Abstract
We have an enormous number of commensal bacteria in our intestine, moreover, the foods that we ingest and the water we drink is sometimes contaminated with pathogenic microorganisms. The intestinal epithelium is always exposed to such microbes, friend or foe, so to contain them our gut is equipped with specialized gut-associated lymphoid tissue (GALT), literally the largest peripheral lymphoid tissue in the body. GALT is the intestinal immune inductive site composed of lymphoid follicles such as Peyer’s patches. M cells are a subset of intestinal epithelial cells (IECs) residing in the region of the epithelium covering GALT lymphoid follicles. Although the vast majority of IEC function to absorb nutrients from the intestine, M cells are highly specialized to take up intestinal microbial antigens and deliver them to GALT for efficient mucosal as well as systemic immune responses. I will discuss recent advances in our understanding of the molecular mechanisms of M-cell differentiation and functions.
Keywords: M cell, gut-associated lymphoid tissue (GALT), antigen uptake, Spi-B, enteroid
The lumen of our gut is colonized with an enormous number of bacteria. These commensal bacteria, collectively called the ‘gut microbiota’, reach a population size of as many as hundreds of trillions, far exceeding ∼40 trillion eukaryotic cells estimated to constitute the human body (1), and have a population complexity of 500–1,000 species (2). Recently developed metagenomic approaches have greatly broadened our knowledge of microbial genomes and it is now estimated that the human gut microbiota possess ∼600,000 genes in total (3), overwhelming the ∼22,000 genes encoded in the human genome (4). In addition to this vast and complex microbial ecosystem present in the gut at homeostasis, gastrointestinal mucosal surfaces are also exposed to pathogenic microorganisms taken in with contaminated food and water.
Our body does not accept these microorganisms unconditionally as inhabitants in our gut. We have developed a special immune system, the gut-associated lymphoid tissue (GALT), which tries to contain or remove these foreign organisms from the gut. An observation supportive of this notion of the importance of GALT has been made studying activation-induced cytidine deaminase (AID)-knockout mice. AID is exclusively expressed in antigen-activated germinal centre B cells giving rise to antibody-producing B cells, or plasma cells. AID is required for somatic hypermutation and class switch recombination necessary for germinal centre B cells to switch the isotype of their immunoglobulin genes from IgM to the other antibody isotypes (such as IgG and IgA) with high affinity for antigen, both in humans (5) and mice (6). As a result, AID-knockout mice lack plasma cells producing IgA (7), crucial for intestinal immune defence (8) (as well as IgG and IgE). At the same time, they suffer from B-cell hyperplasia, most prominent in the intestine, concomitant with an almost 100-fold increase in the number of intestinal bacteria (7), including segmented filamentous bacteria (8), a strong IgA inducing bacteria. Decreasing the intestinal bacteria by treatment with oral antibiotics dampens the B-cell hyperplasia in these mice (7). These observations indicate that the mice can somehow sense the number of intestinal bacteria, which signals GALT to induce IgA-producing plasma cells to contain them.
M cells (where M stands for microfold or membranous) are a unique intestinal epithelial cell (IEC) subset that is responsible for the immune sensing of luminal bacteria. Molecular mechanisms underlying the function and differentiation of M cells will be discussed in this review.
The Intestinal Immune System
To deal with an enormous number of gut commensal bacteria, the gut is equipped with the unique intestinal immune system, which harbours as many as 60–70% of peripheral lymphocytes and constitutes virtually the ‘largest peripheral immune tissue’ in the body (9–11). The intestinal immune system is composed of physically and functionally distinct immune inductive and effector sites (9–11). The immune effector site is the collective designation for the innate immune cells [such as dendritic cells (DCs), macrophages and recently identified innate lymphoid cells] and the adaptive lymphocytes (i.e. effector T cells and IgA-producing plasma cells) diffusely existing in the lamina propria. The immune effector site also includes a unique subpopulation of T cells intercalated in the epithelial layer called IEL, or intraepithelial lymphocytes. By contrast, the immune inductive sites, also called GALT, are organized lymphoid structures consisting of B-cell follicles with germinal centres surrounded by a T-cell zone (11). These lymphoid follicles sometimes exist as aggregated forms, such as Peyer’s patches (PPs) in the small intestine, cecal patches and colonic patches. In humans, PPs consist of hundreds of lymphoid follicles aggregated into an oval shape in the terminal ileum; whereas in mice, 6–8 PPs with 4 or 5 lymphoid follicles each are seen at relatively equal intervals along the entire length of the small intestine. There are also hundreds of isolated lymphoid follicles, in the form of single structures, scattered throughout the small intestine and colon (11).
Follicle-associated epithelium and M cells
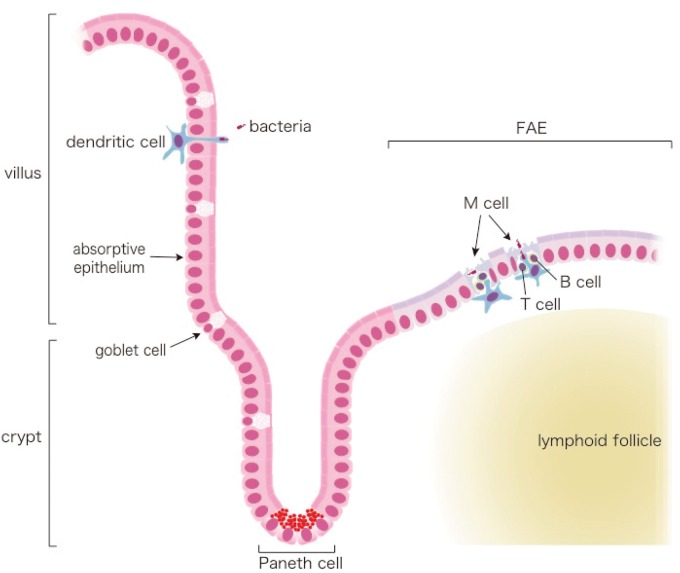
As the immune inductive site, GALT has to sample luminal bacterial and other antigens to evoke immune responses against them, ultimately leading to differentiated plasma cells producing IgA specific to these bacteria (9). Although PP and other organized GALT structures are structurally similar to lymph nodes of the systemic immune system, they do not possess afferent lymphatics via which antigens, more precisely, DCs capturing antigens at peripheral infectious sites, are supplied to lymph nodes; instead, GALT receives its supply of antigens directly from the mucosal surface across the intestinal epithelium overlaying the GALT lymphoid follicles called ‘follicle-associated epithelium’ (FAE) (10–15) (Figs 1 and 2). Villous epithelium mainly consists of absorptive enterocytes, with 10% (in the small intestine) to 20% (in the colon) of mucus-producing goblet cells and a few enteroendocrine cells (15, 16) (Fig. 1). As much as several litters of mucus is secreted a day in adult humans to cover the epithelium and protect it from bacterial encroachment (15, 16). In addition, Paneth cells residing at the base of the small intestinal crypts secrete antimicrobial peptides to further protect the epithelium from bacterial contamination. IgA is also secreted into the mucous layer/lumen for epithelial protection via binding and transcytosis by polymeric immunoglobulin receptors expressed on the basolateral surface of enterocytes (11, 14–16). The FAE possesses characteristics distinct from villous epithelium (10, 12, 14, 15). It is almost devoid of goblet cells and there are few Paneth cells in the crypts surrounding FAE. Moreover, the expression of the IgA-transporting polymeric immunoglobulin receptor is low on enterocytes in and around the FAE. All of these features favour the close association of luminal bacteria with FAE, and hence enhance the opportunity for bacterial delivery to GALT. However, the unquestionably unique feature of FAE must be the presence of M cells, a subset of epithelial cells specialized for antigen uptake.
