SUMMARY
Mice deficient in pregnancy-associated plasma protein-A (PAPP-A) have extended lifespan associated with decreased incidence and severity of degenerative diseases of age, such as cardiomyopathy and nephropathy. In this study, the effect of PAPP-A deficiency on aging skeletal muscle was investigated. Whole-genome expression profiling was performed on soleus muscles from 18-month-old wild-type (WT) and PAPP-A knock-out (KO) mice of the same sex and from the same litter (‘womb-mates’) to identify potential mechanisms of skeletal muscle aging and its retardation in PAPP-A deficiency. Top genes regulated in PAPP-A KO compared to WT muscle were associated with increased muscle function, increased metabolism, in particular lipid metabolism, and decreased stress. Fiber cross-sectional area was significantly increased in solei from PAPP-A KO mice. In vitro contractility experiments indicated increased specific force and decreased fatigue in solei from PAPP-A KO mice. Intrinsic mitochondrial oxidative capacity was significantly increased in skeletal muscle of aged PAPP-A KO compared to WT mice. Moreover, 18-month-old PAPP-A KO mice exhibited significantly enhanced endurance running on a treadmill. Thus, PAPP-A deficiency in mice is associated with indices of healthy skeletal muscle function with age.
Keywords: Pregnancy-associated plasma protein-A, soleus, mitochondria
1. INTRODUCTION
Aging is associated with loss of skeletal muscle mass and compromised function with attendant frailty and increased risk of incapacitating injuries (Marzetti et al., 2009; Rosenberg, 1997). This predisposition to disability and mortality in our ever-growing elderly population is a significant public health issue. A better understanding of the factors involved in this age-related decrease in muscle function could suggest novel approaches to prevent or delay the process.
Lee et al. (1999) used a high-density oligonucleotide array to analyze the genomic profile of the aging process in mouse skeletal muscle, and found a gene expression pattern indicative of a marked stress response and lowered expression of metabolic and biosynthetic genes in old versus young adult mice. Moreover, these changes in gene expression were prevented by caloric restriction, which is known to increase healthy lifespan (Minor et al., 2010). Similarly, Park & Prolla (2005) studied gene expression profiles of aging cardiac and skeletal muscle in mice. The transcriptional pattern suggested that aging increases heat shock response and genes induced by oxidative stress, toxins, and DNA damage, and decreases those involved with energy metabolism. Caloric restriction resulted in a transcriptional shift toward energy metabolism, in particular lipid metabolism, and decreases in mRNA encoding inducible genes involved in metabolic detoxification, oxidative stress, and protein misfolding. Lanza et al. (2012) used whole-genome expression profiling and proteomics to determine the effect of age and caloric restriction on mouse skeletal muscle. In that study, aging was associated with gene expression patterns consistent with up-regulation of pyruvate metabolism, oxidative phosphorylation, and cellular response to oxidative stress. Caloric restriction attenuated or reversed these changes. Thus, several gene expression profile studies using DNA microarrays suggested that skeletal muscle aging is associated with increased stress response genes and diminished energy metabolism. Caloric restriction could prevent or reverse these changes and, thus, preserve muscle function in aging. However, long-term caloric restriction in humans is not a practical approach to the problem of muscle aging.
Pregnancy-associated plasma protein-A (PAPP-A) is a novel zinc metalloproteinase that enhances local insulin-like growth factor (IGF) action through cleavage of inhibitory binding proteins (Conover, 2012). Diminished IGF action is associated with increased lifespan in a variety of species, and may contribute to the extended lifespan with caloric restriction (Barbieri et al., 2003; Berryman et al., 2008). PAPP-A knock-out (KO) mice live 30-40% longer than their wild-type littermates, with decreased incidence and severity of degenerative diseases of age, such as nephropathy and cardiomyopathy (Conover et al., 2010). However, skeletal muscle of PAPP-A KO mice has not been examined in any detail. In this study we compared genome-wide expression profiles in skeletal muscle of aged PAPP-A KO and wild-type mice that were matched for sex, intrauterine environment and postnatal housing. Furthermore, we employed several methodologies to evaluate skeletal muscle phenotype and function in aged PAPP-A KO and wild-type mice.
2. EXPERIMENTAL PROCEDURES
2.1 Mice
All animal procedures followed protocols reviewed and approved by the Institutional Animal Care and Use Committee of Mayo Clinic. Mice heterozygous for PAPP-A gene deletion were crossbred (14 breeder cages) as described previously (Conover et al., 2004). Both male and female offspring were used in experiments, with approximately equal distribution.
2.2 Womb-mate tissue
“Womb-mate” status was assigned to those pups that were from the same litter, of the same sex and paired PAPP-A KO and wild-type (WT). This was to minimize confounding variables of intrauterine development and sex. Womb-mate pairs were then housed together for 18 months to minimize potential confounding variables of environment. At this time tissues were harvested and frozen at −80° C. For this study, we focused on the soleus muscle, which is composed of predominantly oxidative fiber types.
2.3 Illumina Microarray Protocol
RNA was extracted from solei (2 pooled from each mouse) using QIAGEN RNeasy Fibrous Tissue kit (QIAGEN,Valencia, CA). All subsequent procedures and expression analyses were carried out by the Advanced Genomics Technology Center at Mayo Clinic. The quality of total RNA samples was assessed using the Agilent Bioanalyzer 2100 (Santa Clara, CA). Labeling of high quality samples was performed according to manufacturer's instructions for the Illumina Total Prep RNA Amplification Kit (Life Technologies, Grand Island, NY). Briefly, 200 ng of total RNA was reverse transcribed with T7 Oligo d(T) to create second strand cDNA. Subsequently, the products were column-purified and then in vitro transcribed to generate biotin-labeled cRNA. cRNA products were column-purified and hybridized onto Illumina Mouse Whole Genome 6 Beadchips for 16 hours at 58° C. Following hybridization, the arrays were washed, stained with streptavidin-cy3 conjugate, and then scanned in an Illumina BeadArray Reader. All quality assessment parameters were determined to be within normal ranges before proceeding to the final data reduction. Three womb-mate pairs were run simultaneously on a single Beadchip. Six pairs were analyzed (three male and three female).
2.4 Fiber cross-sectional area (CSA)
Tendon-to-tendon soleus muscles were isolated, fixed in 10% formalin and embedded in paraffin. Five μm sections were cut from the middle of the muscle and stained with hematoxylin and eosin. Approximately 70 fiber CSAs were measured from solei from two separate mice of each genotype using Image J software.
2.5 Muscle contractility in vitro
Isometric force and fatigue were measured as previously described (Greising et al., 2011; Greising et al., 2013).
