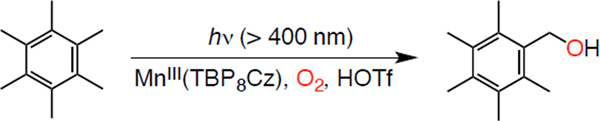Abstract
UV-vis spectral titrations of a manganese(III) corrolazine complex [MnIII(TBP8Cz): TBP8Cz = octakis(p-tert-butylphenyl)corrolazinato3−] with HOTf in benzonitrile (PhCN) indicate mono- and diprotonation of MnIII(TBP8Cz) to give MnIII(OTf)(TBP8Cz(H)) and [MnIII(OTf)(H2O)(TBP8Cz(H)2)][OTf] with the protonation constants of 9.0 × 106 M−1 and 4.7 × 103 M−1, respectively. The protonated site of MnIII(OTf)(TBP8Cz(H)) and [MnIII(OTf)(H2O)-(TBP8Cz(H)2)][OTf] were identified by X-ray crystal structures of the mono- and diprotonated complexes. In the presence of HOTf, the monoprotonated manganese(III) corrolazine complex [MnIII(OTf)(TBP8Cz(H))] acts as an efficient photocatalytic catalyst for the oxidation of hexamethylbenzene and thioanisole by O2 to the corresponding alcohol and sulfoxide with 563 and 902 TON, respectively. Femtosecond laser flash photolysis measurements of MnIII(OTf)(TBP8Cz(H)) and [MnIII(OTf)(H2O)(TBP8Cz(H)2)][OTf] in the presence of O2 revealed the formation of a tripquintet excited state, which was rapidly converted to a tripseptet excited state. The tripseptet excited state of MnIII(OTf)(TBP8Cz(H)) reacted with O2 with a diffusion-limited rate constant to produce the putative MnIV(O2•−)(OTf)(TBP8Cz(H)), whereas the tripseptet excited state of [MnIII(OTf)(H2O)(TBP8Cz(H)2)][OTf] exhibited no reactivity toward O2. In the presence of HOTf, MnV(O)(TBP8Cz) can oxidize not only HMB but also mesitylene to the corresponding alcohols, accompanied by regeneration of MnIII(OTf)(TBP8Cz(H)). This thermal reaction was examined for a kinetic isotope effect, and essentially no KIE (1.1) was observed for the oxidation of mesitylene-d12, suggesting a proton-coupled electron transfer (PCET) mechanism is operative in this case. Thus, the monoprotonated manganese(III) corrolazine complex, MnIII(OTf)(TBP8Cz(H)), acts as an efficient photocatalytic for the oxidation of HMB by O2 to the alcohol.
Graphical Abstract
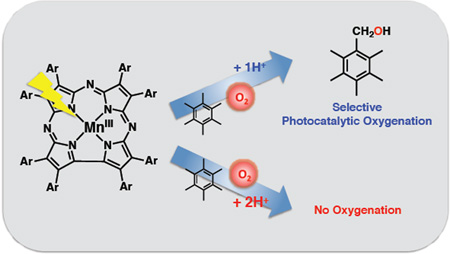
Photocatalytic oxygenation of substrates by a manganese complex in the presence of HOTf has been studied. The protonated site of the mono- and diprotonated MnIII complexes were characterized by single crystal XRD. In the presence of HOTf, the monoprotonated manganese(III) corrolazine complex acts as an efficient photocatalytic catalyst for the oxidation of hexamethylbenzene and thioanisole by O2 whereas the diprotonated manganese(III) corrolazine complex exhibited no reactivity toward O2
INTRODUCTION
Oxidation reactions are essential in the metabolism of organic substrates and many enzymes are known to catalyze these reactions in biological systems. High-valent metal-oxo complexes have been implicated as important reactive intermediates in these reactions for heme and non-heme iron enzymes.1–14 To help clarify related oxidation mechanisms and to control the reactivity, many synthetic high-valent metal-oxo complexes have been prepared using oxidants such as iodosylarenes, peroxy acids, and hydrogen peroxide.3–14 Dioxygen (O2) is an ideal reagent for the production of these metal-oxo complexes, and for the subsequent oxygenation of substrates because of its abundant availability and nontoxicity.15,16 The mechanisms of oxidation of substrates by high-valent metal-oxo complexes have been studied extensively.17–23 High-valent manganese-oxo complexes have attracted special attention because they are postulated as important intermediates for water oxidation in the oxygen-evolving center (OEC) of photosystem II.24–32
We have shown that a well-characterized manganese(V)-oxo complex can be prepared by visible light irradiation of a manganese(III) corrolazine [MnIII(TBP8Cz): TBP8Cz3− = octakis(p-tert-butylphenyl)corrolazinato3−] in the presence of toluene derivatives with dioxygen.15,16 Hexamethylbenzene (HMB) was shown to be oxidized to pentamethylbenzyl alcohol during this reaction, serving as a proton/electron source to assist with O2 activation. However, the produced MnV(O)(TBP8Cz) was unreactive toward HMB, which prevented the regeneration of the MnIII starting complex and removed the possibility for catalytic turnover. Only in the case of the O-atom acceptor PPh3, or 9,10-dihydro-1-methylacridine, which has a very weak C–H bond was photocatalysis observed.16,33 The reactivity of high-valent metal-oxo complexes to oxidize substrates has been reported to be much enhanced by the presence of acids,34–42 suggesting that the addition of an H+ source could activate the MnV(O) complex and possibly lead to catalytic turnover.
We showed that addition of a strong proton source [H(OEt2)2]+[B(C6F5)4]− (H+[B(C6F5)4]−) allowed for the photocatalytic oxidation of HMB by MnIII(TBP8Cz) with O2 as oxidant.43 The addition of one and two equivalents of H+[B(C6F5)4]− to MnIII(TBP8Cz) formed two new species that were characterized by X-ray diffraction (XRD) as the monoprotonated [MnIII(H2O)-(TBP8Cz(H))][B(C6F5)4], and the diprotonated [MnIII(H2O)(TBP8Cz(H)2)][B(C6F5)4]2, respectively. The monoprotonated [MnIII(H2O)(TBP8Cz(H))][B(C6F5)4] is protonated at the meso-N atom adjacent to the direct pyrrole-pyrrole bond of the corrolazine ligand. For the di-protonated [MnIII(H2O)(TBP8Cz(H)2)][B(C6F5)4]2 complex, both H-atoms were found to be located on the opposite meso-N atoms adjacent to the direct pyrrole–pyrrole bond. The mono-protonated MnIII complex was catalytically active with a turnover number (TON) of 18 for pentamethylbenzyl alcohol and 9 for pentamethylbenzaldehyde. Interestingly, the diprotonated [MnIII(H2O)(TBP8Cz(H)2)]2+ complex showed no catalytic activity, indicating that catalytic turnover was highly dependent on the level of protonation.
In this work we present several new findings on the proton-assisted, photoactivated oxygenation chemistry catalyzed by manganese corrolazine. We demonstrate that substitution of the H+[B(C6F5)4]− proton donor with triflic acid (HOTf) leads to dramatic changes in catalytic activity, and generates new protonated Mn(III) corrolazine complexes, MnIII(OTf)(TBP8Cz(H)) and [MnIII(OTf)(H2O)(TBP8Cz(H)2)][OTf], which were characterized by X-ray diffraction. The crystal structures reveal that protonation occurs on the meso nitrogen atoms as seen for H+[B(C6F5)4]−, but significant changes in axial ligation state are observed with HOTf, including coordination of the anionic triflate and formation of both 5- and 6-coordinate species. Coordination of OTf- provides a possible mechanism for enhancing catalytic reactivity through a proposed MnV(O)(TBP8Cz) intermediate.44,45,51 In our previous report on proton-assisted catalysis, we suggested a mechanism that involved a short-lived tripseptet excited state of the monoprotonated MnIII complex as a key intermediate. This mechanism was based on prior photochemical characterization of the parent MnIII complex. However, direct spectroscopic evidence for the proposed photochemical excited states of the protonated MnIII species was absent. Herein we present femtosecond laser flash photolysis measurements on both the monoprotonated MnIII(OTf)(TBP8Cz(H)) and the diprotonated [MnIII(OTf)(H2O)(TBP8Cz(H)2)]-[OTf], which reveal shorter-lived excited states (7T1) in the presence of O2 that provide direct evidence for the proposed mechanism of photocatalytic oxygenation. We also demonstrate new catalytic activity for the monoprotonated complex. This complex was shown to be an excellent catalyst for the selective S-oxygenation of thioanisole with only O2 and light as reagents.
▪ EXPERIMENTAL SECTION
Materials
The starting material MnIII(TBP8Cz) was synthesized according to published procedures.46 The commercially available reagents (hexamethylbenzene and trifluoromethane-sulfonic acid) were purchased with the best available purity and used without further purification. Benzonitrile (PhCN) was purchased with the best available purity from Wako Pure Chemical Industries, Ltd. and dried according to literature procedures47 and distilled under Ar prior to use.
Spectral and Kinetic Measurements
The formation of protonated species of MnIII(TBP8Cz) and MnV(O)(TBP8Cz) were examined from the change in the UV-vis spectra of MnIII(TBP8Cz) (λmax = 695 nm, εmax = 3.5 × 104 M−1 cm−1) and MnV(O)(TBP8Cz) (λmax = 634 nm, εmax = 2.0 × 104 M−1 cm−1)16 by spectral titration with HOTf at 298 K using a Hewlett-Packard HP8453 diode array spectrophotometer. The first and second binding constants of HOTf with MnIII(TBP8Cz) were determined in PhCN from the UV-vis spectral change due to the formation of the protonated species MnIII(OTf)(TBP8Cz(H)) (λmax = 725 nm) and [MnIII(OTf)(H2O)-(TBP8Cz(H)2)][OTf] (λmax = 745 nm). The first binding constant of HOTf with MnV(O)(TBP8Cz) was determined in PhCN from the UV-vis spectral change due to the formation of the protonated species MnIV(OH)(OTf)(TBP8Cz•+) (λmax = 795 nm).
Crystallization of MnIII(OTf)(TBP8Cz(H))
To a solution of MnIII(TBP8Cz) (20 mg, 14 µmol) in CH2Cl2 (1.0 mL) was added Sc(OTf)3 (70 mg, 1.4 × 10−4 mol, 10 equiv) dissolved in CH3CN (0.4 mL). A color change from brown to reddish-brown was observed. The solution was then filtered through celite and eluted with CH2Cl2 (~1.0 mL). X-ray quality crystals were obtained by slow evaporation of this solution for about 4 weeks. Characterization by elemental analysis indicating the monoprotonated MnIII(OTf)(TBP8Cz(H))•CH2Cl2; calcd. for C98H107Cl2F3MnN7O3S: C, 71.52; H, 6.55; N, 5.96. Found: C, 71.65; H, 6.53; N, 6.01.
Crystallization of [MnIII(OTf)(H2O)(TBP8Cz(H)2)][OTf]
To a solution of MnIII(TBP8Cz) (1.5 mg, 1.1 µmol) in CH2Cl2 (0.5 mL) was added 2 equiv HOTf. A color change from brown to red was observed. The solution was transferred to a NMR tube and layered with n-heptane. X-ray quality crystals were obtained after a month. Elemental analysis was performed for [MnIII(OTf)(H2O)(TBP8Cz(H)2)][OTf−]•CH2Cl2•C7H16; calcd. for C106H126Cl2F6MnN7O7S2: C, 66.51; H, 6.64; N, 5.12. Found: C, 66.28; H, 6.32; N, 5.56.
X-ray Crystallography
MnIII(OTf)(TBP8Cz(H))
All reflection intensities were measured at 110(2) K using a KM4/Xcalibur (detector: Sapphire3) with enhance graphite-monochromated Mo Kα radiation (λ = 0.71073 Å) under the program CrysAlisPro (Version 1.171.35.11 Oxford Diffraction Ltd., 2011). The program CrysAlisPro (Version 1.171.35.11, Oxford Diffraction Ltd., 2011) was used to refine the cell dimensions. Data reduction was done using the program CrysAlisPro (Version 1.171.35.11, Oxford Diffraction Ltd., 2011). The structure was solved with the program SHELXS-97 and was refined on F2 with SHELXL-97.48 Analytical numeric absorption corrections based on a multifaceted crystal model were applied using CrysAlisPro (Version 1.171.35.11, Oxford Diffraction Ltd., 2011). The temperature of the data collection was controlled using the system Cryojet (manufactured by Oxford Instruments). The H atoms (except when specified) were placed at calculated positions using the instructions AFIX 43 or AFIX 137 with isotropic displacement parameters having values 1.2 or 1.5 times Ueq of the attached C atoms. The H atom attached to N5 was located from difference Fourier maps, and its atomic coordinates were refined freely. Seven of the eight 4-tert-butylphenyl groups are either wholly disordered (over two orientations) or partially disordered around the tert-butyl groups (over two orientations). The coordinated counterion is also disordered over two orientations.
MnIII(OTf)(TBP8Cz(H))
Fw = 1560.88, black plate, 0.58 × 0.33 × 0.06 mm3, monoclinic, P21/n (no. 14), a = 19.9174(5), b = 21.8922(4), c = 20.3238(5) Å, β = 110.584(3)°, V = 8296.1(3) Å3, Z = 4, Dx = 1.250 g cm−3, µ= 0.247 mm−1, abs. corr. range: 0.923–0.987. 45329 Reflections were measured up to a resolution of (sin θ/λ)max = 0.59 Å−1. 14603 Reflections were unique (Rint = 0.0440), of which 10025 were observed [I > 2σ(I)]. 1482 Parameters were refined using 1634 restraints. R1/wR2 [I > 2σ(I)]: 0.0624/0.1552. R1/wR2 [all refl.]: 0.0977/0.1734. S = 1.041. Residual electron density found between −0.53 and 0.85 e Å−3.
