Supplemental Digital Content is available in the text.
Keywords: clinical trial [publication type], coronary disease, heart failure, primary prevention, safety
Abstract
Background—
Extended follow-up of statin-based low-density lipoprotein cholesterol lowering trials improves the understanding of statin safety and efficacy. Examining cumulative cardiovascular events (total burden of disease) gives a better appreciation of the clinical value of statins. This article evaluates the long-term impact of therapy on mortality and cumulative morbidity in a high-risk cohort of men.
Methods and Results—
The West of Scotland Coronary Prevention Study was a primary prevention trial in 45- to 64-year-old men with high low-density lipoprotein cholesterol. A total of 6595 men were randomized to receive pravastatin 40 mg once daily or placebo for an average of 4.9 years. Subsequent linkage to electronic health records permitted analysis of major incident events over 20 years. Post trial statin use was recorded for 5 years after the trial but not for the last 10 years. Men allocated to pravastatin had reduced all-cause mortality (hazard ratio, 0.87; 95% confidence interval, 0.80–0.94; P=0.0007), attributable mainly to a 21% decrease in cardiovascular death (hazard ratio, 0.79; 95% confidence interval, 0.69–0.90; P=0.0004). There was no difference in noncardiovascular or cancer death rates between groups. Cumulative hospitalization event rates were lower in the statin-treated arm: by 18% for any coronary event (P=0.002), by 24% for myocardial infarction (P=0.01), and by 35% for heart failure (P=0.002). There were no significant differences between groups in hospitalization for noncardiovascular causes.
Conclusion—
Statin treatment for 5 years was associated with a legacy benefit, with improved survival and a substantial reduction in cardiovascular disease outcomes over a 20-year period, supporting the wider adoption of primary prevention strategies.
Lowering of low-density lipoprotein (LDL) cholesterol is accepted as a key objective in the prevention of cardiovascular disease.1,2 Controversies remain, however, as to which kind of subjects to treat, the use of goals, the magnitude of the benefit, and potential harms, especially in the context of primary prevention.3–6 Examination of the long-term (lifetime) consequences of lowering LDL cholesterol can assist greatly in understanding more fully the efficacy and safety of this intervention, and a number of studies have reported extended observations beyond the end of the formal trial.7–11 In the West of Scotland Coronary Prevention Study (WOSCOPS), in which follow-up was first examined ≈10 years after the end of the 5-year trial,7 there was evidence of further reduction in coronary events over the 15-year period and, as indicated by the available data, no emergent safety issues. Overall, there was a reduction in all-cause mortality (hazard ratio [HR]=0.88; 95% confidence interval [CI], 0.79–0.99; P=0.03) and in the outcome of death or hospitalization for coronary heart disease (HR=0.75; 95% CI, 0.68–0.83; P<0.001). Furthermore, over the 15-year period, treatment with pravastatin for 5 years was shown to be cost-saving in terms of overall health service costs,12 adding an important economic dimension to the clinical outcome analysis. Previous work had shown that there was a relatively low uptake of statin treatment in the first 5 years of extended follow-up after the trial, which means that WOSCOPS is uniquely placed to investigate the legacy effects of 5 years of statin treatment in terms of ongoing benefit and potential safety issues later in life.
Clinical Perspective on p 1080
We have now increased the period of follow-up to 20 years to examine a range of mortality and morbidity outcomes as a first event and as a total burden of disease in the form of cumulative hospital admissions. More detailed interrogation of hospitalization rates has allowed a fuller picture of benefits and risks to emerge. Given a mean age at randomization of 55 years, the extended observation period to a mean of 75 years (range, 65–84 years) gives an approximation of the lifetime benefit of this pharmacological intervention.
Methods
The design of WOSCOPS and its long-term follow-up have been described elsewhere.13–15 It was a randomized trial of pravastatin (40 mg once daily) versus placebo in men 45 to 64 years of age (mean age, 55 years) with raised cholesterol who had no evidence of previous myocardial infarction (based on medical history and a baseline, centrally read ECG). Participants had a mean±SD plasma cholesterol level of 272±23 mg/dL (7.0±0.6 mmol/L) and a mean±SD LDL cholesterol level of 192±17 mg/dL (5.0±0.44 mmol/L); 44% were current smokers; 16% had a history of hypertension; and 1% had a history of diabetes mellitus. Between 1989 and 1991, 6595 men gave written informed consent and were enrolled in the trial. The average follow-up was 4.9 years (range, 3.5–6.1 years), with final study visits in May 1995.
After the end of the trial, use of lipid-lowering therapy during the first 5 years of extended follow-up was monitored by review of case records. In the original pravastatin and placebo groups, 28.6% and 24.3% at 1 year after the trial, 33.6% and 29.4% at 3 years, and 38.7% and 35.2% at 5 years, respectively, were found to be on statins.7 No further data on statin treatment were available after this point. Extended follow-up for clinical events was based entirely on linkage to national electronic hospital discharge records held by the Information Services Division in Edinburgh, the Scottish Cancer Registry, and the Scottish General Register Office death records by means of established methods.16,17 Data were extracted from the beginning of the trial to October 2011 and classified with International Classification of Diseases codes and Office of Population, Censuses and Surveys Classification of Surgical Operations and Procedures codes. Cancer Registry Data were available only until November 2010.
The original trial was approved by the ethics committees of the University of Glasgow and participating health boards in Scotland, and the long-term follow-up and associated record linkage were approved by the ethics committee of the Glasgow Royal Infirmary and the Privacy Advisory Committee of the National Health Service for Scotland. All participants gave informed consent to take part in the trial and for the examination of their medical records.
Previous analyses focused on time to first event for deaths, incident cancers, and composite cardiovascular outcomes7,14 and health economics evaluation.12 In the present report, we assessed the impact on mortality, incident cancers, and cumulative number of hospital admissions over 20 years or until death. We report hospital admissions for noncardiovascular causes, cardiovascular causes, coronary heart disease, myocardial infarction, heart failure, and stroke, and we describe the cumulative number of coronary revascularizations (coronary artery bypass graft, percutaneous coronary intervention, or angioplasty).
