Abstract
Human bone marrow multipotent mesenchymal stromal cell (hMSC) number decreases with aging. Subpopulations of hMSCs can differentiate into cells found in bone, vasculature, cartilage, gut, and other tissues and participate in their repair. Maintaining throughout adult life such cell subpopulations should help prevent or delay the onset of age-related degenerative conditions. Low oxygen tension, the physiological environment in progenitor cell-rich regions of the bone marrow microarchitecture, stimulates the self-renewal of marrow-isolated adult multilineage inducible (MIAMI) cells and expression of Sox2, Nanog, Oct4a nuclear accumulation, Notch intracellular domain, notch target genes, neuronal transcriptional repressor element 1 (RE1)-silencing transcription factor (REST), and hypoxia-inducible factor-1 alpha (HIF-1α), and additionally, by decreasing the expression of (i) the proapoptotic proteins, apoptosis-inducing factor (AIF) and Bak, and (ii) senescence-associated p53 expression and β-galactosidase activity. Furthermore, low oxygen increases canonical Wnt pathway signaling coreceptor Lrp5 expression, and PI3K/Akt pathway activation. Lrp5 inhibition decreases self-renewal marker Sox2 mRNA, Oct4a nuclear accumulation, and cell numbers. Wortmannin-mediated PI3K/Akt pathway inhibition leads to increased osteoblastic differentiation at both low and high oxygen tension. We demonstrate that low oxygen stimulates a complex signaling network involving PI3K/Akt, Notch, and canonical Wnt pathways, which mediate the observed increase in nuclear Oct4a and REST, with simultaneous decrease in p53, AIF, and Bak. Collectively, these pathway activations contribute to increased self-renewal with concomitant decreased differentiation, cell cycle arrest, apoptosis, and/or senescence in MIAMI cells. Importantly, the PI3K/Akt pathway plays a central mechanistic role in the oxygen tension-regulated self-renewal versus osteoblastic differentiation of progenitor cells.
Introduction
Human multipotent mesenchymal stromal cells (hMSCs) are a heterogeneous population of cells present in bone marrow [1]. The number of hMSCs with osteogenic potential (CFU-F/ALP+) decreases during human aging [2]. Marrow-isolated adult multilineage inducible (MIAMI) cells represent a homogeneous subpopulation of developmentally immature MSCs [3] expressing sustained levels of pluripotency markers (Oct4, Nanog, Sox2, Rex1, hTeRT, and SSEA-4) under defined growth conditions [4]. Supporting the immature nature of MIAMI cells, we demonstrated in a recent proteomic analysis that MIAMI cells have more proteins in common with embryonic stem cells (ESCs) than regular MSCs do [5]. MIAMI cells expanded at low oxygen consistently respond to osteogenic stimulation independently of donor age [4], suggesting a sustained osteogenic capacity during normal aging. Physiologic oxygen tension ranges from ≤1% in cartilage and regions of the bone marrow to 10%–13% in the main arteries of most tissues [6]. Increasing evidence suggests that oxygen tension is a key regulator of cell growth, proliferation, senescence, and differentiation of stem and progenitor cells [4,7–13]. We reported that low oxygen enhances MIAMI cell pluripotency, proliferation, and maintenance of osteoblastic differentiation capability [4]. However, little is known about the influence of oxygen on the modulation of signaling pathways, particularly, those that influence the interplay between self-renewal, proliferation, differentiation, and senescence.
Stem cell self-renewal involves several pathways, including Notch, Wnt, repressor element (RE)-1-silencing transcription factor (REST), Sirtuin, and the PI3K/Akt/mTOR pathway [13–18]. The highly conserved Notch pathway can regulate stem cell self-renewal [13,19]. Notch releases an intracellular fragment (notch intracellular domain, NICD) [20] that migrates to the nucleus where it regulates expression of target genes. NICD can also recruit HIF-1α to Notch-responsive promoters and elevate expression of Notch downstream targets, while blocking neuronal and myogenic progenitor differentiation [21]. Hypoxia may block this differentiation program by upregulating the stem cell transcription factor, Oct4. Covello et al. used a genetic knock-in strategy to demonstrate that targeted replacement of the oxygen-regulated transcription factor, HIF-1α, with its alternative isoform, HIF-2α, resulted in expanded expression of HIF-2α-specific target genes, including Oct4 [22]. Moreover, Oct4 shRNA reduced β-catenin levels in Hif-2α KI/KI tumors [22]. β-Catenin is a major mediator of the canonical Wnt signaling pathway and is also involved in self-renewal [23].
The Wnt signaling pathway [23,24] regulates downstream targets involved in modulating stem cell proliferation and self-renewal, including cell cycle regulator, Cyclin D1 [25], and pluripotency marker, Oct4 [26]. Pluripotent transcription factors, SOX2, OCT4, and NANOG, key regulators in ESCs, maintain self-renewal and multipotency of adult stem cells [27–30]. Specifically, maintenance of self-renewal and multipotency of hMSCs requires Sox2 [31,32] through mechanisms involving sirtuin [18] and Wnt [31] signaling. Wnt signaling can cross talk with the antiapoptotic/prosurvival pathway, PI3k/Akt, promoting self-renewal and expansion of stem cells [33]. Interestingly, transient hypoxia increases mouse ESC proliferation through activation of the PI3K/Akt pathway [17]. Moreover, proapoptotic protein BAD, an upstream regulator of Bak and others, and mitochondrial-specific caspase-9 are downstream targets of the PI3K/Akt pathway [34].
Repression of differentiation programs also maintains stem cell self-renewal. Neuron-restrictive silencer factor/RE1-silencing transcription factor (NRSF/REST) is an abundantly expressed DNA-binding transcriptional repressor of several neuronal genes in human and mouse pluripotent ESCs [35,36]. REST has both oncogenic and tumor-suppressing roles depending on cellular context [37]. REST may be a component of the transcriptional network involved in maintaining stem cell self-renewal, specifically blocking neuronal differentiation [36], although evidence opposing this proposed mechanism has also been reported [38,39].
To increase our understanding of the pathways and mechanisms by which oxygen tension may stimulate self-renewal of MIAMI cells, we examined the effect of low oxygen on signaling and regulation of self-renewal mediators, cell cycle regulation, apoptosis, and/or senescence gene expression. Furthermore, we used specific inhibitors to determine a mechanistic role of these pathways. This may help develop molecular approaches aimed at promoting stem cell self-renewal, thus preventing or decreasing the observed age-related decline of stem cells and tissue function in vivo [2].
Materials and Methods
Antibodies
Oct4 was from Abcam (Cambridge, MA). FITC/rhodamine secondary for Oct4 was from Invitrogen (Carlsbad, CA). Mounting solution with DAPI was from Vector Laboratories (Burlingame, CA). Antibodies for western blotting, Nanog, hTeRT, Sox2, Oct4a, Oct4b, Lrp5, p53, AIF, Bak, p21, p27, EndoG, Bax, Bid, Bcl-xl, CIAP-2, Mcl-1, XIAP, HIF-1α, NICD, REST, Akt, and α-tubulin were from Abcam.
Isolation and culture of human MIAMI cells
We previously reported the isolation of MIAMI cells in detail [3,4]. Briefly, we isolated MIAMI cells from commercially available human whole bone marrow (BM) (Lonza, Walkersville, MD, BM from a 20-year-old male, a 3-year-old male, or a 7-year-old male donor). For expansion, we plated MIAMI cells at a density of 100 cells/cm2 in expansion medium in a 100% humidified atmosphere of 3% O2, 5% CO2, and 92% N2 (low oxygen tension) and one-half medium changed twice a week. We used only MIAMI cells from passages 3 and 4. Cells receiving exogenous treatments with recombinant mouse Wnt3a underwent a 24-h period of serum starvation before addition of 5 ng/mL of recombinant mouse Wnt3a in the medium. To test Akt activation (phospho-Akt), we plated MIAMI cells in expansion medium and exposed them to atmospheres of 21% or 3% O2. We measured phospho-Akt and total Akt after 30 and 60 min of incubation. We inhibited the PI3K/Akt signaling pathway using different concentrations of wortmannin, 20, 50, 100, 200, and 300 nM, on MIAMI cells incubated for 30 min at 3% O2 in expansion medium.
Quantitative real-time PCR
We performed quantitative real-time PCR (RT-qPCR) as previously described and normalized the fluorescence data to the housekeeping genes, eukaryotic translation elongation factor 1 alpha (EF1α) and/or ribosomal protein large subunit 13a (RPL13a) [40]. Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/scd) lists the DNA oligonucleotide sequences used for RT-qPCR.