[MnIII(OTf)(H2O)(TBP8Cz(H)2)][OTf]
All reflection intensities were measured at 110(2) K using a SuperNova diffractometer (equipped with Atlas detector) with Cu Kα radiation (λ = 1.54178 Å) under the program CrysAlisPro (Version 1.171.36.32 Agilent Technologies, 2013). The same program was used to refine the cell dimensions and for data reduction. The structure was solved and refined on F2 with SHELXL-2013.48 Analytical numeric absorption correction based on a multifaceted crystal model was applied using CrysAlisPro. The temperature of the data collection was controlled using the system Cryojet (manufactured by Oxford Instruments). The H atoms were placed at calculated positions (unless otherwise specified) using the instructions AFIX 23, AFIX 43 or AFIX 137 with isotropic displacement parameters having values 1.2 times Ueq of the attached C or N atoms. The H atoms attached to O1W and O2W/O2W were found from difference fourier maps, and their coordinates were refined freely using the DFIX restraints. The tert-butyl group C53→C56, the tert-butylphenyl group C77→C86, and the hydronium cation are found to be disordered over two orientations, and the occupancy factors of the major components of the disorder refine to 0.50(3), 0.588(6), and 0.634(4), respectively. The crystal lattice contains some disordered solvent molecules (DCM and heptane). All orientations of the three non-fully occupied DCM molecules were successfully modeled, and the asymmetric unit contains ca. 2.53 DCM molecules per Mn complex. The heptane molecule was found to be disordered, and its contribution has been taken out in the final refinement.49
[MnIII(OTf)(H2O)(TBP8Cz(H)2)][OTf]
Fw = 2111.86, dark brown-black block, 0.35 × 0.30 × 0.21 mm3, triclinic, P−1 (no.2), a = 18.3393(4), b = 18.9149(3), c = 21.2275(3), α = 91.1721(13), β = 114.7271(17), γ = 118.8374(19)°, V = 5625.8(2) Å3, Z = 2, Dx = 1.247 g cm−3, µ = 3.176 mm−1, Tmin – Tmax: 0.445–0.619. 72524 Reflections were measured up to a resolution of (sin θ/λ)max = 0.62 Å−1. 22105 Reflections were unique (Rint = 0.0242), of which 20831 were observed [I > 2σ(I)]. 1549 Parameters were refined using 987 restraints. R1/wR2 [I > 2σ( I)]: 0.0500/0.1381. R1/wR2 [all refl.]: 0.0524/0.1403. S = 1.036. Residual electron density found between −0.57 and 1.25 e Å−3.
Photocatalytic Reactivity
The catalytic reactivity of MnIII (TBP8Cz) (1.0 × 10−5 M) was examined under a large excess of a substrate (hexamethylbenzene or thioanisole: 0 – 0.3 M) in the presence of HOTf (0 – 1.0 × 10−4 M) in PhCN (2.0 mL). This solution was transferred to a glass cuvette equipped with a stir bar. The generated products of the reaction solution were analyzed by GC-MS. An aliquot was taken from the product solution and injected directly into the GC-MS for analysis. All peaks of interest were identified by comparison of retention times and co-injection with authentic samples. The amounts of products were quantified by comparison against a known amount of detected products using a calibration curve consisting of a plot of product mole versus area. Mass spectra were recorded on a JEOL JMS-700T Tandem MS station, and the GC-MS analyses were carried out by using a Shimadzu GCMS-QP2000 gas chromatograph mass spectrometer. GC-MS conditions in these experiments were performed as follows: an initial oven temperature of 60 °C was held for 1 min and then raised 30 °C min−1 for 7.3 min until a temperature of 250 °C was reached, which was then held for further 10 min. The products pentamethylbenzyl alcohol and pentamethylbenzaldehyde were identified by comparison with a standard sample. The product yields were quantified by comparison against a known amount of detected products using a calibration curve consisting of a plot of product mole versus area. Calibration curves were prepared by using concentrations in the same range as that observed in the actual reaction mixtures.
Quantum Yield Determination
A standard actinometer (potassium ferrioxalate)50 was used for the quantum yield determination of photocatalytic oxidation of HMB by O2 with MnIII (TBP8Cz) in O2-saturated PhCN. Typically, a square quartz cuvette (10 mm i.d.), which contained an O2-saturated PhCN solution (2.0 mL) of MnIII (TBP8Cz) (1.0 × 10−5 M) and HMB (0.4 M) in the presence of HOTf (4.0 × 10−5 M) was irradiated with monochromatic light of λ =450 nm from a Shimadzu RF-5300PC fluorescence spectrophotometer. Under the conditions of actinometry experiments, the actinometer and MnIII(TBP8Cz) absorbed essentially all of the incident light at λ = 450 nm. The light intensity of monochromatized light at λ = 450 nm was determined to be 3.7 × 10−8 einstein s−1. The quantum yields were determined by the amount of generated a product.
Femtosecond Laser Flash Photolysis Measurements
Measurements of transient absorption spectra of MnIII(OTf)(TBP8Cz(H)) and [MnIII(OTf)(H2O)(TBP8Cz(H)2)][OTf] were performed according to the following procedures. An N2- or O2-saturated PhCN solution containing MnIII(TBP8Cz) (8.4 × 10−5 M) and HOTf (1.7 × 10−4 or 1.2 × 10−2 M) was excited using an ultrafast source, Integra-C (Quantronix Corp.), an optical parametric amplifier, TOPAS (Light Conversion Ltd.), and a commercially available optical detection system, Helios provided by Ultrafast Systems LLC. The source for the pump and probe pulses were derived from the fundamental output of Integra-C (λ = 786 nm, 2 mJ/pulse and fwhm = 130 fs) at a repetition rate of 1 kHz. 75% of the fundamental output of the laser was introduced into a second harmonic generation (SHG) unit: Apollo (Ultrafast Systems) for excitation light generation at λ = 393 nm, while the rest of the output was used for white light generation. The laser pulse was focused on a sapphire plate of 3 mm thickness and then white light continuum covering the visible region from λ = 410 nm to 800 nm was generated via self-phase modulation. A variable neutral density filter, an optical aperture, and a pair of polarizer were inserted in the path in order to generate stable white light continuum. Prior to generating the probe continuum, the laser pulse was fed to a delay line that provides an experimental time window of 3.2 ns with a maximum step resolution of 7 fs. In our experiments, a wavelength at λ = 393 nm of SHG output was irradiated at the sample cell with a spot size of 1 mm diameter where it was merged with the white probe pulse in a close angle (< 10 °). The probe beam after passing through the 2 mm sample cell was focused on a fiber optic cable that was connected to a CMOS spectrograph for recording the time-resolved spectra (λ = 410 – 800 nm). Typically, 1500 excitation pulses were averaged for 3 seconds to obtain the transient spectrum at a set delay time. Kinetic traces at appropriate wavelengths were assembled from the time-resolved spectral data. The decay rate of the tripquintet (5T1) obeyed the first-order kinetics given by eq 1,
| (1) |
where A1 and A2 are pre-exponential factors for the absorbance changes and the final absorbance, and k1 is the rate constant of the decay of the tripquintet (5T1) after irradiation. The slower decay rate of the tripseptet (7T1) also obeyed the first-order kinetics given by eq 2, where A3 is the final absorbance of 7T1 and k2 is the rate constant of the decay of 7T1. All measurements were conducted at room temperature, 298 K.
| (2) |
RESULTS AND DISCUSSION
Protonation of MnIII(TBP8Cz)
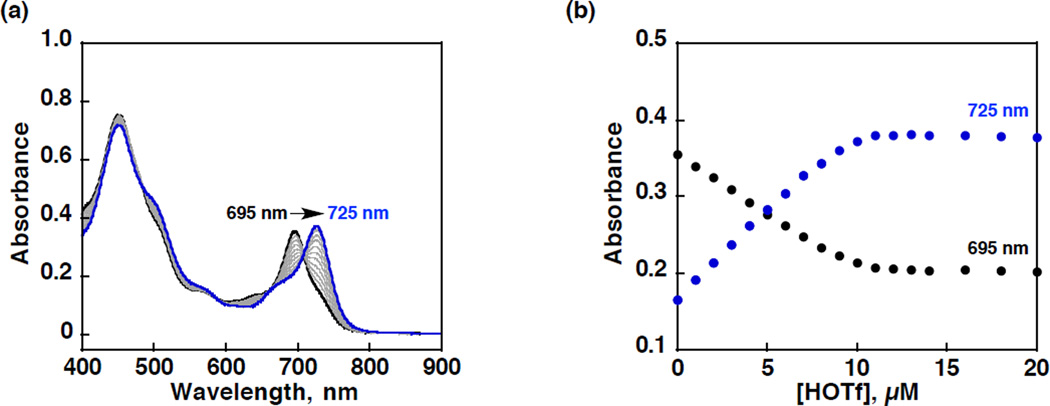
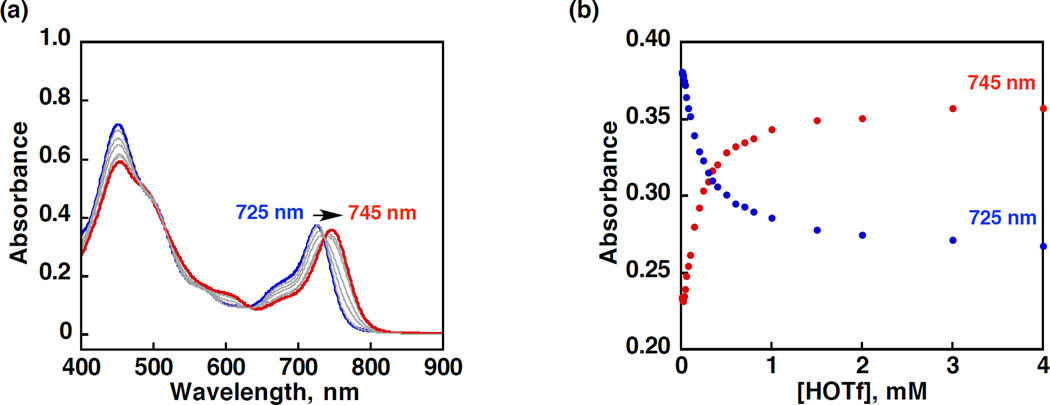
Protonation of MnIII(TBP8Cz) was examined by addition of HOTf to a PhCN solution of MnIII(TBP8Cz) (1.0 × 10−5 M). The formation of a new species with a Q-band at λmax = 725 nm, was monitored by UV-vis as shown in Figure 1a, where HOTf was added up to 1 equiv of MnIII(TBP8Cz). A color change from brown to reddish-brown was observed. This spectral change was similar to that seen for the addition of 1 equiv of H+[B(C6F5)4]−, and indicated the formation of the monoprotonated complex, MnIII(OTf)(TBP8Cz(H)) (1). Addition of excess HOTf (1.0 × 10−5 – 1.0 × 10−2 M) resulted in a change in the Q-band region to give λmax = 745 nm with the isosbestic points as shown in Figure 2. These data were consistent with the formation of the diprotonated, [MnIII(OTf)(TBP8Cz(H)2)]-[OTf] (2) and again similar to the spectral changes observed for H+[B(C6F5)4]−.
Figure 1.
(a) UV-vis spectral changes and (b) plot for determination of the binding constant of the conversion from MnIII(TBP8Cz) (black line, 1.0 × 10−5 M) to MnIII(OTf)(TBP8Cz(H)) (blue line) by spectroscopic titration of HOTf (0 – 2.0 × 10−5 M) monitored at 695 nm (MnIII(TBP8Cz), black dots) and 725 nm (MnIII(OTf)(TBP8Cz(H)), blue dots) in a PhCN solution at room temperature.
Figure 2.
(a) UV-vis spectral changes and (b) plot for determination of the binding constant of the conversion from MnIII(OTf)(TBP8Cz(H)) (blue line, 1.0 × 10−5 M) to [MnIII(OTf)(TBP8-Cz(H)2)][OTf] (red line) by spectroscopic titration of HOTf (1.0 × 10−5 – 1.0 × 10−2 M) monitored at 725 nm (MnIII(OTf)(TBP8Cz(H)), blue dots) and 745 nm ([MnIII(OTf)(TBP8Cz-(H)2)][OTf], red dots) in a PhCN solution at room temperature.
The monoprotonation equilibrium constant (K1) for 1 was determined by fitting of the titration curves in Figure 1b, according to the equilibrium expression in eq 3. This analysis gave
| (3) |
K1 = 9.0 × 106 M−1, indicating strong binding of H+ to MnIII (TBP8Cz). The second equilibrium constant (K2) for the protonation equilibrium in eq 4, was determined to be 4.7 × 103 M−1 by
| (4) |
fitting a plot of reciprocal absorbance difference vs 1/[HOTf] from the titration curve in Figure 2b (see Figure S1 in the Supporting Information (SI)). The K1 value for the first protonation to give 1 is much larger than the K2 value for the second species 2.
