Abstract
Objective
To explore inflammatory factors that influence symptom changes in interstitial cystitis/bladder pain syndrome (IC/BPS). This longitudinal, prospective study examined the association of inflammation elicited by Toll-Like Receptor (TLR) stimulation in peripheral blood mononuclear cells (PBMCs) and diurnal cortisol rhythms with changes in painful and urinary symptoms of IC/BPS and symptom flares over a 48 week period.
Materials and Methods
Participants were 24 women meeting criteria for IC/BPS who supplied blood for isolation of PBMCs and three days of salivary cortisol samples prior to a baseline visit. Participants completed the Genitourinary Pain Index (pain and urinary subscales) and reported symptom flares every 2 weeks for 48 weeks. Mixed effects longitudinal and regression models were used to determine if inflammatory variables were associated with the changes in IC/BPS symptoms (Time × variable interactions), and the probability of a symptom flare.
Results
Elevated TLR-4 inflammation (p = .031) and elevated TLR-2 inflammation (p = .045) from PBMCs, and flattened diurnal cortisol slope (p = .012) were each associated with less improvement in genitourinary pain over time. Additionally, elevated TLR-4 inflammation was associated with less improvement in urinary symptoms (p = .018) while TLR-2 inflammation and cortisol slope were not (both p > .16). In contrast, no inflammatory measure was associated with an increased likelihood of reporting a symptom flare (all p > .25).
Conclusions
TLR-mediated inflammation and diurnal cortisol slope may be useful as markers of symptom changes in IC/BPS.
Keywords: Interstitial Cystitis, Inflammation, Toll-Like Receptor 2, Toll-Like Receptor 4, Hydrocortisone
Introduction
Interstitial Cystitis/Bladder Pain Syndrome (IC/BPS) is a debilitating chronic condition characterized by urinary symptoms such as frequency, urgency, nocturia, and genitourinary pain. Though the condition is prevalent, affecting between 3-8 million women in the United States by some estimates1, the etiology and course of the disease are not well understood. One large scale longitudinal study of IC/BPS sufferers found that most did not experience improvement in symptoms over a long period of observation (up to four years) despite a variety of different treatment modalities.2 Another recent longitudinal investigation found that 62% of IC/BPS sufferers experienced symptom persistence during at least three of four time points when assessed every three months over a one year period.3 However, to date, no biomarkers have been identified that are associated with the course of the syndrome or with short term exacerbation of symptoms, termed “symptom flares.” 4
The clinical presentation of IC/BPS often does not include signs of local inflammation and/or tissue damage (e.g. in the bladder),5 therefore recent investigations have focused on more systemic physiological risk factors for IC/BPS and associated symptoms. We have recently identified Toll-Like Receptor (TLR) inflammatory responses in peripheral blood mononuclear cells (PBMCs) and diurnal cortisol dysregulation as robust correlates of the IC/BPS syndrome.6,7 Specifically, we found that the TLR-2 inflammatory response and diurnal cortisol dysregulation, marked by a flattened diurnal cortisol rhythm, served as possible biomarkers of IC/BPS while TLR-4 inflammatory responses were associated with greater IC/BPS pain severity6 and the extent of comorbid pain.7
The purpose of the current study is to determine whether these inflammatory biomarkers measured at study entry are related to longitudinal IC/BPS symptom changes in women with IC/BPS over a 48 week period of follow-up. We hypothesized that elevated TLR inflammatory responses and flattened diurnal cortisol slope would be associated with less improvement in symptoms over time and with a greater incidence of symptom flares.