2.6 Citrate synthase activity
Citrate synthase (a marker for mitochondrial content) activity was measured spectrophotometrically and expressed relative to tissue wet weight (Lanza et al., 2012).
2.7 Mitochondrial DNA (mtDNA) copy number
DNA was extracted from frozen tissues using a QIAamp DNA mini kit (QIAGEN, Valencia, CA). Relative mtDNA copy numbers were determined by real-time PCR (Applied Biosystems 7900HT Sequence Detection System) using primer/probe sets targeted to mtDNA-encoded NADH dehydrogenase subunits 1 (ND1) and 4 (ND4), as described previously (Lanza et al., 2012). Samples were run in duplicate and normalized for the nuclear reference gene 28S ribosomal DNA.
2.8 Mitochondrial ATP production rate
Mitochondrial ATP production rate was measured in isolated mitochondria with a bioluminescent technique, as described previously (Short et al., 2005).
2.9 Treadmill
Physical function was characterized by measuring running time and distance using a motorized 6-lane treadmill (Columbus Instruments, Columbus, OH), as previously described (LeBrasseur et al., 2009). In brief, 18-20 WT and PAPP-A KO mice were acclimated to the treadmill for three consecutive days for 5 minutes at a speed of 10 m/min at a 5% grade. The next day, mice were run on the treadmill at an initial speed of 10 m/min and grade 5% for 5 minutes, and then every subsequent two minutes the speed was increased by 2 m/min until the mice were exhausted. Exhaustion was defined as the inability of the mouse to remain on the treadmill despite an electric shock stimulus and mechanical prodding for a period of 5 seconds. Mice were run in batches, with WT and KO mice part of each batch. These experiments were performed by experienced personnel in the Aging Animal Phenotyping Core of the Kogod Center on Aging at Mayo Clinic.
2.10 Statistical Methods
Microarray analyses were conducted using log-base2 of the gene expression values, and programming was done in the R statistical language (Team, 2012). Data were normalized using the “fastlo” algorithm (Ballman et al., 2004). Pre- and post-normalization quality controls were assessed visually using minus versus average plots and box-plots. Paired t-tests were used to test for significant differences between the PAPP-A KO and WT mice; fold changes are reported as PAPP-A KO relative to WT. False discovery rates (FDR) were calculated using the Benjamini-Hochberg method (Benjamini and Hochberg, 1995). Complete microarray data have been submitted to the National Center for Biotechnology Informatics (NCBI) Gene Expression Omnibus (GEO) database. The accession number for this data set is pending [submission in process].
Kaplan-Meier survival curves were compared using log-rank test in JMP Pro 9.0.1. Data comparing WT and PAPP-A KO groups, presented as mean ± SEM, were analyzed by unpaired t-test. Significance was set at P < 0.05.
3. RESULTS
3.1 Muscle gene expression
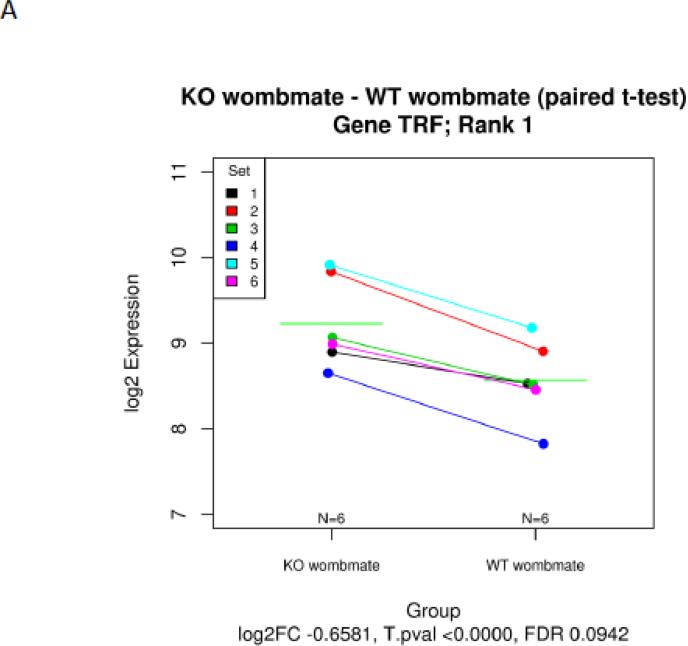
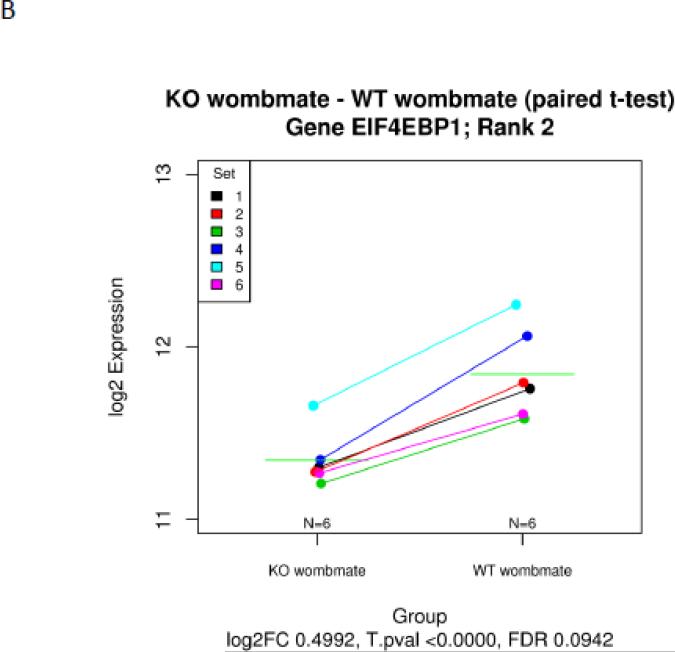
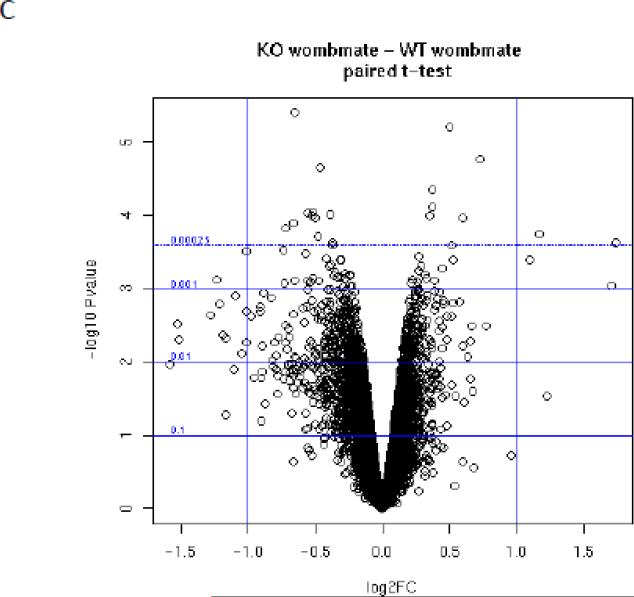
PAPP-A KO and wild-type (WT) womb-mate tissue samples provided robust statistical power for paired t-test analysis of differentially expressed genes. This is illustrated by examples in Figure 1. Each of the six sets (KO and WT) is indicated by a specific color. Transferrin (Rank 1) was up-regulated in the soleus of PAPP-A KO compared to WT in all six pairs (Fig. 1A). Eukaryotic translation initiation factor 4E binding protein-1 (Rank 2) was down-regulated in the soleus of PAPP-A KO compared to WT in all six pairs (Fig. 1B). A volcano plot of statistical significance and fold-change for all of the data is presented in Figure 1C.