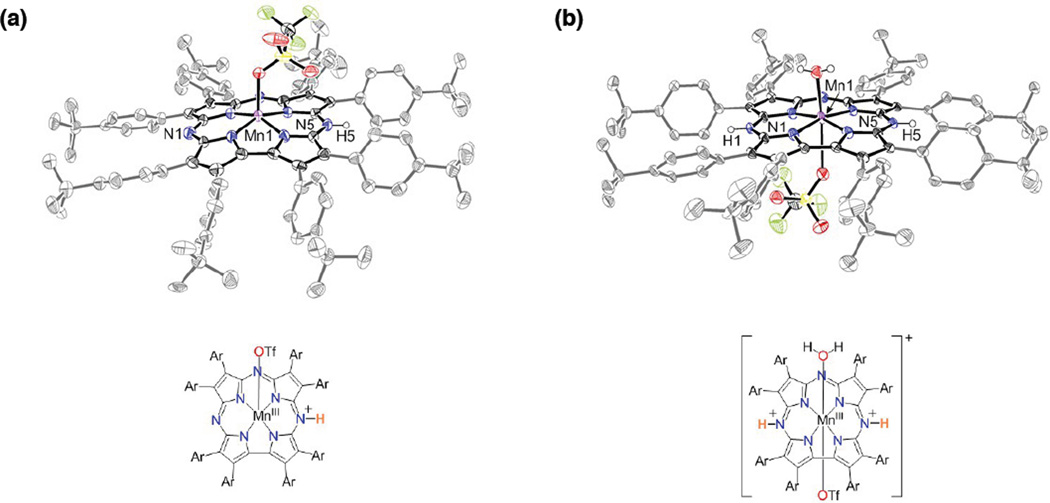
The protonated complexes 1 and 2 were successfully crystallized and characterized by single crystal X-ray Diffraction (XRD) (Figure 3). The monoprotonated complex was originally crystallized from reaction of MnIII (TBP8Cz) with 10 equiv Sc(OTf)3 in CH2Cl2/CH3CN. The strongly Lewis acidic Sc3+ ion presumably reacts with exogenous H2O to generate the proton source, and slow evaporation produced X-ray quality crystals of 1 after 4 weeks. The crystal structure revealed that monoprotonation occurs at one of the meso-N-atoms (N5(H)) adjacent to the direct pyrrole-pyrrole bond of the corrolazine ligand, and that the manganese center is five-coordinate with an axially-ligated triflate (MnIII-O = 2.115(3) Å). The same crystals were subsequently obtained from reaction o MnIII (TBP8Cz) with 1 equiv HOTF and recrystallization from CH2Cl2/heptane. A unit cell measurement matched that for 1.
Figure 3.
Displacement ellipsoid plots (50% probability level) and chemical structures for (a) [MnIII(OTf)(TBP8Cz(H))] and (b) [MnIII(OTf)(H2O)(TBP8Cz(H)2)][OTf] at 110(2) K.
Dissolution of crystalline 1 in PhCN yields the same UV-vis spectrum as seen for the in situ protonation o MnIII (TBP8Cz) by the addition of 1 equiv of HOTF (λmax = 451, 725 nm) (Figure S2). Interestingly dissolution of crystalline 1 in CH2Cl2 (Figure S3) gives a UV-vis spectrum with λmax = 443, 725 nm, similar to what was seen for [MnIII (H2O)(TBP8Cz(H))][B(C6F5)4] (λmax = 446, 730 nm in CH2Cl2)43 although the Q-band is shifted by 5 nm. A similar red-shift in the Q-band occurs upon axial ligation of anionic donors to MnIII(H2O)(TBP8Cz) to give [MnIII(X)(TBP8Cz)]− (e.g. X = CN−, F−)51.
X-ray quality crystals of the diprotonated complex 2 were obtained from the reaction of MnIII(TBP8Cz) with 2 equiv HOTf and recrystallization from CH2Cl2/heptanes (Figure 3). Both H-atoms were unambiguously located on the opposite meso-N atoms (N1 and N5). In contrast to the monoprotonated complex, the diprotonated complex has a six coordinate manganese center with an axially ligated water at 2.144(2) Å and a weakly bound triflate at 2.702(2) Å.
Dissolution of crystalline 2 in CH2Cl2 yielded a spectrum with λmax at 454 and 758 nm (Figure S4). Comparing this spectrum to that seen for the diprotonated [MnIII(H2O)(TBP8Cz(H)2)]-[B(C6F5)4]2 (λmax: 470, 763)43 in the same solvent again reveals a red-shift of 5 nm in the Q-band, matching the differences seen for the two monoprotonated spectra. These results indicate that the triflate (−OTf) anion stays bound in solution for both the mono- and diprotonated complexes. When crystalline 2 was dissolved in PhCN, the UV-vis spectrum matched that for the monoprotonated complex, indicating that this solvent is basic enough to deprotonate the second meso-N atom.
Photocatalytic Oxygenations with O2 by MnIII(TBP8Cz) in the Presence of HOTf
Previously it was shown that the addition of H+[B(C6F5)4]− to MnIII(TBP8Cz) allowed for photo-activated catalysis of HMB under ambient conditions, but only with modest turnover numbers and selectivity.43 Replacement of H+[B(C6F5)4]− with the strong acid HOTf led to the photocatalytic oxygenation of HMB by O2 and MnIII(TBP8Cz) in PhCN.
The addition of HOTf (2 equiv) to MnIII(TBP8Cz) in PhCN gave a spectrum (λmax = 451, 725 nm) indicative of 1. Addition of HMB followed by photoirradiation initiated the catalytic reaction, which occured over 6 h with slow bleaching of the solution (Figure S5 in SI). Regarding the bleaching of the catalyst, we followed the reaction by UV-vis, which showed only the slow loss of the ground state complex. The catalytically active excited state was only present to a small extent at any time, and therefore the slow decomposition of the ground state (which is in large excess) did not change the effective concentration of the catalytically active excited state to a large extent. Analysis of the reaction mixture by removal of aliquots and analysis by GC showed a steady increase in the amount of oxidized products produced over time. Thus, there is an optimized concentration of HOTf for the phtoocatalytic oxygenation of HMB by O2 with 1. The reason why the photocatlaytic oxygenation is prohibited in the presence of large concentrations of HOTf is discussed in relation with the phtoocatalytic reaction mechanism (vide infra). The production of oxidized products seemed only limited by the catalyst stability. The final turnover number (TON) was 563 for the alcohol PMB–OH and 9 for the aldehyde PMB– CHO (Figure S6 in SI), with a quantum yield of 0.125%. The catalytic turnover is dramatically increased by HOTf in comparison to H+[B(C6F5)4]−, which gave only TON(PMB–OH) = 18 and TON(PMB–CHO) = 9.43 In addition, the HOTf reaction is much more selective for production of the alcohol product. When the HMB was replaced by dueturated HMB, the reactivity was lower. The kinetic isotope effect (KIE) was determined to be 3.3 with linear increase of PMB-OH vs time, indicating H-atom abstraction is the rate determining step in the overall photocatalytic reaction.
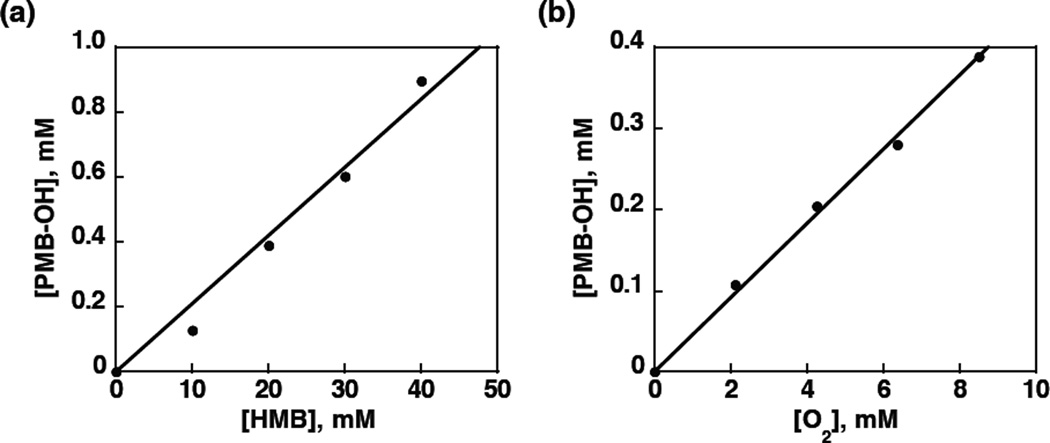
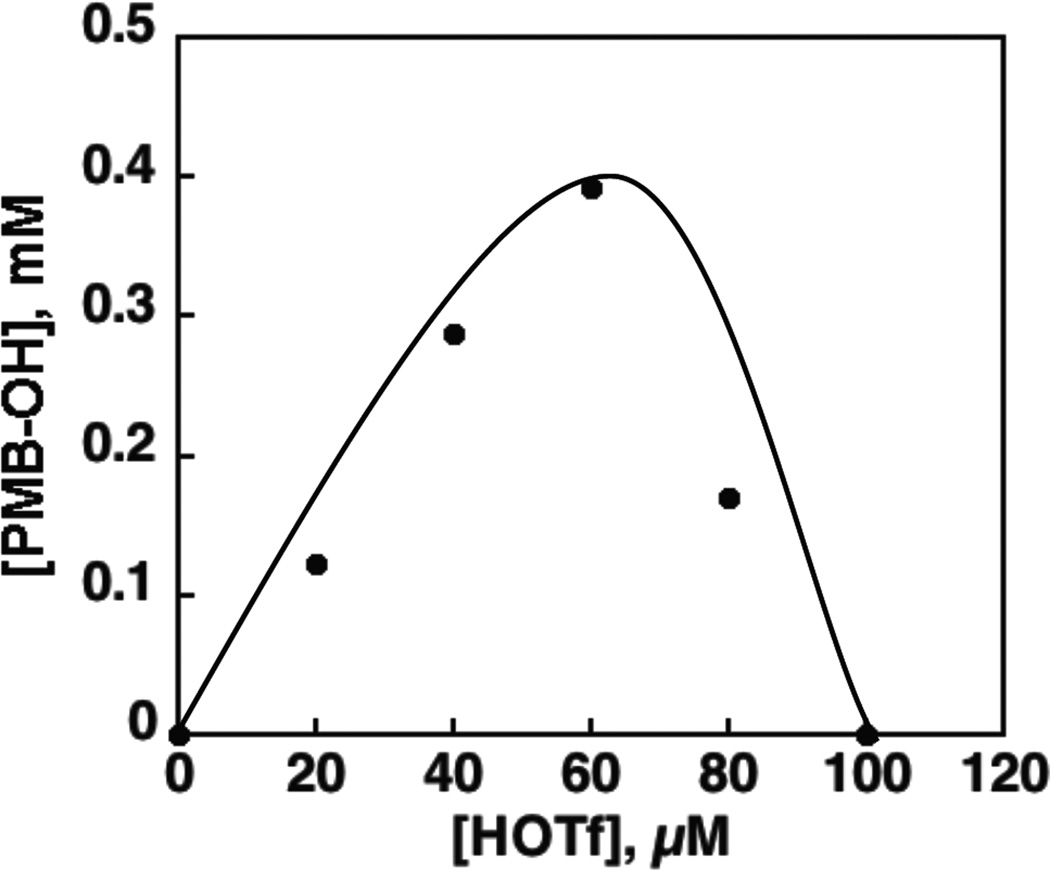
The amount of PMB–OH produced after 1 h photoirradiation of a PhCN solution of MnIII(TBP8Cz) with HMB, O2 and HOTf was proportional to concentrations of HMB (Figure 4a) and O2 (Figure 4b). Thus, the rate-determining step in the photocatalytic oxygenation of HMB by O2 may be the reaction of the photogenerated species from 1 with O2 and HMB. If HOTf is added in excess (10 equiv), 2 is formed as seen by UV-vis. Photoirradiation of this complex in the presence of HMB/O2 shows no oxidized products. This result is consistent with previous observations on the diprotonated MnIII complex generated from H+[B(C6F5)4]−, which also showed no catalytical activity.43 The amount of PMB–OH produced by 30 min photoirradiation with a Xenon lamp increased with increasing concentration of HOTf to reach a maximum and then decreased with further increase in concentrationof HOTf as shown in Figure 5 because of the formation of the diprotonated complex 2 which shows no catalytic activity for oxygenation of HMB by O2 in the presence of large excess HOTf (Figure S6b).
Figure 4.
(a) Plots of the amount of produced pentamethylbenzyl alcohol for photocatalytic oxygenation of HMB (0 – 4.0 × 10−2 M) by MnIII(TBP8Cz) (1.0 × 10−5 M) in the presence of HOTf(4.0 × 10−5 M) in an 02-saturated PhCN solution and (b) Plots of the amount of produced pentamethylbenzyl alcohol for photocatalytic oxygenation of HMB (2.0 × 10−2 M) by MnIII(TBP8Cz) (1.0 × 10−5 M) in the presence of HOTf (4.0 × 10−5 M) in a PhCN solution vs the oxygen concentration (0–8.5 × 10−3 M) under photoirradiation (white light) for 1 h at room temperature.
Figure 5.
Plots of the amount of produced pentamethylbenzyl alcohol for photocatalytic oxygenation of HMB (2.0 × 10−2 M) by MnIII(TBP8Cz) (1.0 × 10−5 M) in the presence of HOTf (0 – 1.0 × 10−4 M) under photoirradiation (white light) for 30 min in an O2-saturated PhCN solution at room temperature.
