Abstract
IMPORTANCE
Little is known about cardiac adverse events among patients with nonobstructive coronary artery disease (CAD).
OBJECTIVE
To compare myocardial infarction (MI) and mortality rates between patients with nonobstructive CAD, obstructive CAD, and no apparent CAD in a national cohort.
DESIGN, SETTING, AND PARTICIPANTS
Retrospective cohort study of all US veterans undergoing elective coronary angiography for CAD between October 2007 and September 2012 in the Veterans Affairs health care system. Patients with prior CAD events were excluded.
EXPOSURES
Angiographic CAD extent, defined by degree (no apparent CAD: no stenosis >20%; nonobstructive CAD: ≥1 stenosis ≥20% but no stenosis ≥70%; obstructive CAD: any stenosis ≥70% or left main [LM] stenosis ≥50%) and distribution (1,2, or 3 vessel).
MAIN OUTCOMES AND MEASURES
The primary outcome was 1-year hospitalization for nonfatal MI after the index angiography. Secondary outcomes included 1-year all-cause mortality and combined 1-year MI and mortality.
RESULTS
Among37 674 patients, 8384 patients (22.3%) had nonobstructive CAD and 20 899 patients (55.4%) had obstructive CAD. Within 1 year, 845 patients died and 385 were rehospitalized for MI. Among patients with no apparent CAD, the 1-year MI rate was 0.11% (n = 8, 95% CI, 0.10%–0.20%) and increased progressively by 1-vessel nonobstructive CAD, 0.24% (n = 10, 95% CI, 0.10%–0.40%); 2-vessel nonobstructive CAD, 0.56% (n = 13, 95% CI, 0.30%–1.00%); 3-vessel nonobstructive CAD, 0.59% (n = 6, 95% CI, 0.30%–1.30%); 1-vessel obstructive CAD, 1.18% (n = 101, 95% CI, 1.00%–1.40%); 2-vessel obstructive CAD, 2.18% (n = 110, 95% CI, 1.80%–2.60%); and 3-vessel or LM obstructive CAD, 2.47% (n = 137, 95% CI, 2.10%–2.90%). After adjustment, 1-year MI rates increased with increasing CAD extent. Relative to patients with no apparent CAD, patients with 1-vessel nonobstructive CAD had a hazard ratio (HR) for 1-year MI of 2.0 (95% CI, 0.8–5.1); 2-vessel nonobstructive HR, 4.6 (95% CI, 2.0–10.5); 3-vessel nonobstructive HR, 4.5 (95% CI, 1.6–12.5); 1-vessel obstructive HR, 9.0 (95% CI, 4.2–19.0); 2-vessel obstructive HR, 16.5 (95% CI, 8.1–33.7); and 3-vessel or LM obstructive HR, 19.5 (95% CI, 9.9–38.2). One-year mortality rates were associated with increasing CAD extent, ranging from 1.38% among patients without apparent CAD to 4.30% with 3-vessel or LM obstructive CAD. After risk adjustment, there was no significant association between 1- or 2-vessel nonobstructive CAD and mortality, but there were significant associations with mortality for 3-vessel nonobstructive CAD (HR, 1.6; 95% CI, 1.1–2.5), 1-vessel obstructive CAD (HR, 1.9; 95% CI, 1.4–2.6), 2-vessel obstructive CAD (HR, 2.8; 95% CI, 2.1–3.7), and 3-vessel or LM obstructive CAD (HR, 3.4; 95% CI, 2.6–4.4). Similar associations were noted with the combined outcome.
CONCLUSIONS AND RELEVANCE
In this cohort of patients undergoing elective coronary angiography, nonobstructive CAD, compared with no apparent CAD, was associated with a significantly greater 1-year risk of MI and all-cause mortality. These findings suggest clinical importance of nonobstructive CAD and warrant further investigation of interventions to improve outcomes among these patients.
Nonobstructive coronary artery disease (CAD) is atherosclerotic plaque that would not be expected to obstruct blood flow or result in anginal symptoms. Although such lesions are relatively common, occurring in 10% to 25% of patients undergoing coronary angiography,1,2 their presence has been characterized as “insignificant” or “no significant CAD” in the medical literature.3–6 However, this perception of nonobstructive CAD may be incorrect, because prior studies have noted that the majority of plaque ruptures and resultant myocardial infarctions (MIs) arise from nonobstructive plaques.7–13
Despite the prevalence of nonobstructive CAD identified by coronary angiography, little is known about its risk of adverse outcomes. The few studies that do exist focus primarily on patients with MI3,4 and thus are less informative about patients with stable nonobstructive CAD. A primary reason behind this lack of knowledge is lack of data. To date, almost all coronary angiography registries include obstructive CAD only.14 The few registries that do include patients with nonobstructive CAD lack longitudinal outcomes data.15 More data on nonobstructive CAD patients and their longitudinal outcomes are essential for understanding their risks for adverse cardiac outcomes and potential therapeutic implications.
This study evaluated the hypothesis that increasing CAD extent across the continuum of nonobstructive and obstructive CAD is associated with increasing rates of MI and all-cause mortality. To test this hypothesis, we used data from the national Veterans Affairs (VA) Clinical Assessment, Reporting, and Tracking (CART) program, which records anatomic data from all coronary angiograms performed in the VA health care system and tracks patients’ longitudinal outcomes. We assessed the prevalence of nonobstructive and obstructive CAD extent and assessed its association with 1-year hospitalization for nonfatal MI and all-cause mortality rates.
Methods
Data for this analysis were from the VA CART program, which is a national clinical quality program for all VA cardiac catheterization laboratories.16 This program uses a clinical software application, embedded in the VA electronic health record (EHR), to capture standardized patient and procedural data for all coronary procedures performed in VA catheterization laboratories nationwide. Data elements in the application are derived from the American College of Cardiology’s National Cardiovascular Data Registry (NCDR) data definitions.15 Quality checks of the CART data are periodically conducted for completeness and accuracy, and its data validity, completeness, and timeliness have been previously demonstrated.17
To capture longitudinal patient data, CART data are combined with other data from the VA patient EHR. These data include vital status, inpatient hospitalizations, outpatient clinic visits, pharmacy prescriptions and refills, and laboratory testing. In addition, the data set is merged with VA claims data from those hospitalizations at non-VA facilities where the VA pays for the veterans’ care. Institutional review board and VA research and development approvals were obtained for the creation of the data set and for this particular study. The Colorado multiple institutional review board provided a waiver of consent and approval for this study.
