Abstract
Purpose
To evaluate tumor response, event-free survival (EFS), overall survival (OS), and toxicity of chemotherapy, children with NF1 and progressive low-grade gliomas (LGG) were enrolled on COG A9952 protocol and treated with carboplatin and vincristine (CV).
Patients and Methods
Non-NF1 patients were randomized to CV versus thioguanine, procarbazine, CCNU, vincristine (TPCV) on COG A9952. NF1 patients were assigned CV only. NF1 and non-NF1 patients, who were treated with CV, were compared on baseline characteristics, toxicity, tumor response, EFS, and OS.
Results
A total of 127 eligible patients with NF1 were non-randomly assigned to CV: 42 (33%) NF1 patients had events and 6 (4.7%) died. The 5-year EFS for the CV-NF1 group was 69% ± 4% versus 39% ± 4% for the CV-non-NF1 group (P<0.001). In univariate analysis, NF1 children had significantly higher tumor response rate and superior EFS and OS compared to CV-treated children without NF1. NF1 and non-NF1 patients differed significantly in amount of residual tumor, extent of resection, tumor location, and pathology. After multivariate analysis, NF1 was independently associated with better EFS (p < 0.001), but not with OS. NF1 patients also had decreased risk of grade 3 or 4 toxicities compared to non-NF1 patients. Three second malignant neoplasms (SMNs) occurred in NF1 patients receiving CV (CV-NF1), at a median of 7.8 years (range 7.3 to 9.4 years) after enrollment, but none in the non-NF1 group.
Conclusions
Children with NF1 tolerated CV well and had tumor response rate and EFS that were superior to children without NF1.
Keywords: Low grade glioma, childhood, pilocytic astrocytoma, Neurofibromatosis 1, carboplatin, vincristine
INTRODUCTION
Optic pathway gliomas occur in 15–20% of children with neurofibromatosis type 1 (NF1) and usually are identified within the first decade of life1, 2. Most have an indolent course and do not require therapy. However, a subset of children with NF1 have low grade glioma (LGG) in the optic chiasmal / hypothalamic region, posterior optic tract, optic nerve, and other locations that progress and become symptomatic, requiring initiation of therapy3. NF1-associated optic pathway gliomas are overwhelmingly pilocytic astrocytoma (PA) and biopsy is unnecessary for intrinsic optic pathway tumors with characteristic appearance on magnetic resonance imaging (MRI)4. Because surgery has limited therapeutic value in tumors intrinsic to the optic pathway and can produce further visual deficit, it is primarily limited to those patients with preexisting loss of vision, bulky disease and/ or at relapse after initial treatment5. Radiotherapy is usually withheld from children with NF1 because of the concerns about vascular damage with subsequent strokes and second malignancies in this group already predisposed to both because of their genetic condition4, 6–9. Therefore, it has become common practice to treat progressive optic pathway and other LGG in children with NF1 initially with chemotherapy. The most common regimen utilized has been carboplatin and vincristine (CV) based on prior clinical trials that primarily utilized this chemotherapy for children without NF1 with LGG10, 11.
The Children’s Oncology Group (COG) study (COG A9952) enrolled all young children with progressive hypothalamic/optic pathway gliomas or other eligible progressive LGG, in a prospective randomized fashion, to either treatment with CV or thioguanine, procarbazine, CCNU, and vincristine (TPCV). Due to concerns over the mutagenic potential of TPCV, children with NF1 were excluded from the randomization and only treated with CV. Results for children without NF1 treated on COG A9952 have been previously reported12. This report focuses on tumor response/control and treatment related toxicities of children with NF1 treated on this protocol and compares results to patients with non-NF1 tumors treated similarly12.
MATERIALS AND METHODS
Patients
Eligibility criteria for this study were the same as previously reported for the randomized non-NF patients12 including: age less than 10 years at study entry; residual LGG (WHO grades I and II13) in any area of the brain except pons and optic nerve without cranial extension; and no previous treatment except surgery. Other eligible diagnoses included pleomorphic xanthoastrocytoma, subependymal giant cell astrocytoma, LG ganglioglioma, LG oligodendroglioma, and infantile desmoplastic astrocytoma. For NF1 patients, objective progression, defined as at least 25% increase in tumor size estimated from the product of the maximal bi-dimensional measurements, was required. Characteristic chiasmatic-hypothalamic tumors intrinsic to the optic pathway were eligible without pathological confirmation. NF1 patients were all treated within 6 weeks after imaging confirmed tumor progression.
All patients/guardians gave written informed consent according to institutional and National Cancer Institute Guidelines, and the protocol was approved by the institutional review boards at all participating centers.
Study Design
Patients without NF1 were randomly assigned to CV versus TPCV. Patients with NF1 were non-randomly assigned only to the same CV regimen as the non-NF1 patients, as previously reported12. The objective response to chemotherapy was determined at end of chemotherapy by the institution and by central review by the study neuroradiologists (Vezina and Booth), who also reviewed the baseline MRI for eligibility. The annual follow-up included notation by the institution about subsequent treatments received after CV. For the purpose of this report, these forms were reviewed and subsequent therapy for all patients with events were collected.
