Abstract
Cancer cells typically display increased rates of aerobic glycolysis that are correlated with tumor aggressiveness and a poor prognosis. Targeting the glycolytic pathway has emerged as an attractive therapeutic route mainly because it should spare normal cells. Here, we evaluate the effects of combining the inhibition of glycolysis with application of the polyphenolic compound resveratrol (RSV) in neuroblastoma (NB) cancer cell lines. Inhibiting glycolysis with 2-deoxy-D-glucose (2-DG) significantly reduced NB cell viability and was associated with increased endoplasmic reticulum (ER) stress and Akt activity. Administration of 2-DG increased the expression of the ER molecular chaperones GRP78 and GRP94, the prodeath protein C/EBP homology protein (CHOP) and the phosphorylation of Akt at S473, T450 and T308. Combined treatment with both RSV and 2-DG reduced GRP78, GRP94 and Akt phosphorylation but increased CHOP and NB cell death when compared with the administration of 2-DG alone. The selective inhibition of Akt activity also decreased 2-DG-induced GRP78 and GRP94 expression and increased CHOP expression, suggesting that Akt can modulate ER stress. Protein phosphatase 1α (PP1α) was activated by RSV, as indicated by a reduction in PP1α phosphorylation at T320. Pretreatment of cells with tautomycin, a selective PP1α inhibitor, prevented the RSV-mediated decrease in Akt phosphorylation, suggesting that RSV enhances 2-DG-induced cell death by activating PP1 and downregulating Akt. The RSV-mediated inhibition of Akt in the presence of 2-DG was not prevented by the selective inhibition of SIRT1, a known target of RSV, indicating that the effects of RSV on this pathway are independent of SIRT1. We propose that RSV inhibits Akt activity by increasing PP1α activity, thereby potentiating 2-DG-induced ER stress and NB cell death.
Introduction
Neuroblastoma (NB), which is presumed to arise from neuronal precursor cells that originate from the neural crest during embryonic development, is the most common pediatric extracranial tumor and the fourth most common malignancy during childhood. NB affects very young children, with approximately one-third of affected children diagnosed in infancy and two-thirds diagnosed by the age of 5 years. More than half of affected children over the age of 1 year have metastatic disease at the time of diagnosis.1 In children without metastatic disease or infants under the age of 18 months, the prognosis is very good. However, the prognosis for high-risk patients is extremely poor, and these include children with MYCN-amplified tumors, unfavorable tumor biology and/or the presence of distant metastasis. Despite aggressive multimodal treatment including surgery, high-dose chemotherapy, radiotherapy and stem cell transplant followed by retinoid maintenance therapy, these children often relapse and succumb to the disease. Newer therapies, including treatment with an anti-GD2 antibody in combination with retinoic acid, and multiple ongoing clinical trials offer some hope; however, the effects of aggressive treatment regimens induce significant morbidity and, as a result, children may not qualify for additional clinical trials.2 This underscores the need for more effective and less toxic treatment options.
The fact that cancer cells display increased aerobic glycolysis compared with normal cells is known as the Warburg effect. This effect has been exploited for imaging tumors using fluorodeoxyglucose positron emission tomography for many years. Fluorodeoxyglucose is an analog of glucose that is rapidly taken up by highly glycolytic cancer cells. The use of fluorodeoxyglucose positron emission tomography is increasing in NB, confirming the glycolytic nature of these tumors.3 The activation of oncogenes, the loss of tumor suppressors and the hypoxic tumor microenvironment all contribute to the upregulation of glycolysis. In addition to generating adenosine triphosphate, glycolysis confers several advantages that contribute to cancer cell survival. Glycolysis generates intermediates such as nucleotides, amino acids and lipids that are needed for rapidly proliferating cells. Furthermore, the secretion of lactic acid, a by-product of glycolysis, leads to an acidic microenvironment that promotes cell death of surrounding cells, extracellular matrix degradation, angiogenesis and the inhibition of immune response.4 Glycolysis also promotes cancer cell survival and chemoresistance by maintaining cellular adenosine triphosphate at levels that are required for drug efflux, drug inactivation, DNA repair and anti-death cell signaling pathways.5 The therapeutic targeting of tumor cell metabolism is a growing area of investigation. Because of the striking metabolic differences between cancer cells and noncancer cells, targeting aspects of cancer metabolism may be the ideal treatment because it should limit toxicity to normal cells.
One of the best-studied glycolytic inhibitors is the glucose analog 2-deoxy-D-glucose (2-DG). Like glucose, 2-DG is taken up by cancer cells via glucose transporters (GLUTs) and is phosphorylated by hexokinase II. However, unlike glucose-6-phosphate, 2-DG-phosphate cannot be further metabolized, and it therefore accumulates in the cell, leading to allosteric inhibition of hexokinase II and glucose utilization. Using 2-DG has been shown to inhibit cell proliferation and to induce cell death both in vitro and in vivo.6, 7, 8 Recently, it was demonstrated that 2-DG could induce endoplasmic reticulum (ER) stress and its adaptive response pathway, known as the unfolded protein response (UPR).9 Owing to the structural similarity between 2-DG and mannose, 2-DG is erroneously incorporated into the oligosaccharide chain of N-linked glycosylation, leading to the accumulation of misfolded proteins and the activation of the UPR.9 The glucose-related proteins (GRPs) GRP78 and GRP94 are ER-localized molecular chaperones that assist in protein folding and assembly and play important roles in cell survival during brief periods of ER stress.10 However, when ER stress is sustained, the UPR switches from adaption to cell death. A key protein in this pathway is the transcription factor C/EBP homology protein (CHOP), also known as growth arrest- and DNA damage-inducible gene 153. CHOP promotes cell death by modulating the expression of members of the BCL-2 family.11 Targeting the UPR by promoting the accumulation of misfolded proteins and/or by inhibiting the adaptive mechanisms of the UPR is currently under investigation for cancer therapy.
In the clinical arena, 2-DG has been shown to be safe and has demonstrated potential when combined with chemotherapy or radiotherapy. Combining 2-DG and radiotherapy resulted in modestly increased survival and a significant enhancement in the quality of life in patients with malignant gliomas.12 More recently, a combination of 2-DG and docetaxel was well tolerated, and of the 34 patients with advanced solid tumors, 1 had a partial response and 11 had stable disease.13 These clinical trials and multiple preclinical studies suggest that 2-DG may be most effective when combined with additional anticancer agents.14
Resveratrol (RSV), a plant-derived polyphenolic compound found in grape skin, some berries and peanuts, has exhibited anticancer effects both in vitro and in vivo. The chemopreventive and therapeutic potential of RSV has been demonstrated in multiple cancers including skin, breast, prostate and lung cancer as well as NB.15 RSV has emerged as a promising anticancer agent because it has the ability to modulate multiple signaling pathways involved in cancer cell proliferation and survival.16 In NB, RSV has been shown to inhibit cell cycle, promote mitochondrial dysfunction, activate caspases and induce apoptosis.17 Furthermore, RSV significantly reduced tumor growth in NB xenograft models and increased the anti-GD2 immunotherapy response in NXS2 tumor-bearing mice.18
One pathway modulated by RSV is the phosphatidylinositol 3-kinase (PI3K)/Akt pathway that has been shown to play a central role in apoptosis avoidance, tumor progression and resistance to chemo- and radiotherapy. The activity of Akt is dependent on its phosphorylation that is regulated by multiple phosphatases. PTEN (phosphatase and tensin homolog) dephosphorylates PIP3 (phosphatidylinositol (3,4,5)-triphosphate), preventing PI3K and subsequent Akt activation. The loss of this tumor suppressor has been observed in several cancers and is often correlated with a poor prognosis.19 However, Akt can also be dephosphorylated directly by protein phosphatase 2A (PP2A), protein phosphatase 1 (PP1) and PHLPP (PH domain leucine-rich repeat protein phosphatase). Recently, it was demonstrated that the adaptor protein PINCH1 (particularly interesting new cysteine-histidine-rich 1) is upregulated in many cancers and contributes to radioresistance by binding to and inhibiting PP1, resulting in increased Akt activity.20
In the present study, we aimed to determine the effects of 2-DG, either alone or in combination with RSV, on multiple NB cell lines generated from children with advanced NB. Clinically relevant concentrations of 2-DG significantly reduced viability in all NB cell lines. Furthermore, combining RSV with 2-DG significantly increased NB cell death compared with using 2-DG alone. The mechanism of action of RSV involves decreased Akt activity and potentiated 2-DG-induced ER stress that is independent of SIRT1 activity.