Study Cohort
The analysis included all VA patients undergoing elective coronary angiography for CAD indications (chest pain, stable angina, ischemic heart disease, or positive functional study) between October 2007 and September 2012 in any of the 79 VA cardiac catheterization laboratories. Positive functional study was defined as any cardiac stress test indicative of ischemia. Patients with known prior CAD events–defined as prior MI, acute coronary syndrome (ACS), or coronary revascularization–were excluded. For patients receiving multiple coronary angiograms during our study time period, the first angiogram was used to characterize CAD extent.
Independent Variables
Consistent with standard definitions of flow-limiting stenoses,18–20 nonobstructive CAD was defined as a coronary artery stenosis 20% or greater but less than 50% in the left main coronary artery or a stenosis 20% or greater but less than 70% in any other epicardial coronary artery, as recorded by the clinician in the catheterization report. Obstructive CAD was defined as any stenosis 50% or greater in the left main coronary artery, 70% or greater in any other coronary artery, or both. No apparent CAD was defined as all coronary stenoses less than 20% or luminal irregularities.
We then categorized each patient by his or her CAD extent. To accomplish this, we categorized each patient by CAD severity in a single, double, or triple-vessel distribution. We defined vessel distribution by the left anterior descending artery and its tributaries, the left circumflex artery and its tributaries, and the right coronary artery and its tributaries. Patients with isolated 20% to 49% left main stenosis were included with the 1-vessel nonobstructive CAD patients. Patients with 50% or greater left main coronary artery stenosis were included with the 3-vessel obstructive CAD patients. For each vascular distribution, we determined the maximal stenosis present and classified that distribution as no apparent CAD, nonobstructive CAD, or obstructive CAD, as defined in the preceding paragraph. In total, we created 7 categories of CAD extent: no apparent CAD; 1-, 2-, and 3-vessel nonobstructive CAD; and 1-, 2-, and 3-vessel obstructive CAD.
Outcomes
The primary outcome was 1-year hospitalization for nonfatal MI after the index angiography. Myocardial infarction was defined by a primary diagnosis International Classification of Diseases, Ninth Revision (ICD-9) code of 410.xx in VA inpatient and VA fee-based data files. To account for those veterans who are “dual covered” with Medicare and VA benefits and may have been hospitalized in a non-VA hospital using their Medicare benefits, we also included all Medicare hospitalizations for MI through calendar year 2011 in our cohort, using the most recent Centers for Medicare & Medicaid Services (CMS) data files. Data files from the VA and CMS were linked using scrambled Social Security numbers for individual patients. Secondary outcomes included 1-year all-cause mortality and combined 1-year MI and mortality. Mortality was measured using VA vital status data.
Statistical Analyses
Characteristics of patients (demographics, comorbidities), procedural data (eg, indications for angiography), postangiography cardiac medications, and postangiography revascularization treatments were collected and compared by CAD extent. Categorical data were compared using χ2 tests and continuous data using Mann-Whitney Wilcoxon non-parametric tests.
Rates of MI, all-cause mortality, and the combined outcome during the full study period were calculated and compared by CAD extent using log-rank tests and Kaplan-Meier curves. Unadjusted outcome rates were calculated using Kaplan-Meier estimates to include the full study cohort with its differential censoring. Cox regression modeling was used to adjust for covariates selected from prior studies and a priori clinical reasoning.15,17 All outcomes were censored at 1 year. Patients with no apparent CAD were used as the reference group.
A robust estimator of the covariance matrix was used to account for clustering by site.21 Model covariates included demographics (age, sex, race), CAD risk factors (hypertension, hyperlipidemia, diabetes, tobacco use, obesity), Framingham 10-year cardiovascular disease risk, other comorbidities (congestive heart failure, chronic obstructive pulmonary disease, cerebrovascular disease, peripheral arterial disease, post-traumatic stress disorder, depression, sleep apnea, chronic kidney disease, dialysis), angiography indication (chest pain, positive functional study, ischemic heart disease, stable angina), postangiography cardiac medications (statins, β-blockers, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers), and postangiography revascularization (none, coronary artery bypass graft surgery, percutaneous coronary intervention).
Race was defined as white or nonwhite. Nonwhite race included American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian, or Other Pacific Islander. Racial and ethnicity classifications were based on patient self-report, where possible, and performed in accord with VA best practices for data classification.22 Obesity was defined as a body mass index of 30 or greater (calculated as weight in kilograms divided by height in meters squared). Framingham risk was calculated using methods previously described20 and defined as low (<10% 10-year predicted risk of cardiac adverse events), intermediate (10%–20% 10-year predicted risk of cardiac adverse events), and high (>20% 10-year predicted risk of cardiac adverse events). Aspirin use could not be reliably calculated, as most veterans obtain those medications from outside, over-the-counter pharmacies rather than from a VA pharmacy.
We conducted several sensitivity analyses to test the robustness of our findings. First, to incorporate all available coronary anatomic data from CART in our classification of CAD extent, we categorized CAD by classifying each major coronary territory as no apparent CAD, nonobstructive, or obstructive using the definitions listed earlier in this section, thus creating 10 CAD categories. We then compared increasing CAD extent, as defined by these 10 categories, to our outcomes using a linear regression model that adjusted for the same covariates used in our primary analysis. Second, our initial analyses noted that a small number of patients with no apparent CAD and nonobstructive CAD (n = 110, 0.3%) underwent subsequent revascularization, indicating that they had at least 1 stenosis that the treating clinician determined appropriate for re-vascularization. To determine if these patients–who could not be definitively classified into no apparent, nonobstructive, or obstructive CAD categories based on the data–affected the overall results, we excluded them and reran our primary analyses. Third, we had CMS data only through the end of calendar year 2011, because of the latency with which CMS makes their data available publicly. To determine whether inclusion of these data altered our primary findings, we reran our primary analyses excluding CMS data.
In addition to our primary and sensitivity analyses, 3 secondary analyses were also conducted. First, to determine if increasing degrees of nonobstructive CAD severity were associated with outcomes in a progressive manner, nonobstructive CAD was subdivided into mild (maximal coronary stenosis of 20%–49%) and moderate nonobstructive CAD (maximal coronary stenosis of 50%–69%), in line with standard definitions regarding CAD severity.23 The analysis was restricted to those patients who had sufficient coronary anatomic information to determine mild and moderate nonobstructive CAD. We then conducted unadjusted and adjusted time-to-event analyses, using no apparent CAD as the referent group.
