Abstract
Neurofeedback is a kind of biofeedback, which teaches self-control of brain functions to subjects by measuring brain waves and providing a feedback signal. Neurofeedback usually provides the audio and or video feedback. Positive or negative feedback is produced for desirable or undesirable brain activities, respectively. In this review, we provided clinical and technical information about the following issues: (1) Various neurofeedback treatment protocols i.e. alpha, beta, alpha/theta, delta, gamma, and theta; (2) Different EEG electrode placements i.e. standard recording channels in the frontal, temporal, central, and occipital lobes; (3) Electrode montages (unipolar, bipolar); (4) Types of neurofeedback i.e. frequency, power, slow cortical potential, functional magnetic resonance imaging, and so on; (5) Clinical applications of neurofeedback i.e. treatment of attention deficit hyperactivity disorder, anxiety, depression, epilepsy, insomnia, drug addiction, schizophrenia, learning disabilities, dyslexia and dyscalculia, autistic spectrum disorders and so on as well as other applications such as pain management, and the improvement of musical and athletic performance; and (6) Neurofeedback softwares. To date, many studies have been conducted on the neurofeedback therapy and its effectiveness on the treatment of many diseases. Neurofeedback, like other treatments, has its own pros and cons. Although it is a non-invasive procedure, its validity has been questioned in terms of conclusive scientific evidence. For example, it is expensive, time-consuming and its benefits are not long-lasting. Also, it might take months to show the desired improvements. Nevertheless, neurofeedback is known as a complementary and alternative treatment of many brain dysfunctions. However, current research does not support conclusive results about its efficacy.
Keywords: Brain diseases, Brain waves, Complementary therapies, Electroencephalography, Neurofeedback
1. Introduction
Neurofeedback is not a new concept. It has been the subject of the study of researchers for several decades. Neurofeedback is a method that assists subjects to control their brain waves consciously. In fact, the electroencephalography (EEG) is recorded during the neurofeedback treatment. Then, its various components are extracted and fed to subjects using online feedback loop in the form of audio, video or their combination. Accordingly, electrophysiological components are separately demonstrated. As an illustration, the power of a signal in a frequency band can be shown by a varying bar graph. During this procedure, the subject becomes aware of the changes occurring during training and will be able to assess his/her progress in order to achieve optimum performance. For instance, the subject tries to improve the brain patterns based on the changes that occur in the sound or movie. Neurofeedback treatment protocols mainly focus on the alpha, beta, delta, theta, and gamma treatment or a combination of them such as alpha/theta ratio, beta/theta ratio, etc. (Dempster, 2012; Vernon, 2005). However, the most commonly used protocols are alpha, beta, theta, and alpha/theta ratio. In this review paper, we discussed various technical and clinical details of different neurofeedback treatment protocols.
2. Various Frequency Components
Activities of cerebral neurons have rich information about neuronal activities. When neurons are activated, they produce electrical pulses. By placing electrodes on the scalp, the electrical activity of the brain, known as EEG, can be recorded. In turn, EEG is generated by a specific type of synchronous activity of neurons which are known as pyramidal neurons and the electrical output is thus reflected in the following areas of the skin where the electrodes are located. Different patterns of electrical activity, known as brain waves, could be recognized by their amplitudes and frequencies. Frequency indicates how fast the waves oscillate which is measured by the number of waves per second (Hz), while amplitude represents the power of these waves measured by microvolt (μV).
Different frequency components are categorized into delta (less than 4 Hz), theta (4–8 Hz), alpha (8–13 Hz), beta (13–30 Hz), and gamma (30–100 Hz) where each represents a particular physiological function. In summary, delta waves are observed in the EEG signal when a person is asleep, theta waves when a person is sleepy, alpha waves when a person is relaxed and his/her muscles are loose but he/she is awake, beta waves when a person is alert and gamma waves are observed when a person is trying to solve a problem (Table 1). However, there are differences in defining the exact range of frequency components in different studies.
Table 1.
Specific brainwaves with their characteristics.
| Common brainwave frequency | Frequency range (Hz) | General characteristics |
|---|---|---|
| Delta | 1–4 | Sleep, repair, complex problem solving, unawareness, deep-unconsciousness |
| Theta | 4–8 | Creativity, insight, deep states, unconsciousness, optimal meditative state, depression, anxiety, distractibility |
| Alpha | 8–13 | Alertness and peacefulness, readiness, meditation, deeply-relaxed |
| Lower alpha | 8–10 | Recalling |
| Upper alpha | 10–13 | Optimize cognitive performance |
| SMR (sensorimotor rhythm) | 13–15 | Mental alertness, physical relaxation |
| Beta | 15–20 | Thinking, focusing, sustained attention, tension, alertness, excitement |
| High beta | 20–32 | Intensity, hyperalertness, anxiety |
| Gamma | 32–100 or 40 | Learning, cognitive processing, problem solving tasks, mental sharpness, brain activity, organize the brain |
These frequency components have subsets. For example, sensorimotor rhythm (SMR) frequency bands (13–15 Hz) are related to the sensorimotor rhythm and entitled as low beta. Some studies claimed that alpha rhythm has two subsets: lower alpha in the range of 8–10 Hz and upper alpha in the range of 10–12 Hz. Whereas some studies indicate that the alpha rhythm has 3 subsets. These definitions indicate that high and low alpha exhibit different behaviors and performances. It is believed that lower alpha is related to remembering action in semantic memory which is not the case for high alpha (Dempster, 2012).
3. EEG Electrode Placement
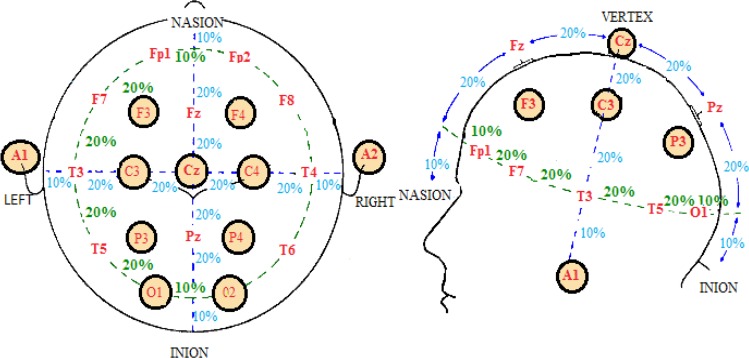
Electrodes (placed on the scalp) can record those cortical activities of the brain regions that are close to them. Electrode System 10–20 is a method for standardizing areas of the skull and comparing data. The term “10–20” refers to the placement of electrodes over 10% or 20% of the total distance between specified skull locations. Studies have shown that these placements correlate with the corresponding cerebral cortical regions. Of 21 electrodes, 19 are used for recording cortical areas and 2 other electrodes as reference electrodes (Figure 1). The skull regions are named using letters and numbers. Letters correspond with the brain regions and numbers to the hemisphere of the brain or the locations of this hemisphere. The letters F, P, T, O, and C are related to frontal, parietal, temporal, occipital, and central areas, respectively. Odd/even numbers are associated with the left/right side of the brain region. The letter z is used as PZ suggests that scalp location falls along the central line running between the nasion and the inion. FP1 and FP2 are respectively related to the left and right poles of the forehead. Also A1 and A2 are the left right regions of vestibular (ear) region that are two common sites for the placement of reference and ground electrodes (Figure 1) (Dempster, 2012; Evans & Abarbanel, 1999).
Figure 1.
The 10–20 electrode placement system and the name of the skull regions.
Traditionally, two types of unipolar and bipolar montage are used in the neurofeedback treatment. In unipolar mode, the active electrode is placed on the skull and the recorded signal by the active electrode is compared to the second electrode entitled as the reference electrode. The activity of the active electrode minus the activity of the reference electrode represents the brain activity at the active electrode.
On the other hand, in the bipolar mode, two active electrodes are used that are separately placed on the skull. The difference between the recorded signals by these 2 electrodes, is the basis of the neurofeedback (Demos, 2005; Dempster, 2012). One of the advantages of the bipolar recording is the common mode rejection that occurs during the recording procedure. It means that any external artifact occurring at both channels and at the same time, its amplitude and phase are subtracted and the spatial selectivity is improved. For example, eye roll and blink artifacts could be reduced in this way (Evans & Abarbanel, 1999).
Neurologists have observed that lesions occurring in specific regions of the brain produce specific symptoms mostly related to these regions. For example, frontal lobes, FP1, FP2, FPZ, FZ, F3, F4, F7 are responsible for immediate and sustained attention, time management, social skills, emotions, empathy, working memory, executive planning, moral fiber or character. Each region represents a specific feeling or task; Thus identification of these areas provides the best and the most accurate neurofeedback treatment. Parietal lobes, PZ, P3 and P4, solve problems conceptualized by the frontal lobes. Complex grammar, naming of the objects, sentence construction, and mathematical processing are identifiable to the left parietal lobe while map orientation, spatial recognition, and knowing the difference between right and left are entirely functions of the right parietal lobe. Temporal lobes, T3, T4, T5 and T6 have various functions. Left hemisphere functions are associated with reading (word recognition), memory, learning and a positive mood, while right hemisphere functions are related to music, anxiety, facial recognition, and sense of direction.
