Abstract
Conessine, a steroidal alkaloid isolated from Holarrhena floribunda, has anti-malarial activity and interacts with the histamine H3 receptor. However, the cellular effects of conessine are poorly understood. Accordingly, we evaluated the involvement of conessine in the regulation of autophagy. We searched natural compounds that modulate autophagy, and conessine was identified as an inhibitor of autophagic flux. Conessine treatment induced the formation of autophagosomes, and p62, an autophagic adapter, accumulated in the autophagosomes. Reactive oxygen species such as hydrogen peroxide (H2O2) result in muscle cell death by inducing excessive autophagic flux. Treatment with conessine inhibited H2O2-induced autophagic flux in C2C12 myoblast cells and also interfered with cell death. Our results indicate that conessine has the potential effect to inhibit muscle cell death by interfering with autophagic flux.
Introduction
Autophagy (specifically, macroautophagy), a catabolic pathway responsible for degrading protein aggregates and organelles [1], can be induced by extracellular stress (e.g., nutrient starvation, hypoxia, high temperature, and microgravity) [2, 3]. The dynamic process of autophagy, including the conversion of autophagosomes into autolysosomes, is termed autophagic flux, and both the activation and inhibition of autophagic flux result in an increase of autophagosomes in the cytoplasm [4]. The relationship between autophagy and diseases is not clear, however excessive autophagic flux or lack of autophagy can contribute to various diseases such as cancer and neurodegenerative diseases [5–7].
Reactive oxygen species are produced in the mitochondria, and several antioxidant enzymes such as catalase and hydrogen peroxidase are responsible for their removal. Treatment with hydrogen peroxide (H2O2) can cause oxidative damage and induce autophagy and autophagic cell death under certain conditions [8]. Silencing experiments on autophagy-related genes show that autophagy is involved in ROS-induced cell death [9, 10]. Accordingly, compounds that interfere with excessive autophagic flux may affect muscle viability.
Conessine is a steroidal alkaloid isolated from the bark of Holarrhena floribunda [11]. Recent research has shown that conessine is a potent histamine H3 receptor antagonist by selectively interacting with the histamine H3 receptor, and there have been several attempts to develop new histamine H3 receptor antagonists based on the structure of conessine [12, 13]. Although conessine is also reported to have anti-malarial activity [14, 15], the effect of conessine on cell signaling has not been studied in detail.
We screened autophagy-modulating agents and identified conessine as a negative modulator of autophagy. We found that conessine treatment inhibited autophagic flux and increased the level of p62. Conessine treatment interfered with H2O2-induced C2C12 cell death. These results suggest that regulation of autophagy by conessine may help prevent muscle cell death.
Materials and Methods
Cell culture and cell proliferation assay
HEK293, MCF7 and C2C12 cells were grown in Dulbecco’s Modified Eagle’s medium (DMEM; Welgene, Korea) supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY, USA). A HEK293 stable cell line expressing GFP-LC3 was generated as described previously using a GFP-LC3 plasmid [16]. Transfection of HEK293 and C2C12 cells was performed using lipofectamine (Invitrogen, Carlsbad, CA, USA). Cell proliferation was measured using the [4,5-dimethylthiazol-2-yl]-2,5-diphenyltrazolium bromide (MTT) assay. Briefly, cells were seeded in a 24-well plate and pretreated with conessine for 24 h. Cells were treated with H2O2 for 24 h and cell proliferation was examined using the MTT assay. Conessine was obtained from the Korea Bioactive Natural Material Bank (KBNMB) and bafilomycin A1 were purchased from Sigma-Aldrich (St. Louis, MO, USA). For cell cycle analysis, transfected cells were washed with phosphate buffered saline (PBS) and fixed with 70% ethanol. After centrifugation, cells were washed and resuspended in PBS containing 0.25 mg/ml propidium iodide (PI) and 10 mg/ml RNase A (Sigma, St. Louis, MO, USA). Cells were analyzed on a FACSCalibur flow cytometer (Beckton-Dickinson, Mountain View, CA, USA). At least 10,000 events per sample were analyzed.