Statistical Methods
Cumulative incidence functions, accounting for the competing risk of death from other causes, were used to describe the incidence of cause-specific deaths or time to first incident cancer. To estimate treatment effects for cause-specific mortality and incident cancers, Cox proportional hazards models were fitted, including the treatment group and baseline risk factors of age, body mass index, systolic and diastolic blood pressures, high-density lipoprotein and LDL cholesterol levels, log-transformed triglyceride level, nitrate use, history of angina, history of diabetes mellitus, history of hypertension (all yes or no), smoking status (current, former, never), and a 7-category social deprivation score.18 Treatment effects (pravastatin versus placebo) were expressed as HRs with 95% CIs and corresponding P values.
The cumulative numbers of hospital admissions of each type were presented without adjustment for the competing risk of death to represent the true difference in healthcare resource use over 20 years (all participants had a potential follow-up of a minimum of 20 years). We also calculated the crude rates of hospital admission of each type, correcting for the different total periods of follow-up in each randomized group resulting from the increased survival and consequent greater exposure to risk in the statin-treated group. These statistics were compared with the use of rerandomization tests (based on 10 000 rerandomizations).
Because of the interest in the long-term impact of statin treatment on diabetes mellitus and its complications, we identified all noncardiovascular hospital admissions that were associated with diabetes mellitus or its complications either as a reason for admission or as a factor complicating the admission. Cardiovascular admissions were omitted from this analysis because of the potential bias associated with the overall reduction in cardiovascular admissions resulting from statin treatment. Similarly, we reported other noncardiovascular hospital admissions grouped by International Classification of Diseases, 10th Revision codes to examine the long-term safety of statin use. These analyses were further subdivided into day cases and non–day cases.
The data analysis was generated with SAS software, version 9.3 of the SAS System for Windows (Cary, NC).
Results
The mean follow-up until censoring date or death in the pravastatin-treated group was 18.6 compared with 18.3 years for the placebo group. Baseline characteristics of the 2 randomized groups have been reported previously.15 There were no differences in characteristics at baseline between the 2 groups, including age, body mass index, blood pressure, cholesterol, alcohol use, smoking, employment, and medical history.
Mortality
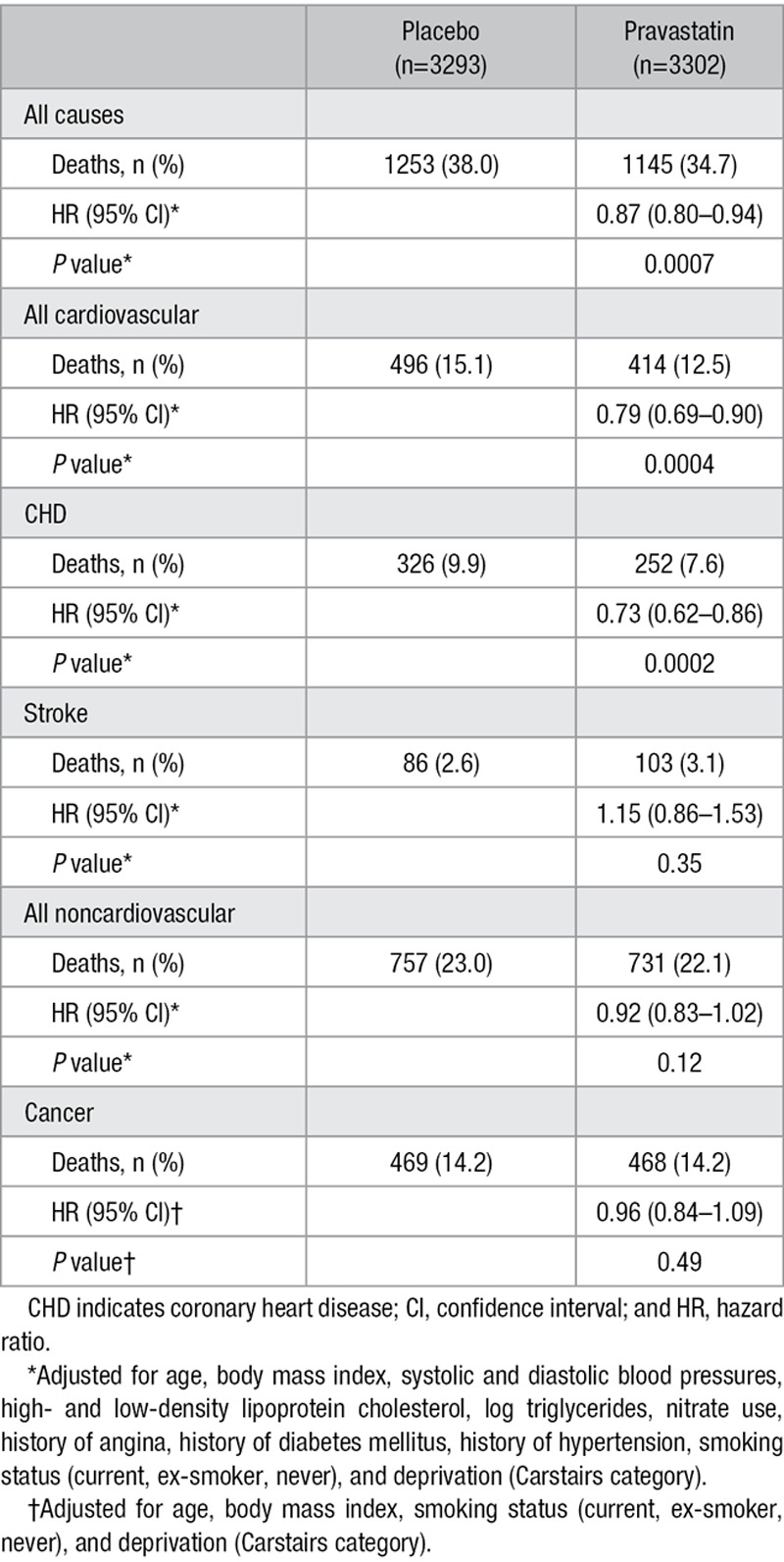
In the total follow-up period, 1253 (38%) of those originally randomized to placebo died compared with 1145 (34.7%) in the pravastatin group (HR=0.87; 95% CI, 0.80–0.94; P=0.0007). There were also reductions in cardiovascular mortality (HR=0.79; 95% CI, 0.69–0.90; P=0.0004) and coronary mortality (HR=0.73; 95% CI, 0.62–0.86; P=0.0002) but not stroke. There was no evidence of an increased risk of noncardiovascular or cancer mortality in the pravastatin group (Table 1, Figure 1A–1D, and Figure Ia in the online-only Data Supplement).
