Abstract
Background
Etiology of hemorrhagic gastroenteritis (HGE) syndrome in dogs is unknown and histopathologic and microbial investigations have only been performed post mortem.
Objective
To identify characteristic intra vitam endoscopic and histologic mucosal lesions, as well as bacterial species, within the mucosa of dogs with HGE.
Animals
Ten dogs diagnosed with HGE were included. Eleven dogs with gastroduodenoscopy and different intestinal diseases were used as controls for microbial changes. Dogs pretreated with antibiotics or diagnosed with any disease known to cause bloody diarrhea were excluded from the study.
Methods
In this prospective study, gastrointestinal biopsies were collected from 10 dogs with HGE. Endoscopic and histologic changes were assessed according to WSAVA guidelines. Biopsies from the stomach, duodenum, ileum, and colon were investigated by histology and by immunohistochemistry for the presence of Clostridium spp. and parvovirus. The first duodenal biopsy taken with a sterile forceps was submitted for bacterial culture.
Results
Acute mucosal lesions were only found in the intestines, not in the stomach. Clostridium spp., identified as Clostridium perfringens in 6/9 cases, were detected on the small intestinal mucosa in all dogs with HGE, either by culture or immunohistopathology. In the control group, C. perfringens could only be cultured in one of 11 dogs.
Conclusions and Clinical Importance
The results of this study demonstrate an apparent association between C. perfringens and the occurrence of acute hemorrhagic diarrhea. The term “HGE,” which implies the involvement of the stomach, should be renamed as “acute hemorrhagic diarrhea syndrome.”
Keywords: Acute emorrhagic diarrhea syndrome, Bloody diarrhea, Clostridium perfringens, Hemorrhagic gastroenteritis
Abbreviations
- AHDS
acute hemorrhagic diarrhea syndrome
- CBC
complete blood count
- CPE
Clostridium perfringens enterotoxin
- CRT
capillary refill time
- ELISA
enzyme‐linked immunosorbent assay
- HGE
hemorrhagic gastroenteritis
- WSAVA
World Small Animal Veterinary Association
A clinical syndrome in dogs, characterized by the acute onset of bloody diarrhea and vomiting, is well known to veterinary practitioners.1, 2 Over the last 40 years, allergic, hereditary, autoimmune, and infectious disorders have been proposed as causes for this syndrome. However, the exact pathogenesis remains unknown.1, 3, 4, 5, 6, 7 In the first description of a large group of dogs with acute hemorrhagic diarrhea, the name “hemorrhagic gastroenteritis” (HGE) was used.1 This terminology has since been questioned, as the intestinal histology of affected dogs investigated at necropsy showed no evidence of an inflammatory reaction in a previous study.6 The principal intestinal lesions of dogs with HGE at necropsy were described as superficial mucosal hemorrhagic necroses.5, 8, 9 An additional histologic finding of the intestinal lesions identified by histopathology was the adherence of large Gram‐positive bacilli, identified as Clostridium perfringens, to the necrotic mucosal surfaces.7, 8, 9 However, all of the histologic and microbiological evaluations of gastrointestinal tissues from dogs with HGE, thus far, had been performed post mortem, which complicates the interpretation of abnormal findings. Autolysis of the gastrointestinal tract begins rapidly, and post mortem degenerative changes can be observed as early as 90 minutes after death. Necrosis and autolysis of mammalian cells present very similar morphological appearances, which can cause difficulties in assessment.10 Because all types of C. perfringens can normally inhabit the intestines of most animals, cultures of this microorganism from the intestinal contents of these animals has no diagnostic value, especially not cultures taken post mortem.11, 12, 13, 14 So far, no studies on the macroscopic appearance or on histologic changes performed intra vitam in dogs with HGE exist.
An association between a clostridial infection and acute hemorrhagic diarrhea is suspected, as 8/27 dogs with acute hemorrhagic diarrhea had positive fecal ELISA results for C. perfringens enterotoxin (CPE), 7/27 for C. difficile toxin A, and 1/27 for both toxins.5 However, C. perfringens can be cultured from the fecal specimens of more than 80% of diarrheic and nondiarrheic dogs, and CPE can also be detected in up to 14% of nondiarrheic, healthy dogs.11, 15 C. difficile can even be found in up to 23% of healthy, nondiarrheic dogs.15, 16, 17 In addition, the moderate‐to‐poor sensitivity and specificity of commercial ELISA, which was used in this previous study, have been reported by comparison with the gold standard of cytotoxicity assays.18
Thus, the aim of this study was to describe endoscopically identifiable gross lesions of the gastrointestinal tract and histologic findings in prospectively collected gastric and intestinal biopsy samples from dogs with HGE. A second goal was to potentially identify bacteria in these biopsy samples. These investigations should provide new insight into the pathogenesis and role of bacteria in this syndrome.
