Significance
Signaling pathways responsive to both external and internal signals are essential for implementation of biological functions in a cell. After perceiving the signal, the signaling needs to be attenuated and terminated, usually mediated by the endocytic trafficking, to ensure a temporal and spatial control of the signaling activities. In mammals, disruption of the vacuolar protein sorting 41 (VPS41)-mediated endocytic pathway was reported to cause pleiotropic diseases such as neurological disorders. However, the role of the endocytic pathway in plant cells is unknown. We report here that a VPS41-mediated late stage of the endocytic pathway is essential for male–female interaction in Arabidopsis.
Keywords: VPS41, pollen tube–stigma interaction, PVC-to-vacuole trafficking, endocytic pathway, Arabidopsis
Abstract
In flowering plants, extensive male–female interactions are required for successful fertilization in which various signaling cascades are involved. Prevacuolar compartments (PVC) and vacuoles are two types of subcellular compartments that terminate signal transduction by sequestrating signaling molecules in yeast and mammalian cells; however, the manner in which they might be involved in male–female interactions in plants is unknown. In this study, we identified Arabidopsis thaliana vacuolar protein sorting 41 (AtVPS41), encoded by a single-copy gene with sequence similarity to yeast Vps41p, as a new factor controlling pollen tube–stigma interaction. Loss of AtVPS41 function disrupted penetration of pollen tubes into the transmitting tissue and thus led to failed male transmission. In the pollen tubes, AtVPS41 protein is associated with PVCs and the tonoplast. We demonstrate that AtVPS41 is required for the late stage of the endocytic pathway (i.e., endomembrane trafficking from PVCs to vacuoles) because internalization of cell-surface molecules was normal in the vps41-deficient pollen tubes, whereas PVC-to-vacuole trafficking was impaired. We further show that the CHCR domain is required for subcellular localization and biological functioning of AtVPS41. These results indicate that the AtVPS41-mediated late stage of the endocytic pathway is essential for pollen tube–stigma interaction in Arabidopsis.
In contrast to the fertilization processes of animals, the sperm cells of which are mobile, the fertilization process in angiosperm plants is more complex and requires more organs to facilitate immotile sperm cells. Pollen grains carrying two sperm cells land on a compatible stigma and, after adhesion and hydration, germinate to form pollen tubes. The germinated pollen tubes then invade the stigma and style, subsequently penetrating into the transmitting tract. Guided by female cues in the transmitting tract tissue, pollen tubes penetrate the septum, climb onto the funiculus, and grow toward the micropyle of the ovule. Two sperm cells are released after the pollen tubes grow into the ovule, after which the sperm fertilize the egg cell and central cell (1–3). During this long process, communication between the male and female gametophytes is very important.
Pollen tube–stigma interaction consists of pollen adhesion, hydration, and germination on the stigma, followed by passaging of growing pollen tubes into the transmitting tract of the style (4). Growth of pollen tubes into the stylar-transmitting tract is an important step in ovule targeting by pollen tubes (4). Pollen tube growth on the stigma is a chemotropic process in which various signaling cascades are involved. Some possible female factors mediating pollen tube–stigma interactions have been identified. Chemocyanin, a small protein, may act as a directional signal on the lily stigma to facilitate entrance of the pollen tube into the style (5). The tobacco glycoprotein, TTS, forms a glycosylation gradient in the transmitting tract, attracting pollen tubes and stimulating pollen tube growth in vivo (6, 7). It is believed that some other female players may also exist, and various signaling pathways are involved in the response of pollen tubes to these directional cues.
Attenuation and termination of signaling activity is an important regulatory mechanism for signaling pathways in animal cells (8, 9). Similar to animal cells, compartmentalization and degradation of signaling molecules, usually mediated by the endocytic pathway, are major regulatory mechanisms in plant cells (10–12). Signaling molecules of extracellular and intracellular origin are degraded in the late stage of the endocytic pathway in which both prevacuolar compartments (PVCs) and vacuoles are involved (13). In mammalian cells, disruption of the late stage of the endocytic pathway often leads to pleiotropic conditions such as immune response deficits, albinism, or neurological disorders (14). However, the manner in which the late stage of the endocytic pathway is involved in regulating signaling pathways during male–female interaction in plants remains unknown.
The endocytic pathway involves cell-surface internalization and PVC-mediated trafficking into vacuoles, which depend mainly on vesicle-mediated transportation. In yeast, studies on vacuolar assembly identified a tethering complex, the homotypic fusion and vacuolar protein sorting (HOPS) complex, which consists of four core subunits and two HOPS-specific subunits, Vps41p and Vps39p (15). Vps41p promotes membrane fusion between late endosomes and lysosomes by working as an effector of Ypt7, a GTP-binding protein (16–18). Loss of Vps41p function led to failure of vacuole membrane fusion and production of fragmented vacuoles (19). Mice lacking vps41 died early in utero because of impaired down-regulation of growth factor signaling (20). In this study, we report the identification of Arabidopsis thaliana vacuolar protein sorting 41 (AtVPS41) as a new factor controlling pollen tube–stigma interaction. Loss of AtVPS41 function resulted in disrupted penetration of pollen tubes into the transmitting tissue, leading to male gametophytic sterility. We demonstrate that the late stage of the endocytic pathway is impaired in vps41 pollen tubes. Our results suggest that the AtVPS41-mediated late stage of the endocytic pathway is required for effective pollen tube–stigma interaction in Arabidopsis.
Results
Loss of VPS41 Function Results in Failure of Pollen Tube Penetration into the Transmission Tract.
To investigate the biological function of VPS41, we obtained two T-DNA alleles of AtVPS41, SALK_076372, and CS857753, which were designated vps41-1 and vps41-2, respectively (Fig. 1A). We observed a distorted segregation ratio in the progenies of the two mutants, which did not produce homozygotes. Reciprocal cross-analysis revealed that, although the female transmission of the mutant alleles was normal, male transmission was nearly absent (Table 1), suggesting that male function was compromised in vps41 mutants. We introduced one of the two constructs into vps41-1 heterozygous mutants, in which the genomic DNA of AtVPS41 was either fused or not fused with GFP driven under its native promoter (e.g., ProVPS41:VPS41 geDNA and ProVPS41:VPS41 geDNA-GFP). The male transmission failure phenotype in vps41-1 was rescued by both constructs (Table 1), demonstrating that the mutation in AtVPS41 (At1g08190) was responsible for the phenotype of vps41 and showing that the VPS41-GFP fusion protein was functional.