Western blot
We prepared protein samples using NP-40 lysis buffer, separated them by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on 10% polyacrylamide gels, and then transferred them onto a PVDF membrane (electrophoresis and transfer apparatus from Bio-Rad; Hercules, CA) for approximately 60–90 min at 100V. We incubated blots with primary antibodies at 1:1,000 overnight at 4°C with gentle rocking, rinsed them with TBS, and incubated them with secondary antibodies at 1:5,000 for 45 min at room temperature with gentle rocking. We normalized protein levels to α-tubulin and/or Coomassie blue-stained membranes. We performed quantitation of blots using ImageJ software from NIH. We normalized data (using either Coomassie blue total protein or β-catenin) to correct for variations in protein loading.
Immunoprecipitation
We aliquoted 25 μL of protein A/G Plus agarose beads (Santa Cruz Biotech., Inc., Santa Cruz, CA) with 2 μg of primary antibody to TCF4 and incubated them for 1 h at room temperature on a rotator. We washed the protein A/G agarose beads 3× with 1 mL PBS (Invitrogen, Carlsbad, CA) and then centrifuged them at 3,000 g for 2 min at 4°C. We adjusted the concentration to 200–400 μg of protein sample to 200 μL PBS and incubated the protein/bead solution overnight at 4°C on a rotator. The next day, we washed the protein/bead solution 3× with PBS before centrifugation at 3,000 g for 2 min at 4°C. We saved the supernatants and separated the proteins using PAGE. Samples run without primary antibody or with isotypic nonimmune (normal rabbit) serum served as negative controls. We incubated the blots in primary antibodies (diluted in either 0.1% BSA or 5% nonfat milk) overnight at 4°C and secondary antibodies conjugated with horseradish peroxidase (diluted in either 0.1% BSA or 5% nonfat milk) for 45 min at room temperature. We finally incubated the blots with ECL chemiluminescence solution (GE Life Sciences) as per the manufacturer's instructions. Final levels of proteins were normalized to Coomassie-stained blots for total protein.
siRNA transfections
We transfected MIAMI cells 1 day after seeding with 50, 100, or 200 nM siRNA ON-TARGET plus SMART pool (siLrp5: CGUCAAAGCCAUCGACUAUUU, CGUCAUGG GUGGUGUCUAUUU, GGACGGACCUACGGAGGAUUU, GUACAGGC CCUACAUCAUUUUU; siLrp6: GCAGAUAU CAGA CGAAUUUUU, CAGAUGAACUGGAUUGUUAUU, CCACAGAGCGAUCACAUUAUU, GCUCAACCGUGAAG UUAUAUU) or siCONTROL Non-Targeting pool (combination of four siRNA sequences that are not targeted to Lrp5 or 6 and found to have little off-target effects, all from Dharmacon, Chicago, IL). We performed the transfections with the Nucleofector electroporator (Amaxa; Walkersville, MD). We trypsinized 1 to 2 × 106 cells for each transfection procedure. We used Nucleofector solution as our transfection reagent according to the Amaxa protocol for the Nucleofector Kit. Immediately following transfections, we placed the cells dropwise into incubated six-well plates containing MIAMI expansion media. Twenty-four hours after the transfections, we replaced all media with fresh MIAMI expansion media. Cells were left undisturbed for 3 days before trypsinization for a second round of transfection using the same procedure stated above. We collected the cells for RNA and protein analysis 3 days after the second transfection. To assess transfection efficiency, we transfected MIAMI cells with 1 μg GFP (green fluorescent protein) plasmid pEGFP-C1 (Clontech, Mountain View, CA) and visualized them on a Nikon fluorescence microscope.
Enzyme-Linked Immunosorbent Assay
We measured Wnt3a concentrations in the media of MIAMI cells cultured in 3% and 21% O2 using enzyme-linked immunosorbent assay (ELISA) as follows: we coated a 96-well plate with capture antibody anti-Wnt3a (R&D Systems, Minneapolis, MN) at a 0.5 μg/mL dilution with PBS. We added media from MIAMI cells grown at 3% and 21% oxygen to the capture antibody preparation. We added fresh media as a negative control. We carefully washed the medium from the plate and added the detection biotinylated anti-Wnt3a (0.5 μg/mL). We finally used a horseradish peroxidase-conjugated streptavidin solution and stopped the colorimetric reaction with 2 M sulfuric acid. Optical density was measured at 450 nm on a microplate reader (BioRad, Hercules, CA).
Oct4 immunofluorescence
For nuclear proteins, we fixed cells with 4% PFA at 4°C for 10 min and permeabilized them with 0.1% Triton X-100 for 10 min. Blocking and diluent solution consisted of PBS with 1% BSA. We blocked the fixed cells for 30 min and incubated them sequentially for 1 h with the primary antibody to Oct4, followed by a 1-h incubation of the fluorescein- or rhodamine-conjugated secondary anti-goat IgG antibody. We used PBS plus 0.3% BSA for the washes between each step. We used isotypic antibodies as controls. Imaging was performed using a Nikon Eclipse TI microscope as well as a Nikon C2 confocal microscope (Nikon Instruments, Melville, NY).
Microarray analysis
We used the Oliogo GEArray® System (SuperArray). The array requires biotin-labeled cRNA to hybridize to the membrane. We synthesized the labeled cRNA needed for hybridization to the array with the TrueLabeling-AMP™ 2.0 (SuperArray) system. We used a total of 3 μg of RNA in the system to optimize for the final cRNA yield. We quantified the cRNA and used the same initial amount for both the 21% and 3% O2 samples. We performed hybridization until chemiluminescent detection performed on the ChemiGenius (Syngene, Frederick, MD) imager and analysis with the GEArray Analysis Suite.
Senescence-associated β-galactosidase activity
We assayed the cells for senescence-associated β-galactosidase (SA-β-Gal) activity using the Cell Signaling kit #9860 (Cell Signaling Technology, Danvers, MA) as per the manufacturer's instructions. In brief, we plated the MIAMI cells (passage 4–6) at 200 cells/cm2 in six-well plates in either 3% or 21% oxygen tension for up to 1 or 3 weeks. To assess for SA-β-Gal, we removed the medium and washed the cells with PBS before fixation for 10–15 min at room temperature. We rinsed the cells again with PBS, followed by application of the β-galactosidase staining solution and incubation at 37°C overnight in a dry incubator in the absence of CO2. Development of a blue color indicated SA-β-Gal activity. Ten fields of cells were counted per sample to determine the percentage of cells in a senescence state.
Osteoblastic differentiation conditions
We plated MIAMI cells at 2,000 cells/cm2 in 24-well plates in α-MEM supplemented with 10% FBS (Hyclone, Logan, UT), 100 U/mL penicillin, 1 mg/mL streptomycin, 10 nM dexamethasone, 100 μM ascorbic acid 2-phosphate, and 10 mM β-glycerophosphate [2].
Alkaline phosphatase histochemical analysis
MIAMI cells in osteogenic medium at 3% and 21% pO2 were rinsed in PBS, fixed with a solution of 2% cold-buffered formaldehyde and 0.2% gluteraldehyde for 30 min at 4°C, rinsed with distilled H2O, and then stained for alkaline phosphatase activity at the times indicated in the corresponding Figure legend. Briefly, we dissolved 8 mg of naphthol AS-TR phosphate in 0.3 mL of N, N-dimethylformamide. Simultaneously, we dissolved 24 mg of fast blue BB in 30 mL of 100 mM Tris-HCl, pH 9.6. We mixed these two solutions, added 10 mg of MgCl2, and then adjusted the pH to 9.0 with 1N HCl. We filtered the final solution through a 0.2-μm filter. We finally incubated the cells at 37°C for 30 min and rinsed them with distilled H2O [2].
Statistical analysis
Results are presented as the mean of three independent experiments ± standard deviations (SD). We tested the differences by one-way ANOVA and calculated the P-values using the Newman–Keuls post hoc test. We considered a threshold P-value ≤ 0.05 as significant and depicted it with an * in the Figures.
