Abstract
Chemical transformation studies were conducted on (−)-8-hydroxymanzamine A (1), (−)-manzamine F (2), manzamine A (3), and (+)-8-hydroxymanzamine A (4), isolated from Indo-Pacific Acanthostrongylophora sponges.1 Thirteen new semisynthetic manzamine derivatives, including four Δ34,35 manzamines (5, 6, 8, and 9) and the unprecedented manzamine derivative 17, are reported. The potent in vitro activities of the obtained semisynthetic manzamines against activated brain microglia and the AIDS opportunistic infection pathogen Mycobacterium tuberculosis are presented.
The manzamines are complex polycyclic marine-derived alkaloids first reported by Higa and co-workers in 1986 from the Okinawan sponge genus Haliclona.2 Manzamines possess a fused and bridged tetra- or pentacyclic ring system, which is attached to a β-carboline moiety. Since the first report of manzamine A, several manzamine-type alkaloids have been reported from 16 sponge species belonging to the Chalinidae, Niphatidae, Petrosidae, Thorectidae, and Irciniidae families.3 These include Acanthostrongylophora, Amphimedon, Cribrochalina, Haliclona, Hyrtios, Ircinia, Pachypellina, Pellina, Petrosia, Prianos, Reniera, and Xestospongia sponge species.1,3–6 Manzamines exhibit a diverse range of bioactivities including cytotoxicity2 and insecticidal,7 antibacterial,8 and antileishmaniasis effects.9 Manzamines also show potent activity against HIV-110 and several AIDS opportunistic infection (OI) pathogens, e.g., Cryptosporidium parvum,1,3 Toxo-plasma gondii,1,3 and Mycobacterium tuberculosis,1,3,9–12 as well as the potential curative activity against malaria in animal models.1 Anti-inflammatory activity of manzamines A–F was also recently reported.13–15
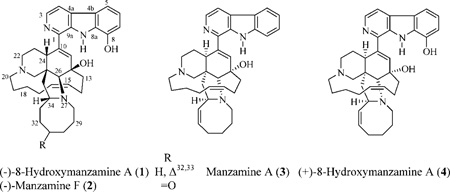
In 2001, the antipodes of 8-hydroxymanzamine A (1) and manzamine F (2) were reported. This was based on comparison of 1H and 13C NMR, optical rotation, TLC, and melting point data for compound 1 and authentic (+)-8-hydroxymanzamine A free bases (both separately and mixed).1 The antipodes of compounds 1 and 2 revealed identical physical properties to 1 and 2 themselves with the exception of opposite optical rotation values. ent-8-Hydroxymanzamine A exhibited improved activity against the P-388 murine lymphocytic leukemia cell line with an IC50 of 0.25 µg/mL, when compared with the previously published value of >20 µg/mL for the (+)-compound, examples further supporting the occurrence of an enantiomeric pair.1 Manzamine A (3) production by a putative symbiotic actinobacterium, Micromonospora sp., supports the possibility of the occurrence of manzamine antipodes due to the wide diversity of microbial enzymes that could be involved in the biosynthesis and later biotransformation of these alkaloids.16
Before the achievement of the total synthesis of manzamine A (3),17 numerous efficient synthetic strategies were conducted to accomplish this goal including the synthesis of manzamine C, the simplest member of the manzamines.5 Herein, we report the first semisynthetic study of the manzamines, which provides important information regarding the chemistry of these alkaloids. The potential importance of the manzamines as antituberculosis and anti-inflammatory leads is also highlighted.
Results and Discussion
The lipophilic extract of different collections of undescribed Indo-Pacific Acanthostronglylophora sponges (order Haplosclerida) afforded manzamines 1–4 in relatively high yields (1.24, 0.055, 0.66, and 1.24%, respectively).1,12
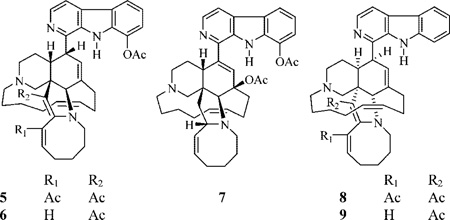
In an attempt to better understand the structure–activity relationship of the manzamine alkaloids, several chemical reactions were performed in parallel starting with 1–4. Reaction of 1 with acetic anhydride in the presence of excess sodium acetate at 80 °C, for 30 min under nitrogen, afforded the minor new Δ34,35 products 5 and 6 (9.8% and 1.5%, respectively) as well as 8,12-di-O-acetyl-8-hydroxymanzamine A (7) as a major product (24%). The same reaction with manzamine A (3) afforded 8 (7.6%) along with the major manzamine analogue 9 (40%).
The IR spectrum of 5 indicated the presence of an acetate ester and α,β-unsaturated ketones. The HRFTMS suggested the molecular formula of C42H49N4O4. The 1H and 13C NMR data displayed close similarities to 1. The methyl carbon resonating at δ 21.6 and correlated to the methyl proton singlet at δ 2.56 was assigned as the 8-O-acetate methyl. This methyl proton singlet showed a 2J-HMBC correlation to the quaternary ester carbonyl carbon at δ 169.5. The proton double-doublets resonating at δ 7.14 correlated to the olefinic methine carbon at δ 144.3 and was assigned to H-32. This proton doublet of doublets displayed COSY coupling to H2-31. Proton H-32 showed a 3J-HMBC correlation to the quaternary carbon at δ 161.8 (C-34) and the ketone carbon at δ 195.0 (C-33 acetyl ketone). The methyl carbon resonating at δ 27.1, which correlated to the methyl singlet at δ 2.21, was assigned as the C-33 acetyl methyl. The latter signal displayed a 2J-HMBC correlation to the ketone carbon at δ 195.0 and a 3J-HMBC correlation to the quaternary olefinic carbon at δ 135.2 (C-33). The assignment of the Δ34,35 was based on the 3J-HMBC correlations with the H-26 proton singlet at δ 4.88 and the 3J-HMBC correlation between H2-36 and C-35, in addition to the disappearance of the H2-35 and H-34 proton signals, as compared to 1. Protons H2-28 also showed a 3J-HMBC correlation to the quaternary C-34 resonance. The location of the C-35 methyl ketone substituent was indicated from the 3J-HMBC correlation of the methyl signal at δ 2.12 to the quaternary olefinic carbon at δ 114.9 (C-35). The latter methyl singlet also showed a 2J-HMBC correlation to the ketone carbon resonating at δ 192.7. The β-orientation of H-10 was proposed on the basis of its NOESY correlation with β-H-24. Final confirmation of the structure 5 was accomplished through a 1H–15N-GHMBC experiment, in which the quaternary nitrogen N-2 resonating at δ 295.9 was correlated to H-3 and H-4, and the protonated N-9 resonating at δ 128.7 correlated to the proton singlet at δ 10.45. The quaternary nitrogen resonating at δ 36.4 (N-27) displayed a 3J-correlation with H2-29, with nitrogen N-21 not observed.
The MS and NMR data of 6 indicated a close structure similarity to 5 except in the lack of one acetyl group. The olefinic proton doublet resonating at δ 6.44 (10.7 Hz) was assigned to H-33, as it showed COSY coupling with the olefinic proton multiplet resonating at δ 5.18 (H-32).
The IR spectrum of 7 displayed two ester absorption bands. The HRFTMS and 1H and 13C NMR data of 7 suggested that it is ent-8,12-di-O-acetyl-8-hydroxymanzamine A. The methyl singlet resonating at δ 2.53 was assigned to the 8-O-acetate methyl. This signal showed a 2J-HMBC correlation to the acetate carbonyl at δ 169.5. Similarly, the methyl singlet resonating at δ 2.17 showed a 2J-HMBC correlation to the acetate carbonyl at δ 171.1 and was assigned to an 8-O-acetate group.
The HRFTMS and NMR data of 8 and 9 indicated their similarity to 5 and 6, respectively, having a manzamine A skeleton instead of that of (−)-8-hydroxymanzamine A. The methyl proton singlets resonating at δ 2.19 and 2.21 in 8 and 9, respectively, were assigned to the 35-acetyl methyl groups. Each of these methyl singlets showed a 2J-HMBC correlation to the methyl ketone carbon resonating at δ 192.6 and 196.8 in 8 and 9, respectively. They also displayed a 3J-HMBC correlation to the quaternary olefinic carbon resonating at δ 114.7 and 114.5 (C-35) in 8 and 9, respectively. Similar correlations were observed for the C-33 acetyl methyl group (δ 2.27) in 8. The proton doublet resonating at δ 6.44, which was assigned to H-33 in 9, showed COSY coupling to the olefinic proton multiplet at δ 5.23 (H-32).
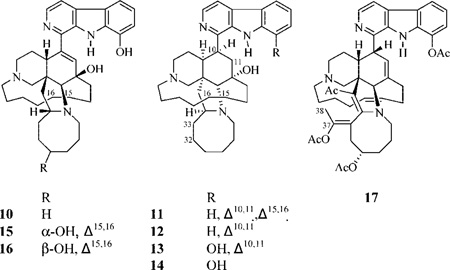
Reduction of 1 in absolute ethanol using hydrazine hydrate in the presence of Pd/carbon for 12 h at room temperature afforded the major product 10 (19%). The same reaction starting with 3 using 18 h reflux afforded 11 (12.8%) and 12 (5.4%). Similarly, reduction of 4 using a 34 h reflux afforded 13 (14.5%) and 14 (3.4%). The order of reduction is as expected, being more favorable for the disubstituted Δ32,33, followed by the Δ15,16 system, and finally for the trisubstituted Δ10,11.
The HRFTMS data of 10–14 indicated reduced derivatives of 1, 3, or 4. The 1H and 13C NMR data suggested that 10 is (−)-15,16,32,33-tetrahydro-8-hydroxymanzamine A, since the olefinic proton and carbon signals C-15, 16, 32, and 33 were replaced by upfield aliphatic signals. Similarly, 11 and 12 were found to be 32,33-dihydro-and 15,16,32,33-tetrahydromanzamine A, respectively. Likewise, the identity of 14 was assigned as 10,11,15,16,32,33-hexahydro-8-hydroxymanzamine A.
