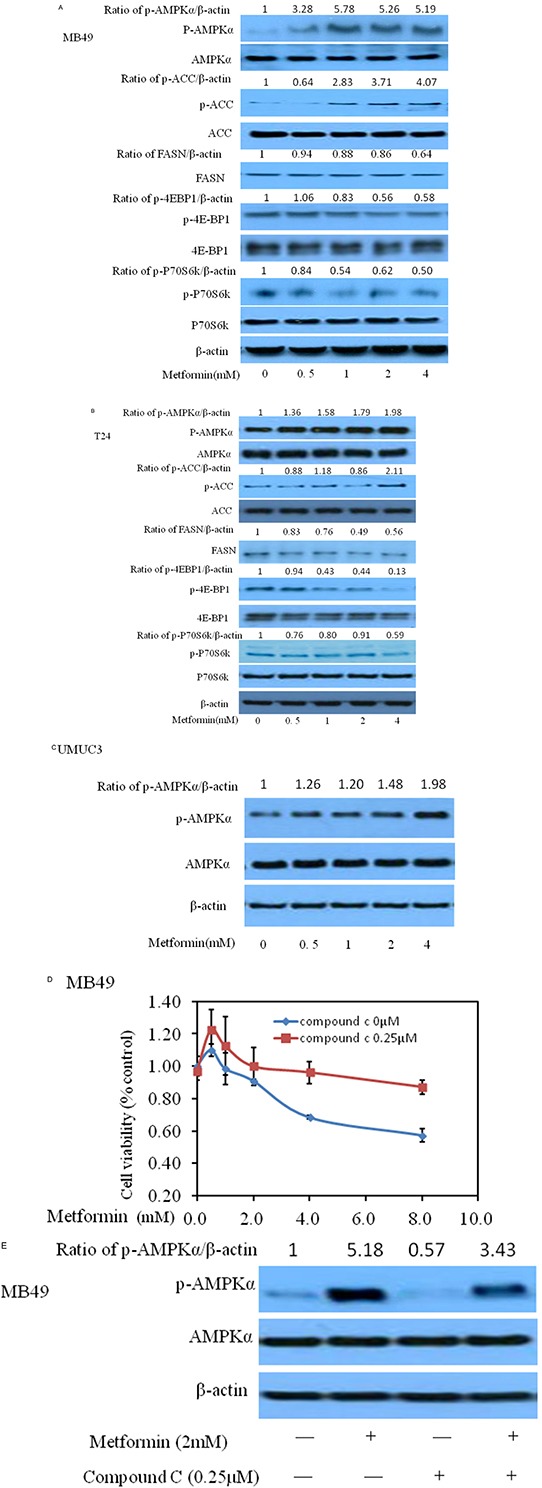
Panels A–C represent western blottings of p-AMPK, p-ACC, FASN, p-p70S6K, p-4EBP1, t-AMPK, t-ACC, t-p70S6K and t-4EBP1 of three bladder cancer cell lines MB49, T24, and UMUC3, respectively. β-actin was included as a loading control. The ratio of different proteins to β-actin was calculated by the band density of Western blots of each cell line using Image J software. D. Cell viability was assessed with 48 hour metformin alone or metformin combined with 0.25μM AMPK antagonist compound C treatment. Compound C significantly reduced the decrease of cell proliferation caused by metformin. E. Western blotting of p-AMPK and t-AMPK after treatment of either metformin or/and compound C.
Next, we added AMPK specific inhibitor compound C to determine whether activating AMPK signaling pathway is essential for metformin to exert anti-tumor effect. As shown in Figure 3D, the ability of metformin to inhibit MB49 cancer cell proliferation was reduced after adding compound C, implying that inhibition of AMPK accelerates proliferation of cancer cells, consistent with western blot results (Figure 3E). Similar trends of human bladder cancer cells T24 and UMUC3 were observed (Supplementary Figures S3 & S4).
