Abstract
Mesenchymal stem cells (MSCs) constitute one of the most powerful tools for therapeutic angiogenesis in infarcted hearts. However, conventional MSC transplantation approaches result in insufficient therapeutic effects due to poor retention of graft cells in severe ischemic diseases. Cell sheet technology has been developed as a new method to prolong graft cell retention even in ischemic tissue. Recently, we demonstrated that hypoxic pretreatment enhances the therapeutic efficacy of cell sheet implantation in infarcted mouse hearts. In this study, we investigated whether hypoxic pretreatment activates the therapeutic functions of bone marrow-derived MSC (BM-MSC) sheets and improves cardiac function in rabbit infarcted hearts following autologous transplantation. Production of vascular endothelial growth factor (VEGF) was increased in BM-MSC monolayer sheets and it peaked at 48 h under hypoxic culture conditions (2% O2). To examine in vivo effects, preconditioned autologous BM-MSC sheets were implanted into a rabbit old myocardial infarction model. Implantation of preconditioned BM-MSC sheets accelerated angiogenesis in the peri-infarcted area and decreased the infarcted area, leading to improvement of the left ventricular function of the infarcted heart. Importantly, the therapeutic efficacy of the preconditioned BM-MSC sheets was higher than that of standardly cultured sheets. Thus, implantation of autologous preconditioned BM-MSC sheets is a feasible approach for enhancing therapeutic angiogenesis in chronically infarcted hearts.
Keywords: Old myocardial infarction, cell-based therapy, mesenchymal stem cells, cell sheet, hypoxic preconditioning
Introduction
Myocardial infarction (MI) causes loss of cardiac tissue and impairment of left ventricular function. Heart transplantation remains the only curative treatment option for end-stage heart failure including MI. However, a critical shortage of donor hearts makes it difficult to save a large number of patients with heart failure. Therefore, alternative options to heart transplantation need to be developed for patients with severe heart failure. Cell-based therapy is thought to be a good alternative to organ transplantation. A variety of cell types have been proposed as possible candidates such as bone marrow cells (BMCs), skeletal myoblasts, and cardiosphere-derived cells (CDCs) [1-3]. Mesenchymal stem cells (MSCs), in particular, possess great potential for treating end-stage heart failure, since grafted MSCs secrete several cardioprotective and regenerative healing factors for the failing myocardium [4]. Indeed, transplantation of MSCs dramatically improved the ventricular functions and pathological condition of heart failure animal models [5-7]. Subsequent clinical trials using MSCs have been conducted on patients with ischemic heart failure; e.g., the Transendocardial Autologous Cells in Ischemic Heart Failure Trial (TAC-HFT) and Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis (POSEIDON). These clinical trials have demonstrated the safety of this therapeutic approach and that MSC transplantation induces reverse remodeling and improves the regional contractility of the infarcted area [8,9]. However, the resulting therapeutic benefits were generally modest and varied depending on the patients. It is thought that these limited therapeutic benefits are due to poor retention of the graft cells in the ischemic heart and in adequate growth factor secretion [10].
The “cell-sheet” technique has been proposed as an advanced cell-delivery method for failing hearts [11]. This technique enables graft cells to maintain cell surface proteins, cell-cell junctions, and the extracellular matrix, leading to higher initial retention of donor cells following transplantation even in ischemic tissues. Epicardial delivery of cell sheets, including MSC sheets, significantly improved cardiac functions in heart failure animal models compared with conventional cell injection methods [12-14]. The paracrine mechanism is thought to be the major therapeutic pathway underlying non-cardiac muscle sheet implantation rather than the replacement of damaged cardiomyocytes by graft cells [12,13].
Hypoxic preconditioning, induced by a brief episode of mild and nonlethal reduced oxygen supply prior to cell transplantation, is known to reinforce multiple cellular functions of “dissociated” graft cells, such as angiogenic activity, oxidative stress resistance, and adhesion capacity [15-19]. Recently, we reported that hypoxic preconditioning was applicable not only for dissociated cells but also for cell sheets; implantation of preconditioned cardiosphere-derived cell sheets dramatically improved left ventricular function in chronically infarcted mouse hearts with reduced fibrosis and enhanced angiogenesis [20]. Based on these results, we sought to use this simple yet powerful cell sheet preconditioning protocol in clinical settings. To do so, however, it is first necessary to determine the therapeutic efficacy of preconditioned sheet implantation in animal models larger than mice.
Here, we used a pre-clinical old myocardial infarction rabbit model and autologous MSC sheets to examine the efficacy of combining cell sheets and hypoxic preconditioning as a therapeutic approach for chronically infarcted hearts.
Materials and methods
Ethics statement
All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC; No. 31-104) of Yamaguchi University. All surgeries were performed under general anesthesia and all efforts were made to minimize animal suffering.
Animals
Male New Zealand white rabbits (2.5-3.0 kg body weight, KBT Oriental) were used for the animal experiments. Animals were housed in a regulated environment (22 ± 2°C), with a 12-h light/dark cycle (light cycle was from 08:00 to 20:00).
Isolation of bone marrow-derived mesenchymal stem cells
Rabbits were anesthetized by an intravenous injection of ketamine (Daiichi Sankyo Propharma, 1 mg/kg) and xylazine (Bayer, 3 mg/kg). Autologous bone marrow-derived cells (3 ml) were aspirated from the ilium with a 5-ml syringe containing 2 ml of heparin [21]. Bone marrow-derived cells were suspended in Dulbecco’s Modified Eagle Medium (DMEM, Thermo Fisher Scientific) containing 10% fetal bovine serum (FBS, Thermo Fisher Scientific) and 1% penicillin/streptomycin (Thermo Fisher Scientific). Following filtration through a 100-μm mesh nylon filter (Corning), bone marrow-derived cells were seeded onto 10-cm culture dishes and incubated at 37 °C under 5% humidified CO2. The mesenchymal population was isolated based on its ability to adhere to the culture plates [22]. At 4 days postplating, non-adherent cells were removed by medium replacement. Thereafter, the medium was changed every two days. Once the cells reached 80-90% confluence, they were trypsinized and further propagated for 2-3 passages.
