Abstract
Molecular mechanism underlying the invasion of oral cancer cells remains to be clarified. We previously demonstrated that transforming growth factor-β1 (TGF-β1) induces the expression of mesenchymal markers in human oral squamous cell carcinoma HSC-4 cells. Intriguingly, the expression of the epithelial–mesenchymal transition-related transcription factor Slug was also significantly upregulated upon TGF-β1 stimulation. However, the mechanism by which Slug transduces the TGF-β1-induced signal to enhance the invasiveness of HSC-4 cells is poorly understood. Proteomic analysis revealed that the expression of matrix metalloproteinase (MMP)-10 was upregulated in TGF-β1-stimulated cells. Additionally, a Boyden chamber assay revealed that the TGF-β1-induced increase in invasiveness of HSC-4 cells was significantly inhibited by MMP-10 small interfering RNA (siRNA). Intriguingly, Slug siRNA suppressed TGF-β1-induced expression of MMP-10. These results suggest that TGF-β1 induces invasion in HSC-4 cells through the upregulation of MMP-10 expression in a Slug-dependent manner. On the other hand, Slug siRNA suppressed TGF-β1-induced Wnt-5b expression. Wnt-5b significantly induced MMP-10 expression, whereas Wnt-5b siRNA suppressed the TGF-β1-induced increase in invasiveness, suggesting that TGF-β1-induced expression of MMP-10 and the resulting upregulation of invasiveness are mediated by Wnt-5b. Overall, these results suggest that TGF-β1 stimulates HSC-4 cell invasion through the Slug/Wnt-5b/MMP-10 signalling axis.
Keywords: invasion, MMP-10, Slug, TGF-β, Wnt-5b
Human oral squamous cell carcinoma (hOSCC) is a common type of cancer (1, 2). Despite the development of advanced treatment, patients suffering from hOSCC are still faced with poor prognosis and high mortality rates. Actually, the mechanisms of hOSCC invasion and metastasis are not well understood (3). Intriguingly, gene expression profiling shows that epithelial–mesenchymal transition (EMT) is a characteristic feature of high-risk head and neck squamous cell carcinoma (2–4). EMT changes the cell phenotype from a polarized epithelium to a mesenchyme, which is characterized by enhanced motility and invasiveness (5). In addition, EMT induces matrix remodelling and thereby promotes tumour metastasis (6, 7). However, the molecular mechanism underlying hOSCC invasion associated with EMT remains to be clarified.
TGF-β produces multiple functions by binding to TGF-β receptor type I (TβR-I) and type II (TβR-II), which are transmembrane serine/threonine kinases and mediate specific intracellular signalling pathways. Receptor-regulated Smads (R-Smads) phosphorylated by a ligand-bound TβR-I/TβR-II hetero-tetramer bind to common-mediator Smad, Smad4 and translocate to the nucleus. The transcriptions of target genes are controlled by the R-Smads/Smad4 complex in cooperation with other transcription factors and transcriptional co-activators or co-repressors (8, 9). TGF-β is related to the malignant transformation and exacerbation of cancer through the induction of EMT (reviewed in Refs. 10–12). Many transcriptional factors, such as Snail (13) and Slug (14), participate in EMT by both activating of mesenchymal genes and repressing of epithelial genes (15, 16).
Recent studies on tumour biology revealed that complex interactions of tumour cells with their adjacent microenvironment, including the extracellular matrix (ECM), are necessary for various mechanisms involved in tumour development and progression (reviewed in Ref. 17). Thus, the interaction between tumour cells and the ECM controls most aspects of tumourigenicity, including EMT and subsequent tumour cell invasion. Several matrix metalloproteinases (MMPs), including MMP-2 and MMP-9, also play a role in tumour invasion and metastasis (18–20). In addition, TGF-β promotes tumour invasion by stimulating the expression of proteases that digest ECM components. Several types of MMPs are upregulated by TGF-β stimulation, such as MMP-1, -3, -9 and -10 (21, 22). MMP-10 (EC3.4.24.22), also known as stromlysin-2, targets a broad range of ECM proteins, including collagen type IV, gelatin, elastin, fibronectin, laminin and proteoglucan, as well as proMMP-1, -7, -8, -9 and -13 (23). In particular, ectopic overexpression of MMP-10 induces invasion of hOSCC cells (24).
The Wnt signalling pathway is important for cell development and cancer (25, 26). The Wnt family of glycoproteins consists of 19 Wnt ligands in humans (27). Wnt ligands bind to membrane-bound receptors, including 10 types of Frizzled (FDZ) receptors, low-density lipoprotein receptor-related protein (LRP) 5/6 and atypical receptor tyrosine kinases (RTKs)-PKT7, ROR2, and RYK (28, 29). Wnt signalling can roughly be divided into two pathways: the β-catenin dependent ‘canonical’ and the β-catenin independent ‘non-canonical’ pathway (30). In canonical signalling, binding of Wnt ligands to both an FDZ receptor and co-receptor (LRP5/6) induces the stabilization of cytosolic β-catenin leading to an augmentation of β-catenin in the cytosol. The cytosolic β-catenin is translocated to the nucleus. Then, the β-catenin binds to T-cell factor/lymphoid enhancer-binding factor and activates target gene transcription. The disruption of E-cadherin-mediated adherens junctions induced by EMT also promotes the translocation of β-catenin from the cell membrane to the cytoplasm. The cytosolic β-catenin allows to enter the nucleus and activates the gene expressions through the canonical Wnt signalling pathway (31, 32). Therefore, the activation of Wnt/β-catenin is essential for EMT (33, 34). β-catenin is predominantly localized in the nucleus at the invasive front of hOSCC cells through Wnt-3 signal (35). On the other hand, other types of Wnt ligands bind to their respective receptors and elicit cellular responses. Wnt-5a (36) and b (24) are the ligands for the activation of the non-canonical signalling pathway. Wnt-5a signalling is stimulated by TGF-β and regulates ECM production in airway smooth muscle cells (36). This signalling pathway consists of the Ca2+-dependent signalling and planar cell polarity pathways. In the Ca2+ pathway, Ca2+-dependent molecules such as calcineurin, Ca2+/calmodulin-dependent protein kinase II and Protein kinase C (PKC) are activated. The planar cell polarity pathway involves the activation of small Rho-GTPases and c-Jun N-terminal kinases or the Rho-kinase signalling (28, 37). Intriguingly, Deraz et al. previously reported that Wnt-5b promotes the upregulation of MMP-10 in hOSCC (24).