Western blotting
For protein immunoblot analysis, polypeptides in whole cell lysates were resolved by SDS-PAGE and transferred to nitrocellulose membrane filters. Detection was conducted with a 1:2,000 or 1:5,000 dilution of primary antibody using an enhanced chemiluminescence (ECL) system. Images were acquired using a Chemidoc-it 410 imaging system (UVP, Upland, CA, USA) and LAS4000 system (GE Healthcare, Uppsala, Sweden). The antibody for LC3 was purchased from Novus Biological (Littleton, CO, USA), and the antibody for p62 was from Sigma-Aldrich.
Immunofluorescence and confocal microscopy
Cells were grown on sterilized glass coverslips. After drug treatment, the cells were fixed with 4% paraformaldehyde. For immunostaining, cells were blocked with 10% bovine serum albumin in PBS and stained with a 1:1,000 dilution of primary antibody in PBS, and then reacted with a 1:1,000 dilution of Alexa 488- or Alexa 568-conjugated secondary antibody (Invitrogen). Finally, the slides were washed three times with PBS, stained with DAPI, and mounted in mounting medium (Vector, Burlingame, CA, USA). Images were captured with a Carl Zeiss LSM710 confocal microscope (Carl Zeiss, Oberkochem, Germany). GFP-mRFP-LC3 (ptfLC3) constructs were purchased from Addgene (Cambridge, MA, USA).
Results
Conessine treatment induces autophagosome formation
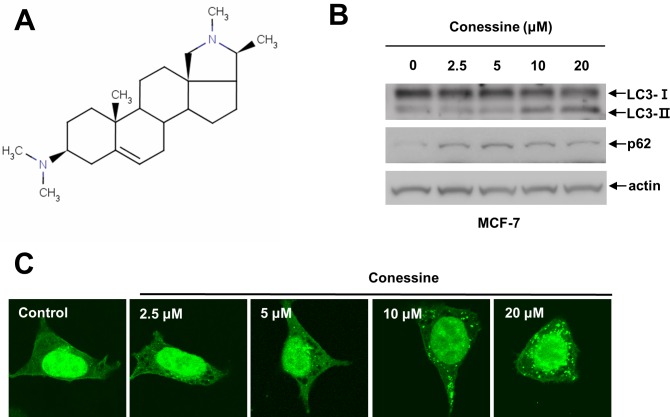
Recently, the regulation of autophagy has shown promise as a new therapeutic strategy to treat many human diseases such as neurodegenerative diseases and cancers. For this reason, we attempted to identify new compounds that might regulate autophagy. We treated HEK293 cells stably expressing GFP-LC3 with natural single compounds for 24 h and then examined whether cytoplasmic GFP-LC3 punctuates were formed using a fluorescent microscope [17, 18]. This screening strategy identified several compounds, and conessine was identified as a potential autophagy regulator (data not shown). To confirm the screening results, MCF7 cells were treated with various concentrations of conessine (0, 2.5, 5, 10, and 20 μM), and the expression of LC3 protein and p62 protein was evaluated. Conessine treatment increased the LC3-II/LC3-I ratio as well as p62, suggesting that it is involved in autophagy regulation (Fig 1B). We next treated GFP-LC3 cells with various concentrations of conessine and examined the cytoplasmic pattern of GFP-LC3 protein. Cytoplasmic punctuates were evident at a concentration of 10 μM conessine (Fig 1C), providing confirmation that conessine is likely involved in autophagy regulation.
Fig 1. Conessine regulates autophagy.
(A) Chemical structure of conessine. (B) Conessine treatment increased the level of LC3-II and p62. MCF-7 cells were incubated with the indicated concentrations of conessine (0, 2.5, 5, 10, and 20 μM) for 24 h, and the cell lysates were subjected to Western blotting with the indicated antibody. (C) Conessine treatment induced autophagosome formation in HEK293 cells. HEK293 cells stably expressing GFP-LC3 were incubated with the indicated concentration of conessine for 24 h, and the cells were analyzed with confocal microscopy.