Both AKT and ERK signaling pathways participate in inhibitory effect of metformin on bladder cancer cell growth
Phosphorylated AKT and ERK are key intracellular mediators of cell survival and proliferation signals. Recent findings have confirmed that activated mutation of PI3k is associated with improved recurrence-free survival [18–20]. Thus, inhibition of Akt, the downstream of PI3K pathway, would be beneficial to block cancer cell growth [21–22]. Examining these molecular pathways during metformin treatment is meaningful for us to elaborate its anticancer mechanism. As shown in Figure 4A–4C, metformin decreased the phosphorylation of Akt and/or Erk1/2 at higher than 0.5 mM with no significant change on the protein levels of total Erk1/2 and AKT, in MB49, T24 and UMUC3, indicating that the inhibitory effect of metformin is correlated to the PI3K and Erk signaling pathways. A slight increase of phosphorylation of Erk1/2 at 0.5 mM in MB49 and UMUC3 cells is associated with the results of increase of colony formation at this concentration in Figure 2A. There was no significant difference of these signaling pathways among these cell lines. These data prompted us to further probe the role of Erk1/2 and AKT pathway by these inhibitors. As shown in Figure 5A and 5C, either AZD6244, the inhibitor of Erk1/2, or MK2206, the inhibitor of AKT exerts synergistic inhibition of cancer cell growth with metformin respectively, in a dose dependent fashion. This indicated that both Erk1/2 and AKT pathways got involved in events of inhibition of metformin. Interestingly, western blot results demonstrate that MK2206 alone increases protein level of phospho-AMPK while AZD6244 does not, indicating that inhibition of Akt may exert a different effect on phospho-AMPK, compared to inhibition of Erk, which warrants further investigation (Figure 5B and 5D). However, both MK2206 and AZD6244 amplify metformin-induced phosphorylation of AMPK (Figure 5B and 5D). Inhibition of either AKT or Erk1/2 will produce synergistical effect with metformin but MK2206 and AZD6244 may exert these effects via different molecular ways which also warrant further investigation.
Figure 4. Effects of metformin on AKT and ERK in three bladder cancer cell lines MB49, T24, and UMUC3.
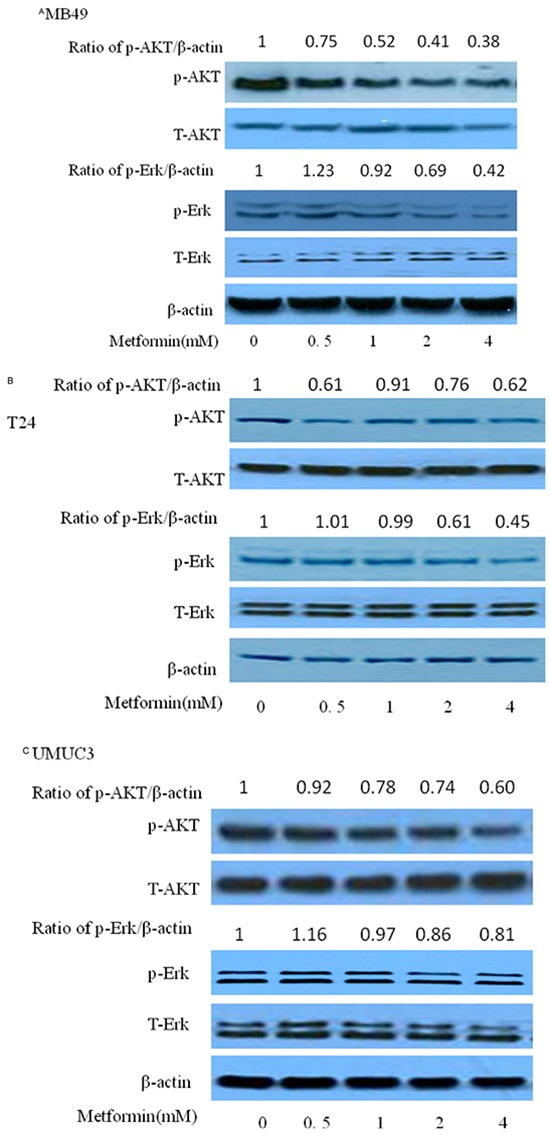
Western blotting of p-AKT, p-ERK, t-AKT and t-ERK of MB49 A. T24 B. and UMUC3 C., respectively. β-actin was included as a loading control.
Figure 5. Effects of metformin on MB49 combined with ERK or AKT specific drugs.