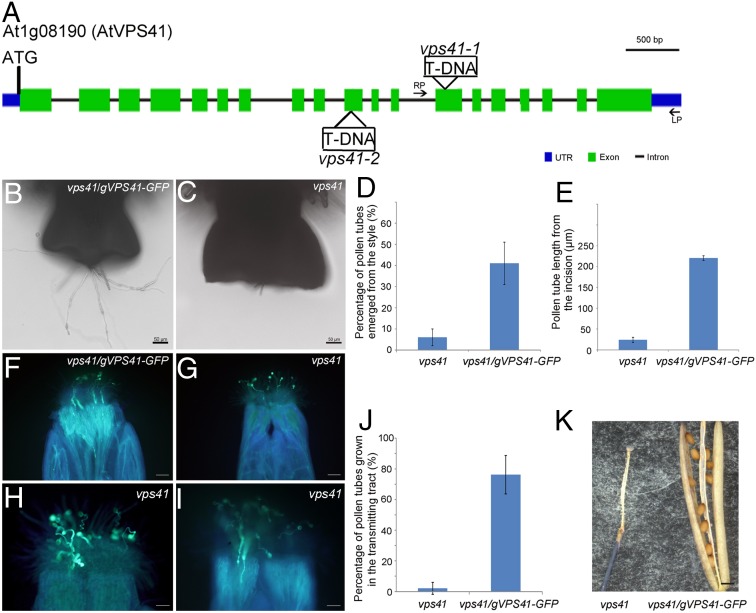
Fig. 1.
Phenotype characterization of vps41 pollen. (A) Diagram of T-DNA insertion sites in the two vps41 alleles. Green box, exons; black line, introns; blue box, untranslated regions. (B and C) vps41 pollen and vps41/gVPS41-GFP pollen germinated semi-in vivo after hand-limited pollination on the emasculated wild-type stigma. Compared with vps41/gVPS41-GFP–complemented pollen tubes, fewer vps41 pollen tubes penetrated the style. (D) Percentage of penetration of vps41 pollen tubes (n = 70) and vps41/gVPS41-GFP pollen tubes (n = 70) into the style 2.5 hag semi-in vivo. Error bars indicate SD; P value < 0.01. (E) Pollen tube length of vps41 pollen (n = 4) and vps41/gVPS41-GFP pollen (n = 29) 2.5 hag semi-in vivo. Error bars indicate SD; P value < 0.01. (F–I) Aniline blue staining assay of emasculated wild-type pistils pollinated with vps41/gVPS41-GFP pollen (F) and vps41 pollen (G–I) 48 h after pollination. (Scale bars: 100 μm in F and G; 50 μm in H and I.) (J) Bar graph of percentage of pollen tubes growing in the transmitting tract; for vps41/gVPS41-GFP pollen, n = 67, and four independent pistils were statistically analyzed; for vps41 pollen, n = 95; and six independent pistils were statistically analyzed. Error bars indicate SD; P value < 0.01. (K) Thirty days after pollination, while there were mature seeds in the siliques pollinated with vps41/gVPS41-GFP pollen (Right), no seed was seen in the siliques pollinated with vps41 pollen (Left). (Scale bar, 500 μm.)
Table 1.
Transmission of the vps41 alleles through male or female gamete
| Progeny | ||||||
| Parents (♀ × ♂) | vps41−/− | vps41+/− | VPS41/VPS41 | Total | TEF | TEM |
| vps41-1+/− selfing | 0 | 209 | 215 | 424 | NA | NA |
| vps41-1+/−♀ × WT♂ | 109 | 111 | 220 | 98.20% | NA | |
| WT♀ × vps41-1+/−♂ | 4 | 695 | 699 | NA | 0.60% | |
| vps41-2+/− selfing | 0 | 315 | 334 | 649 | NA | NA |
| vps41-2+/−♀ × WT♂ | 231 | 240 | 471 | 96.20% | NA | |
| WT♀ × vps41-2+/−♂ | 0 | 405 | 405 | NA | 0% | |
| WT♀ × vps41+/−; ProVPS41:VPS41 geDNA+/+♂ | 275 | 279 | 554 | NA | 98.57% | |
| WT♀ × vps41+/−; ProVPS41:VPS41 geDNA -GFP+/+♂ | 292 | 298 | 590 | NA | 97.99% | |
NA, not applicable; TEF, transmission efficiency of female gametophyte; TEM, transmission efficiency of male gametophyte.
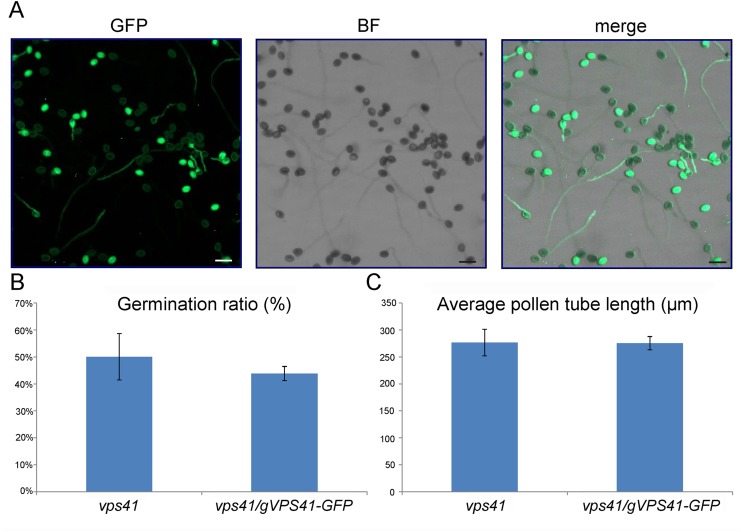
We identified complemented plants with a gVPS41-GFP+/− vps41−/− genotype and collected and separated pollen grains with green fluorescence (complemented) and without green fluorescence (not complemented). Germination of the vps41-1 mutant pollen grains and vps41-1 pollen tube growth were similar to the wild type in vitro (Fig. S1). In semi–in vivo germination assays, vps41-1 mutant pollen grains exhibited normal adhesion and hydration on the stigma (Fig. S2). However, limited pollination assays showed that vps41-1 mutant pollen tubes barely penetrated into the transmitting tissue of the wild-type stigma, and the number of penetrated vps41-1 mutant pollen tubes was significantly less than that of penetrated wild-type pollen tubes (Fig. 1 B–E). This phenotype was further confirmed by in vivo aniline blue staining assays with the siliques 48 h after pollination (Fig. 1 F–J). Hand-pollination of wild-type stigmas with vps41-1 mutant pollen grains resulted in no seed in the siliques (Fig. 1K). These results indicate that, although vps41-1 pollen tubes are competent to grow in vitro, the mutant pollen tubes cannot penetrate into the transmitting tract of the style.
Fig. S1.
Germination ratio and pollen tube length in vitro. (A) Pollen grains from gVPS41-GFP+/− vps41−/− transgenic plants germinate in vitro. (Scale bars, 50 μm.) (B) Percentage of germination of vps41 pollen (n = 435) and vps41/gVPS41-GFP (n = 415) pollen 3 h after germination. Error bars indicate SD; P > 0.1. (C) Pollen tube length of vps41 pollen (n = 48) and vps41/gVPS41-GFP pollen (n = 70) 3 h after germination. Error bars indicate SD; P > 0.1.
Fig. S2.
