Abstract
Previously we established Zygote Electroporation of Nucleases (ZEN) technology as an efficient and high-throughput way to generate genetically modified mouse models. However, there were significant variations of the targeting efficiency among different genomic loci using our previously published protocol. In this study, we improved the ZEN technology by delivering Cas9 protein into mouse zygotes through a series of electroporation. Using this approach, we were able to introduce precise nucleotide substitutions, large segment deletion and short segment insertion into targeted loci with high efficiency.
Keywords: CRISPR-Cas9, Electroporation, Mouse zygote
INTRODUCTION
Bacteria and archaea have evolved an adaptive immune system termed clustered regularly interspaced short palindromic repeats (CRISPR)-associated systems to guide the disruption of the invading viruses or plasmids. Upon optimization, the type II CRISPR-Cas9 system of Streptococcus pyogenes worked efficiently in mammalian cells in generating genome modifications (Cong et al., 2013; Mali et al., 2013; Yang et al., 2015). Previously, we reported that mice carrying specific point mutations as well as reporter and conditional alleles could be generated by delivery of the CRISPR-Cas9 components into zygotes through microinjection (Wang et al., 2013; Yang et al., 2013). Using similar strategies, other genetically engineered animals including rats, monkeys, pigs and dogs were generated (Hai et al., 2014; Ma et al., 2014; Niu et al., 2014; Zou et al., 2015). Although this method is highly versatile and efficient, the delivery procedure depending on microinjection is technically demanding and labor intensive, therefore limiting its adaptation in many labs.
To overcome this limitation, we developed the Zygote Electroporation of Nuclease (ZEN) method (Qin et al., 2015). We demonstrated that the CRISPR-Cas9 components, including Cas9 mRNA, sgRNA, and a DNA oligo donor, could be efficiently delivered into mouse zygotes by electroporation to generate mice with targeted genetic modifications. ZEN is easy to learn, high-throughput and less laborious than microinjection (Qin et al., 2015). It was also reported by other groups that the CRISPR-Cas9 components could be efficiently delivered into the mouse and rat zygotes using different electroporation setups (Kaneko et al., 2014; Hashimoto and Takemoto, 2015). However, our previous ZEN protocol had variations for different target genes, and had a generally lower efficiency when compared with microinjection (Qin et al., 2015). At Aicda and Rosa26 loci, non-homologous end joining (NHEJ)-mediated indel (insertion and deletion) mutations occurred at very low efficiency (0% and 17%, respectively), and we failed to insert LoxP site at the Rosa26 locus using our previous ZEN protocol (data not shown). In another parallel comparative study between microinjection and electroporation, ZEN was not able to generate large segment deletion at Smc1b locus, while the microinjection had high efficiency (Table S1). To further improve our ZEN protocol, we selected these genomic loci as targets to introduce different genetic modifications including precise nucleotide substitutions, large segment deletion and targeted short segment insertion. Here, we report the improvement of ZEN technology, which enables generating mice carrying various targeted genetic modifications with high efficiency.
RESULTS
Multiple times of electroporation improved the mRNA delivery into mouse zygotes
First, we optimized our ZEN protocol through using a series of electroporation instead of just one time of electroporation. To determine the optimal number of electroporation, we tested 1, 4, 6, 8 times of electroporation (3 s interval between electroporation) with the same setting as we previously published (30 V; 1 ms pulse duration; 2 pulses; 100 ms interval) (Qin et al., 2015), and observed the survival rate of embryos in two cell and blastocyst stages. While survival rate was reduced along with increased times of electroporation, 6 times of electroporation still resulted in a reasonable rate of in vitro development of blastocysts (Fig. S1C). Importantly, the delivery of GFP mRNA (350 ng/μL) increased accordingly when more times of electroporation were applied (Fig. S1D).