Figure 1.
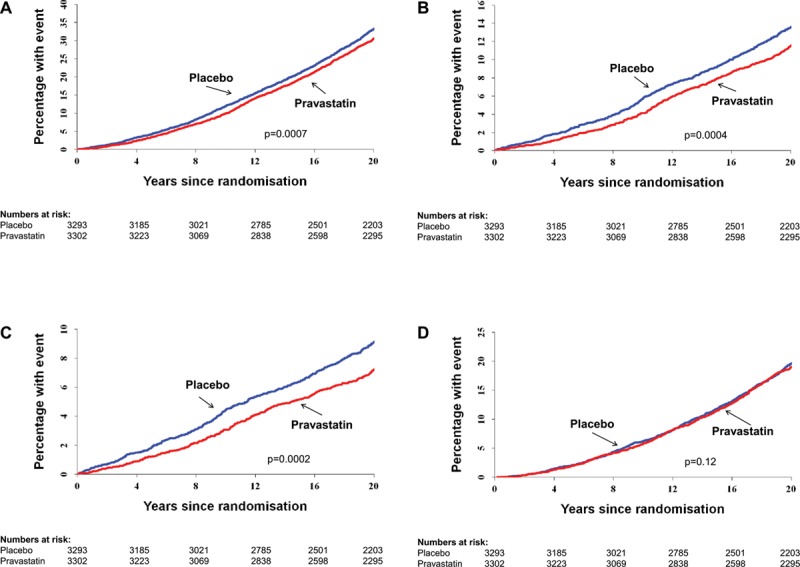
Cumulative events over the 20-year follow-up period. Cumulative incidence functions are provided for the outcomes of death resulting from (A) all causes, (B) cardiovascular disease, (C) coronary heart disease, and (D) noncardiovascular disease. P values were determined by Cox proportional hazards model.
Table 1.
Number of Events and HRs for Pravastatin Treatment Effect for Mortality Outcomes
Incident Cancers
There was no evidence of an increased risk of overall incident cancer (809 [24.6%] participants had events in the placebo-treated group compared with 802 [24.3%] on pravastatin; P=0.24) or of cause-specific cancers (Table I and Figure Ib in the online-only Data Supplement).
Cumulative Hospital Admissions
In the group of 3293 participants originally randomized to placebo, 1546 experienced a total of 4102 cardiovascular admissions compared with 1398 participants (of 3302) in the pravastatin group who had 3436 admissions (P<0.0001; Table 2 and Figure 2A). Similarly, there were significant reductions in recurrent coronary (P=0.0006), myocardial infarction (P=0.0002), and heart failure (P=0.01) admissions (Figure IIa in the online-only Data Supplement and Figure 2B–2C) but not stroke admissions (Figure IIb in the online-only Data Supplement). There was a significant reduction in hospital admissions involving coronary revascularization (percutaneous coronary intervention, coronary artery bypass surgery or angioplasty), with 1210 events in the placebo group and 1029 in the pravastatin group (P=0.0078; Figure 2D).
Table 2.
Subjects With Events, Cumulative Recurrent Events, and Event Rates per 10 Years of Follow-Up Between Randomized Groups
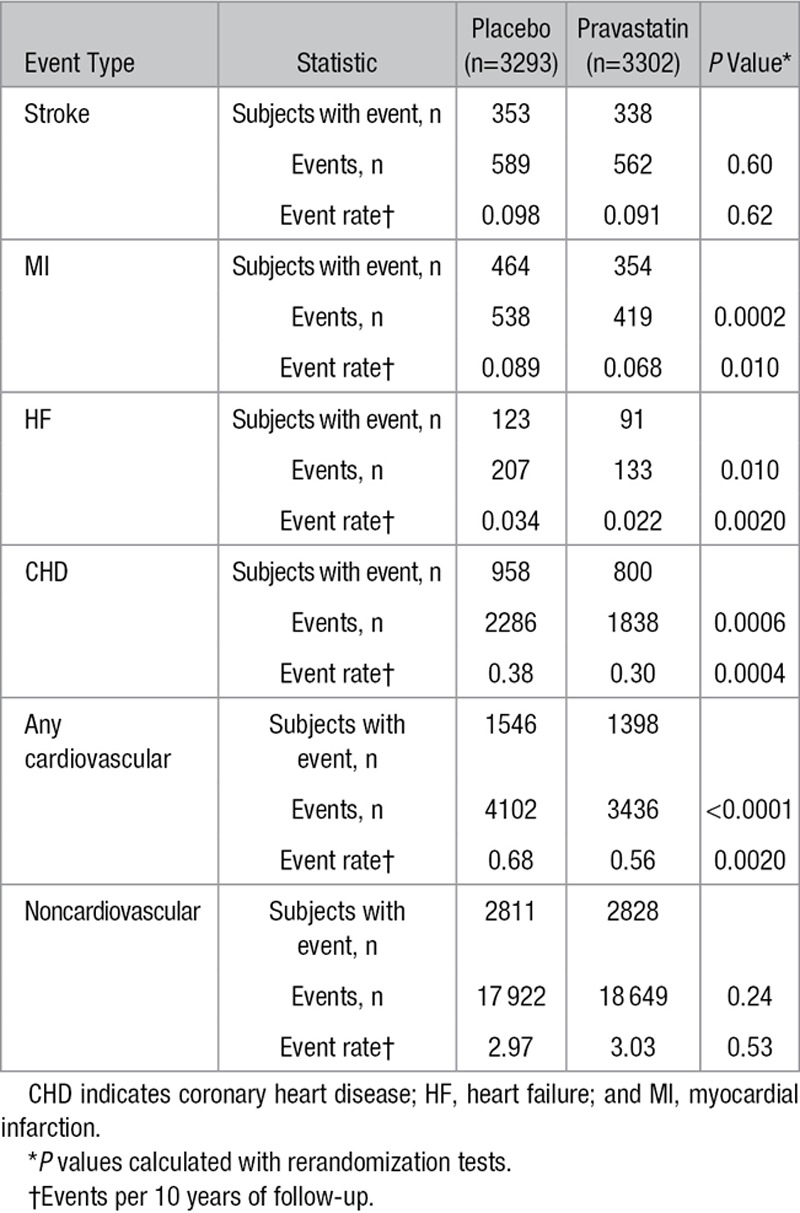
Figure 2.