The adhesion, hydration, and germination of vps41 pollen grains on the stigma. (A and B) Hydration of vps41/gVPS41-GFP pollen (A) and vps41 pollen grains (B) on the emasculated wild-type stigma. Both the complemented pollen and vps41 pollen expand in minutes after pollination on the stigma surface. The examples depicted here are representative of similar observations of 30 pollen grains. (C–F) SEM images of vps41/gVPS41-GFP pollen (C and D) and vps41 pollen grains (E and F) on the emasculated wild-type stigma. After germination on the stigma, the complemented pollen tubes penetrate into the papillar cell wall before traversing down to the base of the cell. Additionally, vps41 pollen is indistinguishable from the complemented pollen in behavior on the stigmatic surface. The examples depicted here are representative of similar observations of 20 pollen grains.
AtVPS41 Is Localized Mainly in the Late Endosomes/PVCs and Tonoplast in Germinating Pollen Tubes.
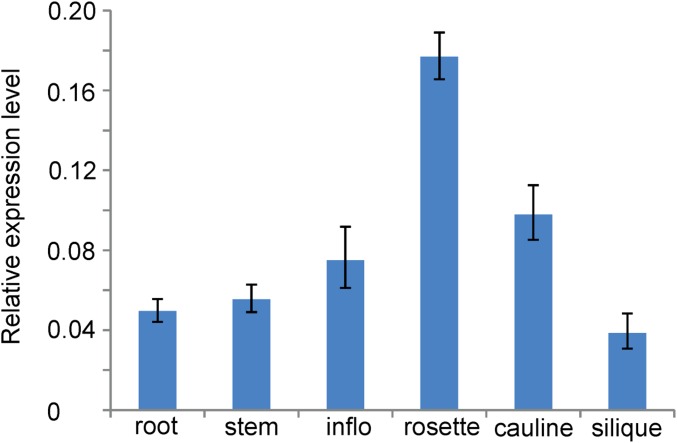
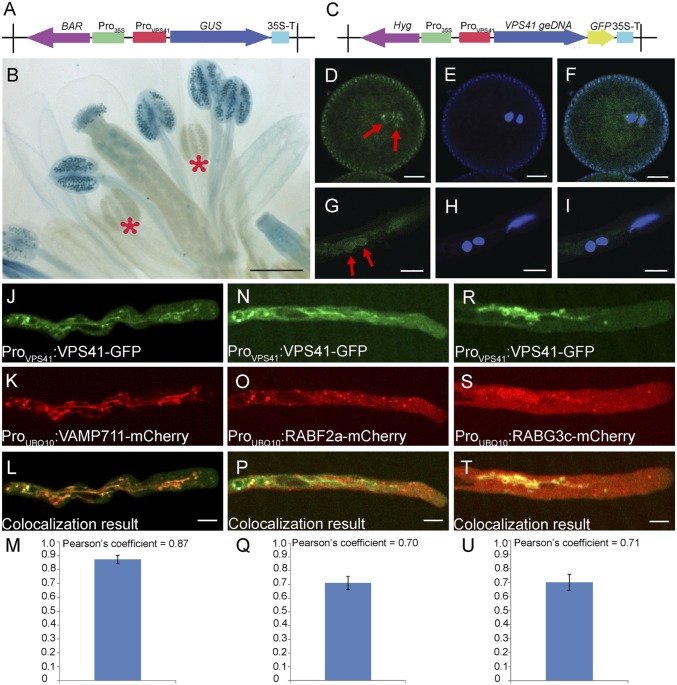
AtVPS41 was ubiquitously expressed in multiple plant organs and tissues (Fig. S3). To investigate the temporal and spatial expression pattern of AtVPS41 during reproduction, we examined the inflorescences of ProVPS41:GUS and ProVPS1:VPS41-GFP transgenic plants. VPS41 transcripts were abundantly detected in mature pollen grains, but not in immature developing pollen (Fig. 2 A and B). Similarly, the VPS41-GFP signal was observed in mature tricellular pollen, but not in microspores and bicellular pollen. In addition, VPS41-GFP was detected in vegetative cells and sperm cells in pollen tubes (Fig. 2 C–I). Interestingly, in sperm cells, the GFP signal formed a ring structure surrounding sperm nuclei (Fig. 2 D and G).
Fig. S3.
Relative transcript levels of VPS41 in various plant organs. Transcription levels of VPS41 in different plant organs including root, stem, inflorescence, rosette leaves, cauline leaves, and siliques are determined by qRT-PCR. Error bars indicate SD.
Fig. 2.
Expression pattern of VPS41 in floral organs and subcellular localization of VPS41 in pollen tubes. (A) Construct diagram of the ProVPS41:GUS reporter. (B) Expression pattern of VPS41 gene in floral organs revealed by GUS assay. Red stars indicate developing pollen. (Scale bar, 500 μm.) (C) Construct diagram of the ProVPS41:VPS41 geDNA-GFP reporter. (D–I) Localization of VPS41 in mature pollen (D–F) and germinating pollen tubes (G–I) of ProVPS41:VPS41 geDNA-GFP transgenic plants. (D and G) GFP signals. (E and H) DAPI-staining images. (F and I) Overlay of the GFP and DAPI images. Red arrows indicate sperm cells. (Scale bars, 5 μm.) (J–M) Localization of VPS41-GFP fusion protein (J) and VAMP711-mCherry fusion protein (K) in pollen tubes. (L) Colocalization result of VPS41-GFP and VAMP711-mCherry. Pearson’s coefficient of the colocalization result in M. Fourteen independent pollen tubes were used for statistical analysis. (Scale bar, 10 μm.) (N–Q) Localization of VPS41-GFP fusion protein (N) and RABF2a-mCherry fusion protein (O) in pollen tubes. (P) Colocalization result of VPS41-GFP and RABF2a-mCherry. Pearson’s coefficient of the colocalization is shown in Q. Nine independent pollen tubes were used for statistical analysis. (Scale bar, 10 μm.) (R–U) Localization of VPS41-GFP fusion protein (R) and RABG3c-mCherry fusion protein (S) in pollen tubes. (T) Colocalization result of VPS41-GFP and RABG3c-mCherry. Pearson’s coefficient of the colocalization is shown in U. Seventeen independent pollen tubes were used for statistical analysis. (Scale bar, 10 μm.)
In the complemented pollen tubes of gVPS41-GFP+/− vps41−/− plants, the GFP signal was highly dynamic and detected in tubular structures and variably sized endosome-like punctate structures (Movie S1). To determine the subcellular localization of AtVPS41, we crossed gVPS41-GFP+/− vps41−/− plants with 18 endomembrane marker lines (wave line markers) (21). In the pollen tubes of the F1 progeny plants, the VPS41-GFP signal was colocalized with vacuole marker ProUBQ10:VAMP711-mCherry (n = 14) and three PVC markers: ProUBQ10:RABG3c-mCherry (n = 17), ProUBQ10:RABG3f-mCherry (n = 12), and ProUBQ10:RABF2a-mCherry (n = 9) (Fig. 2 J–U), but not with other markers (Table S1). These results showed that, in germinating pollen tubes, AtVPS41 resided mainly in the late endosomes/PVCs and tonoplast, similar to the localization profile of its homologs in yeast and mammalian cells (22, 23).
Table S1.