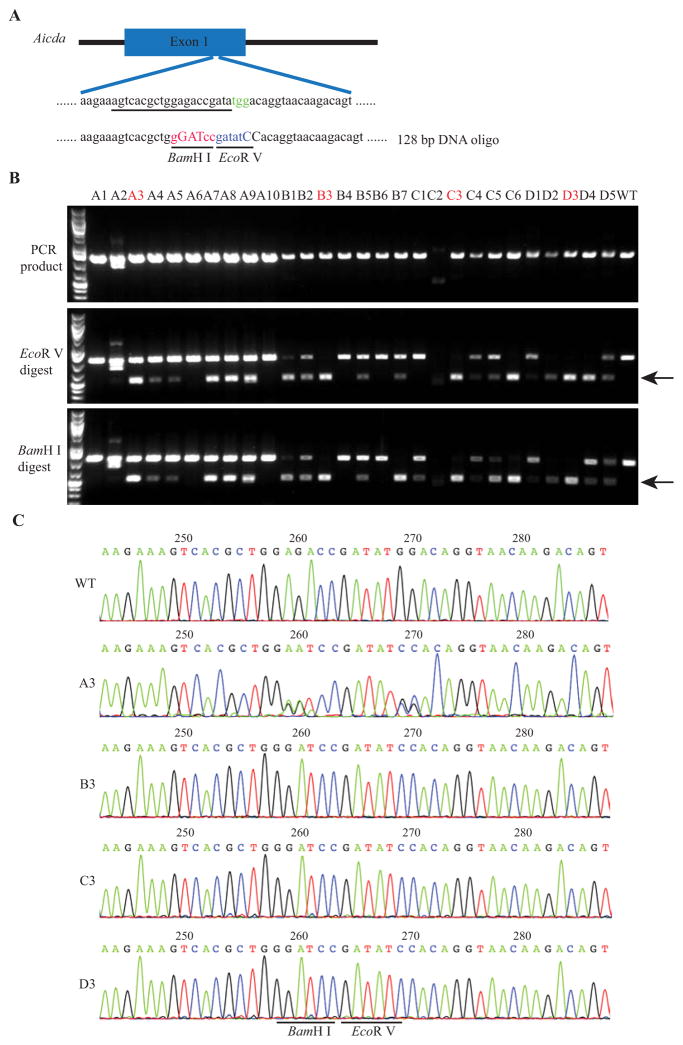
Next, we used Aicda as the target to test whether multiple times of electroporation will improve the targeting efficiency. B6D2F2/J embryos derived from natural mating were treated with acidic Tyrode’s solution for 10 s and then washed extensively before electroporation. Mouse zygotes were loaded into 10 μL of Opti-MEM, and then mixed with 10 μL of CRISPR reagent to achieve final concentration of Cas9 mRNA (600 ng/μL), sgRNA targeting Aicda (300ng/μL), and DNA oligo (1000 ng/μL). The DNA oligo comprises sequence flanking the target region and a few nucleotide changes converting the target sequence (gagaccgatatgg) to the sequence containing a BamH I site (GGATCC) and an EcoR V site (GATATC) (Fig. 1A). Zygotes were electroporated for 1, 4, 6 or 8 times, using the same setting as the GFP mRNA electroporation experiment (Fig. S1B). Afterwards the embryos were transferred into pseudopregnant mice and the pups were born. Tail tip samples were collected for genotyping. The PCR products encompassing the target site were characterized by sequencing and restriction fragment length polymorphism (RFLP) analysis using EcoR V and BamH I. As shown in Fig. S2 and Table S2, there was 1 out of 4 mice containing homology directed repair (HDR) mediated precise nucleotide substitutions after 6 times of electroporation. But all the other groups did not generate HDR allele. We also used commercial Cas9 mRNA for the electroporation with the same setting, and observed better live birth rate upon multiple times of electroporation. As shown in Table S3, although there was no success in most of the groups, there were 3 out of 11 mice containing HDR allele after 8 times of electroporation.
Fig. 1. Delivery of Cas9 protein through electroporation dramatically increased the HDR efficiency.
A: Schematic of the Aicda target sequence and DNA oligo donor. The protospacer sequence is underlined and PAM sequence is colored in green. Oligonucleotides changed in the DNA oligo donor are in uppercase. The BamH I recognition site is colored in red and the EcoR V recognition site is in blue in the DNA oligo donor. B: RFLP analysis of 28 mice from group A (A1–A10), B (B1–B7), C (C1–C6) and D (D1–D5). 1, 4, 6 and 8 times of electroporation were used in group A, B, C and D, respectively. The cleaved bands after EcoR V digestion or BamH I digestion are indicated by black arrow. C: Sequencing traces of PCR products containing the Aicda target region. Clear sequencing traces indicate there is mainly one allele (defined mutation) in the target locus as shown in B3, C3 and D3, while there are both WT and mutant alleles in A3 mouse. WT, wild type.