Materials and Methods
Patients
This study was conducted according to German animal welfare laws. Each owner was informed of the purposes of the study. Between August 2010 and December 2012, 10 dogs with acute hemorrhagic diarrhea without an identifiable cause, whose owners agreed to have endoscopy performed, were presented to the emergency service of the Clinic of Small Animal Medicine, LMU University of Munich, Germany. The inclusion criterion was an acute onset of hemorrhagic diarrhea (<3 days since presentation). Patients pretreated with antibiotics and having hemorrhagic diarrhea caused by a disease etiology unrelated to HGE were excluded from this study. Exclusion diagnoses included nonsteroidal anti‐inflammatory or corticosteroid toxicosis, hypoadrenocorticism, inflammatory bowel disease, severe hepatitis, hepatic neoplasia or hepatic failure, acute and chronic renal failure, pancreatitis, anticoagulant toxicosis, gastrointestinal neoplasia or foreign bodies, and enteric infection with parvovirus, Giardia spp., or endoparasites. To rule out these possible causes of hemorrhagic diarrhea, all dogs underwent a physical examination, abdominal ultrasound examination, CBC, serum biochemistry profile, serum bile acid concentrations, clotting profile, and fecal examination for nematode and protozoan parasites (29.5% natrium nitrate flotation solution,1 Giardia antigen ELISA2) and for parvovirosis (antigen ELISA3). In addition, the presence of parvovirus was excluded by immunohistochemistry (IHC). Immunolabeling of the mucosal tissue was performed through indirect immunostaining using mouse anti‐canine/‐feline parvovirus (MCA 20644) as primary and peroxidase‐labeled rabbit anti‐mouse immunoglobulins (P0s605) as secondary antibodies.
Treatment was standardized for all dogs and included fluid therapy (crystalloids; the fluid amount depended on dehydration, maintenance demands, and ongoing losses) and antiemetics (maropitant6 1 mg/kg SC q24h, on days 1 and 2). Analgesics were administered dependent on clinical judgement of abdominal pain. The clinical assessment was performed by calculating the “canine HGE activity index.”2 This index includes the parameters attitude, appetite, vomiting, stool consistency, stool frequency, and dehydration. Each parameter was scored (0 = normal, 1 = mild, 2 = moderate, 3 = severe), and the sum of scores yielded a total cumulative score (maximum = 18).
Eleven dogs that presented during the same time period and in whom gastroduodenoscopy was performed for other reasons than hemorrhagic diarrhea served as a control group for microbiological evaluations. Endoscopic biopsies in these dogs were used to assess the significance of microbial findings. All samples, including endoscopic biopsies, were collected in a manner identical to that in the HGE patients. Dogs pretreated with antibiotics were excluded from the control population.
Endoscopy
As early, after initial presentation, as anesthesia was considered safe (good pulse quality, heart rate 70–140/min, respiratory rate <25/min, CRT <1 second, rectal temperature 37.5–39.5°C), endoscopy of the upper and lower intestinal tracts of dogs with HGE was performed and evaluated according to the WSAVA International Gastrointestinal Standardization Guidelines.19 The flexible endoscope Olympus GIF Type 1607 was used for dogs <20 kg, and the Olympus PCF Type 140 L7 was used for dogs >20 kg. Each endoscopic parameter (hyperemia/vascularity, discoloration, edema, friability, texture changes, hemorrhage, and erosions/ulcers) was scored as 0 = normal, 1 = mild, 2 = moderate, or 3 = severe by two of the authors performing the endoscopies (SU & KB).
At least 6 biopsy specimens were collected from the stomach and each intestinal segment and then submitted for histology. Because of the size of the dog and the duration of the anesthesia, endoscopy of the ileum and colon was not possible in every patient. In some of these cases, biopsies were obtained blindly. In addition, the first duodenal biopsy was taken with a sterile single‐use biopsy forceps,8 transferred into a sterile tube without transport medium, and immediately submitted for bacterial culture.
Histologic Examination of Biopsies
Tissue samples were fixed in neutral buffered 10% formaldehyde for 24 hours, embedded in paraffin and plastic, sectioned, and stained with the hematoxylin and eosin and Giemsa. Two pathologists (ML and WH) performed the histologic examination of all tissues. The characterization of the histologic changes in the endoscopic biopsy samples was performed according to the histopathologic standards established by the WSAVA Gastrointestinal Standardization Group.20
Immunohistochemistry for C. perfringens
Immunohistochemistry was performed according to standard protocols for indirect IHC assays. All incubations were completed at room temperature. Following manual deparaffinization and rehydration, 4‐μm‐thick tissue sections mounted on positive‐charged glass slides were treated with 1% hydrogen peroxide to quench endogenous peroxidase activity and washed in a bath of Tris‐buffered saline (TBS, 0.5 M, pH 7.6). After incubation with goat normal serum (1 : 10 dilution)9 for 30 minutes, the slides were incubated with polyclonal anti‐Clostridium spp. antibody (polyclonal rabbit antibody against C. perfringens, C. sordellii, C. novyi, C. septicum, and C. chauvoei; 1 : 100 dilution; No. 2119‐2701)4 as the primary antibody for 60 minutes. Subsequently, the slides were washed in baths of TBS, incubated with peroxidase‐labeled goat anti‐rabbit immunoglobulin (1 : 100 dilution; No. P0448)5 as the secondary antibody for 1 hour and washed again in a bath of TBS. The binding of peroxidase coupled to the secondary antibodies was visualized by the reaction of H2O2 and 3′3′‐diaminobenzidinetetrahydrochloride 10 as chromogens. Slides were counterstained with Mayer's hematoxylin.