Fig. 1.
Schematic view of the intestinal mucosa. Mucus-producing goblet cells are scattered throughout the villus region (left), and the epithelium is covered by mucus. (Intraepithelial lymphocytes are not shown here.) DCs in lamina propria sometimes extend their dendrites through epithelium to probe luminal microbes, especially at the end of ileum. Anti-microbial peptide-secreting Paneth cells are found at the crypt base in the villous region. In the FAE covering the GALT lymphoid follicles (right), goblet cells are hardly seen, resulting in the loss of the mucus coat. Instead, 5–10% of FAE cells are M cells. The M-cell pocket is also depicted. Adapted from the webpage of the Laboratory for Intestinal Ecosystem, RIKEN Center for Integrative Medical Sciences (http://leib.rcai.riken.jp/riken/index.html)
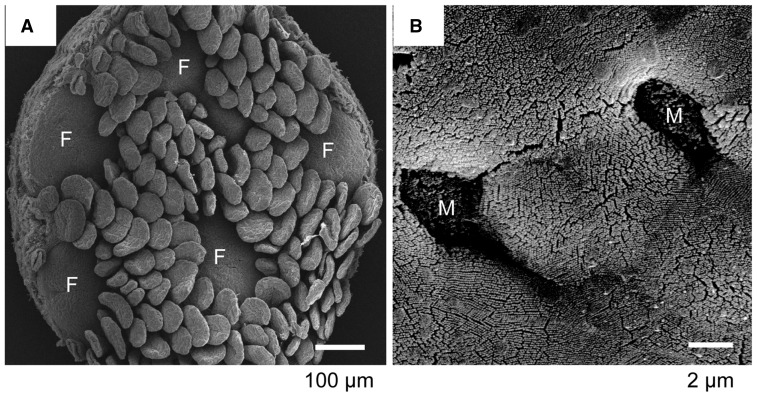
Fig. 2.
Scanning electron micrograph of FAE and M cells. A, Scanning electron micrograph of the mucosal surface of an isolated murine Peyer’s patch. Five FAE regions (F) are surrounded by villi. B, A higher magnification of the FAE. Two M cells (M) are seen. The M-cell surface appears sunken compared with the surrounding enterocytes because of the absence of microvilli. Adapted from the webpage of the Laboratory for Intestinal Ecosystem, RIKEN Center for Integrative Medical Sciences (http://leib.rcai.riken.jp/riken/index.html)
It had already been reported nearly a 100 years ago that FAE is the only region throughout the entire gastrointestinal tract where vigorous uptake of antigenic particles occurs (17). In 1922, Kenzaburo Kumagai, a Japanese physician/microbiologist, found that mycobacteria can only be taken up via FAE. He also noticed that FAE cells can even take up inert particles such as carmine pigments and lyophilized red blood cells, which later turned out to be one notable feature of M-cell mediated antigen transport. However, the resolution of light microscopes in Kumagai’s era was not good enough to differentiate M cells from enterocytes in FAE by morphology, and the discovery of M cells had to wait for some 50 years until the emergence of electron microscopy. In the early 1970s, two groups independently discovered the cells with unique morphology that are responsible for particulate antigen uptake, M cells (18, 19). Unlike surrounding enterocytes in FAE, M cells lack normal microvilli on their luminal (apical) plasma membrane and instead have short fold-like structures, or ‘microfolds’, which are especially obvious in humans (Figs 1 and 2). In addition, their basal plasma membrane is deeply invaginated to form a large sac-like structure, the so-called ‘M-cell pocket’, where DCs and lymphocytes can move in and take up residence, resulting a very thin or ‘membranous’ M cell cytoplasm. M cells are scattered in FAE, accounting for 5–10% of FAE cells both in humans and mice (10–15, 20).
M cells are highly active in phagocytosis and transcytosis, and thereby take up luminal bacteria and antigens and deliver them to DCs in the M-cell pocket for initiation of mucosal immune responses (10–15, 20). M cells have fewer lysosomes than other IEC and those present have low lysosomal enzyme activity. As a result, antigens taken up by M cells are thought not to be processed but instead transferred intact to DCs, which perform the antigen processing and presentation. Since M cells can take up inert particles such as latex beads, their phagocytic recognition capacity can be indiscriminant and non-specific. On the other side of the coin, receptor-mediated specific recognition has been suggested based on the observation that live, but not killed, Vibrio cholerae can be taken up efficiently by M cells (21), and that uptake efficiency by M cells is different among strains of Escherichia coli (22). Despite their significance, identity of these uptake receptors as well as precise mechanisms for antigen uptake by M cells have long been obscure, mainly because the low frequency of M cells and the lack of specific surface markers make it difficult to purify the M cells required for molecular/biochemical analyses. Hence, M-cell studies have largely depended on morphological analyses for more than four decades after their discovery.
Identification of M-cell-specific molecules
The situation has now changed dramatically thanks to technological innovations, such as microarray analysis, enabling exhaustive gene expression data acquisition. We (23) and others (24) independently developed a method to detach epithelial cell sheets from lamina propria and recover FAE and villous regions, and then compare gene expression profiles between FAE and villous epithelium. This strategy was then combined with in situ hybridization and/or immunohistochemistry to identify FAE/M cell-specific genes. Kiyono’s group took a different approach (25) in that M cells were purified by cell sorting with a monoclonal antibody raised by them recognizing a fucose-containing glycan structure specific to M cells (26). M-cell-specific molecules identified by these studies overlap, providing independent lines of evidence that these molecules are indeed expressed in an M-cell-specific manner.
Microbial uptake receptors on M cells
1. Glycoprotein 2 is an M cell-specific cell surface marker that functions as a bacterial uptake receptor
Glycoprotein 2 (GP2) was originally identified as a glycosylphosphatidylinositol (GPI)-anchored protein specifically expressed in secretory (zymogen) granules of pancreatic acinar cells (27, 28). Its function has long been unknown and its expression by M cells was unexpected. GP2 is specifically expressed on M cells among IECs both in humans and mice (25, 29).