On the other hand, visual memories, accurate reading and traumatic memories accompanying visual flashbacks are usually processed in the occipital lobes, O2, O1 and . The other functions of this lobe include helping to locate objects in the environment, seeing colors and recognizing drawings and correctly identifying objects, reading, writing, and spelling. Sensory and motor (sensorimotor) cortex, CZ, C3 and C4 have functions of conscious control of all skeletal movements such as typing, playing musical instruments, handwriting, operation of complex machinery, speaking, and the ability to recognize where bodily sensations originate.
Neurologists have mentioned that the motor cortex helps the cerebral cortex to encode both physical and cognitive tasks. Therefore, subjects who have trouble seeing the logical sequence of cognitive tasks may benefit from neurofeedback training along the left hemisphere sensorimotor cortex (C3). Training along the right hemisphere sensorimotor cortex (C4) may invoke feelings, emotions, or calmness. Training at the median or may facilitate a mixed response. The subjects who suffer from epilepsy are usually trained along the sensorimotor cortex (C3) to increase SMR. Also, training along the sensorimotor cortex could be applied for the treatment of stroke, epilepsy, paralysis, ADHD, and disorders of sensory/motor integration (Table 2) (Demos, 2005).
Table 2.
Brain lobes with their functions and areas (Demos, 2005).
| Sites | Functions | Considerations | |
|---|---|---|---|
| Parietal lobes | Pz, P3, P4 | LH: Problem solving, math, complex grammar, attention, association RH: Spatial awareness, Geometry |
Dyscalculia sense of direction learning disorders |
| Frontal lobes | FP1, FP2, FPZ, FZ, F3, F4, F7, F8 | LH: Working memory, concentration, Executive planning, positive emotions. RH: Episodic memory, social awareness Frontal poles: attention judgment |
LH: Depression RH: Anxiety, fear, executive planning, poor executive functioning |
| Temporal lobes | T3, T4, T5, T6 | LH: Word recognition, reading, language, memory RH: Object recognition, music, social cues Facial recognition |
Anger, rage, dyslexia, long-term memory, closed head injury |
| Occipital lobes | OZ, O1, O2 | Visual learning, reading, parietal- temporal-occipital functions | Learning disorders |
| Sensorimotor cortex | CZ, C3, C4 | LH: Attention, mental processing, RH: Calmness, emotion, Empathy Combined: Fine motor skills, manual dexterity, sensory and motor integration and processing | Paralysis (stroke), seizure disorder, poor handwriting, ADHD symptoms |
| Cingulate gyrus | FPZ, FZ, CZ, PZ, OZ | Mental flexibility, cooperation, attention, motivation, morals | Obsessions, compulsions, tics, perfectionism, worry, ADHD symptoms, OCD & OCD spectrum |
| Broca’s area | F7, T3 | Verbal expression | Dyslexia, poor spelling, poor reading |
| Left hemisphere | All odd numbered sites | Logical sequencing, detail oriented, language abilities, word retrieval, fluency, reading, math, science, problem solving, verbal memory | Depression (underactivation) |
| Right hemisphere | All even numbered sites | Episodic memory encoding, social awareness, eye contact, music, humor, empathy, spatial awareness, art, insight, intuition, non-verbal memory, seeing the whole picture | Anxiety (overactivation) |
Abbreviations: LH, Left hemisphere, RH: Right hemisphere, AHHD: Attention deficit hyperactivity disorder, OCD: Obsessive compulsive disorder.
Generally, electrodes are placed in a way that a particular EEG channel is located on one brain side (Bauer & Pllana, 2014). For instance, low beta and beta are trained on the right (C4) and left (C3) brain side, respectively. If they were switched to the opposite brain side, undesirable results could be obtained. For example, training low beta wave on the left side will result in a depletion of mental energy instead of improvements in concentration. Thus, the location of the EEG electrodes during the neurofeedback procedure is important (Evans, 2007).
4. Types of Neurofeedback
There are 7 types of Neurofeedback for the treatment of various disorders:
The most frequently used neurofeedback is frequency/power neurofeedback. This technique typically includes the use of 2 to 4 surface electrodes, sometimes called “surface neurofeedback”. It is used to change the amplitude or speed of specific brain waves in particular brain locations to treat ADHD, anxiety, and insomnia.
Slow cortical potential neurofeedback (SCP-NF) improves the direction of slow cortical potentials to treat ADHD, epilepsy, and migraines (Christiansen, Reh, Schmidt, & Rief, 2014).
Low-energy neurofeedback system (LENS) delivers a weak electromagnetic signal to change the patient’s brain waves while they are motionless with their eyes closed (Zandi-Mehran, Firoozabadi, & Rostami, 2014). This type of neurofeedback has been used to treat traumatic brain injury, ADHD, insomnia, fibromyalgia, restless legs syndrome, anxiety, depression, and anger.
Hemoencephalographic (HEG) neurofeedback provides feedback on cerebral blood flow to treat migraine (Dias, Van Deusen, Oda, & Bonfim, 2012).
Live Z-score neurofeedback is used to treat insomnia. It introduces the continuous comparison of variables of brain electrical activity to a systematic database to provide continuous feedback (Collura, Guan, Tarrant, Bailey, & Starr, 2010).
Low-resolution electromagnetic tomography (LORE-TA) involves the use of 19 electrodes to monitor phase, power, and coherence (Pascual-Marqui, Michel, & Lehmann, 1994). This neurofeedback technique is used to treat addictions, depression, and obsessive-compulsive disorder.
Functional magnetic resonance imaging (fMRI) is the most recent type of neurofeedback to regulate brain activity based on the activity feedback from deep subcortical areas of the brain (Hurt, Arnold, & Lofthouse, 2014; Lévesque, Beauregard, & Mensour, 2006a).
5. Various Treatment Protocols
5.1. Alpha protocol
The alpha wave of the brain is usually associated with alert relaxation (Evans & Abarbanel, 1999). The alpha mood is described as a calm and pleasant situation. All alpha frequencies describe creative activity of the brain, so that it is used in the process of relaxation (relaxing the muscles), which eventually leads to sleep; Such waves emerge and expand rapidly on the skin. The evidence shows that alpha waves increases during meditation.
Alpha training is usually used for the treatment of various diseases such as pain relief (by 9 Hz simulation), reducing stress and anxiety (by 10 and 30 Hz simulation), memory improvement, improving mental performance, and treatment of brain injuries (by 10.2 Hz simulation). Various studies have been performed on the alpha protocol (Table 3). The most common frequency bandwidth for the alpha treatment is 7–10 Hz frequency range, which is used for meditation, sleep, reducing stress and anxiety. Also frequency of 10 Hz causes deep muscle relaxation, pain reduction, regulating breathing rate, and decreasing heart rate (Dempster, 2012; Vernon, 2005).
Table 3.
Summary of studies using alpha protocol training.
| Site of treatment | Enhance/inhibit | Number of sessions | Outcome | |
|---|---|---|---|---|
| (Allen, Harmon-Jones, & Cavender, 2001) | F3, F4 | Enhance alpha (8–13 Hz) | 5 | Impact of self-reported emotional responses and facial EMG |
| (Angelakis et al., 2007) | FO3 | Enhance peak alpha (8–13 Hz) | 31–36 | Improve cognitive processing speed and executive function |
| (Hanslmayr, Sauseng, Doppelmayr, Schabus, & Klimesch, 2005) | F3, F4, FZ, P3, P4, PZ | Enhance upper alpha | 1 | Improvement in cognitive performance |
| (Hardt & Kamiya, 1978) | OZ, O1, C3 | Enhance alpha (8–13 Hz) | 7 | Decrease anxiety |
| (Hord, Tracy, Lubin, & Johnson, 1975) | O2 | Enhance alpha | Help maintain performance such as counting and auditory discrimination | |
| (Markovska-Simoska et al., 2008) | F3-O1, F4-O2 | Enhance individual upper alpha | 20 | Increasing the quality of musical performance |
| (Martindale & Armstrong, 1974) | O2, P4 | Reduction alpha (7–13) | 1 | High creative |
| (Plotkin & Rice, 1981) | OZ | Enhance alpha | 5–7 | Decrease anxiety |
| (Regestein, Buckland, & Pegram, 1973) | Parietal-occipital | Enhance alpha (8–13 Hz) | 2 | Decrease sleep need |
| (Schmeidler & Lewis, 1971) | Right occipital | both | 2 | Mood changes |
| (Zoefel, Huster, & Herrmann, 2011) | P3, PZ, P4, O1, O2 | Enhance individual upper alpha | 5 | Enhancement of cognitive performance |
Abbreviation: EMG, Electromyogram.