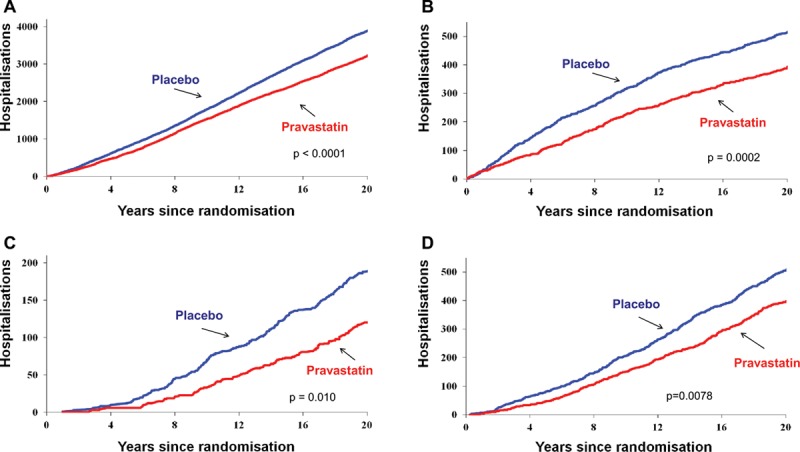
Cumulative numbers of hospital admissions for the outcomes of (A) cardiovascular disease, (B) myocardial infarction, (C) heart failure, and (D) coronary revascularization. P values were computed by rerandomization tests.
There were numerically more noncardiovascular admissions in the pravastatin group, but after adjustment for duration of follow-up, the rates were similar (2.97 events in 10 years for placebo compared with 3.03 events in 10 years for pravastatin). Neither comparison achieved statistical significance (Table 2 and Figure IIc in the online-only Data Supplement).
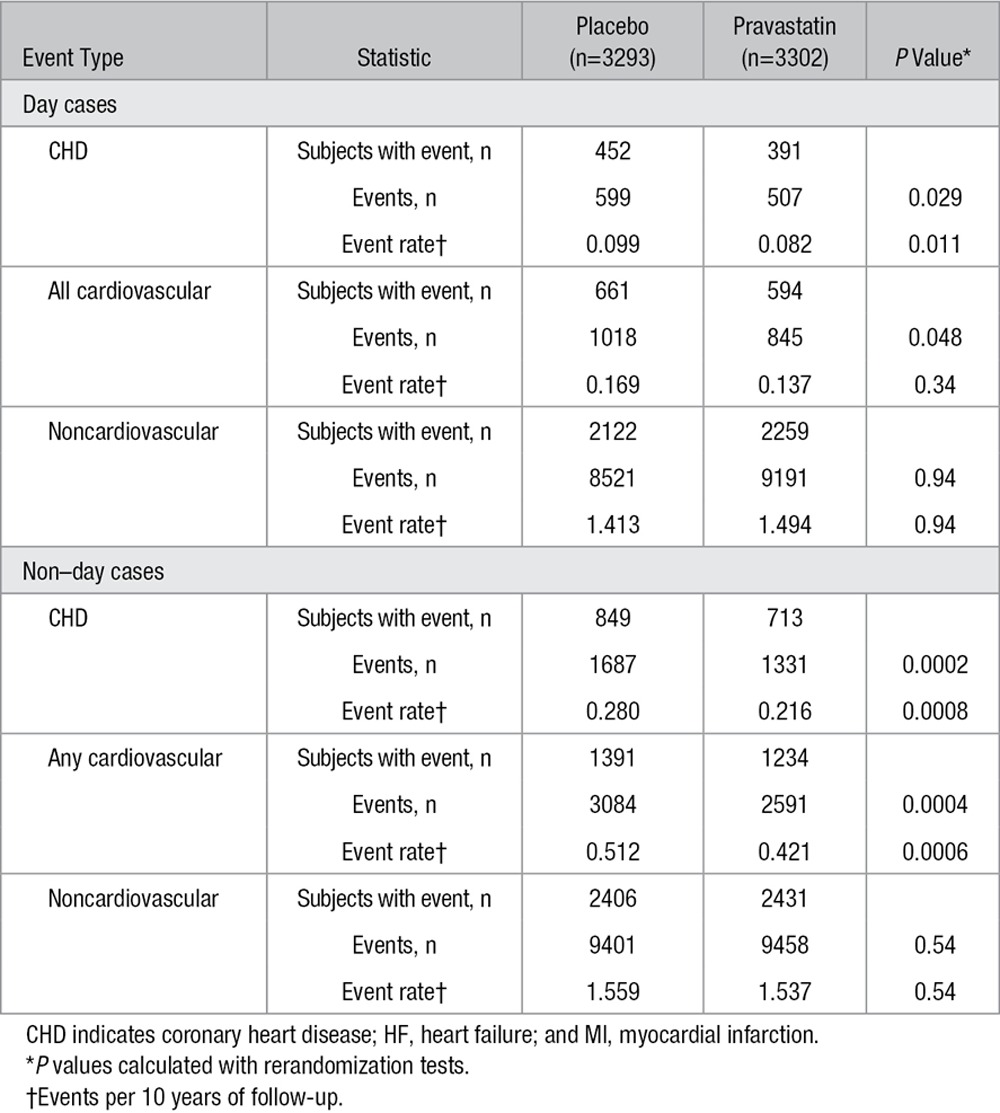
In a further exploration, hospitalizations were divided into day cases and non–day cases (ie, events involving an overnight stay). Subjects in the pravastatin group had fewer hospitalizations for cardiovascular reasons in both categories (Table 3). Tables II and III in the online-only Data Supplement give the frequency of noncardiovascular admissions by International Classification of Diseases body system classification for day cases and non–day cases. Overall, the numbers of subjects and cumulative events were balanced between the 2 groups for non–day cases. For day cases, it was noted that the pravastatin group had an apparent increased risk of events associated with diseases of the eye and adnexa (P=0.03 uncorrected for multiple comparisons), which was attributable for the most part to an excess of admissions for cataract surgery (not individually statistically significant). There were numerically more day-case events but not subjects with events associated with neoplasms and diseases of the digestive system.