Materials and methods
Cell culture and reagents
All cells were maintained at 37 °C in a humidified 5% CO2 incubator. The neuroblastoma cell lines SK-N-SH, SH-SY5Y, SK-N-Be2 and SMS-KCNR were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). NB1691 was generously provided by Dr Andrew Davidoff (St Jude's Children's Research Hospital, Memphis, TN, USA). These cell lines were cultured in RPMI-1640 supplemented with 10% heat-inactivated fetal bovine serum and 1% penicillin–streptomycin. SVBM15 cells were isolated from the bone marrow of a relapsed neuroblastoma patient and cultured in DMEM/F12+GlutaMAX supplemented with 2% B27, 10% heat-inactivated fetal bovine serum and 1% penicillin–streptomycin. Briefly, bone marrow aspirate was mixed 1:3 with phosphate-buffered saline and layered over Ficoll (Sigma-Aldrich, St Louis, MO, USA) and centrifuged at 445 g for 30 min. The cells at the interphase layer were collected, pelleted, washed 1 × with media and plated onto collagen-coated 100 mm plates. Cells were monitored using light microscopy, and identification was verified by staining for the disialoganglioside GD2, an antigen that is expressed on tumors of neuroectodermal origin,21 using NB84 monoclonal antibody from Leica (Supplementary Figure 1). Cell lines were routinely tested for mycoplasma using either a MycoAlert mycoplasma detection kit (Lonza, Walkersville, MD, USA) or a LookOut mycoplasma PCR detection kit (Sigma) according to the manufacturer's instructions. The reagents 2-DG, RSV, mannose and tautomycin were obtained from Sigma; 17-N-allylamino-17-demethoxygeldanamycin (17-AAG) was obtained from LC Laboratories (Woburn, MA, USA); and EX-527 was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Wortmannin, LY294002, insulin-like growth factor (IGF) and insulin were obtained from R&D Systems (Minneapolis, MN, USA).
Cell viability
CellTiter 96 AQueous One Solution Cell Proliferation Assay (MTS) was obtained from Promega (Madison, WI, USA), and viability was determined according to the manufacturer's instructions. At 16 h before drug treatment, ∼5000–10 000 NB cells per well were plated into 96-well plates. RSV and 2-DG were freshly prepared in ethanol and water, respectively, before each experiment. Cell viability was determined after 72 h of drug exposure. Experiments were repeated at least 3 times.
Western blot assays
Cells were lysed in ice-cold RIPA buffer (1% sodium deoxycholate, 0.1% SDS, 1% Triton X-100, 10 mM Tris pH 8 and 140 mM NaCl) supplemented with 250 μnits per ml Benzonase, 1 mM dithiothreitol and phosSTOP phosphatase inhibitor cocktail and a cOmplete protease inhibitor cocktail (both from Roche, Indianapolis, IN, USA). All other reagents were obtained from Sigma. Proteins were quantified using a BCA protein assay (Thermo Scientific, Waltham, MA, USA) and proteins (20 μg per sample) were separated using SDS–polyacrylamide gel electrophoresis and electroblotted to nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). Membranes were stained with Ponceau Red to monitor the transfer of proteins and then blocked with 5% non-fat dry milk (Bio-Rad). Membranes were probed with anti-Grp94 (Enzo Life Sciences, Farmingdale, NY, USA), anti-grp78, anti-CHOP/Gadd153, anti-phospho-Akt (T308, T450 and S473), anti-pan Akt, anti-phospho-GSK3β (S9), anti-GSK3β, anti-phospho-IRS-1 (Y632), anti-IRS-1, anti-survivin, anti-phospho-PP1α (T380), anti-PP1α (all obtained from Cell Signaling Technology, Danvers, MA, USA), anti-α-tubulin (Abcam, Eugene, OR, USA) and anti-β-actin (Chemicon, Temecula, CA, USA) antibodies. Following incubation with primary antibodies, membranes were washed, reacted with horseradish peroxidase–conjugated secondary antibodies and visualized using enhanced chemiluminescence (Thermo Scientific). For some experiments, band intensities were quantified using Image J (NIH, Bethesda, MD, USA).
Co-immunoprecipitation assays
Following treatment, cells were harvested in ice-cold immunoprecipitation buffer (20 mM Tris-HCl pH 8.0, 137 mM NaCl, 2 mM EDTA, 1% Triton X-100 and 10% glycerol). Then, 500 μg of protein was immunoprecipitated overnight at 4 °C using anti-PI3K p85 (EMD Millipore, Billerica, MA, USA). Antibody–protein complexes were incubated for an additional 2 h with TrueBlot (Rockland antibodies and assays, Limerick, PA, USA) and then washed 5 × with immunoprecipitation buffer or phosphate-buffered saline. Co-immunoprecipitated proteins were analyzed using western blot analysis.
Statistical analysis
Cell viability data were analyzed using Student's t-tests for all pairwise comparisons of the different treatments that were tested. The results are presented as the mean±s.e.m.
Results
2-DG causes dose-dependent growth inhibition in NB cell lines
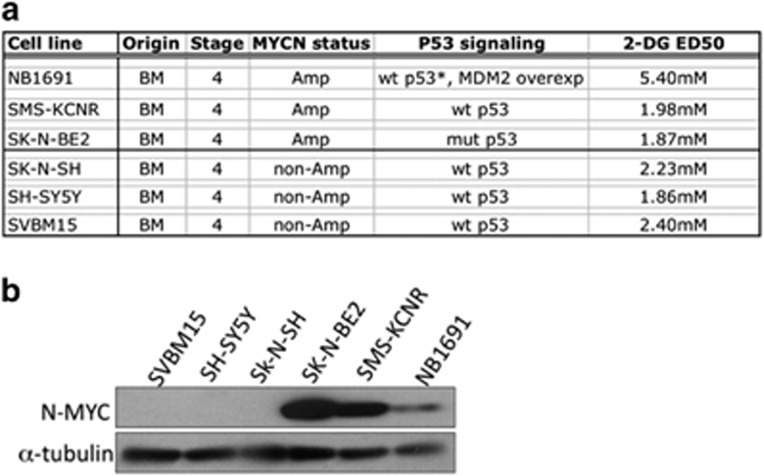
To determine the effect of 2-DG on NB cell viability, we exposed six NB cell lines to increasing concentrations of 2-DG (0–8 mM) and determined the half-maximal inhibitory concentration (IC50) for each cell line. The IC50 was found to range between 1.87 and 5.4 mM, with a median IC50 of 2.1 mM. Cell line information and associated IC50 data are shown in Figure 1a. N-MYC protein levels were examined using western blot analysis (Figure 1b). The amplification of MYCN did not affect sensitivity of the NB cells to 2-DG; this finding is in agreement with a previous study that demonstrated that the rate of glycolysis in NB cells is not related to their MYCN status.22
Figure 1.
2-Deoxy-D-glucose (2-DG) reduces cell viability in neuroblastoma (NB) cell lines independent of NMYC status. (a) Cell line characteristics and the half-maximal inhibitory concentration (IC50) of 2-DG in six NB cell lines. Amp, MYCN amplified; BM, bone marrow. (b) Western blot analysis showing N-Myc protein levels. No correlation was observed between N-Myc status and susceptibility to 2-DG (n=3).
