Abstract
Background
Women living in Africa experience the highest burden of cervical cancer. Research and investment to improve vaccination, screening, and treatment efforts are critically needed. We systematically reviewed and characterized recent research within a broader public health framework to organize and assess the range of cervical cancer research in Africa.
Methods
We searched online databases and the Internet for published articles and cervical cancer reports in African countries. Inclusion criteria included publication between 2004 and 2014, cervical cancer-related content pertinent to one of the four public health categories (primary, secondary, tertiary prevention or quality of life), and conducted in or specifically relevant to countries or regions within the African continent. The study design, geographic region/country, focus of research, and key findings were documented for each eligible article and summarized to illustrate the weight and research coverage in each area. Publications with more than one focus (e.g. secondary and tertiary prevention) were categorized by the primary emphasis of the paper. Research specific to HIV-infected women or focused on feasibility issues was delineated within each of the four public health categories.
Results
A total of 380 research articles/reports were included. The majority (54.6 %) of cervical cancer research in Africa focused on secondary prevention (i.e., screening). The number of publication focusing on primary prevention (23.4 %), particularly HPV vaccination, increased significantly in the past decade. Research regarding the treatment of precancerous lesions and invasive cervical cancer is emerging (17.6 %), but infrastructure and feasibility challenges in many countries have impeded efforts to provide and evaluate treatment. Studies assessing aspects of quality of life among women living with cervical cancer are severely limited (4.1 %). Across all categories, 11.3 % of publications focused on cervical cancer among HIV-infected women, while 17.1 % focused on aspects of feasibility for cervical cancer control efforts.
Conclusions
Cervical cancer research in African countries has increased steadily over the past decade, but more is needed. Tertiary prevention (i.e. treatment of disease with effective medicine) and quality of life of cervical cancer survivors are two severely under-researched areas. Similarly, there are several countries in Africa with little to no research ever conducted on cervical cancer.
Keywords: Cervical cancer, Africa, Systematic review, Prevention, Treatment, Quality of life, Feasibility challenges
Background
Cervical cancer is the second most common cancer among women worldwide, with an estimated 528,000 new cases and 266,000 deaths among women each year [1]. A disproportionate number of these cases (85 %) and deaths (87 %) occur among women living in low and middle income countries [1]. Women living with HIV are at increased risk of developing cervical cancer [2–4] and experience more rapid progression of the disease [5–7]. Since 1993, cervical cancer was classified as an AIDS-defining illness [8].
The World Health Organization (WHO) advocates a comprehensive approach to cervical cancer prevention and control to identify opportunities to deliver effective interventions [9]. Cervical cancer-related research has increased significantly over the past decade, representing biomedical, behavioral, and policy level findings. Existing review papers synthesize knowledge and advancements for multiple areas of focus within the larger effort of cervical cancer prevention and treatment, e.g., biomarkers for cervical cancer [10, 11], HPV vaccination for young adolescent women [12–15], and feasible approaches to screen and treat adult women in low resource settings [16–20]. The purpose of this systematic review is to assess and characterize recent research within a broader public health framework, utilizing well-known public health terminology to organize and assess the range of efforts to respond to cervical cancer. In this context, these include: Primary Prevention (preventing the initial onset of cervical cancer), Secondary Prevention (early detection by screening and treatment of precancerous cervical lesions), Tertiary Prevention (treatment of cervical cancer to reduce morbidity and mortality), and Quality of Life (post-treatment care or palliative care for those without treatment options) among women in African countries. Literature highlighting feasibility considerations (accessibility, affordability, health care infrastructure, and provider training) and findings specific to HIV-infected women are integrated as appropriate in each public health category.
Primary prevention
Vaccination is one of the most commonly used public health strategies to reduce the risk of infection and minimize the prevalence of the disease-causing agent (HPV) in the environment. Nearly all cases (99.7 %) of cervical cancer are caused by human papillomavirus (HPV) [21], particularly types 16 and 18 which cause more than two-thirds of all precancerous cervical lesions and cervical cancers [22, 23]. HPV is one of the most common sexually transmitted infections, with up to 75 % of sexually active people estimated to be infected at some point during their lives [24]. Fortunately, two vaccines are approved for use. The bivalent vaccine protects against HPV types 16 and 18. The quadrivalent vaccine protects against HPV types 16 and 18 and also types 6 and 11, which cause 90 % of genital warts [25]. Since HPV infection often occurs shortly after the onset of sexual activity (over 35 % of women are infected within 2 years of initiating sexual activity) [26–28], vaccination campaigns should target 9–13 year old youth, prior to sexual debut. The vaccines are over 95 % effective at preventing HPV infection caused by vaccine-type HPV when the full three course dose is given over six months [29, 30]. Since 2014, the WHO recommends a two-dose regimen for girls and boys aged 9–13 (quadrivalent vaccine) or aged 9–14 (bivalent vaccine) [31], which is not yet licensed in all countries, but reduces the follow-up burden while maintaining strong protective coverage [32–35].
The WHO recommends the inclusion of HPV vaccination in national immunization programs provided HPV represents a public health priority and vaccine delivery is feasible and cost-effective [31]. Unfortunately, HPV vaccination is not yet available in many African countries. By August 2014, only 58 countries had introduced HPV vaccination for girls into their national immunization program [31]. While the majority of these are high-resource countries, a few low to middle income countries in Africa including Rwanda, South Africa, Lesotho, and Uganda [36] have also introduced national HPV vaccines. In 2013, the Global Alliance for Vaccines and Immunizations (GAVI) began providing support for HPV vaccinations to eligible countries and will support demonstration projects in 23 countries, of which ten have been launched, primarily in sub-Saharan Africa [37, 38]. However, barriers to vaccination (i.e. concerns about the safety of the vaccine, provider reservations about recommending vaccination for younger girls, limited awareness of the relationship between HPV and cervical cancer, and varied parental acceptance of the HPV vaccine [39–42]) result in inconsistent vaccine uptake, globally [13, 43]. Recent efforts to vaccinate young boys have received less focus, but may help indirectly protect girls by reducing the risk of re-infection with HPV and will help prevent other HPV-related morbidities for men including penile cancer, anal cancer, oropharyngeal cancer, and genital warts [44].
Other primary prevention strategies to reduce HPV infection and cervical cancer include delaying sexual debut, reducing the number of lifetime sexual partners, and increasing condom use [45]. In addition to reducing HIV acquisition and transmission, medical male circumcision is also protective for HPV in males [46–48], which reduces the risk of initial or re-infection of HPV among women.
Secondary prevention
Screening for early detection and treatment is a cornerstone of secondary prevention. Early diagnosis and treatment of cervical pre-cancerous lesions prevents up to 80 % of cervical cancers in high resource countries where cervical cancer screening is routine [49]. In higher income countries, cervical cytology (Pap smear) in which cervical cells are examined in order to detect cervical intraepithelial neoplasia (CIN) became part of routine care in the 1940’s [50]. For women who screen positive for premalignant cervical lesions (i.e., CIN), a confirmatory colposcopy is required [51]. Cervical cytology, however, is not a feasible method of screening in many African countries given the required level of medical and laboratory infrastructure and trained personnel, multiple return visits with poor patient tracking strategies, and availability of such services often limited to capital cities. The proportion of women in sub Saharan Africa reporting a pelvic exam and pap test in the previous three years is very low (1.0 % in Ethiopia to 23.2 % in South Africa), with 40 % of women in Tunisia to 94 % of women in Malawi having never received a pelvic exam [52, 53].
The more feasible, and WHO-approved, strategy for cervical cancer screening in low resource settings is visual inspection with acetic acid (VIA) or visual inspection with Lugol’s iodine (VILI). After applying acetic acid or Lugol’s iodine directly on the cervix, pre-cancerous and cancerous lesions turn white, making them visible to the naked eye [54]. This method has high sensitivity among HIV-infected and uninfected women [55, 56]. Results are immediate, thus women who screen positive for precancerous lesions can theoretically be offered cryotherapy treatment during the same visit, or a “screen and treat” approach, if the health facility has the capacity. This strategy has been shown cost-effective, affordable, and an ideal first-line treatment for CIN of any grade when the cervical lesion size and location allows the cryoprobe tip to make adequate contact [57–60]. This ‘screen and treat’ strategy can avoid the burden of costly follow up visits, significant delays in treatment, and loss to follow up [61, 62].
Cryotherapy can be performed at the primary care level by mid-level providers, such as nurses or midwives, who can be trained to perform cryotherapy with a minimum of supplies and equipment [58, 63]. In developed countries, cryotherapy is approximately 90 % effective for all grades of CIN after 1 year [64]. The most commonly employed treatment options for pre-cancerous lesions are removal of diseased tissue using loop electrosurgical excision procedure (LEEP) which requires local anesthetic [65, 66] or by freezing the affected tissue with cryotherapy [57]. These treatments are typically performed by trained providers in outpatient clinics at provincial or referral level hospitals. Cold-knife conization [65] can be used to remove lesions that cannot be effectively treated with LEEP or cryotherapy.
The optimal frequency of screening is every 3–5 years depending on screening method [67], or within 3 years for women living with HIV [68]. If a woman can be screened only once in her lifetime, the most strategic age is between 30 and 39 years [69]. Recent estimates on national rates of cervical cancer screening are not available for many African countries, but a number of studies report self-reported screening rates to be low (8.3–64 %) [70–74], but slightly higher among women accessing HIV-care (9.4–80 %) [72, 75, 76]. A 2008 population-based survey in 57 countries estimated 19 % of women in developing countries were screened for cervical cancer in the preceding three years [77]. In addition to infrastructure and resource-related barriers, awareness of cervical cancer among reproductive-aged women remains low [70, 78–81] and an inadequate proportion of health care providers has been trained to provide high quality screening [82, 83]. Efforts have been made in many countries to integrate cervical cancer screening in HIV care, but routine provision is still limited [77, 84]. HPV DNA testing represents an emerging strategy for early detection of cervical cancer, particularly if technology innovations can permit point-of-care testing which will eliminate requirements for laboratory infrastructure and technical support [68, 85]. HPV DNA testing must be made affordable for widespread use in African countries.
Tertiary prevention
Women with abnormal cervical tissue are diagnosed with either precancerous lesions or invasive cervical cancer, both of which require treatment. Severe cervical dysplasia that remains undiagnosed or untreated can develop into invasive cancer [86]. Unfortunately, a significant proportion of women (56–80.6 %) [(Kenya) [87]; (Tanzania) [88]; (Nigeria) [89]) are identified once their cervical cancer is at an advanced stage [90].
Staging the severity of invasive cervical cancer requires assessment of the vagina, parametrium, urinary bladder and rectum by a combination of clinical and endoscopic procedures to determine the stage of progression (I – IVB) [91]. Inadequate laboratory facilities and personnel shortages may result in treatment decisions being made without proper diagnoses or adequate information. Treatments for invasive cervical cancer can include a range and combination of strategies including hysterectomy (requires surgical facilities), radiotherapy (external and intracavitary radiotherapy infrastructure), and chemotherapy [68, 92, 93]. The availability of these options are typically limited to capital cities in several African countries or, in some cases, not available at all [94]. Consequently, palliative care with symptom control and support may be the most likely option for severely late stage cervical cancer or for women with less advanced disease, but who cannot afford or access treatment. Studies indicate that only between 24–67 % of those diagnosed with cervical cancer in Tanzania, Zimbabwe, Uganda or Nigeria received some form of treatment (either radiotherapy or hysterectomy) [88, 89, 95, 96], with women in advanced stages (III and IV) of disease progression [96] and women co-infected with HIV [88] less likely to be treated.
Cervical cancer mortality rates in low resource countries are nearly three times as high as rates experienced in high resource settings [1, 97, 98]. Survival data for cervical cancer in African countries are limited. Estimated 5-year survival for women diagnosed with cervical cancer in 7 African countries between 2005 – 2009 was 56.3 % (range 19.5–96 %) [99]. Among women receiving treatment (radiotherapy and/or surgery), survival probabilities at one year post diagnosis ranged from 73.9–90.4 % and decreased progressively to 32.5 % by four years [87, 95, 96, 100]. Without treatment, observed survival is 58.6 at 1 year and decreases to 31.1 % by 4 years [95, 96]. Studies from Uganda and Zimbabwe suggest that although treatment with radiotherapy improves patient survival two to three years after diagnosis, this advantage disappears in later years [95, 96].
Quality of life
Cervical cancer is associated with psychological and physical morbidities that negatively impact quality of life [101, 102]. Age-adjusted, daily-adjusted life years (DALY) lost from cancer in African countries is consistently higher than those of high resource countries [103]. The estimated DALY lost from cervical cancer in sub-Saharan Africa is 641 years per 100,000 women [103]. Quality of life is most compromised among patients with inoperable cervical cancer treated by radiotherapy, with a majority reporting deterioration in physical, emotional, social, and economic support [104], and the highest risk for long-term dysfunction of bladder, bowels and psychosocial consequences [105]. Other treatment related side-effects such as extended vaginal bleeding and chronic radiation enteritis can affect physical and social aspects of their quality of life [106, 107].
Significant changes in the sexual domain resulting in marital discordance [104] and waning partner support over the course of treatment and survival [108] have been reported in Kenya and South Africa. While such data are limited for low-resource settings, literature reviews from high resource countries document post-treatment changes in body image, vaginal function, sexual satisfaction, and sexual relationship with partner; indicating a clear need for better integration of sexuality rehabilitation into routine clinical care [109–111]. Furthermore, there are few treatment options available that preserve fertility [92, 112–114], which can have significant implications for young women given the personal and cultural importance of childbearing [115]. Open communication about fertility and sexuality-related issues with cervical cancer patients of reproductive-age should occur prior to treatments to help shape expectations and quality of life during recovery [113].
In most African countries, there is a long historical precedent of providing palliative care at home by family or community members [116]. Although strengthened by the AIDS response, palliative care efforts still fail to provide effective pain relief [91], with the availability and accessibility of opioids for pain relief severely limited in African countries [117]. In a study among Nigerian cancer patients (including cervical cancer patients), the presence of pain was significantly associated with depressive and anxiety symptoms, suicidal ideation, poor sleep, impaired concentration, lack of opportunity for leisure, dissatisfaction with health, poor overall quality of life, poor ability to get around and the need for excessive medical treatment to function in daily life [118]. In 2011, only four African countries had integrated palliative care into their cancer strategic plans and two others had stand-alone national palliative care policies [119]. Pain management for cancer patients should not be neglected as countries develop and adapt their response to cervical and other cancers.
Feasibility considerations
Governments grapple with challenges posed by limited funds and competing healthcare priorities including a heavy burden from both infectious and chronic diseases. While the African Cancer Registry Network (AFCRN), launched in 2012, supports 25 cancer registries in 19 member countries in sub Saharan Africa [120, 121], many still lack established cancer prevention and control health policies [94, 122]. The geographic distribution of cancer treatment centers with cytology laboratories, radiotherapy and chemotherapy infrastructure severely limits accessibility for residents in more rural areas [49, 54, 85]. A 2009 situational analysis of east, central, and southern African countries estimated that only 4 % of institutions had equipment to perform outpatient treatment modalities such as cryosurgery [123]. This demand for scarce services typically results in long waiting periods (median 3.8 months in Ethiopia [100]) for treatment. The costs for return hospital visits, pathology reports, and subsequent treatment are beyond the resources of the majority of women in these settings [124]. According to a recent study, infrastructure investment of approximately $59 million would be required to equip every cervical cancer screening facility with cryotherapy equipment in 23 high-incidence sub-Saharan African countries [125] to be able to employ the recommended screen and treat approach [51]. Estimating the ability to screen nearly 20 million women over a 10 year period in these targeted countries, the costs for screening (VIA) would be less than $10 USD per woman, and the costs for treatment by cryotherapy or LEEP would range between $38 to $71 USD per woman [125]. Systems to track and refer women who test positive and support treatment retention are also lacking; diminishing the return on investment of current screening efforts.
Methods
We searched several online databases including PubMed/MEDLINE (NCBI), Embase (Elsevier), African Index Medicus (AIM), and Google Scholar for published studies. Our search also included highly relevant global and government reports not published in peer-reviewed journals. Inclusion criteria included publication between 2004 and 2014, cervical cancer-related content pertinent to one of the four public health categories (primary, secondary, tertiary prevention or quality of life), and conducted in or specifically relevant to countries or regions within the African continent. Reference sections of articles were reviewed to identify additional eligible articles. Searches for each of the four focus areas were conducted separately, including combinations of the following search words: 1) primary prevention [HPV vaccination, Africa, cervical cancer prevention, HPV prevention, cost effectiveness, medical male circumcision, coverage], 2) secondary prevention [cervical cancer screening, Africa, cervical cancer secondary prevention, HPV screening, screen and treat, male HPV screening], 3) tertiary prevention [cervical cancer treatment, Africa, SSA, pre-cancerous lesions, invasive cervical cancer, management of invasive cervical cancer in Africa, cervical cancer survival, HIV, access to treatment, challenges,], and 4) quality of life [cervical cancer, quality of life, Africa, sexual function, palliative care, relationship challenges, partner support, treatment recovery]. A separate search was conducted to identify research focused on issues of feasibility and infrastructure [health care infrastructure for cervical cancer, Africa, accessibility, affordability, training health care providers, provider knowledge, feasibility, HPV vaccine, screening, screen and treat, treatment]. Rather than creating a section dedicated to feasibility issues, these were integrated into the preceding categories, depending on the focus of the article.
The study design, geographic region/country, focus of research, and key findings were documented for each eligible article. Articles and reports were maintained in four separate spreadsheets (reflecting the four priority categories) and later combined to review and eliminate any duplication and to determine the best grouping for articles that may have covered more than one primary category. The articles and reports under each primary category were then analyzed and refined to highlight sub-themes, type of literature (original research, review article, lessons learned or policy paper) and the country or geographic region of research, Tables 1, 2, 3 and 4.
One thousand five hundred six records were reviewed through database and Internet searches (Fig. 1). Among those 389 met the criteria for inclusion. After eliminating nine duplicates among the categories, a total of 380 records were included in this review. Publications have been organized according to four targeted categories of public health interest to illustrate the weight and coverage of research in each area. Publications that span more than one area of focus (e.g. secondary and tertiary prevention) were categorized by the primary emphasis of the paper.
Fig. 1.
PRISMA flow diagram of the number of searches yielded, excluded, and reviewed
This systematic review did not require approval from an ethical review board or informed consent with participants as research efforts were limited to the review and analysis of previously published or presented cervical cancer research in African countries.
Results
Primary prevention
Table 1 reports the predominate focus of each article describing primary prevention. Of all 89 publications identified, 76 (85 %) focused on the HPV vaccination for the prevention of HPV/cervical cancer. Only 13 (15 %) focused on other prevention methods (mainly male circumcision but also condom use and microbicides), of which eight were performed in Uganda by the same study group. We could not find any study that examined the prevention of HPV and/or cervical cancer by delayed sexual debut, abstinence, and/or limiting the number of sexual partners. Considering the multiple foci of each paper, rather than just the primary focus, 17 talked about the cost-effectiveness or estimated impact of HPV vaccinations; 40 had a focus on acceptability, knowledge, and/or attitudes [12, 39–41, 126–152]; ten had a focus on uptake and/or retention [43, 152–160]; five had a focus on populations affected by HIV [47, 161–163]; four had a focus on males [132, 138, 150, 158]; four had a focus on safety and immunogenicity [161, 162, 164, 165]; one on adverse events [166]; and three on policy [167–169]; ten on male circumcision [46–48, 170–176]; two on lubricants, gels, and/or microbicides [177, 178], one on diaphragms [177]; and one on condoms [179].
Table 1.
Primary Prevention Research, n = 89
| Focus | Literature type | Countries/Geographic Regions Included | ||
|---|---|---|---|---|
| Primary Prevention | HPV Vaccination | Knowledge/Acceptability/Attitudes (N = 29) |
25 Original research [39–41, 126, 127, 130–132, 134–137, 139, 144–151, 158, 180, 256, 257] 2 Review Articles [12, 15] 0 Lessons Learned 2 Policy Papers [167, 258] |
Tanzania [126, 180], Uganda [40, 127], Nigeria [131, 137, 256], South Africa [130, 139, 148, 150, 151, 158], Malawi [41], Mali [132, 147], Cameroon [134, 144], Kenya [39, 145, 257], Botswana [135], Ghana [136], Zambia [146], Morocco [149] |
| Uptake/Retention (N = 10) | 6 Original research [43, 153, 155, 157, 159, 160] 1 Review Articles [259] 3 Lessons Learned [154, 156, 260] 0 Policy |
Uganda [153, 259, 261], Lesotho [43, 155], Cameroon [43, 155, 156], Tanzania [155, 160], Uganda [155, 157], Kenya [155], South Africa [159], Rwanda [154, 260] | ||
| Feasibility (N = 23) Cost/cost effectiveness [14, 170, 181, 214, 261–269] Estimated impact [270–272] Provider knowledge/training [128, 129, 133, 140–143] |
21 Original research [128, 129, 133, 140–143, 170, 181, 214, 261, 263–272] 2 Review Articles [14, 262] 0 Lessons Learned 0 Policy |
Tanzania [14, 181, 267, 269], Uganda [14, 267, 271], Sub-Saharan Africa [262, 264], Mali [263, 270], Nigeria [128, 140, 141, 143, 265], South Africa [129, 142, 214], GAVI-eligible countries [266, 268, 272], Kenya [267, 271], Mozambique [271], Zimbabwe [271], Guinea [170], Cameroon [133] | ||
| HIV (N = 2) | 2 Original research [161, 162] 0 Review Articles 0 Lessons Learned 0 Policy |
South Africa [161, 162] | ||
| Males (N = 1) | 1 Original research [138] 0 Review Articles 0 Lessons Learned 0 Policy |
Uganda [138] | ||
| Other (N = 11) Safety/Immunogenicity (n = 2) [164, 165] Adverse Events (n = 1) [166], Barriers (n = 1) [42], Policy (n = 6) [168, 169, 273–276], Conference Summary (n = 1) [152] |
3 Original research [164–166] 2 Review Articles [42, 152] 0 Lessons Learned 6 Policy [168, 169, 273–276] |
Uganda [166, 168, 274], Senegal [164], Tanzania [164, 165], Low-Middle Income Countries [42, 273, 276], Middle East/North Africa [275], Sub-Saharan Africa [152, 169] | ||
| Primary Prevention (HPV Vaccine) Total = 76 |
59 Original research 6 Review Articles 3 Lessons Learned 8 Policy |
South Africa 12, Uganda 12, Tanzania 10, Nigeria 8, Cameroon 6, Kenya 6, Mali 4, Low-Middle Income Countries 3, GAVI Eligible Countries 3, Lesotho 2, Rwanda 2, sub-Saharan Africa 4, Malawi 1, Botswana 1, Ghana 1, Zambia 1, Morocco 1, Ivory Coast 1, Mozambique 1, Zimbabwe 1, Middle East/North Africa 1, Guinea 1 | ||
| Non-Vaccine Prevention | Male Circumcision (N = 10) | 10 Original research [46–48, 171–176, 277] 0 Review Articles 0 Lessons Learned 0 Policy |
Uganda [47, 48, 171–174, 176, 277], South Africa [46, 175] | |
| Condoms (N = 1) | 1 Original research [179] 0 Review Articles 0 Lessons Learned 0 Policy |
South Africa [179] | ||
| Lubricant Gel, Microbicides and/or Diaphragm (N = 2) | 2 Original research [177, 178] 0 Review Articles 0 Lessons Learned 0 Policy |
South Africa [178], Zimbabwe [177] | ||
| Primary Prevention (Non-Vaccine) Total = 13 |
13 Original research | Uganda (8), South Africa (4), Zimbabwe (1) | ||
Of the 76 publications about HPV vaccination, the researchers and/or study received some support from the pharmaceutical companies that manufacture HPV vaccinations in 16 (21 %) of the studies [43, 126, 142, 153, 155–157, 160–162, 164–166, 170, 180, 181]. All studies that looked at safety/immunogenicity of the vaccines in HIV-infected [161, 162] and HIV-uninfected [164, 165] women were funded by pharmaceutical companies.