Chemotherapy Adjustments for Toxicity
Children with NF1 followed the same dose reduction schedule as those without NF1, as previously reported12.
Evaluation of Tumor Response
Tumor size was estimated from maximal bi-dimensional measurements (i.e., area) by using the product of the longest diameter and its longest perpendicular diameter for solid components of each lesion, excluding cysts using the same criteria as described for the randomized non-NF study12. Because enhancement can vary depending on technique and timing, the fluid attenuated inversion recovery (FLAIR) and T2-weighted images were primarily used by the central reviewers to determine response14.
Statistical Methods
Pearson chi-square test was performed to test comparability of the baseline characteristics, possible prognostic factors, and stratification factors upon enrollment between NF1 and non-NF1 participants who received CV.
Cumulative probabilities of toxicity were estimated using life table methods, with time to an event defined as time from the start of therapy until first occurrence of grade 3 or 4 toxicity, or grade 4 only toxicity or if none of these events occurred, until the end of chemotherapy. A log-rank test was used to test the difference of cumulative probabilities of toxicity between NF1 and non-NF1 patients.
The primary endpoints for analysis of treatment efficacy were EFS and OS. Time to an event was defined as time from enrollment to first disease progression, disease recurrence, deaths from any cause, occurrence of second malignant neoplasm (SMN) or until last contact if no events occurred. OS was defined as time to death from any cause. Patients not experiencing an event or death were censored at the date of last contact. Nonparametric EFS and OS curves were computed using product-limit (Kaplan-Meier) estimates. Point estimates of EFS and OS were reported as estimate ± SE. Cox regression analysis was used to test equality of survivor functions between NF1 and non-NF1 patients, with a P value < 0.05 considered statistically significant. Because of the small number of observed deaths, a permutation test was used to confirm the p-value for the five-year OS comparison. Patients were analyzed according to their original assigned treatment without regard to deviations in treatment that might have occurred after enrollment following intent to treat methods.
Multivariate Cox regression analysis was used to analyze possible prognostic factors. Univariate Cox regression was used to estimate the main effect of each variable. Variables with P value less than 0.1 from univariate models were selected for inclusion in the multivariate model. Likelihood ratio tests were used to compare the full model and the model without individual variables to obtain the P-value for that variable. Variables with P-values less than 0.05 were included in the final model and considered as significant prognostic factors.
RESULTS
Patient Characteristics
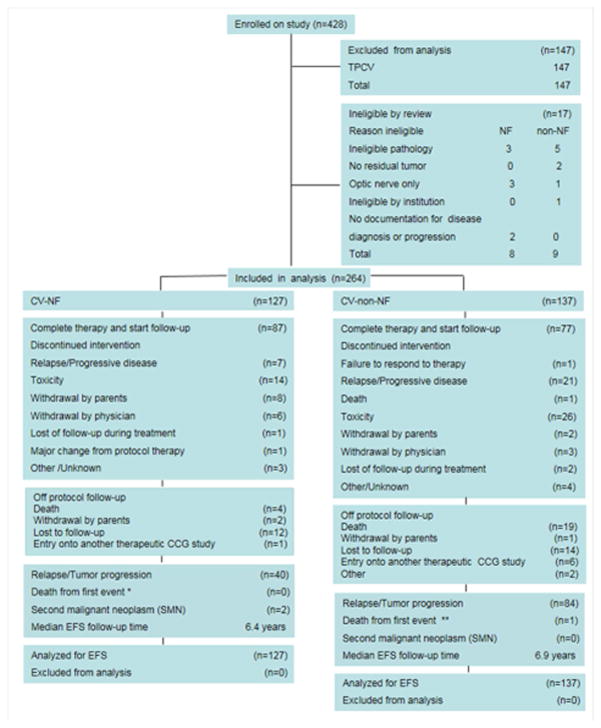
The COG A9952 study enrolled 264 eligible patients who were assigned CV (127 NF1 and 137 Non-NF1). Non-NF1 patients randomized to TPCV were not included in this analysis. No NF1 patients were assigned TPCV. Among those patients assigned to CV, 8 NF1 patients and 9 non-NF1 patients were deemed ineligible by central and chair review, as shown in Figure 1. All other patients were analyzed according to assigned regimen. Characteristics of eligible patients assigned to CV at time of enrollment are shown in Table 1. There were no significant differences between the two groups with respect to sex, race, and age at diagnosis, but tumor characteristics, including amount of residual tumor, extent of resection, results of institutional pathology review, and tumor site, differed significantly between NF1 and non-NF1 patients (P<0.001).
Figure 1.
CONSORT diagram.
(*) 6 patients died: 2 from progressive/persistent disease, 1 from infection, 1 from hemorrhage, 2 from other/unreported reason. None of the deaths were the first event.
(**) 21 patients died: 18 from progressive/persistent disease, 3 from other/unknown reason. The death was the first event in one patient.
Patients with discontinued intervention or lost to follow-up were censored at date last seen. Of the 3 patients with SMN, 2 as shown had the SMN as first event.