Further analyses using GP2-deficient mice and mutant bacteria have revealed that GP2 acts as a receptor for Type I pili on a subset of Gram-negative enterobacilli such as E.coli and Salmonella enterica. Receptor binding results in their efficient uptake into M cells and subsequent intestinal immune responses against them (29). These findings indicate that GP2 can be used as a long-sought universal M cell marker, and that GP2 is important for immnosurveillance at mucosal surfaces by initiating efficient mucosal immune responses against commensal as well as pathogenic bacteria, e.g. E.coli and Salmonella (Fig. 3).
Fig. 3.
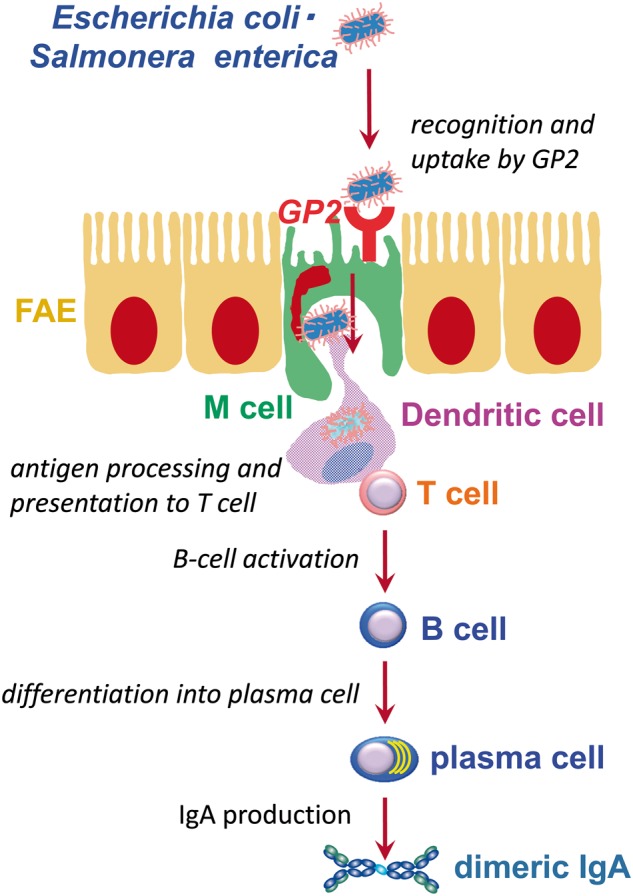
Schematic view of GP2-mediated uptake of bacteria for mucosal immune responses. Adapted from the webpage of the Laboratory for Intestinal Ecosystem, RIKEN Center for Integrative Medical Sciences (http://leib.rcai.riken.jp/riken/index.html)
GP2 possesses a certain degree of homology with Uromodulin, also known as Tamm–Horsfall protein (27). A GPI-anchored protein Uromodulin is expressed on and shed off the apical surface of renal tubular epithelium, and binds to uropahogenic E.coli to prevent urinary tract infection (30). It should be noted that Uromodulin is also specifically expressed on M cells among epithelium and possibly serves as a microbial uptake receptor (31, 32), although the detailed studies are still required to confirm the notion.
2. Cellular prion protein as a Brucella abortus receptor on M cells
Although not specifically expressed on M cells, there are several molecules that act as microbial receptors on these cells. Cellular prion protein (PrPC) is an example. PrPC is a ubiquitously expressed GPI-anchored protein. Its refolding into an amyloidogenic conformation, PrPSc, which templates the conversion of PrPC to PrPSc, causes infectious prion diseases, collectively referred to as transmissible spongiform encephalopathies, a group of neurodegenerative diseases that affect humans as well as other mammals (33). Although the normal physiological role of PrPC is still obscure, it has been shown that various pathogenic microbes and toxins enter host cells by binding to GPI-anchored proteins (34). Brucella abortus is an intracellular pathogen and a causative agent of brucellosis, a re-emerging zoonotic disease responsible for economic damage in the livestock industry, as well as a significant human infectious disease with ∼500,000 annual cases worldwide (35, 36). It has been reported that PrPC on macrophages interacts with B.abortus via binding to its Hsp60, which is secreted via a Type IV secretion system and attached to the bacterial cell surface, and that this interaction is required for bacterial internalization by macrophages (37).
PrPC is also highly expressed on the M-cell apical surface (38), where it functions as a B.abortus uptake receptor (39). Bacterial HSP60 is highly immunogenic and well conserved among bacteria (40). Given that HSP60 has been reported to be present on the cell surface of several other bacteria possessing the various types of secretion system (Table I), PrPC could serve as an uptake receptor for these pathogens as well.
Table I.
Bacteria possessing HSP60 on their surfacea
| Bacteria | Secretion system | Reference |
|---|---|---|
| B.abortus | Type IV | 37 |
| Clostridium difficile | Type III | 41 |
| Helicobacter pylori | Type IV | 42 |
| Haemophilus ducreyi | Type V | 43 |
| Legionella pneumophila | Type IV | 42, 44 |
| Salmonella Typhimurim | Type III | 45 |
| Streptococcus suis | Type II | 43, 46 |
| Mycobacterium avium | Type VII | 47, 48 |
| Actinobacillus actinomycetemcomitans | Type I | 49, 50 |
| Borrelia burgdorferi | Type III | 51, 52 |
aAdapted from the webpage of the Laboratory for Intestinal Ecosystem, RIKEN Center for Integrative Medical Sciences (http://leib.rcai.riken.jp/riken/index.html).
It should also be noted that M cells may play a pivotal role in prion disease pathogenesis. Many prion diseases are orally acquired through ingestion of PrPSc in PPs. It has been reported that M-cell depletion by neutralization of receptor activator of NF-κB ligand (RANKL) signalling blocks the uptake of PrPSc in PPs, suggesting the importance of M cells in the onset of prion disease (53) (the role of RANKL in M-cell development is described in detail in the later section of this review). However, there is also a contradictory publication showing that FAE enterocyte surrounding M cells, rather than M cell itself, is important for uptake of PrPSc in PPs (54). Further studies are required to clarify the point.
3. The IgA receptor on M cells
M cells are also reported to express IgA receptors on their apical plasma membrane and transport secretory IgA (SIgA)-bound antigens into PPs (55, 56). This is also the case for the efficient uptake by M cells of commensal bacteria bound by SIgA (57). Recently, it has been reported that SIgA-antigen complexes are transcytosed by binding to Dectin-1 on the M-cell surface and subsequently transferred to and internalized by DCs via interaction with DC-SIGN on DCs for induction of mucosal and systemic immune responses (58). Consistent with these observations, the composition of the gut commensal microbiota appears altered in mice lacking M cells due to a Spi-B-deficiency (see later) compared with wild-type mice (our unpublished observation), suggesting that M cells are important for intestinal immunosurveillance to contain commensal microbiota.
4. Other microbial receptors
Subcellular localization of membrane proteins is sometimes unique in M cells, in that basolateral membrane proteins on absorptive enterocytes are also expressed on the apical/luminal plasma membrane of M cells. One such example is β1-integrin, which serves as a surface receptor for Yersinia pseudotuberculosis, and its expression on the M-cell apical surface is implicated in bacterial uptake by M cells (59). Another example is CD155; it is a homophilic cellular adhesion molecule at the lateral plasma membrane of epithelium that also serves as the poliovirus receptor, and its apical expression on M cells is suggested to be involved in poliovirus infection (60).
