Abstract
Background
Hyposensitivity to non-drug reward, behaviorally manifested as anhedonia, is a hallmark of chronic substance use. Anhedonia is a transdiagnostic symptom underpinned by neurobiochemical disturbances in the reward circuit, yet an objective measure to assess anhedonia severity still eludes the field. We hypothesized that the Reward Positivity (RewP) component of the event-related potentials (ERPs) will specifically track anhedonia as the RewP is attributed to the same brain regions that are also implicated in anhedonia.
Methods
Forty-six individuals with cocaine use disorders (iCUD) performed a gambling task predicting whether they would win or lose money on each trial, while ERP data was acquired. RewP in response to predicted win trials was extracted from the ERPs using the principal component analysis. State anhedonia and depression severity were assessed using the Cocaine Selective Severity Assessment (CSSA).
Results
Although RewP amplitude correlated with both anhedonia and depression, only the RewP-anhedonia correlation survived a correction for depression severity. Further, a hierarchical multiple regression analysis revealed that anhedonia explained a significant amount of variance in the RewP amplitude, and this variance was significantly greater than that explained by demographics, severity and recency of drug use and even depression.
Conclusions
These results show that RewP amplitude in response to rewarded trials tracks state anhedonia severity in iCUD. We argue that this association is perhaps driven by the activity in the dopaminergic mesocorticolimbic reward pathway that may underlie anhedonia symptomology as well as modulate RewP amplitude.
Keywords: Anhedonia, EEG, Event-related potentials, Reward positivity, Cocaine, Addiction
1. INTRODUCTION
Substance use disorders (SUD) are often characterized by hypersensitivity to drug-related rewards with a concomitant hyposensitivity to non-drug-related rewards (Berridge and Robinson, 1998; Di Chiara, 1998). Preclinical as well as human neuroimaging studies have consistently implicated alterations within the mesocorticolimbic reward circuit (Hyman et al., 2006; Koob and Le Moal, 2001) in mediating such reward dysregulation in SUD. Within this circuit, the ventral striatum (VS) is a critical subcortical node that underlies response to reward behaviors and pleasurable experiences associated with reward (Heinz et al., 1994; Willner et al., 2005). At the cortical level, the medial prefrontal cortex (mPFC) plays a crucial role in the processing of the motivational value of rewards (Grabenhorst and Rolls, 2011; Grabenhorst et al., 2008; Harvey et al., 2007; Keedwell et al., 2005), in goal-directed behavior (Hare et al., 2008) and its adaptive adjustments after reward contingency changes (Hikosaka and Watanabe, 2000). In SUD, however, both the VS and the mPFC show hyposensitivity to non-drug-related rewards, such as food, sex and even to more abstract rewards such as money (Childress et al., 2008; Goldstein et al., 2007; Volkow et al., 2010). Previous studies have posited an attenuated tonic dopamine activity, partially due to a prolonged allostatic overload on the reward circuit by chronic drug use, to underpin such hyposensitivity to non-drug rewards in SUD (e.g., see Koob and Le Moal, 2005).
Similarly, anhedonia, the decreased capacity to experience pleasure, is a clinical symptom that reflects deficits of reward processes. While it is a core symptom of depression (Gorwood, 2008), anhedonia is a salient feature across many neuropsychiatric disorders (Whybrow, 1998), including SUD (Ahmed and Koob, 1998; Volkow et al., 2002). It has been argued that abnormalities in the dopaminergic neuronal reward circuitry underlying anhedonia can be induced by dopamine-inducing drugs, such as cocaine (Volkow et al., 2010; Vollm et al., 2004). Subsequently, this drug-induced dopamine surge influences reward-guided decision making (Bechara, 2005), and plays a crucial role in the development and maintenance of SUD (Ahmed and Koob, 1998; Goldstein and Volkow, 2002, 2011; Koob and Le Moal, 2001). Therefore, the objective quantification of anhedonia in individuals suffering from SUD is critical in gaining better understanding of the impaired reward-related brain functions that are specific to anhedonia. However, anhedonia is a subjective clinical measure, which reflects a wide range of deficits in reward processes.
Anhedonia has traditionally been assessed via self-report questionnaires, either as a composite measure [e.g., using the Snaith-Hamilton Pleasure Scale (SHAPS; Snaith et al., 1995)] or in SUD as a constituent score of a higher-level construct, such as withdrawal [e.g., using the Cocaine Selective Severity Assessment (CSSA, Kampman et al., 1998)]. Indeed, there have been attempts to objectively assess anhedonia behaviorally (Pizzagalli et al., 2005; Treadway et al., 2009) as well as using event-related potential (ERP) components, such as the Oddball P3 component in healthy individuals (Franken et al., 2006), and the feedback-related negativity (FN) (Foti et al., 2014) and reward positivity (RewP; Liu et al., 2014) in individuals with major depressive disorder. The RewP [also termed the feedback correct-related positivity; Holroyd et al., 2008)] is the positive-going deflection in correct (or win/reward) trials; its absence in incorrect (or loss/punishment) trials yields the FN (Baker and Holroyd, 2011; Foti et al., 2011; Holroyd et al., 2008). The RewP amplitude is posited to specifically reflect the activity of the mesencephalic dopamine system underlying reward processing (Foti et al., 2011; Proudfit, 2015), specifically with the activity in the VS and the mPFC (Carlson et al., 2011; Foti et al., 2014).
Here we sought to investigate ERPs correlates of anhedonia in adults with SUD. Given that activity in the VS-mPFC loop contributes to both anhedonia and the RewP amplitude and that chronic drug use is presumed to act as an allostatic overload on this loop (Koob and Le Moal, 2001), we hypothesize that the RewP amplitude would be specifically associated with the severity of anhedonia, and not that of depression, in individuals with cocaine use disorders (iCUD).
