Abstract
Purpose of review
Oxalate is an end product of metabolism excreted via the kidney. Excess urinary oxalate, whether from primary or enteric hyperoxaluria, can lead to oxalate deposition in the kidney. Oxalate crystals are associated with renal inflammation, fibrosis and progressive renal failure. It has long been known that as glomerular filtration rate (GFR) becomes reduced in chronic kidney disease (CKD), there is striking elevation of plasma oxalate. Taken together, these findings raise the possibility that elevation of plasma oxalate in CKD may promote renal inflammation and more rapid progression of CKD independent of primary etiology.
Recent findings
The inflammasome has recently been identified to play a critical role in oxalate-induced renal inflammation. Oxalate crystals have been shown to activate the nucleotide-binding domain, leucine-rich repeat inflammasome 3 (also known as NALP3, NLRP3 or cryopyrin), resulting in release of Interleukin-1β and macrophage infiltration. Deletion of inflammasome proteins in mice protects from oxalate-induced renal inflammation and progressive renal failure.
Summary
The findings reviewed in this article expand our understanding of the relevance of elevated plasma oxalate levels leading to inflammasome activation. We propose that inhibiting oxalate-induced inflammasome activation, or lowering plasma oxalate, may prevent or mitigate progressive renal damage in CKD, and warrants clinical trials.
Keywords: Oxalate, innate immune system, inflammasome, chronic kidney disease
Introduction
Oxalic acid is a potentially toxic dicarboxylic acid that is not further metabolized by mammals [1, 2, 3, 4, 5]. In its ionized form – oxalate – it forms highly insoluble complexes with calcium [1, 2]. When oxalate homeostasis is disturbed, oxalate accumulates in various body tissues and damages primarily the kidney, which serves as its main excretory organ. This in turn leads to further elevations of plasma oxalate levels. Recent studies have directed the focus to oxalate as an activator of inflammatory pathways. Thus, this review aims to provide an impulse to further investigate the prominent role of oxalate in inflammasome activation and the progression of kidney disease.
Oxalate – endogenous production
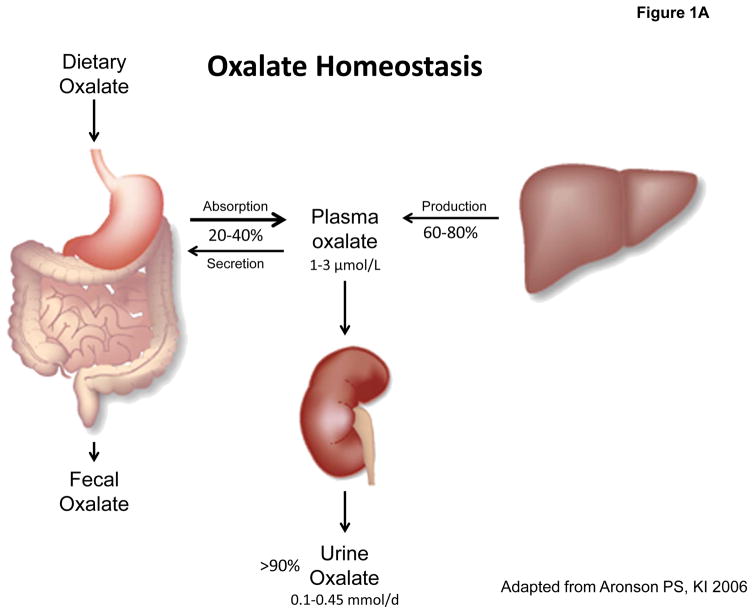
The liver has been identified as major site for the biosynthesis of oxalate as shown in Figure 1A. Endogenous oxalate production is thought to be fairly constant, attributing for up to 60–80% of total plasma oxalate and urinary oxalate excretion [6, 7]. Oxalate biosynthesis evolves from glyoxylate [8, 9, 10, 11] as central precursor molecule. Glyoxylate originates from the oxidation of glycolate by glycolate oxidase (GO) or from the catabolism of hydroxyproline, derived from collagen [12, 13]. Glyoxylate can be eliminated by conversion to glycine (alanine-glyoxylate aminotransferase: AGT) [10] or glycolate (glyoxylate reductase – hydroxpyruvate reductase: GRHPR) [9]. If, however, the glyoxylate supply overflows, oxalate is generated by the activity of either GO or lactate dehydrogenase (LDH). LDH is more likely to be responsible for the conversion in vivo as GO is strongly inhibited by physiological glycolate and lactate concentrations in vitro [10]. The glyoxylate cycle links various metabolic pathways for amino acids [10, 14, 15] and carbohydrates. In recent years glyoxal has been identified as another possible oxalate precursor. Glyoxal is a product of cellular peroxidation and protein glycation. Advanced glycation endproducts (AGEs) are associated with the progression of diabetic nephropathy and increased pro-inflammatory cytokines such as IL-1β, which can both be ameliorated by methylglyoxal trapping [16, 17, 18, 19]. Also, diabetics tend to excrete more oxalate than healthy individuals [20, 21, 22]. In addition, experimental glutathione depletion increases oxalate formation from glyoxal [23, 24]. These findings may suggest links between sugar metabolism, peroxidation and oxalate generation that will require further investigation.
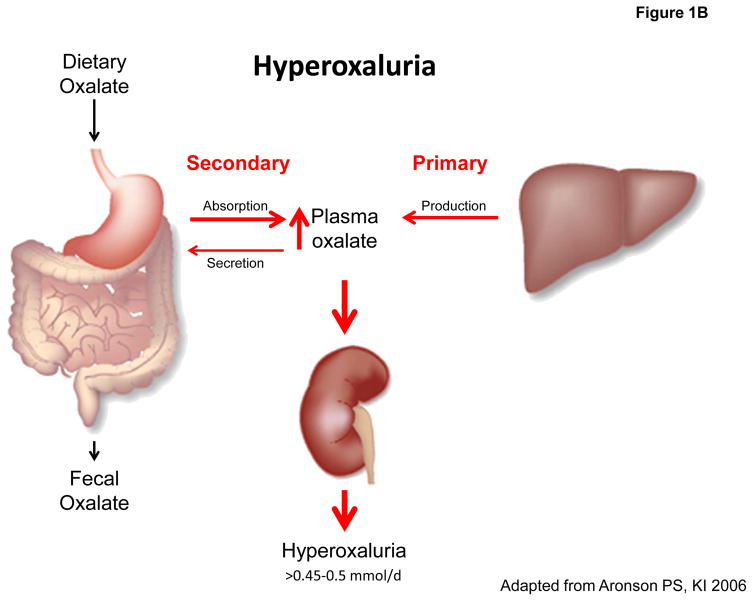
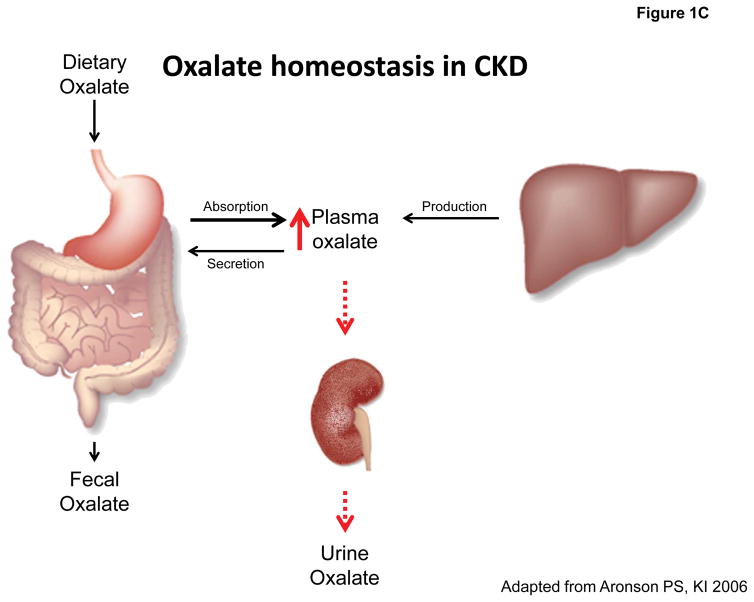
Figure 1. Oxalate homeostasis in health and disease.