Materials and Methods
MAPP study and recruitment
The Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP)), sponsored by The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), is a multi-site research effort intended to characterize various aspects of chronic pelvic pain including the identification of biomarkers associated with the disease and symptom changes.8 Eligible participants were at least 18 years of age, female, not pregnant, and reported chronic pain, pressure, or discomfort in the bladder or pelvic region for the preceding three months or during the majority of the last six months. Further information about the full scope of the Epidemiology and Phenotyping Study within the MAPP Research Network, participants and inclusion/exclusion criteria is available elsewhere.6,8,9
Demographic and symptom information
Participants were 24 women participating in a larger volunteer cohort study (n=66) previously reported on 6,7 who agreed to complete a longitudinal study of symptom changes. Demographic and symptom information was collected during a baseline visit, after which symptom data was collected bi-weekly for 48 weeks via an online module (25 time-points, including baseline). At the baseline visit participants underwent blood draw, urine collection, and physical examination, and completed a battery of questionnaires. Baseline and longitudinal symptom measures included the Symptom and Health Care Utilization Questionnaire,9 and the 9-item Genitourinary Pain Index (GUPI).10 The SYMQ contains a question about symptom flare status (i.e., “…are you currently experiencing symptoms that are much worse than usual?”), and the GUPI contains both pain and urinary subscales. Participants completed the Rome III criteria for irritable bowel syndrome,11 the American College of Rheumatology diagnostic criteria for Fibromyalgia,12 the International Chronic Fatigue Syndrome (CFS) Study Group criteria for CFS,13 an eight question MAPP specific diagnostic tool for symptoms of vulvodynia (e.g. “experience constant burning or raw feeling at the opening of the vagina,”), the Research Diagnostic Criteria for temporomandibular disorder (TMD)14 and the Positive and Negative Affect Scale.15 Medication use at baseline and duration of symptoms in years was collected by participant self-report.
Cortisol
Participants collected saliva in provided salivettes at 9 time points: upon waking (4–9 am), afternoon (5 pm), and bedtime, (8 pm–12 am) for three consecutive days prior to the baseline visit. Samples collected outside this time frame were excluded to maintain homogeneity. Cortisol procedures are described in detail elsewhere.6,16 Salivettes were analyzed by chemiluminescence immunoassay (IBL, Hamburg, Germany) at the Technical University of Dresden. The lower detection limit is 0.41 nmol/L and inter-assay and intra-assay coefficients of variance are less than 10%.
Toll-Like Receptor Inflammatory Responses
Blood draws occurred between approximately 11:30 am and 12:30 pm. PBMCs were separated by Ficoll-paque gradient centrifugation within 30 min of blood collection and cultured in RPMI 1640 medium containing 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin for 3 days with TLR agonists at 37 °C in a humidified incubator with 5% CO2. For stimulation of TLR-4, 50 ng/ml of the classic TLR-4 agonist Lipopolysaccharide (LPS) was used; for TLR-2 stimulation, 0.04 ng/ml of staphylococcus aureus cowan I (SAC) was used. Conditioned media was harvested and frozen at −80 °C prior to batch ELISA analysis. Each well contained 1 × 106 cells in 24 well plates, with one well per subject for TLR-4 and TLR-2 stimulation, respectively. Cytokines interleukin (IL) 6 and IL-1β were assayed in duplicate by DuoSet ELISAs (R&D Systems) according to instructions included with the kit.
Statistical Analyses
Statistical analyses were performed using SPSS v. 21 and R v. 3.1.1. Cytokine and cortisol values were log transformed to normalize their distributions. The inflammatory response to TLR-2 and TLR-4 stimulation in PBMCs was calculated as the sum of each cytokine z-score for each individual: ([individual score – group mean]/group standard deviation) for IL-6 and IL-1β. This inflammatory response was then further standardized for ease of interpretation. Using both cytokine responses is thought to better reflect the inflammatory response to TLR stimulation as both have been implicated in enhanced central pain sensitization and both are controlled, in part, by NFκB transcription.17 Salivary cortisol values at each of the collection points were regressed on the time of collection over the three-day period to calculate cortisol slope, a measure of the average hourly decrease in cortisol over the course of the day16
Primary outcomes included symptom changes in GUPI urinary and pain subscale scores and the probability of self-reported symptom flares over the 48-week period. Missing data points were estimated using the Restricted Estimation of Maximum Likelihood (REML) method.