Differentiation of rabbit BM-MSCs into osteoblasts, adipocytes, chondrocytes
The differentiation potential of passage 3 rabbit BM-MSCs (rBM-MSCs) into tri-lineages (osteogenic, adipogenic, and chondrogenic lineages) was examined. Differentiation into tri-lineages was conducted using commercially available standard differentiation induction media: Advance STEMTM Osteogenic Differentiation Kit (Thermo Fisher Scientific), Mesenchymal Stem Cell Adipogenic Differentiation Medium (PromoCell), and Mesenchymal Stem Cell Chondrogenic Differentiation Medium (PromoCell) in accordance with the manufacturer’s protocols. At day 14, lipid droplets were visualized by Oil red O staining; while at day 21, intracellular alkaline phosphatase (ALP) activity and acidic mucopolysaccharide levels were evaluated using the ALP Assay Kit (Takara Bio) and the Acidic Mucopolysaccaride Assay Kit (Cosmo Bio), respectively.
Preparation of rBM-MSC sheets and hypoxic preconditioning
To develop cell sheets, 6 × 105 rBM-MSCs (passage 3) were seeded in a 6-well temperature-responsive culture dish (UpCell®; Cell Seed)under normoxic conditions (37°C, 20% O2, and 5% CO2). The rBM-MSC sheets on the UpCell® dishes were then cultured under hypoxic conditions (33°C, 2% O2, and 5% CO2) [16-19]. When the culture temperature was decreased to 20-22°C, the rBM-MSC sheets smoothly detached from the culture dish and floated up into the medium as a monolayer cell sheet.
Enzyme-linked immunosorbent assay (ELISA) for VEGF
To assess growth factor production, conditioned medium was collected from the rBM-MSC sheet cultures at 0, 24, 48, 72 h post culture initiation and ELISA targeted at vascular endothelial growth factor (VEGF) was performed (human VEGF Quantikine immunoassay kit; R&D systems).
Western blotting
Rabbit BM-MSC sheets were lysed in radio-immunoprecipitation (RIPA) buffer containing protease/phosphatase inhibitor cocktails. Thirty micrograms of protein was subjected to polyacrylamide gel electrophoresis and then transferred onto a polyvinylidene difluoride (PVDF) membrane. The blotted membranes were reacted with primary antibodies against caspase-7 (Santa Cruz Biotechnology) or β-actin (Santa Cruz Biotechnology). Proteins were visualized using HRP substrate (ECL Prime Western Blotting Detection System; GE Healthcare) and band intensities were quantified using the ImageJ software package.
TUNEL assay
Apoptotic cells were detected using the terminal deoxyribonucleotidyl transferase (TDT)-mediated dUTP-digoxigenin nick end labeling (TUNEL) assay. MSC sheets were cultivated under normoxic or hypoxic conditions. Cells were fixed with 4% paraformaldehyde (Wako), rinsed with phosphate-buffered saline (PBS), permeabilized with 0.1% Triton-X/Protein blocking solution (Dako-Japan), and then incubated with a reaction solution (In Situ Cell Death Detection Kit, Roche). In addition, cells were stained with 6-diamidino-2-phenylindole (DAPI) to visualize the nuclei. Immunofluorescence images were acquired using a BIOREVO microscopy system (BZ-9000 Generation II system; Keyence).
Tube formation assay
Human umbilical endothelial cells (HUVECs) were maintained in EGM2 medium (Lonza). The HUVECs were then trypsinized, resuspended in 10% FBS/DMEM, and then seeded onto Matrigel® (Corning)-coated 96-well plates at 2 × 104 cells/well (75 μl). Next, 75 μl of conditioned medium from the rBM-MSC sheets (hypo or normo) or fresh medium (control) were added into each of the wells. Images of tube formation were captured 9 h later and measured using the Angiogenesis Analyzer for ImageJ software (National Institutes of Health) [23].
Migration assay
Cardiac fibroblasts were isolated from rabbit hearts as previously reported [24] and cultured in Iscove’s modified Dulbecco’s Medium (IMDM, Thermo Fisher Scientific) containing 10% FBS, 1% penicillin/streptomycin, and 1% non-essential amino acids (Thermo Fisher Scientific). Cardiac fibroblasts were seeded into 12-well cell culture dishes at high confluence. Cells were scraped with 1000 μl tips to create a scratch and then further cultured for 12 h in conditioned medium from rBM-MSC sheets (hypo or normo) or in fresh medium (control). Phase contrast images of the scratches were acquired using a BZ-X710 All-in-One fluorescence microscope (Keyence) at 0 and 12 h post incubation. The wound area was measured using the BZ-X analyzer software (Keyence).
Rabbit old myocardial infarction model and sheet implantation
A 24-gauge intravenous catheter was inserted into the marginal ear vein for anesthetics. Rabbits were anesthetized using intravenous injections of ketamine (1 mg/kg) and xylazine (3 mg/kg). Rabbits were intubated with an endotracheal tube (3.0 mm ID) and mechanically ventilated (rate 30/min, tidal volume 20 ml/kg) with 1 l/min oxygen and 1 l/min nitrous oxide. Repeated injections of ketamine and xylazine were administered as required to maintain a deep level of anesthesia. The rabbits were placed on a warm blanket; the chest was opened through the fourth left intercostal space and the left anterior descending artery (LAD) was ligated with 5-0 PROLENE® (Ethicon). An antibiotic (gentamicin, 2.5 mg/kg) was administered both pre- and postoperatively [21]. At 4 weeks post surgery, rabbits were subjected to echocardiography and those showing inappropriate left ventricular ejection fraction (LVEF; >60% or < 40%) were excluded from the study.