For clostridial IHC, positive controls included a clostridial suspension (isolated from dogs with HGE) injected into swine muscle and processed routinely (fixed in formalin and embedded in paraffin).
Bacterial Culture
Within 30 minutes after taking the biopsy, samples were plated onto agar plates using sterile tweezers. Nutrient agar with 5% defibrinated sheep blood for aerobic and Schaedler agar with 5% sheep blood for anaerobic cultivation were used.11 Plates were incubated at 38°C. Colony growth was monitored for 3 days. Every colony type was sampled and differentiated by mass spectrometry using MALDI‐TOF.12 Microbial identification by MALDI‐TOF is considered a very specific method comparable to conventional diagnostics.21
Statistical Evaluation
The numbers of positive bacterial cultures and positive IHC in dogs with HGE and control dogs, respectively, were compared with a Fisher Exact test. Improvement of the HGE index from day 1 to day 3 was compared with a repeated measures ANOVA. For 1 dog that was sent home because of almost complete remission, the value of day 2 was carried forward to day 3 to allow appropriate statistical analysis. For all tests, a P < .05 was considered significant.
Results
Study Population
Dogs with HGE included 5 females and 5 males, of which 3 and 2 were neutered, respectively. The median age and weight were 5 years (range 1–10) and 11.0 kg (range 2.9–30.5), respectively. Breeds included mixed breeds (n = 3), Yorkshire Terrier (n = 2), and one each of Jack‐Russell Terrier, Labrador Retriever, Dachshund, Westhighland White Terrier, and Miniature Australian Shepherd. The median duration of clinical signs until presentation was 12 hours (range 6–36). All 10 dogs also exhibited vomiting (6/10 bloody vomiting) and hemorrhagic diarrhea. In general, a rapid improvement of clinical signs was observed during the first 48 hours (median HGE score at presentation: 12 [range 6–17]; after 48 hours: 5 [range 0–10]). The improvement from day 1 to day 2 and day 1 to day 3 was highly significant (P < .01 and P < .001, respectively). All dogs recovered and were discharged. The median duration of hospitalization was 3 days (range 2–6).
Control Group
Dogs included 2 females and 9 males, of which 2 each were neutered in both groups. The median age and weight was 10 years (range 1–13) and 27.7 kg (range 5.0–62.5), respectively. Breeds included mixed breeds (n = 3), and one each of Yorkshire Terrier, Beagle, Jack‐Russell Terrier, Rhodesian Ridgeback, Leonberger, Malinois, Magyar Viszla, and Dalmatian. Final diagnoses included gastrointestinal foreign body (n = 3), adverse food reaction (n = 2), and one each of esophagitis, gastritis, inflammatory bowel disease, ulcerative colitis, and hypertrophic pylorus.
Endoscopy
In all dogs, an endoscopy was performed within the first 12 hours of presentation. In 6/10 dogs, the mucosa of the esophagus was assessed as normal; 4/10 dogs showed signs of mild hyperemia. The stomach was macroscopically normal in 7/10 dogs; 3/10 showed mild changes characterized by mild‐to‐moderate edema and hyperemia. Gastric erosions or ulcerations could not be detected in any dog, although 6/10 dogs were presented with hemorrhagic vomiting. Duodenoscopy was performed in 10/10, colonoscopy in 8/10, and ileoscopy in 6/10 dogs. The most important macroscopic findings in the intestinal tract, which could be observed in every dog, included hyperemia, increased mucosal friability, hemorrhage, and erosions. Compared with the gross lesions in the macroscopic colon, the lesions in the duodenum appeared to be more prominent (Table 1, Fig 1).
Table 1.
