Abstract
Purpose of review
In recent years, important steps have been taken to improve the treatment of congenital adrenal hyperplasia (CAH), a relatively stagnant area for decades. In this review, we summarize these advances and propose future lines of investigation.
Recent findings
The two main goals of CAH treatment are to replace the deficient hormones when necessary and to dampen the adrenorcorticotropin (ACTH) activation and the ensuing adrenal androgen excess. Glucocorticoids have been the mainstay of CAH treatment, but available preparations only partially meet the clinical needs. Recent efforts have focused on improving the delivery of glucocorticoid replacement agents, to closer mimic the physiologic secretion pattern. Examples include modified-release oral glucocorticoids and continuous subcutaneous hydrocortisone pumps. Furthermore, non-glucocorticoid approaches to address the androgen excess have emerged, such as inhibition of key androgenic enzymes and ACTH secretion blockade by corticotropin-releasing hormone-receptor antagonists.
Summary
The promising recent progress made in CAH treatment brings new perspectives for individualized care in this complex disease.
Keywords: congenital adrenal hyperplasia, 21-hydroxylase deficiency, abiraterone acetate, glucocorticoids
Introduction
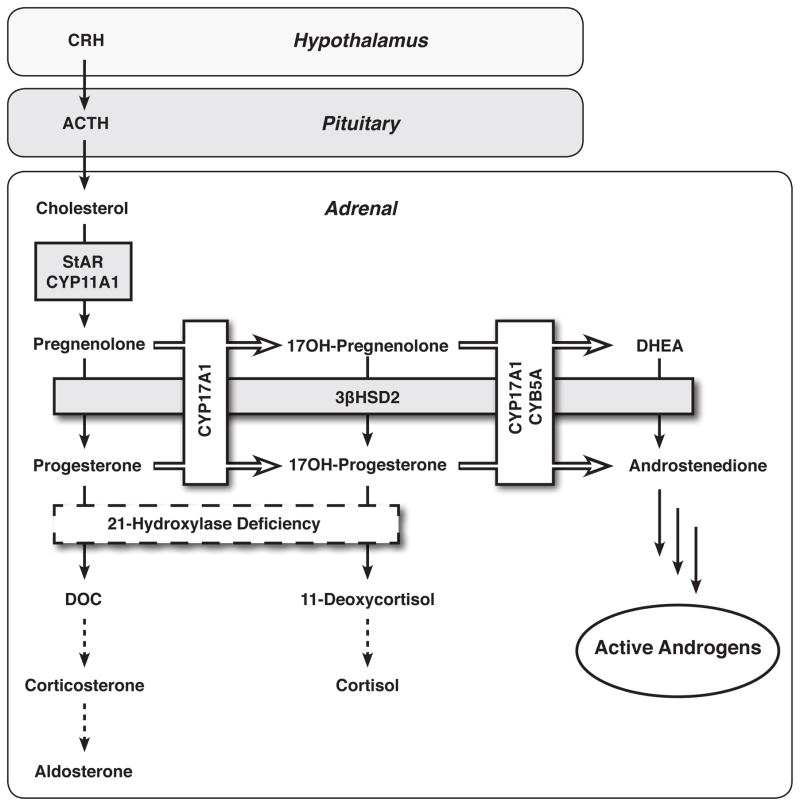
Congenital adrenal hyperplasia (CAH) comprises a group of autosomal recessive enzymatic defects of cortisol synthesis. Over 90% of CAH cases are attributed to 21-hydroxylase (CYP21A2) deficiency (21OHD), one of the most common genetic diseases [1]. The defective cortisol production prompts the hypothalamus and hypophysis to augment the release of corticotropin releasing hormone (CRH) and adrenocorticotropin (ACTH), respectively. In the face of a defective CYP21A2, the upstream steroid precursors, such as 17-hydroxyprogesterone (17OHP), are directed towards formation of androgens (Figure 1). The CYP21A2 defects range from complete to mild, rendering classic forms of 21OHD, in which the cortisol and sometimes mineralocorticoid production is compromised, and nonclassic 21OHD, in which increased ACTH secretion allows compensation to maintain normal cortisol synthesis. The adrenal androgen excess is, at least roughly, inversely correlated with the cortisol production. Girls with classic 21OHD are born with masculinized external genitalia, often managed with corrective surgery. Patients with nonclassic 21OHD might suffer from hirsutism, acne, irregular menses, premature pubarche, rapid somatic growth, advanced bone age, and subfertility [2–5]; however, most nonclassic 21OHD patients never receive this diagnosis.