2. METHODS
2.1. Participants
Fifty-five iCUD (10 females, 43.4 ± 6.9 years old) participated in this study. Forty-six participants (7 females, 43.0 ± 6.9 years old) yielded useable data (Table 1); the other nine participants either did not yield adequate number of trials (< 15 trials, n=6) or their electroencephalography (EEG) data was of low fidelity (n=3). All participants were recruited through advertisements in local newspapers, word of mouth, and local treatment facilities. Additionally, participants were right-handed, native English speakers, and free of sustained/maintenance medications for >30 days prior and throughout the study. Further exclusionary criteria included (A) history of head trauma or loss of consciousness (>30 min) or other neurological diseases of central origin (including seizures); (B) current medical diseases that required hospitalization or regular monitoring; (C) history of major psychiatric disorder [other than substance abuse/dependence and disorders of high comorbidity with substance abuse/dependence (inclusive of depression and post-traumatic stress disorder) and/or nicotine dependence]; (D) positive urine screens for psychoactive drugs or their metabolites (phencyclidine, benzodiazepines, cannabis, opiates, barbiturates, and inhalants) other than cocaine; and (E) more than two standard deviations below the norm on a verbal intelligence measure. Note that data from 28 of the current 46 participants have already been published elsewhere (Parvaz et al., 2015). However, the current study focuses exclusively on previously un-reported data from these participants. All participants were fully informed of all study procedures and risks, and they provided written consent in accordance with the Stony Brook University Institutional Review Board and the associated treatment facility’s Institutional Review Board.
Table 1.
Demographic characteristics and drug use-related measures for iCUD.
| iCUD (N=46) | |
|---|---|
| Demographics | |
| Age (years) | 43.03 ± 6.9 |
| Gender (male/female) | 39/7 |
| Race (African-American/Caucasian/Other) | 28/15/3 |
| Education (years) | 12.83 ± 1.7 |
| Drug Use | |
| Cigarette smokers (current/past/nonsmoker) | 32/10/4 |
| Daily cigarettes in current smokers | 4.02 ± 4.6 |
| Lifetime duration of cocaine use (years) | 15.30 ± 7.9 |
| Duration of current abstinence (days) | 70.89 ± 137.6 |
| Cocaine Craving (Cocaine Craving Questionnaire) | 16.74 ± 12.65 |
| Total Substance Dependence Scale | 7.63 ± 3.8 |
| Cocaine Selective Severity Assessment (CSSA) | |
| CSSA – Anhedonia | 0.70 ± 1.7 |
| CSSA – Depression | 0.39 ± 1.1 |
Note: Values are frequencies or means ± standard deviation (S.D.).
2.2. Diagnostic Interview
All participants underwent a full diagnostic interview including: (A) the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Axis I Disorders (First et al., 1996); (B) the Addiction Severity Index (McLellan et al., 1992), a semi-structured interview instrument that assesses the severity as well as recent and lifetime history of alcohol- and drug-related problems, as they relate to seven problem areas (medical, employment, legal, alcohol, other drug use, family-social functioning, and psychological status); (C) the 5-item Severity of Dependence Scale (Gossop et al., 1992); (D) the 18-item Cocaine Selective Severity Assessment Scale (CSSA; Kampman et al., 1998), designed to evaluate cocaine abstinence/withdrawal signs and symptoms (including anhedonia and depression) 24 hours within the time of interview. Single items on the CSSA were used to quantify anhedonia and depression, using scales from ‘0’ to ‘7’ [0 = “ability to enjoy themselves remain unchanged” (anhedonia), “no feelings related to sadness or depression” (depression); 3–4 = “able to enjoy themselves half the time” (anhedonia), “feels sad or depressed half the time” (depression); 7 = “unable to enjoy themselves at all” (anhedonia), “feels depressed all the time” (depression)].
This interview determined that iCUD met criteria for current cocaine dependence (n=33), or cocaine dependence in partial (n=4) or sustained remission (n=9). Current comorbidities in this sample included marijuana use disorder (n=1), alcohol use disorder (n=1), opiate use disorder (n=1), anti-social personality disorder (n=2), post-traumatic stress disorder (n=1), and major depression disorder (n=1). All other comorbidities were in partial or sustained remission.
2.3. Task
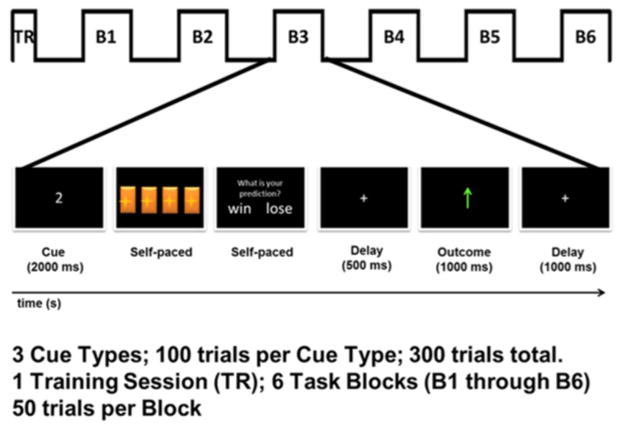
The overall design of the task was similar to what has been previously described (Parvaz et al., 2015) and similar to Experiment 2 reported in a previous study by Hajcak et al. (2007). In brief, participants’ primary objective on each trial was to guess which of four doors hid a prize by pressing one of four keys on a keypad corresponding to each door (Figure 1). At the beginning of each trial, after an initial presentation of a fixation cross (1000ms), a white cue of “1,” “2,” or “3” appeared on the screen for 1000ms, which indicated the number of doors (out of four) that contained a monetary prize (60¢); thus, these cues indicated the probability of reward on the upcoming trial (25, 50, and 75%, respectively). Following the presentation of the cue, an inter-stimulus delay of 500ms was presented followed by a presentation of the graphic of the doors, which remained on-screen until the participant made a selection to indicate their prediction of the winning door. Immediately following their choice, the question “Do you think you won or lost in this trial?” appeared on the screen and remained there until participants indicated via button press a predicted win or loss. Thus, participants were first presented with a cue that conveyed the objective probability of reward on the upcoming trial, and then asked to make a choice and, finally, predict whether or not they thought they chose correctly. Five hundred milliseconds following their prediction, a feedback stimulus appeared on the screen for 1000ms: a green arrow pointing upward indicated a win (i.e., a win of 60¢), or a red arrow pointing downward indicated a loss (i.e., a loss of 30¢; Figure 1). Winning probability was always consistent with the cue type (e.g., 75% winning probability for three-cue trials). Such a task design specifically allows for modulating valence-related variability in FN by explicit prediction errors (Hajcak et al., 2007).