Photocatalytic Sulfoxidation by MnIII(TBP8Cz) in the Presence of HOTf
In our previous studies we focused exclusively on C–H functionalization with toluene derivatives. In the current work we wanted to determine if photocataytic oxygenation could be obtained with an O-atom acceptor substrate, and the thioether substrate PhSMe was examined. There have been some reports involving iron complexes reacting with O2 to give catalytic sulfoxidation of thioether substrates, although they often involved poorly controlled radical-type pathways.52 The photoactivation of diiron(III)-µ-oxo-bridged porphyrins has also lead to catalytic sulfoxidation where dioxygen is used to regenerate the µ-oxo complex.53 Dioxygen has been utilized by iron complexes for catalytic sulfoxidation by the formation of a peracid oxidant.54 However, there remains few examples of sulfoxidation mediated by O2 and well-defined transition metal catalysts that avoid co-additives or radical-type pathways. When HMB was replaced by thioanisole in the photocatalytic reaction with MnIII(OTf)(TBP8Cz(H)), a single S-oxygenated product, methylphenylsulfoxide (PhS(O)Me), was obtained (Scheme 2). Excellent TONs (902) and conversion (98%) of PhSMe to PhS(O)Me was found (Figure S7a) whereas negligible amount of PhS(O)Me (TON: 14) was obtained in the absence of HOTf (Figure S7b). Control experiments confirmed that no corresponding products were observed without photoirradiation or without the MnIII complex (see Figure S8 in SI). These results show that MnIII(TBP8Cz) is an efficient and selective catalyst for the sulfoxidation of thioanisole, and requires only air, light, and an H+ source without the need for a co-reductant.
Scheme 2.
Catalytic Oxygenation of Thioanisole by O2 with MnIII(TBP8Cz) and HOTf under Photoirradiation Conditions
Femtosecond Transient Absorption Measurements
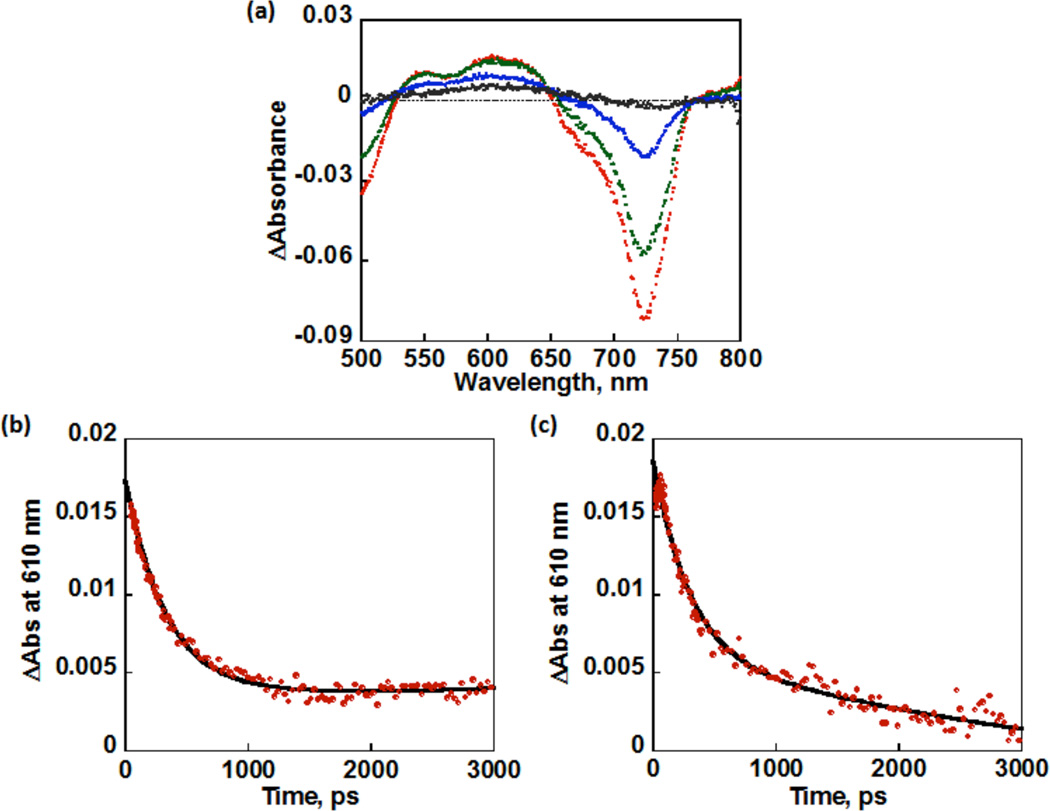
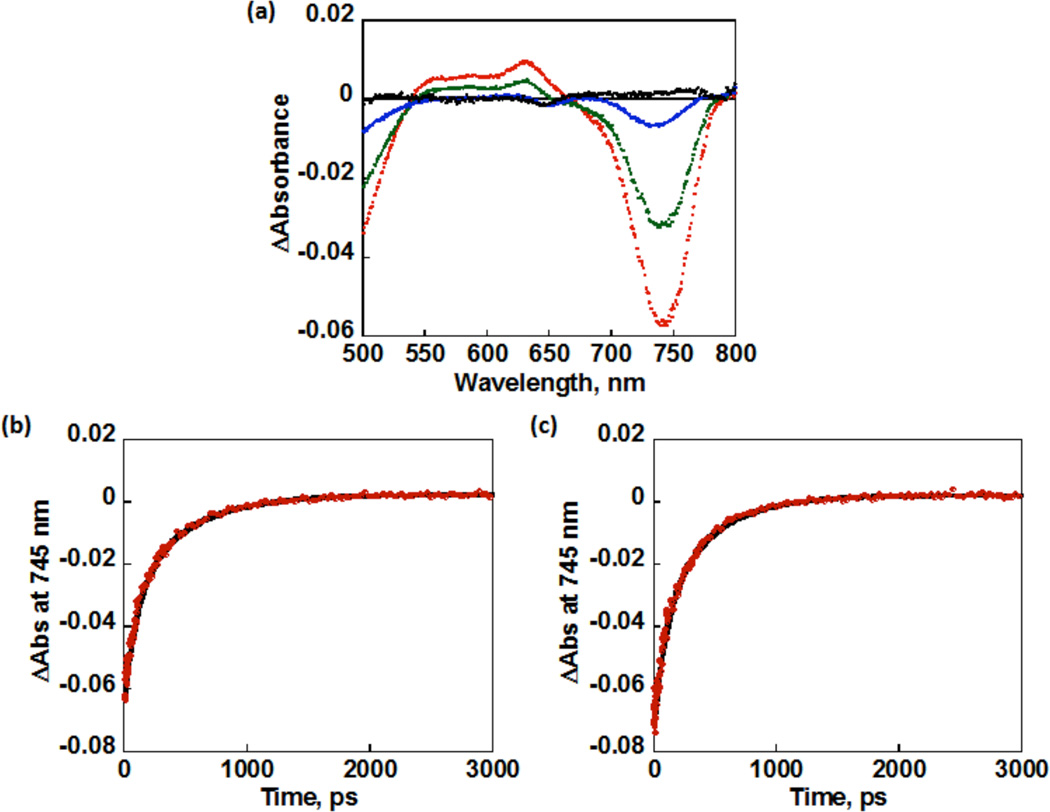
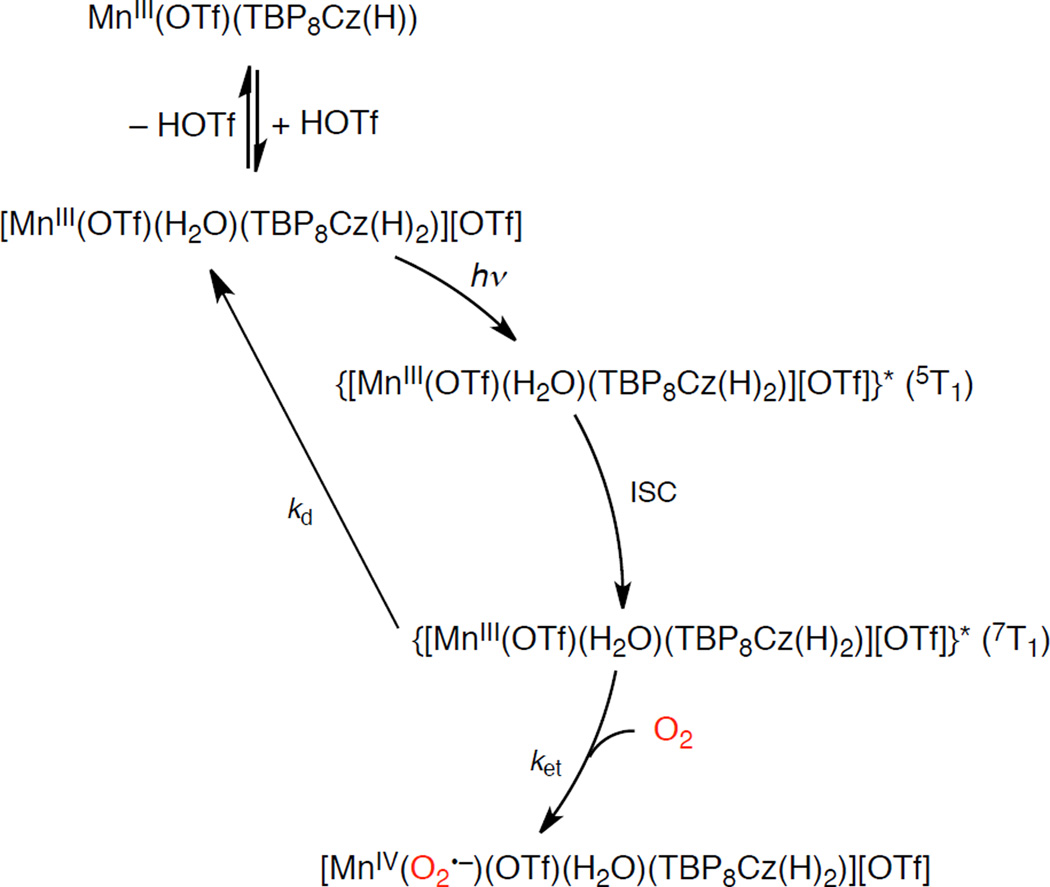
In order to clarify the photodynamics of 1 and 2, femtosecond laser flash photolysis measurements were performed in the absence and presence of O2 in PhCN. The MnIII ion is a high spin (S = 2) species, and coupling between the metal d electrons and the π electrons of the corrolazine ring leads to a singquintet (5S0) ground state derived from the lowest excited ring (π,π*) singlet for 1 and 2 due to the coupling between unpaired electrons of the metal with the π electrons of the corrolazine ring. A “tripmultiplet” manifold (3T1, 5T1, 7T1) is derived from the lowest ring (π,π*) triplet.55 Femtosecond laser excitation of 1 leads to a new maximum absorption at 610 nm, which can be assigned to the tripquintet (5T1) excited state (Figure 6). The absorption due to the tripquintet (5T1) decayed in a few ps with a rate constant of 3.1 × 109 s−1, and there was no effect of O2 on the rate due to the fast intersystem crossing to the tripseptet excited state (7T1) of 1 with a longer lifetime (Figure 6). An extremely rapid intersystem crossing process from the singquintet excited state (5S1) to the tripquintet excited state (5T1) resulting from the presence of unpaired electrons has been seen for a first row paramagnetic complex, MnIII.56 For example, in MnIII porphyrins, the existence of two tripmultiplet levels was suggested where a tripquintet (5T1) relaxes to a long-lived tripseptet (7T1), which requires a spin conversion to go back to the quintet ground state. The absorption at 610 nm due to the tripseptet excited state (7T1) decayed significantly faster in O2-saturated PhCN (Figure 6c) as compared with the decay in the absence of O2 (Figure 6b). The rate constant of the reaction of 7T1 of 1 with O2 was determined to be 5.0 × 109 M−1 s−1, which is the same as that of the reaction of 7T1 of neutral MnIII(TBP8Cz) with O2.16 This reaction should produce the putative superoxo complex ([MnIV(O2•−)(TBP8Cz(H))]+).
Figure 6.
(a) Transient absorbance spectral changes (red after 10 ps, green 100 ps, blue 500 ps, and black 3000 ps) after photoexcitation of MnIII(OTf)(TBP8Cz(H)) in PhCN. Time profile of the generation and decay of {MnIII(OTf)(TBP8Cz(H))}* at λ = 610 nm under (b) N2 and (c) O2. The black lines are exponential fitting given in eqs 1 and 2.
Femtosecond laser flash photolysis measurements of 2 were also performed to characterize the excited state of the diprotonated species in the absence and presence of O2 in PhCN, as shown in Figure 7. The decay rate constant of the intersystem crossing from the tripquintet excited state (5T1) to the tripseptet excited state (7T1) was determined to be 9.4 × 109 s−1 at 745 nm in both the absence and presence of O2. In contrast to the case of MnIII(TBP8Cz) and 1, the decay rate constant for the tripseptet excited state (7T1) of 2 was slower (2.2 × 109 s−1 at 745 nm) and showed no O2 dependence. Thus, the tripseptet excited state (7T1) of 2 does not react with O2, in line with the observation that 2 is not catalytically active. We hypothesize that the one electron oxidation potential of 7T1 of 2 may be too high for reaction with O2.
Figure 7.
(a) Transient absorbance spectral changes (red after 10 ps, green 100 ps, blue 500 ps, and black 3000 ps) after photoexcitation of [MnIII(OTf)(H2O)(TBP8Cz(H)2)][OTf] in PhCN. Time profile of the generation and decay of {[MnIII(OTf)(H2O)(TBP8Cz(H)2)][OTf]}* at λ = 745 nm under (b) N2 and (c) O2. The black lines are exponential fitting given in eqs 1 and 2.