Results
Low oxygen stimulates the expression of transcription factors associated with stem cell self-renewal
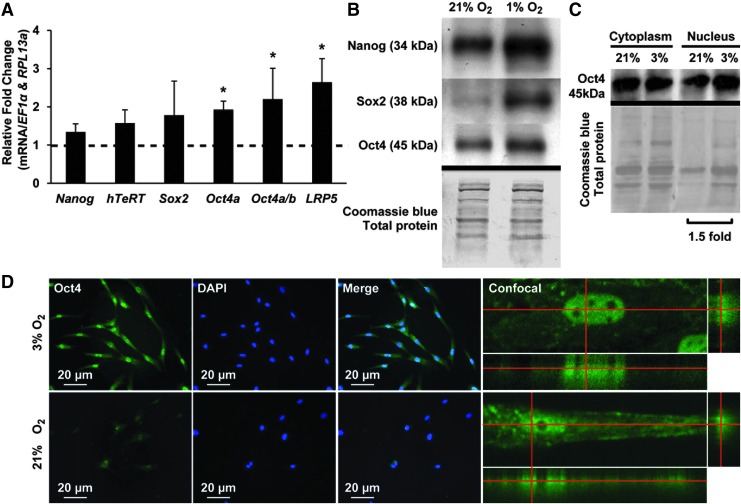
Previously, we reported that low oxygen (3%) upregulated mRNAs for total Oct4 (isoforms a and b), Rex-1, hTeRT, SSEA-4, and HIF-1α under expansion and osteogenic conditions [4]. We hypothesized that low oxygen may also stimulate the expression of other known mediators of self-renewal, such as Nanog, Sox2, and Oct4a (the active Oct4 splice variant, which localizes to the nucleus [41]). In cells grown at 3% versus 21% oxygen for 7 days, we found increased levels of transcripts for these genes and Lrp5 (Fig. 1A), a key coreceptor and modulator of the Wnt signaling pathway implicated in the maintenance of mouse embryonic stem cell (mESC) pluripotency. To determine whether low oxygen tension treatment would acutely increase the protein level, we exposed to 21% O2 overnight cells previously expanded at 3% O2 and then switched to 1% O2 for 2 h (acute setting). Western blot analysis showed that protein levels of Oct4a/b, Nanog, and, more dramatically, Sox2 increased after acute low oxygen (Fig. 1B).
FIG. 1.
Low oxygen tension increases key self-renewal transcription factors and LRP5. (A) Graph depicts relative fold change of mRNA expression of Wnt mediators and self-renewal markers (Nanog, hTeRT, Sox2, Oct4a, Oct4a/b) and Lrp5 in MIAMI cells grown for 7 days at low oxygen compared with cells kept at 21% O2 for 7 days (dashed line set to 1). (B) Western blot of MIAMI cells after switch from 21% to 1% oxygen for 1 h. Specific antibodies to Nanog (top panel), Sox2 (second panel), and Oct4 (third panel) were used to detect proteins. (C) Western blot analysis shows increased levels of nuclear Oct4 (45 kDa) at 3% compared with 21% O2. Coomassie blue stain (bottom panel) shown as loading control for (B) and (C). (D) DAPI-stained nuclei and merged Oct4-FITC/DAPI image of MIAMI cells at 3% (top) and 21% (bottom) oxygen (left). We confirmed the nuclear localization of Oct4 at 3% oxygen using confocal microscopy (right). MIAMI, marrow-isolated adult multilineage inducible. *P ≤ 0.05.
Low oxygen increases the nuclear levels of Oct4a
To determine whether Oct4 localized to the nucleus and to distinguish between the Oct4a/b isoforms, we examined cytoplasmic and nuclear fractions and used immunofluorescence to assess subcellular Oct4 localization. Western blot analysis demonstrated increased levels of nuclear Oct4 after 1 week at 3% versus 21% O2 (Fig. 1C). We further confirmed nuclear localization by immunofluorescence and confocal studies that showed stronger Oct4 nuclear staining in cells at low (3%) versus ambient (21%) oxygen (Fig. 1D).
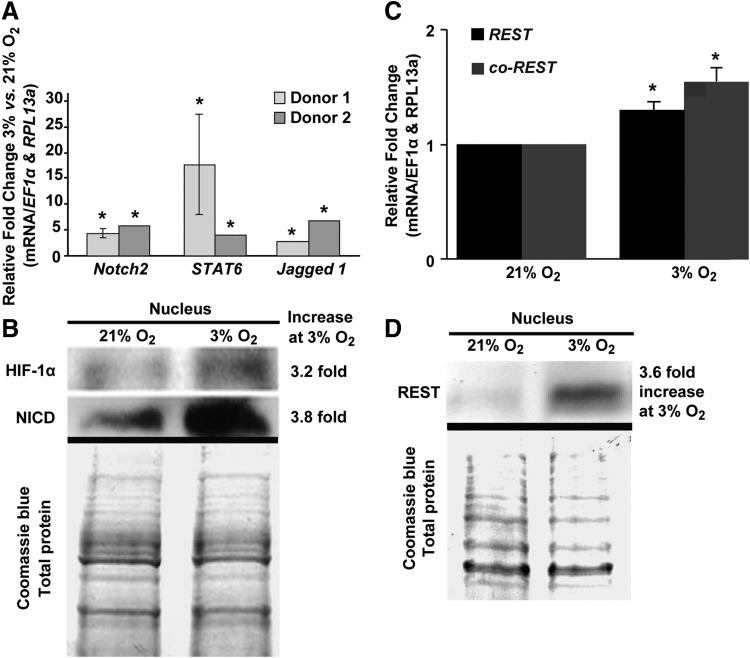
Low oxygen increases expression of Notch signaling pathway mediators
To examine potential signaling pathways mediating the low oxygen-stimulated self-renewal program of MIAMI cells, we performed a signaling pathway array on cells cultured for 7 days at 3% versus 21% O2 (Supplementary Fig. S1). Genes upregulated at 3% O2 are listed in Supplementary Table S2. The data revealed upregulation of key Notch mediators among others. RT-qPCR confirmed gene expression stimulation of key genes: Notch2, STAT6, and Jagged 1 (Fig. 2A). To determine involvement of Notch signaling activation, we examined low oxygen effects on NICD nuclear levels. Western blot analyses demonstrated that NICD protein levels increased (3.8-fold) in the nucleus at low oxygen along with a simultaneous nuclear increase of HIF-1α (3.2-fold), a low-oxygen transcriptional mediator and NICD cofactor for the transactivation of specific target genes [21] (Fig. 2B).
FIG. 2.
Low oxygen activates Notch signaling in MIAMI cells and increases REST and co-REST expression. (A) Relative fold change of Notch mediator (Notch2, Stat6, and Jagged1) mRNA levels after 7 days at 3% compared with 21% O2 measured by RT-qPCR and normalized to housekeeping genes, EF1α and RPL13a. (B) Western blot analysis: lane 1 shows protein lysates from MIAMI cells grown at 21% O2, lane 2 cells were grown at 3% O2. Top panel shows HIF-1α protein, center panel shows NICD protein. (C) mRNA levels of REST and co-REST as measured by RT-qPCR from MIAMI cells grown at high and low oxygen for 7 days. (D) Protein levels of REST and co-REST were measured by western blot from the nuclear fraction of MIAMI cells grown at high and low oxygen for 7 days (top panel). Coomassie blue staining used as loading control (bottom panel). REST, repressor element-1-silencing transcription factor; RT-qPCR, quantitative real-time PCR. *P ≤ 0.05.
Low oxygen increases REST/NRSF levels
MIAMI cells can be induced to differentiate into neuron-like cells [3,42–45]. REST/NRSF repressed neuronal differentiation in mouse ESCs [36]. Thus, we sought to examine whether REST/NRSF may contribute to MIAMI stem cell self-renewal by inhibiting neuronal differentiation. RT-qPCR showed that MIAMI cell mRNA levels of REST and co-REST increased in 3% versus 21% O2 conditions (Fig. 2C). Furthermore, the REST protein accumulated at 3.6-fold higher levels in the nucleus of cells under low oxygen conditions (Fig. 2D).
