Abstract
The progressive transformation of one organ system into another is a fundamental signature of fibrodysplasia ossificans progressiva (FOP), the most catastrophic form of extraskeletal bone formation in humans. In all affected individuals, FOP is caused by heterozygous missense gain-of-function mutations in Activin receptor A type I (ACVR1), a bone morphogenetic protein (BMP) type I receptor. Loss of autoinhibition of the mutant receptor (mACVR1) results in dysregulated BMP pathway signaling, and is necessary for the myriad developmental features of FOP, but does not appear sufficient to induce the episodic flare-ups that lead to disabling post-natal heterotopic endochondral ossification (HEO) and that are a hallmark of the disease. Post-natal FOP flare-ups strongly implicate an underlying immunological trigger involving inflammation and the innate immune system. Recent studies implicate canonical and non-canonical TGFβ/BMP family ligands in the amplification of mACVR1 signaling leading to the formation of FOP lesions and resultant HEO. BMP and Activin ligands that stimulate mACVR1 signaling also have critical regulatory functions in the immune system. Cross-talk between the morphogenetic and immunological pathways that regulate tissue maintenance and wound healing identifies potential robust therapeutic targets for FOP. Here we review current evidence for an immunological trigger for flare-ups and HEO in FOP, propose a working schema for the pathophysiology of observed phenomena, and highlight outstanding questions under investigation.
Keywords: fibrodysplasia ossificans progressiva (FOP), heterotopic ossification, bone morphogenetic protein (BMP), bone morphogenetic protein signaling, innate immune system, ACVR1, Activin A, Toll-like receptor (TLR)
1. Introduction
Fibrodysplasia ossificans progressiva (FOP; MIM#135100) is the most aggressive form of heterotopic ossification (HO) in humans [1]. In FOP, this extra-skeletal bone tissue forms through heterotopic endochondral ossification (HEO) [2]. As with nearly all forms of HEO, FOP lesion formation is associated with inflammatory triggers suggesting that the innate immune system participates in the pathophysiology of the disease [3]. However, unlike other forms of HEO, FOP is caused by missense mutations in a BMP type I receptor that impart an altered basal threshold of dysregulated BMP pathway signaling [4]. Recently, ligands that sensitize the mutant receptor have gained renewed attention due to their established role in both heterotopic ossification and inflammation [5-7].
Here we review evidence for an immunological basis for progressive episodic HEO in FOP. First, we briefly review FOP and the putative immunological features of this catastrophic disorder. Next, we review known mechanisms for dysregulation of the BMP pathway in FOP, with special attention to the mutant ACVR1 (mACVR1) receptor as well as to selective sensitivity to ligands in the BMP and Activin families. We then review the nominal roles of these ligands as modulators of innate immunity. Finally, we propose a working schema for the pathophysiology of FOP, and highlight outstanding enigmas under investigation.
2. Fibrodysplasia ossificans progressiva
2.1 Clinical overview of FOP
FOP is a rare, debilitating autosomal dominant disorder that leads to progressive postnatal endochondral bone formation at extra-skeletal sites. HEO begins in childhood and is induced by trauma or can occur spontaneously [1, 8, 9]. Bone formation is episodic and progressive, and forms in well-defined spatial and temporal patterns that cause ankylosis of the joints of the axial and appendicular skeleton, immobilizing the patient in a “second skeleton” of heterotopic bone [10-12].
Embryonic malformations of the normotopic skeleton precede the post-natal development of HEO in FOP. Characteristic malformations of the great toes are present in all classically affected individuals [8]. Common sites of variable malformations include the facet joints of the cervical spine [8, 13], the costovertebral joints [8] and the interphalangeal joints of the thumbs [8]. Osteochondromas are seen throughout the normotopic skeleton [14] as are short broad femoral necks and acetabular dysplasias [8]. Hearing impairment is commonly associated with conductive hearing loss due to ankylosis of the auditory ossicles [15].
FOP is an extremely rare disorder affecting approximately one in every two million individuals worldwide. There is no ethnic, racial, gender or geographic predilection [16]. Misdiagnosis is common and leads to iatrogenic harm [17, 18]. Most cases arise as spontaneous new mutations [16]. Genetic transmission is through an autosomal dominant mechanism [19]. Reproductive fitness is low [16]. Early mortality from cardiovascular complications of restrictive chest wall disease is common [20-22]. Presently, there is no definitive treatment. Management is symptomatic [23-27].
2.2 Molecular features of FOP
A classic clinical presentation of FOP (characteristic malformations of the great toes and post-natal progressive HEO) is caused by a recurrent heterozygous activating mutation of Activin receptor A type I (ACVR1), a bone morphogenetic protein (BMP) type I receptor [28]. Phenotypic and genotypic variants of FOP occur in ~3% of all FOP patients worldwide [29]. All classic and variant forms of FOP exhibit loss of autoinhibition of ACVR1 and dysregulated ligand-independent and ligand-stimulated BMP signaling [4, 9, 30].
DNA sequence analysis of ACVR1 in patients who have classic FOP reveals the identical single nucleotide mutation in ACVR1 (c.617G>A) which results in the substitution of arginine by histidine at codon 206 (p.R206H) in the GS domain of the receptor [28]. Protein structural homology modeling correctly predicted that this amino acid substitution results in a conformational change in the receptor which alters its sensitivity and activity leading to loss of autoinhibition with mild basal activation as well as ligand-dependent hyperactivity of the downstream bone morphogenetic protein (BMP) signaling pathway [28, 30-34].
All of the ACVR1 mutations identified in individuals with classic or variant FOP occur in highly conserved amino acids, indicating their functional importance [9, 29]. Protein structure homology modeling of the resulting ACVR1 proteins predicted that these mutant receptors activate ACVR1 and enhance receptor signaling – now a well-confirmed finding [28-35]. Many studies have demonstrated that signal transduction through the BMP pathway is altered in cells from individuals with FOP, with decreased expression of BMP antagonists, increased phosphorylation of BMP pathway signaling mediators (BMP-specific SMAD proteins and p38MAPK), dysregulated trafficking of BMP receptors, and increased expression of BMP transcriptional targets in the absence of exogenous BMP ligand [36-43]. Although mutant ACVR1 (mACVR1) exhibits dysregulated basal activity and increased ligand sensitivity, binding of the type II receptor is necessary for signaling. In fact, a key determinant for ACVR1 hyperactivity is a functional type II receptor [44, 45].
The interaction of mACVR1 with FKBP12, a glycine/serine domain–binding protein that prevents leaky BMP type I receptor activation in the absence of ligand has been investigated [4, 30, 32-34, 46]. mACVR1 exhibits reduced binding to FKBP12 in in vitro assays, suggesting that increased BMP pathway activity in cells with mACVR1 is due, at least in part, to decreased binding of this inhibitory factor [46].
Interaction of the FKBP12 with type I receptors of the TGFβ superfamily has been hypothesized to act as a gradient reader, playing a role in the morphogenetic activities of the signal ligands (TGFβ, BMP, GDF, Activin, and Nodal) [47]. Diminished FKBP12 binding could plausibly perturb the read-out of the gradient, aberrantly increasing the signaling output and leading to developmental defects or homeostatic aberrations such as the congenital developmental defects and heterotopic ossification of FOP.