To determine whether symptom changes differed by inflammatory markers, mixed effects longitudinal models were used. The effect of time was evaluated with both linear and quadratic terms and the results compared by likelihood testing. The linear effect of time was retained following those results. Optimal random effects structures were determined by likelihood testing of nested models with and without each random term, beginning with maximum complexity. Modeling subject specific variance in intercepts and slopes can allow more accurate estimation of fixed effects.18 For models of symptom changes (both GUPI subscales) random intercepts for subjects and random slopes for the effect of time for each subject were retained. Covariate selection was conducted by using age, BMI, duration of symptoms, PANAS negative affect score, and use of medications (SSRI/SNRIs, opioids, NSAIDs, and pentosan polysulfate) as predictors of GUPI subscales with a time and a time by variable interaction.
Significant main effects indicated an effect of the variable on the outcome measure across the period of observation, while significant time by variable interaction terms indicate an effect on the slope of the symptom change. Significant main and interaction effects were retained in multivariable models that included TLR-4 inflammation scores, TLR-2 inflammation score, and cortisol slope. To determine whether self-reported symptom flares were associated with increases on the GUPI pain and urinary subscales, symptom flare status was used as a predictor of GUPI pain and urinary subscale scores across the 48-week period of observation.
For models of flare probability, mixed effects logistic regression models were used with random intercepts for subject retained following model evaluation. Covariate selection was conducted as above, with significant covariates retained in multivariable models that included TLR-4 inflammation scores, TLR-2 inflammation score, and cortisol slope.
Results
Participants
Participants were mostly White and non-Hispanic (96%), and well educated (67% with college or graduate degrees). A majority of participants were using tricyclic antidepressants (62%) or pentosan polysulfate (67%). Baseline demographic and medical information is presented in Table 1. On average, participants completed 89% of longitudinal symptom questionnaire time-points (range 56%-100%). Of 24 participants, 22 (92%) experienced at least one symptom flare during the period of observation (Average = 4.29, SD = 3.30).
Table 1.
Demographic and medical characteristics of participants.
| Participant Characteristics | N=24 |
|---|---|
|
| |
| Mean (SD) | |
| Age | 42.09 (13.23) |
| BMI | 27.43 (5.66) |
| PANAS negative affect | 21.67 9.74 |
| Duration of symptoms in years | 7.21 (7.97) |
| GUPI Score Total, 0-45 | 23.85 (8.69) |
| GUPI pain subscale, 0-23, mean (SD) | 11.35 (5.03) |
| GUPI urinary subscale, 0-10 | 6.33 (2.94) |
|
| |
| Race % (n) | |
| White | 96 (23) |
| Asian | 4 (1) |
|
| |
| Ethnicity % (n) | |
| Non-Hispanic | 100 (24) |
|
| |
| Education % (n) | |
| High School or GED | 4 (1) |
| Some College | 30 (7) |
| Graduated College | 33 (8) |
| Graduate Degree | 33 (8) |
|
| |
| Employment % (n) | |
| Employed | 83 (20) |
| Disabled | 8 (2) |
| Retired | 4 (1) |
| Full Time Homemaker | 4 (1) |
|
| |
| Annual Income/$ % (n) | |
| <10,000 | 21 (5) |
| <25,000 | 4 (1) |
| <50,000 | 17 (4) |
| <100,000 | 46 (11) |
| >100,000 | 12 (3) |
|
| |
| Comorbid Conditions % (n) | |
| None | 33 (8) |
| One or More (IBS, Fibromyalgia, TMD, vulvodynia, CFS) | 67 (16) |
|
| |
| Tricyclic anti-depressants | |
| No | 38 (9) |
| Yes | 62 (15) |
|
| |
| Opioids | |
| No | 75 (18) |
| Yes | 25 (6) |
|
| |
| Pentosan Polysulfate | |
| No | 33 (8) |
| Yes | 67 (16) |
|
| |
| NSAIDs | |
| No | 96 (23) |
| Yes | 4 (1) |
|
| |
| SSRI/SNRIs | |
| No | 88 (21) |
| Yes | 12 (3) |
|
| |
| Experiencing flare at baseline visit? | |
| No | 67 (16) |
| Yes | 33 (8) |
|
| |
| GUPI Score Total, 0-45 | 23.85 (8.69) |
|
| |
| GUPI pain subscale, 0-23, mean (SD) | 11.35 (5.03) |
|
| |
| GUPI urinary subscale, 0-10 | 6.33 (2.94) |
BMI= Body Mass Index
CFS = Chronic Fatigue Syndrome
IBS= Irritable Bowel Syndrome
GUPI = Genitourinary Pain Index
NSAID = Non-Steroidal Anti-Inflammatory Drug
PANAS = Positive and Negative Affect Scale
TMD= Temporomandibular Disorder
SSRI/SNRI= Selective Serotonin/Norepinephrine Reuptake Inhibitor
Symptom Changes
Patient age, BMI, duration of symptoms in years, and use of any medication were not associated with levels of symptoms across the 48-week period or symptom change (all p > .08). Levels of negative affect were associated with higher average levels of GUPI pain and urinary subscales (both p < .01) but were not associated with symptom changes for either (both p > .74). Comorbid status was associated with higher average levels of GUPI urinary subscale scores (p = .045) but not symptom change (p = .13). Therefore, the main effect of negative affect was retained as a covariate for the multivariate models predicting pain subscale change, and main effects of negative affect and comorbid condition status were retained as covariates for the multivariate models predicting urinary subscale change.