Second, we conducted prespecified subgroup analyses among patients with diabetes in our cohort, because prior cardiac computed tomography (CT) investigations among patients with nonobstructive CAD found that diabetes significantly modified outcomes.24 Diabetes was determined from VA data files. Stratified analyses and interaction testing were performed. To assess for interaction between diabetes and CAD extent,andbetween symptoms and CAD extent, separate Cox models were fitted with the interaction term and main effect, adjusting for covariates. Wald tests with 6 df were used to test the interaction term.
Third, we conducted a similar analysis among symptomatic patients in our cohort, again because prior CT literature demonstrated effect modification by symptoms among patients with nonobstructive CAD.25,26 Cardiac symptoms were determined by the presence of either stable angina or chest pain as the primary angiography indication. Stratified analyses and interaction testing were performed.
Because we cross-referenced CART data with VA patient data files, most variables were missing in less than 5% of cases. One exception was data on race, which we imputed using the SAS procedure PROC MI. All analyses were done in SAS version 9.3 (SAS Institute). Significance testing was 2-sided, and all P values <.05 were considered statistically significant.
Results
During the study period, 37 674 patients underwent elective coronary angiography for indications related to CAD as characterized by the treating clinician (Table 1 and Table 2). Of those, 8391 (22.3%) patients had no apparent CAD; 8384 (22.3%) patients had nonobstructive CAD (1-vessel: 4646 [12.3% of total patients], 2-vessel: 2605 [6.9%], 3-vessel: 1133 [3.0%]); and 20 899 (55.4%) patients had obstructive CAD (1-vessel: 9411 [25.0% of total patients], 2-vessel: 5452 [14.5%], 3-vessel or left main [LM]: 6036 [16.0%]). The majority of patients underwent angiography for chest pain. Age, cardiovascular risk factors (eg, hypertension, hyperlipidemia, and diabetes), and Framingham risk scores all increased with increasing CAD extent. In addition, the frequency of prescriptions for postangiography cardiovascular medications and rates of coronary revascularization also increased with CAD extent.
Table 1.
Patient Characteristics by No Apparent and Nonobstructive CAD
| No Apparent CAD, No. (%) | Nonobstructive CAD, No. (%) | P Value | |||
|---|---|---|---|---|---|
| 1-Vessel | 2-Vessel | 3-Vessel | |||
| Patients, No. | 8391 | 4646 | 2605 | 1133 | |
| Age, median (IQR), y | 58.5 (51.3–63.3) | 61.9 (57.1–66) | 62.3 (57.6–66.7) | 63.0 (58.7–67.8) | <.001 |
| Male sex | 7456 (88.9) | 4401 (94.7) | 2505 (96.2) | 1103 (97.4) | <.001 |
| White race | 6055 (72.2) | 3731 (80.3) | 2115 (81.2) | 923 (81.5) | <.001 |
| Clinical comorbidities | |||||
| Hypertension | 6452 (76.9) | 3905 (84.1) | 2256 (86.6) | 1004 (88.6) | <.001 |
| Hyperlipidemia | 6200 (73.9) | 3801 (81.8) | 2212 (84.9) | 966 (85.3) | <.001 |
| Diabetes | 2721 (32.4) | 1854 (39.9) | 1149 (44.1) | 542 (47.8) | <.001 |
| Tobacco use (ever) | 4703 (56.0) | 2823 (60.8) | 1639 (62.9) | 720 (63.5) | <.001 |
| Obese | 3301 (39.3) | 1743 (37.5) | 990 (38.0) | 442 (39.0) | .19 |
| CHF | 674 (8.0) | 441 (9.5) | 274 (10.5) | 134 (11.8) | <.001 |
| COPD | 1567 (18.7) | 1047 (22.5) | 628 (24.1) | 295 (26.0) | <.001 |
| CVD | 647 (7.7) | 508 (10.9) | 302 (11.6) | 150 (13.2) | <.001 |
| PAD | 608 (7.2) | 513 (11.0) | 380 (14.6) | 204 (18.0) | <.001 |
| PTSD | 2155 (25.7) | 1033 (22.2) | 580 (22.3) | 258 (22.8) | <.001 |
| Depression | 3960 (47.2) | 1967 (42.3) | 1088 (41.8) | 464 (41.0) | <.001 |
| Sleep apnea | 2075 (24.7) | 1126 (24.2) | 631 (24.2) | 248 (21.9) | .22 |
| Chronic kidney disease | 697 (8.3) | 485 (10.4) | 287 (11.0) | 157 (13.9) | <.001 |
| Dialysis | 113 (1.3) | 59 (1.3) | 57 (2.2) | 26 (2.3) | .001 |
| Framingham risk score | |||||
| Low (10-y risk <10%) | 3657 (43.6) | 1234 (26.6) | 594 (22.8) | 254 (22.4) | <.001 |
| Medium (10-y risk 10%–20%) | 3920 (46.7) | 2624 (56.5) | 1442 (55.4) | 632 (55.8) | |
| High (10-y risk >20%) | 814 (9.7) | 788 (17.0) | 569 (21.8) | 247 (21.8) | |
| Angiography indication | |||||
| Chest pain | 5693 (67.8) | 3063 (65.9) | 1753 (67.3) | 714 (63.0) | <.001 |
| Positive functional study | 2317 (27.6) | 1318 (28.4) | 705 (27.1) | 346 (30.5) | |
| Ischemic heart disease | 100 (1.2) | 102 (2.2) | 57 (2.2) | 29 (2.6) | |
| Stable angina | 281 (3.3) | 163 (3.5) | 90 (3.5) | 44 (3.9) | |
| Postangiography cardiac medications | |||||
| Statins | 3758 (44.8) | 2745 (59.1) | 1641 (63.0) | 710 (62.7) | <.001 |
| β-Blockers | 3142 (37.4) | 2186 (47.1) | 1322 (50.7) | 577 (50.9) | <.001 |
| ACEIs/ARBs | 2848 (33.9) | 2071 (44.6) | 1188 (45.6) | 556 (49.1) | <.001 |
| Postangiography revascularization | |||||
| None | 8374 (99.8) | 4603 (99.1) | 2573 (98.8) | 1115 (98.4) | <.001 |
| CABG | 2 (<0.05) | 6 (0.1) | 3 (0.1) | 2 (0.2) | |
| PCI | 15 (0.2) | 37 (0.8) | 29 (1.1) | 16 (1.4) | |
Abbreviations: ACEIs/ARBs, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers; CABG, coronary artery bypass graft surgery; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVD, cerebrovascular disease; IQR, interquartile range; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; PTSD, posttraumatic stress disorder.