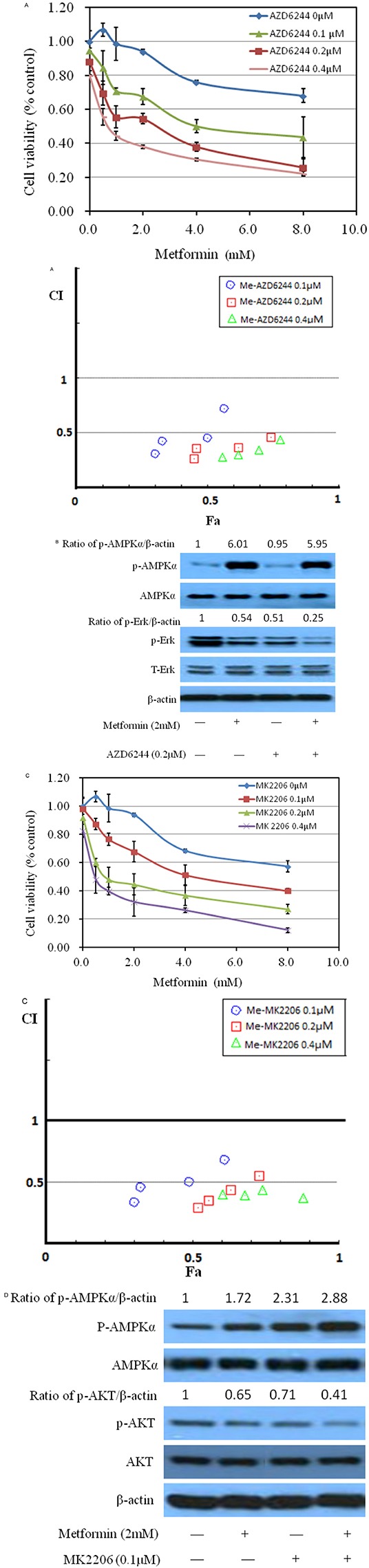
A. Metformin combined with ERK specific inhibitor AZD6244 inhibited MB49 proliferation synergistically. Above: Cell viability was assessed with 48 hour metformin at 0, 1, 2, 4, 8mM alone or metformin combined with various concentrations (0.1, 0.2, 0.4μM) of AZD6244 treatment. Below: Combination index (CI) among the combinations of two drugs were calculated using CompuSyn software. If CI = 1, it denotes additivity; if CI > 1, it denotes antagonism; if CI < 1, it denotes synergism. CI values in the vast majority of combinations weres less than 0.5, indicating moderately strong synergism. B. Western blotting of p-ERK, t-ERK, p-AMPK and t-AMPK following treatment with metformin at 2mM and AZD6244 at 0.2μM in MB49. β-actin was included as a loading control. C. Metformin combined with AKT specific inhibitor MK2206 inhibited MB49 proliferation synergistically. Cell viability was assessed with 48 hour metformin at 0, 1, 2, 4, 8mM alone or metformin combined with indicated concentrations of MK2206 treatment. Combination index (CI) were calculated. D. Western blotting of p-AKT, p-AMPK, t-AKT and t-AMPK following treatment with the indicated concentration of metformin and AZD6244 in MB49. β-actin was included as a loading control.
Intravesical rather than oral administration of metformin exert potent anti-cancer effects on orthotopic bladder tumors
Orthotopic mouse model was established to provide the useful tool to determine the effect of intravesical localized treatment [23]. Syngeinic tumor implantation provided better tumor take rate compared to the xenograft implantation [24].
Further, experimental trials on cancer cell implantation were conducted with harder but safe scratching on bladder wall to guarantee 100% tumor take rate. After achieving this rate, we designed intravesical and oral administrations, comparing efficacy and toxicity between the two different routes.
In total, five groups were designed, 2 control groups: I-control with tumor and V- control without tumor, and 3 treatment groups: II- oral-treated group (800mg/kg/d), III- intravesical treated group (80mM, 50μL, equivalent to 26 mg/kg, twice per week), and IV- intravesical treated group (320mM, 50μL, equivalent to 104 mg/kg, twice per week). All treatments started at day 2 post tumor implantation for two weeks. Orthotopic bladder cancer implantation was processed for mice in Groups I, II, III, and IV except Group V, control mice without treatment in the absence of tumor. Figure 6A shows cumulative survival curves of five groups. Cancer cell implantation induced death of mice (Group I) but intravesical treatment dramatically enhanced life span with better survival in higher dose (Group IV vs Group I, p<0.001). Identical cumulative survival curves were observed in Groups IV and V, suggesting a potent anticancer efficiency of high dose of intravesical metformin. Oral treatment exerts very mild benefit in improving survival (Group II vs Group I, p = 0.015). Furthermore, there is a close correlation between the decrease of mouse body weight and the progression of tumor, based on observations of tumor implantation trials (data not shown). Therefore, decrease of mouse body weight could be a surrogate for tumor progression. As shown in Figure 6B, the decrease of body weight in Group I is seen, indicating the toxicity induced by tumor implantation. This decrease in body weight is attenuated by oral metformin at dose of 800mg/kg/d (Group II). However, oral administration is not able to reverse this decrease. In contrast, intravesical treatments at both doses reverse this decrease with better efficiency at higher dose (320mM). Furthermore, at the end of experiment, the weight of bladders was examined. The weight of every bladder in Group I was much bigger than that in Group V, indicating 100% existence of tumors in all mice in Group I (Figure 6C). Intravesical treatment profoundly diminished bladder weights at both doses. The bladder weights with higher dose are almost back to normal (Group IV vs Group V, p = 0.081). Oral administration reduced the bladder weights as well but this reduction was the mildest (132.8 mg vs 80.1 mg, Figure 6C). Furthermore, H&E results show the complete absence of tumor in Group IV (Figure 6D). In contrast, oral administration (Group II) causes much more residue of tumor. Ki67 staining demonstrates similar efficiency pattern Figure 6E. In all, intravesical treatment exhibited potent anti-cancer effect.