A: Oxalate homeostasis is determined by the contribution of dietary (20–40%) and endogenous oxalate (60–80%) to plasma oxalate concentration (normal: 1–3 μmol/L), which is maintained low by > 90% of plasma oxalate excreted by the kidney (normal: 0.1–0.45 mmol/d). B: Hyperoxaluria is defined by a urinary oxalate excretion of >0.45–0.5 mmol/d and may occur as a consequence of endogenous overproduction (primary) or exogenous oversupply (secondary). C: Oxalate homeostasis in CKD is impaired secondary to reduced renal clearance of oxalate leading to elevated plasma oxalate levels.
Oxalate – exogenous supply
Dietary sources of oxalate include e.g. green leafy vegetables, different seeds and roots, cocoa and tea [2, 25]. Reports from different countries average daily oxalate intake to 100–200 mg/d (1.14–2.28 mmol/d) in healthy subjects [5, 7, 26]. Following dietary oxalate loads plasma levels peak at 2–4 hours. At 6 hours post-ingestion more than 75% of the ingested oxalate is excreted. This time course implicates the small intestine as the primary location for oxalate absorption [27, 28, 29]. Additional evidence suggests a role for the stomach and large intestine in physiological oxalate absorption [30, 31, 32]. The amount of oxalate that is absorbed from a dietary load can be extrapolated from an increase in urinary oxalate excretion as indicated, for example, by a 13C–oxalate absorption test. 5–15% of the ingested oxalate load reaches the systemic circulation in healthy children and adults [27, 28, 33, 34, 35, 36]. In total, exogenous oxalate is estimated to account for approximately 20–40% of urinary oxalate as shown in Figure 1A [7, 37, 38]. However, both oxalate intake and intestinal absorption are subject to a significant intra- and inter-individual variability: in some regional and seasonal diets oxalate ingestion may be considerably higher [25, 39]. In addition, oxalate bioavailability is an important factor [40] as dietary components such as Ca2+ or Mg2+ can reduce the amount of soluble oxalate in the intestinal lumen by complex formation and precipitation, impede its intestinal absorption and thereby reduce its urinary excretion. Conversely, reduced availability of Ca2+ enhances oxalate absorption (see below). Additional factors influencing oxalate absorption such as fiber have been discussed but their relevance remains controversial [7, 29, 35, 41, 42, 43]. While oxalate absorption is largely passive and paracellular across the tight junction [44], studies using knockout mice suggest that apical transporter SLC26A3 (DRA) may also play a role in oxalate absorption [45]. Knockout mice studies suggest a pivotal role of apical transporter SLC26A6 in back-secretion of oxalate that limits its net intestinal absorption, as gene deletion of SLC26A6 results in significant hyperoxaluria [46, 47]. Likewise, knockout of the basolateral transporter SLC26A1 also results in hyperoxaluria [48]. However, the contribution of these oxalate transporters to oxalate homeostasis and risk for hyperoxaluria in humans needs to be further defined [49].
Hyperoxaluria – disturbed oxalate homeostasis
Oxalate is mainly excreted by the kidney [3, 50]. Several studies have demonstrated almost complete recovery of radiolabeled oxalate in urine following infusion into healthy subjects or given as dietary load [3, 28]. In addition to glomerular filtration, there is net tubular secretion of oxalate [51, 52, 53], mainly in the proximal tubule, although there is also evidence for oxalate transport in collecting duct and papillary cells [54, 55]. Total daily oxalate excretion by the kidney is estimated at 10–40 mg per 24 h (0.1–0.45 mmol per 24 h) in healthy children and adults, with the average excretion being slightly higher in males than in females [34, 37, 56, 57, 58]. Only a minor part is eliminated through the gastrointestinal tract [6]. Marengo et al. reported fecal oxalate excretion to account for only 5–7% of the oxalate administered in rats treated with subcutaneously implanted minipumps [37].
When oxalate homeostasis is disturbed (Figure 1B), hyperoxaluria ensues defined by a urinary excretion > 40–45 mg per 24 h (0.45–0.5 mmol per 24 h) [37, 56]. Primary hyperoxalurias (PH) are caused by mutations in the genes encoding key enzymes of hepatic oxalate biosynthesis with many of these patients presenting with end-stage renal disease (ESRD) already at time of diagnosis [59, 60, 61, 62]. Secondary or enteric hyperoxalurias are characterized by a pathological hyperabsorption of dietary oxalate that in turn increases plasma and urine oxalate (Figure 1B) [7, 63]. Secondary hyperoxalurias can have a broad range of etiologies. Fat malabsorption is a very common side effect following small bowel resection or bariatric surgery. Enteric hyperoxaluria is thought to be mediated by two mechanisms: 1) by increasing the permeability of the colonic mucosa for oxalate 2) by complexation of luminal calcium with fatty acids, increasing the amount of soluble oxalate available for absorption [64, 65, 66]. Humans are not able to degrade oxalate. In contrast, the bacterial species Oxalobacter, a gram-negative obligate anaerobic bacterium that colonizes the colon, takes up oxalate through an oxalate-formate-antiport carrier and metabolizes it to formate and CO2 for exclusive energy supply [67]. Several human and animal studies hypothesize that a lack of colonic Oxalobacter formigenes favors enteric hyperoxaluria, kidney disease and adverse cardiovascular outcomes [7, 63, 68, 69, 70, 71].
Oxalate-induced inflammasome activation
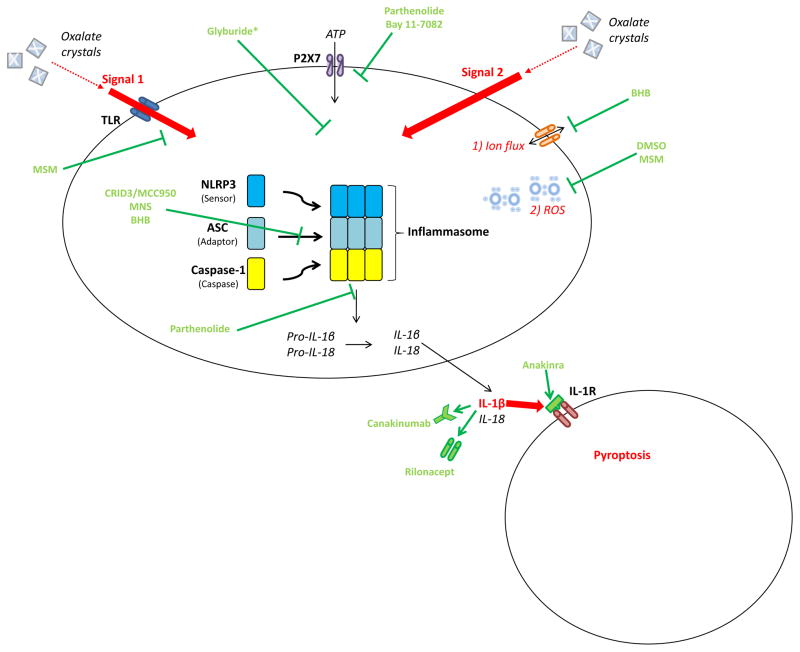
The inflammasome is a cytosolic multiprotein complex that assembles to promote Caspase-1 and thus IL-1β and IL-18 activation [72, 73]. A wide variety of immune cells, such as dendritic cells (DCs) and macrophages, have been shown to use the inflammasome machinery to release cytokines [74]. Several studies suggest an additional role in tubular epithelial cells (TECs) [75, 76, 77] and podocytes [78, 79]. The NLRP3 inflammasome is the most extensively studied so far. As shown in Figure 2, it consists of three subunits: 1) a sensor protein (Nod-like receptor pyrin-domain containing protein 3), 2) an adaptor protein (apoptosis-associated speck-like protein (ASC)) and 3) pro-inflammatory Caspase-1 [80]. For inflammasome activation, first a priming signal is required to induce transcription of NLRP3, pro IL-1β, and pro IL-18. In vitro experiments have primarily shown TOLL-like receptor (TLR) ligands to provide signal 1. A broad spectrum of pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), likely released secondary to oxalate crystals, can then serve as signal 2 which eventually triggers the formation of the inflammasome complex and secretion of IL-1β, IL-18 and pyroptosis [74, 81, 82]. The importance of oxalate-induced inflammasome activation in nephropathy has been shown in murine models of acute [74] and chronic kidney injury [81]. Calcium-oxalate (CaOx) treatment leads to a NLRP3-activation in DCs by either 1) CaOx-phagocytosis or 2) CaOx–induced K+-efflux or 3) CaOx-induced ATP-release from necrotic TECs in co-culture with DCs [74]. In vitro DCs responded with a marked IL-1β-release [74] and in vivo plasma BUN and creatinine indicated prominent renal failure [74, 81]. A recent study found that ROS might trigger oxalate-induced NLRP3-activation, as an inhibition of the former decreased renal oxalate deposition and injury [83]. Moreover, a cornucopia of experimental models has illustrated the protective effect of NLRP3 gene deletion in different types of renal disease, such as necrotic cell induced sterile inflammation, diabetic nephropathy, renal ischemia-reperfusion-injury (IRI), unilateral ureteral obstruction and glomerular disease [76, 77, 78, 84, 85].