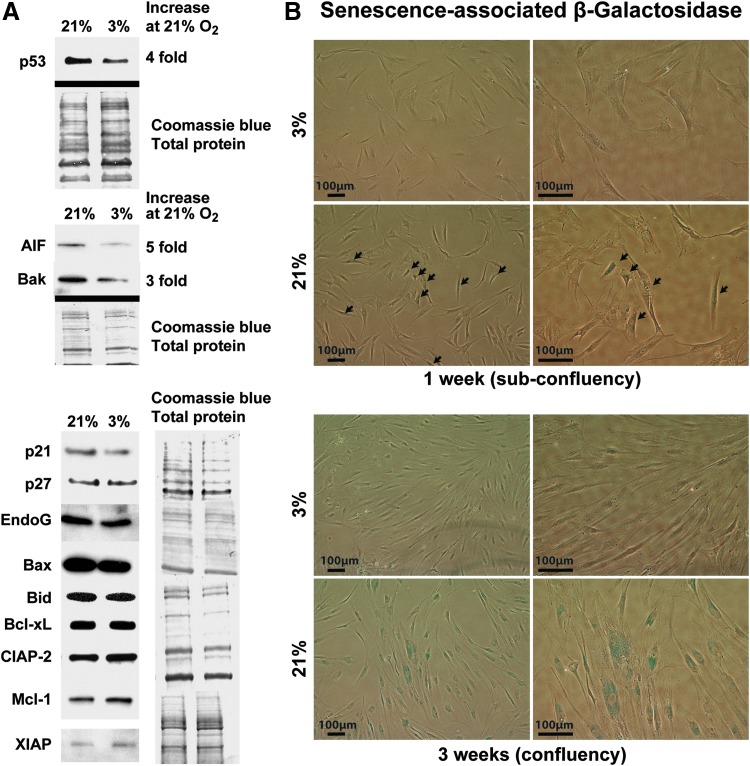
Low oxygen regulates expression of specific apoptosis/senescence pathway genes
We examined whether the expression of cell cycle control, apoptosis, and senescence pathway-associated genes was affected by low oxygen. The tumor suppressor p53 is a master regulator of these programs by differentially modulating target genes controlling cell cycle arrest, apoptosis, or senescence [46,47]. We found that low oxygen decreased protein expression of p53 by approximately fourfold (Fig. 3A), while p21 and p27 levels did not appear to be affected. Furthermore, expression of the proapoptotic proteins, apoptosis-inducing factor (AIF) and Bak, was reduced five- and threefold, respectively (Fig. 3A). Expression of endoG, Bax, Bid, Bcl-xL, and cIAP-2 was not affected, while the inhibitors of apoptosis, XIAP and Mcl-1, appeared to increase slightly (Fig. 3A). These results are consistent with an overall cell prosurvival program at low oxygen. To further assess an effect on cell senescence, we quantified the number of cells with detectable senescence-associated β-galactosidase (SA-βgal) activity and determined that 3% O2 dramatically decreased positive cells versus 21% O2 (5% ± 2% vs. 48% ± 8% at 3 weeks, respectively, P ≤ 0.01; Fig. 3B).
FIG. 3.
Low oxygen decreases markers of cell cycle arrest, apoptosis, and senescence. (A) Left panels show increased proapoptotic proteins; p53, AIF, and Bak in high (21% O2) versus low oxygen (3% O2). Right panels show no change in cell cycle proteins, p21 and p27, and apoptosis-related proteins, EndoG, Bax, Bid, Bcl-XL, CIAP-2, Mcl-1, and XIAP, at high versus low oxygen. Coomassie blue staining was used as loading control for western blots. (B) MIAMI cells were stained for senescence-associated β-galactosidase (SA-βgal) activity after 1 and 3 weeks at 3% O2 versus 21% O2. As early as after 1 week of culture, a blue stain was observed in a significant fraction of the cells exposed to 21% O2 (48% ± 8% positive cells) compared with the cells exposed to 3% O2 (5% ± 2% positive cells). Black arrows point to positive cells. After 3 weeks of culture, almost all of the cells exposed to 21% O2 expressed SA-βgal (bottom). AIF, apoptosis-inducing factor.
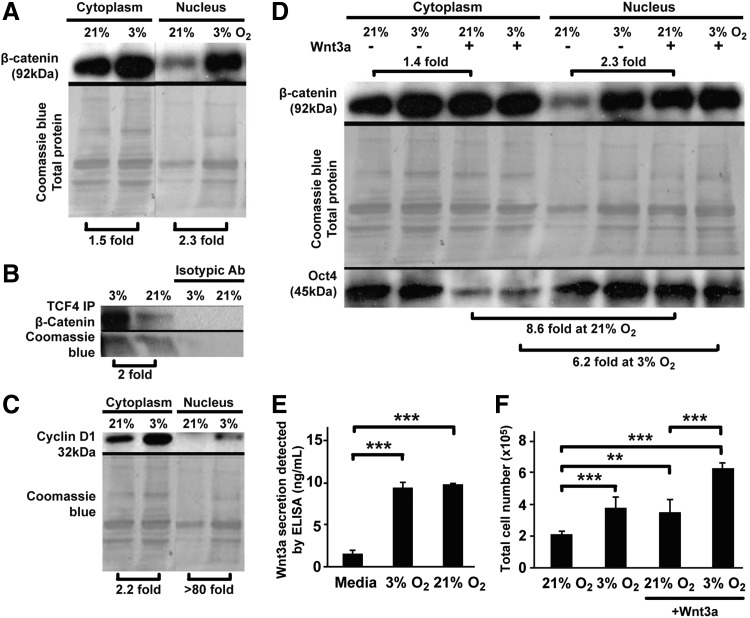
Low oxygen stimulates the canonical Wnt pathway
Since we observed 3% O2 stimulation of Lrp5 (Fig. 1A) and frizzled 1 and 2 (Supplementary Fig. S1B), we further examined canonical Wnt pathway activation. Western blot analysis of nuclear and cytoplasmic extracts of MIAMI cells demonstrated that 3% oxygen stimulated cytoplasmic β-catenin (1.5-fold) and, to a larger extent, nuclear β-catenin (2.3-fold, Fig. 4A). Once in the nucleus, β-catenin binds a member of the high mobility group (HMG) DNA-binding protein family of transcription factors. We investigated canonical Wnt signaling specific protein–protein interaction in MIAMI cells. We pulled down proteins bound by T-cell Factor 4 (TCF4) antibody with protein-A/G beads. Subsequently, we size separated, blotted, and probed the immunoprecipitated proteins with β-catenin antibody. Samples from cells at 3% O2 displayed more than twofold greater association between β-catenin and TCF4 versus 21% O2 (Fig. 4B). Subsequently, we examined downstream targets of canonical Wnt activation. The cell cycle protein and direct Wnt target, Cyclin D1, was increased by 3% O2 about 2.2-fold in the cytoplasm and by more than 80-fold in the nucleus (Fig. 4C).
FIG. 4.
Low oxygen tension stimulates the canonical Wnt pathway. Low oxygen increases (A) nuclear accumulation of β-catenin. (B) β-Catenin/TCF4 interactions: immunoprecipitation of 200 μg protein samples with TCF4, which were size separated and probed with β-catenin Abs in MIAMI cells grown at 3% oxygen (lane 1) and 21% oxygen (lane 2). Isotypic negative controls (normal rabbit serum); 3% samples (lane 3) and 21% (lane 4) Coomassie blue stain (bottom panel) as loading control. (C) CyclinD1 cytoplasmic (lanes 1–2) and nuclear proteins (lanes 3–4) at 3% and 21% oxygen. Coomassie stain is loading control. (D) Changes in the levels of cytoplasmic and nuclear β-catenin in response to oxygen and Wnt3a stimulation. Changes in the levels of cytoplasmic and nuclear Oct4 in response to oxygen and Wnt3a stimulation. (E) Low oxygen fails to stimulate Wnt3a secretion: ELISA from MIAMI cell-conditioned media taken from cells grown for 7 days at 3% and 21% oxygen. A media-only sample was run to determine baseline levels of Wnt3a. (F) Exogenous Wnt3a increased the cell number at low and high oxygen tension: total cell numbers of cells seeded at 100 cells/cm2 and grown at 3% or 21% oxygen with or without rmWnt3a treatment for 7 days. **P ≤ 0.01 and ***P ≤ 0.001.
To determine the magnitude of the canonical Wnt signaling activation by low oxygen, we compared β-catenin nuclear localization at 3% and 21% O2 in the absence and presence of recombinant Wnt3a. Both 3% O2 and Wnt3a increased to similar levels of cytoplasmic (1.5 vs. 1.4-fold) and nuclear (2.3-fold) β-catenin, while the combination did not have an additive effect (Fig. 4D). To determine the effect of Wnt3a on the nuclear localization of a different low oxygen-sensitive protein, we examined the levels of Oct4 in the cytoplasm and nucleus at 3% and 21% O2 in the presence and absence of Wnt3a. Surprisingly, we found that in contrast to low oxygen that stimulated nuclear Oct4 localization by 1.5-fold (Fig. 1C), Wnt3a increased Oct4 nuclear translocation by 8.6-fold at 21% O2 and by 6.2-fold at 3% O2 (Fig. 4D).
Since low oxygen induced canonical Wnt pathway stimulation, we sought to determine whether this was a paracrine effect accomplished through a low oxygen-stimulated secretion of canonical Wnt signaling activators. We found that 3% O2 significantly stimulated Wnt3a mRNA (20-fold), while Wnt7 and Wnt11 mRNAs were only minimally stimulated (Supplementary Table S2). Interestingly, low oxygen failed to stimulate Wnt3a secretion, quantified by ELISA (Fig. 4E). Furthermore, we demonstrated that either low oxygen or Wnt3a stimulation increased cell numbers to similar extents; however, when combined, the effect was additive (Fig. 4F).