Rabbit BM-MSC sheets were placed on Seprafilm® (Sanofi US), which is used to prevent adhesion post surgery, and then carefully implanted onto the heart surface to cover the infarct and surrounding border areas [20]. Seprafilm® alone was implanted onto MI hearts in the control group. Rabbits were sacrificed at 4 weeks post sheet implantation and the hearts were harvested for histological analysis.
Echocardiography
Transthoracic echocardiography was assessed during pretreatment (4 weeks post LAD ligation) and 28 days post sheet implantation using Vivid i (GE Medical Systems) under intravenous anesthesia with pentobarbital sodium (20 mg/kg). LVEF and left ventricular fractional shortening (LVFS) were measured with the M-mode.
Histological analysis
Harvested hearts were fixed with 10% formalin overnight at room temperature. Following paraffin embedding, tissue sections (3 μm thickness) were prepared for histological analysis. Masson’s trichrome staining was performed to assess cardiac fibrosis in the infarcted hearts. The images were taken using a BIOREVO microscopy system and calculated using a BZ-II analyzer (Keyence). The infarcted area in the left ventricle was measured and compared between the groups. The heart sections were also stained with DyeLight 488-conjugated Tomato-Lectin (Vector Laboratories) to assess capillary density, which was calculated as the number of positively stained capillary vessels in 10 randomly selected fields in the peri-infarct area.
Statistical analysis
All values are expressed as mean ± SD. Statistical comparison between two groups was conducted using the student’s unpaired t-test. Statistical analysis was performed using Stata 12.1 (Lightstone Corp). P-values < 0.05 or 0.01 were considered statistically significant.
Results
Isolation of bone marrow-derived mesenchymal stem cells and differentiation into tri-lineages
Rabbit bone marrow-derived mesenchymal stem cells (rBM-MSCs) were isolated based on their ability to adhere to the culture plates [22]. Colonies of spindle-shaped MSCs attached to the surface of cell culture dish were observed at day 4 post seeding. These spindle-shaped MSCs reached 80-90% confluence at 2 weeks post seeding (Figure 1A) and the expression of CD44, a cell surface marker for rabbit BM-MSCs [25], was confirmed in the isolated cells (Figure 1B).
Figure 1.
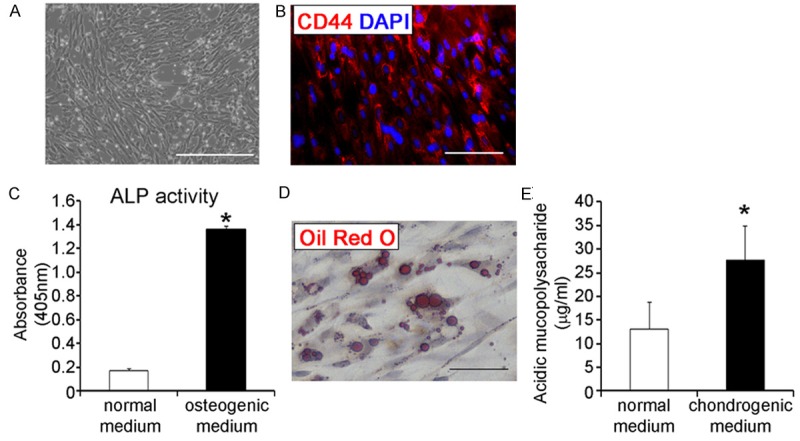
Isolation of bone marrow-derived mesenchymal stem cells and differentiation into tri-lineages. (A) Fibroblast-like cells at 2 weeks post seeding; scale bar = 500 μm (B) Osteogenic, adipogenic, and chondrogenic differentiation of isolated BM-MSCs was confirmed by intracellular alkaline phosphatase (ALP) activity (C; n = 9 for each), intracellular accumulation of lipid droplets (D), and the concentration of acidic mucopolysaccharide (E; n = 5 for each), respectively. Scale bar = 100 μm. *p < 0.01.
BM-MSCs have the capacity to differentiate into osteoblasts, adipocytes, and chondrocyte lineages [4]. Therefore, we examined the multi-potency of the isolated rBM-MSCs to determine their quality. Under osteogenic differentiation conditions, the rBM-MSCs exhibited higher alkaline-phosphatase (ALP) activity at day 21 compared to the untreated rBM-MSC controls (p < 0.01, Figure 1B), indicating that the rBM-MSCs possess osteogenic differentiation potential. Next, we examined the adipogenic differentiation capacity of isolated rBM-MSCs; the rBM-MSCs were cultivated under adipogenic differentiation conditions and then stained with Oil Red O to visualize lipid droplets. Lipid-rich vacuole formation was confirmed in the culture at 2 weeks post adipogenic induction, demonstrating the adipogenic potential of the rBM-MSCs (Figure 1C). Finally, the rBM-MSCs were also cultured under chondrogenic differentiation conditions; following 3 weeks of differentiation, the level of acidic mucopolysaccharide, a marker for chondrogenic differentiation, was significantly higher in the differentiation group than the untreated control, demonstrating the chondrogenic potential of the rBM-MSCs (p < 0.01, Figure 1D). Based on these results, we determined that the rBM-MSCs could be used for further experiments because of their typical MSC cellular characteristics.