Figure 2. Model of the NLRP3-inflammasome with specific inhibitors targeting its activation.
Two distinct signals (Signal 1 and Signal 2) are required for inflammasome and caspase-1 activation: Signal 1, a priming signal, is mainly provided by TLR ligands, such as LPS. Signal 2 is an activating signal. A variety of stimulating PAMPs or DAMPs, likely released secondary to oxalate crystals, can trigger Signal 2 by one of three mechanisms: 1) ion flux: potassium, calcium or protons 2) ROS generation [3) lysosomal rupture induced by uptake of crystalline or aggregated materials (not shown). NLRP3 inflammasome activation results in processing and release of IL-1β and IL-18 which communicate mainly with inflammatory cells (adjacent cell) via the IL-1 receptor. Several pharmacological approaches have been developed targeting Signal 1, Signal 2, inflammasome assembly, cytokines, and their respective receptors as detailed in the main body of the text. * Exact target not identified. Glyburide acts downstream of the P2X7 ATP receptor and upstream of cryopyrin.
Current treatment options for auto-inflammatory diseases (Figure 2) targeting inflammasome activation are directed against IL-1, principally through their central agent IL-1 receptor antagonist (IL-1ra) Anakinra. Administration of IL-1ra protects rats from renal IRI [86] and reduces plasma levels of inflammatory biomarkers in HD patients [87]. In addition, it has been demonstrated to attenuate oxalate-induced AKI [74]. Two newcomers among the IL-1-blockers were first approved for the treatment of cryopyrin-associated periodic syndrome (CAPS): the neutralizing IL-1β antibody canakinumab and the soluble decoy IL-1 receptor rilonacept. They are now reported to remarkably reduce symptoms also in other rare autoinflammatory syndromes and widespread diseases such as gout and diabetes [88, 89, 90, 91, 92]. Presently the multinational phase III CANTOS study is evaluating treatment benefits of canakinumab in cardiovascular disease, which is the No. 1 cause of death in advanced CKD [93, 94, 95]. In a preliminary phase IIb trial enrolling well controlled diabetic patients with high cardiovascular risk, canakinumab significantly reduced CRP, IL-6 and fibrinogen [96]. Pharmacokinetically, canakinumab and rilonacept bear advantages over anakinra: 1) Their long half-life allows for less frequent applications (anakinra: daily, rilonacept: weekly, canakinumab: once every 8 weeks) and 2) due to their large molecular size elimination is mainly non-renal, which makes dosage adjustments in kidney disease as required with anakinra treatment unnecessary [91, 97, 98]. As a result of their favorable pharmacologic profile in ESRD and promising effects in autoinflammatory diseases, canakinumab and rilonacept might have the potential to emerge as future therapeutic drugs in CKD as well.
Most recently, investigations have shifted towards specific NLRP3 inhibitors (Figure 2). Glyburide, a sulfonylurea-containing diabetic drug known to inhibit ATP-sensitive K+ - channels in the pancreas, also impedes NLRP3-activation and IL-1β production at micromolar concentrations upstream of inflammasome assembly and independent of effects on K+ channels [99]. Parthenolide, an herbal compound, and Bay 11–7082, a synthetic molecule, target ATP-mediated NLRP3-activation downstream of the P2X7 receptor. Parthenolide has also been shown to directly inhibit caspase-1 by alkylation of the enzyme’s p20 subunit [100]. Ahn et al. revealed two new selective NLRP3 inhibitors targeting multiple steps during inflammasome activation in the past two years: dimethyl sulfoxide (DMSO) [101] and its oxidized metabolite methylsulfonylmethane (MSM) [102]. Both substances 1) restrain NLRP3 activation through blockage of mitochondrial ROS generation, and 2) dose-dependently attenuate IL-1β-release in models of LPS-primed bone marrow-derived macrophages (BMDMs). MSM also interferes with the priming step (Signal 1) by downregulating NLRP3 and pro-inflammatory cytokines [101, 102]. 3,4-methylenedioxy-β-nitrostyrene (MNS) [103] and MCC950, also known as CRID3 (cytokine release inhibitory drug CP-456,773) [104], interfere primarily with ASC oligomerization at micromolar levels [103]. MCC950 is a small synthetic diarylsulfonylurea antagonizing the cysteinyl leukotriene–receptor that has been known to block IL-1β for over a decade [105, 106]. Only very recently it has been demonstrated that MCC950 specifically inhibits NLRP3-dependent pyroptotic cell death in vivo, beginning at concentrations as low as 10 nM in human cells ex vivo [88]. While MCC950 blocks ASC oligomerization and inhibits both canonical and noncanonical inflammasome pathways, β-hydroxybutyrate (BHB) additionally hinders intracellular K+-depletion and specifically obstructs the canonical route [107]. BHB is a ketone body used as alternative energy source in metabolic states of energy depletion, such as those simulated by the experimental concentrations starting at 1 mM BHB [107]. This discovery proposes an interesting link between metabolism and the regulation of systemic inflammation. Given the profound protection against oxalate-induced progressive renal insufficiency in mice deficient for NLRP3, future studies should examine these compounds in the established murine models of oxalate-induced CKD and crystal-induced progressive renal failure [81, 108].
Oxalate and CKD
In primary hyperoxalurias the unbounded oxalate overproduction causes sheer CaOx supersaturation in plasma by exceeding the oxalosis cut-off > 30 μmol/L(βCaOx>1) [109, 110, 111] and extreme urinary excretion ranging between 1–2 mmol/1.73 m2 in 24 hours [112]. In enteric hyperoxaluria the high oxalate absorption is often associated with diarrhea-induced volume depletion, metabolic acidosis and hypocitraturia, providing favorable conditions for oxalate accumulation and precipitation in renal tissue [5, 7, 65]. PH patients usually progress to CKD rapidly: mean age at ESRD onset in PH1 patients is 24–27 years. 70% of adults and 29% of kids are in renal failure already at time of diagnosis [62, 112]. In secondary hyperoxaluria progression to acute or chronic kidney disease is less frequently reported – possibly in part because it is under recognized. Glew et al. recently provided an extensive review of nephropathy resulting from enteric hyperoxaluria [5]. Hence, both primary and secondary hyperoxaluria provide the “proof of principle” that increased plasma oxalate can lead to progressive CKD in humans.
Plasma or serum oxalate levels in healthy children and adults are mainly reported to be in the range of 1–3 μmol/L [57, 58, 113, 114, 115, 116]; depending on the method used, however, higher normal limits of up to 11 μmol/L have been reported [117, 118]. As GFR declines, renal oxalate clearance is decreasing, which leads to increased plasma oxalate as depicted in Figure 1C [59, 119, 120, 121, 122, 123, 124, 125]. Mean plasma oxalate levels reported for different kinds of chronic renal disease are above normal limits and inversely correlate with GFR [123, 126]. Moreover, in patients on dialysis maximal oxalate elimination of 6–10 mmol/1.73 m2 per week in routine HD or peritoneal dialysis (PD) is insufficient to address supersaturation. Consequently, ESRD patients on dialysis tend to have even higher plasma oxalate levels ranging around 45 μmol/l [111, 119, 126, 127, 128]. These extreme plasma oxalate levels are only exceeded by those in PH patients with levels of 80–125 μmol/L commonly found [110, 111]. In mouse models of oxalate-induced CKD, there is clear evidence for activation of inflammasome pathways by oxalate crystals. Translating this concept to a clinical setting, it becomes possible or even likely that high plasma oxalate levels as observed in advanced CKD patients launch a vicious cycle of inflammasome mediated systemic inflammation and kidney damage resulting in progressive renal disease. In particular, the cardiovascular implications of such high oxalate levels in the circulation are of great concern: in our murine model of dietary oxalate-induced CKD, mice develop a clear presentation of cardiovascular disease including cardiac fibrosis and profound arterial hypertension [108]. Whether these findings are solely related to reduced renal function or elevated plasma oxalate levels remains to be defined.