Table 3.
Subjects With Events, Cumulative Recurrent Events, and Event Rates per 10 Years of Follow-Up Between Randomized Groups, Subdivided by Day Case/Non–Day Case
Hospital Admissions Associated With Complications of Diabetes Mellitus
In the placebo group, a total of 221 participants experienced 911 noncardiovascular hospital admissions that contained a diabetes-related International Classification of Diseases code compared with 201 participants (770 admissions) in the pravastatin-treated group (HR=0.81; 95% CI, 0.67–0.98; P=0.030). For hospital admissions involving complications of diabetes mellitus, 29 participants in the placebo group experienced 80 admissions compared with 12 participants (44 admissions) in the pravastatin-treated group (HR=0.33; 95% CI, 0.16–0.66; P=0.0016). There were 23 deaths for which diabetes mellitus was given as the cause (15 patients on placebo and 8 patients on pravastatin).
Discussion
This 20-year follow-up of the WOSCOPS identified a continued legacy benefit from 5 years of LDL cholesterol lowering with a statin through improved survival resulting from decreased mortality from cardiovascular causes and an ongoing reduction in cardiovascular hospital admissions. Cumulative event rates are presented for both treatment arms to assess the impact of therapy on the total burden of disease. We observed a substantial and significant benefit: Cumulative event rates were 18% lower for cardiovascular disease and 24% lower for myocardial infarction in the pravastatin group. Our focus on recurrent events reflects current interest in the impact of interventions on the total burden of disease. We also observed continuing divergence of the cumulative event curves for heart failure hospitalization over 20 years, with a 35% lower rate in the pravastatin arm.
More than 2 decades since the publication of the first successful primary prevention trial of a statin,15 with subsequent studies19,20 and meta-analyses5,21,22 also confirming the benefits of LDL cholesterol reduction, there are still concerns about side effects of treatment, long-term safety, impact on all-cause mortality, and cost-effectiveness3–6,23,24 that lead a number of commentators to continue to express caution when wider use of statins in primary prevention strategies is promoted.25,26 The present study found no increased incidence of cancer overall, and enhanced site-specific data show no imbalance between the 2 groups (Note the balanced rates for prostate cancer compared with the previous report).7 Examination of noncardiovascular hospital admissions also showed no differences, with the exception of a possible increased risk of day-case hospital admissions associated with diseases of the eye for patients using pravastatin. The significance of this latter finding, which should be treated cautiously because of the borderline significance and the multiple adverse effects investigated, lies in its link to historical concern that inhibition of cholesterol synthesis would lead to risk of corneal opacity.27,28 Epidemiological studies have suggested both increased and decreased risk of cataracts linked to statin use.29–31 However, these studies will not have the length of follow-up available in WOSCOPS or the benefits of randomization in minimizing confounding factors. It should be noted that any treatment that improves cardiovascular survival will inevitably result in an increase in hospital admissions for noncardiovascular causes. We saw no evidence of this for non–day-case admissions. However, the trend toward increased day-case admissions, including treatment for cancer but not number of participants with cancer, particularly for events associated with advancing age, in the later years of follow-up could be early evidence of this survival bias effect.
Reduction in heart failure as an outcome has been reported recently in a meta-analysis of 14 trials of statin-based LDL lowering with a risk reduction of 10%.32 The mean duration of observation in these studies was 4.3 years. Data presented here suggest that this additional clinical benefit may be underestimated in short-term studies, particularly in primary prevention. As Preiss et al32 noted, we found that the number of heart failure admissions was reduced in subjects who had and in those who did not have an antecedent myocardial infarction (data not shown). The overall mechanism by which LDL lowering leads to a reduced incidence of heart failure is not fully clear. However, we note that much of the WOSCOPS follow-up was before the use of troponin assays and certainly before newer high-sensitivity assays. Hence, many of the events classified as other coronary hospitalizations would today be classified as myocardial infarction. Likewise, the reduced need for revascularization in statin-treated participants indicates lower levels of ischemia, the repeated occurrence of which could be a mechanism for the development of heart failure.
