Abstract
Telomeres progressively shorten throughout life. A hallmark of advanced malignancies is the ability for continuous cell divisions that almost universally correlates with the stabilization of telomere length by the reactivation of telomerase. The repression of telomerase and shorter telomeres in humans may have evolved in part as an anti-cancer protection mechanism. While there is still much we do not understand about the regulation of telomerase, it remains a very attractive and novel target for cancer therapeutics. This review focuses on the current state of advances in the telomerase area, identifies outstanding questions, and addresses areas and methods that need refinement.
Keywords: TERT promoter mutations, Peto’s paradox, telomerase inhibitors
Introduction
Historical Background
Telomere terminal transferase (telomerase) enzyme activity (not the identification of the genes involved in telomerase) was discovered in 1985 in the single cell organism, Tetrahymena (1). Almost a decade later telomerase was described as an almost universal marker in advanced human cancers (2, 3), but it was not until 1997 that the catalytic protein component was isolated first in yeast (4) and shortly thereafter in humans (5, 6). It is well recognized that telomeres progressively shorten with increased age in vitro and in vivo, (7–14) and, in combination with a series of oncogenic changes, cells with short telomeres escape senescence and become immortal (Figure 1), generally by activating or upregulating telomerase. Most human tumors (85–90%) not only constitutively express telomerase (2) but also have short telomeres, whereas telomerase activity is absent in most normal tissues or is highly regulated in normal transit amplifying stem-like cells, making the inhibition of telomerase an attractive target for cancer therapeutics (2).
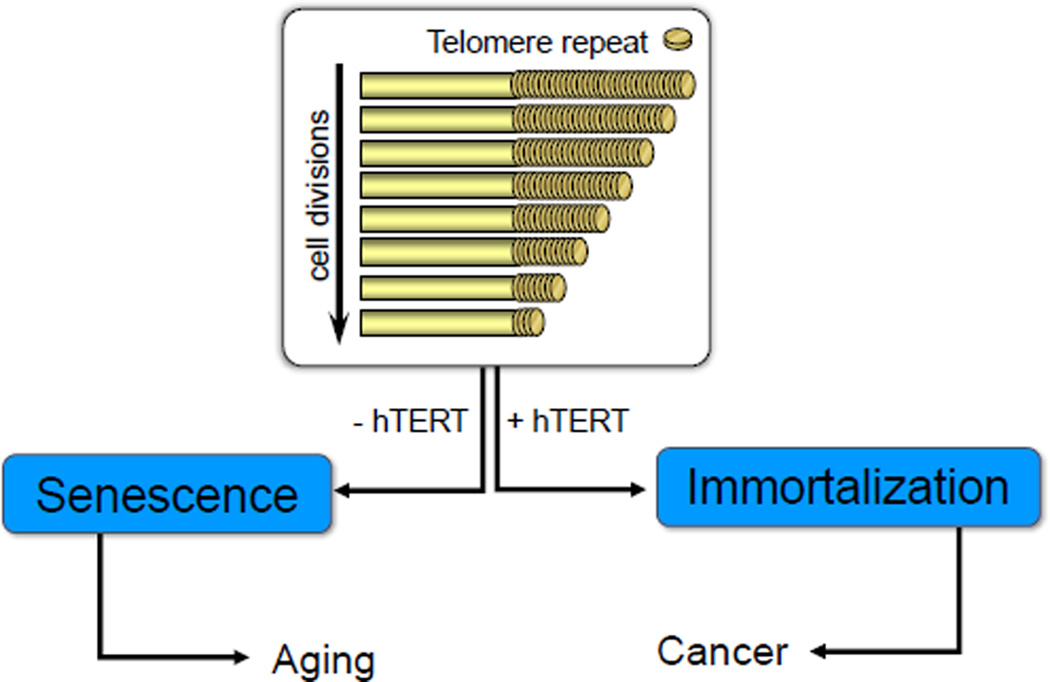
Figure 1.
All somatic normal human cells display progressive telomere shortening with increased cell divisions. In the absence of a mechanism to maintain telomeres, cells eventually undergo replicative senescence (aging). Ectopically expressing just the catalytic subunit (TERT) of the telomerase holoenzyme complex is sufficient to maintain telomere length and immortalize normal cells. While normal cells with or without telomerase activity are not transformed, in the background of additional oncogenic changes, normal cells not only upregulate or reactivate telomerase but can become fully malignant.
Telomerase is a cellular reverse transcriptase (molecular motor) that adds new DNA onto the telomeres that are located at the ends of chromosomes (1, 15–17). While the importance of telomeres has been recognized for a long time (18–19), the DNA sequence of telomeres was somewhat more recent (20–21). Telomeres in mammals consist of long tracts of the hexameric TTAGGG nucleotide repeat and an associated protein complex, termed shelterin (22–23). The shelterin complex protects chromosomes from end-to-end fusions and degradation by forming special t-loop like structures (24), thus masking the very ends of chromosomes from being recognized as double-strand DNA breaks. The TTAGGG repeats shorten with each cell division due to the “end replication problem” (25, 26), oxidative damage, and other still poorly understood end-processing events. When a few telomeres become critically shortened there is a growth arrest state, at which time a DNA damage signal and cellular senescence is normally triggered (27–29). In the absence of other changes, cells can remain in a quiescent/senescent state for years which can be considered a tumor suppressor mechanism at least for long-lived species such as humans. It is a common misconception that normal senescent cells undergo apoptosis and die. It is now recognized that senescent cells can secrete factors that can influence age-associated diseases (30) and remain viable but not dividing for long periods of time. Thus, with increased age it is believed that there is a gradual accumulation of senescent cells that may affect some aspects of aging.
In contrast, human carcinomas (tumors derived from epithelial tissues) almost universally bypass cellular senescence and DNA damage-induced inhibitory signaling pathways by up-regulating telomerase. Regulated telomerase activity is present in a subset of normal transit amplifying stem-like cells but upon differentiation telomerase is again silenced. However, some transit amplifying cells may accumulate oncogenic changes, become tumorigenic, and express telomerase. In human cells the bypass or escape from senescence can be experimentally demonstrated by abrogating important cell cycle checkpoint genes [such as p53 (TP53), p21 (CDKN1A), p16INK4a (CDKN2A) and pRb (RB1)], leading to increased numbers of cell divisions of potentially initiated premalignant cells (31–34). Eventually cells enter a state termed “crisis”, which is a period where cell division and death are in balance. In crisis, due to chromosome end fusions, there are chromosome breakage-fusion-bridge events, leading to genomic instability, rearrangements of chromosomes, and eventually activation or upregulation of telomerase and progression to malignant cancers (Figure 2). Telomerase is detected in approximately 85–90% of all malignant tumors (2, 3) making it a highly attractive target for the development of more precision mechanism-based cancer therapeutics. The hope is that such therapies may have minimal or no toxicities on normal telomerase silent cells and perhaps limited effects on telomerase expressing transit amplifying stem cells. However, it has been over two decades since telomerase was recognized as an excellent target for cancer therapy but there are no approved telomerase targeted therapies. In this review, some of the reasons for this lack of progress will be discussed. In addition, while there have been some recent major advances in understanding the structure of telomerase (16–17, 35), there remain a number of critical issues that have not been addressed adequately or have been misinterpreted. To advance the telomere and telomerase field these areas need to be carefully considered.