Colocalization result of VPS41-GFP with different wave line marker lines
| Assignment localization | Name | VPS41 colocalization |
| Endoplasmic reticulum | NIP1;1 | Nonsignificant |
| Golgi | Got1p | Nonsignificant |
| SYP32 | Nonsignificant | |
| RABD2a | Nonsignificant | |
| MEMB12 | Nonsignificant | |
| Post-Golgi/endosomal | RABC1 | Nonsignificant |
| RABD1 | Nonsignificant | |
| RABE1d | Nonsignificant | |
| Trans-Golgi network/early endosome | VTI12 | Nonsignificant |
| Late endosome/prevacuolar compartment/vacuole | RABG3f | Strong |
| RABF2b | Weak | |
| RABF2a | Strong | |
| RABG3c | Strong | |
| Vacuole | VAMP711 | Strong |
| Endosomal/recycling endosome | RABA5d | Nonsignificant |
| RABA1e | Nonsignificant | |
| RABA1g | Nonsignificant | |
| Cytosol/nucleus | Tag only | NA |
| Plasma membrane | NPSN12 | NA |
AtVPS41 Is Recruited to PVCs and the Tonoplast by Interacting with RABF2a, RABG3c, and VAMP711.
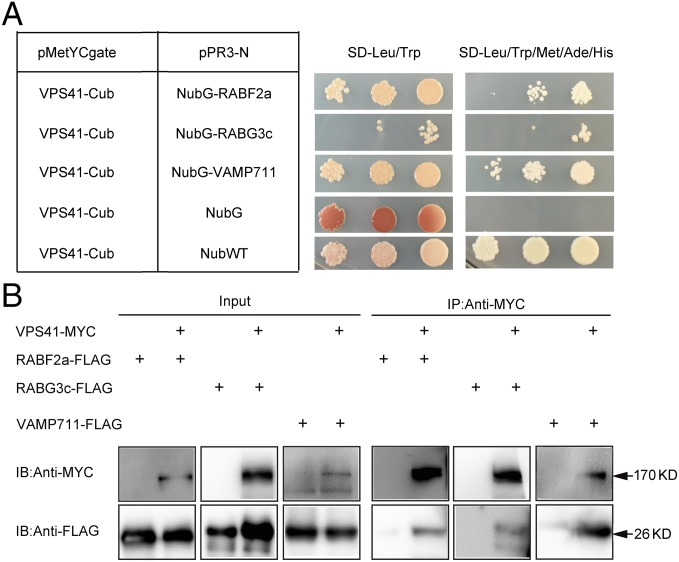
In yeast cells, the HOPS complex is recruited by the active form of RAB GTPase Ypt7/RAB7 to mediate fusion between the prevacuolar compartment membrane and vacuole/lysosome membrane with the assistance of SNARE proteins (16–18). In Arabidopsis, RAB7/RABG GTPases and a Rab5 homolog Rha1/RABF2a were also involved in vacuolar trafficking of cargo proteins (24–26). To test whether recruitment of AtVPS41 to PVCs/tonoplast is also mediated by interaction between AtVPS41 and the RAB GTPases and SNARE proteins, we performed split-ubiquitin yeast two-hybrid assays (27, 28) using AtVPS41 as a bait protein and RABF2a, RABG3c, and VAMP711 as prey proteins. As shown in Fig. 3A, we found that AtVPS41 interacted with RABF2a, RABG3c, and VAMP711. We further generated constructs of the VPS41-MYC fusion protein and RABF2a-FLAG/RABG3c-FLAG/VAMP711-FLAG and transiently coexpressed them in tobacco (Nicotiana benthamiana) leaves by agro-infiltration (29). We found that RABF2a-FLAG, RABG3c-FLAG, and VAMP711-FLAG were coimmunoprecipitated with VPS41 (Fig. 3B). Taken together, these results indicate that AtVPS41 is recruited to PVCs by interacting with RAB GTPases RABF2a and RABG3c, whereas AtVPS41 is recruited to the tonoplast through interacting with the SNARE protein VAMP711.
Fig. 3.
AtVPS41 interacts with RABG3c, RABF2a, and VAMP711. (A) Yeast two-hybrid assays of VPS41 with RABG3c, RABF2a, and VAMP711. NubWT represents the wild-type N-terminal half of ubiquitin. NubG represents the mutated N-terminal half of ubiquitin that was not able to interact with the C-terminal half of ubiquitin. Interactions between two proteins were tested using Met, Ade, and His reporter genes. (B) Co-IP assays. VPS41-MYC with RABF2a-FLAG, RABG3c-FLAG, and VAMP711-FLAG were transiently coexpressed in Nicotiana benthamiana leaves. Total proteins were subjected to immunoprecipitation with MYC beads followed by immunoblot analysis.
PVC-to-Vacuole Trafficking Is Greatly Impaired in vps41-1 Pollen Tubes.
The dual localization of AtVPS41 in PVCs and vacuoles suggests that AtVPS41 is probably involved in PVC-to-vacuole trafficking in pollen tubes. To verify this hypothesis, we transformed ProLAT52:2S albumin-mRFP, which encodes a fusion protein to be transported into vacuoles through a PVC-mediated transport pathway in cultured plant cells (30), into gVPS41-GFP+/− vps41−/− plants. At 1.5 h after germination (hag), the 2S albumin-mRFP signal was found mainly in tonoplast-like tubular membrane structures, as well as in endosome-like punctate structures in complemented pollen tubes (Fig. 4 A and B), whereas in vps41-1 mutant pollen tubes the signal was observed mainly in endosome-like punctate structures (Fig. 4 C and D). At 3.5 hag, the 2S albumin–monomeric red fluorescent protein (mRFP) signal was observed mostly in vacuoles in complemented pollen tubes (Fig. 4 E and F), whereas in vps41-1 mutant pollen tubes the mRFP signal was retained mostly in endosome-like structures (Fig. 4 G and H), suggesting that vacuolar transport of the 2S albumin-mRFP signal was greatly compromised in vps41-1 mutant pollen tubes.
Fig. 4.
Endosomal trafficking to vacuoles was greatly compromised in vps41 pollen tubes. (A–H) Endosomal trafficking to vacuoles revealed by a 2S albumin–mRFP fusion protein marker. Fluorescent and bright-field images of wild-type and vps41 pollen tubes are shown. (A and B) Fluorescent and bright-field images of wild-type pollen tubes at 1.5 hag. (C and D) Fluorescent and bright-field images of vps41 pollen tubes at 1.5 hag. (E and F) Fluorescent and bright-field images of wild-type pollen tubes at 3.5 hag. (G and H) Fluorescent and bright-field images of vps41 pollen tubes at 3.5 hag. At least 20 independent pollen tubes were observed for each genotype and each time point. (Scale bar, 5 μm.) (I–M) FM4-64 uptake assays. Intracellular accumulation of FM4-64 is shown within 10 min after incubation in 5 μM FM4-64 in a wild-type pollen tube (I) and vps41 pollen tube (J). (K) Statistical quantification of relative FM4-64 uptake within 10 min. At least 50 independent pollen tubes were used for statistical analysis for each genotype. Error bars indicate SD. Distribution of FM4-64 is shown after 240 min of incubation in 5 μM FM4-64 in a wild-type pollen tube (L) and a vps41 pollen tube (M). (N) Statistical quantification of relative FM4-64 transport to tonoplast at 240 min; 20 independent pollen tubes were used for statistical analysis for vps41 pollen tubes, and 14 independent pollen tubes were used for statistical analysis for wild-type pollen tubes. (Scale bar, 5 μm.)