Reduction of 2 using sodium borohydride in anhydrous tetrahydrofuran at room temperature afforded 15 (27.5%) and 16 (6%). The HRFTMS of 15 and 16 displayed molecular ion peaks [M + H]+ at m/z 583.3600 and 583.3615, respectively, suggesting the same molecular formula, C36H47N4O3, and 16 degrees of unsaturation. The broad proton singlets resonating at δ 3.77 and 3.86, respectively, were assigned to the new oxygenated H-31 in 15 and 16. The β-orientation of H-31 in 15 was attributed on the basis of comparison of its NOESY correlation with the β-oriented H-34. Compound 16 must then be the C-31 epimer of 15, since the only spectroscopic difference between these compounds was evident at C-31.
Reaction of the semisynthetic product 15 with (Ac)2O/pyridine at 100 °C for 12 h afforded 17 (56%). The HRFTMS data of 17 displayed a molecular ion peak [M + H]+ at m/z 775.4271, suggesting the molecular formula C46H55N4O7 and 22 unsaturation units. The IR spectrum of 17 showed the presence of both acetate esters and an α,β-unsaturated ketone functionality. The 1H and 13C NMR data of 17 demonstrated the presence of three O-acetates, one methyl ketone, and one methyl group located on a tetrasubstituted double bond. The methyl singlet resonating at δ 2.58 was 2J-HMBC correlated to the acetate carbonyl at δ 169.8 and assigned to an 8-O-acetate unit. In turn, the methyl singlet resonating at δ 2.07 that showed a 2J-HMBC correlation to the acetate carbonyl at δ 170.3 was assigned as 31-O-acetate. The Δ34,35 formation was confirmed through the 3J-HMBC correlation between the H-26 proton singlet and the quaternary olefinic carbons at δ 163.9 (C-34) and 111.7 (C-35), in addition to the 3J-HMBC correlations of H2-28 with C-34. The methyl singlet resonating at δ 2.29, which exhibited a 2J-HMBC correlation to the ketone at δ 192.0 and a 3J-HMBC correlation to C-35, was assigned as the 35-acetyl functionality. The methyl singlet resonating at δ 1.85 that correlated to the methyl carbon at δ 16.8 was assigned to C-38, on the basis of its 2J- and 3J-HMBC correlations to the quaternary oxygenated olefinic carbons resonating at δ 148.1 (C-37) and 116.9 (C-33). The assignment of the system Δ33,37 rather than Δ32,37 was based on the COSY correlation between H-31 and H2-32, the 3J-HMBC correlation between H2-32 and C-30, C-34, and C-37, and the 2J-HMBC correlation of H2-32 to C-33. The only remaining acetate methyl singlet resonated at δ 2.21 and showed a 2J-HMBC correlation with the acetate carbonyl resonating at δ 168.8; it was assigned as the C-37-O-acetate. This was further supported by the downfield resonance of C-37 (δ 148.1) due to the additional oxygenation. The configuration at C-31 was assigned similar to the parent compound 15.
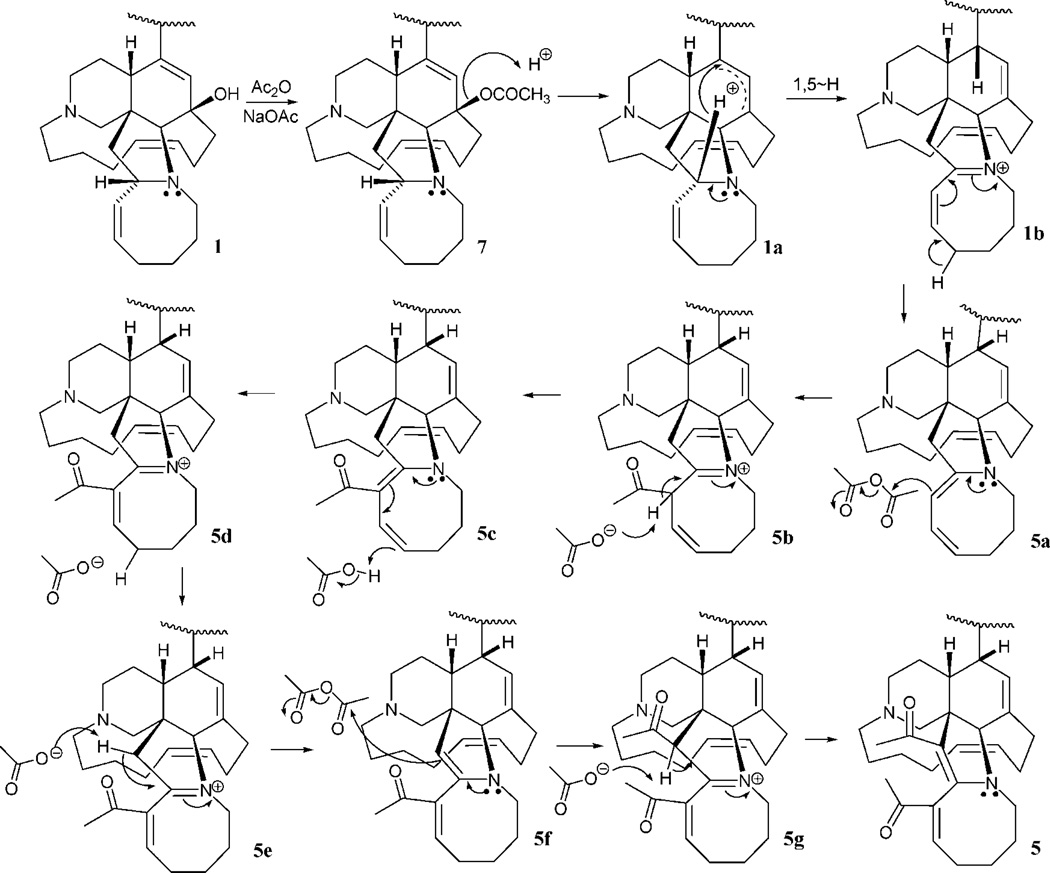
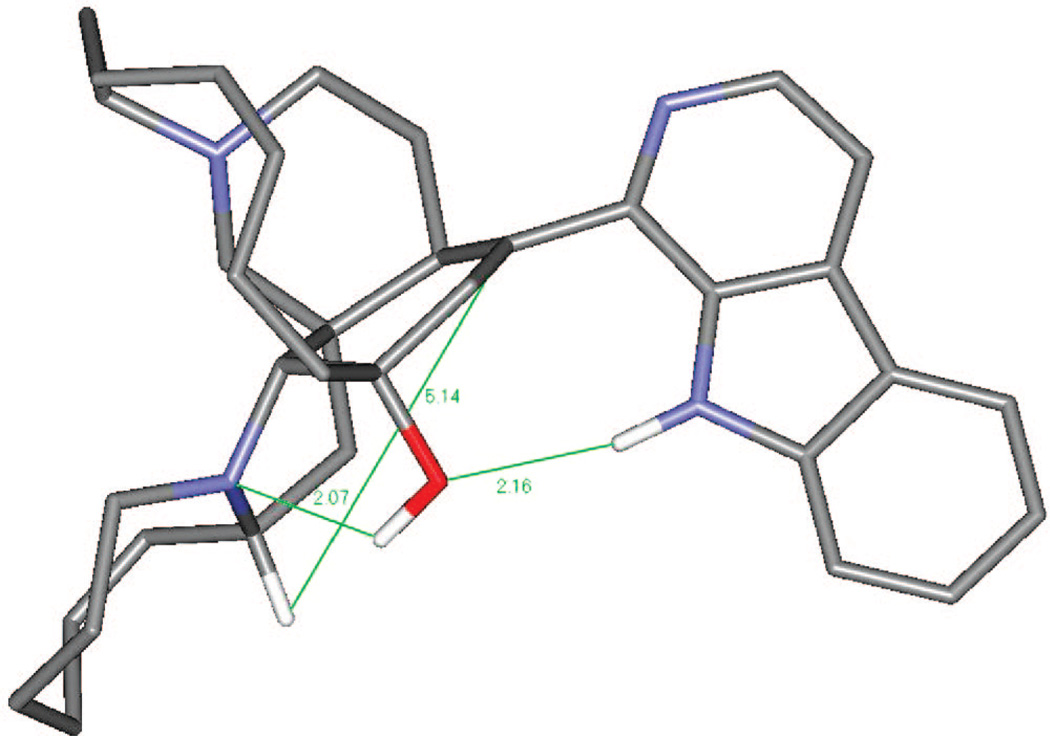
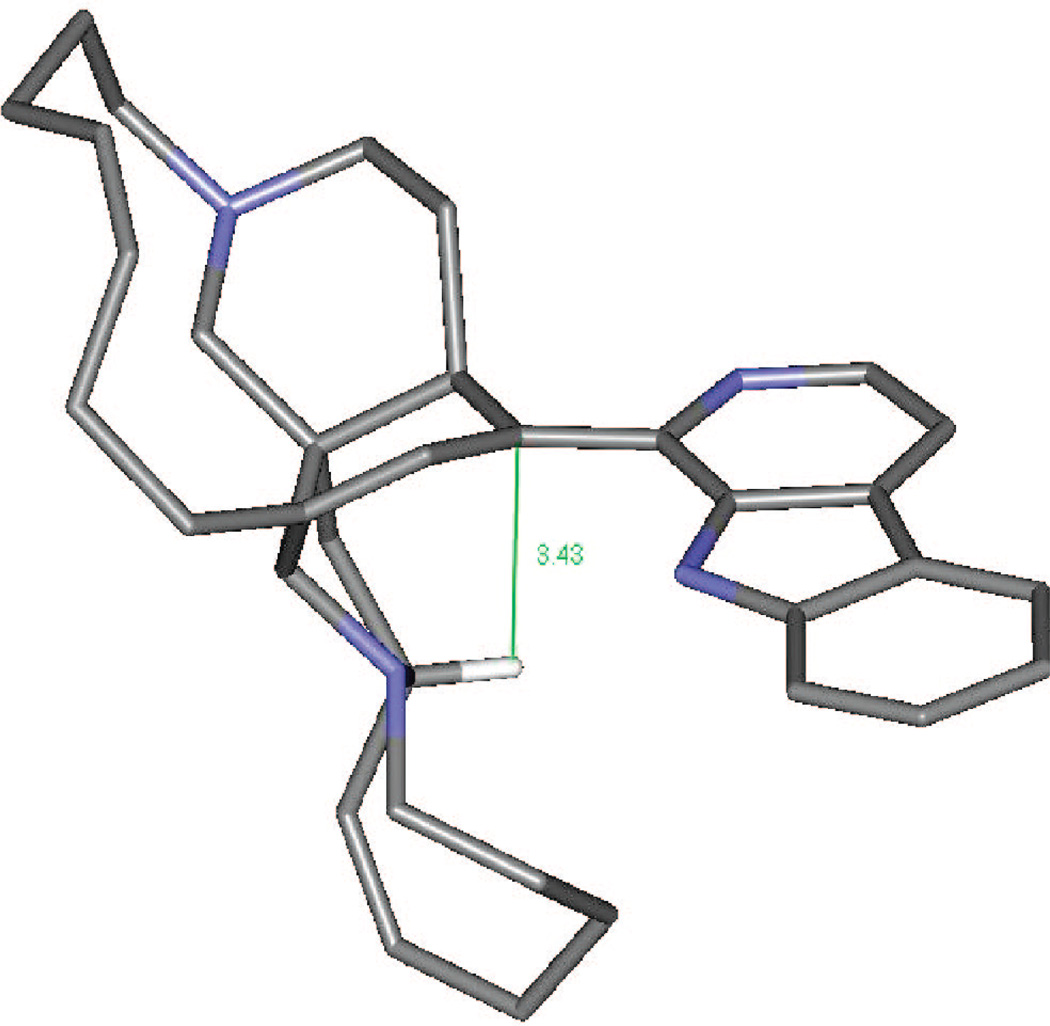
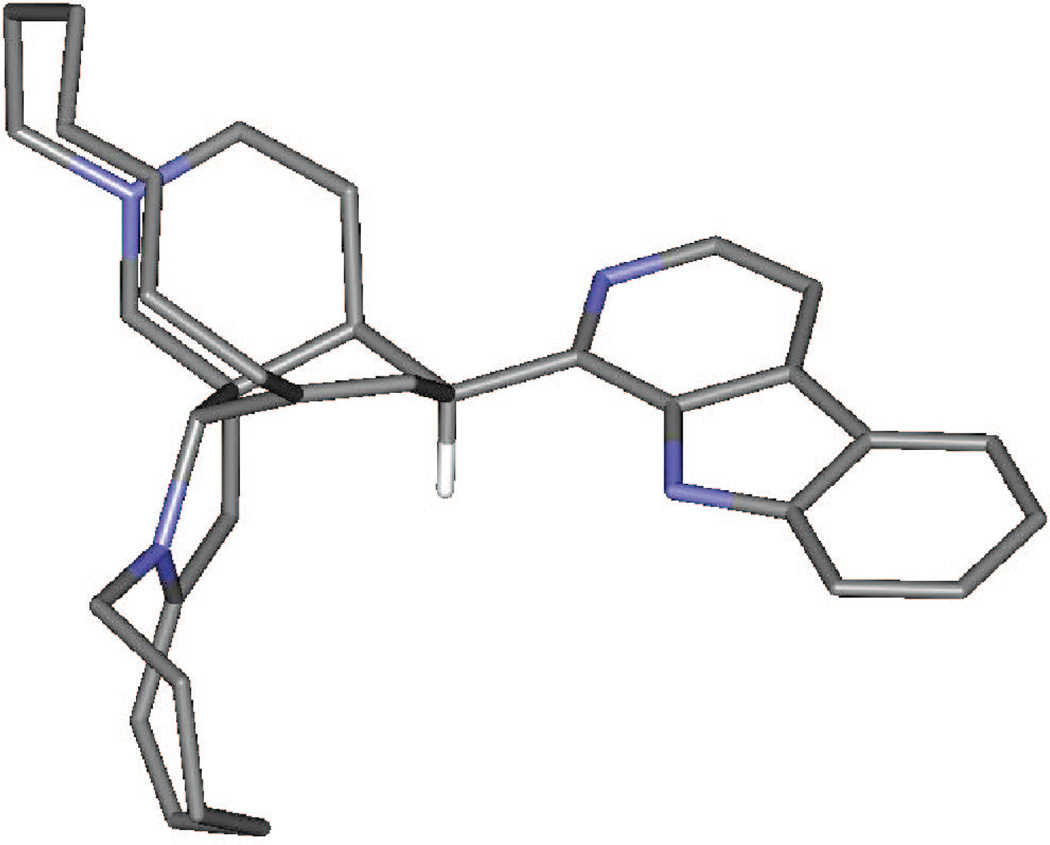
Plausible mechanistic schemes of the generation of compounds 5–7, 9, and 17 are proposed in Scheme 1 as well as Schemes S1 and S2 found in the Supporting Information. It is noteworthy that a stereospecific hydride transfer with reductive elimination of water occurred via the C-10–11–12 allylic alcohol system. On the basis of manzamine A (3), which produced only compounds with an α-H at C-10, and (−)-8-hydroxymanzamine A (1), producing only compounds with a β-H at C-10, it is presumed that the incorporation of a hydrogen atom at C-10 might well occur by an intramolecular H atom transfer, or one controlled by the substrate in some other way. To examine the feasibility of such an unusual process occurring by intramolecular H atom transfer, a molecular modeling study was performed. Careful examination of the manzamine A (4) model showed a distance of 5.30 Å between the C-34–H and the C-10/C-9 double bond (Figure 1). This hydrogen was close enough to produce an intramolecular H transfer, but the distance was not optimal for such a process. Also, hydrogen bonds between N-9–H---(C-12)O–H---N-27 anchoring the aromatic heterocycle almost perpendicular to the bicyclic A,B-ring system could be proposed. To produce such a transfer, the OH must be lost, generating a carbocation