Figure 1.
Adrenal steroidogenic pathways in 21-hydroxylase deficiency. CRH, corticotropin-releasing hormone; ACTH, adrenocorticotropic hormone; StAR, steroidogenic acute regulatory protein; CYP11A1, cholesterol side-chain cleavage enzyme; 3βHSD2, 3β-hydroxysteroid dehydrogenase/isomerase type 2; CYP17A1, 17α-hydroxylase/17,20-lyase; CYB5A, cytochrome b5 type A; DHEA, dehydroepinadrosterone; DOC, 11-deoxycorticosterone.
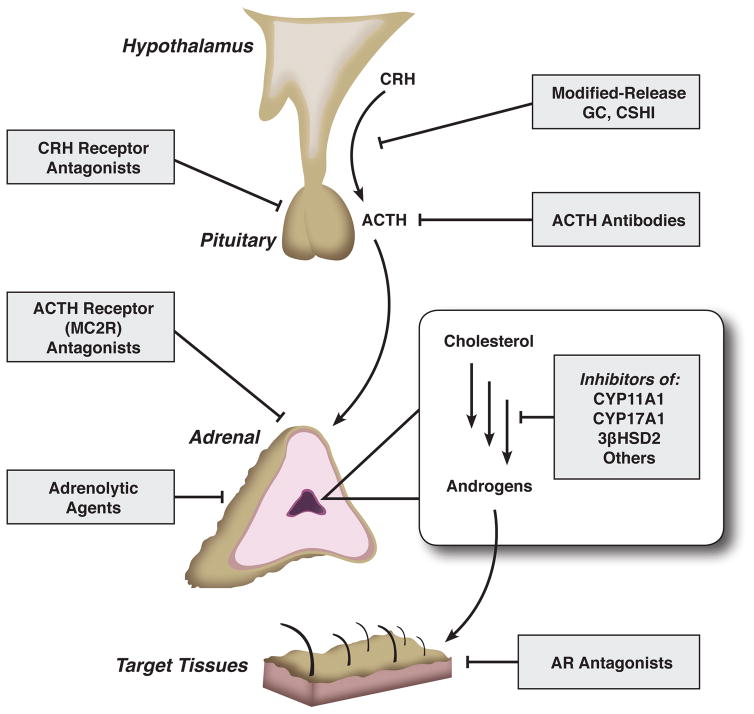
Glucocorticoid therapy did not become available until the 1950s, almost a century after the first description of the disease. While these drugs have prolonged survival routinely into adulthood, CAH treatment has remained relatively stagnant since then and has failed to meet all of the complex clinical needs of these patients. Unlike other forms of adrenal insufficiency, treatment of 21OHD aims not only to replace the deficient hormones but also to blunt the ACTH secretion, the major driver of the adrenal androgen production. Current guidelines for CAH treatment are flexible but emphasize the importance of minimizing potential iatrogenic Cushing syndrome [6]. Adequate adrenal androgen suppression, however, is often difficult to achieve without supraphysiologic doses of glucocorticoids and their associated detrimental effects. Alternative, non-glucocorticoid treatment strategies to target the adrenal androgen excess have only recently begun to emerge. Figure 2 depicts potential therapeutic approaches to manage the adrenal androgen excess in CAH. Such strategies include: interference with the synthesis or function of CRH, ACTH, or key androgenic enzymes; non-selective adrenal steroidogenesis blockade; and androgen receptor antagonists. In the recent years, investigators have strived to improve CAH treatment by optimizing the glucocorticoid delivery systems, as well as by exploring non-glucocorticoid therapeutic strategies. Herein, we review recent and long overdue, evolving advances in CAH treatment.
Figure 2.
Potential therapeutic targets in congenital adrenal hyperplasia. CRH, corticotropin-releasing hormone; ACTH, adrenocorticotropin hormone; GC, glucocorticoids; CSHI, continuous subcutaneous hydrocortisone infusion; CYP11A1, cholesterol side-chain cleavage enzyme; CYP17A1, 17α-hydroxylase/17,20-lyase; 3βHSD2, 3β-hydroxysteroid dehydrogenase/isomerase type 2; AR, androgen receptor.