Figure 1.
Task schematic during a win trial of the gambling task. Based on a cue (1, 2, or 3), the participants select a door and then identify if they expect to win or lose in that trial. A feedback of their accuracy is provided at the end of each trial.
This task was administered using Presentation software (Neurobehavioral Systems) to control the presentation and timing of all stimuli. All stimuli were positioned in the center of the screen. The cue and feedback stimuli occupied 21° of visual angle horizontally and 21° vertically. A fixation cross was then presented during each intertrial interval for 500ms. The task consisted of six blocks of 50 pseudorandom trials (i.e., a total of 300 trials; 100 trials per cue type) interspaced by a brief break. Participants were told that the task earnings would be given to them at the end of the EEG session. Unbeknownst to the participants, the task earnings were not dependent on their choice and they were always paid $75 for the task.
2.4. Psychophysiological recordings and data reduction
Electroencephalogram (EEG; Neuroscan) recordings were obtained using a 64 silver–silver chloride electrode cap positioned according to the International 10/20 system. Electro-oculogram electrodes at the left supraorbital and infraorbital sites and the right and left outer canthi recorded the blinks (and vertical eye movements) and horizontal eye movements, respectively. EEG recordings were sampled at 500 Hz and bandpass filtered from DC to 70 Hz. Electrode impedances were kept <50 kΩ.
Offline preprocessing of the EEG signal was performed using SPM8 (Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm) and custom Matlab (The MathWorks) scripts. Data were first bandpass filtered (0.01–30 Hz) and re-referenced to the averaged electrical activity from all 64-scalp sites. To evaluate the effect of the participant’s actual predictions on the feedback-locked ERP and to maximize the number of trials for each analysis across the objective winning probabilities (25, 50, and 75%), averaged waveforms were created for all feedback trial types: Predicted Win, Unpredicted Win, Predicted Loss, and Unpredicted Loss. Therefore, the continuous EEG data were segmented beginning 200ms before the feedback onset and continuing for 1000ms. For each trial, the 200ms baseline was subtracted from the post-stimulus data for baseline correction. Eye-blink and ocular corrections were performed using the partial signal space projection method proposed by Nolte and Hamalainen (2001), such that the contribution to the estimated spatial structure of eye-blink artifact was removed only from the artifact-ridden epochs, leaving as much information as possible in the data. This artifact-rejection procedure identified a voltage step of >75 μV between sample points and a peak-to-peak voltage difference of 150 μV within an epoch. Additional artifacts were identified through visual inspection, and the contaminated epochs were subsequently rejected. Finally, robust averaging was used to create artifact-free ERPs (Wager et al., 2005) separately for each of the four conditions. To test our hypothesis, we only used data from the Predicted-Win condition, as this condition signals reward outcome without being influenced by reward prediction error (in contrast to the unpredicted-win condition). Nevertheless, to inspect for specificity of results, data from the other conditions were also extracted and the results are presented online as Supplementary Material1. The average number of artifact-free epochs used for ERP averaging of the Predicted Win condition was 110.85 (Range: 60 – 134).
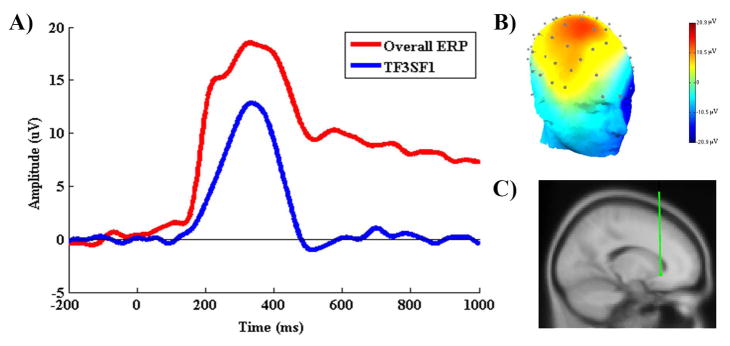
Following recently published studies, temporospatial principal components analysis (PCA) [EP-Toolkit, version 2.51b (Dien, 2010)] was used to isolate RewP amplitude from the Predicted Win ERP waveform (Foti et al., 2011; Liu et al., 2014). First, a temporal PCA was performed to capture variance across time and maximize the initial separation of the ERP components (Dien et al., 2005). A Promax rotation was used (Dien, 2010; Dien et al., 2007) and 11 temporal factors were extracted based on the resulting Scree plot (Cattell, 1966). Following the temporal PCA, a separate spatial PCA was performed for all 11 temporal factors obtained in the previous step. The Infomax rotation was used and based on the results of the parallel Scree plot five spatial factors were extracted from each temporal factor, yielding a total of 55 temporospatial factor combinations. To directly assess timing and spatial voltage distributions, we then translated the factors back into voltages. Seventeen factor combinations accounted for more than 1% of the variance each. Of these, one factor combination was identified to reflect the RewP (Spatial factor 1 of the temporal Factor 3, or TF3SF1; Figure 2) based on its latency (between 250 – 400ms), scalp topography (frontocentral maxima) and statistically computed source location (medial orbitofrontal cortex), consistent with proposed characteristics of RewP (see, Proudfit, 2015). The latency and amplitude of PCA factors in other task conditions are presented online as Table S12.
Figure 2.
(A) The time series waveforms of the ERP (red) in response to the predicted win condition, and its derived PCA factor (TF3SF1) that reflects RewP (blue). (B) The scalp topography with frontocentral maxima and (C) the dipole source location in the medial orbitofrontal cortex (Tailarach coordinates: x=0, y=21, and z=−6), are consistent with previous reports of RewP.