Knowledge/awareness and acceptability of the HPV vaccine was often reported together and assessed in many different countries in SSA and among many different populations. While acceptability of HPV vaccination tends to be high, knowledge and awareness of the vaccination is low, even amongst health care workers. There is a glaring lack of literature regarding HPV vaccination among HIV-positive populations and among males. Despite some data indicating HPV’s role in other cancers (i.e. penile, anal, throat and mouth), all vaccination programs have focused solely on cervical cancer prevention. The few studies that focused on males emphasized their ability to influence HPV vaccine decision making in females or HPV vaccination in males to help protect females from cervical cancer. Direct benefits for men’s health (i.e. reductions in genital warts, and penile, anal, throat/mouth cancers) were not highlighted.
Geographically, South Africa and Uganda contributed the most research, representing 18 and 23 % of all primary prevention articles, respectively, and were represented in both vaccination and non-vaccination articles.
Secondary prevention
We identified a significant number of publications (n = 208) focused on various aspects of secondary prevention of cervical cancer. Table 2 reports the predominant focus of each article. The primary focus of the 208 published articles include: 46 (22 %) on screening test performance; 16 (8 %) on screen-and-treat program implementation and/or outcomes; 28 (13 %) on screening feasibility including infrastructure, provider knowledge and attitudes, and costs; 59 (29 %) on women’s knowledge about, attitudes towards, and uptake of cervical cancer; 18 (8 %) on cervical cancer screening specifically among HIV+ women; 3 (1.5 %) on aspects of HPV/cervical cancer screening relevant to males; 23 on program (excluding screen-and-treat programs) or intervention implementation and/or outcomes; and 15 focused on other categories. A total of 65 (32 %) reported on observed or self-reported uptake (ranging between 0–100 %; mean = 25 %; median = 15 %) among different populations.
Table 2.
Secondary prevention research, n = 208
| Primary focus | Literature type | Countries/Geographic Regions Included | |
|---|---|---|---|
| Secondary Prevention | Screening Test Performance (N = 46) 11 VIA/VILI [194, 196, 197, 232, 278–284] 15 HPV Testing [163, 182–192, 285–287] 3 Cytology [288–290] 1 Colposcopy [291] 16 Comparison of 2+ tests [16, 193, 195, 198, 292–303] |
41 Original Research [182, 184–186, 188–198, 232, 278–293, 295, 296, 298–303] 4 Review Article [16, 187, 294, 297] Lessons Learned 1 Policy Papers [163] |
Africa (2) [285, 297], Developing Countries (4) [16, 284, 294, 302], Angola (1) [197], Botswana (1) [232], Burkina Faso (5) [194, 280, 282, 291, 298], Cameroon (4) [182, 193, 286, 300], Democratic Republic of Congo (3) [195, 198, 296], Republic of the Congo (6) [191, 194, 280, 282, 291, 298], Egypt (2) [186, 278], Gambia (1) [192], Ghana (1) [288], Guinea (5) [194, 280, 282, 291, 298], Ivory Coast (1) [190], Kenya (2) [184, 301], Mali (5) [194, 280, 282, 291, 298], Niger (5) [194, 280, 282, 291, 298], Nigeria (4) [279, 287, 289, 293], Rwanda (1) [294], South Africa (7) [163, 183, 187, 188, 290, 292, 299], Tanzania (2) [196, 295] |
| Screen-and-Treat (SNT) (N = 16) 8 VIA then Cryotherapy [63, 199–201, 304–307] 5 Other SNT methods [61, 308–311] 3 Guidelines/Overview [51, 202, 203] |
10 Original Research [61, 63, 200, 201, 305, 307–311] 2 Review [203, 304] 2 Lessons Learned [199, 202] 2 Policy [51,283 1,217] |
Global (1) [51], Africa (1) [203], Botswana (1) [307], Ghana (1) [63], Kenya (1) [61], Madagascar (1) [306], Malawi (1) [306], Nigeria (2) [306, 311],South Africa (2) [308, 310], Tanzania (1) [306], Uganda (2) [200, 306], Zambia (7) [199, 201, 202, 304–306, 309] | |
| Feasibility (N = 28) 2 Infrastructure [215, 220] 18 Provider Knowledge/Training/Attitudes [60, 82, 83, 208–211, 312–322] 8 Cost/Cost-effectiveness [59, 125, 212–214, 323–325] |
26 Original Research [59, 83, 125, 208–215, 220, 312–325] 2 Review [60, 82] 0 Lessons Learned 0 Policy |
Africa (3) [82, 125, 212], Cameroon (1) [313], Ghana (1) [323], Ivory Coast (1) [208], Kenya (2) [59, 325], Nigeria (11) [83, 211, 220, 312, 315–317, 319–321], South Africa (6) [59, 213, 214, 318, 324, 325], Tanzania (1) [209], LRC [60] | |
| Awareness and Utilization (N = 59) 44 Knowledge, Attitudes, Uptake [53, 70, 72, 74, 78–80, 204–207, 225, 326–357] 15 Determinants of Uptake [62, 73, 358–370] |
59 Original Research [53, 62, 70, 72–74, 78–80, 204–207, 225, 326–370] 0 Review 0 Lessons Learned 0 Policy |
Global (1) [368], Developing Countries (1) [328], Botswana (3) [72, 205, 349], Cameroon (1) [74], Ghana (2) [70, 326], Kenya (5) [204, 335, 340, 355, 358], Malawi (1) [53], Mozambique (1) [207], Morocco (1) [365], Nigeria (18) [78, 79, 225, 332–334, 337, 338, 342, 344–346, 348, 351, 352, 363, 366, 367], South Africa (12) [80, 327, 329, 330, 341, 347, 353, 356, 360, 361, 369], Sudan (1) [343], Tanzania (5) [62, 336, 362, 364, 370], Tunisia (1) [331] | |
| HIV-Positive Women (N = 18) 5 Service Integration [77, 84, 229–231] 9 Test Performance [371–379] 3 Knowledge, Attitudes, Uptake [75, 227, 228] 1 Screen-and-Treat [226] |
17 Original Research [75, 77, 84, 226–230, 371–379] 1 Review [231] 0 Lessons Learned 0 Policy |
Global (1) [231], Burkina Faso (1) [373], Cameroon (1) [230], Kenya (4) [229, 374, 376, 378], Nigeria (3) [75, 84, 227], South Africa (6) [226, 228, 371, 373, 375, 377], Uganda (1) [379], Tanzania (1) [77] | |
| Males and HPV Screening (N = 3) 1 Knowledge and Beliefs [221], 1 Male Partner Support [222], 1 Penile HPV Detection [223] |
3 Original Research [221–223] 0 Review 0 Lessons Learned 0 Policy |
Ghana (1) [221], Kenya (1) [223], Uganda (1) [222] | |
| Other (N = 38) 23 Program/Policy Implementation [54, 71, 216–219, 224, 380–395] 3 Screening Follow-Up [396, 397] and Side-effects [398] 5 Overview of Current Situation [123, 399–402] 7 Other Ethical Issues,[403] message development, [404] overviews,[69, 85, 405, 406] publication output [407] |
17 Original Research [71, 216, 217, 219, 224, 380, 381, 383–385, 388, 395–398, 403, 404] 14 Review [54, 69, 85, 123, 382, 391, 392, 394, 399, 400, 402, 405–407] 4 Lessons Learned [218, 386, 387, 393] 3 Policy [389, 390, 401] |
Africa (3) [391, 400, 405], Developing Countries (7) [54, 69, 85, 123, 382, 392, 406], Middle East/North Africa (1) [399], Ghana (2) [217, 401], Ivory Coast (1) [224], Kenya (2) [384, 403], Mali (1) [383], Mozambique (1) [216], Nigeria (6) [386–388, 393, 396, 398], South Africa (11) [71, 218, 219, 381, 385, 390, 394, 395, 402, 404, 407], Sudan (1) [389] | |
| Total N = 208 |
173 Original Research 23 Review Article 6 Lessons Learned 6 Policy Papers |
Global (3), Africa (9), Developing Countries (11), Middle East/North Africa (1), Angola (1), Botswana (5), Burkina Faso (6), Cameroon (7), Congo (9), Egypt (2), Gambia (1), Ghana (8), Guinea (5), Ivory Coast (3), Kenya (17), Madagascar (1), Malawi (2), Mali (6), Morocco (1), Mozambique (2), Niger (5), Nigeria (43), Rwanda (1), South Africa (45), Sudan (2),Tanzania (9), Tunisia (1), Uganda (4), Zambia (7), |
VIA/VILI and HPV testing were the most commonly evaluated screening tests, both individually and when compared with other tests. Of the 15 articles examining the performance of HPV testing, 12 compared different sample collection methods (i.e. self vs. physician collected samples [182–189]; dried cervical spots [190, 191]; tampons [188, 191, 192]). Sensitivity and specificity of each test ranged from 25–91.7 % [193, 194] and 64.6–98.2 % [195, 196] for VIA, 44–97.7 % [194, 195] and 68.9–97.3 % [196, 197] for VILI, 31–90.4 % [193, 195] and 84.5–96.9 % [193, 198] for cytology, and 63.9–100 % [192, 198] and 73–96.6 % [184, 198] for HPV DNA testing, respectively. The sensitivity and specificity of self-collected and physician collected HPV samples were similar, making it useful for women who may be reluctant to undergo a pelvic exam.
Most (53 %) of the articles focusing on a screen-and-treat approach assessed VIA/VILI and then treatment with cryotherapy. While many reported on barriers, challenges, and lessons learned from this approach [199–202], most indicated that screen-and-treat methods were safe, acceptable, and feasible in African settings and reduced loss-to-follow up after a positive screening test. One commentary [203] was critical of screen-and-treat approaches, stating a lack of evidence of safety which can compromise acceptability and potential effectiveness of all screening programs. Articles focusing on awareness and utilization were mostly cross-sectional studies and reported low levels of knowledge and awareness of cervical cancer screening, but generally positive attitudes [204, 205]. This was supported by higher uptake (59.6–100 %) of screening among women who were offered the test as part of an intervention [71, 75, 84, 206, 207].
The majority of articles in the feasibility section focused on provider knowledge, awareness, and acceptability. Although knowledge of cervical cancer and screening methods was higher amongst health care workers compared to the general population [208–211], utilization of screening among female health care workers remained low (4–41 %). Cervical cancer screening programs were extremely cost-effective, either implemented by themselves [59, 212, 213] or in combination with other cervical cancer prevention programs [214]. However, infrastructure challenges reported in both feasibility and program/policy implementation articles (long travel distances to screening and care centers, lack of gynecologists and laboratory pathologists and other manpower shortages, equipment problems, poor record keeping, inadequate patient follow-up, and delayed testing results [215–220]) make wide scale implementation and utilization challenging in many African settings.
Only three articles were identified that focused primarily on males and cervical cancer screening in Africa [221–223]. Similarly, while several articles included HIV-positive women in their analyses [72, 224, 225], only 16 focused exclusively on cervical cancer screening among HIV-positive women. Despite a higher positivity rate among HIV-infected women [224, 226], knowledge of cervical cancer screening [75, 225, 227, 228] and uptake [72, 75, 227, 228] is still low. Integrating HIV care and cervical cancer screening [229–231] and utilizing telemedicine [232] may provide viable methods for providing cervical cancer screening to HIV-positive women.
Geographically, the countries with the most research on secondary prevention were South Africa (n = 45), Nigeria (n = 43), and Kenya (n = 17). Countries were not evenly represented by category of secondary prevention. In several cases, a country was well-represented within one category but not in the others. For example, well represented in the screening test performance category were Republic of the Congo (n = 6) Guinea (n = 5), and Niger (n = 5), yet these countries were not represented within any of the other categories.
Tertiary prevention
We identified n = 67 articles highlighting aspects of tertiary prevention of cervical cancer in African countries, reported in Table 3. The primary focus of published studies and reports included: 7 (10 %) on treatment of precancerous lesions, 8 (12 %) on diagnosis and staging, 6 (9 %) on treatment for invasive cervical cancer, 8 (12 %) on survival outcomes, 23 (34 %) on HIV and cervical cancer, and 14 (21 %) regarding the feasibility of providing cervical cancer treatment. Treatment programs for cervical cancer are limited in most countries in Africa, as is research contributing outcome data, lessons learned, and implementation recommendations. Late diagnosis was routinely documented in several African countries among both HIV+ and HIV-uninfected women, with the proportion diagnosed with stage III or higher ranging from 56 to 90 % [87, 88, 233, 234]. Suboptimal management of symptomatic patients (in frequent gynecologic exams and appropriate referrals) further exacerbates the challenge of late diagnosis [234].
Table 3.
Tertiary prevention research, n = 67
| Focus | Literature type | Countries/Geographic Regions Included | |
|---|---|---|---|
| Tertiary Prevention | Treatment of Pre-Cancerous lesions 3 LLETZ [408–410] 2 Cryotherapy [57, 411] 2 Comparison LEEP/Cryotherapy [311, 412] N = 7 |
5 Original research [311, 408–411] 2 Review Articles [57, 412] 0 Lessons Learned 0 Policy |
Kenya (1) [411], South Africa (3) [408–410], SSA (2) [57, 412], Nigeria (1) [311] |
| Late Diagnosis/Staging N = 8 |
8 Original research [88, 233, 234, 365, 413–416] 0 Review Articles 0 Lessons Learned 0 Policy |
Nigeria (3) [413–415], Morocco (1) [365], Tunisia (1) [416], Zimbabwe (1) [233], Tanzania (1) [88], South Africa (1) [234] | |
| Treatment of Invasive Cervical Cancer 2 Surgery [417, 418] 3 Radiotherapy [419–421] 2 Combination therapy [422, 423] N = 7 |
5 Original research [417, 418, 420, 421, 423] 1 Review Articles [422] 1 Lessons Learned [419] 0 Policy |
SSA (1) [422], South Africa (3) [418, 420, 423], Uganda (1) [421], Senegal (1) [419], Nigeria (1) [417] | |
| Survival N = 8 |
6 Original research [87, 95, 100, 424–426] 2 Review Articles [427, 428] 0 Lessons Learned 0 Policy |
Malawi (1) [424], Uganda (2) [425, 428], Gambia (1) [428], Ethiopia (1) [100], South Africa (1) [426], Zimbabwe (1) [95], Kenya (1) [87], Egypt (1) [427] | |
| HIV and treatment 8 HIV and CIN [235, 236, 240–242, 244, 429, 430] 8 HIV and ICC [238, 239, 243, 246, 431–434] 7 HIV/Treatment of CIN and ICC [239, 245, 247, 248, 435–437] N = 23 |
22 Original research [235, 236, 238–248, 429–433, 435–437] 1 Review Articles [434] 0 Lessons Learned 0 Policy |
South Africa (11) [240, 242, 243, 245, 247, 248, 429, 432, 433, 436, 437], Kenya (5) [235, 236, 239, 244, 430], Uganda (1) [238], Global/SSA (3) [241, 246, 434], Cote ‘Ivoire (1) [431], Senegal (1) [435] | |
| Feasibility 1 Access/Awareness/Health Policy [255] 6 Training needs [92, 438–442] 5 Infrastructure needs [443–447] 2 Costs [448, 449] N = 14 |
10 Original research 1 Review Articles [445] 0 Lessons Learned 0 Policy Papers 2 Meeting report [92, 442, 447] 1 Book Chapter [255] |
Africa (4) [441–443, 447], Kenya (1) [438], Botswana (1) [444], Nigeria (1) [439], SSA (4) [92, 255, 445, 446], Morocco (1) [448], Tunisia (1) [449], South Africa (1) [440] | |
| N = 67 | 57 Original research 7 Review Articles 1 Lessons Learned 0 Policy Papers 2 Meeting report 1 Book Chapter |
Kenya (8), South Africa (20), SSA (10), Nigeria (6), Morocco (2), Tunisia (2), Zimbabwe (2), Tanzania (1), Uganda (4), Senegal (2), Malawi (1), Ethiopia (1), Gambia (1), Egypt (1), Cote d’Ivoire (1), Botswana (1) | |
The treatment sections for precancerous lesions and invasive cervical cancer are delineated by treatment strategy to indicate areas receiving more or less research focus. Studies describing outcomes or evaluating treatment strategies for precancerous lesions included loop electrical excision procedure (LEEP) vs cryotherapy (n = 2), large loop excision of the transformation zone (LEETZ) (n = 3), and cryotherapy (n = 2). These treatment strategies were found to be well accepted [235, 236]. Studies regarding treatment for invasive cervical cancer included surgery (n = 2), radiotherapy (n = 3) and combined radiochemotherapy (n = 2). This dearth of research reflects the limited capacity for cervical cancer treatment in African countries. According to the African Organization for Research and Training in Cancer (AORTIC) published in 2013, 22 % of the 54 countries in Africa have no access to any form of anti-cancer therapies, which include surgical oncology, chemotherapy and radiation [237]. Among women who do begin treatment, loss to follow up continues to be a problem [61, 238, 239].
The largest proportion of articles was specific to conditions and outcomes of HIV+ women with CIN or cervical cancer, followed by an emphasis on feasibility considerations for providing treatment. HIV+ women were more likely to present with CIN or cervical cancer at an earlier age [240], have higher grade CIN [241, 242], faster progression [243], more likely to experience CIN reoccurrence after treatment [236], and have poorer treatment and survival outcomes [238, 243, 244]. Antiretroviral therapy was shown to improve treatment outcomes for CIN and cervical cancer [245–248]. While feasibility articles were more comprehensive in nature, we categorized them according to the primary feasibility issue discussed: training needs of health care personnel on cervical cancer management (n = 6), the lack of medical infrastructure and scarcity of equipment to provide treatment (n = 5), and more generally, issues of access and awareness (n = 1) and costs (n = 2). While provider training for cervical cancer screening is slowly improving, multidisciplinary clinicians (pathologist, oncologists, radiologists) for the treatment of advanced cancer are far too few to meet the current demand [92, 237]. It’s important to note that many of the tertiary prevention articles also addressed feasibility concerns in the discussion sections, but this subsection reflects articles with a primary focus on feasibility.
The majority of research on tertiary prevention for cervical cancer was conducted in South Africa and Nigeria, yet research from countries in West, East and Southern Africa were also represented. 82 % were original research articles, 11 % review articles and the remaining 7 % were a combination of reports and commentary articles.
Quality of life
While there were a significant number of publications focusing on various aspects of quality of life among cervical cancer patients and survivors in the United States, Europe, and an increasing number from Taiwan, we found only n = 16 publications from countries in Africa. Publications were organized into the following areas of focus: 8 (50 %) on quality of life (physical, psychological, emotional, social, and spiritual), 5 (31 %) on palliative care, 1 (6 %) on partner support, and 2 (13 %) on symptoms and complications, see Table 4.
Table 4.
Quality of life research, n = 16
| Focus | Literature type | Countries/Geographic Regions Included | |
|---|---|---|---|
| Quality of Life | Quality of Life Outcomes N = 8 |
6 Original research [101, 104, 118, 450–452] 2 Review Articles [103, 249] 0 Lessons Learned 0 Policy Papers |
South Africa (2) [450, 451], Kenya (1) [104], Nigeria (2) [101, 118], Sudan (1) [452], Global-12 world regions (1) [103], Uganda (3) [249, 450, 451] |
| Palliative Care N = 5 |
3 Original research [250, 252, 253] 1 Review Articles [251] 0 Lessons Learned 1 Policy [117] |
Nigeria (1) [250], sub-Saharan Africa (1) [253], Africa (2) [117, 251], South Africa (1) [252], Uganda (1) [252] | |
| Partner Support N = 1 |
1 Original research [108] 0 Review Articles 0 Lessons Learned 0 Policy |
South Africa (1) [108] | |
| Symptoms and complications N = 2 |
2 Original research [107, 254] 0 Review Articles 0 Lessons Learned 0 Policy |
Nigeria (1) [107], Uganda (1) [254] | |
| Total N = 16 |
12 Original research 3 Review Articles 0 Lessons Learned 1 Policy Paper |
Global (1), SSA (3), South Africa (4), Kenya (1), Nigeria (4), Sudan (1), Uganda (5) | |
Among the seven publications describing quality of life outcomes, five studies reported the patient perspective and 1 study included data from both cervical cancer patients and caregivers. In regard to the timing of quality of life assessments, approximately half of these research studies included patients actively receiving treatment (typically radiotherapy) and the other half were receiving palliative care. A recent review article estimated age-adjusted, daily-adjusted life years lost attributed to various types of cancer, including cervical cancer, in sub-Saharan Africa and other global regions. Five studies focused on palliative care for cancer patients (specific to or including cervical cancer patients) in Africa highlighting challenges to provide adequate pain management [117, 249, 250], and training to optimize palliative care for cancer patients [251, 252], including one review article [253]. Only one study directly addressed partner support for cervical cancer patients [108]. One study assessed urologic complications among women with advanced cervical cancer in Uganda prior to treatment [254], and another assessed Cisplatin based chemotherapy for treatment of long term vaginal bleeding [107]. The majority of these studies was conducted in Nigeria, South Africa, and Uganda or had a sub-Saharan Africa regional focus.
Despite the heavy burden of disease and limited access to cervical cancer treatment, research regarding multiple quality of life issues for women, their partners, and caregivers is still limited for countries in Africa. A notable gap in the literature is attention to fertility-related concerns and the impact of the disease and its treatment on women’s bodies, sexual function, and relationships.
Distribution of cervical cancer research in Africa
Eligible articles and reports either focused globally (n = 4), on low or middle income countries (LMIC) (n = 14), the sub Saharan/African region (n = 27), and targeted multiple (n = 27) or single (n = 308) countries in Africa. A total of n = 30 countries in Africa were represented by at least one publication. Figure 2 illustrates the density of cervical cancer research publications by country. This excludes publications with a regional focus. Five categories ranging from 0 to >15 publications are delineated by a color gradient per country.
Fig. 2.
