Abstract
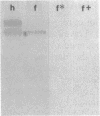
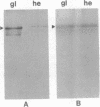
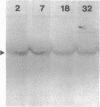
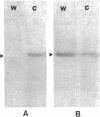
The rhizoid cell of the two-celled embryo of the brown alga Fucus is structurally and functionally differentiated from the thallus cell. The rhizoid cell is highly polar and transports directionally components of the cell wall to its elongating tip, which attaches the developing embryo to the substratum. Polyclonal antibodies to human vitronectin (Vn) recognize a vitronectin-like glycoprotein (Vn-F) in extracts of zygotes and two-celled embryos of Fucus, with a molecular mass (approximately 62 kDa) similar to that of human Vn. The specificity of the immunological cross-reactivity of Vn-F to rabbit polyclonal antibodies made to human Vn is demonstrated by competition experiments using pure human Vn and monospecific antibodies generated toward Vn-F. Vn-F possesses affinities for glass and heparin that are identical to those of human Vn. Immunolocalization and subcellular fractionation results demonstrate that Vn-F is localized first in the cytoplasm of the zygote, which is followed by the polar transport of Vn-F to its exclusive localization in the cell wall of the elongating rhizoid tip. Vn does not localize to the rhizoid tip under culture conditions that prevent two-celled embryos from attaching. Furthermore, an adhesion assay demonstrates that two-celled Fucus embryos do not adhere to the substratum in the presence of the Vn antibody, suggesting that the Vn-F in this brown alga not only possesses structural similarity to mammalian Vn but may also have a similar functional role in adhesion. The presence of Vn-F in brown algae suggests a high degree of evolutionary conservation of its structural and functional characteristics.
Full text
PDF


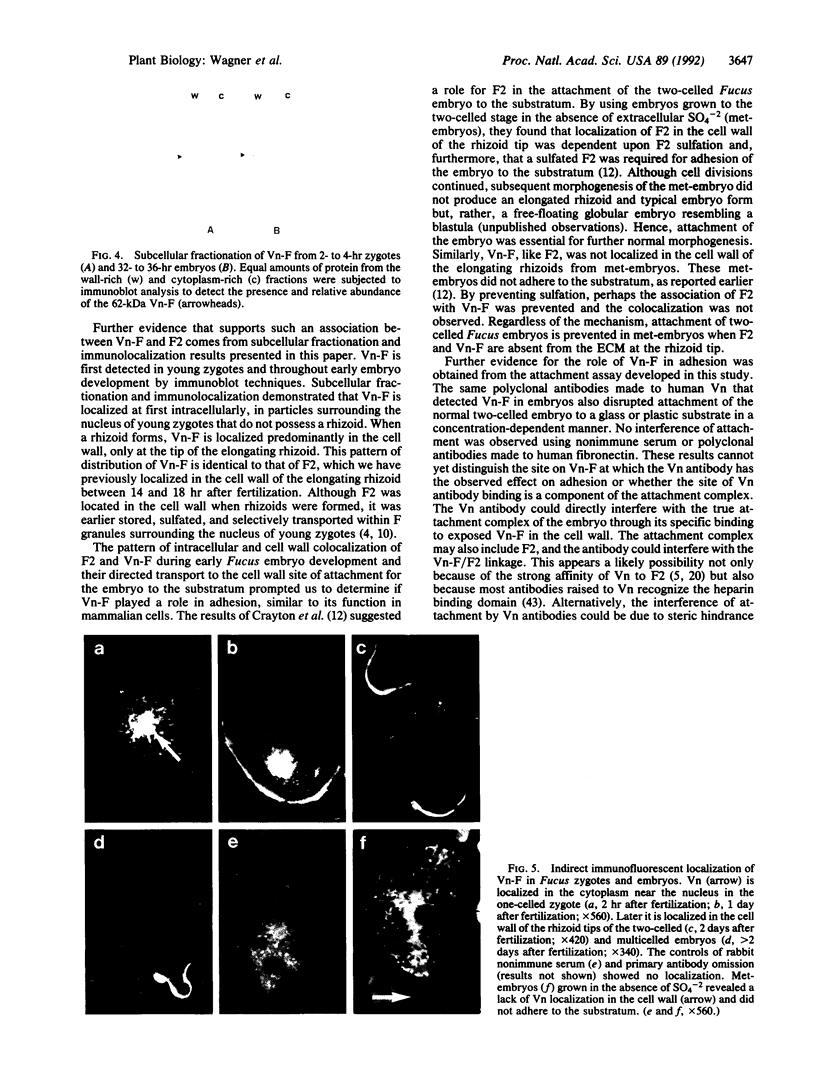

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akama T., Yamada K. M., Seno N., Matsumoto I., Kono I., Kashiwagi H., Funaki T., Hayashi M. Immunological characterization of human vitronectin and its binding to glycosaminoglycans. J Biochem. 1986 Nov;100(5):1343–1351. doi: 10.1093/oxfordjournals.jbchem.a121840. [DOI] [PubMed] [Google Scholar]
- Brawley S. H., Quatrano R. S. Sulfation of fucoidin in Fucus embryos. IV. Autoradiographic investigations of fucoidin sulfation and secretion during differentiation and the effect of cytochalasin treatment. Dev Biol. 1979 Dec;73(2):193–205. doi: 10.1016/0012-1606(79)90063-0. [DOI] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Callow M. E., Coughlan S. J., Evans L. V. The role of Golgi bodies in polysaccharide sulphation in Fucuszygotes. J Cell Sci. 1978 Aug;32:337–356. doi: 10.1242/jcs.32.1.337. [DOI] [PubMed] [Google Scholar]
- Crayton M. A., Wilson E., Quatrano R. S. Sulfation of fucoidan in Fucus embryos. II. Separation from initiation of polar growth. Dev Biol. 1974 Jul;39(1):164–167. doi: 10.1016/s0012-1606(74)80018-7. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion molecules in the regulation of animal form and tissue pattern. Annu Rev Cell Biol. 1986;2:81–116. doi: 10.1146/annurev.cb.02.110186.000501. [DOI] [PubMed] [Google Scholar]