In the context of the gradient reader mechanism, a modest three-fold decrease in affinity for FKBP12 could account for the basal dysregulation of BMP signaling by mACVR1 in classic FOP [46]. Because phosphorylation of the regulatory GS subdomain of ACVR1 abrogates binding by FKBP12 [48, 49], the effect of diminished interaction may be amplified, increasing over time or in certain subcellular microenvironments such as hypoxia that might exist in lesional tissues, or in response to extracellular ligands that have immunogenic properties.
In addition to numerous in vitro studies, the BMP signaling pathway has been studied in several highly informative animal models including Drosophila melanogaster and the zebrafish Danio Rerio providing important insight into the cellular and molecular mechanisms of BMP signaling and the activities of the evolutionarily conserved ACVR1 receptor, its orthologs and ligands in vivo [32, 44, 50-52]. As in vertebrates, elevated basal BMP pathway signaling associated with mACVR1 in Drosophila is BMP ligand-independent [51]. Wild type ACVR1 can antagonize, as well as promote BMP signaling while mACVR1 can only promote signaling with or without ligand [44,45].
2.3 Immunological features of FOP
Despite the occurrence of germline activating mutations of ACVR1 in FOP patients, and the presence of mild ligand-independent elevation of basal BMP signaling, individuals with FOP do not form bone continuously, but rather episodically and often following trivial injury - a finding that suggests that innate immune-related triggers induce tissue metamorphosis in the setting of altered micro-environmental thresholds [3, 53]. Many clinical and pathologic features of FOP strongly point to an underlying immunological component to heterotopic ossification:
Episodic disease flare-ups are triggered by soft tissue injury, muscle fatigue, viruses, and immunizations [3, 8, 10, 12, 54-57].
Local and systemic activation of flare-ups occur following antigenic re-challenge by intramuscular immunizations [54].
Ongoing flare-ups are exacerbated by intercurrent immunizations [54].
Trauma induced by surgical removal of heterotopic bone leads to new bone formation [10, 12].
Sudden and massive soft tissue edema occurs at the clinical onset of many flare-ups [10, 12, 58].
Massive migratory edema is noted during early flare-ups [8, 10].
Perivascular accumulation of lymphocytes, mast cells, and macrophages occurs in affected skeletal muscle during the earliest phases of disease flare-ups in FOP patients and in mouse models of FOP [2, 59-62].
Infiltration of lymphocytes, mast cells and macrophages occurs between the fascicles of skeletal muscle during the early phases of disease flare-ups in patients and in mouse models of FOP [59-62].
Dramatic clinical response to corticosteroids is noted in the first 12 to 36 hours following the onset of a flare-up [12, 23-26, 63].
Early use of high-dose corticosteroids during flare-ups improves symptoms in FOP patients [12, 23-26, 63].
Prophylactic use of high-dose corticosteroids abrogates the formation of heterotopic bone in a mouse model of FOP [64].
Long periods of disease quiescence can occur between flare-ups, reminiscent of the exacerbation-remission cycles of patients who have multiple sclerosis [3, 8, 10-12].
Long periods of disease quiescence occur following immuno-ablation/immunosuppression [65].
Increased sensitivity of mACVR1 to auto-inflammatory ligands (BMP4 and Activin A) in mouse models of FOP is observed [5, 6, 42, 66, 67].
Targeted ablation of macrophages and mast cells impairs heterotopic ossification in mouse models of FOP [68, 69].
There is a notable absence of heterotopic ossification prenatally [10-12].
Sensory nerves regulate the innate immune system and amplify the formation of heterotopic bone in FOP mouse models [70-72].
Blocking any major signaling hub in the sensory pathway – the TRPV1 ion channel, the dorsal root ganglion cells, the preprotachykinin (PPTA) gene that encodes substance P, the neurokinin1 receptor (Nk1) for substance P, the tissue mast cells that express Nk1r, or the c-Kit gene (required for mast cell development) profoundly abrogates heterotopic ossification in an FOP mouse model [70, 71].
These myriad epidemiologic, clinical, pathological and molecular features of FOP support that the innate immune system plays a prominent and provocative role in the pathophysiology of FOP.
3. Heterotopic Ossification and the innate immune system in FOP
3.1 BMP Ligands and heterotopic ossification in FOP
Despite the autonomous loss of autoinhibition of mACVR1 in FOP, the mutant receptor is exquisitely sensitive to BMP ligands [4, 30, 32-34, 42, 43]. A recent study highlighted the differential effect of BMP ligands on wtACVR1 and mACVR1 and showed that mACVR1 was significantly more sensitive than wtACVR1 to BMP4-induction of the BMP-Smad1/5/8 pathway [5]. The basis for the increased sensitivity of the mutant receptor kinase activity in response to BMP or any of the canonical or non-canonical ligands was not discussed, but the observation supports previously published work suggesting that aberrant hetero-dimerization and/or membrane trafficking of the mutant receptor might be a factor [39, 41].
Importantly, BMP4 is highly expressed in injured muscle tissue [73-75] and in inflammatory and fibroproliferative cells from early FOP lesions [36, 37]. A comprehensive study determined that mRNA levels of Smads1/5 were increased in response to muscle injury in vivo. Because heterotopic bone formation in FOP commonly occurs following soft tissue injury, these data support that the stimulation of Smad1/5 phosphorylation following injury further enhances BMP signaling downstream of mACVR1 and leads to HEO [42]. Importantly, HEO in mouse models of FOP is effectively blocked by soluble BMP inhibitors and BMP ligand traps [60, 62, 64].
The development of mACVR1 knock-in mouse (ACVR1R206H/+) models has propelled in vivo research on FOP [5, 66, 67]. Although germline transmission of mACVR1 in the mouse leads to perinatal lethality, mice that are 70-90% chimeric for mACVR1 cells exhibit clinical features of FOP, including embryonic skeletal malformations and postnatal heterotopic ossification that is identical to that seen in the human condition [66]. Importantly, knock-in mACVR1 mice also develop spontaneous and injury-induced FOP-like lesions that progress to mature heterotopic bone through a cellular cascade identical to that seen in individuals with FOP [66]. These mice validate that mACVR1 is a direct genetic cause of FOP and that mild basal activity and increased ligand sensitivity of mACVR1 reproduce the heterotopic ossification seen in humans [66].
Recently, mACVR1 was shown to directly regulate early chondrogenic fate in FOP. Importantly chondrogenic differentiation was accelerated in mACVR1 cells due, in part, to enhanced sensitivity to BMP ligand [43]. Importantly, BMP4 ligand is detected in patient lesions prior to the appearance of chondrocytes, suggesting that the mutation, together with endogenous BMPs and perhaps other non-canonical ligands, may direct lineage decisions towards cartilage, a critical scaffold of HEO in FOP [36, 37].
3.2 BMP ligands and the innate immune system in FOP
Complementary to their effect on stimulating HEO, native and recombinant BMPs are potent pro-inflammatory proteins at heterotopic sites [76, 77]. Soon after the BMP genes were cloned, Cunningham et al. showed that BMP4 was a potent chemoattractant to monocytes in vitro and may promote HEO through its profound effects on monocyte recruitment and cytokine synthesis [78].
