Abstract
The important roles of miR-124 in the development and progression of various diseases are being increasing recognized. This study was aimed to investigate the potential roles of miR-124 in dopaminergic (DA) neuronal apoptosis and autophagy in Parkinson’s disease (PD) and to explore their mechanisms. Human SH-SY5Y cells that are treated with MPTP were transfected with mature miR-124 vector and control empty vector. The effect of MPTP on miR-124 mRNA level was analyzed using RT-PCR analysis. Furthermore, the effects of miR-124 expression on neuronal apoptosis and autophagy, as well as the expression of proteins in the AMPK/mTOR pathway, were analyzed using RT-PCR and western blotting. This study found that miR-124 was down-regulated in the MPTP-treated (100 μM) neurons, and miR-124 suppression significantly increased cell apoptosis and induced autophagy-associated protein expression, including that of Beclin 1 and increased the ratio of LC3 II/LC3 I compared with that in controls. In addition, in vitro rescue of miR-124 significantly decreased the percentage of apoptotic cells and the ratio of LC3 II/LC3 I, findings that were approximately equal to the controls. Moreover, miR-124 suppression increased p-AMPK but decreased p-mTOR levels in neurons. Our study suggested that miR-124 functions as a protector of DA neurons during PD through the involvement of cell apoptosis and autophagy by regulating the AMPK/mTOR pathway.
Keywords: Parkinson’s disease, dopaminergic nerve cells, miR-124, AMPK/mTOR pathway, cell apoptosis and autophagy
Introduction
Parkinson’s disease (PD) is a degenerative disease of the nervous system that occurs frequently among the elderly, and has become the second largest killer of the elderly, ranking only second to Alzheimer’s disease (AD) [1]. Statistics has shown that the morbidity for PD is high and with a younger trend in recent years [2]. Although treatment methods including drugs and surgery have produced certain effects on attenuating the symptoms of PD, controlling PD development and progression remains challenging due to its complicated pathogenesis, which also places a huge economic burden on society and patients’ families [3]. Previous evidence has shown that progressive lesions of the dopaminergic neurons in the midbrain are the major pathological features of PD [4]. Loss of DA neurons within the substantia nigra pars compacta of the basal ganglia is the visible sign of PD [5]. Activated microglia inflicts huge injury to nerve cells, and massive apoptosis of DA nerve cells is observed in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced mouse PD model [6]. Therefore, strategies focused on explaining the pathogenic mechanism at the molecular level may provide an effective cure for PD.
microRNAs (miRNAs) are endogenous 20- to 22- nt in length and are highly conserved non-coding RNAs, that function in a variety of biological processes at the transcriptional or post-transcriptional level through targeting the 3’UTR of genes [7]. Increasing evidence has demonstrated that various of miRNAs are involved in the progression and biology of neurodegeneration [8-10]. For example, miR-7 protects nerve cells from damage caused by α-Syn (SNCA)-induced proteins by targeting the 3’UTR of SNCA [11], and miR-133b expression is abnormal in case of PD with missing dopaminergic neurons (DN) and regulates the homologous structure domain transcription factor 3 (Pitx3) [12]. In recent years, studies have shown that miR-124 is overexpressed in the brain compared with other organs [13-15]. For example, miR-124 is abundant in the brain in case of PD, and the down-regulation of miR-124 may provide a therapeutic target for MPTP-induced PD in mice [16]. In addition, Wang et al reported that miR-124 could regulate MPTP-induced PD nerve cell apoptosis and autophagy by targeting Bim [17]. Although several researches have investigated the role and mechanism of miR-124 in PD, few have reported the mechanism of miR-124 in regulating PD nerve cell apoptosis and autophagy by regulating the AMPK/mTOR pathway.
In the current study, we investigated the potential effects of miR-124 expression on the apoptosis and autophagy of PD DA cells and on the AMPK/mTOR pathway which were induced by MPTP using SH-SY5Y cells and siRNA-mediated gene silencing. Comprehensive experimental methods were used to assess the effects of miR-124 suppression on AMPK/mTOR pathway related protein expression. This study was aimed to investigate the possible effects of miR-124 on DA cell apoptosis and autophagy and to elucidate its potential mechanism of action.
Material and methods
Cell lines
Human neuroblastoma SH-SY5Y and SK-N-SH cell lines were cultured in DMEM (Dulbecco’s Modified Eagle Medium) solutions supplemented with 10% fetal bovine serum (FBS) and 100 U/mL penicillin (Sigma-Aldrich, St Louis, MO, USA) in an atmosphere of 5% CO2 at 37°C. For the PD model construction, cells were incubated with MPTP at different concentration, as indicated, and then harvested at the indicated time points for further analysis.
Cell transfection
The miRNA vectors including miR-124 mimic, miR-124 negative, miR-124 inhibitor, and miR-124 inhibitor control were purchased from Ambion (Foster City, CA, USA). Cells transfected with miR-124 negative or miR-124 inhibitor control vectors are the control for cells transfected with miR-124 mimic or miR-124 inhibitor, respectively. Cell transfections were conducted based on the Lipofection 2000 protocol.