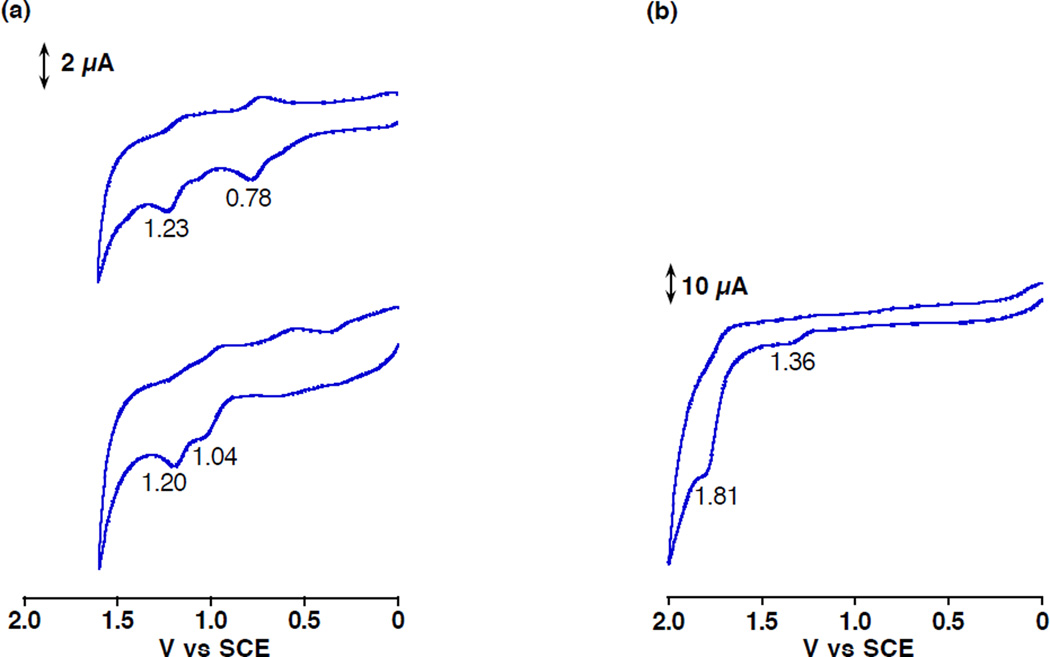
Oxidation Potentials of MnIII(TBP8Cz), MnIII(OTf)(TBP8Cz(H)) and [MnIII(OTf)-(H2O)(TBP8Cz(H)2)][OTf]
The effects of protonation of MnIII(TBP8Cz) on the one-electron oxidation potentials were examined by cyclic voltammetry measurements of MnIII(TBP8Cz), 1, and 2, and the peak potentials (referenced to SCE) are shown in Figure 8. The first one-electron oxidation potential of MnIII(TBP8Cz) was determined to be Eox = 0.78 V vs SCE in PhCN, which was shifted to the positive direction by the addition of HOTf: Eox = 1.04 V for 1 and Eox = 1.36 V for 2. However, the second one-electron oxidation potential was not positively shifted by the addition of HOTf: Eox = 1.23 V for MnIII(TBP8Cz) and Eox = 1.20 V for 1 probably because of the counter anion effect of OTf−. Although the energies of 7T1 of MnIII(TBP8Cz), 1 and 2 have yet to be determined because of the absence of phosphorescence, the Eox value of 2 in the ground state, which is 0.32 V higher than that of 1, is consistent with the observation that the 7T1 of 2 is unreactive towards O2 in contrast to 1.
Figure 8.
Cyclic voltammograms of (a) MnIII(TBP8Cz) (upper, 1.0 × 10−3 M) in the absence of HOTf and in the presence of HOTf (below, 1.0 × 10−3 M), and (b) MnIII(TBP8Cz) (1.0 × 10−3 M) in the presence of HOTf (0.1 M) in PhCN containing 0.1 M TBAP. Scan rate = 0.10 V s−1.
Reaction Mechanism
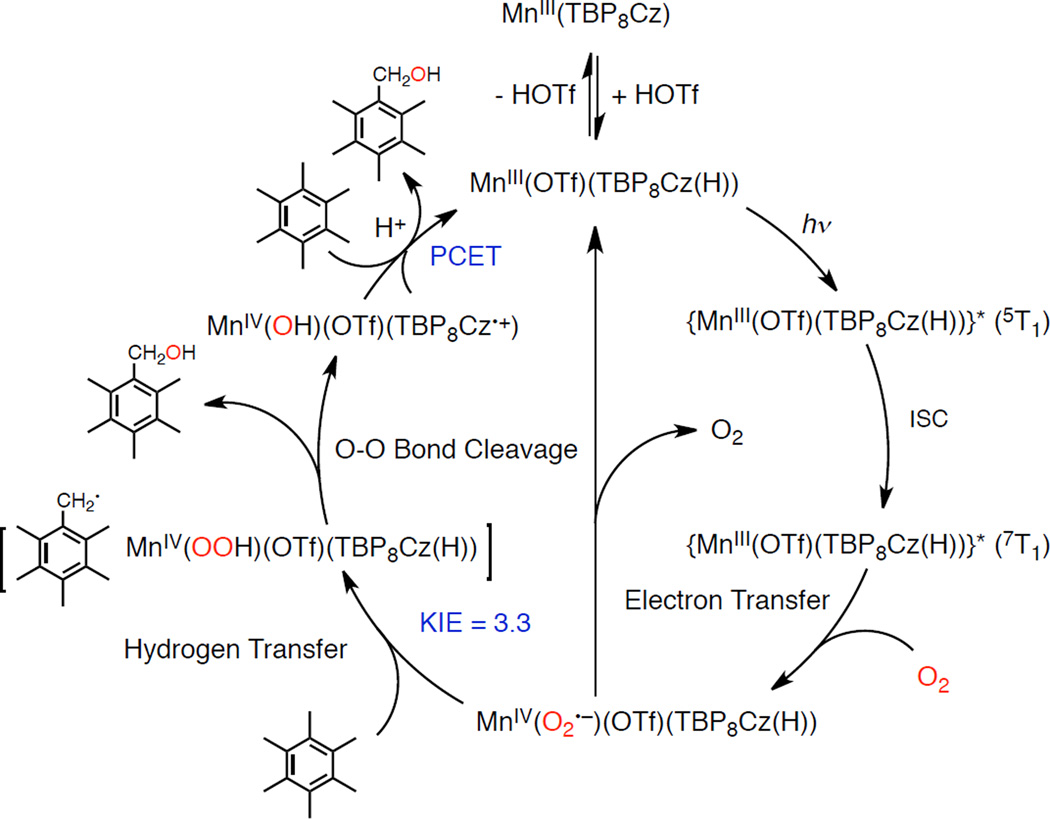
Based on the study of the photocatalytic oxygenation of HMB by O2 with 1 and photodynamics of 1 (vide supra), the mechanism of photocatalytic oxygenation of HMB by O2 with 1 is proposed as shown in Scheme 3.
Scheme 3.
Mechanism of Photocatalytic Oxygenation of HMB by 1 with O2
In the presence of 1 equiv of HOTf, MnIII(TBP8Cz) is converted to the monoprotonated complex 1, which is then excited to the tripquintet state {MnIII(OTf)(TBP8Cz(H))}* (5T1) upon photoirradiation. The 5T1 of 1 is converted rapidly by intersystem crossing (ISC) to the tripseptet excited state (7T1). Electron transfer from 7T1 to O2 occurs to produce the superoxo complex [MnIV(O2•−)(OTf)(TBP8Cz(H))], which abstracts a hydrogen atom from HMB to produce the hydroperoxo complex [MnIV(OOH)(OTf)(TBP8Cz(H))] and pentamethylbenzyl radical, in competition with the back electron-transfer (k–et) to regenerate the ground state 1 and O2. C–H activation by a metal superoxo species has been demonstrated with chromium and copper non-heme model complexes.57 In our case, the subsequent homolytic O–O bond cleavage and combination with benzyl radical to yield PMB–OH is accompanied by generation of high-valent MnV(O)(OTf)(TBP8Cz(H)), followed by conversion to MnIV(OH)(OTf)(TBP8Cz•+).43 This species, in the presence of excess H+, is proposed to react with another equivalent of substrate to produce PMB–OH, regenerate 1, and close the catalytic cycle.
When 1 is further protonated to 2 in the presence of a large excess of HOTf no catalytic activity is observed. The tripseptet excited state (7T1) of 2 is favored to go back to the ground state rather than react with O2, possibly due to the higher oxidation potential for 2 as compared with that of 1 (vide supra) as shown in Scheme 4. As a result, the photocatalytic oxygenation of HMB by O2 with MnIII(TBP8Cz) does not occur in the presence of large excess HOTf (10 equiv HOTf). In such a case, the involvement of singlet oxygen is unlikely, because the formation of singlet oxygen may not be affected by the acid. In addition, the efficient photocatalytic oxygenation of thioanisole by O2 with MnIII(TBP8Cz) and HOTf in PhCN (Scheme 2) also suggests no involvment of singlet oxygen, because the reactions of alkyl and aryl sulfides with singlet oxygen in aprotic solvents such as PhCN is known to be sluggish.58
Scheme 4.
Mechanism of Photocatalytic Oxygenation of HMB by 2 with O2
Protonation of MnV(O)(TBP8Cz)
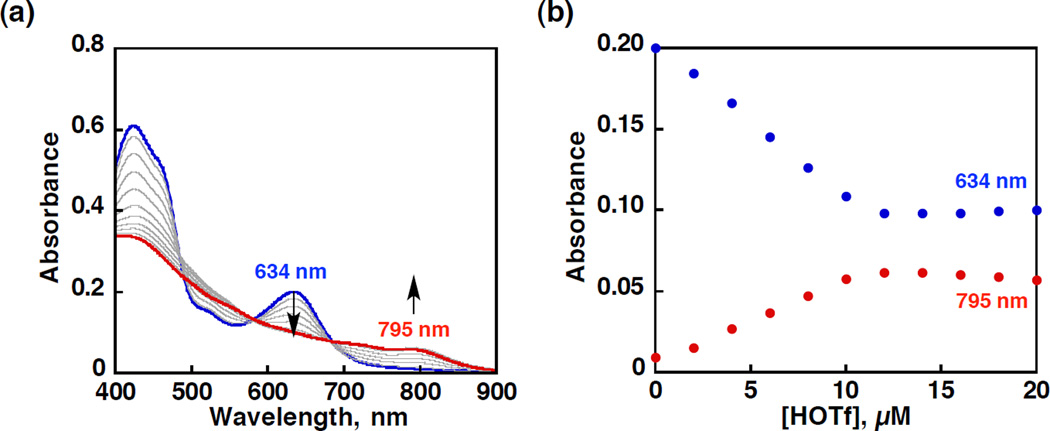
To further study the proposed final step in the catalysis to regenerate the MnIII resting state, the reaction of isolated MnV(O)(TBP8Cz) with acid was studied. Protonation of MnV(O)(TBP8Cz) was examined by reaction of MnV(O)(TBP8Cz) with HOTf in PhCN as shown in Figure 9a. The broadening and decrease in the intensity of the Soret band at 420 nm and the Q band at 634 nm together with the appearance of a relatively weak band in the near-IR region at 795 nm is characteristic of the formation of a porphyrinoid π-radical cation.43 The new spectrum matches that observed previously upon the addition of the Lewis or Brønsted acids (ZnII(OTf)2, B(C6F5)3, H+) to the MnV(O) complex.42,43 These acids stabilize a high-spin triplet (S = 1) (or quintet S = 2) state with an electronic configuration best described as a manganese(IV) corrolazine π-radical cation.42 Protonation of the terminal oxo ligand should weaken the Mn-O π -bonding and destabilize the MnV oxidation state, favoring an MnIV(O)(π-cation-radical) configuration. The formation of MnIV(OH)(OTf)(TBP8Cz•+) (3) (λmax = 795 nm) was monitored by UV-vis spectroscopy as shown in Figure 9, where HOTf was added up to 1 equiv. The equilibrium constant for the addition of acid to MnV(O)(TBP8Cz) was determined to be 1.0 × 107 M−1 by a plot of absorbance vs concentration of HOTf in Figure 9b. No further spectral changes were observed in the presence of large excess HOTf (Figure S9).
Figure 9.
(a) UV-vis spectral changes and (b) plot for the conversion of MnV(O)(TBP8Cz) (blue line, 1.0 × 10−5 M) to MnIV(OH)(OTf)(TBP8Cz•+) (red line) by spectroscopic titration of HOTf (0 – 2.0 × 10−5 M) monitored at 634 nm (MnV(O)(TBP8Cz), blue dots) and 795 nm (MnIV(OH)(OTf)(TBP8Cz•+), red dots) in PhCN at 298 K.
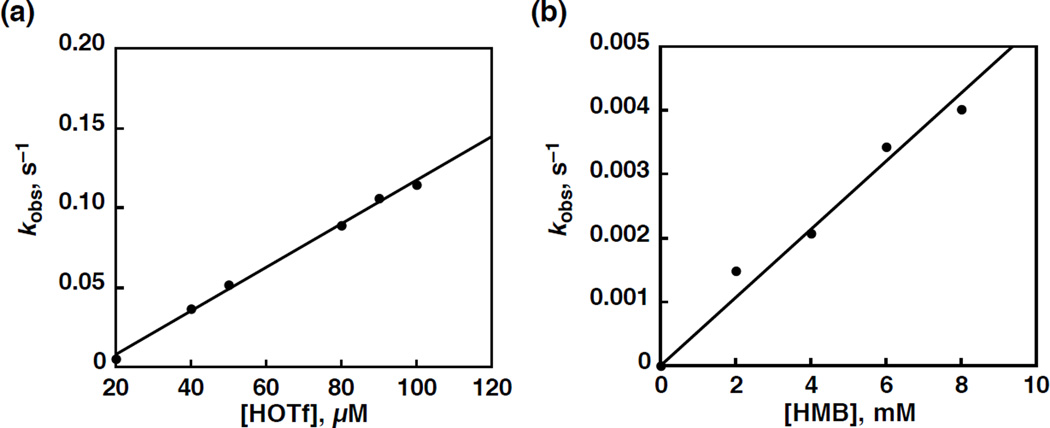
The addition of HMB to 3 and monitoring the reaction by UV-Vis showed slow decay of 3 and the growth of MnIII over 14 hours. In the presence of excess of HOTf, however, 3 is reduced by HMB to produce 1 (Figure S10b in SI) and PMB-OH (yield: 85%) by GC-MS. Rates of the oxidation of HMB by MnV(O)(TBP8Cz) in the presence of excess HOTf were determined from an increase in absorbance at 725 nm due to 1 in a PhCN at 298 K, obeying pseudo-first-order kinetics in the presence of large exess HMB and HOTf (Figure S11 and Figure S12 in SI). The observed pseudo-first-order rate constants are proportional to concentrations of HOTf and HMB to afford the second-order rate constants from the slopes of the linear plots as 1.4 × 10−3 M−1 s−1 (Figure 10a) and 5.3 × 10−4 M−1 s−1 (Figure 10b), respectively.