We did not demonstrate a reduction in stroke at 20 years. There was no effect in the original trial,15 although we saw evidence of stroke reduction in the 15-year follow-up.7 However, WOSCOPS was a primary prevention trial in relatively young subjects compared with the majority of trials in the Cholesterol Treatment Trialists Collaborators (CTTC) analysis, which were secondary prevention studies in participants ≈10 years older at randomization and had shorter periods of follow-up.22 It is therefore difficult to compare directly the findings in CTTC with extended observations made when the original treatment arms are predicted to be receiving statin therapy at the same level after the trial.
In the assessment of the long-term impact of interventions in primary prevention, it is essential that there is a balanced evaluation of the benefits resulting from reduction in cardiovascular events and the potential for adverse clinical outcomes. Muscle-related side effects of statins have been studied in detail.33 They can lead to intolerance to the medication and, in rare instances, rhabdomyolysis.1,2 Statin therapy has also been shown in a number of studies to increase the propensity to develop type 2 diabetes mellitus,34,35 with an HR of ≈1.09 compared with placebo; a similar increase in diabetes risk is associated with the use of high- versus low-dose statin therapy.35 This is one of the issues raised by those concerned about the more widespread use of statins in the prevention of disease in lower-risk subjects.3–6 Diabetes mellitus is defined on the basis of blood glucose levels and is a disorder associated with macrovascular but also microvascular/noncardiovascular complications. Statins reduce cardiovascular disease, and it has been estimated that their use will prevent 5 incidences of myocardial infarction for every new case of diabetes mellitus.36 In this study, we were able to report that statin treatment was also associated over a 20-year follow-up with significantly fewer hospital admissions associated with noncardiovascular complications of diabetes mellitus. This raises the possibility that although statin use may affect blood glucose levels, this does not necessarily translate into deleterious noncardiovascular pathologies. It should be noted, however, that WOSCOPS was unusual in that the incidence of type 2 diabetes mellitus was lower in the actively treated arm during the original trial.37
There are limitations to this form of long-term follow-up based on electronic health records as noted previously.7,16 Critically, we do not know what lipid-regulating therapy was being used by the participants for the last 10 years of the study, and this limits the interpretation of data with regard to the magnitude of the 20-year benefit. If in the second half of the extended follow-up more subjects originally assigned to the placebo arm compared with the pravastatin arm were placed on a statin (as a result of having a coronary event or as a primary prevention measure by the general practitioner), then the difference in event rates between the 2 groups would be diminished, and the observed HR would be an underestimate of the long-term risk reduction attributable to statin therapy. In addition, we are dependent on the stability of the population to allow comprehensive capture of hospitalizations within the healthcare information systems. However, Scotland is an area of relatively low social mobility after people reach middle age, which allowed us to achieve 100% follow-up at the end of the study. We also flagged all of our participants with the death registry in England and Wales and identified only 15 deaths (0.6%) there from a total of 2398 deaths. We have no current method for identifying hospital admissions outside Scotland but do not feel this would have altered our findings. Finally, as a substantial number of participants die, analyses are complicated by competing risks.
Conclusions
In this primary prevention trial in high-risk men with elevated LDL cholesterol but without a history of myocardial infarction, we observed a long-term legacy benefit of LDL lowering by statin therapy. Because over the 20 years the cohort aged from an average of 55 to 75 years, the cumulative event rate is an estimate of the total burden of disease (more specifically of premature morbidity and mortality; male life expectancy in Scotland is 76.8 years), and the reduction in cardiovascular events is a measure of the lifetime benefit of the intervention. The reduction in cumulative cardiovascular events is substantial both numerically (with attendant economic savings) and in terms of relative risk (especially for heart failure). The observation that 5 years of statin therapy led to a prolonged risk reduction raises the issue that treatment might not need to be lifelong. That is, the legacy risk reduction after a 5- to 10-year treatment period may be sufficient to produce a clinically meaningful benefit while limiting lifetime exposure to the drug. We cannot address this question fully using the information in the present study, although it is clear that therapy would have to be maintained for subjects to experience maximum risk reduction because we saw a diminution in the treatment effect in the posttrial compared with the in-trial phases. The data on diabetes-associated noncardiovascular events indicate that further work is required to understand the clinical consequences of the statin-induced increase in the incidence of this disorder. These long-term efficacy findings, particularly on all-cause mortality, and detailed safety data should allay concerns over strategies to promote the more widespread use of statins in the population.
Acknowledgments
Dr Ford and H. Murray had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Sources of Funding
The study was supported by a grant from Merck, Sharp & Dohme, Kenilworth, NJ, as part of an Investigator Initiated Program. The funder had no role in the design or conduct of the study or in the analysis of the data and preparation of the manuscript. The funder was provided with a draft for comment and made nonbinding suggestions.
Disclosures
Dr Packard reports research grants from Roche and honoraria from Merck, Sharpe & Dohme, and Sanofi. The other authors report no conflicts.
Supplementary Material
Footnotes
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.115.019014/-/DC1.
CLINICAL PERSPECTIVE
Adoption of statin therapy for the primary prevention of cardiovascular disease is an area of controversy in clinical practice. There is perceived uncertainty about long-term efficacy with respect to vascular and all-cause mortality and the long-term safety of treatment. The findings of the present study should help alleviate at least some of these concerns. Extended follow-up of this primary prevention trial in high-risk men with raised low-density lipoprotein cholesterol but without a history of myocardial infarction demonstrated a long-term legacy benefit of low-density lipoprotein lowering by statin therapy with a significant reduction in cardiovascular mortality and improved survival when viewed over the entire 20 years of follow-up. Furthermore, the reduction in cumulative cardiovascular events, representing the total burden of disease, was substantial both numerically (with attendant economic savings) and clinically in terms of relative risk across a range of cardiovascular outcomes. The finding of a late posttrial benefit of a reduced risk of heart failure gives further impetus to the need to start treatment early. These long-term efficacy findings, particularly for all-cause mortality, and detailed safety data should allay concerns over strategies to promote the more widespread use of statins in the population.