Table 2.
Patient Characteristics by Obstructive CAD
| Obstructive CAD, No. (%) | P Value | |||
|---|---|---|---|---|
| 1-Vessel | 2-Vessel | 3-Vessel or Left Main | ||
| Patients, No. | 9411 | 5452 | 6036 | |
| Age, median (IQR), y | 63.2 (59.3–67.8) | 64.0 (60.2–69.1) | 64.6 (60.9–70.8) | <.001 |
| Male sex | 9191 (97.7) | 5371 (98.5) | 5981 (99.1) | <.001 |
| White race | 8073 (85.8) | 4763 (87.4) | 5249 (87.0) | .01 |
| Clinical comorbidities | ||||
| Hypertension | 8125 (86.3) | 4781 (87.7) | 5314 (88.0) | .004 |
| Hyperlipidemia | 8041 (85.4) | 4801 (88.1) | 5340 (88.5) | <.001 |
| Diabetes | 4026 (42.8) | 2553 (46.8) | 2854 (47.3) | <.001 |
| Tobacco use (ever) | 5781 (61.4) | 3243 (59.5) | 3583 (59.4) | .01 |
| Obese | 3189 (33.9) | 1773 (32.5) | 1820 (30.2) | <.001 |
| CHF | 948 (10.1) | 543 (10.0) | 566 (9.4) | .35 |
| COPD | 1943 (20.6) | 1020 (18.7) | 970 (16.1) | <.001 |
| CVD | 1250 (13.3) | 838 (15.4) | 1038 (17.2) | <.001 |
| PAD | 1569 (16.7) | 1126 (20.7) | 1351 (22.4) | <.001 |
| PTSD | 1777 (18.9) | 898 (16.5) | 863 (14.3) | <.001 |
| Depression | 3322 (35.3) | 1707 (31.3) | 1653 (27.4) | <.001 |
| Sleep apnea | 1823 (19.4) | 916 (16.8) | 855 (14.2) | <.001 |
| Chronic kidney disease | 1114 (11.8) | 756 (13.9) | 870 (14.4) | <.001 |
| Dialysis | 163 (1.7) | 115 (2.1) | 101 (1.7) | .16 |
| Framingham risk score | ||||
| Low (10-y risk <10%) | 1734 (18.4) | 787 (14.4) | 776 (12.9) | <.001 |
| Medium (10-y risk 10%–20%) | 5397 (57.3) | 3099 (56.8) | 3242 (53.7) | |
| High (10-y risk >20%) | 2280 (24.2) | 1566 (28.7) | 2018 (33.4) | |
| Angiography indication | ||||
| Chest pain | 5944 (63.2) | 3390 (62.2) | 3792 (62.8) | <.001 |
| Positive functional study | 2384 (25.3) | 1348 (24.7) | 1462 (24.2) | |
| Ischemic heart disease | 650 (6.9) | 452 (8.3) | 548 (9.1) | |
| Stable angina | 433 (4.6) | 262 (4.8) | 234 (3.9) | |
| Postangiography cardiac medications | ||||
| Statins | 6992 (74.3) | 4083 (74.9) | 4410 (73.1) | .07 |
| β-Blockers | 6348 (67.5) | 3926 (72.0) | 4487 (74.3) | <.001 |
| ACEIs/ARBs | 4854 (51.6) | 2893 (53.1) | 2961 (49.1) | <.001 |
| Postangiography revascularization | ||||
| None | 4628 (49.2) | 2274 (41.7) | 2408 (39.9) | <.001 |
| CABG | 323 (3.4) | 879 (16.1) | 2815 (46.6) | |
| PCI | 4460 (47.4) | 2299 (42.2) | 813 (13.5) | |
Abbreviations: ACEIs/ARBs, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers; CABG, coronary artery bypass graft surgery; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVD, cerebrovascular disease; IQR, interquartile range; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; PTSD, posttraumatic stress disorder.
Outcomes
In unadjusted analyses, 1-year MI rates progressively increased with increasing CAD extent, ranging from 0.11% among patients with no apparent CAD to 2.47% among patients with 3-vessel or LM obstructive CAD (Table 3 and Figure 1). After risk adjustment using the covariates described in the “Methods” section, there was no association between 1-vessel nonobstructive CAD and MI, but there were significant associations with MI for 2-vessel nonobstructive; 3-vessel nonobstructive; and 1-, 2-, and 3-vessel or LM obstructive CAD (Figure 2).
Table 3.
Kaplan-Meier Estimates of 1-Year MI, All-Cause Mortality, and Combined Outcome Rates by CAD Extent
| No Apparent CAD, % (95% CI) | Nonobstructive CAD, % (95% CI) | Obstructive CAD, % (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| 1-Vessel | 2-Vessel | 3-Vessel | 1-Vessel | 2-Vessel | 3-Vessel/Left Main | ||
| 1-Year MI | 0.11 (0.10–0.20) | 0.24 (0.10–0.40) | 0.56 (0.30–1.00) | 0.59 (0.30–1.30) | 1.18 (1.00–1.40) | 2.18 (1.80–2.60) | 2.47 (2.10–2.90) |
| 1-Year mortality | 1.38 (1.10–1.70) | 2.02 (1.60–2.50) | 1.50 (1.10–2.10) | 2.72 (1.90–3.90) | 2.25 (2.00–2.60) | 3.33 (2.90–3.90) | 4.30 (3.80–4.90) |
| 1-Year combined outcomes | 1.48 (1.20–1.80) | 2.18 (1.80–2.70) | 1.88 (1.40–2.50) | 3.18 (2.30–4.50) | 3.13 (2.80–3.50) | 4.94 (4.40–5.60) | 6.19 (5.60–6.90) |
Abbreviations: CAD, coronary artery disease; MI, myocardial infarction.
Figure 1. Time-to-Event Plots for 1-Year Myocardial Infarction, Mortality, and Combined Myocardial Infarction and Mortality,by CAD Extent.