Endoscopically visualized lesions and histologic changes in the gastrointestinal mucosa of dogs with acute hemorrhagic diarrhea syndrome
| Endoscopic Changes | Duodenum (n = 10) | Ileum (n = 6) | Colon (n = 8) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Severity | No | Mild | Mod. | Severe | No | Mild | Mod. | Severe | Norm. | Mild | Mod | Severe |
| Hyperemia | 1 | 0 | 7 | 2 | 1 | 1 | 4 | 0 | 0 | 4 | 4 | 0 |
| Friability | 0 | 3 | 5 | 2 | 0 | 2 | 4 | 0 | 1 | 5 | 2 | 0 |
| Hemorrhage | 3 | 3 | 2 | 2 | 0 | 3 | 3 | 0 | 2 | 2 | 3 | 1 |
| Erosion/ulcers | 3 | 3 | 2 | 2 | 1 | 2 | 3 | 0 | 2 | 2 | 4 | 0 |
| Histologic Changes | Duodenum (n = 10; ns = 64) | Ileum (n = 8; ns = 29) | Colon (n = 9; ns = 55) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Severity | Norm. | Mild | Mod. | Severe | Norm. | Mild | Mod. | Severe | Norm. | Mild | Mod | Severe |
| Epith. injury | 2 | 3 | 3 | 2 | 2 | 4 | 2 | 0 | 0 | 3 | 2 | 4 |
| L. p. neutroph. | 2 | 6 | 1 | 1 | 1 | 3 | 3 | 1 | 0 | 4 | 5 | 0 |
| Vill. stunting | 4 | 2 | 2 | 2 | 1 | 2 | 4 | 1 | ||||
n, number of dogs; ns, number of adequate samples per location in total; epith. injury, epithelial injury; L. p. neutroph., Lamina propria neutrophils; vill. stunting, villous stunting; norm., no changes; mod., moderate changes.
Figure 1.
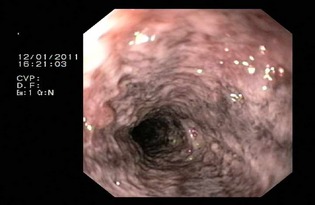
Dog #4. Endoscopic appearance of the duodenum, showing edema, hyperemia, increased vascularity, and erosions.
Histology
Lesions were restricted to the small and large intestine and were not found in the stomach. Neither relevant signs of acute inflammation nor destruction of the mucosal epithelial surface could be detected in gastric biopsy samples. The most important histologic changes in the intestine included acute mucosal necrosis (10/10) and neutrophilic infiltration (10/10). Histologic lesions reflecting acute mucosal intestinal destruction in the intestine are presented in Table 1.
A prominent feature of the small intestinal lesions was the adherence of large rod‐shaped bacteria to the necrotic mucosal surfaces, which could be observed in 9/10 dogs (6/10 in duodenal, 5/8 in ileal biopsies; Table 2, Fig 2). The bacteria built a dense layer on the surface of the necrotic lesions. Bacteria adhering to the duodenal mucosa were not detected by histology in any dog of the control group. There was a significant difference in the microscopic detection rate of clostridial‐type bacteria in duodenal biopsies between patients (6/10) and control dogs (0/11) (P = .004) (Table 3).
Table 2.
Detection of Clostridium spp. in small intestinal biopsy samples by histology and culture (all confirmed as C. perfringens by mass spectrometry using MALDI‐TOF) in the 10 individual patients with acute hemorrhagic diarrhea syndrome. The first duodenal biopsy was taken with a sterile forceps and submitted for bacterial culture
| Dog Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Histology duodenum | + | + | − | + | − | − | + | + | − | + |
| Culture duodenum | Ne | + | + | + | + | − | − | + | − | + |
| Histology ileum | − | + | − | Ne | + | + | Ne | + | + | − |
| Either histology or culture | + | + | + | + | + | + | + | + | + | + |
+, visualization of large rod‐shaped bacteria/culture of C. perfringens; −, no visualization of large rod‐shaped bacteria/no culture of C. perfringens; Ne, not evaluated.
Figure 2.
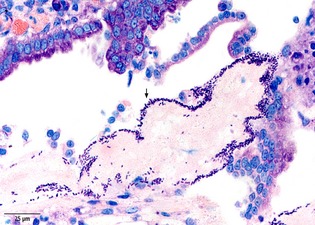
Giemsa staining of a duodenal section collected from Dog #2. A dense layer of large rod‐shaped bacteria adherent to a necrotic villous tip (black arrow).
Table 3.
Comparison of Clostridium spp. detection on the duodenal mucosa between dogs with hemorrhagic gastroenteritis and control dogs
| Patient Group | Control Group | ||||
|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | P value | |
| Culture duodenum | 6 | 3 | 1 | 10 | .017 |
| Histology duodenum | 6 | 4 | 0 | 11 | .004 |
| Either histology or culture duodenum | 8 | 2 | 1 | 11 | .002 |
All cultured Clostridium spp. were identified as C. perfringens by mass spectrometry using MALDI‐TOF.
Immunohistochemistry
The histologically detected bacteria were identified as clostridial antigen positive by immunohistochemical staining (Fig 3).
Figure 3.
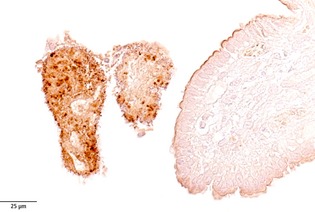
Immunohistochemical staining of a duodenal section collected from Dog #2. Many rod‐shaped bacteria present on the necrotic villous tissue were strongly positive for a polyclonal chicken antibody raised against Clostridium spp.