PVCs and vacuoles are involved in the late stage of the endocytic pathway, which down-regulates signaling activity by sequestrating activated signaling components into degradative organelles (11, 12, 20). To examine endocytic activity in Arabidopsis pollen tubes, we treated both the wild-type and vps41-1 mutant pollen tubes with FM4-64, an endocytic tracer for internalization and endosomal trafficking (31). Uptake and intracellular accumulation of FM4-64 were evident within 10 min in wild-type (n = 50) and vps41-1 pollen tubes (n = 50) (Fig. 4 I and J) with clearly labeled endosomes, whereas the endocytosis rates of the groups were statistically indistinguishable (Fig. 4K), indicating that the early stage of the endocytic pathway was not affected in vps41-1 mutant pollen tubes. After 4 h, FM4-64 had accumulated in the tonoplast in most (42/50) of the wild-type pollen tubes, but was still mainly present in punctate endosome-like structures in most (36/50) vps41-1 mutant pollen tubes (Fig. 4 L and M). The relative FM4-64 transport to tonoplast was dramatically decreased in vps41-1 pollen tubes in comparison with the wild-type pollen tubes (Fig. 4N). These results demonstrate that loss of AtVPS41 disturbs the late stage of the endocytic pathway in pollen tubes.
The CHCR Domain of AtVPS41 Is Required for Subcellular Localization and Biological Functioning.
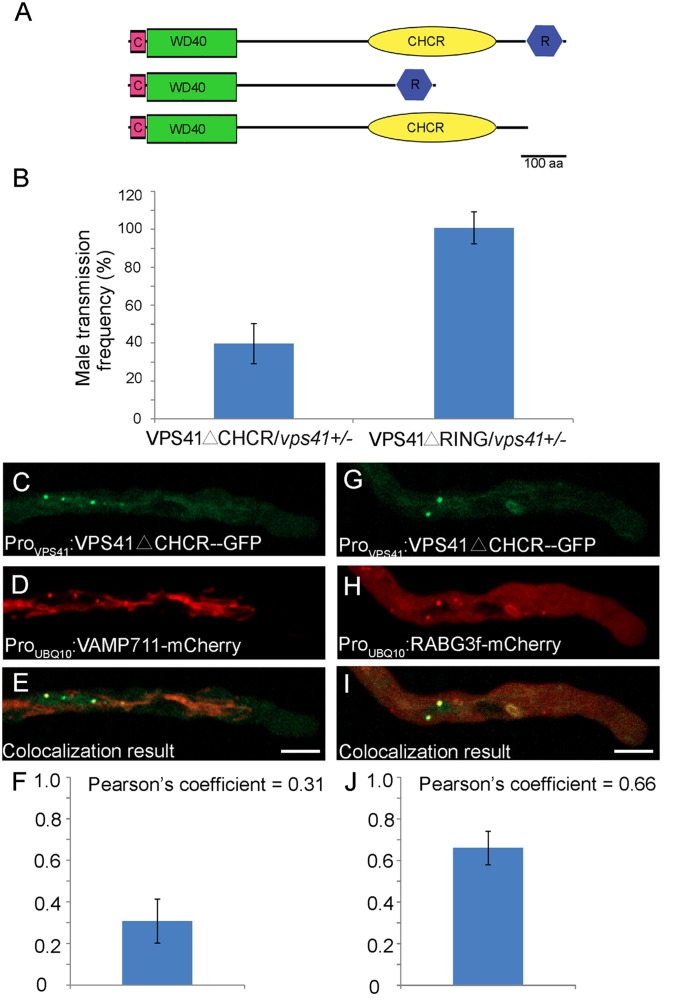
To determine the importance of each domain of VPS41 for subcellular localization and functioning of the protein, we introduced two truncated forms of AtVPS41, lacking the RING domain or CHCR domain and designated ProVPS41:VPS41 geDNAΔRING-GFP and ProVPS41:VPS41 geDNAΔCHCR-GFP, respectively, into vps41-1+/− heterozygous mutant plants (Fig. 5A). ProVPS41:VPS41 geDNAΔRING-GFP+/+ vps41-1+/− (hereafter, VPS41ΔRING/vps41-1+/− complemented plants) and ProVPS41:VPS41 geDNAΔCHCR-GFP+/+ vps41-1+/− (hereafter, VPS41ΔCHCR/vps41-1+/− complemented plants) transgenic T2 plants were crossed with wild-type plants and calculated for the Kanr/Kans ratio in the resulting progeny, which reflected male transmission of the vps41-1 allele. Whereas pollination with pollen from VPS41ΔRING/vps41-1+/−–complemented plants produced a Kanr/Kans ratio of almost 1:1 (395:390) in the progeny, pollination with pollen from VPS41ΔCHCR/vps41-1+/−–complemented plants produced a Kanr/Kans ratio of 0.40:1 (138:347) (Fig. 5B). This result indicates that the CHCR domain, but not the RING domain, is required for AtVPS41 to function in male transmission. The VPS41ΔCHCR-GFP signal was partially colocalized with PVC marker RABG3f (21) (n = 3), but not with tonoplast marker VAMP711 (n = 6) (Fig. 5 C–J). Therefore, the CHCR domain is required for proper localization of AtVPS41 in the tonoplast and for functioning of the protein.
Fig. 5.
CHCR domain is essential for subcellular localization and biological function of VPS41. (A) Schematic diagram representing full-length and two truncated forms of AtVPS41: VPS41△CHCR and VPS41△RING. (B) VPS41△CHCR and VPS41△RING were introduced into vps41+/− heterozygous plants. Although the VPS41△RING could fully rescue the male transmission frequency, the VPS41△CHCR could rescue the ratio to only about 40%, indicating that the CHCR domain is essential for the biological function of AtVPS41. Error bars indicate SD. (C–F) Localization of VPS41△CHCR-GFP fusion protein (C) and VAMP△711-mCherry fusion protein (D) in pollen tubes. Colocalization result of VPS41△CHCR-GFP and VAMP711-mCherry is shown in E. Pearson’s coefficient of the colocalization result is shown in F. Three independent pollen tubes were statistically analyzed. (Scale bar, 10 μm.) (G–J) Localization of VPS41CHCR-GFP fusion protein (G) and RABG3f-mCherry fusion protein (H) in pollen tubes. (I) Colocalization result of VPS41△CHCR-GFP and RABG3f-mCherry. Pearson’s coefficient of the colocalization result in J. Six independent pollen tubes were statistically analyzed. (Scale bar, 10 μm.)
Taken together, our results demonstrate that AtVPS41-mediated PVC-to-vacuole trafficking is an important process in the endocytic pathway in pollen tubes that is essential for pollen tube–stigma interaction in Arabidopsis (Fig. 6).
Fig. 6.