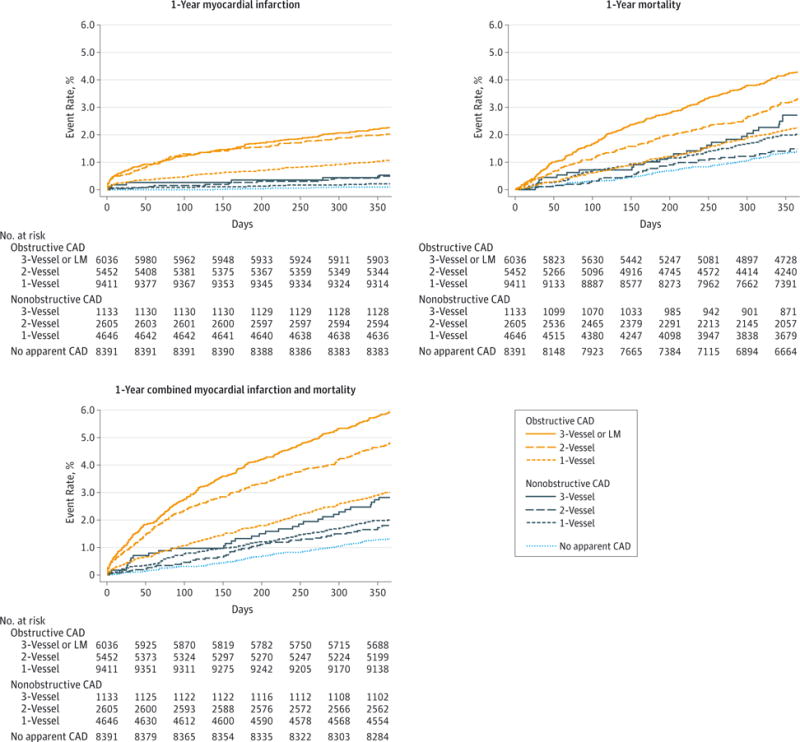
CAD indicates coronary artery disease; LM, left main.
Figure 2. Adjusted Cox Model Results for 1-Year Myocardial Infarction, Mortality, and Combined Myocardial Infarction and Mortality by CAD Extent, Relative to No Apparent CAD.
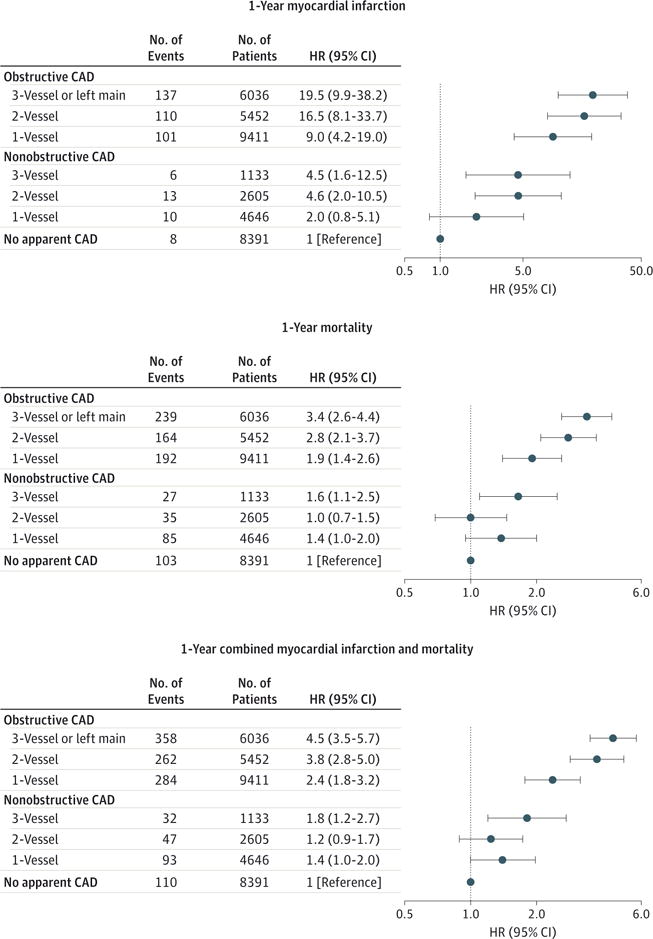
CAD indicates coronary artery disease; HR, hazard ratio.
Similar relationships were noted when both 1-year all-cause mortality and combined MI and mortality outcomes were examined. One-year mortality rates demonstrated a largely progressive relationship with increasing CAD extent, ranging from 1.38% among patients without apparent CAD to 4.30% among patients with 3-vessel or LM obstructive CAD (Table 3 and Figure 1). After risk adjustment, there was no association between 1-vessel or 2-vessel nonobstructive CAD and mortality, but there were significant associations with mortality for 3-vessel nonobstructive and 1-, 2-, and 3-vessel or LM obstructive CAD (Figure 2).
Similarly, combined MI and mortality outcomes also demonstrated a largely progressive relationship with increasing CAD extent, ranging from 1.48% for patients with no apparent CAD to 6.19% for patients with 3-vessel or LM obstructive CAD (Table 3 and Figure 1). After risk adjustment, there was no association between 1-vessel or 2-vessel nonobstructive CAD and mortality, but there were significant associations with combined outcomes for 3-vessel non-obstructive and 1-, 2-, and 3-vessel or LM obstructive CAD (Figure 2). Sensitivity analyses exploring additional categorization of CAD extent, exclusion of nonobstructive CAD patients undergoing coronary revascularization, and exclusion of Medicare outcomes were conducted as described in the “Methods” section. None of the analyses materially affected our findings for any of the outcomes.
Secondary Analyses
Three subgroup analyses were conducted: outcomes among patients with mild/moderate nonobstructive CAD, patients with diabetes, and symptomatic patients. Among 8740 patients with nonobstructive CAD, 4913 (56.2%) had mild nonobstructive CAD, and 3827 (43.8%) had moderate non-obstructive CAD. All unadjusted outcomes increased in progressive fashion in association with mild to moderate non-obstructive CAD (eTable in the Supplement). After risk adjustment, all outcomes, with the exception of mild non-obstructive CAD and 1-year mortality, significantly increased with increasing nonobstructive CAD extent (eFigure 1 in the Supplement). Among 37 674 patients in the study cohort, 15 699 (41.7%) had diabetes. Adjusted outcome rates increased with increasing CAD extent but did not significantly differ by diabetes status in interaction testing (eFigure 2 in the Supplement). Among 37 674 patients in the study cohort, 25 856 (68.6%) were symptomatic. Adjusted outcome rates increased with increasing CAD extent but did not significantly differ by symptomatic status in interaction testing (eFigure 3 in the Supplement).