References
- 1.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(suppl 2):S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 2.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvänne M, Scholte op Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad F European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG) European guidelines on cardiovascular disease prevention in clinical practice (version 2012): the Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635–1701. doi: 10.1093/eurheartj/ehs092. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 3.D’Agostino RB, Sr, Ansell BJ, Mora S, Krumholz HM. The guidelines battle on starting statins. N Engl J Med. 2014;370:1652–1658. doi: 10.1056/NEJMclde1314766. doi: 10.1056/NEJMclde1314766. [DOI] [PubMed] [Google Scholar]
- 4.Redberg RF, Katz MH. Healthy men should not take statins. JAMA. 2012;307:1491–1492. doi: 10.1001/jama.2012.423. doi: 10.1001/jama.2012.423. [DOI] [PubMed] [Google Scholar]
- 5.Taylor FC, Huffman M, Ebrahim S. Statin therapy for primary prevention of cardiovascular disease. JAMA. 2013;310:2451–2452. doi: 10.1001/jama.2013.281348. doi: 10.1001/jama.2013.281348. [DOI] [PubMed] [Google Scholar]
- 6.Yeboah J, Sillau S, Delaney JC, Blaha MJ, Michos ED, Young R, Qureshi WT, McClelland R, Burke GL, Psaty BM, Herrington DM. Implications of the new American College of Cardiology/American Heart Association cholesterol guidelines for primary atherosclerotic cardiovascular disease event prevention in a multi ethnic cohort: Multi-Ethnic Study of Atherosclerosis (MESA). Am Heart J. 2015;169:387–395.e3. doi: 10.1016/j.ahj.2014.12.018. doi: 10.1016/j.ahj.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ford I, Murray H, Packard CJ, Shepherd J, Macfarlane PW, Cobbe SM West of Scotland Coronary Prevention Study Group. Long-term follow-up of the West of Scotland Coronary Prevention Study. N Engl J Med. 2007;357:1477–1486. doi: 10.1056/NEJMoa065994. doi: 10.1056/NEJMoa065994. [DOI] [PubMed] [Google Scholar]
- 8.Pedersen TR, Wilhelmsen L, Faergeman O, Strandberg TE, Thorgeirsson G, Troedsson L, Kristianson J, Berg K, Cook TJ, Haghfelt T, Kjekshus J, Miettinen T, Olsson AG, Pyörälä K, Wedel H. Follow-up study of patients randomized in the Scandinavian Simvastatin Survival Study (4S) of cholesterol lowering. Am J Cardiol. 2000;86:257–262. doi: 10.1016/s0002-9149(00)00910-3. [DOI] [PubMed] [Google Scholar]
- 9.Heart Protection Study Collaborative Group. Effects on 11-year mortality and morbidity of lowering LDL cholesterol with simvastatin for about 5 years in 20,536 high risk individuals: a randomised controlled trial. Lancet. 2011;378:2013–2020. doi: 10.1016/S0140-6736(11)61125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LIPID Study Group (Long-term Intervention with Pravastatin in Ischaemic Disease) Long-term effectiveness and safety of pravastatin in 9014 patients with coronary disease and average cholesterol levels: the LIPID trial follow-up [published correction appears in Lancet. 2002;360:1430]. Lancet. 2002;359:1379–1387. doi: 10.1016/S0140-6736(02)08351-4. [DOI] [PubMed] [Google Scholar]
- 11.Sever PS, Chang CL, Gupta AK, Whitehouse A, Poulter NR ASCOT Investigators. The Anglo-Scandinavian Cardiac Outcomes Trial: 11-year mortality follow-up of the lipid-lowering arm in the U.K. Eur Heart J. 2011;32:2525–2532. doi: 10.1093/eurheartj/ehr333. doi: 10.1093/eurheartj/ehr333. [DOI] [PubMed] [Google Scholar]
- 12.McConnachie A, Walker A, Robertson M, Marchbank L, Peacock J, Packard CJ, Cobbe SM, Ford I. Long-term impact on healthcare resource utilization of statin treatment, and its cost effectiveness in the primary prevention of cardiovascular disease: a record linkage study. Eur Heart J. 2014;35:290–298. doi: 10.1093/eurheartj/eht232. doi: 10.1093/eurheartj/eht232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.West of Scotland Coronary Prevention Study Group. A coronary primary prevention study of Scottish men aged 45–64 years: trial design. J Clin Epidemiol. 1992;45:849–860. doi: 10.1016/0895-4356(92)90068-x. [DOI] [PubMed] [Google Scholar]
- 14.WOSCOPS Study Group. Screening experience and baseline characteristics in the West of Scotland Coronary Prevention Study. Am J Cardiol. 1995;76:485–491. doi: 10.1016/s0002-9149(99)80135-0. [DOI] [PubMed] [Google Scholar]
- 15.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, McKillop JH, Packard CJ. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia: West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 16.Kendrick S, Clarke J. The Scottish Record Linkage System. Health Bull (Edinb) 1993;51:72–79. [PubMed] [Google Scholar]
- 17.West of Scotland Coronary Prevention Study Group. Computerised record linkage: comparison with traditional patient follow-up methods in clinical trials and illustrated in a prospective epidemiological study. J Clin Epidemiol. 1995;48:1441–1452. doi: 10.1016/0895-4356(95)00530-7. [DOI] [PubMed] [Google Scholar]