Differentiation of M cells
Differentiation of M cells is thought to be regulated by the interaction of FAE with GALT cells, especially a thin layer of cells in the region between FAE and the B-cell follicle called the subepithelial dome (SED). Epithelial cells in the crypt region surrounding GALT follicles have been reported to display a gene expression profile distinct from those in the villous region, which is thought to be due to their interaction with GALT (13).
RANKL-RANK interaction for M-cell differentiation
RANKL, also known as tumour necrosis factor ligand superfamily member 11 (TNFSF11), TNF-related activation-induced cytokine, osteoprotegerin ligand and osteoclast differentiation factor, was originally recognized for its key roles in bone metabolism and the immune system, but has now been shown to be critical for the formation of mammary epithelia during lactation, thermoregulation of the central nervous system, osteoporosis and also for some cancers (61). A clue for its molecular mechanisms has come from the discovery that RANKL is expressed only by stromal cells in the SED region of GALT throughout the gastrointestinal tract (62). It has further been reported that RANKL deficiency as well as RANKL-neutralizing antibody treatment eliminates most M cells in mice (63). Intraperitoneal administration of recombinant RANKL protein in RANKL-deficient mice corrects the M cell defect and also induces emergence of ectopic M cells in villous regions (63). Considering the expression of RANK, the receptor for RANKL, throughout the intestine in mice (63), together these observations suggest that RANKL signalling on epithelium triggers M-cell differentiation.
The Spi-B transcription factor is critical for M-cell differentiation
Exogenous RANKL-induced ectopic M cells in the villous epithelium express several previously identified M-cell markers at different times after RANKL exposure (20, 64) (Fig. 4). Furthermore, the location where these M-cell markers are initially expressed moves from the crypt zone towards the tips of villi from day 1 to 3 after RANKL treatment. Given that the position of the epithelial cells along the crypt-villus axis reflects their degree of maturation, RANKL likely signals the immature epithelial cells in the crypt region to initiate M-cell maturation, and those cells differentiate to become mature M cells along with their movement towards the tips of villi. The order of appearance of these M cells markers is phenocopied in their order of detection during M-cell differentiation in mouse ontogeny (64) (Fig. 4), suggesting the usefulness of RANKL treatment as a means for studying M-cell differentiation in vivo.
Fig. 4.
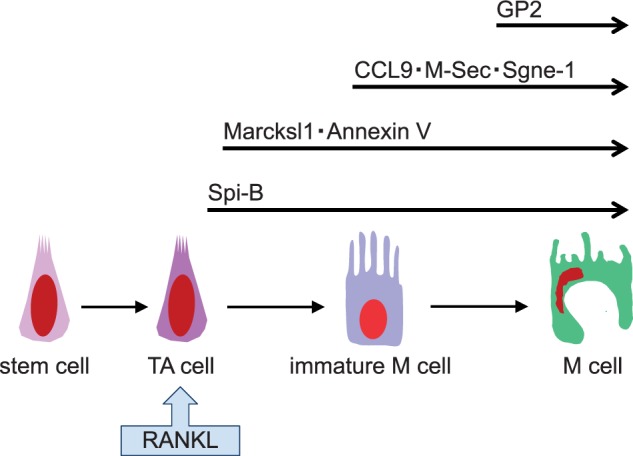
Schematic model of RANKL-induced M-cell differentiation. Upon RANKL stimulation of immature IECs, most likely transit-amplifying cells, the Spi-B transcription factor is induced to initiate M-cell differentiation. Some M-cell markers, such as Marcksl1 and Annexin V appear early after RANKL stimulation and their expression is RANKL-independent (64). By contrast, expression of the other M-cell markers after RANKL stimulation takes a longer time and is Spi-B-dependent (64). Among the markers examined, GP2 expression occurs last and is thought to be a marker of fully mature M cells. Adapted from the webpage of the Laboratory for Intestinal Ecosystem, RIKEN Center for Integrative Medical Sciences (http://leib.rcai.riken.jp/riken/index.html)
Taking advantage of that approach, we identified Spi-B as a highly induced transcription factor in villous epithelium upon RANKL treatment in mice (20, 64). Spi-B is a member of the E26 transformation-specific (Ets) transcriptional factor family originally described as a key regulator for B cells (65, 66), and is also reported to play important roles in the development and function of plasmacytoid DCs (67).
We (64) and others (31, 32) have shown that Spi-B is expressed specifically in M cells among IECs, and that mature M cells are absent in mice lacking Spi-B in IECs but not in haematopoietic cells. In the absence of Spi-B and hence M cells, immune responses against luminal bacteria such as S.enterica serovar Typhimurium and Yersinia enterocoiltica are defective, suggesting that epithelium-specific expression of Spi-B is required for mature M-cell differentiation, which is prerequisite for efficient uptake of luminal bacterial antigens and their delivery into PPs and subsequent intestinal anti-bacterial immune responses.
Other factors affecting M-cell differentiation or maintenance
There have been several studies reporting decreases in M-cell numbers using genetically engineered mice. In mice lacking B cells, the area of the FAE as well as the number of M cells decreases significantly along with a marked decease in the number and size of PPs (68). RAG1-deficient mice, which lack both B and T cells, have an even more severe defect in PPs and M cells are hardly observed (68). By contrast, mice lacking T cells possess PPs and M cells comparable to wild-type mice (68), suggesting the importance of B cells for the formation of both FAE and M cells. However, a conflicting observation has been made in another study of RAG1-deficient mice where there was no difference in M-cell frequency despite a drastic decrease in the area of FAE as well as in absolute numbers of M cells compared with wild-type mice (69).
Regarding the molecules mediating localization and migration of FAE and GALT immune cells, CCL20, a chemokine expressed throughout FAE, and its receptor CCR6 could be among the candidates. A decrease in the size of PPs and the number of M cells has been reported in CCR6-deficient mice (70). Subsequently, it was reported that a unique CCR6-high CD11c-intermediate B-cell subset (CCR6hiCD11cint B cells) is present in the SED region in wild-type mice, and that adoptive transfer of these cells into CCR6-deficient mice restores the number of M cells (71). Considering the comparable expression of RANKL in SED stromal cells in wild-type and CCR6-deficient, hence CCR6hiCD11cint B cell-deficient, mice (71), it is unlikely that CCR6hiCD11cint B cells affect the number of M cells through upregulation of RANKL expression on SED stromal cells. Furthermore, as described later, M-cell differentiation can be induced upon RANKL treatment of intestinal crypt ‘enteroid’ in vitro cultures without addition of any immune cells (31), suggesting that CCR6hiCD11cint B cells are dispensable for M-cell differentiation. With this in mind, it has been postulated that CCR6hiCD11cint B cells may play a role in the maintenance of existing M cells rather than in induction of M-cell differentiation. Lymphotoxin-β signalling has also been suggested to be involved in interactions between FAE and SED cells, since SED immune cells including DCs and follicular DCs disappear in conjunction with a decrease in M cells upon blockade of lymphotoxin-β receptor signalling (69).