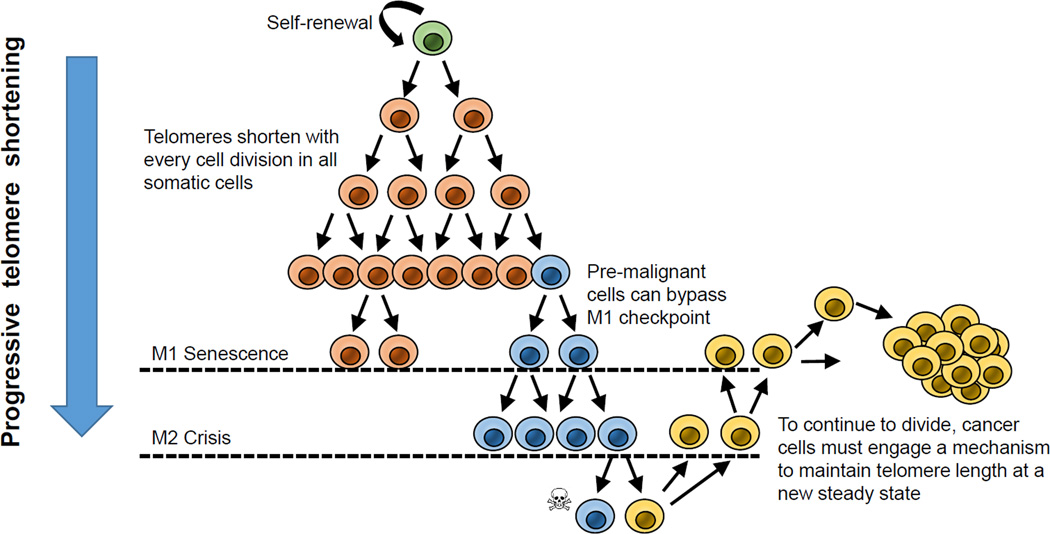
Figure 2.
With increasing cell divisions, telomeres progressively shorten. Even stem cell s that self-renew, there is a gradual shorterning of telomeres. After a finite number of cell doublings, eventually the cells have sufficient short telomeres that they undergo a growth arrest called senescence or the Mortality Stage I (M1). This has also been termed the Hayflick limit. Premalignant cells that have obtained a number of oncogenic changes can bypass M1 and enter into an extended lifespan period. This has been termed the extended lifespan period but vventually these cells also slow down in proliferation and enter a period called crisis. In crisis there is a balance between cell growth and apoptosis and the vast majority of the cell population dies. A rare cell can upregulate telomerase or the much rarer ALT pathway and continue to growth. The hallmark of cells escaping crisis is almost universally, stable but short telomere lengths and telomerase activity.
Areas of Current Studies and Controversy
The following topics and questions in this review will be discussed (Table 1). For other background information the reader is referred to the following recent review (36)
Table 1. Outstanding Questions in the Telomere/Telomerase Field.
What are some of the outstanding questions in the telomere/telomerase field?
|
Cancer and Telomerase
Nearly the complete spectrum of human tumor types has been shown to be telomerase positive (2, 3). In general, malignant tumors are characterized by telomerase expression, correlating with the capacity for unlimited cell proliferation while most benign, premalignant tumors are characterized by the absence of telomerase (3). Somatic mutations in the proximal promoter of the human telomerase reverse transcriptase gene (TERT) is now considered the most common noncoding mutation in cancer. For example, the vast majority of primary melanomas (67–85%), glioblastomas (28–84%), liposarcomas (74–79%), and urothelial cancers (47%) contain TERT promoter mutations, and additional tumors types are being reported almost weekly (37–44). It is not known why some common cancers such as lung, colon, ovarian, esophageal, pancreatic, breast and prostate cancers do not have a high frequency of TERT promoter mutations (42). Generally there are no or a very small percent (<10%) of promoter mutations in these cancer types. While this may change with additional future studies, it may also be the specific constellation of oncogenic changes that predispose cells in the premalignant lesions to TERT promoter mutations. Alternatively, TERT promoter mutations appear to be somewhat more common in tissues that do not have a high rate of cell turnover (self-renewal) (40), but exceptions to this such as carcinomas of the oral cavity already exist (40). While it is believed that these mutations activate telomerase activity (by converting conserved regions to an ETS transcription factor binding site) to permit the continuous cell divisions required for advanced cancers, much of the molecular steps remain unknown about the causal relationship of promoter mutations to telomere length maintenance. Previously it was reported that some cancers do not have detectable telomerase activity and these often result in spontaneous cancer remission (45–46). These examples demonstrate that one does not need to have telomerase activity to develop cancer, but a mechanism to maintain telomeres is required for the continuous growth of the advanced tumor (45–47). In almost all human cancers immortalization of emergent cancer cells occurs by the reactivation or up-regulation of telomerase, however, another mechanism can also reverse telomere attrition in order to bypass senescence that is termed ALT (alternative lengthening of telomeres) that involves DNA recombination between telomeres (48). The ALT pathway is not common in carcinomas but does appears in soft tissue sarcomas and some other less common tumor types but at present there are no directed therapies to the ALT pathway (48).
In addition, it requires very little telomerase to maintain the shortest telomeres. Previously it was shown that even 1% of typical advanced cancer levels of telomerase is sufficient to maintain the shortest telomeres (49) and that a short-term (~2 weeks) expression of telomerase in normal cells is sufficient to double the proliferative lifespan of cells (50). These studies indicate that telomerase is recruited to the shortest telomeres and very little telomerase enzyme activity is required to maintain these short telomeres. These issues have important implications for the development of telomerase therapeutics (discussed in a later section). Thus, one possibility is that high levels of telomerase and significant elongation of telomeres may not be required for the sustained growth of emerging malignant cells. Indeed, almost all malignant tumors have very short telomeres. One could speculate that if telomerase was expressed at very high levels, then telomeres might elongate greatly and this could have detrimental consequences. Alternatively, if telomerase was not activated sufficiently, then telomeres would continue to shorten with continuing cell divisions and the cells would eventually stop dividing. Thus, there is unlikely to be a selective advantage to have more than sufficient telomerase to work on a very small number of the shortest telomeres. In addition, there may be other mechanisms to activate telomerase such as genomic amplifications, rearrangements (51) or alterations in TERT splicing (52). Finally, there is the possibility that the TERT gene may have functions independent of maintaining telomeres.
Recently it was shown that active chromatin marks in cells with TERT promoter mutations correlate with TERT expression (44). It was reported that mutant TERT promoters exhibit the H3K4me2/3 mark of active chromatin and recruit the GABPA/B1 transcription factor, while the wild-type TERT allele retains the H3K27me3 mark of epigenetic silencing and do not recruit the GABPA/B1 transcription factor. Interestingly, TERT promoter mutations in telomerase expressing normal human embryonic stem cells (hESC) only modestly increase telomerase activity (39). While wild type hESCs silence telomerase activity when induced to differentiate, telomerase remains active in hESCs with TERT promoter mutations under differentiation conditions (39). Thus, monoallelic TERT promoter mutations must provide a selective advantage in specific tumor types such as glioblastomas, urothelial carcinomas and melanomas possibly by retaining an active chromatin state (44), to perhaps bypass telomere-based senescence permitting extra cell divisions for other oncogenic changes to occur. In contrast, many common solid tumor types do not have frequent TERT promoter mutations and very little is presently known why there is such large variations in frequencies of promoter mutations or if TERT promoter mutations are sufficient for the formation of tumors. Most, but not all, carcinomas undergo dramatic telomere shortening prior to telomerase activation, so one possibility is that the greatly shortened telomeres also change the chromatin state in the TERT promoter (which is about 1.2 Mb from the 5p telomere) in cancers that do not contain TERT promoter mutations. There still remain many fundamental questions that are unresolved about telomerase in cancer.
Are TERT promoter mutations sufficient for cell immortality in normal human cells that are silenced for telomerase activity (e.g. normal fibroblasts)?
What is the basis of tissue specificity for TERT promoter mutations?