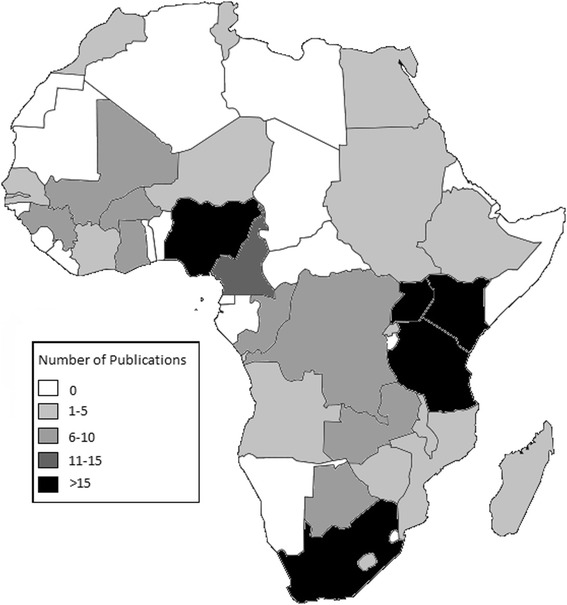
Geographic Distribution of Cervical Cancer Research within Africa. Figure 2 is a map of Africa illustrating the geographic distribution of cervical cancer research. The map represents individual countries only and does not clearly illustrate some of the smaller African countries. Fifty articles reporting on a geographic region were excluded (Excluded articles reported on the following geographic regions: Middle East/North Africa (2); 3 GAVI-eligible countries (3); Global (6); LMIC countries/developing countries (14); Africa/Sub-Saharan Africa (25). Image modified from: Tourbillon (Own work) [Public domain], via Wikimedia Commons, available at https://commons.wikimedia.org/wiki/File:Colored_map_of_Africa.png#
South Africa had the highest number of cervical cancer publications included in this review (n = 85), followed by Nigeria (n = 61), and Uganda and Kenya, with 33 and 32 publications, respectively. These same four countries also have cervical cancer publications representing each of the four target categories: primary, secondary, tertiary prevention and quality of life. Botswana, Cote d’Ivoire, Malawi and Morocco had publications from each of the three types of prevention, but none related to quality of life. Eligible cervical cancer publications were missing from a total of 24 African countries, including: Algeria, Benin, Burundi, Cape Verde, Central African Republic, Chad, Comoros, Djibouti, Equatorial Guinea, Eritrea, Gabon, Guinea-Bissau, Liberia, Libya, Mauritania, Mauritius, Namibia, Sao Tome and Principe, Seychelles, Sierra Leone, Somalia, South Sudan, Swaziland, and Togo.
Discussion
This study reviewed cervical cancer related research from countries in Africa over the past decade and categorized them according to primary focus areas within public health. Among a total of 380 research articles/reports eligible for inclusion in this systematic review, the majority (54.6 %) focused on secondary prevention. The number of publication focusing on primary prevention (23.4 %), particularly HPV vaccination, increased significantly in the past decade. Research regarding the treatment of precancerous lesions and invasive cervical cancer are emerging (17.6 %), but infrastructure and feasibility challenges in many countries have impeded efforts to provide and evaluate such treatment services. Studies assessing varying aspects of quality of life among women living with cervical cancer are limited, representing only 4.1 % of eligible publications. Across all categories, 11.3 % of publications focused on cervical cancer prevention or treatment among HIV-infected women. While integrated throughout, n = 65 (17.1 %) publications focused on various aspects of feasibility for cervical cancer control efforts, appealing for increased financial, human and political investments to adequately address the existing and increasing need for cervical cancer prevention and treatment services.
Limitations
While a wide range of articles are referenced in the introduction of this manuscript, the results section of the review excludes a significant number of articles describing the epidemiology of cervical cancer in sub Saharan Africa or a focus on genetic biomarkers, which were beyond the scope of this review. Some publications had significant overlap in the topics presented. This was particularly true for findings on secondary and tertiary prevention. For the purpose of this review, publications were assigned to only one category, but this does not mean other articles or reports did not give a degree of focus to additional categories as well.
Conclusion
The number of publications focused on cervical cancer control and treatment in African countries has increased over the past decade. Tertiary prevention (i.e. treatment of disease with effective medicine) and quality of life of cervical cancer survivors are two under-researched areas in African countries. Similarly, there are several countries in Africa with little to no research ever conducted on cervical cancer. Given the significant burden and high morbidity and mortality experienced by women with cervical cancer in this setting, targeted research is critically needed, particularly implementation science research to inform feasible and sustainable strategies to maximize the number of women reached with services. Lessons learned reports and publications describing and evaluating service implementation are highly relevant, as are cost-effectiveness studies to guide service strategies for scale-up. There is a need to support national capacity for developing population-based cancer registries to track cases and project needs [255], strategies to increase the availability of cervical cancer screening and treatment, and innovative strategies to retain cervical cancer patient in treatment.
Abbreviations
AFCRN, African Cancer Registry Network; AIDS, autoimmune deficiency syndrome; AIM, African index medicus; AORTIC, African Organization for Research and Training Center; CIN, cervical intraepithelial neoplasia; DALY, daily adjusted life years; DNA, deoxyribonucleic acid; GAVI, global alliance for vaccines and immunizations; HIV, human immunodeficiency virus; HPV, human papilloma virus; LEEP, loop electrical excision procedure; LEETZ, large loop excision of the transformation zone; LMIC, low and middle income countries; NCBI, National Center for Biotechnology Information; SSA, Sub Saharan Africa; VIA, visual inspection with acetic acid; VILI, visual inspection with lugol’s iodine; WHO, World Health Organization
Acknowledgements
Not applicable.
Funding
This systematic review was not directly funded; however, effort for authors SFK and CW was partially funded by R01HD076673.
Availability of data and materials
Data used for this study is limited to previously published or presented cervical cancer research and, thus, is publicly available by journal and conference review.
Authors’ contributions
SFK conceived of the idea for the systematic review, outlined the results and wrote the Introduction and Methods. CW, MM, NM and FNM systematically reviewed and summarized research meeting the eligibility criteria. MM and EB provided technical review and quality assurance given their clinical expertise in cervical cancer screening and treatment in low resource settings. CW compiled the extensive list of citations. All authors contributed to drafting and reviewing of the final manuscript. All authors read and approved the final manuscript.
Authors’ information
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This systematic review did not require approval from an ethical review board or informed consent with participants as research efforts were limited to the review and analysis of previously published or presented cervical cancer research in African countries.
References
- 1.Globocan. Cervical Cancer: Estimated Incidence, Mortality and Prevalence Worldwide in 2012 [Internet]. International Agency for Research on Cancer (IARC); 2012. Available from: http://globocan.iarc.fr/old/FactSheets/cancers/cervix-new.asp. Accessed 6 July 2015.
- 2.Frisch M. Human Papillomavirus-Associated Cancers in Patients With Human Immunodeficiency Virus Infection and Acquired Immunodeficiency Syndrome. J Natl Cancer Inst. 2000;92(18):1500–10. doi: 10.1093/jnci/92.18.1500. [DOI] [PubMed] [Google Scholar]
- 3.Hawes SE, Critchlow CW, Faye Niang MA, Diouf MB, Diop A, Touré P, et al. Increased Risk of High-Grade Cervical Squamous Intraepithelial Lesions and Invasive Cervical Cancer among African Women with Human Immunodeficiency Virus Type 1 and 2 Infections. J Infect Dis. 2003;188(4):555–63. doi: 10.1086/376996. [DOI] [PubMed] [Google Scholar]
- 4.Hawes SE, Critchlow CW, Sow PS, Touré P, N’Doye I, Diop A, et al. Incident high-grade squamous intraepithelial lesions in Senegalese women with and without human immunodeficiency virus type 1 (HIV-1) and HIV-2. J Natl Cancer Inst. 2006;98(2):100–9. doi: 10.1093/jnci/djj010. [DOI] [PubMed] [Google Scholar]
- 5.Clarke B, Chetty R. Postmodern cancer: the role of human immunodeficiency virus in uterine cervical cancer. Mol Pathol MP. 2002;55(1):19–24. doi: 10.1136/mp.55.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maiman M, Fruchter RG, Serur E, Remy JC, Feuer G, Boyce J. Human immunodeficiency virus infection and cervical neoplasia. Gynecol Oncol. 1990;38(3):377–82. doi: 10.1016/0090-8258(90)90077-X. [DOI] [PubMed] [Google Scholar]
- 7.Petry KU, Scheffel D, Bode U, Gabrysiak T, Köchel H, Kupsch E, et al. Cellular immunodeficiency enhances the progression of human papillomavirus-associated cervical lesions. Int J Cancer J Int Cancer. 1994;57(6):836–40. doi: 10.1002/ijc.2910570612. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention; National Center for Infectious Diseases Division of HIV/AIDS. 1993 Revised Classification System for HIV Infection and Expanded Surveillance Case Definition for AIDS Among Adolescents and Adults [Internet]. [cited 2014 Dec 12]. Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/00018871.htm. Accessed 6 July 2015.
- 9.World Health Organization (WHO). Comprehensive cervical cancer prevention and control: a healthier future for girls and women [Internet]. 2013. Available from: http://apps.who.int/iris/bitstream/10665/78128/3/9789241505147_eng.pdf?ua=1. Accessed 6 July 2015.
- 10.de Freitas AC, Coimbra EC, Leitão Mda CG. Molecular targets of HPV oncoproteins: potential biomarkers for cervical carcinogenesis. Biochim Biophys Acta. 2014;1845(2):91–103. doi: 10.1016/j.bbcan.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Flepisi BT, Bouic P, Sissolak G, Rosenkranz B. Biomarkers of HIV-associated Cancer. Biomark Cancer. 2014;3:11–20. doi: 10.4137/BIC.S15056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunningham MS, Davison C, Aronson KJ. HPV vaccine acceptability in Africa: a systematic review. Prev Med. 2014;69:274–9. doi: 10.1016/j.ypmed.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 13.Hopkins TG, Wood N. Female human papillomavirus (HPV) vaccination: Global uptake and the impact of attitudes. Vaccine. 2013;31(13):1673–9. doi: 10.1016/j.vaccine.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 14.Levin A, Wang SA, Levin C, Tsu V, Hutubessy R. Costs of Introducing and Delivering HPV Vaccines in Low and Lower Middle Income Countries: Inputs for GAVI Policy on Introduction Grant Support to Countries. PLoS ONE. 2014;9(6):e101114. doi: 10.1371/journal.pone.0101114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perlman S, Wamai RG, Bain PA, Welty T, Welty E, Ogembo JG. Knowledge and Awareness of HPV Vaccine and Acceptability to Vaccinate in Sub-Saharan Africa: A Systematic Review. PLoS ONE. 2014;9(3):e90912. doi: 10.1371/journal.pone.0090912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anorlu RI, Ola ER, Abudu OO. Low cost methods for secondary prevention of cervical cancer in developing countries. Niger Postgrad Med J. 2007;14(3):242–6. [PubMed] [Google Scholar]
- 17.Boisen M, Diedrich JT, Lonky NM, Guido R. Secondary prevention of cervical cancer part 1: screening for cervical cancer and its precursors. Clin Obstet Gynecol. 2014;57(2):279–91. doi: 10.1097/GRF.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 18.Guido R. Secondary prevention of cervical cancer part 2: initial management of abnormal cervical cancer screening test. Clin Obstet Gynecol. 2014;57(2):292–301. doi: 10.1097/GRF.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 19.Guido R, Lonky NM, Diedrich J. Secondary prevention of cervical cancer part 3: evidence-based management of women with cervical intraepithelial neoplasia. Clin Obstet Gynecol. 2014;57(2):302–15. doi: 10.1097/GRF.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 20.Roque DR, Wysham WZ, Soper JT. The surgical management of cervical cancer: an overview and literature review. Obstet Gynecol Surv. 2014;69(7):426–41. doi: 10.1097/OGX.0000000000000089. [DOI] [PubMed] [Google Scholar]
- 21.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 22.Clifford GM, Smith JS, Plummer M, Muñoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. 2003;88(1):63–73. doi: 10.1038/sj.bjc.6600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li N, Franceschi S, Howell-Jones R, Snijders PJF, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J Cancer. 2011;128(4):927–35. doi: 10.1002/ijc.25396. [DOI] [PubMed] [Google Scholar]
- 24.WHO. Human papillomavirus laboratory manual [Internet]. Geneva, Switzerland; 2010. Available from: http://whqlibdoc.who.int/hq/2010/WHO_IVB_10.12_eng.pdf?ua=1. Accessed 6 July 2015.
- 25.Centers for Disease Control and Prevention (CDC). HPV Vaccine Information for Clinicians - Fact Sheet [Internet]. 2012 [cited 2014 Dec 17]. Available from: http://www.cdc.gov/std/hpv/stdfact-hpv-vaccine-hcp.htm. Accessed 6 July 2015.
- 26.for the HPV PATRICIA Study Group, Castellsagué X, Paavonen J, Jaisamrarn U, Wheeler CM, Skinner SR, et al. Risk of first cervical HPV infection and pre-cancerous lesions after onset of sexual activity: analysis of women in the control arm of the randomized, controlled PATRICIA trial. BMC Infect Dis [Internet]. 2014 [cited 2014 Dec 16];14(1). Available from: http://www.biomedcentral.com/1471-2334/14/551. Accessed 6 July 2015. [DOI] [PMC free article] [PubMed]
- 27.Collins S, Mazloomzadeh S, Winter H, Blomfield P, Bailey A, Young LS, et al. High incidence of cervical human papillomavirus infection in women during their first sexual relationship. BJOG Int J Obstet Gynaecol. 2002;109(1):96–8. doi: 10.1111/j.1471-0528.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- 28.Kjaer SK, Chackerian B, van den Brule AJ, Svare EI, Paull G, Walbomers JM, et al. High-risk human papillomavirus is sexually transmitted: evidence from a follow-up study of virgins starting sexual activity (intercourse) Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2001;10(2):101–6. [PubMed] [Google Scholar]
- 29.Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Quadrivalent Vaccine against Human Papillomavirus to Prevent Anogenital Diseases. N Engl J Med. 2007;356(19):1928–43. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 30.The Future II Study Group Quadrivalent Vaccine against Human Papillomavirus to Prevent High-Grade Cervical Lesions. N Engl J Med. 2007;356(19):1915–27. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization (WHO). Human papillomavirus vaccines: WHO position paper, October 2014 [Internet]. 2014. Available from: http://www.who.int/wer/2014/wer8943.pdf?ua=1. Accessed 6 July 2015.
- 32.Dobson SRM, McNeil S, Dionne M, Dawar M, Ogilvie G, Krajden M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA. 2013;309(17):1793–802. doi: 10.1001/jama.2013.1625. [DOI] [PubMed] [Google Scholar]
- 33.Kreimer AR, Rodriguez AC, Hildesheim A, Herrero R, Porras C, Schiffman M, et al. Proof-of-Principle Evaluation of the Efficacy of Fewer Than Three Doses of a Bivalent HPV16/18 Vaccine. JNCI J Natl Cancer Inst. 2011;103(19):1444–51. doi: 10.1093/jnci/djr319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romanowski B, Schwarz TF, Ferguson LM, Ferguson M, Peters K, Dionne M, et al. Immune response to the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose or 3-dose schedule up to 4 years after vaccination: results from a randomized study. Hum Vaccines Immunother. 2014;10(5):1155–65. doi: 10.4161/hv.28022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. Evidence based recommendations on Human Papilloma Virus (HPV) Vaccines Schedules [Internet]. 2014. Available from: http://www.who.int/immunization/sage/meetings/2014/april/1_HPV_Evidence_based_recommendationsWHO_with_Appendices2_3.pdf. Accessed 6 July 2015.
- 36.Cervical Cancer Action. Cervical Cancer Action 2007 to 2014 [Internet]. 2014. Available from: http://www.cervicalcanceraction.org/pubs/CCA_accomplishments_2007-2014.pdf. Accessed 6 July 2015.
- 37.Cagney H. GAVI to fund HPV vaccines in low-income countries. Lancet Oncol. 2013;14(3):e92. doi: 10.1016/S1470-2045(13)70050-0. [DOI] [PubMed] [Google Scholar]
- 38.HPV Subteam, Gavi Alliance . HPV Demonstration Programme Update and initial lessons learnt on programme design. GAVI Alliance Board Technical Briefing Session. 2014. [Google Scholar]
- 39.Friedman AL, Oruko KO, Habel MA, Ford J, Kinsey J, Odhiambo F, et al. Preparing for human papillomavirus vaccine introduction in Kenya: implications from focus-group and interview discussions with caregivers and opinion leaders in Western Kenya. BMC Public Health. 2014;14:855. doi: 10.1186/1471-2458-14-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katahoire RA, Jitta J, Kivumbi G, Murokora D, Arube WJ, Siu G, et al. An assessment of the readiness for introduction of the HPV vaccine in Uganda. Afr J Reprod Health. 2008;12(3):159–72. [PubMed] [Google Scholar]
- 41.Ports KA, Reddy DM, Rameshbabu A. Barriers and Facilitators to HPV Vaccination: Perspectives from Malawian Women. Women Health. 2013;53(6):630–45. doi: 10.1080/03630242.2013.809046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wigle J, Coast E, Watson-Jones D. Human papillomavirus (HPV) vaccine implementation in low and middle-income countries (LMICs): Health system experiences and prospects. Vaccine. 2013;31(37):3811–7. doi: 10.1016/j.vaccine.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ladner J, Besson M-H, Hampshire R, Tapert L, Chirenje M, Saba J. Assessment of eight HPV vaccination programs implemented in lowest income countries. BMC Public Health. 2012;12(1):370. doi: 10.1186/1471-2458-12-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stanley M. HPV vaccination in boys and men. Hum Vaccines Immunother. 2014;10(7):2109–11. doi: 10.4161/hv.29137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plummer M, Franceschi S. Strategies for HPV prevention. Virus Res. 2002;89(2):285–93. doi: 10.1016/S0168-1702(02)00197-1. [DOI] [PubMed] [Google Scholar]
- 46.Auvert B, Sobngwi‐Tambekou J, Cutler E, Nieuwoudt M, Lissouba P, Puren A, et al. Effect of Male Circumcision on the Prevalence of High-Risk Human Papillomavirus in Young Men: Results of a Randomized Controlled Trial Conducted in Orange Farm, South Africa. J Infect Dis. 2009;199(1):14–9. doi: 10.1086/595566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serwadda D, Wawer MJ, Makumbi F, Kong X, Kigozi G, Gravitt P, et al. Circumcision of HIV-Infected Men: Effects on High-Risk Human Papillomavirus Infections in a Randomized Trial in Rakai, Uganda. J Infect Dis. 2010;201(10):1463–9. doi: 10.1086/652185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tobian AAR, Serwadda D, Quinn TC, Kigozi G, Gravitt PE, Laeyendecker O, et al. Male Circumcision for the Prevention of HSV-2 and HPV Infections and Syphilis. N Engl J Med. 2009;360(13):1298–309. doi: 10.1056/NEJMoa0802556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sankaranarayanan R, Budukh AM, Rajkumar R. Effective screening programmes for cervical cancer in low- and middle-income developing countries. Bull World Health Organ. 2001;79(10):954–62. [PMC free article] [PubMed] [Google Scholar]