We previously demonstrated that TGF-β1 promotes the EMT of hOSCC cells (38). More specifically, TGF-β1 upregulates the expression levels of mesenchymal markers, such as N-cadherin, vimentin and integrin α3β1-targeted proteins, and also enhances cell migratory activity in HSC-4, a hOSCC cell line. Intriguingly, the expression level of the EMT-related transcription factor Slug was also significantly upregulated upon TGF-β1 stimulation, suggesting that Slug may control the EMT of HSC-4 cells stimulated with TGF-β1. However, it remains to be clarified how Slug, the different Wnt family members and MMPs cooperate in the transduction of TGF-β-induced signals to stimulate the invasion ability of hOSCC cells. In this article, we discuss the functional relationship between Slug, MMP-10 and Wnt-5b with regard to the upregulation of HSC-4 cellular invasion in response to TGF-β stimulation.
Materials and Methods
Materials
Cultured cell lines were obtained from the Human Science Resource Cell Bank (Osaka, Japan). Recombinant human TGF-β1 was purchased from PEPROTECH (Rocky Hill, NJ, USA). Dvl-PDZ Domain Inhibitor II, which disrupts FZD-dishevelled (Dvl) interactions in Wnt signalling, was also purchased from Merck-Millipore. Human recombinant DKK1 protein, which inhibits non-canonical Wnt signalling by preventing LRP5/6 interaction with Wnt, was provided by ATGen (Seongnam-si, South Korea). Recombinant human Wnt-5b was purchased from R&D systems (Minneapolis, MN, USA). Protease inhibitor cocktail for use with mammalian cell and tissue extracts and phosphatase inhibitor cocktail 1 and 2 were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other reagents were analytical grade.
Cell culture
All cell lines were grown at 37°C and 5% CO2. Human HSC-2 and HSC-4 squamous cell carcinoma cells were cultured in Eagle’s minimum essential medium (MEM; Sigma-Aldrich) supplemented with 10% foetal bovine serum (FBS; Gibco BRL, Rockville, MD, USA). HSC-3 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco BRI) containing 10% FBS. SAS cells were cultured in PRIM1640 medium (Gibco BRL) supplemented with 10% FBS. The culture medium was removed and replaced with serum-free medium 24 h prior to TGF-β1 stimulation. In the experiments pertaining to the production of secreted proteins, such as MMP-10 and Wnt-5b, 4 × 105 cells (HSC-4) were cultured in six-well plates for 48 or 72 h with 3.0 ml serum-free medium, containing 10 ng/ml TGF-β1. A fraction of the conditioned medium (500 µl) was harvested and then concentrated by ultrafiltration using Microcon-10 filters (cut-off, 10 kDa; Merck) to a volume of 20 µl. An equal volume of sample buffer (Laemmli 2 × concentrate; Sigma-Aldrich) was added to the concentrated medium and the samples were separated by SDS-PAGE. Based on our previous work (38), we compared the expression of ECM proteins in conditioned medium for an equal number of cells, without detection of a loading control (Figs 1C and 2B).
Fig. 1.
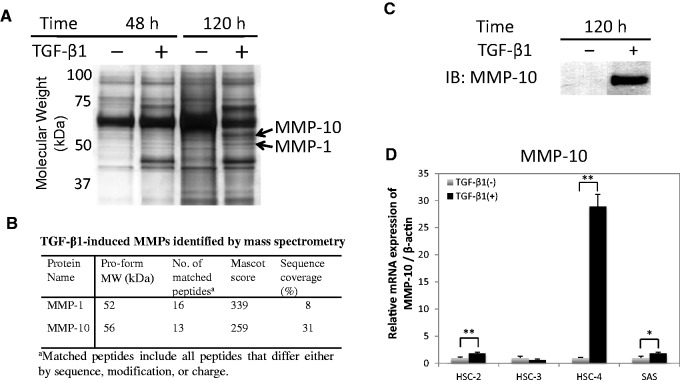
TGF-β1 induces expression of MMP-10 in HSC-4 cells. (A) HSC-4 cells were treated with 10 ng/ml TGF-β1. The secreted proteins, present in the culture medium, were separated via SDS-PAGE and analysed by LC-MS/MS. Proteomic analysis identified MMP-1 and MMP-10 (bands indicated by arrows) in the TGF-β1-stimulated cells. (B) The mass data were analysed using Mascot software against a protein database for protein identification. (C) The conditioned medium was also analysed by western blotting with anti-MMP-10 antibodies (LA-12 clone). (D) The expression of MMP-10 in four TGF-β-stimulated hOSCC cell lines was examined by qRT-PCR. The values have been normalized to the β-actin mRNA level. Data represent the mean ± SD of three wells for each time point (*P < 0.05; **P < 0.01).
Fig. 2.
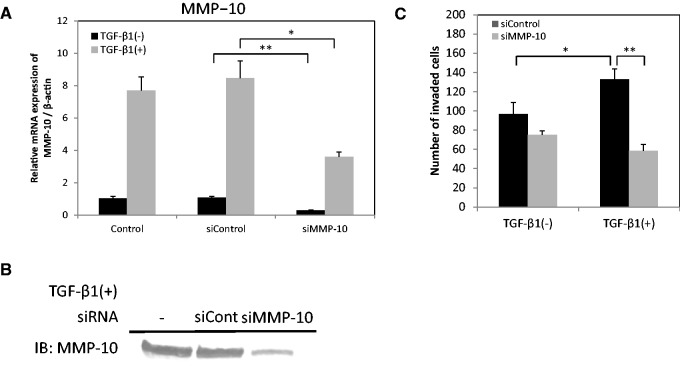
TGF-β1 promotes invasion in HSC-4 cells through MMP-10 expression. (A) HSC-4 cells were transfected with MMP-10 siRNA (siMMP-10) or control siRNA (siControl). The expression of MMP-10 mRNA was examined by qRT-PCR. The values have been normalized to the β-actin mRNA levels. Data are represented as the mean ± SD of three wells for each time point (*P < 0.05; **P < 0.01). (B) For the detection of MMP-10 protein, culture media were collected 72 h after TGF-β1 (10 ng/ml) stimulation, and then subjected to western blotting analysis. (C) First, the cells were transfected with control siRNA (black bars) or MMP-10 siRNA (grey bars). Then, the invasiveness of the cells was examined in a Boyden chamber assay. Data are represented as the mean ± SD of three wells for each time point (*P < 0.05; **P < 0.01).