2-DG induces UPR in neuroblastoma cell lines
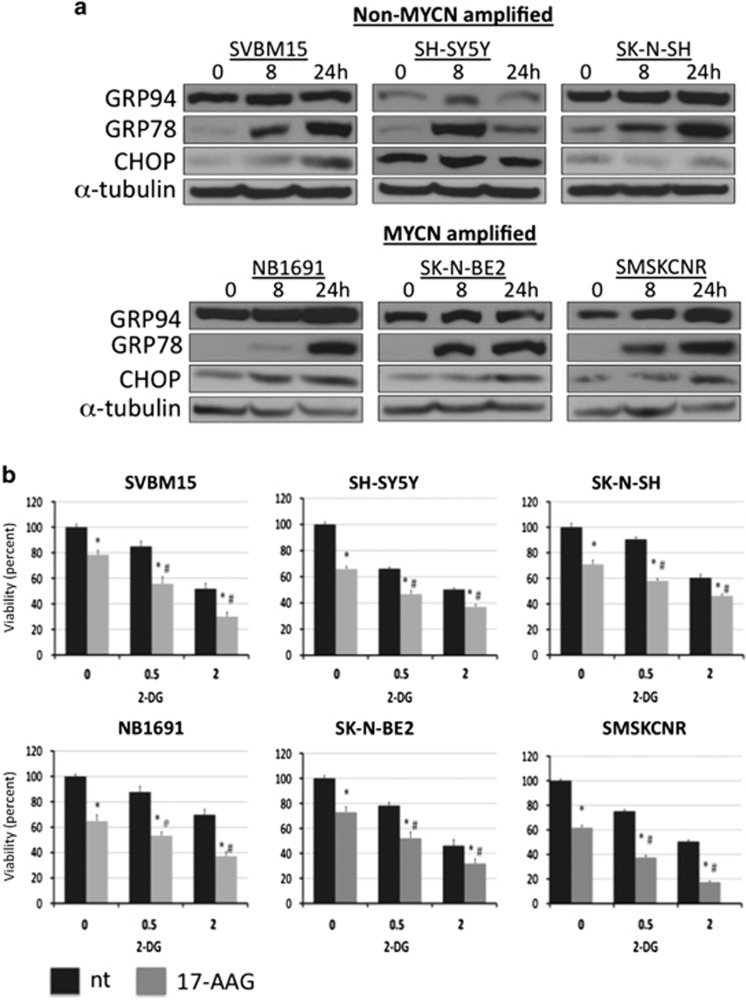
Phase II clinical trials have indicated that 2-DG is minimally effective as a single agent. Therefore, to identify other possible therapeutic targets that may enhance the effectiveness of 2-DG in NB, we examined cell stress and survival signaling pathways that were induced by 2-DG treatment. In certain cell types, a low dose of 2-DG induced ER stress and the UPR.9, 23 To examine the effects of 2-DG on ER stress and the UPR, NB cells were exposed to 2 mM 2-DG for 8 or 24 h, and the levels of the known UPR markers GRP78, GRP94 and CHOP were quantified using western blot analysis. An increase in at least two of these markers was observed in NB cells, with GRP78 being robustly induced in all of the cell lines (Figure 2a), indicating that 2-DG induces the UPR in NB. To determine whether 2-DG induces UPR by interfering with N-linked glycosylation, NB1691 and SK-N-BE2 cells were exposed to 2-DG with or without mannose, an N-linked glycosylation precursor. Exogenously supplied mannose prevented 2-DG-induced induction of GRP78, suggesting that 2-DG induces the UPR by interfering with N-linked glycosylation (Supplementary Figure 2). GRP78 and GRP94 are ER-localized molecular chaperones that play important roles in cell survival following ER stress.10 To determine whether inhibition of the UPR potentiated the 2-DG-induced loss in cell viability, NB cells were treated with 17-AAG, a GRP94 inhibitor, in combination with 2-DG, and cell viability was analyzed after 72 h of treatment. The addition of 17-AAG to 2-DG significantly decreased cell viability compared with the effect of 2-DG alone in all NB cell lines (P<0.05; n=3). Combined application of 2-DG and 17-AAG (500 nM) decreased cell viability by an average of 35±1.9% (0.5 mM 2-DG) and 31±5.5% (2 mM 2-DG) compared with the viability of cells treated with only 2-DG (Figure 2b).
Figure 2.
2-Deoxy-D-glucose (2-DG) induces endoplasmic reticulum (ER) stress. (a) Neuroblastoma (NB) cell lines were treated with 2 mM 2-DG for 8 or 24 h, and the levels of the unfolded protein response (UPR) markers GRP78, GRP94 and C/EBP homology protein (CHOP) were then determined using western blot analysis. (b) NB cells were treated with 500 nM 17-N-allylamino-17-demethoxygeldanamycin (17-AAG), 2-DG (0.5 and 2 mM) or both, and cell viability was determined using MTS assays (n=3). *P<0.05 compared with vehicle-treated controls, #P<0.05 for treatment with 17-AAG+2-DG compared with treatment with 2-DG alone.
2-DG induces the IRS-1/PI3K/Akt signaling pathway
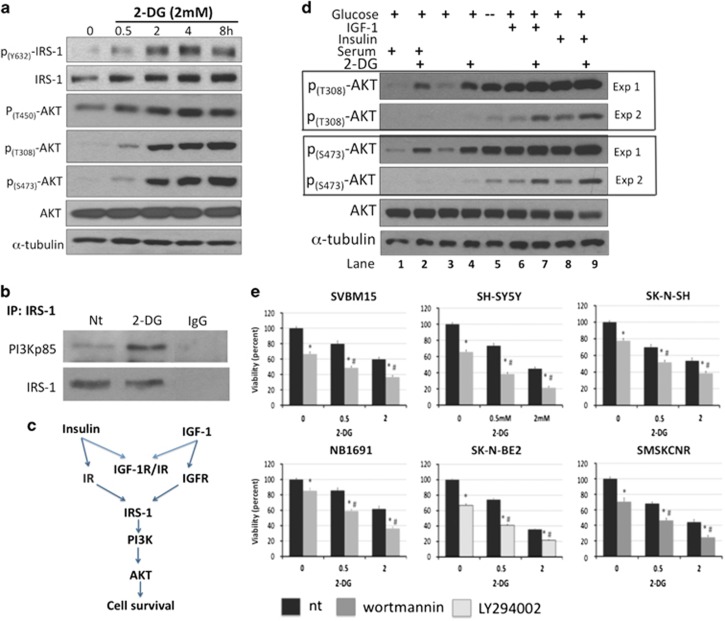
The serine/threonine kinase Akt regulates key cellular processes, including cell growth, survival and metabolism, and is therefore an attractive therapeutic target for NB. In different studies, glycolytic inhibitors have been shown to either increase or decrease Akt activity.24, 25 To examine the effect of 2-DG on Akt signaling in NB, we treated NB1691 cells with 2 mM 2-DG for 0–8 h and examined Akt phosphorylation at threonine 308 (T308), 450 (T450) and serine 473 (S473) using western blot analysis. Application of 2-DG induced a rapid and robust increase in the phosphorylation of Akt at all examined residues (Figure 3a). An increase in Akt phosphorylation was observed as early as 30 min after exposure to 2 mM 2-DG, and it remained elevated for at least 8 h. Furthermore, after 2 h of 2-DG treatment, we observed an increase in the phosphorylation of insulin receptor substrate 1 (IRS-1) at tyrosine 632 (Y632), a site that is necessary for full IRS-1 activation.26 IRS-1 is a major substrate for both the insulin receptor and IGF-1R (Figure 3c). Activated IRS-1 binds and activates PI3K, leading to an increase in the level of PIP3 that is followed by the recruitment and activation of PDK-1 (phosphoinositide-dependent kinase-1) and Akt. To determine whether 2-DG promoted IRS-1 binding to PI3K in NB cells, we performed immunoprecipitation pulldown assays using IRS-1 and the PI3K regulatory subunit p85. After 2 h of 2-DG treatment, PI3K pulldown was significantly increased, confirming the activation of the IRS-1/PI3K/Akt pathway (Figure 3b).
Figure 3.