Apoptosis assay
Cell apoptosis was performed using Annexin V-Cy5 and propidium iodide (PI) staining and analyzed by flow cytometry [18]. Briefly, after being transfected with siRNA or a control vector for 24 h, cells were harvested and washed 3 times with PBS buffer. Subsequently, cells were pelleted and resuspended in 5 μL Annexin V-binding buffer containing Annexin V-Cy5 (1:1000) and 5 μL PI at room temperature for 10 min. Then cells were analyzed using an FACS Calibur flow cytometer (Becton-Dickinson, CA, USA). Annexin V-positive and PI-negative cells were considered to be apoptotic.
Real-time PCR
Total RNA was extracted from cells using TRIzol Reagent (Invitrogen) as previously described [19]. The isolate RNA was treated with RNase-free DNase I (Promega Biotech, USA), and the concentration and purity of the isolated RNA were detected using SMA 400 UV-VIS (Merinton, Shanghai, China). cDNA was synthesized using the reverse transcriptase PrimerScript 1st Strand cDNA Synthesis Kit (Invitrogen, USA). Expressions for targets were detected using SYBR ExScript RT-qPCR Kit (Takara, China). Phosphoglyceraldehyde dehydrogenase (GAPDH) was chosen as the internal control. The primers used for targets amplification are shown in Table 1.
Table 1.
Primers used for targets amplification in this study
| Target | Primer | Sequence (5’-3’) |
|---|---|---|
| GAPDH | Sense | GGGTGGAGCCAAACGGGTC |
| Anti-sense | GGAGTTGCTGTTGAAGTCGCA | |
| Beclin 1 | Sense | CTGGACACGAGTTTCAAGATCCTG |
| Anti-sense | GGGCATGGTAGCACACAGACCTC | |
| LC3 | Sense | GGAAGAATGACAGATGAC |
| Anti-sense | CTTTCAATCTGTTGGCTG | |
| miR-124 | GCGAGGATCTGTGAATGCCAAA | |
| U6 | GCTTCGGCAGCACATATACTAAAAT |
Western blotting analysis
Cells cultured for 48 h were lapped with RIPA (radioimmunoprecipitation, Sangon Biotech, China) lysate containing PMSF (phenylmethanesufonyl fluoride, Sigma, USA), and then were centrifuged at 12,000 rpm for 10 min at 4°C. Supertanant was collected for the protein concentrations were determined using a BCA protein assay kit (Pierce, Rochford, IL). Proteins were separated on a 10% sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by transferred onto a Polyvinylidencefluoride (PVDF) membrane (Mippore) [20]. Membrane was blocked in Tris Buffered Saline Tween (TBST) containing 5% non-fat milk for 1 h at room temperature, and then incubated with rabbit anti-human antibodies (Beclin 1, LC3 II and LC3 I, 1:100 dilution, Invitrogen) overnight at 4°C. Subsequently, membrane was incubated with a horseradish-peroxidase labeled goat anti-rat secondary antibody (1:1000 dilution) for 1 h at room temperature. Finally, PVDF membrane was washed with 1×TBST buffer for 10 min with 3 times. Detection was conducted using the development of X-ray film after chromogenic substrate with an enhanced chemiluminescence (ECL) method. GAPDH served as the internal control.
Statistical analysis
All of the experiments were conducted independently 3 times. The data were expressed as the mean ± SD. Statistical analyses were performed using graph prism 5.0 software (GraphPad Prism, San Diego, CA). Significant differences in the data of the two groups were analyzed using Student’s t test. The P<0.05 was considered to be statistically significant.
Results
miR-124 expression in DA cells
To assess the influence of MPTP on miR-124 expression in DA cells, the mRNA expression of miR-124 in DA cells was analyzed using RT-PCR (Figure 1). miR-124 expression was significantly suppressed by 100 μM (P<0.05) and 200 μM MPTP (P<0.01) compared to that in controls. Interestingly, the results showed that 50 μM MPTP slightly, but not significantly, suppressed miR-124 expression in DA cells.
Figure 1.
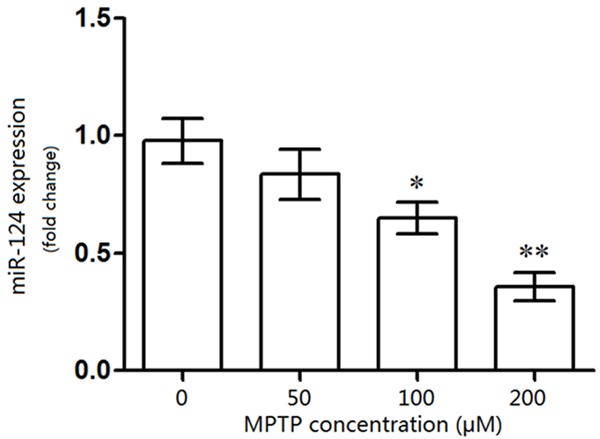
Influence of MPTP on miR-124 expression in dopaminergic nerve (DA) cells. The DA neurons were treated with different concentration of MPTP, and compared with the controls (0 μM of MPTP), expression of miR-124 was significantly decreased in MPTP-treated (100 μM) DA cells. *P<0.05, **P<0.01, compared with the control (0 μM of MPTP).
miR-124 suppression increased cell autophagy-related protein expression
Because LC3 II is a marker for the autophagosome, as is the autophagy gene Beclin 1, the protein levels of LC3 II and Beclin 1 represent the level of autophagosome activity [21,22]. To determine whether miR-124 expression was correlated with cell autophagy, the autophagy related-protein expression was assessed using western blotting (Figure 2). The relative expression of Beclin 1 was significantly increased by miR-124 suppression in the MPTP group compared with the control (P<0.05, Figure 2A), but its expression was significantly increased by miR-124 inhibitor compared to the negative control (P<0.05, Figure 2A and 2C). In addition, the LC3 II/LC3 I ratio was significantly increased by miR-124 suppression in the MPTP group compared with the control group (P<0.01, Figure 2B), but this ratio was also significantly increased by miR-124 inhibitor compared to the negative control, which elicited a slight decrease (P<0.05, Figure 2B and 2C).