Hypoxic preconditioning accelerates secretion of vascular endothelial growth factor (VEGF) in BM-MSC sheets
Previously, we demonstrated that VEGF expression in roughly isolated bone marrow-derived cells, including MSCs, was increased by pre-incubation under hypoxia and peaked at 24 h of culturing [26]. In this study, we examined whether hypoxic treatment reinforced the functions of the rBM-MSC sheet as well as bone marrow-derived cells. Cell sheets composed of rBM-MSCs were developed in temperature-responsive cell culture dishes and then cultured under low oxygen conditions (2% O2; hypoxia) for 24, 48, and 72 h. In contrast to bone marrow-derived cells, rBM-MSC sheets exhibited different reactivity to hypoxic treatment and VEGF levels reached their peak at 48 h, not 24 h (Figure 2A). These results suggest that the appropriate hypoxic culture conditions should be determined for all cell types and structures. Importantly, extended exposure to hypoxia did not induce apoptosis in the rBM-MSC sheets, as determined by the expression of cleaved caspase and TUNEL (Figure 2B, 2C). Taken together, these results indicate that the appropriate culturing period for rBM-MSC sheets is 48 h under hypoxia.
Figure 2.
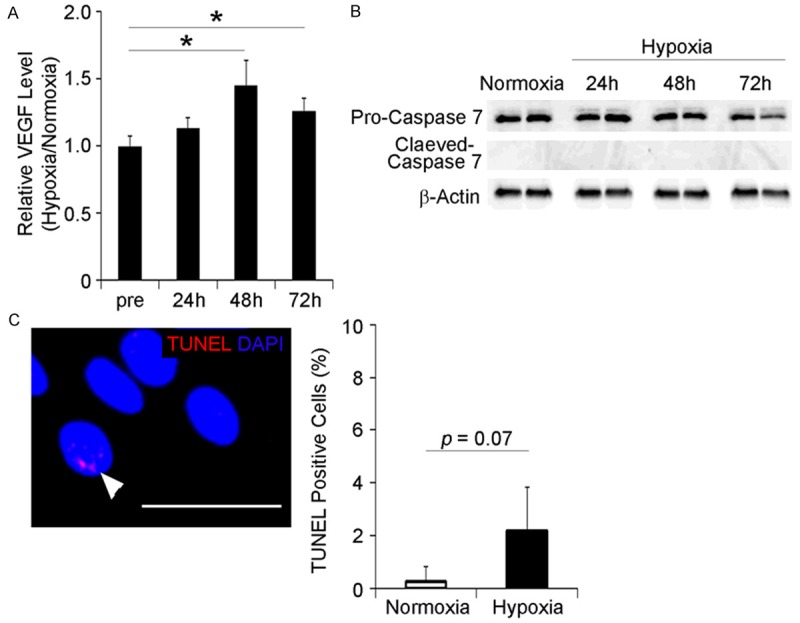
Hypoxic preconditioning enhances VEGF secretion from rBM-MSC sheets. A. Rabbit BM-MSC sheets were cultivated under hypoxic conditions (2% oxygen) for 24, 48, and 72 h and the concentration of VEGF in the conditioned medium was measured by ELISA; *p < 0.05. MSC sheets secreted the highest level of VEGF under hypoxic culture conditions (2% oxygen) at 48 h. (n = 5 for each groups). B. Hypoxic preconditioning does not cause an apoptotic response in rBM-MSC sheets; cleaved Caspase 7 was undetectable in cultured rBM-MSC sheets. C. No significant differences in the number of TUNEL-positive cells were observed between normoxic and hypoxic conditions. The arrowhead indicates the TUNEL-positive cell; scale bar = 50 μm. (n = 4 for each).
Next, we investigated whether hypoxic preconditioning enhanced the angiogenic activity of the rBM-MSC sheet using the tube formation assay. Human umbilical vascular endothelial cells (HUVECs) were cultured in conditioned media (CM) from preconditioned (hypo) or non-preconditioned (normo) rBM-MSC sheets and tube formation was then analyzed after 9 h. Human endothelial cell tube formation was increased with CMs from both normo and hypo sheets. Interestingly, hypo CMs induced higher human endothelial cell tube formation than normo CM (Figure 3A). These results suggest that hypoxic preconditioning augments the angiogenic activity of rBM-MSC sheets in addition to increasing VEGF production.
Figure 3.
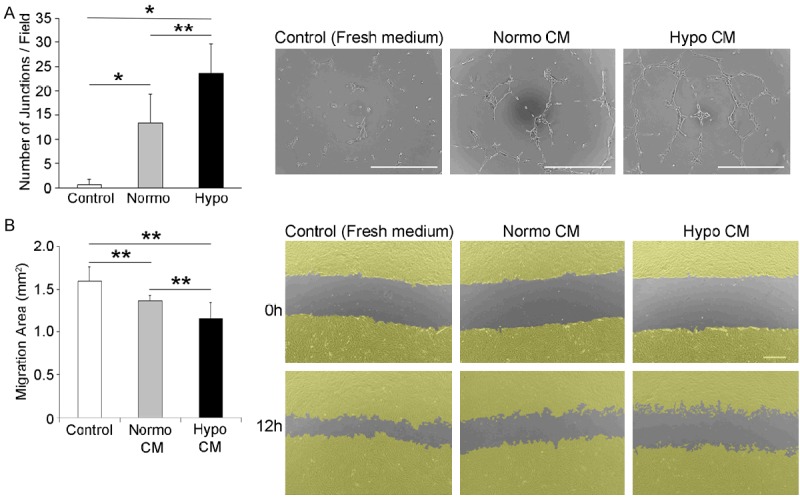
Hypoxic preconditioning enhances the angiogenic activity of rBM-MSC sheets. Human umbilical vascular endothelial cells (HUVECs) were cultured in fresh medium or in conditioned medium (CM) from rBM-MSC sheets (Normoxia or Hypoxia). The number of junctions in the field was counted at 10 × microscopic magnification; scale bar = 500 μm. (n = 3 for each groups). B. The anti-fibrotic activity of preconditioned cell sheets was analyzed using the scratch assay. Confluent rabbit cardiac fibroblasts were scraped to create a scratch approximately 1.2-mm in size and cells were then further cultivated in conditioned medium for 12 h. Wound closure was measured and compared between the experimental groups. Cells were virtually colored in yellow to visualize the wound closure area; scale bar = 500 μm. (n = 6 for each groups). Normo CM; conditioned medium from normo sheet. Hypo CM; conditioned medium from hypo sheet. *p < 0.01, **p < 0.05.