Immunohistochemical localization of BMPs was examined during induced HEO in pre-FOP models. BMPs were expressed in perivascular and periosteal cells suggesting that osteoprogenitor cells express BMP [79]. These findings supported previous studies showing robust BMP4 production in connective tissue progenitor cells (CTPCs) of early FOP lesions [37]. In another study, BMP6 induced expression of pro-inflammatory inducible nitric oxide synthase (iNOS) and tumor necrosis factor alpha (TNFα) in macrophages. Over-expression as well as knock-down studies demonstrated that ACVR1 is a functional BMP6 receptor in macrophages [80].
Kan and colleagues utilized an Nse-BMP4 transgenic animal model of FOP to examine the cellular mechanisms underlying HEO and found that HEO in these animals was triggered by injury and mediated by macrophages [68, 81]. In a recent study, Convente and colleagues reported that targeted ablation of macrophages and mast cells in ACVR1R206H+ mice dramatically impaired HEO [69].
Epstein Barr virus, a lymphotrophic virus that has been associated with the induction of FOP flare-ups, unmasks the dysregulation of BMP4 signaling pathway in FOP lymphoblastoid cell lines but not in control lymphoblastoid cell lines, suggesting that the virus may directly or indirectly autoregulate BMP signaling [3, 36]. Several studies have shown that BMP4 causes early lymphocytic infiltration in mouse models similar to that seen in FOP flare-ups, suggesting that overexpression of BMP4 or dysregulated BMP signaling may be central to lymphocytic infiltration in early FOP lesions [62, 81].
Immune cells of hematopoietic origin have been implicated in the HEO of FOP [3]. Clinical observation and in vivo murine transplantation studies precisely determined the contribution of hematopoietic cells to HEO [65]. A patient with FOP who had undergone bone marrow transplantation for the treatment of intercurrent aplastic anemia twenty-five years earlier was evaluated to determine whether the clinical course of his FOP had been influenced by bone marrow replacement or immunosuppression, or both. In complementary studies, hematopoietic stem cells were transplanted from constitutively expressing LacZ transgenic mice to identify the contribution of hematopoietic cells to BMP4-induced heterotopic ossification, an early histopathologic model of FOP [65].
Replacement of hematopoietic cells was not sufficient to prevent HEO in the patient with FOP, but pharmacologic suppression of the normal donor immune system following transplantation in the new host modulated the activity of the postnatal FOP phenotype and diminished the formation of HEO [65]. In complementary murine transplantation studies, cells of hematopoietic origin contributed to the early inflammatory and late marrow-repopulating stages of BMP4-induced heterotopic ossification but were not represented in the fibroproliferative, chondrogenic, or osteogenic stages of heterotopic ossification. Interestingly, both recombinant human BMP4 induction of HO in an animal model and the dysregulated BMP signaling pathway in a patient with FOP were sufficient to recruit at least two populations of cells, one of hematopoietic origin and at least one of non-hematopoietic origin, that contribute to the formation of an ectopic skeleton [65].
Taken together, these findings demonstrated that bone marrow transplantation did not cure FOP in the patient, most likely because the hematopoietic cells were not the population, or at least not the dominant target population, of the intrinsic dysregulation of the BMP signaling pathway in FOP. However, following transplantation of bone marrow from a presumably normal donor, immunosuppression of the immune system appeared to ameliorate HEO in a genetically susceptible host. Thus, cells of hematopoietic origin may initiate the formation of an ectopic skeleton, although they may not be sufficient to complete the process alone. Furthermore, while cells of hematopoietic origin may contribute robustly to FOP flare-ups, wild-type hematopoietic cells may be sufficient to stimulate the process in resident FOP osteoprogenitor cells [65].
A central challenge in FOP research is to construct a unified theory that explains both the tissue metamorphic and immunological features of the condition. ECSIT (evolutionarily conserved signal intermediate in the Toll pathway) modulates critical cross-talk between the Toll-like receptors (TLRs) of the innate immune system and the BMP signaling pathway, and provides an important evolutionary clue into the correspondence of these two ancient and highly conserved systems [82-84]. The ECSIT complex, including TAK1 and TRAF6, plays a pivotal role in TLR4-mediated signals to activate NF-κB and the Smad 1/5/8 pathway [85].
3.3. Activin A (Act A) and heterotopic ossification in FOP
Two studies recently highlighted the role of non-canonical ligands in the pathogenesis of HEO in FOP [5, 6]. In the first study, using a series of in vitro analyses, investigators showed that Act A, a non-canonical ligand for the BMP signaling pathway that was not previously thought to play a role in the disease, potently stimulated the Smad 1/5/8 BMP signaling pathway in the presence of mACVR1 but not wild type (wt) ACVR1 [5]. Additionally, the investigators showed that Act A induced HEO in mice expressing mACVR1 but not in mice expressing only wtACVR1. Inhibition of Act A with a fully humanized monoclonal antibody completely blocked formation of HEO in the conditional knock-in model of classic FOP [5]. Thus, mACVR1 unexpectedly sensitized mACVR1 cells to Act A, a ligand that normally inhibits BMP signaling.
In their paper, the authors caution that there are a paucity of data implicating Act A as a driver of heterotopic ossification in FOP patients; largely due to the inability to safely acquire relevant human tissues and cells for testing. Nevertheless, due to the high degree of evolutionary conservation in the signaling system, together with the high fidelity of the genetically-humanized mouse model, the scientists were optimistic about exploring therapeutic Act A blockade in FOP patients [5]. Verification of the findings of this latest study in primary human cells from FOP patients will be critical before clinical development of this new antibody approach would be warranted.
In the second study, Hino and colleagues showed that Act A enhanced chondrogenesis in induced mesenchymal stromal cells (FOP-iMSCs) derived from FOP induced pluripotent stem cells [6]. BMP pathway signaling (pSmad1/5/8) was aberrantly activated, in addition to activation of TGFβ pathway signaling (pSmad2/3), and induced endochondral ossification of FOP-iMSCs in vivo [6].
The molecular, physiologic and structural basis for the dramatic dimorphic sensitivity of mACVR1 and wtACVR1 to Act A is presently unknown but may plausibly involve the role of type II receptors in the signaling complex [5, 6, 86]. The unexpected discovery of Act A in the pathogenesis of FOP flare-ups identifies a refined therapeutic target for FOP in the broad context of the mACVR1-Smad1/5/8 - BMP signaling pathway and excavates a foundation for future clinical development.
In summary, the recent findings from Hatsell, Hino, and colleagues suggest that mACVR1 causes FOP by imparting responsiveness to Act A through the BMP-specific Smad1/5/8 pathway [5, 6]. The studies further highlight that:
Act A is a potent activator in vivo of mACVR1-driven HEO.
mACVR1-driven HEO is a ligand dependent process.
mACVR1 is an Act A responsive receptor that transmits its signals through Smad1/5/8, although whether this occurs through direct or indirect binding remains to be established.
Enhanced chondrogenesis of FOP-iMSCs by Act A occurs via BMP and TGFβ signaling.
Act A normally binds to but acts as an antagonist of ACVR1 by binding Activin type 2 receptors and preventing their binding to ACVR1 in a heterotetrameric complex [86].