2-Deoxy-D-glucose (2-DG) activates the Akt signaling pathway independent of serum growth factors. (a) NB1691 cells were treated with 2 mM 2-DG for the indicated times, and the levels of phosphorylation of insulin receptor substrate 1 (IRS-1; Y632) and Akt (T308, T450, S473) were determined using western blot analysis. (b) NB1691 cells were treated with and without 2 mM 2-DG for 2 h. Cell lysates were immunoprecipitated using an anti-IRS-1 antibody and then immunoblotted using an anti-PI3K p85 antibody. (c) Schematic showing the activation of the Akt pathway by insulin and insulin-like growth factor 1 (IGF-1). (d) The effects of serum growth factors, insulin or IGF-1 on 2-DG-induced Akt phosphorylation were evaluated in NB1691 cells. In the serum-free experiments, cells were starved overnight in serum-free media and then treated with 2 mM 2-DG (in serum-free media) for 4 h with or without insulin (100 ng ml−1) or IGF-1 (100 ng ml−1). A no-glucose control, shown in lane 5 (−), has been included. These cells were incubated in serum-free and glucose-free medium for 4 h before harvest. Two exposures are shown, for better clarity. (e) Neuroblastoma (NB) cells were treated with phosphatidylinositol 3-kinase (PI3K) inhibitors (10 μM wortmannin or 10 μM LY294002), 2-DG (0.5 and 2 mM) or both, and viability was determined using MTS assays (n=3). *P<0.05 compared with vehicle-treated controls, #P<0.05 for treatment with Akt inhibitor+2-DG compared with treatment with 2-DG alone.
The role of growth factor signaling in 2-DG-mediated activation of Akt is controversial.27, 28 To determine whether growth factors are necessary for 2-DG-induced phosphorylation of Akt in NB, cells were serum starved overnight, washed again in serum-free media and treated with 2 mM 2-DG for 4 h, and the levels of phospho-Akt were examined using western blot analysis. Akt phosphorylation (at T308 and S473) was induced by 2-DG irrespective of whether serum was present or absent. As a control, we included a glucose-free condition (Figure 3d, lane 5 that is indicated by a negative sign (−)). Here, cells were serum starved overnight and then incubated in serum- and glucose-free media for 4 h. Similar to the effect of 2-DG, the lack of glucose also induced Akt phosphorylation in the absence of serum growth factors, indicating that serum growth factors are not required to activate the Akt pathway under glucose-limiting conditions. However, the addition of either IGF-1 or insulin greatly enhanced 2-DG-induced levels of phospho-Akt (Figure 3d). These data suggest that although serum growth factors are not required for the 2-DG-induced activation of Akt, its response is amplified by IGF-1 or insulin.
Inhibiting Akt increases the cytotoxicity of 2-DG
To determine the functional consequences of 2-DG-induced activation of Akt in NB, cell lines were exposed to 0.5 or 2 mM 2-DG with or without the PI3 kinase inhibitors wortmannin or LY294002. In NB1691 cells, PI3K inhibition by either wortmannin or LY294002 attenuated 2-DG-induced phosphorylation of Akt and its downstream target GSK3β (Supplementary Figure 3A) and reduced cell viability more than application of 2-DG alone (Supplementary Figure 3B). Furthermore, PI3K inhibition significantly decreased cell viability compared with application of 2-DG alone in all tested NB cell lines (P<0.05; n=3). PI3K inhibition decreased viability by an average of 34±2.3% and 39±4.6% in cells treated with 0.5 and 2 mM 2-DG (Figure 3e), respectively, compared with the use of 2-DG alone.
RSV attenuates 2-DG-induced Akt activation and potentiates ER stress
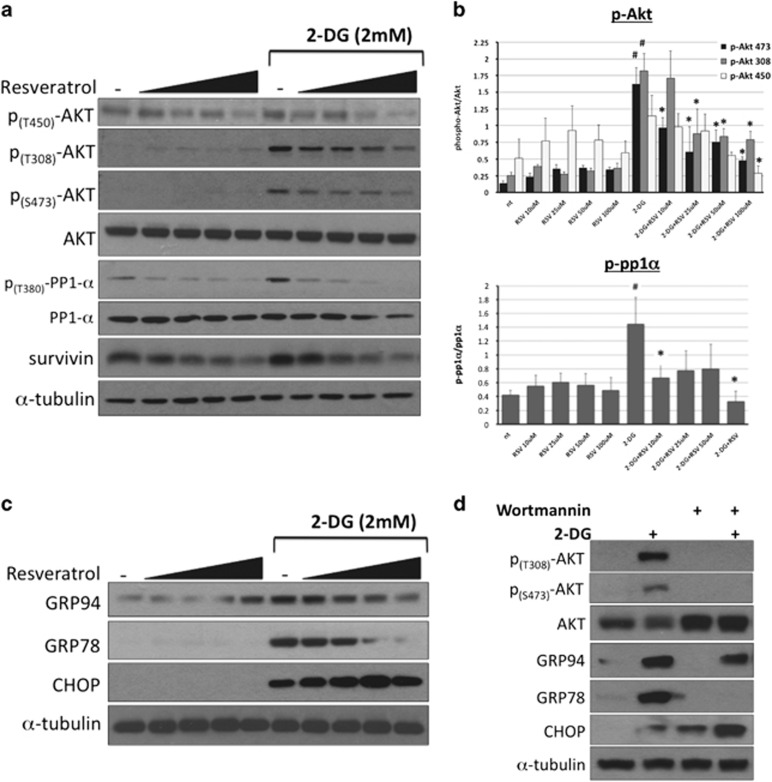
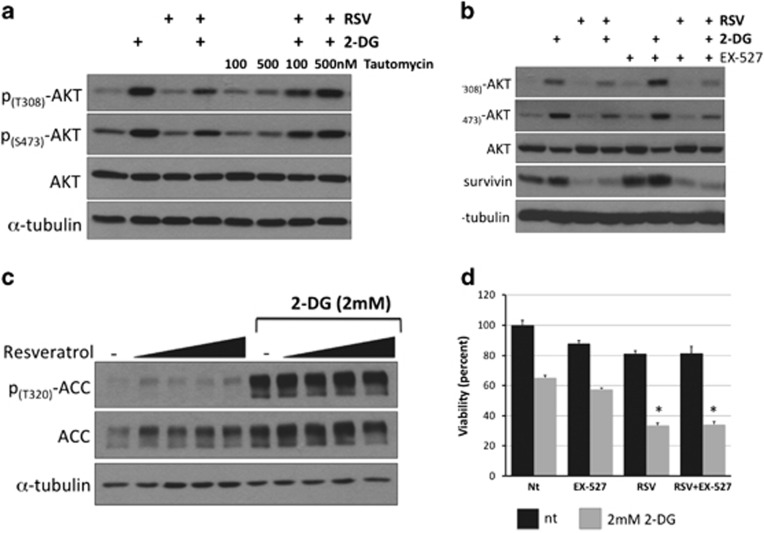
RSV has been shown to promote cell death by modulating cell survival signaling pathways and the levels of apoptosis regulators, such as Akt, ERK (extracellular signal-regulated kinase), p53, Bcl-2, IAP (inhibitor of apoptosis) and TRAIL (tumor necrosis factor-related apoptosis-inducing ligand).16 To determine the effect of RSV on 2-DG-induced Akt phosphorylation, we exposed NB1691 cells to increasing concentrations of RSV (10–100 μM) in the presence or absence of 2-DG for 8 h and then examined phospho-Akt levels. RSV decreased 2-DG-induced phosphorylation of Akt at T308, T450 and S473 and decreased the level of survivin, a prosurvival protein that has previously been shown to be regulated by Akt (Figure 4a). Quantification of Akt phosphorylation revealed that 2-DG significantly increased the level of phospho-T308 (by 7.2-fold) and phospho-S473 (by 11.6-fold) compared with untreated controls. Furthermore, RSV treatment reduced T450, T308 and S473 phosphorylation. Importantly, all concentrations of RSV significantly reduced phosphorylation at S473, and this site is known to be required for the full activation of Akt (Figure 4b). To determine whether this was a cell line-specific effect, we also examined the effect of RSV on 2-DG-induced Akt phosphorylation and the level of survivin in three additional NB cell lines, SH-SY5Y, SK-N-SH and SK-N-BE2, and observed similar results (Supplementary Figures 4A–C).