Mass spectrometry analysis
SDS-PAGE was carried out on a 10–20% acrylamide gradient gel (ATTO Co., Tokyo, Japan). Protein bands were stained with silver stain (Ez Stain silver, ATTO). Digestion of proteins in the gel pieces was carried out according to a previously described method (38). The peptides were eluted with a gradient of 10–65% acetonitrile in 0.1% formic acid by capillary HPLC (Agilent 1100 System; Agilent). Mass spectrometry (MS) was carried out on an HCT ultra si (Bruker Daltonics, Bremen, Germany), according to the manufacturer’s instructions. Protein sequence database searches were performed with Mascot (Matrix Science, Boston, MA, USA) using the MS/MS peptide ions.
Quantitative real-time reverse transcriptase polymerase chain reaction
For total RNA preparation, 1 × 105 cells were cultured in 24-well tissue culture plates. Total RNA was isolated using the ISOGEN reagent (Nippon Gene, Toyama, Japan) according to the manufacturer’s instructions. The first-strand cDNA was prepared from 4 µg of total RNA using the poly-A primer provided in the RT-PCR System Kit (Takara Bio Inc., Shiga, Japan). Quantitative real-time reverse transcriptase polymerase chain reaction (qRT-PCR) was performed on a Thermal Cycler Dice Real Time System (Takara Bio) using SYBR Premix Ex Taq II (Takara Bio) with gene-specific primers (listed in Table I). The mRNA expression levels of the target genes were normalized to those of the endogenous reference gene β-actin and are shown in terms of fold increase or decrease relative to the level of the control sample.
Table I.
Sequence of primers for qRT-PCR
| Target mRNA | Oligonucleotide sequence (5′–3′) | Predicted size (bp) |
|---|---|---|
| MMP-10 | (F) CTGGACCTGGGCTTTATGGAGA | 100 |
| (R) AGTTCATGAGCAGCAACGAGGA | ||
| Slug | (F) TGTTGCAGTGAGGGCAAGAA | 158 |
| (R) GACCCTGGTTGCTTCAAGGA | ||
| Wnt-3a | (F) TCAGCCTCTGCCACTGTGAA | 169 |
| (R) GTTGGACAGTGGATATAGCAGCAT | ||
| Wnt-5a | (F) TTACCACTGCAACTATTGCACCTC | 146 |
| (R) CACAATGAACCTTTAGTTTCCAACC | ||
| Wnt-5b | (F) GGGTGCTCATGAACCTGCAA | 71 |
| (R) GCAGGCTACGTCTGCCATCTT | ||
| β-Actin | (F) GGAGATTACTGCCCTGGCTCCTA | 89 |
| (R) GACTCATCGTACTCCTGCTTGCTG |
Western blot analysis
A total of 5 × 105 cells were lysed in RIPA (radio-immunoprecipitation assay) buffer (50 mM Tris–HCl pH 7.2, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate and 0.1% SDS) containing protease and phosphatase inhibitors (Sigma-Aldrich). The protein content of the samples was measured using bicinchoninic acid reagent (Pierce, Rockford, IL, USA). To examine marker proteins in cell lysate, 4 × 105 cells were cultured in a six-well plate in serum-free MEM with or without 10 ng/ml TGF-β1 for the indicated times. The cells were dissolved in SDS sample buffer containing protease and phosphatase inhibitors (Sigma-Aldrich). Acrylamide gels of 12.5% (ATTO Co.) were used for protein separation via SDS-PAGE, and the proteins were subsequently transferred onto polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). The membranes were probed with the following primary antibodies: anti-MMP-10 mouse (LA-12) and anti-MMP-10 goat (I-18) antibody (Figs 1C, 2B, and 3B) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and anti-Wnt-5b rabbit antibody (ab124818; Abcam, Cambridge, UK) (Fig. 5B). The volume of medium used in the analysis was determined by the corresponding amount of protein in cell lysate. The levels of β-actin in cell lysates were monitored as a loading control. Anti-β-actin mouse antibody (clone C4; Santa Cruz) was used in the experiments (Figs 3B and 5B). All blots were incubated with alkaline phosphatase-conjugated secondary antibody and signals were detected using an alkaline phosphatase substrate kit (BCIP/NBT Substrate Kit; Vector Laboratories Inc., Burlingame, CA, USA).
Fig. 3.
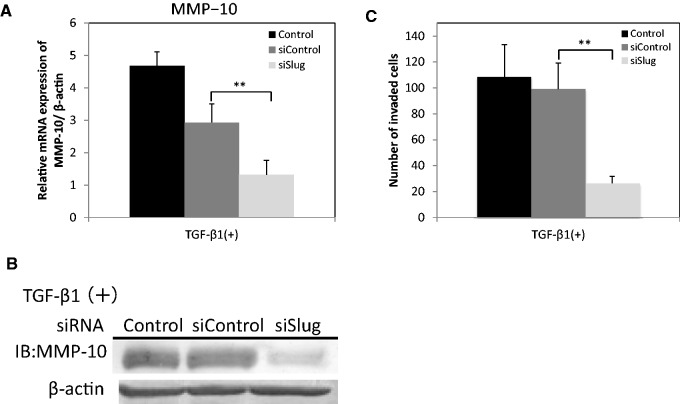
TGF-β1 induces MMP-10 expression in a Slug-dependent manner. (A) HSC-4 cells were transfected with Slug siRNA (siSlug) or control siRNA (siControl). The expression of MMP-10 mRNA was examined by qRT-PCR. Data are represented as the mean ± SD of three wells for each time point (**P < 0.01). (B) The protein expression levels of MMP-10 were determined by western blotting with anti-MMP-10 (LA-12) antibodies. β-actin in cell lysate was used as a loading control. (C) The invasiveness of HSC-4 cells was evaluated in a Boyden chamber assay after transfection with siSlug (light grey bars) or siControl (dark grey bars). After a 24-h incubation, the penetrated cells on the lower side of the membrane were stained by haematoxylin. Data are represented as the mean ± SD of three wells for each time point (*P < 0.05; **P < 0.01).
Fig. 5.