Glucocorticoid treatment
Glucocorticoids, with or without mineralocorticoids and sodium supplementation, have constituted the cornerstone of 21OHD management at all ages. All glucocorticoid drugs substitute the deficient endogenous cortisol synthesis in classic 21OHD and partially restore the negative feedback to the hypothalamus and the pituitary, to dampen their exaggerated secretion of CRH and ACTH, respectively, thus decreasing adrenal androgen synthesis. While exogenous glucocorticoids satisfactorily substitute for the cortisol deficiency in most patients, sufficient suppression of ACTH, particularly early in the morning, has been challenging with the currently available formulations. Under physiologic conditions, ACTH is secreted with a circadian rhythmicity, typically rising about 0300 h in early morning, reaching a peak by 0600–0800 h and declining to nadir in the evening [7, 8]. Oral glucocorticoids regimens aim to roughly mimic this diurnal pattern but allow the ACTH suppression to escape in between doses [9]. The effects on ACTH secretion vary widely with the different types of glucocorticoids and their frequency of administration. Traditional substitution regimens include hydrocortisone, administered two or three times daily, the intermediate-acting prednisone or prednisolone, or the long-acting dexamethasone. Hydrocortisone is usually preferred in children, and the lowest possible doses are used in order to avoid growth suppression [10]. In adults, the intermediate and long-acting synthetic glucocorticoids are often preferred, due to a more convenient dosing schedule, although with a higher risk of unfavorable effects, such as obesity, dermal atrophy, insulin resistance, hypertension and bone loss [11–16].
Novel oral glucocorticoid preparations
In the recent years, investigators have worked on developing new glucocorticoid delivery systems, which could better replicate the physiologic circadian rhythm. A dual-release hydrocortisone, consisting of an extended-release core surrounded by an immediate-release coating, was initially developed for once-daily first-morning administration in patients with primary adrenal insufficiency (Plenadren; ViroPharma, Maidenhead, UK) [17]. This dual-release hydrocortisone at 30 mg/day achieved improved body weight, glucose metabolism, and both systolic and diastolic blood pressure, compared with the similar total daily hydrocortisone, administered in 3 divided doses, after 3 months of treatment [18]. This pharmacologic strategy, however, does not provide any early morning cortisol rise and thus is not designed to suppress the ACTH and the adrenal androgens during this window. Although small trials of this product have been conducted in CAH patients, no studies have been published at this writing.
Another oral modified-release multiparticulate hydrocortisone, Chronocort® (Diurnal, UK), was formulated to reproduce the physiologic early morning rise in cortisol when administered at bedtime [19]. In a phase 2, open-label, crossover study of 14 patients (7 males, ages 17 to 55) with classic 21OHD, 30 mg Chronocort administered at 2200 h, led to a single cortisol peak approximately 8 h later, leading to significantly lower 0800 h 17OHP compared with conventional hydrocortisone administered three times daily [20]. When Chronocort was administered as a single bedtime dose, however, the average 17OHP, androstenedione, and ACTH over 24 hours were not suppressed as well as during conventional hydrocortisone replacement. More recently, a phase 2, open-label trial of 16 adults (8 females) with classic CAH studied a reformulated Chronocort product administered twice daily, initially 10 mg at 0700 h and 20 mg at 2300 h, and titrated over six months based on androstenedione, 17OHP and clinical symptomatology [21**]. Serum steroids and other clinical parameters were compared for each patient on their various glucocorticoid replacement regimens prescribed prior to entering this study. Compared with conventional therapy, this Chronocort regimen resulted in lower 24 h (p = 0.003), morning (0700–1500 h; p = 0.0008) and afternoon (1500–2300 h; p = 0.009) area under the curve (AUC) for androstenedione and lower 24 h (p = 0.021) and morning (0700–1500 h; p = 0.018) AUC for 17OHP, despite of a lower hydrocortisone dose equivalent (28±11.8 vs 25.9±7.1 mg/d). Along with the lower glucocorticoid exposure, patients also experienced an increase in lean body mass, morning HOMA-IR, and osteocalcin. Interestingly, the bone mineral density declined slightly but significantly during Chronocort treatment in women, a change attributed to the improved androgen control. Long-term studies of more patients, including children, would help clarify the effects of such modified-release glucocorticoid preparations on various metabolic parameters and on clinically relevant outcomes, such as cardiovascular and fracture risks.