Figure 10.
(a) Plot of the observed pseudo-first-order rate constant (kobs) vs concentrations of HOTf for the oxidation of HMB (2.0 × 10−2 M) by MnV(O)(TBP8Cz) (1.0 × 10−5 M) in the presence of HOTf (2.0 × 10−5 – 1.0 × 10−4 M) in PhCN at 298 K. (b) Plot of the observed pseudo-first-order rate constant (kobs) vs concentrations of HMB for the oxidation of HMB (0 – 1.0 × 10−2 M) by MnV(O)(TBP8Cz) in the presence of HOTf (3.0 × 10−5 M) in PhCN at 298 K.
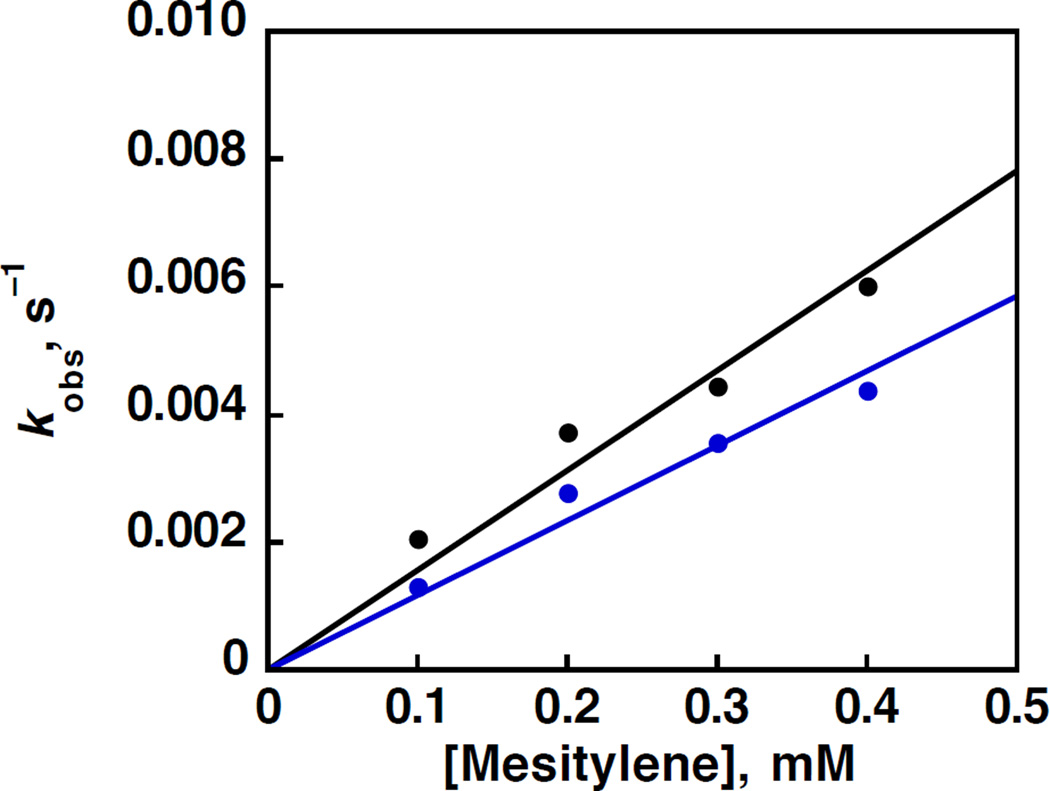
When HMB was replaced by mesitylene (C6H3(CH3)3), 3 was also reduced by mesitylene in the presence of excess HOTf. A very small deuterium kinetic isotope effect (KIE) close to unity (KIE = 1.1) was observed when C6H3(CH3)3 was replaced by the deuterated compound (C6D3(CD3)3) as shown in Figure 11. The KIE close to unity indicates that the oxidation of mesitylene as well as HMB by 3 proceeds via proton-coupled electron transfer from mesitylene and HMB to 3 rather than hydrogen atom transfer from mesitylene and HMB to 3.
Figure 11.
Plots of pseudo-first-order rate constants vs concentrations of mesitylene (C6H3(CH3)3) (black) and mesitylene-d12 (C6D3(CD3)3) (blue) in the oxidation of C6H3(CH3)3 and (C6D3(CD3)3) by MnV(O)(TBP8Cz) (3.0 × 10−5 M) in the presence of HOTf (1.5 × 10−4 M) in PhCN at 298 K.
CONCLUSIONS
In conclusion, MnIII(OTf)(TBP8Cz(H)) is capable of the photocatalytic oxygenation of HMB and PhSMe under ambient conditions, and was found to be a more robust, efficient and selective catalyst than the monoprotonated [MnIII(H2O)(TBP8Cz(H))][B(C6F5)4]. Thus changing the acid from H+[B(C6F5)4]− to an acid with a different conjugate base resulted in a dramatic change in catalytic reactivity. The major difference in conjugate bases is the ability of OTf− to coordinate to the Mn center, and suggests that OTf− coordination has a significant influence on the catalytic activity of the system. The dynamics of the photoexcited states were similar for both acids, and the same photoexcited state mechanism can be invoked for both H+[B(C6F5)4]− and HOTf. The proton-coupled electron transfer from HMB to MnIV(OH)(OTf)(TBP8Cz•+) occurs to yield pentamethylbenzyl alcohol, accompanied by regeneration of MnIII(OTf)(TBP8Cz(H)) to complete the catalytic cycle. The PCET oxidation of HMB by MnIV(OH)(OTf)(TBP8Cz•+) is enhanced with increasing concentration of HOTf. In the presence of large excess HOTf, however, MnIII(OTf)(TBP8Cz(H)) is converted the diprotonated complex [MnIII(OTf)(TBP8Cz(H)2)][OTf], when 7T1 of [MnIII(OTf)(TBP8Cz(H)2)][OTf] cannot react with O2 to produce the superoxo complex because of the high oxidation potential of [MnIII(OTf)(TBP8Cz(H)2)][OTf] and thereby the catalytic cycle is stopped. It would be of interest in future efforts to examine other acids in order to elucidate the effect of the H+ source and conjugate base on the catalysis.
Supplementary Material
Scheme 1.
Catalytic Oxygenation of Hexamethylbenzene by O2 with MnIII(TBP8Cz) and HOTf under Photoirradiation Conditions
Acknowledgments
This work was supported by Grants-in-Aid (No. 20108010 to S.F. and 23750014 to K.O.), the NSF (CHE0909587, CHE121386 to D.P.G.), the NIH (GM101153 to D.P.G.), and by a Grant-in-Aid (20108010) and an ALCA project (to S.F.) from JST, Japan.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge via the Internet at http://pubs.acs.org.
UV-vis absorption spectral data (Figure S1–S5 and S9–S10), product analysis data (Figure S6–S8), kinetic analyses (Figure S11–S12), and X-ray crystallographic data (Figure S13–S14)
REFERENCES
- 1.(a) Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry. 3rd. New York: Kluwer Academic/Plenum Publishers; 2005. [Google Scholar]; (b) Meunier B, editor. Metal-Oxo and Metal-Peroxo Species in Catalytic Oxidations. Berlin: Springer-Verlag; 2000. [Google Scholar]; (c) Terner J, Gold A. J. Am. Chem. Soc. 2007;129:16279–16280. [Google Scholar]; (d) Guengerich FP. Chem. Res. Toxicol. 2001;14:611–650. doi: 10.1021/tx0002583. [DOI] [PubMed] [Google Scholar]; (e) Denisov IG, Makris TM, Sligar SG, Schlichting I. Chem. Rev. 2005;105:2253–2278. doi: 10.1021/cr0307143. [DOI] [PubMed] [Google Scholar]
- 2.(a) Coelho PS, Brustad EM, Kannan A, Arnold FH. Science. 2013;339:307–310. doi: 10.1126/science.1231434. [DOI] [PubMed] [Google Scholar]; (b) Zhang R, Newcomb M. Acc. Chem. Res. 2008;41:468–477. doi: 10.1021/ar700175k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Meunier B, Visser SP, de Shaik S. Chem. Rev. 2004;104:3947–3980. doi: 10.1021/cr020443g. [DOI] [PubMed] [Google Scholar]; (d) Franke A, Hessenauer-Ilicheva N, Meyer D, Stochel G, Woggon W-D, van Eldik R. J. Am. Chem. Soc. 2006;128:13611–13624. doi: 10.1021/ja060650o. [DOI] [PubMed] [Google Scholar]
- 3.(a) Nam W. Acc. Chem. Res. 2007;40:522–531. doi: 10.1021/ar700027f. [DOI] [PubMed] [Google Scholar]; (b) Czernuszewicz RS, Mody V, Czader A, Gałęzowski M, Gryko DT. J. Am. Chem. Soc. 2009;131:14214–14215. doi: 10.1021/ja906393r. [DOI] [PubMed] [Google Scholar]; (c) Shaik S, Hirao H, Kumar D. Acc. Chem. Res. 2007;40:532–542. doi: 10.1021/ar600042c. [DOI] [PubMed] [Google Scholar]; (d) Schmidt A-C, Heinemann FW, Lukens WW, Jr, Meyer K. J. Am. Chem. Soc. 2014;136:11980–11993. doi: 10.1021/ja504528n. [DOI] [PubMed] [Google Scholar]
- 4.(a) Collman JP, Slaughter LM, Eberspacher TA, Strassner T, Brauman JI. Inorg. Chem. 2001;40:6272–6280. doi: 10.1021/ic010639j. [DOI] [PubMed] [Google Scholar]; (b) Crestoni ME, Fornarini S. Inorg. Chem. 2005;44:5379–5387. doi: 10.1021/ic048595c. [DOI] [PubMed] [Google Scholar]; (c) Zhou M, Balcells D, Parent AR, Crabtree RH, Eisenstein O. ACS Catal. 2012;2:208–218. [Google Scholar]
- 5.(a) Edwards NY, Eikey RA, Loring MI, Abu-Omar MM. Inorg. Chem. 2005;44:3700–3708. doi: 10.1021/ic0484506. [DOI] [PubMed] [Google Scholar]; (b) Tahsini L, Bagherzadeh M, Nam W, de Visser SP. Inorg. Chem. 2009;48:6661–6669. doi: 10.1021/ic900593c. [DOI] [PubMed] [Google Scholar]; (c) Lai S, Lepage CJ, Lee DG. Inorg. Chem. 2002;41:1954–1957. doi: 10.1021/ic0108336. [DOI] [PubMed] [Google Scholar]
- 6.(a) Ramdhanie B, Telser J, Caneschi A, Zakharov LN, Rheingold AL, Goldberg DP. J. Am. Chem. Soc. 2004;126:2515–2525. doi: 10.1021/ja036983s. [DOI] [PubMed] [Google Scholar]; (b) Buda BEF, Gribnau MCM, Baerends EJ. J. Am. Chem. Soc. 2004;126:4355–4365. doi: 10.1021/ja038865a. [DOI] [PubMed] [Google Scholar]; (c) Gupta R, Lacy DC, Bominaar EL, Borovik AS, Hendrich MP. J. Am. Chem. Soc. 2012;134:9775–9784. doi: 10.1021/ja303224p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Yoshizawa K. Acc. Chem. Res. 2006;39:375–382. doi: 10.1021/ar050194t. [DOI] [PubMed] [Google Scholar]
- 7.(a) Kumar D, Hirao H, Shaik S, Kozlowski PM. J. Am. Chem. Soc. 2006;128:16148–16158. doi: 10.1021/ja064611o. [DOI] [PubMed] [Google Scholar]; (b) Comba P, Maurer M, Vadivelu P. Inorg. Chem. 2009;48:10389–10396. doi: 10.1021/ic901702s. [DOI] [PubMed] [Google Scholar]; (c) Kumar D, Derat E, Khenkin AM, Neumann R, Shaik S. J. Am. Chem. Soc. 2005;127:17712–17718. doi: 10.1021/ja0542340. [DOI] [PubMed] [Google Scholar]
- 8.(a) Bart SC, Anthon C, Heinemann FW, Bill E, Edelstein NM, Meyer K. J. Am. Chem. Soc. 2008;130:12536–12546. doi: 10.1021/ja804263w. [DOI] [PubMed] [Google Scholar]; (b) Abbina S, Bian S, Oian C, Du G. ACS Catal. 2013;3:678–684. [Google Scholar]; (c) Comba P, Maurer M, Vadivelu P. J. Am. Chem. Soc. 2008;112:13028–13036. doi: 10.1021/jp8037895. [DOI] [PubMed] [Google Scholar]; (d) Lacy DC, Gupta R, Stone KL, Greaves J, Ziller JW, Hendrich MP, Borovik AS. J. Am. Chem. Soc. 2010;132:12188–12190. doi: 10.1021/ja1047818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Jensen MP, Costas M, Ho RYN, Kaizer J, Payeras AMI, Münck E, Que L, Jr, Rohde J-U, Stubna A. J. Am. Chem. Soc. 2005;127:10512–10525. doi: 10.1021/ja0438765. [DOI] [PubMed] [Google Scholar]; (b) Tooyama Y, Braband H, Spingler B, Abram U, Alberto R. Inorg. Chem. 2008;47:257–264. doi: 10.1021/ic701908q. [DOI] [PubMed] [Google Scholar]; (c) Fernandes AC, Fernandes JA, Romão CC, Veiros LF, Calhorda MJ. Organometallics. 2010;29:5517–5525. [Google Scholar]
- 10.(a) Slep LD, Mijovilovich A, Meyer-Klaucke W, Weyhermüller T, Bill E, Bothe E, Neese E, Wieghardt K. J. Am. Chem. Soc. 2013;125:15554–15570. doi: 10.1021/ja030377f. [DOI] [PubMed] [Google Scholar]; (b) Krebs C, Fujimori DC, Walsh CT, Bollinger JM., Jr Acc. Chem. Res. 2007;40:484–492. doi: 10.1021/ar700066p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Martinho M, Banse F, Bartoli J-F, Mattioli TA, Battioni P, Horner O, Bourcier S, Girerd J-J. Inorg. Chem. 2005;44:9592–9596. doi: 10.1021/ic051213y. [DOI] [PubMed] [Google Scholar]
- 11.(a) Gunay A, Theopold KH. Chem. Rev. 2010;110:1060–1081. doi: 10.1021/cr900269x. [DOI] [PubMed] [Google Scholar]; (b) Yi CS, Zeczycki TN, Guzei IA. Organometallics. 2006;25:1047–1051. doi: 10.1021/om0510674. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Bakac A. J. Am. Chem. Soc. 2000;122:1092–1097. [Google Scholar]; (d) Czernuszewicz RS, Mody V, Zareba AA, Zaczek MB, Gałęzowski M, Sashuk V, Grela K, Gryko DT. Inorg. Chem. 2007;46:5616–5624. doi: 10.1021/ic070275g. [DOI] [PubMed] [Google Scholar]