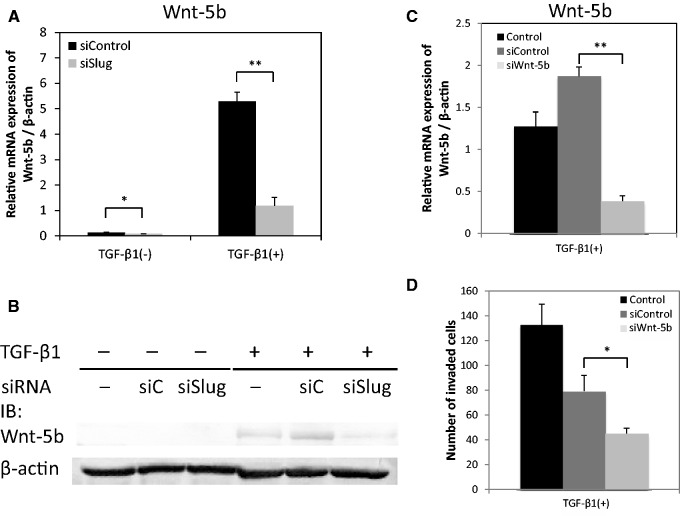
TGF-β1 increases the invasiveness of HSC-4 cells by induction of Wnt-5b expression through Slug. (A) HSC-4 cells were transfected with Slug siRNA (siSlug) or control siRNA (siControl). The expression of Wnt-5b mRNA was examined by qRT-PCR. Data are represented as the mean ± SD of three wells for each time point (*P < 0.05; **P < 0.01). (B) HSC-4 cells were transfected with Slug siRNA (siSlug) or control siRNA (siC). The protein expression levels of Wnt-5b were determined by western blotting using anti-Wnt-5b (ab124818) antibodies. β-actin in cell lysate was used as a loading control. (C) The cells were transfected with Wnt-5b siRNA (siWnt-5b) or siControl and mRNA expression of Wnt-5b was examined by qRT-PCR. Data are represented as the mean ± SD of three wells for each time point (**P < 0.01). (D) The invasiveness of HSC-4 cells transfected with siWnt-5b (light grey bars) or siControl (dark grey bars) was evaluated in a Boyden chamber assay. After 24 h of incubation, the penetrated cells on the lower side of the membrane were stained by haematoxylin. Data are represented as the mean ± SD of three wells for each time point (*P < 0.05).
Suppression of gene expression by small interfering RNA
The sense sequence of human Slug small interfering RNA (siRNA) (MISSION siRNA; Sigma-Aldrich) was previously described (38). The siRNAs of MMP-10 (Stealth™ RNAi, MMP10HSS106682) and Wnt5b (Stealth™ RNAi, WNT5BHSS129775) were purchased from Life Technologies (Carlsbad, CA). Logarithmically growing cells were seeded at a density of 1 × 105 cells per well in a 24-well tissue culture plate and transfected with 10 nM siRNA using Lipofectamine RNAiMAX (Life Technologies) according to the manufacturer’s instructions. Forty-eight hours after transfection, cells were stimulated using 10 ng/ml TGF-β1 and subsequently used for qRT-PCR analysis of gene expression or in an invasion assay as described below. Stealth™ RNAi Negative Control Low GC Duplex (Life Technologies) was used as a negative control. Suppression of gene expression by siRNA was evaluated by qRT-PCR and western blotting analyses for the targeted molecules.
Cell invasion assay with a Boyden chamber
Polycarbonate membranes coated with Matrigel were used as the insert of the Boyden chamber for invasion assays (Corning Inc., Corning, NY, USA). First, the cells were transfected with siRNA as described above. Then, they were treated with 10 ng/ml TGF-β1 under serum-free conditions for 72 h. Subsequently, the cells were plated in the upper compartment of a Boyden chamber apparatus at a density of 1 × 105 cells in serum-free medium with or without inhibitor. Cells were allowed to migrate into medium containing 10% FBS in the lower chamber for 24 h at 37°C. Following the 24-h incubation, the filter was fixed in 4% paraformaldehyde and stained with haematoxylin (Wako Pure Chem. Ind., Osaka, Japan) for 16 h. Cells that had migrated onto the lower side of the membrane were counted. The values are averages of experiments conducted in triplicate.
Statistical analysis
At least three independent replicates were performed for all experiments. The results are expressed as the mean ± standard deviation (SD). Data were analysed using the unpaired Student’s t-test. In all statistical analyses, a P value < 0.05 was considered statistically significant, and all P values are two-sided.
Results
TGF-β1 upregulates MMP-10 expression in HSC-4 cells
We examined which proteins are involved in cell invasion by performing a proteomic analysis on the conditioned media from HSC-4 cells stimulated with TGF-β1 (Fig. 1A and B). We found that MMP-1 and -10 were highly expressed in conditioned media upon TGF-β1 stimulation, as compared with that in the control media. The increase in MMP-10 protein expression induced by TGF-β1 stimulation was confirmed by western blotting (Fig. 1C). MMP-10 was of particular interest because this protease digests type IV collagen, an important constituent of the basal membrane that blocks tumour cell invasion into the fibrous tissue under the epithelial tissue, and activates other MMPs (23). We confirmed the increase in MMP-10 expression upon stimulation with TGF-β1 at the mRNA level in HSC-4 cells via qRT-PCR analysis (Fig. 1D). HSC-4 cells show a strong upregulation in MMP-10 expression in response to TGF-β1 stimulation, whereas other hOSCC cells, including HSC-2, HSC-3 and SAS cells, did not (Fig. 1D).
TGF-β1 promotes invasion ability of HSC-4 cells through expression of MMP-10
To understand whether MMP-10 expression affects the invasiveness of HSC-4 cells, we used siRNA to knockdown MMP-10. First, we confirmed that MMP-10 siRNA indeed reduced MMP-10 expression at the mRNA level, using qRT-PCR (Fig. 2A), and at the protein level, via western blot analysis (Fig. 2B). Intriguingly, MMP-10 siRNA inhibited the TGF-β1-induced increase in HSC-4 invasion ability (Fig. 2C). These results suggest that the TGF-β1-induced increase in invasion ability of HSC-4 cells is mediated by MMP-10 expression.
TGF-β1 upregulates MMP-10 expression in a Slug-dependent manner
We next examined whether Slug affected the TGF-β1-induced expression of MMP-10 and the invasion ability of HSC-4 cells. Therefore, we downregulated Slug using siRNA, which was previously demonstrated to downregulate the expression of Slug in HSC-4 cells at both the mRNA and protein level (38). We also confirmed the efficient downregulation of Slug in this study (data not shown). The TGF-β1-induced expression of MMP-10 was significantly downregulated in HSC-4 cells by administration of Slug siRNA as compared with that of control siRNA at the mRNA (Fig. 3A) and protein (Fig. 3B) levels. Intriguingly, Slug siRNA significantly suppressed the TGF-β1-induced invasion ability of HSC-4 cells, whereas control siRNA did not (Fig. 3C). These results indicate that TGF-β1 stimulates MMP-10 expression in HSC-4 cells in a Slug-dependent manner.