2.5. Statistical analyses
First, the association between the amplitude of the isolated PCA factor (i.e., TF3SF1) denoting RewP and the severity of anhedonia and depression (assessed via CSSA) were ascertained using the Spearman Rank correlation. Next, a hierarchical multiple regression analysis was conducted to assess whether anhedonia and depression predicted unique and significant variance in the RewP amplitude. The first model included participants’ demographic characteristics (age and gender), the second included the measures of severity and recency of drug-use [i.e., severity of cocaine dependence (assessed via the severity of dependence scale), current cocaine abstinence (in days) and cigarette smoking status (current, past, or non-smoker)]. Anhedonia and depression (assessed via CSSA) were entered in the last two models. This way the predictive value of either anhedonia or depression on RewP amplitude was tested beyond the predictive value of participants’ demographics and drug-use measures.
3. RESULTS
3.1. Correlations
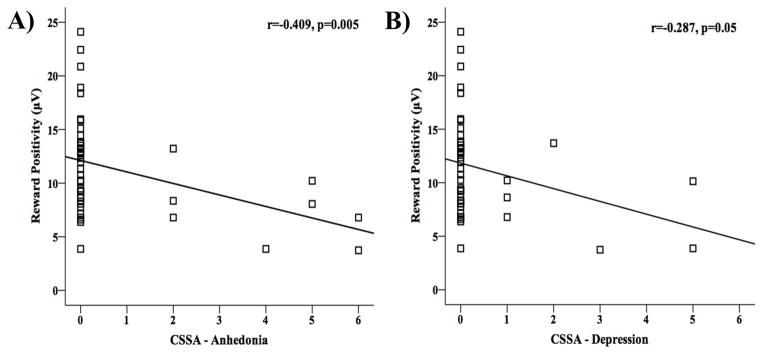
As hypothesized, the RewP amplitude was negatively correlated with anhedonia (rs=−0.409, p=0.005) and depression (rs=−0.287, p=0.05) scores, such that reduced RewP amplitude was associated with greater anhedonia and depression severity in iCUD (Figure 3). As expected, both anhedonia and depression severity scores were significantly intercorrelated (rs=0.389, p=0.003).
Figure 3.
The correlations between the (PCA derived) RewP amplitude and subjectively assessed (via CSSA) (A) anhedonia and (B) depression scores show that iCUD who presented higher scores on anhedonia and depression also presented lower RewP amplitudes.
3.2. Hierarchical Multiple Regression
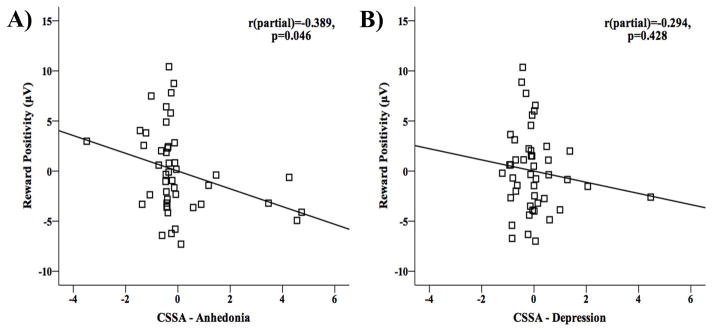
The hierarchical multiple regression analyses revealed that the RewP amplitude were significantly associated with the anhedonia scores (β=−0.32, p=0.046) and not with the depression scores (β=−0.14, p=0.428) (Table 2). Further, a total of 24.9% of the variance in the RewP amplitude was explained by the full regression model. Demographic (i.e., age and gender) and drug use variables [i.e., severity of cocaine dependence, current cocaine abstinence (in days) and cigarette smoking status (current or past/non-smoker)] explained 2.3% (Fchange(2,43)=0.50, p=0.607) and 7.7% (Fchange(3,40)=1.13, p=0.347) of the variance in the RewP amplitude, respectively. Anhedonia score significantly predicted 13.7% of the variance (Fchange(1,39)=6.97, p=0.012), whereas depression score only predicted 1.3% of the variance (Fchange(1,38)=0.64, p=0.428) in the RewP amplitude. Standardized and unstandardized regression coefficients and the test statistic for each independent variable in the model are listed in Table 2.
Table 2.
Summary of Hierarchical Regression Analysis for Variables predicting RewP.
| Variable | B | B-SE | β | t | p | R | R2 | ΔR2 | sig. ΔF |
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | 0.15 | 0.02 | 0.02 | 0.607 | |||||
| Age | −0.08 | 0.10 | −0.13 | −0.84 | 0.405 | ||||
| Gender | 1.98 | 1.99 | 0.16 | 0.99 | 0.328 | ||||
| Model 2 | 0.32 | 0.10 | 0.08 | 0.347 | |||||
| Severity of dependence score | 0.28 | 0.18 | 0.23 | 1.50 | 0.141 | ||||
| Length of abstinence of cocaine | 0.00 | 0.01 | −0.01 | −0.05 | 0.964 | ||||
| Cigarette smokers | 0.51 | 1.41 | 0.06 | 0.36 | 0.719 | ||||
| Model 3 | 0.49 | 0.24 | 0.14* | 0.012 | |||||
| CSSA – Anhedonia | −0.89 | 0.43 | −0.32 | −2.06* | 0.046 | ||||
| Model 4 | 0.50 | 0.25 | 0.01 | 0.428 | |||||
| CSSA – Depression | −0.56 | 0.67 | −0.14 | −0.80 | 0.428 |
p<0.05
To rule out order effects in model specification, a similar hierarchical multiple regression was conducted again, but with depression and anhedonia scores in the third and the fourth model, respectively. This time depression marginally predicted 6.6% of the variance (Fchange(1,39)=3.06, p=0.088), whereas anhedonia still significantly predicted 8.4% of the variance in the RewP amplitude (Fchange(1,38)=4.24, p=0.046), highlighting the specificity of the association between anhedonia and the RewP amplitude in iCUD.
4. DISCUSSION
The goal of this study was to determine an objective and specific electrocortical marker of anhedonia in individuals with SUD. Using ERPs in response to a monetarily rewarded condition in a probabilistic gambling task, the current study titrated the specific association between RewP amplitude and anhedonia severity. Indeed, recent studies have reported that modulation in FN (a complimentary ERP component of RewP) amplitude stems from stimuli that signal reward (Foti et al., 2011; Holroyd et al., 2011, 2008) and is associated with depressive symptoms (Foti et al., 2014; Foti and Hajcak, 2009), including anhedonia (Liu et al., 2014). Thus, the current study replicates these findings by showing an association between the RewP amplitude in response to rewarding feedback and the severity of anhedonia and depression (both assessed via the CSSA) in individuals with SUD. More importantly, this study extends the existing literature to further highlight the specificity of the association between RewP amplitude and anhedonia, irrespective of the depressed mood, severity and recency of drug use and demographic characteristics in iCUD.