Microbial interactions may also affect M cells. For example, relocation of mice from a ‘clean’ specific pathogen-free facility to a ‘dirtier’ conventional one has been reported to increase M-cell numbers (72). It has also been reported that oral administration of a single pathogen, S.Typhimurium, to germ-free mice increases M cells (73). Even though the detection of M cells in these studies depended on a relatively lower expression of alkaline phosphatase, a phenotypic characterization of M cells that leaves room for inaccurate conclusions, the observed increases in M-cell number occurred 7 days after the animal facility relocation or bacterial administration, suggesting the probable promotion of M-cell differentiation by bacteria, although precise mechanisms remain to be elucidated. More rapid (within one to several hours) M-cell increase has also been reported using S.Typhimurium in mice (73) and Streptococcus pneumoniae in rabbits (74). Tahoun et al. (75) have used bovine organoid cultures to define molecular mechanisms and suggest that an S.Typhimurium virulence factor SopB is responsible for the bacterium-induced acute M-cell induction. Such a rapid appearance of M cells cannot be explained by the physiological differentiation of M cells, which takes a few days in vivo before maturation (64), and may reflect phenotypic transition of FAE enterocytes or final differentiation of immature to mature M cells (76). Further studies are required to clarify this situation.
Culture systems for M cells
Even though molecules involved in M-cell functions and differentiation have recently been identified, the molecular mechanisms underlying phagocytosis and transcytotiosis of antigens as well as the differentiation of M cells are still largely unknown. To understand the molecular mechanisms for M-cell functions and differentiation in detail, an in vitro M-cell culture system would be very useful.
In 1997, Kernéis et al. (77) reported that a human adenocarcinoma cell line Caco-2 transdifferentiated into cells with M-cell like morphology and transcytotic capacity for beads and V.cholerae upon coculture with murine PP immune cells. A dozen or two articles using this culture system have since been published. Recently it has been shown with this in vitro M-like cell culture system that the SRC family tyrosine kinase HCK is involved in transcytosis of antigens into M cells (78). This study also suggested that Spi-B regulates HCK-dependent transcytosis.
Another culture model for M-cell study came from ‘enteroid’ cultures treated with RANKL (31). Sato et al. (79) first described the establishment of long-term cultures of isolated single crypts, devoid of mesenchymal cells including stromal and immune cells. The cultured crypts undergo multiple crypt fission events and eventually give rise to organoids with protruding crypts and spherical villus-like epithelial domains containing all the differentiated cell types. When RANKL is added to this so-called ‘intestinal organoid’ or ‘enteroid’ culture, some cells in the villous-like epithelial domains become GP2+ M cells (31). Recently, a method has been developed to convert the spherical enteroids into epithelial monolayer cultures, with which IgA transcytosis can be analysed (80).
Future perspectives
A major function of M cells is particulate antigen delivery to GALT. As described here, several receptors on M cells have been identified, and the molecular basis for antigen uptake has been partially clarified. The M-cell-specific cell surface molecules could serve as targets for developing efficient mucosal vaccine delivery systems (81, 82).
By contrast, there is much less information with respect to the molecular basis of transcytosis of antigens by M cells. Possible involvement of HCK downstream of Spi-B in M-cell transcytosis (78) suggests that determination of Spi-B targets in M cells may provide important new insights into the mechanisms of M-cell transcytosis. RANKL-mediated M-cell induction in the enteroid-derived epithelial monolayer cultures should also facilitate M-cell study at the molecular level.
Finally, detailed examination of M-cell-deficient mice, such as the Spi-B-knockout, should shed light on the in vivo significance of M cells.
Acknowledgement
I would like to thank Dr. Peter D Burrows (University of Alabama at Birmingham) for critical reading and English editing of the manuscript.
Funding
The author’s work cited in this review was supported in part by Grants-in-Aid for Scientific research (A) (24249029) and (B) (21390155), and Scientific Research on Innovative Areas (20113003) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Conflict of Interest
None declared.
Glossary
Abbreviations
- FAE
follicle-associated epithelium
- GALT
gut-associated lymphoid tissue
- IEC
intestinal epithelial cell
- M cell
microfold cell
References
- 1.Bianconi E., Piovesan A., Facchin F., Beraudi A., Casadei R., Frabetti F., Vitale L., Pelleri M.C., Tassani S., Piva F., Perez-Amodio S., Strippoli P., Canaider S. (2013) An estimation of the number of cells in the human body. Ann. Hum. Biol. 40, 463–471 [DOI] [PubMed] [Google Scholar]
- 2.Peterson C.T., Sharma V., Elmén L., Peterson S.N. (2015) Immune homeostasis, dysbiosis and therapeutic modulation of the gut microbiota. Clin. Exp. Immunol. 179, 363–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qin J. Li R. Raes J. Arumugam M. Burgdorf K.S. Manichanh C. Nielsen T. Pons N. Levenez F. Yamada T. Mende D.R. Li J. Xu J. Li S. Li D. Cao J. Wang B. Liang H. Zheng H. Xie Y. Tap J. Lepage P. Bertalan M. Batto J.M. Hansen T. Le Paslier D. Linneberg A. Nielsen H.B. Pelletier E. Renault P. Sicheritz-Ponten T. Turner K. Zhu H. Yu C. Li S. Jian M. Zhou Y. Li Y. Zhang X. Li S. Qin N. Yang H. Wang J. Brunak S. Doré J. Guarner F. Kristiansen K. Pedersen O. Parkhill J. Weissenbach J MetaHIT Consortium, Bork P. Ehrlich S.D. Wang J. (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Human Genome Sequencing Consortium. (2004) Finishing the euchromatic sequence of the human genome. Nature 431, 931–945 [DOI] [PubMed] [Google Scholar]
- 5.Revy P., Muto T., Levy Y., Geissmann F., Plebani A., Sanal O., Catalan N., Forveille M., Dufourcq-Labelouse R., Gennery A., Tezcan I., Ersoy F., Kayserili H., Ugazio A.G., Brousse N., Muramatsu M., Notarangelo L.D., Kinoshita K., Honjo T., Fischer A., Durandy A. (2000) Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2). Cell 102, 565–575 [DOI] [PubMed] [Google Scholar]