Conclusion
Progressive kidney failure and early mortality due to adverse cardiovascular outcomes make CKD a very serious disease. Oxalate is strongly involved in inflammatory pathways, which makes it a prime candidate to contribute to progression of CKD and systemic inflammation. In animal models, members of our and other research groups have uncovered part of the complex interplay between oxalate, inflammasome activation and the progression of kidney disease. Accordingly, inhibiting oxalate-induced inflammasome activation, or lowering plasma oxalate, may prevent or mitigate progressive renal damage in CKD, and also reduce morbidity and mortality due to systemic inflammation. Possibly some of the new anti-inflammatory drugs presented in this review might provide novel therapeutic options of tomorrow for patients with primary or secondary hyperoxaluria or CKD in general.
Key points.
Oxalate can accumulate as a result of endogenous overproduction (primary hyperoxaluria), exogenous oversupply (secondary hyperoxaluria) or decreasing renal clearance (declining GFR in CKD).
Primary hyperoxaluria proves the principle that excess plasma oxalate can lead to CKD.
Oxalate is a potent activator of the NLRP3-inflammasome that mediates inflammation in murine models of oxalate-induced CKD.
NLRP3-knockouts are protected from oxalate-induced progressive kidney disease.
Novel NLRP3- and IL-1-inhibitors might be promising therapeutic options of tomorrow for primary or secondary hyperoxaluria or CKD in general to prevent progressive loss of kidney function and systemic inflammatory complications of CKD.
Acknowledgments
Dr. Knauf is supported by grants KN 1148/2-1 from the Deutsche Forschungsgemeinschaft, Gessler Foundation, Oxalosis and Hyperoxaluria Foundation and he and K.-U. Eckardt have received research grant support from Dicerna Pharmaceuticals, Cambridge, MA, U.S.A. Theresa Ermer is recipient of a TRENAL medical student scholarship from the German Academic Exchange Service (DAAD). Peter Aronson is supported by NIH grant R37DK33793 and the George M. O’Brien Kidney Center at Yale (P30DK079310).
References and recommended reading
- 1.Hagler L, Herman RH. Oxalate Metabolism. I. Am J Clin Nutr. 1973;26:758–765. doi: 10.1093/ajcn/26.6.758. [DOI] [PubMed] [Google Scholar]
- 2.Williams HE. Oxalic Acid and the Hyperoxaluric Syndromes. Kidney Int. 1978;13:410–417. doi: 10.1038/ki.1978.59. [DOI] [PubMed] [Google Scholar]
- 3.Elder TD, Wyngaarden JB. The Biosynthesis and Turnover of Oxalate in Normal and Hyperoxaluric Subjects. J Clin Invest. 1960;39:1337–1344. doi: 10.1172/JCI104151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schepers MSJ, van Ballegooijen ES, Bangma CH, Verkoelen CF. Crystals Cause Acute Necrotic Cell Death in Renal Proximal Tubule Cells, but Not in Collecting Tubule Cells. Kidney Int. 2005;68:1543–1553. doi: 10.1111/j.1523-1755.2005.00566.x. [DOI] [PubMed] [Google Scholar]
- **5.Glew RH, Sun Y, Horowitz BL, et al. Nephropathy in Dietary Hyperoxaluria: A Potentially Preventable Acute or Chronic Kidney Disease. World J Nephrol. 2014;3:122–142. doi: 10.5527/wjn.v3.i4.122. This paper provides a detailed analysis of cases where dietary hyperoxaluria led to AKI or CKD. It urges us to reconsider the importance of nutritional factors as possible contributors to the development of oxalate-induced chronic kidney disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brzica H, Breljak D, Burckhardt Birgitta C, et al. Oxalate: From the Environment to Kidney Stones. Archives of Industrial Hygiene and Toxicology. 2013:609. doi: 10.2478/10004-1254-64-2013-2428. [DOI] [PubMed] [Google Scholar]
- *7.Asplin J. The Management of Patients with Enteric Hyperoxaluria. Urolithiasis. 2015:1–11. doi: 10.1007/s00240-015-0846-5. This review concisely and very up-to-date summarizes the current knowledge on the pathogenesis and therapy of enteric hyperoxaluria and presents potential future developments. [DOI] [PubMed] [Google Scholar]
- 8.Baker PRS, Cramer SD, Kennedy M, et al. Glycolate and Glyoxylate Metabolism in Hepg2 Cells. Am J Physiol Cell Physiol. 2004;287:C1359–C1365. doi: 10.1152/ajpcell.00238.2004. [DOI] [PubMed] [Google Scholar]
- 9.Holmes RP, Assimos DG. Glyoxylate Synthesis and Its Modulation and Influence on Oxalate Synthesis. J Urol. 1998;160:1617–1624. [PubMed] [Google Scholar]
- 10.Poore RE, Hurst CH, Assimos DG, Holmes RP. Pathways of Hepatic Oxalate Synthesis and Their Regulation. Am J Physiol Cell Physiol. 1997;272:C289–C294. doi: 10.1152/ajpcell.1997.272.1.C289. [DOI] [PubMed] [Google Scholar]
- 11.Farinelli MP, Richardson KE. Oxalate Synthesis from [14c1]Glycollate and [14c1]Glyoxylate in the Hepatectomized Rat. Biochim Biophys Acta. 1983;757:8–14. doi: 10.1016/0304-4165(83)90146-0. [DOI] [PubMed] [Google Scholar]
- 12.Knight J, Jiang J, Assimos DG, Holmes RP. Hydroxyproline Ingestion and Urinary Oxalate and Glycolate Excretion. Kidney Int. 2006;70:1929–1934. doi: 10.1038/sj.ki.5001906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takayama T, Fujita K, Suzuki K, et al. Control of Oxalate Formation from L-Hydroxyproline in Liver Mitochondria. J Am Soc Nephrol. 2003;14:939–946. doi: 10.1097/01.asn.0000059310.67812.4f. [DOI] [PubMed] [Google Scholar]
- 14.Knight J, Assimos DG, Callahan MF, Holmes RP. Metabolism of Primed, Constant Infusions of [1,2–13c2] Glycine and [1–13c1] Phenylalanine to Urinary Oxalate. Metabolism. 2011;60:950–956. doi: 10.1016/j.metabol.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gambardella RL, Richardson KE. The Pathways of Oxalate Formation from Phenylalanine, Tyrosine, Tryptophan and Ascorbic Acid in the Rat. Biochim Biophys Acta. 1977;499:156–168. doi: 10.1016/0304-4165(77)90238-0. [DOI] [PubMed] [Google Scholar]
- 16.Beisswenger PJ, Howell SK, Russell GB, et al. Early Progression of Diabetic Nephropathy Correlates with Methylglyoxal-Derived Advanced Glycation End Products. Diabetes Care. 2013;36:3234–3239. doi: 10.2337/dc12-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodrigues L, Matafome P, Crisostomo J, et al. Advanced Glycation End Products and Diabetic Nephropathy: A Comparative Study Using Diabetic and Normal Rats with Methylglyoxal-Induced Glycation. J Physiol Biochem. 2014;70:173–184. doi: 10.1007/s13105-013-0291-2. [DOI] [PubMed] [Google Scholar]
- 18.Zhu D, Wang L, Zhou Q, et al. (+)-Catechin Ameliorates Diabetic Nephropathy by Trapping Methylglyoxal in Type 2 Diabetic Mice. Mol Nutr Food Res. 2014;58:2249–2260. doi: 10.1002/mnfr.201400533. [DOI] [PubMed] [Google Scholar]