Act A is a potential therapeutic target for FOP
3.4. Act A and the innate immune system in FOP
In addition to its unexpected role in the pathogenesis of HEO in FOP, Act A is a powerful cytokine that acts as a key regulator of the immune system in mammals [7]. Early studies established a role for Act A in the regulation of immune cell function. In recent years, a plethora of evidence suggests its versatility in the control of immune responses especially in the inflammatory pathways [7]. Notably, Act A regulates the interface between tolerance and immunity. Importantly, many studies have shown that Act A exhibits both pro- and anti-inflammatory properties depending upon the cellular and temporal context [7]. Selective activation of mACVR1 by Act A highlights an intriguing link between inflammation and HEO in FOP.
A recent review examined the role of Activin signaling in immune cells [7]. Act A has a major regulatory role in innate immune cells including monocytes, macrophages, microglial cells, mast cells, NK cells, and dendritic cells, as well as B-cell and T-cells. Importantly, Act A regulates cell growth and maturation of mast cells, a critical innate immune cell involved in all stages of FOP flare-ups. Act A can be induced in many cell types under inflammatory conditions, and can stimulate multiple toll-like receptors (TLRs) [7], a likely component of HEO induction in FOP lesions.
Act A is highly relevant to the pathogenesis of HEO in FOP [5, 6]. Act A expression is induced in skeletal muscle after cardiotoxin-induced injury and overexpression of Act A causes significant damage to skeletal muscle [87]. Additionally, Act A neutralization improves repair and regeneration and restores normal muscle function in cardiotoxin-induced skeletal muscle injury. Importantly, Act A inhibition after cardiotoxin-induced skeletal muscle injury is associated with histological improvements and alterations in inflammatory biomarkers [87].
4. Perspectives and outstanding questions
BMP ligand-independent basal activation of the BMP signaling pathway in FOP is a well-noted molecular signature of the disease and is plausibly responsible for the myriad developmental features of FOP such as the congenital malformations of the great toes, thumbs, cervical facet, costovertebral, and hip joints; osteochondromas and hearing loss, as well as numerous variant and congenital phenotypic features. While the molecular basis of the congenital features of FOP is intriguing and will likely elucidate additional therapeutic targets, it is the post-natal, episodic, progressive and disabling formation of HEO that commands greatest attention.
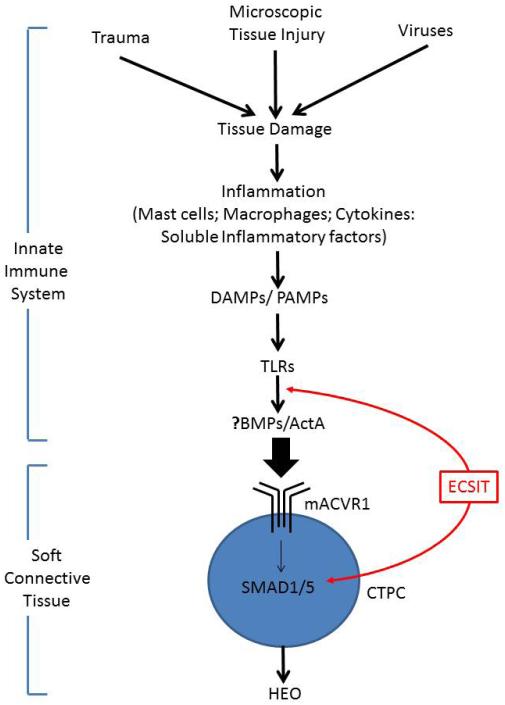
Clinical observations strongly suggest that inflammatory triggers, initiated through the innate immune system are responsible for trauma-induced flare-ups and subsequent HEO in FOP [3, 53]. Our findings and those of others allow us to construct a working hypothesis of the pathophysiology of flare-ups and resultant HEO in FOP (Figure 1). It is intriguing to speculate that perhaps all flare-ups, even those that appear spontaneous, are activated by the innate immune system through damage-associated molecular patterns (DAMPs), pathogen associated molecular patterns (PAMPs), and attendant stimulation of toll-like receptors (TLRs) of the innate immune system [82-85]. Clearly, the innate immune system is ubiquitously active and functional postnatally in vertebrates even in the absence of overt injury. Ongoing studies will elucidate the role of down-stream and re-entrant activation of cognate and orphan ligands such as BMP4 and Activin A in this process, their crosstalk with the inflammatory pathways, and their impingement on resident CTPCs capable of orchestrating the requisite pathologic cascade of HEO [66, 81, 88-91].
Figure-1. Hypothetical schema for activation of FOP flare-ups.
DAMPS = Damage-associated molecular patterns;
PAMPS = Pathogen-associated molecular patterns;
TLRs = Toll-like receptors;
ECSIT = Evolutionarily conserved signal intermediate in the Toll pathway;
CTPC = Connective tissue progenitor cell;
HEO = Heterotopic endochondral ossification
Numerous studies suggest that the BMP signaling pathway and Toll-like signaling pathway of the innate immune system are fundamentally and intimately co-regulated [83-85]. Signaling intermediates in the TLR-pathway are also required for BMP signaling [83-85]. The discovery of a TLR-BMP signaling network may have unanticipated implications for understanding the pathophysiology of FOP. It is plausible that in FOP, soft tissue injury triggered by macroscopic or microscopic trauma or viruses stimulates innate immune cells to increase TLR-agonist-induced pro-inflammatory cytokine and morphogen production that stimulate CTPCs to induce progressive and disabling HEO.
Important outstanding questions remain:
How do injury and inflammation trigger FOP flare-ups?
What is the role of the innate immune system in injury-induced and spontaneous FOP flare-ups?
How is cross-talk mediated between the innate immune system and the BMP signaling pathway in FOP?
What are relative roles of ligand stimulation (BMP4 and Act A, for example) in the induction of FOP flare-ups?
How exactly does Act A sensitize mACVR1 to signal through the BMP pathway?
Are ligands of the Act A class necessary for basal activity of mACVR1 (even in the absence of BMP ligands) or is basal activity of mACVR1 truly ligand-independent?
How do the innate immune system and the lesional microenvironment influence the progression of FOP [92]?
How does the innate immune system communicate with CTPCs that initiate FOP flare-ups?
How do CTPCs feed-back to modulate the immune response?
Why do some individual with FOP have virtually no flare-ups or post-natal HEO?
What are the serum, urine and exhaled biomarkers of the immediate pre-clinical flare-up?
How does FOP progress in the absence of flare-ups?
Is FOP an autoimmune or auto-inflammatory disease?
These and other questions are the focus of intense ongoing investigation. As Tom Maeder wrote in The Atlantic Monthly, “FOP and its problems lie at the crossroads of several seemingly unrelated disciplines. Answers to questions that FOP poses will also address grander issues of how the body first creates its shape, and knows where to stop; how tissues decide to become what they are, and why they don’t be turn into something else [93].”