Figure 4.
Resveratrol (RSV) attenuates 2-deoxy-D-glucose (2-DG)-induced Akt and potentiates endoplasmic reticulum (ER) stress. To determine the effects of combining 2-DG with RSV on the Akt pathway and unfolded protein response (UPR) markers, NB1691 cells were treated with 2 mM 2-DG and RSV (10, 25, 50 or 100 μM) for 8 h and then examined using western blot analysis. (a) Western blot analysis showing the levels of phospho-Akt (T450, T308 and S473), phospho-PP1α (T320) and survivin. (b) Densitometry results showing the ratio of phosphorylated to total Akt and the ratio of phosphorylated protein phosphatase 1α (PP1α) to total PP1α arbitrary units (n=3). #P<0.05 compared with nontreated controls; *P<0.05 compared with treatment with 2-DG treated alone. (c) Western blot analysis showing the levels of the UPR markers GRP78, GRP94 and C/EBP homology protein (CHOP). (d) To determine the effect of Akt on the UPR, NB1691 cells were treated with 10 μM wortmannin before treatment with 2 mM 2-DG treatment for 8 h, and the levels of phospho-Akt (T308, S473), GRP78, GRP94 and CHOP were evaluated using western blot analysis.
RSV has been demonstrated to both inhibit and promote ER stress.29, 30, 31, 32 To determine the effect of RSV on 2-DG-induced ER stress, NB1691 cells were exposed to 10–100 μM RSV in the presence or absence of 2-DG for 8 h, and the levels of GRP94, GRP78 and CHOP were then evaluated using western blot analysis. As shown in Figure 4c, RSV reduced the 2-DG-mediated induction of GRP94 and GRP78 but increased CHOP expression. This result is consistent with potentiation of ER stress. Recently, it was demonstrated that Akt modulates ER stress by increasing the expression of GRP78.33 To determine the role of Akt in 2-DG-induced ER stress, NB1691 cells were pretreated with the PI3K inhibitor wortmannin and then exposed to 2-DG for 8 h. The levels of GRP78, GRP94 and CHOP were then examined using western blot analysis (Figure 4d). PI3K inhibition prevented the 2-DG-induced increase in Akt phosphorylation and the level of GRP78. Simultaneously, PI3K inhibition attenuated the 2-DG-induced increase in GRP94 but greatly increased the level of CHOP. These data indicate that Akt enhances the prosurvival arm of the UPR and confirm that PI3K inhibitors partially mimic RSV and suggest a mechanism wherein RSV downregulates the UPR by suppressing the Akt pathway and its activation by 2-DG.
Combined application of 2-DG and RSV induces apoptotic cell death in NB cells
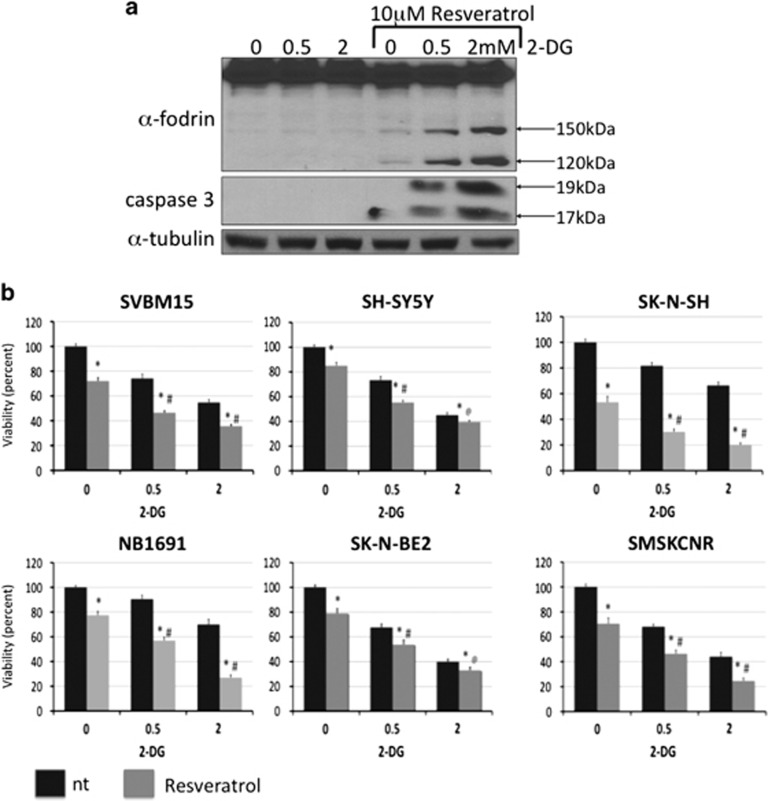
RSV has been shown to induce both caspase- and calpain-mediated apoptotic cell death.34 α-Fodrin is a cytoskeletal protein that is cleaved by both calpains and caspases, and therefore we used western blot analysis to examine α-fodrin cleavage in NB1691 cells that were exposed to 2-DG and RSV. As shown in Figure 5a, combined treatment with RSV and 2-DG generated 150 and 120 kDa fragments that are characteristic of caspase activation, but not the 145 and 150 kDa fragments that are characteristic of calpain activation. Caspase activation was confirmed by the presence of cleaved caspase-3 fragments in the lysates of cells exposed to both 2-DG and RSV but not in lysates of cells exposed to 2-DG alone. To determine the functional consequences of combined RSV and 2-DG treatment in NB, NB cell lines were exposed to 0.5 or 2 mM 2-DG in the presence or absence of 10 μM RSV, and viability was examined after 72 h. Combined treatment with 2-DG and RSV significantly reduced viability compared with treatment with 2-DG alone in all NB cell lines (P<0.05; n=3). RSV decreased viability by an average of 41+6.9% and 41+10% for cells treated with 0.5 and 2 mM 2-DG, respectively (Figure 5b).
Figure 5.
Resveratrol (RSV) enhances 2-deoxy-D-glucose (2-DG)-induced cell death. The effect of 10 μM RSV on 2-DG (0.5 and 2 mM)-treated NB1691 cells was examined using western blot analysis after 48 h. (a) RSV promoted the cleavage of α-fodrin (indicated by 150 and 120 kDa fragments) and caspase-3 (indicated by 19 and 17 kDa fragments), demonstrating the activation of caspase activity. (b) The effect of combining RSV (10 μM) and 2-DG (0.5 and 2 mM) on cell viability was analyzed using MTS assays after 72 h. RSV significantly increased 2-DG-induced cell death (n=3). *P<0.05 compared with vehicle-treated controls, #P<0.05 for treatment with RSV+2-DG compared with treatment with 2-DG alone.