At what stage of cancer development do TERT promoter mutations activate telomerase? Does it depend on telomere length, rate of cell turnover, or other genomic rearrangements at the time of telomerase activation?
Role of telomerase in malignant transformation
Almost all pre-neoplastic lesions have critically shortened telomeres and this may be an initial protective mechanism limiting the maximum number of divisions human cells can undergo. Thus, a short telomere senescence-based mechanism would be a potent initial tumor suppressor mechanism since a large number of genetic and epigenetic alterations are required for a normal cell to become malignant. One can imagine however, limiting the maximal number of cellular divisions in human cells would eventually result in a pre-neoplastic proliferative growth arrest state referred to as replicative aging or senescence (Figure 3). Thus, senescence may have evolved as an anti-cancer molecular mechanism in large long-lived mammals to avoid cancer at an early age (53–54). In cells that acquire a series of oncogenic changes, replicative senescence can be bypassed and eventually cells enter a state known as crisis (31,55). In crisis telomeres are so short that end-end chromosome fusions occur followed by bridge-breakage-fusion cycles and then rarely in humans (55) a cell engages a mechanism to escape from crisis. The molecular mechanisms to bypass crisis are not well understood and in some instances a DNA recombination mechanism is engaged instead of telomerase (48, 56). In addition, it is likely that what is often being called replicative (telomere-based) senescence is in fact a DNA damage response that may not be due to terminally shortened telomeres but perhaps inadequate cell culture conditions (57).
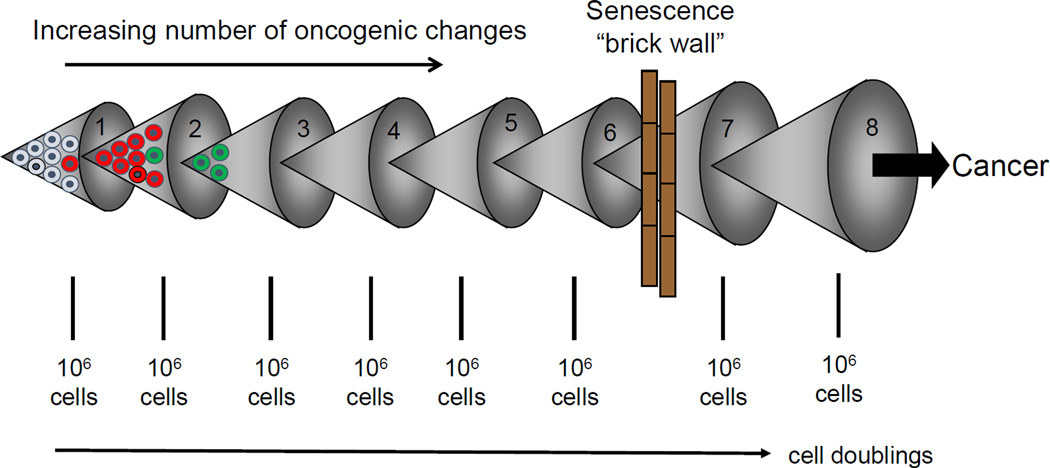
Figure 3.
If one assume spontaneous mutations can occur approximately each 20 cell divisions (about 1 million cells), and assuming that mutations provide a premalignant cell with a slight growth advantage, then after 60–100 doubling (at least in cell culture conditions) the cells would contain some very short telomeres that are uncapped and initiate DNA damage signaling. This is a potential potent initial anti-cancer senescence “brick wall” that protect large long-lived species such as humans from the early onset on cancer. It is now believed that it requires 8–15 key oncogenic changes for a normal cell to become a cancer cell, so senescence could have evolved in humans to prevent most cancer until later in life. Eventually, however, senescence can be bypassed and this can lead to telomerase activation and cancer progression.
Multiple mechanisms have been proposed for engaging telomerase activity. These include mutations/deletions in the TERT promoter (36–44), engagement of TERT alternative splicing (58–59), TERT gene amplification (60), and epigenetic changes (44). Another possibility is that the human TERT gene may autoregulate itself since it is located very close to the telomere end of chromosome 5 (61). In most large long-lived species TERT is also close to a telomere but in small short-lived species such as mice TERT is not located near a telomere. Telomerase by necessity would have to be carefully regulated in large long-lived species to avoid the early onset of cancer while in smaller mammals, such as mice, telomerase is known to be more promiscuous and most inbred strains of mice have very long telomeres compared to humans but the reasons for this are not well understood. One could speculate that the TERT gene being located near a telomere in large and long-lived species may have been selected for over evolutionary time to regulate telomerase and thus the maximal telomere length permitted during human development (62). It is known that telomerase is active during early human fetal development, then becomes silenced in most tissues at approximately 3–4 months gestation (62). Thus, when telomeres reach a certain initial length (~15–20 kb) during human development, three-dimensional chromatin structures involving telomere position effects over long distances (TPE-OLD) (63–64) may silence the TERT gene. As part of cancer progression, as telomeres shorten the chromatin silencing effects may become relaxed resulting in a permissive environment for telomerase promoter mutations and telomerase reactivation (Figure 4). This is consistent with the observation that almost 70% of all cancers are in the 65 and older segment of the population. TPE-OLD (long-distance chromatin loops involving telomeres) has now been demonstrated for several genes including interferon stimulating gene 15 (ISG15), desmoplakin, complement component 1s subcomplement (C1S) and several genes thought to be important in the human disease facioscapulohmeral dystrophy (FSHD) (63–64). Thus it is entirely possible that TERT is regulated at multiple levels including long-distance telomere looping and chromatin modifications.
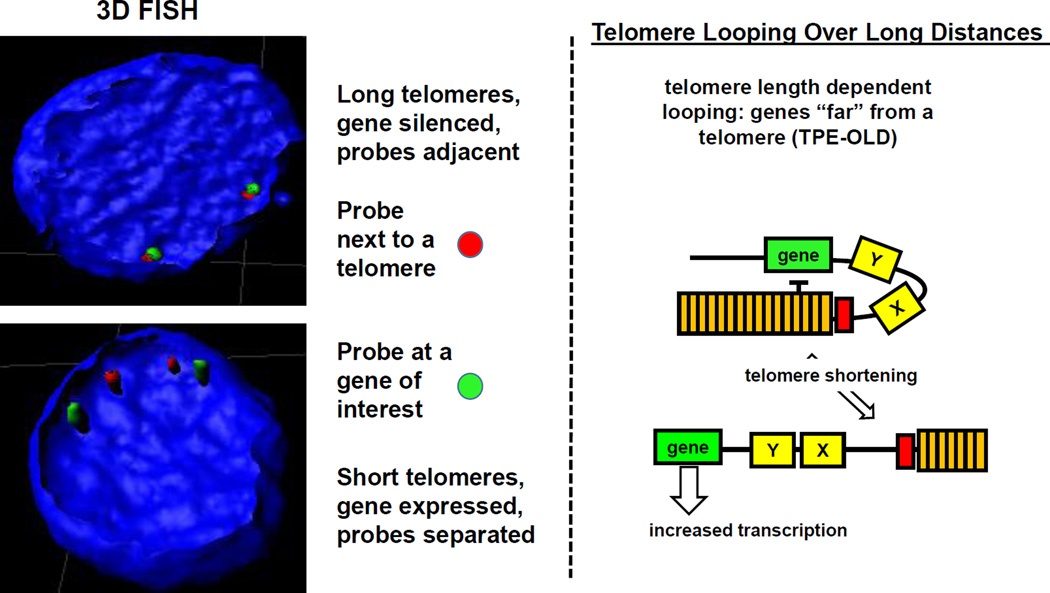
Figure 4.