Delivery of Cas9 protein through series of electroporation dramatically increased the HDR efficiency
To further improve the efficiency, we delivered Cas9 protein together with other components at the same setting as we delivered Cas9 mRNA. Cas9 protein at 250 ng/μL was used for all the experimental groups. Embryos were immediately transferred into pseudopregnant mice after electroporation and live mice were derived. Some embryos were also cultured to blastocyst stage and harvested for genotyping using RFLP and DNA sequencing. Surprisingly, genotyping of the blastocyst samples indicated that the efficiencies of generating indel and HDR alleles were both extremely high after delivery of Cas9 protein through electroporation. Even one time of electroporation had around 70% HDR efficiency (Fig. S3 and Table S4). To confirm the precise nucleotide substitutions, some of the samples were randomly selected for TOPO® cloning and DNA sequencing (Fig. S3C). As shown in Fig. 1B, the correct HDR was confirmed by the genotyping results of the live born mice. Using one time of electroporation, we had 60.00% (6/10) HDR efficiency. Increasing the times of electroporation to 4 times had the similarly high efficiency 71.43% (5/7); further increase of the times of electroporation to 6 times and 8 times yielded 81.33% (5/6) and 100% HDR efficiency (5/5), respectively (Fig. 1 and Table 1). Importantly, when more times of electroporation were used, more mice containing only the HDR allele were identified, suggesting a higher efficiency of generating homozygous HDR animals.
Table 1.
CRISPR/Cas9 mediated genome editing of Aicda gene locus through electroporation of Cas9 protein.
| Time of Electroporation | Number of embryos transferred | Number of mice born | Total mice analyzed | Live birth rate (%) | Percentage of Indela/HDRb (%) | Percentage of mutantc (%) |
|---|---|---|---|---|---|---|
| 1 | 15 | 10 | 10 | 66.67 | 50.00/60.00 | 80.00 |
| 4 | 15 | 7 | 7 | 46.67 | 71.43/71.43 | 100.00 |
| 6 | 15 | 6 | 6 | 40.00 | 50.00/81.33 | 100.00 |
| 8 | 15 | 5 | 5 | 33.33 | 40.00/100.00 | 100.00 |
The percentage of mice with indel mutation at the targeting site.
The percentage of mice with precise nucleotide substitutions at the targeting site.
The percentage of mice with any targeted mutation (indel or HDR, or both).
Delivery of Cas9 protein through electroporation achieved high efficiency of large genomic fragment deletion
Using our previously published protocol (Qin et al., 2015), ZEN did not work as well as microinjection for 2.2 kb deletion at Smc1b locus (Table S1). To test whether delivery of Cas9 protein with a series of electroporation is efficient to generate large DNA segment deletion, we co-electroporated Cas9 protein and two sgRNAs targeting sites flanking exon 2–4 of Smc1b (Fig. 2A). B6D2F2/J embryos were prepared through IVF (in vitro fertilization) in which GSH (glutathione) was used to weaken the zona pellucida. The fertilized embryos were graded, selected and washed. The CRISPR reagent was added to the embryos to reach final concentration of Cas9 protein (250 ng/μL) and two sgRNAs (300 ng/μL each). After 1, 4 or 6 times of electroporation, the embryos were transferred and pups were genotyped. As shown in Fig. S4A, 3/9 mice had the 2.2 kb deletion at the Smc1b locus with one time of electroporation. 4 and 6 times of electroporation yielded 20% (1/5) and 33.33% (2/6) deletion efficiency, respectively (Fig. S4 and Table S5). The correct deletion was confirmed by Sanger sequencing.
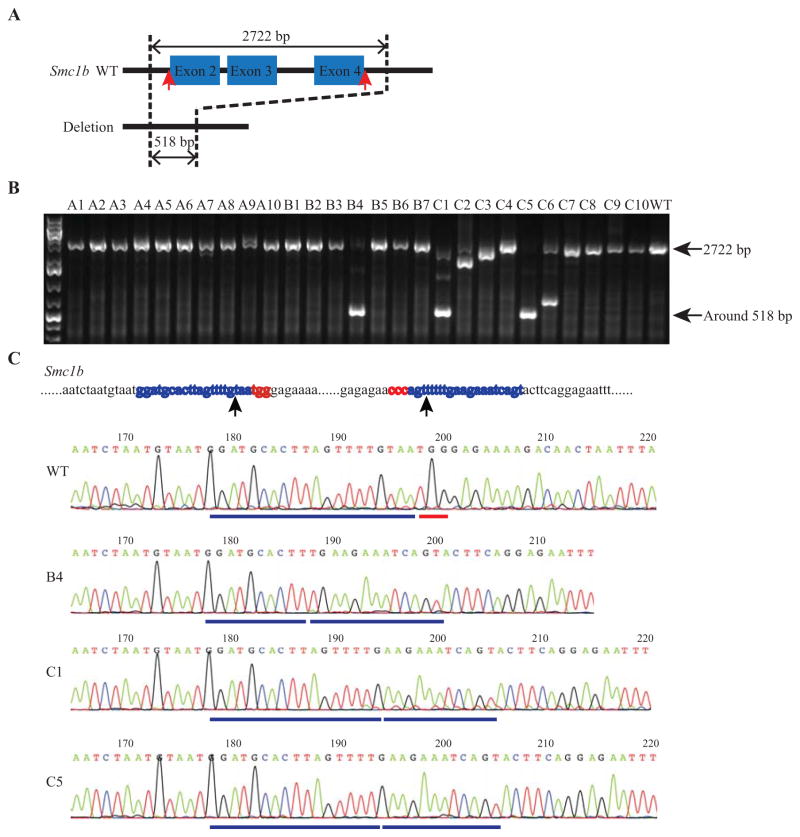
Fig. 2. Delivery of Cas9 protein through electroporation achieved high efficiency of genome deletion in an inbred strain.