Parenteral glucocorticoids
A more precise titration of glucocorticoid delivery can be attained with parenteral administration, although this approach has practical limitations. A small study conducted a decade ago showed that intravenous administration of hydrocortisone in a pattern similar to physiologic cortisol secretion brings ACTH and 17OHP much closer to normal than conventional therapy, in both adrenal insufficiency and CAH patients [22]. In 2009, hydrocortisone continuously administered via a subcutaneous insulin pump was reported for the first time in a CAH patient, a 14.5-year old boy with poor bioavailability and increased clearance of oral hydrocortisone [23]. This patient achieved better control on a lower total daily dose of hydrocortisone with subcutaneous versus oral hydrocortisone, which allowed normal pubertal progression over several years.
More recently, continuous subcutaneous hydrocortisone infusion was found to be superior to conventional oral delivery in a prospective crossover, randomized, multicenter clinical trial of 33 patients with Addison disease [24*]. The subcutaneous infusion was adjusted based on salivary cortisol levels (obtained between 0600–0800 h and 1100–1200 h) and morning serum cortisol after 3–5 days, to achieve a morning salivary cortisol in the middle to upper reference range, an evening salivary cortisol in the lower reference range, and a normal morning serum cortisol. During the three months of treatment, patients on continuous subcutaneous hydrocortisone infusion had significantly lower mean ACTH concentrations, and their morning serum and 24 h salivary cortisol profiles were closer to physiologic patterns, with improved quality of life metrics as compared to the conventional oral hydrocortisone treatment. Nevertheless, a double-blind placebo-controlled trial did not show any improvement with continuous subcutaneous hydrocortisone infusion in subjective health status in patients with Addison disease and good baseline health status [25].
Another group attempted to further improve the dynamics of hydrocortisone administration by using a portable pulsatile continuous subcutaneous delivery system along with real-time sampling of ACTH and cortisol [26*]. This paradigm was achieved through an automated blood sampling system connected to a venous cannula inserted in the antecubital fossa, through which cortisol and ACTH were sampled every 10 and 60 minutes, respectively. The study was conducted in healthy volunteers in whom the intrinsic adrenal function was suppressed with dexamethasone or metyrapone. Using this system, physiological circadian and ultradian rhythmicity was replicated. While these concepts are promising, future studies in patients with CAH are important for determining the patterns of glucocorticoid administration necessary not only in physiologic circumstances, but also to adequately suppress ACTH and androgen synthesis.
The widespread implementation of parenteral hydrocortisone for CAH patients faces several hurdles. Similar to the use of insulin pumps in diabetic patients, these devices involve uninterrupted equipment wear, high cost, considerable patient sophistication, and the potential for local irritation and malfunction, which is particularly risky in patients with complete glucocorticoid deficiency. In addition, the commercial hydrocortisone formulations (hydrocortisone semisuccinate, Solu-cortef, Pfizer) are not designed for subcutaneous administration, and frequent infusion site reactions have been anecdotally reported, possibly due to the preservatives or other components of the preparation.
Non-glucocorticoid treatment
A radical approach intended to eradicate the adrenal androgen excess is surgical removal of the adrenal glands. While surgery often achieves this objective in the short term [27], bilateral adrenalectomy leaves patients fully depended on exogenous glucocorticoid replacement and puts them at risk for adrenal crises [28, 29], which can be fatal. Patients and parents should never be counseled that adrenalectomy will permanently cure the hyperandrogenemia, due to the development of adrenal rest tumors in the testes [29]in men or in the ovaries [30] and retroperitoneum [31] in women. In one study, hyperandrogenemia recurred in 8 of 18 patients following adrenalectomy [29]. Other complications following adrenalectomy include hyperpigmentation and pituitary corticotrope adenoma formation requiring more surgery [32]. For these reasons, the initial enthusiasm from short-term results has given way to a more tempered long-term view, and bilateral adrenalectomy has fallen out of favor in the past 2 decades.