Lrp5-mediated inhibition of the canonical Wnt signaling pathway decreases self-renewal capacity of MIAMI cells, self-renewal marker mRNAs, and Oct4a nuclear accumulation
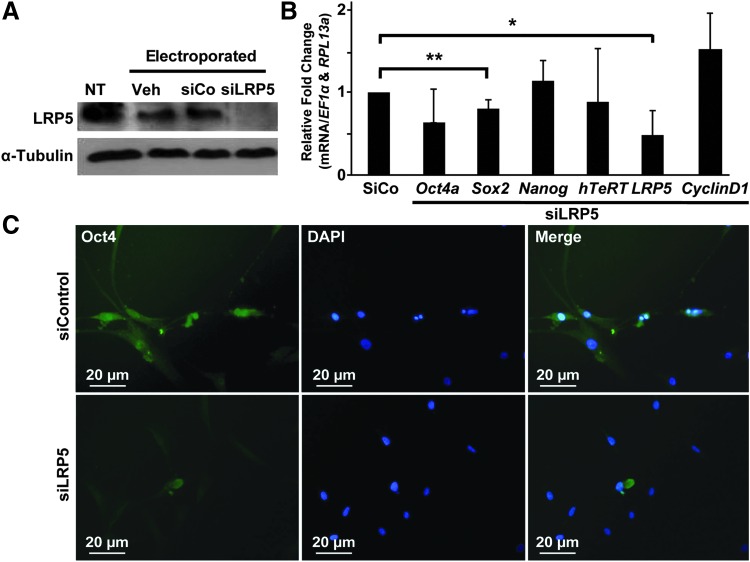
Canonical Wnt activity requires receptors, Lrp5/6. Basal mammary cells express both Lrp5 and Lrp6 similarly. Interestingly, high Lrp5 expression identifies the stem cell-enriched population and is required for maintenance of the basal cell layer. The absence of Lrp5 specifically depletes adult regenerative stem cell activity to less than 1% [48]. It has been shown that an increased expression of Lrp5 is sufficient for activation of the canonical Wnt signaling pathway [49]. In light of our data showing that low oxygen increased Lrp5 mRNA transcript levels (Fig. 1A) and our ELISA data showing equal secretion of Wnt3a at low and high oxygen tension (Fig. 4E), we sought to examine the possibility that Lrp5 could be the rate-limiting step in the low oxygen-stimulated Wnt activity we observed in our cells and that it may be essential for MIAMI cell self-renewal. We found that exposure of cells to siLrp5 (100-nM) decreased Lrp5 protein levels (Fig. 5A) as well as Oct4a, Sox2, and Lrp5 mRNA levels, although only the last two with statistical significance (Fig. 5B, *P ≤ 0.05 and **P ≤ 0.01, respectively). The number of cells was decreased by 50% after comparing the cell number of cells exposed to siControl versus siLrp5 for 2 consecutive periods of 3 days each (not shown). Since the results for Oct4 were not conclusive, we used immunofluorescence to determine the effect of siLrp5 on the subcellular localization of the Oct4 protein. We observed nuclear localization of Oct4 in siControl cells (Fig. 5C) consistent with untreated cells in Fig. 1D. In siLrp5-treated cells, Oct4 protein did not appear to accumulate in the nucleus or cytoplasm (Fig. 5C); an indication that Oct4a-specific nuclear levels decrease in the absence of Lrp5. We sporadically observed a diffuse area of Oct4 protein outside the nucleus in cells transfected with siLrp5 compared with the negative siControl (Fig. 5C) with an overall reduced Oct4 signal similar to that seen in untreated cells grown at 21% O2 in Fig. 1D.
FIG. 5.
LRP5 knockdown by siLRP5 decreased mRNA of self-renewal markers and nuclear accumulation of Oct4a. (A) Western blot of LRP5 (top panel) from siLrp5-treated cells at 3% oxygen: Nontransfected cells (NT, lane 1). Cells electroporated with no siRNA (lane 2); siControl (siCo, lane 3); and siLRP5 (lane 4). Tubulin (bottom panel) is used as loading control. (B) RT-qPCR determined mRNA levels for Lrp5, Oct4a, Sox2, Nanog, Cyclin D1, and hTeRT in siLRP5 cells treated for 3 days compared with siControl-treated cells (siCo, set as 1 arbitrarily, *P ≤ 0.05 and **P ≤ 0.01). All genes normalized to EF1α and RPL13a. (C) ICF of cells treated with siControl or siLrp5 with anti-Oct4-FITC; DAPI-stained nucleus; images merged as indicated.
Low oxygen transiently upregulates Akt expression
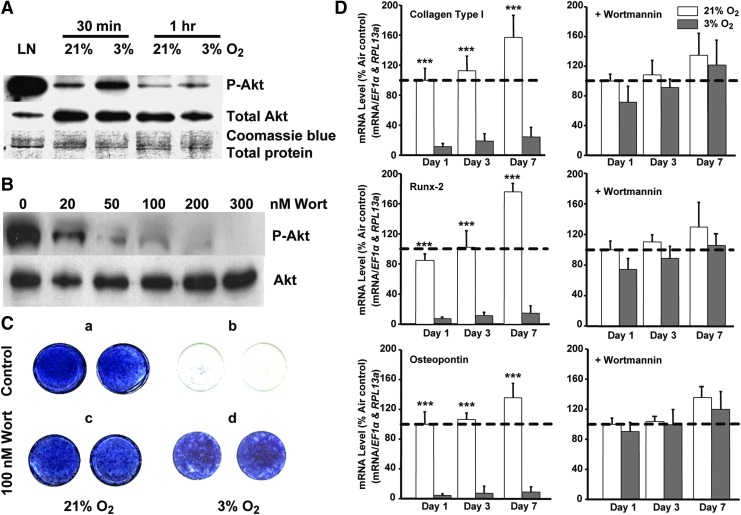
Akt is a cytosolic serine/threonine kinase critical for cell survival under adverse conditions [34]. Akt blocks apoptosis induced by many death stimuli and is required for growth factor-mediated cell survival in different cell types [34]. Akt may therefore play a key role in promoting self-renewal, and thus we assessed whether oxygen tension affects Akt signaling. We incubated cells at 3% and 21% O2 for 30 and 60 min and observed strong Akt activation (phospho-Akt) by low oxygen after a 30-min transient exposure, which then returned to basal levels. Total Akt was unaffected (Fig. 6A). Furthermore, we assessed the effect of PI3K/Akt inhibitor, wortmannin, on MIAMI cells. We tested different concentrations of wortmannin, 20, 50, 100, 200, and 300 nM, and demonstrated that 100 nM is most effective to inhibit phospho-Akt protein (Fig. 6B) without cell damage (not shown).
FIG. 6.
The Akt/PI3K pathway plays a central mechanistic role in the oxygen tension-regulated osteoblastic differentiation of MIAMI cells. (A) Akt was activated (phospho-Akt) by low oxygen after 30 min and p-Akt was back to the basal condition after 60 min. Akt did not change at 21% pO2. Total Akt did not show any changes in both conditions. (B) MIAMI cells were cultured in the presence of expansion medium at 21% and 3% O2 for 30 min in the presence and absence of wortmannin (PI3K/AKT inhibitor). (C) Wortmannin reverses the osteogenic inhibition induced by low oxygen. MIAMI cells were grown in 21% and 3% O2 in the presence or in the absence of 100 nM wortmannin in osteogenic differentiation medium for 14 days. (a) MIAMI cells at 21% O2 showed a high level of alkaline phosphatase. (b) In contrast, no alkaline phosphatase activity was detected in cells grown in the absence of the 100 nM PI3K/AKT inhibitor at 3% O2. (c) 100 nM wortmannin did not have any effect on MIAMI cells grown in 21% O2. (d) MIAMI cells grown at 3% O2 in the presence of 100 nM wortmannin showed high alkaline phosphatase activity. (D) Left panels: the expression of Runx-2, collagen type I-α1, and osteopontin genes was inhibited in MIAMI cells exposed to osteogenic induction at low oxygen tension (3% O2). (D) Right panels: Wortmannin (100 nM)-mediated inhibition of the Akt/PI3K pathway released the low oxygen tension-mediated inhibition of osteoblastic gene expression.