Bacterial Culture
A bacterial culture from duodenal biopsies was performed in 9 dogs with HGE and 11 control dogs. In 6/9 dogs with HGE, C. perfringens could be cultured. In 1/11 control dogs, a growth of C. perfringens was observed. There was a significant difference in the number of positive C. perfringens cultures from duodenal biopsies between patient and control group (P = .016) (Table 3).
Discussion
Results of this study demonstrate an association between clostridial overgrowth and HGE. Necrotic epithelial lesions were restricted to the small and large intestine. Biopsy samples from dogs with HGE showed a lack of gastric mucosal involvement in this study. In addition to profuse, watery‐mucoid, bloody diarrhea, many dogs with HGE demonstrate acute vomiting.1 Therefore, it was assumed that the stomach is involved in this disorder. Because the clinical signs could most likely be explained by an acute inflammation of the entire gastrointestinal tract, the name “HGE” was given to this syndrome.1
A very early report about the microscopic evaluation of the gastrointestinal mucosa from dogs with acute hemorrhagic diarrhea (obtained post mortem) mentioned that “no evidence of an inflammatory reaction” could be detected.6 A description of 2 other cases with pre‐acute hemorrhagic enteritis and their necropsy results was published 6 years later. One dog died within 15 minutes after admission, whereas the second dog was found dead, surrounded by pools of bloody diarrhea and vomitus, at home by his owners before being presented for necropsy. Microscopic changes in the gastric mucosa could only be detected in the second dog. Gastric lesions were described as superficial epithelial necrosis, and it remains unclear whether autolytic changes that were present at the time of necropsy caused difficulties in the assessment of the post mortem lesions.8 In all other reports on the histologic changes of the gastrointestinal tract in necropsied dogs with acute hemorrhagic diarrhea, descriptions concerning gastric changes are lacking, or the results are conflicting. One explanation is that all previously reported histopathologic evaluations were based on necropsied dogs, and post mortem changes can modify the microscopic appearance of the gastrointestinal mucosa of animals very rapidly after their deaths. In pigs, significant epithelial loss of the stomach begins to occur only 90 minutes after death.10 Gastric necrosis observed in necropsied dogs with hemorrhagic diarrhea could therefore represent autolytic morphological changes. This study, for the first time, evaluated the gastrointestinal histopathologic changes from samples collected intra vitam and immediately fixed in formalin. The results clearly show no signs of significant acute inflammation or destruction of the gastric mucosal epithelial surface. This finding was surprising because all of the dogs exhibited vomiting, even hemorrhagic vomiting in 6/10 cases, indicative of stomach involvement. Because (1) no acute gastric lesions could be detected macroscopically or histologically, (2) the prominent lesions found in the intestines may themselves cause vomiting, and (3) the presence of blood in the vomitus could be explained by the duodenal erosions, it is likely that lesions associated with this syndrome are generally restricted to the intestines and that the stomach is not primarily involved in the disease process.
Some reports have mentioned that lesions in dogs with HGE mainly affect the small intestine,5, 9 whereas other reports have stated that these lesions are particularly severe in the colon.8 In the present patient population, we demonstrated that the entire intestinal tract was affected in dogs with HGE and that mucosal damage is generally more severe in the large intestine. This study confirms, for the first time intra vitam, that intestinal mucosal hemorrhagic necrosis is the principal histologic lesion found in dogs with HGE. In contrast to previous histologic studies,5, 6 some infiltration of neutrophilic granulocytes in the intestinal mucosa was observed; however, in relation to the severe epithelial destruction, the influx of inflammatory cells was disproportionally minor. Acute mucosal destruction, in general, could be explained by ischemia,22 hyperthermia,23 acute parvoviral infection,24 and enterotoxins.25, 26, 27, 28 In this study population, systemic hypoxia and hyperthermia were ruled out by patient evaluation and history. Local thrombosis in the intestine was not likely, as no underlying disease predisposing for thromboembolism could be identified in any of the cases. Parvovirus infection was ruled out by fecal ELISA and IHC. Therefore, enterotoxins are the most logical explanation of the destructive lesions found in the dogs.
Clostridium spp., identified by culture as C. perfringens in 6/9 cases, were detected in the small intestinal biopsies of all dogs with HGE either via culture or via IHC of formalin‐fixed histologic samples. In contrast, clostridial‐type bacteria were not detected by histology or IHC in any dog of the control group, and only a mild growth of C. perfringens could be observed in a single duodenal biopsy sample. Dogs have highly diverse duodenal microflora that differ markedly among individual dogs.29 Although a high number of Clostridium spp. can be a part of the normal colonic flora,11 C. perfringens can only be rarely cultured from the duodenal juice,30 and bacterial layers on the surface of the duodenum are normally not present in healthy dogs.31 Dogs with parvovirus enteritis show similar clinical signs (acute hemorrhagic diarrhea) and similar histologic small intestinal lesions (epithelial necrosis) as dogs with HGE. However, no adherence of bacteria to necrotic villuos tips is observed in necropsied dogs with parvovirosis.32 Therefore, the microscopic detection of a large number of clostridial‐type bacteria in the small intestine of a majority of the patients in this study was judged as clostridial overgrowth on the mucosal surface. It is known that inflammation drives dysbiosis33 and dogs with acute nonhemorrhagic diarrhea have profound alterations in their microbiome.34 In the presented group of dogs, samples were obtained after the onset of diarrhea. Thus, it is possible that the clostridial overgrowth is a sequela of the disease rather than the cause.