Figure 2.
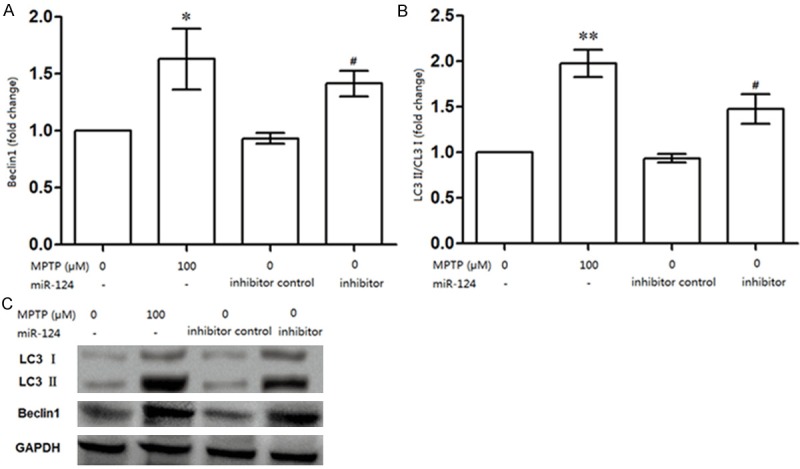
miR-124 expression was correlated with cell autophagy in DA cells. A: miR-124 suppression could significantly increase the relative expression of Beclin 1; B: miR-124 suppression significantly increased the ratio of LC3 II/LC3 I in DA cells compared to that in controls. C: Western blot analysis about the protein expressions of Beclin 1, LC3 II and LC3 I in DA cells. *P<0.05, ** P<0.01, compared with the control (0 μM MPTP); #P<0.05, compared with the inhibitor contro.
miR-124 suppression was correlated with DA cell apoptosis
Further experiments were performed to identify whether miR-124 suppression was correlated with DA cell apoptosis (Figure 3). When miR-124 was down-regulated in the MPTP-treated neurons, the percentage of apoptotic DA cells was significantly greater (approximately 15.5%) than that in the control (approximately 6.2%) (P<0.05). Additionally, a similar difference in the apoptotic DA cell percentage was observed between the negative (approximately 5.01%) and the miR-124 inhibitor group (approximately 12.2%) (Figure 3A and 3B).
Figure 3.

Effects of miR-124 suppression on DA cell apoptosis. When miR-124 was significantly suppressed in MPTP-treated (100 μM) neurons, the percentage of apoptotic DA cells was increased (15.5%) compared to the controls (6.2%). Besides, when miR-124 expression was suppressed by an inhibitor, percentage of apoptotic DA cells was increased (12.2%) than that in negative control (5.01%). *P<0.05, compared with the control (0 μM MPTP); #P<0.05; compared with the inhibitor control.
In vitro effect of miR-124 expression on DA cell autophagy and apoptosis
To rescue the damage evoked by MPTP in DA cells, miR-124 was overexpressed in vitro (Figure 4). Interestingly, the LC3 II/LC3 I ratio in the MPTP + negative control group was significantly increased compared to that in the MPTP + miR-124 group (P<0.01), indicating that miR-124 overexpression could alleviate the damage induced by MPTP in DA cells (Figure 4A and 4B). Furthermore, the percentage of apoptotic DA cells was significantly decreased by miR-124 overexpression (from 15.7% to 5.28 %, P<0.01, Figure 4C and 4D), suggesting that miR-124 overexpression could rescue the harmful influence of MPTP on DA cell apoptosis.
Figure 4.