We further examined whether hypoxic treatment enhances the anti-fibrotic activity of rBM-MSC sheets. Primary rabbit cardiac fibroblasts were seeded onto cell culture dishes and scratches were created in confluent cultures. Next, the cells were cultured in conditioned medium from rBM-MSC sheets (normo or hypo) or in fresh medium; 12 h later, cardiac fibroblast migration was inhibited in the conditioned hypo rBM-MSC sheet medium, suggesting that hypoxic preconditioning enhances both the angiogenic and anti-fibrotic activity of rBM-MSC sheets (Figure 3B).
Functional recovery of failing heart following preconditioned cell-sheet implantation
To evaluate the in vivo therapeutic effects of preconditioned BM-MSC sheets, autologous preconditioned rBM-MSC sheets were implanted onto chronically infarcted hearts. In this experiment, the autologous rBM-MSC sheets were preconditioned under hypoxia 2 days prior to sheet delivery (Figure 4A); rBM-MSC sheets detached from cell culture dishes were placed on Seprafilm® for delivery to the heart (Figure 4B).
Figure 4.
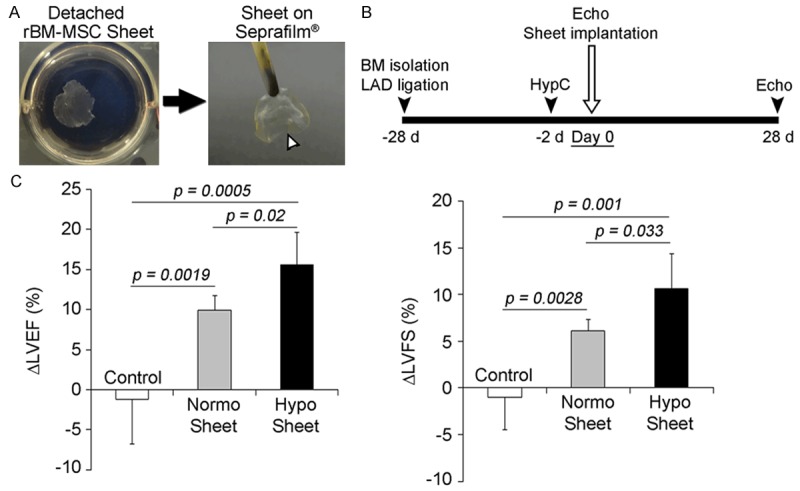
Implantation of rBM-MSC sheet onto the old myocardial infarction (OMI) heart. A. Rabbit BM-MSC sheetswere detached from temperature-responsive culture dishes incubated at < 20°C and the rBM-MSC sheets were thenplaced on Seprafilm® for delivery to the heart (arrowhead). B. Protocol for sheet implantation onto rabbit OMI. C. Cardiac performance was improved by preconditioned sheet implantation. The change in LVEF (deltaLVEF) and LVFS (deltaLVFS) at 4 weeks post sheet implantation compared to pre-implantation was measured by echocardiograms (n = 5 for each group).
Echocardiography was performed immediately prior to and on day 28 post sheet implantation to determine the change ratio of left ventricular ejection fraction (LVEF) and left ventricular fractioning shortening (LVFS) (Figure 4A). Implantation of autologous BM-MSC sheets significantly improved both LVEF and LVFS compared to the non-sheet delivered control (Figure 4C). In particular, implantation of hypo sheets resulted in greater improvement of the left ventricle than normo sheets, indicating that hypoxic preconditioning enhanced the therapeutic efficacy of BM-MSC sheets for old myocardial infarction.
Autologous implantation of preconditioned BM-MSC sheets reduces the infarcted area and induces angiogenesis
Histological changes in the infarcted hearts caused by preconditioned BM-MSC sheets were evaluated post implantation. At 4 weeks post sheet implantation, the infarcted hearts were collected to assess the effects of sheet implantation on the infarcted area and new vessel formation. Masson’s trichrome staining was performed to quantify the infarct size in the left ventricle (LV). The infarct size in the LVs was significantly reduced in sheet-delivered groups in contrast to the large infarcts identified in the non-sheet delivered control group (Figure 5A). Next, we assessed the effect of MSC sheet implantation on new vessel formation in infarcted hearts. A greater number of vessels was observed in the peri-infarcted myocardium in sheet delivered groups (normo and hypo sheet) than in the non-sheet delivered control group. Furthermore, vessel formation was significantly increased in the preconditioned BM-MSC sheet group compared to the normo sheet group (Figure 5B), indicating that preconditioned BM-MSC sheets exhibited higher angiogenic activity in infarcted hearts, consistent with the results of the in vitro tube formation assay (Figure 3).
Figure 5.