Although novel, these results are not surprising. Both anhedonia and depressed mood are key symptoms of depression that is highly comorbid in SUD, but they are empirically distinct – each can occur without co-occurrence of the other (Zimmerman et al., 2006), have distinct underlying neural correlates (Wacker et al., 2009) and are posited to be distinct endophenotypes of the depression syndrome (Hasler et al., 2004). Having an objective and unique marker of anhedonia (RewP) is clinically significant in SUD as more severe anhedonia is associated with increased likelihood of relapse among nicotine dependent individuals (Cook et al., 2010; Leventhal et al., 2009) and with increased drug craving among alcohol (Martinotti et al., 2008a, 2008b), opioid (Janiri et al., 2005; Martinotti et al., 2008a) and nicotine dependent individuals (Cook et al., 2004; Leventhal et al., 2009). Increased anhedonia severity has also been reported in recent as well as in protracted abstinent substance-dependent populations (Hatzigiakoumis et al., 2011).
A substantial body of research has suggested that psychostimulants (i.e., drugs that activate mesolimbic dopamine system, such as cocaine) usurp the dopamine-mediated process of assigning salience to reinforcers by reducing tonic dopaminergic transmission in the striato-cortical loop, resulting in hyposensitivity to non-drug-related reward and related motivational disturbances such as anhedonia (Di Chiara and Bassareo, 2007; Gorwood, 2008; Melis et al., 2005; Romer Thomsen et al., 2015). Similar dopaminergic neural substrates that are implicated in reward sensitivity, mainly in the striato-cortical loop, are also posited to generate the RewP amplitude (mainly from the findings in FN; Carlson et al., 2011; Foti et al., 2014). Thus, it can be speculated that the specific association between the RewP amplitude and anhedonia in our sample is mediated by dopaminergic signaling in the mesocorticolimbic reward circuit, especially in individuals with chronic stimulant use disorders, such as the iCUD.
Indeed, anhedonia-related modulations of ERP amplitude have been studied previously. For example, studies in healthy individuals have shown that, compared to those with low anhedonia, those with higher anhedonia score [assessed via the Chapman’s Physical Anhedonia Scale (PAS; Chapman et al., 1976), the Beck-Weissman’s Dysfunctional Attitude Scale (DAS; Weissman, 1979), or the SHAPS] manifest deficits in the orienting response and motor preparation processes quantified via the contingent negative variation (Pierson et al., 1987) and P3 amplitude (elicited using an Oddball paradigm; Franken et al., 2006). Similarly, a recent multimodal study in healthy individuals has shown a unique association between anhedonia [assessed via the anhedonic depression scale of the Mood and Anxiety Symptoms Questionnaire (MASQ-AD; Watson et al., 1995a, 1995b)] and the resting-state EEG delta band power reflecting the activity in the rostral anterior cingulate cortex (Wacker et al., 2009). Compared to healthy controls, a recent study has shown reduced FN amplitude as correlated with increased anhedonia and depressive symptoms in a clinical cohort of patients with major depressive disorder (Liu et al., 2014). However, the significant anhedonia-FN correlation was reduced to a trend significance when controlled for overall depression severity (Liu et al., 2014). In another recent clinical study, more pronounced N2 and reduced P3 amplitudes during successful response inhibition (in a Go/No-Go task) were associated with more severe anhedonia, but only in healthy control and not in iCUD (Morie et al., 2014). The authors speculated that the diverging results might be driven by the interaction between diagnostic groups and the distinct neural substrates underlying No-Go N2 and P3 amplitudes (i.e., response inhibition) and anhedonia severity (i.e., reward processing deficits; Morie et al., 2014). Thus, the current results extend the literature by using RewP amplitude that is specifically elicited by reward-signaling stimuli (i.e., Predicted Win condition), and by using data-driven approaches (i.e., temporospatial PCA and dipole source modelling) to systematically show the specificity of the RewP-anhedonia association in a population with specific impairments in reward processing (Di Chiara and Bassareo, 2007; Parvaz et al., 2012).
Here, it is important to distinguish the RewP from what has previously been termed as the reward-related P3. The RewP is a positive-going deflection that is elicited only in response to reward-related stimuli and shows peak amplitude between 250 – 400 msec at fronto-central scalp location (Holroyd et al., 2011, 2008). The RewP has specifically been implicated as a functional marker of dopaminergic reward processing (Carlson et al., 2011; Foti et al., 2014, 2011; Proudfit, 2015). In contrast, the P3, also a positive-going deflection, is elicited by both reward- and loss-related trials (or outcomes of motivational importance; Wu and Zhou, 2009; Yeung and Sanfey, 2004), showing a comparatively delayed maxima (350 – 600 msec) at centro-parietal scalp locations (Goldstein et al., 2006; Sato et al., 2005). Although there have been reports of increased P3 amplitude to positive compared to non-positive feedback (Hajcak et al., 2007), perhaps due to increased motivational salience attributed to positive feedback, functionally, the reward-related P3 is sensitive to outcome magnitude (Bellebaum et al., 2010; Parvaz et al., 2012; Sato et al., 2005), regardless of its valence (win or loss; Yeung and Sanfey, 2004).