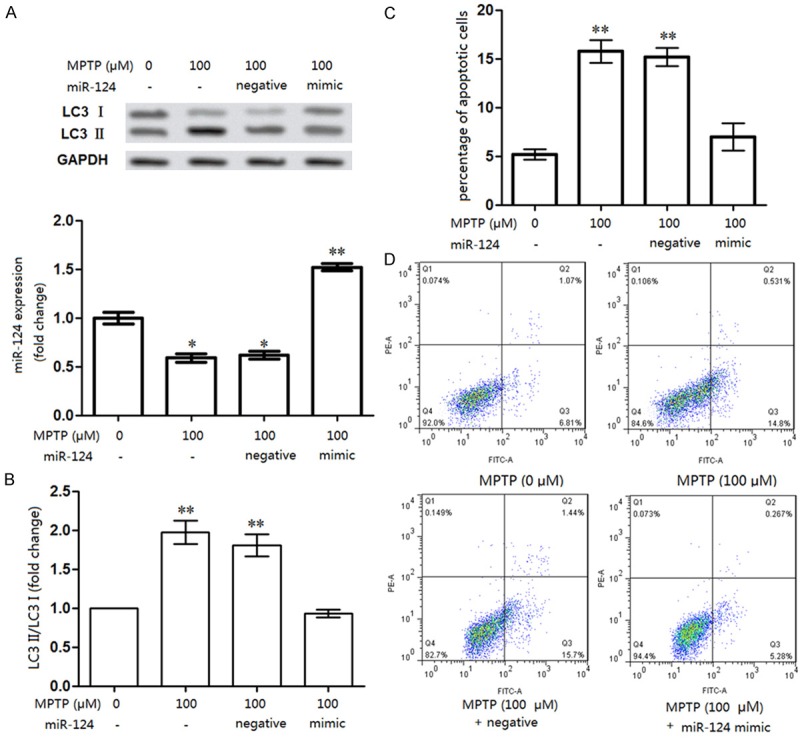
Rescue experiments for miR-124 on DA neurons apoptosis and autophagy. A, B: mRNA and protein levels of ratio of LC3 II/LC3 I in neurons were significantly decreased when miR-124 was rescued in vitro; C, D: Percentage of apoptotic neurons was significantly decreased by miR-124 rescuing in vitro. Compared to the control (6.81%), the percentage of apoptotic cells was increased to 14.8% by MPTP treatment (miR-124 suppression), but declined to 5.28% when miR-124 was rescued (miR-124 mimic transfection) in vitro. **P<0.01, compared to the control (0 μM MPTP).
Effects of miR-124 suppression on the AMPK/mTOR pathway
When miR-124 expression was significantly inhibited by MPTP treatment in DA cells, phosphorylated (p)-AMPK level was significantly increased, whereas p-mTOR expression was significantly decreased in MPTP group compared to that in the control group (Figure 5A). Additionally, similar differences in p-MAPK and p-mTOR expressions were observed between the negative and the miR-124 inhibitor group. Besides, to further determine whether AMPK or mTOR expression could block the effects of MPTP on cell apoptosis, the experiment of AMPK inhibitor group on cell apoptosis should be conducted. Compound C has been reported to be an inhibitor for AMPK by blocking the phosphorylation process [23,24], we therefore choose compound C as the AMPK inhibitor to analyze whether AMPK and mTOR could block the MPTP-induced (miR-124 down-regulation) effects on cell apoptosis. Figure 5B showed that the p-AMPK level was decreased by the inhibitor of compound C application. In addition, compared to that in control cells, 1 μM compound C slightly but not significantly, increased the percentage of apoptotic cells (Figure 5C), the application of AMPK inhibitor suggesting that AMPK expression showed no significant influence on apoptosis affected by the miR-124 regulation (MPTP-induced). Taken together, these results suggested the promote effect of miR-124 down-regulation in SH-SY5Y cells.
Figure 5.

Influence of miR-124 expression on AMPK/mTOR pathway associated proteins expression. A: The p-AMPK was increased but p-mTOR was decreased by the suppressed miR-124 in MPTP-treated neurons than the control, the same tendency was observed between the negative and anti-miR-124 group; B: The application of AMPK inhibitor, 1 μM compound C, decreased the p-AMPK level; C: When cells were treated with compound C, the percentage of apoptotic cells was slightly, but not significantly increased. *P<0.05, compared to the control cells (treated with 0 μM MPTP), #P<0.05, compared to the inhibitor control.
Effects of miR-124 expression on cell apoptosis in SK-N-SH cells
To further determine the effects of miR-124 suppression on DA cell apoptosis and to reveal its potential mechanism, another PD cell line of SK-N-SH was used to assess the miR-124 expression on cell apoptosis (Figure 6). The treatment on SK-N-SH cells was similar to that on SH-SY5Y cells. Similar to that in SH-SY5Y cells, miR-124 expression was down-regulated in the MPTP-treated SK-N-SH cells (Figures 6A and 4A). Accordingly, the influence of miR-124 expression on SK-N-SH cell apoptosis was also similar to that in SH-SY5Y cells (Figures 6B and 5C). Additionally, the effects of the overexpressed miR-124 on AMPK/mTOR pathway-related protein expression in SK-N-SH cells was also as the same as that in SH-SY5Y cells (Figures 6C and 5A). These data might be the validation on confirming our investigated results about the miR-124 expression on DA cell apoptosis on another angle.
Figure 6.