A: Schematic of the strategy to delete DNA fragment from the Smc1b gene locus. A DNA segment including exon 2, exon 3 and exon 4 was deleted from the genome. B: Analysis of the sizes of the PCR products through DNA electrophoresis. 1, 4, and 6 times of electroporation were used in group A (A1–A10), B (B1–B7) and C (C1–C10), respectively. A small band in B4, C1, C5 and C6, which is around 518 bp long, indicates the successful deletion. The WT band and the band after deletion were indicated by black arrow. C: Upper panel, the Smc1b target sequence. The two protospace sequences of the two gRNAs are labeled in blue and PAM sequences are in red. The two arrowheads indicate the position of DNA double strand breaks induced by Cas9. Lower panels, sequencing traces of PCR products containing the Smc1b target region. The blue line and the red line under the WT sequencing trace indicate the upstream gRNA sequence and the PAM sequence, respectively. The two blue lines under the sequencing traces of B4, C1, and C5 indicate the remaining upstream and downstream gRNAs. Clear sequencing traces indicate that mainly one allele was amplified as shown in B4, C1 and C5. WT, wild type.
To evaluate the new protocol on inbred strains, we repeated the experiment using C57BL/6NJ IVF-derived embryos. Two embryo transfers were performed for each group. Genotyping results showed that 1 out of 7 mice from 4 times of electroporation, 3 out of 10 mice from 6 times of electroporation had expected large segment deletion (Fig. 2B and Table 2). One time of electroporation did not produce large segment deletion, while it indeed generated indel mutations at both target sites with high efficiency (Table 2). In addition, multiple times of electroporation did not influence the live birth rate of the inbred strain. The above results demonstrated that Cas9 protein delivery through a series of electroporation was efficient in generating large DNA segment deletion in the mouse genome.
Table 2.
CRISPR/Cas9 mediated large segment deletion of Smc1b through electroporation of Cas9 protein in an inbred strain.
| Time of electroporation | Pseudo-ID | Number of embryos transferred | Number of mice born | Live birth rate (%) | Percentage of indela (%) (gRNA1/gRNA2) | Percentage of 2.2 kb deletionb (%) |
|---|---|---|---|---|---|---|
| 1 | 1 | 15 | 6 | 33.33 | 80.00/60.00 | 0 |
| 2 | 15 | 4 | ||||
| 4 | 3 | 15 | 4 | 23.33 | 28.57/42.85 | 14.29 |
| 4 | 15 | 3 | ||||
| 6 | 5 | 15 | 5 | 33.33 | 70.00/70.00 | 30.00 |
| 6 | 15 | 5 |
The percentage of mice with indel mutation at the targeting site of either gRNA1 or gRNA2.
The percentage of mice with the desired 2.2 kb deletion at the targeting site.