It is important to note that the current study used individual items from the CSSA to assess state anhedonia and depression severity in iCUD. The CSSA is a clinician-administered instrument that measures early cocaine abstinence signs and symptoms. The literature-informed questions seek to quantify severity of symptoms that are most often associated with early cocaine abstinence, including depression, fatigue, anhedonia, anxiety, irritability, sleep disturbance, and inability to concentrate (Brower et al., 1988; Cottler et al., 1993; Watson et al., 1992). Thus, similar to using the Beck Depression Inventory to quantify depression severity in patients with mood disorders, the CSSA provides a targeted quantification of anhedonia and depression in iCUD. Individual items of the CSSA have been previously used to investigate unique withdrawal-related state measures, such as craving (Hendricks and Greenway, 2011; Reid and Thakkar, 2009). Nevertheless, the use of individual items of a questionnaire instead of a composite score, and the resulting limited variability in the scores (evident in Figures 3 and 4), is a limitation of this study. Therefore, it is important that future studies test if this unique association of the RewP amplitude with the CSSA-derived anhedonia score also generalizes to anhedonia measured using other scales (i.e., PAS, SHAPS, and MASQ-AD). Another limitation of the current study is the absence of a healthy control group. However, since differences between iCUD and healthy controls have consistently been reported in anhedonia severity (see, Leventhal et al., 2010) and the RewP (or FN) amplitude (Franken et al., 2007; Parvaz et al., 2015; Torres et al., 2013), inclusion of a control group might only have served the purpose of yet another validation of group differences, which was not the focus of the current study.
Figure 4.
The partial correlation plots (from the hierarchical multiple regression) between the RewP amplitude and (A) anhedonia, and (B) depression scores, show that unlike depression scores, anhedonia scores significantly predict the RewP amplitude.
In sum, the current study reported a unique association between reduced RewP amplitude in response to rewarding feedback stimuli and increased anhedonia severity in iCUD, even after controlling for demographics, drug use variables and, importantly, depression severity. Since RewP amplitude has been posited to be a marker of depression (Proudfit, 2015), the current results help titrate this association specifically to anhedonia, a core transdiagnostic neuropsychiatric symptom. Although anhedonia is typically assessed via psychometrically validated subjective (self-report) scales, their utility in populations with known self-awareness and insight impairments, such as in individuals with SUD (Goldstein et al., 2009), remains questionable. Therefore, our results suggest that the RewP amplitude can potentially serve as an objective biomarker that reliably tracks anhedonia severity in iCUD. These results also speak to the overall utility of EEG markers in clinical settings to facilitate objective assessments of clinical symptomology.
Supplementary Material
Highlights.
Anhedonia is a core symptom of drug addiction but assessed only subjectively.
Reward positivity (RewP) is a reward-sensitive event-related potential.
RewP amplitude is correlated with anhedonia and depression severity
However, anhedonia explains a significant variance in RewP amplitude
Significantly more than demographics, drug-use, and depression.
Acknowledgments
Role of Funding: This study was supported by grants from the National Institute on Drug Abuse (to MAP: F32DA033088 and to RZG: R01DA023579) and from the National Institute on Mental Health (to VG: R01MH095807 and R01MH101479).
The authors thank Tom Maloney and Naomi Spilka for their help with data mining and conducting preliminary analyses.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Contributors: MAP, VG and RZG designed the study. MAP and PM analyzed the data and MAP penned the manuscript with the help of VG, PM and RZG. All authors approve of the final version of the manuscript.
Conflict of Interest: No conflict declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Baker TE, Holroyd CB. Dissociated roles of the anterior cingulate cortex in reward and conflict processing as revealed by the feedback error-related negativity and N200. Biol Psychol. 2011;87:25–34. doi: 10.1016/j.biopsycho.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bellebaum C, Polezzi D, Daum I. It is less than you expected: the feedback-related negativity reflects violations of reward magnitude expectations. Neuropsychologia. 2010;48:3343–3350. doi: 10.1016/j.neuropsychologia.2010.07.023. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Maddahian E, Blow FC, Beresford TP. A comparison of self-reported symptoms and DSM-III-R criteria for cocaine withdrawal. Am J Drug Alcohol Abuse. 1988;14:347–356. doi: 10.3109/00952998809001556. [DOI] [PubMed] [Google Scholar]
- Carlson JM, Foti D, Mujica-Parodi LR, Harmon-Jones E, Hajcak G. Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: a combined ERP and fMRI study. Neuroimage. 2011;57:1608–1616. doi: 10.1016/j.neuroimage.2011.05.037. [DOI] [PubMed] [Google Scholar]
- Cattell RB. Scree Test for Number of Factors. Multivar Behav Res. 1966;1:245–276. doi: 10.1207/s15327906mbr0102_10. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. J Abnorm Psychol. 1976;85:374–382. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- Childress AR, Ehrman RN, Wang Z, Li Y, Sciortino N, Hakun J, Jens W, Suh J, Listerud J, Marquez K, Franklin T, Langleben D, Detre J, O’Brien CP. Prelude to passion: limbic activation by “unseen” drug and sexual cues. PLoS One. 2008;3:e1506. doi: 10.1371/journal.pone.0001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J, Spring B, McChargue D, Doran N. Effects of anhedonia on days to relapse among smokers with a history of depression: a brief report. Nicotine Tob Res. 2010;12:978–982. doi: 10.1093/ntr/ntq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D, Hedeker D. Hedonic capacity, cigarette craving, and diminished positive mood. Nicotine Tob Res. 2004;6:39–47. doi: 10.1080/14622200310001656849. [DOI] [PubMed] [Google Scholar]