Table 1.
Characteristics of patients who received carboplatin and vincristine
| NF1 (N=127) | Non-NF1 (N=137) | P | |||
|---|---|---|---|---|---|
| Characteristic | # | % | # | % | |
| Sex | 0.47 | ||||
| Male | 64 | 50 | 63 | 46 | |
| Female | 63 | 50 | 74 | 54 | |
| Age, years | |||||
| <3 | 43 | 34 | 50 | 36 | 0.57 |
| 3–6 | 48 | 38 | 56 | 41 | |
| >6–10 | 36 | 28 | 31 | 23 | |
| Race | 0.26 | ||||
| White | 108 | 85 | 104 | 76 | |
| Hispanic | 8 | 6 | 15 | 11 | |
| African American | 7 | 6 | 9 | 7 | |
| Other/unknown | 4 | 3 | 9 | 7 | |
| Amount of residual tumor | |||||
| <1.5 cm2 | 12 | 9 | 15 | 11 | <0.001 |
| 1.5–3.0 cm2 | 36 | 28 | 28 | 20 | |
| >3 cm2 | 42 | 33 | 79 | 58 | |
| Unknown | 37 | 29 | 15 | 11 | |
| Extent of resection | |||||
| No surgery | 104 | 82 | 24 | 18 | <0.001 |
| Biopsy only (<10%) | 12 | 9 | 48 | 35 | |
| Partial/subtotal (10–95%) | 7 | 6 | 57 | 42 | |
| Radical subtotal (>95%) | 2 | 2 | 7 | 5 | |
| Unknown | 2 | 2 | 1 | 1 | |
| Institutional Pathology | |||||
| Pilocytic astrocytoma | 12 | 9 | 60 | 44 | <0.001 |
| LG fibrillary astrocytoma | 2 | 2 | 12 | 9 | |
| LG astrocytoma, NOS | 2 | 2 | 15 | 11 | |
| Other eligible diagnosis | 0 | 0 | 10 | 7 | |
| No surgery | 111 | 87 | 40 | 29 | |
| Tumor site | |||||
| Optic chiasm/hypothalamic | 110 | 87 | 71 | 52 | <0.001 |
| Thalamus | 3 | 2 | 11 | 8 | |
| Other supratentorial | 4 | 3 | 20 | 15 | |
| Posterior fossa/brainstem | 6 | 5 | 25 | 18 | |
| Spinal cord | 1 | 1 | 6 | 4 | |
| Unknown | 3 | 2 | 4 | 3 | |
Treatment Events
Of the 127 eligible NF1 patients, 42 experienced an event, as identified by their treating institution, and 6 died: 2 NF patients died from progressive/persistent disease, 1 from infection, 1 from hemorrhage, and 2 from unreported causes. There were no deaths on treatment. The 2 NF1 deaths due to tumor were in patients who had extensive tumor in the hypothalamus-optic chiasm with extension either into the frontal lobe or basal ganglion and thalamus. A total of three SMNs occurred in three NF1 patients at a median of 7.8 years (range 7.3 to 9.4 years) after enrollment: anaplastic astrocytoma at remote location from primary, acute myeloid leukemia, and undifferentiated sarcoma. Each diagnosis was observed in one patient. All three patients who developed SMNs had received additional therapy after CV for either recurrent tumor (1) and or carboplatin allergy (2) that included temozolomide, prior to development of their SMNs. None of these patients received radiotherapy. Of 42 NF1 patients with events, 27 reported 1–3 subsequent chemotherapy regimens after CV (Table 2). As shown in Figure 1, the two patients who had allergy and change in treatment had a SMN as first event, while the other patient had recurrence as first event. Patients who did not experience an EFS event were followed for a median of 6.4 years.
Table 2.
Subsequent treatments in 27 NF1 patients who received 1–3 other chemotherapy regimens after CV and # with subsequent malignant neoplasms (SMN).