- 6.Muramatsu M., Kinoshita K., Fagarasan S., Yamada S., Shinkai Y., Honjo T. (2000) Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102, 553–563 [DOI] [PubMed] [Google Scholar]
- 7.Fagarasan S., Muramatsu M., Suzuki K., Nagaoka H., Hiai H., Honjo T. (2002) Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science 298, 1424–1427 [DOI] [PubMed] [Google Scholar]
- 8.Suzuki K., Meek B., Doi Y., Muramatsu M., Chiba T., Honjo T., Fagarasan S. (2004) Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc. Natl. Acad. Sci. U. S. A. 101, 1981–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macpherson A.J., McCoy K.D., Johansen F.-E., Brandtzaeg P. (2008) The immune geography of IgA induction and function. Mucosal Immunol. 1, 11–22 [DOI] [PubMed] [Google Scholar]
- 10.Neutra M.R., Pringault E., Kraehenbuhl J.-P. (1996) Antigen sampling across epithelial barriers and induction of mucosal immune responses. Annu. Rev. Immunol. 14, 275–300 [DOI] [PubMed] [Google Scholar]
- 11.Brandtzaeg P., Kiyono H., Pabst R., Russell M.W. (2008) Terminology: nomenclature of mucosa-associated lymphoid tissue. Mucosal Immunol. 1, 31–37 [DOI] [PubMed] [Google Scholar]
- 12.Owen R.L. (1999) Uptake and transport of intestinal macromolecules and microorganisms by M cells in Peyer’s patches–a personal and historical perspective. Semin. Immunol. 11, 157–163 [DOI] [PubMed] [Google Scholar]
- 13.Kraehenbuhl J.P., Neutra M.R. (2000) Epithelial M cells: differentiation and function. Annu. Rev. Cell. Dev. Biol. 16, 301–332 [DOI] [PubMed] [Google Scholar]
- 14.Neutra M.R., Kraehenbuhl J.-P. (2005) Cellular and molecular basis for antigen transport across epithelial barriers in Mucosal Immunology (Mestecky J., Lamm M.E., Strober W., Bienenstock J., McGhee J.R., Mayer L., eds.) pp. 111–130, Elsevier Academic Press, San Diego [Google Scholar]
- 15.Kato T., Owen R.L. (2005) Structure and function of intestinal mucosal epithelium in Mucosal Immunology (Mestecky J., Lamm M.E., Strober W., Bienenstock J., McGhee J.R., Mayer L., eds.) pp. 131–151, Elsevier Academic Press, San Diego [Google Scholar]
- 16.Vijay-Kumar M., Gewirtz A.T. (2005) Role of epithelium in mucosal immunity in Mucosal Immunology (Mestecky J., Lamm M.E., Strober W., Bienenstock J., McGhee J.R., Mayer L., eds.) pp. 423–434, Elsevier Academic Press, San Diego [Google Scholar]
- 17.Kumagai K. (1922) Über den Resorptionsvergang der corpusculären Bestandteile im Darm. Kekkaku-Zassi 4, 429–431 [Google Scholar]
- 18.Bockman D.E., Cooper M.D. (1973) Pinocytosis by epithelium associated with lymphoid follicles in the bursa of Fabricius, appendix, and Peyer’s patches. An electron microscopic study. Am. J. Anat. 136, 455–477 [DOI] [PubMed] [Google Scholar]
- 19.Owen R.L., Jones A.L. (1974) Epithelial cell specialization within human Peyer’s patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology 66, 189–203 [PubMed] [Google Scholar]
- 20.Kanaya T., Ohno H. (2014) The mechanisms of M-cell differentiation. Biosci. Microbiota Food Health 33, 91–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owen R.L., Pierce N.F., Apple R.T., Cray W.C., Jr. (1986) M cell transport of Vibrio cholerae from the intestinal lumen into Peyer’s patches: a mechanism for antigen sampling and for microbial transepithelial migration. J. Infect. Dis. 153, 1108–1118 [DOI] [PubMed] [Google Scholar]
- 22.Von Moll L.K., Cantey J.R. (1997) Peyer’s patch adherence of enteropathogenic Escherichia coli strains in rabbits. Infect. Immun. 65, 3788–3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hase K., Ohshima S., Kawano K., Hashimoto N., Matsumoto K., Saito H., Ohno H. (2005) Distinct gene expression profiles characterize cellular phenotypes of follicle-associated epithelium and M cells. DNA Res. 12, 127–137 [DOI] [PubMed] [Google Scholar]
- 24.Verbrugghe P., Waelput W., Dieriks B., Waeytens A., Vandesompele J., Cuvelier C.A. (2006) Murine M cells express annexin V specifically. J. Pathol. 209, 240–249 [DOI] [PubMed] [Google Scholar]
- 25.Terahara K., Yoshida M., Igarashi O., Nochi T., Pontes G.S., Hase K., Ohno H., Kurokawa S., Mejima M., Takayama N., Yuki Y., Lowe A.W., Kiyono H. (2008) Comprehensive gene expression profiling of Peyer’s patch M cells, villous M-like cells, and intestinal epithelial cells. J. Immunol. 180, 7840–7846 [DOI] [PubMed] [Google Scholar]
- 26.Nochi T., Yuki Y., Matsumura A., Mejima M., Terahara K., Kim D.Y., Fukuyama S., Iwatsuki-Horimoto K., Kawaoka Y., Kohda T., Kozaki S., Igarashi O., Kiyono H. (2007) A novel M cell-specific carbohydrate-targeted mucosal vaccine effectively induces antigen-specific immune responses. J. Exp. Med. 204, 2789–2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoops T.C., Rindler M.J. (1991) Isolation of the cDNA encoding glycoprotein-2 (GP-2), the major zymogen granule membrane protein. Homology to uromodulin/Tamm-Horsfall protein J. Biol. Chem. 266, 4257–4263 [PubMed] [Google Scholar]
- 28.Fukuoka S., Freedman S.D., Scheele G.A. (1991) A single gene encodes membrane-bound and free forms of GP-2, the major glycoprotein in pancreatic secretory (zymogen) granule membranes. Proc. Natl. Acad. Sci. U. S. A. 88, 2898–2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hase K., Kawano K., Nochi T., Pontes G.S., Fukuda S., Ebisawa M., Kadokura K., Tobe T., Fujimura Y., Kawano S., Nakato G., Kimura S., Murakami T., Iimura M., Hamura K., Fukuoka S.I., Lowe A.W., Waguri S., Itoh K., Kiyono H., Ohno H. (2009) Uptake through Glycoprotein 2 of FimH+ bacteria by M cells initiates mucosal immune response. Nature 462, 226–230 [DOI] [PubMed] [Google Scholar]
- 30.Bates J.M., Raffi H.M., Prasadan K., Mascarenhas R., Laszik Z., Maeda N., Hultgren S.J., Kumar S. (2004) Tamm-Horsfall protein knockout mice are more prone to urinary tract infection: rapid communication. Kidney Int. 65, 791–797 [DOI] [PubMed] [Google Scholar]
- 31.de Lau W., Kujala P., Schneeberger K., Middendorp S., Li V.S.W., Barker N., Martens A., Hofhuis F., DeKoter R.P., Peters P.J., Nieuwenhuis E., Clevers H. (2012) Peyer’s patch M cells derived from Lgr5(+) stem cells require SpiB and are induced by RankL in cultured “miniguts”. Mol. Cell. Biol. 32, 3639–3647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato S., Kaneto S., Shibata N., Takahashi Y., Okura H., Yuki Y., Kunisawa J., Kiyono H. (2013) Transcription factor Spi-B-dependent and -independent pathways for the development of Peyer’s patch M cells. Mucosal Immunol. 6, 838–846 [DOI] [PubMed] [Google Scholar]