- Cottler LB, Shillington AM, Compton WM, 3rd, Mager D, Spitznagel EL. Subjective reports of withdrawal among cocaine users: recommendations for DSM-IV. Drug Alcohol Depend. 1993;33:97–104. doi: 10.1016/0376-8716(93)90051-q. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. A motivational learning hypothesis of the role of mesolimbic dopamine in compulsive drug use. J Psychopharmacol. 1998;12:54–67. doi: 10.1177/026988119801200108. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn’t do. Curr Opin Pharmacol. 2007;7:69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Dien J. The ERP PCA Toolkit: an open source program for advanced statistical analysis of event-related potential data. J Neurosci Methods. 2010;187:138–145. doi: 10.1016/j.jneumeth.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Dien J, Beal DJ, Berg P. Optimizing principal components analysis of event-related potentials: matrix type, factor loading weighting, extraction, and rotations. Clin Neurophysiol. 2005;116:1808–1825. doi: 10.1016/j.clinph.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Dien J, Khoe W, Mangun GR. Evaluation of PCA and ICA of simulated ERPs: Promax vs. Infomax rotations. Hum Brain Mapp. 2007;28:742–763. doi: 10.1002/hbm.20304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinial Interview for DSM-IV-R Axis I Disorders (SCID-I), Clinican Version, Users Guide. American Psychiatric Publishing, Inc; Arlington: 1996. [Google Scholar]
- Foti D, Carlson JM, Sauder CL, Proudfit GH. Reward dysfunction in major depression: multimodal neuroimaging evidence for refining the melancholic phenotype. Neuroimage. 2014;101:50–58. doi: 10.1016/j.neuroimage.2014.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Hajcak G. Depression and reduced sensitivity to non-rewards versus rewards: evidence from event-related potentials. Biol Psychol. 2009;81:1–8. doi: 10.1016/j.biopsycho.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Foti D, Weinberg A, Dien J, Hajcak G. Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: temporospatial principal components analysis and source localization of the feedback negativity. Hum Brain Mapp. 2011;32:2207–2216. doi: 10.1002/hbm.21182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken IH, van Strien JW, Franzek EJ, van de Wetering BJ. Error-processing deficits in patients with cocaine dependence. Biol Psychol. 2007;75:45–51. doi: 10.1016/j.biopsycho.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Franken IH, Van Strien JW, Nijs IM. Effect of hedonic tone on event-related potential measures of cognitive processing. Psychiatry Res. 2006;142:233–239. doi: 10.1016/j.psychres.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Cottone LA, Jia Z, Maloney T, Volkow ND, Squires NK. The effect of graded monetary reward on cognitive event-related potentials and behavior in young healthy adults. Int J Psychophysiol. 2006;62:272–279. doi: 10.1016/j.ijpsycho.2006.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, Volkow ND. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci. 2009;13:372–380. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Alia-Klein N, Cottone LA, Zhang L, Telang F, Volkow ND. Subjective sensitivity to monetary gradients is associated with frontolimbic activation to reward in cocaine abusers. Drug Alcohol Depend. 2007;87:233–240. doi: 10.1016/j.drugalcdep.2006.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorwood P. Neurobiological mechanisms of anhedonia. Dialogues Clin Neurosci. 2008;10:291–299. doi: 10.31887/DCNS.2008.10.3/pgorwood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossop M, Griffiths P, Powis B, Strang J. Severity of dependence and route of administration of heroin, cocaine and amphetamines. Br J Addict. 1992;87:1527–1536. doi: 10.1111/j.1360-0443.1992.tb02660.x. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET. Value, pleasure and choice in the ventral prefrontal cortex. Trends Cogn Sci. 2011;15:56–67. doi: 10.1016/j.tics.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET, Bilderbeck A. How cognition modulates affective responses to taste and flavor: top-down influences on the orbitofrontal and pregenual cingulate cortices. Cereb Cortex. 2008;18:1549–1559. doi: 10.1093/cercor/bhm185. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF. It’s worse than you thought: the feedback negativity and violations of reward prediction in gambling tasks. Psychophysiology. 2007;44:905–912. doi: 10.1111/j.1469-8986.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- Hare TA, O’Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci. 2008;28:5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PO, Pruessner J, Czechowska Y, Lepage M. Individual differences in trait anhedonia: a structural and functional magnetic resonance imaging study in non-clinical subjects. Mol Psychiatry. 2007;12:703, 767–775. doi: 10.1038/sj.mp.4002021. [DOI] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Hatzigiakoumis DS, Martinotti G, Giannantonio MD, Janiri L. Anhedonia and substance dependence: clinical correlates and treatment options. Front Psychiatry. 2011;2:10. doi: 10.3389/fpsyt.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Schmidt LG, Reischies FM. Anhedonia in schizophrenic, depressed, or alcohol-dependent patients--neurobiological correlates. Pharmacopsychiatry. 1994;27(Suppl 1):7–10. doi: 10.1055/s-2007-1014317. [DOI] [PubMed] [Google Scholar]
- Hendricks EJ, Greenway FL. A study of abrupt phentermine cessation in patients in a weight management program. American Journal of Therapeutics. 2011;18:292–299. doi: 10.1097/MJT.0b013e3181d070d7. [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Watanabe M. Delay activity of orbital and lateral prefrontal neurons of the monkey varying with different rewards. Cereb Cortex. 2000;10:263–271. doi: 10.1093/cercor/10.3.263. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Krigolson OE, Lee S. Reward positivity elicited by predictive cues. Neuroreport. 2011;22:249–252. doi: 10.1097/WNR.0b013e328345441d. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Pakzad-Vaezi KL, Krigolson OE. The feedback correct-related positivity: sensitivity of the event-related brain potential to unexpected positive feedback. Psychophysiology. 2008;45:688–697. doi: 10.1111/j.1469-8986.2008.00668.x. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Janiri L, Martinotti G, Dario T, Reina D, Paparello F, Pozzi G, Addolorato G, Di Giannantonio M, De Risio S. Anhedonia and substance-related symptoms in detoxified substance-dependent subjects: a correlation study. Neuropsychobiology. 2005;52:37–44. doi: 10.1159/000086176. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, McGinnis DE, Alterman AI, Weinrieb RM, D’Angelo L, Epperson LE. Reliability and validity of the Cocaine Selective Severity Assessment. Addict Behav. 1998;23:449–461. doi: 10.1016/s0306-4603(98)00011-2. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58:843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Brightman M, Ameringer KJ, Greenberg J, Mickens L, Ray LA, Sun P, Sussman S. Anhedonia associated with stimulant use and dependence in a population-based sample of American adults. Exp Clin Psychopharmacol. 2010;18:562–569. doi: 10.1037/a0021964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Kahler CW, Ray LA, Sussman S. Relations between anhedonia and smoking motivation. Nicotine Tob Res. 2009;11:1047–1054. doi: 10.1093/ntr/ntp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WH, Wang LZ, Shang HR, Shen Y, Li Z, Cheung EF, Chan RC. The influence of anhedonia on feedback negativity in major depressive disorder. Neuropsychologia. 2014;53:213–220. doi: 10.1016/j.neuropsychologia.2013.11.023. [DOI] [PubMed] [Google Scholar]
