Abstract
Neurons and neural stem cells are sensitive to their mechanical and topographical environment, and cell–substrate binding contributes to this sensitivity to activate signaling pathways for basic cell functions. Many transmembrane proteins transmit signals into and out of the cell, including integrins, growth factor receptors, G-protein-coupled receptors, cadherins, cell adhesion molecules, and ion channels. Specifically, integrins are one of the main transmembrane proteins that transmit force across the cell membrane between a cell and its extracellular matrix, making them critical in the study of cell–material interactions. This review focuses on mechanotransduction, defined as the conversion of force a cell generates through cell–substrate bonds to a chemical signal, of neural cells. The chemical signals relay information via pathways through the cellular cytoplasm to the nucleus, where signaling events can affect gene expression. Pathways and the cellular response initiated by substrate binding are explored to better understand their effect on neural cells mechanotransduction. As the results of mechanotransduction affect cell adhesion, cell shape, and differentiation, knowledge regarding neural mechanotransduction is critical for most regenerative strategies in tissue engineering, where novel environments are developed to improve conduit design for central and peripheral nervous system repair in vivo.
Introduction
Terminally differentiated nerves and neural stem cells (NSCs) are sensitive to their surrounding environment or niche, and they interact with this environment through cell surface receptors. Niche properties, such as substrate-bound molecules, stiffness, extracellular matrix (ECM) proteins, and topography, affect cell adhesion, survival, proliferation, migration, morphology, and differentiation.1–5 These outcomes are often used to draw conclusions on a general cellular response to a given material. While they are each valid measures of cell behavior, the specific signaling pathways responsible for each event, and their potential links to each other, are still not fully understood.6
The study of mechanotransduction as defined by Alenghat and Ingber is “cellular signal transduction in response to mechanical stimuli7.” Mechanical stimuli include substrate properties that affect cellular behavior of adherent cells. Integrins are a leading molecule of interest for mechanotransduction because they are one of the primary transmembrane proteins that permit force transmission between the cellular cytoskeleton and the ECM, hence their designation as a mechanosensing protein.8 Integrins are composed of an alpha and a beta subunit, with the beta subunit serving as the component that relays force across the cell membrane.8,9
However, many questions remain unanswered for neural cells: How are signaling pathways involved in mechanotransduction? How do two-dimensional (2D) versus three-dimensional (3D) culture systems affect neuronal behavior? How does surface topography and stiffness affect neuronal behavior?
In this review, the area of mechanobiology, with a focus on the force that is transmitted across the cell membrane by cell–substrate interactions, will be discussed. The force can mediate the formation of protein complexes within the cell that affect signaling pathways, controlling behavior, such as alignment and neuronal differentiation.9,10 Extracellular physical signals are critical in embryonic development and stem cell differentiation; many of these integrin-based signals can be mimicked by substrate-bound molecules and physical properties of scaffolds in tissue engineering to guide cell growth, maintain stem cell self-renewal, or promote differentiation.11 Therefore, the goal of this review was to examine the influence of mechanotransduction via substrate interactions in neural cell response to examine how this interaction influences applications in neural tissue engineering. Improving our knowledge of mechanotransduction in the study of neural cells has the potential to advance material design for regenerative medicine, investigate neurodegenerative diseases, and better understand NSC self-renewal and differentiation.
While mesenchymal stem cells (MSCs) have been differentiated to neuron-like cells,12–14 they will not be discussed because it is questioned if they behave as mature neurons.15 Due to the uncertainty of cells differentiating across germ lines, the cell sources covered here will be restricted to those derived from the nervous system or pluripotent stem cells. Because this article focuses on mechanotransduction through cell–substrate binding, mechanosensitive ion channels will not be discussed (see the review by Poole et al. for information on calcium channels and their role in signaling).16 For an overview of stem cell biology and biomaterials to study mechanotransduction, see McMurray et al.17 By focusing the review, we hope to inspire new avenues of research of the application of mechanosensing to improve engineered regeneration of the nervous system.
Mechanotransduction Pathways
The force generated through cell–substrate interactions is important to initiate a cellular response to any adherent substrate. Forces are developed in the actomyosin cytoskeleton, a dynamic structure on the inner side of the cell membrane,8 that affects protein conformation by exposing or blocking binding sites18 and controlling cell shape.1,3,19 For cell–substrate interactions, integrin binding to an ECM protein, commonly fibronectin, laminin, or collagen or peptides derived from them, is a primary contributor to cell recognition. Binding of the cell to the substrate is the first stage to transmitting force across the cell membrane,3,9,18,20 and through this binding, integrins interact with the extracellular environment.21 Next, adaptor proteins bind to actin in the cytoskeleton, linking it to the cell membrane. Then, forces are transmitted from the actin filaments through the myosin head.18 Integrins and adaptor proteins, such as those that form focal adhesions, initiate signaling pathways for various cell functions.
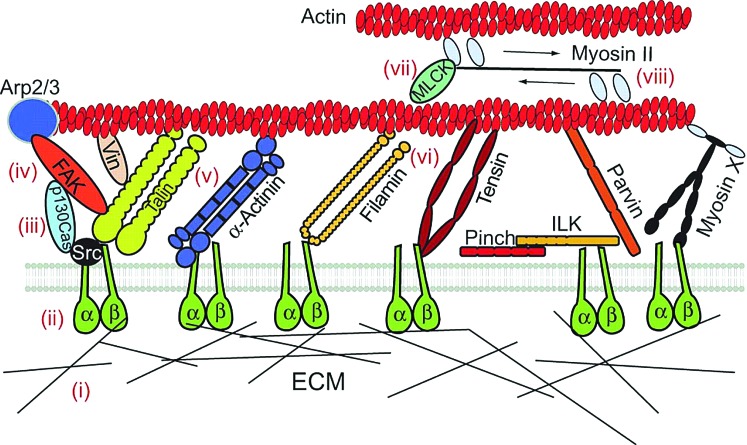
The clustering of integrin attachments affects the formation of focal adhesions and, ultimately, the force that the cell can generate to pull on the ligand.18,22 Figure 1 shows the possible routes intracellular proteins use to transmit force from the ECM–integrin bond (ii) to the cytoskeleton (vii and viii).18 Many proteins bind to actin and form complexes that transmit force across the cell membrane. When these complexes increase in size, they are called focal adhesions, which are composed of more than 100 proteins, including talin, paxillin, filamin A, and vinculin.23 Much focus is placed on focal adhesions because they link the beta subunit of the integrin to actin in the cytoskeleton, and they are commonly immunolabeled to observe cell–material binding.24,25 Focal adhesions are sensitive to mechanical force; for example, tensile force increases vinculin recruitment, which increases the strength of the adhesion.24 This link between integrins and actin is important because changes to the cytoskeleton structure affect the signal as it is passed to the nucleus, where mechanical tension can alter the centromere shape to regulate gene expression, cell survival, migration, proliferation, and differentiation.19,26
FIG. 1.