| Subsequent Treatments | # Patients | # SMN |
|---|---|---|
| PCV or TPCV | 4 | 0 |
| Temozolomide | 9 | 2 |
| Temozolomide + thalidomide | 1 | 1 |
| Carboplatin + Vincristine | 5 | 0 |
| Vinblastine | 6 | 0 |
| Radiation | 8 | 0 |
| Rapamycin | 2 | 0 |
| Lenalidomide | 2 | 0 |
| Thalidomide | 1 | 0 |
| Etoposide | 2 | 0 |
| Cyclophosphamide + celecoxib | 1 | 0 |
Overall Outcome and Effect of NF1 Status
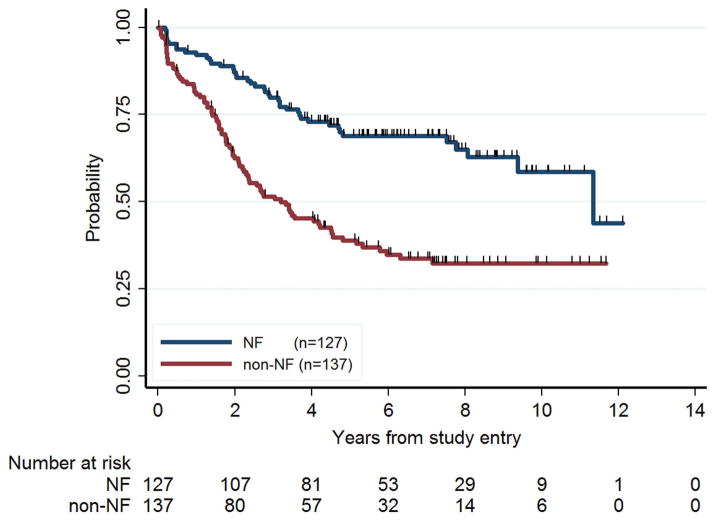
NF1 patients had a better EFS than non-NF1 patients. In univariate analysis, five-year EFS ± SE was 69% ± 4% for NF1 patients and 39% ± 4% for non-NF patients (P<0.001, Figure 2). NF1 patients also had better overall survival. Five-year OS was 98% ± 1% for NF1 patients and 87% ± 3% for non-NF1 patients (P=0.003; permutation test P=0.002). Among patients with hypothalamic/optic chiasmal tumors, five-year EFS was 68% ± 5% for NF1 and 38% ± 6% for non-NF1 (P<0.001), and five-year OS was 99% ± 1% for NF1 and 86% ± 4% for non-NF1 (P=0.01).
Figure 2.
Event-free survival (EFS) for NF1 and non-NF1 children. NF1 patients had significantly better prognosis than non-NF1 patients in terms of EFS (P<0.001).
A multivariate analysis was performed to see whether the apparent difference in NF/non-NF outcome was due to other factors. While age and amount of residual were found to be independent prognostic factors, the NF1/non-NF1 difference in EFS nonetheless remained significant (HR 2.45, p<0.001). However, the OS difference was no longer significant after controlling for significant prognostic factors sex, age, residual, pathology, and tumor site (Table 4). Restricting these multivariate analyses to optic pathway/hypothalamic tumors, which comprised the majority of NF1 patients, did not change these conclusions.
Table 4.
Cox multivariate analysis of EFS and OS in all patients.
| Prognostic Factor | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| Affecting event-free survival (EFS) | |||
| Age, years | 0.02 | ||
| < 3 | 1 | --- | |
| 3 to 6 | 0.55 | 0.37–0.83 | |
| >=6 | 0.68 | 0.43–1.06 | |
| Residual tumor | 0.05 | ||
| < = 1.5 cm2 | 1 | --- | |
| 1.5–3.0 cm2 | 1.47 | 0.66–3.27 | |
| >3 cm2 | 2.25 | 1.08–4.69 | |
| Unknown | 1.64 | 0.71–3.78 | |
| NF1 status | <0.001 | ||
| NF1 | 1 | --- | |
| Non-NF1 | 2.45 | 1.64–3.66 | |
| Affecting overall survival (OS) | |||
| Sex | 0.05 | ||
| Male | 1 | --- | |
| Female | 2.33 | 0.99–5.51 | |
| Age, years | 0.04 | ||
| < 3 | 1 | --- | |
| 3–6 | 0.29 | 0.094–0.88 | |
| >= 6 | 0.35 | 0.12–1.03 | |
| Residual tumor | 0.02 | ||
| <=1.5 cm2 | 1 | --- | |
| 1.5–3.0 cm2 | 0.17 | 0.01–2.04 | |
| >3.0 cm2 | 1.78 | 0.36–8.73 | |
| Unknown | 0.86 | 0.15–5.06 | |
| Institutional path | 0.01 | ||
| Pilocytic astrocytoma | 1 | --- | |
| LG fibrillary astrocytoma | 3.83 | 0.91–16.17 | |
| LG astrocytoma, NOS | 1.33 | 0.30–6.0 | |
| Other eligible diagnoses | 16.20 | 2.87–91.25 | |
| No surgery/insufficient | 0.78 | 0.25–2.40 | |
| Tumor site | 0.02 | ||
| Optic chiasm/hypothalamic | 1 | --- | |
| Thalamus | 5.64 | 1.42–22.42 | |
| Other supratentorial | 0.35 | 0.06–2.06 | |
| Posterior fossa/brainstem | 0.47 | 0.06–3.86 | |
| Spinal cord | 3.69 | 0.37–37.12 | |
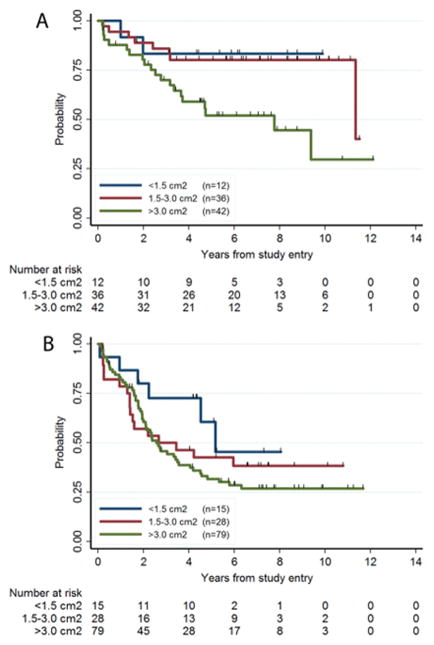
There was evidence that the influence of amount of residual tumor and age on EFS differed between NF1 and non-NF1 patients. The effect of amount of residual on EFS was larger for NF1 patients (P=0.02, log-rank trend test P=0.01), than for non-NF1 patients (P=0.18) (Fig. 3).