Blocking the Akt signaling pathway reverses the osteogenic inhibition induced by low oxygen tension
Akt plays central mechanistic roles in the regulation of proliferation, differentiation, and survival, in addition to cell survival, in several systems. This is achieved, in part, through functional cross talk with other signaling pathways (including canonical Wnt). We assessed whether the Akt pathway played a mechanistic role in maintaining the oxygen-dependent balance between MIAMI cell self-renewal and differentiation. To accomplish this, we assessed its role in the osteoblastic differentiation program of MIAMI cells. We previously demonstrated that MIAMI cells fail to progress toward the osteoblastic differentiation program under low oxygen tension conditions [4]. Thus, we sought to determine if blocking Akt activation reversed the effect of low oxygen tension on osteoblastic differentiation. We grew cells at 21% and 3% O2 in osteogenic differentiation medium for 14 days in the presence or absence of wortmannin (100 nM). Cells grown at 21% O2 showed high levels of alkaline phosphatase activity (Fig. 6Ca). We did not detect alkaline phosphatase activity in cells grown at 3% O2 (Fig. 6Cb). However, when we added 100 nM wortmannin to cells growing at low oxygen, blocking Akt activation reversed the low oxygen-dependent osteogenic inhibition and alkaline phosphatase activity was high (Fig. 6Cd) with no detectable effect on cells grown at 21% O2. To further confirm the Akt/PI3K functional role, we analyzed the expression of key osteoblastic gene markers on cells grown for 1, 3, and 7 days under osteogenic conditions at 21% and 3% O2 in the presence or absence of wortmannin. Osteoblastic marker (Runx-2, collagen type I-α1, and osteopontin) expression increased in cells at 21% O2, while in cells at 3% O2, the markers did not increase (Fig. 6D, left panels). Blocking Akt activation using 100-nM wortmannin in cells grown at low oxygen reversed the inhibition of Runx-2, collagen I-α1, and osteopontin genes induced by 3% O2 (Fig. 6D, right panels).
Discussion
Stemness of a cell can be defined as the capability of self-renewal and developing into more differentiated cell types [50]. This distinctive state of the cell is assigned to various cell types, including ESCs, cancer stem cells, and adult stem cells such as the stromal MIAMI cells studied here. Decline in tissue homeostasis and repair during adult life may indeed be associated with a decrease in the number and/or function of stem cells [51]. Thus, a better understanding of the molecular drivers regulating the balance between self-renewal and proliferation of primitive MSCs can help design strategies to maintain a stem cell pool capable of tissue repair as we age as well as implement needed improvements in current stem cell culture techniques for proper ex vivo expansion and subsequent clinical use. MSCs are multipotential cells giving rise to various differentiated cells [52–57] and display in vivo functional reconstitution of injured tissues [58–60]. The development of strategies to prevent decline of endogenous MSCs in the bone marrow during adult life will have a positive impact in delaying age-related tissue function decline.
Oxygen is a key regulator of gene expression programs and a potential modulator of the balance between stemness and differentiation [7]. Oxygen homeostasis is maintained by complex mechanisms that have evolved to sense oxygen levels and alter physiological responses accordingly [61]. Distribution of hematopoietic progenitor cells is not random throughout the marrow space as many interrelated spatial and temporal cues regulate their proliferation and differentiation [62]. Committed precursors localize in close proximity to oxygen-rich blood vessels (vascular niche), while less mature progenitors preferentially reside in areas of lower oxygen tension [63]. A model suggesting that human stem cells can self-renew without engaging in differentiation when located in a specific anatomical area with a unique architecture defines what is known as the stem cell niche [64].
We here demonstrated that Nanog, Sox2, Oct4a, and Wnt coreceptor Lrp5 increased at low oxygen (Fig. 1A). Our results suggest that low oxygen maintains MIAMI cell stemness, in part, by increasing Sox2 expression and Oct4a nuclear localization. Additionally, the data suggest that both transcriptional and post-transcriptional events may be mediating these low oxygen-stimulated effects, and transient exposure (≤2 h.) to atmospheric oxygen appears to be at least partially reversible (Fig. 1B–D). Moreover, we found that multiple pathways appear to mediate the low oxygen-stimulated self-renewal maintenance. Notch pathway activation is suggested by increased nuclear NICD levels and target genes (ie, Hes1). Additionally, HIF-1α costimulation raises possible HIF-1α/NICD interactions in targeting gene promoters, which may mediate self-renewal [21].
It is generally accepted that self-renewal mechanisms require increased expression of stemness genes with repression of differentiation programs. MIAMI cells develop an immature neuronal phenotype in response to neurotrophic factors at higher oxygen levels [43], while at low oxygen, neuronal differentiation is inhibited. REST appears to block neuronal differentiation of stem cells as a component of the transcriptional network maintaining stem cell self-renewal [36]. We observed a low oxygen-dependent stimulation of both REST/NRFS transcripts and nuclear localized protein (Fig. 2), suggesting that low oxygen activates pathways stimulating repressors of differentiation.
Furthermore, low oxygen appears to contribute to a prosurvival program in MIAMI cells, evidenced by the activation of the prosurvival Akt pathway and the downregulation of proapoptotic factors, p53, AIF, and Bak, with a concomitant dramatic decrease in SA-βgal activity, increasing self-renewal, while decreasing cell cycle arrest, senescence, and apoptosis.
Low oxygen simultaneously activates the canonical Wnt pathway through increased Lrp5 expression, which in turn may play a key role in self-renewal. Other studies provide precedence for an Lrp5-directed activation of the canonical Wnt signaling [49], suggesting that the low oxygen-stimulated Lrp5 gene expression may be sufficient for the observed activation of this pathway in MIAMI cells. Wnt signaling is particularly complex, involving a large number of mediators, some of which may be directly involved in different aspects of cellular physiology, including self-renewal and differentiation. Thus, the specific mechanistic context of these specific molecular mediators, together with their interaction with other simultaneously activated signaling pathways, will ultimately result in the final phenotypic or functional outcome.
An intracellular regulatory protein central to mediating the effect of complex extracellular signaling pathways (ie, canonical Wnt), self-renewal, and differentiation, p53, senescence, and apoptosis is the serine/threonine kinase, Akt. The strong transient activation of Akt in response to low oxygen tension (Fig. 6A) provides evidence that it may be playing a central mechanistic role. Having established that low oxygen tension inhibited osteoblastic differentiation under strong cytokine-mediated stimulatory conditions [4], it sets the stage to test such a role. We found that inhibition of Akt activation released the low oxygen-mediated block of the osteoblastic gene expression program and phenotypic determination (Fig. 6C, D). Thus, these results place Akt as a central mechanistic player in regulating the oxygen tension-controlled balance between self-renewal and osteoblastic differentiation in MIAMI cells.
Importantly, a recent study performed in the context of cancer stem cells suggests that nuclear localization of Akt is a key factor promoting cell stemness [65]. Our study corroborates this observation, and future studies will examine the distribution of Akt in MIAMI cells when exposed to low oxygen tension. Future studies will extend our analysis to differentiation pathways other than the osteoblastic lineage and will examine the role of Akt in mediating other processes involved in maintaining a stem cell pool during adult life. It has recently been reported that PFKFB3 plays a critical role in controlling the cell cycle entrance decision [66], and it would therefore be interesting to assess changes of PFKFB3 expression in MIAMI cells when exposed to various levels of oxygen.
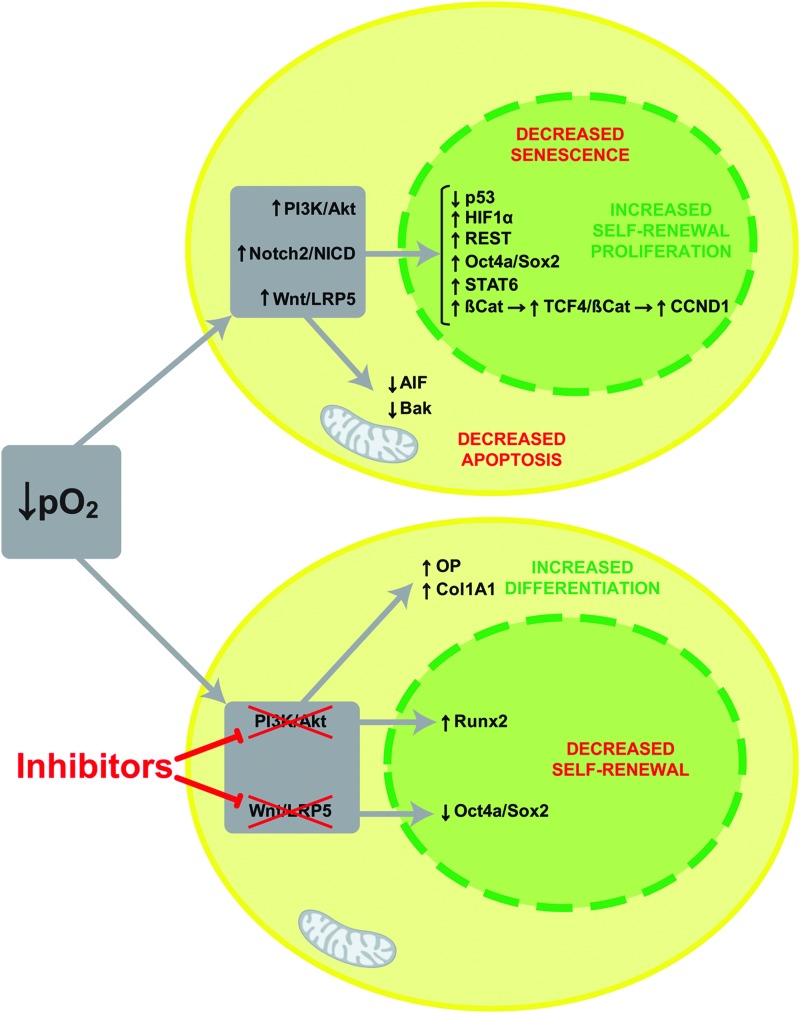
In summary, we demonstrate that low oxygen stimulates the activity of the Notch, Akt/PI3K, and canonical Wnt pathways. The latter appears to be mechanistically dependent on Lrp5 function. Low oxygen also increased the nuclear localization of the transcriptional repressor REST/NRSF. We have identified key pathways activated in MIAMI cells that are similar to those responsible for ESC self-renewal and stemness. Collectively, Notch and canonical Wnt pathways, in association with REST, may lead to increased self-renewal and concomitant decreased differentiation, cell cycle arrest, apoptosis, and/or senescence in MIAMI cells in which Akt appears to play a convergence role, setting the stage for further analyses of mechanistic roles and potential cross talk of these pathways (Fig. 7). Importantly, we demonstrated that the PI3K/Akt pathway plays a central mechanistic role in the oxygen tension-regulated self-renewal versus osteoblastic differentiation of MIAMI cells.