Proposed mechanotransduction mechanisms. First, (i) binding sites are exposed on the extracellular matrix (ECM), so (ii) integrins, formed by alpha and beta subunits, can bind. Next, (iii) p130Cas phosphorylation increases (iv) tyrosine kinase activity of focal adhesion kinase (FAK). This exposes (v) vinculin binding sites on talin, and (vi) the β-integrin tail on filamin. MLCK autoinhibition is released, (vii) and myosin II is activated. (viii) Adapted from Roca-Cusachs et al.18 Color images available online at www.liebertpub.com/teb
In the study of these various cellular responses, the formation of focal adhesions through integrin clustering has been thought to regulate the Rho signaling pathway, which controls myosin II phosphorylation and affects the cytoskeleton tension in adherent cells,18,21,23 making it an important component of substrate-mediated mechanotransduction. Other mechanotransduction pathways involve Src family kinases (SFKs), which respond to integrin clustering to activate the Rho GTPases, Rac, Cdc42, and RhoA, and control cytoskeleton organization by regulating actin at focal adhesions18,22 and ERK1/2, which is activated by focal adhesion kinase (FAK) to participate in actin filament formation.10,23 FAK, one of the first mechanosensitive proteins within the cell, is known to be activated by integrin binding27 and has recently been demonstrated as a link between mechanotransduction and neural differentiation, where increased FAK phosphorylation correlated with increased MAP2 expression, a mature neuronal marker.27
In addition to examination of FAK phosphorylation, crosstalk between Hippo/Yes-associated protein (YAP) and Rho GTPase has been shown to influence the maintenance of stem cell pluripotency and neural differentiation through substrate rigidity.11 Maintenance of stem cell pluripotency is affected by phosphorylation of YAP and TAZ, proteins within the Hippo pathway, that mediate nuclear accumulation of Smads, which are required for maintenance of stem cell pluripotency. Loss of Smad nuclear accumulation leads to differentiation.11 Musah et al. investigated YAP as a regulator for neuronal differentiation of pluripotent stem cells and found that soft substrates (∼0.23 kPa shear modulus) decreased nuclear YAP expression and cellular differentiation even in media lacking soluble differentiation factors.28 Figure 2 shows an overview of pathways that can affect gene expression. Together, these pathways transmit the chemical signal initiated by force transferred across cell–substrate bonds.
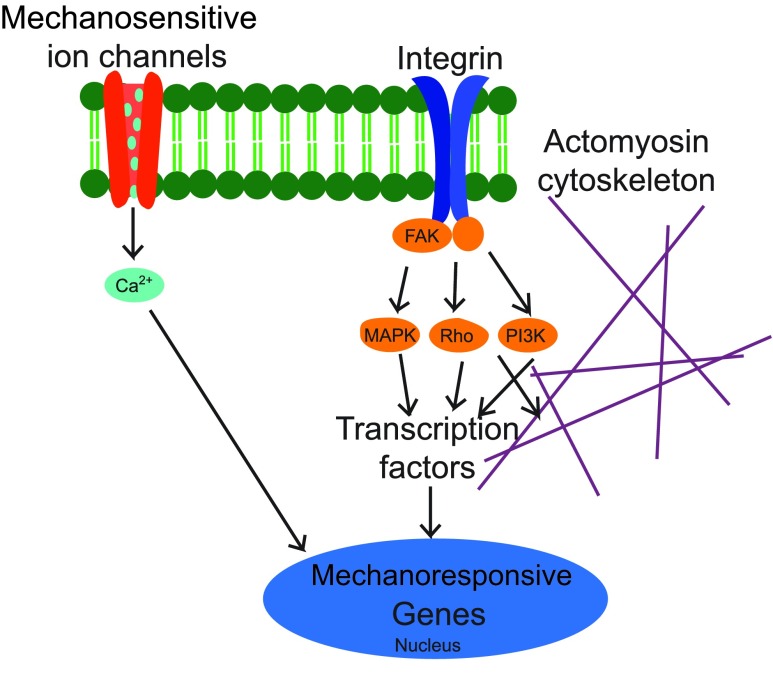
FIG. 2.
Basic overview of transmembrane mechanosensitive molecules. Mechanosensitive calcium channels control the release of calcium into the cell that affects mechanoresponsive genes in the nucleus. Integrins bind to FAK within the cell that initiates several pathways, including MAPK, Rho, and P13K. Rho also binds to the actomyosin cytoskeleton. The integrin-related signaling pathways also affect mechanoresponsive genes within the nucleus. Reprinted by permission from Macmillan Publishers Ltd: Wong et al.29 Copyright (2011). Color images available online at www.liebertpub.com/teb
Effect of the Physical Environment on Neuronal Mechanotransduction
Understanding neuronal and NSC mechanotransduction is important to design materials that promote the intended response, such as neurite extension, neurite alignment, or neuronal differentiation. It is clear that neural cells respond sensitively to their physical environment, but mechanotransduction has not been widely studied for these types of cells.30–32 Although studies may not directly discuss mechanotransduction, many target the effects of substrate-bound ECM proteins or peptides, scaffold stiffness, cell shape, and 2D versus 3D culture on the outcomes listed above. Therefore, results from these studies can increase our understanding of the effects of cell–substrate interactions. Agents, such as blebbistatin and Y27632, block myosin II and Rho kinase (ROCK), respectively, and they confirm interactions between the cell–material interface and the cytoskeleton.33 However, they block the entire pathway, so downstream effects cannot be elucidated. The link between the physical environment and cellular response is critical to further connect results to cell–substrate interactions, so materials can be rationally designed to target specific results. Better understanding the effect of niche properties on mechanotransduction with respect to integrin binding might also give clues as to why there are differences in proliferation and migration between subventricular zone (SVZ)-derived NSCs from different species. For example, neuronal migration to the olfactory bulb is present in mice but not in humans.34 It can also aid in material design to guide cells toward specific behavior, such as proliferation, migration, or differentiation.
Substrate-bound interactions
Cell–ECM interactions
Since cells feel their environment through integrin binding, ECM proteins that integrins bind are an essential aspect of mechanotransduction. For tissue engineering, cell adhesion peptides are of particular interest due to the ease of manufacture, ease of tethering to biomaterials, and their increased stability compared to the whole protein.35,36 In vivo, NSCs remain in their niche by binding to the ECM or neighboring cells, and the lack of adhesion is a proposed reason for the low survival of injected stem cell therapy.37 Another aspect of the NSC niche is its vasculature. Mature neurons follow close paths to blood vessels, and similarly, NSCs are found in close proximity to blood vessels within their niche,38,39 and an integrin specific for laminin, α6β1, may aid NSC adhesion to the vascular niche. Adult mice NSCs closer in proximity to blood vessels were found to have a higher expression of α6β1 compared to those farther away.38 Integrins that bind to laminin, fibronectin, collagen, and their corresponding binding peptides are listed in Table 1.