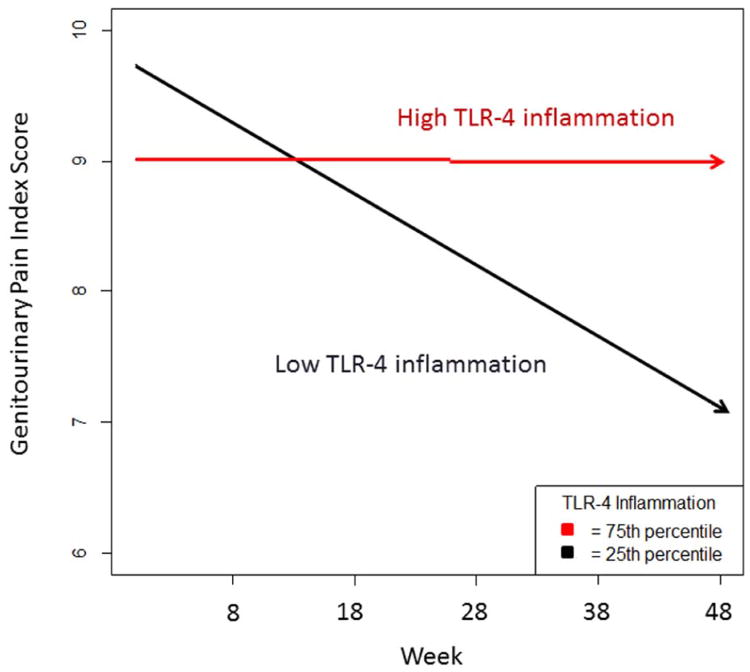
Higher TLR-4 inflammation scores (p = 0.031), higher TLR-2 inflammation scores (p = .045), and flatter diurnal cortisol slopes (p = .012) were each associated with less symptom improvement on the GUPI pain subscale over the 48 week period. See Table 2 for parameters of multivariable models. These findings predict that a patient one standard deviation above the mean on TLR-4 inflammation would experience a slight worsening of symptoms over the period (.36 points) while a patient one standard deviation below the mean would experience moderate improvement (-2.89 points) on the 23 point GUPI pain subscale. Results for TLR-2 inflammation and diurnal cortisol slope were similar. See Figure 1- for fitted symptom changes on the GUPI pain subscale of patients at the 25th and 75th percentile of TLR-2 inflammation scores.
Table 2.