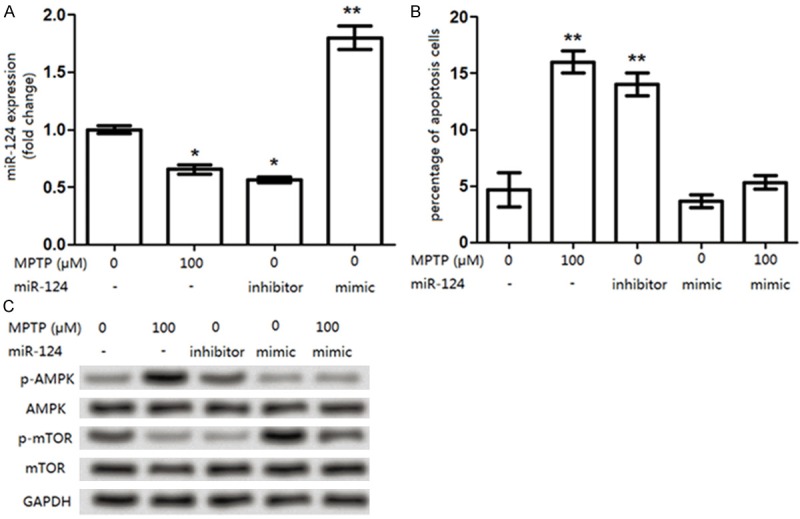
Effects of miR-124 expression on cell apoptosis in in SK-N-SH cells. A: When SK-N-SH cells were treated with 100 μM MPTP, miR-124 expression was significantly deceased compared to that in control cells (0 μM MPTP), but was significantly increased by the miR-124 mimic transfection; B: The percentage of apoptotic SK-N-SH cells was significantly increased by the MPTP treatment (miR-124 suppression) compared with that in control cells (0 μM MPTP), similar results was found in cells treated with the miR-124 inhibitor. However, there was no significant difference for the apoptotic cell percentage between the miR-124 mimic group and the MPTP + miR-124 mimic group; C: The levels for p-AMPK, p-mTOR, AMPK, and mTOR in each were analyzed in each group. The MPTP treatment increased the p-AMPK expression but decreased the p-mTOR level in SK-N-SH cells, similar results were found in the miR-124 inhibitor group. *P<0.05 and **P<0.01, compared to the control cells (0 μM MPTP).
Discussion
Increasing evidence has demonstrated that miRNAs play pivotal roles in neuron biology and miR-124 is abundantly expressed in neurons and during CNS development [25,26]. To date, miR-124 alterations have been reported to be involved in a variety of diseases such as cancers, embryonic CNS development, and medulloblastoma [27,28]. Besides, miR-124 expression is down-regulated in neurons from the MPTP-induced PD [16]. On the other hand, previous review reported that there are various kinds of cell models for the PD research [29]. For example, the non-neuronal tumor PC12 cell line [30], the neuronal tumor SK-N-SH and SH-SY5Y cell lines [31], and the original generation of brain cells. Hence, based on the former theoretical basis, PC12 cell line has been widely used for the research of neuron functions including differentiation, apoptosis, the neurotransmitter secretion, and the potential molecular mechanism, whereas the SH-SY5Y cell line has been widely used as the dopaminergic neuron cell model in the research of PD, as well as the SK-N-SH cell line. Additionally, there are several methods for the construction of PD cell model, such as the permanent DA depletion model induced by neurotoxicity including MPTP, OHDA (6-hydroxy dopamine) [32], and MA (methamphetamine) [33] 4-methylenedioxymethamphetamine (MDMA, and the reversible drug model including reserpine and rotenone. Taken together, we choose the two kinds of human neuroblastoma SH-SY5Y and SK-N-SH cell lines and treated them with the MPTP to construct the PD cell model. In this study, we analyzed the role of miR-124 expression in DA SH-SY5Y cells treated with MPTP. In agreement with previous data, our results showed that miR-124 was down-regulated in the MPTP-treated SH-SY5Y [17]. Moreover, our data showed that miR-124 suppression significantly increased cell apoptosis and autophagy-related proteins expression, but these effects were reversed by miR-124 rescue in vitro. In addition, miR-124 suppression significantly increased AMPK/mTOR pathway-related protein expression, including that of p-AMPK and p-mTOR.
When MPTP enters the brain through penetrating the blood-brain barrier, it can be catalyzed into MPDP+ by MAO-B and then transformed to MPP+ via spontaneous oxidation, leading to its high affinity for the dopamine transporter (DAT) in DA neurons [34]. The entered of MPP+ causes extensive damage to DA neurons by blocking ATP synthesis in mitochondria or destroying the normal cell metabolism [35]. Previous evidence has shown that the brain-abundant miR-124 may play significant roles in PD, and its expression can be suppressed by MPTP in MPP+-induced PD mouse model. In this study, miR-124 was down-regulated in MPTP-treated SH-SY5Y cells, and high dose of MPTP (from 100 μM to 200 μM) presented excellent suppression of miR-124 expression, suggesting a correlation between miR-124 expression and PD; and the MPTP-induced miR-124 suppressed in SH-SY5Y cells may be similar to that in the previously described the MPP+ mouse model.
Accordingly, we investigated the effects of miR-124 suppression on DA neurons apoptosis and autophagy in SH-SY5Y cells, which were treated with 100 μM MPTP. Beclin 1, a mammalian orthologue of yeast Atg6, plays a central role in cell autophagy, a process of programmed cell survival [36], while LC3, a microtubule-associated protein 1 light chain 3, Atg8, which localizes on the autophagosome membrane, is another autophagy marker [21]. Soluble LC3 (LC3 I) can be converted to the autophagic vesicle-associated form (LC3 II) during autophagosome formation; thus, the ratio of LC3 II/LC3 I is an vital marker for autophagy [21]. Cell autophagy together with apoptosis are the necessary types of programmed cell death in cell survival in PD [37,38]. Beclin 1 mediates autophagy in the neurodegenerative pathology of PD [39], while LC3 involves in PD autophagy through binding externalized cardiolipin on injured mitochondria [40], indicating the symbiotic roles of Beclin 1 and LC3 in PD autophagy. The associations between miR-124 and autophagy has not been fully discussed. However, Frankel et al proved that miR-124 may regulate autophagy through targeting 52 target genes including Beclin 1 [41]. In this study, when miR-124 was down-regulated in MPTP-treated SH-SY5Y cells, both Beclin 1 and the ratio of LC3 II/LC3 I ratio were significantly increased compared with that in the controls, indicating that miR-124 suppression may play a role in inducing autophagy in DA neurons autophagy. However, our data showed that the percentage of SH-SY5Y cell apoptosis was significantly increased by miR-124 suppression compared to the control, indicating that miR-124 suppression may play an accelerating role in PD development via induction of cell apoptosis. Moreover, we performed rescue experiments to verify the effects of miR-124 suppression on neurons apoptosis and autophagy in reverse. Our results revealed that when miR-124 expression was rescued in vitro, the percentage of cell apoptosis and the ratio of LC3 II/LC3 I were both significantly decreased to the original levels which were approximately to that in controls (Figure 4), suggesting that neurons apoptosis and autophagy could be affected by miR-124 suppression.