intermediate. This could occur via protonation of the acetylated intermediate such as 7 (OAc-8, -12 from 1; and OAc-12 from 3) or by protonation of the free hydroxyl group, leading to loss of either acetic acid or water and the generation of an allylic carbonium ion. The carbocation intermediate 1a would rearrange by hydride transfer through a bridge transition state to produce intermediate 1b. This is evident from the modeled structure of manzamine 3 that supplied an approach of C-34–H to C-10 from 5.14 Å, whereas the allylic carbocation 1a showed this distance as 3.43 Å due to the conformational change associated with the loss of OH (Scheme 1, Figure 2). This loss produced an alteration of the B ring from an almost planar ring to a boat conformation in which the hydrogen more closely approached the double bond, facilitating the transfer. The rearrangement could now proceed by a transannular 1,5-hydride shift, transferring the hydride to C-10 while shifting the electrons to generate the Δ8,9 double bond. Similar transannnular 1,5-hydride shifts have been observed,18,19 where the molecular orbital demand requires a migrating σ-bond to be perpendicular to the plane of the sp2 carbon. An examination of the lowest unoccupied molecular orbital (LUMO) of this cation 1a showed that the hydrogen atom directed to the p-orbital over C-10 was in accordance with the orbital demand for this type of rearrangement. Therefore, the carbocation intermediate 1b was modeled showing no significant conformational change, but this cation was 37.4 kcal/mol more stable than 1a.20 This was undoubtedly due to resonance stabilization of the C-34 carbocation via the adjacent (α) nitrogen as an iminium ion and the release of the unfavorable boat conformation of the B ring to a more energetically favorable twist-boat conformation (Figure 3).
Scheme 1.
Plausible Mechanism of Formation of Compounds 5 and 7
Figure 1.
Manzamine A (3) global minimum energy by 3–21G ab initio methods. Note the green hydrogen bonds between heterocycle 9-NH, the 12-OH lone pair, and the OH with the tertiary 27-N lone pair.
Figure 2.
3–21G-calculated manzamine-derived allylic carbonium ion 3a showing a pretransition state transannular H-34 to C-10 distance of 3.43 Å. Of interest, the H-34 to C-10 distance in Figure 1 (manzamine A, 3) is 5.14 Å, the shortened distance arising as a result of conformational transmission.
Figure 3.
Intermediate 3b after transannular 1,5-H transfer from H-34 to C-10 cation, calculated by ab initio methods at the 3–21G level. This stable iminium ion (over 35 kcal/M more stable than 3a) can lose a proton to generate different nucleophilic enamines, as shown in Scheme 1.
With the formation of the iminium ion 1b stereospecifically rationalized by molecular modeling, incorporation of acetate units into the intermediate was examined next (Scheme 1). Iminium ions generated in the presence of base are known to undergo elimination to a neutral enamine or, in this case, a dienamine, 5a. Reaction of the nucleophilic enamine 5a with the electrophile acetic anhydride can then proceed via attack of the C-33–34 double bond upon the electrophilic carbonyl carbon in an accepted mechanistic fashion for reactions of enamines with electrophiles. With acetate now present at C-33 and an iminium regenerated between the N and C-34, the system 5b is ideally suited to undergo deprotonation to give 5c. To envisage formation of the product 5, one must now place another unsaturated acetate unit at C-35. This may be achieved rationally by protonation–deprotonation to equilibrate 5c with 5f via the intermediates 5d and 5e. Then, 5f would be suited to undergo another acetylation at C-35 via attack of the C-34–35 double bond on acetic anhydride. As before, the resultant iminium ion 5g now simply undergoes deprotonation to furnish the isolated product 5.
To explain the formation of 6 (Scheme S1, Supporting Information), 1b could be deprotonated to afford the intermediate enamine 6a. Monoacetylation of 6a would give the iminium ion 6b, which deprotonates to furnish the observed product 6. By analogy to the formation of 5 and 6 (not shown), the enantiomeric manzamine 3 would lead to products 8 and 9.