- 19.Waris S, Winklhofer-Roob BM, Roob JM, et al. Increased DNA Dicarbonyl Glycation and Oxidation Markers in Patients with Type 2 Diabetes and Link to Diabetic Nephropathy. J Diabetes Res. 2015;2015:915486. doi: 10.1155/2015/915486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lapolla A, Flamini R, Vedova Antonio D, et al. Glyoxal and Methylglyoxal Levels in Diabetic Patients: Quantitative Determination by a New Gc/Ms Method. Clin Chem Lab Med. 2003:1166. doi: 10.1515/CCLM.2003.180. [DOI] [PubMed] [Google Scholar]
- 21.Eisner BH, Porten SP, Bechis SK, Stoller ML. Diabetic Kidney Stone Formers Excrete More Oxalate and Have Lower Urine Ph Than Nondiabetic Stone Formers. J Urol. 2010;183:2244–2248. doi: 10.1016/j.juro.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Taylor EN, Curhan GC. Determinants of 24-Hour Urinary Oxalate Excretion. Clin J Am Soc Nephrol. 2008;3:1453–1460. doi: 10.2215/CJN.01410308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knight J, Assimos DG, Easter L, Holmes RP. Metabolism of Fructose to Oxalate and Glycolate. Horm Metab Res. 2010;42:868–873. doi: 10.1055/s-0030-1265145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *24.Knight J, Wood KD, Lange JN, et al. Oxalate Formation from Glyoxal in Erythrocytes. Urology. 2015 doi: 10.1016/j.urology.2015.10.014. This study illustrates how glutathione depletion can favor endogenous oxalate synthesis from oxalate, highlighting new links between sugar metabolism, peroxidation and oxalate synthesis that will require further investigations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noonan SC, Savage GP. Oxalate Content of Foods and Its Effect on Humans. Asia Pac J Clin Nutr. 1999;8:64–74. [PubMed] [Google Scholar]
- 26.Holmes RP, Kennedy M. Estimation of the Oxalate Content of Foods and Daily Oxalate Intake. Kidney Int. 2000;57:1662–1667. doi: 10.1046/j.1523-1755.2000.00010.x. [DOI] [PubMed] [Google Scholar]
- 27.Holmes RP, Ambrosius WT, Assimos DG. Dietary Oxalate Loads and Renal Oxalate Handling. J Urol. 2005;174:943–947. doi: 10.1097/01.ju.0000169476.85935.e2. [DOI] [PubMed] [Google Scholar]
- 28.Chai W, Liebman M, Kynast-Gales S, Massey L. Oxalate Absorption and Endogenous Oxalate Synthesis from Ascorbate in Calcium Oxalate Stone Formers and Non-Stone Formers. Am J Kidney Dis. 2004;44:1060–1069. doi: 10.1053/j.ajkd.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 29.Hatch M, Freel R. Intestinal Transport of an Obdurate Anion: Oxalate. Urol Res. 2005;33:1–16. doi: 10.1007/s00240-004-0445-3. [DOI] [PubMed] [Google Scholar]
- 30.Chen Z, Ye Z, Zeng L, Yang W. Clinical Investigation on Gastric Oxalate Absorption. Chin Med J (Engl) 2003;116:1749–1751. [PubMed] [Google Scholar]
- 31.Hautmann RE. The Stomach: A New and Powerful Oxalate Absorption Site in Man. J Urol. 1993;149:1401–1404. doi: 10.1016/s0022-5347(17)36400-5. [DOI] [PubMed] [Google Scholar]
- 32.Hatch M, Freel RW, Goldner AM, Earnest DL. Oxalate and Chloride Absorption by the Rabbit Colon: Sensitivity to Metabolic and Anion Transport Inhibitors. Gut. 1984;25:232–237. doi: 10.1136/gut.25.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voss S, Hesse A, Zimmermann DJ, et al. Intestinal Oxalate Absorption Is Higher in Idiopathic Calcium Oxalate Stone Formers Than in Healthy Controls: Measurements with the [13c2]Oxalate Absorption Test. J Urol. 2006;175:1711–1715. doi: 10.1016/S0022-5347(05)01001-3. [DOI] [PubMed] [Google Scholar]
- 34.Sikora P, von Unruh GE, Beck B, et al. [Lsqb]13c2[Rsqb]Oxalate Absorption in Children with Idiopathic Calcium Oxalate Urolithiasis or Primary Hyperoxaluria. Kidney Int. 2008;73:1181–1186. doi: 10.1038/ki.2008.63. [DOI] [PubMed] [Google Scholar]
- 35.von Unruh GE, Voss S, Sauerbruch T, Hesse A. Reference Range for Gastrointestinal Oxalate Absorption Measured with a Standardized [13c2]Oxalate Absorption Test. J Urol. 2003;169:687–690. doi: 10.1097/01.ju.0000051637.63068.92. [DOI] [PubMed] [Google Scholar]
- 36.Zimmermann D, Hesse A, von Unruh G. Influence of a High-Oxalate Diet on Intestinal Oxalate Absorption. World J Urol. 2005;23:324–329. doi: 10.1007/s00345-005-0028-0. [DOI] [PubMed] [Google Scholar]
- 37.Marengo SR, Romani AMP. Oxalate in Renal Stone Disease: The Terminal Metabolite That Just Won t Go Away. Nat Clin Pract Nephrol. 2008;4:368–377. doi: 10.1038/ncpneph0845. [DOI] [PubMed] [Google Scholar]
- 38.Holmes RP, Goodman HO, Assimos DG. Contribution of Dietary Oxalate to Urinary Oxalate Excretion. Kidney Int. 2001;59:270–276. doi: 10.1046/j.1523-1755.2001.00488.x. [DOI] [PubMed] [Google Scholar]
- 39.Singh PP, Kothari LK, Sharma DC, Saxena SN. Nutritional Value of Foods in Relation to Their Oxalic Acid Content. Am J Clin Nutr. 1972;25:1147–1152. doi: 10.1093/ajcn/25.11.1147. [DOI] [PubMed] [Google Scholar]
- 40.Chai W, Liebman M. Assessment of Oxalate Absorption from Almonds and Black Beans and with and without the Use of an Extrinsic Label. J Urol. 2004;172:953–957. doi: 10.1097/01.ju.0000135918.00761.8a. [DOI] [PubMed] [Google Scholar]
- 41.Borghi L, Schianchi T, Meschi T, et al. Comparison of Two Diets for the Prevention of Recurrent Stones in Idiopathic Hypercalciuria. N Engl J Med. 2002;346:77–84. doi: 10.1056/NEJMoa010369. [DOI] [PubMed] [Google Scholar]
- 42.Liebman M, Costa G. Effects of Calcium and Magnesium on Urinary Oxalate Excretion after Oxalate Loads. J Urol. 2000;163:1565–1569. [PubMed] [Google Scholar]
- 43.Hess B, Jost C, Zipperle L, et al. High-Calcium Intake Abolishes Hyperoxaluria and Reduces Urinary Crystallization During a 20-Fold Normal Oxalate Load in Humans. Nephrol Dial Transplant. 1998;13:2241–2247. doi: 10.1093/ndt/13.9.2241. [DOI] [PubMed] [Google Scholar]
- 44.Knauf F, Ko N, Jiang Z, et al. Net Intestinal Transport of Oxalate Reflects Passive Absorption and Slc26a6-Mediated Secretion. J Am Soc Nephrol. 2011;22:2247–2255. doi: 10.1681/ASN.2011040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freel RW, Whittamore JM, Hatch M. Transcellular Oxalate and Cl- Absorption in Mouse Intestine Is Mediated by the Dra Anion Exchanger Slc26a3, and Dra Deletion Decreases Urinary Oxalate. Am J Physiol Gastrointest Liver Physiol. 2013;305:G520–527. doi: 10.1152/ajpgi.00167.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang Z, Asplin JR, Evan AP, et al. Calcium Oxalate Urolithiasis in Mice Lacking Anion Transporter Slc26a6. Nat Genet. 2006;38:474–478. doi: 10.1038/ng1762. [DOI] [PubMed] [Google Scholar]
- 47.Freel RW, Hatch M, Green M, Soleimani M. Ileal Oxalate Absorption and Urinary Oxalate Excretion Are Enhanced in Slc26a6 Null Mice. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2006;290:G719–G728. doi: 10.1152/ajpgi.00481.2005. [DOI] [PubMed] [Google Scholar]