Parameters of multivariable models examining the relationship between inflammatory variables and and IC/BPS symptoms over the 48 week observation period.
| GUPI Pain | Estimate | S.E. | DF | Z value | p |
|---|---|---|---|---|---|
| Time | -0.053 | 0.029 | 22.294 | -1.791 | 0.087 |
| TLR-4 composite score | 0.519 | 0.871 | 21.586 | -0.596 | 0.56 |
| Negative Affect | 0.445 | 0.087 | 21.131 | 5.096 | <.001 |
| Time*TLR-4 composite scorea | 0.068 | 0.030 | 23.347 | 2.289 | 0.031 |
| GUPI Pain | Estimate | S.E. | DF | Z value | p |
| Time | -0.054 | 0.030 | 22.089 | -1.825 | 0.082 |
| TLR-2 composite score | -0.784 | 0.838 | 21.245 | -0.936 | 0.36 |
| Negative Affect | .450 | 0.085 | 21.204 | 5.291 | <.001 |
| Time*TLR-2 composite score | 0.063 | 0.030 | 22.078 | 2.123 | 0.045 |
| GUPI Pain | Estimate | S.E. | DF | Z value | p |
| Time | -0.046 | 0.030 | 20.174 | -1.555 | 0.14 |
| Diurnal Cortisol Slope | -0.692 | - 0.802 | 19.332 | 0.864 | 0.40 |
| Negative Affect | 0.445 | 0.079 | 19.229 | 5.622 | <.001 |
| Time*Slope | 0.083 | 0.030 | 20.702 | 2.763 | 0.01 |
| GUPI Urinary Symptoms | Estimate | S.E. | DF | Z value | p |
| Time | -0.017 | 0.011 | 22.153 | -1.561 | 0.13 |
| Comorbid Condition | 2.015 | 1.212 | 19.999 | 1.663 | 0.11 |
| TLR-4 composite score | -0.446 | 0.559 | 20.128 | -0.797 | 0.44 |
| Negative Affect | 0.122 | 0.059 | 20.074 | 2.073 | 0.051 |
| Time*TLR-4 composite score | 0.027 | 0.011 | 23.677 | 2.521 | 0.019 |
GUPI= Genitourinary Pain Index
TLR= Toll-Like Receptor
Significant Time × variable interactions indicate differential symptom changes.
Figure 1.
Predicted change in genitorurinary pain for a participant at the 25th and 75th percentile of TLR-4 inflammation over the 25 visit (48 week) period controlling for negative affect. P = .031 for Time × TLR-4 interaction. TLR= Toll-Like Receptor.
Elevated TLR-4 inflammation (p = .018) was associated with less symptom improvement on the GUPI urinary subscale, while TLR-2 inflammation (p = .16) and diurnal cortisol slope were not (p = .17). See Table 2. This finding predicts that a patient one standard deviation above the mean on TLR-4 inflammation would experience slight worsening of symptoms over the 48 week period (.25 point increase) while a patient one standard deviation below the mean would experience mild improvement (-1 point) on the 10 point scale.
Probability of Symptom Flares
Age, BMI, duration of symptoms, and use of any medication were not associated with an increased probability of reporting symptom flares over the full 48-week period (all p > .12). During self-reported symptom flares, GUPI pain subscale and urinary scores were modestly elevated (estimated increase on GUPI pain subscale= 3.11 points, S.E. = .32;estimated increase on GUPI urinary subscale = .88 points, S.E. = .15; both p <.01). Higher levels of negative affect (p < .001) and the presence of a comorbid condition (p = .013) were each associated with a higher probability of reporting a symptom flare over the 48 week period and were retained as covariates for multivariable models of flare probability over the 48 weeks.
In multivariate models, neither TLR-4 inflammation, TLR-2 inflammation, nor diurnal cortisol slope was significantly associated with greater flare probability over the 48 week period (all p > .29)
Discussion
The primary findings of this study are that elevated levels of TLR-mediated inflammation and dysregulated diurnal cortisol slope are associated with less improvement in genitourinary pain in a sample of women suffering from IC/BPS over a 48 week period. Elevated TLR-4 inflammation was also associated with less improvement in urinary symptoms. In contrast, these inflammatory markers were not associated with the probability of self-reported symptom flares over the period of observation. These findings support our previous cross-sectional investigations demonstrating that TLR-4 inflammatory responses are associated with genitourinary pain 6 and extend them by demonstrating that TLR-4 mediated inflammation is associated with less improvement in IC/BPS symptoms over time. Participants one standard deviation below the mean on TLR-4 inflammation experienced an estimated 30% decrease in self-reported pain from that reported at study entry, indicating that TLR-mediated inflammation may be associated with clinically relevant changes in symptoms in this sample. The association of TLR-4 inflammation and urologic symptoms warrants further investigation; our previous cross-sectional work found a marginal relationship between TLR-4 inflammation and urinary symptoms.6 It is possible that TLR-4 serves as a marker of both amplified pain and non-painful interoceptive processes.