Table 1.
Neural ECM Proteins, Peptides, and Their Integrins
| ECM proteins and peptide derivatives | Integrins | References |
|---|---|---|
| Laminin (IKVAV, RGD, YIGSR) | α3β1 | 40–42 |
| α4β1 | 41,43 | |
| α6β1 | 20,41,42 | |
| α7β1 | 42 | |
| αvβ3 | 44 | |
| α6β1 | 44 | |
| Fibronectin (RGD) | α5β1 | 40–42 |
| αvβ1 | 40–42 | |
| αvβ3 | 40,41 | |
| αvβ5 | 41 | |
| αvβ6 | 41 | |
| αvβ8 | 40 | |
| Collagen (RGD) | α1β1 | 35,42 |
| α2β1 | 35,42 | |
| α10β1 | 35,42 | |
| α10β11 | 35,42 |
Integrin expression has been determined by flow cytometry and applying antibodies against specific subunits to block integrin activity.20
ECM, extracellular matrix.
In addition to α6β1,9,34 several integrins have been explicitly studied for expression in neuronal cells. The β1 integrin subunit is strongly expressed in neural progenitor cells, with 98% ± 1.5% expression in cells cultured on poly-D-lysine/laminin-coated tissue culture plastic compared to 15% ± 3% of the cells expressing β4.20 Several studies have demonstrated that the β1 subunit helps maintain stem cell self-renewal and is involved with NSC adhesion within its niche.38,45,46 For example, neural progenitor adhesion is mediated through α5β1 and α6β1 on fibronectin and laminin, respectively.9 Downstream signaling from β1 has also been found to control neural cell functions, including survival,9,47 migration,9 neurite growth,9 and glial myelination.9,47
Peptides that have been found to bind to integrins include IKVAV, YIGSR, and RGD (Table 1).48 RGD is common to many ECM proteins, such as fibronectin, fibrinogen, vitronectin, Von Willebrand factor,49 and laminin,50 but has been widely studied as a fibronectin-derived peptide.35 While it is well accepted that YIGSR binds to the 67-kDa laminin receptor, mixed evidence suggests that YIGSR binding may also bind to β1 integrin.41,43 For example, the percentage of cell spreading was decreased on YIGSR-coated surfaces when incubated with anti-α4 and anti-β1 antibodies, indicating that YIGSR can at least partially bind to α4β1 integrin.43 IKVAV, present on the laminin α1 chain,51 affects ESC adhesion, neuronal differentiation, and neurite length52 and binds the 110-kDa laminin receptor and possibly the α3β1, α4β1, and α6β1 integrins.41,51 Mixed evidence is reported for the optimal IKVAV concentration and resulting cell response, potentially due to differences in cell types, substrates, and IKVAV concentrations between studies.52 After culturing ESCs on a concentration gradient of IKVAV, it was determined that 570-μM IKVAV promoted neuronal mRNA markers in 2D, while 60-μM IKVAV was sufficient to promote neuronal differentiation in 3D culture within the same stiffness gel.52 Therefore, YIGSR and IKVAV are examples of cell adhesion peptides that can potentially bind to both integrin and nonintegrin receptors, leaving open the question of how the interaction through this peptide drives the cellular response. Ongoing research on neural mechanotransduction will likely shed more light on the potential for mechanosensing through these peptides.
Substrate-bound molecules, either proteins or peptides, are important when functionalizing biomaterials for interactions with neural cells. However, it is also clear that the response of cell–substrate binding can be improved through combinations with soluble factors. Synergism of ECM proteins and soluble stromal cell-derived factor-1α (SDF-1α) was studied for neural progenitor stem cells. Significant increases in migration and enhanced neuronal differentiation were reported after 6 days on laminin- and Matrigel®-coated surfaces compared to poly-L-lysine or vitronectin, even in chemotactic studies of SDF-1α.53 Ultimately, the combined influence of cell–substrate binding and soluble factors will likely influence the cellular pathways and the behavior of the cells. The relative influence of ECM versus soluble interactions, however, must be further studied to determine which factors can be incorporated into tissue-engineered material designs.
Cell–cell interactions
Cadherins, another mechanosensitive protein, link the cytoskeletons of neighboring cells, typically through homophilic interactions. It is this homophilic binding that is thought to elicit a mechanoresponse54 through pathways similar to integrin–substrate binding,55 and as such, N-cadherin mimic peptide (Ac-HAVDIGGGC) has been tethered to substrates to promote chondrogenesis of MSCs.56,57 Additionally, cadherins can work with integrins through the Src and P13K cellular pathways55 to increase traction force generation. When S180 cells, a murine fibroblast carcinoma cell line, expressing E-cadherin were in contact with a cluster of 3–5 neighboring cells, the traction strength increased compared to a single cell culture. In addition, no change in the traction force of individual cells was measured when cultured in medium from cell clusters, demonstrating the importance of cell–cell communication rather than a paracrine effect.55
This crosstalk between cell adhesion molecules and ECM adhesion molecules is potentially significant in the neural environment because neurons and glia intimately interact with each other, and with ECM, in carrying out regenerative functions. Neurons also bind to other cell adhesion molecules, including L1 cell adhesion molecule and neural cell adhesion molecules (NCAMs),58,59 and therefore, it may be important to consider how these molecules are influenced by synchronous integrin binding. For example, substrate-bound L1 CAM, which plays a role in neuron migration and axonal guidance,60 was specific for neuron binding in the presence of astrocytes and meningeal cells.61 Importantly, neither fibronectin nor lysine offered this specificity to neurons over the other cells61; however, it is not clear if the neuronal response can be further manipulated with mixed presentation of substrate-bound molecules. Further investigation into the influence of binding both cell and matrix adhesion molecules to substrates will be an interesting method to characterize the importance of cell–cell contacts, and their role in mechanotransduction, within the neural niche as single cells act differently compared to interconnected networks of cells.62
Scaffold stiffness
Scaffold stiffness is a widely studied parameter to increase neurite extension and focal adhesion formation of terminally differentiated neurons. Engler et al. were the first to demonstrate the sensitivity of MSCs to the stiffness of their substrate.63 It is now well accepted that soft materials, close to that of neural tissue or <1 kPa elastic modulus,40 encourage neuron viability and neurite extension.4 For example, cortical neurons extended longer neurites on 0.2-kPa shear modulus, approximately equivalent to 0.6-kPa elastic modulus, laminin-coated acrylamide gels, while astrocytes were rounded and had disrupted actin fibers on the same scaffolds.4 In contrast, neurite extension was decreased on 9-kPa shear modulus gels, approximately equivalent to 27 kPa elastic modulus, yet astrocytes formed distinct actin stress fibers.4 Chen et al. conducted a similar study but further investigated the effects of varying stiffness and protein coating on hippocampal neuron extension by focusing on actin filament formation, FAK, growth cone formation, and neurite outgrowth through the ERK1/2 pathway.10,23 Hippocampal neurons had the greatest neurite extension on 88-kPa elastic modulus fibronectin-coated polydimethylsiloxane (PDMS), which correlated to increased FAK and ERK1/2 phosphorylation compared to poly-L-lysine-coated surfaces, a nonselective focal adhesion activator.10 Adding complexity, the age of the tissue that NSCs are isolated from affects their commitment to neural lineages when cultured on varying stiffness substrates.64 Embryonic NSCs differentiated to neurons and astrocytes on PDMS of a wide range of elastic moduli, but softer scaffolds, ∼12 kPa elastic modulus, promoted neuronal maturation as determined by neurite length.64 Softer scaffolds, 0.1–0.5 kPa, were necessary to drive neuronal differentiation of adult NSCs, while more astrocytes formed on 1- to 10-kPa gels that had previously promoted embryonic neuronal differentiation.64,65 Although the absolute range of scaffold stiffness between studies varies, an overall trend emerges, where neurons prefer soft environments with similar stiffness to brain tissue (<1 kPa) and glia prefer stiffer environments (>5 kPa) when cultured on 2D substrates.