5.2 Beta protocol
Beta activity is a good indicator for mental performance and inappropriate beta activity represents mental and physical disorders like depression, ADHD, and insomnia (Egner & Gruzelier, 2004). Beta brain waves are associated with conscious precision, strong focus, and ability to solve problems. Medications that are used to stimulate alertness and concentration such as Ritalin and Adderall also cause the brain to produce beta brainwaves.
Beta training is used to improve focus and attention (simulation of increased beta 12-14 Hz), improve the reading ability (simulation of 7–9 Hz), and introduce positive changes in school performance. It also improves the computational performance, cognitive processing, reduction of worries, over-thinking, obsessive compulsive disorder (OCD), alcoholism, and insomnia (simulation of 14–22 Hz and 12–15 Hz). Meanwhile, this type of neurofeedback improves sleep cognitive performance as well as reducing fatigue and stress (simulation of light and sound of beta) (Table 4). The beta waves in the range of 12–15 Hz (SMR) reduce anxiety, epilepsy, anger and stress (Egner & Gruzelier, 2004; Vernon, 2005).
Table 4.
Summary of studies using beta protocol training.
| Site of treatment | Enhance/inhibit | Number of sessions | Outcome | |
|---|---|---|---|---|
| (Rasey, Lubar, McIntyre, Zoffuto, & Abbott, 1995) | Central-posterior region (CPZ, PCZ) | Enhance beta (16–22 Hz) and inhibit high theta and low alpha | 20 | Improvement in attentional performance |
| (Egner & Gruzelier, 2001) | (12–15 Hz) at right central region (C4) and (15–18 Hz) at the left central region (C3) | Enhance low beta (12–15 and 15–18 Hz), inhibiting theta (4–7 Hz) and high beta (22–30 Hz) | 10 | Successful enhancement of attentional performance |
| (Vernon et al., 2003) | CZ | Enhance low beta (12–15 Hz), inhibiting theta (4–8 Hz) and high beta (18–23 Hz) | 15 | Enhance cognitive performance |
| (Egner & Gruzelier, 2001) | CZ | Enhance SMR (12–15 Hz) and inhibit theta (4–7 Hz) and high beta (22–30 Hz) | 10 | Improve perceptual sensitivity |
| (Egner & Gruzelier, 2001) | CZ | Enhance low beta (15–18 Hz), inhibiting theta (4–7 Hz) and high beta (22–30 Hz) | 10 | Increase cortical arousal |
| (Vernon et al., 2003) | CZ | Enhance SMR (12–15 Hz) and inhibit theta (4–7 Hz) and high beta (18–22 Hz) | 8 | Increased recall in semantic working memory |
| (Lubar, Swartwood, Swart-wood, & O’Donnell, 1995) | FCZ, CPZ | Enhance beta (16–20 Hz) and inhibit theta | 40 | Reduction of inattention, hyperactivity and impulsivity |
| (Fuchs, Birbaumer, Lutzenberger, Gruzelier, & Kaiser, 2003) | C3, C4 | Enhance beta (15–18 Hz) and SMR (12–15), inhibit theta | 36 | Improvement in attention and intelligence |
| (Heinrich, Gevensleben, & Strehl, 2007) | C4, CZ | Enhance SMR and inhibit theta | Treatment epilepsy disorder and ADHD | |
| (Heinrich, Gevensleben, & Strehl, 2007) | CZ, C3 | Enhance beta (13–20 Hz) and inhibit theta | Treatment ADHD |
Abbreviation: SMR, Sensorimotor rhythm.
5.3. Alpha/theta protocol
Alpha/theta is an indicator between awareness and sleep. Alpha/theta training is one of the most popular neurofeedback trainings for stress reduction (Gruzelier, 2009; Raymond, Varney, Parkinson, & Gruzelier, 2005). Also, this treatment is used for deep levels of depression, addiction, anxiety while it increases creativity, relaxation, musical performance, and promotes healing from trauma reactions. The electrodes are usually located on O1, O2, CZ and PZ. Alpha/theta frequency range is 7–8.5 Hz with the typical value of 7.8 Hz. This treatment is done under eyes-closed condition that increases the ratio of theta to alpha waves using auditory feedback (Demos, 2005; Egner & Gruzelier, 2003; Thompson & Thompson, 2003). The summary of the studies using alpha/theta protocol training are presented in Table 5.
Table 5.
Summary of studies using alpha/theta protocol training.
| Site of treatment | Enhance/inhibit | Number of sessions | Outcome | |
|---|---|---|---|---|
| (Raymond, Sajid, Parkinson, & Gruzelier, 2005) | P4 | Enhance theta (4–7 Hz) over alpha (8–11 Hz) | 10 | Improvement in artistic performance |
| (Egner & Gruzelier, 2003) | C4, C3, PZ | Enhance theta (5–8 Hz) over alpha (8–11 Hz) | 10 | Improvement of music performance |
| (Gruzelier, 2009) | Enhance theta (4–7 Hz) over alpha (8–11 Hz) | Half-hour sessions, twice a week | Enhancement of artistic performance and mood | |
| (Gruzelier, 2009) | Enhance theta (4–7 Hz) over alpha (8–11 Hz) | 10 | Enhancement of music performance |
5.4. Delta protocol
Delta waves are the slowest brain waves, which are associated with stages 3 and 4 of the sleep (Sürmeli & Ertem, 2007). They represent increased comfort, reduced pain, and sleep. Thus, they are used to alleviate headaches, traumatic brain injury, learning disorders, and to treatment hard and sharp contraction of muscles (by simulation of 1–3 Hz delta wave). They also reduce concerns and improve sleep (Vernon, 2005).
5.5. Gamma protocol
Gamma waves have the highest frequency, and they are associated with cognitive processing and memory (Staufenbiel, Brouwer, Keizer, & Van Wouwe, 2014). Thus, when these waves are faster, the speed of recalling memory is faster. Gamma waves are fast rhythms that are responsible for the brain’s neural connections and data transfer to the outside world.
They are mainly observed in the hippocampus (an area of the brain which is responsible for converting short-term to long-term memory). Also, these rapid rhythms are observed in sudden attacks like seizure and spasm. Hence, gamma training is used for promoting cognition, mental sharpness, brain activity, and problem-solving tasks. It not only improves poor calculation, but also organizes the brain, improves the speed of information processing, short-term memory, and reduces the number of migraine attacks (Hughes, Vernon, 2005).
5.6. Theta protocol
Theta brain waves are related to a number of brain activities such as memory, emotion, creativity, sleep, meditation, and hypnosis. These waves are also associated with the first phase of sleep when the sleep is light and the person easily wakes up. Theta treatment reduces anxiety, depression, day dreaming, distractibility, emotional disorders, and ADHD (Beatty, Greenberg, Deibler, & O’Hanlon, 1974; Vernon, 2005).
5.7. Low frequency versus high frequency training
Basically, there are two classical directions in neurofeedback training. It is either focusing on low frequencies (alpha or theta) to strengthen relaxation and focus (Gruzelier, 2009) or emphasizing on high frequencies (low beta, beta, and theta) for reinforcing activation, organizing, and inhibiting distractibility (Ros et al., 2009).
A suitable comparison between these two directions could be found at Thomas F. Collura (2000), and Kropotov (2010) studies. For example, in the former strategy eyes are closed while in the later one, eyes are open. Also, children are not involved in the first strategy while children and adult could undergo the second training procedure.
6. Clinical Applications of Neurofeedback Training in the Treatment of Diseases and Disorders
Antisocial behavior of individuals, have an undesirable impact on the society. In recent years, with advances in brain science, the cause of abnormal brain function and mental illness has been attributed to the low activity of the anterior brain lobe that presents itself in different types of psychological damages (Gil, Li, & Lee, 2009). The neuro-feedback training has been widely used in the treatment of many diseases and disorders; some of which are mentioned below.
6.1. Attention deficit/hyperactivity disorder
Evidence suggests that the malfunction of the right frontal lobe, is the cause of attention deficit/hyperactivity disorder (ADHD) (Hynd et al., 1991). The resulting symptoms are inattention, distractibility, hyperactivity, and extreme dispassionateness. Neurofeedback therapy is a rehabilitation approach for its treatment. Its goal is to normalize the behavior without dependence on medications or behavioral therapy. For a long time, such drugs as Ritalin, Concerta, and Dexedrine have been used for treating ADHD. But, recent research showed that these drugs do not have any effect on the clinical treatment of ADHD on some of children. Also, these drugs have the side effects such as anxiety, irritability, abdominal pain, decreased appetite, insomnia, and headache. However, using neurofeedback is associated with their long-term improvement (Yan et al., 2008). Studies showed that people with ADHD disorder have slower brain wave activity (theta) and less beta activity compared to normal people.