A proposed model for endocytic endomembrane trafficking of pollen tube plasma membrane-localized receptors and female cues. Upon stimulation with the female cues, the receptors are phosphorylated and then internalized into the trans-Golgi network/early endosome (TGN/EE) where the signals are transmitted to downstream intracellular mediators. The receptors are further delivered to RABF2a-positive PVCs, and through maturation of endosomal organelles, the receptors are transferred into the RABG3c-positive PVCs where the pH is lower and the ligands dissociate with the receptors. Finally, the receptors and ligands are transported into vacuoles for degradation, thus realizing the attenuation of the signaling. In vps41 pollen tubes, although the internalization of the receptors is normal, the PVC-to-vacuole trafficking of receptors is greatly impaired. Thus, the vacuoles are not assembled normally and the PVC cannot be fused with vacuoles efficiently, which leads to accumulation of PVCs in pollen tubes. Meanwhile, these active receptors may also be accumulated in the TGN/EE, which may cause the excessive signaling in pollen tubes. The deregulated signaling in pollen tubes may eventually result in the failure of pollen tube penetration into the transmitting tract.
Discussion
Successful fertilization requires extensive pollen–pistil interaction, which occurs immediately after a pollen grain contacts a stigmatic papillae. In this study, we identified AtVPS41 as an essential male factor participating in pollen tube–stigma interaction based on genetic evidence and the results of in vitro and semi-in vivo biological studies. First, vps41 mutants are male-gametophyte–sterile because of the failure of pollen tubes to penetrate into the transmitting tract of the stigma. Second, VPS41 is dynamically localized in the PVCs and vacuoles in pollen tubes. Third, the late stage of endocytic trafficking is compromised in vps41 mutant pollen tubes. Fourth, disrupting VPS41 localization in the tonoplast compromised VPS41 function in pollen tubes. Therefore, the AtVPS41-mediated late stage of the endocytic pathway in pollen tubes is essential for pollen tube–stigma interaction in Arabidopsis.
Pollen tube growth in pistils is directional and requires extensive cell–cell communication between pollen tubes and pistil tissues, including signal perception at the plasma membrane of pollen tubes, signal transduction into the cytoplasm of pollen tubes, and signaling cue/receptor complex internalization and turnover, in which guidance signal cues from sporophytic and female gametophytic tissues are required and endomembrane trafficking plays an essential role (Fig. 6). Because in mammals loss of Vps41 resulted in deficient down-regulation of growth factor signaling and thus led to embryonic lethality (20) and because Vps41 is highly conserved among eukaryotes, Vps41 may also be implicated in attenuation of signaling cascades involved in cell–cell communication between pollen tubes and female tissues in Arabidopsis. It is very possible that defective penetration of vps41 pollen tubes into the stylar-transmitting tract is due to the failure of such pollen tubes to respond to female signal cues that need to be internalized and transported through the AtVPS41-mediated endocytic pathway (Fig. 6). Another possibility, however, also exists: the mutation of VPS41 might affect the pollen tube growth, the defective phenotype of which is likely masked in vitro by a component of the medium.
Another possible cause is associated with enzymes. After germinating on the stigma, pollen tubes have to penetrate the cuticle covering the stigmatic surface (32–34). Pollen-held enzymes, such as pectin-degrading enzymes and enzymes that modify the cell wall, must be secreted to degrade the stigmatic cuticle and loosen the stigmatic cell wall. For example, serine esterases have been reported to be required for pollen tube penetration into dry Brassica stigmas (34). Therefore, it is also possible that AtVPS41 is involved in the regulated release of these enzymes from pollen tubes.
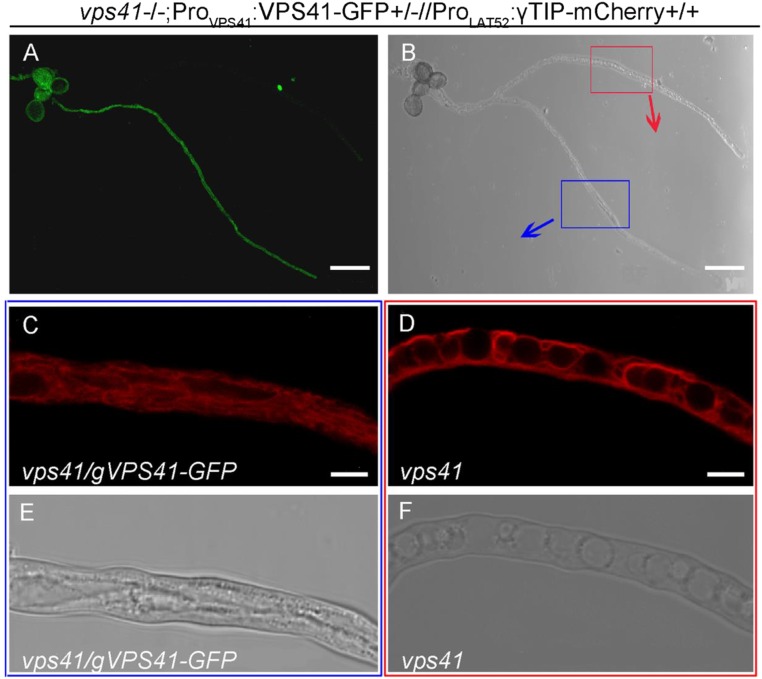
The finding that the CHCR domain of AtVPS41 is needed for proper localization and biological functioning of the protein is consistent with the fact that VPS41 is an essential subunit of the HOPS complex, a tethering complex that mediates heterotypic late endosome vacuole fusion and homotypic vacuolar fusion in yeast and mammalian cells (22). This is also supported by the observation that multiple small vacuoles occurred in the vps41-1 mutant pollen tubes (Fig. S4), showing that membrane fusion associated with endomembrane trafficking is impaired in such plants. The compromised vacuole biogenesis may affect the buildup of the turgor pressure in vps41 pollen tubes and consequently affects the pollen tube penetration. In mammalian cells, VPS41 is involved in sorting proteins to the regulated secretory pathway in a process that is also dependent on the CHCR domain, which interacts with adaptor protein 3 (AP-3) (35). This process is highly specific for VPS41 and operates independently of the HOPS complex (35). Therefore, another possibility is that AtVPS41 may also be involved in regulating a secretory pathway independent of the HOPS complex in pollen tubes.
Fig. S4.
The structure of small vacuoles in vps41 pollen tubes. Vacuole structure revealed by a tonoplast marker γTIP-mCherry fusion protein. Fluorescent (A) and bright-field (B) images of vps41 (without GFP signal) and complemented (with GFP signal) pollen tubes are shown. Blue box, magnification of a complemented pollen tube carrying ProLAT52:γTIP-mCherry construct in which mCherry fluorescent (C) and bright-field (E) images of one complemented vps41 pollen tube are shown. Red box, magnification of vps41 pollen tube carrying ProLAT52:γTIP-mCherry construct in which mCherry fluorescent (D) and bright-field (F) images of one vps41 pollen tube are shown. (Scale bars: 50 μm in A and B; 5 μm in C–F.)