Conessine treatment inhibits autophagic flux
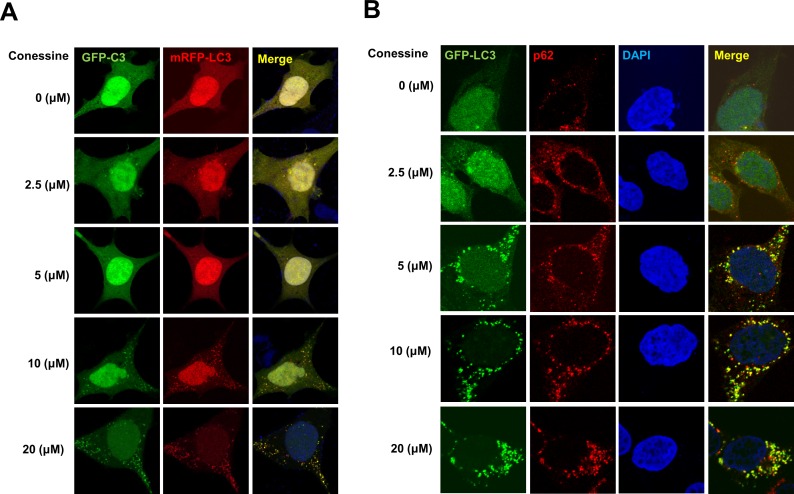
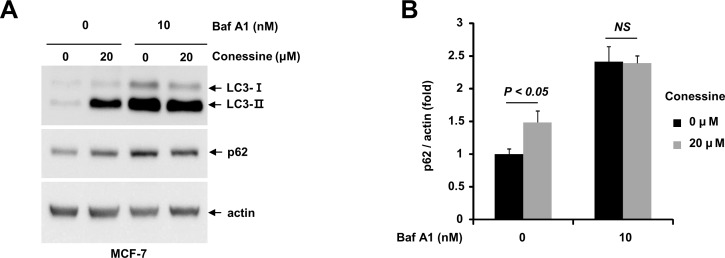
Autophagosome formation can be induced either by activation of the autophagy process or by inhibition of autophagic flux [19]. Because we observed increased levels of p62 by Western blot, we hypothesized that autophagic flux was interrupted by conessine treatment. To test our hypothesis, we used GFP-mRFP-LC3 protein to analyze autophagic flux. While both GFP and mRFP are active in autophagosomes, GFP loses its fluorescence in autolysosomes [20]. Treatment with conessine induced the formation of GFP-LC3 punctuates (autophagosomes), however mRFP punctuates (autolysosomes) were rarely detected, suggesting that autophagic flux was interrupted by conessine treatment (Fig 2A). Next, we examined the localization of p62, which accumulated with GFP-LC3 punctuates, indicating that autophagic flux was interrupted (Fig 2B). Finally, we examined autophagic flux using bafilomycin A1, a lysosomal inhibitor. Conessine treatment (10 μM) significantly increased the level of p62, but co-treatment with bafilomycin A1 eliminated the difference in p62 levels between the control and conessine treated samples (Fig 3A and 3B). These results confirm that conessine inhibited autophagic flux (Fig 3A and 3B).
Fig 2. Autolysosomes were not formed by conessine treatment.
(A) Autophagosome formation by conessine. HEK293 cells were transfected with a plasmid encoding mRFP-GFP-LC3. After 24h, the transfected cells were incubated with the indicated concentrations of conessine (0, 2.5, 5, 10, and 20 μM) for 24 h. Cells were analyzed with confocal microscopy. (B) p62 accumulated in autophagosomes after conessine treatment. HEK293 cells were transfected with a plasmid encoding GFP-LC3, and the cells were treated with indicated concentrations of conessine, followed by immunostaining with anti-p62 antibody. Cells were analyzed with confocal microscopy.
Fig 3. Conessine interferes with autophagic flux.
(A) Conessine treatment suppressed with autophagic flux. MCF-7 cells were treated with either mock or conessine (10 μM) in the presence or absence of Bafilomycin A1 (10 nM). (B) The levels of p62 were analyzed. Autophagic flux experiments were performed in triplicate, and the mean and standard deviations are shown in the graph.
Conessine attenuates H2O2-induced myoblast cell death.