Figure 6. Bladders of female C57/BL6 mice were orthotopically implanted with MB49 cells (1.2×105) and divided into 4 groups randomly (n = 12 each group).
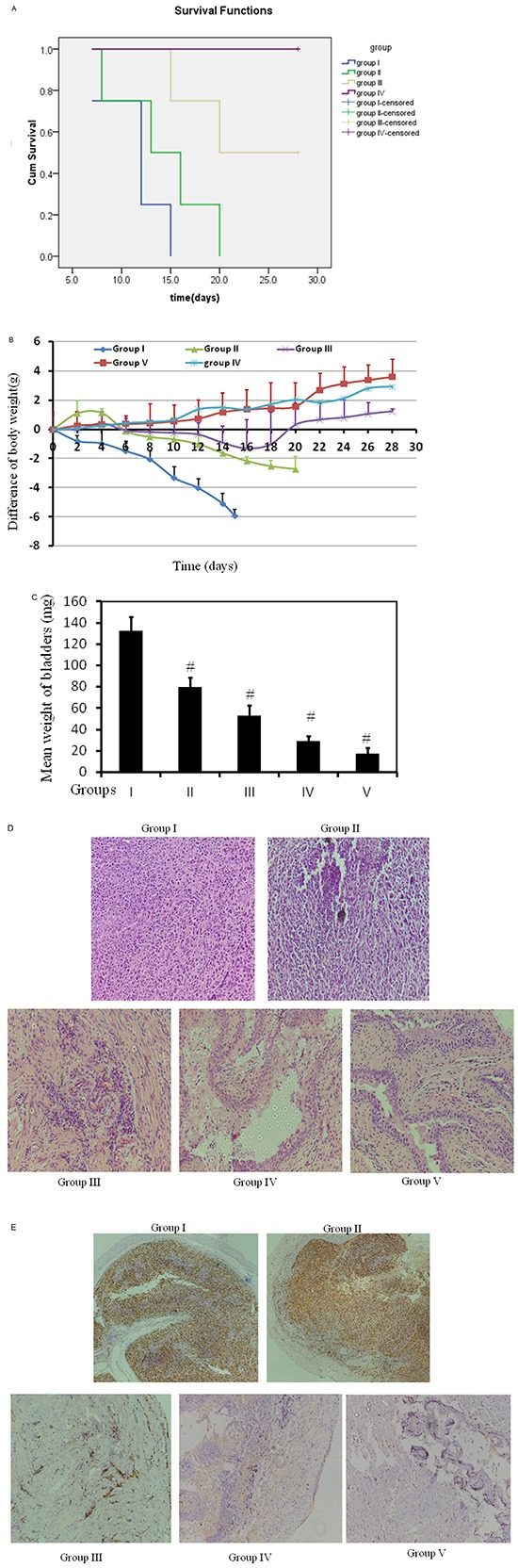
Orthotopic bladder cancer implantation was processed for mice in groups I, II, III, and IV. Group V was mice without treatment without cancer cell implantation. Groups I and II were treated with either PBS or 800mg/kg/d metformin in PBS orally. Oral treatment started at day 2 and treated daily. Groups III and IV were intravesically treated with 50 μl either 80mM or 320mM metformin, respectively in PBS per mouse, starting at day 2, twice per week for two weeks. A. Kaplan-Meier survival analysis of five groups. Death of mice was checked daily and cumulative survival rate was plotted against the time course. Life span was 12.5 days vs 16 days in groups I and II mice, respectively. The weight of mice was measured daily B. and all living mice were sacrificed at day 28. C. Weights of mouse bladders including those died before the end of experiment were measured. All bladder tissues were collected and fixed. Histological sections from these tissues were subjected to H&E stain or immunohistochemistry for Ki67 to confirm the presence or absence of tumors D. & E.
Lactic acid and toxicity
Previous report has shown that the oral metformin in mice was 200mg/ml in drinking water [25], approximately equivalent to around 1000 mg/kg/d with gavage administration. To detect the toxicity of oral treatment, 1600mg/kg/d in mice was applied to determine the tolerance of mice. However, mice died successively at this dose with acute toxicity and behaved severe gastrointestinal side effects (data not shown). In contrast, neither visual vomiting, gastrointestinal side effects nor hematuria were observed in 800 mg/kg/d oral and two intravesical groups (II, III and IV) although hematuria is seen in Group I. Lactic acid and metformin in blood samples increased markedly in 800 mg/kg/d oral treatment (Group II) with maximum increase within two hours on day 2 and 9 respectively. Amount of lactic acid maintained within 7 hours whereas metformin disappeared sharply (Figure 7). In contrast, lactic acid in Group IV does not increase (Figure 7) and metformin is not detectable (data not shown), indicating that intravesical administration does neither increase any lactic acid nor any metformin leak into blood circulating system.