Formation of the remaining semisynthetic manzamine 17 from its precursor 15 is shown in Scheme S2, Supporting Information. After acetylation of the sterically accessible C-31 hydroxyl group to give 15a, loss of water to give the allylic carbonium 15b is consistent with Scheme 1, as is the intramolecular H atom transfer to afford 15c. Enamine formation (15d) results from deprotonation of 15c and would lead to attack on acetic anhydride as before to give 15e. This iminium ion-acetate could undergo enamine formation again to the available C-35 position by loss of a C-35 H, affording 15f. Attack on acetic anhydride again leads to an iminium ion, 15g, that then undergoes deprotonation to give 15h. Finally, enolization of 15h favorably places another double bond in conjugation with the enone system at C-34–35 via deprotonation of the acidic proton at C-33 by pyridine. Acetylation of the enolate affords the observed reaction product 17.
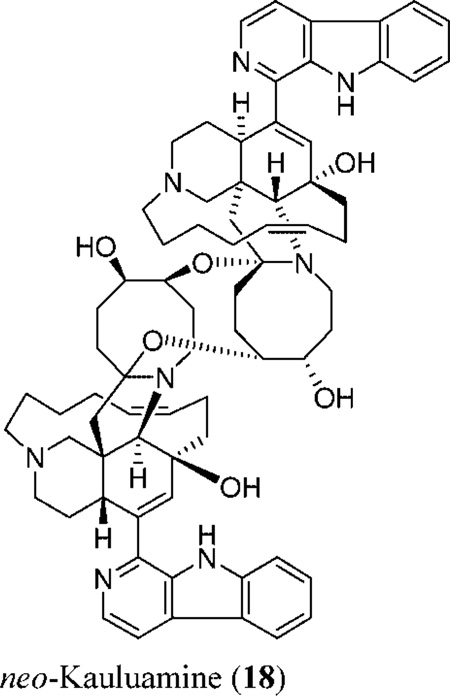
The enormous potential of the marine environment to provide new antituberculous leads has been documented.21 The in vitro activity of natural and semisynthetic manzamines against M. tuberculosis (H37Rv) was evaluated in the present investigation. Most manzamines induced 95–99% inhibition of M. tuberculosis with MIC values of <12.5 µg/mL, except for manzamines 9, 16, and neo-kauluamine (18).1 Rifampin, the positive drug control, induced 100% inhibition at 0.5 µg/mL. (+)-8-Hydroxymanzamine A induced 100% inhibition with a MIC of <6.25 µg/mL, suggesting the precedence of the (+)-over the (−)-enantiomer for TB activity. While 15 induced 100% inhibition with a MIC of <6.25 µg/mL, its C-31 epimer (manzamine 16) was less active (61% inhibition). neo-Kauluamine (18), the second known manzamine dimer with two β-oriented ether bonds bridging both monomers at C-31/C-34′ and C-30′/C-34 carbons of the azacyclooctane site, in addition to two free α- and β-hydroxyl groups at C-30 and C-31′, respectively,1 also displayed less activity (44% inhibition). This suggests that α-hydroxylation at C-31 maintains activity, while β-hydroxylation at this position reduces activity. Reduction of the Δ10,11, Δ15,16, and Δ32,33 systems has no effect on the activity, as suggested by the activities of semisynthetic manzamines 10, 11, and 13. Substitution at C-33 improves the activity, as indicated by the higher bioactivity of 5 as compared to that of 6 and 9.
Neuroinflammatory conditions appear to involve release of the eicosanoid thromboxane B2 (TXB2) and the free radical superoxide anion (O2−) by activated brain microglia BMΦ.22 Escherichia coli lipopolysaccharide-activated rat neonatal microglia represent a convenient in vitro model to study natural products that could potentially inhibit production of neurotoxic TXB2 and O2−.23 Using this model, we have investigated the effect of natural and semi-synthetic manzamines on the release of BMΦ O2− and TXB2. Manzamines 2, 4–13, and 15–17 did not inhibit BMΦ O2− and TXB2 release even at 30 µM, the highest concentration tested. However, (−)-8-hydroxymanzamine A (1), manzamine A (3), and tetrahydro-8-hydroxymanzamine A (14) inhibited TXB2 (IC50 0.25, <0.1, and 1.97 µM, respectively), but in contrast, did not affect O2−. Furthermore, because in vitro toxicity by these manzamines, determined as lactate dehydrogenase release, was observed to be minimal, the current data appear to support the potential of 1, 3, and 14 as lead compounds for the development of novel anti-inflammatory agents to modulate activated brain microglia.13–15
On the basis of the results reported herein and the absence of in vivo toxicity from previous reports, the manzamine alkaloids are viable antituberculosis and antineuroinflammatory leads.1 β-Hydroxylation at position C-31 of the azacyclooctane ring significantly reduces the resultant activity, as opposed to α-hydroxylation, which has little effect. Double-bond systems Δ10,11, Δ15,16, and Δ32,33 are not essential for the antimycobacterial activity. Substitution at C-33 was shown to enhance activity, as indicated by the high potency of manzamine analogue 5.
Experimental Section
General Experimental Procedures
Melting points were determined on a Thomas-Hoover capillary melting point apparatus and are uncorrected. Optical rotations were measured at room temperature with a JASCO DIP-370 digital polarimeter. UV spectra were aquired using a Perkin-Elmer Lambda 3B UV/vis spectrophotometer. The IR spectra were recorded on an ATI Mattson Genesis Series FTIR spectrophotometer. The 1H and 13C NMR spectra were recorded in CDCl3 on Bruker DRX- or AMX-NMR spectrometers operating at 400 or 500 MHz for 1H NMR and 100 or 125 MHz for 13C NMR. The HRMS were measured using a Bioapex FTMS with electrospray ionization. TLC was carried out on precoated silica gel G254 or aluminum oxide ALOX-100 UV254 500 µm (E. Merck), with the following developing systems: n-hexanes/acetone (65–35) or cyclohexane/EtOAc/NH4OH (100:50:0.1) or C18-reversed phase plates, 200 µm using MeOH/H2O/NH4OH (80:20:0.1). For column chromatography, Si gel 60 (40 µm) was used.
Biological Material
The sponge was collected in November 1994, in Manado Bay, Sulawesi, Indonesia, from a depth of 20 m.1,9 It is irregularly massive with a rough surface, the texture is tough and crumbly, and the colors in life are maroon externally and yellow internally. The skeleton is very irregular and composed of small, round meshes set in irregular curving fascicles. The spicules are irregularly curved strongyles, 80–140 µm.1,9 The sponge was identified as a species of the genus Acanthostrongylophora (order Haplosclerida, family Petrosiidae) by Michelle Kelly of the National Institute of Water and Atmospheric Research Ltd., Auckland, New Zealand. A voucher specimen designated BMNH 1997.11.11.9 is deposited at the Natural History Museum, London, UK.1,9
15N NMR Spectroscopy
Inverse-detected 15N NMR spectra were recorded using a 500 MHz NMR spectrometer equipped with a 3 mm inverse-detection gradient probe. A gradient HMBC pulse sequence with 1 ms Gaussian Z-axis gradient pulses (70:30:50) was used. Referencing of the indirectly detected 15N dimension was accomplished using nitromethane as an external standard. A GHMBC experiment was performed with nitromethane, and the 15N correlation was calibrated to 380.2 ppm. This same calibration value was then used for the manzamines. The acquisition time was 12 to 24 h for each compound.
Computational Methods
A full conformational search with no symmetry constraints using hybrid MonteCarlo simulations was done using MOE, or Molecular Operating Environment. Many conformations were generated, minimized, and compared to each other to discard duplicates. Energetically and conformationally, most representative conformations were selected and minimized at the semiempirical level using the AM1 force field as implemented on HyperChem 4.5 using a conjugate gradient algorithm (Polak-Riviere) with a gradient of 0.01. Then, global minima were used as initial conformations to minimize the structure at the ab initio level, using HF and 3–21G basis sets as implemented in Gaussian 98.20 The nature of all the minima at semiempirical and ab initio levels was established by a complete vibrational analysis of each conformation. Final edition of the pictures was performed using Viewer Pro 4.0 (Accelrys).
Reaction of Acetic Anhydride/NaOAc with 1 and 3
A solution of 100 mg of 1 or 3 in 2 mL of acetic anhydride was added to 30 mg of anhydrous NaOAc under nitrogen. Each reaction mixture was stirred for 30 min at 80 °C. The reaction mixture was brought to room temperature, brine solution (10 mL) was then added, and the solution was made alkaline by aqueous NH4OH (1.5 mL). Each reaction mixture was extracted with CHCl3 (2 × 15 mL). The organic layer was washed with H2O (2 × 20 mL), dried over anhydrous Na2SO4, and evaporated under reduced pressure. The reaction residue of 1 (90 mg) was fractionated on Si gel 60 (10 g) using n-hexanes/EtOAc, gradient elution, followed by preparative TLC on Si gel G254, using cyclohexane/EtOAc/NH4OH (100/50/0.1) to afford 5 (9.8 mg, Rf 0.25), 6 (1.5 mg, Rf 0.36), and 7 (24.0 mg, Rf 0.88). The reaction residue of 3 was chromatographed on a Si gel 60 column (20 g), by isocratic elution with n-hexanes/acetone (80/20) as solvent system, to afford 8 (7.6 mg, Rf 0.26) and 9 (40.0 mg, Rf 0.41).
Compound 5
yellowish powder from EtOH; mp 128–130 °C; [α]D25 −14.0 (c 0.10, CHCl3); UV λmax (log ε) (MeOH) 267 (2.17), 368 (2.24), 400 (2.12) nm; IR νmax (CHCl3) 3631–3260 (NH), 3011–2838, 1730, 1702 (C═O) 1690, 1563, 1472, 1421, 1232, 1017 cm−1; 1H and 13C NMR, see Table 1; HRFTMS m/z 673.3714 (calcd for C42H49N4O4, 673.3754 [M + H]+).