Taking into account our observations in Figs 1 and 2, we propose that Slug-dependent induction of MMP-10 expression by TGF-β1 plays an important role in the regulation of the invasion ability of HSC-4 cells.
TGF-β1-induced MMP-10 expression seems to be mediated through non-canonical Wnt signalling activated by Wnt-5b
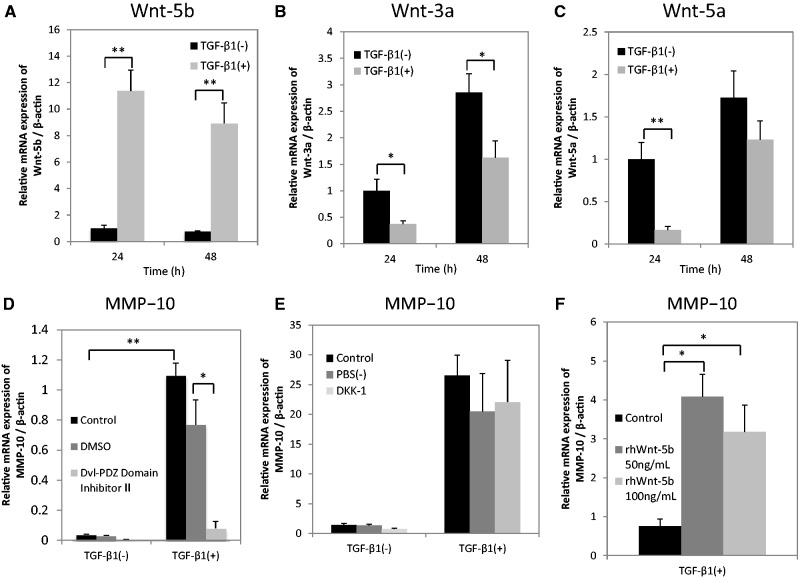
In order to investigate whether TGF-β1 affects canonical or non-canonical Wnt signalling in HSC-4 cells, we evaluated the mRNA expression levels of different players in the canonical (Wnt-3a) and non-canonical (Wnt-5a and -5b) pathway after TGF-β1 stimulation. TGF-β1 significantly upregulated Wnt-5b expression (Fig. 4A), but did not affect Wnt-3a (Fig. 4B) and Wnt-5a (Fig. 4C) expression. In addition, a universal Wnt signal inhibitor, Dvl-PDZ domain inhibitor II, significantly suppressed the TGF-β1-induced expression of MMP-10 mRNA (Fig. 4D). On the other hand, DKK-1 inhibitor, a protein that binds co-receptor LRP5/6 and specifically inhibits canonical Wnt signalling, did not affect the expression levels of MMP-10 in HSC-4 cells stimulated with TGF-β1 (Fig. 4E). We also confirmed that recombinant human Wnt-5b (rhWnt-5b) significantly upregulated the expression of MMP-10 in HSC-4 cells (Fig. 4F), as previously described (24).
Fig. 4.
TGF-β1-induced MMP-10 expression seems to be mediated through non-canonical Wnt signalling possibly activated by Wnt-5b. (A–C) HSC-4 cells with (grey bars) or without (black bars) stimulation with 10 ng/ml TGF-β1 for 24 or 48 h in serum-free medium were analysed by qRT-PCR. The expression levels of (A) Wnt-5b, (B) Wnt-3a and (C) Wnt-5a were examined. Data are represented as the mean ± SD of three wells for each time point (*P < 0.05; **P < 0.01). HSC-4 cells with or without a 48-h stimulation using 10 ng/ml TGF-β1 were treated with (D) Dvl-PDZ Domain Inhibitor II (10 µM) or (E) DKK-1 (10 µg/ml) 60 min prior to TGF-β1 treatment. The expression levels of MMP-10 were examined by qRT-PCR. Data are represented as the mean ± SD of three wells for each time point (*P < 0.05; **P < 0.01). (F) HSC-4 cells were stimulated with 0, 50, or 100 ng/ml rhWnt-5b for 24 h in serum-free medium. The expression levels of MMP-10 were assessed by qRT-PCR. Data are represented as the mean ± SD of three wells for each time point (*P < 0.05).
These data suggest that non-canonical Wnt signalling, possibly activated by Wnt-5b, mediates the induction of MMP-10 expression by TGF-β1.
TGF-β1 induces Wnt-5b expression through Slug, which increases the invasiveness of HSC-4 cells
The TGF-β1-induced expression of Wnt-5b was significantly downregulated by Slug siRNA in HSC-4 cells at both the mRNA (Fig. 5A) and protein (Fig. 5B) levels. These results suggest that TGF-β1 induces the expression of Wnt-5b in a Slug-dependent manner. In addition, HSC-4 cells in which Wnt-5b was downregulated by siWnt-5b (Fig. 5C) exhibited a decrease in invasion ability in our Boyden chamber assay upon TGF-β1 treatment (Fig. 5D).
Time course of the mRNA expressions of Slug, Wnt-5b and MMP-10 after TGF-β1 stimulation in HSC-4 cells
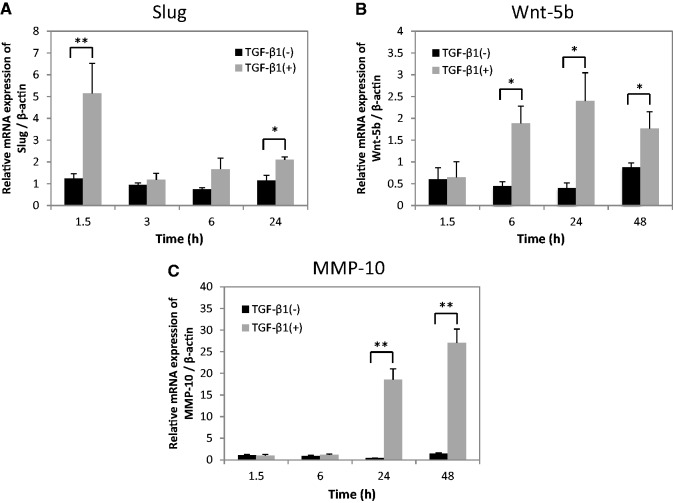
We found that the time-dependent mRNA expressions of genes related to tumour invasion in the order of Slug, Wnt-5b and MMP-10 after TGF-β1 stimulation using with qRT-PCR (Fig. 6). Namely, at first, Slug expression level was significantly upregulated at 1.5 h after TGF-β1 stimulation in HSC-4 cells (Fig. 6A). Secondly, the expression of Wnt-5b was significantly upregulated from 6 to 24 h after TGF-β1 stimulation (Fig. 6B). Finally, MMP-10 was significantly upregulated from 24 to 48 h after TGF-β1 stimulation (Fig. 6C).