In the present study, we provide evidence demonstrating that AtVPS41-mediated endomembrane trafficking, possibly PVC-to-vacuole trafficking via the endocytic pathway, is essential for pollen tube–stigma interaction in Arabidopsis. Future studies should aim to investigate the molecular mechanisms by which AtVPS41 mediates endomembrane trafficking in pollen tubes.
Materials and Methods
VPS41 Sequence Analysis.
The sequences of VPS41 homologs in various species were obtained from the National Center for Biotechnology Information using BLAST (blast.ncbi.nlm.nih.gov/Blast.cgi). Protein domains were analyzed using the Protein Homology/Analogy Recognition Engine (Phyre) (www.sbg.bio.ic.ac.uk/∼phyre/).
Plasmid Construction.
To generate the ProVPS41:GUS construct and the ProVPS41:VPS41 geDNA construct for complementation, GATEWAY-compatible destination vector pCB308R or pCB2003 was used, respectively (36). For the ProVPS41:VPS41 geDNA-GFP construct, a reconstructed vector pH7FWG0, which was made from the GATEWAY-compatible destination vector pH7FWG2 (VIB-Ghent University), was used.
ProLAT52:γTIP-mCherry was constructed from the Pro35S:γTIP-mCherry construct (The Arabidopsis Information Resource stock no. CD3-975) by replacing the 35S promoter with a LAT52 promoter. For generation of co-immunoprecipitation (Co-IP) constructs, a GATEWAY-compatible pBA-Myc vector was used for Pro35S:VPS41-6×Myc construction and a GATEWAY-compatible pB7FLAGWG2 vector was used for Pro35S:RABF2a/RABG3c/VAMP711-3×FLAG construction. To generate the ProVPS41:VPS41 geDNAΔRING-GFP construct for complementation assay, the pENTRY-ProVPS41:VPS41 geDNAΔRING and pENTRY-ProVPS41:VPS41 geDNAΔCHCR-GFP plasmids were then cloned into pH7FWG0.
In Vitro Pollen Germination Analysis.
In vitro pollen germination assays were conducted according to previously reported protocols (37, 38). The measurement of pollen tube length and pollen tube germination percentages were made with Image J software (rsbweb.nih.gov/ij/).
Semi-in Vivo Pollen Germination Assay, Aniline Blue Staining, and GUS Staining.
Semi-in vivo pollen germination assay and aniline blue staining were conducted according to previously reported protocols (39). The histochemical GUS assay was performed according to a reported protocol (40).
FM4-64 Uptake Quantification Assay and Colocalization Analysis.
Pollen tubes 2 hag were stained with 5 μM FM4-64 (Molecular Probes) as according to ref. 31. Fluorescence intensities of 50 independent pollen tubes were calculated and standardized for each genotype. Fluorescence images were observed according to a previously reported protocol (41).
Co-IP and Immunoblotting.
Co-IP assays were conducted as previously described (42, 43). Immunoblotting was performed as previously described (39) using anti-MYC and anti-FLAG antibody (Santa Cruz).
Supplementary Material
Acknowledgments
We thank Dr. Zhenbiao Yang (University of California, Riverside) for suggestions and discussions; Dr. Sheila McCormick (University of California, Berkeley) for the pENTRY-ARA6 and pENTRY-ARA7 plasmids; Dr. Qi Xie (Institute of Genetics and Developmental Biology, China) for the Y2H cDNA library; Dr. Liwen Jiang (Chinese University of Hong Kong) for the plasmid carrying a 2S albumin gene; Dr. Fred Berger (Temasek Life Sciences, Singapore) for HTR10-mRFP seeds; and Dr. Jerry Kaplan (University of Utah) for the Δvps41 yeast strain. This work was supported by the National Basic Research Program of China (Grant 2011CB915402) and the National Natural Science Foundation of China Grant 31370344 (to L.-J.Q.). This work was partially supported by the 111 Project.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1602757113/-/DCSupplemental.
References
- 1.Lord EM, Russell SD. The mechanisms of pollination and fertilization in plants. Annu Rev Cell Dev Biol. 2002;18:81–105. doi: 10.1146/annurev.cellbio.18.012502.083438. [DOI] [PubMed] [Google Scholar]
- 2.Palanivelu R, Tsukamoto T. Pathfinding in angiosperm reproduction: Pollen tube guidance by pistils ensures successful double fertilization. Wiley Interdiscip Rev Dev Biol. 2012;1(1):96–113. doi: 10.1002/wdev.6. [DOI] [PubMed] [Google Scholar]
- 3.Qu LJ, Li L, Lan Z, Dresselhaus T. Peptide signalling during the pollen tube journey and double fertilization. J Exp Bot. 2015;66(17):5139–5150. doi: 10.1093/jxb/erv275. [DOI] [PubMed] [Google Scholar]
- 4.Hiscock SJ, Allen AM. Diverse cell signalling pathways regulate pollen-stigma interactions: The search for consensus. New Phytol. 2008;179(2):286–317. doi: 10.1111/j.1469-8137.2008.02457.x. [DOI] [PubMed] [Google Scholar]
- 5.Kim S, et al. Chemocyanin, a small basic protein from the lily stigma, induces pollen tube chemotropism. Proc Natl Acad Sci USA. 2003;100(26):16125–16130. doi: 10.1073/pnas.2533800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu HM, Wang H, Cheung AY. A pollen tube growth stimulatory glycoprotein is deglycosylated by pollen tubes and displays a glycosylation gradient in the flower. Cell. 1995;82(3):395–403. doi: 10.1016/0092-8674(95)90428-x. [DOI] [PubMed] [Google Scholar]
- 7.Cheung AY, Wang H, Wu HM. A floral transmitting tissue-specific glycoprotein attracts pollen tubes and stimulates their growth. Cell. 1995;82(3):383–393. doi: 10.1016/0092-8674(95)90427-1. [DOI] [PubMed] [Google Scholar]
- 8.Jékely G, Sung HH, Luque CM, Rørth P. Regulators of endocytosis maintain localized receptor tyrosine kinase signaling in guided migration. Dev Cell. 2005;9(2):197–207. doi: 10.1016/j.devcel.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141(7):1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah K, Russinova E, Gadella TW, Jr, Willemse J, De Vries SC. The Arabidopsis kinase-associated protein phosphatase controls internalization of the somatic embryogenesis receptor kinase 1. Genes Dev. 2002;16(13):1707–1720. doi: 10.1101/gad.220402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robatzek S, Chinchilla D, Boller T. Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes Dev. 2006;20(5):537–542. doi: 10.1101/gad.366506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geldner N, Hyman DL, Wang X, Schumacher K, Chory J. Endosomal signaling of plant steroid receptor kinase BRI1. Genes Dev. 2007;21(13):1598–1602. doi: 10.1101/gad.1561307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Roy C, Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol. 2005;6(2):112–126. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- 14.Di Pietro SM, Dell’Angelica EC. The cell biology of Hermansky-Pudlak syndrome: Recent advances. Traffic. 2005;6(7):525–533. doi: 10.1111/j.1600-0854.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- 15.Price A, Wickner W, Ungermann C. Proteins needed for vesicle budding from the Golgi complex are also required for the docking step of homotypic vacuole fusion. J Cell Biol. 2000;148(6):1223–1229. doi: 10.1083/jcb.148.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cabrera M, et al. Vps41 phosphorylation and the Rab Ypt7 control the targeting of the HOPS complex to endosome-vacuole fusion sites. Mol Biol Cell. 2009;20(7):1937–1948. doi: 10.1091/mbc.E08-09-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ostrowicz CW, et al. Defined subunit arrangement and rab interactions are required for functionality of the HOPS tethering complex. Traffic. 2010;11(10):1334–1346. doi: 10.1111/j.1600-0854.2010.01097.x. [DOI] [PubMed] [Google Scholar]