Delivery of Cas9 protein through electroporation achieved high efficiency of targeted small DNA fragment insertion
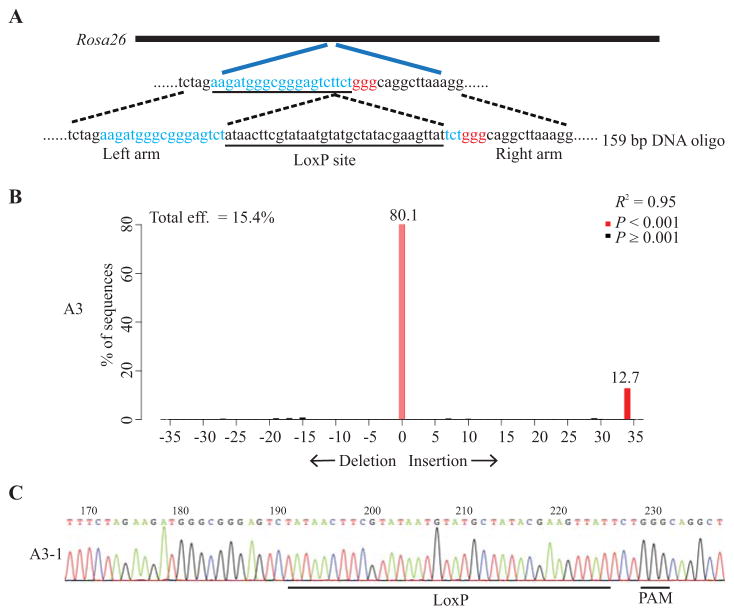
To test whether delivery of Cas9 protein with a series of electroporation is efficient to generate targeted insertion of small DNA fragments, we prepared IVF embryos from the C57BL/6NJ strain and did the electroporation with a mixture of Cas9 protein (250 ng/μL), sgRNA (300 ng/μL) targeting the Rosa26 locus and a DNA oligo with a LoxP site flanked by homology sequence (1000 ng/μL) (Fig. 3A). The embryos were then transferred and live mice were recovered. TIDE (Tracking of Indels by DEcomposition) analysis of PCR products encompassing the target site showed that 1, 4, and 6 times of electroporation yielded 4 out of 10 mice, 0 out of 5 mice and 2 out of 3 mice carrying correct LoxP site in Rosa26 locus, respectively (Table 3). Those samples were subcloned and independent clones were sequenced. The sequencing results confirmed the insertion of correct LoxP site into the Rosa26 locus. Representative images showed the result of TIDE analysis and Sanger sequencing of one sample (Fig. 3). These results indicated that the small DNA fragment could be efficiently inserted into the target region through electroporation of Cas9 protein in C57BL/6NJ inbred strain mice.
Fig. 3. Delivery of Cas9 protein through electroporation achieved high efficiency of targeted small DNA fragment insertion.
A: Schematic of the Rosa26 target sequence and DNA oligo donor. The protospacer sequence is colored in blue and PAM sequence is in red. The LoxP site in the DNA oligonucleotide is underlined. B: A representative image shows the analysis result of the sizes of the PCR products using TIDE. The red bar at the 34 bp insertion indicates potential insertion of LoxP site. The result of one of the pups with one time of electroporation was shown (A3). C: PCR products were cloned and individual clones were sequenced. Sequencing trace from clones A3-1 is shown. The successfully inserted LoxP site is underlined.
Table 3.
CRISPR/Cas9 mediated small segment insertion of Rosa26 through electroporation of Cas9 protein in an inbred strain.
| Time of electroporation | Pseudo-ID | Number of embryos transferred | Number of mice born | Total mice analyzed | Live birth rate (%) | Percentage of Indela/KIb (%) | Percentage of mutantc (%) |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 15 | 6 | 6 | 33.33 | 60.00/40.00 | 90.00 |
| 2 | 15 | 4 | 4 | ||||
| 4 | 3 | 15 | 4 | 4 | 20.00 | 83.33/0 | 83.33 |
| 4 | 15 | 2 | 2 | ||||
| 6 | 5 | 15 | 3 | 3 | 10.00 | 66.67/66.67 | 100 |
| 6 | 15 | 1bdd | 0 |
The percentage of mice with indel mutation at the targeting site.
The percentage of mice with LoxP insertion at the targeting site.
The percentage of mice with any targeted mutation (indel or KI, or both).
bd represents born dead.
DISCUSSION
Although the ZEN protocol we reported previously worked efficiently for some target genes, such as Tet2, there were significant variations among different targets, which limited its application (Qin et al., 2015). For Aicda, Rosa26, and Smc1b loci, the old ZEN protocol failed to generate desired model, while microinjection worked well. By applying a series of electroporation of Cas9 mRNA, sgRNA and DNA oligo, we were able to make the precise nucleotide substitutions at Aicda locus, but the efficiency was still low.
Delivery of Cas9 protein into mammalian primary cells by electroporation produced genome modification with higher efficiency than delivery of Cas9 mRNA or plasmids (Liang et al., 2015; Schumann et al., 2015). It has also been shown that Cas9 protein delivered to mouse zygotes by microinjection could induce HDR with high efficiency (Aida et al., 2015). To further improve the ZEN technology, we performed electroporation using Cas9 protein instead of Cas9 mRNA, and the HDR efficiency was dramatically improved (Fig. 1). Although delivery of Cas9 protein using one time of electroporation worked efficiently for generating HDR alleles (Fig. 1 and Table 3), 6 times of electroporation achieved higher efficiency in generating homozygous HDR alleles at Aicda locus, and in producing large DNA segment deletion at Smc1b locus (Fig. 2). For various genetic modifications (precise nucleotide changes, targeted short segment insertion, and large segment deletion) in all three genomic loci, our new ZEN protocol achieved much higher efficiency than the old protocol.