Figure 1.
Microarray expression data of solei from paired sets of PAPP-A KO and WT mice. (A) transferrin, (B) eukaryotic translation initiation factor 4E binding protein, (C) Volcano plot of statistical significance versus fold-change (FC).
N = 6 womb-mate sets.
Green horizontal lines in A and B indicate means.
The top up-regulated and down-regulated genes identified in the soleus muscle of 18-month-old PAPP-A KO compared to WT womb-mates are presented in Table 1 (the complete Illumina microarray data set will be made available at GEO). The majority of these genes fell into three main groupings: regulation of muscle mass and function, alteration in fuel metabolism, and involvement in stress response (Table 2). Activated genes in the soleus muscle of aged PAPP-A KO mice were associated with preservation of muscle mass and function with up-regulation (compared to WT) in transcripts for transferrin, myosin light chain and aminovulinate dehydrogenase, and down-regulation of eukaryotic translation initiation factor 4E binding protein 1. Differentially expressed genes in the PAPP-A KO soleus also reflected increased energy metabolism, in particular lipid metabolism, with up-regulation of cell-death-inducing DFFA-like effector, pyruvate dehydrogenase phosphatase, adiponectin, and down-regulation of cholesterol 25-hydroxylase. Furthermore, there were decreases in genes induced with stress, such as heat shock protein.
Table 1.
Differential Gene Expression in Soleus Muscle from Aged PAPP-A KO Mice Compared to WT Mice.
| Up-Regulated Genes | |||||
|---|---|---|---|---|---|
| Rank | Gene | Description | Accession | P-Value | FC |
| 1 | Trf | Transferrin | NM_133977.2 | <0.00001 | 1.66 |
| 4 | Hdhd3 | Haloacid dehydrogenase-like hydrolase | NM_024257.1 | <0.00001 | 1.46 |
| 7 | Myl10 | Myosin light chain 10 | NM_001085387.1 | <0.0001 | 1.52 |
| 8 | Cidec | Cell death-inducing DFFA-like effector | NM_178373.3 | <0.0001 | 1.56 |
| 9 | Prkg2 | cGMP-dependent protein kinase, type II | NM_008926.4 | <0.0001 | 1.39 |
| 13 | Pdp2 | Pyruvate dehydrogenase phosphatase catalytic subunit 2 | NM_001024606.1 | <0.0001 | 1.50 |
| 14 | Adipoq | Adiponectin | NM_009605.4 | 0.0001 | 1.66 |
| 15 | Alad | Aminolevulinate dehydratase | NM_008525.3 | 0.0001 | 1.72 |
| 17 | Gng10 | G-protein gamma 10 | NM_025277.3 | 0.0002 | 1.49 |
| 20 | Myl4 | Myosin light chain 4 | NM_010858.4 | 0.0003 | 1.37 |
| Down-Regulated Genes | |||||
| 2 | Eif4ebp1 | Eukaryotic translation initiation factor 4E binding protein 1 | NM_007918.3 | <0.00001 | 0.50 |
| 5 | Ch25h | Cholesterol 25-hydroxylase | NM_009890.1 | <0.0001 | 0.63 |
| 6 | Panx1 | Pannexin 1 | NM_019482.2 | <0.0001 | 0.63 |
| 10 | Hspa1l | Heat shock protein 1-like | NM_013558.2 | <0.0001 | 0.65 |
| 12 | Cacng1 | Calcium channel, voltage-dependent gamma subunit-1 | NM_007582.2 | 0.0001 | 0.60 |
| 18 | Pvalb | Parvalbumin | NM_013645.3 | 0.0002 | 0.73 |
See Experiment Procedures for description of tissues and analyses
FC, fold change (KO/WT)
Table 2.
Categories of Differential Gene Expression in Soleus Muscle from Aged PAPP-A KO Compared to WT Mice.
| Gene Expression | Function |
|---|---|
| Preservation Muscle Mass and Function | |
| ↑ Transferrin | Iron-binding muscle tropic factor |
| ↑ Myosin light chain | Muscle contraction |
| ↑ Aminovulinate dehydrogenase | Heme biosynthesis and cellular protein quality control |
| ↓ Eukaryotic translation initiation factor 4E binding protein 1 | Translation initiation repressor |
| Increased Energy Metabolism | |
| ↑ Cell death-inducing DFFA-like effector | Fatty acid metabolism |
| ↑ Pyruvate dehydrogenase phosphatase | Oxidative phosphorylation |
| ↑ Adiponectin | β-Oxidation lipids |
| ↑ Fatty acid synthase | Fatty acid metabolism |
| ↓ Cholesterol 25-hydroxylase | Cholesterol metabolism |
| Decreased Stress Response | |
| ↓ Heart shock protein | Chaperone, misfolded protein |
The other gene transcripts did not fit neatly into any of these three categories. Interestingly, haloacid dehydrogenase-like hydrolase (Rank 4) was highly up-regulated in PAPP-A KO soleus. Its function is unknown but was shown to be significantly decreased in aging rat gastrocnemious muscle (Piec et al., 2005). Pannexin 1 was down-regulated in the soleus of PAPP-A KO mice. Little is known about pannexin 1, but it may be involved in pathophysiological ATP release (Bao et al., 2004). Thus, its down-regulation may represent less muscle dysregulation in aged PAPP-A KO mice. Parvalbumin is a calcium-binding albumin protein primarily localized to fast-contracting muscle (Olive et al., 1994). It may be down-regulated in soleus muscle of PAPP-A KO mice because of fiber switching. Aging has been shown to change composition from fast twitch to slow twitch in rat skeletal muscle (Piec et al., 2005). A possible role for cGMP-dependent protein kinase II in skeletal muscle is unknown.