- 50.Sahasrabuddhe VV, Parham GP, Mwanahamuntu MH, Vermund SH. Cervical cancer prevention in low- and middle-income countries: feasible, affordable, essential. Cancer Prev Res Phila Pa. 2012;5(1):11–7. doi: 10.1158/1940-6207.CAPR-11-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.World Health Organization. WHO guidelines for screening and treatment of precancerous lesions for cervical cancer prevention. [Internet]. 2013 [cited 2015 Jan 7]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK195239/. Accessed 6 July 2015. [PubMed]
- 52.Gakidou E, Nordhagen S, Obermeyer Z. Coverage of cervical cancer screening in 57 countries: low average levels and large inequalities. PLoS Med. 2008;5(6):e132. doi: 10.1371/journal.pmed.0050132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ports KA, Reddy DM, Rameshbabu A. Cervical Cancer Prevention in Malawi: A Qualitative Study of Women’s Perspectives. J Health Commun. 2015;20(1):97–104. doi: 10.1080/10810730.2014.908986. [DOI] [PubMed] [Google Scholar]
- 54.Denny L, Quinn M, Sankaranarayanan R. Chapter 8: Screening for cervical cancer in developing countries. Vaccine. 2006;24(Suppl 3):S3/71–7. doi: 10.1016/j.vaccine.2006.05.121. [DOI] [PubMed] [Google Scholar]
- 55.Huchko MJ, Sneden J, Leslie HH, Abdulrahim N, Maloba M, Bukusi E, et al. A comparison of two visual inspection methods for cervical cancer screening among HIV-infected women in Kenya. Bull World Health Organ. 2014;92(3):195–203. doi: 10.2471/BLT.13.122051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huchko MJ, Sneden J, Sawaya G, Smith-McCune K, Maloba M, Abdulrahim N, et al. Accuracy of visual inspection with acetic acid to detect cervical cancer precursors among HIV-infected women in Kenya. Int J Cancer J Int Cancer. 2015;136(2):392–8. doi: 10.1002/ijc.28996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jacob M, Broekhuizen FF, Castro W, Sellors J. Experience using cryotherapy for treatment of cervical precancerous lesions in low-resource settings. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2005;89(Suppl 2):S13–20. doi: 10.1016/j.ijgo.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 58.Gaffikin L, Blumenthal PD, Emerson M, Limpaphayom K, Royal Thai College of Obstetricians and Gynaecologists (RTCOG)/JHPIEGO Corporation Cervical Cancer Prevention Group [corrected] Safety, acceptability, and feasibility of a single-visit approach to cervical-cancer prevention in rural Thailand: a demonstration project. Lancet Lond Engl. 2003;361(9360):814–20. doi: 10.1016/S0140-6736(03)12707-9. [DOI] [PubMed] [Google Scholar]
- 59.Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD, Gordillo-Tobar A, Levin C, Mahé C, et al. Cost-effectiveness of cervical-cancer screening in five developing countries. N Engl J Med. 2005;353(20):2158–68. doi: 10.1056/NEJMsa044278. [DOI] [PubMed] [Google Scholar]
- 60.Blumenthal PD, Lauterbach M, Sellors JW, Sankaranarayanan R. Training for cervical cancer prevention programs in low-resource settings: focus on visual inspection with acetic acid and cryotherapy. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2005;89(Suppl 2):S30–7. doi: 10.1016/j.ijgo.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 61.Khozaim K, Orang’o E, Christoffersen-Deb A, Itsura P, Oguda J, Muliro H, et al. Successes and challenges of establishing a cervical cancer screening and treatment program in western Kenya. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2014;124(1):12–8. doi: 10.1016/j.ijgo.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 62.Perng P, Perng W, Ngoma T, Kahesa C, Mwaiselage J, Merajver SD, et al. Promoters of and barriers to cervical cancer screening in a rural setting in Tanzania. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2013;123(3):221–5. doi: 10.1016/j.ijgo.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blumenthal PD, Gaffikin L, Deganus S, Lewis R, Emerson M, Adadevoh S. Cervical cancer prevention: safety, acceptability, and feasibility of a single-visit approach in Accra, Ghana. Am J Obstet Gynecol. 2007;196(4):407. doi: 10.1016/j.ajog.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 64.Castro W, Gage J, Gaffikin L, Sellors J, Sherris J. Effectiveness, Safety, and Acceptability of Cryotherapy: A Systematic Literature Review. [Internet] Seattle: Path; 2003. [Google Scholar]
- 65.Apgar BS, Kaufman AJ, Bettcher C, Parker-Featherstone E. Gynecologic procedures: colposcopy, treatments for cervical intraepithelial neoplasia and endometrial assessment. Am Fam Physician. 2013;87(12):836–43. [PubMed] [Google Scholar]
- 66.Muruka K, Nelly MR, Gichuhi W, Anne-Beatrice K, Eunice CJ, Rose KJ. Same day colposcopic examination and loop electrosurgical excision procedure (LEEP) presents minimal overtreatment and averts delay in treatment of cervical intraepithelial neoplasia in Kenyatta National Hospital, Kenya. Open J Obstet Gynecol. 2013;03(03):313–8. doi: 10.4236/ojog.2013.33058. [DOI] [Google Scholar]
- 67.U.S. Preventive Services Task Force . ervical Cancer Screening: Summary of Recommendations and Evidence [Internet] 2012. [Google Scholar]
- 68.World Health Organization (WHO) Comprehensive Cervical Cancer Control: A guide to essential practice - Second edition [Internet] 2014. [PubMed] [Google Scholar]
- 69.Sherris J, Wittet S, Kleine A, Sellors J, Luciani S, Sankaranarayanan R, et al. Evidence-Based, Alternative Cervical Cancer Screening Approaches in Low-Resource Settings. Int Perspect Sex Reprod Health. 2009;35(03):147–54. doi: 10.1363/3514709. [DOI] [PubMed] [Google Scholar]
- 70.Abotchie PN, Shokar NK. Cervical Cancer Screening Among College Students in Ghana: Knowledge and Health Beliefs. Int J Gynecol Cancer. 2009;19(3):412–6. doi: 10.1111/IGC.0b013e3181a1d6de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maree JE, Lu XM, Wright SCD. Combining breast and cervical screening in an attempt to increase cervical screening uptake. An intervention study in a South African context: Cervical screening uptake. Eur J Cancer Care (Engl) 2012;21(1):78–86. doi: 10.1111/j.1365-2354.2011.01292.x. [DOI] [PubMed] [Google Scholar]
- 72.Mingo AM, Panozzo CA, DiAngi YT, Smith JS, Steenhoff AP, Ramogola-Masire D, et al. Cervical cancer awareness and screening in Botswana. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. 2012;22(4):638–44. doi: 10.1097/IGC.0b013e318249470a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mupepi SC, Sampselle CM, Johnson TRB. Knowledge, attitudes, and demographic factors influencing cervical cancer screening behavior of Zimbabwean women. J Womens Health. 2002;20(6):943–52. doi: 10.1089/jwh.2010.2062. [DOI] [PubMed] [Google Scholar]
- 74.Tebeu P-M, Major AL, Rapiti E, Petignat P, Bouchardy C, Sando Z, et al. The attitude and knowledge of cervical cancer by Cameroonian women; a clinical survey conducted in Maroua, the capital of Far North Province of Cameroon. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. 2008;18(4):761–5. doi: 10.1111/j.1525-1438.2007.01066.x. [DOI] [PubMed] [Google Scholar]
- 75.Ezechi OC, Gab-Okafor CV, Ostergren PO, Odberg PK. Willingness and acceptability of cervical cancer screening among HIV positive Nigerian women. BMC Public Health. 2013;13(1):46. doi: 10.1186/1471-2458-13-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rosser JI, Njoroge B, Huchko MJ. Cervical Cancer Screening Knowledge and Behavior among Women Attending an Urban HIV Clinic in Western Kenya. J Cancer Educ [Internet]. 2015 [cited 2015 Mar 12]; Available from: http://link.springer.com/10.1007/s13187-014-0787-7. Accessed 6 July 2015. [DOI] [PMC free article] [PubMed]
- 77.Plotkin M, Besana GV, Yuma S, Kim Y, Kulindwa Y, Kabole F, et al. Integrating HIV testing into cervical cancer screening in Tanzania: an analysis of routine service delivery statistics. BMC Womens Health. 2014;14(1):120. doi: 10.1186/1472-6874-14-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ayinde OA, Omigbodun AO, Ilesanmi AO. Awareness of cervical cancer, Papanicolaou’s smear and its utilisation among female undergraduates in Ibadan. Afr J Reprod Health. 2004;8(3):68–80. doi: 10.2307/3583394. [DOI] [PubMed] [Google Scholar]
- 79.Eze JN, Umeora OU, Obuna JA, Egwuatu VE, Ejikeme BN. Cervical cancer awareness and cervical screening uptake at the Mater Misericordiae Hospital, Afikpo, Southeast Nigeria. Ann Afr Med. 2012;11(4):238–43. doi: 10.4103/1596-3519.102856. [DOI] [PubMed] [Google Scholar]
- 80.Hoque M. Cervical Cancer Awareness and Preventive Behaviour among Female University Students in South Africa. Asian Pac J Cancer Prev. 2010;11:127–30. [PubMed] [Google Scholar]
- 81.Kivuti-Bitok LW, Pokhariyal GP, Abdul R, McDonnell G. An exploration of opportunities and challenges facing cervical cancer managers in Kenya. BMC Res Notes. 2013;6(1):136. doi: 10.1186/1756-0500-6-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Asonganyi E, Vaghasia M, Rodrigues C, Phadtare A, Ford A, Pietrobon R, et al. Factors affecting compliance with clinical practice guidelines for pap smear screening among healthcare providers in africa: systematic review and meta-summary of 2045 individuals. PLoS ONE. 2013;8(9):e72712. doi: 10.1371/journal.pone.0072712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Awodele O, Adeyomoye AA, Awodele DF, Kwashi V, Awodele IO, Dolapo DC. A study on cervical cancer screening amongst nurses in Lagos University Teaching Hospital, Lagos, Nigeria. J Cancer Educ Off J Am Assoc Cancer Educ. 2011;26(3):497–504. doi: 10.1007/s13187-010-0187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Odafe S, Torpey K, Khamofu H, Oladele E, Adedokun O, Chabikuli O, et al. Integrating cervical cancer screening with HIV care in a district hospital in Abuja, Nigeria. Niger Med J J Niger Med Assoc. 2013;54(3):176–84. doi: 10.4103/0300-1652.114590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wright TC, Kuhn L. Alternative approaches to cervical cancer screening for developing countries. Best Pract Res Clin Obstet Gynaecol. 2012;26(2):197–208. doi: 10.1016/j.bpobgyn.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 86.Crum CP, Abbott DW, Quade BJ. Cervical cancer screening: from the Papanicolaou smear to the vaccine era. J Clin Oncol Off J Am Soc Clin Oncol. 2003;21(10 Suppl):224s–30. doi: 10.1200/JCO.2003.01.116. [DOI] [PubMed] [Google Scholar]
- 87.Maranga IO, Hampson L, Oliver AW, Gamal A, Gichangi P, Opiyo A, et al. Analysis of Factors Contributing to the Low Survival of Cervical Cancer Patients Undergoing Radiotherapy in Kenya. PLoS ONE. 2013;8(10):e78411. doi: 10.1371/journal.pone.0078411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mosha D, Mahande M, Ahaz J, Njau B, Kitali B, Obure J. Factors associated with management of cervical cancer patients at KCMC Hospital, Tanzania: a retrospective cross-sectional study. Tanzan J Health Res [Internet]. 2009;11(2). Available from: http://www.ajol.info/index.php/thrb/article/view/45204/28692. Accessed 6 July 2015.
- 89.Gaya S, Muhammad A, Yakasai I, Galadanci H, Garba I. Cancer of the cervix in unscreened West African women. J Basic Clin Reprod Sci. 2012;1(1):44. doi: 10.4103/2278-960X.104296. [DOI] [Google Scholar]
- 90.Chirenje ZM, Rusakaniko S, Akino V, Mlingo M. A review of cervical cancer patients presenting in Harare and Parirenyatwa Hospitals in 1998. Cent Afr J Med. 2000;46(10):264–7. doi: 10.4314/cajm.v46i10.8566. [DOI] [PubMed] [Google Scholar]
- 91.International Agency for Research on Cancer, World Health Organization . Cervical Cancer Screening [Internet] 2005. [Google Scholar]
- 92.Southern Africa Litigation Centre. Tackling Cervical Cancer: Improving Access to Cervical Cancer Services for Women in Southern Africa [Internet]. 2012 [cited 2015 May 14]. Available from: http://www.southernafricalitigationcentre.org/1/wp-content/uploads/2012/11/CERVICAL-CANCER-Report1.pdf. Accessed 6 July 2015.
- 93.Abu-Rustum NR, Sonoda Y. Fertility-sparing surgery in early-stage cervical cancer: indications and applications. J Natl Compr Cancer Netw JNCCN. 2010;8(12):1435–8. doi: 10.6004/jnccn.2010.0107. [DOI] [PubMed] [Google Scholar]
- 94.World Health Organization . Cervical Cancer - Issues and Challenges [Internet] 2015. [Google Scholar]
- 95.Chokunonga E, Ramanakumar AV, Nyakabau AM, Borok MZ, Chirenje ZM, Sankila R, et al. Survival of cervix cancer patients in Harare, Zimbabwe, 1995–1997. Int J Cancer J Int Cancer. 2004;109(2):274–7. doi: 10.1002/ijc.11670. [DOI] [PubMed] [Google Scholar]
- 96.Wabinga H, Ramanakumar AV, Banura C, Luwaga A, Nambooze S, Parkin DM. Survival of cervix cancer patients in Kampala, Uganda: 1995–1997. Br J Cancer. 2003;89(1):65–9. doi: 10.1038/sj.bjc.6601034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012: Globocan 2012. Int J Cancer. 2015;136(5):E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 98.Arbyn M, Castellsague X, de Sanjose S, Bruni L, Saraiya M, Bray F, et al. Worldwide burden of cervical cancer in 2008. Ann Oncol. 2011;22(12):2675–86. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- 99.Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang X-S, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015;385(9972):977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kantelhardt EJ, Moelle U, Begoihn M, Addissie A, Trocchi P, Yonas B, et al. Cervical cancer in Ethiopia: survival of 1,059 patients who received oncologic therapy. Oncologist. 2014;19(7):727–34. doi: 10.1634/theoncologist.2013-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nuhu FT, Adebayo KO, Adejumo O. Quality of life of people with cancers in Ibadan, Nigeria. J Ment Health Abingdon Engl. 2013;22(4):325–33. doi: 10.3109/09638237.2012.734644. [DOI] [PubMed] [Google Scholar]
- 102.Vistad I, Fosså SD, Dahl AA. A critical review of patient-rated quality of life studies of long-term survivors of cervical cancer. Gynecol Oncol. 2006;102(3):563–72. doi: 10.1016/j.ygyno.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 103.Soerjomataram I, Lortet-Tieulent J, Parkin DM, Ferlay J, Mathers C, Forman D, et al. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet. 2012;380(9856):1840–50. doi: 10.1016/S0140-6736(12)60919-2. [DOI] [PubMed] [Google Scholar]
- 104.Kamau RK, Osoti AO, Njuguna EM. Effect of diagnosis and treatment of inoperable cervical cancer on quality of life among women receiving radiotherapy at Kenyatta National Hospital. East Afr Med J. 2007;84(1):24–30. doi: 10.4314/eamj.v84i1.9487. [DOI] [PubMed] [Google Scholar]
- 105.Pfaendler KS, Wenzel L, Mechanic MB, Penner KR. Cervical cancer survivorship: long-term quality of life and social support. Clin Ther. 2015;37(1):39–48. doi: 10.1016/j.clinthera.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Abayomi J, Kirwan J, Hackett A, Bagnall G. A study to investigate women’s experiences of radiation enteritis following radiotherapy for cervical cancer. J Hum Nutr Diet Off J Br Diet Assoc. 2005;18(5):353–63. doi: 10.1111/j.1365-277X.2005.00633.x. [DOI] [PubMed] [Google Scholar]
- 107.Adewuyi SA, Shittu OS, Rafindadi AH, Zayyan MS, Samaila MOA, Oguntayo AO. Cisplatin chemotherapy for haemostasis in bleeding cervical cancer: experience from a resource-poor setting. Niger Postgrad Med J. 2010;17(2):122–7. [PubMed] [Google Scholar]
- 108.Maree JE, Mosalo A, Wright SCD. “It depends on how the relationship was before you became ill”: black South African women’s experiences of life partner support through the trajectory of cervical cancer. Eur J Cancer Care (Engl) 2013;22(4):459–67. doi: 10.1111/ecc.12051. [DOI] [PubMed] [Google Scholar]
- 109.Levin AO, Carpenter KM, Fowler JM, Brothers BM, Andersen BL, Maxwell GL. Sexual morbidity associated with poorer psychological adjustment among gynecological cancer survivors. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. 2010;20(3):461–70. doi: 10.1111/IGC.0b013e3181d24ce0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Herzog TJ, Wright JD. The impact of cervical cancer on quality of life--the components and means for management. Gynecol Oncol. 2007;107(3):572–7. doi: 10.1016/j.ygyno.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 111.Ye S, Yang J, Cao D, Zhu L, Lang J, Shen K. Quality of life and sexual function of cervical cancer patients following radical hysterectomy and vaginal extension. Zhonghua Fu Chan Ke Za Zhi. 2014;49(8):609–15. [PubMed] [Google Scholar]
- 112.Eftekhar M, Pourmasumi S, Karimi-Zarchi M. Preservation of ovarian function during chemotherapy and radiotherapy in young women with malignancies. Iran J Reprod Med. 2014;12(6):377–82. [PMC free article] [PubMed] [Google Scholar]
- 113.Karimi Zarchi M, Mousavi A, Gilani MM, Barooti E, Amini Rad O, Ghaemmaghami F, et al. Fertility sparing treatments in young patients with gynecological cancers: Iranian experience and literature review. Asian Pac J Cancer Prev APJCP. 2011;12(8):1887–92. [PubMed] [Google Scholar]
- 114.Rodriguez-Wallberg KA, Oktay K. Options on fertility preservation in female cancer patients. Cancer Treat Rev. 2012;38(5):354–61. doi: 10.1016/j.ctrv.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 115.Dyer SJ. Psychological distress among women suffering from couple infertility in South Africa: a quantitative assessment. Hum Reprod. 2005;20(7):1938–43. doi: 10.1093/humrep/deh845. [DOI] [PubMed] [Google Scholar]
- 116.Downing J, Powell RA, Mwangi-Powell F. Home-Based Palliative Care in sub-Saharan Africa. Home Healthc Nurse J Home Care Hosp Prof. 2010;28(5):298–307. doi: 10.1097/NHH.0b013e3181dbf2b6. [DOI] [PubMed] [Google Scholar]
- 117.Cleary J, Powell RA, Munene G, Mwangi-Powell FN, Luyirika E, Kiyange F, et al. Formulary availability and regulatory barriers to accessibility of opioids for cancer pain in Africa: a report from the Global Opioid Policy Initiative (GOPI) Ann Oncol. 2013;24(suppl 11):xi14–23. doi: 10.1093/annonc/mdt499. [DOI] [PubMed] [Google Scholar]
- 118.Nuhu FT, Odejide OA, Adebayo KO, Yusuf AJ. Psychological and physical effects of pain on cancer patients in Ibadan, Nigeria. Afr J Psychiatry. 2009;12(1):64–70. doi: 10.4314/ajpsy.v12i1.30281. [DOI] [PubMed] [Google Scholar]
- 119.Mwangi-Powell F, Dix O. Palliative care in Africa: an overview. Afr Health [Internet] 2011. [Google Scholar]
- 120.AFCRN. AFCRN: African Cancer Registry Network [Internet]. 2015 [cited 2015 Mar 11]. Available from: http://www.afcrn.org/. Accessed 6 July 2015.
- 121.Morhason-Bello IO, Odedina F, Rebbeck TR, Harford J, Dangou J-M, Denny L, et al. Challenges and opportunities in cancer control in Africa: a perspective from the African Organisation for Research and Training in Cancer. Lancet Oncol. 2013;14(4):e142–51. doi: 10.1016/S1470-2045(12)70482-5. [DOI] [PubMed] [Google Scholar]
- 122.Busolo DS, Woodgate RL. Cancer prevention in Africa: a review of the literature. Glob Health Promot. 2014;22:31–9. doi: 10.1177/1757975914537094. [DOI] [PubMed] [Google Scholar]
- 123.Denny L, Wright T. Strategies for Overcoming the Barriers to Cervical Cancer Screening in Low-Resource Settings. Glob Libr Womens Med [Internet]. 2009 [cited 2015 Jul 8]; Available from: http://www.glowm.com/index.html?p=glowm.cml/section_view&articleid=22. Accessed 6 July 2015.