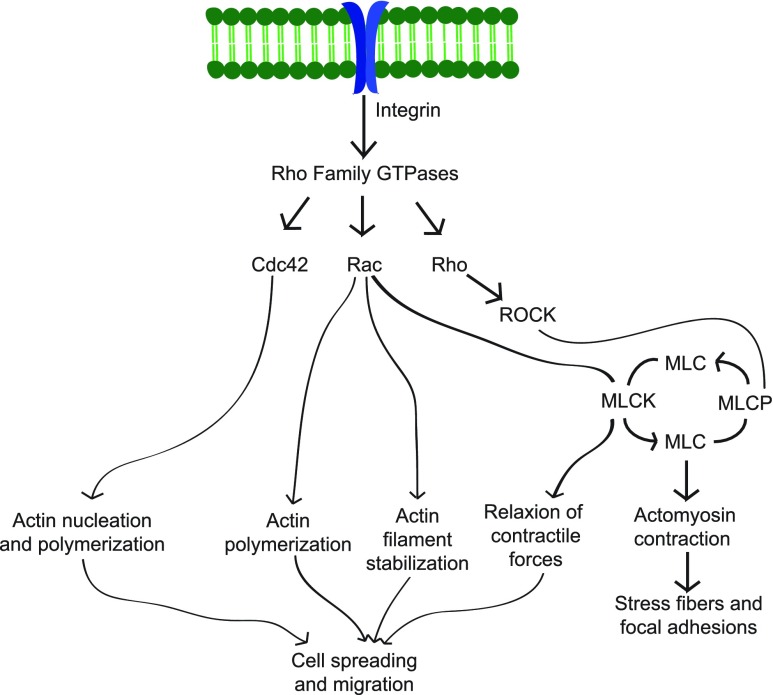
While substrate stiffness has been used as a driving force for NSC differentiation,33,66 if and how mechanotransduction events regulate this differentiation are less understood. Keung et al. studied the correlation between cell contractility, relating to the cytoskeleton shape, and NSC neuronal differentiation on 0.1- to 75-kPa laminin-coated PDMS for 6 days.33 They determined that specific Rho GTPase, RhoA, and Cdc42, but not Rac 1, activation in vitro and in vivo regulated cell fate by promoting neuronal differentiation over astrocytes or oligodendrocytes. It is these Rho GTPases that may be acting by means of transduction (Fig. 3). As Rho GTPases are widely studied in somatic cells for regulating cell shape and contractile forces, Keung et al. suggest that ROCK, myosin II, MLCK, Src, and FAK could be downstream factors that modulate cytoskeleton contraction.33 As previously mentioned, the Rho GTPases are connected to the Hippo/YAP pathway. Future work investigating connections with the Hippo/YAP pathway could lead to better understanding of the significance of substrate stiffness for NSC fate.
FIG. 3.
Rho family GTPase pathway. The Rho family GTPases, Cdc42, Rac, and Rho, control the cell shape, focal adhesions, and migration through signaling mechanisms to regulate actin filaments and the cytoskeleton. This image is a modification of QIAGENs original copyrighted image by Jessica M. Stukel. The original image may be found at www.QIAGEN.com/de/products/genes%20and%20pathways/pathway%20details/?pwid=12. Color images available online at www.liebertpub.com/teb
Interestingly, the NSCs adapted their own cellular stiffness in response to their substrate. Increased substrate stiffness resulted in increased NSC stiffness, as measured by atomic force microscopy. The stiffer substrate also resulted in differentiation to a mixture of neurons and astrocytes compared to the softer substrates that drove greater neuronal differentiation.33 Based on other cell types,67 cell stiffening is likely due to changes in the cytoskeleton based on integrin binding and/or focal adhesions. Future studies to validate these hypotheses, and the related links to mechanotransduction, are necessary but clearly point to the importance of material properties and cell sourcing for tissue engineering applications.
2D versus 3D culture
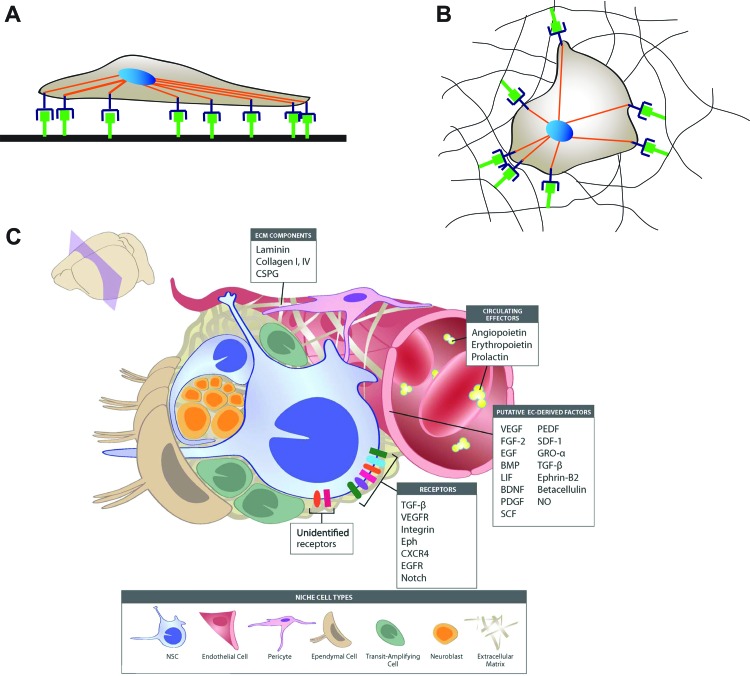
Mechanotransduction studies in 2D provide essential information about cellular interactions with a material, but a 3D culture niche may better mimic the in vivo environment. Comparing results in 2D and 3D studies is challenging because cells have access to different adhesion points to interact with the material as shown in Figure 4.