To our knowledge, this is the first study to report on a biomarker predicting symptom changes in IC/BPS. Previous research has primarily focused on whether individuals met case definitions at subsequent visits following initial diagnosis with IC/BPS and demographic correlates of this likelihood.3 The main effect of time was not statistically significant in any of the final multivariable models. This is consistent with the results of a large prospective study of IC/BPS patients assessed at approximately three-month intervals for up to four years that found no significant change in symptoms over the period of observation.2 Examining predicted symptom changes of patients at high and low levels of TLR-4 and TLR-2 inflammation, as well as cortisol slope, indicates that dysregulated inflammatory factors were primarily associated with a lack of symptom improvement over time, while low levels of TLR inflammation and more normal cortisol slopes were associated with symptom improvement. This suggests that these inflammatory factors could have utility in identifying patients who will struggle to improve with conventional treatment. However, a prospective study with treatment-matched participants, or an investigation of treatment modalities on inflammatory markers, would be necessary to address this question.
Flatter diurnal cortisol slopes and loss of pulsatility in glucorticoid rhythms have previously been linked to glucocorticoid insensitivity 19 and difficulty responding to acute stressors with sufficient glucocorticoid release.20 Therefore it is possible that IC/BPS patients with flattened cortisol slopes have a compromised ability to control endogenous levels of inflammation. The release of inflammatory cytokines following stimulation of TLR-4 in spinal glia has been identified as mechanistic component of centrally mediated pain sensitization.17, 21, 22 and a recent investigation found some concordance between levels of inflammatory cytokines following TLR stimulation in both spinal glia and PBMCs.23 It is possible that centrally acting treatment strategies, particularly those that might target TLR-mediated inflammation, could be effective in a subset of IC/BPS patients.
Strengths of this study include the frequency of longitudinal time points (bi-weekly for 48 weeks), inclusion of data on medication use, and the use of relatively sophisticated modeling techniques. Limitations include the relatively small sample size, the heterogeneous nature of study entry and treatment (i.e. different symptom durations and treatment modalities) and the ethnic/racial homogeneity of the sample. While the period of observation was relatively long, regression-to-the-mean effects during the earlier period of observation could have influenced the results. This study did not examine symptoms of comorbid conditions, which limits inferences about factors that affect a large proportion of IC/BPS sufferers.
Future MAPP research network prospective studies are designed to determine if centrally acting treatments (e.g. tricyclic antidepressants) reduce markers of inflammation while improving pain and urinary symptoms. Greater frequency of inflammatory data may reveal associations with symptom flares, as transient increases in stress that may impact HPA axis function have previously been shown to precipitate symptom flares.24
Acknowledgments
The authors gratefully acknowledge the assistance of Mary Eno in carrying out this project. The authors gratefully acknowledge the MAPP research network. Please see MAPP masthead.
Funding: This work was supported in part by grants UO1DK082344 and RO1DK100891 from the National Institute of Diabetes Digestive and Kidney Diseases.