RSV-mediated downregulation of Akt is dependent on PP1α activity but independent of SIRT1
Although RSV is known to downregulate Akt activity, the mechanism for this action is not clear. However, it may involve phosphatase activation.34, 35 To investigate this possibility, we quantified the effects of 2-DG and RSV on PP1 activation by measuring the phosphorylation status of the catalytic subunit PP1α. As demonstrated in Figures 5a and b, 2-DG treatment induced a 3.4-fold increase in the phosphorylation of PP1α at threonine 380 (T380). Combined treatment with 2-DG and RSV significantly reduced the phosphorylation of PP1α at T380 compared with treatment with 2-DG alone. Phosphorylation of PP1α at T380 has previously been shown to inhibit phosphatase activity,36 suggesting that RSV treatment increases PP1α activity. To determine whether PP1α activity is necessary for RSV-mediated downregulation of Akt activity, NB1691 cells were pretreated with tautomycin, a specific inhibitor of PP1α, and then subjected to treatment with 2-DG and RSV, as described above. The level of Akt phosphorylation was examined using western blot analysis (Figure 6a). Tautomycin treatment attenuated RSV-mediated downregulation of Akt phosphorylation in the presence of 2-DG, consistent with the hypothesis that PP1α is an intermediate in RSV-mediated downregulation of Akt. RSV has been shown to activate members of the silent information regulator family (sirtuins or SIRTS). SIRTs are NAD-dependent histone deacetylases that play key roles in cellular stress and aging.37 In 3T3-L1 pre-adipocytes, RSV-mediated downregulation of Akt was itself mediated by the activation of AMPK (AMP-activated protein kinase) and SIRT1.38 RSV has been shown to activate AMPK that can indirectly regulate SIRT1 activity by modulating intracellular NAD+ levels.39, 40 To determine whether the SIRT1 activity is required for RSV-mediated downregulation of Akt, NB1691 cells were pretreated with EX-527, a specific SIRT1 inhibitor, and then exposed to 2-DG and RSV, as described above. As shown in Figure 6b, Akt phosphorylation was reduced by treatment with RSV+EX527 to levels similar to those observed following treatment with RSV+vehicle in the control groups. These data confirm that RSV downregulates Akt independently of SIRT1. Treatment with 2-DG alone induced robust activation of AMPK, as indicated by the increased phosphorylation of acetyl-CoA carboxylase (at T320), a downstream target of AMPK, whereas treatment with RSV alone failed to activate AMPK or affect phospho-acetyl-CoA carboxylase levels, even when it was combined with 2-DG (Figure 6c). In addition, the potentiating effects of RSV on 2-DG-mediated cytotoxicity were not affected by treatment with EX-527 (Figure 6d). To confirm EX-527 activity in our experiments, NB1691 cells were exposed to 10 μM EX-527 for increasing period of time, and the level of acetylated lysine was examined using western blot analysis. EX-527 rapidly increased the levels of acetylated lysine, suggesting that EX-527 did inhibit SIRT1 activity in our cell lines (Supplementary Figure 5).
Figure 6.
The resveratrol (RSV)-mediated reduction of Akt activation is dependent on protein phosphatase 1α (PP1α) but independent of SIRT1. (a) NB1691 cells were treated with the PP1α inhibitor tautomycin at the concentrations shown before 8 h of exposure to 2-deoxy-D-glucose (2-DG) and RSV. Akt phosphorylation levels were examined using western blot analysis. (b) NB1691 cells were treated with the specific SIRT1 inhibitor EX-527 before 8 h of exposure to 2-DG and RSV. Akt phosphorylation levels were examined using western blot analysis. (c) To determine the effect of combining 2-DG with RSV on AMPK (AMP-activated protein kinase), NB1691 cells were treated with 2 mM 2-DG and RSV (10, 25, 50 or 100 μM) for 8 h and the level of ACC phosphorylation examined using western blot analysis. (d) To examine whether SIRT1 inhibition affected 2-DG+RSV-induced cell death, NB1691 cells were treated with 2-DG+RSV with or without EX-527, and viability was determined using MTS assays. *P<0.05 compared with treatment with 2-DG alone. No significant difference was observed between 2-DG+RSV-treated and EX-527+2-DG+RSV-treated cells.
Discussion
Our work confirms the results of previous studies indicating that the glycolytic pathway is a valid target for treatment of NB. Furthermore, we demonstrate, for the first time, that combining RSV treatment with 2-DG significantly reduces NB cell viability in response to 2-DG by downregulating Akt signaling and inhibiting the UPR. We independently demonstrate that these two pathways together confer a survival advantage in responses to 2-DG, and that inhibiting their activity can increase the effect of 2-DG on cell viability. We also propose a novel mechanism of action in which 2-DG inhibits PP1 activity, leading to increased Akt phosphorylation and cell survival, and we show that RSV restores PP1 activity, leading to the dephosphorylation of Akt and cell death.
NB is the most common extracranial solid tumor in children, and it accounts for 15% of pediatric cancer deaths. Because it originates from the neural crest, it is both clinically and genetically heterogeneous. The prognosis for high-risk patients remains poor. Most high-risk NB patients will relapse after an initial response to therapy, and treatment options are limited in these patients because of the cumulative toxicities associated with conventional chemotherapy. Because cancer cells depend on glucose, the glycolytic pathway has emerged as an attractive target: damage to normal, less glycolytic cells should be minimal when using such therapies. Recently, it was demonstrated that elevated GLUT1 expression was a strong predictor of NB patient outcomes, suggesting that high-risk patients might benefit from the use of glycolytic inhibitors.41 Furthermore, Matsushita et al.41 demonstrated that NB cell lines with high GLUT1 expression were sensitive to the glycolysis inhibitor 3-bromopyruvate acid. Here, we examined the effects of 2-DG in six NB cell lines, all of which were derived from patients with advanced-stage NB. Application of 2-DG decreased cell viability in all of the cell lines, with IC50 values ranging from 1.86 to 5.40 mM. We found that the IC50 for most cell lines was ∼2 mM, within the reported range for clinically achievable treatments.42 Furthermore, we observed a significant decrease in NB cell viability when 2-DG was applied at concentrations as low as 0.5 mM. Although these treatments have been demonstrated to be safe in multiple clinical trials, 2-DG is only minimally effective when used as a single agent. We therefore sought ways to increase its effectiveness as an NB therapeutic agent.
The polytrophic nature of RSV makes it appealing because it can attenuate multiple cancer-promoting pathways.43 Extensive preclinical analyses have indicated that RSV regulates cell survival pathways and cell cycle progression in addition to having effects on tumor cell invasion, metastasis and angiogenesis.16 In NB, RSV has been shown to induce apoptotic cell death and decrease tumor volume.17, 18 Here, we found that RSV augmented 2-DG-induced cell death in all of the NB cell lines that were examined. Furthermore, we found that in the NB cell line NB1691, a combination treatment including both 2-DG and RSV induced robust caspase-3 activity, indicating the activation of apoptotic cell death pathways. In examining cell survival and cell stress pathways, we found that RSV potentiated the effects of 2-DG by modulating the activity of Akt. In our experiments, 2-DG treatment activated Akt by increasing Akt phosphorylation at T450, T308 and S473. This effect has previously been observed in other cancer cell lines, including those derived from breast, colorectal and non-small-cell lung cancer.25 DeSalvo et al.24 recently reported that inhibition of Akt sensitized acute lymphoblastic leukemia cells to 2-DG-induced apoptosis. Similar to these results, we found that inhibiting Akt greatly increased 2-DG-induced NB cell death. We also found that 2-DG induced ER stress and UPR, as indicated by observed increases in the levels of GRP78, GRP94 and CHOP. GRP78 and GRP94 play key roles in mediating cell survival following ER stress.10 Reducing GRP78 levels using a GRP78-specific small interfering RNA significantly increased 2-DG-induced apoptosis in acute lymphoblastic leukemia.24 Because GRP78 inhibitors are not clinically available, we inhibited UPR survival activity using 17-AAG that inhibits GRP94. In pediatric clinical trials, 17-AAG was found to be safe and well tolerated.44 Treating NB cells with 17-AAG significantly increased 2-DG-induced cell death.
Akt was previously shown to regulate the UPR during ER stress by promoting the accumulation of GRP78.33 Our data indicate that in NB cells, Akt increased the accumulation of GRP78 in response to 2-DG. We found that inhibiting Akt signaling blocked the 2-DG-induced accumulation of GRP78 and increased CHOP levels. Finally, RSV treatment decreased Akt phosphorylation and GRP78 and GRP94 levels and increased the level of CHOP in a dose-dependent manner, indicating that RSV both attenuates Akt activity and downregulates the UPR. Our data suggest that RSV potentiates 2-DG-induced NB cell death by reducing Akt activity and inhibiting the UPR.
The mechanism by which 2-DG activates Akt has not been determined. Zhong et al.25 reported that 2-DG-induced activation of Akt was independent of LBK1/AMPK activation or glucose inhibition. Some reports have suggested that 2-DG induces Akt phosphorylation via growth factor receptor-mediated signaling. Estan et al.45 found that 2-DG induced the phosphorylation of IGF-1R and the activation of Akt in myeloid leukemia cell lines. Although we observed a modest increase in the level of phospho-IRS-1 and an increase in PI3K binding to IRS-1, which indicates the activation of the IRS-1/PI3K/Akt pathway, we found that 2-DG, like glucose deprivation, activated Akt in a serum growth factor-independent manner. These results are in contrast to a report by Estan et al.45 who showed that the induction of phospho-Akt by 2-DG required serum factors. Therefore, the mechanism of Akt activation by 2-DG appears to be cell type dependent, and it may differ between different types of cancers.