In one‐third of the dogs, Clostridium spp. was detected by IHC of the small intestine, but there was no clostridial growth upon duodenal culture. These negative cultures could be attributable to dehydration or the exposure of the anaerobic bacteria to lethal concentrations of oxygen. Alternatively, it is possible that Clostridium spp. were not equally distributed or present at each site within the small intestinal tract. In 3/10 cases, clostridial bacteria could be histologically detected in the ileum, but not in the duodenum, which was the only part of the small intestine from which the samples for culture were taken.
Enterotoxemia caused by C. perfringens infection causes necrotic enteritis as a result of several toxins in many animal species and humans. It has now been clearly established that certain C. perfringens strains are capable of inducing necrotic enteritis in broilers, pigs, lambs, horses, felines, and humans.13, 35, 36, 37, 38 Currently, the cause of C. perfringens overgrowth in the gastrointestinal tracts of humans or other mammals, including dogs, is not always known. It has been proposed that physical stress, decreased immunoreactivity, and intestinal hypermotility reduce the normal anaerobic bacterial flora and may predispose the subject to the bacterial overgrowth of C. perfringens in the small intestine.9 In the present patient population, no history of previous physical exhaustion was evident, and no underlying disorder predisposing for immunosuppression could be found. High‐carbohydrate rations and diet changes have also been associated with the overgrowth of C. perfringens in different species.39, 40, 41 Owners reported dietary changes at the onset of hemorrhagic diarrhea in only 8/111 dogs in 1 study.1 A specific dietary factor could not be identified as the cause for the acute intestinal signs in this study population. Therefore, it seems unlikely that components of the diet are predisposing dogs with acute hemorrhagic diarrhea to clostridial overgrowth. However, C. perfringens is the third most common cause of food‐borne illness in humans in the United States,42 and food poisoning cannot be completely ruled out among the dogs in this study. C. perfringens can cause self‐resolving enteritis in dogs,11 and it is possible that hemorrhagic diarrhea represents the extreme end of a spectrum of infection. Additional research might uncover virulence factors and toxin production caused by C. perfringens, as well as host factors predisposing for acute hemorrhagic diarrhea in dogs.
The following facts suggest a primary pathogenic role for C. perfringens in dogs with HGE: (1) the characteristic lesions in the intestine that have been observed in different species after infection with C. perfringens could also be identified in the patient population of this study; (2) Clostridium spp. (identified in 6/9 patients as C. perfringens by mass spectrometry using MALDI‐TOF) was detected in the small intestinal biopsy samples of every dog with HGE; (3) the presence of bacteria and epithelial lesions was closely associated; (4) histologic changes were not influenced by autolysis or microbial overgrowth after post mortem changes in the prospectively intra vitam‐collected specimens; and (5) no other explanation of the necrosis of the superficial intestinal epithelium could be identified.
The rather small number of dogs in the patient group is a possible limitation of this study. The overwhelming majority of dogs showed the same findings, thus it seems unlikely that the results would have changed with a larger patient group.
Dogs with different intestinal disorders, in which intestinal biopsies were diagnostically indicated, were used as controls for microbial changes. To evaluate if clostridial overgrowth is a sequela of the disease rather than the cause, it would have been more informative to include dogs with known causes of hemorrgahic diarrhea as control dogs.
Another limitation was the fact that ileoscopy was not routinely performed in dogs of the control group. Therefore, data for comparison of microscopic detection of Clostridium spp. in the ileum were not available.
In conclusion, this report describes endoscopically visualized changes, histologically confirmed lesions, and microbial changes intra vitam in dogs diagnosed with the so‐called HGE syndrome. The results of this study demonstrate an apparent association between C. perfringens and the occurrence of acute hemorrhagic diarrhea. The mucosal lesions were restricted to the large and small intestines. Therefore, the term “HGE,” which is often used in the current literature and implies the involvement of the stomach, is misleading. We suggest that the syndrome should be renamed “acute hemorrhagic diarrhea syndrome.”