In ADHD, the goal is to decrease the brain activity in the theta band and to increase its activity in the beta band (or to decrease theta/beta ratio) at the vertex (electrode) (Heinrich, Gevensleben, & Strehl, 2007). This treatment is effective in reducing hyperactivity; Increasing focus, grades, and parental consent from children’s behavior; and improving indicators of sustained attention (Gnecchi, Herrera Garcia, & de Dios Ortiz Alvarado, 2007; Karimi, Haghshenas, & Rostami, 2011; Wang & Sourina, 2013). The studies on the neurofeedback treatment of ADHD in children are listed in Table 6. According to this Table, theta/beta protocol and the area for locating the EEG electrode are the most commonly used neurofeedback strategy in ADHD treatment.
Table 6.
Summary of neurofeedback treatment studies on ADHD.
| Site of treatment | Neurofeedback Protocol | Number of sessions | The age range (year) | Outcome | |
|---|---|---|---|---|---|
| (Linden, Habib, & Radojevic, 1996) | CZ | Enhance beta Inhibit theta | 20 | 5–15 | Improvement in mental functions and accuracy |
| (Palsson et al., 2001) | CZ | Theta/beta, SMR | 40 | 9–13 | Improvement in effects of ADHD |
| (Orlandi, 2004) | CZ | Theta/beta, SMR | 40 | 9–11 | Improvement in attention, focus and memory |
| (Lévesque, Beauregard, & Mensour, 2006b) | CZ | Theta/beta, SMR | 40 | 8–12 | Improving performance of anterior cingulate cortex |
| (Leins et al., 2007) | CZ | Theta/beta | 30 | 8–13 | Improvement in attention, hyperactivity and distraction |
| (Gevensleben et al., 2009) | CZ | Theta/beta | 18 | 9–12 | Improvement in combined treatment of neurofeedback protocols |
| (Perreau-Linck, Lessard, Lévesque, & Beauregard, 2010) | CZ | Theta/SMR | 40 | 8–13 | Improvement in the effects of ADHD |
Abbreviations: ADHA: Attention deficit hyperactivity disorder, SMR: Sensorimotor rhythm.
6.1.1. Schizophrenia
Schizophrenia is known as the most unbearable mental illness (Surmeli, Ertem, Eralp, & Kos, 2012). People with schizophrenia have the illusion of auditory disorders, restlessness, non-flexible muscles, confusion, delirium, and depression. Based on several papers on the treatment of schizophrenia, Minnesota Multiphasic Personality Inventory (MMPI) and Test of Variables of Attention (TOVA), positive effect of neurofeedback training on the treatment of this disease is expressed in such a way that the person with schizophernia is able to adjust his/her brain activity on specific frequencies (McCarthy-Jones, 2012; Surmeli, Ertem, Eralp, & Kos, 2012; Wenya et al., 2012; Gil, Li, & Lee, 2009).
6.1.2. Insomnia
Insomnia is known as an epidemic disorder. The first change observed in patients, who are treated with neuro-feedback training is the change and improvement in their sleep pattern. Hence, the neurofeedback training is used in the treatment of sleep disorders (Hammer, Colbert, Brown, & Ilioi, 2011). For example, the following process is used to improve sleep. One electrode is placed on and the treatment is done for 30 minutes at a frequency of 15–18 Hz. This method makes the waking state, alert and active and assist people in waking up faster. The calmness treatment is done at frequencies of 12–15 Hz and in location. Using neurofeedback helps the people who normally take about an hour in order to prepare their body and mind for sleep, go to sleep faster.
6.1.3. Learning disabilities, dyslexia and dyscalculia
Neurofeedback has created a big change in the treatment of these disorders. These disorders are more common at school age and patients with dyslexia have trouble in reading and spelling the characters (Breteler, Arns, Peters, Giepmans, & Verhoeven, 2010). People having dyscalculia, are unable to understand and solve math problems. These disorders are treated with increased alpha wave activity using neurofeedback (Wang & Sourina, 2013).
6.1.4. Drug addiction
Studies have shown that neurofeedback training is a good way to quit drug addiction whereas long-term use of the drug has a profound effect on the individual’s EEG. Temptation and craving of drugs could be reduced by neurofeedback in patients addicted to cocaine (Horrell et al., 2010). This treatment can also be used to treat alcoholism and addiction to computer games (Moradi et al., 2011).
6.1.5. Enhancing the performance of athletes, artists, and surgeons
Studies have shown that professional athletes have different patterns of brain activity compared to those of the beginners. Recognition of the status of the professional’s EEG before and during performance, provides a rationale for the use of neurofeedback training to create or emulate these patterns and to improve the performance of unprofessional individuals (Vernon, 2005). In fact the purpose of neurofeedback on athletes is improving the athlete’s psychomotor and self-regulation ability, their confidence, and subsequent performance in important competitions of the year (Edmonds & Tenenbaum, 2011).
6.1.6. Autistic spectrum disorder
Autistic spectrum disorder (ASD) is a neurodevelopmental disorder with challenges that maintain in adulthood. Children with autism have difficulty in functions such as social interaction, verbal and nonverbal communication, behavior and interests. ASD may be associated with emotional problems, mental retardation, or seizure disorders. These children may also have extreme sensitivity to sounds and smells. Also, children with autism may show idiosyncratic behaviors, obsessive rumination, poor social interrelatedness, and flat affect. Researchers found out that individuals with autism differ from normative samples with regard to impediments in empathy or theory of mind (TOM) tasks, weak central coherence, and executive functioning.
One of the primary symptoms of ASD is a qualitative impairment in social interactions related to mutual interest, understanding others’ intentions, empathy, emotional reciprocity, and the underlying concepts of TOM. Empathizing deficits are consistent with problems in reciprocating communication, difficulty in predicting thoughts and feelings of others, interpreting abstract emotions of others, and an appearance of social insensitivity. Individuals with autism are also often seen to have interest in system details and pursue careers in engineering, construction, clocks, machines, puzzles, or computers, which are often obsessive interests in ASD (Lucido, 2012).
There are several diagnostic tools designed to show abnormalities in brain’s function for autism. They are (1) High-beta activity related to anxiety; (2) The high activity of delta/theta corresponding with the slow cortex, lack of attention, impulsivity and hyperactivity; and (3) Abnormal EEG/seizure activity. High beta type is the most common one seen among children with ASD (approximately 50–60% of individuals with ASD) (Coben, Linden, & Myers, 2010; Kouijzer, van Schie, de Moor, Gerrits, & Buitelaar, 2010). The goal of neurofeedback in children with autism is to inhibit theta-alpha ratio while enhancing beta wave. Efficacy of neurofeedback in children diagnosed with autism has been well researched in qualitative case studies summarized in Table 7.
Table 7.
Summary of neurofeedback treatment studies on autistic spectrum disorder (ASD).
| Site of treatment | Enhance/inhibit | Number of sessions | Outcome | |
|---|---|---|---|---|
| (Cowan & Markham, 1994) | Parietal and occipital lobes | Enhance (16–20 HZ) Inhibit (4–10 HZ) | 21 | Improvement in focus, attention, and relax |
| (Thompson & Thompson, 2003) | Sensorimotor cortex (C2, C4) | Enhance (13–15 Hz) Inhibit (3–10 Hz) | 40–100 | Improvement in neuropsychological functioning, improved educational performance, decrease anxiety and impulsivity |
| (Sichel, Fehmi, & Goldstein, 1995) | Sensorimotor strip and parietal lobe | Enhance SMR (12–15 Hz) Inhibit theta (4–8 Hz) | 31 | Improvement in sleep, social behaviors Increase in appropriate eye contact Reduction in self-simulation |
| (Othmer, 2007) | P4, T4, T3, F2, FP1 | Enhance SMR (12–15 Hz) | 28–100 | Decreased need for special education services and autism symptoms |
| (Thompson, Thompson, & Reid, 2010) | Central sites | Enhance SMR (12–15 or 13–15 Hz) Inhibit theta (3–7 Hz) and beta (23–35 Hz) | 40–60 | Improvement in intelligence testing and psychological assessments |
| (Cowan & Markham, 1994) | Enhance beta (16–20 Hz) Inhibit theta-alpha (4–10 Hz) | Improvement in autistic behaviors, social, academic functioning and attention |
Abbreviation: SMR: Sensorimotor rhythm.
6.1.8. Epilepsy
In about one-third of patients with epilepsy, medical treatment is ineffective. Neurofeedback training was shown to be a good alternative treatment for these patients. Research has been focused on increasing SMR (12–15 Hz) and synchronous or asynchronous reduction of slow rhythms (4–7 Hz) for diagnosing this disorder. Also, observing low-amplitude gamma wave after surgery is a good sign for the improvement of epilepsy. The results of studies on the treatment of epilepsy by neurofeedback indicated that continuous SMR treatment reduces the rate of seizures in severe and uncontrolled epilepsy (Table 8) (Hughes et al., 2009; Walker, 2010).
Table 8.