In addition to the top-regulated transcripts noted in Tables 1 and 2, transcripts for fatty acid synthase (Fasn) (P = 0.002, Rank 115) were up-regulated in soleus of 18-month-old PAPPA KO compared to WT mice (Table 2).
Differential expression was confirmed by real-time PCR in a subset of the genes identified in the microarray (Table 3). Transferrin, myosin light chain and adiponectin mRNA levels were significantly increased, and eif4ebp1 and heat shock protein mRNA levels were significantly decreased in soleus muscles from 18-month-old PAPP-A KO compared to WT mice. Cidec was also increased in soleus muscle from PAPP-A KO mice, but this difference did not reach statistical significance.
Table 3.
Gene Expression in Soleus Muscle from Aged WT and PAPP-A KO Mice
| mRNA abundance (copy # × 105) | |||
|---|---|---|---|
| WT | PAPP-A KO | P-Value | |
| Transferrin | 5.0 ± 0.66 | 7.1 ± 0.68 | 0.036 |
| Myosin, light chain | 16.0 ± 1.60 | 22.0 ± 2.20 | 0.045 |
| Cidec | 1.4 ± 0.16 | 1.9 ± 0.23 | 0.086 |
| Adiponectin | 2.5 ± 0.20 | 4.2 ± 0.53 | 0.007 |
| Eif4ebp1 | 0.84 ± 0.081 | 0.64 ± 0.040 | 0.036 |
| Hsp | 7.5 ± 0.38 | 5.9 ± 0.39 | 0.008 |
Gene expression by real-time PCR
Results are means ± S.E.M of solei from 12-14 mice per group.
3.2 Muscle phenotype and functional analyses
Muscle fiber cross-sectional area (CSA) was determined in soleus muscles from 18-month-old WT and PAPP-A KO mice. Soleus fiber CSA was significantly increased (P = 0.049) in PAPP-A KO (1219 ± 36 μm2) compared to WT (1110 ± 41 μm2) mice.
Soleus muscle contractility suggested increased specific force (18.7 ± 2.7 vs. 16.8 ± 2.7 N/cm2) and decreased fatigue index (27.3 ± 8.4 vs 38.8 ± 10.9 %) in PAPP-A KO vs WT mice. The group sizes (n = 4) were too small for statistical evaluation.
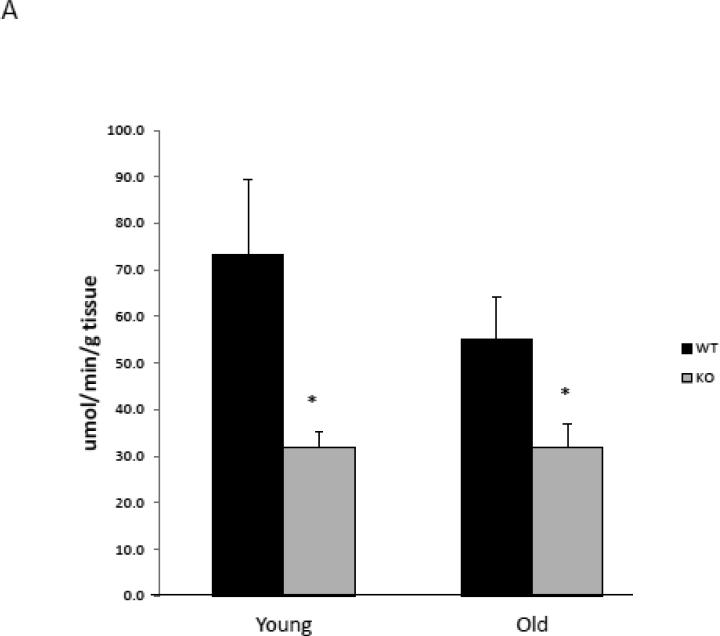
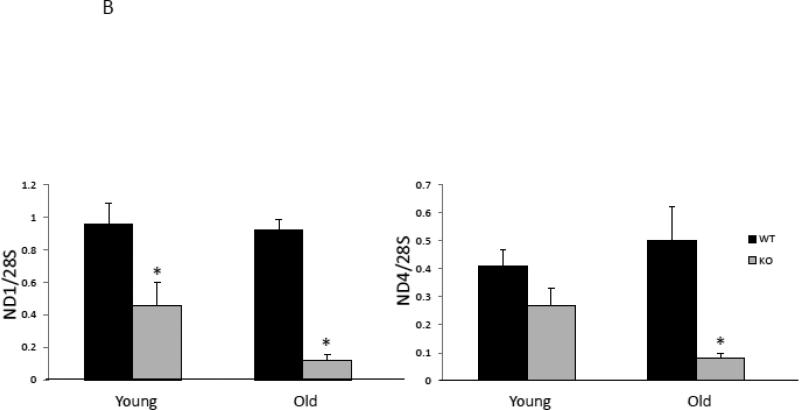
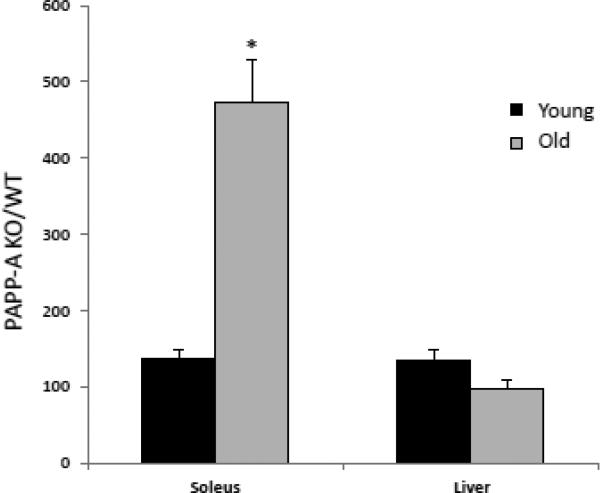
With aging, mitochondrial ATP production correlates with mitochondrial abundance (Johnson et al., 2013; Short et al., 2005). Therefore, mitochondrial abundance was assessed by citrate synthase activity and mitochondrial DNA (mtDNA) copy number in solei from young and aged PAPP-A KO and WT mice. Interestingly, citrate synthase activity was significantly decreased in soleus muscle from young and old PAPP-A KO mice (Fig 2A). Similar decreases were seen in mtDNA copy number in solei from PAPP-A KO mice (Fig. 2B), which were accentuated with age. These data on their own might suggest diminished mitochondrial function in soleus muscle from PAPP-A KO mice. However, mitochondrial ATP production rates normalized to mtDNA copy number in solei from the same mouse were significantly increased in aged PAPP-A KO mice compared to WT (Fig. 3). This increase was not seen in liver from these same mice. It is of note that liver, unlike soleus muscle, expresses very little PAPP-A (Conover et al., 2004).