Subsequently, p-AMPK protein levels were markedly increased, while p-mTOR protein levels were significantly decreased when miR-124 expression was suppressed in neurons (Figure 5). AMPK is an important receptor that helps cells recognize the changes in energy regulation while mTOR functions as a vital signaling complex in many cellular processes; the roles of AMPK and mTOR in autophagy has become the hot spot in recent years [42]. Both mTOR and AMPK in autophagy and apoptosis have significant roles in tumors progression, such as AMPK induced apoptosis and autophagy in breast cancer or during myocardial ischemia [43,44]. In addition, the activated p-AMPK and p-mTOR have been suggested to participate in neurons differentiation and anti-oxidative stress [45]. Basgupta et al proved that the p-AMPK level was increased by resveratrol in neurons protection [46]. Besides, increasing p-AMPK activation would induce colon cell apoptosis through suppressing p-mTOR in colon cancer [47]. On the other side, Ikeda et al used 2 μM compound C to analyze the effects of heparin cofactor II on angiogenesis by the activated-AMPK signal pathway [23]. Therefore, we used the AMPK inhibitor, 1 μM compound C, to analyze the effects of AMPK on the MPTP-induced DA cell apoptosis and our results showed that compound C application decreased the p-AMPK level and the apoptotic cells were also increased (Figure 5), implying the influence of AMPK on MPTP-induced cell apoptosis on the opposite side. In addition, we further analyzed the potential effects of miR-124 expression on another DA cell line of SK-N-SH. Our results revealed that miR-124 suppression induced DA cell apoptosis, and increased the levels of p-AMPK and p-mTOR, which is similar to the results observed in SH-SY5Y cells (Figure 6). Based on our results, we speculated that miR-124 suppression may induce neurons apoptosis and autophagy through activating the p-AMPK pathway and then resulted in the inhibition of the mTOR signaling in PD.
In conclusion, the data presented herein suggest that miR-124 suppression increases neuronal apoptosis and autophagy by regulating the AMPK/mTOR signaling pathway in PD. miR-124 is down-regulated in MPTP-treated PD cells, and the inhibition of miR-124 increases neurons apoptosis and autophagy, as well as the Beclin 1 and the LC3 II/LC3 I ratio. The mechanisms underlined in our study may provide a theoretical basis for the possible application of miR-124 in neuronal protection in PD.
Acknowledgements
This work was supported by National Natural Science Foundation of China (81371397) and The Guangdong Provincial Clinical Medical Centre for Neurosurgery (2013B020400005).
References
- 1.Beitz JM. Parkinson’s disease: a review. Front Biosci. 2014;6:65–74. doi: 10.2741/s415. [DOI] [PubMed] [Google Scholar]
- 2.Homma T, Mochizuki Y, Takahashi K, Komori T. Medial temporal regional argyrophilic grain as a possible important factor affecting dementia in Parkinson’s disease. Neuropathology. 2015;16:12208. doi: 10.1111/neup.12208. [DOI] [PubMed] [Google Scholar]
- 3.Gambaryan PY, Kondrasheva IG, Severin ES, Guseva AA, Kamensky AA. Increasing the Efficiency of Parkinson’s Disease Treatment Using a poly (lactic-co-glycolic acid) (PLGA) Based L-DOPA Delivery System. Exp Neurobiol. 2014;23:246–252. doi: 10.5607/en.2014.23.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le PG. Progressive loss of dopaminergic neurons in the ventral midbrain of adult mice heterozygote for Engrailed1 : A new genetic model for Parkinson’s disease? Parkinsonism Relat Disord. 2008;14:S107–S111. doi: 10.1016/j.parkreldis.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Mcnaught KS, Belizaire R, Jenner P, Olanow CW, Isacson O. Selective loss of 20S proteasome alpha-subunits in the substantia nigra pars compacta in Parkinson’s disease. Neurosci Lett. 2002;326:155–158. doi: 10.1016/s0304-3940(02)00296-3. [DOI] [PubMed] [Google Scholar]
- 6.Nicotra A. Apoptotic molecules and MPTP-induced cell death. Neurotoxicol Teratol. 2002;24:599–605. doi: 10.1016/s0892-0362(02)00213-1. [DOI] [PubMed] [Google Scholar]