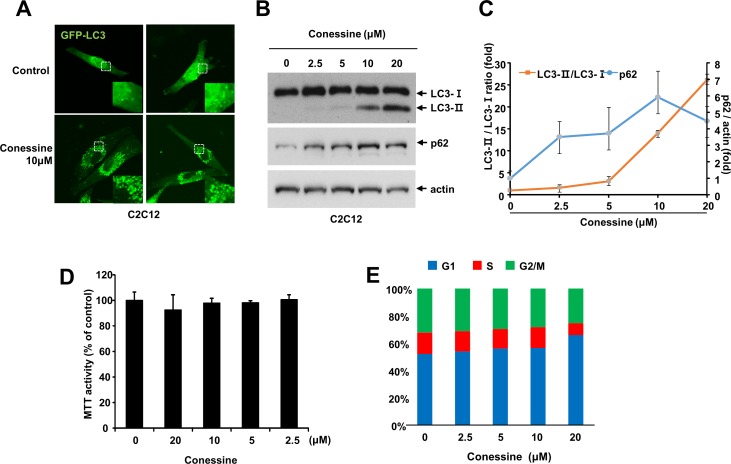
Since conessine interferes with autophagic flux in MCF-7 cells, we wanted to examine whether conessine would also work in muscle cells. For this purpose, we used the C2C12 myoblast cell line. First, we evaluated whether conessine induced the formation of autophagosomes in C2C12 cells. C2C12 cells were transfected with GFP-LC3 plasmids and either mock treated or treated with conessine. Conessine treatment induced the formation of GFP-LC3 punctuates in the cytoplasm (Fig 4A). Next, we examined the expression of autophagic markers LC3 and p62 upon conessine treatment. As expected, conessine treatment increased both the LC3-II/LC-3-I ratio and the level of p62 protein (Fig 4B and 4C). These results indicate conessine inhibited autophagic flux in C2C12 cells.
Fig 4. Conessine treatment induces autophagosome formation in C2C12 cells.
(A) C2C12 cells were transfected with a plasmid encoding GFP-LC3 and cells were treated with either mock or conessine (10 μM). (B) Conessine treatment increased the level of LC3-II and p62. C2C12 cells were incubated with the indicated concentration of conessine for 24 h, and the cell lysates were subjected to Western blotting with the indicated antibody. (C) The levels of LC3-II/LC3-I and p62 were analyzed. The experiments were performed in triplicate, and the means and standard deviations are shown in the graph. (D) Conessine less than 10 μM did not affect proliferation of C2C12 cells. C2C12 cells were treated with the indicated concentration of conessine, and cell viability were measured by MTT assay (E) Conessine treated cells were analyzed with flow cytometry. The percentage of cells at G1, S and G2/M was measured, and is shown in the graph.
Oxidative stress can induce muscle cell death by inducing excessive autophagy [9, 10]. Because conessine inhibits autophagic flux, we hypothesized that it interferes with oxidative stress-induced cell death. First, we determined the optimal concentration of conessine treatment. C2C12 cells were incubated with various concentrations of conessine and cell proliferation was examined. Cell proliferation of C2C12 was marginally lower at 20 μM, and cell viability was not affected by conessine up to 10 μM (Fig 4D). Next, we used flow cytometry to analyze the cell cycle after conessine treatment. While there were fewer cells in S and G2/M phase with 20 μM of conessine, the cell cycle was not affected by conessine up to 10 μM (Fig 4E). In addition, the cells in sub G1 phase did not increase after treatment with conessine, indicating that conessine does not induce cell death (Data not shown). These results indicate that conessine up to 10 μM did not affect C2C12 cell viability.