Figure 2.
Mitochondrial abundance in soleus muscle from PAPP-A KO and WT mice.
(A) Citrate synthase activity.
(B) mtDNA copy number.
N = 8
* Indicates significant difference between PAPP-A KO and WT, P < 0.05.
Figure 3.
Intrinsic mitochondrial oxidative capacity.
Mitochondrial ATP production rate per mtDNA copy number. Results are expressed as PAPP-A KO/WT.
N = 8
* Indicates significant difference between PAPP-A KO and WT, P < 0.05.
3.3 Physical function in vivo
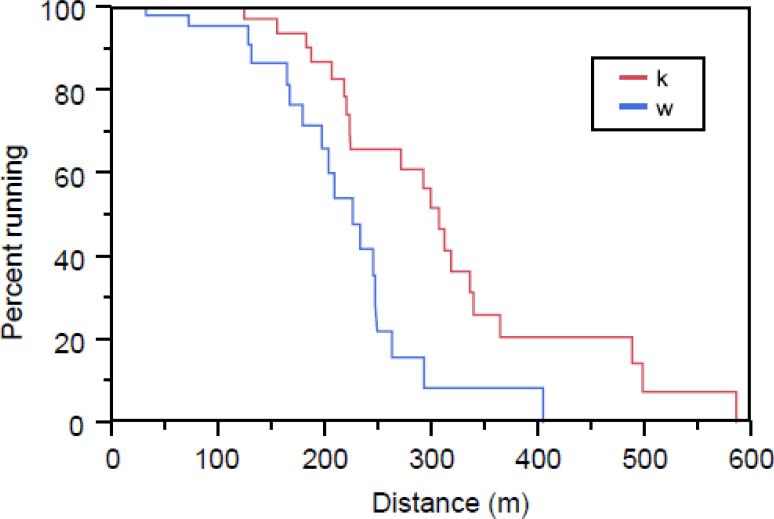
PAPP-A KO and WT mice underwent treadmill testing at 4-, 11-, and 18-months-of-age. The results at 18 months are presented in Figure 4. Survival curves for running distance were significantly different (P < 0.0001) between PAPP-A KO and WT mice. Median running distance to exhaustion for PAPP-A KO mice was 75 m (32%) farther than for WT mice. There were no significant differences between groups at 4 or 11 months.
Figure 4.
Treadmill testing
18-month-old WT mice (N = 18) and PAPP-A KO mice (N = 20) were run to exhaustion on a motorized treadmill, as described in the Methods.
4. DISCUSSION
In this study, we present gene expression profiling, phenotype, and mitochondrial function assessment for the soleus muscle from 18-month-old WT and PAPP-A KO mice, the latter being a mouse model of extended lifespan with multiple beneficial effects on health span (Conover et al., 2010). All-in-all, the data are consistent with preservation of healthy muscle function with aging in PAPP-A KO mice. In particular, the differential gene expression pattern was indicative of higher expression of metabolic and biosynthetic genes important in muscle function, and lower expression of genes induced with stress. Thus, PAPP-A deficiency attenuated many of the age-associated transcriptional changes in skeletal muscle. Fiber size, strength and mitochondrial function in the solei were increased in aging PAPP-A KO mice compared to WT mice. Moreover, 18-month-old PAPP-A KO mice were significantly more physically fit than WT mice, as assessed by treadmill running to exhaustion.
4.1 Gene expression
4.1.1 Muscle function genes
Aging in muscle is associated with decreased mass, strength, and contraction rate. Down-regulation of eukaryotic translation initiation factor 4E binding protein 1 (eif4ebp1), a key translation initiation repressor, would favor increased protein synthesis and maintenance of muscle mass of aged PAPP-A KO mice. Myosin light chain subunit functions in actin binding and ATP hydrolysis, thereby playing an important role in muscle contraction. Myosin light chain protein was shown to be decreased with aging in rat skeletal muscle (Piec et al., 2005). Thus, up-regulated myosin light chain expression in skeletal muscle from old PAPP-A KO mice may reflect preservation of efficient muscle function.
The top-ranked gene transcript that was differentially expressed (up-regulated) in soleus from PAPP-A KO compared to WT mice was transferrin, which encodes for a critical iron-binding muscle trophic factor and is involved in several biosynthetic processes. Although transferrin has not been reported to be regulated in aging muscle, circulating levels of transferrin are decreased in inflammation (Ritchie et al., 1999). Thus, this up-regulation of transferrin gene expression may reflect a lesser inflammatory state in soleus muscle of PAPP-A KO mice compared with WT mice. This would also fit with the down-regulation of stress-related gene activation in PAPP-A KO muscle.
Aminovulinate dehydrogenase is involved in heme biosynthesis and participates in several metabolic pathways. It has recently been shown to be a proteasome-interacting protein that may modulate proteasome activity (Bardag-Gorce and French, 2011). Proteasomes are responsible for degrading altered and oxidized proteins thereby providing cellular quality control. Thus, its up-regulation in PAPP-A KO soleus may promote efficient muscle function and fuel metabolism.
4.1.2 Metabolism genes
In aging skeletal muscle there tends to be a shift from oxidative phosphorylation and lipid metabolism to glycolytic metabolism. Cell death-inducing DNA fragmentation factor-A-like effector (Cidec), an up-regulated gene in aged soleus muscle from PAPP-A KO mice, promotes lipid droplet formation in adipocytes and optimal energy storage in humans, tracks intrahepatic fat in humans, and regulates fatty acid metabolism (Matsusue et al., 2008; Puri et al., 2007). A similar role may be speculated in skeletal muscle. Fatty acid synthase was also up-regulated in PAPP-A KO muscle, as was seen with caloric restriction (Dhahbi et al., 2012). The decrease in cholesterol 25-hydroxylase may also relate to lipid metabolism in the soleus muscle, as it can serve as an intermediate in cholesterol catabolism (Lund et al., 1998).
The pyruvate dehydrogenase complex is central to mitochondrial fuel metabolism (Stacpoole, 2012). Aging up-regulates pyruvate metabolism in muscle (Lanza et al., 2012). Thus, increased pyruvate dehydrogenase phosphatase in PAPP-A KO muscle may foster a switch in metabolic flux from glycolysis towards oxidative phosphorylation.