- 12.(a) Konar S, Clearfield A. Inorg. Chem. 2008;47:3489–3491. doi: 10.1021/ic8001493. [DOI] [PubMed] [Google Scholar]; (b) King AE, Nippe M, Atanasov M, Chantarojsiri T, Wray CA, Bill E, Neese F, Long JR, Chang CJ. Inorg. Chem. 2014;53:11388–11395. doi: 10.1021/ic5010177. [DOI] [PubMed] [Google Scholar]; (c) Vasbinder MJ, Bakac A. Inorg. Chem. 2007;46:2921–2928. doi: 10.1021/ic070015z. [DOI] [PubMed] [Google Scholar]
- 13.(a) Bigi JP, Harman WH, Lassalle-Kaiser B, Robles DM, Stich TA, Yano J, Britt RD, Chang CJ. J. Am. Chem. Soc. 2012;134:1536–1542. doi: 10.1021/ja207048h. [DOI] [PubMed] [Google Scholar]; (b) Lovell T, Han W-G, Liu T, Noodleman L. J. Am. Chem. Soc. 2002;124:5890–5894. doi: 10.1021/ja0121282. [DOI] [PubMed] [Google Scholar]; (c) Price JC, Barr EW, Tirupati B, Bollinger JM, Jr, Krebs C. Biochemistry. 2003;42:7497–7508. doi: 10.1021/bi030011f. [DOI] [PubMed] [Google Scholar]; (d) Leeladee P, Jameson GNL, Siegler MA, Kumar D, Visser SP, de Goldberg DP. Inorg. Chem. 2013;52:4668–4682. doi: 10.1021/ic400280x. [DOI] [PubMed] [Google Scholar]
- 14.(a) Liu S, Mase K, Bougher C, Hicks SD, Abu-Omar MM, Fukuzumi S. Inorg. Chem. 2014;53:7780–7788. doi: 10.1021/ic5013457. [DOI] [PubMed] [Google Scholar]; (b) Zall CM, Clouston LJ, Young VG, Jr, Ding K, Kim HJ, Zherebetskyy D, Chen YS, Bill E, Gagliardi L, Lu CC. Inorg. Chem. 2013;52:9216–9228. doi: 10.1021/ic400292g. [DOI] [PubMed] [Google Scholar]; (c) Groysman S, Villagrán D, Nocera DG. Inorg. Chem. 2010;49:10759–10761. doi: 10.1021/ic101968s. [DOI] [PubMed] [Google Scholar]
- 15.Prokop KA, Goldberg DP. J. Am. Chem. Soc. 2012;134:8014–8017. doi: 10.1021/ja300888t. [DOI] [PubMed] [Google Scholar]
- 16.Jung J, Ohkubo K, Prokop KA, Neu HM, Goldberg DP, Fukuzumi S. Inorg. Chem. 2013;52:13594–13604. doi: 10.1021/ic402121j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.(a) Goldberg DP. Acc. Chem. Res. 2007;40:626–634. doi: 10.1021/ar700039y. [DOI] [PubMed] [Google Scholar]; (b) Conradie J, Swarts JC, Ghosh A. J. Phys. Chem. B. 2004;108:452–456. [Google Scholar]; (c) Venkataramanan NS, Premsingh S, Rajagopal S, Pitchumani K. J. Org. Chem. 2003;68:7460–7470. doi: 10.1021/jo034558b. [DOI] [PubMed] [Google Scholar]; (d) Sugimoto H, Kitayama K, Ashikari K, Matsunami C, Ueda N, Umakoshi K, Hosokoshi Y, Sasaki Y, Itoh S. Inorg. Chem. 2011;50:9014–9023. doi: 10.1021/ic201230k. [DOI] [PubMed] [Google Scholar]
- 18.(a) Meier-Callahan AE, Gray HB, Gross Z. Inorg. Chem. 2000;39:3605–3607. doi: 10.1021/ic000180d. [DOI] [PubMed] [Google Scholar]; (b) Vanover E, Huang Y, Xu L, Newcomb M, Zhang R. Org. Lett. 2010;12:2246–2249. doi: 10.1021/ol1005938. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Chung LW, Lee HG, Lin Z, Wu Y-D. J. Org. Chem. 2006;71:6000–6009. doi: 10.1021/jo060654b. [DOI] [PubMed] [Google Scholar]; (d) Harischandra DN, Lowery G, Zhang R, Newcomb M. Org. Lett. 2009;11:2089–2092. doi: 10.1021/ol900480p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.(a) McGown AJ, Kerber WD, Fujii H, Goldberg DP. J. Am. Chem. Soc. 2009;131:8040–8048. doi: 10.1021/ja809183z. [DOI] [PubMed] [Google Scholar]; (b) Kang M-J, Song WJ, Han A-R, Choi YS, Jang HG, Nam W. J. Org. Chem. 2007;72:6301–6304. doi: 10.1021/jo070557y. [DOI] [PubMed] [Google Scholar]; (c) Nam W, Lee Y-M, Fukuzumi S. Acc. Chem. Res. 2014;47:1146–1154. doi: 10.1021/ar400258p. [DOI] [PubMed] [Google Scholar]
- 20.(a) Mitić N, Clay MD, Saleh L, Bollinger JM, Jr, Solomon EI. J. Am. Chem. Soc. 2007;129:9049–9065. doi: 10.1021/ja070909i. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chen H, Lai W, Shaik S. J. Phys. Chem. Lett. 2010;1:1533–1540. [Google Scholar]; (c) Chiavarino B, Cipollini R, Crestoni ME, Fornarini S, Lanucara S, Lapi A. J. Am. Chem. Soc. 2008;130:3208–3217. doi: 10.1021/ja077286t. [DOI] [PubMed] [Google Scholar]
- 21.(a) Pan Z, Newcomb M. Inorg. Chem. 2007;46:6767–6774. doi: 10.1021/ic700395j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chung LW, Li X, Hirao H, Morokuma K. J. Am. Chem. Soc. 2011;133:20076–20079. doi: 10.1021/ja2084898. [DOI] [PubMed] [Google Scholar]; (c) Pan Z, Wang Q, Sheng X, Horner JH, Newcomb M. J. Am. Chem. Soc. 2009;131:2621–2628. doi: 10.1021/ja807847q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.(a) Pestovsky O, Bakac O. J. Am. Chem. Soc. 2003;125:14714–14715. doi: 10.1021/ja0382213. [DOI] [PubMed] [Google Scholar]; (b) Betley TA, Wu Q, Voorhis TV, Nocera DG. Inorg. Chem. 2008;47:1849–1861. doi: 10.1021/ic701972n. [DOI] [PubMed] [Google Scholar]
- 23.(a) Taguchi T, Gupta R, Lassalle-Kaiser B, Boyce DW, Yachandra VK, Tolman WB, Yano J, Hendrich MP, Borovik AS. J. Am. Chem. Soc. 2012;134:1996–1999. doi: 10.1021/ja210957u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Harischandra DN, Zhang R, Newcomb M. J. Am. Chem. Soc. 2005;127:13776–13777. doi: 10.1021/ja0542439. [DOI] [PubMed] [Google Scholar]
- 24.(a) McEvoy JP, Brudvig GW. Chem. Rev. 2006;106:4455–4483. doi: 10.1021/cr0204294. [DOI] [PubMed] [Google Scholar]; (b) Umena Y, Kawakami K, Shen J-R, Kamiya N. Nature. 2011;473:55–60. doi: 10.1038/nature09913. [DOI] [PubMed] [Google Scholar]; (c) Vaddypally S, Kondaveeti SK, Zdilla MJ. Inorg. Chem. 2012;51:3950–3952. doi: 10.1021/ic300502s. [DOI] [PubMed] [Google Scholar]; (d) Suga M, Akita F, Hirata K, Ueno G, Murakami H, Nakajima Y, Shimizu T, Yamashita K, Yamamoto M, Ago H, Shen J-R. Nature. 2015;517:99. doi: 10.1038/nature13991. [DOI] [PubMed] [Google Scholar]
- 25.(a) Lansky DE, Goldberg DP. Inorg. Chem. 2006;45:5119–5125. doi: 10.1021/ic060491+. [DOI] [PubMed] [Google Scholar]; (b) Cho K, Leeladee P, McGown AJ, DeBeer S, Goldberg DP. J. Am. Chem. Soc. 2012;134:7392–7399. doi: 10.1021/ja3018658. [DOI] [PubMed] [Google Scholar]; (c) Prokop KA, Neu HM, Visser SP, de Goldberg DP. J. Am. Chem. Soc. 2011;133:15874–15877. doi: 10.1021/ja2066237. [DOI] [PubMed] [Google Scholar]; (d) Leeladee P, Goldberg DP. Inorg. Chem. 2010;49:3083–3085. doi: 10.1021/ic902517j. [DOI] [PubMed] [Google Scholar]
- 26.(a) Zhang R, Newcomb M. J. Am. Chem. Soc. 2003;125:12418–12419. doi: 10.1021/ja0377448. [DOI] [PubMed] [Google Scholar]; (b) Kumar A, Goldberg I, Botoshansky M, Buchman Y, Gross Z. J. Am. Chem. Soc. 2010;132:15233–15245. doi: 10.1021/ja1050296. [DOI] [PubMed] [Google Scholar]; (c) Parsell TH, Behan RK, Green MT, Hendrich MP, Borovik AS. J. Am. Chem. Soc. 2006;128:8728–8729. doi: 10.1021/ja062332v. [DOI] [PubMed] [Google Scholar]
- 27.(a) Shirin Z, Hammes BS, Young VG, Jr, Borovik AS. J. Am. Chem. Soc. 2000;122:1836–1837. [Google Scholar]; (b) Zdilla MJ, Abu-Omar MM. J. Am. Chem. Soc. 2006;128:16971–16979. doi: 10.1021/ja0665489. [DOI] [PubMed] [Google Scholar]
- 28.(a) Fukuzumi S, Fujioka N, Kotani H, Ohkubo K, Lee Y-M, Nam W. J. Am. Chem. Soc. 2009;131:17127–17134. doi: 10.1021/ja9045235. [DOI] [PubMed] [Google Scholar]; (b) Lee JY, Lee Y-M, Kotani H, Nam W, Fukuzumi S. Chem. Commun. 2009:704–706. doi: 10.1039/b814928c. [DOI] [PubMed] [Google Scholar]; (c) Prokop KA, Visser SP, de Goldberg DP. Angew. Chem. 2010;2010;122:5217–5221. [Google Scholar]; Angew. Chem. Int. Ed. 2010;49:5091–5095. doi: 10.1002/anie.201001172. [DOI] [PubMed] [Google Scholar]; (d) Fukuzumi S, Kotani H, Prokop KA, Goldberg DP. J. Am. Chem. Soc. 2011;133:1859–1869. doi: 10.1021/ja108395g. [DOI] [PubMed] [Google Scholar]
- 29.(a) Yin G. Acc. Chem. Res. 2013;46:483–492. doi: 10.1021/ar300208z. [DOI] [PubMed] [Google Scholar]; (b) Zhang R, Horner JH, Newcomb M. J. Am. Chem. Soc. 2005;127:6573–6582. doi: 10.1021/ja045042s. [DOI] [PubMed] [Google Scholar]; (c) Angelis FD, Jin N, Car R, Groves JT. Inorg. Chem. 2006;45:4268–4276. doi: 10.1021/ic060306s. [DOI] [PubMed] [Google Scholar]; (d) Arunkumar C, Lee Y-M, Lee JY, Fukuzumi S, Nam W. Chem.-Eur. J. 2009;15:11482–11489. doi: 10.1002/chem.200901362. [DOI] [PubMed] [Google Scholar]
- 30.(a) Mukhopadhyay S, Gandhi BA, Kirk ML, Armstrong WH. Inorg. Chem. 2003;42:8171–8180. doi: 10.1021/ic034641h. [DOI] [PubMed] [Google Scholar]; (b) Kurahashi T, Kikuchi A, Shiro Y, Hada M, Fujii H. Inorg. Chem. 2010;49:6664–6672. doi: 10.1021/ic100673b. [DOI] [PubMed] [Google Scholar]; (c) Pecoraro VL, Hsieh W-Y. Inorg. Chem. 2008;47:1765–1778. doi: 10.1021/ic7017488. [DOI] [PubMed] [Google Scholar]
- 31.(a) Lansky DE, Kosack JR, Sarjeant AAN, Goldberg DP. Inorg. Chem. 2006;45:8477–8479. doi: 10.1021/ic0609251. [DOI] [PubMed] [Google Scholar]; (b) Wang SH, Mandimutsira BS, Todd R, Ramdhanie B, Fox JP, Goldberg DP. J. Am. Chem. Soc. 2004;126:18–19. doi: 10.1021/ja038951a. [DOI] [PubMed] [Google Scholar]; (c) Lewis RA, Wu G, Hayton TW. Inorg. Chem. 2011;50:4660–4668. doi: 10.1021/ic200490v. [DOI] [PubMed] [Google Scholar]