Fig. 6.
Time course of the expressions of Slug, Wnt-5b and MMP-10 after TGF-β1 stimulation in HSC-4 cells. HSC-4 cells with (grey bars) or without (black bars) stimulation with 10 ng/ml TGF-β1 for various periods up to 48 h in serum-free medium were analysed by qRT-PCR. The mRNA expression levels of (A) Slug, (B) Wnt-5b and (C) MMP-10 were examined and normalized to β-actin. Data are represented as the mean ± SD of four wells for each time point (*P < 0.05; **P < 0.01).
Discussion
Our data support a model in which the transcription-related factor Slug induces Wnt-5b expression upon TGF-β1 stimulation (Figs 4A, 5B and 6). Wnt-5b in turn activates the non-canonical Wnt pathway in HSC-4 cells through autocrine and/or paracrine signalling, and subsequently upregulates MMP-10 expression (Figs 4 and 6). In summary, we propose that the expression level of MMP-10 is increased by TGF-β1 treatment in HSC-4 cells through a Slug/Wnt-5b signalling axis. On the other hand, the invasiveness of HSC-4 cells was dependent on the expression level of MMP-10, as downregulation of MMP-10 clearly decreased invasion in HSC-4 cells (Fig. 2C). In addition, Wnt-5b siRNA downregulated the TGF-β1-induced invasion ability of HSC-4 cells (Fig. 5D), suggesting that TGF-β1 indirectly affects HSC-4 cell invasion through the expression of Wnt-5b and MMP-10. Thus, TGF-β1 stimulates the invasion ability of HSC-4 cells through the Slug/Wnt-5b/MMP-10 signalling axis.
Slug is an important transcription factor in EMT, as it regulates the expression of EMT-related genes (14, 39). We actually showed that Slug induced the expression of vimentin, but not of N-cadherin, during EMT in HSC-4 cells (38). It has not been elucidated whether Slug regulates the expression of MMP-10 and Wnt-5b in hOSCC cells. In this article, we demonstrate for the first time that Slug stimulates the expression of both MMP-10 (Fig. 3A and B) and Wnt-5b (Fig. 5A and B) in HSC-4 cells.
There are a few reports that have shown the upregulation of Wnt-5b expression in tumour cells stimulated by TGF-β1, for example in human pituitary tumour cells (40); however, the function of Wnt-5b in cancer remains poorly understood. In contrast, the function of Wnt-5a in cancer has been relatively well investigated (reviewed in Ref. 41). Several reports correlate increased Wnt-5a expression with decreased patient survival and increased invasion ability in melanoma, gastric, ovarian and colorectal cancer (41, 42). In addition, Kumawat et al. (36) reported that TGF-β1 induced the expression of Wnt-5a, but not Wnt-5b, in airway smooth muscle cells.
There are also a few reports that demonstrate the upregulation of MMP-10 expression during TGF-β1-induced EMT (43, 44). TGF-β1 plus EGF stimulation induces expression of MMP-10 and MMP-1 in HaCaT II-4 keratinocytes and promotes the invasion ability of cells growing on collagen type I gels (43), presumably through enhanced collagen degradation. Another report showed that the expression of MMP-10 is upregulated by TGF-β through myocyte enhancer factor 2A in mouse and human mammary epithelial cells (NMuMG and MCF10A, respectively) (44).
It was previously reported that MMP-10 induces tumour progression and invasion in cervical tumours (45) and in hOSCCs (46). We compared the invasiveness of three cell lines derived from hOSCCs (HSC-2, HSC-4 and SAS) after TGF-β1 stimulation and found that TGF-β1 promoted the invasion in HSC-4 cells and SAS cells, but not in HSC-2 cells (data not shown). In addition, as shown in Fig. 1D, MMP-10 expression in HSC-4 cells was significantly induced by TGF-β1, whereas in HSC-2, HSC-3 and SAS cells, this was not the case. These results suggest that other factors besides MMP-10 also play important roles in the TGF-β1-induced increase in invasion in hOSCC cells.
As MMP-2 and MMP-9 are also involved in invasion and metastasis (18–20), we evaluated expression of other MMPs besides MMP-1 and MMP-10 in HSC-4 cells. MMP-2 and MMP-9 expression levels were significantly upregulated 24 and 48 h after TGF-β1 stimulation (Supplementary Fig. S1A and B). Therefore, there is a possibility that MMP-2 and MMP-9 cooperatively promote invasion activity induced by MMP-10. However, MMP-14 expression levels were not changed in HSC-4 cells upon TGF-β1 treatment (data not shown). The roles of MMP-1, MMP-2 and MMP-9 in the invasion ability of TGF-β1-stmimulated HSC-4 cells should be elucidated in future studies.
The protein levels of MMP-10, as a secreted form in the medium used for HSC-4 cell culture and as a non-secreted form in the cell lysates, were evaluated by western blot. TGF-β1 treatment induced a remarkable upregulation of MMP-10 protein expression and almost all protein was secreted into the medium (Supplementary Fig. S1C). MMP-1 protein levels were lower than that of MMP-10 (Fig. 1A), suggesting that MMP-10 is a major MMP in TGF-β1-stimulated HSC-4. Intriguingly, the expression levels of tissue inhibitor of metalloproteinase (TIMP)1 and TIMP2, two MMP inhibitors, were significantly downregulated 24 h after TGF-β1 stimulation (data not shown). This suggests that the TGF-β1-induced increase in invasion in HSC-4 cells is reciprocally controlled by the upregulation of MMPs and the downregulation of TIMPs.
Although it was previously suggested that increased MMP-10 expression in hOSCC depends on the pathologic processes and the invasion abilities of tumours (46), our findings show that the invasion ability of the cells also depends on the expression level of MMP-10. A specific MMP-10 inhibitor could be valuable as a research tool and for clinical applications in hOSCC. However, to date, a specific and useful inhibitor of MMP-10 has not been developed. Our observations on the molecular mechanisms that regulate invasion in HSC-4 cells led to the identification of a Slug/Wnt-5b/MMP-10 signalling axis, which can aid in finding novel therapeutic molecular targets to inhibit invasion of cancer cells.