Figure 3.
The amount of residual tumor as a prognostic factor in NF1 (Fig. 3A) or non-NF1 (Fig. 3B) patients. The EFS outcomes for these 3 groups were significantly different for NF1 patients (Fig. 3A, P=0.02, log-rank trend test P=0.01), but not for non-NF1 patients (Fig. 3B, P=0.18).
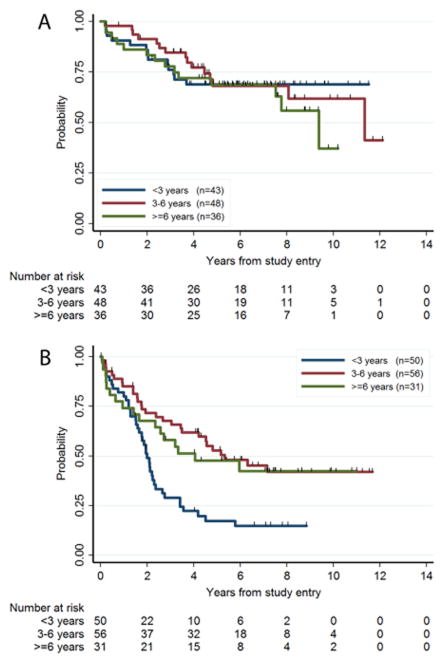
In contrast, the effect of age on EFS was larger in non-NF1 patients (P=0.0015, log rank trend test P=0.004) than in NF1 patients (P=0.73) (Fig. 4). In multivariate analysis these interaction effects did not quite reach statistical significance (p=0.20 for amount of residual, p=0.10 for age).
Figure 4.
Age as a prognostic factor in NF1 (A) or non-NF1 (B) patients. The EFS for the 3 age groups were significantly different for non-NF1 patients (Fig. 4B, P=0.0015, log rank trend test P=0.004), but not for NF1 patients (Fig 4A, P=0.73).
Tumor Response
Tumor responses at end of chemotherapy as coded by central review are shown in Table 3. There were 44 (32%) patients on non-NF1-CV and 45 (35%) NF1-CV respectively who were not evaluable for response because they went off therapy for allergic reactions to carboplatin, parent/ physician choice, or did not submit MRI scans at the appropriate time point. The test for trend demonstrates significantly better response rate for patients with NF1 (p=0.018).
Table 3.
Tumor response at end of chemotherapy as determined by central review. The test for trend demonstrates significantly better response rate for patients with NF1 (p=0.018).
| Review Response | CV-non-NF1 | CV – NF1 | ||
|---|---|---|---|---|
| # | % | # | % | |
| Partial Response | 33 | 35.4% | 38 | 46.3% |
| Minor Response | 14 | 15.1% | 16 | 19.5% |
| Stable disease | 16 | 17.2% | 16 | 19.5% |
| Progressive disease/recurrence/ Off by PD | 30 | 32.3% | 12 | 14.6% |
| TOTAL | 93 | 100.0% | 82 | 100.0% |
Toxicity
The cumulative probability of toxicity by end of chemotherapy for non-NF1 patients with CV was previously reported for the randomized study12. All allergic reactions reported were attributed to carboplatin. Compared to non-NF1 patients, NF1 patients had statistically significant lower probability of experiencing ANC toxicity, regardless of grade 3 or 4 (P=0.02) or grade 4 only (P=0.01). They also had significantly lower probability of experiencing grade 3 or 4 HGB toxicity (P=0.01). For all other toxicities there were no significant differences. Both groups had less than 5 % cumulative grade 3 or 4 toxicity for SGPT, bilirubin, creatinine, pulmonary, calcium, and magnesium. Both groups had over 5% reported cumulative grade 3 or 4 toxicity in peripheral nervous system (19% CV and 23% CV-NF1, p=0.46), central nervous system (12% CV and 5% NF1-CV, p=0.07), allergy (10% CV and 8% NF1-CV, p=0.31), and infection (23% CV and 18% CV-NF1, p=0.34).
DISCUSSION
The A9952 trial was initiated to validate the approach of using chemotherapy for LGG to improve survival and delay radiotherapy in young children and compare the effectiveness and toxicity of the two regimens12. NF1 patients were not randomized because of concerns about an increased risk of second malignancies from the genetic background and alkylator therapy included in the alternative TPCV regimen of this randomized study15. The vast majority of children with NF1 were entered on study without biopsy.