Recent evidence suggests telomere length can regulate genes over long distances. There are genes several megabases from a telomere that are silenced in young cells, expressed in old cells and repressed again when TERT is introduced into old cells. Using 3D FISH with a subtelomeric probe and a distal gene of interest, one can observe adjacent probe signals in young cells and separated signals in old cells with short telomeres. This model provide an explanation for how gene expression changes can occur during aging without initiating a DNA damage signal. As an example, the model (right side) shows a schematic of how telomeres when long could repress the expression of a specific gene over long distances and when telomeres shorten as part of normal aging, expression of that specific gene could change. Genes (X and Y in the illustration) although closer to the telomere are not regulated by this mechanism (TPE-OLD). Previously it has been shown that ISG15, desmoplaskin, C1S, and SORBS2 are regulated by this mechanism (62, 63).
While introduction and expression of telomerase has been shown to immortalize cells (65), it does not by itself induce a transformed phenotype (66). In human fibroblasts, many factors are required to experimentally transform telomerase positive cells. Normal cells only expressing ectopically introduced hTERT exhibit normal cell cycle activities, maintain contact inhibition, anchorage dependent growth requirements, and maintain a normal karyotype (66).
Thus, there is a diverse system of cellular mechanisms in place to suppress the early development of neoplastic cells in humans. One could further postulate that the multiplicity of these anti-cancer defenses explains the relative rarity of adult human cancers in the first four decades of life. Given that human cancer incidence increases with age, older individuals, whose telomeres in somatic cells are shorter than in younger ones, should have an increased propensity to major cancers. While this is correlative, and certainly does not prove a cause and effect relationship, these findings suggest that individuals with inherently short telomeres should be at increased risk for cancer. It is widely believed that short telomeres in combination with other oncogenic changes leads to genomic instability, which is typically observed in most human cancers. However, recent studies have shown that in the general population individuals with inherently long telomeres are also at a higher risk for major cancers (67–71). How do we explain this apparent paradox?
Peto’s Paradox: Why do most large long-lived species not get cancer at a higher frequency compared to small short-lived species?
It is well established that most large mammals also have a more cells and generally longer lifespans that require more cell replications, which theoretically should increase the mutational burden and augment cancer risk. However, Peto pointed out that cancer risk does not always scale with size (72). Large, long-living mammals show no increase in cancer risk compared to small, short-lived ones. Known as Peto’s paradox (73–74), these findings suggest a role of evolutionary forces, part of which might be mediated through telomere biology. Large, long-living mammals typically repress telomerase in somatic tissues and have short telomeres compared to small, short lifespan mammals (e.g. telomerase activity inversely correlates with body mass not necessarily lifespan) (75–76). Repressed telomerase and short telomeres would thus diminish the maximal number of replication-mediated mutations that would occur prior to engaging telomere-based senescence. It was reported that short telomere length correlated with increased lifespan and that telomerase repression correlated with increased body size (mass) in over 50 mammalian species covering most of the mammalian radiation (75). This paradigm has led to the concept of evolutionary tradeoffs. Cancer resistance due to repressed telomerase and short telomeres might limit regenerative capacity, thus increasing the likelihood of age-dependent degenerative diseases, particularly as animals get older and their telomeres undergo further shortening. However, there are exceptions such as the small long-lived mole rat (77) which show increases in tumor suppressor p15/p16 variants, decreased inflammation, and increases in high–molecular-mass hyaluronan, perhaps influencing cell adhesion. In contrast to somewhat rare exceptions, the concept of shorter inherited telomere length being an anti-cancer protection mechanism has been experimentally tested in a large series of mammals and remains a viable explanation (75).
Thus, the overarching question is how do large mammals reduce their risk of cancer? In two recent papers on the elephant (78) and the bowhead whale (79) there are emerging findings that mechanisms to reduce cancer risk in large mammals may have evolved. For example, in the African and Asian elephant approximately 20 TP53-related sequences (p53) are detected by DNA sequencing (78), and while some of these may be pseudogenes, others produce functional protein. Thus, cancer-free longevity in the elephant may be due to acquiring extra copies of functional ancestral TP53 (78). While p53 protein is generally thought of as a tumor suppressor pathway, it is more difficult to understand in evolutionary terms how these extra copies could have been selected for to protect against cancer. TP53 is also a cell stressor responsive gene and this could possibly explain the evolutionary acquisition of extra copies of TP53. One possibility that was recently demonstrated (80) is that an ancestral function of wild type p53, but not mutant p53, is to restrain retrotransposon mobility and thus extra copies of wild type p53 could serve as a tumor suppressor mechanism by reducing transposable elements from moving around in the normal genome. In contrast, the bowhead whale, which lives almost 200 years and is believed to be the longest-living mammal, has ~1000 times more cells compared to humans. Similar to elephants, whales are rarely found to develop cancer. The bowhead whale genome was also recently sequenced and the investigators proposed that increased copies or variants in DNA damage repair genes (mutations in ERCC1 and PCNA and FEN1 duplications) may account for cancer-free longevity in whales (79).
Man versus mouse cancer paradox
If whales and elephants have evolved anti-cancer protection mechanisms what occurs in humans? An average human weighs about 60–80kg and lives about 75–80 years compared to inbred strains of mice that weighs about 20–25 grams and live approximately 2–3 years. Yet humans and mice get about the same incidence of cancer. For this to make sense, humans would have to be at least 100,000 times more resistant to cancer compared to mice (53). Perhaps humans have better DNA repair mechanisms or perhaps inbred strains of mice are inappropriate to compare to wild type mice. In addition, inbred strains of mice have probably been inadvertently selected for fast growth, big litter sizes and rapid maturation which may have discarded slow-aging genes including anti-cancer genes. Indeed, wild type mice in captivity have been shown to live longer compared to inbred strains. Many wild type mouse strains also have somewhat shorter telomeres compared to inbred strains, which generally have very long telomeres. Finally, if one deletes TERT or TERC (functional RNA template component of telomerase) from inbred strains of mice (81), telomeres do progressively shorten and in later generations mice develop aging phenotypes (stem cell dysfunction, cardiomyopathies, insulin resistance, diminished stress responses and only a modest increase in cancer) similar to humans (81). Thus, inbred strains of mice in a normal lifespan probably do not use telomere-based replicative aging as an anti-cancer protection mechanism (53). While this large difference in protection from cancer may be true when comparing inbred mice to humans, it is not true for humans when compared to elephants and whales. Thus, while humans may have evolutionarily evolved more efficient DNA repair or other mechanisms to reduce cancer incidence, humans still appear to be less protected from developing cancer when compared to other large long-lived mammalian species.
Why are humans more susceptible to cancer compared to elephants and whales?
So one could ask, why are humans are especially vulnerable to cancer? While some anti-cancer mechanisms may have evolved in evolutionary terms such as dark pigmented skin to protect against UVB-induced cancers, humans in the modern era get a reasonably large tumor incidence [some estimate close to 50% in more developed, Western societies (82–83)]. One explanation is that humans historically died in childbirth, of accidents, infectious diseases and/or starvation and never had the evolutionary pressures to develop even better anti-cancer protection mechanisms. With the improvement in sanitation, the development of vaccines and antibiotics, safer working environments, and improved medicines and surgical procedures, humans have essentially doubled their average lifespan in the last 150 years. In addition, humans have also dramatically changed their lifestyles from our ancestral hunter gatherer, low fat and active environment, to a more sedentary, high fat, smoking, sun exposed, polluted environment. Some have estimated that the vast majority of human cancers are indeed associated with lifestyle factors that do not occur in other large long-lived mammals (83). Thus, since humans are living longer and most cancers occur in the 65 year-old and older segment of the population (e.g. post reproduction), evolutionary adaptations have yet to occur in humans to the extent they have occurred in elephants and whales even though humans have shorter telomeres and repress telomerase in somatic tissues similarly to elephants and whales. Since humans now live in a vastly different environment, it is possible that inflammatory responses are driving human cells past senescence into an extended lifespan phase so cells have additional divisions to engage additional oncogenic changes. When cells then enter crisis, in combination with other genetic and epigenetic changes, instead of engaging senescence, cells develop genomic instability and an increased risk of cancer and activation of telomerase. One way to think about this is that the rapid lifespan increases has most likely put most humans out of balance with evolution.