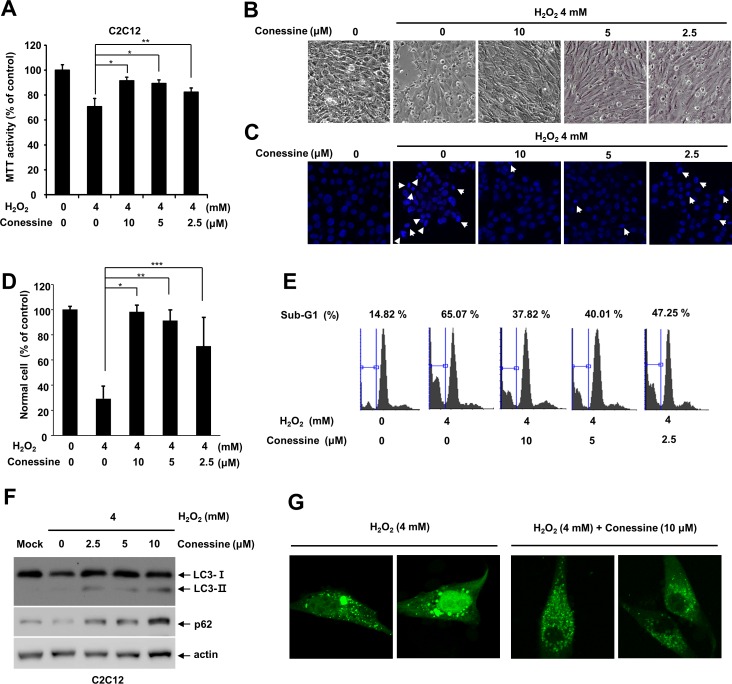
Next, we examined whether conessine interferes with H2O2-induced myoblast cell death. C2C12 cells were either mock-treated or treated with various concentrations of conessine, followed by treatment with H2O2. While mock-treated cells showed a significant decline in cell viability, conessine treatment attenuated cell death in a dose-dependent manner (Fig 5A and 5B). In addition, we examined whether conessine interferes with H2O2-induced cell death in other cell lines. We used mouse fibroblast cell line, NIH3T3, and conessine treatment attenuated H2O2-induced cell death in NIH3T3 cells (S1 Fig).
Fig 5. Conessine treatment protects C2C12 myoblast cells from H2O2-induced cell death.
(A) Conessine treatment interferes with H2O2-induced C2C12 cell death. C2C12 cells were treated with the indicated concentration of conessine, followed by H2O2 treatment (4 mM) for 24 h. Relative cell viability was measured by MTT assay. Control versus conessine treatment, * P < 0.01; ** P <0.05 (B) Cellular morphological changes were observed under a phase contrast microscope. (C, D) Apoptotic cells were quantified by counting DAPI-stained nuclei, and apoptotic cells showed nuclei and chromatin condensation. The number of normal cells were shown in graph. At least 160 cells were counted in each samples. Control versus conessine treatment, * P < 0.00005; ** P <0.0005; *** P<0.05. (E) Conessine decreased the sub-G1 cell population, which was induced by H2O2 treatment. C2C12 cells were treated with conessine and H2O2, and the cell cycle was analyzed with flow cytometry. (F) Conessine attenuates H2O2 induced autophagy. C2C12 cells were sequentially treated with conessine and H2O2, and the cell lysates were subject to Western blot with the indicated antibodies. (G) Conessine alleviates H2O2-induced excessive autophagy. C2C12 cells were transfected with GFP-LC3 and sequentially treated with conessine and H2O2. The cells were analyzed by confocal microscopy.
To confirm our results, we examined DNA condensation, a feature of apoptosis. C2C12 cells were pretreated with conessine and then incubated with H2O2 and fixed for DAPI staining. H2O2 treatment induced up to 70% apoptotic cells with enhanced DAPI staining and conessine treatment decreased the number of apoptotic cells (Fig 5C and 5D). We also examined the cell cycle. While treatment with H2O2 increased the sub-G1 population up to 71%, conessine treatment decreased the sub-G1 population in a dose-dependent manner (Fig 5E). These results indicate that conessine treatment alleviated H2O2-induced myoblast cell death.
Conessine interferes with H2O2-induced autophagic flux activation
Since conessine attenuated H2O2-induced cell death, we examined the level of LC3 and p62 after treatment with conessine and H2O2. H2O2 treatment decreased p62, suggesting that activation of autophagic flux was induced by H2O2, but incubation with both conessine and H2O2 increased the level of p62 to a greater extent (Fig 5F). In addition, the LC3-II/LC3-I ratio also increased after conessine treatment, suggesting that autophagy was modulated by conessine in the presence of H2O2. These results indicate that conessine interfered with the activation of autophagic flux by H2O2. Next, we examined GFP-LC3 localization. H2O2 treatment induced various sizes of GFP-LC3 punctuates in the cytoplasm, and we often observed enlarged autophagosomes, indicating excessive autophagy. However, conessine treatment resulted in uniformly sized autophagosomes and interfered with the formation of enlarged autophagosomes (Fig 5G). These results collectively indicate that conessine interfered with the activation of H2O2-induced autophagic flux in the myoblast cell line.