Discussion
This study assessed the risk of patients with nonobstructive CAD for 1-year MI and all-cause mortality rates, compared with those with no apparent CAD and obstructive CAD. The 1-year MI risk progressively increased by CAD extent, rather than abruptly increasing between nonobstructive and obstructive CAD. Moreover, patients with nonobstructive CAD had an associated risk of MI that was 2- to 4.5-fold greater than among those with no apparent CAD. Similar observations were noted with 1-year mortality and combined outcomes. These findings highlight a need to recognize that nonobstructive CAD is associated with significantly increased risk for MI, consistent with prior biologic studies indicating that a majority of MIs are related to nonobstructive stenoses.7–13 Correspondingly, these results reveal the limitations of a dichotomous characterization of angiographic CAD into “obstructive” and “nonobstructive” to predict MI and highlight the importance of preventive strategies such as pharmacotherapy treatments and lifestyle modifications to mitigate these risks.
Historically, obstructive CAD has been the primary focus in CAD management because of its role in causing cardiac ischemia and accompanying anginal symptoms.27,28 In addition, obstructive CAD usually corresponds to extensive CAD, which is associated with higher MI rates. However, the recognition that ruptured plaque, rather than occlusive plaque, is the genesis for most MIs,7,9,12,13,29 along with the recognition that the majority of ruptured plaques arise from nonobstructive CAD,8,10,11,30 suggests that nonobstructive CAD is associated with significant risk for MI and all-cause mortality and provided the rationale for this investigation.2,31
The ability to explore cardiac outcomes among patients with nonobstructive CAD has been limited by insufficient data about both the disease and its outcomes. Most trials and registries in CAD have been limited to patients with obstructive CAD.14,32 Furthermore, those registries that do collect data about patients with nonobstructive disease, such as the American College of Cardiology’s NCDR CathPCI clinical registry, are limited to in-hospital outcomes and cannot assess long-term adverse clinical events.15 The VA CART database overcomes these limitations by collecting patient and procedural information about all coronary angiograms conducted in the national VA health care system and links that information to long-term outcomes. Accordingly, this database provides a unique opportunity to study the association between nonobstructive CAD and longer-term adverse events.
To our knowledge, this study provides the most comprehensive assessment of the risks associated with non-obstructive CAD demonstrated during elective coronary angiography. Prior studies have assessed outcomes among MI patients with nonobstructive CAD noted during diagnostic angiography, but this patient population is small (approximately 5%–10% of all MI patients) and clinically very different from stable patients undergoing elective angiography.3,4 Studies of nonobstructive CAD have also been conducted among patients undergoing cardiac CT imaging. Although some studies are conflicting, the majority of cardiac CT studies suggest a significant, progressive increase in the risk of major adverse cardiac events with increasing extent of CAD.33–36 Our study complements these findings by demonstrating the association between nonobstructive CAD and adverse cardiac outcomes using the predominant method of CAD diagnosis in current clinical practice—coronary angiography.
The results of this study support the concept that nonobstructive CAD is not “insignificant”37 but rather is associated with a significant and quantifiable risk for cardiovascular morbidity and mortality. This suggests that the traditional dichotomous framework for CAD–useful for characterizing and managing ischemia and cardiac symptoms–should not be applied to MI and mortality risks inherent in CAD. Rather, overall CAD extent should be considered a better proxy for both prognosis and management decisions. Some investigators have previously proposed angiographic burden scores and correlated increasing scores with increasing CAD risk.38,39 Further investigations should focus on the best methods of quantifying CAD extent and correlating it with subsequent MI and mortality rates.
In addition to risk characterization, efforts are needed to understand the best methods for risk mitigation. To date, the major cardiac prevention studies have required either obstructive CAD or a cardiac clinical event for inclusion. The stable nonobstructive CAD patient population was systematically excluded from these studies. Thus, empirical evidence is lacking as to whether these patients benefit from the prevention therapies recommended for their obstructive CAD counterparts. Prior observational studies have found that CAD secondary prevention therapies are prescribed for patients with nonobstructive CAD, although less frequently than for patients with obstructive CAD.37,40 However, randomized clinical studies of therapies such as antiplatelet agents and statins in patients with clearly defined nonobstructive CAD are needed.
Several potential limitations of this study deserve consideration. First, CART data are recorded directly by the clinician performing the angiogram. As such, misclassification of the degree of CAD severity and its distribution is possible. However, this individual characterization of CAD extent, with its inherent inaccuracies, is standard clinical care, accurately reflects real-world CAD categorization, and thus informs contemporary clinical practice. Second, criteria by which patients are selected to undergo coronary angiograms are variable and likely do not reflect the prevalence of nonobstructive CAD among patients not undergoing angiography. However, the intent of our study was to provide information about the association of adverse outcomes among nonobstructive CAD patients identified at angiography, rather than this broader population.
Third, our association between CAD extent and MI and mortality rates could be confounded by other factors than CAD burden. We used regression modeling that incorporated major demographic, clinical, and treatment variables known to correspond to both CAD and adverse events. Nonetheless,several variables, such as aspirin use, were not available. As with all observational studies, there is a possibility of unmeasured confounding. Fourth, because cause of death is not available in VA data sets, all-cause mortality was assessed, but cardiac-specific mortality could not be separately evaluated as an additional outcome. Fifth, classification of patients as symptomatic or asymptomatic in secondary analysis relied on the angiographic indication recorded in CART, rather than a direct assessment of symptoms.
Sixth, given the latency with which CMS hospitalization data are reported, we were unable to measure CMS MI rates after December 2011 in our cohort. As a result, we likely under-reported MI rates. However, sensitivity analyses excluding all CMS data from our cohort did not materially change our primary findings, supporting that the CMS MI rates were not differential by CAD extent. Seventh, our findings among VA patients undergoing angiography may not generalize to other populations. As such, our analyses should be replicated in other populations.
Conclusions
In this cohort of patients undergoing elective coronary angiography, nonobstructive CAD, compared with no apparent CAD, was associated with a significantly greater 1-year risk of MI and all-cause mortality. These findings suggest clinical importance of nonobstructive CAD and warrant further investigation of interventions to improve outcomes among these patients.
Supplementary Material
Acknowledgments
Funding/Support: The CART program is an operational program of the Department of Veterans Affairs Office of Information and Analytics. Drs Maddox and Bradley are supported with VA Health Services Research and Development career development awards.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: We acknowledge Megan Petrich, MPH, at the Department of Veterans Affairs, for her editorial support. She did not receive compensation for her contribution.