- Giloh H., Sedat J. W. Fluorescence microscopy: reduced photobleaching of rhodamine and fluorescein protein conjugates by n-propyl gallate. Science. 1982 Sep 24;217(4566):1252–1255. doi: 10.1126/science.7112126. [DOI] [PubMed] [Google Scholar]
- Hayman E. G., Pierschbacher M. D., Suzuki S., Ruoslahti E. Vitronectin--a major cell attachment-promoting protein in fetal bovine serum. Exp Cell Res. 1985 Oct;160(2):245–258. doi: 10.1016/0014-4827(85)90173-9. [DOI] [PubMed] [Google Scholar]
- Hogsett W. E., Quatrano R. S. Sulfation of fucoidin in Fucus embryos. III. Required for localization in the rhizoid wall. J Cell Biol. 1978 Sep;78(3):866–873. doi: 10.1083/jcb.78.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe L. F. Localization in the developing Fucus egg and the general role of localizing currents. Adv Morphog. 1968;7:295–328. doi: 10.1016/b978-1-4831-9954-2.50012-4. [DOI] [PubMed] [Google Scholar]
- Jamrich M., Mahon K. A., Gavis E. R., Gall J. G. Histone RNA in amphibian oocytes visualized by in situ hybridization to methacrylate-embedded tissue sections. EMBO J. 1984 Sep;3(9):1939–1943. doi: 10.1002/j.1460-2075.1984.tb02073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenne D., Stanley K. K. Nucleotide sequence and organization of the human S-protein gene: repeating peptide motifs in the "pexin" family and a model for their evolution. Biochemistry. 1987 Oct 20;26(21):6735–6742. doi: 10.1021/bi00395a024. [DOI] [PubMed] [Google Scholar]
- Kitagaki-Ogawa H., Yatohgo T., Izumi M., Hayashi M., Kashiwagi H., Matsumoto I., Seno N. Diversities in animal vitronectins. Differences in molecular weight, immunoreactivity and carbohydrate chains. Biochim Biophys Acta. 1990 Jan 29;1033(1):49–56. doi: 10.1016/0304-4165(90)90193-z. [DOI] [PubMed] [Google Scholar]
- Kropf D. L., Hopkins R., Quatrano R. S. Protein synthesis and morphogenesis are not tightly linked during embryogenesis in Fucus. Dev Biol. 1989 Aug;134(2):451–461. doi: 10.1016/0012-1606(89)90118-8. [DOI] [PubMed] [Google Scholar]
- Marcantonio E. E., Hynes R. O. Antibodies to the conserved cytoplasmic domain of the integrin beta 1 subunit react with proteins in vertebrates, invertebrates, and fungi. J Cell Biol. 1988 May;106(5):1765–1772. doi: 10.1083/jcb.106.5.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki K., Hamano T., Hayashi M. Physarum vitronectin-like protein: an Arg-Gly-Asp-dependent cell-spreading protein with a distinct NH2-terminal sequence. Exp Cell Res. 1992 Mar;199(1):106–110. doi: 10.1016/0014-4827(92)90467-m. [DOI] [PubMed] [Google Scholar]
- Preissner K. T. Structure and biological role of vitronectin. Annu Rev Cell Biol. 1991;7:275–310. doi: 10.1146/annurev.cb.07.110191.001423. [DOI] [PubMed] [Google Scholar]
- Quatrano R. S., Brian L., Aldridge J., Schultz T. Polar axis fixation in Fucus zygotes: components of the cytoskeleton and extracellular matrix. Dev Suppl. 1991;1:11–16. [PubMed] [Google Scholar]