- 33.Prusiner S.B. (1998) Prions. Proc. Natl. Acad. Sci. U. S. A. 95, 13363–13383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shin J.S., Abraham S.N. (2001) Glycosylphosphatidylinositol-anchored receptor-mediated bacterial endocytosis. FEMS Microbiol. Lett. 197, 131–138 [DOI] [PubMed] [Google Scholar]
- 35.Avila-Calderón E.D., Lopez-Merino A., Sriranganathan N., Boyle S.M., Contreras-Rodríguez A. (2013) A history of the development of Brucella vaccines. Biomed. Res. Int. 2003: 743509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carvalho Neta A.V., Mol J.P., Xavier M.N., Paixão T.A., Lage A.P., Santos R.L. (2010) Pathogenesis of bovine brucellosis. Vet. J. 184, 146–155 [DOI] [PubMed] [Google Scholar]
- 37.Watarai M., Kim S., Erdenebaatar J., Makino S., Horiuchi M., Shirahata T., Sakaguchi S., Katamine S. (2003) Cellular prion protein promotes Brucella infection into macrophages. J. Exp. Med. 198, 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakato G., Fukuda S., Hase K., Goitsuka R., Cooper M.D., Ohno H. (2009) New approach for M-cell-specific molecules screening by comprehensive transcriptome analysis. DNA Res. 16, 227–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakato G., Hase K., Suzuki M., Kimura M., Ato M., Hanazato M., Tobiume M., Horiuchi| M., Atarashi R., Nishida N., Watarai M., Imaoka K., Ohno H. (2012) Cutting Edge: Brucella abortus exploits a cellular prion protein on intestinal M cells as an invasive receptor. J. Immunol. 189, 1540–1544 [DOI] [PubMed] [Google Scholar]
- 40.Kaufmann S.H. (1990) Heat shock proteins and the immune response. Immunol. Today 11, 129–136 [DOI] [PubMed] [Google Scholar]
- 41.Hennequin C., Porcheray F., Waligora-Dupriet A., Collignon A., Barc M., Bourlioux P., Karjalainen T. (2001) GroEL (Hsp60) of Clostridium difficile is involved in cell adherence. Microbiology 147, 87–96 [DOI] [PubMed] [Google Scholar]
- 42.Hoffman P.S., Garduno R.A. (1999) Surface-associated heat shock proteins of Legionella pneumophila and Helicobacter pylori: roles in pathogenesis and immunity. Infect. Dis. Obstet. Gynecol. 7, 58–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frisk A., Ison C.A., Lagergård T. (1998) GroEL heat shock protein of Haemophilus ducreyi: association with cell surface and capacity to bind to eukaryotic cells. Infect. Immun. 66, 1252–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garduño R.A., Faulkner G., Trevors M.A., Vats N., Hoffman P.S. (1998) Immunolocalization of Hsp60 in Legionella pneumophila. J. Bacteriol. 180, 505–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ensgraber M., Loos M. (1992) A 66-kilodalton heat shock protein of Salmonella typhimurium is responsible for binding of the bacterium to intestinal mucus. Infect. Immun. 60, 3072–3078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benkirane R., Gottschalk MG., Jacques M., Dubreuil J.D. (1998) Immunochemical characterization of an IgG-binding protein of Streptococcus suis. FEMS Immunol Med Microbiol. 20, 121–127 [DOI] [PubMed] [Google Scholar]
- 47.Rao S.P., Ogata K., Morris S.L., Catanzaro A. (1994) Identification of a 68 kd surface antigen of Mycobacterium avium that binds to human macrophages. J. Lab. Clin. Med. 123, 526–535 [PubMed] [Google Scholar]
- 48.Houben E.N.G., Korotkov K.V., Bitter W. (2014) Take five — Type VII secretion systems of Mycobacteria. Biochem. Biophys. Acta 1843, 1707–1716 [DOI] [PubMed] [Google Scholar]
- 49.Goulhen F., Hafezi A., Uitto V.J., Hinode D., Nakamura R., Grenier D., Mayrand D. (1998) Subcellular localization and cytotoxic activity of the GroEL-like protein isolated from Actinobacillus actinomycetemcomitans. Infect. Immun. 66, 5307–5313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee M., Jun S.Y., Yoon B.Y., Song S., Lee K., Ha N.C. (2012) Membrane fusion proteins of type I secretion system and tripartite efflux pumps share a binding motif for TolC in gram-negative bacteria. PLoS One 7, e40460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scopio A., Johnson P., Laquerre A., Nelson D.R. (1994) Subcellular localization and chaperone activities of Borrelia burgdorferi Hsp60 and Hsp70. J. Bacteriol. 176, 6449–6456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin T., Gao L., Zhao X., Liu J., Norris S.J. (2015) Mutations in the Borrelia burgdorferi flagellar type III secretion system genes fliH and fliI profoundly affect spirochete flagellar assembly, morphology, motility, structure, and cell division. MBio. 6, e00579–e00515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Donaldson D.S., Kobayashi A., Ohno H., Yagita H., Williams I.R., Mabbott N.A. (2012) M cell-depletion blocks oral prion disease pathogenesis. Mucosal Immunol. 5, 216–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kujala P., Raymond C.R., Romeijn M., Godsave S.F., van Kasteren S.I., Wille H., Prusiner S.B., Mabbott N.A., Peters P.J. (2011) Prion uptake in the gut: identification of the first uptake and replication sites. PLoS Pathog. 7, e1002449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mantis N.J., Cheung M.C., Chintalacharuvu K.R., Rey J., Corthésy B., Neutra M.R. (2002) Selective adherence of IgA to murine Peyer’s patch M cells: evidence for a novel IgA receptor. J. Immunol. 169, 1844–1851 [DOI] [PubMed] [Google Scholar]
- 56.Rey J., Garin N., Spertini F., Corthésy B. (2004) Targeting of secretory IgA to Peyer’s patch dendritic and T cells after transport by intestinal M cells. J. Immunol. 172, 3026–3033 [DOI] [PubMed] [Google Scholar]