- 48.Dawson PA, Russell CS, Lee S, et al. Urolithiasis and Hepatotoxicity Are Linked to the Anion Transporter Sat1 in Mice. The Journal of Clinical Investigation. 2010;120:706–712. doi: 10.1172/JCI31474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monico CG, Weinstein A, Jiang Z, et al. Phenotypic and Functional Analysis of Human Slc26a6 Variants in Patients with Familial Hyperoxaluria and Calcium Oxalate Nephrolithiasis. Am J Kidney Dis. 2008;52:1096–1103. doi: 10.1053/j.ajkd.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hautmann R, Osswald H. Pharmacokinetic Studies of Oxalate in Man. Invest Urol. 1979;16:395–398. [PubMed] [Google Scholar]
- 51.Weinman EJ, Frankfurt SJ, Ince A, Sansom S. Renal Tubular Transport of Organic Acids: Studies with Oxalate and Para-Aminohippurate in the Rat. J Clin Invest. 1978;61:801–806. doi: 10.1172/JCI108994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Senekjian HO, Weinman EJ. Oxalate Transport by Proximal Tubule of the Rabbit Kidney. Am J Physiol. 1982;243:F271–275. doi: 10.1152/ajprenal.1982.243.3.F271. [DOI] [PubMed] [Google Scholar]
- 53.Knight TF, Sansom SC, Senekjian HO, Weinman EJ. Oxalate Secretion in the Rat Proximal Tubule. Am J Physiol. 1981;240:F295–298. doi: 10.1152/ajprenal.1981.240.4.F295. [DOI] [PubMed] [Google Scholar]
- 54.Sigmon D, Kumar S, Carpenter B, et al. Oxalate Transport in Renal Tubular Cells from Normal and Stone-Forming Animals. Am J Kidney Dis. 1991;17:376–380. doi: 10.1016/s0272-6386(12)80626-3. [DOI] [PubMed] [Google Scholar]
- 55.Chandhoke PS, Fan JIE. Transport of Oxalate across the Rabbit Papillary Surface Epithelium. The Journal of Urology. 2000;164:1724–1728. [PubMed] [Google Scholar]
- 56.Robijn S, Hoppe B, Vervaet BA, et al. Hyperoxaluria: A Gut-Kidney Axis[Quest] Kidney Int. 2011;80:1146–1158. doi: 10.1038/ki.2011.287. [DOI] [PubMed] [Google Scholar]
- 57.Wilson DM, Liedtke RR. Modified Enzyme-Based Colorimetric Assay of Urinary and Plasma Oxalate with Improved Sensitivity and No Ascorbate Interference: Reference Values and Sample Handling Procedures. Clin Chem. 1991;37:1229–1235. [PubMed] [Google Scholar]
- 58.Fry ID, Starkey BJ. The Determination of Oxalate in Urine and Plasma by High Performance Liquid Chromatography. Ann Clin Biochem. 1991;28( Pt 6):581–587. doi: 10.1177/000456329102800607. [DOI] [PubMed] [Google Scholar]
- 59.Cochat P, Rumsby G. Primary Hyperoxaluria. N Engl J Med. 2013;369:649–658. doi: 10.1056/NEJMra1301564. [DOI] [PubMed] [Google Scholar]
- 60.Williams EL, Acquaviva C, Amoroso A, et al. Primary Hyperoxaluria Type 1: Update and Additional Mutation Analysis of the Agxt Gene. Hum Mutat. 2009;30:910–917. doi: 10.1002/humu.21021. [DOI] [PubMed] [Google Scholar]
- 61.Rumsby DG. University College London Hospitals. Primary hyperoxaluria mutation database. 2015 [Google Scholar]
- **62.Mandrile G, van Woerden CS, Berchialla P, et al. Data from a Large European Study Indicate That the Outcome of Primary Hyperoxaluria Type 1 Correlates with the Agxt Mutation Type. Kidney Int. 2014;86:1197–1204. doi: 10.1038/ki.2014.222. This analysis provides perspectives on the disease burden of PH and its devastating course towards CKD. [DOI] [PubMed] [Google Scholar]
- 63.Allison MJ, Cook HM, Milne DB, et al. Oxalate Degradation by Gastrointestinal Bacteria from Humans. J Nutr. 1986;116:455–460. doi: 10.1093/jn/116.3.455. [DOI] [PubMed] [Google Scholar]
- 64.Rankin AC, Walsh SB, Summers SA, et al. Acute Oxalate Nephropathy Causing Late Renal Transplant Dysfunction Due to Enteric Hyperoxaluria. Am J Transplant. 2008;8:1755–1758. doi: 10.1111/j.1600-6143.2008.02288.x. [DOI] [PubMed] [Google Scholar]
- 65.Whittamore J, Hatch M. Chronic Metabolic Acidosis Reduces Urinary Oxalate Excretion and Promotes Intestinal Oxalate Secretion in the Rat. Urolithiasis. 2015;43:489–499. doi: 10.1007/s00240-015-0801-5. [DOI] [PubMed] [Google Scholar]
- 66.Kumar R, Lieske JC, Collazo-Clavell ML, et al. Fat Malabsorption and Increased Intestinal Oxalate Absorption Are Common after Roux-En-Y Gastric Bypass Surgery. Surgery. 2011;149:654–661. doi: 10.1016/j.surg.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stewart CS, Duncan SH, Cave DR. Oxalobacter Formigenes and Its Role in Oxalate Metabolism in the Human Gut. FEMS Microbiol Lett. 2004;230:1–7. doi: 10.1016/S0378-1097(03)00864-4. [DOI] [PubMed] [Google Scholar]
- **68.Gulhan B, Turkmen K, Aydin M, et al. The Relationship between Serum Oxalic Acid, Central Hemodynamic Parameters and Colonization by Oxalobacter Formigenes in Hemodialysis Patients. Cardiorenal Med. 2015;5:164–174. doi: 10.1159/000381219. This study directly links the absence of Oxalobacter formigenes and high serum oxalate in HD patients to cardiovascular parameters predicting increased cardiovascular morbidity and mortality. Thus, it highlights the relevance of monitoring oxalate levels for patient outcomes in ESRD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hatch M, Freel RW. A Human Strain of Oxalobacter (Hc-1) Promotes Enteric Oxalate Secretion in the Small Intestine of Mice and Reduces Urinary Oxalate Excretion. Urolithiasis. 2013;41:379–384. doi: 10.1007/s00240-013-0601-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lefaucheur C, Hill GS, Amrein C, et al. Acute Oxalate Nephropathy: A New Etiology for Acute Renal Failure Following Nonrenal Solid Organ Transplantation. Am J Transplant. 2006;6:2516–2521. doi: 10.1111/j.1600-6143.2006.01485.x. [DOI] [PubMed] [Google Scholar]
- 71.Hoppe B, Dittlich K, Fehrenbach H, et al. Reduction of Plasma Oxalate Levels by Oral Application of Oxalobacter Formigenes in 2 Patients with Infantile Oxalosis. Am J Kidney Dis. 2011;58:453–455. doi: 10.1053/j.ajkd.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 72.Gross O, Thomas CJ, Guarda G, Tschopp J. The Inflammasome: An Integrated View. Immunol Rev. 2011;243:136–151. doi: 10.1111/j.1600-065X.2011.01046.x. [DOI] [PubMed] [Google Scholar]
- 73.Lorenz G, Darisipudi MN, Anders H-J. Canonical and Non-Canonical Effects of the Nlrp3 Inflammasome in Kidney Inflammation and Fibrosis. Nephrol Dial Transplant. 2014;29:41–48. doi: 10.1093/ndt/gft332. [DOI] [PubMed] [Google Scholar]
- 74.Mulay SR, Kulkarni OP, Rupanagudi KV, et al. Calcium Oxalate Crystals Induce Renal Inflammation by Nlrp3-Mediated Il-1β Secretion. J Clin Invest. 2013;123:236–246. doi: 10.1172/JCI63679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *75.Kasimsetty SG, DeWolf SE, Shigeoka AA, McKay DB. Regulation of Tlr2 and Nlrp3 in Primary Murine Renal Tubular Epithelial Cells. Nephron Clin Pract. 2014;127:119–123. doi: 10.1159/000363208. This paper elucidates a role for TLR2 in NLRP3-inflammasome-mediated cell death in primary murine TECs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shigeoka AA, Mueller JL, Kambo A, et al. An Inflammasome-Independent Role for Epithelial-Expressed Nlrp3 in Renal Ischemia-Reperfusion Injury. J Immunol. 2010;185:6277–6285. doi: 10.4049/jimmunol.1002330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhuang Y, Hu C, Ding G, et al. Albumin Impairs Renal Tubular Tight Junctions Via Targeting the Nlrp3 Inflammasome. Am J Physiol Renal Physiol. 2015;308:F1012–F1019. doi: 10.1152/ajprenal.00509.2014. [DOI] [PubMed] [Google Scholar]