Footnotes
Author Contributions: Drs Maddox and Rumsfeld had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Maddox, Stanislawski, Tsai, Patel, Sandhu, Magid, Fihn, Rumsfeld.
Acquisition, analysis, or interpretation of data: Maddox, Stanislawski, Grunwald, Bradley, Ho, Patel, Valle, Leon, Bhatt.
Drafting of the manuscript: Maddox, Grunwald, Sandhu.
Critical revision of the manuscript for important intellectual content: Maddox, Stanislawski, Bradley, Ho, Tsai, Patel, Sandhu, Valle, Magid, Leon, Bhatt, Fihn, Rumsfeld.
Statistical analysis: Maddox, Stanislawski, Grunwald, Sandhu.
Obtained funding: Maddox.
Administrative, technical, and material support: Stanislawski, Tsai, Fihn, Rumsfeld.
Study supervision: Maddox, Patel, Rumsfeld.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Patel reported having received grants from the National Heart, Lung, and Blood Institute–PROMISE Trial, Johnson and Johnson, and the Agency for Healthcare Research and Quality. Dr Bhatt reported having served on an advisory board for Elsevier Practice Update Cardiology, Medscape Cardiology, and Regado Biosciences; on the board of directors for Boston VA Research Institute and Society of Cardiovascular Patient Care; as a chair for the American Heart Association Get With The Guidelines Steering Committee; and on data monitoring committees for Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, and Population Health Research Institute; having received honoraria from the American College of Cardiology (Editor, Clinical Trials, Cardiosource), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), and WebMD (CME steering committees); having served as editor for Clinical Cardiology (Associate Editor) and the Journal of the American College of Cardiology (Section Editor, Pharmacology); having received research grants from Amarin, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Medtronic, Roche, sanofi-aventis, and The Medicines Company; and having performed unfunded research for FlowCo, PLx Pharma, and Takeda. No other disclosures were reported.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government.
References
- 1.Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362(10):886–895. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bugiardini R, Bairey Merz CN. Angina with “normal” coronary arteries: a changing philosophy. JAMA. 2005;293(4):477–484. doi: 10.1001/jama.293.4.477. [DOI] [PubMed] [Google Scholar]
- 3.Patel MR, Chen AY, Peterson ED, et al. Prevalence, predictors, and outcomes of patients with non-ST-segment elevation myocardial infarction and insignificant coronary artery disease: results from the Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines (CRUSADE) initiative. Am Heart J. 2006;152(4):641–647. doi: 10.1016/j.ahj.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 4.Roe MT, Harrington RA, Prosper DM, et al. Clinical and therapeutic profile of patients presenting with acute coronary syndromes who do not have significant coronary artery disease: the Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy (PURSUIT) Trial Investigators. Circulation. 2000;102(10):1101–1106. doi: 10.1161/01.cir.102.10.1101. [DOI] [PubMed] [Google Scholar]
- 5.Hung M-J, Cherng W-J. Comparison of white blood cell counts in acute myocardial infarction patients with significant versus insignificant coronary artery disease. Am J Cardiol. 2003;91(11):1339–1342. doi: 10.1016/s0002-9149(03)00325-4. [DOI] [PubMed] [Google Scholar]
- 6.Wang C-H, Kuo L-T, Hung M-J, Cherng W-J. Coronary vasospasm as a possible cause of elevated cardiac troponin I in patients with acute coronary syndrome and insignificant coronary artery disease. Am Heart J. 2002;144(2):275–281. doi: 10.1067/mhj.2002.123843. [DOI] [PubMed] [Google Scholar]
- 7.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111(25):3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 8.Little WC, Constantinescu M, Applegate RJ, et al. Can coronary angiography predict the site of a subsequent myocardial infarction in patients with mild-to-moderate coronary artery disease? Circulation. 1988;78(5 pt 1):1157–1166. doi: 10.1161/01.cir.78.5.1157. [DOI] [PubMed] [Google Scholar]
- 9.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92(3):657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- 10.Ambrose JA, Tannenbaum MA, Alexopoulos D, et al. Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol. 1988;12(1):56–62. doi: 10.1016/0735-1097(88)90356-7. [DOI] [PubMed] [Google Scholar]
- 11.Giroud D, Li JM, Urban P, Meier B, Rutishauer W. Relation of the site of acute myocardial infarction to the most severe coronary arterial stenosis at prior angiography. Am J Cardiol. 1992;69(8):729–732. doi: 10.1016/0002-9149(92)90495-k. [DOI] [PubMed] [Google Scholar]
- 12.Fuster V. Lewis A. Conner Memorial Lecture: Mechanisms leading to myocardial infarction: insights from studies of vascular biology. Circulation. 1994;90(4):2126–2146. doi: 10.1161/01.cir.90.4.2126. [DOI] [PubMed] [Google Scholar]
- 13.Shah PK. Molecular mechanisms of plaque instability. Curr Opin Lipidol. 2007;18(5):492–499. doi: 10.1097/MOL.0b013e3282efa326. [DOI] [PubMed] [Google Scholar]
- 14.Mancini GBJ, Hartigan PM, Shaw LJ, et al. Predicting outcome in the COURAGE trial (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation): coronary anatomy versus ischemia. JACC Cardiovasc Interv. 2014;7(2):195–201. doi: 10.1016/j.jcin.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Brindis RG, Fitzgerald S, Anderson HV, Shaw RE, Weintraub WS, Williams JF. The American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR): building a national clinical data repository. J Am Coll Cardiol. 2001;37(8):2240–2245. doi: 10.1016/s0735-1097(01)01372-9. [DOI] [PubMed] [Google Scholar]
- 16.Box TL, McDonell M, Helfrich CD, Jesse RL, Fihn SD, Rumsfeld JS. Strategies from a nationwide health information technology implementation: the VA CART story. J Gen Intern Med. 2010;25(suppl 1):72–76. doi: 10.1007/s11606-009-1130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Byrd JB, Vigen R, Plomondon ME, et al. Data quality of an electronic health record tool to support VA cardiac catheterization laboratory quality improvement: the VA Clinical Assessment, Reporting, and Tracking System for Cath Labs (CART) program. Am Heart J. 2013;165(3):434–440. doi: 10.1016/j.ahj.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Wilson RF, Marcus ML, White CW. Prediction of the physiologic significance of coronary arterial lesions by quantitative lesion geometry in patients with limited coronary artery disease. Circulation. 1987;75(4):723–732. doi: 10.1161/01.cir.75.4.723. [DOI] [PubMed] [Google Scholar]