- 124.Bingham A, Bishop A, Coffey P, Winkler J, Bradley J, Dzuba I, et al. Factors affecting utilization of cervical cancer prevention services in low-resource settings. Salud Pública México. 2003;45:408–16. doi: 10.1590/S0036-36342003000900015. [DOI] [PubMed] [Google Scholar]
- 125.Mvundura M, Tsu V. Estimating the costs of cervical cancer screening in high-burden Sub-Saharan African countries. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2014;126(2):151–5. doi: 10.1016/j.ijgo.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 126.Remes P, Selestine V, Changalucha J, Ross DA, Wight D, de Sanjosé S, et al. A qualitative study of HPV vaccine acceptability among health workers, teachers, parents, female pupils, and religious leaders in northwest Tanzania. Vaccine. 2012;30(36):5363–7. doi: 10.1016/j.vaccine.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bingham A, Drake JK, LaMontagne DS. Sociocultural Issues in the Introduction of Human Papillomavirus Vaccine in Low-Resource Settings. Arch Pediatr Adolesc Med. 2009;163(5):455. doi: 10.1001/archpediatrics.2009.50. [DOI] [PubMed] [Google Scholar]
- 128.Audu BM, Bukar M, Ibrahim AI, Swende TZ. Awareness and perception of human papilloma virus vaccine among healthcare professionals in Nigeria. J Obstet Gynaecol J Inst Obstet Gynaecol. 2014;12:1–4. doi: 10.3109/01443615.2014.925431. [DOI] [PubMed] [Google Scholar]
- 129.Hoque ME, Monokoane S, Van Hal G. Knowledge of and attitude towards human papillomavirus infection and vaccines among nurses at a tertiary hospital in South Africa. J Obstet Gynaecol. 2014;34(2):182–6. doi: 10.3109/01443615.2013.861395. [DOI] [PubMed] [Google Scholar]
- 130.Hoque ME, Ghuman S, Hal GV. Human Papillomavirus vaccination acceptability among female university students in South Africa. Asian Pac J Cancer Prev APJCP. 2013;14(8):4865–9. doi: 10.7314/APJCP.2013.14.8.4865. [DOI] [PubMed] [Google Scholar]
- 131.Makwe CC, Anorlu RI, Odeyemi KA. Human papillomavirus (HPV) infection and vaccines: knowledge, attitude and perception among female students at the University of Lagos, Lagos, Nigeria. J Epidemiol Glob Health. 2012;2(4):199–206. doi: 10.1016/j.jegh.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Poole DN, Tracy JK, Levitz L, Rochas M, Sangare K, Yekta S, et al. A Cross-Sectional Study to Assess HPV Knowledge and HPV Vaccine Acceptability in Mali. PLoS ONE. 2013;8(2):e56402. doi: 10.1371/journal.pone.0056402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wamai RG, Ayissi CA, Oduwo GO, Perlman S, Welty E, Welty T, et al. Awareness, knowledge and beliefs about HPV, cervical cancer and HPV vaccines among nurses in Cameroon: An exploratory study. Int J Nurs Stud. 2013;50(10):1399–406. doi: 10.1016/j.ijnurstu.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 134.Ayissi CA, Wamai RG, Oduwo GO, Perlman S, Welty E, Welty T, et al. Awareness, Acceptability and Uptake of Human Papilloma Virus Vaccine Among Cameroonian School-Attending Female Adolescents. J Community Health. 2012;37(6):1127–35. doi: 10.1007/s10900-012-9554-z. [DOI] [PubMed] [Google Scholar]
- 135.DiAngi YT, Panozzo CA, Ramogola-Masire D, Steenhoff AP, Brewer NT. A Cross-Sectional Study of HPV Vaccine Acceptability in Gaborone, Botswana. PLoS ONE. 2011;6(10):e25481. doi: 10.1371/journal.pone.0025481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Coleman MA, Levison J, Sangi-Haghpeykar H. HPV vaccine acceptability in Ghana, West Africa. Vaccine. 2011;29(23):3945–50. doi: 10.1016/j.vaccine.2011.03.093. [DOI] [PubMed] [Google Scholar]
- 137.Iliyasu Z, Abubakar IS, Aliyu MH, Galadanci HS. Cervical cancer risk perception and predictors of human papilloma virus vaccine acceptance among female university students in northern Nigeria. J Obstet Gynaecol. 2010;30(8):857–62. doi: 10.3109/01443615.2010.511724. [DOI] [PubMed] [Google Scholar]
- 138.Muhwezi WW, Banura C, Turiho AK, Mirembe F. Parents’ Knowledge, Risk Perception and Willingness to Allow Young Males to Receive Human Papillomavirus (HPV) Vaccines in Uganda. PLoS ONE. 2014;9(9):e106686. doi: 10.1371/journal.pone.0106686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Francis SA, Battle-Fisher M, Liverpool J, Hipple L, Mosavel M, Soogun S, et al. A qualitative analysis of South African women’s knowledge, attitudes, and beliefs about HPV and cervical cancer prevention, vaccine awareness and acceptance, and maternal-child communication about sexual health. Vaccine. 2011;29(47):8760–5. doi: 10.1016/j.vaccine.2011.07.116. [DOI] [PubMed] [Google Scholar]
- 140.Morhason-Bello IO, Oladokun A, Adedokun B, Adesina O, Awolude O, Aimakhu C, et al. Introduction of human papilloma virus vaccine in a low resource setting: a survey of the views of Nigerian gynaecologists. Int J Gynecol Obstet. 2009;107S2:S93–396. [Google Scholar]
- 141.Ugwu EO, Obi SN, Ezechukwu PC, Okafor II, Ugwu AO. Acceptability of human papilloma virus vaccine and cervical cancer screening among female health-care workers in Enugu, Southeast Nigeria. Niger J Clin Pract. 2013;16(2):249–52. doi: 10.4103/1119-3077.110141. [DOI] [PubMed] [Google Scholar]
- 142.Nelson JA, Francis SA, Liverpool J, Soogun S, Mofammere N. Healers in a non-traditional role; a focus group study of Sangoma’s knowledge of and attitudes to cervical cancer prevention and screening in Johannesburg, South Africa. Sex Reprod Healthc Off J Swed Assoc Midwives. 2010;1(4):195–6. doi: 10.1016/j.srhc.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 143.Makwe CC, Anorlu RI. Knowledge of and attitude toward human papillomavirus infection and vaccines among female nurses at a tertiary hospital in Nigeria. Int J Womens Health. 2011;3:313–7. doi: 10.2147/IJWH.S22792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wamai RG, Ayissi CA, Oduwo GO, Perlman S, Welty E, Manga S, et al. Assessing the effectiveness of a community-based sensitization strategy in creating awareness about HPV, cervical cancer and HPV vaccine among parents in North West Cameroon. J Community Health. 2012;37(5):917–26. doi: 10.1007/s10900-012-9540-5. [DOI] [PubMed] [Google Scholar]
- 145.Becker-Dreps S, Otieno WA, Brewer NT, Agot K, Smith JS. HPV vaccine acceptability among Kenyan women. Vaccine. 2010;28(31):4864–7. doi: 10.1016/j.vaccine.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.W Liu F, Vwalika B. Cervical Cancer and HPV Vaccination: Knowledge and Attitudes of Adult Women in Lusaka, Zambia. J Vaccines Vaccin [Internet]. 2012 [cited 2014 Oct 2];03(03). Available from: http://www.omicsonline.org/2157-7560/2157-7560-3-138.digital/2157-7560-3-138.html. Accessed 6 July 2015. [DOI] [PMC free article] [PubMed]
- 147.Poole D, Tracy K, Levitz L, Yekta S, Kossow E, Huang T, et al. Knowledge/attitude/practices of HPV & cervical cancer, willingness to participate in vaccine trial in preparation for HIV & HPV vaccine trials in Mali. Retrovirology. 2012;9(Suppl 2):O27. doi: 10.1186/1742-4690-9-S2-O27. [DOI] [Google Scholar]
- 148.Hoque ME, Van Hal G. Acceptability of Human Papillomavirus Vaccine: A Survey among Master of Business Administration Students in KwaZulu-Natal, South Africa. BioMed Res Int. 2014;2014:257807. doi: 10.1155/2014/257807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mouallif M, Bowyer HL, Festali S, Albert A, Filali-Zegzouti Y, Guenin S, et al. Cervical cancer and HPV: Awareness and vaccine acceptability among parents in Morocco. Vaccine. 2014;32(3):409–16. doi: 10.1016/j.vaccine.2013.10.069. [DOI] [PubMed] [Google Scholar]
- 150.Harries J, Moodley J, Barone MA, Mall S, Sinanovic E. Preparing for HPV vaccination in South Africa: key challenges and opinions. Vaccine. 2009;27(1):38–44. doi: 10.1016/j.vaccine.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 151.Francis SA, Katz ML. The HPV Vaccine: A Comparison of Focus Groups Conducted in South Africa and Ohio Appalachia. Matern Child Health J. 2013;17(7):1222–9. doi: 10.1007/s10995-012-1116-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.El-Khatib Z, Tota JE, Kaufmann AM. Progress on human papillomavirus (HPV) infection and cervical cancer prevention in sub-Saharan Africa: Highlights of the 27th International Papillomavirus Conference in Berlin, 17–22 September 2011. J Epidemiol Glob Health. 2012;2(2):99–102. doi: 10.1016/j.jegh.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Galagan SR, Paul P, Menezes L, LaMontagne DS. Influences on parental acceptance of HPV vaccination in demonstration projects in Uganda and Vietnam. Vaccine. 2013;31(30):3072–8. doi: 10.1016/j.vaccine.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 154.Binagwaho A, Wagner C, Gatera M, Karema C, Nutt C, Ngaboa F. Achieving high coverage in Rwanda’s national human papillomavirus vaccination programme. Bull World Health Organ. 2012;90(8):623–8. doi: 10.2471/BLT.11.097253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ladner J, Besson M-H, Rodrigues M, Audureau E, Saba J. Performance of 21 HPV vaccination programs implemented in low and middle-income countries, 2009–2013. BMC Public Health. 2014;14(1):670. doi: 10.1186/1471-2458-14-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ogembo JG, Manga S, Nulah K, Foglabenchi LH, Perlman S, Wamai RG, et al. Achieving high uptake of human papillomavirus vaccine in Cameroon: Lessons learned in overcoming challenges. Vaccine. 2014;32(35):4399–403. doi: 10.1016/j.vaccine.2014.06.064. [DOI] [PubMed] [Google Scholar]
- 157.LaMontagne DS, Barge S, Le NT, Mugisha E, Penny ME, Gandhi S, et al. Human papillomavirus vaccine delivery strategies that achieved high coverage in low- and middle-income countries. Bull World Health Organ. 2011;89(11):821–30B. doi: 10.2471/BLT.11.089862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Katz IT, Nkala B, Dietrich J, Wallace M, Bekker L-G, Pollenz K, et al. A Qualitative Analysis of Factors Influencing HPV Vaccine Uptake in Soweto, South Africa among Adolescents and Their Caregivers. PLoS ONE. 2013;8(8):e72094. doi: 10.1371/journal.pone.0072094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Moodley I, Tathiah N, Mubaiwa V, Denny L. High uptake of Gardasil vaccine among 9–12-year-old schoolgirls participating in an HPV vaccination demonstration project in KwaZulu-Natal, South Africa. South Afr Med J Suid-Afr Tydskr Vir Geneeskd. 2013;103(5):318–21. doi: 10.7196/samj.6414. [DOI] [PubMed] [Google Scholar]
- 160.Watson-Jones D, Baisley K, Ponsiano R, Lemme F, Remes P, Ross D, et al. Human Papillomavirus Vaccination in Tanzanian Schoolgirls: Cluster-Randomized Trial Comparing 2 Vaccine-Delivery Strategies. J Infect Dis. 2012;206(5):678–86. doi: 10.1093/infdis/jis407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kojic EM, Kang M, Cespedes MS, Umbleja T, Godfrey C, Allen RT, et al. Immunogenicity and Safety of the Quadrivalent Human Papillomavirus Vaccine in HIV-1-Infected Women. Clin Infect Dis. 2014;59(1):127–35. doi: 10.1093/cid/ciu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Denny L, Hendricks B, Gordon C, Thomas F, Hezareh M, Dobbelaere K, et al. Safety and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine in HIV-positive women in South Africa: a partially-blind randomised placebo-controlled study. Vaccine. 2013;31(48):5745–53. doi: 10.1016/j.vaccine.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 163.Botha H, Cooreman B, Dreyer G, Lindeque G, Mourton A, Guidozzi F, et al. Cervical cancer and human papillomavirus: South African guidelines for screening and testing. South Afr J Gynaecol Oncol. 2010;2(1):23–6. doi: 10.1080/20742835.2010.11441154. [DOI] [Google Scholar]
- 164.Sow PS, Watson-Jones D, Kiviat N, Changalucha J, Mbaye KD, Brown J, et al. Safety and Immunogenicity of Human Papillomavirus-16/18 AS04-Adjuvanted Vaccine: A Randomized Trial in 10-25-Year-Old HIV-Seronegative African Girls and Young Women. J Infect Dis. 2013;207(11):1753–63. doi: 10.1093/infdis/jis619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Brown J, Baisley K, Kavishe B, Changalucha J, Andreasen A, Mayaud P, et al. Impact of malaria and helminth infections on immunogenicity of the human papillomavirus-16/18 AS04-adjuvanted vaccine in Tanzania. Vaccine. 2014;32(5):611–7. doi: 10.1016/j.vaccine.2013.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jain KM, Paul P, LaMontagne DS. Monitoring adverse events following immunisation in developing countries: experience from human papillomavirus vaccination demonstration projects. Sex Health. 2013;10(1):57–63. doi: 10.1071/SH11161. [DOI] [PubMed] [Google Scholar]
- 167.Botha MH, Dochez C. Introducing human papillomavirus vaccines into the health system in South Africa. Vaccine. 2012;30(Suppl 3):C28–34. doi: 10.1016/j.vaccine.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 168.Gulland A. Uganda launches HPV vaccination programme to fight its commonest cancer. BMJ. 2012;345(sep10 1):e6055–5. [DOI] [PubMed]
- 169.Biddlecom A, Bankole A, Patterson K. Vaccine for cervical cancer: reaching adolescents in sub-Saharan Africa. Lancet. 2006;367:1299–300. doi: 10.1016/S0140-6736(06)68555-3. [DOI] [PubMed] [Google Scholar]
- 170.Baussano I, Lazzarato F, Ronco G, Dillner J, Franceschi S. Benefits of catch-up in vaccination against human papillomavirus in medium- and low-income countries. Int J Cancer J Int Cancer. 2013;133(8):1876–81. doi: 10.1002/ijc.28197. [DOI] [PubMed] [Google Scholar]
- 171.Gray RH, Serwadda D, Kong X, Makumbi F, Kigozi G, Gravitt PE, et al. Male Circumcision Decreases Acquisition and Increases Clearance of High-Risk Human Papillomavirus in HIV-Negative Men: A Randomized Trial in Rakai, Uganda. J Infect Dis. 2010;201(10):1455–62. doi: 10.1086/652184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tobian AAR, Kong X, Gravitt PE, Eaton KP, Kigozi G, Serwadda D, et al. Male circumcision and anatomic sites of penile high-risk human papillomavirus in Rakai, Uganda. Int J Cancer. 2011;129(12):2970–5. doi: 10.1002/ijc.25957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wawer MJ, Tobian AA, Kigozi G, Kong X, Gravitt PE, Serwadda D, et al. Male Circumcision Reduces Human Papillomavirus Transmission to HIV-Negative Female Partners: A Randomized Trial in Rakai, Uganda. Lancet. 2011;377(9761):209–18. doi: 10.1016/S0140-6736(10)61967-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tobian AAR, Kong X, Wawer MJ, Kigozi G, Gravitt PE, Serwadda D, et al. Circumcision of HIV-infected men and transmission of human papillomavirus to female partners: analyses of data from a randomised trial in Rakai, Uganda. Lancet Infect Dis. 2011;11(8):604–12. doi: 10.1016/S1473-3099(11)70038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tarnaud C, Lissouba P, Cutler E, Puren A, Taljaard D, Auvert B. Association of low-risk human papillomavirus infection with male circumcision in young men: results from a longitudinal study conducted in Orange Farm (South Africa) Infect Dis Obstet Gynecol. 2011;2011:567408. doi: 10.1155/2011/567408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Davis M-A, Gray RH, Grabowski MK, Serwadda D, Kigozi G, Gravitt PE, et al. Male circumcision decreases high-risk human papillomavirus viral load in female partners: a randomized trial in Rakai, Uganda. Int J Cancer J Int Cancer. 2013;133(5):1247–52. doi: 10.1002/ijc.28100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sawaya GF, Chirenje MZ, Magure MT, Tuveson JL, Ma Y, Shiboski SC, et al. Effect of Diaphragm and Lubricant Gel Provision on Human Papillomavirus Infection Among Women Provided With Condoms: A Randomized Controlled Trial. Obstet Gynecol. 2008;112(5):990–7. doi: 10.1097/AOG.0b013e318189a8a4. [DOI] [PubMed] [Google Scholar]
- 178.Marais D, Gawarecki D, Allan B, Ahmed K, Altini L, Cassim N, et al. The effectiveness of Carraguard, a vaginal microbicide, in protecting women against high-risk human papillomavirus infection. Antivir Ther. 2011;16(8):1219–26. doi: 10.3851/IMP1890. [DOI] [PubMed] [Google Scholar]
- 179.Maree JE. “No condom, no sex”: Easy to say, but not possible for all South African women. Health SA Gesondheid [Internet]. 2010 Mar 17 [cited 2015 May 14];15(1). Available from: http://www.hsag.co.za/index.php/HSAG/article/view/506. Accessed 6 July 2015.
- 180.Watson-Jones D, Tomlin K, Remes P, Baisley K, Ponsiano R, Soteli S, et al. Reasons for Receiving or Not Receiving HPV Vaccination in Primary Schoolgirls in Tanzania: A Case Control Study. PLoS ONE. 2012;7(10):e45231. doi: 10.1371/journal.pone.0045231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Quentin W, Terris-Prestholt F, Changalucha J, Soteli S, Edmunds WJ, Hutubessy R, et al. Costs of delivering human papillomavirus vaccination to schoolgirls in Mwanza Region, Tanzania. BMC Med. 2012;10:137. doi: 10.1186/1741-7015-10-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Untiet S, Vassilakos P, McCarey C, Tebeu P-M, Kengne-Fosso G, Menoud P-A, et al. HPV self-sampling as primary screening test in sub-Saharan Africa: implication for a triaging strategy. Int J Cancer J Int Cancer. 2014;135(8):1911–7. doi: 10.1002/ijc.28834. [DOI] [PubMed] [Google Scholar]
- 183.Adler DH, Laher F, Lazarus E, Grzesik K, Gray GE, Allan B, et al. A Viable and Simple Self-Sampling Method for Human Papillomavirus Detection among South African Adolescents. J Immunol Tech Infect Dis. 2013;18:2(3). doi: 10.4172/2329-9541.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ting J, Mugo N, Kwatampora J, Hill C, Chitwa M, Patel S, et al. High-risk human papillomavirus messenger RNA testing in physician- and self-collected specimens for cervical lesion detection in high-risk women, Kenya. Sex Transm Dis. 2013;40(7):584–9. doi: 10.1097/OLQ.0b013e31828e5a91. [DOI] [PubMed] [Google Scholar]
- 185.Safaeian M, Kiddugavu M, Gravitt PE, Ssekasanvu J, Murokora D, Sklar M, et al. Comparability of self-collected vaginal swabs and physician-collected cervical swabs for detection of human papillomavirus infections in Rakai, Uganda. Sex Transm Dis. 2007;34(7):429–36. doi: 10.1097/01.olq.0000243623.67673.22. [DOI] [PubMed] [Google Scholar]
- 186.Kamal EM, El Sayed GA, El Behery MM, El Shennawy GA. HPV detection in a self-collected vaginal swab combined with VIA for cervical cancer screening with correlation to histologically confirmed CIN. Arch Gynecol Obstet. 2014;290(6):1207–13. doi: 10.1007/s00404-014-3321-6. [DOI] [PubMed] [Google Scholar]
- 187.Adler DH, Almudevar A, Gray GE, Allan B, Williamson A-L. High level of agreement between clinician-collected and self-collected samples for HPV detection among South African adolescents. J Pediatr Adolesc Gynecol. 2012;25(4):280–1. doi: 10.1016/j.jpag.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jones HE, Allan BR, van de Wijgert JHHM, Altini L, Taylor SM, de Kock A, et al. Agreement between self- and clinician-collected specimen results for detection and typing of high-risk human papillomavirus in specimens from women in Gugulethu, South Africa. J Clin Microbiol. 2007;45(6):1679–83. doi: 10.1128/JCM.02369-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Taylor S, Wang C, Wright TC, Denny L, Kuhn L. A comparison of human papillomavirus testing of clinician-collected and self-collected samples during follow-up after screen-and-treat. Int J Cancer. 2011;129(4):879–86. doi: 10.1002/ijc.25731. [DOI] [PubMed] [Google Scholar]
- 190.Charbonneau V, Garrigue I, Jaquet A, Horo A, Minga A, Recordon-Pinson P, et al. Dried cervical spots for human papillomaviruses identification. J Med Virol. 2013;85(7):1222–8. doi: 10.1002/jmv.23573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Alidjinou EK, Ebatetou-Ataboho E, Sané F, Moukassa D, Dewilde A, Hober D. Cervical samples dried on filter paper and dried vaginal tampons can be useful to investigate the circulation of high-risk HPV in Congo. J Clin Virol Off Publ Pan Am Soc Clin Virol. 2013;57(2):161–4. doi: 10.1016/j.jcv.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 192.Lack N, West B, Jeffries D, Ekpo G, Morison L, Soutter WP, et al. Comparison of non-invasive sampling methods for detection of HPV in rural African women. Sex Transm Infect. 2005;81(3):239–41. doi: 10.1136/sti.2004.010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bigoni J, Gundar M, Tebeu P-M, Bongoe A, Schäfer S, Fokom-Domgue J, et al. Cervical cancer screening in sub-Saharan Africa: A randomized trial of VIA versus cytology for triage of HPV-positive women. Int J Cancer J Int Cancer. 2015;137(1):127–34. doi: 10.1002/ijc.29353. [DOI] [PubMed] [Google Scholar]
- 194.Stock EM, Stamey JD, Sankaranarayanan R, Young DM, Muwonge R, Arbyn M. Estimation of disease prevalence, true positive rate, and false positive rate of two screening tests when disease verification is applied on only screen-positives: a hierarchical model using multi-center data. Cancer Epidemiol. 2012;36(2):153–60. doi: 10.1016/j.canep.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 195.Sangwa-Lugoma G, Mahmud S, Nasr SH, Liaras J, Kayembe PK, Tozin RR, et al. Visual inspection as a cervical cancer screening method in a primary health care setting in Africa. Int J Cancer. 2006;119(6):1389–95. doi: 10.1002/ijc.21972. [DOI] [PubMed] [Google Scholar]
- 196.Ngoma T, Muwonge R, Mwaiselage J, Kawegere J, Bukori P, Sankaranarayanan R. Evaluation of cervical visual inspection screening in Dar es Salaam, Tanzania. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2010;109(2):100–4. doi: 10.1016/j.ijgo.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 197.Muwonge R, Manuel Mda G, Filipe AP, Dumas JB, Frank MR, Sankaranarayanan R. Visual screening for early detection of cervical neoplasia in Angola. Int J Gynecol Obstet. 2010;111(1):68–72. doi: 10.1016/j.ijgo.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 198.Hovland S, Arbyn M, Lie AK, Ryd W, Borge B, Berle EJ, et al. A comprehensive evaluation of the accuracy of cervical pre-cancer detection methods in a high-risk area in East Congo. Br J Cancer. 2010;102(6):957–65. doi: 10.1038/sj.bjc.6605594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mwanahamuntu MH, Sahasrabuddhe VV, Kapambwe S, Pfaendler KS, Chibwesha C, Mkumba G, et al. Advancing Cervical Cancer Prevention Initiatives in Resource-Constrained Settings: Insights from the Cervical Cancer Prevention Program in Zambia. PLoS Med. 2011;8(5):e1001032. doi: 10.1371/journal.pmed.1001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Paul P, Winkler JL, Bartolini RM, Penny ME, Huong TT, Nga LT, et al. Screen-and-treat approach to cervical cancer prevention using visual inspection with acetic acid and cryotherapy: experiences, perceptions, and beliefs from demonstration projects in Peru, Uganda, and Vietnam. Oncologist. 2013;18(Suppl):6–12. doi: 10.1634/theoncologist.18-S2-6. [DOI] [PubMed] [Google Scholar]
- 201.White HL, Mulambia C, Sinkala M, Mwanahamuntu MH, Parham GP, Kapambwe S, et al. Motivations and experiences of women who accessed “see and treat” cervical cancer prevention services in Zambia. J Psychosom Obstet Gynaecol. 2012;33(2):91–8. doi: 10.3109/0167482X.2012.656161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mwanahamuntu MH, Sahasrabuddhe VV, Pfaendler KS, Mudenda V, Hicks ML, Vermund SH, et al. Implementation of “see-and-treat” cervical cancer prevention services linked to HIV care in Zambia. AIDS Lond Engl. 2009;23(6):N1–5. doi: 10.1097/QAD.0b013e3283236e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Fokom-Domgue J, Vassilakos P, Petignat P. Is screen-and-treat approach suited for screening and management of precancerous cervical lesions in Sub-Saharan Africa? Prev Med. 2014;65:138–40. doi: 10.1016/j.ypmed.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 204.Rositch AF, Gatuguta A, Choi RY, Guthrie BL, Mackelprang RD, Bosire R, et al. Knowledge and Acceptability of Pap Smears, Self-Sampling and HPV Vaccination among Adult Women in Kenya. PLoS ONE. 2012;7(7):e40766. doi: 10.1371/journal.pone.0040766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ibekwe CM, Hoque ME, Ntuli-Ngcobo B. Perceived benefits of cervical cancer screening among women attending Mahalapye District Hospital, Botswana. Asian Pac J Cancer Prev APJCP. 2010;11(4):1021–7. [PubMed] [Google Scholar]
- 206.Busingye P, Nakimuli A, Nabunya E, Mutyaba T. Acceptability of cervical cancer screening via visual inspection with acetic acid or Lugol’s iodine at Mulago Hospital, Uganda. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2012;119(3):262–5. doi: 10.1016/j.ijgo.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 207.Audet CM, Silva Matos C, Blevins M, Cardoso A, Moon TD, Sidat M. Acceptability of cervical cancer screening in rural Mozambique. Health Educ Res. 2012;27(3):544–51. doi: 10.1093/her/cys008. [DOI] [PubMed] [Google Scholar]
- 208.Tchounga BK, Jaquet A, Coffie PA, Horo A, Sauvaget C, Adoubi I, et al. Cervical cancer prevention in reproductive health services: knowledge, attitudes and practices of midwives in Côte d’Ivoire, West Africa. BMC Health Serv Res. 2014;14:165. doi: 10.1186/1472-6963-14-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Urasa M, Darj E. Knowledge of cervical cancer and screening practices of nurses at a regional hospital in Tanzania. Afr Health Sci. 2011;11(1):48–57. [PMC free article] [PubMed] [Google Scholar]
- 210.Mutyaba T, Mmiro FA, Weiderpass E. Knowledge, attitudes and practices on cervical cancer screening among the medical workers of Mulago Hospital, Uganda. BMC Med Educ. 2006;6:13. doi: 10.1186/1472-6920-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Anya SE, Oshi DC, Nwosu SO, Anya AE. Knowledge, attitude, and practice of female health professionals regarding cervical cancer and Pap smear. Niger J Med J Natl Assoc Resid Dr Niger. 2005;14(3):283–6. [PubMed] [Google Scholar]
- 212.Ginsberg GM, Lauer JA, Zelle S, Baeten S, Baltussen R. Cost effectiveness of strategies to combat breast, cervical, and colorectal cancer in sub-Saharan Africa and South East Asia: mathematical modelling study. BMJ. 2012;344:e614. doi: 10.1136/bmj.e614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Vijayaraghavan A, Efrusy M, Lindeque G, Dreyer G, Santas C. Cost effectiveness of high-risk HPV DNA testing for cervical cancer screening in South Africa. Gynecol Oncol. 2009;112(2):377–83. doi: 10.1016/j.ygyno.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 214.Sinanovic E, Moodley J, Barone MA, Mall S, Cleary S, Harries J. The potential cost-effectiveness of adding a human papillomavirus vaccine to the cervical cancer screening programme in South Africa. Vaccine. 2009;27(44):6196–202. doi: 10.1016/j.vaccine.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 215.Mwaka AD, Wabinga HR, Mayanja-Kizza H. Mind the gaps: a qualitative study of perceptions of healthcare professionals on challenges and proposed remedies for cervical cancer help-seeking in post conflict northern Uganda. BMC Fam Pract. 2013;14:193. doi: 10.1186/1471-2296-14-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Moon TD, Silva-Matos C, Cordoso A, Baptista AJ, Sidat M, Vermund SH. Implementation of cervical cancer screening using visual inspection with acetic acid in rural Mozambique: successes and challenges using HIV care and treatment programme investments in Zambézia Province. J Int AIDS Soc. 2012;15(2):17406. doi: 10.7448/IAS.15.2.17406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Sanghvi H, Limpaphayom KK, Plotkin M, Charurat E, Kleine A, Lu E, et al. Cervical cancer screening using visual inspection with acetic acid: operational experiences from Ghana and Thailand. Reprod Health Matters. 2008;16(32):67–77. doi: 10.1016/S0968-8080(08)32401-X. [DOI] [PubMed] [Google Scholar]
- 218.Kawonga M, Fonn S. Achieving effective cervical screening coverage in South Africa through human resources and health systems development. Reprod Health Matters. 2008;16(32):32–40. doi: 10.1016/S0968-8080(08)32403-3. [DOI] [PubMed] [Google Scholar]
- 219.Sibiya MN, Grainger L. An assessment of the implementation of the provincial cervical screening programme in selected Primary Health Care Clinics in the Ilembe Region, KwaZulu-Natal. Curationis. 2007;30(1):48–55. doi: 10.4102/curationis.v30i1.1050. [DOI] [PubMed] [Google Scholar]
- 220.Anorlu RI, Ribiu KA, Abudu OO, Ola ER. Cervical cancer screening practices among general practitioners in Lagos Nigeria. J Obstet Gynaecol J Inst Obstet Gynaecol. 2007;27(2):181–4. doi: 10.1080/01443610601124398. [DOI] [PubMed] [Google Scholar]
- 221.Williams MS, Amoateng P. Knowledge and beliefs about cervical cancer screening among men in Kumasi, Ghana. Ghana Med J. 2012;46(3):147–51. [PMC free article] [PubMed] [Google Scholar]
- 222.Mutyaba T, Mirembe F, Sandin S, Weiderpass E. Male partner involvement in reducing loss to follow-up after cervical cancer screening in Uganda. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2009;107(2):103–6. doi: 10.1016/j.ijgo.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 223.Smith JS, Moses S, Hudgens MG, Agot K, Franceschi S, Maclean IW, et al. Human papillomavirus detection by penile site in young men from Kenya. Sex Transm Dis. 2007;34(11):928–34. doi: 10.1097/OLQ.0b013e318065b8ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Horo A, Jaquet A, Ekouevi DK, Toure B, Coffie PA, Effi B, et al. Cervical cancer screening by visual inspection in Côte d’Ivoire, operational and clinical aspects according to HIV status. BMC Public Health. 2012;12:237. doi: 10.1186/1471-2458-12-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Dim CC, Dim NR, Ezegwui HU, Ikeme AC. An unmet cancer screening need of HIV-positive women in southeastern Nigeria. Medscape J Med. 2009;11(1):19. [PMC free article] [PubMed] [Google Scholar]
- 226.Kuhn L, Wang C, Tsai W-Y, Wright TC, Denny L. Efficacy of human papillomavirus-based screen-and-treat for cervical cancer prevention among HIV-infected women. AIDS Lond Engl. 2010;24(16):2553–61. doi: 10.1097/QAD.0b013e32833e163e. [DOI] [PubMed] [Google Scholar]
- 227.Rabiu KA, Akinbami AA, Adewunmi AA, Akinola OI, Wright KO. The need to incorporate routine cervical cancer counselling and screening in the management of HIV positive women in Nigeria. Asian Pac J Cancer Prev APJCP. 2011;12(5):1211–4. [PubMed] [Google Scholar]
- 228.Wake RM, Rebe K, Burch VC. Patient perception of cervical screening among women living with human immuno-deficiency virus infection attending an antiretroviral therapy clinic in urban South Africa. J Obstet Gynaecol J Inst Obstet Gynaecol. 2009;29(1):44–8. doi: 10.1080/01443610802484070. [DOI] [PubMed] [Google Scholar]
- 229.Huchko MJ, Bukusi EA, Cohen CR. Building capacity for cervical cancer screening in outpatient HIV clinics in the Nyanza province of western Kenya. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2011;114(2):106–10. doi: 10.1016/j.ijgo.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Atashili J, Smith JS, Adimora AA, Eron J, Miller WC, Myers E. Potential impact of antiretroviral therapy and screening on cervical cancer mortality in HIV-positive women in sub-Saharan Africa: a simulation. PLoS ONE. 2011;6(4):e18527. doi: 10.1371/journal.pone.0018527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Franceschi S, Jaffe H. Cervical Cancer Screening of Women Living with HIV Infection: A Must in the Era of Antiretroviral Therapy. Clin Infect Dis. 2007;45(4):510–3. doi: 10.1086/520022. [DOI] [PubMed] [Google Scholar]
- 232.Quinley KE, Gormley RH, Ratcliffe SJ, Shih T, Szep Z, Steiner A, et al. Use of mobile telemedicine for cervical cancer screening. J Telemed Telecare. 2011;17(4):203–9. doi: 10.1258/jtt.2011.101008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Mushosho EY, Ndlovu N, Engel-Hills P, Wyrley-Birch B. Presentation patterns of invasive cancer of the cervix: results from Parirenyatwa Oncology and Radiotherapy Centre, Harare, Zimbabwe 1998–2010. Cent Afr J Med. 2011;57(9–12):43–9. [PubMed] [Google Scholar]
- 234.Snyman LC, Herbst U. Reasons why unscreened patients with cervical cancer present with advanced stage disease. South Afr J Gynaecol Oncol. 2013;5(1):16–20
- 235.Woo VG, Cohen CR, Bukusi EA, Huchko MJ. Loop Electrosurgical Excision Procedure: Safety and Tolerability Among Human Immunodeficiency Virus–Positive Kenyan Women. Obstet Gynecol. 2011;118(3):554–9. doi: 10.1097/AOG.0b013e31822b0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Mungo C, Cohen CR, Maloba M, Bukusi EA, Huchko MJ. Prevalence, characteristics, and outcomes of HIV-positive women diagnosed with invasive cancer of the cervix in Kenya. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2013;123(3):231–5. doi: 10.1016/j.ijgo.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.AORTIC. Cancer Plan for the African Continent [Internet]. 2013. Available from: http://www.iccp-portal.org/sites/default/files/plans/AORTIC_CANCER_PLAN%20AFRICA.pdf. Accessed 6 July 2015.