Acknowledgment
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Part of the results were presented at the ECVIM Congress, Maastricht, the Netherlands, September 6–8, 2012
Footnotes
Natriumnitratflotationslösung; Janssen‐Cilag, Neuss, Germany
ProSpecT Giardia Microplate Assay; Remel Inc, Lenexa, KS
Snap Parvo Test; IDEXX Laboratories Inc, Westbrook, ME
AbD Serotec, Duesseldorf, Germany
Dako, Glostrup, Denmark
Cerenia; Pfizer Pharma GmbH, Hamburg, Germany
Olympus Flexible Medizinische Endoskopie, Hamburg, Germany
Wieser GmbH Medizintechnik & Geräte, Egenhofen, Germany
No. 08642921, MP Biomedicals, Illkirch, France
No. 4170, Biotrend Chemikalien, Köln, Germany
Anaerocult P and Anaerocult C, Merck, Darmstadt, Germany
Microflex LT, Flex Anaysis Software, Bruker‐Daltonics, Bremen, Germany
References
- 1. Burrows C. Canine Hemorrhagic gastroenteritis. J Am Anim Hosp Assoc 1977;13:451–458. [Google Scholar]
- 2. Unterer S, Strohmeyer K, Kruse BD, et al. Treatment of aseptic dogs with hemorrhagic gastroenteritis with amoxicillin/clavulanic acid: A prospective blinded study. J Vet Intern Med 2011;25:973–979. [DOI] [PubMed] [Google Scholar]
- 3. Spielman B, Garvey M. Hemorrhagic gastroenteritis in 15 Dogs. J Am Anim Hosp Assoc 1993;29:341–344. [Google Scholar]
- 4. Holt PE. Haemorrhagic gastroenteritis in a dog. Vet Rec 1979;104:150. [DOI] [PubMed] [Google Scholar]
- 5. Cave NJ, Marks SL, Kass PH, et al. Evaluation of a routine diagnostic fecal panel for dogs with diarrhea. J Am Vet Med Assoc 2002;221:52–59. [DOI] [PubMed] [Google Scholar]
- 6. Hill F. Acute intestinal haemorrhage syndrome in dogs. Vet Anual 1972;98–101. [Google Scholar]
- 7. Badcoe L. Haemorrhagic enteritis in a dog associated with Clostridium sp. NZVJ 1992;40:34. [Google Scholar]
- 8. Prescott JF, Johnson JA, Patterson JM, et al. Haemorrhagic gastroenteritis in the dog associated with Clostridium welchii . Vet Rec 1978;103:116–117. [DOI] [PubMed] [Google Scholar]
- 9. Sasaki J, Goryo M, Asahina M, et al. Hemorrhagic enteritis associated with Clostridium perfringens type A in a dog. J Vet Med Sci 1999;61:175–177. [DOI] [PubMed] [Google Scholar]
- 10. Thorpe E, Thomlinson JR. Autolysis and post‐mortem bacteriological changes in the alimentary tract of the pig. J Pathol Bacteriol 1967;93:601–610. [DOI] [PubMed] [Google Scholar]
- 11. Weese JS, Staempfli HR, Prescott JF, et al. The roles of Clostridium difficile and enterotoxigenic Clostridium perfringens in diarrhea in dogs. J Vet Intern Med 2001;15:374–378. [PubMed] [Google Scholar]
- 12. Clooten JK, Kruth SA, Weese JS. Genotypic and phenotypic characterization of Clostridium perfringens and Clostridium difficile in diarrheic and healthy dogs. J Vet Intern Med 2003;17:123; author reply 123. [DOI] [PubMed] [Google Scholar]
- 13. Niilo L. Clostridium perfringens in animal disease: A review of current knowledge. Can Vet J 1980;21:141–148. [PMC free article] [PubMed] [Google Scholar]
- 14. Uzal FA. Diagnosis of Clostridium perfringens intestinal infections in sheep and goats. Anaerobe 2004;10:135–143. [DOI] [PubMed] [Google Scholar]
- 15. Marks SL, Kather EJ, Kass PH, et al. Genotypic and phenotypic characterization of Clostridium perfringens and Clostridium difficile in diarrheic and healthy dogs. J Vet Intern Med 2002;16:533–540. [DOI] [PubMed] [Google Scholar]
- 16. Borriello SP, Honour P, Turner T, et al. Household pets as a potential reservoir for Clostridium difficile infection. J Clin Pathol 1983;36:84–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weber A, Kroth P, Heil G. The occurrence of Clostridium difficile in fecal samples of dogs and cats. Zentralbl Veterinarmed B 1989;36:568–576. [PubMed] [Google Scholar]
- 18. Chouicha N, Marks SL. Evaluation of five enzyme immunoassays compared with the cytotoxicity assay for diagnosis of Clostridium difficile‐associated diarrhea in dogs. J Vet Diagn Invest 2006;18:182–188. [DOI] [PubMed] [Google Scholar]
- 19. Washabau RJ, Day MJ, Willard MD, et al. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J Vet Intern Med 2010;24:10–26. [DOI] [PubMed] [Google Scholar]
- 20. Day MJ, Bilzer T, Mansell J, et al. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: A report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J Comp Pathol 2008;138:S1–S43. [DOI] [PubMed] [Google Scholar]