- Quatrano R. S., Crayton M. A. Sulfation of fucoidan in Fucus embryos. I. Possible role in localization. Dev Biol. 1973 Jan;30(1):29–41. doi: 10.1016/0012-1606(73)90045-6. [DOI] [PubMed] [Google Scholar]
- Quatrano R. S., Griffing L. R., Huber-Walchli V., Doubet R. S. Cytological and biochemical requirements for the establishment of a polar cell. J Cell Sci Suppl. 1985;2:129–141. doi: 10.1242/jcs.1985.supplement_2.7. [DOI] [PubMed] [Google Scholar]
- Quatrano R. S., Stevens P. T. Cell wall assembly in fucus zygotes: I. Characterization of the polysaccharide components. Plant Physiol. 1976 Aug;58(2):224–231. doi: 10.1104/pp.58.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts K. Structures at the plant cell surface. Curr Opin Cell Biol. 1990 Oct;2(5):920–928. doi: 10.1016/0955-0674(90)90093-t. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Suzuki S., Hayman E. G., Ill C. R., Pierschbacher M. D. Purification and characterization of vitronectin. Methods Enzymol. 1987;144:430–437. doi: 10.1016/0076-6879(87)44192-x. [DOI] [PubMed] [Google Scholar]
- Sanders L. C., Wang C. S., Walling L. L., Lord E. M. A Homolog of the Substrate Adhesion Molecule Vitronectin Occurs in Four Species of Flowering Plants. Plant Cell. 1991 Jun;3(6):629–635. doi: 10.1105/tpc.3.6.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Schindler M., Meiners S., Cheresh D. A. RGD-dependent linkage between plant cell wall and plasma membrane: consequences for growth. J Cell Biol. 1989 May;108(5):1955–1965. doi: 10.1083/jcb.108.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiffert D., Keeton M., Eguchi Y., Sawdey M., Loskutoff D. J. Detection of vitronectin mRNA in tissues and cells of the mouse. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9402–9406. doi: 10.1073/pnas.88.21.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Oldberg A., Hayman E. G., Pierschbacher M. D., Ruoslahti E. Complete amino acid sequence of human vitronectin deduced from cDNA. Similarity of cell attachment sites in vitronectin and fibronectin. EMBO J. 1985 Oct;4(10):2519–2524. doi: 10.1002/j.1460-2075.1985.tb03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasini B. R., Mosher D. F. Conformational states of vitronectin: preferential expression of an antigenic epitope when vitronectin is covalently and noncovalently complexed with thrombin-antithrombin III or treated with urea. Blood. 1988 Sep;72(3):903–912. [PubMed] [Google Scholar]
- Tomasini B. R., Mosher D. F. Vitronectin. Prog Hemost Thromb. 1991;10:269–305. [PubMed] [Google Scholar]
- Underwood P. A., Bennett F. A. A comparison of the biological activities of the cell-adhesive proteins vitronectin and fibronectin. J Cell Sci. 1989 Aug;93(Pt 4):641–649. doi: 10.1242/jcs.93.4.641. [DOI] [PubMed] [Google Scholar]
- Yatohgo T., Izumi M., Kashiwagi H., Hayashi M. Novel purification of vitronectin from human plasma by heparin affinity chromatography. Cell Struct Funct. 1988 Aug;13(4):281–292. doi: 10.1247/csf.13.281. [DOI] [PubMed] [Google Scholar]