ACKNOWLEDGEMENTS
This work was supported in part by the International Fibrodysplasia Ossificans Progressiva Association (IFOPA), the Center for Research in FOP and Related Disorders, the Ian Cali Endowment for FOP Research, the Whitney Weldon Endowment for FOP Research, the Isaac and Rose Nassau Professorship of Orthopaedic Molecular Medicine (to FSK), the Cali-Weldon Professorship of FOP Research (to EMS), the Ian Cali Distinguished Clinician-Scientist (to RJP), the Penn Center for Musculoskeletal Disorders, and the National Institutes of Health (NIH R01-AR41916). The authors thank Mr. Robert Caron and Mrs. Kamlesh Rai for their invaluable technical and administrative assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Shore EM, Kaplan FS. Inherited human diseases of heterotopic bone formation. Nat Rev Rheumatol. 2010;6:518–527. doi: 10.1038/nrrheum.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaplan FS, Tabas J, Gannon FH, Finkel G, Hahn GV, Zasloff MA. The histopathology of fibrodysplasia ossificans progressiva: an endochondral process. J Bone Joint Surg. 1993;75-A:220–230. doi: 10.2106/00004623-199302000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan FS, Shore EM, Gupta R, Billings PC, Glaser DL, Pignolo RJ, Graf D, Kamoun M. Immunological features of fibrodysplasia ossificans progressiva and the dysregulated BMP4 Pathway. Clin Rev Bone Miner Metab. 2005;3:189–193. [Google Scholar]
- 4.Kaplan FS, Pignolo RJ, Shore EM. The FOP metamorphogene encodes a novel type I receptor that dysregulates BMP signaling. Cytokine Growth Factor Rev. 2009;20:399–407. doi: 10.1016/j.cytogfr.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hatsell SJ, Idone V, Wolken DM, Huang L, Kim HJ, Wang L, Wen X, Nannuru KC, Jimenez J, Xie L, Das N, Makhoul G, Chernomorsky R, D'Ambrosio D, Corpina RA, Schoenherr CJ, Feeley K, Yu PB, Yancopoulos GD, Murphy AJ, Economides AN. ACVR1R206H receptor mutation causes fibrodysplasia ossificans progressiva by imparting responsiveness to activin A. Sci Transl Med. 2015 Sep 2;7(303):303ra137. doi: 10.1126/scitranslmed.aac4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hino K, Ikeya M, Horigome K, Matsumoto Y, Ebise H, Nishio M, Sekiguchi K, Shibata M, Nagata S, Matsuda S, Toguchida J. Neofunction of ACVR1 in fibrodysplasia ossificans progressiva. PNAS. 2015 doi: 10.1073/pnas.1510540112. doi/10.1073/pnas.1510540112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aleman-Muench GR, Soldevila G. When versatility matters: activins/inhibins as key regulators of immunity. Immunol Cell Biol. 2012;90:137–48. doi: 10.1038/icb.2011.32. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan FS, Glaser DL, Shore EM, Deirmengian GK, Gupta R, Delai P, Morhart R, Smith R, Le Merrer M, Rogers JG, Connor JM, Kitterman JA. The phenotype of fibrodysplasia ossificans progressiva. Clin Rev Bone Miner Metab. 2005;3:183–188. [Google Scholar]
- 9.Hüning I, Gillessen-Kaesbach G. Fibrodysplasia ossificans progressiva: clinical course, genetic mutations and genotype-phenotype correlation. Mol Syndromol. 2014;5:201–211. doi: 10.1159/000365770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen RB, Hahn GV, Tabas J, Peeper J, Levitz CL, Sando A, Sando N, Zasloff M, Kaplan FS. The natural history of heterotopic ossification in patients who have fibrodysplasia ossificans progressiva. J Bone Joint Surg. 1993;75-A:215–219. doi: 10.2106/00004623-199302000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Rocke DM, Zasloff M, Peeper J, Cohen RB, Kaplan FS. Age and joint-specific risk of initial heterotopic ossification in patients who have fibrodysplasia ossificans progressiva. Clin Orthop Rel Res. 1994;301:243–248. [PubMed] [Google Scholar]
- 12.Pignolo RJ, Bedford-Gay C, Liljesthröm M, Durbin-Johnson BP, Shore EM, Rocke DM, Kaplan FS. The Natural History of Flare-Ups in Fibrodysplasia Ossificans Progressiva (FOP): A Comprehensive Global Assessment. J Bone Miner Res. 2015 doi: 10.1002/jbmr.2728. doi: 10.1002/jbmr.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaffer AA, Kaplan FS, Tracy MR, O’Brien ML, Dormans JP, Shore EM, Harland RM, Kusumi K. Developmental anomalies of the cervical spine in patients with fibrodysplasia ossificans progressiva are distinctly different from those in patients with Klippel-Feil syndrome. Spine. 2005;30:1379–1385. doi: 10.1097/01.brs.0000166619.22832.2c. [DOI] [PubMed] [Google Scholar]
- 14.Deirmengian GK, Hebela NM, O’Connell M, Glaser DL, Shore EM, Kaplan FS. Proximal tibial osteochondromas in patients with fibrodysplasia ossificans progressiva. J Bone Joint Surg Am. 2008;90:366–374. doi: 10.2106/JBJS.G.00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy CE, Lash AT, Janoff HB, Kaplan FS. Conductive hearing loss in individuals with fibrodysplasia ossificans progressiva. Am J Audiol. 1999;8:29–33. doi: 10.1044/1059-0889(1999/011). [DOI] [PubMed] [Google Scholar]
- 16.Shore EM, Feldman GJ, Xu M, Kaplan FS. The genetics of fibrodysplasia ossificans progressiva. Clin Rev Bone Miner Metab. 2005;3:201–204. [Google Scholar]
- 17.Kitterman JA, Kantanie S, Rocke DM, Kaplan FS. Iatrogenic harm caused by diagnostic errors in fibrodysplasia ossificans progressiva. Pediatrics. 2005;116:654–661. doi: 10.1542/peds.2005-0469. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan FS, Xu M, Glaser DL, Collins F, Connor M, Kitterman J, Sillence D, Zackai E, Ravitsky V, Zasloff M, Ganguly A, Shore EM. Early diagnosis of fibrodysplasia ossificans progressiva. Pediatrics. 2008;121:e1295–e1300. doi: 10.1542/peds.2007-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan FS, McCluskey W, Hahn G, Tabas J, Muenke M, Zasloff MA. Genetic transmission of fibrodysplasia ossificans progressiva. J Bone Joint Surg. 1993;75-A:1214–1220. doi: 10.2106/00004623-199308000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Kussmaul WG, Esmail AN, Sagar Y, Ross J, Gregory S, Kaplan FS. Pulmonary and cardiac function in advanced fibrodysplasia ossificans progressiva. Clin Orthop Rel Res. 1998;346:104–109. [PubMed] [Google Scholar]
- 21.Kaplan FS, Glaser DL. Thoracic insufficiency syndrome in patients with fibrodysplasia ossificans progressiva. Clin Rev Bone Miner Metab. 2005;3:213–216. [Google Scholar]