The CRISPR-Cas9 system is widely used to generate animal models (Bassett and Liu, 2014; Liu et al., 2014; Yin et al., 2014; Ma et al., 2014; Niu et al., 2014; Chapman et al., 2015; Ruan et al., 2015; Chen et al., 2015; Zou et al., 2015), and microinjection is still the main method to deliver CRISPR-Cas9 components into the embryos. In addition to being technically demanding and low in throughput, microinjection requires significant investment in equipment and personnel training. In comparison, our improved ZEN method enables efficient genome editing in early embryos, while requires much less investment and is much easier to learn. It is likely the methods reported here could be adapted to facilitate the generation of genetically modified animal models in other species with high efficiency and ease.
MATERIALS AND METHODS
Preparation of the reagents for electroporation
The px330 plasmid was used as the template to amplify the Cas9 coding sequence. T7 promoter sequence was added to the forward primer. In vitro transcription (IVT) was performed using mMESSAGE mMACHINE T7 ULTRA Transcription kit (Life Technologies, USA). For sgRNA synthesis, the T7 promoter sequence was added to sgRNA sequence through PCR amplification using 2 universal primers (Table S6) and one primer with various gRNA sequences (Table S7). The T7-sgRNA PCR product was purified using Qiagen PCR product purification kit (Qiagen, Germany) and used as the template for IVT. The sgRNAs were synthesized using MEGAshortscript T7 kit (Life Technologies) and purified using MEGAclear kit (Life Technologies). Single-stranded DNA oligos were ordered as Ultramer DNA oligos from Integrated DNA Technologies (IDT, USA). The DNA oligo sequences were listed in Table S8. Commercial Cas9 mRNA was ordered from Trilink (L-6125, USA). Cas9 protein was ordered from PNA Bio (CP01-50, USA) or Thermo Fisher Scientific (B25641, USA)
To prepare the reagent for electroporation, all the components at the required amount were mixed, and lyophilized if the total volume is bigger than 12 μL. If the volume is lower, TE will be added to achieve 12 μL volume. The mixture was then centrifuged at 20,000g for 15 min at 4 °C. After centrifugation, 10 μL supernatant was transferred to a new nuclease free tube. For the preparation of Cas9 RNP (recombinant Cas9 protein binding with an sgRNA), the solution including Cas9 protein, sgRNA with or without DNA oligo were incubated at 37 °C for 15 min. The samples were then put on ice and used for electroporation immediately.
Zygote isolation, culture and transfer
All animal work was approved by the Jackson Laboratory Animal Care and Use Committee and adhered to the standards of Guide for the Care and Use of Laboratory Animals set forth by the NIH. When generating embryos via natural mating, the mouse embryos were isolated and cultured as described previously (Qin et al., 2015). The IVF was performed following a standard protocol with some minor changes (Byers et al., 2006). In brief, Cook RVF medium was used as the fertilization medium. Cumulus oocyte masses were pre-incubated in 1.0 mmol/L GSH for 30 min to improve fertilization rate and weaken the zona pellucida. The embryos were graded for fertilization and viability and deposited into pre-equilibrated microdrops of K-RVCL-50 media in COOK MINC bench top incubator. The PMSG was bought from ProSpec (USA) or EMD Millipore (Germany). The hCG was bought from ProSpec (USA) or Sigma-Aldrich (USA).
Zygote electroporation
Electroporation was performed as described before (Qin et al., 2015). In brief, zygotes were treated with the acidic Tyrode’s solution (T1788, Sigma-Aldrich, USA) for 10 s and washed extensively in pre-warmed M2 media. The embryos produced by IVF were not treated with the acidic Tyrode’s solution. To weaken the zona pellucida, they were previously treated with GSH before fertilization in the modified IVF protocol(Byers et al., 2006). Zygotes were then placed in 10 μL drops of Opti-MEM media (P/N 31985, Gibco, USA). 10 μL of solution including the Cas9 mRNA or protein/sgRNA/donor DNA oligo was mixed with the Opti-MEM drops with the embryos and deposited into a 1 mm electroporation cuvette (P/N 45-0124, Harvard Apparatus, USA). Electroporation was performed in an ECM830 Square Wave Electroporation System (BTX Harvard Apparatus). The electroporation setting is the same for all the experiments using 1 ms pulse duration and two pulses with 100 ms pulse interval at 30V. Following the electroporation, a pre-warmed 100 μL aliquot of M2 media was deposited into the cuvette with a sterile plastic pipette to recover the embryos. The zygotes were removed from the cuvette and washed in pre-warmed M2 media. Embryos were then cultured in vitro to blastocysts in a MINC benchtop incubator (COOK; 37°C, 5% CO2 and 5% O2/Nitrogen) or transferred into pseudopregnant female mice (CByB6F1/J). For the IVF embryos, they were cultured to the two-cell stage and then transferred.