- 18.McLoone P, Boddy FA. Deprivation and mortality in Scotland, 1981 and 1991. BMJ. 1994;309:1465–1470. doi: 10.1136/bmj.309.6967.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, Langendorfer A, Stein EA, Kruyer W, Gotto AM., Jr Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS: Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 21.Taylor F, Huffman MD, Macedo AF, Moore TH, Burke M, Davey Smith G, Ward K, Ebrahim S. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;1:CD004816. doi: 10.1002/14651858.CD004816.pub5. doi: 10.1002/14651858.CD004816.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cholesterol Treatment Trialists Collaborators. The effects of lowering LDL cholesterol in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ray KK, Seshasai SR, Erqou S, Sever P, Jukema JW, Ford I, Sattar N. Statins and all-cause mortality in high-risk primary prevention: a meta-analysis of 11 randomized controlled trials involving 65,229 participants. Arch Intern Med. 2010;170:1024–1031. doi: 10.1001/archinternmed.2010.182. doi: 10.1001/archinternmed.2010.182. [DOI] [PubMed] [Google Scholar]
- 24.Lazar LD, Pletcher MJ, Coxson PG, Bibbins-Domingo K, Goldman L. Cost-effectiveness of statin therapy for primary prevention in a low-cost statin era. Circulation. 2011;124:146–153. doi: 10.1161/CIRCULATIONAHA.110.986349. doi: 10.1161/CIRCULATIONAHA.110.986349. [DOI] [PubMed] [Google Scholar]
- 25.National Institute for Health and Care Excellence. Lipid Modification: Cardiovascular Risk Assessment and the Modification of Blood Lipids for the Primary and Secondary Prevention of Cardiovascular Disease. London, UK: National Institute for Health and Care Excellence; 2014. Jul, [PubMed] [Google Scholar]
- 26.Abramson JD, Rosenberg HG, Jewell N, Wright JM. Should people at low risk of cardiovascular disease take a statin? BMJ. 2013;347:f6123. doi: 10.1136/bmj.f6123. doi: 10.1136/bmj.f6123. [DOI] [PubMed] [Google Scholar]
- 27.Gerson RJ, MacDonald JS, Alberts AW, Chen J, Yudkovitz JB, Greenspan MD, Rubin LF, Bokelman DL. On the etiology of subcapsular lenticular opacities produced in dogs receiving HMG-CoA reductase inhibitors. Exp Eye Res. 1990;50:65–78. doi: 10.1016/0014-4835(90)90012-j. [DOI] [PubMed] [Google Scholar]
- 28.Harris ML, Bron AJ, Brown NA, Keech AC, Wallendszus KR, Armitage JM, MacMahon S, Snibson G, Collins R. Absence of effect of simvastatin on the progression of lens opacities in a randomised placebo controlled study: Oxford Cholesterol Study Group. Br J Ophthalmol. 1995;79:996–1002. doi: 10.1136/bjo.79.11.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ. 2010;340:c2197. doi: 10.1136/bmj.c2197. doi: 10.1136/bmj.c2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kostis JB, Dobrzynski JM. Prevention of cataracts by statins: a meta-analysis. J Cardiovasc Pharmacol Ther. 2014;19:191–200. doi: 10.1177/1074248413511690. doi: 10.1177/1074248413511690. [DOI] [PubMed] [Google Scholar]
- 31.Wise SJ, Nathoo NA, Etminan M, Mikelberg FS, Mancini GBJ. Statin use and risk for cataract: a nested case-control study of 2 populations in Canada and the United States. Can J Cardiol. 2014;30:1613e1619. doi: 10.1016/j.cjca.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 32.Preiss D, Campbell RT, Murray HM, Ford I, Packard CJ, Sattar N, Rahimi K, Colhoun HM, Waters DD, LaRosa JC, Amarenco P, Pedersen TR, Tikkanen MJ, Koren MJ, Poulter NR, Sever PS, Ridker PM, MacFadyen JG, Solomon SD, Davis BR, Simpson LM, Nakamura H, Mizuno K, Marfisi RM, Marchioli R, Tognoni G, Athyros VG, Ray KK, Gotto AM, Clearfield MB, Downs JR, McMurray JJ. The effect of statin therapy on heart failure events: a collaborative meta-analysis of unpublished data from major randomized trials. Eur Heart J. 2015;36:1536–1546. doi: 10.1093/eurheartj/ehv072. doi: 10.1093/eurheartj/ehv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stroes ES, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, Roden M, Stein E, Tokgözoğlu L, Nordestgaard BG, Bruckert E, De Backer G, Krauss RM, Laufs U, Santos RD, Hegele RA, Hovingh GK, Leiter LA, Mach F, März W, Newman CB, Wiklund O, Jacobson TA, Catapano AL, Chapman MJ, Ginsberg HN European Atherosclerosis Society Consensus Panel. Statin-associated muscle symptoms: impact on statin therapy: European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36:1012–1022. doi: 10.1093/eurheartj/ehv043. doi: 10.1093/eurheartj/ehv043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW, Macfarlane PW, Packard CJ, Stott DJ, Westendorp RG, Shepherd J, Davis BR, Pressel SL, Marchioli R, Marfisi RM, Maggioni AP, Tavazzi L, Tognoni G, Kjekshus J, Pedersen TR, Cook TJ, Gotto AM, Clearfield MB, Downs JR, Nakamura H, Ohashi Y, Mizuno K, Ray KK, Ford I. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–742. doi: 10.1016/S0140-6736(09)61965-6. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 35.Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, DeMicco DA, Barter P, Cannon CP, Sabatine MS, Braunwald E, Kastelein JJ, de Lemos JA, Blazing MA, Pedersen TR, Tikkanen MJ, Sattar N, Ray KK. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305:2556–2564. doi: 10.1001/jama.2011.860. doi: 10.1001/jama.2011.860. [DOI] [PubMed] [Google Scholar]
- 36.Preiss D, Sattar N. Statins and the risk of new-onset diabetes: a review of recent evidence. Curr Opin Lipidol. 2011;22:460–466. doi: 10.1097/MOL.0b013e32834b4994. [DOI] [PubMed] [Google Scholar]
- 37.Freeman DJ, Norrie J, Sattar N, Neely RD, Cobbe SM, Ford I, Isles C, Lorimer AR, Macfarlane PW, McKillop JH, Packard CJ, Shepherd J, Gaw A. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation. 2001;103:357–362. doi: 10.1161/01.cir.103.3.357. [DOI] [PubMed] [Google Scholar]