- 22.Kaplan FS, Zasloff MA, Kitterman JA, Shore EM, Hong CC, Rocke DM. Early mortality and cardiorespiratory failure in patients with fibrodysplasia ossificans progressiva. J Bone Joint Surg Am. 2010;92:686–691. doi: 10.2106/JBJS.I.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplan FS, LeMerrer M, Glaser DL, Pignolo RJ, Goldsby RE, Kitterman JA, Groppe J, Shore EM. Fibrodysplasia ossificans progressiva. Best Pract Res Clin Rheumatol. 2008;22:191–205. doi: 10.1016/j.berh.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaplan FS, Shore EM, Pignolo RJ, editors. The International Clinical Consortium on FOP. The medical management of fibrodysplasia ossificans progressiva: current treatment considerations. Clin Proc Intl Clin Consort FOP. 2011;4:1–100. [Google Scholar]
- 25.Pignolo RJ, Shore EM, Kaplan FS. Fibrodysplasia ossificans progressiva: clinical and genetic aspects. Orphanet J Rare Dis. 2011;6:80. doi: 10.1186/1750-1172-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pignolo RJ, Shore EM, Kaplan FS. Fibrodysplasia ossificans progressiva: diagnosis management, and therapeutic horizons. In Emerging Concepts in Pediatric Bone Disease. Pediatric Endocrinology Rev. 2013;10(S-2):437–448. [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplan FS, Pignolo RJ, Shore EM. From mysteries to medicines: drug development for fibrodysplasia ossificans progressiva. Expert Opinion on Orphan Drugs. 2013;1:637–649. doi: 10.1517/21678707.2013.825208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho T-J, Choi IH, Connor JM, Delai P, Glaser DL, Le Merrer M, Morhart R, Rogers JG, Smith R, Triffitt JT, Urtizberea JA, Zasloff M, Brown MA, Kaplan FS. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nature Genetics. 2006;38:525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan FS, Xu M, Seemann P, Connor JM, Glaser DL, Carroll L, Delai P, Fastnacht-Urban E, Forman SJ, Gillessen-Kaesbach G, Hoover-Fong J, Köster B, Pauli RM, Reardon W, Zaidi S-A, Zasloff M, Morhart R, Mundlos S, Groppe J, Shore EM. Classic and atypical fibrodysplasia ossificans progressiva (FOP) phenotypes are caused by mutations in the bone morphogenetic protein (BMP) type I receptor ACVR1. Hum Mutat. 2009;30:379–390. doi: 10.1002/humu.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaikuad A, Alfano I, Kerr G, Sanvitale CE, Boergermann JH, Triffitt JT, von Delft F, Knapp S, Knaus P, Bullock AN. Structure of the bone morphogenetic protein receptor ALK2 and implications for fibrodysplasia ossificans progressiva. J Biol Chem. 2012;287:36990–369998. doi: 10.1074/jbc.M112.365932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Groppe JC, Shore EM, Kaplan FS. Functional modeling of the ACVR1 (R206H) mutation in FOP. Clin Orthop Rel Res. 2007;462:87–92. doi: 10.1097/BLO.0b013e318126c049. [DOI] [PubMed] [Google Scholar]
- 32.Shen Q, Little SC, Xu M, Haupt J, Ast C, Katagiri T, Mundlos S, Seemann P, Kaplan FS, Mullins MC, Shore EM. The fibrodysplasia ossificans progressiva R206H ACVR1 mutation activates BMP-independent chondrogenesis and zebrafish embryo ventralization. J Clin Invest. 2009;119:3462–3472. doi: 10.1172/JCI37412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song GA, Kim HJ, Woo KM, Baek JH, Kim GS, Choi JY, Ryoo HM. Molecular consequences of the ACVR1(R206H) mutation of fibrodysplasia ossificans progressiva. J Biol Chem. 2010;285:22542–22553. doi: 10.1074/jbc.M109.094557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Dinther M, Visser N, de Gorter DJ, Doorn J, Goumans MJ, de Boer J, ten Dijke P. ALK2 R206H mutation linked to fibrodysplasia ossificans progressiva confers constitutive activity to the BMP type I receptor and sensitizes mesenchymal cells to BMP-induced osteoblast differentiation and bone formation. J Bone Miner Res. 2010;25:1208–1215. doi: 10.1359/jbmr.091110. [DOI] [PubMed] [Google Scholar]
- 35.Kaplan FS, Seemann P, Haupt J, Xu M, Mullins M, Shore EM. Investigations of activated ACVR1/ALK2, a bone morphogenetic protein type I receptor that causes fibrodysplasia ossificans progressiva. Methods Enzymol. 2010;484:357–373. doi: 10.1016/B978-0-12-381298-8.00018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shafritz AB, Shore EM, Gannon FH, Zasloff MA, Taub R, Muenke M, Kaplan FS. Over-expression of an osteogenic morphogen in fibrodysplasia ossificans progressiva. N Engl J Med. 1996;335:555–561. doi: 10.1056/NEJM199608223350804. [DOI] [PubMed] [Google Scholar]
- 37.Gannon FH, Kaplan FS, Olmsted E, Finkel G, Zasloff MA, Shore EM. Bone morphogenetic protein 2/4 in early fibromatous lesions of fibrodysplasia ossificans progressiva. Hum Pathol. 1997;28:339–343. doi: 10.1016/s0046-8177(97)90133-7. [DOI] [PubMed] [Google Scholar]
- 38.Ahn J, Serrano de la Peña L, Shore EM, Kaplan FS. Paresis of a bone morphogenetic protein-antagonist response in a genetic disorder of heterotopic skeletogenesis. J Bone Joint Surg. 2003;85-A:667–674. doi: 10.2106/00004623-200304000-00013. [DOI] [PubMed] [Google Scholar]
- 39.Serrano de la Peña L, Billings PC, Fiori JL, Ahn J, Shore EM, Kaplan FS. Fibrodysplasia ossificans progressiva (FOP), a disorder of ectopic osteogenesis, misregulates cell surface expression and trafficking of BMPRIA. J Bone Miner Res. 2005;20:1168–1176. doi: 10.1359/JBMR.050305. [DOI] [PubMed] [Google Scholar]
- 40.Fiori JL, Billings PC, Serrano de la Peña L, Kaplan FS, Shore EM. Dysregulation of the BMP-p38 MAPK signaling pathway in cells from patients with fibrodysplasia ossificans progressiva (FOP) J Bone Miner Res. 2006;21:902–909. doi: 10.1359/jbmr.060215. [DOI] [PubMed] [Google Scholar]