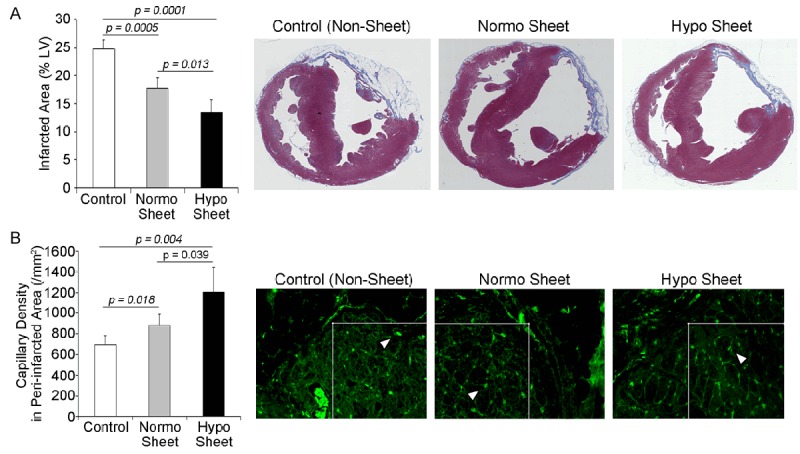
Implantation of autologous hypoxia-primed rBM-MSC sheets reduced the infarcted area and promoted angiogenesis. A. Representative macroscopic images of Masson’s trichrome-stained heart tissue (right three panels). The infarcted area in the preconditioned sheet group (hypo sheet) was markedly reduced compared to the normo sheet and control groups (n = 5 for each group). B. Lectin+ capillaries (arrowheads) in the peri-infarcted area were evaluated at 4 weeks post sheet implantation. Implantation of preconditioned rBM-MSC sheets accelerated angiogenesis in infarcted hearts. The capillary density in the peri-infarcted area was measured within the 200-µm square (white box); (n = 5 for each group).
Discussion
Recently, we demonstrated the versatility of hypoxic preconditioning of cell sheets and its therapeutic effects on infarcted hearts [20]. However, these results were obtained using a small animal model; the therapeutic applicability of the hypoxic reinforcement of cell sheets for clinical use was needed to be confirmed in larger animal models. Therefore, we used mid-size animals to determine whether implantation of autologous preconditioned cell sheets improves the left ventricular function of chronically infarcted hearts.
BM-MSCs possess great potential for curing ischemic heart diseases, since engrafted MSCs are able to secrete various biological factors including VEGF, platelet-derived growth factor (PDGF), matrix metalloproteinases (MMPs), and stem cell-derived factor (SDF). These secreted factors promote angiogenesis, remodeling of the extracellular matrix, and recruitment of stem cells [27,28]. Consistent with our previous study, rabbit BM-MSC sheets were stimulated by hypoxic preconditioning, resulting in up-regulation of VEGF. The therapeutic benefit of cell sheet VEGF up-regulation for heart failure is further confirmed by a recent study demonstrating that virus-mediated VEGF overexpression enhanced the therapeutic efficacy of rabbit MSC sheet implantation in infarcted hearts [29]. However, genetic therapies such as these bear the risk of tumorigenesis and immune-response post implantation, concerns which necessitate biosafety measures. In contrast, hypoxic preconditioning naturally enhances VEGF expression in cell sheets without any apparent cellular damage. Taken together, these findings indicate that hypoxic preconditioning is a much more feasible approach for reinforcing the angiogenic activity of cell sheets compared to genetic modification techniques. The enhancement of anti-fibrotic activity further promotes the use of hypoxic preconditioning of cell sheets; autologous implantation of preconditioned BM-MSC sheets resulted in enhanced therapeutic angiogenesis, reduced fibrosis, and improvement of left ventricular function of rabbit infarcted hearts.
Although the detailed molecular mechanism underlying the enhancement of angiogenic activity by hypoxic preconditioning in rBM-MSC sheets is not fully understood, our results indicate that the extracellular regulated kinase (Erk)-mediated pathway was activated in rBM-MSC sheets by hypoxic preconditioning (Figure S1). Since the Erk-mediated signaling pathway regulates hypoxia-inducible factor (HIF) dependent VEGF induction under hypoxic conditions [30], hypoxic preconditioning may augment the angiogenic activity of rBM-MSC sheets through the Erk-mediated pathway. However, we have also previously shown that hypoxic preconditioning activates the phosphoinositide 3-kinase (PI3K)/Akt pathway to accelerate VEGF expression in human CDC sheets [20]. Interestingly, Akt phosphorylation was not increased in rBM-MSC sheets in response to hypoxic stimulation (Figure S1). These results suggest that multiple pathways are involved in the induction of VEGF expression in different cell types under hypoxia.Additional experiments are required to elucidate the precise molecular mechanisms of hypoxic preconditioning of cell sheets. Preconditioning also enhanced the anti-fibrotic activity of rBM-MSC sheets and actually improved fibrosis in chronically infarcted hearts. Recent studies have proposed a possible mechanism by which hypoxia-induced leptin enhances the anti-fibrotic activity of MSCs by blocking both the Smad2 and MRTF-A -mediated signaling pathways [1,31]. Although we did not examine leptin expression and leptin-mediated inhibition of fibrosis in this study, it is possible that hypoxic preconditioning accelerates leptin expression and enhances the anti-fibrotic activity of rBM-MSC sheets. Endoglin, which antagonizes the TGF-β signaling pathway, is another potential secreted anti-fibrotic factor since the endoglin promoter region contains the hypoxia response element [32]. However, the results of our preliminary study showed that expression of the endoglin gene was not increased in preconditioned rBM-MSC sheets, suggesting that endoglin is not essential for rBM-MSC sheets to inhibit fibrosis of infarcted hearts (data not shown).