- 18.Balderhaar HJ, Ungermann C. CORVET and HOPS tethering complexes: Coordinators of endosome and lysosome fusion. J Cell Sci. 2013;126(Pt 6):1307–1316. doi: 10.1242/jcs.107805. [DOI] [PubMed] [Google Scholar]
- 19.Radisky DC, Snyder WB, Emr SD, Kaplan J. Characterization of VPS41, a gene required for vacuolar trafficking and high-affinity iron transport in yeast. Proc Natl Acad Sci USA. 1997;94(11):5662–5666. doi: 10.1073/pnas.94.11.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aoyama M, et al. Spatial restriction of bone morphogenetic protein signaling in mouse gastrula through the mVam2-dependent endocytic pathway. Dev Cell. 2012;22(6):1163–1175. doi: 10.1016/j.devcel.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Geldner N, et al. Rapid, combinatorial analysis of membrane compartments in intact plants with a multicolor marker set. Plant J. 2009;59(1):169–178. doi: 10.1111/j.1365-313X.2009.03851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bröcker C, et al. Molecular architecture of the multisubunit homotypic fusion and vacuole protein sorting (HOPS) tethering complex. Proc Natl Acad Sci USA. 2012;109(6):1991–1996. doi: 10.1073/pnas.1117797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pols MS, ten Brink C, Gosavi P, Oorschot V, Klumperman J. The HOPS proteins hVps41 and hVps39 are required for homotypic and heterotypic late endosome fusion. Traffic. 2013;14(2):219–232. doi: 10.1111/tra.12027. [DOI] [PubMed] [Google Scholar]
- 24.Sohn EJ, et al. Rha1, an Arabidopsis Rab5 homolog, plays a critical role in the vacuolar trafficking of soluble cargo proteins. Plant Cell. 2003;15(5):1057–1070. doi: 10.1105/tpc.009779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui Y, et al. Activation of the Rab7 GTPase by the MON1-CCZ1 complex is essential for PVC-to-vacuole trafficking and plant growth in Arabidopsis. Plant Cell. 2014;26(5):2080–2097. doi: 10.1105/tpc.114.123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ebine K, et al. Plant vacuolar trafficking occurs through distinctly regulated pathways. Curr Biol. 2014;24(12):1375–1382. doi: 10.1016/j.cub.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Johnsson N, Varshavsky A. Split ubiquitin as a sensor of protein interactions in vivo. Proc Natl Acad Sci USA. 1994;91(22):10340–10344. doi: 10.1073/pnas.91.22.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stagljar I, Korostensky C, Johnsson N, te Heesen S. A genetic system based on split-ubiquitin for the analysis of interactions between membrane proteins in vivo. Proc Natl Acad Sci USA. 1998;95(9):5187–5192. doi: 10.1073/pnas.95.9.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu L, et al. An efficient system to detect protein ubiquitination by agroinfiltration in Nicotiana benthamiana. Plant J. 2010;61(5):893–903. doi: 10.1111/j.1365-313X.2009.04109.x. [DOI] [PubMed] [Google Scholar]
- 30.Miao Y, Li KY, Li HY, Yao X, Jiang L. The vacuolar transport of aleurain-GFP and 2S albumin-GFP fusions is mediated by the same pre-vacuolar compartments in tobacco BY-2 and Arabidopsis suspension cultured cells. Plant J. 2008;56(5):824–839. doi: 10.1111/j.1365-313X.2008.03645.x. [DOI] [PubMed] [Google Scholar]
- 31.Vida TA, Emr SD. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol. 1995;128(5):779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hiscock S, Dewey F, Coleman JD, Dickinson H. Identification and localization of an active cutinase in the pollen of Brassica napus L. Planta. 1994;193(3):377–384. [Google Scholar]
- 33.Shayk M, Kolattukudy PE. Production of a novel extracellular cutinase by the pollen and the chemical composition and ultrastructure of the stigma cuticle of nasturtium (Tropaeolum majus) Plant Physiol. 1977;60(6):907–915. doi: 10.1104/pp.60.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hiscock S, Bown D, Gurr S, Dickinson H. Serine esterases are required for pollen tube penetration of the stigma in Brassica. Sex Plant Reprod. 2002;15(2):65–74. [Google Scholar]
- 35.Asensio CS, et al. Self-assembly of VPS41 promotes sorting required for biogenesis of the regulated secretory pathway. Dev Cell. 2013;27(4):425–437. doi: 10.1016/j.devcel.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lei ZH, et al. High-throughput binary vectors for plant gene function analysis. J Integr Plant Biol. 2007;49(4):556–567. [Google Scholar]
- 37.Boavida LC, McCormick S. Temperature as a determinant factor for increased and reproducible in vitro pollen germination in Arabidopsis thaliana. Plant J. 2007;52(3):570–582. doi: 10.1111/j.1365-313X.2007.03248.x. [DOI] [PubMed] [Google Scholar]
- 38.Guan Y, Lu J, Xu J, McClure B, Zhang S. Two mitogen-activated protein kinases, MPK3 and MPK6, are required for funicular guidance of pollen tubes in Arabidopsis. Plant Physiol. 2014;165(2):528–533. doi: 10.1104/pp.113.231274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu J, et al. Membrane-bound RLCKs LIP1 and LIP2 are essential male factors controlling male-female attraction in Arabidopsis. Curr Biol. 2013;23(11):993–998. doi: 10.1016/j.cub.2013.04.043. [DOI] [PubMed] [Google Scholar]
- 40.Guo Y, Qin G, Gu H, Qu LJ. Dof5.6/HCA2, a Dof transcription factor gene, regulates interfascicular cambium formation and vascular tissue development in Arabidopsis. Plant Cell. 2009;21(11):3518–3534. doi: 10.1105/tpc.108.064139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J, et al. Targeted degradation of the cyclin-dependent kinase inhibitor ICK4/KRP6 by RING-type E3 ligases is essential for mitotic cell cycle progression during Arabidopsis gametogenesis. Plant Cell. 2008;20(6):1538–1554. doi: 10.1105/tpc.108.059741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.English JJ, Davenport GF, Elmayan T, Vaucheret D, Baulcombe C. Requirement of sense transcription for homology-dependent virus resistance and trans-inactivation. Plant J. 1997;12(3):597–603. [Google Scholar]
- 43.Voinnet O, Rivas S, Mestre P, Baulcombe D. An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J. 2003;33(5):949–956. doi: 10.1046/j.1365-313x.2003.01676.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.