Are the commonly used methods for measuring telomerase and telomere length being interpreted correctly?
While it is well established that the vast majority of human tumors express telomerase activity, assays for measuring this activity are varied making comparisons between studies difficult. Telomerase can be assayed using a variety of methods, some more reliable and reproducible than others. For example, the TRAP protocol, which uses PCR to amplify the extension products of the telomerase enzyme is quite sensitive and can detect as few as 0.01% positive cells (2, 49). Recently, more quantitative telomerase assays using droplet digital PCR (ddPCR) have been described (84) and ddTRAP can potentially provide more exact numbers of molecules of telomerase per cell instead of semi-quantitative information using other methods. Indeed, the standard TRAP assay can vary widely in semi-quantitating telomerase activity levels in tumor specimens so most studies indicating that telomerase activity levels are prognostic indicators of outcome may be suspect. Many investigators also use mRNA for TERT as a surrogate for telomerase enzyme activity but since there is now evidence that mRNA for TERT does exist in normal cells, caution is needed in using indirect methods for assuming enzyme activity.
There are also many methods to measure telomere length including TRF (terminal restriction fragment) analysis (6), in situ Q-FISH (85–86), Flow FISH (87), Q-PCR (88), chromosome specific single telomere length analysis (STELA) (89), and Universal STELA (90–91). In addition, there is now whole genome sequencing (TelSeq) to estimate average or mean telomere length that is quantitative but somewhat still expensive compared to other methods (92). All these methods for measuring telomere lengths have their strengths and limitations. For example, depending on the number of restriction enzymes used for the TRF Southern blot analysis one gets very different ranges of average telomeres sizes and no standardizations in the field exist. Perhaps the most popular and widely used method for determine average telomere length is the Q-PCR method since it is quite easy to conduct and provides an average telomere length compared to a single copy gene (88). The problem in using this technique in cancer cells, as opposed to normal diploid cells, is the global aneuploidy that exists in cancers raising the very real possibility that the single copy reference gene may not be accurate and almost nothing is mentioned about this in published studies.
Perhaps even more importantly, it is not certain what average telomere length actually means when it is well established that the shortest telomeres lead to senescence and genomic instability (93). While in situ Q-FISH and Flow-FISH can provide information about the shorter telomere lengths in normal and tumor cells, both methods rely on probe hybridization kinetics to the telomeres which may not hybridize to the very shortest telomeres. For example, signal-free ends using in situ telomere Q-FISH does not mean these chromosome ends do not have telomeric repeats. Thus, quantitation of the very shortest telomeres require more sensitive assays. Both single chromosome and universal STELA are methods to identify the percent of telomeres that are the very shortest (e.g. less than 1–2 Kb). Single chromosome STELA is perhaps less useful in cancer since there is a great variation and losses in chromosome numbers. Universal STELA (90–91) has recently emerged to measure the shortest telomeres on all chromosomes, but neither single cell nor universal STELA are high throughput methods, so large scale studies would be more difficult. Issues to consider when conducting telomere testing for disease susceptibility and aging are provided in Table 2.
Table 2. Telomere Testing Considerations.
What are the strengths and weaknesses of the different types of telomere length tests?
|
Targeting telomerase: therapeutic potential
While there have been several comprehensive reviews on the approaches being considered to inhibit telomerase in cancer (94–99), there have yet to be any approved anti-telomerase therapies approved for any indication. This is certainly not from lack of trying and some approaches have recently led to Phase 2 clinical trials (100–102). Telomerase inhibitors remain an attractive approach to targeting cancer cells, largely because of the specificity of the activity in tumor cells. However, a key to understanding the role for this class of agents is that the inhibitory effects are only apparent after the cancer cells shorten their telomeres sufficiently through continued proliferation to cause them to enter crisis and die. Therefore, time to effectiveness in halting tumor growth is theoretically dependent on the original length of the telomeres in the cancer cells. Because the cancer cells will continue to proliferate before signals to initiate growth arrest or die is “sensed” by the cell, they are less likely to be as effective in first-line therapy but more likely to play a supportive role to control residual disease (maintenance therapy) after initial control is accomplished through conventional surgery, radiotherapy, general chemotherapy, and even targeted therapy. In addition, since some hematopoietic proliferative cells have regulated telomerase, toxicities have been observed (100–102) such as thrombocytopenia (e.g. low platelet counts). These toxicities require patients going off the telomerase inhibitor and very quickly the telomeres regain their length.
Indeed, the thrombocytopenia side effects of one therapy, imetelstat, has now been re-purposed to treat patients with essential thrombocythemia (101) and myelofibrosis (102) with excellent initial results, even though there are still many side effects. Importantly, the impressive response rates may be non-specific since there were no changes in telomere lengths over the course of the treatments and initial telomere lengths did not predict clinical responses. Alternatively, imetelstat may block terminal maturation in megakaryocyte precursors by inhibiting telomerase. A new approach to targeting telomerase expressing cancer cells is to develop telomerase-mediated, telomere uncapping compounds (103). This would have the advantage of rapidly shrinking tumor size but largely not affecting telomerase silent normal cells. While there is still the possibility of some side effects with this approach, it does avoid the long lag period from initiation of therapy to tumor shrinkage.
In summary, telomerase activity is detected in the vast majority of human cancers. The bottleneck at present is that additional validation studies and clinical trials will be required before knowledge of telomerase activity will be useful in a practical sense for decisions regarding patient management. This remains an area of intense investigation and several additional classes of potential agents have been developed (reviewed in 94–99).
Conclusions
There is mounting evidence that cellular senescence acts as a "cancer brake" because it takes many divisions to accumulate all the changes needed to become a cancer cell. In addition to the accumulation of several mutations in oncogenes and tumor suppressor genes, almost all advanced cancer cells are immortal and have overcome the normal cellular signals that prevent continued cell division. Young normal cells can divide many times; but these cells are not cancer cells since they have not accumulated all the other changes needed to make a cell malignant. In most instances cells become senescent before they can become a cancer cell. Therefore, aging and cancer are two ends of the same spectrum. Inhibition of telomerase in cancer cells may be a viable target for anti-cancer therapeutics while expression of telomerase in normal cells may extend healthy lifespan especially for patients with inherited telomere spectrum disorders (104). This may be particularly important in specific age-related diseases in which increased cell turnover due to the pathologic processes results in replicative senescence and a failure to maintain physiologic function (104). In summary, telomerase and its regulation of telomere length is both an important target for cancer therapy and for the treatment of age-related disease. The telomerase gene will likely have many important applications in the future of medicine and cellular engineering.
Significance.
Despite many recent advances, telomerase remains a challenging target for cancer therapy. There are few telomerase directed therapies and many of the assays used to measure telomeres and telomerase have serious limitations. This review provides an overview of the current state of the field and how recent advances could affect future research and treatment approaches.
Acknowledgments
I would like to thank Abraham Aviv (New Jersey Medical School, Rutgers) and Woodring E. Wright (UT Southwestern) for valuable discussions
Financial Support:
The lab is supported by the National Cancer Institute (Lung SPORE P50CA70907); RO1 AG001228, and a distinguished chair from the Southland Financial Foundation in Geriatrics Research. This work was performed in laboratories constructed with support from National Institute of Health grant C06 RR30414.