- 41.Billings PC, Fiori JL, Bentwood JL, O’Connell MP, Jiao X, Nussbaum B, Caron RJ, Shore EM, Kaplan FS. Dysregulated BMP signaling and enhanced osteogenic differentiation of connective tissue progenitor cells from patients with fibrodysplasia ossificans progressiva. J Bone Miner Res. 2008;23:305–313. doi: 10.1359/JBMR.071030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukuda T, Kohda M, Kanomata K, Nojima J, Nakamura A, Kamizono J, Noguchi Y, Iwakiri K, Kondo T, Kurose J, Endo KI, Awakura T, Fukushi J, Nakashima Y, Chiyonobu T, Kawara A, Nishida Y, Wada I, Akita M, Komori T, Nakayama K, Nanba A, Yoda T, Tomoda H, Yu PB, Shore EM, Kaplan FS, Miyazono K, Matsuoka M, Ikebuchi K, Ohtake A, Oda H, Jimi E, Owan I, Okazaki Y, Katagiri T. Constitutively activated ALK2 and increased SMAD 1/5 cooperatively induce bone morphogenetic protein signaling in fibrodysplasia ossificans progressiva. J Biol Chem. 2009;284:7149–7156. doi: 10.1074/jbc.M801681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Culbert AL, Chakkalakal SA, Theosmy EG, Brennan TA, Kaplan FS, Shore EM. Alk2 regulates early chondrogenic fate in fibrodysplasia ossificans progressiva heterotopic endochondral ossification. Stem Cells. 2014;32:1289–1300. doi: 10.1002/stem.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le VQ, Wharton KA. Hyperactive BMP signaling induced by ALK2 (R206H) requires type II receptor function in a Drosophila model for classic fibrodysplasia ossificans progressiva. Dev Dynamics. 2012;241:200–214. doi: 10.1002/dvdy.22779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bagarova J, Vonner AJ, Armstrong KA, Börgermann J, Lai CS, Deng DY, Beppu H, Alfano I, Filippakopoulos P, Morrell NW, Bullock AN, Knaus P, Mishina Y, Yu PB. Constitutively active ALK2 receptor mutants require type II receptor cooperation. Mol Cell Biol. 2013;33:2413–2424. doi: 10.1128/MCB.01595-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Groppe JC, Wu J, Shore EM, Kaplan FS. In vitro analysis of dysregulated R206H ALK2 kinase-FKBP12 interaction associated with heterotopic ossification in FOP. Cells Tissues Organs. 2011;194:291–295. doi: 10.1159/000324230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang T, Donahoe PK. The immunophilin FKBP12: a molecular guardian of the TGF-beta family type I receptors. Front Biosci. 2004;9:619–631. doi: 10.2741/1095. [DOI] [PubMed] [Google Scholar]
- 48.Huse M, Chen YG, Massagué J, Kuriyan J. Crystal structure of the cytoplasmic domain of the type I TGF beta receptor in complex with FKBP12. Cell. 1999;96:425–436. doi: 10.1016/s0092-8674(00)80555-3. [DOI] [PubMed] [Google Scholar]
- 49.Huse M, Muir TW, Xu L, Chen YG, Kuriyan J, Massagué J. The TGF beta receptor activation process: an inhibitor- to substrate-binding switch. Mol Cell. 2001;8:671–682. doi: 10.1016/s1097-2765(01)00332-x. [DOI] [PubMed] [Google Scholar]
- 50.Hong CC, Yu PB. Applications of small molecule BMP inhibitors in physiology and disease. Cytokine Growth Factor Rev. 2009;20:409–418. doi: 10.1016/j.cytogfr.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Twombly V, Bangi E, Le V, Malnic B, Singer MA, Wharton KA. Functional analysis of saxophone, the Drosophila gene encoding the BMP type I receptor ortholog of human ALK1/ACVR1 and ACVR1/ALK2. Genetics. 2009;183:563–579. doi: 10.1534/genetics.109.105585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Little SC, Mullins MC. Bone morphogenetic protein heterodimers assemble heteromeric type I receptor complexes to pattern the dorsoventral axis. Nat Cell Biol. 2009;11:637–643. doi: 10.1038/ncb1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Convente MR, Wang H, Pignolo RJ, Kaplan FS, Shore EM. The immunological contribution to heterotopic ossification disorders. Curr Osteoporos Res. 2015;13:116–124. doi: 10.1007/s11914-015-0258-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lanchoney TF, Cohen RB, Rocke DM, Zasloff MA, Kaplan FS. Permanent heterotopic ossification at the injection site after diphtheria-tetanus-pertussis immunizations in children who have fibrodysplasia ossificans progressiva. J Pediatrics. 1995;126:762–764. doi: 10.1016/s0022-3476(95)70408-6. [DOI] [PubMed] [Google Scholar]
- 55.Luchetti W, Cohen RB, Hahn GV, Rocke DM, Helpin M, Zasloff M, Kaplan FS. Severe restriction in jaw movement after routine injection of local anesthetic in patients who have fibrodysplasia ossificans progressiva. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:21–25. doi: 10.1016/s1079-2104(96)80141-7. [DOI] [PubMed] [Google Scholar]
- 56.Glaser DL, Rocke DM, Kaplan FS. Catastrophic falls in patients who have fibrodysplasia ossificans progressiva. Clin Orthop Rel Res. 1998;346:110–116. [PubMed] [Google Scholar]
- 57.Scarlett RF, Rocke DM, Kantanie S, Patel JB, Shore EM, Kaplan FS. Influenza-like viral illnesses and flare-ups of fibrodysplasia ossificans progressiva (FOP) Clin Orthop Rel Res. 2004;423:275–279. doi: 10.1097/01.blo.0000129557.38803.26. [DOI] [PubMed] [Google Scholar]
- 58.Moriatis JM, Gannon FH, Shore EM, Bilker W, Zasloff MA, Kaplan FS. Limb swelling in patients who have fibrodysplasia ossificans progressiva. Clin Orthop Rel Res. 1997;336:247–253. doi: 10.1097/00003086-199703000-00033. [DOI] [PubMed] [Google Scholar]
- 59.Gannon FH, Valentine BA, Shore EM, Zasloff MA, Kaplan FS. Acute lymphocytic infiltration in an extremely early lesion of fibrodysplasia ossificans progressiva. Clin Orthop Rel Res. 1998;346:19–25. [PubMed] [Google Scholar]
- 60.Gannon FH, Glaser D, Caron R, Thompson LD, Shore EM, Kaplan FS. Mast cell involvement in fibrodysplasia ossificans progressiva. Hum Pathol. 2001;32:842–848. doi: 10.1053/hupa.2001.26464. [DOI] [PubMed] [Google Scholar]
- 61.Hegyi L, Gannon FH, Glaser DL, Shore EM, Kaplan FS, Shanahan CM. Stromal cells of fibrodysplasia ossificans progressiva lesions express smooth muscle lineage markers and the osteogenic transcription factor Runx2/Cbfa-1: clues to a vascular origin of heterotopic ossification. J Pathol. 2003;201:141–148. doi: 10.1002/path.1413. [DOI] [PubMed] [Google Scholar]
- 62.Glaser DL, Economides AN, Wang L, Liu X, Kimble RD, Fandl JP, Wilson JM, Stahl N, Kaplan FS, Shore EM. In vivo somatic cell gene transfer of an engineered noggin mutein prevents BMP4-induced heterotopic ossification. J Bone Joint Surg. 2003;85-A:2332–2342. doi: 10.2106/00004623-200312000-00010. [DOI] [PubMed] [Google Scholar]
- 63.Janoff HB, Zasloff MA, Kaplan FS. Submandibular swelling in patients with fibrodysplasia ossificans progressiva. Otolaryngol Head Neck Surg. 1996;114:599–604. doi: 10.1016/S0194-59989670253-X. [DOI] [PubMed] [Google Scholar]
- 64.Yu PB, et al. BMP type receptor inhibition reduces heterotopic ossification. Nat Med. 2008;14:1363–1369. doi: 10.1038/nm.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaplan FS, Glaser DL, Shore EM, Pignolo RJ, Xu M, Zhang Y, Senitzer D, Forman SJ, Emerson SG. Hematopoietic stem-cell contribution to ectopic skeletogenesis. J Bone Joint Surg Am. 2007;89:347–357. doi: 10.2106/JBJS.F.00472. [DOI] [PubMed] [Google Scholar]