- 32.(a) Shen D, Miao C, Wang S, Xia C, Sun W. Org. Lett. 2014;16:1108–1111. doi: 10.1021/ol4037083. [DOI] [PubMed] [Google Scholar]; (b) Gómez-Hortigüela L, Corà F, Catlow CRA. ACS Catal. 2011;1:18–28. [Google Scholar]; (c) Krewald V, Neese F, Pantazis DA. J. Am. Chem. Soc. 2013;135:5726–5739. doi: 10.1021/ja312552f. [DOI] [PubMed] [Google Scholar]
- 33.Jung J, Ohkubo K, Goldberg DP, Fukuzumi S. J. Phys. Chem. A. 2014;118:6223–6229. doi: 10.1021/jp505860f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.(a) Morimoto Y, Kotani H, Park J, Lee Y, Nam W, Fukuzumi S. J. Am. Chem. Soc. 2011;133:403–405. doi: 10.1021/ja109056x. [DOI] [PubMed] [Google Scholar]; (b) Fukuzumi S, Morimoto Y, Kotani H, Naumov P, Lee Y, Nam W. Nat. Chem. 2010;2:756–759. doi: 10.1038/nchem.731. [DOI] [PubMed] [Google Scholar]; (c) Fukuzumi S, Ohkubo K, Morimoto Y. Phys. Chem. Chem. Phys. 2012;14:8472–8484. doi: 10.1039/c2cp40459a. [DOI] [PubMed] [Google Scholar]; (d) Fukuzumi S, Ohkubo K. Coord. Chem. Rev. 2010;254:372–385. [Google Scholar]
- 35.(a) Chen J, Lee Y, Davis KM, Wu X, Seo MS, Cho K, Yoon H, Park YJ, Fukuzumi S, Pushkar YN, Nam W. J. Am. Chem. Soc. 2013;135:6388–6391. doi: 10.1021/ja312113p. [DOI] [PubMed] [Google Scholar]; (b) Yoon H, Lee Y-M, Wu X, Cho K-B, Sarangi R, Nam W, Fukuzumi S. J. Am. Chem. Soc. 2013;135:9186–9194. doi: 10.1021/ja403965h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Li F, Heuvelen KM, van Meier KK, Münck E, Que L., Jr J. Am. Chem. Soc. 2013;135:10198–10201. doi: 10.1021/ja402645y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park YJ, Ziller JW, Borovik AS. J. Am. Chem. Soc. 2011;133:9258–9261. doi: 10.1021/ja203458d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.(a) Park YJ, Cook SA, Sickerman NS, Sano Y, Ziller JW, Borovik AS. Chem. Sci. 2013;4:717–726. doi: 10.1039/C2SC21400H. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lee YM, Bang S, Kim YM, Cho J, Hong S, Nomura T, Ogura T, Troeppner O, Ivanovic- Burmazovic I, Sarangi R, Fukuzumi S, Nam W. Chem. Sci. 2013;4:3917–3923. doi: 10.1039/C3SC51864G. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Yin G, Danby AM, Kitko D, Carter JD, Scheper WM, Busch DH. J. Am. Chem. Soc. 2008;130:16245–16253. doi: 10.1021/ja804305x. [DOI] [PubMed] [Google Scholar]
- 38.(a) Park J, Morimoto Y, Lee Y-M, Nam W, Fukuzumi S. Inorg. Chem. 2014;53:3618–3628. doi: 10.1021/ic403124u. [DOI] [PubMed] [Google Scholar]; (c) Park J, Lee Y-M, Nam W, Fukuzumi S. J. Am. Chem. Soc. 2013;135:5052–5061. doi: 10.1021/ja311662w. [DOI] [PubMed] [Google Scholar]
- 39.(a) Du H, Lo P, Hu Z, Liang H, Lau K, Wang YN, Lam WWY, Lau TC. Chem. Commun. 2011;47:7143–7145. doi: 10.1039/c1cc12024g. [DOI] [PubMed] [Google Scholar]; (b) Yiu S-M, Man W-L, Lau T-C. J. Am. Chem. Soc. 2008;130:10821–10827. doi: 10.1021/ja802625e. [DOI] [PubMed] [Google Scholar]; (c) Lam WWY, Yiu S-M, Lee JMN, Yau SKY, Kwong H-K, Lau T-C, Liu D, Lin Z. J. Am. Chem. Soc. 2006;128:2851–2858. doi: 10.1021/ja0552951. [DOI] [PubMed] [Google Scholar]; (d) Yiu S-M, Wu Z-B, Mak C-K, Lau T-C. J. Am. Chem. Soc. 2004;126:14921–14929. doi: 10.1021/ja0487832. [DOI] [PubMed] [Google Scholar]
- 40.(a) Herbert DE, Lionetti D, Rittle J, Agapie T. J. Am. Chem. Soc. 2013;135:19075–19078. doi: 10.1021/ja4104974. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Smeltz JL, Lilly CP, Boyle PD, Ison EA. J. Am. Chem. Soc. 2013;135:9433–9441. doi: 10.1021/ja401390v. [DOI] [PubMed] [Google Scholar]; (c) Asao N, Sato K. Org. Lett. 2006;8:5361–5363. doi: 10.1021/ol062268m. [DOI] [PubMed] [Google Scholar]
- 41.(a) Tsui EY, Tran R, Yano J, Agapie T. Nat. Chem. 2013;5:293–299. doi: 10.1038/nchem.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Das D, Lee Y-M, Ohkubo K, Nam W, Karlin K, D; Fukuzumi S. J. Am. Chem. Soc. 2013;135:4018–4026. doi: 10.1021/ja312256u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Das D, Lee Y-M, Ohkubo K, Nam W, Karlin KD, Fukuzumi S. J. Am. Chem. Soc. 2013;135:2825–2834. doi: 10.1021/ja312523u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.(a) Leeladee P, Baglia RA, Prokop KA, Latifi R, de Visser SP, Goldberg DP. J. Am. Chem. Soc. 2012;134:10397–10400. doi: 10.1021/ja304609n. [DOI] [PubMed] [Google Scholar]; (b) Baglia RA, Dürr M, Ivanovic-Burmazovic I, Goldberg DP. Inorg. Chem. 2014;53:5893–5895. doi: 10.1021/ic500901y. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zaragoza JPT, Baglia RA, Siegler MA, Goldberg DP. J. Am. Chem. Soc. 2015;137:6531–6540. doi: 10.1021/jacs.5b00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neu HM, Jung J, Baglia RA, Siegler MA, Ohkubo K, Fukuzumi S, Goldberg DP. J. Am. Chem. Soc. 2015;137:4614–4617. doi: 10.1021/jacs.5b00816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.(a) Rauchfuss TB. Acc. Chem. Res. 2015;48:2107–2116. doi: 10.1021/acs.accounts.5b00177. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Fukuzumi S, Tahsini L, Lee Y-M, Ohkubo K, Nam W. J. Am. Chem. Soc. 2012;134:7025–7035. doi: 10.1021/ja211656g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Svetlanova-Larsen A, Zoch CR, Hubbard JL. Organometallics. 1996;15:3076–3087. [Google Scholar]
- 45.(a) Labios LA, Heiden ZM, Mock MT. Inorg. Chem. 2015;54:4409–4422. doi: 10.1021/acs.inorgchem.5b00209. [DOI] [PubMed] [Google Scholar]; (b) Yue NLS, Jennings MC, Puddephatt RJ. Inorg. Chem. 2005;44:1125–1131. doi: 10.1021/ic048549c. [DOI] [PubMed] [Google Scholar]; (c) del Río I, Gossage RA, Hannu MS, Lutz M, Spek AL, van Koten G. Organometallics. 1999;18:1097–1105. [Google Scholar]
- 46.(a) Lansky DE, Mandimutsira B, Ramdhanie B, Clausen M, Penner-Hahn J, Zvyagin SA, Telser J, Krzystek J, Zhan R, Ou Z, Kadish KM, Zakharov L, Rheingold AL, Goldberg DP. Inorg. Chem. 2005;44:4485–4498. doi: 10.1021/ic0503636. [DOI] [PubMed] [Google Scholar]; (b) Ramdhanie B, Stern CL, Goldberg DP. J. Am. Chem. Soc. 2001;123:9447–9448. doi: 10.1021/ja011229x. [DOI] [PubMed] [Google Scholar]; (c) Mandimutsira BS, Ramdhanie B, Todd RC, Wang HL, Zareba AA, Czernuszewicz RS, Goldberg DP. J. Am. Chem. Soc. 2002;124:15170–15171. doi: 10.1021/ja028651d. [DOI] [PubMed] [Google Scholar]
- 47.Armarego WLF, Perrin DD, editors. Purification of Laboratory Chemicals. Oxford: Pergamon Press; 1997. [Google Scholar]
- 48.(a) Sheldrick GM. Acta Crystallogr. C. 2008;A64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]; (b) Sheldrick GM. Acta Crystallogr. C. 2015;71:3–8. doi: 10.1107/S2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spek AL. J. Appl. Cryst. 2003;36:7–13. [Google Scholar]
- 50.Hatchard CG, Parker CA. Proc. R. Soc. London, Ser. A. 1956;235:518–536. [Google Scholar]
- 51.(a) Neu HM, Quesne MG, Yang T, Prokop-Prigge KA, Lancaster KM, Donohoe J, DeBeer S, de Visser SP, Goldberg DP. Chem.-Eur. J. 2014;20:14584–14588. doi: 10.1002/chem.201404349. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Neu HM, Yang T, Baglia RA, Yosca TH, Green MT, Quesne MG, de Visser SP, Goldberg DP. J. Am. Chem. Soc. 2014;136:13845–13852. doi: 10.1021/ja507177h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Punniyamurthy T, Velusamy S, Iqbal J. Chem. Rev. 2005;105:2329–2363. doi: 10.1021/cr050523v. [DOI] [PubMed] [Google Scholar]
- 53.Pistorio BJ, Chang CJ, Nocera DG. J. Am. Chem. Soc. 2002;124:7884–7885. doi: 10.1021/ja026017u. [DOI] [PubMed] [Google Scholar]
- 54.Zhou X-T, Ji H-B. Catal. Commun. 2014;53:29–32. [Google Scholar]
- 55.Kim Y, Choi JR, Yoon M. J. Phys. Chem. B. 2001;105:8513–8518. [Google Scholar]
- 56.(a) Humphrey JL, Kuciauskas D. J. Am. Chem. Soc. 2006;128:3902–3903. doi: 10.1021/ja0588353. [DOI] [PubMed] [Google Scholar]; (b) Spänig F, Ruppert M, Dannhäuser J, Hirsch A, Guldi DM. J. Am. Chem. Soc. 2009;131:9378–9388. doi: 10.1021/ja9029686. [DOI] [PubMed] [Google Scholar]; (c) Gonçalves PJ, De Boni L, Borissevitch IE, Zílio SC. J. Phys. Chem. A. 2008;112:6522–6526. doi: 10.1021/jp800589j. [DOI] [PubMed] [Google Scholar]; (d) Krokos E, Spänig F, Ruppert M, Hirsch A, Guldi DM. Chem.-Eur. J. 2012;18:1328–1341. doi: 10.1002/chem.201102851. [DOI] [PubMed] [Google Scholar]
- 57.(a) Cho J, Sarangi R, Nam W. Acc. Chem. Res. 2012;45:1321–1330. doi: 10.1021/ar3000019. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Peterson RL, Himes RA, Kotani H, Suenobu T, Tian L, Siegler MA, Solomon EI, Fukuzumi S, Karlin KD. J. Am. Chem. Soc. 2011;133:1702–1705. doi: 10.1021/ja110466q. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Cho J, Woo J, Nam W. J. Am. Chem. Soc. 2010;132:5958–5959. doi: 10.1021/ja1015926. [DOI] [PubMed] [Google Scholar]; (d) Nemes A, Bakac A. Inorg. Chem. 2001;40:746–749. doi: 10.1021/ic001063l. [DOI] [PubMed] [Google Scholar]
- 58.(a) Clennan EL, Greer AJ. Org. Chem. 1996;61:4793–4797. doi: 10.1021/jo9604999. [DOI] [PubMed] [Google Scholar]; (b) Bonesi SM, Albini AJ. Org. Chem. 2000;65:4532–4536. doi: 10.1021/jo000069p. [DOI] [PubMed] [Google Scholar]; (c) Baciocchi E, Chiappe C, Fasciani C, Lanzalunga O, Lapi A. Org. Lett. 2010;12:5116–5119. doi: 10.1021/ol102263w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.