- 78.Gao P, He FF, Tang H, et al. Nadph Oxidase-Induced Nalp3 Inflammasome Activation Is Driven by Thioredoxin-Interacting Protein Which Contributes to Podocyte Injury in Hyperglycemia. J Diabetes Res. 2015;2015:504761. doi: 10.1155/2015/504761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haque S, Lan X, Wen H, et al. Hiv Promotes Nlrp3 Inflammasome Complex Activation in Murine Hiv-Associated Nephropathy. Am J Pathol. 2015 doi: 10.1016/j.ajpath.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chang A, Ko K, Clark MR. The Emerging Role of the Inflammasome in Kidney Diseases. Curr Opin Nephrol Hypertens. 2014;23:204–210. doi: 10.1097/01.mnh.0000444814.49755.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Knauf F, Asplin JR, Granja I, et al. Nalp3-Mediated Inflammation Is a Principal Cause of Progressive Renal Failure in Oxalate Nephropathy. Kidney Int. 2013;84:895–901. doi: 10.1038/ki.2013.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *82.Darisipudi M, Knauf F. An Update on the Role of the Inflammasomes in the Pathogenesis of Kidney Diseases. Pediatr Nephrol. 2015:1–10. doi: 10.1007/s00467-015-3153-z. This review summarizes the current knowledge on NLRP3-inflammasome activation and its link to kidney disease in detail. [DOI] [PubMed] [Google Scholar]
- **83.Joshi S, Wang W, Peck AB, Khan SR. Activation of the Nlrp3 Inflammasome in Association with Calcium Oxalate Crystal Induced Reactive Oxygen Species in Kidneys. J Urol. 2015;193:1684–1691. doi: 10.1016/j.juro.2014.11.093. This paper demonstrates that ROS-generation for inflammasome activation is a a major theme not only in diabetic, but also in oxalate nephropathy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Iyer SS, Pulskens WP, Sadler JJ, et al. Necrotic Cells Trigger a Sterile Inflammatory Response through the Nlrp3 Inflammasome. Proc Natl Acad Sci U S A. 2009;106:20388–20393. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vilaysane A, Chun J, Seamone ME, et al. The Nlrp3 Inflammasome Promotes Renal Inflammation and Contributes to Ckd. J Am Soc Nephrol. 2010;21:1732–1744. doi: 10.1681/ASN.2010020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rusai K, Huang H, Sayed N, et al. Administration of Interleukin-1 Receptor Antagonist Ameliorates Renal Ischemia-Reperfusion Injury. Transpl Int. 2008;21:572–580. doi: 10.1111/j.1432-2277.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- 87.Hung AM, Ellis CD, Shintani A, et al. Il-1β Receptor Antagonist Reduces Inflammation in Hemodialysis Patients. J Am Soc Nephrol. 2011;22:437–442. doi: 10.1681/ASN.2010070760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **88.Coll RC, Robertson AAB, Chae JJ, et al. A Small-Molecule Inhibitor of the Nlrp3 Inflammasome for the Treatment of Inflammatory Diseases. Nat Med. 2015;21:248–255. doi: 10.1038/nm.3806. This group introduces a new specific NLRP3 inhibitor with impressive protective effects in autoinflammatory diseases. Investigations for a possible role in kidney disease are warranted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Scarpioni R, Rigante D, Cantarini L, et al. Renal Involvement in Secondary Amyloidosis of Muckle-Wells Syndrome: Marked Improvement of Renal Function and Reduction of Proteinuria after Therapy with Human Anti-Interleukin-1beta Monoclonal Antibody Canakinumab. Clin Rheumatol. 2015;34:1311–1316. doi: 10.1007/s10067-013-2481-2. [DOI] [PubMed] [Google Scholar]
- 90.Kuemmerle-Deschner JB, Haug I. Canakinumab in Patients with Cryopyrin-Associated Periodic Syndrome: An Update for Clinicians. Ther Adv Musculoskelet Dis. 2013;5:315–329. doi: 10.1177/1759720X13502629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dubois EA, Rissmann R, Cohen AF. Rilonacept and Canakinumab. Br J Clin Pharmacol. 2011;71:639–641. doi: 10.1111/j.1365-2125.2011.03958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jesus AA, Goldbach-Mansky R. Il-1 Blockade in Autoinflammatory Syndromes. Annu Rev Med. 2014;65:223–244. doi: 10.1146/annurev-med-061512-150641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1β Inhibition and the Prevention of Recurrent Cardiovascular Events: Rationale and Design of the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (Cantos) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 94.Thompson S, James M, Wiebe N, et al. Cause of Death in Patients with Reduced Kidney Function. J Am Soc Nephrol. 2015;26:2504–2511. doi: 10.1681/ASN.2014070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Navaneethan SD, Schold JD, Arrigain S, et al. Cause-Specific Deaths in Non–Dialysis-Dependent Ckd. J Am Soc Nephrol. 2015;26:2512–2520. doi: 10.1681/ASN.2014101034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ridker PM, Howard CP, Walter V, et al. Effects of Interleukin-1β Inhibition with Canakinumab on Hemoglobin A1c, Lipids, C-Reactive Protein, Interleukin-6, and Fibrinogen: A Phase Iib Randomized, Placebo-Controlled Trial. Circulation. 2012;126:2739–2748. doi: 10.1161/CIRCULATIONAHA.112.122556. [DOI] [PubMed] [Google Scholar]
- 97.Chakraborty A, Tannenbaum S, Rordorf C, et al. Pharmacokinetic and Pharmacodynamic Properties of Canakinumab, a Human Anti-Interleukin-1β Monoclonal Antibody. Clin Pharmacokinet. 2012;51:e1–e18. doi: 10.2165/11599820-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Radin A, Marbury T, Osgood G, Belomestnov P. Safety and Pharmacokinetics of Subcutaneously Administered Rilonacept in Patients with Well-Controlled End-Stage Renal Disease (Esrd) J Clin Pharmacol. 2010;50:835–841. doi: 10.1177/0091270009351882. [DOI] [PubMed] [Google Scholar]
- 99.Lamkanfi M, Mueller JL, Vitari AC, et al. Glyburide Inhibits the Cryopyrin/Nalp3 Inflammasome. J Cell Biol. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Juliana C, Fernandes-Alnemri T, Wu J, et al. Anti-Inflammatory Compounds Parthenolide and Bay 11–7082 Are Direct Inhibitors of the Inflammasome. J Biol Chem. 2010;285:9792–9802. doi: 10.1074/jbc.M109.082305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ahn H, Kim J, Jeung E-B, Lee G-S. Dimethyl Sulfoxide Inhibits Nlrp3 Inflammasome Activation. Immunobiology. 2014;219:315–322. doi: 10.1016/j.imbio.2013.11.003. [DOI] [PubMed] [Google Scholar]
- **102.Ahn H, Kim J, Lee M-J, et al. Methylsulfonylmethane Inhibits Nlrp3 Inflammasome Activation. Cytokine. 2015;71:223–231. doi: 10.1016/j.cyto.2014.11.001. This group presents us with two synthetic molecules that specifically target the NLRP3 inflammasome at various points of its activation process. [DOI] [PubMed] [Google Scholar]