- 66.Chakkalakal SA, Zhang D, Culbert AL, Convente MR, Caron RJ, Wright AC, Maidment AD, Kaplan FS, Shore EM. An Acvr1 Knock-in mouse has fibrodysplasia ossificans progressiva. J Bone Miner Res. 2012;27:1746–1756. doi: 10.1002/jbmr.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaplan FS, Chakkalakal SA, Shore EM. Fibrodysplasia ossificans progressiva: mechanisms and models of skeletal metamorphosis. Dis Model Mech. 2012;5:756–762. doi: 10.1242/dmm.010280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kan L, Liu Y, McGuire TL, Berger DM, Awatramani RB, Dymecki SM, Kessler JA. Dysregulation of local stem/progenitor cells as a common cellular mechanism for heterotopic ossification. Stem Cells. 2009;27:150–156. doi: 10.1634/stemcells.2008-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Convente MR, Yang E, Chakkalakal SA, Zhang D, Caron RJ, Perrien DS, Kambayashi T, Kaplan FS, Shore EM. Targeted Ablation of Macrophages and Mast Cells Impairs Heterotopic Ossification in a Mouse Model of Fibrodysplasia Ossificans Progressiva. Abstract, 2015 [Google Scholar]
- 70.Kan L, Lounev VY, Pignolo RJ, Duan L, Liu Y, Stock SR, McGuire TL, Lu B, Gerard NP, Shore EM, Kaplan FS, Kessler JA. Substance P signaling mediates BMP-dependent heterotopic ossification. J Cell Biochem. 2011;112:2759–2772. doi: 10.1002/jcb.23259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salisbury E, Rodenberg E, Sonnet C, Hipp J, Gannon FH, Vadakkan TJ, Dickinson ME, Olmsted-Davis EA, Davis AR. Sensory nerve induced inflammation contributes to heterotopic ossification. J Cell Biochem. 2011;112:2748–2758. doi: 10.1002/jcb.23225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tracey KJ. Understanding immunity requires more than immunology. Nat Immunol. 2010;11:561–564. doi: 10.1038/ni0710-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakase T, Nomura S, Yoshikawa H, Hashimoto J, Hirota S, Kitamura Y, Oikawa S, Ono K, Takaoka K. Transient and localized expression of bone morphogenetic protein 4 messenger RNA during fracture healing. J Bone Miner Res. 1994;9:651–659. doi: 10.1002/jbmr.5650090510. [DOI] [PubMed] [Google Scholar]
- 74.Clever JL, Sakai Y, Wang RA, Schneider DB. Inefficient skeletal muscle repair in inhibitor of differentiation knockout mice suggests a crucial role for BMP signaling during adult muscle regeneration. Am J Physiol Cell Physiol. 2010;298:C1087–1099. doi: 10.1152/ajpcell.00388.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kluk MW, Ji Y, Shin EH, Amrani O, Onodera J, Jackson WM, Nesti LJ. Fibroregulation of mesenchymal progenitor cells by BMP-4 after traumatic muscle injury. J Orthop Trauma. 2012;26:693–698. doi: 10.1097/BOT.0b013e3182712adf. [DOI] [PubMed] [Google Scholar]
- 76.Urist MR. Bone: Formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 77.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 78.Cunningham NS, Paralkar V, Reddi AH. Osteogenin and recombinant bone morphogenetic protein 2B are chemotactic for human monocytes and stimulate transforming growth factor beta 1 mRNA expression. Proc Natl Acad Sci USA. 1992;89:11740–11744. doi: 10.1073/pnas.89.24.11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McCullough KA, Waits CA, Garimella R, Tague SE, Sipe JB, Anderson HC. Immunohistochemical localization of bone morphogenetic proteins (BMPs) 2, 4, 6, and 7 during induced heterotopic bone formation. J Orthop Res. 2007;25:465–472. doi: 10.1002/jor.20340. [DOI] [PubMed] [Google Scholar]
- 80.Hong JH, Lee GT, Lee JH, Kwon SJ, Park SH, Kim SJ, Kim IY. Effect of bone morphogenetic protein-6 on macrophages. Immunology. 2009;128(1 Suppl):e442–450. doi: 10.1111/j.1365-2567.2008.02998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kan L, Hu M, Gomes WA, Kessler JA. Transgenic mice overexpressing BMP4 develop a fibrodysplasia ossificans progressiva (FOP)-like phenotype. Am J Pathol. 2004;165:1107–1115. doi: 10.1016/S0002-9440(10)63372-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kopp E, Medzhitov R, Carothers J, Xiao C, Douglas I, Janeway CA, Ghosh S. ECSIT is an evolutionarily conserved intermediate in the Toll/IL-1 signal transduction pathway. Genes Dev. 1999;13:2059–2071. doi: 10.1101/gad.13.16.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xiao C, Shim JH, Klüppel M, Zhang SS, Dong C, Flavell RA, Fu XY, Wrana JL, Hogan BL, Ghosh S. Ecsit is required for Bmp signaling and mesoderm formation during mouse embryogenesis. Genes Dev. 2003;17:2933–2949. doi: 10.1101/gad.1145603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moustakas A, Heldin CH. Ecsit-ement on the crossroads of Toll and BMP signal transduction. Genes Dev. 2003;17:2855–2859. doi: 10.1101/gad.1161403. [DOI] [PubMed] [Google Scholar]
- 85.Wi SM, Moon G, Kim J, Kim ST, Shim JH, Chun E, Lee KY. TAK1-ECSIT-TRAF6 complex plays a key role in the TLR4 signal to activate NF-κB. J Biol Chem. 2014;289:35205–35214. doi: 10.1074/jbc.M114.597187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Olsen OE, Wader KF, Hella H, Mylin AK, Turesson I, Nesthus I, Waage A, Sundan A, Holien T. Activin A inhibits BMP-signaling by binding ACVR2A and ACVR2B. Cell Commun Signal. 2015;13:27. doi: 10.1186/s12964-015-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yaden BC, Wang YX, Wilson JM, Culver AE, Milner A, Datta-Mannan A, Shetler P, Croy JE, Dai G, Krishnan V. Inhibition of activin A ameliorates skeletal muscle injury and rescues contractile properties by inducing efficient remodeling in female mice. Am J Pathol. 2014;184:1152–1166. doi: 10.1016/j.ajpath.2013.12.029. [DOI] [PubMed] [Google Scholar]
- 88.Lounev V, Ramachandran R, Wosczyna MN, Yamamoto M, Maidment ADA, Shore EM, Glaser DL, Goldhamer DJ, Kaplan FS. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J Bone Joint Surg Am. 2009;91:652–663. doi: 10.2106/JBJS.H.01177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BJ. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med. 2010;16:1400–1406. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fukuda T, Scott G, Komatsu Y, Araya R, Kawano M, Ray MK, Yamada M, Mishina Y. Generation of a mouse with conditionally activated signaling through the BMP receptor, ALK2. Genesis. 2006;44:159–167. doi: 10.1002/dvg.20201. [DOI] [PubMed] [Google Scholar]
- 91.Wosczyna MN, Biswas AA, Cogswell CA, Goldhamer DJ. Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification. J Bone Miner Res. 2012;27:1004–1017. doi: 10.1002/jbmr.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maeder T. A few hundred people turned to bone. The Atlantic Monthly. 1998;281:81–89. [Google Scholar]