- Martinotti G, Cloninger CR, Janiri L. Temperament and character inventory dimensions and anhedonia in detoxified substance-dependent subjects. Am J Drug Alcohol Abuse. 2008a;34:177–183. doi: 10.1080/00952990701877078. [DOI] [PubMed] [Google Scholar]
- Martinotti G, Nicola MD, Reina D, Andreoli S, Foca F, Cunniff A, Tonioni F, Bria P, Janiri L. Alcohol protracted withdrawal syndrome: the role of anhedonia. Subst Use Misuse. 2008b;43:271–284. doi: 10.1080/10826080701202429. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Melis M, Spiga S, Diana M. The dopamine hypothesis of drug addiction: hypodopaminergic state. Int Rev Neurobiol. 2005;63:101–154. doi: 10.1016/S0074-7742(05)63005-X. [DOI] [PubMed] [Google Scholar]
- Morie KP, De Sanctis P, Garavan H, Foxe JJ. Executive dysfunction and reward dysregulation: a high-density electrical mapping study in cocaine abusers. Neuropharmacology. 2014;85:397–407. doi: 10.1016/j.neuropharm.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte G, Hamalainen MS. Partial signal space projection for artefact removal in MEG measurements: a theoretical analysis. Physics in Medicine and Biology. 2001;46:2873–2887. doi: 10.1088/0031-9155/46/11/308. [DOI] [PubMed] [Google Scholar]
- Parvaz MA, Konova AB, Proudfit GH, Dunning JP, Malaker P, Moeller SJ, Maloney T, Alia-Klein N, Goldstein RZ. Impaired neural response to negative prediction errors in cocaine addiction. J Neurosci. 2015;35:1872–1879. doi: 10.1523/JNEUROSCI.2777-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvaz MA, Maloney T, Moeller SJ, Woicik PA, Alia-Klein N, Telang F, Wang GJ, Squires NK, Volkow ND, Goldstein RZ. Sensitivity to monetary reward is most severely compromised in recently abstaining cocaine addicted individuals: a cross-sectional ERP study. Psychiatry Res. 2012;203:75–82. doi: 10.1016/j.pscychresns.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson A, Ragot R, Ripoche A, Lesevre N. Electrophysiological changes elicited by auditory stimuli given a positive or negative value: a study comparing anhedonic with hedonic subjects. Int J Psychophysiol. 1987;5:107–123. doi: 10.1016/0167-8760(87)90015-8. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2005;57:319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfit GH. The reward positivity: from basic research on reward to a biomarker for depression. Psychophysiology. 2015;52:449–459. doi: 10.1111/psyp.12370. [DOI] [PubMed] [Google Scholar]
- Reid MS, Thakkar V. Valproate treatment and cocaine cue reactivity in cocaine dependent individuals. Drug Alcohol Depend. 2009;102:144–150. doi: 10.1016/j.drugalcdep.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer Thomsen K, Whybrow PC, Kringelbach ML. Reconceptualizing anhedonia: novel perspectives on balancing the pleasure networks in the human brain. Front Behav neurosci. 2015;9:49. doi: 10.3389/fnbeh.2015.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Yasuda A, Ohira H, Miyawaki K, Nishikawa M, Kumano H, Kuboki T. Effects of value and reward magnitude on feedback negativity and P300. Neuroreport. 2005;16:407–411. doi: 10.1097/00001756-200503150-00020. [DOI] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995;167:99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- Torres A, Catena A, Candido A, Maldonado A, Megias A, Perales JC. Cocaine dependent individuals and gamblers present different associative learning anomalies in feedback-driven decision making: a behavioral and ERP study. Front Psychol. 2013;4:122. doi: 10.3389/fpsyg.2013.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH. Worth the ‘EEfRT’? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS One. 2009;4:e6598. doi: 10.1371/journal.pone.0006598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem. 2002;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F, Baler R. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. Bioessays. 2010;32:748–755. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollm BA, de Araujo IE, Cowen PJ, Rolls ET, Kringelbach ML, Smith KA, Jezzard P, Heal RJ, Matthews PM. Methamphetamine activates reward circuitry in drug naive human subjects. Neuropsychopharmacology. 2004;29:1715–1722. doi: 10.1038/sj.npp.1300481. [DOI] [PubMed] [Google Scholar]
- Wacker J, Dillon DG, Pizzagalli DA. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. Neuroimage. 2009;46:327–337. doi: 10.1016/j.neuroimage.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Keller MC, Lacey SC, Jonides J. Increased sensitivity in neuroimaging analyses using robust regression. Neuroimage. 2005;26:99–113. doi: 10.1016/j.neuroimage.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. J Abnorm Psychol. 1995a;104:15–25. doi: 10.1037//0021-843x.104.1.15. [DOI] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol. 1995b;104:3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- Watson R, Bakos L, Compton P, Gawin F. Cocaine use and withdrawal: the effect on sleep and mood. Am J Drug Alcohol Abuse. 1992;18:21–28. doi: 10.3109/00952999209001608. [DOI] [PubMed] [Google Scholar]
- Weissman AN. The Dysfunctional Attitude Scale; A Validation Study. University of Michigan; Ann Arbor, MI: 1979. [Google Scholar]
- Whybrow PC. A Mood Apart; The Thinkers Guide to Emotion and its Disorder. Harper Perennial; New York: 1998. [Google Scholar]
- Willner P, Hale AS, Argyropoulos S. Dopaminergic mechanism of antidepressant action in depressed patients. J Affect Disord. 2005;86:37–45. doi: 10.1016/j.jad.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhou X. The P300 and reward valence, magnitude, and expectancy in outcome evaluation. Brain Res. 2009;1286:114–122. doi: 10.1016/j.brainres.2009.06.032. [DOI] [PubMed] [Google Scholar]
- Yeung N, Sanfey AG. Independent coding of reward magnitude and valence in the human brain. J Neurosci. 2004;24:6258–6264. doi: 10.1523/JNEUROSCI.4537-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M, McGlinchey JB, Young D, Chelminski I. Diagnosing major depressive disorder: II: is there justification for compound symptom criteria? J Nerv Ment Dis. 2006;194:235–240. doi: 10.1097/01.nmd.0000207423.36765.89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.