- 21. El‐Bouri K, Johnston S, Rees E, et al. Comparison of bacterial identification by MALDI‐TOF mass spectrometry and conventional diagnostic microbiology methods: Agreement, speed and cost implications. Br J Biomed Sci 2012;69:47–55. [PubMed] [Google Scholar]
- 22. Han YM, Lee JM, Jin KY, et al. Embolization of superior mesenteric artery branches in dogs. Ischemic bowel changes depend on location of vessel occlusion and embolic materials. Invest Radiol 1999;34:629–635. [DOI] [PubMed] [Google Scholar]
- 23. Ger R, Ravo B, Harris M, et al. Mucosal destruction and regeneration of the colon by local hyperthermia. An experimental preliminary study. Dis Colon Rectum 1986;29:177–181. [DOI] [PubMed] [Google Scholar]
- 24. Macartney L, McCandlish IA, Thompson H, et al. Canine parvovirus enteritis 1: Clinical, haematological and pathological features of experimental infection. Vet Rec 1984;115:201–210. [DOI] [PubMed] [Google Scholar]
- 25. Keusch GT, Grady GF, Takeuchi A, et al. The pathogenesis of shigella diarrhea. II. Enterotoxin‐induced acute enteritis in the rabbit ileum. J Infect Dis 1972;126:92–95. [DOI] [PubMed] [Google Scholar]
- 26. Cushing AH. Necrotizing enterocolitis with Escherichia coli heat‐labile enterotoxin. Pediatrics 1983;71:626–630. [PubMed] [Google Scholar]
- 27. Santos RL, Zhang S, Tsolis RM, et al. Animal models of Salmonella infections: Enteritis versus typhoid fever. Microbes Infect 2001;3:1335–1344. [DOI] [PubMed] [Google Scholar]
- 28. Isaacson RE. Enteric bacterial pathogens, villus atrophy and microbial growth. Vet Q 1998;20(Suppl 3):S68–S72. [PubMed] [Google Scholar]
- 29. Suchodolski JS, Ruaux CG, Steiner JM, et al. Application of molecular fingerprinting for qualitative assessment of small‐intestinal bacterial diversity in dogs. J Clin Microbiol 2004;42(10):4702–4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Benno Y, Nakao H, Uchida K, et al. Impact of the advances in age on the gastrointestinal microflora of Beagle dogs. J Vet Med Sci 1992;54:703–706. [DOI] [PubMed] [Google Scholar]
- 31. Davis CP, Cleven D, Balish E, et al. Bacterial association in the gastrointestinal tract of Beagle dogs. Appl Environ Microbiol 1977;34:194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meunier PC, Cooper BJ, Appel MJ, et al. Pathogenesis of canine parvovirus enteritis: Sequential virus distribution and passive immunization studies. Vet Pathol 1985;22:617–624. [DOI] [PubMed] [Google Scholar]
- 33. Craven M, Egan CE, Dowd SE, et al. Inflammation drives dysbiosis and bacterial invasion in murine models of ileal Crohn's disease. PLoS One 2012;7:e41594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suchodolski JS, Markel ME, Garcia‐Mazcorro JF, et al. The fecal microbiome in dogs with acute diarrhea and idiopathic inflammatory bowel disease. PLoS One 2012;7:e51907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miclard J, Jaggi M, Sutter E, et al. Clostridium perfringens beta‐toxin targets endothelial cells in necrotizing enteritis in piglets. Vet Microbiol 2009;137:320–325. [DOI] [PubMed] [Google Scholar]
- 36. Diab SS, Kinde H, Moore J, et al. Pathology of Clostridium perfringens type C enterotoxemia in horses. Vet Pathol 2012;49:255–263. [DOI] [PubMed] [Google Scholar]
- 37. Sobel J, Mixter CG, Kolhe P, et al. Necrotizing enterocolitis associated with Clostridium perfringens type A in previously healthy north american adults. J Am Coll Surg 2005;201:48–56. [DOI] [PubMed] [Google Scholar]
- 38. Zhang Y, Hou Z, Ma J. Hemorrhagic enterocolitis and death in two felines (Panthera tigris altaica and Panthera leo) associated with Clostridium perfringens type A. J Zoo Wildl Med 2012;43:394–396. [DOI] [PubMed] [Google Scholar]
- 39. Krugner‐Higby L, Girard I, Welter J, et al. Clostridial enteropathy in lactating outbred swiss‐derived (ICR) mice. J Am Assoc Lab Anim Sci 2006;45:80–87. [PubMed] [Google Scholar]
- 40. Ewoldt JM, Anderson DE. Determination of the effect of single abomasal or jejunal inoculation of Clostridium perfringens type A in dairy cows. Can Vet J 2005;46:821–824. [PMC free article] [PubMed] [Google Scholar]
- 41. Lawrence G, Walker PD. Pathogenesis of enteritis necroticans in Papula New Guinea. Lancet 1976;1:125–126. [DOI] [PubMed] [Google Scholar]
- 42. Scallan E, Hoekstra RM, Angulo FJ, et al. Foodborne illness acquired in the United States—Major pathogens. Emerg Infect Dis 2011;17:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]