FIG. 4.
Two-dimensional (2D) versus three-dimensional (3D) culture. Cartoon of a cell cultured on a protein-coated (A) 2D surface and in a (B) 3D scaffold. The cell shape is determined by the distribution of integrins (purple) binding to the adhesion motif of an ECM protein (green), not drawn to scale. When cells are cultured on 2D surfaces, they tend to spread over the substrate and are flatter. In contrast, when cells are seeded within a 3D scaffold, they take on a spherical shape based on interactions with an ECM protein or peptide tethered or adsorbed to the scaffold. Three-dimensional culture better mimics (C) the in vivo neural stem cell (NSC) niche. (C) Reused from © 2013 Joshua S. Goldberg and Karen K. Hirschi. Originally published in Joshua S. Goldberg and Karen K. Hirschi (2013). A Vascular Perspective on Neurogenesis, Neural Stem Cells—New Perspectives, Dr. Luca Bonfanti (Ed.), ISBN: 978-953-51-1069-9, InTech, DOI: 10.5772/54980. Available from: www.intechopen.com/books/neural-stem-cells-new-perspectives/a-vascular-perspective-on-neurogenesis under CC BY 3.0 license. Available from: 10.5772/54980.39 Color images available online at www.liebertpub.com/teb
As previously mentioned, cell–cell contacts within the neural niche are important for cell signaling, and a proper 3D in vitro environment might better mimic this mechanism of cell–cell communication. However, 3D culture to study the influence of mechanotransduction is limiting because mechanical and biochemical properties of materials are coupled, making it difficult to determine the matrix or stiffness factors that are truly important. The Mahoney group studied neural progenitor cell differentiation in 2D and 3D poly(ethylene glycol) (PEG) hydrogels with a compressive modulus range from approximately 1 to 300 kPa.68 After 16 days, metabolic activity was not dependent on stiffness in 2D; however, the scaffold stiffness affected both metabolic and apoptotic activity in 3D.68 In these scaffolds, however, the pore size changes with the stiffness, and therefore, other factors may be a factor in the cellular response. The Schmidt group showed similar trends in neural progenitor cells isolated from E13.5 mouse ventral midbrain preference for stiffness of 2D versus 3D culture for progenitor cell differentiation for up to 3 weeks in hyaluronic acid (HA) hydrogels.66,69 A 3-kPa compressive modulus gel had increased β-III tubulin-positive cells (neuronal) compared to stiffer gels with a compressive moduli of 4 and 5 kPa. In addition, neurons in the 3-kPa hydrogels had branching neurites extending up to 600 μm, while those in stiffer gels had shorter (∼200 μm; 4 kPa) or no (5 kPa) neurites, indicating that the stiffer gel might impede extension of narrow neurites.66 Overall, the researchers hypothesized that CD44, the HA cell receptor, activated the Rho and Rac pathways for cell migration and gene expression and controlled progenitor cell differentiation.66 As other work has demonstrated an even greater affinity for neurons in softer gels (<1 kPa), it would be interesting to further study the effects in softer HA gels4 or to further characterize if these interactions with CD44 are mechanosensitive. As HA is a critical matrix molecule in the NSC niche, future studies could be particularly influential in the design of materials for neural regenerative medicine.
One approach to studying 3D mechanotransduction would be to observe the interaction between labeled integrins and proteins in the adhesion complexes.25 Cukierman et al. analyzed the involvement of α5, β1, β3, and proteins present in focal adhesions, and significant differences were noted between 2D and 3D matrices for initial attachment, cell morphology at 5 and 18 h, cell velocity, and proliferation. This work demonstrates differences in 2D and 3D cell–material interactions at the integrin level.25 Additionally, the extension of traction force microscopy to 3D70 and the capability of extending FRET to 3D71 open new areas of investigation for quantifying the interactions between cells and substrates.
Topography
Cell shape and neurite extension on patterned ridges and grooves
While the specific mechanisms for neuronal signaling remain unknown, it is clear that there is a link between mechanosensitive molecules and the cytoskeleton. Differences in the cytoskeleton structure influence the cell shape, including the shape of the nucleus,72 and it has been proposed that changing the cell shape affects signaling events and cell behavior.2,3,19 Aligned topography guides neurite extension longitudinally, modeling the in vivo environment when axons extend along the Bands of Büngner formed by glial support cells.73 Topography can also affect the direction and length of neurite outgrowth, an important parameter for neuronal regeneration, which is often observed through growth cones. Growth cones form the leading edge of extending neurites and are composed of dense polymerized actin.74 The growth cone extends by interacting with myosin II motors and naturally puts the neurite under tension.74,75 These events mechanically activate FAK, which activates the ERK2 pathway as the axon grows.17,74
Cell shape has been exogenously manipulated by culturing cells on substrates with defined topographical features or by stretching cells. Patterned ridges and grooves are a common topography for neural research because they mimic the aligned patterns of glial cells and neurons in vivo.2,19,26,76,77 PDMS is often used because well-defined patterns are formed with well-established photolithography techniques.2,78 Many studies report neuron alignment along the patterned substrate, but few probe signaling mechanisms responsible for the change in cell shape or how the cell–substrate interactions play a role in the alignment. Most often, neurons cultured on these substrates are analyzed based on the effect of the anisotropic topography on cell polarity, focal adhesions, and growth cones. Neurons align along the ridges and grooves by contact guidance, even when the pattern dimensions are less than the diameter of a round cell.76,77 One study investigating the mechanism regulating focal adhesions at neuronal growth cones determined that neuron polarity is mediated by ROCK though focal adhesions.74,76 These ideas of contact guidance, cell alignment, and potential links to gene expression have led to investigation of stem cell differentiation based on topography.1–3
Pattern dimensions cited for stem cell differentiation are on the nanometer to micron scale, similar to that used for neurons.79,80 For example, NSCs cultured on fibronectin-coated PDMS with parallel 1.5-μm-wide grooves with 10-nm-diameter pores had increased focal adhesions, integrin subunit β1, and NCAM expression compared to substrates with only with grooves and flat surfaces.19 In this study, NSCs differentiated into all potential fates, including functional neurons, astrocytes, and oligodendrocytes, but neuronal differentiation was the greatest. By blocking interactions with integrin subunit β1, Tuj1 and glial fibrillary acidic protein (GFAP) expression, markers for neurons and astrocytes, decreased; cell alignment decreased with application of a ROCK inhibitor.19 To determine if mechanical memory from the differentiation culture conditions was preserved; neurons that were differentiated on the patterned surfaces were then transferred to a flat substrate. The quantity and length of neurites were greater after being transferred compared to those differentiated and grown on flat topography alone.19 Future work to investigate if NSCs also retained their differentiation state after implantation for in vivo regeneration applications or on how mechanosensing pathways play a role in the differentiation would provide further insight.