Table 1.
13C and 1H NMR Data of Compounds 5 and 7a
| 5 | 7 | |||
|---|---|---|---|---|
| position | δC | δH | δC | δH |
| 1 | 146.5, s | 146.3, s | - | |
| 3 | 137.9, d | 8.41, d (5.3) | 138.5, d | 8.38, d (5.6) |
| 4 | 113.0, d | 7.81, d (5.3) | 113.5, d | 7.82, d (5.6) |
| 4a | 128.3, s | 128.9, s | ||
| 4b | 124.2, s | 124.4, s | ||
| 5 | 118.9, d | 7.96, d (7.5) | 119.1, d | 7.97, d (7.8) |
| 6 | 119.5, d | 7.14, dd (7.6, 7.5) | 119.5, d | 7.23, dd (7.8, 7.6) |
| 7 | 120.2, d | 7.30, d (7.6) | 120.2, d | 7.29, d (7.6) |
| 8 | 136.5, s | 136.7, s | ||
| 8a | 133.3, s | 133.2, s | ||
| 9a | 134.6, s | 133.9, s | ||
| 10 | 40.9, d | 4.41, brs | 144.0, s | |
| 11 | 125.8, d | 6.60, s | 135.0, d | 6.50, s |
| 12 | 138.7, s | 84.0, s | ||
| 13 | 34.3, t | 2.65, m 2.40, m | 40.3, t | 1.79, m 1.68, m |
| 14 | 21.6, t | 2.12, m | 21.5, t | 2.21, m 1.80, m |
| 15 | 130.6, d | 5.43, m | 127.4, d | 5.56, m |
| 16 | 131.2, d | 5.35, m | 133.1, d | 5.50, m |
| 17 | 23.7, t | 1.89, m 1.73, m | 25.6, t | 1.65, m |
| 18 | 24.7, t | 1.38, m | 27.0, t | 1.42, m 1.30, m |
| 19 | 27.7, t | 1.92, m | 26.7, t | 1.40, m 1.32, m |
| 20 | 59.6, t | 2.62, m 2.51, m | 53.1, t | 2.60, m 2.43, m |
| 22 | 51.2, t | 2.71, m 1.98, m | 49.4, t | 2.83, m 1.95, m |
| 23 | 28.0, t | 2.73, m 2.41, m | 34.2, t | 2.81, m |
| 24 | 38.7, d | 3.96, m | 41.7, d | 2.44, m |
| 25 | 52.2, s | 47.7, s | ||
| 26 | 64.3, d | 4.88, s | 72.3, d | 3.50, s |
| 28 | 45.6, t | 3.85, dd (14.7, 4.7) 3.34, dd (14.7, 10.8) |
50.5, t | 3.14, dd (11.9, 8.7) 2.99, dd (11.8, 4.7) |
| 29 | 24.8, t | 1.38, m | 25.7 | 1.76, m |
| 30 | 28.4, t | 1.88, m 1.60, m | 27.7, t | 2.15, m 2.05, m |
| 31 | 29.5, t | 2.15, m | 31.7, t | 2.42, m |
| 32 | 144.3, d | 7.14, dd (8.5, 7.0) | 132.8, d | 5.84, dd (7.7, 7.6) |
| 33 | 135.2, s | 131.1, d | 5.21, m | |
| 34 | 161.8, s | 53.7, d | 3.99, dd (8.3, 8.2) | |
| 35 | 114.9, s | 44.8, t | 2.42, m 1.49, d (12.9) | |
| 36 | 62.7, t | 2.62, d (10.9) 2.51, d (10.9) |
68.6, t | 2.76, d (11.5) 2.30, m |
| OAc-8 | 169.5, s 21.6, q |
2.56, s | 169.5, s 21.4, q |
2.53, s |
| OAc-12 | 171.1, s 23.2, q |
2.17, s | ||
| Ac-33 | 195.0, s 27.1, q |
2.21, s | ||
| Ac-35 | 192.7, s 29.3, q |
2.12, s | ||
In CDCl3, 400 or 500 MHz for 1H and 100 or 125 MHz for 13C NMR. Carbon multiplicities were determined by DEPT135° experiments.
s = quaternary, d = methine, t = methylene, q = methyl carbons. Coupling constants (J) are in Hz.
Compound 6
yellowish powder from EtOH; mp 134 °C; [α]D25 −26.0 (c 0.12, CHCl3); UV λmax (log ε) (MeOH) 267 (2.26), 356 (2.20), 368 (2.32) nm; IR νmax (CHCl3) 3631–3260 (NH), 3011–2838, 1729, 1702 (C═O) 1633, 1503, 1472, 1236, 1011 cm−1; 1H NMR CDCl3, 400 MHz, δ 8.44 (1H, d, J = 5.6 Hz, H-3); 7.81 (1H, d, J = 5.6 Hz, H-4); 7.95, d (1H, d, J = 7.7 Hz, H-5); 7.21 (1H, dd, J = 7.7 and 7.6 Hz, H-6); 7.29 (1H, d, J = 7.6 Hz, H-7); 4.61 (1H, brs, H-10); 6.34 (1H, s, H-11); 5.48 (1H, m, H-15); 5.39 (1H, m, H-16); 2.71 (1 H, m, H-20a); 2.46 (1H, m, H-20b); 2.72 (2H, m, 2.30, H2-22); 3.52 (1H, m, H-24); 4.25 (1H, s, H-26); 4.20 (1H, m, H-28a); 4.09 (1H, brd J = 13.5 Hz, H-28b); 5.18 (1H, m, H-32); 6.44 (1H, d, J = 10.7 Hz, H-33); 2.56 (3H, s, H3-8-O-acetate); 2.15 (3H, s, H3-35-acetyl); HRFTMS m/z 631.3619 (calcd for C40H47N4O3, 631.3648 [M + H]+).
(−)-8,12-Di-O-acetyl-8-hydroxymanzamine A (7)
yellowish-green powder (EtOH); mp 168–170 °C; [α]D25 −22.0 (c 0.10, CHCl3); UV λmax (log ε) (MeOH) 266 (2.35), 356 (2.29), 368 (2.42) nm; IR νmax (CHCl3) 3458 (NH), 3100–2930, 1740, 1714 (C═O) 1660, 1637, 1561, 1466, 1240, 1013 cm−1; 1H and 13C NMR, see Table 1; HRFTMS m/z 649.3678 (calcd for C40H49N4O4, 649.3754 [M + H]+).
Compound 8
yellowish powder (EtOH); mp 136–138 °C; [α]D25 +21.0 (c 0.09, CHCl3); UV λmax (log ε) (MeOH) 266 (2.61), 356 (2.65), 367 (2.75) nm; IR νmax (CHCl3) 3640–3250 (NH), 3010–2840, 1725 (C═O) 1660, 1620, 1500, 1460, 1365, 1020 cm−1; 1H and 13C NMR, see Table 2; HRFTMS m/z 615.3658 (calcd for C40H47N4O2, 615.3699 [M + H]+).
Table 2.
13C and 1H NMR Data of Compounds 8 and 9a
| 8 | 9 | |||
|---|---|---|---|---|
| position | δC | δH | δC | δH |
| 1 | 146.9, s | 147.0, s | ||
| 3 | 137.5, d | 8.42, d (5.5) | 138.0, d | 8.43, d (5.6) |
| 4 | 112.8, d | 7.85, d (5.5) | 113.3, d | 7.87, d (5.6) |
| 4a | 127.9, s | 129.5, s | ||
| 4b | 121.4, s | 121.8, s | ||
| 5 | 121.2, d | 8.11, d (7.5) | 122.0, d | 8.10, d (7.7) |
| 6 | 119.3, d | 7.25, dd (7.6, 7.5) | 121.5, d | 7.26, dd (7.7, 7.4) |
| 7 | 127.6, d | 7.55, dd (8.0, 7.8) | 128.9, d | 7.54, dd (7.7, 7.4) |
| 8 | 112.4, d | 7.71, d (8.0) | 112.5, d | 7.63, d (7.7) |
| 8a | 140.5, s | 139.8, s | ||
| 9a | 134.5, s | 134.8, s | ||
| 10 | 41.2, d | 4.49, brs | 40.1, d | 4.63, brs |
| 11 | 126.2, d | 6.62, s | 128.2, d | 6.40, s |
| 12 | 138.6, s | 140.1, s | ||
| 13 | 35.0, t | 2.78, m 1.88, m | 32.0, t | 2.66, m 2.40, m |
| 14 | 29.6, t | 2.78, m | 21.1, t | 2.10, m |
| 15 | 131.1, d | 5.38, m | 130.4, d | 5.48, m |
| 16 | 130.6, d | 5.51, m | 131.2, d | 5.39, m |
| 17 | 27.7, t | 2.45, m 2.32, m | 24.0, t | 1.88, m |
| 18 | 25.0, t | 1.42, m | 24.7, t | 1.40, m |
| 19 | 28.3, t | 1.84, m | 27.5, t | 1.92, m |
| 20 | 59.6, t | 2.45, m | 59.0, t | 2.70, m 2.47, m |
| 22 | 51.9, t | 2.78, m 2.02, m | 51.0, t | 2.71, m 2.00, m |
| 23 | 24.2, t | 1.79, m 0.88, m | 28.0, t | 2.20, m |
| 24 | 39.2, d | 3.98, m | 36.9, d | 3.62, m |
| 25 | 52.6, s | 52.0, s | ||
| 26 | 64.9, d | 4.89, s | 66.0, d | 4.27, s |
| 28 | 45.7, t | 3.83, dd (15.0, 4.0) 3.35, dd (14.5, 11.0) |
45.5, t | 4.05, brd (17.7) 3.49, brd (17.7) |
| 29 | 28.9, t | 1.91, m 1.66, m | 24.6, t | 1.38, m |
| 30 | 23.2, t | 2.03, m 1.48, m | 29.7, t | 1.88, m 1.60, m |
| 31 | 30.7, t | 2.55, m 2.20, m | 30.1, t | 2.72, m 2.40, m |
| 32 | 144.3, d | 7.16, dd (8.5, 7.0) | 128.5, d | 5.19, m |
| 33 | 135.1, s | 135.5, d | 6.46, d (10.7) | |
| 34 | 161.9, s | 155.1, s | ||
| 35 | 114.7, s | 114.5, s | ||
| 36 | 62.8, t | 2.63, d (10.9) 2.55, d (10.9) |
62.0, t | 2.98, d (11.9) 2.69, d (11.9) |
| Ac-33 | 194.7, s 27.4, q |
2.27, s | ||
| Ac-35 | 192.6, s 30.1, q |
2.19, s | 197.0, s 29.8, q |
2.19, s |
In CDCl3, 400 or 500 MHz for 1H and 100 or 125 MHz for 13C NMR. Carbon multiplicities were determined by DEPT 135° experiments.
s = quaternary, d = methine, t = methylene, q = methyl carbons. Coupling constants (J) are in Hz.