FIG. 7.
Oxygen tension modulates multiple signaling pathways regulating self-renewal, differentiation, senescence, and apoptosis in MIAMI cells. Diagrammatic representation of the pathways, targets, and biological responses modulated by oxygen tension in MIAMI stem cells, leading to functional changes. Color images available online at www.liebertpub.com/scd
Supplementary Material
Acknowledgments
The authors thank David Vazquez and Blanca Rodriguez for their assistance throughout this study, as well as the entire GRECC staff.
This study was supported by a Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development (Biomedical Laboratory Research and Development) Merit Review award (BX000952) to P.C.S and by the James and Esther King Biomedical Research Program from the State of Florida to G.D.I.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D. and Horwitz E. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317 [DOI] [PubMed] [Google Scholar]
- 2.D'Ippolito G, Schiller PC, Ricordi C, Roos BA. and Howard GA. (1999). Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res 14:1115–1122 [DOI] [PubMed] [Google Scholar]
- 3.D'Ippolito G, Diabira S, Howard GA, Menei P, Roos BA. and Schiller PC. (2004). Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci 117:2971–2981 [DOI] [PubMed] [Google Scholar]
- 4.D'Ippolito G, Diabira S, Howard GA, Roos BA. and Schiller PC. (2006). Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone 39:513–522 [DOI] [PubMed] [Google Scholar]
- 5.Roche S, D'Ippolito G, Gomez LA, Bouckenooghe T, Lehmann S, Montero-Menei CN. and Schiller PC. (2013). Comparative analysis of protein expression of three stem cell populations: models of cytokine delivery system in vivo. Int J Pharm 440:72–82 [DOI] [PubMed] [Google Scholar]
- 6.Chow DC, Wenning LA, Miller WM. and Papoutsakis ET. (2001). Modeling pO(2) distributions in the bone marrow hematopoietic compartment. II. Modified Kroghian models. Biophys J 81:685–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salim A, Nacamuli RP, Morgan EF, Giaccia AJ. and Longaker MT. (2004). Transient changes in oxygen tension inhibit osteogenic differentiation and Runx2 expression in osteoblasts. J Biol Chem 279:40007–40016 [DOI] [PubMed] [Google Scholar]
- 8.Bassett CA. and Herrmann I. (1961). Influence of oxygen concentration and mechanical factors on differentiation of connective tissues in vitro. Nature 190:460–461 [DOI] [PubMed] [Google Scholar]
- 9.Ma T, Grayson WL, Frohlich M. and Vunjak-Novakovic G. (2009). Hypoxia and stem cell-based engineering of mesenchymal tissues. Biotechnol Prog 25:32–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panchision DM. (2009). The role of oxygen in regulating neural stem cells in development and disease. J Cell Physiol 220:562–568 [DOI] [PubMed] [Google Scholar]
- 11.Okazaki K. and Maltepe E. (2006). Oxygen, epigenetics and stem cell fate. Regen Med 1:71–83 [DOI] [PubMed] [Google Scholar]
- 12.Holzwarth C, Vaegler M, Gieseke F, Pfister SM, Handgretinger R, Kerst G. and Muller I. (2010). Low physiologic oxygen tensions reduce proliferation and differentiation of human multipotent mesenchymal stromal cells. BMC Cell Biol 11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moriyama H, Moriyama M, Isshi H, Ishihara S, Okura H, Ichinose A, Ozawa T, Matsuyama A. and Hayakawa T. (2014). Role of notch signaling in the maintenance of human mesenchymal stem cells under hypoxic conditions. Stem Cells Dev 23:2211–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Artavanis-Tsakonas S, Matsuno K. and Fortini ME. (1995). Notch signaling. Science 268:225–232 [DOI] [PubMed] [Google Scholar]
- 15.Boland GM, Perkins G, Hall DJ. and Tuan RS. (2004). Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem 93:1210–1230 [DOI] [PubMed] [Google Scholar]
- 16.Kidder BL, Palmer S. and Knott JG. (2009). SWI/SNF-Brg1 regulates self-renewal and occupies core pluripotency-related genes in embryonic stem cells. Stem Cells 27:317–328 [DOI] [PubMed] [Google Scholar]
- 17.Lee SH, Lee MY. and Han HJ. (2008). Short-period hypoxia increases mouse embryonic stem cell proliferation through cooperation of arachidonic acid and PI3K/Akt signalling pathways. Cell Prolif 41:230–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoon DS, Choi Y, Jang Y, Lee M, Choi WJ, Kim SH. and Lee JW. (2014). SIRT1 directly regulates SOX2 to maintain self-renewal and multipotency in bone marrow-derived mesenchymal stem cells. Stem Cells 32:3219–3231 [DOI] [PubMed] [Google Scholar]
- 19.Artavanis-Tsakonas S, Rand MD. and Lake RJ. (1999). Notch signaling: cell fate control and signal integration in development. Science 284:770–776 [DOI] [PubMed] [Google Scholar]
- 20.Martinez Arias A, Zecchini V. and Brennan K. (2002). CSL-independent Notch signalling: a checkpoint in cell fate decisions during development? Curr Opin Genet Dev 12:524–533 [DOI] [PubMed] [Google Scholar]
- 21.Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U. and Bondesson M. (2005). Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell 9:617–628 [DOI] [PubMed] [Google Scholar]
- 22.Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ, Labosky PA, Simon MC. and Keith B. (2006). HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev 20:557–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Logan CY. and Nusse R. (2004). The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20:781–810 [DOI] [PubMed] [Google Scholar]
- 24.Espada J, Calvo MB, Diaz-Prado S. and Medina V. (2009). Wnt signalling and cancer stem cells. Clin Transl Oncol 11:411–427 [DOI] [PubMed] [Google Scholar]
- 25.Benhaj K, Akcali KC. and Ozturk M. (2006). Redundant expression of canonical Wnt ligands in human breast cancer cell lines. Oncol Rep 15:701–707 [PubMed] [Google Scholar]
- 26.Hochedlinger K, Yamada Y, Beard C. and Jaenisch R. (2005). Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell 121:465–477 [DOI] [PubMed] [Google Scholar]
- 27.Seo E, Basu-Roy U, Gunaratne PH, Coarfa C, Lim DS, Basilico C. and Mansukhani A. (2013). SOX2 regulates YAP1 to maintain stemness and determine cell fate in the osteo-adipo lineage. Cell Rep 3:2075–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsai CC, Su PF, Huang YF, Yew TL. and Hung SC. (2012). Oct4 and Nanog directly regulate Dnmt1 to maintain self-renewal and undifferentiated state in mesenchymal stem cells. Mol Cell 47:169–182 [DOI] [PubMed] [Google Scholar]
- 29.Liu TM, Wu YN, Guo XM, Hui JH, Lee EH. and Lim B. (2009). Effects of ectopic Nanog and Oct4 overexpression on mesenchymal stem cells. Stem Cells Dev 18:1013–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pierantozzi E, Gava B, Manini I, Roviello F, Marotta G, Chiavarelli M. and Sorrentino V. (2011). Pluripotency regulators in human mesenchymal stem cells: expression of NANOG but not of OCT-4 and SOX-2. Stem Cells Dev 20:915–923 [DOI] [PubMed] [Google Scholar]