- 238.Kigula-Mugambe JB, Kavuma A. Effect of HIV serological status on outcome in patients with cancer of cervix treated with radiotherapy. East Afr Med J. 2006;83(8):416–23. doi: 10.4314/eamj.v83i8.9455. [DOI] [PubMed] [Google Scholar]
- 239.Gichangi P, Bwayo J, Estambale B, Rogo K, Njuguna E, Ojwang S, et al. HIV impact on acute morbidity and pelvic tumor control following radiotherapy for cervical cancer. Gynecol Oncol. 2006;100(2):405–11. doi: 10.1016/j.ygyno.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 240.Moodley M, Mould S. Invasive cervical cancer and human immunodeficiency virus (HIV) infection in KwaZulu-Natal, South Africa. J Obstet Gynaecol J Inst Obstet Gynaecol. 2005;25(7):706–10. doi: 10.1080/01443610500294599. [DOI] [PubMed] [Google Scholar]
- 241.Denslow SA, Rositch AF, Firnhaber C, Ting J, Smith JS. Incidence and progression of cervical lesions in women with HIV: a systematic global review. Int J STD AIDS. 2014;25(3):163–77. doi: 10.1177/0956462413491735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.van Bogaert L-J. The impact of human immunodeficiency virus infection on cervical preinvasive and invasive neoplasia in South Africa. Ecancermedicalscience. 2013;7:334. doi: 10.3332/ecancer.2013.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Simonds HM, Wright JD, du Toit N, Neugut AI, Jacobson JS. Completion of and early response to chemoradiation among human immunodeficiency virus (HIV)-positive and HIV-negative patients with locally advanced cervical carcinoma in South Africa. Cancer. 2012;118(11):2971–9. doi: 10.1002/cncr.26639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Huchko MJ, Leslie H, Maloba M, Bukusi EA, Cohen CR. Factors associated with recurrence of cervical intraepithelial neoplasia 2+ after treatment among HIV-infected women in Western Kenya. J Acquir Immune Defic Syndr. 2014;66(2):188–92. doi: 10.1097/QAI.0000000000000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Hank E, Hoque ME, Zungu L. Cervical precancerous lesions and cancer among patients in the gynaecology outpatient department at a tertiary hospital in South Africa. Asian Pac J Cancer Prev APJCP. 2013;14(8):4903–6. doi: 10.7314/APJCP.2013.14.8.4903. [DOI] [PubMed] [Google Scholar]
- 246.Flepisi BT, Bouic P, Sissolak G, Rosenkranz B. Drug–drug interactions in HIV positive cancer patients. Biomed Pharmacother. 2014;68(5):665–77. doi: 10.1016/j.biopha.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 247.Zeier MD, Botha MH, van der Merwe FH, Eshun-Wilson I, van Schalkwyk M, la Grange M, et al. Progression and persistence of low-grade cervical squamous intraepithelial lesions in women living with human immunodeficiency virus. J Low Genit Tract Dis. 2012;16(3):243–50. doi: 10.1097/LGT.0b013e3182403d18. [DOI] [PubMed] [Google Scholar]
- 248.Zeier MD, Nachega JB, Van Der Merwe FH, Eshun-Wilson I, Van Schalkwyk M, La Grange M, et al. Impact of timing of antiretroviral therapy initiation on survival of cervical squamous intraepithelial lesions: a cohort analysis from South Africa. Int J STD AIDS. 2012;23(12):890–6. doi: 10.1258/ijsa.2012.012040. [DOI] [PubMed] [Google Scholar]
- 249.Logie DE, Harding R. An evaluation of a morphine public health programme for cancer and AIDS pain relief in Sub-Saharan Africa. BMC Public Health. 2005;5:82. doi: 10.1186/1471-2458-5-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Omoyeni N, Soyannwo O, Aikomo O, Iken O. Home-based palliative care for adult cancer patients in Ibadan-a three year review. Ecancermedicalscience. 2014;8:490. doi: 10.3332/ecancer.2014.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Rawlinson F. The current situation in education and training of health-care professionals across Africa to optimise the delivery of palliative care for cancer patients. ecancermedicalscience [Internet]. 2014 Dec 11 [cited 2015 May 14];8. Available from: http://www.ecancer.org/journal/8/full/492-the-current-situation-in-education-and-training-of-health-care-professionals-across-africa-to-optimise-the-delivery-of-palliative-care-for-cancer-patients.php. Accessed 6 July 2015. [DOI] [PMC free article] [PubMed]
- 252.Selman L, Higginson IJ, Agupio G, Dinat N, Downing J, Gwyther L, et al. Meeting information needs of patients with incurable progressive disease and their families in South Africa and Uganda: multicentre qualitative study. BMJ. 2009;338:b1326. doi: 10.1136/bmj.b1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Harding R, Higginson IJ. Palliative care in sub-Saharan Africa. Lancet. 2005;365(9475):1971–7. doi: 10.1016/S0140-6736(05)66666-4. [DOI] [PubMed] [Google Scholar]
- 254.Atuhairwe S, Busingye RB, Sekikubo M, Nakimuli A, Mutyaba T. Urologic complications among women with advanced cervical cancer at a tertiary referral hospital in Uganda. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2011;115(3):282–4. doi: 10.1016/j.ijgo.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 255.Ntekim A. Cervical Cancer in Sub Sahara Africa. In: R. Rajamanickam, editor. Topics on Cervical Cancer With an Advocacy for Prevention [Internet]. InTech; 2012 [cited 2015 May 14]. Available from: http://www.intechopen.com/books/topics-on-cervical-cancer-with-an-advocacy-for-prevention/cervical-cancer-in-sub-sahara-africa. Accessed 6 July 2015.
- 256.Ezeanochie MC, Olagbuji BN. Human papilloma virus vaccine: determinants of acceptability by mothers for adolescents in Nigeria. Afr J Reprod Health. 2014;18(3):154–8. [PubMed] [Google Scholar]
- 257.Omondi-Ogutu, M’Imunya J. Parental Acceptance of Human Papilloma Virus Vaccine for Their Pre-Pubertal and Teenage Daughters. East Afr Med J. 2011;88(5):163–70.
- 258.Kane MA, Serrano B, de Sanjosé S, Wittet S. Implementation of Human Papillomavirus Immunization in the Developing World. Vaccine. 2012;30:F192–200. doi: 10.1016/j.vaccine.2012.06.075. [DOI] [PubMed] [Google Scholar]
- 259.Tsu VD, Cernuschi T, LaMontagne DS. Lessons Learned From HPV Vaccine Delivery in Low-Resource Settings and Opportunities for HIV Prevention, Treatment, and Care Among Adolescents. JAIDS J Acquir Immune Defic Syndr. 2014;66:S209–16. doi: 10.1097/QAI.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 260.Binagwaho A, Ngabo F, Wagner CM, Mugeni C, Gatera M, Nutt CT, et al. Integration of comprehensive women’s health programmes into health systems: cervical cancer prevention, care and control in Rwanda. Bull World Health Organ. 2013;91(9):697–703. doi: 10.2471/BLT.12.116087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Levin CE, Van Minh H, Odaga J, Rout SS, Ngoc DNT, Menezes L, et al. Delivery cost of human papillomavirus vaccination of young adolescent girls in Peru, Uganda and Viet Nam. Bull World Health Organ. 2013;91(8):585–92. doi: 10.2471/BLT.12.113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Louie KS, De Sanjose S, Mayaud P. Epidemiology and prevention of human papillomavirus and cervical cancer in sub-Saharan Africa: a comprehensive review: Epidemiology of human papillomavirus. Trop Med Int Health. 2009;14(10):1287–302. doi: 10.1111/j.1365-3156.2009.02372.x. [DOI] [PubMed] [Google Scholar]
- 263.Tracy JK, Schluterman NH, Greene C, Sow SO, Gaff HD. Planning for human papillomavirus (HPV) vaccination in sub-Saharan Africa: a modeling-based approach. Vaccine. 2014;32(26):3316–22. doi: 10.1016/j.vaccine.2014.03.067. [DOI] [PubMed] [Google Scholar]
- 264.Kim JJ, Sharma M, O’Shea M, Sweet S, Diaz M, Sancho-Garnier H, et al. Model-based impact and cost-effectiveness of cervical cancer prevention in the Extended Middle East and North Africa (EMENA) Vaccine. 2013;31(Suppl 6):G65–77. doi: 10.1016/j.vaccine.2012.06.096. [DOI] [PubMed] [Google Scholar]
- 265.Demarteau N, Morhason-Bello IO, Akinwunmi B, Adewole IF. Modeling optimal cervical cancer prevention strategies in Nigeria. BMC Cancer. 2014;14:365. doi: 10.1186/1471-2407-14-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Kim S-Y, Sweet S, Chang J, Goldie SJ. Comparative evaluation of the potential impact of rotavirus versus HPV vaccination in GAVI-eligible countries: a preliminary analysis focused on the relative disease burden. BMC Infect Dis. 2011;11:174. doi: 10.1186/1471-2334-11-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Campos NG, Kim JJ, Castle PE, Ortendahl JD, O’Shea M, Diaz M, et al. Health and economic impact of HPV 16/18 vaccination and cervical cancer screening in Eastern Africa. Int J Cancer J Int Cancer. 2012;130(11):2672–84. doi: 10.1002/ijc.26269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Goldie SJ, O’Shea M, Campos NG, Diaz M, Sweet S, Kim S-Y. Health and economic outcomes of HPV 16,18 vaccination in 72 GAVI-eligible countries. Vaccine. 2008;26(32):4080–93. doi: 10.1016/j.vaccine.2008.04.053. [DOI] [PubMed] [Google Scholar]
- 269.Hutubessy R, Levin A, Wang S, Morgan W, Ally M, John T, et al. A case study using the United Republic of Tanzania: costing nationwide HPV vaccine delivery using the WHO Cervical Cancer Prevention and Control Costing Tool. BMC Med. 2012;10:136. doi: 10.1186/1741-7015-10-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Tracy L, Gaff HD, Burgess C, Sow S, Gravitt PE, Tracy JK. Estimating the impact of human papillomavirus (HPV) vaccination on HPV prevalence and cervical cancer incidence in Mali. Clin Infect Dis Off Publ Infect Dis Soc Am. 2011;52(5):641–5. doi: 10.1093/cid/ciq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Kiatpongsan S, Campos NG, Kim JJ. Potential benefits of second-generation human papillomavirus vaccines. PLoS ONE. 2012;7(11):e48426. doi: 10.1371/journal.pone.0048426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Lee LA, Franzel L, Atwell J, Datta SD, Friberg IK, Goldie SJ, et al. The estimated mortality impact of vaccinations forecast to be administered during 2011–2020 in 73 countries supported by the GAVI Alliance. Vaccine. 2013;31:B61–72. doi: 10.1016/j.vaccine.2012.11.035. [DOI] [PubMed] [Google Scholar]
- 273.Kane MA. Preventing Cancer with Vaccines: Progress in the Global Control of Cancer. Cancer Prev Res (Phila Pa) 2012;5(1):24–9. doi: 10.1158/1940-6207.CAPR-11-0533. [DOI] [PubMed] [Google Scholar]
- 274.Banura C, Mirembe FM, Katahoire AR, Namujju PB, Mbidde EK. Universal routine HPV vaccination for young girls in Uganda: a review of opportunities and potential obstacles. Infect Agent Cancer. 2012;7(1):24. doi: 10.1186/1750-9378-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Jumaan AO, Ghanem S, Taher J, Braikat M, Awaidy SA, Dbaibo GS. Prospects and Challenges in the Introduction of Human Papillomavirus Vaccines in the Extended Middle East and North Africa Region. Vaccine. 2013;31:G58–64. doi: 10.1016/j.vaccine.2012.06.097. [DOI] [PubMed] [Google Scholar]
- 276.van Bogaert L. Are the currently existing anti-human papillomavirus vaccines appropriate for the developing world? Ann Med Health Sci Res. 2013;3(3):306–12. doi: 10.4103/2141-9248.117924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Wilson LE, Gravitt P, Tobian AAR, Kigozi G, Serwadda D, Nalugoda F, et al. Male circumcision reduces penile high-risk human papillomavirus viral load in a randomised clinical trial in Rakai, Uganda. Sex Transm Infect. 2013;89(3):262–6. doi: 10.1136/sextrans-2012-050633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Sanad AS, Ibrahim EM, Gomaa W. Evaluation of cervical biopsies guided by visual inspection with acetic acid. J Low Genit Tract Dis. 2014;18(1):21–5. doi: 10.1097/LGT.0b013e31828ce581. [DOI] [PubMed] [Google Scholar]
- 279.Ajenifuja KO, Gage JC, Adepiti AC, Wentzensen N, Eklund C, Reilly M, et al. A population-based study of visual inspection with acetic acid (VIA) for cervical screening in rural Nigeria. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. 2013;23(3):507–12. doi: 10.1097/IGC.0b013e318280f395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Muwonge R, Walter SD, Wesley RS, Basu P, Shastri SS, Thara S, et al. Assessing the gain in diagnostic performance when two visual inspection methods are combined for cervical cancer prevention. J Med Screen. 2007;14(3):144–50. doi: 10.1258/096914107782066158. [DOI] [PubMed] [Google Scholar]
- 281.Gaffikin L, McGrath JA, Arbyn M, Blumenthal PD. Visual inspection with acetic acid as a cervical cancer test: accuracy validated using latent class analysis. BMC Med Res Methodol. 2007;7:36. doi: 10.1186/1471-2288-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Sankaranarayanan R, Basu P, Wesley RS, Mahe C, Keita N, Mbalawa CCG, et al. Accuracy of visual screening for cervical neoplasia: Results from an IARC multicentre study in India and Africa. Int J Cancer J Int Cancer. 2004;110(6):907–13. doi: 10.1002/ijc.20190. [DOI] [PubMed] [Google Scholar]
- 283.Gaffikin L, McGrath J, Arbyn M, Blumenthal PD. Avoiding verification bias in screening test evaluation in resource poor settings: a case study from Zimbabwe. Clin Trials Lond Engl. 2008;5(5):496–503. doi: 10.1177/1740774508096139. [DOI] [PubMed] [Google Scholar]
- 284.Sauvaget C, Fayette J-M, Muwonge R, Wesley R, Sankaranarayanan R. Accuracy of visual inspection with acetic acid for cervical cancer screening. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2011;113(1):14–24. doi: 10.1016/j.ijgo.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 285.Godfrey CC, Michelow PM, Godard M, Sahasrabuddhe VV, Darden J, Firnhaber CS, et al. Improving diagnostic capability for HPV disease internationally within the NIH-NIAID Division of AIDS Clinical Trial Networks. Am J Clin Pathol. 2013;140(6):881–9. doi: 10.1309/AJCPIBIS19QIYHJY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Berner A, Hassel SB, Tebeu P-M, Untiet S, Kengne-Fosso G, Navarria I, et al. Human papillomavirus self-sampling in Cameroon: women’s uncertainties over the reliability of the method are barriers to acceptance. J Low Genit Tract Dis. 2013;17(3):235–41. doi: 10.1097/LGT.0b013e31826b7b51. [DOI] [PubMed] [Google Scholar]
- 287.Gage JC, Ajenifuja KO, Wentzensen N, Adepiti AC, Stoler M, Eder PS, et al. Effectiveness of a simple rapid human papillomavirus DNA test in rural Nigeria. Int J Cancer J Int Cancer. 2012;131(12):2903–9. doi: 10.1002/ijc.27563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Handlogten KS, Molitor RJ, Roeker LE, Narla NP, Bachman MJ, Quayson S, et al. Cervical cancer screening in Ghana, west Africa: prevalence of abnormal cytology and challenges for expanding screening. Int J Gynecol Pathol Off J Int Soc Gynecol Pathol. 2014;33(2):197–202. doi: 10.1097/PGP.0b013e318298a9e6. [DOI] [PubMed] [Google Scholar]
- 289.Akinremi TO, Nazeer S, Tötsch M. Reduced alcohol use in the staining of Pap smears: a satisfactory low-cost protocol for cervical cancer screening. Acta Cytol. 2005;49(2):169–72. doi: 10.1159/000326127. [DOI] [PubMed] [Google Scholar]
- 290.Taylor S, Kuhn L, Dupree W, Denny L, De Souza M, Wright TC. Direct comparison of liquid-based and conventional cytology in a South African screening trial. Int J Cancer J Int Cancer. 2006;118(4):957–62. doi: 10.1002/ijc.21434. [DOI] [PubMed] [Google Scholar]
- 291.Muwonge R, Mbalawa CG, Keita N, Dolo A, Nouhou H, Nacoulma M, et al. Performance of colposcopy in five sub-Saharan African countries. BJOG Int J Obstet Gynaecol. 2009;116(6):829–37. doi: 10.1111/j.1471-0528.2009.02122.x. [DOI] [PubMed] [Google Scholar]
- 292.van Bogaert L-J. Influence of knowledge of human immunodeficiency virus serostatus on accuracy of cervical cytologic diagnosis. Cancer Cytopathol. 2014;122(12):909–13. doi: 10.1002/cncy.21487. [DOI] [PubMed] [Google Scholar]
- 293.Olagbuji BN, Okonkwo CA, Akhiwu W. Evaluation of diagnostic performances of visual cervical inspection with acetic acid and colposcopy in Benin city, Nigeria. West Afr J Med. 2014;33(1):26–31. [PubMed] [Google Scholar]
- 294.Mukakalisa I, Bindler R, Allen C, Dotson J. Cervical cancer in developing countries: effective screening and preventive strategies with an application in Rwanda. Health Care Women Int. 2014;35(7–9):1065–80. doi: 10.1080/07399332.2014.909433. [DOI] [PubMed] [Google Scholar]
- 295.Dartell MA, Rasch V, Iftner T, Kahesa C, Mwaiselage JD, Junge J, et al. Performance of visual inspection with acetic acid and human papillomavirus testing for detection of high-grade cervical lesions in HIV positive and HIV negative Tanzanian women. Int J Cancer J Int Cancer. 2014;135(4):896–904. doi: 10.1002/ijc.28712. [DOI] [PubMed] [Google Scholar]
- 296.Mahmud SM, Sangwa-Lugoma G, Nasr SH, Kayembe PK, Tozin RR, Drouin P, et al. Comparison of human papillomavirus testing and cytology for cervical cancer screening in a primary health care setting in the Democratic Republic of the Congo. Gynecol Oncol. 2012;124(2):286–91. doi: 10.1016/j.ygyno.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 297.Cronjé HS. Cervical screening strategies in resourced and resource-constrained countries. Best Pract Res Clin Obstet Gynaecol. 2011;25(5):575–84. doi: 10.1016/j.bpobgyn.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 298.Arbyn M, Sankaranarayanan R, Muwonge R, Keita N, Dolo A, Mbalawa CG, et al. Pooled analysis of the accuracy of five cervical cancer screening tests assessed in eleven studies in Africa and India. Int J Cancer J Int Cancer. 2008;123(1):153–60. doi: 10.1002/ijc.23489. [DOI] [PubMed] [Google Scholar]
- 299.Allan BR, Marais DJ, Denny L, Hoffman M, Shapiro S, Williamson A-L. The agreement between cervical abnormalities identified by cytology and detection of high-risk types of human papillomavirus. South Afr Med J Suid-Afr Tydskr Vir Geneeskd. 2006;96(11):1186–90. [PubMed] [Google Scholar]
- 300.Doh AS, Nkele NN, Achu P, Essimbi F, Essame O, Nkegoum B. Visual inspection with acetic acid and cytology as screening methods for cervical lesions in Cameroon. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2005;89(2):167–73. doi: 10.1016/j.ijgo.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 301.De Vuyst H, Claeys P, Njiru S, Muchiri L, Steyaert S, De Sutter P, et al. Comparison of pap smear, visual inspection with acetic acid, human papillomavirus DNA-PCR testing and cervicography. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2005;89(2):120–6. doi: 10.1016/j.ijgo.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 302.Cuzick J, Arbyn M, Sankaranarayanan R, Tsu V, Ronco G, Mayrand M-H, et al. Overview of Human Papillomavirus-Based and Other Novel Options for Cervical Cancer Screening in Developed and Developing Countries. Vaccine. 2008;26:K29–41. doi: 10.1016/j.vaccine.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 303.Jeronimo J, Bansil P, Lim J, Peck R, Paul P, Amador JJ, et al. A Multicountry Evaluation of careHPV Testing, Visual Inspection With Acetic Acid, and Papanicolaou Testing for the Detection of Cervical Cancer. Int J Gynecol Cancer. 2014;24(3):576–85. doi: 10.1097/IGC.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Mwanahamuntu MH, Sahasrabuddhe VV, Blevins M, Kapambwe S, Shepherd BE, Chibwesha C, et al. Monitoring the performance of “screen-and-treat” cervical cancer prevention programs. Int J Gynecol Obstet. 2014;126(1):88–9. doi: 10.1016/j.ijgo.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Mwanahamuntu MH, Sahasrabuddhe VV, Blevins M, Kapambwe S, Shepherd BE, Chibwesha C, et al. Utilization of cervical cancer screening services and trends in screening positivity rates in a “screen-and-treat” program integrated with HIV/AIDS care in Zambia. PLoS ONE. 2013;8(9):e74607. doi: 10.1371/journal.pone.0074607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.WHO. Prevention of cervical cancer through screening using visual inspection with acetic acid (VIA) and treatment with cryotherapy: A demonstration project in six African countries: Malawi, Madagascar, Nigeria, Uganda,the United Republic of Tanzania, and Zambia [Internet]. 2012. Available from: http://apps.who.int/iris/bitstream/10665/75250/1/9789241503860_eng.pdf. Accessed 6 July 2015.