Chemical adrenalectomy
Chemical adrenalectomy with mitotane was used as a measure of last resort after failure of supraphysiologic doses of glucocorticoids in a 29-year old man with classic 21OHD and testicular adrenal rest tumors, causing azoospermia and infertility [33]. During two years of treatment with mitotane, serum 17OHP and androstenedione, as well as TART volume, decreased substantially despite a further increase in ACTH. Conversely, pituitary gonadotropins, inhibin B and sperm count increased significantly, leading to a successful in vitro fertilization. Side effects of mitotane include anorexia and nausea, fatigue and depression, confusion and ataxia [34], and increased hydrocortisone requirement due to profound induction of CYP3A4 enzyme and enhanced cortisol metabolism [35]. This patient experienced hyponatremia and weight loss, probably due to reduced cortisol exposure, which resolved with higher doses of hydrocortisone and fludrocortisone. Recent studies suggest that the adrenolytic effect of mitotane is due to its inhibition of sterol-O-acyltransferase (SOAT1, also known as acyl-coenzyme A:cholesterol acyltransferase or ACAT1), which increases free cholesterol and oxysterols, and subsequently induces an endoplasmic reticulum stress response in NCI-H295 cells [36].
Androgen biosynthesis inhibitors
With the current formulations available, physiologic glucocorticoid doses are not sufficient to normalize CRH and ACTH production, resulting in sustained androgen excess despite adequate cortisol replacement. Thus, alternative therapies that obviate the need for supraphysiologic exogenous glucocorticoids are highly desirable for all CAH patients. One potential strategy is to inhibit key enzymes of androgen biosynthesis (Figure 2), such as 17α-hydroxylase/17,20-lyase (CYP17A1, P450c17). Abiraterone is a potent inhibitor of CYP17A1 [37], an enzyme required for the synthesis of all androgens. Abiraterone acetate, the oral prodrug of abiraterone, profoundly decreases serum testosterone and improves survival in men with castration-resistant prostate cancer [38–40]. In a recent phase I open-label, multiple-dose clinical study, abiraterone acetate was administered for 6 days to adult women with classic 21OHD and elevated androgen production while receiving physiological hydrocortisone doses of approximately 20 mg/day [41**]. All 6 participants enrolled had serum androstenedione concentrations >1.5 times above the upper limit of normal (>345 ng/dL or >12 nmol/L). Abiraterone acetate at 100 mg/day decreased the mean predose androstenedione on day 6 from 764 to 254 ng/dL and further decreased androstendione during the 8 h after the final dose. At 250 mg/day, abiraterone acetate normalized the androstenedione 24 h after the final dose in all 6 participants. Serum testosterone and urinary metabolites (androsterone and etiocholanolone glucuronides) declined in parallel with androstenedione, and the response correlated well with the abiraterone acetate exposure. Because the 17-hydroxylase activity of CYP17A1 is needed for cortisol synthesis, blockade with abiraterone acetate in patients with normal adrenal function causes a marked increase of 11-deoxycorticosterone (DOC, Figure 1), leading to hypertension and hypokalemia unless a glucocorticoid, such as prednisolone, is co-administered [42]. In 21OHD, however, the inherited enzymatic defect prevents DOC accumulation and the ensuing side effects, as was demonstrated in this study. While no serious side effects were observed in this short-term phase I clinical trial, one concern is the potential for adrenal rest expansion if ACTH is allowed to remain high. Furthermore, only adult women taking oral contraception therapy were allowed to enter this trial, because abiraterone acetate will also inhibit gonadal steroid production. Consequently, the use of this drug might be limited to pre-pubertal children and patients taking gonadal replacement therapy who do not desire fertility. In theory, CYP17A1 inhibitors might be effective in nonclassic CAH as well; however, inhibition of cortisol synthesis complicates therapy. Selective 17,20-lyase inhibitors, which would not alter the glucocorticoid pathway, might be a superior alternative to complete CYP17A1 blockade, but these drugs would also impair gonadal function.