Compound 9
yellowish powder (EtOH); mp 130–132 °C; [α]D25 +13.0 (c 0.10, CHCl3); UV λmax (log ε) (MeOH) 267 (2.65), 355 (2.60), 368 (2.73) nm; IR νmax (CHCl3) 3631–3260 (NH), 3011–2838, 1729 (C═O) 1660, 1626, 1504, 1466, 1364, 1013 cm−1; 1H and 13C NMR, see Table 2; HRFTMS m/z 573.3571 (calcd for C38H45N4O, 573.3593 [M + H]+).
Diimide Reduction of 1 and 3
To a solution of 50 mg of 1 in absolute EtOH (5 mL) was added 10 mg of Pd on carbon. Hydrazine hydrate (0.5 mL) was gradually added to the mixture with stirring, over a 30 min period. After the addition was complete, the reaction mixture was refluxed for 12 h until TLC indicated a complete reaction. The reaction mixture was filtered while hot through Celite, treated with brine solution (5 mL), alkalinized with NH4OH to pH 9, and extracted with CHCl3 (2 × 10 mL). The organic layer was washed with H2O (2 × 10 mL), dried over anhydrous Na2SO4, and evaporated under vacuum. Preparative TLC of the residue on Si gel G254 plates using cyclohexane/EtOAc/NH4OH (100:50:0.1) afforded 10 (9.5 mg, Rf 0.51). The same reaction was repeated starting with 50 mg of 3 or 100 mg of 4. Final purification of the reaction residue of 3 was conducted using preparative Si gel G254TLC, using n-hexanes/acetone (70:30), to afford 11 (6.4 mg, Rf 0.48) and 12 (2.7 mg, Rf 0.53). Similarly, preparative TLC of the reaction residue of 4, using n-hexanes/acetone (65:35), afforded 13 (14.5 mg, Rf 0.42) and 14 (3.4 mg, Rf 0.18).
15,16,32,33-Tetrahydro-8-hydroxymanzamine A (10)
yellowish-brown powder (EtOH); mp 162–164 °C dec; [α]D25 −41.0 (c 0.10, CHCl3); UV λmax (log ε) (MeOH) 267 (1.98), 355 (1.92), 368 (2.04) nm; IR νmax (CHCl3) 3620–3230 (NH and OH), 3110–2854, 1637, 1561, 1468, 1364, 1019 cm−1; 1H and 13C NMR, see Table 3; HRFTMS m/z 569.3844 (calcd for C36H49N4O2, 569.3856 [M + H]+).
Table 3.
13C and 1H NMR Data of Compounds 10 and 11a
| 10 | 11 | |||
|---|---|---|---|---|
| position | δC | δH | δC | δH |
| 1 | 142.8, s | 144.8, s | ||
| 3 | 138.1, d | 8.40, d (5.6) | 138.6, d | 8.64, d (5.0) |
| 4 | 113.8, d | 7.78, d (5.6) | 113.6, d | 7.83, d (5.1) |
| 4a | 130.0, s | 129.6, s | ||
| 4b | 123.2, s | 121.8, s | ||
| 5 | 112.5, d | 7.51, d (7.7) | 122.0, d | 8.09, d (7.7) |
| 6 | 120.7, d | 7.06, dd (7.7, 7.6) | 120.0, d | 7.26, dd (7.6, 7.5) |
| 7 | 112.4, d | 6.98, d (7.6) | 128.3, d | 7.53, dd (7.6, 7.5) |
| 8 | 144.0, s | 112.0, d | 7.58, d (7.5) | |
| 8a | 130.4, s | 141.8, s | ||
| 9a | 133.3, s | 133.5, s | ||
| 10 | 139.5, s | 144.2, s | ||
| 11 | 136.5, d | 6.77, s | 137.3, d | 6.61, s |
| 12 | 69.1, s | 68.7, s | ||
| 13 | 37.6, t | 2.10, m | 41.2, t | 2.02, m 1.83, m |
| 14 | 20.3, t | 1.65, m | 21.3, t | 2.30, m 2.21, m |
| 15 | 23.7, t | 1.45, m | 128.5, d | 5.65, m |
| 16 | 25.5, t | 1.70, m | 132.7, d | 5.54, m |
| 17 | 27.1, t | 1.55, m | 25.8, t | 2.57, m 1.57, m |
| 18 | 24.6, t | 1.50, m | 26.9, t | 1.70, m |
| 19 | 26.0, t | 1.62, m,, 1.40, m | 25.6, t | 1.72, m |
| 20 | 58.9, t | 2.45, dd (12.2, 5.3) 2.32, m |
53.1, t | 2.68, m 2.43, m |
| 22 | 52.3, t | 2.97, m 2.34, m | 49.9, t | 2.80, m 1.99, m |
| 23 | 32.7, t | 2.05, m 1.71, m | 33.8, t | 2.30, m |
| 24 | 41.1, d | 3.29, m | 42.0, d | 3.14, m |
| 25 | 45.9, s | 46.0, s | ||
| 26 | 81.5, d | 3.76, s | 81.3, d | 3.78, s |
| 28 | 45.6, t | 3.44, m 2.97, m | 52.7, t | 3.38, m 2.95, m |
| 29 | 29.7, t | 1.70, m | 25.9, t | 1.80, m 1.65, m |
| 30 | 25.7, t | 2.03, m 1.70, m | 28.5,b t | 1.55, m |
| 31 | 28.6, t | 1.58, m | 25.4,b t | 1.77, m |
| 32 | 25.2, t | 1.70, m | 28.9,b t | 2.15, m 1.55, m |
| 33 | 30.5, t | 1.72, m | 29.9, t | 1.65, m |
| 34 | 63.8, d | 3.05, m | 63.6, d | 3.11, m |
| 35 | 51.8, t | 2.40, m | 49.3, t | 1.85, m 1.56, m |
| 36 | 69.5, t | 2.64, d (11.3) 2.21, d (11.3) |
69.2, t | 2.62, brd (11.9) 2.30, d (12.0) |
In CDCl3, 400 MHz for 1H, 100 MHz for 13C NMR. Carbon multiplicities were determined by DEPT 135° experiments.
s = quaternary, d = methine, t = methylene, q = methyl carbons. Coupling constants (J) are in Hz.
Interchangeable in the same column.
32,33-Dihydromanzamine A (11)
colorless powder (EtOH); mp 131–134 °C; [α]D25 +51.0 (c 0.24, CHCl3); UV λmax (log ε) (MeOH) 265 (2.00), 353 (1.80), 366 (2.01) nm; IR νmax (CHCl3) 3500–3100 (NH and OH), 2926, 2854, 1658, 1625, 1564, 1460, 1360 cm−1; 1H and 13C NMR, see Table 3; HRFTMS m/z 551.3752 (calcd for C36H47N4O, 551.3750 [M + H]+).
15,16,32,33-Tetrahydromanzamine A (12)
colorless powder (EtOH); mp 145–147 °C; [α]D25 +41.0 (c 0.05, CHCl3); UV λmax (log ε) (MeOH) 262 (1.99), 350 (1.77), 365 (2.00) nm; IR νmax (CHCl3) 3500–3100 (NH and OH), 2920, 2850, 1625, 1560, 1450, 1355 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.46 (1H, brs, NH), 8.41 (1H, d, J = 5.0 Hz, H-3); 7.83 (1H, d, J = 5.1 Hz, H-4); 8.09 (1H, d, J = 7.9 Hz, H-5); 7.26 (1H, dd, J = 7.9, 7.6 Hz, H-6); 7.53 (1H, dd, J = 7.6, 7.4 Hz, H-7); 7.58 (1H, d, J = 7.4 Hz, H-8); 6.66 (1H, s, H-11); 2.97 (1H, m, H-20a); 2.45 (1H, m, H-20b); 2.93 (1H, m, H-22a); 2.28 (1H, m, H-22b); 3.09 (1H, m, H-24); 3.68 (1H, s, H-26); 3.38 (1H, dd, J = 13.4, 12.7 Hz, H-28a); 2.42 (1H, dd, J = 12.5, 4.9 Hz, H28b); 3.11 (1H, m, H-34); 2.67 (1H, brd, J = 11.1 Hz, H-36a); 2.19 (1H, d, J = 11.4 Hz, H-36b); HRFTMS m/z 553.3902 (calcd for C36H49N4O, 553.3906 [M + H]+).
15,16,32,33-Tetrahydro-8-hydroxymanzamine A (13)
colorless powder (EtOH); mp 122–124 °C; [α]D25 +7.0 (c 0.4, CHCl3); UV λmax (log ε) (MeOH) 264 (1.94), 351 (1.70), 363 (1.99) nm; IR νmax (CHCl3) 3475, 3196 (NH and OH), 2929, 2850, 1633, 1593, 1562, 1460, 1160 cm−1; 1H and 13C NMR, see Table 4; HRFTMS m/z 569.3838 (calcd for C36H49N4O2, 569.3856 [M + H]+).
Table 4.