Footnotes
Conflicts of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berry SH, Elliott MN, Suttorp M, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011;186:540–544. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Propert KJ, Schaeffer AJ, Brensinger CM, et al. A prospective study of interstitial cystitis: results of longitudinal followup of the interstitial cystitis data base cohort. J Urol. 2000;163:1434–1439. doi: 10.1016/s0022-5347(05)67637-9. [DOI] [PubMed] [Google Scholar]
- 3.Suskind AM, Berry SH, Suttorp MJ, et al. Symptom persistence in a community cohort of women with interstitial cystitis/bladder pain syndrome (IC/BPS): 3-, 6-, 9-, and 12-month follow-up from the RICE cohort. Int Urogynecol J. 2014;25:1639–1643. doi: 10.1007/s00192-014-2420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutcliffe S, Bradley CS, Clemens JQ, et al. Urological chronic pelvic pain syndrome flares and their impact: qualitative analysis in the MAPP network. Int Urogynecol J. 2015;26:1047–1060. doi: 10.1007/s00192-015-2652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Potts JM, Payne CK. Urologic chronic pelvic pain. Pain. 2012;153:755–758. doi: 10.1016/j.pain.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Schrepf A, O'Donnell M, Luo Y, et al. Inflammation and inflammatory control in interstitial cystitis/bladder pain syndrome: Associations with painful symptoms. Pain. 2014;155:1755–1761. doi: 10.1016/j.pain.2014.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schrepf A, Bradley CS, O'Donnell M, et al. Toll-like receptor 4 and comorbid pain in Interstitial Cystitis/Bladder Pain Syndrome: a multidisciplinary approach to the study of chronic pelvic pain research network study. Brain Behav Immun. 2015;49:66–74. doi: 10.1016/j.bbi.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clemens JQ, Mullins C, Kusek JW, et al. The MAPP research network: a novel study of urologic chronic pelvic pain syndromes. BMC Urol. 2014;14:57. doi: 10.1186/1471-2490-14-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landis JR, Williams DA, Lucia MS, et al. The MAPP research network: design, patient characterization and operations. BMC Urol. 2014;14:58. doi: 10.1186/1471-2490-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clemens JQ, Calhoun EA, Litwin MS, et al. Validation of a modified National Institutes of Health chronic prostatitis symptom index to assess genitourinary pain in both men and women. Urology. 2009;74:983–987. doi: 10.1016/j.urology.2009.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drossman DA, Dumitrascu DL. Rome III: New standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis. 2006;15:237–241. [PubMed] [Google Scholar]
- 12.Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62:600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 13.Fukuda K, Straus SE, Hickie I, et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121:953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 14.Dworkin SF, Sherman J, Mancl L, et al. Reliability, validity, and clinical utility of the research diagnostic criteria for Temporomandibular Disorders Axis II Scales: depression, non-specific physical symptoms, and graded chronic pain. J Orofac Pain. 2002;16:207–220. [PubMed] [Google Scholar]
- 15.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 16.Kraemer HC, Giese-Davis J, Yutsis M, et al. Design decisions to optimize reliability of daytime cortisol slopes in an older population. Am J Geriatr Psychiatry. 2006;14:325–333. doi: 10.1097/01.JGP.0000201816.26786.5b. [DOI] [PubMed] [Google Scholar]
- 17.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baayen RH, Davidson DJ, Bates DM. Mixed-effects modeling with crossed random effects for subjects and items. J Mem Lang. 2008;59:390–412. [Google Scholar]
- 19.Jarcho MR, Slavich GM, Tylova-Stein H, et al. Dysregulated diurnal cortisol pattern is associated with glucocorticoid resistance in women with major depressive disorder. Biol Psychol. 2013;93:150–158. doi: 10.1016/j.biopsycho.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheff JD, Calvano SE, Lowry SF, et al. Transcriptional implications of ultradian glucocorticoid secretion in homeostasis and in the acute stress response. Physiol Genomics. 2011;44:121–129. doi: 10.1152/physiolgenomics.00128.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutchinson MR, Zhang Y, Brown K, et al. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4) Eur J Neurosci. 2008;28:20–29. doi: 10.1111/j.1460-9568.2008.06321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grace PM, Hutchinson MR, Maier SF, et al. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014;14:217–231. doi: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwok YH, Tuke J, Nicotra LL, et al. TLR 2 and 4 responsiveness from isolated peripheral blood mononuclear cells from rats and humans as potential chronic pain biomarkers. PLoS One. 2013;8:e77799. doi: 10.1371/journal.pone.0077799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothrock NE, Lutgendorf SK, Kreder KJ, et al. Stress and symptoms in patients with interstitial cystitis: a life stress model. Urology. 2001;57:422–427. doi: 10.1016/s0090-4295(00)00988-2. [DOI] [PubMed] [Google Scholar]