Footnotes
Conflict of Interest: Life Length, Inc (Madrid) consultant and scientific advisor; Elizabeth Therapeutics, Inc, Founding Scientist.
References
- 1.Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43(2 Pt 1):405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 2.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266(5193):2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 3.Shay JW, Bacchetti S. A survey of telomerase in human cancer. Eur. J. Cancer. 1997;33:787–791. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 4.Lingner J, Hughes TR, Shevchenko A, Mann M, Lundblad V, Cech TR. Reverse transcriptase motifs in the catalytic subunit of telomerase. Science. 1997;276(5312):561–567. doi: 10.1126/science.276.5312.561. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, et al. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277(5328):955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- 6.Meyerson M, Counter CM, Eastonn EN, Ellisen LW, Steiner P. hEST2, the putative human telomerase catalytic subunit gene, is upregulated in tumor cells and during immortalization. Cell. 1997;90(4):785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- 7.Harley CB, Futcher BA, Greider CW. Telomeres shorten during aging of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 8.Hastie ND, Dempster M, Dunlop MG, Thompson AM, Green DK, Allshire RC. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990;346:866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- 9.Lindsey J, McGill NI, Lindsey LA, Green DK, Cooke HJ. In vivo loss of telomeric repeats with age in humans. Mut Res. 1991;256:45–48. doi: 10.1016/0921-8734(91)90032-7. [DOI] [PubMed] [Google Scholar]
- 10.de Lange T, Shiue L, Myers RM, Cox DR, Naylor SL, Killery AM, et al. Structure and variability of human chromosome ends. Mol Cell Biol. 1990;10(2):518–527. doi: 10.1128/mcb.10.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shay JW, Wright WE. The reactivation of telomerase activity in cancer progression. Trends Genet. 1996;12:129–131. doi: 10.1016/0168-9525(96)30018-8. [DOI] [PubMed] [Google Scholar]
- 12.Kim N-W, Harley CB, Prowse KR, Weinrich SL, Piatyszek MA, Wright WE, et al. Telomeres, telomerase and cancer. Science. 1995;268:1115–1117. doi: 10.1126/science.268.5214.1116. [DOI] [PubMed] [Google Scholar]
- 13.Wright WE, Pereira-Smith OM, Shay JW. Reversible cellular senescence: A two-stage model for the immortalization of normal human diploid fibroblasts. Mol Cell Biol. 1989;9:3088–3092. doi: 10.1128/mcb.9.7.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Counter CM. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992;11:1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blackburn E. Telomerases. Annual Review of Biochemistry. 1992;61:113–129. doi: 10.1146/annurev.bi.61.070192.000553. [DOI] [PubMed] [Google Scholar]
- 16.Jiang J, Chan H, Cash DD, Miracco EJ, Ogorzalek RR, Loo HE, et al. Structure of Tetrahymena telomerase reveals previously unknown subunits, functions and interactions. Science. 2015;350(6260):529–534. doi: 10.1126/science.aab4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu RA, Dagdas YS, Yilmaz ST, Yildiz A, Collins K. Single-molecule imaging of telomerase reverse transcriptase in human telomerase holoenzyme and minimal RNP complexes. eLife. 2015;4:e08363. doi: 10.7554/eLife.08363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller HJ. Studies of genetics: The selected papers of HJ Muller. Bloomington: Indiana University Press; 1962. The remaking of chromosomes; pp. 384–408. [Google Scholar]
- 19.McClintock B. The stability of broken ends of chromosomes in Zea mays. Genetics. 1941;26:234–282. doi: 10.1093/genetics/26.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blackburn EH, Gall JG. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J Mol Biol. 1978;120:33–53. doi: 10.1016/0022-2836(78)90294-2. [DOI] [PubMed] [Google Scholar]
- 21.Moyzis RK, Buckingham JM, Cram LS, Dani M, Deaven LL, Jones MD, et al. A highly conserved repetitive DNA sequence (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci USA. 1988;85:6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19(18):2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 23.Palm W, de Lange T. How shelterin protects mammalian telomeres. Ann Rev Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 24.Griffith JD, Comeau L, Rosenfield S, Standel RM, Bianchi A, Moss H, et al. Mammalian telomeres end in a large duplex loop. Cell. 1999;97:503–514. doi: 10.1016/s0092-8674(00)80760-6. [DOI] [PubMed] [Google Scholar]
- 25.Watson JD. Origin of concatemeric T7 DNA. Nature, New Biol. 1972;239:197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- 26.Olovnikov AM. A theory of margintomy: The incomplete copying of template margin in enzymes synthesis of polynucleotides and biological significance of the problem. J Theoret Biol. 1973;41:181–190. doi: 10.1016/0022-5193(73)90198-7. [DOI] [PubMed] [Google Scholar]
- 27.Zou Y, Sfeir A, Gryaznov SM, Shay JW, Wright WE. Does a sentinel or groups of short telomere determine replicative senescence? Mol Biol Cell. 2004;15:3709–3718. doi: 10.1091/mbc.E04-03-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fumagalli M, Rossiello F, Clerici M, Barozzi S, Cittaro D, Kaplunov JM, et al. DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat Cell Bio. 2012;14(4):355–365. doi: 10.1038/ncb2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Von Zglinicki T, Saretzki G, Ladhoff J, d'Adda di Fagagna F, Jackson SP. Human cell senescence as a DNA damage responses. Mechan Ageing Devel. 2005;26(1):111–117. doi: 10.1016/j.mad.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 30.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966–972. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shay JW, Wright WE. Historical claims and current interpretations of replicative aging. Nature Biotech. 2002;20:682–688. doi: 10.1038/nbt0702-682. [DOI] [PubMed] [Google Scholar]
- 32.Wright WE, Shay JW. The two-stage mechanism controlling cellular senescence and immortalization. Exp Gerontol. 1992;27:383–389. doi: 10.1016/0531-5565(92)90069-c. [DOI] [PubMed] [Google Scholar]
- 33.Shay JW, Wright WE, Werbin H. Defining the molecular mechanism of human cell immortalization. Biochim Biophys Acta. 1991;1072:1–7. doi: 10.1016/0304-419x(91)90003-4. [DOI] [PubMed] [Google Scholar]
- 34.Harley CB. Telomere loss: mitotic clock or genetic time bomb? Mut Res. 1991;256:271–282. doi: 10.1016/0921-8734(91)90018-7. [DOI] [PubMed] [Google Scholar]
- 35.Sandin S, Rhodes D. Telomerase Structure. Curr Opin Struct Biol. 2014;25:104–110. doi: 10.1016/j.sbi.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blackburn EH, Epel ES, Lin J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science. 2015;350:1193–1198. doi: 10.1126/science.aab3389. [DOI] [PubMed] [Google Scholar]