Summary of neurofeedback treatment studies on epilepsy that the results was the remission.
| Neurofeedback protocol | Measuring results | Length of treatment | The age range (year) | |
|---|---|---|---|---|
| (Sterman, Macdonald, & Stone, 1974) | SMR (11–15 Hz) | Seizure frequency, EEG | 6–18 months | 6–46 |
| (Kaplan, 1975) | SMR | The number of seizures per day | 20–25 weeks | 20–30 |
| (Lubar & Bahler, 1976) | SMR | The number of seizures | 80–260 days | 12–29 |
| (Kuhlman & Allison, 1977) | SMR (4–9 Hz) | The number of seizures, EEG | 24 sessions | 17–42 |
| (Sterman & Macdonald, 1978) | SMR | The number of seizures per month, EEG | 12 months | 10–40 |
| (Cott, Pavloski, & Black, 1979) | SMR | The number of seizures per month | 210 days | 16–31 |
| (Quy, Hutt, & Forrest, 1979) | SMR | The number of seizures per week, EEG | 12 months | 23–49 |
| (Lubar et al., 1981) | SMR | Seizure frequency, EEG | 10 months | 13–52 |
| (Tozzo, Elfner, & May, 1988) | SMR | The number of seizures | 5 weeks | 18–29 |
Abbreviation: EEG, Electroencephalogram, SMR, Sensorimotor rhythm.
6.1.9. Depression
Depression is associated with hypometabolism in the cingulate and occasionally in the frontal cortex, insula, anterior temporal cortices, amygdala, basal ganglia, and thalamus. Along with the frontal electrophysiology findings in depression, there seems to be an inverse relationship between frontal alpha asymmetry and parietal asymmetries. More specifically, depressed patients who do not have significant anxiety, appear to have decreased right parietal activation (alpha wave at P4). Neurofeedback training is used to increase alpha and theta, while inhibit faster beta frequencies, produces significant improvements in depression (Budzynski, 2009a; Hurt, Arnold, & Lofthouse, 2014).
6.1.10. Anxiety
In clinical medicine, anxiety is often defined, at least in part, as high level of muscle tension. Researchers found out that decreasing frontal electromyogram (EMG) levels by EMG biofeedback could alleviate both generalized and specific anxiety patterns. It was believed that anxiety inhibits alpha waves, so alpha training would relieve the anxiety (Budzynski, 2009a; Demos, 2005; Moore, 2000).
6.1.11. Pain management
Pain is considered a symptom associated with physical damage, purportedly having an objective element connected with the sensation. Neurofeedback methodology proposes that by teaching self-regulation, a patient can reduce or even eliminate pain sensations. Studies suggested that brain changes its functional organization at the level of the somatosensory cortex in chronic pain patients. Researchers recommend the use of biofeedback/neurofeedback for pain management. Biofeedback protocols are designed to address the peripheral correlation of arousal, such as temperature, heart rate variability, and muscle tension while neurofeedback directly affects the processing of pain perception (Ibric & Dragomirescu, 2009).
6.2. Other uses of neurofeedback
Other applications of neurofeedback include the recovery from an injury and stroke problems, improvement of memory by increasing alpha activity (Escolano, Aguilar, & Minguez, 2011; Klimesch, 1999; Vernon, 2005; Wenya et al., 2012), treatment of headache and migraines (Walker, 2011), distraction, confusion, attention problems, withdrawal (Escolano, Aguilar, & Minguez, 2011; Gnecchi, Herrera Garcia, & de Dios Ortiz Alvarado, 2007), health promotion (Escolano, Olivan, Lopez-del-Hoyo, Garcia-Campayo, & Minguez, 2012), treatment of mental illness (Heinrich, Gevensleben, & Strehl, 2007), eating disorders (Bartholdy, Musiat, Campbell, & Schmidt, 2013) Parkinson disease (Rossi-Izquierdo et al., 2013), fibromyalgia, restless legs syndrome (Hurt, Arnold, & Loft-house, 2014), obsessive compulsive disorder (Sürmeli & Ertem, 2011), and obsession (Markovska-Simoska, Pop-Jordanova, & Georgiev, 2008; Surmeli & Ertem, 2011). Meanwhile, artists and surgeons use neurofeedback to improve their music performance (Markovska-Simoska et al., 2008) and microsurgical operations (Ros et al., 2009), respectively.
Alpha-EEG/EMG biofeedback is capable of increasing voluntary self-regulation and the quality of musical performance (Budzynski, 2009b; Markovska-Simoska et al., 2008).
7. Neurofeedback Softwares
Brain-computer interface systems (BCI) are widely used in clinical and research applications. BCI can propose a new aim for playing videogames or interacting with 3D virtual environments (VE). Interaction with VE includes tasks such as navigating to modify the selection and manipulation of virtual objects.
There are several examples of VE feedback games used in sports, puzzles, or trainings. Nowadays, many universities and laboratories are trying to provide more interactions with the virtual world through the BCI. Here, we describe some of the BCI VE feedback software.
Researchers at University College Dublin and Media Lab Europe manufactured Mind Balance videogame that uses BCI to interact with the virtual world. The game was designed to move an animated character in a 3D virtual environment. The purpose is to control the balance of an animated character on a thin rope, based on the EEG signals of a player.
In the other computer game, designed jointly by the University College London and Graz University of Technology, a disabled person in a virtual street controls the movements of the simulated wheelchair (GRAZ-BC). These results indicated that a disabled person sitting in a wheelchair can control his/her movement in the VE using asynchronous BCI based on signal EEG.
University of Tokyo performed several tests using a “virtual joystick” to navigate 3-D VE. Researchers provided two virtual buttons on the left and right sides of the VE. The participants were asked to gaze at either side to move the camera to the other side. The detection enabled the system to identify the button at which the user gazed.
Researchers at the University of Tokyo also worked on a system to keep the alertness level of car drivers. In this project, the driver’s state of concentration was illustrated when placed in a virtual driving environment. Accordingly, the BCI hearing system actively monitors the state of alertness of drivers and warns them when loss of consciousness occurs.
In the field of promotion of neurofeedback in VE, INRIA designed several BCI systems. In one of them, called “use-the-force”, subjects were asked to control the launch of a virtual spaceship by using real or imagined foot movements. They studied the response of the subjects in challenging situations (Lecuyer et al., 2008). In another system (Gnecchi, Herrera Garcia, & de Dios Ortiz Alvarado, 2007), neurofeedback was examined in order to diagnose ADHD and hyperactivity disorder. In this system, there are two graphical interfaces.
In the first interface, when the ratio of beta/theta goes higher than a predetermined threshold, dolphins are moving to an area where there are fish. Having maintained the focus, dolphin intercepts a fish. When the number of trapped fish increases, it reflects advances in process of treatment. In the second graphical interface, the speed of a racing car increases when subject’s attention improved. There are various available neurofeedback softwares in the market whose information such as operating systems, developers, and supported devices could be assessed via Wikipedia (“Comparison of neurofeedback software”, April 11, 2015).
8. Conclusion
In this paper, we reviewed the clinical applications of neurofeedback, various protocols of treatment and some of the systems designs by BCI and VR technology.
In neurofeedback, EEG is usually recorded, and various brain-activity components are extracted and feedbacked to subjects. During this procedure, subjects become aware of the changes that occur during training and are able to assess their progress in order to achieve optimal performance. Electrode placement is performed according to specific brain functions and specific symptoms. Considering information about these skull regions, the entire treatment process is simplified. There are several protocols in neurofeedback training, but alpha, beta, theta, and alpha/theta protocol are the most commonly used ones.
BCI is an EEG-based communication device. VE is a human-computer interface system with which users can virtually move their viewpoint freely in real time. The purpose of using VE is to construct a virtual environment with natural interactivity and to create a real sensation from multimodality. Three-dimensional VR is much more attractive and interesting than most of two-dimensional environments.
To date, many studies have been conducted on the neuro-feedback therapy and its effectiveness on the treatment of many diseases. However, there are some methodological limitations and clinical ambiguities. For example, considering the alpha treatment protocols, there are some issues to deal with such as how many sessions are needed before participants can learn to exert an alert control over their own alpha waves, or how many sessions are needed before such training procedures produce the expected effect on the optimal performance, and how long the desired effects last without feedback (long-term effects). Thus, it is necessary to provide standard protocols to perform neurofeedback.
Similar to other treatments, neurofeedback has its own pros and cons. Although it is a safe and non-invasive procedure that showed improvement in the treatment of many problems and disorders such as ADHD, anxiety, depression, epilepsy, ASD, insomnia, drug addiction, schizophrenia, learning disabilities, dyslexia and dyscalculia, its validity has been questioned in terms of conclusive scientific evidence of its effectiveness. Moreover, it is an expensive procedure which is not covered by many insurance companies. It is also time-consuming and its benefits are not long-lasting. Finally, it might take several months to see the desired improvements (Mauro & Cermak, 2006).