- 307.Ramogola-Masire D, de Klerk R, Monare B, Ratshaa B, Friedman HM, Zetola NM. Cervical cancer prevention in HIV-infected women using the “see and treat” approach in Botswana. J Acquir Immune Defic Syndr. 1999;59(3):308–13. doi: 10.1097/QAI.0b013e3182426227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Denny L, Kuhn L, Hu C-C, Tsai W-Y, Wright TC. Human Papillomavirus-Based Cervical Cancer Prevention: Long-term Results of a Randomized Screening Trial. JNCI J Natl Cancer Inst. 2010;102(20):1557–67. doi: 10.1093/jnci/djq342. [DOI] [PubMed] [Google Scholar]
- 309.Pfaendler KS, Mwanahamuntu MH, Sahasrabuddhe VV, Mudenda V, Stringer JSA, Parham GP. Management of cryotherapy-ineligible women in a “screen-and-treat” cervical cancer prevention program targeting HIV-infected women in Zambia: lessons from the field. Gynecol Oncol. 2008;110(3):402–7. doi: 10.1016/j.ygyno.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Denny L, Kuhn L, De Souza M, Pollack AE, Dupree W, Wright TC. Screen-and-treat approaches for cervical cancer prevention in low-resource settings: a randomized controlled trial. JAMA. 2005;294(17):2173–81. doi: 10.1001/jama.294.17.2173. [DOI] [PubMed] [Google Scholar]
- 311.Chigbu CO, Onyebuchi AK. See-and-treat management of high-grade squamous intraepithelial lesions in a resource-constrained African setting. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2014;124(3):204–6. doi: 10.1016/j.ijgo.2013.07.040. [DOI] [PubMed] [Google Scholar]
- 312.Arulogun OS, Maxwell OO. Perception and utilization of cervical cancer screening services among female nurses in University College Hospital, Ibadan, Nigeria. Pan Afr Med J. 2012;11:69. [PMC free article] [PubMed] [Google Scholar]
- 313.McCarey C, Pirek D, Tebeu P, Boulvain M, Doh A, Petignat P. Awareness of HPV and cervical cancer prevention among Cameroonian healthcare workers. BMC Womens Health. 2011;11(1):45. doi: 10.1186/1472-6874-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Levine LD, Chudnoff SG, Taylor K, Baganizi M, Banks E. A 5-day educational program for teaching cervical cancer screening using visual inspection with acetic acid in low-resource settings. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2011;115(2):171–4. doi: 10.1016/j.ijgo.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 315.Dim CC, Ekwe E, Madubuko T, Dim NR, Ezegwui HU. Improved awareness of Pap smear may not affect its use in Nigeria: a case study of female medical practitioners in Enugu, southeastern Nigeria. Trans R Soc Trop Med Hyg. 2009;103(8):852–4. doi: 10.1016/j.trstmh.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 316.Miller D, Okolo CA, Mirabal Y, Guillaud M, Arulogun OS, Oladepo O, et al. Knowledge dissemination and evaluation in a cervical cancer screening implementation program in Nigeria. Gynecol Oncol. 2007;107(1 Suppl 1):S196–207. doi: 10.1016/j.ygyno.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 317.Udigwe GO. Knowledge, attitude and practice of cervical cancer screening (pap smear) among female nurses in Nnewi, South Eastern Nigeria. Niger J Clin Pract. 2006;9(1):40–3. [PubMed] [Google Scholar]
- 318.Moodley J, Kawonga M, Bradley J, Hoffman M. Challenges in implementing a cervical screening program in South Africa. Cancer Detect Prev. 2006;30(4):361–8. doi: 10.1016/j.cdp.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 319.Gharoro EP, Ikeanyi EN. An appraisal of the level of awareness and utilization of the Pap smear as a cervical cancer screening test among female health workers in a tertiary health institution. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. 2006;16(3):1063–8. doi: 10.1111/j.1525-1438.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- 320.Nwobodo EI, Malami SA. Knowledge and practice of cervical screening among female health workers in Sokoto, North Western Nigeria. Niger Postgrad Med J. 2005;12(4):255–7. [PubMed] [Google Scholar]
- 321.Adefuye P. Knowledge and practice of cervical cancer screening among female professional health workers in a sub-urban district of Nigeria. Niger Med Pract. 2006;50(1):19–22. [Google Scholar]
- 322.Mutyaba T, Faxelid E, Mirembe F, Weiderpass E. Influences on uptake of reproductive health services in Nsangi community of Uganda and their implications for cervical cancer screening. Reprod Health. 2007;4(1):4. doi: 10.1186/1742-4755-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Quentin W, Adu-Sarkodie Y, Terris-Prestholt F, Legood R, Opoku BK, Mayaud P. Costs of cervical cancer screening and treatment using visual inspection with acetic acid (VIA) and cryotherapy in Ghana: the importance of scale. Trop Med Int Health TM IH. 2011;16(3):379–89. doi: 10.1111/j.1365-3156.2010.02722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Goldhaber-Fiebert JD, Denny LE, De Souza M, Wright TC, Kuhn L, Goldie SJ. The costs of reducing loss to follow-up in South African cervical cancer screening. Cost Eff Resour Alloc CE. 2005;3:11. doi: 10.1186/1478-7547-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Goldhaber-Fiebert JD, Goldie SJ. Estimating the cost of cervical cancer screening in five developing countries. Cost Eff Resour Alloc. 2006;4:13. doi: 10.1186/1478-7547-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Williams M, Kuffour G, Ekuadzi E, Yeboah M, ElDuah M, Tuffour P. Assessment of psychological barriers to cervical cancer screening among women in Kumasi, Ghana using a mixed methods approach. Afr Health Sci. 2013;13(4):1054–61. doi: 10.4314/ahs.v13i4.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Hoque ME, Ghuman S, Coopoosmay R, Van Hal G. Cervical cancer screening among university students in South Africa: a theory based study. PLoS ONE. 2014;9(11):e111557. doi: 10.1371/journal.pone.0111557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Pengpid S, Peltzer K. Attitudes and practice of cervical cancer screening among female university students from 25 low, middle income and emerging economy countries. Asian Pac J Cancer Prev APJCP. 2014;15(17):7235–9. doi: 10.7314/APJCP.2014.15.17.7235. [DOI] [PubMed] [Google Scholar]
- 329.Adonis L, An R, Luiz J, Mehrotra A, Patel D, Basu D, et al. Provincial screening rates for chronic diseases of lifestyle, cancers and HIV in a health-insured population. South Afr Med J Suid-Afr Tydskr Vir Geneeskd. 2013;103(5):309–12. doi: 10.7196/samj.6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Hoque ME. Awareness of cervical cancer, Papanicolau’s smear and its utilization among female, final year undergraduates in Durban, South Africa. J Cancer Res Ther. 2013;9(1):25–8. doi: 10.4103/0973-1482.110350. [DOI] [PubMed] [Google Scholar]
- 331.El Mhamdi S, Bouanene I, Mhirsi A, Bouden W, Soussi SM. Cervical cancer screening: women’s knowledge, attitudes, and practices in the region of Monastir (Tunisia) Rev Dépidémiologie Santé Publique. 2012;60(6):431–6. doi: 10.1016/j.respe.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 332.Ndikom CM, Ofi BA. Awareness, perception and factors affecting utilization of cervical cancer screening services among women in Ibadan, Nigeria: a qualitative study. Reprod Health. 2012;9:11. doi: 10.1186/1742-4755-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Hyacinth HI, Adekeye OA, Ibeh JN, Osoba T. Cervical cancer and pap smear awareness and utilization of pap smear test among Federal civil servants in North Central Nigeria. PLoS ONE. 2012;7(10):e46583. doi: 10.1371/journal.pone.0046583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Balogun MR, Odukoya OO, Oyediran MA, Ujomu PI. Cervical cancer awareness and preventive practices: a challenge for female urban slum dwellers in Lagos, Nigeria. Afr J Reprod Health. 2012;16(1):75–82. [PubMed] [Google Scholar]
- 335.Ngugi CW, Boga H, Muigai AWT, Wanzala P, Mbithi JN. Factors affecting uptake of cervical cancer early detection measures among women in Thika, Kenya. Health Care Women Int. 2012;33(7):595–613. doi: 10.1080/07399332.2011.646367. [DOI] [PubMed] [Google Scholar]
- 336.Lyimo FS, Beran TN. Demographic, knowledge, attitudinal, and accessibility factors associated with uptake of cervical cancer screening among women in a rural district of Tanzania: three public policy implications. BMC Public Health. 2012;12:22. doi: 10.1186/1471-2458-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Mbamara SU, Ikpeze OC, Okonkwo JEN, Onyiaorah IV, Ukah CO. Knowledge, attitude and practice of cervical cancer screening among women attending gynecology clinics in a tertiary level medical care center in southeastern Nigeria. J Reprod Med. 2011;56(11–12):491–6. [PubMed] [Google Scholar]
- 338.Asuzu CC, Unegbu J, Akin-Odanye E. Knowledge, attitude and behaviour of the University of Ibadan women towards cancer of the cervix and its prevention. Psychooncology. 2012;21(9):1010–5. doi: 10.1002/pon.2007. [DOI] [PubMed] [Google Scholar]
- 339.Mitchell S, Ogilvie G, Steinberg M, Sekikubo M, Biryabarema C, Money D. Assessing women’s willingness to collect their own cervical samples for HPV testing as part of the ASPIRE cervical cancer screening project in Uganda. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2011;114(2):111–5. doi: 10.1016/j.ijgo.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 340.Were E, Nyaberi Z, Buziba N. Perceptions of risk and barriers to cervical cancer screening at Moi Teaching and Referral Hospital (MTRH), Eldoret, Kenya. Afr Health Sci. 2011;11(1):58–64. [PMC free article] [PubMed] [Google Scholar]
- 341.Mosavel M. Health promotion and cervical cancer in South Africa: why adolescent daughters can teach their mothers about early detection. Health Promot Int. 2012;27(2):157–66. doi: 10.1093/heapro/dar014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Nwankwo KC, Aniebue UU, Aguwa EN, Anarado AN, Agunwah E. Knowledge attitudes and practices of cervical cancer screening among urban and rural Nigerian women: a call for education and mass screening. Eur J Cancer Care (Engl) 2011;20(3):362–7. doi: 10.1111/j.1365-2354.2009.01175.x. [DOI] [PubMed] [Google Scholar]
- 343.Hassan FM, Khirelseed M. Cervical cancer screening among Sudanese women. Gulf J Oncolog. 2009;6:28–34. [PubMed] [Google Scholar]
- 344.Obiechina NJA, Mbamara SU. Knowledge attitude and practice of cervical cancer screening among sexually active women in Onitsha, southeast Nigeria. Niger J Med J Natl Assoc Resid Dr Niger. 2009;18(4):384–7. doi: 10.4314/njm.v18i4.51248. [DOI] [PubMed] [Google Scholar]
- 345.Aniebue PN, Aniebue UU. Awareness and practice of cervical cancer screening among female undergraduate students in a Nigerian university. J Cancer Educ Off J Am Assoc Cancer Educ. 2010;25(1):106–8. doi: 10.1007/s13187-009-0023-z. [DOI] [PubMed] [Google Scholar]
- 346.Dim CC, Nwagha UI, Ezegwui HU, Dim NR. The need to incorporate routine cervical cancer counselling and screening in the management of women at the outpatient clinics in Nigeria. J Obstet Gynaecol J Inst Obstet Gynaecol. 2009;29(8):754–6. doi: 10.3109/01443610903225323. [DOI] [PubMed] [Google Scholar]
- 347.Mosavel M, Simon C, Oakar C, Meyer S. Cervical cancer attitudes and beliefs-a Cape Town community responds on World Cancer Day. J Cancer Educ Off J Am Assoc Cancer Educ. 2009;24(2):114–9. doi: 10.1080/08858190902854590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Akujobi CN, Ikechebelu JI, Onunkwo I, Onyiaorah IV. Knowledge, attitude and practice of screening for cervical cancer among female students of a tertiary institution in South Eastern Nigeria. Niger J Clin Pract. 2008;11(3):216–9. [PubMed] [Google Scholar]
- 349.Hoque M, Ibekwe CM, Ntuli-Ngcobo B. Screening and perceived severity of cervical cancer among women attending Mahalapye District Hospital, Botswana. Asian Pac J Cancer Prev APJCP. 2009;10(6):1095–100. [PubMed] [Google Scholar]
- 350.Hoque M, Hoque E, Kader SB. Evaluation of cervical cancer screening program at a rural community of South Africa. East Afr J Public Health. 2008;5(2):111–6. [PubMed] [Google Scholar]
- 351.Ezem BU. Awareness and uptake of cervical cancer screening in Owerri, South-Eastern Nigeria. Ann Afr Med. 2007;6(3):94–8. doi: 10.4103/1596-3519.55727. [DOI] [PubMed] [Google Scholar]
- 352.Oladepo O, Ricketts OL, John-Akinola Y. Knowledge and utilization of cervical cancer screening services among Nigerian students. Int Q Community Health Educ. 2008–2009;29(3):293–304. [DOI] [PubMed]
- 353.Cronjé HS, Beyer E. Screening for cervical cancer in an African setting. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2007;98(2):168–71. doi: 10.1016/j.ijgo.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 354.Mangoma JF, Chirenje MZ, Chimbari MJ, Chandiwana SK. An assessment of rural women’s knowledge, constraints and perceptions on cervical cancer screening: the case of two districts in Zimbabwe. Afr J Reprod Health. 2006;10(1):91–103. doi: 10.2307/30032448. [DOI] [PubMed] [Google Scholar]
- 355.Gatune JW, Nyamongo IK. An ethnographic study of cervical cancer among women in rural Kenya: is there a folk causal model? Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. 2005;15(6):1049–59. doi: 10.1111/j.1525-1438.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- 356.Hoque E, Hoque M. Knowledge of and attitude towards cervical cancer among female university students in South Africa. South Afr J Epidemiol Infect. 2009;24(1):21–4. [Google Scholar]
- 357.Ahmed S, Ahmed R, Idris S, Sabitu K. Knowledge, attitude and practice of cervical cancer screening among market women in Zaria, Nigeria. Niger Med J. 2013;54(5):316. doi: 10.4103/0300-1652.122337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Morema EN, Atieli HE, Onyango RO, Omondi JH, Ouma C. Determinants of cervical screening services uptake among 18–49 year old women seeking services at the Jaramogi Oginga Odinga Teaching and Referral Hospital, Kisumu, Kenya. BMC Health Serv Res. 2014;14:335. doi: 10.1186/1472-6963-14-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Osingada CP, Ninsiima G, Chalo RN, Muliira JK, Ngabirano T. Determinants of uptake of cervical cancer screening services at a no-cost reproductive health clinic managed by nurse-midwives. Cancer Nurs. 2015;38(3):177–84. doi: 10.1097/NCC.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 360.Peltzer K, Phaswana-Mafuya N. Breast and cervical cancer screening and associated factors among older adult women in South Africa. Asian Pac J Cancer Prev APJCP. 2014;15(6):2473–6. doi: 10.7314/APJCP.2014.15.6.2473. [DOI] [PubMed] [Google Scholar]
- 361.Adonis L, Basu D, Luiz J. Predictors of adherence to screening guidelines for chronic diseases of lifestyle, cancers, and HIV in a health-insured population in South Africa. Glob Health Action. 2014;7:23807. doi: 10.3402/gha.v7.23807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Kahesa C, Kjaer S, Mwaiselage J, Ngoma T, Tersbol B, Dartell M, et al. Determinants of acceptance of cervical cancer screening in Dar es Salaam, Tanzania. BMC Public Health. 2012;12:1093. doi: 10.1186/1471-2458-12-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Chigbu CO, Onyebuchi AK, Ajah LO, Onwudiwe EN. Motivations and preferences of rural Nigerian women undergoing cervical cancer screening via visual inspection with acetic acid. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2013;120(3):262–5. doi: 10.1016/j.ijgo.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 364.Kahesa C, Kjaer SK, Ngoma T, Mwaiselage J, Dartell M, Iftner T, et al. Risk factors for VIA positivity and determinants of screening attendances in Dar es Salaam, Tanzania. BMC Public Health. 2012;12:1055. doi: 10.1186/1471-2458-12-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Berraho M, Obtel M, Bendahhou K, Zidouh A, Errihani H, Benider A, et al. Sociodemographic factors and delay in the diagnosis of cervical cancer in Morocco. Pan Afr Med J. 2012;12:14. [PMC free article] [PubMed] [Google Scholar]
- 366.Chigbu CO, Aniebue U. Why southeastern Nigerian women who are aware of cervical cancer screening do not go for cervical cancer screening. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. 2011;21(7):1282–6. doi: 10.1097/IGC.0b013e31822bd139. [DOI] [PubMed] [Google Scholar]
- 367.Chigbu CO, Aniebue UU. Non-uptake of colposcopy in a resource-poor setting. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2011;113(2):100–2. doi: 10.1016/j.ijgo.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 368.McKinnon B, Harper S, Moore S. Decomposing income-related inequality in cervical screening in 67 countries. Int J Public Health. 2011;56(2):139–52. doi: 10.1007/s00038-010-0224-6. [DOI] [PubMed] [Google Scholar]
- 369.Bradley J, Risi L, Denny L. Widening the cervical cancer screening net in a South African township: who are the underserved? Health Care Women Int. 2004;25(3):227–41. doi: 10.1080/07399330490272732. [DOI] [PubMed] [Google Scholar]
- 370.Peters LM, Soliman AS, Bukori P, Mkuchu J, Ngoma T. Evidence for the need of educational programs for cervical screening in rural Tanzania. J Cancer Educ Off J Am Assoc Cancer Educ. 2010;25(2):153–9. doi: 10.1007/s13187-009-0018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Mahomed K, Evans D, Sauls C, Richter K, Smith J, Firnhaber C. Human papillomavirus (HPV) testing on self-collected specimens: perceptions among HIV positive women attending rural and urban clinics in South Africa. Pan Afr Med J. 2014;17:189. doi: 10.11604/pamj.2014.17.189.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Bateman AC, Parham GP, Sahasrabuddhe VV, Mwanahamuntu MH, Kapambwe S, Katundu K, et al. Clinical performance of digital cervicography and cytology for cervical cancer screening in HIV-infected women in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2014;67(2):212–5. doi: 10.1097/QAI.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Ngou J, Magooa MP, Gilham C, Djigma F, Didelot M-N, Kelly H, et al. Comparison of careHPV and hybrid capture 2 assays for detection of high-risk human Papillomavirus DNA in cervical samples from HIV-1-infected African women. J Clin Microbiol. 2013;51(12):4240–2. doi: 10.1128/JCM.02144-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Chung MH, McKenzie KP, De Vuyst H, Richardson BA, Rana F, Pamnani R, et al. Comparing Papanicolau smear, visual inspection with acetic acid and human papillomavirus cervical cancer screening methods among HIV-positive women by immune status and antiretroviral therapy. AIDS Lond Engl. 2013;27(18):2909–19. doi: 10.1097/01.aids.0000432472.92120.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Firnhaber C, Mayisela N, Mao L, Williams S, Swarts A, Faesen M, et al. Validation of cervical cancer screening methods in HIV positive women from Johannesburg South Africa. PLoS ONE. 2013;8(1):e53494. doi: 10.1371/journal.pone.0053494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Mabeya H, Khozaim K, Liu T, Orango O, Chumba D, Pisharodi L, et al. Comparison of conventional cervical cytology versus visual inspection with acetic acid among human immunodeficiency virus-infected women in Western Kenya. J Low Genit Tract Dis. 2012;16(2):92–7. doi: 10.1097/LGT.0b013e3182320f0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Kitchener H, Nelson L, Adams J, Mesher D, Sasieni P, Cubie H, et al. Colposcopy is not necessary to assess the risk to the cervix in HIV-positive women: an international cohort study of cervical pathology in HIV-1 positive women. Int J Cancer J Int Cancer. 2007;121(11):2484–91. doi: 10.1002/ijc.22947. [DOI] [PubMed] [Google Scholar]