- 37.Bojesen SE, Pooley KA, Johnatty SE, Beesley J, Michailidou K, Tyrer JP, et al. Multiple independent variants at the TERT locus are associated with telomere length and risks of breast and ovarian cancer. Nat Genet. 2013;45:371–384. doi: 10.1038/ng.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heidenreich B, Rachakonda PS, Hemminki K, Kumar R. TERT promoter mutations in cancer development. Current Opin Genet Develop. 2014;24:30–37. doi: 10.1016/j.gde.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957–959. doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiba K, Johnson JZ, Vogan JM, Wagner T, Boyle JM, Hockemeyer D. Cancer-associated TERT promoter mutations abrogate telomerase silencing. eLife. 2015;4:e07918. doi: 10.7554/eLife.07918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, Diaz LA, Jr, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci USA. 2013;110:6021–6026. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vinagre J, Almeida A, Pópulo H, Batista R, Lyra J, Pinto V, et al. Frequency of TERT promoter mutations in human cancers. Nature Comm. 2013;4:2185. doi: 10.1038/ncomms3185. [DOI] [PubMed] [Google Scholar]
- 43.Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339:959–961. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 44.Stern JL, Theodorescu D, Vogelstein B, Papadopoulos N, Cech TR. Mutation of the TERT promoter switch to active chromatin, and monoallelic TERT expression in multiple cancers. Genes Dev. 2015;29:1–6. doi: 10.1101/gad.269498.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hiyama E, Hiyama K, Yokoyama T, Matsuura Y, Piatyszek MA, Shay JW. Correlating telomerase activity levels with human neuroblastoma outcomes. Nature Med. 1995;1:249–257. doi: 10.1038/nm0395-249. [DOI] [PubMed] [Google Scholar]
- 46.Tabori U, Vukovic B, Zielenska M, Hawkins C, Braude I, Rutka J, et al. The role of telomere maintenance in the spontaneous growth arrest of pediatric low-grade gliomas. Neoplasia. 2006;8(2):136–142. doi: 10.1593/neo.05715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hiyama E, Hiyman K, Ohtsu K, Yamaoka H, Ichikawa T, Shay JW, Yokoyama T. Telomerase activity in neuroblastoma: Is it a prognostic indicator of clinical behavior? Eur. J. Cancer. 1997;33:1932–1936. doi: 10.1016/s0959-8049(97)00226-8. [DOI] [PubMed] [Google Scholar]
- 48.Shay JW, Reddel RR, Wright WE. Cancer and telomerase: An ALTernative to telomerase. Science. 2012;336:1388–1390. doi: 10.1126/science.1222394. [DOI] [PubMed] [Google Scholar]
- 49.Ouellette MM, Liao M, Herbert B-S, Johnson M, Holt SE, Liss HS, et al. Senescent telomere lengths in fibroblasts immortalized by limiting amounts of telomerase. J. Biol. Chem. 2000;275:10072–10076. doi: 10.1074/jbc.275.14.10072. [DOI] [PubMed] [Google Scholar]
- 50.Steinert S, Shay JW, Wright WE. Transient expression of human telomerase extends the lifespan of normal human fibroblasts. Biochem Biophys Res Commun. 2000;273:1095–1098. doi: 10.1006/bbrc.2000.3080. [DOI] [PubMed] [Google Scholar]
- 51.Peifer M, Hertwig F, Roel F, Dreidax D, Gartlgruber M, Menon R, et al. Telomerase activation by genomic rearrangements in high-risk neuroblastoma. Nature. 2015;526(7575):700–704. doi: 10.1038/nature14980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong MS, Wright WE, Shay JW. Alternative splicing regulation of telomerase: a new paradigm? Trends in Genetics. 2015;30(10):430–438. doi: 10.1016/j.tig.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shay JW, Wright WE. When do telomeres matter? Science. 2001;291:839–840. doi: 10.1126/science.1058546. [DOI] [PubMed] [Google Scholar]
- 54.Wright WE, Shay JW. Cellular senescence as a tumor-protection mechanism: the essential role of counting. Curr Opin Genet Develop. 2001;11:98–103. doi: 10.1016/s0959-437x(00)00163-5. [DOI] [PubMed] [Google Scholar]
- 55.Shay JW, Wright WE. Quantitation of the frequency of immortalization of normal diploid fibroblasts by SV40 large T-antigen. Exp Cell Res. 1989;184:109–118. doi: 10.1016/0014-4827(89)90369-8. [DOI] [PubMed] [Google Scholar]
- 56.Cesare AJ, Reddel RR. Alternative lengthening of telomeres: models, mechanisms and implications. Nature Rev Genet. 2010;11:319–330. doi: 10.1038/nrg2763. [DOI] [PubMed] [Google Scholar]
- 57.Ramirez R, Morales CP, Herbert B-S, Rhode JM, Passon C, Shay JW, et al. Putative telomere-independent mechanisms of replicative aging reflect inadequate growth conditions. Genes & Development. 2001;15:398–403. doi: 10.1101/gad.859201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong MS, Chen L, Foster C, Kainthla R, Shay JW, Wright WE. Regulation of hTERT (telomerase) alternative splicing: a new target for chemotherapy. Cell Reports. 2013;3:1028–1035. doi: 10.1016/j.celrep.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wong MS, Shay JW, Wright WE. Regulation of human telomerase splicing by RNA:RNA pairing. Nature Commun. 2014 Feb 28;5:3306. doi: 10.1038/ncomms4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cao Y, Bryan TM, Reddel RR. Increased copy number of the TERT and TERC telomerase subunit genes in cancer cells. Cancer Sci. 2008;99:1092–1099. doi: 10.1111/j.1349-7006.2008.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shay JW, Wright WE. Implications of mapping the human telomerase genes (hTERT) as the most distal gene on chromosome 5p. Neoplasia. 2000;2:195–196. doi: 10.1038/sj.neo.7900093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW. Telomerase activity in human germline and embryonic tissues and cells. Dev. Genet. 1996;18:173–179. doi: 10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 63.Robin JD, Ludlow AT, Chen M, Magdinier F, Batten K, Holohan B, et al. Telomere looping: a new paradigm for the regulation of gene expression with progressive telomere shortening. Genes Dev. 2014;28:2464–2476. doi: 10.1101/gad.251041.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robin JD, Ludlow AT, Batten K, Gaillard M-C, Stadler G, Magdinier F, et al. SORBS2 transcription is activated by telomere position effect-over long distance upon telomere shortening in muscle cells from patients with fasciscapulohumeral dystrophy. Genome Res. 2015;25:1781–1790. doi: 10.1101/gr.190660.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279(5349):349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 66.Morales CP, Holt SE, Ouellette M, Kaur KJ, Wilson KS, White MA, et al. Absence of cancer-associated changes in human fibroblasts immortalized with telomerase. Nature Genetics. 1999;21:115–118. doi: 10.1038/5063. [DOI] [PubMed] [Google Scholar]
- 67.Julin B, Shui I, Heaphy CM, Joshu CE, Meeker AK, Giovannucci E, et al. Circulating leukocyte telomere length and risk of overall and aggressive prostate cancer. Br J Cancer. 2015;112:769–776. doi: 10.1038/bjc.2014.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nan H, Du M, De Vivo I, Manson JE, Liu S, McTiernan A, Curb J, et al. Shorter telomeres associate with a reduced risk of melanoma development. Cancer Res. 2011;71:6758–6763. doi: 10.1158/0008-5472.CAN-11-1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lynch SM, Major JM, Cawthon R, Weinstein SJ, Virtamo J, Lan Q, et al. A prospective analysis of telomere length and pancreatic cancer in the alpha-tocopherol beta-carotene cancer (ATBC) prevention study. Int J Cancer. 2013;133:2672–2680. doi: 10.1002/ijc.28272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qu S, Wen W, Shu XO, Chow WH, Xiang YB, Wu J, et al. Association of leukocyte telomere length with breast cancer risk: nested case-control findings from the Shanghai Women's Health Study. Am J Epidemiol. 2013;177:617–624. doi: 10.1093/aje/kws291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seow WJ, Cawthon RM, Purdue MP, Hu W, Gao YT, Huang WY, et al. Telomere length in white blood cell DNA and lung cancer: a pooled analysis of three prospective cohorts. Cancer Res. 2014;74:4090–4098. doi: 10.1158/0008-5472.CAN-14-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peto R. Quantitative implications of the approximate irrelevance of mammalian body size and lifespan to lifelong cancer risk. Philos Trans R Soc Lond B Biol Sci. 2015;370(1673) doi: 10.1098/rstb.2015.0198. 