- 31.Park SB, Seo KW, So AY, Seo MS, Yu KR, Kang SK. and Kang KS. (2012). SOX2 has a crucial role in the lineage determination and proliferation of mesenchymal stem cells through Dickkopf-1 and c-MYC. Cell Death Differ 19:534–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basu-Roy U, Ambrosetti D, Favaro R, Nicolis SK, Mansukhani A. and Basilico C. (2010). The transcription factor Sox2 is required for osteoblast self-renewal. Cell Death Differ 17:1345–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perry JM, He XC, Sugimura R, Grindley JC, Haug JS, Ding S. and Li L. (2011). Cooperation between both Wnt/{beta}-catenin and PTEN/PI3K/Akt signaling promotes primitive hematopoietic stem cell self-renewal and expansion. Genes Dev 25:1928–1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Datta SR, Brunet A. and Greenberg ME. (1999). Cellular survival: a play in three Akts. Genes Dev 13:2905–2927 [DOI] [PubMed] [Google Scholar]
- 35.Chen ZF, Paquette AJ. and Anderson DJ. (1998). NRSF/REST is required in vivo for repression of multiple neuronal target genes during embryogenesis. Nat Genet 20:136–142 [DOI] [PubMed] [Google Scholar]
- 36.Gupta SK, Gressens P. and Mani S. (2009). NRSF downregulation induces neuronal differentiation in mouse embryonic stem cells. Differentiation 77:19–28 [DOI] [PubMed] [Google Scholar]
- 37.Majumdar MK, Thiede MA, Mosca JD, Moorman M. and Gerson SL. (1998). Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol 176:57–66 [DOI] [PubMed] [Google Scholar]
- 38.Jorgensen HF, Chen ZF, Merkenschlager M. and Fisher AG. (2009). Is REST required for ESC pluripotency? Nature 457:E4–5; discussion E7 [DOI] [PubMed] [Google Scholar]
- 39.Buckley NJ, Johnson R, Sun YM. and Stanton LW. (2009). Is REST a regulator of pluripotency? Nature 457:E5–E6; discussion E7 [DOI] [PubMed] [Google Scholar]
- 40.Curtis KM, Gomez LA, Rios C, Garbayo E, Raval AP, Perez-Pinzon MA. and Schiller PC. (2010). EF1alpha and RPL13a represent normalization genes suitable for RT-qPCR analysis of bone marrow derived mesenchymal stem cells. BMC Mol Biol 11:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atlasi Y, Mowla SJ, Ziaee SA, Gokhale PJ. and Andrews PW. (2008). OCT4 spliced variants are differentially expressed in human pluripotent and nonpluripotent cells. Stem Cells 26:3068–3074 [DOI] [PubMed] [Google Scholar]
- 42.Tatard VM, D'Ippolito G, Diabira S, Valeyev A, Hackman J, McCarthy M, Bouckenooghe T, Menei P, Montero-Menei CN. and Schiller PC. (2007). Neurotrophin-directed differentiation of human adult marrow stromal cells to dopaminergic-like neurons. Bone 40:360–373 [DOI] [PubMed] [Google Scholar]
- 43.Curtis KM, Gomez LA. and Schiller PC. Rac1b regulates NT3-stimulated Mek-Erk signaling, directing marrow-isolated adult multilineage inducible (MIAMI) cells toward an early neuronal phenotype. Mol Cell Neurosci 49:138–148 [DOI] [PubMed] [Google Scholar]
- 44.Delcroix GJ, Curtis KM, Schiller PC. and Montero-Menei CN. (2010). EGF and bFGF pre-treatment enhances neural specification and the response to neuronal commitment of MIAMI cells. Differentiation 80:213–227 [DOI] [PubMed] [Google Scholar]
- 45.Delcroix GJ, Garbayo E, Sindji L, Thomas O, Vanpouille-Box C, Schiller PC. and Montero-Menei CN. (2011). The therapeutic potential of human multipotent mesenchymal stromal cells combined with pharmacologically active microcarriers transplanted in hemi-parkinsonian rats. Biomaterials 32:1560–1573 [DOI] [PubMed] [Google Scholar]
- 46.Zhao T. and Xu Y. (2009). p53 and stem cells: new developments and new concerns. Trends Cell Biol 20:170–175 [DOI] [PubMed] [Google Scholar]
- 47.Liu Y, Elf SE, Asai T, Miyata Y, Sashida G, Huang G, Di Giandomenico S, Koff A. and Nimer SD. (2009). The p53 tumor suppressor protein is a critical regulator of hematopoietic stem cell behavior. Cell Cycle 8:3120–3124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Badders NM, Goel S, Clark RJ, Klos KS, Kim S, Bafico A, Lindvall C, Williams BO. and Alexander CM. (2009). The Wnt receptor, Lrp5, is expressed by mouse mammary stem cells and is required to maintain the basal lineage. PLoS One 4:e6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang M, Yan Y, Lim YB, Tang D, Xie R, Chen A, Tai P, Harris SE, Xing L, Qin YX. and Chen D. (2009). BMP-2 modulates beta-catenin signaling through stimulation of Lrp5 expression and inhibition of beta-TrCP expression in osteoblasts. J Cell Biochem 108:896–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farahani E, Patra HK, Jangamreddy JR, Rashedi I, Kawalec M, Rao Pariti RK, Batakis P. and Wiechec E. (2014). Cell adhesion molecules and their relation to (cancer) cell stemness. Carcinogenesis 35:747–759 [DOI] [PubMed] [Google Scholar]
- 51.Jones DL. and Rando TA. (2011). Emerging models and paradigms for stem cell ageing. Nat Cell Biol 13:506–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Owen M. (1988). Marrow stromal stem cells. J Cell Sci Suppl 10:63–76 [DOI] [PubMed] [Google Scholar]
- 53.Caplan AI. (1991). Mesenchymal stem cells. J Orthop Res 9:641–650 [DOI] [PubMed] [Google Scholar]
- 54.Prockop DJ. (1997). Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276:71–74 [DOI] [PubMed] [Google Scholar]
- 55.Bianco P, Riminucci M, Gronthos S. and Robey PG. (2001). Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 19:180–192 [DOI] [PubMed] [Google Scholar]
- 56.Dennis JE. and Charbord P. (2002). Origin and differentiation of human and murine stroma. Stem Cells 20:205–214 [DOI] [PubMed] [Google Scholar]
- 57.Minguell JJ, Erices A. and Conget P. (2001). Mesenchymal stem cells. Exp Biol Med (Maywood) 226:507–520 [DOI] [PubMed] [Google Scholar]
- 58.Pereira RF, Halford KW, O'Hara MD, Leeper DB, Sokolov BP, Pollard MD, Bagasra O. and Prockop DJ. (1995). Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci U S A 92:4857–4861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pereira RF, O'Hara MD, Laptev AV, Halford KW, Pollard MD, Class R, Simon D, Livezey K. and Prockop DJ. (1998). Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci U S A 95:1142–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Muul L. and Hofmann T. (2002). Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci U S A 99:8932–8937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bruick RK. (2003). Oxygen sensing in the hypoxic response pathway: regulation of the hypoxia-inducible transcription factor. Genes Dev 17:2614–2623 [DOI] [PubMed] [Google Scholar]
- 62.Wright EG. and Lord BI. (1986). Haemopoietic stem cell proliferation: spatial and temporal considerations. Br J Cancer Suppl 7:130–132 [PMC free article] [PubMed] [Google Scholar]
- 63.Kopp HG, Avecilla ST, Hooper AT. and Rafii S. (2005). The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda) 20:349–356 [DOI] [PubMed] [Google Scholar]
- 64.Abkowitz JL, Robinson AE, Kale S, Long MW. and Chen J. (2003). Mobilization of hematopoietic stem cells during homeostasis and after cytokine exposure. Blood 102:1249–1253 [DOI] [PubMed] [Google Scholar]
- 65.Jain MV, Jangamreddy JR, Grabarek J, Schweizer F, Klonisch T, Cieslar-Pobuda A. and Los MJ. (2015). Nuclear localized Akt enhances breast cancer stem-like cells through counter-regulation of p21(Waf1/Cip1) and p27(kip1). Cell Cycle 14:2109–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cieslar-Pobuda A, Jain MV, Kratz G, Rzeszowska-Wolny J, Ghavami S. and Wiechec E. (2015). The expression pattern of PFKFB3 enzyme distinguishes between induced-pluripotent stem cells and cancer stem cells. Oncotarget 6:29753–29770 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.