- 7.Bartel DP. MicroRNAs: Target Recognition and Regulatory Functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bushati N, Cohen SM. MicroRNAs in neurodegeneration. Curr Opin Neurobiol. 2008;18:292–296. doi: 10.1016/j.conb.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Sonntag KC. MicroRNAs and deregulated gene expression networks in neurodegeneration. Brain Res. 2010;1338:48–57. doi: 10.1016/j.brainres.2010.03.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abe M, Bonini NM. MicroRNAs and neurodegeneration: role and impact. Trends Cell Biol. 2013;23:30–36. doi: 10.1016/j.tcb.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Junn E, Lee KW, Jeong BS, Chan TW, Im JY, Mouradian MM. Repression of α-synuclein expression and toxicity by microRNA-7. Proc Natl Acad Sci U S A. 2009;106:13052–13057. doi: 10.1073/pnas.0906277106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J, Inoue K, Ishii J, Vanti WB, Voronov SV, Murchison E, Hannon G, Abeliovich A. A MicroRNA Feedback Circuit in Midbrain Dopamine Neurons. Science. 2007;317:1220–1224. doi: 10.1126/science.1140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia H, Cheung WK, Ng SS, Jiang X, Jiang S, Sze J, Leung GK, Lu G, Chan DT, Bian XW. Loss of brain-enriched miR-124 microRNA enhances stem-like traits and invasiveness of glioma cells. J Biol Chem. 2012;287:9962–9971. doi: 10.1074/jbc.M111.332627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu K, Liu Y, Mo W, Qiu R, Wang X, Wu JY, He R. MiR-124 regulates early neurogenesis in the optic vesicle and forebrain, targeting NeuroD1. Nucleic Acids Res. 2011;39:2869–2879. doi: 10.1093/nar/gkq904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilje P, Gidlöf O, Rundgren M, Cronberg T, Al-Mashat M, Olde B, Friberg H, Erlinge D. The brain-enriched microRNA miR-124 in plasma predicts neurological outcome after cardiac arrest. Crit Care. 2014;18:206–214. doi: 10.1186/cc13753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanagaraj N, Beiping H, Dheen ST, Tay SSW. Downregulation of miR-124 in MPTP-treated mouse model of Parkinson’s disease and MPP iodide-treated MN9D cells modulates the expression of the calpain/cdk5 pathway proteins. Neuroscience. 2014;272:167–179. doi: 10.1016/j.neuroscience.2014.04.039. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Ye Y, Zhu Z, Mo L, Lin C, Wang Q, Gong X, He X, Lu G, Lu F, Zhang S. MiR-124 Regulates Apoptosis and Autophagy Process in MPTP Model of Parkinson’s Disease by Targeting to Bim. Brain Pathol. 2015;15:12267. doi: 10.1111/bpa.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shynkar VV, Klymchenko AS, Kunzelmann C, Duportail G, Muller CD, Demchenko AP, Freyssinet JM, Mely Y. Fluorescent biomembrane probe for ratiometric detection of apoptosis. J Am Chem Soc. 2007;129:2187–2193. doi: 10.1021/ja068008h. [DOI] [PubMed] [Google Scholar]
- 19.Fransson L, Rosengren V, Saha TK, Grankvist N, Islam T, Honkanen RE, Sjöholm Å, Ortsäter H. Mitogen-activated protein kinases and protein phosphatase 5 mediate glucocorticoid-induced cytotoxicity in pancreatic islets and β-cells. Mol Cell Endocrinol. 2014;383:126–136. doi: 10.1016/j.mce.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y, Li Q, Chen XZ. Detecting protein-protein interactions by far western blotting. Nat Protoc. 2007;2:3278–3284. doi: 10.1038/nprot.2007.459. [DOI] [PubMed] [Google Scholar]
- 21.Xiao J, Zhu X, He B, Zhang Y, Kang B, Wang Z, Ni X. MiR-204 regulates cardiomyocyte autophagy induced by ischemia-reperfusion through LC3-II. J Biomed Sci. 2011;18:1423–0127. doi: 10.1186/1423-0127-18-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikeda Y, Aihara K, Yoshida S, Iwase T, Tajima S, Izawa-Ishizawa Y, Kihira Y, Ishizawa K, Tomita S, Tsuchiya K, Sata M, Akaike M, Kato S, Matsumoto T, Tamaki T. Heparin cofactor II, a serine protease inhibitor, promotes angiogenesis via activation of the AMP-activated protein kinase-endothelial nitric-oxide synthase signaling pathway. J Biol Chem. 2012;287:34256–34263. doi: 10.1074/jbc.M112.353532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ljubica V, Maja M, Kristina J, Urosh V, Emina S, Esma I, Marko P, Ljubica HT, Tamara KS, Vladimir B. Compound C induces protective autophagy in cancer cells through AMPK inhibition-independent blockade of Akt/mTOR pathway. Autophagy. 2011;7:40–50. doi: 10.4161/auto.7.1.13883. [DOI] [PubMed] [Google Scholar]
- 25.Maiorano NA, Mallamaci A. The pro-differentiating role of miR-124: indicating the road to become a neuron. RNA Biol. 2010;7:528–533. doi: 10.4161/rna.7.5.12262. [DOI] [PubMed] [Google Scholar]