Up-regulation of adiponectin gene expression in aged PAPP-A KO soleus muscle was of particular interest, since it was originally thought that adiponectin expression was limited to adipocytes. However, recent studies indicate that skeletal muscle cells can synthesize and secrete adiponectin (Krause et al., 2008; Liu et al., 2009). Adiponectin is known to increase β-oxidation of lipids within skeletal muscle and may play a role in maintaining healthy muscle function and insulin sensitivity. Adiponectin KO mice show impaired skeletal muscle peak force production (Krause et al., 2008).
4.1.3 Stress response genes
Muscle aging often associates with increased oxidative and endoplasmic reticulum stress. Thus, there are several reports of increased levels of inducible molecular chaperones, such as heat shock proteins, in aged animals (Laschober et al., 2010). Our finding of lowered expression of heat shock protein gene expression may reflect diminished levels of stressors in soleus muscle of PAPP-A KO mice.
Thus, PAPP-A deficiency attenuated many of the age-associated transcriptional changes in skeletal muscle as seen in earlier microarray-based studies of caloric restriction, such as up-regulation of genes associated with increased protein turnover and lipid biosynthesis, and down-regulation of genes associated with pyruvate metabolism and cellular response to stress (Dhahbi et al., 2012; Lanza et al., 2012; Lee et al., 1999; Park and Prolla, 2005; Selman et al., 2006).
In addition, there were a number of novel observations in our study that were not noted in the caloric restriction studies, in particular the up-regulation of transferrin and adiponectin in the soleus muscle of PAPP-A KO mice. These could represent real differences in the two models, different platforms for the analyses, and/or different statistical analyses. It is unlikely to reflect the use of two different muscle types, since we have seen significantly increased transferrin and adiponectin expression in soleus and quadriceps muscles (Table 3 and Suppl. Table 1). Further studies are necessary to understand the physiologic relevance of these and other findings from the expression profile.
4.2 Muscle phenotype
Reduced fiber size, loss of contractile force, and reduced resistance to fatigue are associated with muscle aging. Soleus muscles from 18-month-old PAPP-A KO mice had increased fiber CSA, generated increased specific force, and were more resistant to contractile fatigue than muscle from WT mice. Among the proposed causes of diminished muscle function with age is mitochondrial dysfunction (Johnson et al., 2013). Decreased mitochondrial function particularly compromises highly oxidative tissues. In solei from PAPP-A KO mice, mitochondrial abundance was significantly reduced, as measured both by citrate synthase activity and mtDNA copy number. However, mitochondrial ATP production, expressed relative to mtDNA copy number in solei from the same animal, was markedly elevated in aged PAPP-A KO muscle. These data indicate increased intrinsic bioenergetic efficiency. At this time, we do not have a mechanistic explanation for the decreased mitochondrial abundance in soleus muscle of PAPP-A KO mice. One possibility is an increase in autophagy to remove defective mitochondria, thereby increasing efficiency. Mizushima et al. (2004) demonstrated autophagic flux in β-oxidative fibers of the mouse soleus and speculated a role in muscle mass maintenance. Autophagy can be inhibited by IGF-I (Gu et al., 2004), therefore it is plausible that PAPP-A deficiency could reduce this inhibition. Further studies are necessary to answer questions about the role of PAPP-A in mitochondrial regulation. In other mouse models of extended healthy lifespan, i.e., Ames dwarf and growth hormone receptor KO mice, skeletal muscle mitochondrial function was better preserved with age compared to WT mice (Brown-Borg and Bartke, 2012).
4.3 Physical function
Overall physical function in vivo was assessed by treadmill running to exhaustion. Aged PAPP-A KO mice had significantly enhanced resistance to fatigue than WT mice, which might be predicted by the transcriptional, phenotypic and mitochondrial function analyses in skeletal muscle in vitro. However, unlike isolated skeletal muscle, treadmill running is impacted by other physical structures, such as tendons, and the endocrine/paracrine environment of the mouse. Thus, these results are consistent with healthier muscles in aged PAPP-A KO mice, but further studies will be necessary to identify contributing factors.
4.4 PAPP-A and skeletal muscle
This is the first study to investigate skeletal muscle in the long-lived PAPP-A KO mouse model. Soleus was chosen for this initial study because of the relative homogeneity of fiber type relative to other muscles, such as quadriceps (Augusto et al., 2004). In preliminary studies, real-time PCR was performed for the differentially expressed genes in quadriceps of 18-month-old WT and PAPP-A KO mice (Supplemental Table 1), as shown for the soleus in Table 3. In the quadriceps, there were significant differences between WT and PAPP-A KO mice for transferrin, myosin light chain, and adiponectin. Cidec, Eif4ebp1 and heat shock protein showed the same differences as in the soleus, but they didn't reach statistical significance. Therefore, there are similarities in the findings of soleus and quadriceps muscle in regard to gene expression profiles.
Diminished IGF signaling has been proposed to underlie, at least in part, the extended healthspan of PAPP-A KO and calorically-restricted mice (Conover et al., 2010; Gems and Partridge, 2001; Yamaza et al., 2007). In this study, PAPP-A KO mice had indications of extended healthy muscle function. On the other hand, IGFs and PAPP-A have been shown to play a role in muscle regeneration in response to injury (Clemmons, 2009; Kumar et al., 2005). Furthermore, transgenic overexpression of PAPP-A in skeletal muscle of mice increased muscle mass (Rehage et al., 2007). The reasons for these apparently discrepant findings are unclear. There are likely to be differences in acute response to injury and chronic modulation of expression and signaling (Bale et al., 2013; Clemmons, 2007). And it is possible that reduced IGF signaling in pluripotent stem cells residing in skeletal muscle protects against premature depletion (Ratajczak et al., 2013). Furthermore, there are different effects of moderate versus extreme changes in IGF-I (and PAPP-A) levels. Indeed, there is a “U-Shape” relationship for IGF-I levels in cancer and cardiovascular mortality (Burgers et al., 2011). It has been emphasized that limited impairment, but not complete loss, of IGF-I signaling is important for longevity and increased metabolic health (Ristow and Zarse, 2010). Along these lines, the PAPP-A KO mouse represents a model of moderate reduction in local IGF signaling.
4.5 Conclusion
PAPP-A deficiency in old mice is associated with markers of preserved healthy muscle metabolism and function. These data suggest PAPP-A as a potential target to prevent/reverse skeletal muscle dysfunction of age.
Supplementary Material
HIGHLIGHTS.
PAPP-A deficiency in aged mice is associated with healthy skeletal muscle function:
Increased muscle function and lipid metabolism genes
Increased fiber cross-sectional area and specific force
Increased intrinsic mitochondrial oxidative capacity
Enhanced endurance running on treadmill
ACKNOWLEDGMENTS
This work was supported by NIH grant RO1 AG028141 to CAC.