Discussion
Autophagy is involved in various diseases including cancer and neurodegenerative diseases, making autophagy modulators potentially useful for treatment. Rapamycin, an autophagy activator, has been extensively studied for its potential to treat cancer and neurodegenerative diseases [21, 22]. In addition, chloroquine, an autophagic flux inhibitor, has been studied for its potential use for cancer treatment [6]. For this reason, we searched for autophagy modulators using a stable cell line expressing GFP-LC3. With this system, we previously found that amurensin G, an autophagy activator, can attenuate rotenone-induced neuronal cell death, the in vitro Parkinson’s disease model system [17]. Moreover, we showed that reserpine, an anti-hypertensive drug, can contribute to Parkinson’s disease by modulating autophagy [18]. In this report, we demonstrated that conessine has the potential to inhibit autophagic flux. Conessine treatment induces the accumulation of enlarged autophagosomes in the cytoplasm by impairing the fusion of autophagosome with a lysosome (Fig 2). Although we did not identify the molecular targets of conessine to regulate autophagic flux, these findings suggest that conessine potentially interferes with the regulatory proteins which mediate the fusion process of autophagosome with a lysosome, such as Rab proteins, SNARE proteins and v-ATPase [1, 19]. Because muscle cell death and atrophy is related to accelerated autophagic flux, autophagic flux inhibitors like conessine can be useful to prevent muscle cell death. Recently, we also reported that microgravity in space can potentially affect muscle weakening by regulating autophagy, and an autophagic flux inhibitor like conessine would be potentially useful in such a setting [3].
Autophagy is often called a “double-edged sword,” as both excessive autophagy and lack of autophagy can affect cell viability. Likewise, the relationship between autophagy and muscle diseases such as muscle atrophy is not clear yet. Some reports indicate that autophagy is required to prevent muscle atrophy, while others show that autophagy contributes to the induction of muscle atrophy [23, 24]. In this report, we found that conessine, an autophagy inhibitor, interfered with H2O2-induced myoblast cell death. Because muscle atrophy involves breakdown of aberrant proteins, the inhibition of protein breakdown may delay the onset of muscle atrophy. Further study will be required to determine the long term effect of conessine on muscle atrophy.
Supporting Information
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by a grant from the Leading Space Core Technology Development Program through the NRF funded by the Ministry of Science, ICT & Future Planning (2013M1A3A3A02042433) (http://www.nrf.re.kr). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy. 2016;12(1):1–222. 10.1080/15548627.2015.1100356 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6(4):463–77. Epub 2004/04/08. S1534580704000991 [pii]. . [DOI] [PubMed] [Google Scholar]
- 3.Ryu HW, Choi SH, Namkoong S, Jang IS, Seo DH, Choi I, et al. Simulated microgravity contributes to autophagy induction by regulating AMP-activated protein kinase. DNA Cell Biol. 2014;33(3):128–35. Epub 2014/01/07. 10.1089/dna.2013.2089 . [DOI] [PubMed] [Google Scholar]
- 4.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140(3):313–26. Epub 2010/02/11. 10.1016/j.cell.2010.01.028 S0092-8674(10)00063-2 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306(5698):990–5. 10.1126/science.1099993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimura T, Takabatake Y, Takahashi A, Isaka Y. Chloroquine in cancer therapy: a double-edged sword of autophagy. Cancer Res. 2013;73(1):3–7. 10.1158/0008-5472.CAN-12-2464 . [DOI] [PubMed] [Google Scholar]
- 7.Kesidou E, Lagoudaki R, Touloumi O, Poulatsidou KN, Simeonidou C. Autophagy and neurodegenerative disorders. Neural Regen Res. 2013;8(24):2275–83. 10.3969/j.issn.1673-5374.2013.24.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. 2008;15(1):171–82. 10.1038/sj.cdd.4402233 . [DOI] [PubMed] [Google Scholar]