- 378.De Vuyst H, Chung MH, Baussano I, Mugo NR, Tenet V, van Kemenade FJ, et al. Comparison of HPV DNA testing in cervical exfoliated cells and tissue biopsies among HIV-positive women in Kenya. Int J Cancer J Int Cancer. 2013;133(6):1441–6. doi: 10.1002/ijc.28131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Bansil P, Lim J, Byamugisha J, Kumakech E, Nakisige C, Jeronimo JA. Performance of Cervical Cancer Screening Techniques in HIV-Infected Women in Uganda. J Low Genit Tract Dis. 2015;19(3):215–9. [DOI] [PubMed]
- 380.Ogilvie GS, Mitchell S, Sekikubo M, Biryabarema C, Byamugisha J, Jeronimo J, et al. Results of a community-based cervical cancer screening pilot project using human papillomavirus self-sampling in Kampala, Uganda. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2013;122(2):118–23. doi: 10.1016/j.ijgo.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 381.Tum SJ, Maree JE, Clarke M. Creating awareness and facilitating cervical and breast cancer screening uptake through the use of a Community Health Worker: a pilot intervention study. Eur J Cancer Care (Engl) 2013;22(1):107–16. doi: 10.1111/ecc.12005. [DOI] [PubMed] [Google Scholar]
- 382.Sankaranarayanan R, Sauvaget C, Ramadas K, Ngoma T, Teguete I, Muwonge R, et al. Clinical trials of cancer screening in the developing world and their impact on cancer healthcare. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2011;22 Suppl 7:vii20–8. doi: 10.1093/annonc/mdr422. [DOI] [PubMed] [Google Scholar]
- 383.Teguete I, Muwonge R, Traore CB, Dolo A, Bayo S, Sankaranarayanan R. Can visual cervical screening be sustained in routine health services? Experience from Mali, Africa. BJOG Int J Obstet Gynaecol. 2012;119(2):220–6. doi: 10.1111/j.1471-0528.2011.03122.x. [DOI] [PubMed] [Google Scholar]
- 384.Were E, Nyaberi Z, Buziba N. Integrating cervical cancer and genital tract infection screening into mother, child health and family planning clinics in Eldoret, Kenya. Afr Health Sci. 2010;10(1):58–65. [PMC free article] [PubMed] [Google Scholar]
- 385.Goldhaber-Fiebert JD, Denny LA, De Souza M, Kuhn L, Goldie SJ. Program spending to increase adherence: South African cervical cancer screening. PLoS ONE. 2009;4(5):e5691. doi: 10.1371/journal.pone.0005691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Shinn E, Basen-Engquist K, Crain B, Follen M. A theory-aided dissemination strategy for emerging technologies in cervical cancer screening. Gynecol Oncol. 2007;107(1):S35–9. doi: 10.1016/j.ygyno.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 387.Roblyer D, Richards-Kortum R, Park S-Y, Adewole I, Follen M. Objective screening for cervical cancer in developing nations: lessons from Nigeria. Gynecol Oncol. 2007;107(1 Suppl 1):S94–7. doi: 10.1016/j.ygyno.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 388.Obi SN, Ozumba BC, Nwokocha AR, Waboso PA. Participation in highly subsidized cervical cancer screening by women in Enugu, South-east Nigeria. J Obstet Gynaecol J Inst Obstet Gynaecol. 2007;27(3):305–7. doi: 10.1080/01443610701227976. [DOI] [PubMed] [Google Scholar]
- 389.Hamad HMA. Cancer initiatives in Sudan. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2006;17 Suppl 8:viii32–6. doi: 10.1093/annonc/mdl985. [DOI] [PubMed] [Google Scholar]
- 390.Knight B. Project: screen South Africa. Diagn Cytopathol. 2005;33(5):356–8. doi: 10.1002/dc.20295. [DOI] [PubMed] [Google Scholar]
- 391.Adewole IF, Benedet JL, Crain BT, Follen M. Evolving a strategic approach to cervical cancer control in Africa. Gynecol Oncol. 2005;99(3 Suppl 1):S209–12. doi: 10.1016/j.ygyno.2005.07.086. [DOI] [PubMed] [Google Scholar]
- 392.Cronjé HS. Screening for cervical cancer in the developing world. Best Pract Res Clin Obstet Gynaecol. 2005;19(4):517–29. doi: 10.1016/j.bpobgyn.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 393.Thomas JO, Babarinsa IA, Ajayi IO, Fawole O, Ojemakinde KO, Omigbodun AO. Mobilization for cervical cancer screening: lessons from a poor-urban Yoruba community in Nigeria. Afr J Med Med Sci. 2005;34(1):81–5. [PubMed] [Google Scholar]
- 394.Richter K. Understanding and incorporating human papillomavirus testing in cervical cancer screening : a South African perspective : review. South Afr J Gynaecol Oncol. 2011;3(1):9–14. doi: 10.1080/20742835.2011.11441169. [DOI] [Google Scholar]
- 395.Risi L, Bindman JP, Campbell OMR, Imrie J, Everett K, Bradley J, et al. Media interventions to increase cervical screening uptake in South Africa: an evaluation study of effectiveness. Health Educ Res. 2004;19(4):457–68. doi: 10.1093/her/cyg044. [DOI] [PubMed] [Google Scholar]
- 396.Ezechi OC, Petterson KO, Gbajabiamila TA, Idigbe IE, Kuyoro O, Ujah IAO, et al. Predictors of default from follow-up care in a cervical cancer screening program using direct visual inspection in south-western Nigeria. BMC Health Serv Res. 2014;14:143. doi: 10.1186/1472-6963-14-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Knegt Y. Audit of cervical cancer screening and colposcopy attendance in rural South Africa. Afr J Reprod Health. 2014;18(4):70–8. [PubMed] [Google Scholar]
- 398.Okonkwo CA, Ezeanochie MC, Olagbuji BN. Physical after effects and clients satisfaction following colposcopy and cervical biopsy in a Nigerian population. Afr Health Sci. 2013;13(2):402–6. doi: 10.4314/ahs.v13i2.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Sancho-Garnier H, Khazraji YC, Cherif MH, Mahnane A, Hsairi M, El Shalakamy A, et al. Overview of cervical cancer screening practices in the extended Middle East and North Africa countries. Vaccine. 2013;31(Suppl 6):G51–7. doi: 10.1016/j.vaccine.2012.06.046. [DOI] [PubMed] [Google Scholar]
- 400.Brower V. AIDS-Related Cancers Increase in Africa. JNCI J Natl Cancer Inst. 2011;103(12):918–9. doi: 10.1093/jnci/djr235. [DOI] [PubMed] [Google Scholar]
- 401.Bosu WK. A comprehensive review of the policy and programmatic response to chronic non-communicable disease in Ghana. Ghana Med J. 2012;46(2 Suppl):69–78. [PMC free article] [PubMed] [Google Scholar]
- 402.Denny L. Cervical cancer: the South African perspective. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2006;95 Suppl 1:S211–4. doi: 10.1016/S0020-7292(06)60036-2. [DOI] [PubMed] [Google Scholar]
- 403.Omondi Aduda DS, Mkhize N. Ethical issues evolving from patients’ perspectives on compulsory screening for syphilis and voluntary screening for cervical cancer in Kenya. BMC Med Ethics. 2014;15:27. doi: 10.1186/1472-6939-15-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Maree JE, Wright SCD. Cervical cancer: does our message promote screening? A pilot study in a South African context. Eur J Oncol Nurs Off J Eur Oncol Nurs Soc. 2011;15(2):118–23. doi: 10.1016/j.ejon.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 405.Kennedy A. Integrating cervical cancer prevention in HIV/AIDS treatment and care programmes. Bull World Health Organ. 2008;86(8):656–6. [DOI] [PMC free article] [PubMed]
- 406.Ditzian LR, David-West G, Maza M, Hartmann B, Shirazian T, Cremer M. Cervical Cancer Screening in Low- and Middle-Income Countries. Mt Sinai J Med J Transl Pers Med. 2011;78(3):319–26. doi: 10.1002/msj.20263. [DOI] [PubMed] [Google Scholar]
- 407.Maree J, Schmollgruber S. An integrative review of South African cancer nursing research published from 2002–2012. Curationis. 2014;37(1):E1–10. doi: 10.4102/curationis.v37i1.1193. [DOI] [PubMed] [Google Scholar]
- 408.Adam Y, van Gelderen CJ, de Bruyn G, McIntyre JA, Turton DA, Martinson NA. Predictors of persistent cytologic abnormalities after treatment of cervical intraepithelial neoplasia in Soweto, South Africa: a cohort study in a HIV high prevalence population. BMC Cancer. 2008;8:211. doi: 10.1186/1471-2407-8-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Adam Y, van Gelderen CJ, Newell K. “Look and Lletz”--a Chris Hani Baragwanath Hospital experience. South Afr Med J Suid-Afr Tydskr Vir Geneeskd. 2008;98(2):119–22. [PubMed] [Google Scholar]
- 410.Lindeque BG. Management of cervical premalignant lesions. Best Pract Res Clin Obstet Gynaecol. 2005;19(4):545–61. doi: 10.1016/j.bpobgyn.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 411.Lewis KDC, Sellors JW, Dawa A, Tsu VD, Kidula NA. Report on a cryotherapy service for women with cervical intraepithelial neoplasia in a district hospital in western Kenya. Afr Health Sci. 2011;11(3):370–6. [PMC free article] [PubMed] [Google Scholar]
- 412.Chamot E, Kristensen S, Stringer JSA, Mwanahamuntu MH. Are treatments for cervical precancerous lesions in less-developed countries safe enough to promote scaling-up of cervical screening programs? A systematic review. BMC Womens Health. 2010;10:11. doi: 10.1186/1472-6874-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Anorlu RI, Orakwue CO, Oyeneyin L, Abudu OO. Late presentation of patients with cervical cancer to a tertiary hospital in Lagos: what is responsible? Eur J Gynaecol Oncol. 2004;25(6):729–32. [PubMed] [Google Scholar]
- 414.Anunobi CC, Jagun OE, Agboola AO, Akintola PA, Andu BA. Clinico-pathological study of female genital malignancies in Olabisi Onabanjo University Teaching Hospital, Sagamu, Ogun State, Nigeria. West Afr J Med. 2014;33(1):7–11. [PubMed] [Google Scholar]
- 415.Ikechebelu JI, Onyiaorah IV, Ugboaja JO, Anyiam DCD, Eleje GU. Clinicopathological analysis of cervical cancer seen in a tertiary health facility in Nnewi, south-east Nigeria. J Obstet Gynaecol J Inst Obstet Gynaecol. 2010;30(3):299–301. doi: 10.3109/01443610903531394. [DOI] [PubMed] [Google Scholar]
- 416.Missaoui N, Hmissa S, Trabelsi A, Frappart L, Mokni M, Korbi S. Cervix cancer in Tunisia: clinical and pathological study. Asian Pac J Cancer Prev APJCP. 2010;11(1):235–8. [PubMed] [Google Scholar]
- 417.Onah HE, Iyoke CA. Abnormal Pap smears: a comparison of total abdominal hysterectomy and cone biopsy in management. J Obstet Gynaecol J Inst Obstet Gynaecol. 2006;26(1):48–51. doi: 10.1080/01443610500378640. [DOI] [PubMed] [Google Scholar]
- 418.Dreyer G. Operative management of cervical cancer. Best Pract Res Clin Obstet Gynaecol. 2005;19(4):563–76. doi: 10.1016/j.bpobgyn.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 419.Einck JP, Hudson A, Shulman AC, Yashar CM, Dieng MM, Diagne M, et al. Implementation of a high-dose-rate brachytherapy program for carcinoma of the cervix in Senegal: a pragmatic model for the developing world. Int J Radiat Oncol Biol Phys. 2014;89(3):462–7. doi: 10.1016/j.ijrobp.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 420.Dreyer G, Snyman LC, Mouton A, Lindeque BG. Management of recurrent cervical cancer. Best Pract Res Clin Obstet Gynaecol. 2005;19(4):631–44. doi: 10.1016/j.bpobgyn.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 421.Mubangizi L, Namusoke F, Mutyaba T. Aerobic cervical bacteriology and antibiotic sensitivity patterns in patients with advanced cervical cancer before and after radiotherapy at a national referral hospital in Uganda. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2014;126(1):37–40. doi: 10.1016/j.ijgo.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 422.Denny L. Cervical cancer treatment in Africa. Curr Opin Oncol. 2011;23(5):469–74. doi: 10.1097/CCO.0b013e3283495a3f. [DOI] [PubMed] [Google Scholar]
- 423.Nyongesa C, Ruff P, Donde B, Kotzen J. A phase I study of concurrent cisplatin chemotherapy in patients with carcinoma of the cervix receiving pelvic radiotherapy. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. 2006;16(4):1614–9. doi: 10.1111/j.1525-1438.2006.00635.x. [DOI] [PubMed] [Google Scholar]
- 424.Msyamboza KP, Manda G, Tembo B, Thambo C, Chitete L, Mindiera C, et al. Cancer survival in Malawi: a retrospective cohort study. Pan Afr Med J. 2014;19:234. doi: 10.11604/pamj.2014.19.234.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Gondos A, Brenner H, Wabinga H, Parkin DM. Cancer survival in Kampala, Uganda. Br J Cancer. 2005;92(9):1808–12. doi: 10.1038/sj.bjc.6602540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Alleyne-Mike K, van Wijk L, Hunter A. A retrospective review of patients with stage IB2 cervical cancer treated with radical radiation versus radical surgery as a primary modality. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. 2013;23(7):1287–94. doi: 10.1097/IGC.0b013e31829fb834. [DOI] [PubMed] [Google Scholar]
- 427.Osman M. The role of neoadjuvant chemotherapy in the management of locally advanced cervix cancer: a systematic review. Oncol Rev. 2014;8(2):250. doi: 10.4081/oncol.2014.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Sankaranarayanan R, Swaminathan R, Brenner H, Chen K, Chia KS, Chen JG, et al. Cancer survival in Africa, Asia, and Central America: a population-based study. Lancet Oncol. 2010;11(2):165–73. doi: 10.1016/S1470-2045(09)70335-3. [DOI] [PubMed] [Google Scholar]
- 429.Kabir F, van Gelderen C, McIntyre J, Michelow P, Turton D, Adam Y. Cervical intra-epithelial neoplasia in HIV-positive women after excision of transformation zone - does the grade change? South Afr Med J Suid-Afr Tydskr Vir Geneeskd. 2012;102(9):757–60. doi: 10.7196/samj.5050. [DOI] [PubMed] [Google Scholar]
- 430.Woo VG, Liegler T, Cohen CR, Sawaya GF, Smith-McCune K, Bukusi EA, et al. Association of cervical biopsy with HIV type 1 genital shedding among women on highly active antiretroviral therapy. AIDS Res Hum Retroviruses. 2013;29(7):1000–5. doi: 10.1089/aid.2012.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Jaquet A, Horo A, Ekouevi DK, Toure B, Coffie PA, Effi B, et al. Risk factors for cervical intraepithelial neoplasia in HIV-infected women on antiretroviral treatment in Côte d’Ivoire, West Africa. PLoS ONE. 2014;9(3):e90625. doi: 10.1371/journal.pone.0090625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Omar T, Schwartz S, Hanrahan C, Modisenyane T, Tshabangu N, Golub JE, et al. Progression and regression of premalignant cervical lesions in HIV-infected women from Soweto: a prospective cohort. AIDS Lond Engl. 2011;25(1):87–94. doi: 10.1097/QAD.0b013e328340fd99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.van Bogaert L-JJ. Age at diagnosis of preinvasive and invasive cervical neoplasia in South Africa: HIV-positive versus HIV-negative women. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. 2011;21(2):363–6. doi: 10.1097/IGC.0b013e3182094d78. [DOI] [PubMed] [Google Scholar]
- 434.Odendal L. Cervical cancer in women with HIV [Internet]. HIV & AIDS Treatment in Practice; 2011 [cited 2015 Jul 10]. Available from: http://www.aidsmap.com/pdf/HATIP-174-February-17th-2011/page/1669154/. Accessed 6 July 2015.
- 435.Holmes RS, Hawes SE, Touré P, Dem A, Feng Q, Weiss NS, et al. HIV infection as a risk factor for cervical cancer and cervical intraepithelial neoplasia in Senegal. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2009;18(9):2442–6. doi: 10.1158/1055-9965.EPI-08-0956. [DOI] [PubMed] [Google Scholar]
- 436.Moodley M. Radical hysterectomy for cervical cancer amongst women infected with the human immunodeficiency virus. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. 2007;17(6):1264–5. doi: 10.1111/j.1525-1438.2007.00936.x. [DOI] [PubMed] [Google Scholar]
- 437.Moodley M. Cervical cancer in Southern Africa: The challenges. SA J Gynaecol Oncol. 2009;1:1. [Google Scholar]
- 438.Elit LM, Rosen B, Jimenez W, Giede C, Cybulska P, Sinasac S, et al. Teaching cervical cancer surgery in low- or middle-resource countries. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. 2010;20(9):1604–8. [PubMed] [Google Scholar]
- 439.Nwogu C, Mahoney M, George S, Dy G, Hartman H, Animashaun M, et al. Promoting cancer control training in resource limited environments: Lagos, Nigeria. J Cancer Educ Off J Am Assoc Cancer Educ. 2014;29(1):14–8. doi: 10.1007/s13187-013-0581-y. [DOI] [PubMed] [Google Scholar]
- 440.van Schalkwyk SL, Maree JE, Wright SCD. Cervical cancer: the route from signs and symptoms to treatment in South Africa. Reprod Health Matters. 2008;16(32):9–17. doi: 10.1016/S0968-8080(08)32399-4. [DOI] [PubMed] [Google Scholar]
- 441.Williams CK, Cristina Stefan D, Rawlinson F, Simbiri K, Mbulaiteye SM. The African Organisation for Research and Training in Cancer and its conferences: a historical perspective and highlights of the Ninth International Conference, Durban, South Africa, 21–24 November 2013. Ecancermedicalscience. 2014;8:396. doi: 10.3332/ecancer.2014.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Kerr D, FIander A. Towards Prevention of Cervical Cancer in Africa Report from Meeting at St. Catherine’s College, Oxford [Internet]. Oxford, UK: AFROX; 2009 [cited 2015 Jul 10]. Available from: http://www.afrox.org/uploads/asset_file/Towards%20the%20Prevention%20of%20Cervical%20Cancer%20in%20Africa%20-%20Conference%20Report.pdf
- 443.Abdel-Wahab M, Bourque J-M, Pynda Y, Iżewska J, Van der Merwe D, Zubizarreta E, et al. Status of radiotherapy resources in Africa: an International Atomic Energy Agency analysis. Lancet Oncol. 2013;14(4):e168–75. doi: 10.1016/S1470-2045(12)70532-6. [DOI] [PubMed] [Google Scholar]
- 444.Bvochara-Nsingo M, Grover S, Gierga DP, Makufa R, Efstathiou JA, Dixit N, et al. Cervical Brachytherapy Exchange: Steps Toward Oncology Capacity Building in Botswana. Oncologist. 2014;19(7):e1–2. doi: 10.1634/theoncologist.2013-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Anorlu RI. Cervical cancer: the sub-Saharan African perspective. Reprod Health Matters. 2008;16(32):41–9. doi: 10.1016/S0968-8080(08)32415-X. [DOI] [PubMed] [Google Scholar]
- 446.McArdle O, Kigula-Mugambe JB. Contraindications to cisplatin based chemoradiotherapy in the treatment of cervical cancer in Sub-Saharan Africa. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2007;83(1):94–6. doi: 10.1016/j.radonc.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 447.Government of the Republic of Zambia. The 6th Stop Cervical Cancer in Africa Conference Report [Internet]. Lusaka, Zambia; 2013. Available from: http://pinkribbonredribbon.org/wp-content/uploads/6th-SCCA-Report.pdf. Accessed 6 July 2015.
- 448.Berraho M, Najdi A, Mathoulin-Pelissier S, Salamon R, Nejjari C. Direct costs of cervical cancer management in Morocco. Asian Pac J Cancer Prev APJCP. 2012;13(7):3159–63. doi: 10.7314/APJCP.2012.13.7.3159. [DOI] [PubMed] [Google Scholar]
- 449.Ben Gobrane H, Aounallah-Skhiri H, Oueslati F, Frikha H, Achour N, Hsairi M. Estimated cost of managing invasive cervical cancer in Tunisia. Santé Publique Vandoeuvre-Lès-Nancy Fr. 2009;21(6):561–9. [PubMed] [Google Scholar]
- 450.Selman L, Siegert RJ, Higginson IJ, Agupio G, Dinat N, Downing J, et al. The “Spirit 8” successfully captured spiritual well-being in African palliative care: factor and Rasch analysis. J Clin Epidemiol. 2012;65(4):434–43. doi: 10.1016/j.jclinepi.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 451.Selman LE, Higginson IJ, Agupio G, Dinat N, Downing J, Gwyther L, et al. Quality of life among patients receiving palliative care in South Africa and Uganda: a multi-centred study. Health Qual Life Outcomes. 2011;9:21. doi: 10.1186/1477-7525-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Awadalla AW, Ohaeri JU, Gholoum A, Khalid AOA, Hamad HMA, Jacob A. Factors associated with quality of life of outpatients with breast cancer and gynecologic cancers and their family caregivers: a controlled study. BMC Cancer. 2007;7:102. doi: 10.1186/1471-2407-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used for this study is limited to previously published or presented cervical cancer research and, thus, is publicly available by journal and conference review.