When we compare results of NF1 patients and non-NF1 patients receiving CV, the data revealed some notable differences (Table 1). However, the NF status remained significant (p< 0.001) for EFS when adjusted for significant prognostic variables in the multivariate analysis. The NF1 children also had a higher objective tumor response rate. The percentage of patients not evaluable for response with NF1 (35%) and non-NF1 (32%) was a weakness, but were similar between groups allowing valid comparison on this study. In the multivariate analysis for OS, NF status was not an independent prognostic variable. For NF1, the size of residual tumor had a larger effect on EFS than for non-NF1, whereas age had a larger effect on EFS for non-NF1 than for NF1.
For unclear reasons, the hematological toxicity occurred less frequently in NF1 compared to the non-NF1 subjects; other organ toxicity did not differ between the groups. Ototoxicity was not evaluated on our study. While ototoxicity with carboplatin is much less common than with cisplatin, in a recent study, very young children treated for retinoblastoma with a CV containing regimen were found to have ototoxicity16. Despite lack of evidence that carboplatin causes cognitive issues17, children treated for LGG with CV should be monitored long term for both cognitive deficits and ototoxicity. Both may affect quality of life in children with NF1 and brain tumors, especially those with visual deficits18.
Eligibility criteria for our study included only those NF1 patients who demonstrated 25% or greater increase in size of the tumor. Patients were not enrolled based on the initial finding of the tumor or optic pathway abnormality. This is different than other reports in the literature19–21. We were concerned about over-treatment of children with NF1, as had been suggested by others1, 4. In a cooperative group study being performed in a similar time period, the HIT-LGG study, 65 of 108 children with NF1 were treated at diagnosis. For EFS from initial diagnosis, they found that NF1 was an unfavorable prognostic factor, probably due to their methodology of designating a patient as having an event when they were enrolled on chemotherapy at diagnosis. 19
Despite restricting the regimen to CV, the majority of the second malignancies on A9952 study occurred in the NF1 population, likely due to their genetic risk and possible interaction with subsequent chemotherapy, such as temozolomide. In the non-NF1 population treated with TPCV previously reported, there were only two patients with SMNs, one of whom also received temozolomide as subsequent treatment12. While some studies suggest that radiation therapy is associated with SMNs in NF1 patients, the concern about alkylator therapy is supported by 2 patients reported in the literature with NF1 who had transformation of PA to anaplastic astrocytoma after treatment with carboplatin, vincristine, and oral temozolomide without radiotherapy22.
On the positive side, this study demonstrated that the NF1 children tolerated the CV chemotherapy well and had a higher tumor response and control rate than the non-NF1 children. There were no deaths or serious adverse events from the chemotherapy during the treatment period. However, the tolerance of the chemotherapy should not be used as justification in using it in children with NF1 where it is not necessary. Determining the indication for initiation of therapy in NF1 children remains controversial4. Physicians should be aware that the natural history of low grade gliomas in children with NF1 is more variable with prolonged stable disease and even spontaneous regressions reported 23. Also tumor enhancement can fluctuate depending on the timing of imaging14, so increase in enhancement may not be adequate change to warrant treatment. It is possible that spontaneous regression could have increased the response rate in the NF1 patients on our study.
Recent retrospective studies have suggested that despite response to chemotherapy, children with NF1 do not necessarily improve their vision with treatment20, 24, 25. However, Fisher et al. recently reported a multicenter retrospective analysis of visual outcomes in children with NF1 treated with chemotherapy20. On this study, 88 subjects were evaluable for visual acuity outcome. At the completion of chemotherapy, visual acuity had improved or remained stable in 72% of subjects. A limitation of our study was that ophthalmological evaluation was not mandated. Although such examinations can be difficult in very young children, their inclusion in future studies is required to evaluate efficacy of treatment.
There are relatively few other chemotherapy regimens that have been tested in LGG in children with NF1. Bouffet et al studied weekly vinblastine in recurrent/ refractory LGG and reported some objective responses in children with NF126–28. Packer et al reported objective responses to bevacizumab and irinotecan in 10 children with multiply recurrent LGG, including 3 patients with NF129. Targeted therapies have been investigated in LGG with mixed results. A phase II study of sorafenib in progressive LGG showed unexpected acceleration of tumor growth in both NF1 and BRAF fusion LGG, with the only response in a BRAF wild-type LGG; likely related to paradoxical ERK activation.30 More promising, a phase I trial of MEK inhibitor AZD6244 had 8/38 sustained responses in LGG31.
In summary, this large centrally reviewed study of LGG treated with chemotherapy validates that carboplatin and vincristine given in the weekly regimen is efficacious in producing chemotherapy response and tumor control in children with NF1. Development of SMNs, presumably related to germline susceptibility, is more frequent among NF1 than non-NF1 patients treated for these tumors, and appears to be associated with the use of secondary regimens, particularly alkylators such as temozolomide.
Acknowledgments
The authors wish to recognize the neuropathological review for this study provided by Dr. Allan Yates, who passed away in August 2010.