CRH receptor antagonists
Another strategy to decrease the adrenal androgen excess is to directly prevent the hypophyseal ACTH production. This approach was recently explored with a selective CRH receptor type 1 antagonist, NBI-77860 [43**]. In a single-blind, placebo-controlled, fixed-sequence, single-dose trial, 8 women with classic 21OHD were administered placebo or NBI-77860, 300 mg or 600 mg, at 2200 h during three distinct treatment periods separated by 3-week washout intervals. NBI-77860 achieved dose-dependent reductions of ACTH and/or 17OHP in 6 of 8 participants. Relative to placebo, NBI-77860 decreased ACTH and 17OHP by a mean of 43% and 0.7% for the 300 mg dose, respectively, and by 41% and 27% for the 600 mg dose, respectively. Variable reductions of androstenedione and testosterone were noted in this single dose study. A more sustained ACTH assuaging might be required to suppress these downstream steroids, and future longer-term studies to validate this hypothesis are needed.
Conclusion
Recent years have brought some long-awaited progress in the treatment of CAH, a relatively stagnant area for decades. Some of the advances made include: 1. improved glucocorticoid delivery systems, both oral and parenteral, designed to better replicate the physiologic secretion and to diminish the exaggerated ACTH elevations; 2. the development of non-glucocorticoid therapeutic alternatives, such as inhibitors of key androgen biosynthetic enzymes and direct reduction of ACTH via CRH receptor antagonists. Each of these options has different advantages and pitfalls, as summarized in Table 1. Larger trials to determine the long-term outcomes and safety profiles of these novel therapies in both adults and children should follow. Additional, yet unexplored, treatment options are theoretically plausible solutions (Figure 2), including ACTH receptor (melanocortin type 2 receptor, MC2R) antagonists or second-generation androgen receptor antagonists (enzalutamide, ARN-509). It is important to emphasize that cortisol replacement therapy will always be required in patients with classic CAH, unless gene therapy or adrenal transplantation becomes feasible. Nevertheless, these new treatment approaches, alone or in combination, hold promise to bring additional benefits to CAH care.
Table 1.
Novel treatment strategies in congenital adrenal hyperplasia
| Treatment | Administration | Advantages | Disadvantages | |
|---|---|---|---|---|
| Glucocortiocoids | Modified-release hydrocortisone (Plenadren, Chronocort) | Twice daily | Oral administration Early morning ACTH suppression |
Potential for iatrogenic Cushing syndrome |
| Subcutaneous hydrocortisone infusion | Continuous | Close replication of circadian secretory patterns for ACTH and cortisol Potential for ultradian pulsatility replication if a real-time ACTH/cortisol monitoring is added. |
Continuous device wear Local irritation Potential for malfunctioning High fixed costs & complexity |
|
| Androgen biosynthetic enzyme blockage | CYP17A1 inhibitors (Abiraterone acetate) | Once daily | Highly effective Well-tolerated |
Does not lower ACTH Gonadal sex-steroid inhibition |
| CRH receptor type 1 antagonists | NBI-77860 | Once daily | Prevention of ACTH secretion | Long-term safety data are lacking |
Key points.
The goals of CAH treatment are to achieve adequate androgen suppression without inducing iatrogenic Cushing syndrome and associated comorbidities.
Glucocorticoid replacement can be optimized by preparations that replicate the physiologic secretion pattern, such as modified-release oral hydrocortisone and continuous subcutaneous delivery devices.
Non-glucocorticoid strategies to address the excessive androgen production in CAH include drugs that inhibit key androgen biosynthetic enzymes (CYP17A1 inhibitors) or ACTH secretion (corticotropin-releasing hormone receptor type 1 antagonists).
Long-term clinical studies of recently developed treatments and exploration of novel therapeutic strategies in both children and adults with CAH are essential to determine optimal management for the future.
Acknowledgments
Funding: This work was supported by the MICHR Pilot U046500 to AFT and by R01GM086596 to RJA. AFT was supported by 1F32DK103461-01A1.
We thank Kayla Capper for her graphic design of our figures.
This work was supported by the MICHR Pilot U046500 to AFT and by R01GM086596 to RJA. AFT was supported by 1F32DK103461-01A1.
R.J.A. discloses contracted research support from Neurocrine Biosciences and consulting fees from Janssen Pharmaceuticals and Alder Biopharmaceuticals.
Footnotes
Disclosure Statement: R.J.A. discloses contracted research support from Neurocrine Biosciences and consulting fees from Janssen Pharmaceuticals.