2015.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schiffman J, Maley CC, Nunney L, Hochberg M, Breen M, editors. Cancer across life: Peto’s paradox and the promise of comparative oncology. Philos Trans R Soc Lond B Biol Sci. (theme issue) 2015;370(1673) doi: 10.1098/rstb.2014.0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caulin AF, Maley CC. Peto’s paradox: evolution’s prescription for cancer prevention. Trends Ecol Evol. 2011;26(4):175–182. doi: 10.1016/j.tree.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gomes NMV, Ryder OA, Houck ML, Charter SJ, Walker W, Forsyth NR, et al. The comparative biology of mammalian telomeres: ancestral states and functional transitions. Aging Cell. 2011;10:761–768. doi: 10.1111/j.1474-9726.2011.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seluanov A, Chen Z, Hine C, Sasahara TH, Ribeiro AA, Catania KC, et al. Telomerase activity coevolves with body mass not lifespan. Aging Cell. 2007;6:45–52. doi: 10.1111/j.1474-9726.2006.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gorbunova V, Seluanov A, Zhang Z, Gladyshev VN, Vijg J. Comparative genetics of longevity and cancer: insights from long-lived rodents. Nat Rev Genet. 2014;15(8):531–540. doi: 10.1038/nrg3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abegglen LM, Caulin AF, Chan A, Lee K, Robinson R, Campbell MS, et al. Potential mechanisms for cancer resistance in elephants and comparative cellular response to DNA damage in humans. JAMA. 2015;314(17):1850–1860. doi: 10.1001/jama.2015.13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Keane M, Semeiks J, Webb AE, Li YI, Quesada V, Craig T, et al. Insights into the evolution of longevity from the bowhead whale genome. Cell Rep. 2015;10(1):112–122. doi: 10.1016/j.celrep.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wylie A, Jones AE, D’Brot A, Lu W-J, Kurtz P, Moran JV, et al. p53 genes function to restrain mobile elements. Genes Dev. 2015;30:1–14. doi: 10.1101/gad.266098.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sahin E, DePinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464:520–528. doi: 10.1038/nature08982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Greaves M. Evolutionary determinants of cancer. Cancer Discov. 2015;5(8):806–820. doi: 10.1158/2159-8290.CD-15-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Greaves M, Ermini L. Evolutionary adaptation to risk of cancer: evidence from cancer resistance in elephants. JAMA. 2015;314(17):1806–1807. doi: 10.1001/jama.2015.13153. [DOI] [PubMed] [Google Scholar]
- 84.Ludlow AT, Robin JD, Sayed M, Litterst CM, Shelton DN, Shay JW, et al. Quantitative telomerase enzyme activity determination using droplet digital PCR with single cell resolution. Nucleic Acids Res. 2014;42:e104. doi: 10.1093/nar/gku439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meeker AK, Gage WR, Hicks JL, Simon I, Coffman JR, Platz EA, et al. Telomere length assessment in human archival tissues: combined telomere fluorescence in situ hybridization and immunostaining. Am J Pathol. 2002;160(4):1259–1268. doi: 10.1016/S0002-9440(10)62553-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Canela A, Vera E, Klatt P, Blasco MA. High-throughput telomere length quantification by FISH and its application to human populations studies. Proc Natl Acd Sci USA. 2006;104(13):5300–5305. doi: 10.1073/pnas.0609367104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rufer N, Dragowska W, Thornbury G, Roosnek E, Lansdorp PM. Telomere length dynamics in human lymphocytes subpopulations measured by flow cytometry. Nature Biotech. 1998;16:743–747. doi: 10.1038/nbt0898-743. [DOI] [PubMed] [Google Scholar]
- 88.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baird DM, Rowson J, Wynford-Thomas D, Kipling D. Extensive allelic variation and ultrashort telomeres in senescent human cells. Nature Genet. 2003;33:203–207. doi: 10.1038/ng1084. [DOI] [PubMed] [Google Scholar]
- 90.Bendix L, Horn PB, Jensen UB, Rubelj I, Kolvraa S. The load of short telomeres, estimated by a new method, Universal STELA, correlates with number of senescent cells. Aging Cell. 2010;9(3):383–397. doi: 10.1111/j.1474-9726.2010.00568.x. [DOI] [PubMed] [Google Scholar]
- 91.Holohan B, Hagiopian MM, Lai TP, Huang E, Friedman DR, Wright WE, et al. Perifosine as a potential novel anti-telomerase therapy. Oncotarget. 2015 doi: 10.18632/oncotarget.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ding Z, Mangino M, Aviv A, Spector T, Durbin R UK10K Consortium. Estimating telomere length from whole genome sequence data. Nucl. Acids Res. 2014;42(9):e75. doi: 10.1093/nar/gku181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hemann MT, Strong MA, Hao LY, Greider CW. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001;107(1):67–77. doi: 10.1016/s0092-8674(01)00504-9. [DOI] [PubMed] [Google Scholar]
- 94.Artandi SE, DePinho RA. Telomeres and telomerase in cancer. Carcinogenesis. 2009;31(1):9–18. doi: 10.1093/carcin/bgp268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Buseman CM, Wright WE, Shay JW. Is telomerase a viable target in cancer? Mutat Res. 2012;730(102):90–97. doi: 10.1016/j.mrfmmm.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.White LK, Wright WE, Shay JW. Telomerase Inhibitors. Trends in Biotechnolog. 2001;19(3):114–120. doi: 10.1016/s0167-7799(00)01541-9. [DOI] [PubMed] [Google Scholar]
- 97.Harley CB. Telomerase and cancer therapeutics. Nature Rev Cancer. 2008;8:167–179. doi: 10.1038/nrc2275. [DOI] [PubMed] [Google Scholar]
- 98.Autexier C, Greider CW. Telomerase and cancer: revisiting the telomere hypothesis. Trend in Bioch Sci. 1996;21(10):387–391. [PubMed] [Google Scholar]
- 99.Agrawal A, Dang S, Gabrani R. Recent patents on anti-telomerase cancer therapy. Recent Pat Anticancer Drug Discov. 2012;7(1):102–117. doi: 10.2174/157489212798357958. [DOI] [PubMed] [Google Scholar]
- 100.Chiappori AA, Kolevska T, Spigel DR, Hager S, Rarick M, Gadgeel S, et al. A randomized phase II study of the telomerase inhibitor imetelstat as maintenance therapy for advanced non-small-cell lung cancer. Annal Oncol. 2015;26:354–362. doi: 10.1093/annonc/mdu550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baerlocker GM, Leibundgut EO, Ottmann OG, Spitzer G, Odenike O, McDivitt MA, et al. Telomerase inhibitor imetelstat in patients with essential thrombocythemia. New Engl J Med. 2015;373(10):920–928. doi: 10.1056/NEJMoa1503479. [DOI] [PubMed] [Google Scholar]
- 102.Tefferi A, Lasho TL, Begna KH, Patnaik MM, Zblewski DL, Finke CM, et al. A pilot study of the telomerase inhibitor imetelstat for myelofibrosis. New Engl J Med. 2015;373(10):908–919. doi: 10.1056/NEJMoa1310523. [DOI] [PubMed] [Google Scholar]
- 103.Mender I, Gryaznov S, Dikmen ZG, Wright WE, Shay JW. Induction of telomere dysfunction mediated by the telomerase substrate precursor, 6-thio-2-deoxyguanosine. Cancer Discovery. 2015;5(1):82–95. doi: 10.1158/2159-8290.CD-14-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Holohan B, Wright WE, Shay JW. Impaired telomere maintenance spectrum disorders. J Cell Biology. 2014;205(3):289–299. doi: 10.1083/jcb.201401012. [DOI] [PMC free article] [PubMed] [Google Scholar]