FUNDING SUPPORT
Supported by the COG Chairman’s Grant U10 CA98543 from NIH
Footnotes
Author Contributions: Joann L. Ater: Conceptualization, methodology, formal analysis, investigation, writing – original draft, visualization, and supervision. Caihong Xia: Software, formal analysis, data curation, writing – original draft, writing – review and editing, and visualization. Claire M. Mazewski: Conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, writing – review and editing, visualization, supervision, and project administration. Timothy N. Booth: Methodology, investigation, and writing – review and editing. David R. Freyer: Investigation, writing – review and editing, and visualization. Roger J. Packer: Conceptualization, methodology, formal analysis, investigation, writing – original draft, and writing – review and editing. Richard Sposto: Formal analysis, writing – review and editing, and supervision. Gilbert Vezina: Conceptualization, methodology, validation, investigation, and writing – review and editing. Ian F. Pollack: Conceptualization, resources, and writing – review and editing.
CONFLICT OF INTEREST DISCLOSURES
There are no financial disclosures or conflicts of interest from any authors, except the following:
Roger Packer, MD has had travel expenses supported by AstraZeneca
Contributor Information
Joann L. Ater, Email: jater@mdanderson.org, Division of Pediatrics, the University of Texas MD Anderson Cancer Center, Houston, TX
Caihong Xia, Email: caihongx@usc.edu, Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA
Claire M. Mazewski, Email: Claire.Mazewski@choa.org, Children’s Health Care Atlanta, Emory University School of Medicine, Atlanta, GA
Timothy N. Booth, Email: tim.booth@childrens.com, Univ. of Texas Southwestern Med. Center, Dallas, TX
David R. Freyer, Email: dfreyer@chla.usc.edu, Children’s Hospital Los Angeles
Roger J. Packer, Email: RPACKER@childrensnational.org, Children’s National Medical Center, Washington, DC
Richard Sposto, Email: RSposto@chla.usc.edu, Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA
Gilbert Vezina, Email: gvezina@cnmc.org, Children’s National Medical Center, Washington, DC
Ian F. Pollack, Email: ian.pollack@chp.edu, Children’s Hospital of Pittsburgh, Pittsburgh, PA
References
- 1.Listernick R, Charrow J, Gutmann DH. Intracranial gliomas in neurofibromatosis type 1. Am J Med Genet. 1999;89:38–44. doi: 10.1002/(sici)1096-8628(19990326)89:1<38::aid-ajmg8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 2.Listernick R, Charrow J, Greenwald MJ, Esterly NB. Optic gliomas in children with neurofibromatosis type 1. J Pediatr. 1989;114:788–792. doi: 10.1016/s0022-3476(89)80137-4. [DOI] [PubMed] [Google Scholar]
- 3.Janss AJ, Grundy R, Cnaan A, et al. Optic pathway and hypothalamic/chiasmatic gliomas in children younger than age 5 years with a 6-year follow-up. Cancer. 1995;75:1051–1059. doi: 10.1002/1097-0142(19950215)75:4<1051::aid-cncr2820750423>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 4.Listernick R, Ferner RE, Liu GT, Gutmann DH. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann Neurol. 2007;61:189–198. doi: 10.1002/ana.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawamura Y, Kamada K, Kamoshima Y, et al. Role of surgery for optic pathway/hypothalamic astrocytomas in children. Neuro Oncol. 2008;10:725–733. doi: 10.1215/15228517-2008-033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guillamo JS, Creange A, Kalifa C, et al. Prognostic factors of CNS tumours in Neurofibromatosis 1 (NF1): a retrospective study of 104 patients. Brain. 2003;126:152–160. doi: 10.1093/brain/awg016. [DOI] [PubMed] [Google Scholar]
- 7.Sharif S, Ferner R, Birch JM, et al. Second primary tumors in neurofibromatosis 1 patients treated for optic glioma: substantial risks after radiotherapy. J Clin Oncol. 2006;24:2570–2575. doi: 10.1200/JCO.2005.03.8349. [DOI] [PubMed] [Google Scholar]
- 8.Grill J, Couanet D, Cappelli C, et al. Radiation-induced cerebral vasculopathy in children with neurofibromatosis and optic pathway glioma. Ann Neurol. 1999;45:393–396. doi: 10.1002/1531-8249(199903)45:3<393::aid-ana17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 9.Merchant TE, Conklin HM, Wu S, Lustig RH, Xiong X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009;27:3691–3697. doi: 10.1200/JCO.2008.21.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Packer RJ, Ater J, Allen J, et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86:747–754. doi: 10.3171/jns.1997.86.5.0747. [DOI] [PubMed] [Google Scholar]
- 11.Packer RJ, Lange B, Ater J, et al. Carboplatin and vincristine for recurrent and newly diagnosed low-grade gliomas of childhood. J Clin Oncol. 1993;11:850–856. doi: 10.1200/JCO.1993.11.5.850. [DOI] [PubMed] [Google Scholar]