The present study using an autologous implantation model with larger animals clearly demonstrates the feasibility of transient hypoxic stimulation of cell sheets and its therapeutic potential for patients with heart failure. However, the viability of allogeneic transplantation of preconditioned cell sheets for treatment of myocardial infarction will need to be assessed prior to implementing this technique in clinical settings. Although autologous therapy is a practical technique for treating heart failure, significant technical, economic, and timing constraints remain. For example, the preparation of autologous BM-MSC sheets requires several weeks; therefore, autologous sheet therapy is not appropriate for patients with acute heart failure. The POSEIDON randomized study conducted on patients with myocardial infarction demonstrated the safety of allogeneic BM-MSC transplantation with a low rate of immune rejection of the graft cells [6]. The results of the current study indicate that implantation of allogeneic preconditioned cell sheets can be used for treating patients with heart failure, producing better therapeutic results than transplantation of BM-MSCs in clinical settings.
In conclusion, using a mid-size heart failure animal model we have demonstrated that autologous BM-MSC sheets improve contractile function in rabbit infarcted hearts. Importantly, VEGF secretion in BM-MSC sheets was increased by hypoxic preconditioning without considerable damage to the cell sheet, resulting in functional improvement of left ventricle post implantation with enhanced angiogenesis and reduced fibrosis in the infarcted hearts. Although the precise therapeutic mechanisms underlying the preconditioned MSC sheets remain unclear, enhanced angiogenic activity might affect cardiac regeneration in chronically infarcted hearts. Thus, hypoxic preconditioning can enhance therapeutic capacity not only in dissociated cells but also in cell sheets; therefore this technique is applicable for reinforcing cell sheets. Future studies will be aimed at applying this protocol to early phase trials on healthy volunteers. Ultimately, we hope that preconditioned autologous BM-MSC sheets will be used to improve the LV function and condition of end-stage heart failure patients in the near future.
Acknowledgements
All authors have approved the final version of the manuscript and read the journal’s authorship agreement. We wish to thank Kazuko Tanaka, Yukari Hironaka, Masakatsu Yamashita, and Atsushi Hidano for technical assistance. This work was partly supported by a JSPS-KAKENHI (no.15K10217 to B.S.).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Cheng K, Blusztajn A, Shen D, Li TS, Sun B, Galang G, Zarembinski TI, Prestwich GD, Marban E, Smith RR, Marban L. Functional performance of human cardiosphere-derived cells delivered in an in situ polymerizable hyaluronan- gelatin hydrogel. Biomaterials. 2012;33:5317–5324. doi: 10.1016/j.biomaterials.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hagege AA, Marolleau JP, Vilquin JT, Alheritiere A, Peyrard S, Duboc D, Abergel E, Messas E, Mousseaux E, Schwartz K, Desnos M, Menasche P. Skeletal myoblast transplantation in ischemic heart failure: long-term follow-up of the first phase I cohort of patients. Circulation. 2006;114:I108–113. doi: 10.1161/CIRCULATIONAHA.105.000521. [DOI] [PubMed] [Google Scholar]
- 3.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 4.Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK, Branco RV, Oliveira EM, He R, Geng YJ, Willerson JT, Perin EC. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150–156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 6.Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, Pittenger MF, Martin BJ. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg. 2002;73:1919–1925. doi: 10.1016/s0003-4975(02)03517-8. [DOI] [PubMed] [Google Scholar]
- 7.Price MJ, Chou CC, Frantzen M, Miyamoto T, Kar S, Lee S, Shah PK, Martin BJ, Lill M, Forrester JS, Chen PS, Makkar RR. Intravenous mesenchymal stem cell therapy early after reperfused acute myocardial infarction improves left ventricular function and alters electrophysiologic properties. Int J Cardiol. 2006;111:231–239. doi: 10.1016/j.ijcard.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 8.Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, Mushtaq M, Williams AR, Suncion VY, McNiece IK, Ghersin E, Soto V, Lopera G, Miki R, Willens H, Hendel R, Mitrani R, Pattany P, Feigenbaum G, Oskouei B, Byrnes J, Lowery MH, Sierra J, Pujol MV, Delgado C, Gonzalez PJ, Rodriguez JE, Bagno LL, Rouy D, Altman P, Foo CW, da Silva J, Anderson E, Schwarz R, Mendizabal A, Hare JM. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311:62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, Palasis M, Wilensky RL. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27:1114–1122. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- 11.Shimizu T, Yamato M, Isoi Y, Akutsu T, Setomaru T, Abe K, Kikuchi A, Umezu M, Okano T. Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circ Res. 2002;90:e40. doi: 10.1161/hh0302.105722. [DOI] [PubMed] [Google Scholar]