Figure 7. Toxicity of metformin.
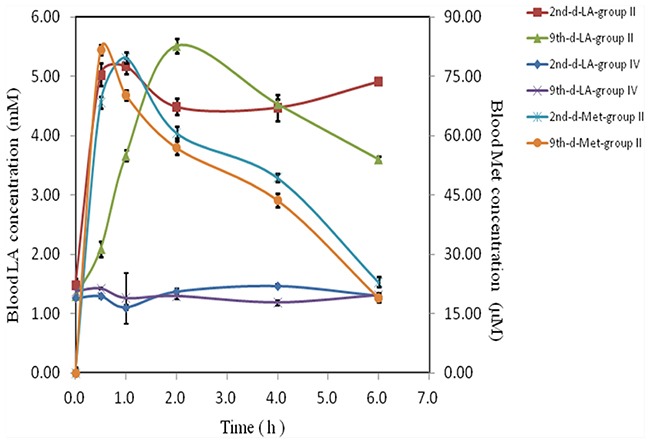
Nausea, hematuria, vomiting or other gastrointestinal reactions of mice with various treatments were recorded. Blood samples from oral and high dose intravesical treatment groups (Groups II an IV) at various time points (0-6 hours) on either day 2 or day 9, were collected, lactic acid (LA) and metformin (Met) were measured (n = 12 per group).
DISCUSSION
In this study, we report pre-clinical results of intravesical treatment using a syngeneic orthotopic bladder cancer model and the molecular mechanism of mode of action is elaborated.
Intravesical treatment of metformin showed a rapid elimination of the implanted tumor twice per week after 2 weeks without any evidence of toxicity. Furthermore, mice are very tolerated with these doses. Interestingly, our in vitro data show that metformin at 0.5 mM enhances rather than blocks colony formation (Figure 2A), indicating that the sufficient dose is essential to exhibit anticancer effect. A recent negative report of oral metformin on pancreatic cancer from clinical trial strongly highlights the importance of dose concentration on target organ [26]. At the other hand, increasing oral metformin does not help to enhance its blood concentration rather than generating more side effect due to pharmacokinetics of metformin [27]. Higher dose of oral metformin arise gastrointestinal discomfort and lactic acidosis [28], as evidenced in this study as well. Markedly, intravesical administration is able to overcome these obstacles and exhibit potent effects.
The basis of molecular anticancer mechanism of metformin is activation of AMPK, proven by previous reports [29]. Interestingly, metformin is able to increase the longevity which is related to AMPK signaling pathway as well [30–33]. Another new proposal is that metformin functions primarily as antiforlate agent and secondarily as AMK activator [34]. We present new findings that AKT and ERK signaling participates in this event.
In summary, the intravesical administration of metformin yields a high rate of tumor clearance after 2 weeks therapy. In addition, intravesical treatment limits systemic exposure and reduces non-specific toxicities. This study has demonstrated the promising result of anti-bladder cancer effects in orthotopic preclinical animal model. This will encourage us to further explore its clinical application and/or combine metformin with other chemotherapy.
MATERIALS AND METHODS
Reagents
Metformin was purchased from Aladdin chemistry Co.Ltd and was diluted across a range of concentrations in culture media. Antibodies against total Akt, phosphor-Akt(Ser473), total Erk1/2, phosphor-Erk1/2, phosphor-Acetyl-CoA Carboxylase (Ser79), total Acetyl-CoA Carboxylase (Ser79), total p70 S6 kinase (Thr389), phosphor-p70 S6 kinase (Thr389), total AMPKα (Thr172), phosphor-AMPKα (Thr172), total 4E-BP1, phosphor-4EBP1, FASN and β actin were purchased from Cell Signaling Technology. AZD6244 and MK-2206 were from Selleckchem (Houston, Texas, USA).
Cell lines and culture conditions
Murine and human bladder cancer cell lines provided by Dr. P Guo were cultured in DMEM supplemented (Hyclone, Logan, UT, USA) with 10% of FBS (Hyclone, Logan, UT, USA) and 1% of penicillin-streptomycin at 37°C, in humidified air containing 5% of CO2.
Cell viability and cologenic assay
Cell viability was assessed using a tetrazolium-based assay using microplate reader (Biotek, SYNERGY HTX, Vermont, USA). IC50 values were determined through the dose-response curves.
Cologenic survival was defined as the ability of the cells to form colonies. Images were taken and analyzed by microscopy (Leica, DFC450C, Wetzlar, Germany) and microplate reader (Biotek, SYNERGY HTX, Vermont, USA).