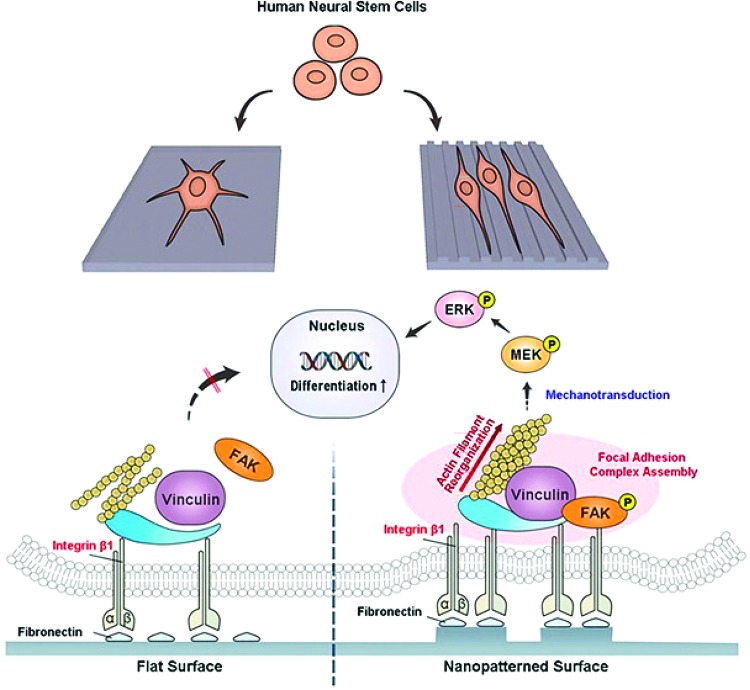
To investigate the effect of topography, groups have used ridges and grooves of varying dimensions. Neurons dominated the differentiated cell population with few astrocytes on gelatin-coated 350 × 350 nm ridges and grooves; oligodendrocytes were not labeled.2 Lee et al. hypothesized that changes in the cytoskeleton shape based on cell alignment and stretching, along the narrow parallel topography, transferred tensile forces to the nucleus and influenced signaling pathways and gene expression.2 However, details on specific mechanisms to confirm these mechanotransduction hypotheses have not been investigated. Another study from the Cho group investigated differences in NSC differentiation on two patterns of varying dimensions. Grooves and pillars of the same dimensions were fibronectin coated and seeded with NSCs for 5 days.26 The greatest neuron and astrocyte differentiation was reported on the 300-nm grooves and ridges,26 similar dimensions to the gelatin-coated substrate with greater neuronal differentiation.2 Yang et al. used nanotopography to study changes in focal adhesions between pattern dimensions. Vinculin expression was quantified by quantitative polymerase chain reaction (qPCR) and had the greatest relative expression on the surfaces with 300-nm ridges, 600-nm grooves, and 300-nm-diameter pillars with 300-nm gaps.26 Phosphorylated FAK was correlated with vinculin gene expression, shown in Figure 5, to demonstrate that patterns with smaller dimensions increased focal adhesions and FAK activation.26 Differentiation was analyzed based on integrin binding to fibronectin-coated substrates with varying nanotopographies, specifically with the α5β1 integrin. Although the integrin expression was not analyzed, an increase in focal adhesions, likely due to increased integrin binding or clustering, resulted in increased neuron and astrocyte differentiation.26 The MEK/ERK pathway was examined for downstream signaling from FAK because it affected neurite extension and stem cell differentiation.26 Blocking β1 and the MEK/ERK pathway decreased cell alignment, focal adhesions, cell spreading, neurite outgrowth, and differentiation.26 This work strongly indicates that topography, including that on the nanoscale, affects signaling of mechanotransduction events in NSCs.
FIG. 5.
Mechanism driving NSC differentiation based on topographical patterning. The influence of nanopatterned substrates affects FAK phosphorylation and focal adhesion complex assembly. Actin filaments reorganize and activate the MEK and ERK pathways to increase differentiation by nuclear changes. Differences in flat and patterned surfaces are shown. Reprinted (adapted) with permission from Yang et al.26 Copyright (2013) American Chemical Society. Color images available online at www.liebertpub.com/teb
Cell shape guided by electrospun fibers
Another method of promoting cell orientation is by the alignment of electrospun fibers. Many groups have compared aligned and random fibers on neurite orientation, and some have used aligned fibers as a driving force for neuronal differentiation. The fiber diameter ranges from 0.25 to 2.2 μm,79–81 but a consistent trend is fiber diameter less than that of the cell body to mimic the ECM structure and topographical features.81 Aligned electrospun fibers promote neuron orientation along the direction of alignment based on adhesion and contact guidance,81 although mixed results are noted on the effect of aligned fibers on neurite length compared to neurons on random fibers or flat substrates.81,82 Aligned fibers have also been investigated as a topographical driving force of neuronal differentiation of NSCs.79,80 Lim et al. treated NSCs with retinoic acid, and the neuronal differentiation was greater on aligned fibers compared to random fibers, indicating that fiber alignment has a role in NSC fate.79 Functionalizing aligned fibers can also affect the time required for differentiation. Pluripotency markers decreased after only 1 day of culture on aligned YIGSR-tethered PCL fibers, followed closely by increased neural differentiation markers of Tuj1 and MAP2 by day 3. Controls contained fibers that were aligned, with no peptide, or randomly arranged with YIGSR.83 These results demonstrated that neural cells respond to fiber alignment in similar ways to patterned ridges and grooves but noted that peptides may also be critical for differentiation.
Potential for Neural Tissue Engineering Therapies
As we progress in the neural tissue engineering field, the links between the microenvironment and cell behavior will lead to an informed and a rational design of new scaffolds. To date, work exploring the effect of ECM proteins or peptides, substrate stiffness, topography, and 3D culture has provided insight on increasing neural response. Understanding the mechanisms responsible for cell fate, proliferation, and migration will further progress the field for improved treatment for spinal cord injury, use in a peripheral nerve conduit, guiding growing neurons, and driving differentiation. Treatments can be improved by designing scaffolds to interact with or recruit endogenous cells for neural regeneration and using scaffold mechanical and biochemical properties to drive NSC differentiation. These improvements are significant because they have the potential to reduce the need for excess soluble factors to guide cell growth or differentiation. Additionally, knowledge of mechanotransduction can lead to a better prediction of the cell response, so materials can be rationally designed. Streamlining the process from scaffold design to in vivo testing has the potential to bring new treatments to patients in less time.