13C and 1H NMR Data of Compounds 13 and 14a
| 13 | 14 | |||
|---|---|---|---|---|
| position | δC | δH | δC | δH |
| 1 | 142.9, s | 142.5, s | ||
| 3 | 138.2, d | 8.40, d (5.2) | 138.3, d | 8.31, d (5.2) |
| 4 | 114.0, d | 7.78, d (5.2) | 113.5, d | 7.81, d (5.2) |
| 4a | 130.2, s | 129.9, s | ||
| 4b | 123.4, s | 123.1, s | ||
| 5 | 112.6, d | 7.56, d (7.7) | 113.2, d | 7.58, d (7.6) |
| 6 | 121.0, d | 7.03, dd (7.7, 7.5) | 121.2, d | 7.12, dd (7.7, 7.6) |
| 7 | 112.4, d | 6.96, d (7.5) | 112.2, d | 7.05, d (7.6) |
| 8 | 144.0, s | 143.3 | ||
| 8a | 130.8, s | 133.4, s | ||
| 9a | 133.6, s | 134.0, s | ||
| 10 | 140.7, s | 32.5, d | 3.01, m | |
| 11 | 137.7, d | 6.78, s | 30.6, t | 1.50, m |
| 12 | 69.2, s | 69.3, s | ||
| 13 | 37.9, t | 2.07, m | 30.1, t | 1.55, m |
| 14 | 20.6, t | 1.60, m | 20.3, t | 1.45, m |
| 15 | 23.8, t | 1.42, m | 24.3, tb | 1.46, m |
| 16 | 25.4, t | 1.73, m | 25.0, tb | 1.55, m |
| 17 | 27.3, t | 1.58, m | 24.9, tb | 1.57, m |
| 18 | 25.7, t | 1.55, m | 26.3, t | 1.66, m |
| 19 | 26.3, t | 1.64, m, 1.43, m | 25.4, t | 1.70, m |
| 20 | 59.1, t | 2.45, dd (12.0, 5.3) 2.32, m |
59.3, t | 2.58, m 2.25, m |
| 22 | 52.5, t | 2.97, m 2.34, m | 51.3, t | 3.11, m 2.03, m |
| 23 | 33.2, t | 2.05, m 1.74, m | 33.6, t | 2.34, m 1.67, m |
| 24 | 41.2, d | 3.36, m | 38.1, d | 1.85, m |
| 25 | 46.8, s | 45.5, s | ||
| 26 | 81.4, d | 3.76, s | 78.5, d | 4.93, s |
| 28 | 45.8, t | 3.44, m 2.97, m | 50.1, t | 4.65, brd (14.9) 4.24, m |
| 29 | 29.9, t | 1.70, m | 27.3, t | 1.70, m |
| 30 | 25.9, t | 2.05, m 1.75, m | 29.2, t | 2.02, m 1.65, m |
| 31 | 28.7, tb | 1.58, m | 28.8, tc | 1.50, m |
| 32 | 25.2, tb | 1.75, m | 26.0, tc | 1.70, m |
| 33 | 30.8, t | 1.72, m | 29.2, t | 1.75, m |
| 34 | 64.3, d | 2.99, m | 63.0, d | 2.99, m |
| 35 | 52.0, t | 2.40, m | 48.2, t | 2.60, m |
| 36 | 69.6, t | 2.62, d (11.2) 2.18, d (11.2) |
67.9, t | 2.52, d (11.3) 2.20, m |
In CDCl3, 400 MHz for 1H, 100 MHz for 13C NMR. Carbon multiplicities were determined by DEPT 135° experiments.
s = quaternary, d = methine, t = methylene, q = methyl carbons. Coupling constants (J) are in Hz.
Interchangeable in the same column.
Interchangeable in the same column.
10,11,15,16,32,33-Hexahydro-8-hydroxymanzamine A (14)
colorless powder (EtOH); mp 162–164 °C dec; [α]D25 +17.0 (c 0.10, CHCl3); UV λmax (log ε) (MeOH) 265 (1.88), 353 (1.90), 364 (2.00) nm; IR νmax (CHCl3) 3475, 3200 (NH and OH), 3110–2850, 1550, 1460, 1360, 1100 cm−1; 1H and 13C NMR, see Table 4; HRFTMS m/z 571.4009 (calcd for C36H51N4O2, 571.4012 [M + H]+).
Reduction of 2 with NaBH4
To a solution of 20 mg of 2 in anhydrous THF was added 2 mg of NaBH4. The reaction was stirred at room temperature for 9 h until TLC indicated a complete reaction. Then, brine solution was added (10 mL), and the mixture was alkalinized by NH4OH to pH 9 and extracted with CHCl3 (2 × 10 mL). The organic layer was washed by H2O (2 × 10 mL), dried over anhydrous Na2SO4, and evaporated under vacuum. Preparative TLC of the residue on Si gel G254, using cyclohexane/EtOAc/NH4OH (100: 50:0.1) afforded 15 (5.5 mg, Rf 0.36) and 16 (1.2 mg, Rf 0.30).
31β-Hydroxy-32,33-dihydro-8-hydroxymanzamine A (15)
yellowish powder (EtOH); mp 134–136 °C; [α]D25 −26.0 (c 0.10, CHCl3); UV λmax (log ε) (MeOH) 267 (2.26), 368 (2.33), 389 (2.21) nm; IR νmax (CHCl3) 3650–3250 (NH and OH), 3100–2820, 1636, 1559, 1466, 1013 cm−1; 1H and 13C NMR, see Table 5; HRFTMS m/z 583.3600 (calcd for C36H47N4O3, 583.3648 [M + H]+).
Table 5.
13C and 1H NMR Data of Compounds 15 and 17a
| 15 | 17 | |||
|---|---|---|---|---|
| position | δC | δH | δC | δH |
| 1 | 142.5, s | 146.7, s | ||
| 3 | 137.2, d | 8.37, d (5.6) | 137.9, d | 8.39, d (5.6) |
| 4 | 113.7, d | 7.83, d (5.6) | 112.9, d | 7.79, d (5.6) |
| 4a | 128.9, s | 128.2, s | ||
| 4b | 123.1, s | 124.1, s | ||
| 5 | 113.5, d | 7.59, d (7.7) | 118.9, d | 7.97, d (7.8) |
| 6 | 120.9, d | 7.13, dd (7.7, 7.6) | 119.4, d | 7.23, dd (7.8, 7.6) |
| 7 | 112.2, d | 7.04, d (7.6) | 120.1, d | 7.29, d (7.6) |
| 8 | 143.3 | 136.6, s | ||
| 8a | 133.4, s | 133.3, s | ||
| 9a | 134.1, s | 134.7, s | ||
| 10 | 137.9, s | 40.8, d | 4.36, brs | |
| 11 | 127.9, d | 6.81, s | 126.0, d | 6.53, s |
| 12 | 68.3, s | 138.4, s | ||
| 13 | 36.5, t | 2.20, m | 34.4, t | 2.52, m 2.37, m |
| 14 | 21.3, t | 2.15, m | 28.1, t | 2.42, 2H m |
| 15 | 130.1, d | 5.46, m | 130.5, d | 5.47, m |
| 16 | 132.3, d | 5.39, m | 131.4, d | 5.35, m |
| 17 | 24.9, t | 1.57, m | 24.7, t | 1.37, 2H m |
| 18 | 26.5, t | 1.66, m | 27.8, t | 2.70, m 2.42, m |
| 19 | 25.4, t | 1.60, m | 27.3, t | 1.95, 2H m |
| 20 | 52.7, t | 2.88, m 2.41, m | 59.4, t | 2.39, m 2.30, m |
| 22 | 49.6, t | 2.87, m 2.59, m | 51.2, t | 2.89, 2H m |
| 23 | 32.6, t | 2.44, m 2.15, m | 23.6, t | 1.68, 2H m |
| 24 | 40.1, d | 3.16, m | 37.9, d | |
| 25 | 45.5, s | 52.9, s | ||
| 26 | 81.5, d | 3.88, s | 63.3, d | 4.83, s |
| 28 | 41.1, t | 3.15, m 2.99, m | 45.8, t | 3.77, 2H m |
| 29 | 25.3, t | 1.60, m | 29.7, t | 1.81, 2H m |
| 30 | 29.1, t | 2.12, m 1.65, m | 30.4, t | 1.89, 2H m |
| 31 | 64.0, d | 3.77, brs | 71.7, d | 4.91, ddd (11.9, 8.2, 3.6) |
| 32 | 31.1, t | 2.15, m 1.55, m | 36.9, t | 3.04, dd (14.1, 8.2) 2.41, m |
| 33 | 29.4, t | 1.75, m | 116.9, s | |
| 34 | 64.0, d | 4.24, brs | 163.9, s | |
| 35 | 36.5, t | 2.30, m 2.15, m | 111.7, s | |
| 36 | 68.9, t | 2.68, d (11.9) 2.35, d (11.9) |
62.1, t | 2.62, d (11.9) 2.51,d (11.9) |
| 37 | 148.1, s | |||
| 38 | 16.8, q | 1.85, s | ||
| OAc-8 | 169.8, s 21.7, q |
2.58, s | ||
| OAc-31 | 170.3, s 20.7, q |
2.07, s | ||
| Ac-35 | 192.0, s 27.3, q |
2.29, s | ||
| OAc-37 | 168.8, s 21.2, q |
2.21, s | ||
In CDCl3, 400 or 500 MHz for 1H and 100 or 125 MHz for 13C NMR. Carbon multiplicities were determined by DEPT 135° experiments.
s = quaternary, d = methine, t = methylene, q = methyl carbons. Coupling constants (J) are in Hz.