Because the activation of Akt by 2-DG was independent of growth factor signaling, we next examined the effect of 2-DG on phosphatase activity. Akt activity is regulated by multiple phosphatases, including PTEN, PP2a and PP1α, and recent data have indicated that PP1α is the major phosphatase that targets Akt at T450 that modulates the activity of Akt.46 PP1α is a subunit of PP1, and its activity negatively regulates Akt activation by targeting PDK1 and other kinases, leading to decreased Akt activity. PP1α was also reported to cause Akt dephosphorylation and apoptosis in sphingosine-treated Jurkat cells and geldanamycin-treated breast cancer cells.47, 48 We found that 2-DG increased the level of PP1α that was phosphorylated at T320 that is thought to inactivate PP1α.36 RSV blocked this increase, resulting in attenuated phospho-Akt levels (Figure 5). This provides a mechanism by which RSV can mediate Akt dephosphorylation by modulating the phosphatase activity of PP1 and PP1α. Furthermore, inhibiting PP1α activity using tautomycin blocked RSV-mediated Akt dephosphorylation, confirming the role of PP1α. Interestingly, it was recently reported that 2-DG activated Akt via the NOX4-mediated generation of H2O2.49 This is consistent with our findings because reactive oxygen species are known to inhibit the activity of protein phosphatases.50
Many of the health-promoting effects of RSV, including neuroprotection, have been shown to be mediated by the activation of SIRT1.51 However, RSV-mediated anticancer effects have been found to be both SIRT1 dependent and SIRT1 independent. RSV inhibited gastric cancer cell proliferation and tumor growth in a SIRT1-dependent manner.52 In NB, RSV inhibited the cell cycle and induced apoptosis independent of SIRT1.53 Similarly, using the SIRT1-specific inhibitor EX-527, we found that RSV decreased phospho-Akt levels and increased 2-DG-induced cell death independent of SIRT1 activity, and inhibiting SIRT1 did not affect RSV+2-DG-induced cell death.
The results of this study indicate that NB cells are sensitive to clinically relevant concentrations of 2-DG and that the efficacy of 2-DG is significantly increased by the addition of RSV. Our experiments suggest that the modulation of PP1α activity by both 2-DG and RSV may be responsible for modulating the activity of Akt and, therefore, the subsequent 2-DG-induced ER stress and cell death. To our knowledge, this is the first report to examine the potential of combining 2-DG and RSV, two relatively nontoxic agents, for NB therapy. We propose a mechanism by which 2-DG increases Akt activity that can be quenched by RSV, resulting in much more powerful cytotoxicity.
Acknowledgments
This work was supported by the Mystic Force Foundation.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Experimental & Molecular Medicine website (http://www.nature.com/emm)
Supplementary Material
References
- Ara T, DeClerck YA. Mechanisms of invasion and metastasis in human neuroblastoma. Cancer Metastasis Rev 2006; 25: 645–657. [DOI] [PubMed] [Google Scholar]
- Matthay KK, George RE, Yu AL. Promising therapeutic targets in neuroblastoma. Clin Cancer Res 2012; 18: 2740–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller WP, Coppenrath E, Pfluger T. Nuclear medicine and multimodality imaging of pediatric neuroblastoma. Pediatr Radiol 2013; 43: 418–427. [DOI] [PubMed] [Google Scholar]
- Gatenby RA, Gawlinski ET, Gmitro AF, Kaylor B, Gillies RJ. Acid-mediated tumor invasion: a multidisciplinary study. Cancer Res 2006; 66: 5216–5223. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Tozzi F, Chen J, Fan F, Xia L, Wang J et al. Intracellular ATP levels are a pivotal determinant of chemoresistance in colon cancer cells. Cancer Res 2012; 72: 304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwarakanath BS. Cytotoxicity, radiosensitization, and chemosensitization of tumor cells by 2-deoxy-D-glucose in vitro. J Can Res Ther 2009; 5: S27–S31. [DOI] [PubMed] [Google Scholar]
- Gupta S, Farooque A, Adhikari JS, Singh S, Dwarakanath BS. Enhancement of radiation and chemotherapeutic drug responses by 2-deoxy-D-glucose in animal tumors. J Can Res Ther 2009; 5: S16–S20. [DOI] [PubMed] [Google Scholar]
- Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene 2006; 25: 4633–4646. [DOI] [PubMed] [Google Scholar]
- Kurtoglu M, Gao N, Shang J, Maher JC, Lehman MA, Wangpaichitr M et al. Under normoxia, 2-deoxy-D-glucose elicits cell death in select tumor types not by inhibition of glycolysis but by interfering with N-linked glycosylation. Mol Cancer Ther 2007; 6: 3049–3058. [DOI] [PubMed] [Google Scholar]
- Wang M, Wey S, Zhang Y, Ye R, Lee AS. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal 2009; 11: 2307–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov 2008; 7: 1013–1030. [DOI] [PubMed] [Google Scholar]
- Dwarakanath BS, Singh D, Banerji AK, Sarin R, Venkataramana NK, Jalali R et al. Clinical studies for improving radiotherapy with 2-deoxy-D-glucose: present status and future prospects. J Cancer Res Ther 2009; 5: S21–S26. [DOI] [PubMed] [Google Scholar]
- Raez LE, Papadopoulos K, Ricart AD, Chiorean EG, Dipaola RS, Stein MN et al. A phase I dose-escalation trial of 2-deoxy-D-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol 2013; 71: 523–530. [DOI] [PubMed] [Google Scholar]
- El Mjiyad N, Caro-Maldonado A, Ramirez-Peinado S, Munoz-Pinedo C. Sugar-free approaches to cancer cell killing. Oncogene 2011; 30: 253–264. [DOI] [PubMed] [Google Scholar]
- Bishayee A. Cancer prevention and treatment with resveratrol: from rodent studies to clinical trials. Cancer Prev Res 2009; 2: 409–418. [DOI] [PubMed] [Google Scholar]
- Athar M, Back JH, Kopelovich L, Bickers DR, Kim AL. Multiple molecular targets of resveratrol: Anti-carcinogenic mechanisms. Arch Biochem Biophys 2009; 486: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MA, Kim NH, Kim SH, Oh SM, Huh SO. Antiproliferative and cytotoxic effects of resveratrol in mitochondria-mediated apoptosis in rat b103 neuroblastoma cells. Korean J Physiol Pharmacol 2012; 16: 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto BL, Hank JA, Van De Voort TJ, Subramanian L, Polans AS, Rakhmilevich AL et al. The anti-tumor effect of resveratrol alone or in combination with immunotherapy in a neuroblastoma model. Cancer Immunol Immunother 2011; 60: 731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocana A, Vera-Badillo F, Al-Mubarak M, Templeton AJ, Corrales-Sanchez V, Diez-Gonzalez L et al. Activation of the PI3K/mTOR/AKT pathway and survival in solid tumors: systematic review and meta-analysis. PloS ONE 2014; 9: e95219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke I, Koch U, Hehlgans S, Sandfort V, Zips D, Baumann M et al. PINCH1 regulates Akt1 activation and enhances radioresistance by inhibiting PP1alpha. J Clin Invest 2010; 120: 2516–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujoo K, Cheresh DA, Yang HM, Reisfeld RA. Disialoganglioside GD2 on human neuroblastoma cells: target antigen for monoclonal antibody-mediated cytolysis and suppression of tumor growth. Cancer Res 1987; 47: 1098–1104. [PubMed] [Google Scholar]