Protein characterization
Western blot assessment was performed using regular procedure in previous work [23]. Primary antibody was added in BSA and allowed to incubate overnight at 4°C, then washed with TBS/0.05% Tween-20 before secondary antibody was added and incubated for an additional hour at room temperature. The membrane was again washed 3 times before adding Pierce Super Signal chemiluminescent substrate (Rockford, IL, USA) and then immediately imaged on Chemi Doc (Bio-Rad, Hercules, CA, USA). The films were scanned using EPSON PERFECTION V500 PHOTO and quantified by Image J (NIH, Bethesda, MD, USA).
Animals
Female C57BL/6 mice were purchased from Hunan SJA Laboratory Animal Co., Ltd (Changsha, Hunan, China). Animals were housed 4 per cage in a specific pathogen-free animal facility. The experimental protocol was reviewed and approved by the Institutional Animal Care and Use Committee at Hunan Normal University.
Orthotopic implantation and intravesical treatment
Exponential growth of MB49 cells [35] were harvested and cell density in collection tube was counted by cell counter. Female mice 6 to 8 weeks of age were used for cancer cell implantation. The entire procedure of orthotopic implantation and intravesical treatment was similar to the previous published work [23] except that catheter scratching is much harder to guarantee 100% tumor cell implantation rate.
Histologic analysis
Tissue processing, H&E and Ki67 staining of 7-μm tissue sections were conducted by Department of Pathology, Hunan Provincial Cancer Hospital, Changsha, Hunan, P.R. China. The slides were reviewed by a pathologist, Lei Xue. The pathology evaluation was done to confirm the presence or absence of tumor.
Metformin and lactic acid measurement
Blood samples of mice were collected using the process described previously [36] from 0.5 to 6h after metformin was given via oral gavage. Blood lactic acid levels were measured by lactic acid assay kit (Sigma-Aldrich) and metformin concentrations were determined by HPLC, as reported previously [37].
Statistical analysis
All data are presented as mean±SEM. Statistical analyses were carried out using ANOVA analysis and statistical significance was assumed at a value of p<0.05.
SUPPLEMENTARY FIGURES
Acknowledgments
All authors thank to Department of Pathology, Hunan Provincial Cancer Hospital, Changsha, Hunan, P. R. China to share the Facility of Pathology Analysis of all specimens and Mr. Kwame Oteng Darko to proof reading for language.
Footnotes
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
GRANT SUPPORT
This work is in part supported by Hunan Normal University for Xiaoxiang Endowed University Professor (840140-008) to X. Y., and by the National Basic Research Program (973 Program) 2014CB542400 and the National Science Foundation of China (81130004, 81370359, and 913392019) to A.F.C.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: A Cancer Journal for Clinicians. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Sternberg CN, Bellmunt J, Sonpavde G, Siefker-Radtke AO, Stadler WM, Bajorin DF, Dreicer R, George DJ, Milowsky MI, Theodorescu D, Vaughn DJ, Galsky MD, Soloway MS, Quinn DI. International Consultation on Urologic Disease-European Association of Urology Consultation on Bladder Cancer 2012. ICUD-EAU International Consultation on Bladder Cancer 2012: Chemotherapy for urothelial carcinoma-neoadjuvant and adjuvant settings. European Urology. 2013;63:58–66. doi: 10.1016/j.eururo.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Rouprêt M, Babjuk M, Compérat E, Zigeuner R, Sylvester RJ, Burger M, Cowan NC, Böhle A, Van Rhijn BW, Kaasinen E, Palou J, Shariat SF. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Cell Carcinoma: 2015 Update. European Urology. 2015;S0302-2838:00550–00553. doi: 10.1016/j.eururo.2015.06.044. [DOI] [PubMed] [Google Scholar]
- 4.Serretta V, Morgia G, Altieri V, Di Lallo A, Ruggiero G, Salzano L, Battaglia M, Falsaperla M, Zito A, Sblendorio D, Melloni D, Allegro R, members of Gruppo Studi Tumori Urologici (GSTU) Foundation A 1-year maintenance after early adjuvant intravesical chemotherapy has a limited efficacy in preventing recurrence of intermediate risk non-muscle-invasive bladder cancer. British Journal of Urology International. 2010;106:212–217. doi: 10.1111/j.1464-410X.2009.09153.x. [DOI] [PubMed] [Google Scholar]