- 26.Tapocik JD, Luu TV, Mayo CL, Wang BD, Doyle E, Lee AD, Lee NH, Elmer GI. Neuroplasticity, axonal guidance and micro-RNA genes are associated with morphine self-administration behavior. Addict Biol. 2013;18:480–495. doi: 10.1111/j.1369-1600.2012.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li KK, Pang JC, Ching AK, Wong CK, Kong X, Wang Y, Zhou L, Chen Z, Ng HK. miR-124 is frequently down-regulated in medulloblastoma and is a negative regulator of SLC16A1. Hum Pathol. 2009;40:1234–1243. doi: 10.1016/j.humpath.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Visvanathan J, Lee S, Lee B, Lee JW, Lee SK. The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes Dev. 2007;21:744–749. doi: 10.1101/gad.1519107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlesinger HR, Gerson JM, Moorhead PS, Maguire H, Hummeler K. Establishment and characterization of human neuroblastoma cell lines. Cancer Res. 1976;36:3094–3100. [PubMed] [Google Scholar]
- 30.Tischler AS, Greene LA. Morphologic and cytochemical properties of a clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Lab Invest. 1978;39:77–89. [PubMed] [Google Scholar]
- 31.Lombet A, Zujovic V, Kandouz M, Billardon C, Carvajal-Gonzalez S, Gompel A, Rostène W. Resistance to induced apoptosis in the human neuroblastoma cell line SK-N-SH in relation to neuronal differentiation. Role of Bcl-2 protein family. Eur J Biochem. 2001;268:1352–1362. doi: 10.1046/j.1432-1327.2001.02002.x. [DOI] [PubMed] [Google Scholar]
- 32.Ungerstedt U. 6-hydroxy-dopamine induced degeneration of central monoamine neurons. Eur J Pharmacol. 1968;5:107–110. doi: 10.1016/0014-2999(68)90164-7. [DOI] [PubMed] [Google Scholar]
- 33.Shankaran M, Yamamoto BK, Gudelsky GA. Ascorbic acid prevents 3,4-methylenedioxymethamphetamine (MDMA)-induced hydroxyl radical formation and the behavioral and neurochemical consequences of the depletion of brain 5-HT. Synapse. 2001;40:55–64. doi: 10.1002/1098-2396(200104)40:1<55::AID-SYN1026>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 34.Gainetdinov RR, Fumagalli F, Jones SR, Caron MG. Dopamine transporter is required for in vivo MPTP neurotoxicity: evidence from mice lacking the transporter. J Neurochem. 1997;69:1322–1325. doi: 10.1046/j.1471-4159.1997.69031322.x. [DOI] [PubMed] [Google Scholar]
- 35.Alvarez-Fischer D, Noelker C, Grünewald A, Vulinović F, Guerreiro S, Fuchs J, Lu L, Lombès A, Hirsch EC, Oertel WH. Probenecid potentiates MPTP/MPP+ toxicity by interference with cellular energy metabolism. J Neurochem. 2013;127:782–792. doi: 10.1111/jnc.12343. [DOI] [PubMed] [Google Scholar]
- 36.Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18:571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chew KCM, Ang ET, Tai YK, Tsang F, Lo SQ, Ong E, Ong WY, Shen HM, Lim KL, Dawson VL. Enhanced autophagy from chronic toxicity of iron and mutant A53T α-synuclein: Implications for neuronal cell death in Parkinson’s disease. J Biol Chem. 2011;286:33380–33389. doi: 10.1074/jbc.M111.268409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirsch EC. Apoptosis, Glial Cells and Parkinson’s Disease. Res Perspect Neurosci. 2001:97–107. [Google Scholar]
- 39.Spencer B, Potkar R, Trejo M, Rockenstein E, Patrick C, Gindi R, Adame A, Wyss-Coray T, Masliah E. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson’s and Lewy body diseases. J Neurosci. 2009;29:13578–13588. doi: 10.1523/JNEUROSCI.4390-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chu CT, Bayır H, Kagan VE. LC3 binds externalized cardiolipin on injured mitochondria to signal mitophagy in neurons: Implications for Parkinson disease. Autophagy. 2014;10:376–378. doi: 10.4161/auto.27191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frankel LB, Lund AH. MicroRNA regulation of autophagy. Carcinogenesis. 2012;33:2018–2025. doi: 10.1093/carcin/bgs266. [DOI] [PubMed] [Google Scholar]
- 42.Kim J. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takagi H, Matsui Y, Hirotani S, Sakoda H, Asano T, Sadoshima J. AMPK mediates autophagy during myocardial ischemia in vivo. Autophagy. 2007;3:405–407. doi: 10.4161/auto.4281. [DOI] [PubMed] [Google Scholar]
- 44.Lu C, Wang W, Jia Y, Liu X, Tong Z, Li B. Inhibition of AMPK/autophagy potentiates parthenolide-induced apoptosis in human breast cancer cells. J Cell Biochem. 2014;115:1458–1466. doi: 10.1002/jcb.24808. [DOI] [PubMed] [Google Scholar]
- 45.Zeng M, Zhou JN. Roles of autophagy and mTOR signaling in neuronal differentiation of mouse neuroblastoma cells. Cell Signal. 2008;20:659–665. doi: 10.1016/j.cellsig.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 46.Dasgupta B. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim GT, Lee SH, Kim YM. Quercetin Regulates Sestrin 2-AMPK-mTOR Signaling Pathway and Induces Apoptosis via Increased Intracellular ROS in HCT116 Colon Cancer Cells. J Cancer Prev. 2013;18:264–270. doi: 10.15430/JCP.2013.18.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]