Conclusions
Mechanotransduction is a key factor in the cell response to its environment, and better understanding of the response has great significance for neural tissue engineering. Work in investigating signaling pathways, especially those that are sensitive to the extracellular mechanical environment, has provided clues to how cells sense and respond to varying stiffness substrates and topographical features. Recent work to understand signaling pathways, especially the Hippo/YAP pathway, has advanced our understanding of mechanotransduction. However, there are still many unknowns with respect to directing NSC differentiation. More work in this area is likely to result in improved control of stem cell fate for differentiation before or after in vivo implantation. Future work that clearly defines universal parameters and mechanisms for regulating the neural differentiation of stem cells in 3D environments will significantly advance the field.
Acknowledgments
This work was supported by the University of Akron (J.M.S.) and NIH 1R15GM113155-01 and the Margaret F. Donovan Endowed Chair for Women in Engineering (R.K.W.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Guilak F., et al. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 5, 17, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee M.R., et al. Direct differentiation of human embryonic stem cells into selective neurons on nanoscale ridge/groove pattern arrays. Biomaterials 31, 4360, 2010 [DOI] [PubMed] [Google Scholar]
- 3.McBeath R., et al. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 6, 483, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Georges P.C., et al. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys J 90, 3012, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev 13, 543, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Kshitiz , et al. Control of stem cell fate and function by engineering physical microenvironments. Integr Biol (Camb) 4, 1008, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alenghat F.J., and Ingber D.E. Mechanotransduction: all signals point to cytoskeleton, matrix, and integrins. Sci STKE 2002, pe6, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Ingber D.E. From cellular mechanotransduction to biologically inspired engineering. Ann Biomed Eng 38, 1148, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tate M.C., et al. Specific beta(1) integrins mediate adhesion, migration, and differentiation of neural progenitors derived from the embryonic striatum. Mol Cell Neurosci 27, 22, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Chen W.H., et al. Probing relevant molecules in modulating the neurite outgrowth of hippocampal neurons on substrates of different stiffness. PLoS One 8, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Y., et al. Hippo/YAP-mediated rigidity-dependent motor neuron differentiation of human pluripotent stem cells. Nat Mater 13, 599, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J., et al. Directing stem cell fate on hydrogel substrates by controlling cell geometry, matrix mechanics and adhesion ligand composition. Biomaterials 34, 8140, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Taupin P. Adult neural stem cells, neurogenic niches, and cellular therapy. Stem Cell Rev 2, 213, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Nava M.M., Raimondi M.T., and Pietrabissa R. Controlling self-renewal and differentiation of stem cells via mechanical cues. J Biomed Biotechnol 2012, 797410, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neirinckx V., et al. Concise review: adult mesenchymal stem cells, adult neural crest stem cells, and therapy of neurological pathologies: a state of play. Stem Cells Transl Med 2, 284, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poole K., Moroni M., and Lewin G.R. Sensory mechanotransduction at membrane-matrix interfaces. Pflugers Arch 467, 121, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMurray R.J., Dalby M.J., and Tsimbouri P.M. Using biomaterials to study stem cell mechanotransduction, growth and differentiation. J Tissue Eng Regen Med 9, 528, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Roca-Cusachs P., Iskratsch T., and Sheetz M.P. Finding the weakest link: exploring integrin-mediated mechanical molecular pathways. J Cell Sci 125, 3025, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang K., et al. Multiscale, hierarchically patterned topography for directing human neural stem cells into functional neurons. ACS Nano 8, 7809, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Ma W., et al. Cell-extracellular matrix interactions regulate neural differentiation of human embryonic stem cells. BMC Dev Biol 8, 90, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nampe D., and Tsutsui H. Engineered micromechanical cues affecting human pluripotent stem cell regulations and fate. J Lab Autom 18, 482, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Legate K.R., Wickstrom S.A., and Fassler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev 23, 397, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Mammoto A., Mammoto T., and Ingber D.E. Mechanosensitive mechanisms in transcriptional regulation. J Cell Sci 125, 3061, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geiger B., Spatz J.P., and Bershadsky A.D. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol 10, 21, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Cukierman E., et al. Taking cell-matrix adhesions to the third dimension. Science 294, 1708, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Yang K., et al. Nanotopographical manipulation of focal adhesion formation for enhanced differentiation of human neural stem cells. ACS Appl Mater Interfaces 5, 10529, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Teo B.K.K., et al. Nanotopography modulates mechanotransduction of stem cells and induces differentiation through focal adhesion kinase. ACS Nano 7, 4785, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Musah S., et al. Substratum-induced differentiation of human pluripotent stem cells reveals the coactivator YAP is a potent regulator of neuronal specification. Proc Natl Acad Sci U S A 111, 13805, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong V.W., et al. Pushing back: wound mechanotransduction in repair and regeneration. J Invest Dermatol 131, 2186, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Delmas P., Hao J., and Rodat-Despoix L. Molecular mechanisms of mechanotransduction in mammalian sensory neurons. Nat Rev Neurosci 12, 139, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Loverde J.R., Tolentino R.E., and Pfister B.J. Axon stretch growth: the mechanotransduction of neuronal growth. J Vis Exp 54, 2753, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiang L.-Y., et al. Laminin-332 coordinates mechanotransduction and growth cone bifurcation in sensory neurons. Nat Neurosci 14, 993, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Keung A.J., et al. Rho GTPases mediate the mechanosensitive lineage commitment of neural stem cells. Stem Cells 29, 1886, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quinones-Hinojosa A., et al. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol 494, 415, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Hersel U., Dahmen C., and Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials 24, 4385, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Li X., et al. Engineering neural stem cell fates with hydrogel design for central nervous system regeneration. Prog Polym Sci 37, 1105, 2012 [Google Scholar]