- 5.Gontero P, Bohle A, Malmstrom PU, O'Donnell MA, Oderda M, Sylvester R, Witjes F. the role of bacillus Calmette-Guerin in the treatment of non-muscle-invasive bladder cancer. European Urology. 2010;57:410–429. doi: 10.1016/j.eururo.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D., Weinberg R. A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. British Medical Journal. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruiter R, Visser LE, van Herk-Sukel MP, Coebergh JW, Haak HR, Geelhoed-Duijvestijn PH, Straus SM, Herings RM, Stricker BH. Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: Results from a large population-based follow-up study. Diabetes Care. 2012;35:119–124. doi: 10.2337/dc11-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes: Impact of metformin on survival. Diabetes Care. 2012;35:299–304. doi: 10.2337/dc11-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gandini S, Puntoni M, Heckman-Stoddard BM, Dunn BK, Ford L, DeCensi A, Szabo E. Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prevention Research (Phila) 2014;7:867–885. doi: 10.1158/1940-6207.CAPR-13-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iliopoulos D, Hirsch HA, Struhl K. Metformin decreases the dose of chemotherapy for prolonging tumor remission in mouse xenograft involving multiple cancer cell types. Cancer Research. 2011;71:3196–3201. doi: 10.1158/0008-5472.CAN-10-3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalva-Aydemir S, Bajpai R, Martinez M, Adekola KU, Kandela I, Wei C, Singhal S, Koblinski JE, Raje NS, Rosen ST, Shanmugam M. Targeting the metabolic plasticity of multiple myeloma with FDA-approved ritonavir and metformin. Clinical Cancer Research. 2015;21:1161–1171. doi: 10.1158/1078-0432.CCR-14-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menendez JA, Quirantes-Piné R, Rodríguez-Gallego E, Cufí S, Corominas-Faja B, Cuyàs E, Bosch-Barrera J, Martin-Castillo B, Segura-Carretero A, Joven J. Oncobiguanides: Paracelsus' law and nonconventional routesfor administering diabetobiguanides for cancer treatment. Oncotarget. 2014;5:2344–2348. doi: 10.18632/oncotarget.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao Z, Sperl B, Ullrich A, Knyazev P. Metformin and salinomycin as the best combination for the eradication of NSCLC monolayer cells and their alveospheres (cancer stem cells) irrespective of EGFR, KRAS, EML4/ALK and LKB1 status. Oncotarget. 2014;5:12877–12890. doi: 10.18632/oncotarget.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jalving M, Gietema JA, Lefrandt JD, de Jong S, Reyners AK, Gans RO, de Vries EG. Metformin: taking away the candy for cancer? European Journal of Cancer. 2010;46:2369–2380. doi: 10.1016/j.ejca.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Loubière C, Goiran T, Laurent K, Djabari Z, Tanti JF, Bost F. Metformin-induced energy deficiency leads to the inhibition of lipogenesis in prostate cancer cells. Oncotarget. 2015 Jun 20;6:15652–15661. doi: 10.18632/oncotarget.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Brien AJ, Villani LA, Broadfield LA, Houde VP, Galic S, Blandino G, Kemp BE, Tsakiridis T, Muti P, Steinberg GR. Salicylate activates AMPK and synergizes with metformin to reduce the survival of prostate and lung cancer cells ex vivo through inhibition of de novo lipogenesis. Biochem J. 2015;469:177–187. doi: 10.1042/BJ20150122. [DOI] [PubMed] [Google Scholar]
- 18.Kim PH, Cha EK, Sfakianos JP, Iyer G, Zabor EC, Scott SN, Ostrovnaya I, Ramirez R, Sun A, Shah R, Yee AM, Reuter VE, Bajorin DF, Rosenberg JE, Schultz N, Berger MF, Al-Ahmadie HA, Solit DB, Bochner BH. Genomic predictors of survival in patients with high-grade urothelial carcinoma of the bladder. European Urology. 2015;67:198–201. doi: 10.1016/j.eururo.2014.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Cancer Genome Atlas Research Network Comprehensive Molecular Characterization of Urothelial BladderCarcinoma. Nature. 2014;507:315–322. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nature Reviews Cancer. 2015;15:25–41. doi: 10.1038/nrc3817. [DOI] [PubMed] [Google Scholar]
- 21.Toren P, Kim S, Cordonnier T, Crafter C, Davies BR, Fazli L, Gleave ME, Zoubeidi A. Combination AZD5363 with Enzalutamide Significantly Delays Enzalutamide-resistant Prostate Cancer in Preclinical Models. European Urology. 2015;67:986–990. doi: 10.1016/j.eururo.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Marques RB, Aghai A, de Ridder CM, Stuurman D, Hoeben S, Boer A. High Efficacy of Combination Therapy Using PI3K/AKT Inhibitors with Androgen Deprivation in Prostate Cancer Preclinical Models. European Urology. 2015;67:1177–1185. doi: 10.1016/j.eururo.2014.08.053. [DOI] [PubMed] [Google Scholar]