- 57.Rol N., Favre L., Benyacoub J., Corthésy B. (2012) The role of secretory immunoglobulin A in the natural sensing of commensal bacteria by mouse Peyer’s patch dendritic cells. J. Biol. Chem. 287, 40074–40082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rochereau N., Drocourt D., Perouzel E., Pavot V., Redelinghuys P., Brown G.D., Tiraby G., Roblin X., Verrier B., Genin C., Corthésy B., Paul S. (2013) Dectin-1 is essential for reverse transcytosis of glycosylated SIgA-antigen complexes by intestinal M cells. PLoS Biol. 11, e1001658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clark M.A., Hirst B.H., Jepson M.A. (1998) M-cell surface beta1 integrin expression and invasin-mediated targeting of Yersinia pseudotuberculosis to mouse Peyer’s patch M cells. Infect. Immun. 66, 1237–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iwasaki A., Welker R., Mueller S., Linehan M., Nomoto A., Wimmer E. (2002) Immunofluorescence analysis of poliovirus receptor expression in Peyer’s patches of humans, primates, and CD155 transgenic mice: implications for poliovirus infection. J. Infect. Dis. 186, 585–592 [DOI] [PubMed] [Google Scholar]
- 61.Nagy V., Penninger J.M. (2015) The RANKL-RANK Story. Gerontology 61, 534–542 [DOI] [PubMed] [Google Scholar]
- 62.Taylor R.T., Patel S.R., Lin E., Butler B.R., Lake J.G., Newberry R.D., Williams I.R. (2007) Lymphotoxin-independent expression of TNF-related activation-induced cytokine by stromal cells in cryptopatches, isolated lymphoid follicles, and Peyer’s patches. J. Immunol. 178, 5659–5667 [DOI] [PubMed] [Google Scholar]
- 63.Knoop K.A., Kumar N., Butler B.R., Sakthivel S.K., Taylor R.T., Nochi T., Akiba H., Yagita H., Kiyono H., Williams I.R. (2009) RANKL is necessary and sufficient to initiate development of antigen-sampling M cells in the intestinal epithelium. Immunology 183, 5738–5747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kanaya T., Hase K., Takahashi D., Fukuda S., Hoshino K., Sasaki I., Hemmi H., Knoop K.A., Kumar N., Sato M., Katsuno T., Yokosuka O., Toyooka K., Nakai K., Sakamoto A., Kitahara Y., Jinnohara T., McSorley S.J., Kaisho T., Williams I.R., Ohno H. (2012) The Ets transcription factor Spi-B is essential for the differentiation of intestinal M cells. Nat. Immunol. 13, 729–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Su G.H., Chen H.M., Muthusamy N., Garrett-Sinha L.A., Baunoch D., Tenen D.G., Simon M.C. (1997) Defective B cell receptor-mediated responses in mice lacking the Ets protein, Spi-B. EMBO J. 16, 7118–7129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gallant S., Gilkeson G. (2006) ETS transcription factors and regulation of immunity. Arch. Immunol. Ther. Exp. 54, 149–163 [DOI] [PubMed] [Google Scholar]
- 67.Sasaki I., Hoshino K., Sugiyama T., Yamazaki C., Yano T., Iizuka A., Hemmi H., Tanaka T., Saito M., Sugiyama M., Fukuda Y., Ohta T., Sato K., Ainai A., Suzuki T., Hasegawa H., Toyama-Sorimachi N., Kohara H., Nagasawa T., Kaisho T. (2012). Spi-B is critical for plasmacytoid dendritic cell function and development. Blood 120, 4733–4743 [DOI] [PubMed] [Google Scholar]
- 68.Golovkina T.V., Shlomchik M., Hannum L., Chervonsky A. (1999) Organogenic role of B lymphocytes in mucosal immunity. Science 286, 1965–1968 [DOI] [PubMed] [Google Scholar]
- 69.Debard N., Sierro F., Browning J., Kraehenbuhl J.P. (2001) Effect of mature lymphocytes and lymphotoxin on the development of the follicle-associated epithelium and M cells in mouse Peyer’s patches. Gastroenterology 120, 1173–1182 [DOI] [PubMed] [Google Scholar]
- 70.Lügering A., Floer M., Westphal S., Maaser C., Spahn T.W., Schmidt M.A., Domschke W., Williams I.R., Kucharzik T. (2005) Absence of CCR6 inhibits CD4+ regulatory T-cell development and M-cell formation inside Peyer’s patches. Am. J. Pathol. 166, 1647–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ebisawa M., Hase K., Takahashi D., Kitamura H., Knoop K.A., Williams I.R., Ohno H. (2011) CCR6hiCD11cint B cells promote M-cell differentiation in Peyer’s patch. Int. Immunol. 23, 261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith M.W., James P.S., Tivey D.R. (1987) M cell numbers increase after transfer of SPF mice to a normal animal house environment. Am. J. Pathol. 128, 385–389 [PMC free article] [PubMed] [Google Scholar]
- 73.Savidge T.C., Smith M.W., James P.S., Aldred P. (1991) Salmonella-induced M-cell formation in germ-free mouse Peyer’s patch tissue. Am. J. Pathol. 139,177–184 [PMC free article] [PubMed] [Google Scholar]
- 74.Borghesi C., Regoli M., Bertelli E., Nicoletti C. (1996) Modifications of the follicle-associated epithelium by short-term exposure to a non-intestinal bacterium. J. Pathol. 180, 326–332 [DOI] [PubMed] [Google Scholar]
- 75.Tahoun A., Mahajan S., Paxton E., Malterer G., Donaldson D.S., Wang D., Tan A., Gillespie T.L., O’Shea M., Roe A.J., Shaw D.J., Gally D.L., Lengeling A., Mabbott N.A., Haas J., Mahajan M. (2012) Salmonella transforms follicle-associated epithelial cells into M cells to promote intestinal invasion. Cell Host Microbe 12, 645–656 [DOI] [PubMed] [Google Scholar]
- 76.Ohno H., Kanaya T., Williams I.R. (2012) M cell differentiation: distinct lineage or phenotypic transition? Salmonella provides answers. Cell Host Microbe 12, 607–609 [DOI] [PubMed] [Google Scholar]
- 77.Kernéis S., Bogdanova A., Kraehenbuhl J.P., Pringault E. (1997) Conversion by Peyer’s patch lymphocytes of human enterocytes into M cells that transport bacteria. Science 277, 949–952 [DOI] [PubMed] [Google Scholar]
- 78.Asai T., Morrison S.L. (2013) The SRC family tyrosine kinase HCK and the ETS family transcription factors SPIB and EHF regulate transcytosis across a human follicle-associated epithelium model. J. Biol. Chem. 288, 10395–10405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E., van Es J.H., Abo A., Kujala P., Peters P.J., Clevers H. (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 [DOI] [PubMed] [Google Scholar]
- 80.Moon C., VanDussen K.L., Miyoshi H., Stappenbeck T.S. (2014) Development of a primary mouse intestinal epithelial cell monolayer culture system to evaluate factors that modulate IgA transcytosis. Mucosal Immunol. 7, 818–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nochi T., Yuki Y., Matsumura A., Mejima M., Terahara K., Kim D.Y., Fukuyama S., Iwatsuki-Horimoto K., Kawaoka Y., Kohda T., Kozaki S., Igarashi O., Kiyono H. (2007) A novel M cell-specific carbohydrate-targeted mucosal vaccine effectively induces antigen-specific immune responses. J. Exp. Med. 204, 2789–2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shima H., Watanabe T., Fukuda S., Fukuoka S., Ohara O., Ohno H. (2014) A novel mucosal vaccine targeting Peyer’s patch M cells induces protective antigen-specific IgA responses. Int. Immunol. 26, 619–625 [DOI] [PubMed] [Google Scholar]