- 37.Discher D.E., Mooney D.J., and Zandstra P.W. Growth factors, matrices, and forces combine and control stem cells. Science 324, 1673, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen Q., et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell 3, 289, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldberg J.S., and Hirschi K.K. Diverse roles of the vasculature within the neural stem cell niche. Regen Med 4, 879, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arulmoli J., et al. Static stretch affects neural stem cell differentiation in an extracellular matrix-dependent manner. Sci Rep 5, 8499, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frith J.E., et al. Tailored integrin-extracellular matrix interactions to direct human mesenchymal stem cell differentiation. Stem Cells Dev 21, 2442, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayashi Y., et al. Integrins regulate mouse embryonic stem cell self-renewal. Stem Cells 25, 3005, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Maeda T., Titani K., and Sekiguchi K. Cell-adhesive activity and receptor-binding specificity of the laminin-derived YIGSR sequence grafted onto staphylococcal protein-A. J Biochem 115, 182, 1994 [DOI] [PubMed] [Google Scholar]
- 44.Saha K., et al. Biomimetic interfacial interpenetrating polymer networks control neural stem cell behavior. J Biomed Mater Res A 81, 240, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Solozobova V., Wyvekens N., and Pruszak J. Lessons from the embryonic neural stem cell niche for neural lineage differentiation of pluripotent stem cells. Stem Cell Rev 8, 813, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brizzi M.F., Tarone G., and Defilippi P. Extracellular matrix, integrins, and growth factors as tailors of the stem cell niche. Curr Opin Cell Biol 24, 645, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Doe C.Q. Neural stem cells: balancing self-renewal with differentiation. Development 135, 1575, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Santiago L.Y., et al. Peptide-surface modification of poly(caprolactone) with laminin-derived sequences for adipose-derived stem cell applications. Biomaterials 27, 2962, 2006 [DOI] [PubMed] [Google Scholar]
- 49.D'Souza S.E., Ginsberg M.H., and Plow E.F. Arginyl-glycyl-aspartic acid (RGD): a cell adhesion motif. Trends Biochem Sci 16, 246, 1991 [DOI] [PubMed] [Google Scholar]
- 50.Li X., et al. Short laminin peptide for improved neural stem cell growth. Stem Cells Transl Med 3, 662, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kleinman H.K., et al. Identification of a 110-kda nonintegrin cell-surface laminin-binding protein which recognizes an A chain neurite-promoting peptide. Arch Biochem Biophys 290, 320, 1991 [DOI] [PubMed] [Google Scholar]
- 52.Yang Y.-H., et al. Optimization of adhesive conditions for neural differentiation of murine embryonic stem cells using hydrogels functionalized with continuous Ile-Lys-Val-Ala-Val concentration gradients. Acta Biomater 21, 55, 2015 [DOI] [PubMed] [Google Scholar]
- 53.Addington C.P., et al. The role of SDF-1 alpha-ECM crosstalk in determining neural stem cell fate. Biomaterials 35, 3263, 2014 [DOI] [PubMed] [Google Scholar]
- 54.Tabdili H., et al. Cadherin-dependent mechanotransduction depends on ligand identity but not affinity. J Cell Sci 125, 4362, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jasaitis A., et al. E-cadherin-dependent stimulation of traction force at focal adhesions via the Src and PI3K signaling pathways. Biophys J 103, 175, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bian L., et al. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proc Natl Acad Sci U S A 110, 10117, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lamond A.I. Molecular biology of the cell, 4th edition. Nature 417, 383, 2002 [Google Scholar]
- 58.Kiryushko D., Berezin V., and Bock E. Regulators of neurite outgrowth: role of cell adhesion molecules. Ann N Y Acad Sci 1014, 140, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Ardini E., et al. Co-regulation and physical association of the 67-kDa monomeric laminin receptor and the alpha 6 beta 4 integrin. J Biol Chem 272, 2342, 1997 [DOI] [PubMed] [Google Scholar]
- 60.Buhusi M., et al. Close homolog of L1 is an enhancer of integrin-mediated cell migration. J Biol Chem 278, 25024, 2003 [DOI] [PubMed] [Google Scholar]
- 61.Webb K., et al. Substrate-bound human recombinant L1 selectively promotes neuronal attachment and outgrowth in the presence of astrocytes and fibroblasts. Biomaterials 22, 1017, 2001 [DOI] [PubMed] [Google Scholar]
- 62.Campos L.S. Neurospheres: insights biology into neural stem cell biology. J Neurosci Res 78, 761, 2004 [DOI] [PubMed] [Google Scholar]
- 63.Engler A.J., et al. Matrix elasticity directs stem cell lineage specification. Cell 126, 677, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Teixeira A.I., et al. The promotion of neuronal maturation on soft substrates. Biomaterials 30, 4567, 2009 [DOI] [PubMed] [Google Scholar]
- 65.Saha K., et al. Substrate modulus directs neural stem cell behavior. Biophys J 95, 4426, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seidlits S.K., et al. The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials 31, 3930, 2010 [DOI] [PubMed] [Google Scholar]
- 67.Krishnan R., et al. Substrate stiffening promotes endothelial monolayer disruption through enhanced physical forces. Am J Physiol Cell Physiol 300, C146, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lampe K.J., et al. Effect of macromer weight percent on neural cell growth in 2D and 3D nondegradable PEG hydrogel culture. J Biomed Mater Res A 94, 1162, 2010 [DOI] [PubMed] [Google Scholar]
- 69.Banerjee A., et al. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials 30, 4695, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Franck C., et al. Three-dimensional traction force microscopy: a new tool for quantifying cell-matrix interactions. PLoS One 6, e17833, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kong H.J., Boontheekul T., and Mooney D.J. Quantifying the relation between adhesion ligand-receptor bond formation and cell phenotype. Proc Natl Acad Sci U S A 103, 18534, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mazumder A., and Shivashankar G.V. Emergence of a prestressed eukaryotic nucleus during cellular differentiation and development. J R Soc Interface 7, S321, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ribeiro-Resende V.T., et al. Strategies for inducing the formation of bands of Bungner in peripheral nerve regeneration. Biomaterials 30, 5251, 2009 [DOI] [PubMed] [Google Scholar]
- 74.Franze K. The mechanical control of nervous system development. Development 140, 3069, 2013 [DOI] [PubMed] [Google Scholar]
- 75.VanEssen D.C. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature 385, 313, 1997 [DOI] [PubMed] [Google Scholar]
- 76.Tonazzini I., et al. Neuronal differentiation on anisotropic substrates and the influence of nanotopographical noise on neurite contact guidance. Biomaterials 34, 6027, 2013 [DOI] [PubMed] [Google Scholar]
- 77.Lopez-Fagundo C., et al. Navigating neurites utilize cellular topography of Schwann cell somas and processes for optimal guidance. Acta Biomater 9, 7158, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wieringa P., et al. Nanotopography induced contact guidance of the F11 cell line during neuronal differentiation: a neuronal model cell line for tissue scaffold development. Nanotechnology 23, 275102, 2012 [DOI] [PubMed] [Google Scholar]
- 79.Lim S.H., et al. The effect of nanofiber-guided cell alignment on the preferential differentiation of neural stem cells. Biomaterials 31, 9031, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang F., et al. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials 26, 2603, 2005 [DOI] [PubMed] [Google Scholar]
- 81.Lee J.Y., et al. Enhanced polarization of embryonic hippocampal neurons on micron scale electrospun fibers. J Biomed Mater Res A 92, 1398, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McMurtrey R.J. Patterned and functionalized nanofiber scaffolds in three-dimensional hydrogel constructs enhance neurite outgrowth and directional control. J Neural Eng 11, 066009, 2014 [DOI] [PubMed] [Google Scholar]
- 83.Callahan L.A.S., et al. Directed differentiation and neurite extension of mouse embryonic stem cell on aligned poly(lactide) nanofibers functionalized with YIGSR peptide. Biomaterials 34, 9089, 2013 [DOI] [PubMed] [Google Scholar]