Supplementary Data
Supplementary data are available at JB Online.
Funding
This study was supported in part by Grand-in-Aid for Scientific Research (Grant nos 26293426 to T.S., 22592076 to M.K., 26462823 to S.K. and 26670852 to A.I.) and by a Grant-in-Aid for the Strategic Medical Science Research Centre from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, 2010–14.
Conflict of Interest
None declared.
Supplementary Material
Glossary
Abbreviations
- DMEM
Dulbecco’s modified Eagle’s medium
- Dvl
dishevelled
- ECM
extracellular matrix
- EMT
epithelial–mesenchymal transition
- FBS
foetal bovine serum
- FDZ
Frizzled
- hOSCC
human oral squamous cell carcinoma
- LRP
low-density lipoprotein receptor-related protein
- MEM
Eagle’s minimum essential medium
- MMP
matrix metalloproteinase
- MS
Mass spectrometry
- PKC
Protein kinase C
- qRT-PCR
quantitative real-time reverse transcriptase polymerase chain reaction
- RIPA
radio-immunoprecipitation assay
- R-Smad
receptor-regulated Smad
- SD
standard deviation
- siRNA
small interfering RNA
- TGF-β
transforming growth factor-β
- TβR
transforming growth factor-β receptor
- TβR-I
TGF-β receptor type I
- TβR-II
TGF-β receptor type II
- TIMP
tissue inhibitor of metalloproteinase
References
- 1.Lambert R., Sauvaget C., de Camargo Cancela M., Sankaranarayanan R. (2011) Epidemiology of cancer from the oral cavity and oropharynx. Eur. J. Gastroenterol. Hepatol. 23, 633–641 [DOI] [PubMed] [Google Scholar]
- 2.Graves C.A., Abboodi F.F., Tomar S., Wells J., Pirisi L. (2014) The translational significance of epithelial-mesenchymal transition in head and neck cancer. Clin. Transl. Med. 3, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith A., Teknos T.N., Pan Q. (2013) Epithelial to mesenchymal transition in head and neck squamous cell carcinoma. Oral. Oncol. 49, 287–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung C.H., Parker J.S., Ely K., Carter J., Yi Y., Murphy B.A., Ang K.K., El-Naggar A.K., Zanation A.M., Cmelak A.J., Levy S., Slebos R.J., Yarbrough W.G. (2006) Gene expression profiles identify epithelial-to-mesenchymal transition and activation of nuclear factor-kappaB signaling as characteristics of a high-risk head and neck squamous cell carcinoma. Cancer Res. 66, 8210–8218 [DOI] [PubMed] [Google Scholar]
- 5.Waldmeier L., Meyer-Schaller N., Diepenbruck M., Christofori G. (2012) Py2T murine breast cancer cells, a versatile model of TGFβ-induced EMT in vitro and in vivo. PLoS One 7, e48651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Micalizzi D.S., Farabaugh S.M., Ford H.L. (2010) Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J. Mammary Gland Biol. Neoplasia 15, 117–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jing Y., Han Z., Zhang S., Liu Y., Wei L. (2011) Epithelial-mesenchymal transition in tumor microenvironment. Cell Biosci. 1, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mu Y., Gudey S.K., Landström M. (2012) Non-Smad signaling pathways. Cell Tissue Res. 347, 11–20 [DOI] [PubMed] [Google Scholar]
- 9.Miyazawa K., Shinozaki M., Hara T., Furuya T., Miyazono K. (2002) Two major Smad pathways in TGF-beta superfamily signalling. Genes Cells 7, 1191–1204 [DOI] [PubMed] [Google Scholar]
- 10.Saitoh M. (2015) Epithelial-mesenchymal transition is regulated at post-transcriptional levels by transforming growth factor-β signaling during tumor progression. Cancer Sci. 106, 481–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saitoh M., Miyazawa K. (2012) Transcriptional and post-transcriptional regulation in TGF-β-mediated epithelial-mesenchymal transition. J. Biochem. 151, 563–571 [DOI] [PubMed] [Google Scholar]
- 12.Papageorgis P. (2015) TGFβ signaling in tumor initiation, epithelial-to-mesenchymal transition, and metastasis. J. Oncol. 2015, 587193–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batlle E., Sancho E., Franci C., Dominguez D., Monfar M., Baulida J., Garcia De Herreros A. (2000) The transcription factor Snail is a repressor of E-cadherin gene expression in the epithelial tumour cells. Nat. Cell Biol. 2, 84–89 [DOI] [PubMed] [Google Scholar]
- 14.Medici D., Hay E.D., Olsen B.R. (2008) Snail and Slug promote epithelial-mesenchymal transition through beta-catenin-T-cell factor-4-dependent expression of transforming growth factor-beta3. Mol. Biol. Cell 19, 4875–4887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeisberg M., Neilson E.G. (2009) Biomarkers for epithelial-mesenchymal transitions. J. Clin. Invest. 119, 1429–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peinado H., Olmeda D., Cano A. (2007) Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat . Rev. Cancer 7, 415–428 [DOI] [PubMed] [Google Scholar]
- 17.Ungefroren H., Sebens S., Seidl D., Lehnert H., Hass R. (2011) Interaction of tumor cells with the microenvironment. Cell Commun. Signal 9, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duffy M.J., Maguire T.M., Hill A., McDermott E., O’Higgins N. (2000) Metalloproteinases: role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res. 2, 252–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chien M.-H., Lin C.-W., Cheng C.-W., Wen Y.-C., Yang S.-F. (2013) Matrix metalloproteinase-2 as a target for head and neck cancer therapy. Expert Opin. Ther. Targets 17, 203–216 [DOI] [PubMed] [Google Scholar]
- 20.Lamar J.M., Iyer V., DiPersio C.M. (2008) Integrin alpha3beta1 potentiates TGFbeta-mediated induction of MMP-9 in immortalized keratinocytes. J. Invest. Dermatol. 128, 575–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madlener M., Mauch C., Conca W., Brauchle M., Parks W.C., Werner S. (1996) Regulation of the expression of stromelysin-2 by growth factors in keratinocytes: implications for normal and impaired wound healing. Biochem. J. 320, 659–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim H.-S., Shang T., Chen Z., Pflugfelder S.C., Li D.-Q. (2004) TGF-beta1 stimulates production of gelatinase (MMP-9), collagenases (MMP-1, -13) and stromelysins (MMP-3, -10, -11) by human corneal epithelial cells. Exp. Eye Res. 79, 263–274 [DOI] [PubMed] [Google Scholar]