31α-Hydroxy-32,33-dihydro-8-hydroxymanzamine A (16)
yellowish powder (EtOH); mp 140–142 °C, [α]D25 −34.0 (c 0.10, CHCl3); UV λmax (log ε) (MeOH) 267 (2.94), 356 (2.88), 368 (3.01) nm; IR νmax (CHCl3) 3650–3250 (NH and OH), 3100–2800, 1636, 1561, 1459, 1015 cm−1; 1H NMR (CDCl3, 400 MHz) δ 8.35 (1H, d, J = 5.6 Hz, H-3); 7.82 (1H, d, J = 5.6 Hz); 7.59 (1H, d, J = d 7.7 Hz, H-5); 7.13 (1H, dd, J = 7.7, 7.6 Hz, H-6); 7.06 (1H, d, J = 7.6 Hz, H-7); 6.88 (1H, s, H-11); 5.60 (1H, m, H-15); 5.54 (1H, m, H-16); 2.61 (1H, m, H-20a); 2.41 (1H, m, H-20b); 2.59 (1H m, H-22a); 2.32 (1H, m, H-22b); 3.08 (1H, m, H-24); 3.86 (1H, s, H-26); 3.48 (1H, m, H-28a); 2.86 (1H, m, H-28b); 3.86 (1H, brs, H-31); 4.22 (1H, brs, H-34); HRFTMS m/z 583.3615 (calcd for C36H47N4O3, 583.3648 [M + H]+).
Acetylation of Manzamine 15
To a solution of 5 mg of 15 in 2.5 mL of acetic anhydride was added 1.0 mL of pyridine. The reaction mixture was stirred at 100 °C for 12 h. About 5 mL of a saturated NaCl solution was added, and the mixture was alkalinized by NH4OH to pH 9 and extracted with CHCl3 (2 × 10 mL). The organic layer was washed by H2O (2 × 10 mL), dried over anhydrous Na2SO4, and evaporated under a vacuum. Preparative TLC of the residue on Si gel G254, using cyclohexane/EtOAc/NH4OH (100:50:0.1), afforded 17 (2.8 mg, Rf 0.69).
Compound 17
yellowish powder (EtOH); mp 118 °C; [α]D25 −48.0 (c 0.12, CHCl3); UV λmax (log ε) (MeOH) 266 (2.68), 355 (2.63), 368 (2.76) nm; IR νmax (CHCl3) 3412 (NH), 3100–2820, 1753, 1730, 1690 (C═O), 1638, 1568, 1473, 1213, 1011 cm−1; 1H and 13C NMR, see Table 5; HRFTMS m/z 775.4271 (calcd for C46H55N4O7, 775.4231 [M + H]+).
Antituberculosis Assays
Compounds were tested in the microplate Alamar Blue assay as previously published.24
Anti-inflammatory Assays
Rat neonatal microglia (2 × 105 cells/24-well cell culture clusters) were stimulated with Escherichia coli lipopolysaccharide (LPS) (0.3 ng/mL) in 1 mL of Dulbecco’s modified Eagle medium, with 10% fetal bovine serum, penicillin, and streptomycin, for 17 h in a humidified 5% CO2 incubator at 37 °C.23 The medium was then removed, and the microglia cells were washed with warm (37 °C) Hanks’ balanced salt solution (HBSS) and then incubated with manzamines 1–17 (0.1–10 µM) or vehicle (DMSO) for 15 min prior to stimulation with phorbol 12-myristate 13-acetate (PMA) (1 µM). All experimental treatments were run in triplicate and in a final volume of 1 mL. The cells were stimulated for 70 min with PMA, after which, HBSS was aspirated, and O2−, TXB2, and LDH release were determined, as described elsewhere.23
Supplementary Material
Table 6.
In Vitro Inhibitory Activity and Minimum Inhibitory Concentrations (MIC) of Natural and Semisynthetic Manzamines against M. tuberculosis (H37Rv)
| compound | MIC (µg/mL) | % inhibition |
|---|---|---|
| 1 | 0.91 | 100 |
| 2 | <12.5 | 99 |
| 3 | 1.56 | 99 |
| 5 | <6.25 | 98 |
| 6 | <6.25 | 95 |
| 7 | >6.25 | 96 |
| 8 | NTa | NTa |
| 9 | >6.25 | 58 |
| 10 | <6.25 | 99 |
| 11 | 7.41 | 99 |
| 12 | NTa | NTa |
| 13 | 1.84 | 98 |
| 14 | NTa | NTa |
| 15 | <6.25 | 100 |
| 16 | >6.25 | 61 |
| 17 | <6.25 | 99 |
| 181 | >12.5 | 44 |
| rifampin | 0.5 | 100 |
NT = not tested.
Acknowledgments
This work was supported by the Public Health Service, grant number R29 AI 36596-01A1 from the National Institute of Allergy and Infectious Diseases, and, in part, by the Center for Disease Control, Cooperative Agreement No. U50/CCU418839 (M.A.A.). Antimycobacterial data were provided by the U.S. Tuberculosis Facility (TAACF), NIAID/NIH Contract No. NO1-AI-45246. We also thank the Monsanto Corporation and The Mississippi-Alabama Sea Grant College Program for financial support, and the Mississippi Center for Supercomputing Research (MCSR). The antineuroinflammatory studies described were supported by intramural funding from the Office of Research & Sponsored Programs, Midwestern University. We are grateful to the late Dr. P. J. Scheuer, of the University of Hawaii at Manoa, for his valuable editorial guidance and for providing authentic (+)-8-hydroxymanzamine A, Drs. S. Wahyuono, A. Mursyidi, S. Bobzin, and M. Slattery, for assistance with sample collection, J. Trott and S. Sanders for in vitro malaria assays, and Drs. D. C. Dunbar, S. Duke, A. Rimando, and J. O’Neal for 15N NMR and mass spectrometric data. The expert technical assistance for the O2−, TXB2, and LDH assays by M. Hall from the Pharmacology Department, Chicago College of Osteopathic Medicine, Midwestern University, is gratefully acknowledged.
Footnotes
Supporting Information Available: Schemes S1 and S2, plausible mechanisms of formation for compounds 6, 9, and 17, are available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- 1.El Sayed KA, Kelly M, Kara UAK, Ang KKH, Katsuyama I, Dunbar DC, Khan AA, Hamann MT. J. Am. Chem. Soc. 2001;123:1804–1808. doi: 10.1021/ja002073o. [DOI] [PubMed] [Google Scholar]
- 2.Sakai R, Higa T, Jefford CW, Bernardinelli G. J. Am. Chem. Soc. 1986;108:6404–6405. [Google Scholar]
- 3.Hu JF, Hamann MT, Hill R, Kelly M. In: The Alkaloids. Cordell GA, editor. Vol. 60. New York: Elsevier Science; 2003. pp. 207–285. [Google Scholar]
- 4.Tsuda M, Kobayashi J. Heterocycles. 1997;46:765–794. [Google Scholar]
- 5.Magnier E, Langlois Y. Tetrahedron. 1998;54:6201–6258. [Google Scholar]
- 6.Urban S, Hickford SJH, Blunt JW, Munro MHG. Curr. Org. Chem. 2000;4:765–807. [Google Scholar]
- 7.Edrada RA, Proksch P, Wray V, Witte L, Müller WEG, Van Soest RWM. J. Nat. Prod. 1996;59:1056–1060. doi: 10.1021/np9604083. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura H, Deng S, Kobayashi J, Ohizumi Y, Tomotake Y, Matsuzaki T, Hirata Y. Tetrahedron Lett. 1987;28:621–624. [Google Scholar]
- 9.Rao KV, Santarsiero BD, Mesecar AD, Schinazi RF, Tekwani BL, Hamann MT. J. Nat. Prod. 2003;66:823–828. doi: 10.1021/np020592u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng J, Hu JF, Kazi AB, Li Z, Avery M, Peraud O, Hill R, Franzblau SG, Zhang F, Schinazi RF, Wirtz SS, Tharnish P, Kelly M, Wahyuono S, Hamann MT. J. Am. Chem. Soc. 2003;125:13382–13386. doi: 10.1021/ja030087z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamann MT, El Sayed KA. PCT WO02/017917 A1
- 12.Yousaf M, El Sayed KA, Rao KV, Lim CW, Hu J, Kelly M, Franzblau SG, Zhang F, Peraud O, Hill RT, Hamann MT. Tetrahedron. 2002;58:7397–7402. [Google Scholar]
- 13.Mayer AMS, Hall ML, Lynch SM, Gunasekera SP, Sennett SH, Pomponi SA. BioMedCentral Pharmacol. 2005;5:6–18. doi: 10.1186/1471-2210-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayer AMS, Gunasekera SP, Pomponi SA, Sennett SH. 6,387,916. U.S. Patent. 2002
- 15.Mayer AMS, Gunasekera SP, Pomponi SA, Sennett SH. 6,602,881. U.S. Patent. 2003
- 16.Hill RT, Hamann MT, Peraud O, Kasanah N. PCT WO 2004/013297 A3
- 17.Winkler JD, Axten JM. J. Am. Chem. Soc. 1998;120:6425–6426. doi: 10.1021/ja981303k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lansbury PT, Bieber JB, Saeva FD, Fountain KR. J. Am. Chem. Soc. 1969;91:399–405. [Google Scholar]
- 19.Prelog V, Trayham JG. In: Molecular Rearrangements. de Mayo P, editor. New York: Wiley-Interscience; 1963. p. 593. [Google Scholar]
- 20.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA, Jr, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo CS, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin R, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson BG, Chen W, Wong MW, Andres JL, Head-Gordon M, Replogle E, Pople JA. Gaussian. Vol. 98. Pittsburgh, PA: Gaussian, Inc.; 1998. [Google Scholar]
- 21.El Sayed KA, Bartyzel P, Shen X, Perry TL, Zjawiony JK, Hamann MT. Tetrahedron. 2000;56:949–953. [Google Scholar]
- 22.Mayer AMS. Medicina (Buenos Aires) 1998;58:377–385. [PubMed] [Google Scholar]
- 23.Mayer AMS, Oh S, Ramsey KH, Jacobson PB, Glaser KB, Romanic AM. SHOCK. 1999;11:180–186. [PubMed] [Google Scholar]
- 24.Collins L, Franzblau SG. Antimicrob. Agents Chemother. 1997;41:1004–1009. doi: 10.1128/aac.41.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.