Footnotes
Conflicts of Interest:
None declared.
References
- Allen J. J., Harmon-Jones E., Cavender J. H. ( 2001). Manipulation of frontal EEG asymmetry through biofeedback alters self-reported emotional responses and facial EMG. Psychophysiology, 38( 4), 685– 693. [PubMed] [Google Scholar]
- Angelakis E., Stathopoulou S., Frymiare J. L., Green D. L., Lubar J. F., Kounios J. ( 2007). EEG neurofeedback: a brief overview and an example of peak alpha frequency training for cognitive enhancement in the elderly. Clinical Neuropsychologist, 21( 1), 110– 129. [DOI] [PubMed] [Google Scholar]
- Bartholdy S., Musiat P., Campbell I. C., Schmidt U. ( 2013). The potential of neurofeedback in the treatment of Eating Disorders: A Review of the Literature. European Eating Disorders Review, 21( 6), 456– 463. [DOI] [PubMed] [Google Scholar]
- Bauer H., Pllana A. ( 2014). EEG-based local brain activity feedback training-tomographic neurofeedback. Frontiers Human Neuroscience, 8, 1005. doi: 10.3389/fnhum.2014.01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty J., Greenberg A., Deibler W. P., O’Hanlon J. F. ( 1974). Operant control of occipital theta rhythm affects performance in a radar monitoring task. Science, 183( 4127), 871– 873. [DOI] [PubMed] [Google Scholar]
- Breteler M. H., Arns M., Peters S., Giepmans I., Verhoeven L. ( 2010). Improvements in spelling after QEEG-based neurofeedback in dyslexia: a randomized controlled treatment study. Applied Psychophysiol Biofeedback, 35( 1), 5– 11. doi: 10.1007/s10484-009-9105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budzynski T. ( 2009a). Introduction to quantitative EEG and neurofeedback: Advanced theory and applications ( 2nd ed.). Amsterdam, Elsevier: Academic Press. [Google Scholar]
- Budzynski T. ( 2009b). Introduction to quantitative EEG and neurofeedback: Advanced theory and applications ( 2nd ed.). Amsterdam, Elsevier: Academic Press. [Google Scholar]
- Christiansen H., Reh V., Schmidt M. H., Rief W. ( 2014). Slow cortical potential neurofeedback and self-management training in outpatient care for children with ADHD: Study protocol and first preliminary results of a randomized controlled trial. Frontiers Human Neuroscience, 8, 943. doi: 10.3389/fnhum.2014.00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coben R., Linden M., Myers T. E. ( 2010). Neurofeedback for autistic spectrum disorder: A review of the literature. Applied Psychophysiol Biofeedback, 35( 1), 83– 105. doi: 10.1007/s10484-009-9117-y. [DOI] [PubMed] [Google Scholar]
- Collura T. F. ( 2000). Practical Issues Concerning EEG Biofeedback Devices, Protocols and Methods (Doctoral Dissertation). Retrieved from http://openeeg.sourceforge.net/arch/att-0944/01-part.
- Collura T. F., Guan J., Tarrant J., Bailey J., Starr F. ( 2010). EEG biofeedback case studies using live Z-score training and a normative database. Journal of Neurotherapy, 14( 1), 22– 46. [Google Scholar]
- Comparison of neurofeedback software ( 2015, April 11). Retrieved from http://en.wikipedia.org/w/index.php?title=Comparison_of_neurofeedback_software&oldid=656032961.
- Cott A., Pavloski R. P., Black A. H. ( 1979). Reducing epileptic seizures through operant conditioning of central nervous system activity: Procedural variables. Science, 203( 4375), 73– 75. [DOI] [PubMed] [Google Scholar]
- Cowan J., Markham L. ( 1994, March). EEG biofeedback for the attention problems of autism: A case study. In 25th the Annual Meeting of the Association for applied Psychophysiology and Biofeedback. [Google Scholar]
- Demos J. N. ( 2005). Getting started with neurofeedback ( 1th ed.). New York: W.W. Norton. [Google Scholar]
- Dempster T. ( 2012). An investigation into the optimum training paradigm for alpha electroencephalographic biofeedback (PhD Thesis). U.K.: Canterbury Christ Church University. [Google Scholar]
- Dias A. M., Van Deusen A. M., Oda E., Bonfim M. R. ( 2012). Clinical efficacy of a new automated hemoencephalographic neurofeedback protocol. Spanish Journal of Psychology, 15( 3), 930– 941. [DOI] [PubMed] [Google Scholar]
- Edmonds W. A., Tenenbaum G. ( 2011). Case studies in applied psychophysiology: Neurofeedback and biofeedback treatments for advances in human performance. New Jersey: John Wiley & Sons. [Google Scholar]
- Egner T., Gruzelier J. H. ( 2001). Learned self-regulation of EEG frequency components affects attention and event-related brain potentials in humans. Neuroreport, 12( 18), 4155– 4159. [DOI] [PubMed] [Google Scholar]
- Egner T., Gruzelier J. H. ( 2003). Ecological validity of neuro-feedback: modulation of slow wave EEG enhances musical performance. Neuroreport, 14( 9), 1221– 1224. [DOI] [PubMed] [Google Scholar]
- Egner T., Gruzelier J. H. ( 2004). EEG Biofeedback of low beta band components: frequency-specific effects on variables of attention and event-related brain potentials. Clinical Neurophysiology, 115( 1), 131–139. doi: 10.1016/S1388-2457(03)00353-5. [DOI] [PubMed] [Google Scholar]
- Escolano C., Aguilar M., Minguez J. ( 2011). EEG-based upper alpha neurofeedback training improves working memory performance. International Conference of the IEEE Engineering in Medicine and Biology Society, 2011, 2327–2330. doi: 10.1109/IEMBS.2011.6090651. [DOI] [PubMed] [Google Scholar]
- Escolano C., Olivan B., Lopez-del-Hoyo Y., Garcia-Campayo J., Minguez J. ( 2012). Double-blind single-session neuro-feedback training in upper-alpha for cognitive enhancement of healthy subjects. Conference Proceedings of the IEEE Engineering in Medicine and Biology Society, 2012, 4643–4647. doi: 10.1109/EMBC.2012.6347002. [DOI] [PubMed] [Google Scholar]
- Evans J. R. ( 2007). Handbook of neurofeedback: dynamics and clinical applications. New York: Haworth Medical Press. [Google Scholar]
- Evans J. R., Abarbanel A. ( 1999). Introduction to quantitative EEG and neurofeedback. San Diego, Calif: Academic Press. [Google Scholar]
- Fuchs T., Birbaumer N., Lutzenberger W., Gruzelier J. H., Kaiser J. ( 2003). Neurofeedback treatment for attention-deficit/hyperactivity disorder in children: A comparison with methylphenidate. Applied Psychophysiol Biofeedback, 28( 1), 1– 12. [DOI] [PubMed] [Google Scholar]
- Gevensleben H., Holl B., Albrecht B., Vogel C., Schlamp D., Kratz O., et al. ( 2009). Is neurofeedback an efficacious treatment for ADHD? A randomised controlled clinical trial. Journal of Child Psychology and Psychiatry, 50( 7), 780– 789. doi: 10.1111/j.1469-7610.2008.02033. [DOI] [PubMed] [Google Scholar]
- Gil Y., Li G., Lee J. ( 2009). Integrated real-time neurofeedback system to raise the frontal lobe activity: Design and Implementation. Institute of Electrical and Electronics Engineers, 2009, 845– 848. [DOI] [PubMed] [Google Scholar]
- Gnecchi J. A. G., Herrera Garcia J. C., de Dios Ortiz Alvarado J. ( 2007, September). Auxiliary Neurofeedback System for Diagnostic of Attention Deficit Hyperactivity Disorder. Proceedings of the Electronics, Robotics and Automotive Mechanics Conference (pp. 135–138). Mexico: Morelos. doi: 10.1109/CERMA.2007.4367674. [DOI] [Google Scholar]
- Gruzelier J. ( 2009). A theory of alpha/theta neurofeedback, creative performance enhancement, long distance functional connectivity and psychological integration. Cognitive Processing, 10( 1), 101–109. doi: 10.1007/s10339-008-0248-5. [DOI] [PubMed] [Google Scholar]
- Hammer B. U., Colbert A. P., Brown K. A., Ilioi E. C. ( 2011). Neurofeedback for insomnia: a pilot study of Z-score SMR and individualized protocols. Applied Psychophysiol Biofeedback, 36( 4), 251– 264. doi: 10.1007/s10484-011-9165-y. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S., Sauseng P., Doppelmayr M., Schabus M., Klimesch W. ( 2005). Increasing Individual Upper Alpha Power by Neurofeedback Improves Cognitive Performance in Human Subjects. Applied Psychophysiol Biofeedback, 30( 1), 1– 10. doi: 10.1007/s10484-005-2169-8. [DOI] [PubMed] [Google Scholar]