- 103.He Y, Varadarajan S, Muñoz-Planillo R, et al. 3,4-Methylenedioxy-B-Nitrostyrene Inhibits Nlrp3 Inflammasome Activation by Blocking Assembly of the Inflammasome. J Biol Chem. 2014;289:1142–1150. doi: 10.1074/jbc.M113.515080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Coll RC, Robertson AAB, Butler MS, et al. The Cytokine Release Inhibitory Drug Crid3 Targets Asc Oligomerisation in the Nlrp3 and Aim2 Inflammasomes. PLoS One. 2011;6:e29539. doi: 10.1371/journal.pone.0029539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Perregaux DG, McNiff P, Laliberte R, et al. Identification and Characterization of a Novel Class of Interleukin-1 Post-Translational Processing Inhibitors. J Pharmacol Exp Ther. 2001;299:187–197. [PubMed] [Google Scholar]
- 106.Laliberte RE, Perregaux DG, Hoth LR, et al. Glutathione S-Transferase Omega 1-1 Is a Target of Cytokine Release Inhibitory Drugs and May Be Responsible for Their Effect on Interleukin-1β Posttranslational Processing. J Biol Chem. 2003;278:16567–16578. doi: 10.1074/jbc.M211596200. [DOI] [PubMed] [Google Scholar]
- **107.Youm Y-H, Nguyen KY, Grant RW, et al. The Ketone Metabolite [Beta]-Hydroxybutyrate Blocks Nlrp3 Inflammasome-Mediated Inflammatory Disease. Nat Med. 2015;21:263–269. doi: 10.1038/nm.3804. This group finds an intriguing relation between a ketone body and NLRP3-inflammasome activation, presenting an endogenous NLRP3 inhibitor and raising the question whether nutritional therapy might have broader implications than previously thought. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *108.Mulay SR, Eberhard JN, Pfann V, et al. Oxalate-Induced Chronic Kidney Disease with Its Uremic and Cardiovascular Complications in C57bl/6 Mice. Am J Physiol Renal Physiol. 2016 doi: 10.1152/ajprenal.00488.2015. ajprenal.00488.02015. This paper presents a new reliable and technically simple murine model to cause oxalate-induced CKD. The results demonstrate the serious cardiovascular implications of oxalate-induced progressive renal disease in detail. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Elgstoen KBP, Johnsen LF, Woldseth B, et al. Plasma Oxalate Following Kidney Transplantation in Patients without Primary Hyperoxaluria. Nephrol Dial Transplant. 2010;25:2341–2345. doi: 10.1093/ndt/gfq065. [DOI] [PubMed] [Google Scholar]
- 110.Hoppe B, Kemper MJ, Bokenkamp A, et al. Plasma Calcium Oxalate Supersaturation in Children with Primary Hyperoxaluria and End-Stage Renal Failure. Kidney Int. 1999;56:268–274. doi: 10.1046/j.1523-1755.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- 111.Ogawa Y, Machida N, Ogawa T, et al. Calcium Oxalate Saturation in Dialysis Patients with and without Primary Hyperoxaluria. Urol Res. 2006;34:12–16. doi: 10.1007/s00240-005-0004-6. [DOI] [PubMed] [Google Scholar]
- **112.Hopp K, Cogal AG, Bergstralh EJ, et al. Phenotype-Genotype Correlations and Estimated Carrier Frequencies of Primary Hyperoxaluria. J Am Soc Nephrol. 2015;26:2559–2570. doi: 10.1681/ASN.2014070698. This study highlights a role for molecular analyses in PH diagnostics and prognostics by genotype-phenotype correlation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Porowski T, Zoch-Zwierz W, Konstantynowicz J, et al. Reference Values of Plasma Oxalate in Children and Adolescents. Pediatr Nephrol. 2008;23:1787–1794. doi: 10.1007/s00467-008-0889-8. [DOI] [PubMed] [Google Scholar]
- 114.Harris AH, Freel RW, Hatch M. Serum Oxalate in Human Beings and Rats as Determined with the Use of Ion Chromatography. J Lab Clin Med. 2004;144:45–52. doi: 10.1016/j.lab.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 115.Ogura H. Determinations of Oxalate in Urine and Plasma by Capillary Electrophoresis. Jap J Urol. 2000;91:547–555. doi: 10.5980/jpnjurol1989.91.547. [DOI] [PubMed] [Google Scholar]
- 116.Manoharan M, Schwille PO. Measurement of Oxalate in Human Plasma Ultrafiltrate by Ion Chromatography. J Chromatogr B Biomed Sci Appl. 1997;700:261–268. doi: 10.1016/s0378-4347(97)00310-1. [DOI] [PubMed] [Google Scholar]
- 117.Elgstoen KBP. Liquid Chromatography–Tandem Mass Spectrometry Method for Routine Measurement of Oxalic Acid in Human Plasma. Journal of Chromatography B. 2008;873:31–36. doi: 10.1016/j.jchromb.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 118.Hoppe B, Kemper MJ, Hvizd MG, et al. Simultaneous Determination of Oxalate, Citrate and Sulfate in Children’s Plasma with Ion Chromatography. Kidney Int. 1998;53:1348–1352. doi: 10.1046/j.1523-1755.1998.00891.x. [DOI] [PubMed] [Google Scholar]
- 119.Ogi M, Abe R, Nishitani T, et al. The Oxalate Level in Ultrafiltrate Fluid Collected from a Dialyzer Is Useful for Estimating the Plasma Oxalate Level in Hemodialysis Patients. Clin Exp Nephrol. 2006;10:118–123. doi: 10.1007/s10157-006-0406-y. [DOI] [PubMed] [Google Scholar]
- 120.Mori S, Beppu T. Secondary Renal Oxalosis. A Statistical Analysis of Its Possible Causes. Acta Pathol Jpn. 1983;33:661–669. [PubMed] [Google Scholar]
- 121.Chen SM, Chen TW, Lee YH, et al. Renal Excretion of Oxalate in Patients with Chronic Renal Failure or Nephrolithiasis. J Formos Med Assoc. 1990;89:651–656. [PubMed] [Google Scholar]
- 122.Tomson CR, Channon SM, Ward MK, Laker MF. Oxalate Retention in Chronic Renal Failure: Tubular Vs Glomerular Diseases. Clin Nephrol. 1989;32:87–95. [PubMed] [Google Scholar]
- 123.Hoppe B, Kemper MJ, Bokenkamp A, Langman CB. Plasma Calcium-Oxalate Saturation in Children with Renal Insufficiency and in Children with Primary Hyperoxaluria. Kidney Int. 1998;54:921–925. doi: 10.1046/j.1523-1755.1998.00066.x. [DOI] [PubMed] [Google Scholar]
- 124.Prenen JAC, Mees EJD, Boer P. Plasma Oxalate Concentration and Oxalate Distribution Volume in Patients with Normal and Decreased Renal Function. Eur J Clin Invest. 1985;15:45–49. doi: 10.1111/j.1365-2362.1985.tb00142.x. [DOI] [PubMed] [Google Scholar]
- 125.Gershman B, Sheth S, Dretler SP, et al. Relationship between Glomerular Filtration Rate and 24-Hour Urine Composition in Patients with Nephrolithiasis. Urology. 2012;80:38–42. doi: 10.1016/j.urology.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 126.Marangella M, Cosseddu D, Petrarulo M, et al. Thresholds of Serum Calcium Oxalate Supersaturation in Relation to Renal Function in Patients with or without Primary Hyperoxaluria. Nephrol Dial Transplant. 1993;8:1333–1337. [PubMed] [Google Scholar]
- 127.Watts RWE, Veall N, Purkiss P. Oxalate Dynamics and Removal Rates During Haemodialysis and Peritoneal Dialysis in Patients with Primary Hyperoxaluria and Severe Renal Failure. Clin Sci. 1984;66:591–597. doi: 10.1042/cs0660591. [DOI] [PubMed] [Google Scholar]
- 128.Tomson CRV, Channon SM, Ward MK, Laker MF. Plasma Oxalate Concentration, Oxalate Clearance and Cardiac Function in Patients Receiving Haemodialysis. Nephrol Dial Transplant. 1989;4:792–799. [PubMed] [Google Scholar]