- 23.Nakamura H., Fujii Y., Ohuchi E., Yamamoto E., Okada Y. (1998) Activation of the precursor of human stromelysin 2 and its interactions with other matrix metalloproteinases. Eur. J. Biochem. 253, 67–75 [DOI] [PubMed] [Google Scholar]
- 24.Deraz E.M., Kudo Y., Yoshida M., Obayashi M., Tsunematsu T., Tani H., Siriwardena S.B.S.M., Kiekhaee M.R., Qi G., Iizuka S., Ogawa I., Campisi G., Lo Muzio L., Abiko Y., Kikuchi A., Takata T. (2011) MMP-10/stromelysin-2 promotes invasion of head and neck cancer. PLoS One 6, e2543857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Logan C.Y., Nusse R. (2004) The WNT signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20, 781–810 [DOI] [PubMed] [Google Scholar]
- 26.Clevers H. (2006) Wnt/beta-catenin signaling in development and disease. Cell 127, 469–480 [DOI] [PubMed] [Google Scholar]
- 27.Kim W., Kim M., Jho E.-H. (2013) WNT/β-catenin signalling: from plasma membrane to nucleus. Biochem. J. 450, 9–21 [DOI] [PubMed] [Google Scholar]
- 28.Angers S., Moon R.T. (2009) Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell Biol. 10, 468–477 [DOI] [PubMed] [Google Scholar]
- 29.Peradziryi H., Kaplan N.A., Podleschny M., Liu X., Wehner P., Borchers A., Tolwinski N.S. (2011) PTK7/Otk interacts with WNTS and inhibits canonical Wnt signalling. EMBO J. 30, 3729–3740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kikuchi A., Yamamoto H., Sato A., Matsumoto S. (2011) WNT5A: its signalling, functions and implication in diseases. Acta Physiol. 204,17–33 [DOI] [PubMed] [Google Scholar]
- 31.Kim K., Lu Z., Hay E.D. (2002) Direct evidence for a role of beta-catenin/LEF-1 signaling pathway in induction of EMT. Cell Biol. Int. 26, 463–476 [DOI] [PubMed] [Google Scholar]
- 32.Chilosi M., Poletti V., Zamò A., Lestani M., Montagna L., Piccoli P., Pedron S., Bertaso M., Scarpa A., Murer B., Cancellieri A., Maestro R., Semenzato G., Dogurioni C. (2003) Aberrant Wnt/beta-catenin pathway activation in idiopathic pulmonary fibrosis. Am. J. Pathol. 162, 1495–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaw S.Y., Abdul Majeed A., Dalley A.J., Chan A., Stein S., Farah C.S. (2012) Epithelial to mesenchymal transition (EMT) biomarkers–E-cadherin, beta-catenin, APC and vimentin–in oral squamous cell carcinogenesis and transformation. Oral Oncol. 48, 997–1006 [DOI] [PubMed] [Google Scholar]
- 34.Nelson W.J., Nusse R. (2004) Convergence of Wnt, beta-catenin, and cadherin pathways. Science 303, 1483–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uraguchi M., Morikawa M., Shirakawa M., Sanada K., Imai K. (2004) Activation of WNT family expression and signaling in squamous cell carcinomas of the oral cavity. J. Dent. Res. 83, 327–332 [DOI] [PubMed] [Google Scholar]
- 36.Kumawat K., Menzen M.H., Bos I. S.T., Baarsma H.A., Borger P., Roth M., Tamm M., Halayko A.J., Simoons M., Prins A., Postma D.S., Schmidt M., Gosens R. (2013) Noncanonical WNT-5A signaling regulates TGF-β-induced extracellular matrix production by airway smooth muscle cells. FASEB J. 27, 1631–1643 [DOI] [PubMed] [Google Scholar]
- 37.Komiya Y., Habas R. (2008) Wnt signal transduction pathways. Organogenesis 4, 68–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saito D., Kyakumoto S., Chosa N., Ibi M., Takahashi N., Okubo N., Sawada S., Ishisaki A., Kamo M. (2013) Transforming growth factor-β1 induces epithelial-mesenchymal transition and integrin α3β1-mediated cell migration of HSC-4 human squamous cell carcinoma cells through Slug. J. Biochem. 153, 303–315 [DOI] [PubMed] [Google Scholar]
- 39.Lamouille S., Xu J., Derynck R. (2014) Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 15, 178–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruebel K.H., Leontovich A.A., Tanizaki Y., Jin L., Stilling G.A., Zhang S., Coonse K., Scheithauer B.W., Lombardero M., Kovacs K., Lloyd R.V. (2008) Effects of TGFβ1 on gene expression in the HP75 human pituitary tumor cell line identified by gene expression profiling. Endocrine 33 62–76 [DOI] [PubMed] [Google Scholar]
- 41.Anastas J.N., Moon R.T. (2013) WNT signalling pathways as therapeutic targets in cancer. Nat. Rev. Cancer 13, 11–26 [DOI] [PubMed] [Google Scholar]
- 42.Weeraratna A.T., Jiang Y., Hostetter G., Rosenblatt K., Duray P., Bittner M., Trent J. M. (2002) Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell 1, 279–288 [DOI] [PubMed] [Google Scholar]
- 43.Wilkins-Port C.E., Ye Q., Mazurkiewicz J.E., Higgins P.J. (2009) TGF-beta1 + EGF-initiated invasive potential in transformed human keratinocytes is coupled to a plasmin/MMP-10/MMP-1-dependent collagen remodeling axis: role for PAI-1. Cancer Res. 69, 4081–4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ishikawa F., Miyoshi H., Nose K., Shibanuma M. (2010) Transcriptional induction of MMP-10 by TGF-β, mediated by activation of MEF2A and downregulation of class IIa HDACs. Oncogene 29, 909–919 [DOI] [PubMed] [Google Scholar]
- 45.Zhang G., Miyake M., Lawton A., Goodison S., Rosser C.J. (2014) Matrix metalloproteinase-10 promotes tumor progression through regulation of angiogenic and apoptotic pathways in cervical tumors. BMC Cancer 14, 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mashhadiabbas F., Mahjour F., Mahjour S.B., Fereidooni F., Hosseini F.S. (2012) The immunohistochemical characterization of MMP-2, MMP-10, TIMP-1, TIMP-2, and podoplanin in oral squamous cell carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 114, 240–250 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.