- Hardt J. V., Kamiya J. ( 1978). Anxiety change through electroencephalographic alpha feedback seen only in high anxiety subjects. Science, 201( 4350), 79– 81. [DOI] [PubMed] [Google Scholar]
- Heinrich H., Gevensleben H., Strehl U. ( 2007). Annotation: Neurofeedback-train your brain to train behaviour. Journal of Child Psychology and Psychiatry, 48( 1), 3– 16. doi: 10.1111/j.1469-7610.2006.01665.x. [DOI] [PubMed] [Google Scholar]
- Hord D. J., Tracy M. L., Lubin A., Johnson L. ( 1975). Effect of Self-Enhanced EEG Alpha on Performance and Mood After Two Nights of Sleep Loss. Psychophysiology, 12( 5), 585– 590. [DOI] [PubMed] [Google Scholar]
- Horrell T., El-Baz A., Baruth J., Tasman A., Sokhadze G., Stewart C., et al. ( 2010). Neurofeedback Effects on Evoked and Induced EEG Gamma Band Reactivity to Drug-related Cues in Cocaine Addiction. Journal of Neurotherapy, 14( 3), 195–216. doi: 10.1080/10874208.2010.501498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. R. ( 2008). Gamma, fast, and ultrafast waves of the brain: Their relationships with epilepsy and behavior. Epilepsy & Behavior, 13( 1), 25– 31. doi: 10.1016/j.yebeh.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Hurt E., Arnold L. E., Lofthouse N. ( 2014). Quantitative EEG Neurofeedback for the Treatment of Pediatric Attention-Deficit/Hyperactivity Disorder, Autism Spectrum Disorders, Learning Disorders, and Epilepsy. Child and Adolescent Psychiatric Clinics of North America, 23( 3), 465– 486. [DOI] [PubMed] [Google Scholar]
- Hynd G. W., Lorys A. R., Semrud-Clikeman M., Nieves N., Huettner M. I., Lahey B. B. ( 1991). Attention deficit disorder without hyperactivity: a distinct behavioral and neurocognitive syndrome. Journal of Child Neurology, 6( 1), S37–43. [DOI] [PubMed] [Google Scholar]
- Ibric V. L., Dragomirescu L. G. ( 2009). Neurofeedback in pain management. In Budzyknski T. H., Budzynski H. K., Evans J. R., Abarbanel A. (Eds.). Introduction to quantitative EEG and neurofeedback: Advanced theory and applications ( 2nd ed.) (pp. 417–451). Amsterdam, Elsevier: Academic Press. [Google Scholar]
- Kaplan B. J. ( 1975). Biofeedback in Epileptics: Equivocal Relationship of Reinforced EEG Frequency to Seizure Reduction. Epilepsia, 16( 3), 477–485. doi: 10.1111/j.1528-1157.1975.tb06076.x. [DOI] [PubMed] [Google Scholar]
- Karimi M., Haghshenas S., Rostami R. ( 2011). Neurofeedback and autism spectrum: A case study. Procedia-Social and Behavioral Sciences, 30, 1472– 1475. doi: 10.1016/j.sbspro.2011.10.285. [DOI] [Google Scholar]
- Klimesch W. ( 1999). EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Research Reviews, 29( 2–3), 169– 195. doi: 10.1016/S0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Kouijzer M. E. J., van Schie H. T., de Moor J. M. H., Gerrits B. J. L., Buitelaar J. K. ( 2010). Neurofeedback treatment in autism. Preliminary findings in behavioral, cognitive, and neurophysiological functioning. Research in Autism Spectrum Disorders, 4( 3), 386– 399. doi: 10.1016/j.rasd.2009.10.007. [DOI] [Google Scholar]
- Kropotov J. ( 2010). Introduction to Quantitative EEG, event-related potentials and neurotherapy. Amsterdam, Elsevier: Academic Press. [Google Scholar]
- Kuhlman W. N., Allison T. ( 1977). EEG feedback training in the treatment of epilepsy: Some questions and some answers. The Pavlovian Journal of Biological Science, 12( 2), 112– 122. doi: 10.1007/bf03004498. [DOI] [PubMed] [Google Scholar]
- Lecuyer A., Lotte F., Reilly R. B., Leeb R., Hirose M., Slater M. ( 2008). Brain-Computer Interfaces, Virtual Reality, and Videogames. Computer, 41( 10), 66– 72. doi: 10.1109/mc.2008.410. [DOI] [Google Scholar]
- Leins U., Goth G., Hinterberger T., Klinger C., Rumpf N., Strehl U. ( 2007). Neurofeedback for Children with ADHD: A Comparison of SCP and Theta/Beta Protocols. Applied Psychophysiol Biofeedback, 32( 2), 73– 88. doi: 10.1007/s10484-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Lévesque J., Beauregard M., Mensour B. ( 2006a). Effect of neurofeedback training on the neural substrates of selective attention in children with attention-deficit/hyperactivity disorder: A functional magnetic resonance imaging study. Neuroscience Letters, 394( 3), 216– 221. [DOI] [PubMed] [Google Scholar]
- Lévesque J., Beauregard M., Mensour B. ( 2006b). Effect of neurofeedback training on the neural substrates of selective attention in children with attention-deficit/hyperactivity disorder: A functional magnetic resonance imaging study. Neuroscience Letters, 394( 3), 216– 221. doi: 10.1016/j.neulet.2005.10.100. [DOI] [PubMed] [Google Scholar]
- Linden M., Habib T., Radojevic V. ( 1996). A controlled study of the effects of EEG biofeedback on cognition and behavior of children with attention deficit disorder and learning disabilities. Biofeedback & Self Regulation, 21( 3), 297. doi: 10.1007/bf02214740. [DOI] [PubMed] [Google Scholar]
- Lubar J., Bahler W. W. ( 1976). Behavioral management of epileptic seizures following EEG biofeedback training of the sensorimotor rhythm. Biofeedback and Self-regulation, 1( 1), 77–104. doi: 10.1007/bf00998692. [DOI] [PubMed] [Google Scholar]
- Lubar J. F., Shabsin H. S., Natelson S. E., Holder G. S., Whitsett S. F., Pamplin W. E., et al. ( 1981). EEG operant conditioning in intractable epileptics. Archives of Neurology, 38( 11), 700– 704. [DOI] [PubMed] [Google Scholar]
- Lubar J. F., Swartwood M. O., Swartwood J. N., O’Donnell P. H. ( 1995). Evaluation of the effectiveness of EEG neurofeedback training for ADHD in a clinical setting as measured by changes in TOVA scores, behavioral ratings, and WISC-R performance. Biofeedback and Self-regulation, 20( 1), 83– 99. [DOI] [PubMed] [Google Scholar]
- Lucido M. ( 2012). College of social and behavioural sciences. Minneapolis, Minnesota: Walden University. [Google Scholar]
- Markovska-Simoska S., Pop-Jordanova N., Georgiev D. ( 2008). Simultaneous EEG and EMG biofeedback for peak performance in musicians. Prilozi, 29( 1), 239– 252. [PubMed] [Google Scholar]
- Martindale C., Armstrong J. ( 1974). The relationship of creativity to cortical activation and its operant control. Journal of Genetic Psychology, 124( 2), 311– 320. [DOI] [PubMed] [Google Scholar]
- Mauro T., Cermak S. A. ( 2006). The Everything Parent’s Guide to Sensory Integration Disorder: Get the Right Diagnosis, Understand Treatments, and Advocate for Your Child. USA: Adams Media Corporation. [Google Scholar]
- McCarthy-Jones S. ( 2012). Taking back the brain: could neuro-feedback training be effective for relieving distressing auditory verbal hallucinations in patients with schizophrenia? Schizophrenia Bulletin, 38( 4), 678– 682. doi: 10.1093/schbul/sbs006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore N. ( 2000). A review of EEG biofeedback treatment of anxiety disorders. Clinical EEG and Neuroscience (Electroencephalography), 31( 1), 1– 6. [DOI] [PubMed] [Google Scholar]
- Moradi A., Pouladi F., Pishva N., Rezaei B., Torshabi M., Mehrjerdi Z. A. ( 2011). Treatment of Anxiety Disorder with Neurofeedback: Case Study. Procedia-Social and Behavioral Sciences, 30, 103–107. doi: j.sbspro.2011.10.021. [Google Scholar]
- Orlandi M. A., Greco D. ( 2004, August). A randomized, doubleblind clinical trial of EEG neurofeedback treatment for attention-deficit/hyperactivity disorder. Paper presented at the meeting of the International Society for Neuronal Regulation, Fort Lauderdale, FL. [Google Scholar]