The authors thank Suban Chrakraborty, Sally West, Jessica Mader, Katherine Klaus, and Jill Schimke from the Conover and Nair labs for excellent technical support, and acknowledge Vernadette Simon and the Mayo Medical Genome Facility Gene Expression Core for the microarray analyses. We also thank Dr. Sarah Greising for performing the muscle contractility assays (supported by T32-HL105355).
Glossary
- PAPP-A
pregnancy-associated plasma protein-A
- WT
wild-type
- KO
knock-out
- IGF
insulin-like growth factor
- CSA
cross-sectional area
- mtDNA
mitochondrial DNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest.
REFERENCES
- 1.Augusto V, Padovani CR, Campos GR. Skeletal muscle fiber types in C57BL6J mice. Braz J Morphol Sci. 2004;21:89–94. [Google Scholar]
- 2.Bale LK, Resch ZT, Harstad SL, Overgaard MT, Conover CA. Constitutive expression of pregnancy-associated plasma protein-A in arterial smooth muscle reduces the vascular response to injury in vivo. Am J Physiol Endocrinol Metab. 2013;304:E139–E144. doi: 10.1152/ajpendo.00376.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballman KV, Grill DE, Oberg AL, Therneau TM. Faster cyclic loess: normalizing RNA arrays via linear models. Bioinformatics. 2004;20:2778–2786. doi: 10.1093/bioinformatics/bth327. [DOI] [PubMed] [Google Scholar]
- 4.Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Barbieri M, Bonafe M, Franceschi C, Paolisso G. Insulin/IGF-I-signaling pathway: an evolutionarily conserved mechanism of longevity from yeast to humans. Am J Physiol Endocrinol Metab. 2003;285:E1064–E1071. doi: 10.1152/ajpendo.00296.2003. [DOI] [PubMed] [Google Scholar]
- 6.Bardag-Gorce F, French SW. Delta-aminolevulinic dehydratase is a proteasome interacting protein. Experimental and Molecular Pathology. 2011;91:485–9. doi: 10.1016/j.yexmp.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Statistical Soc Series B. 1995;57:289–300. [Google Scholar]
- 8.Berryman DE, Christiansen JS, Johannsson G, Thorner MO, Kopchick JJ. Role of the GH/IGF-1 axis in lifespan and healthspan: lessons from animal models. Growth Horm IGF Res. 2008;18:455–471. doi: 10.1016/j.ghir.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown-Borg HM, Bartke A. GH and IGF1: roles in energy metabolism of long-living GH mutant mice. J Gerontol A Biol Sci Med Sci. 2012;67:652–660. doi: 10.1093/gerona/gls086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgers AMG, Biermasz NR, Schoones JW, Pereira AM, Renehan AG, Zwahlen M, Egger M, Dekkers OM. Meta-analysis and dose-response metaregression: circulating insulin-like growth factor I (IGF-I) and mortality. J Clin Endocrinol Metab. 2011;96:2912–2920. doi: 10.1210/jc.2011-1377. [DOI] [PubMed] [Google Scholar]
- 11.Clemmons DR. Modifying IGF1 activity: an approach to treat endocrine disorders, atherosclerosis and cancer. Nat Rev Drug Discov. 2007;6:821–833. doi: 10.1038/nrd2359. [DOI] [PubMed] [Google Scholar]
- 12.Clemmons DR. Role of IGF-I in skeletal muscle mass maintenance. Trends Endocrinol Metab. 2009;20:349–356. doi: 10.1016/j.tem.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Conover CA. Key questions and answers about pregnancy-associated plasma protein-A. Trends Endocrinol Metab. 2012;23:242–249. doi: 10.1016/j.tem.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conover CA, Bale LK, Mader JR, Mason MA, Keenan KP, Marler RJ. Longevity and age-related pathology of mice deficient in pregnancy-associated plasma protein-A. J Gerontol A Biol Sci Med Sci. 2010;65:590–599. doi: 10.1093/gerona/glq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conover CA, Bale LK, Overgaard MT, Johnstone EW, Laursen UH, Fuchtbauer E-M, Oxvig C, van Deursen J. Metalloproteinase pregnancy-associated plasma protein A is a critical growth regulatory factor during fetal development. Development. 2004;131:1187–1194. doi: 10.1242/dev.00997. [DOI] [PubMed] [Google Scholar]
- 16.Dhahbi JM, Atamna H, Boffelli D, Martin DI, Spindler SR. mRNA-Seq reveals complex patterns of gene regulation and expression in the mouse skeletal muscle transcriptome associated with calorie restriction. Physiol Genomics. 2012;44:331–344. doi: 10.1152/physiolgenomics.00129.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gems D, Partridge L. Insulin/IGF signalling and ageing: seeing the bigger picture. Curr Opin Genet Dev. 2001;11:287–292. doi: 10.1016/s0959-437x(00)00192-1. [DOI] [PubMed] [Google Scholar]
- 18.Greising SM, Baltgalvis KA, Kosir AM, Moran AL, Warren GL, Lowe DA. Estradiol's beneficial effect on murine muscle function is independent of muscle activity. J Appl Physiol. 2011;110:109–15. doi: 10.1152/japplphysiol.00852.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greising SM, Mantilla CB, Gorman BA, Ermilov LG, Sieck GC. Diaphragm muscle sarcopenia in aging mice. Exp Gerontol. 2013;48:881–7. doi: 10.1016/j.exger.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu Y, Wang C, Cohen A. Effect of IGF-1 on the balance between autophagy of dysfunctional mitochondria and apoptosis. FEBS Lett. 2004;577:357–360. doi: 10.1016/j.febslet.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 21.Johnson ML, Robinson MM, Nair KS. Skeletal muscle aging and the mitochondrion. Trends Endocrinol Metab. 2013;24:247–256. doi: 10.1016/j.tem.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krause MP, Liu Y, Vu V, Chan L, Xu A, Riddell MC, Sweeney G, Hawke TJ. Adiponectin is expressed by skeletal muscle fibers and influences muscle phenotype and function. Am J Physiol Cell Physiol. 2008;295:C203–C212. doi: 10.1152/ajpcell.00030.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar A, Mohan S, Newton J, Rehage M, Tran K, Baylink DJ, Qin X. Pregnancy-associated plasma protein-A regulates myoblast proliferation and differentiation through an insulin-like growth factor-dependent mechanism. J Biol Chem. 2005;280:37782–37789. doi: 10.1074/jbc.M505278200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanza IR, Zabielski P, Klaus KA, Morse DM, Heppelmann CJ, Bergen HR, III, Dasari S, Walrand S, Short KR, Johnson ML, Robinson MM, Schimke JM, Jakaitis DR, Asmann YW, Sun Z, Nair KS. Chronic caloric restriction preserves mitochondrial function in senescence without increasing mitochondrial biogenesis. Cell Metab. 