For testing of the survival rate of the embryos after a series of electroporation, we delivered GFP mRNA at 350 ng/μL final concentration. 25 embryos from each group were used. To quantify GFP signal, we randomly drew 4 circles with the diameter 10μm in each embryos. The GFP intensity was analyzed using Image J. The sum of the values from four circled areas were used to represent the GFP level in each embryo, and the average GFP level from each group is plotted in Fig. S1D.
RFLP analysis and Sanger sequencing
Genomic DNA from tissue or embryos was extracted using alkaline lysis as described before (Qin et al., 2015). PCR with specific primers was performed under the following conditions: 98°C for 5 min, 34 cycles (98°C for 30 s, 58°C (or other annealing temperature) for 30 s, 68°C for 30 s), 68°C for 2 min, and hold at 4°C. 4 μL PCR products were digested with restriction enzymes and separated on an agarose gel (1.0%) with GelRed (Biotium, USA). PCR products were sequenced and cloned into the pCR4 blunt vector from the Zero Blunt TOPO® PCR Cloning Kit (Invitrogen, USA) to identify the correct single clones. The sequences of the individual clones were determined by Sanger sequencing.
For all experiments, the presence of indel allele was determined by analyzing the sequences of genotyping PCR products using TIDE software. Mouse containing at least one allele with small insertion or deletion at target site will be counted as an indel founder. The percentage of indel is calculated as the percentage of mice containing indel allele among all mice analyzed in the same experimental group. For Aicda locus, the founders containing HDR allele were identified by RFLP analysis (both EcoR V and BamH I digestion), followed by subcloning and sequencing. For the Rosa26 KI experiments, successful LoxP knock-in (KI) was identified as containing 34 bp insertion at the target site by TIDE analysis, followed by sub-cloning and sequencing. The percentage of KI is calculated as the percentage of mice containing KI allele among all mice analyzed in the same experimental group. The percentage of mutant is calculated as the percentage of mice containing indel, KI, or both types of mutant alleles among all mice analyzed in the same experimental group.
Supplementary Material
Acknowledgments
We thank the Microinjection Service group, Molecular Biology Service group and Reproductive Sciences & Transgenic Genotyping Services group in the Jackson laboratory for their excellent assistance with embryo generation, transfer, and sample preparation (Vance Dyer, April Lee, Christina Battis, Jerri Reynolds, Kristen Bourland, Sabrina Hatch, Thomas O’Rourke, Greg Perry, Jeffrey Lamont, Rick Maser, Lindsay Ware). This work was in part supported by the National Cancer Institute (grant No. P30CA034196), and an institutional grant from the Jackson Laboratory (H.W.). H.W. was supported by the National Natural Science Foundation of China (No. 31471215), Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDA01010409), and the National High Technology Research and Development Program (“863”Program) of China (No. 2015AA020307).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aida T, Chiyo K, Usami T, Ishikubo H, Imahashi R, Wada Y, Tanaka KF, Sakuma T, Yamamoto T, Tanaka K. Cloning-free CRISPR/Cas system facilitates functional cassette knock-in in mice. Genome Biol. 2015;16:87. doi: 10.1186/s13059-015-0653-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AR, Liu JL. CRISPR/Cas9 and genome editing in Drosophila. J. Genet. Genomics. 2014;41:7–19. doi: 10.1016/j.jgg.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Byers SL, Payson SJ, Taft RA. Performance of ten inbred mouse strains following assisted reproductive technologies (ARTs) Theriogenology. 2006;65:1716–1726. doi: 10.1016/j.theriogenology.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Chapman KM, Medrano GA, Jaichander P, Chaudhary J, Waits AE, Nobrega MA, Hotaling JM, Ober C, Hamra FK. Targeted germline modifications in rats using CRISPR/Cas9 and spermatogonial stem cells. Cell Rep. 2015;10:1828–1835. doi: 10.1016/j.celrep.2015.