References
- 1.Speiser PW, White PC. Congenital adrenal hyperplasia. N Engl J Med. 2003;349:776–788. doi: 10.1056/NEJMra021561. [DOI] [PubMed] [Google Scholar]
- 2.Stikkelbroeck NM, Hermus AR, Braat DD, Otten BJ. Fertility in women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Obstet Gynecol Surv. 2003;58:275–284. doi: 10.1097/01.OGX.0000062966.93819.5B. [DOI] [PubMed] [Google Scholar]
- 3.Cabrera MS, Vogiatzi MG, New MI. Long term outcome in adult males with classic congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2001;86:3070–3078. doi: 10.1210/jcem.86.7.7668. [DOI] [PubMed] [Google Scholar]
- 4.Reisch N, Flade L, Scherr M, et al. High prevalence of reduced fecundity in men with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2009;94:1665–1670. doi: 10.1210/jc.2008-1414. [DOI] [PubMed] [Google Scholar]
- 5.Claahsen-van der Grinten HL, Otten BJ, Hermus AR, et al. Testicular adrenal rest tumors in patients with congenital adrenal hyperplasia can cause severe testicular damage. Fertil Steril. 2008;89:597–601. doi: 10.1016/j.fertnstert.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 6.Speiser PW, Azziz R, Baskin LS, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:4133–4160. doi: 10.1210/jc.2009-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veldhuis JD, Iranmanesh A, Johnson ML, Lizarralde G. Amplitude, but not frequency, modulation of adrenocorticotropin secretory bursts gives rise to the nyctohemeral rhythm of the corticotropic axis in man. J Clin Endocrinol Metab. 1990;71:452–463. doi: 10.1210/jcem-71-2-452. [DOI] [PubMed] [Google Scholar]
- 8.Weitzman ED, Fukushima D, Nogeire C, et al. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J Clin Endocrinol Metab. 1971;33:14–22. doi: 10.1210/jcem-33-1-14. [DOI] [PubMed] [Google Scholar]
- 9.Debono M, Mallappa A, Gounden V, et al. Hormonal circadian rhythms in patients with congenital adrenal hyperplasia: identifying optimal monitoring times and novel disease biomarkers. Eur J Endocrinol. 2015;173:727–737. doi: 10.1530/EJE-15-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonfig W, Pozza SB, Schmidt H, et al. Hydrocortisone dosing during puberty in patients with classical congenital adrenal hyperplasia: an evidence-based recommendation. J Clin Endocrinol Metab. 2009;94:3882–3888. doi: 10.1210/jc.2009-0942. [DOI] [PubMed] [Google Scholar]
- 11.Horrocks PM, London DR. Effects of long term dexamethasone treatment in adult patients with congenital adrenal hyperplasia. Clin Endocrinol (Oxf) 1987;27:635–642. doi: 10.1111/j.1365-2265.1987.tb02945.x. [DOI] [PubMed] [Google Scholar]
- 12.Young MC, Hughes IA. Dexamethasone treatment for congenital adrenal hyperplasia. Arch Dis Child. 1990;65:312–314. doi: 10.1136/adc.65.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Maouche D, Collier S, Prasad M, et al. Cortical bone mineral density in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Clin Endocrinol (Oxf) 2015;82:330–337. doi: 10.1111/cen.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falhammar H, Filipsson Nystrom H, Wedell A, et al. Bone mineral density, bone markers, and fractures in adult males with congenital adrenal hyperplasia. Eur J Endocrinol. 2013;168:331–341. doi: 10.1530/EJE-12-0865. [DOI] [PubMed] [Google Scholar]
- 15.Arlt W, Willis DS, Wild SH, et al. Health status of adults with congenital adrenal hyperplasia: a cohort study of 203 patients. J Clin Endocrinol Metab. 2010;95:5110–5121. doi: 10.1210/jc.2010-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finkielstain GP, Kim MS, Sinaii N, et al. Clinical characteristics of a cohort of 244 patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2012;97:4429–4438. doi: 10.1210/jc.2012-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johannsson G, Bergthorsdottir R, Nilsson AG, et al. Improving glucocorticoid replacement therapy using a novel modified-release hydrocortisone tablet: a pharmacokinetic study. Eur J Endocrinol. 2009;161:119–130. doi: 10.1530/EJE-09-0170. [DOI] [PubMed] [Google Scholar]