- 23.Yang X, Kessler E, Su LJ, Thorburn A, Frankel AE, Li Y, La Rosa FG, Shen J, Li CY, Varella-Garcia M, Glodé LM, Flaig TW. Diphtheria toxin-epidermal growth factor fusion protein DAB389EGF for the treatment of bladder cancer. Clinical Cancer Research. 2013;19:148–157. doi: 10.1158/1078-0432.CCR-12-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Günther JH, Jurczok A, Wulf T, Brandau S, Deinert I, Jocham D, Böhle A. Optimizing syngeneic orthotopic murine bladder cancer (MB49) Cancer Research. 1999;59:2834–2837. [PubMed] [Google Scholar]
- 25.Morgillo F, Sasso FC, Della Corte CM, Vitagliano D, D'Aiuto E, Troiani T, Martinelli E, De Vita F, Orditura M, De Palma R, Ciardiello F. Synergistic Effects of Metformin Treatment in Combination with Gefitinib, a Selective EGFR Tyrosine Kinase Inhibitor, inLKB1 Wild-type NSCLC Cell Lines. Clinical Cancer Research. 2013;19:3508–3519. doi: 10.1158/1078-0432.CCR-12-2777. [DOI] [PubMed] [Google Scholar]
- 26.Kordes S, Pollak MN, Zwinderman AH, Mathôt RA, Weterman MJ, Beeker A, Punt CJ, Richel DJ, Wilmink JW. Metformin in patients with advanced pancreatic cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncology. 2015;16:839–847. doi: 10.1016/S1470-2045(15)00027-3. [DOI] [PubMed] [Google Scholar]
- 27.Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, Furlong TJ, Greenfield JR, Greenup LC, Kirkpatrick CM, Ray JE, Timmins P, Williams KM. Clinical pharmacokinetics of metformin. Clinical Pharmacokinetics. 2011;50:81–98. doi: 10.2165/11534750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 28.Quaile MP, Melich DH, Jordan HL, Nold JB, Chism JP, Polli JW, Smith GA, Rhodes MC. Toxicity and toxicokinetics of metformin in rats. Toxicology and Applied Pharmacology. 2010;243:340–347. doi: 10.1016/j.taap.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 29.Faubert B, Boily G, Izreig S, Griss T, Samborska B, Dong Z, Dupuy F, Chambers C, Fuerth BJ, Viollet B, Mamer OA, Avizonis D, DeBerardinis RJ, Siegel PM, Jones RG. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metabolism. 2013;17:113–124. doi: 10.1016/j.cmet.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halicka HD, Zhao H, Li J, Lee YS, Hsieh TC, Wu JM, Darzynkiewicz Z. Potential anti-aging agents suppress the level of constitutive mTOR- and DNA damage- signaling. Aging (Albany NY) 2012;4:952–965. doi: 10.18632/aging.100521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menendez JA, Joven J. One-carbon metabolism: an aging-cancer crossroad for the gerosuppressant metformin. Aging (Albany NY) 2012;4:894–898. doi: 10.18632/aging.100523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moiseeva O, Deschênes-Simard X, Pollak M, Ferbeyre G. Metformin, aging and cancer. Aging (Albany NY) 2013;5:330–331. doi: 10.18632/aging.100556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anisimov VN. Metformin and rapamycin are master-keys for understanding the relationship between cell senescent, aging and cancer. Aging (Albany NY) 2013;5:337–338. doi: 10.18632/aging.100561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corominas-Faja B, Quirantes-Piné R, Oliveras-Ferraros C, Vazquez-Martin A, Cufí S, Martin-Castillo B, Micol V, Joven J, Segura-Carretero A, Menendez JA. Metabolomic fingerprint reveals that metformin impairs one-carbon metabolism in a manner similar to the antifolate class of chemotherapy drugs. Aging (Albany NY) 2012;4:480–498. doi: 10.18632/aging.100472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu YT, Zhao Z, Fu XY, Luo Y, Lei CY, Chen W, Li F, Pang SY, Chen SS, Tan WL. The granulocyte macrophage-colony stimulating factor surface modified MB49 bladder cancer stem cells vaccine against metastatic bladder cancer. Stem Cell Research. 2014;13:111–122. doi: 10.1016/j.scr.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Yang X, Bemis L, Su LJ, Gao D, Flaig TW. miR-125b Regulation of Androgen Receptor Signaling Via Modulation of the Receptor Complex Co-Repressor NCOR2. Biores Open Access. 2012;1:55–62. doi: 10.1089/biores.2012.9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toyama K, Yonezawa A, Masuda S, Osawa R, Hosokawa M, Fujimoto S, Inagaki N, Inui K, Katsura T. Loss of multidrug and toxin extrusion 1 (MATE1) is associated with metformin-induced lactic acidosis. British Journal of Pharmacology. 2012;166:1183–1191. doi: 10.1111/j.1476-5381.2012.01853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