- Smith DJ, Cossins LR, Hatzinisiriou I, Haber M, Nagley P. Lack of correlation between MYCN expression and the Warburg effect in neuroblastoma cell lines. BMC Cancer 2008; 8: 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchan JR, Kovacs K, Railsback JW, Kurtoglu M, Jing Y, Gao N et al. Antiangiogenic activity of 2-deoxy-D-glucose. PloS ONE 2010; 5: e13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSalvo J, Kuznetsov JN, Du J, Leclerc GM, Leclerc GJ, Lampidis TJ et al. Inhibition of Akt potentiates 2-DG-induced apoptosis via downregulation of UPR in acute lymphoblastic leukemia. Mol Cancer Res 2012; 10: 969–978. [DOI] [PubMed] [Google Scholar]
- Zhong D, Liu X, Schafer-Hales K, Marcus AI, Khuri FR, Sun SY et al. 2-Deoxyglucose induces Akt phosphorylation via a mechanism independent of LKB1/AMP-activated protein kinase signaling activation or glycolysis inhibition. Mol Cancer Ther 2008; 7: 809–817. [DOI] [PubMed] [Google Scholar]
- Esposito DL, Li Y, Cama A, Quon MJ. Tyr(612) and Tyr(632) in human insulin receptor substrate-1 are important for full activation of insulin-stimulated phosphatidylinositol 3-kinase activity and translocation of GLUT4 in adipose cells. Endocrinology 2001; 142: 2833–2840. [DOI] [PubMed] [Google Scholar]
- Zhong D, Xiong L, Liu T, Liu X, Liu X, Chen J et al. The glycolytic inhibitor 2-deoxyglucose activates multiple prosurvival pathways through IGF1R. J Biol Chem 2009; 284: 23225–23233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mireuta M, Hancock MA, Pollak M. Binding between insulin-like growth factor 1 and insulin-like growth factor-binding protein 3 is not influenced by glucose or 2-deoxy-D-glucose. J Biol Chem 2011; 286: 16567–16573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon DH, Kwon OY, Mang JY, Jung MJ, Kim do Y, Park YK et al. Protective potential of resveratrol against oxidative stress and apoptosis in Batten disease lymphoblast cells. Biochem Biophys Res Com 2011; 414: 49–52. [DOI] [PubMed] [Google Scholar]
- Li C, Wang L, Huang K, Zheng L. Endoplasmic reticulum stress in retinal vascular degeneration: protective role of resveratrol. Invest Ophthalmol Vis Sci 2012; 53: 3241–3249. [DOI] [PubMed] [Google Scholar]
- Wang FM, Galson DL, Roodman GD, Ouyang H. Resveratrol triggers the pro-apoptotic endoplasmic reticulum stress response and represses pro-survival XBP1 signaling in human multiple myeloma cells. Exp Hematol 2011; 39: 999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Gao YY, Liu BQ, Niu XF, Zhuang Y, Wang HQ. Resveratrol-induced cytotoxicity in human Burkitt's lymphoma cells is coupled to the unfolded protein response. BMC Cancer 2010; 10: 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai RY, Chen SK, Yan DM, Chen R, Lui YP, Duan CY et al. PI3K/Akt promotes GRP78 accumulation and inhibits endoplasmic reticulum stress-induced apoptosis in HEK293 cells. Folia Biol 2010; 56: 37–46. [PubMed] [Google Scholar]
- Sareen D, Darjatmoko SR, Albert DM, Polans AS. Mitochondria, calcium, and calpain are key mediators of resveratrol-induced apoptosis in breast cancer. Mol Pharmacol 2007; 72: 1466–1475. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rodriguez A, Mas Gutierrez JA, Sanz-Gonzalez S, Ros M, Burks DJ, Valverde AM. Inhibition of PTP1B restores IRS1-mediated hepatic insulin signaling in IRS2-deficient mice. Diabetes 2010; 59: 588–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon YG, Lee SY, Choi Y, Greengard P, Nairn AC. Cell cycle-dependent phosphorylation of mammalian protein phosphatase 1 by cdc2 kinase. Proc Natl Acad Sci USA 1997; 94: 2168–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis MC, Yankner BA. The aging stress response. Mol Cell 2010; 40: 333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Xiao X, Feng X, Li W, Zhou N, Zheng L et al. Resveratrol induces Sirt1-dependent apoptosis in 3T3-L1 preadipocytes by activating AMPK and suppressing AKT activity and survivin expression. J Nutr Biochem 2012; 23: 1100–1112. [DOI] [PubMed] [Google Scholar]
- Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW et al. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes 2010; 59: 554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009; 458: 1056–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K, Uchida K, Saigusa S, Ide S, Hashimoto K, Koike Y et al. Glycolysis inhibitors as a potential therapeutic option to treat aggressive neuroblastoma expressing GLUT1. J Pediatr Surg 2012; 47: 1323–1330. [DOI] [PubMed] [Google Scholar]
- Stein M, Lin H, Jeyamohan C, Dvorzhinski D, Gounder M, Bray K et al. Targeting tumor metabolism with 2-deoxyglucose in patients with castrate-resistant prostate cancer and advanced malignancies. Prostate 2010; 70: 1388–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gescher A, Steward WP, Brown K. Resveratrol in the management of human cancer: how strong is the clinical evidence? Ann NY Acad Sci 2013; 1290: 12–20. [DOI] [PubMed] [Google Scholar]
- Weigel BJ, Blaney SM, Reid JM, Safgren SL, Bagatell R, Kersey J et al. A phase I study of 17-allylaminogeldanamycin in relapsed/refractory pediatric patients with solid tumors: a Children's Oncology Group study. Clin Cancer Res 2007; 13: 1789–1793. [DOI] [PubMed] [Google Scholar]
- Estan MC, Calvino E, de Blas E, Boyano-Adanez Mdel C, Mena ML, Gomez-Gomez M et al. 2-Deoxy-D-glucose cooperates with arsenic trioxide to induce apoptosis in leukemia cells: involvement of IGF-1R-regulated Akt/mTOR, MEK/ERK and LKB-1/AMPK signaling pathways. Biochem Pharmacol 2012; 84: 1604–1616. [DOI] [PubMed] [Google Scholar]
- Xiao L, Gong LL, Yuan D, Deng M, Zeng XM, Chen LL et al. Protein phosphatase-1 regulates Akt1 signal transduction pathway to control gene expression, cell survival and differentiation. Cell Death Differ 2010; 17: 1448–1462. [DOI] [PubMed] [Google Scholar]
- Thayyullathil F, Chathoth S, Shahin A, Kizhakkayil J, Hago A, Patel M et al. Protein phosphatase 1-dependent dephosphorylation of Akt is the prime signaling event in sphingosine-induced apoptosis in Jurkat cells. J Cell Biochem 2011; 112: 1138–1153. [DOI] [PubMed] [Google Scholar]
- Xu W, Yuan X, Jung YJ, Yang Y, Basso A, Rosen N et al. The heat shock protein 90 inhibitor geldanamycin and the ErbB inhibitor ZD1839 promote rapid PP1 phosphatase-dependent inactivation of AKT in ErbB2 overexpressing breast cancer cells. Cancer Res 2003; 63: 7777–7784. [PubMed] [Google Scholar]
- Owada S, Shimoda Y, Tsuchihara K, Esumi H. Critical role of H2O2 generated by NOX4 during cellular response under glucose deprivation. PLoS ONE 2013; 8: e56628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagishi Y, Matsuda S. Redox regulation of tumor suppressor PTEN in cancer and aging (Review). Int J Mol Med 2013; 31: 511–515. [DOI] [PubMed] [Google Scholar]
- Pallas M, Porquet D, Vicente A, Sanfeliu C. Resveratrol: new avenues for a natural compound in neuroprotection. Curr Pharml Des 2013; 19: 6726–6731. [DOI] [PubMed] [Google Scholar]
- Yang Q, Wang B, Zang W, Wang X, Liu Z, Li W et al. Resveratrol inhibits the growth of gastric cancer by inducing g1 phase arrest and senescence in a sirt1-dependent manner. PloS ONE 2013; 8: e70627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro JG, Verdaguer E, Ancrenaz V, Junyent F, Sureda F, Pallas M et al. Resveratrol inhibits proliferation and promotes apoptosis of neuroblastoma cells: role of sirtuin 1. Neurochem Res 2011; 36: 187–194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.