- 19.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58(24):e44–e122. doi: 10.1016/j.jacc.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Bradley SM, Maddox TM, Stanislawski MA, et al. Normal coronary rates for elective angiography in the Veterans Affairs Healthcare System: insights from the VA CART program (Veterans Affairs Clinical Assessment Reporting and Tracking) J Am Coll Cardiol. 2014;63(5):417–426. doi: 10.1016/j.jacc.2013.09.055. [DOI] [PubMed] [Google Scholar]
- 21.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84(408):1074–1078. doi: 10.1080/01621459.1989.10478874. [DOI] [Google Scholar]
- 22.Assessing race and ethnicity. Center for Health Equity Research and Promotion, VA Pittsburgh Healthcare System; http://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/763-notes.pdf. Accessed October 14, 2014. [Google Scholar]
- 23.Raff GL, Abidov A, Achenbach S, et al. Society of Cardiovascular Computed Tomography SCCT guidelines for the interpretation and reporting of coronary computed tomographic angiography. J Cardiovasc Comput Tomogr. 2009;3(2):122–136. doi: 10.1016/j.jcct.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Otaki Y, Arsanjani R, Gransar H, et al. What have we learned from CONFIRM? prognostic implications from a prospective multicenter international observational cohort study of consecutive patients undergoing coronary computed tomographic angiography. J Nucl Cardiol. 2012;19(4):787–795. doi: 10.1007/s12350-012-9582-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin FY, Shaw LJ, Dunning AM, et al. Mortality risk in symptomatic patients with nonobstructive coronary artery disease: a prospective 2-center study of 2,583 patients undergoing 64-detector row coronary computed tomographic angiography. J Am Coll Cardiol. 2011;58(5):510–519. doi: 10.1016/j.jacc.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 26.Jespersen L, Hvelplund A, Abildstrøm SZ, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33(6):734–744. doi: 10.1093/eurheartj/ehr331. [DOI] [PubMed] [Google Scholar]
- 27.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60(24):e44–e164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Montalescot G, Sechtem U, Achenbach S, et al. Task Force Members; ESC Committee for Practice Guidelines; Document Reviewers 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34(38):2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 29.Steg PG, Greenlaw N, Tendera M, et al. Prospective Observational Longitudinal Registry of Patients With Stable Coronary Artery Disease (CLARIFY) Investigators Prevalence of anginal symptoms and myocardial ischemia and their effect on clinical outcomes in outpatients with stable coronary artery disease: data from the international observational CLARIFY Registry. JAMA Intern Med. 2014;174(10):1651–1659. doi: 10.1001/jamainternmed.2014.3773. [DOI] [PubMed] [Google Scholar]
- 30.Tian J, Dauerman H, Toma C, et al. Prevalence and characteristics of TCFA and degree of coronary artery stenosis: an OCT, IVUS, and angiographic study. J Am Coll Cardiol. 2014;64(7):672–680. doi: 10.1016/j.jacc.2014.05.052. [DOI] [PubMed] [Google Scholar]
- 31.Bugiardini R, Manfrini O, De Ferrari GM. Unanswered questions for management of acute coronary syndrome: risk stratification of patients with minimal disease or normal findings on coronary angiography. Arch Intern Med. 2006;166(13):1391–1395. doi: 10.1001/archinte.166.13.1391. [DOI] [PubMed] [Google Scholar]
- 32.Newby LK, LaPointe NM, Chen AY, et al. Long-term adherence to evidence-based secondary prevention therapies in coronary artery disease. Circulation. 2006;113(2):203–212. doi: 10.1161/CIRCULATIONAHA.105.505636. [DOI] [PubMed] [Google Scholar]
- 33.Segev A, Beigel R, Goitein O, et al. Non-obstructive coronary artery disease upon multi-detector computed tomography in patients presenting with acute chest pain: results of an intermediate term follow-up. Eur Heart J Cardiovasc Imaging. 2012;13(2):169–173. doi: 10.1093/ejechocard/jer189. [DOI] [PubMed] [Google Scholar]
- 34.Min JK, Dunning A, Lin FY, et al. CONFIRM Investigators Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings: results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol. 2011;58(8):849–860. doi: 10.1016/j.jacc.2011.02.074. [DOI] [PubMed] [Google Scholar]
- 35.Ahmadi N, Nabavi V, Hajsadeghi F, et al. Mortality incidence of patients with non-obstructive coronary artery disease diagnosed by computed tomography angiography. Am J Cardiol. 2011;107(1):10–16. doi: 10.1016/j.amjcard.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 36.De Ferrari GM, Fox KA, White JA, et al. Outcomes among non-ST-segment elevation acute coronary syndromes patients with no angiographically obstructive coronary artery disease: observations from 37,101 patients. Eur Heart J Acute Cardiovasc Care. 2014;3(1):37–45. doi: 10.1177/2048872613489315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maddox TM, Ho PM, Roe M, Dai D, Tsai TT, Rumsfeld JS. Utilization of secondary prevention therapies in patients with nonobstructive coronary artery disease identified during cardiac catheterization: insights from the National Cardiovascular Data Registry Cath-PCI Registry. Circ Cardiovasc Qual Outcomes. 2010;3(6):632–641. doi: 10.1161/CIRCOUTCOMES.109.906214. [DOI] [PubMed] [Google Scholar]
- 38.Bigi R, Cortigiani L, Colombo P, Desideri A, Bax JJ, Parodi O. Prognostic and clinical correlates of angiographically diffuse non-obstructive coronary lesions. Heart. 2003;89(9):1009–1013. doi: 10.1136/heart.89.9.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sullivan DR, Marwick TH, Freedman SB. A new method of scoring coronary angiograms to reflect extent of coronary atherosclerosis and improve correlation with major risk factors. Am Heart J. 1990;119(6):1262–1267. doi: 10.1016/s0002-8703(05)80173-5. [DOI] [PubMed] [Google Scholar]
- 40.Dwyer JP, Redfern J, Freedman SB. Low utilisation of cardiovascular risk reducing therapy in patients with acute coronary syndromes and non-obstructive coronary artery disease. Int J Cardiol. 2008;129(3):394–398. doi: 10.1016/j.ijcard.2007.12.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