- 12.Memon IA, Sawa Y, Fukushima N, Matsumiya G, Miyagawa S, Taketani S, Sakakida SK, Kondoh H, Aleshin AN, Shimizu T, Okano T, Matsuda H. Repair of impaired myocardium by means of implantation of engineered autologous myoblast sheets. J Thorac Cardiovasc Surg. 2005;130:1333–1341. doi: 10.1016/j.jtcvs.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 13.Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K, Sano S, Okano T, Kitamura S, Mori H. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 14.Zakharova L, Mastroeni D, Mutlu N, Molina M, Goldman S, Diethrich E, Gaballa MA. Transplantation of cardiac progenitor cell sheet onto infarcted heart promotes cardiogenesis and improves function. Cardiovasc Res. 2010;87:40–49. doi: 10.1093/cvr/cvq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akita T, Murohara T, Ikeda H, Sasaki K, Shimada T, Egami K, Imaizumi T. Hypoxic preconditioning augments efficacy of human endothelial progenitor cells for therapeutic neovascularization. Lab Invest. 2003;83:65–73. doi: 10.1097/01.lab.0000050761.67879.e4. [DOI] [PubMed] [Google Scholar]
- 16.Kubo M, Li TS, Kamota T, Ohshima M, Qin SL, Hamano K. Increased expression of CXCR4 and integrin alphaM in hypoxia-preconditioned cells contributes to improved cell retention and angiogenic potency. J Cell Physiol. 2009;220:508–514. doi: 10.1002/jcp.21803. [DOI] [PubMed] [Google Scholar]
- 17.Kubo M, Li TS, Suzuki R, Shirasawa B, Morikage N, Ohshima M, Qin SL, Hamano K. Hypoxic preconditioning increases survival and angiogenic potency of peripheral blood mononuclear cells via oxidative stress resistance. Am J Physiol Heart Circ Physiol. 2008;294:H590–595. doi: 10.1152/ajpheart.00856.2007. [DOI] [PubMed] [Google Scholar]
- 18.Kudo T, Hosoyama T, Samura M, Katsura S, Nishimoto A, Kugimiya N, Fujii Y, Li TS, Hamano K. Hypoxic preconditioning reinforces cellular functions of autologous peripheral blood-derived cells in rabbit hindlimb ischemia model. Biochem Biophys Res Commun. 2014;444:370–375. doi: 10.1016/j.bbrc.2014.01.054. [DOI] [PubMed] [Google Scholar]
- 19.Kudo T, Kubo M, Katsura S, Nishimoto A, Ueno K, Samura M, Fujii Y, Hosoyama T, Hamano K. Hypoxically preconditioned human peripheral blood mononuclear cells improve blood flow in hindlimb ischemia xenograft model. Am J Transl Res. 2014;6:570–579. [PMC free article] [PubMed] [Google Scholar]
- 20.Hosoyama T, Samura M, Kudo T, Nishimoto A, Ueno K, Murata T, Ohama T, Sato K, Mikamo A, Yoshimura K, Li TS, Hamano K. Cardiosphere- derived cell sheet primed with hypoxia improves left ventricular function of chronically infarcted heart. Am J Transl Res. 2015;7:2738–2751. [PMC free article] [PubMed] [Google Scholar]
- 21.Shintani S, Murohara T, Ikeda H, Ueno T, Sasaki K, Duan J, Imaizumi T. Augmentation of postnatal neovascularization with autologous bone marrow transplantation. Circulation. 2001;103:897–903. doi: 10.1161/01.cir.103.6.897. [DOI] [PubMed] [Google Scholar]
- 22.Qu Z, Xu H, Tian Y, Jiang X. Atorvastatin improves microenvironment to enhance the beneficial effects of BMSCs therapy in a rabbit model of acute myocardial infarction. Cell Physiol Biochem. 2013;32:380–389. doi: 10.1159/000354445. [DOI] [PubMed] [Google Scholar]
- 23.Arnaoutova I, George J, Kleinman HK, Benton G. The endothelial cell tube formation assay on basement membrane turns 20: state of the science and the art. Angiogenesis. 2009;12:267–274. doi: 10.1007/s10456-009-9146-4. [DOI] [PubMed] [Google Scholar]
- 24.Hutchinson KR, Lord CK, West TA, Stewart JA Jr. Cardiac fibroblast-dependent extracellular matrix accumulation is associated with diastolic stiffness in type 2 diabetes. PLoS One. 2013;8:e72080. doi: 10.1371/journal.pone.0072080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee BH, Kim WH, Choi MJ, Rho JR, Kim WG. Chronic heart failure model in rabbits based on the concept of the bifurcation/trifurcation coronary artery branching pattern. Artif Organs. 2002;26:360–365. doi: 10.1046/j.1525-1594.2002.06881.x. [DOI] [PubMed] [Google Scholar]
- 26.Li TS, Hamano K, Suzuki K, Ito H, Zempo N, Matsuzaki M. Improved angiogenic potency by implantation of ex vivo hypoxia prestimulated bone marrow cells in rats. Am J Physiol Heart Circ Physiol. 2002;283:H468–473. doi: 10.1152/ajpheart.00261.2002. [DOI] [PubMed] [Google Scholar]
- 27.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 28.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 29.Yeh TS, Fang YH, Lu CH, Chiu SC, Yeh CL, Yen TC, Parfyonova Y, Hu YC. Baculovirus-transduced, VEGF-expressing adipose-derived stem cell sheet for the treatment of myocardium infarction. Biomaterials. 2014;35:174–184. doi: 10.1016/j.biomaterials.2013.09.080. [DOI] [PubMed] [Google Scholar]
- 30.Mukhopadhyay D, Tsiokas L, Zhou XM, Foster D, Brugge JS, Sukhatme VP. Hypoxic induction of human vascular endothelial growth factor expression through c-Src activation. Nature. 1995;375:577–581. doi: 10.1038/375577a0. [DOI] [PubMed] [Google Scholar]
- 31.Hu X, Wu R, Jiang Z, Wang L, Chen P, Zhang L, Yang L, Wu Y, Chen H, Chen H, Xu Y, Zhou Y, Huang X, Webster KA, Yu H, Wang J. Leptin signaling is required for augmented therapeutic properties of mesenchymal stem cells conferred by hypoxia preconditioning. Stem Cells. 2014;32:2702–2713. doi: 10.1002/stem.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanchez-Elsner T, Botella LM, Velasco B, Langa C, Bernabeu C. Endoglin expression is regulated by transcriptional cooperation between the hypoxia and transforming growth factor-beta pathways. J Biol Chem. 2002;277:43799–43808. doi: 10.1074/jbc.M207160200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