2012;16:777–788. doi: 10.1016/j.cmet.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laschober GT, Ruli D, Hofer E, Muck C, Carmona-Gutierrez D, Ring J, Hutter E, Ruckenstuhl C, Micutkova L, Brunauer R, Jamnig A, Trimmel D, Herndler-Brandstetter D, Brunner S, Zenzmaier C, Sampson N, Breitenbach M, Frohlich KU, Grubeck-Loebenstein B, Berger P, Wieser M, Grillari-Voglauer R, Thallinger GG, Grillari J, Trajanoski Z, Madeo F, Lepperdinger G, Jansen-Durr P. Identification of evolutionarily conserved genetic regulators of cellular aging. Aging Cell. 2010;9:1084–1097. doi: 10.1111/j.1474-9726.2010.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LeBrasseur NK, Schelhorn TM, Bernardo BL, Cosgrove PG, Loria PM, Brown TA. Myostatin inhibition enhances the effects of exercise on performance and metabolic outcomes in aged mice. J Gerontol A Biol Sci Med Sci. 2009;64:940–8. doi: 10.1093/gerona/glp068. [DOI] [PubMed] [Google Scholar]
- 27.Lee CK, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285:1390–1393. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Chewchuk S, Lavigne C, Brule S, Pilon G, Houde V, Xu A, Marette A, Sweeney G. Functional significance of skeletal muscle adiponectin production, changes in animal models of obesity and diabetes, and regulation by rosiglitazone treatment. Am J Physiol Endocrinol Metab. 2009;297:E657–E664. doi: 10.1152/ajpendo.00186.2009. [DOI] [PubMed] [Google Scholar]
- 29.Lund EG, Kerr TA, Sakai J, Li WP, Russell DW. cDNA cloning of mouse and human cholesterol 25-hydroxylases, polytopic membrane proteins that synthesize a potent oxysterol regulator of lipid metabolism. The Journal of biological chemistry. 1998;273:34316–27. doi: 10.1074/jbc.273.51.34316. [DOI] [PubMed] [Google Scholar]
- 30.Marzetti E, Lees HA, Wohlgemuth SE, Leeuwenburgh C. Sarcopenia of aging: underlying cellular mechanisms and protection by calorie restriction. BioFactors. 2009;35:28–35. doi: 10.1002/biof.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsusue K, Kusakabe T, Noguchi T, Takiguchi S, Suzuki T, Yamano S, Gonzalez FJ. Hepatic steatosis in leptin-deficient mice is promoted by the PPARgamma target gene Fsp27. Cell Metab. 2008;7:302–311. doi: 10.1016/j.cmet.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minor RK, Allard JS, Younts CM, Ward TM, de Cabo R. Dietary interventions to extend life span and health span based on calorie restriction. J Gerontol A Biol Sci Med Sci. 2010;65:695–703. doi: 10.1093/gerona/glq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olive M, Rivera R, Ferrer I. Parvalbumin immunocytochemistry and calcium deposition in muscle fiber necrosis and subsequent regeneration following intramuscular injection of metoclopramide. Muscle Nerve. 1994;17:494–499. doi: 10.1002/mus.880170505. [DOI] [PubMed] [Google Scholar]
- 35.Park S-K, Prolla TA. Gene expression profiling studies of aging in cardiac and skeletal muscles. Cardiovasc Res. 2005;66:205–212. doi: 10.1016/j.cardiores.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Piec I, Listrat A, Alliot J, Chambon C, Taylor RG, Bechet D. Differential proteome analysis of aging in rat skeletal muscle. FASEB J. 2005;19:1143–1145. doi: 10.1096/fj.04-3084fje. [DOI] [PubMed] [Google Scholar]
- 37.Puri V, Konda S, Ranjit S, Aouadi M, Chawla A, Chouinard M, Chakladar A, Czech MP. Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage. J Biol Chem. 2007;282:34213–34218. doi: 10.1074/jbc.M707404200. [DOI] [PubMed] [Google Scholar]
- 38.Ratajczak MZ, Shin DM, Schneider G, Ratajczak J, Kucia M. Parental imprinting regulates insulin-like growth factor signaling: a Rosetta Stone for understanding the biology of pluripotent stem cells, aging and cancerogenesis. Leukemia. 2013;27:773–779. doi: 10.1038/leu.2012.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rehage M, Mohan S, Wergedal JE, Bonafede B, Tran K, Hou D, Phang D, Kumar A, Qin X. Transgenic overexpression of pregnancy-associated plasma protein-A increases the somatic growth and skeletal muscle mass in mice. Endocrinology. 2007;148:6176–6185. doi: 10.1210/en.2007-0274. [DOI] [PubMed] [Google Scholar]
- 40.Ristow M, Zarse K. How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis). Exp Gerontol. 2010;45:410–418. doi: 10.1016/j.exger.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 41.Ritchie RF, Palomaki GE, Neveux LM, Navolotskaia O, Ledue TB, Craig WY. Reference distributions for the negative acute-phase serum proteins, albumin, transferrin and transthyretin: a practical, simple and clinically relevant approach in a large cohort. J Clin Lab Anal. 1999;13:273–279. doi: 10.1002/(SICI)1098-2825(1999)13:6<273::AID-JCLA4>3.0.CO;2-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127:990S–991S. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- 43.Selman C, Kerrison ND, Cooray A, Piper MD, Lingard SJ, Barton RH, Schuster EF, Blanc E, Gems D, Nicholson JK, Thornton JM, Partridge L, Withers DJ. Coordinated multitissue transcriptional and plasma metabonomic profiles following acute caloric restriction in mice. Physiol Genomics. 2006;27:187–200. doi: 10.1152/physiolgenomics.00084.2006. [DOI] [PubMed] [Google Scholar]
- 44.Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA. 2005;102:5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stacpoole PW. The pyruvate dehydrogenase complex as a therapeutic target for age-related diseases. Aging Cell. 2012;11:371–377. doi: 10.1111/j.1474-9726.2012.00805.x. [DOI] [PubMed] [Google Scholar]
- 46.Team RDC. A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. [Google Scholar]
- 47.Yamaza H, Komatsu T, To K, Toyama H, Chiba T, Higami Y, Shimokawa I. Involvement of insulin-like growth factor-1 in the effect of caloric restriction: regulation of plasma adiponectin and leptin. J Gerontol A Biol Sci Med Sci. 2007;62:27–33. doi: 10.1093/gerona/62.1.27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.