- 12.Ater JL, Zhou T, Holmes E, et al. Randomized Study of Two Chemotherapy Regimens for Treatment of Low-Grade Glioma in Young Children: A Report From the Children's Oncology Group. J Clin Oncol. 2012;30:2641–2647. doi: 10.1200/JCO.2011.36.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaudino S, Quaglio F, Schiarelli C, et al. Spontaneous modifications of contrast enhancement in childhood non-cerebellar pilocytic astrocytomas. Neuroradiology. 2012;54:989–995. doi: 10.1007/s00234-012-1010-3. [DOI] [PubMed] [Google Scholar]
- 15.Maris JM, Wiersma SR, Mahgoub N, et al. Monosomy 7 myelodysplastic syndrome and other second malignant neoplasms in children with neurofibromatosis type 1. Cancer. 1997;79:1438–1446. [PubMed] [Google Scholar]
- 16.Qaddoumi I, Bass JK, Wu J, et al. Carboplatin-associated ototoxicity in children with retinoblastoma. J Clin Oncol. 2012;30:1034–1041. doi: 10.1200/JCO.2011.36.9744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruggiero A, Trombatore G, Triarico S, et al. Platinum compounds in children with cancer: toxicity and clinical management. Anticancer Drugs. 2013;24:1007–1019. doi: 10.1097/CAD.0b013e3283650bda. [DOI] [PubMed] [Google Scholar]
- 18.Moore BD, 3rd, Ater JL, Needle MN, Slopis J, Copeland DR. Neuropsychological profile of children with neurofibromatosis, brain tumor, or both. J Child Neurol. 1994;9:368–377. doi: 10.1177/088307389400900406. [DOI] [PubMed] [Google Scholar]
- 19.Gnekow AK, Falkenstein F, von Hornstein S, et al. Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro Oncol. 2012;14:1265–1284. doi: 10.1093/neuonc/nos202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher MJ, Loguidice M, Gutmann DH, et al. Visual outcomes in children with neurofibromatosis type 1-associated optic pathway glioma following chemotherapy: a multicenter retrospective analysis. Neuro Oncol. 2012;14:790–797. doi: 10.1093/neuonc/nos076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernaiz Driever P, von Hornstein S, Pietsch T, et al. Natural history and management of low-grade glioma in NF-1 children. J Neurooncol. 2010;100:199–207. doi: 10.1007/s11060-010-0159-z. [DOI] [PubMed] [Google Scholar]
- 22.Peters KB, Cummings TJ, Gururangan S. Transformation of juvenile pilocytic astrocytoma to anaplastic pilocytic astrocytoma in patients with neurofibromatosis type I. J Pediatr Hematol Oncol. 2011;33:e198–201. doi: 10.1097/MPH.0b013e318205e230. [DOI] [PubMed] [Google Scholar]
- 23.Piccirilli M, Lenzi J, Delfinis C, Trasimeni G, Salvati M, Raco A. Spontaneous regression of optic pathways gliomas in three patients with neurofibromatosis type I and critical review of the literature. Childs Nerv Syst. 2006;22:1332–1337. doi: 10.1007/s00381-006-0061-3. [DOI] [PubMed] [Google Scholar]
- 24.Campagna M, Opocher E, Viscardi E, et al. Optic pathway glioma: long-term visual outcome in children without neurofibromatosis type-1. Pediatr Blood Cancer. 2010;55:1083–1088. doi: 10.1002/pbc.22748. [DOI] [PubMed] [Google Scholar]
- 25.Shofty B, Ben-Sira L, Freedman S, et al. Visual outcome following chemotherapy for progressive optic pathway gliomas. Pediatr Blood Cancer. 2011;57:481–485. doi: 10.1002/pbc.22967. [DOI] [PubMed] [Google Scholar]
- 26.Jakacki RI, Bouffet E, Adamson PC, et al. A phase 1 study of vinblastine in combination with carboplatin for children with low-grade gliomas: a Children's Oncology Group phase 1 consortium study. Neuro Oncol. 2011;13:910–915. doi: 10.1093/neuonc/nor090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouffet E, Jakacki R, Goldman S, et al. Phase II study of weekly vinblastine in recurrent or refractory pediatric low-grade glioma. J Clin Oncol. 2012;30:1358–1363. doi: 10.1200/JCO.2011.34.5843. [DOI] [PubMed] [Google Scholar]
- 28.Lafay-Cousin L, Holm S, Qaddoumi I, et al. Weekly vinblastine in pediatric low-grade glioma patients with carboplatin allergic reaction. Cancer. 2005;103:2636–2642. doi: 10.1002/cncr.21091. [DOI] [PubMed] [Google Scholar]
- 29.Packer RJ, Jakacki R, Horn M, et al. Objective response of multiply recurrent low-grade gliomas to bevacizumab and irinotecan. Pediatr Blood Cancer. 2009;52:791–795. doi: 10.1002/pbc.21935. [DOI] [PubMed] [Google Scholar]
- 30.Karajannis MA, Legault G, Fisher MJ, et al. Phase II study of sorafenib in children with recurrent or progressive low-grade astrocytomas. Neuro Oncol. 2014;16:1408–1416. doi: 10.1093/neuonc/nou059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banjerjee A, JR, Onar-Thomas j, et al. A phase 1 study of AZD6244 in children with refractory low-grade gliomas: A Pediatric Brain Tumor Consortium Report. Journal of Clinical Oncology. 2014;32:15s. [Google Scholar]