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Sanjana NE, Zheng K, Shalem O, Lee K, Shi X, Scott DA, Song J, Pan JQ, Weissleder R, Lee H, Zhang F, Sharp PA. Genome-wide CRISPR screen in a mouse model of tumor growth and metastasis. Cell. 2015;160:1246–1260. doi: 10.1016/j.cell.2015.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin SL, Barretto R, Habib N, Hsu PD, Wu XB, Jiang WY, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai T, Teng F, Guo R, Li W, Zhou Q. One-step generation of knockout pigs by zygote injection of CRISPR/Cas system. Cell Res. 2014;24:372–375. doi: 10.1038/cr.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Takemoto T. Electroporation enables the efficient mRNA delivery into the mouse zygotes and facilitates CRISPR/Cas9-based genome editing. Sci Rep. 2015;5:11315. doi: 10.1038/srep11315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Sakuma T, Yamamoto T, Mashimo T. Simple knockout by electroporation of engineered endonucleases into intact rat embryos. Sci Rep. 2014;4:6382. doi: 10.1038/srep06382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Potter J, Kumar S, Zou Y, Quintanilla R, Sridharan M, Carte J, Chen W, Roark N, Ranganathan S, Ravinder N, Chesnut JD. Rapid and highly efficient mammalian cell engineering via Cas9 protein transfection. J Biotechnol. 2015;208:44–53. doi: 10.1016/j.jbiotec.2015.04.024. [DOI] [PubMed] [Google Scholar]
- Liu D, Wang Z, Xiao A, Zhang Y, Li W, Zu Y, Yao S, Lin S, Zhang B. Efficient gene targeting in zebrafish mediated by a zebrafish-codon-optimized cas9 and evaluation of off-targeting effect. J Genet Genomics. 2014;41:43–46. doi: 10.1016/j.jgg.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Ma Y, Zhang X, Shen B, Lu Y, Chen W, Ma J, Bai L, Huang X, Zhang L. Generating rats with conditional alleles using CRISPR/Cas9. Cell Res. 2014;24:122–125. doi: 10.1038/cr.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y, Shen B, Cui Y, Chen Y, Wang J, Wang L, Kang Y, Zhao X, Si W, Li W, Xiang AP, Zhou J, Guo X, Bi Y, Si C, Hu B, Dong G, Wang H, Zhou Z, Li T, Tan T, Pu X, Wang F, Ji S, Zhou Q, Huang X, Ji W, Sha J. Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell. 2014;156:836–843. doi: 10.1016/j.cell.2014.01.027. [DOI] [PubMed] [Google Scholar]
- Qin W, Dion SL, Kutny PM, Zhang Y, Cheng A, Jillette NL, Malhotra A, Geurts AM, Chen YG, Wang H. Efficient CRISPR/Cas9-mediated genome editing in mice by zygote electroporation of nuclease. Genetics. 2015;200:423–430. doi: 10.1534/genetics.115.176594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan J, Li H, Xu K, Wu T, Wei J, Zhou R, Liu Z, Mu Y, Yang S, Ouyang H, Yanru Chen-Tsai R, Li K. Highly efficient CRISPR/Cas9-mediated transgene knockin at the H11 locus in pigs. Sci Rep. 2015;5:14253. doi: 10.1038/srep14253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann K, Lin S, Boyer E, Simeonov DR, Subramaniam M, Gate RE, Haliburton GE, Ye CJ, Bluestone JA, Doudna JA, Marson A. Generation of knock-in primary human T cells using Cas9 ribonucleoproteins. Proc Natl Acad Sci USA. 2015;112:10437–10442. doi: 10.1073/pnas.1512503112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153:910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Wang H, Shivalila CS, Cheng AW, Shi L, Jaenisch R. One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell. 2013;154:1370–1379. doi: 10.1016/j.cell.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Guell M, Niu D, George H, Lesha E, Grishin D, Aach J, Shrock E, Xu W, Poci J, Cortazio R, Wilkinson RA, Fishman JA, Church G. Genome-wide inactivation of porcine endogenous retroviruses (PERVs) Science. 2015;350:1101–1104. doi: 10.1126/science.aad1191. [DOI] [PubMed] [Google Scholar]
- Yin H, Xue W, Chen S, Bogorad RL, Benedetti E, Grompe M, Koteliansky V, Sharp PA, Jacks T, Anderson DG. Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype. Nat Biotechnol. 2014;32:551–553. doi: 10.1038/nbt.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Q, Wang X, Liu Y, Ouyang Z, Long H, Wei S, Xin J, Zhao B, Lai S, Shen J, Ni Q, Yang H, Zhong H, Li L, Hu M, Zhang Q, Zhou Z, He J, Yan Q, Fan N, Zhao Y, Liu Z, Guo L, Huang J, Zhang G, Ying J, Lai L, Gao X. Generation of gene-target dogs using CRISPR/Cas9 system. J Mol Cell Biol. 2015;7:580–583. doi: 10.1093/jmcb/mjv061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.