- 9.Seo G, Kim SK, Byun YJ, Oh E, Jeong SW, Chae GT, et al. Hydrogen peroxide induces Beclin 1-independent autophagic cell death by suppressing the mTOR pathway via promoting the ubiquitination and degradation of Rheb in GSH-depleted RAW 264.7 cells. Free Radic Res. 2011;45(4):389–99. 10.3109/10715762.2010.535530 . [DOI] [PubMed] [Google Scholar]
- 10.Rahman M, Mofarrahi M, Kristof AS, Nkengfac B, Harel S, Hussain SN. Reactive oxygen species regulation of autophagy in skeletal muscles. Antioxid Redox Signal. 2014;20(3):443–59. 10.1089/ars.2013.5410 . [DOI] [PubMed] [Google Scholar]
- 11.Paris R. [On a new acquisition in phytotherapy: Holarrhena floribunda and its principal alkaloid: conessine]. Gaz Med Fr. 1951;Spec. No.:79–83. [PubMed]
- 12.Zhao C, Sun M, Bennani YL, Gopalakrishnan SM, Witte DG, Miller TR, et al. The alkaloid conessine and analogues as potent histamine H3 receptor antagonists. J Med Chem. 2008;51(17):5423–30. 10.1021/jm8003625 . [DOI] [PubMed] [Google Scholar]
- 13.Santora VJ, Covel JA, Hayashi R, Hofilena BJ, Ibarra JB, Pulley MD, et al. A new family of H3 receptor antagonists based on the natural product Conessine. Bioorg Med Chem Lett. 2008;18(4):1490–4. 10.1016/j.bmcl.2007.12.059 . [DOI] [PubMed] [Google Scholar]
- 14.Dua VK, Verma G, Singh B, Rajan A, Bagai U, Agarwal DD, et al. Anti-malarial property of steroidal alkaloid conessine isolated from the bark of Holarrhena antidysenterica. Malar J. 2013;12:194 10.1186/1475-2875-12-194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zirihi GN, Grellier P, Guede-Guina F, Bodo B, Mambu L. Isolation, characterization and antiplasmodial activity of steroidal alkaloids from Funtumia elastica (Preuss) Stapf. Bioorg Med Chem Lett. 2005;15(10):2637–40. 10.1016/j.bmcl.2005.03.021 . [DOI] [PubMed] [Google Scholar]
- 16.Yoon JH, Her S, Kim M, Jang IS, Park J. The expression of damage-regulated autophagy modulator 2 (DRAM2) contributes to autophagy induction. Mol Biol Rep. 2011. Epub 2011/05/18. 10.1007/s11033-011-0835-x . [DOI] [PubMed] [Google Scholar]
- 17.Ryu HW, Oh WK, Jang IS, Park J. Amurensin G induces autophagy and attenuates cellular toxicities in a rotenone model of Parkinson's disease. Biochem Biophys Res Commun. 2013;433(1):121–6. 10.1016/j.bbrc.2013.02.053 . [DOI] [PubMed] [Google Scholar]
- 18.Lee KI, Kim MJ, Koh H, Lee JI, Namkoong S, Oh WK, et al. The anti-hypertensive drug reserpine induces neuronal cell death through inhibition of autophagic flux. Biochem Biophys Res Commun. 2015;462(4):402–8. 10.1016/j.bbrc.2015.04.145 . [DOI] [PubMed] [Google Scholar]
- 19.Namkoong S, Lee KI, Lee JI, Park R, Lee EJ, Jang IS, et al. The integral membrane protein ITM2A, a transcriptional target of PKA-CREB, regulates autophagic flux via interaction with the vacuolar ATPase. Autophagy. 2015;11(5):756–68. 10.1080/15548627.2015.1034412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimura S, Noda T, Yoshimori T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy. 2007;3(5):452–60. . [DOI] [PubMed] [Google Scholar]
- 21.Zaytseva YY, Valentino JD, Gulhati P, Evers BM. mTOR inhibitors in cancer therapy. Cancer Lett. 2012;319(1):1–7. 10.1016/j.canlet.2012.01.005 . [DOI] [PubMed] [Google Scholar]
- 22.Pan T, Rawal P, Wu Y, Xie W, Jankovic J, Le W. Rapamycin protects against rotenone-induced apoptosis through autophagy induction. Neuroscience. 2009;164(2):541–51. Epub 2009/08/18. 10.1016/j.neuroscience.2009.08.014 S0306-4522(09)01294-9 [pii]. . [DOI] [PubMed] [Google Scholar]
- 23.Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, et al. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007;6(6):472–83. 10.1016/j.cmet.2007.11.004 . [DOI] [PubMed] [Google Scholar]
- 24.Masiero E, Sandri M. Autophagy inhibition induces atrophy and myopathy in adult skeletal muscles. Autophagy. 2010;6(2):307–9. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.