- Othmer S. ( 2007). Progress in neurofeedback for the autism spectrum. Paper presented at the 38th Annual Meeting of the Association for Applied Psychophysiology & Biofeedback Monterey, Canada, 15–18 February 2007. [Google Scholar]
- Palsson O. S., Pope A. T., Ball J. D., Turner M. J., Nevin S., deBeus R. ( 2001). Neurofeedback videogame ADHD technology: Results of the first concept study. Paper presented at the annual meeting of Association for Applied Psychophysiology & Biofeedback, Research Triangle Park, NC. [Google Scholar]
- Pascual-Marqui R. D., Michel C. M., Lehmann D. ( 1994). Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. International Journal of Psychophysiology, 18( 1), 49– 65. [DOI] [PubMed] [Google Scholar]
- Perreau-Linck E., Lessard N., Lévesque J., Beauregard M. ( 2010). Effects of neurofeedback training on inhibitory capacities in ADHD children: A single-blind, randomized, placebo-controlled study. Journal of Neurotherapy, 14( 3), 229– 242. doi: 10.1080/10874208.2010.501514. [DOI] [Google Scholar]
- Plotkin W. B., Rice K. M. ( 1981). Biofeedback as a placebo: anxiety reduction facilitated by training in either suppression or enhancement of alpha brainwaves. Journal of Consulting and Clinical Psychology, 49( 4), 590. [DOI] [PubMed] [Google Scholar]
- Quy R. J., Hutt S. J., Forrest S. ( 1979). Sensorimotor rhythm feedback training and epilepsy: Some methodological and conceptual issues. Biological Psychology, 9( 2), 129–149. doi: 10.1016/0301-0511(79)90059-0. [DOI] [PubMed] [Google Scholar]
- Rasey H., Lubar J. F., McIntyre A., Zoffuto A., Abbott P. L. ( 1995). EEG biofeedback for the enhancement of attentional processing in normal college students. Journal of Neurotherapy, 1( 3), 15– 21. doi: 10.1300/J184v01n03_03. [DOI] [Google Scholar]
- Raymond J., Sajid I., Parkinson L. A., Gruzelier J. H. ( 2005). Biofeedback and dance performance: A preliminary investigation. Applied Psychophysiology Biofeedback, 30( 1), 65– 73. [DOI] [PubMed] [Google Scholar]
- Raymond J., Varney C., Parkinson L. A., Gruzelier J. H. ( 2005). The effects of alpha/theta neurofeedback on personality and mood. Cognitive Brain Research, 23( 2), 287– 292. [DOI] [PubMed] [Google Scholar]
- Regestein Q., Buckland G., Pegram G. ( 1973). Effect of daytime alpha rhythm maintenance on subsequent sleep. Psychosomatic Medicine, 35( 5), 415– 418. [DOI] [PubMed] [Google Scholar]
- Ros T., Moseley M. J., Bloom P. A., Benjamin L., Parkinson L. A., Gruzelier J. H. ( 2009). Optimizing microsurgical skills with EEG neurofeedback. BMC Neuroscience, 10( 1), 87. doi: 10.1186/1471-2202-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi-Izquierdo M., Ernst A., Soto-Varela A., Santos-Pérez S., Faraldo-García A., Sesar-Ignacio Á., Basta D. ( 2013). Vibrotactile neurofeedback balance training in patients with Parkinson’s disease: Reducing the number of falls. Gait & Posture, 37( 2), 195– 200. [DOI] [PubMed] [Google Scholar]
- Schmeidler G., Lewis L. ( 1971). Mood changes after alpha feedback training. Perceptual and Motor Skills, 32( 3), 709– 710. [DOI] [PubMed] [Google Scholar]
- Sichel A. G., Fehmi L. G., Goldstein D. M. ( 1995). Positive outcome with neurofeedback treatment in a case of mild autism. Journal of Neurotherapy, 1( 1), 60– 64. doi: 10.1300/J184v01n01_08. [DOI] [Google Scholar]
- Staufenbiel S., Brouwer A. M., Keizer A., Van Wouwe N. ( 2014). Effect of beta and gamma neurofeedback on memory and intelligence in the elderly. Biological Psychology, 95, 74– 85. [DOI] [PubMed] [Google Scholar]
- Sterman M. B., Macdonald L. R. ( 1978). Effects of central cortical EEG feedback training on incidence of poorly controlled seizures. Epilepsia, 19( 3), 207– 222. [DOI] [PubMed] [Google Scholar]
- Sterman M. B., Macdonald L. R., Stone R. K. ( 1974). Biofeedback Training of the Sensorimotor Electroencephalogram Rhythm in Man: Effects on Epilepsy. Epilepsia, 15( 3), 395– 416. doi: 10.1111/j.1528-1157.1974.tb04016.x. [DOI] [PubMed] [Google Scholar]
- Sürmeli T., Ertem A. ( 2007). EEG neurofeedback treatment of patients with Down Syndrome. Journal of Neurotherapy, 11( 1), 63– 68. [Google Scholar]
- Sürmeli T., Ertem A. ( 2011). Obsessive Compulsive Disorder and the Efficacy of qEEG-Guided Neurofeedback Treatment: A Case Series. Clinical EEG and Neuroscience, 42( 3), 195– 201. doi: 10.1177/155005941104200310. [DOI] [PubMed] [Google Scholar]
- Surmeli T., Ertem A., Eralp E., Kos I. H. ( 2012). Schizophrenia and the efficacy of qEEG-guided neurofeedback treatment: a clinical case series. Clinical EEG and Neuroscience, 43( 2), 133– 144. doi: 10.1177/1550059411429531. [DOI] [PubMed] [Google Scholar]
- Thompson L., Thompson M., Reid A. ( 2010). Neurofeedback outcomes in clients with Asperger’s syndrome. Applied Psychophysiol Biofeedback, 35( 1), 63– 81. [DOI] [PubMed] [Google Scholar]
- Thompson M., Thompson L. ( 2003). The neurofeedback book: An introduction to basic concepts in applied psychophysiology. Wheat Ridge, CO: Association for Applied Psychophysiology and Biofeedback. [Google Scholar]
- Tozzo C. A., Elfner L. F., May J. G. ( 1988). EEG biofeedback and relaxation training in the control of epileptic seizures. International Journal of Psychophysiology, 6( 3), 185– 194. [DOI] [PubMed] [Google Scholar]
- Vernon D., Egner T., Cooper N., Compton T., Neilands C., Sheri A., et al. ( 2003). The effect of training distinct neurofeedback protocols on aspects of cognitive performance. International Journal of Psychophysiology, 47( 1), 75– 85. doi: 10.1016/S0167-8760(02)00091-0. [DOI] [PubMed] [Google Scholar]
- Vernon D. J. ( 2005). Can neurofeedback training enhance performance? An evaluation of the evidence with implications for future research. Applied Psychophysiol Biofeedback, 30( 4), 347–364. doi: 10.1007/s10484-005-8421-4. [DOI] [PubMed] [Google Scholar]
- Walker J. E. ( 2010). Using QEEG-guided neurofeedback for epilepsy versus standardized protocols: Enhanced effectiveness? Applied Psychophysiol Biofeedback, 35( 1), 29– 30. doi: 10.1007/s10484-009-9123-0. [DOI] [PubMed] [Google Scholar]
- Walker J. E. ( 2011). QEEG-guided neurofeedback for recurrent migraine headaches. Clinical EEG and Neuroscience, 42( 1), 59– 61. [DOI] [PubMed] [Google Scholar]
- Wang Q., Sourina O. ( 2013). Real-time mental arithmetic task recognition from EEG signals. IEEE Transactions on Neural Systems and Rehabilitation Engineering, 21( 2), 225– 232. doi: 10.1109/TNSRE.2012.2236576. [DOI] [PubMed] [Google Scholar]
- Wenya N., Lanshin C., Rodrigues J. P., Feng W., Peng Un M., Pui-In M., et al. ( 2012). Neurofeedback for the treatment of schizophrenia: Case study. Paper presented at the Virtual Environments Human-Computer Interfaces and Measurement Systems (VECIMS), 2012 IEEE International Conference on, Tianjin, China, 2–4 July 2012. [Google Scholar]
- Yan N., Wang J., Liu M., Zong L., Jiao Y., Yue J., et al. ( 2008). Designing a Brain-computer Interface Device for Neurofeedback Using Virtual Environments. Medical and Biological Engineering, 28( 3), 167–172. [Google Scholar]
- Zandi-Mehran Y., Firoozabadi M., Rostami R. ( 2014). Improvement of Neurofeedback Therapy for Improved Attention Through Facilitation of Brain Activity Using Local Sinusoidal Extremely Low Frequency Magnetic Field Exposure. Clinical EEG and Neuroscience, 46( 2), 100– 12. doi: 10.1177/1550059414524403. [DOI] [PubMed] [Google Scholar]
- Zoefel B., Huster R. J., Herrmann C. S. ( 2011). Neurofeedback training of the upper alpha frequency band in EEG improves cognitive performance. NeuroImage, 54( 2), 1427– 1431. doi: 10.1016/j.neuroimage.2010.08.078. [DOI] [PubMed] [Google Scholar]