- 18.Johannsson G, Nilsson AG, Bergthorsdottir R, et al. Improved cortisol exposure-time profile and outcome in patients with adrenal insufficiency: a prospective randomized trial of a novel hydrocortisone dual-release formulation. J Clin Endocrinol Metab. 2012;97:473–481. doi: 10.1210/jc.2011-1926. [DOI] [PubMed] [Google Scholar]
- 19.Debono M, Ghobadi C, Rostami-Hodjegan A, et al. Modified-release hydrocortisone to provide circadian cortisol profiles. J Clin Endocrinol Metab. 2009;94:1548–1554. doi: 10.1210/jc.2008-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verma S, Vanryzin C, Sinaii N, et al. A pharmacokinetic and pharmacodynamic study of delayed- and extended-release hydrocortisone (Chronocort) vs. conventional hydrocortisone (Cortef) in the treatment of congenital adrenal hyperplasia. Clin Endocrinol (Oxf) 2010;72:441–447. doi: 10.1111/j.1365-2265.2009.03636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21**.Mallappa A, Sinaii N, Kumar P, et al. A phase 2 study of Chronocort, a modified-release formulation of hydrocortisone, in the treatment of adults with classic congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2015;100:1137–1145. doi: 10.1210/jc.2014-3809. In this three-month phase 2, open-label study of 16 adults with classic congenital adrenal hyperplasia, the modified-release hydrocortisone Chronocort led to lower androstenedione and 17-hydroxyprogesterone as compared with conventional oral therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merza Z, Rostami-Hodjegan A, Memmott A, et al. Circadian hydrocortisone infusions in patients with adrenal insufficiency and congenital adrenal hyperplasia. Clin Endocrinol (Oxf) 2006;65:45–50. doi: 10.1111/j.1365-2265.2006.02544.x. [DOI] [PubMed] [Google Scholar]
- 23.Bryan SM, Honour JW, Hindmarsh PC. Management of altered hydrocortisone pharmacokinetics in a boy with congenital adrenal hyperplasia using a continuous subcutaneous hydrocortisone infusion. J Clin Endocrinol Metab. 2009;94:3477–3480. doi: 10.1210/jc.2009-0630. [DOI] [PubMed] [Google Scholar]
- 24*.Oksnes M, Bjornsdottir S, Isaksson M, et al. Continuous subcutaneous hydrocortisone infusion versus oral hydrocortisone replacement for treatment of addison’s disease: a randomized clinical trial. J Clin Endocrinol Metab. 2014;99:1665–1674. doi: 10.1210/jc.2013-4253. In this prospective crossover, randomized, multicenter clinical trial of 33 patients with Addison disease, a continuous subcutaneous hydrocortsione infusion was superior to conventional oral substitution treatment in achieving lower ACTH concentrations and cortisol profiles closer to physiologic patterns. [DOI] [PubMed] [Google Scholar]
- 25.Gagliardi L, Nenke MA, Thynne TR, et al. Continuous subcutaneous hydrocortisone infusion therapy in Addison’s disease: a randomized, placebo-controlled clinical trial. J Clin Endocrinol Metab. 2014;99:4149–4157. doi: 10.1210/jc.2014-2433. [DOI] [PubMed] [Google Scholar]
- 26*.Russell GM, Durant C, Ataya A, et al. Subcutaneous pulsatile glucocorticoid replacement therapy. Clin Endocrinol (Oxf) 2014;81:289–293. doi: 10.1111/cen.12470. This study used a portable pulsatile continuous subcutaneous delivery system along with a real-time sampling of ACTH and cortisol in healthy individuals in whom the cortisol secretion was suppresed by dexamethasone or metirapone, and showed that both circadian and ultradian rhythmicity can be obtained. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Wyk JJ, Gunther DF, Ritzen EM, et al. The use of adrenalectomy as a treatment for congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1996;81:3180–3190. doi: 10.1210/jcem.81.9.8784066. [DOI] [PubMed] [Google Scholar]
- 28.Ogilvie CM, Rumsby G, Kurzawinski T, Conway GS. Outcome of bilateral adrenalectomy in congenital adrenal hyperplasia: one unit’s experience. Eur J Endocrinol. 2006;154:405–408. doi: 10.1530/eje.1.02096. [DOI] [PubMed] [Google Scholar]
- 29.Van Wyk JJ, Ritzen EM. The role of bilateral adrenalectomy in the treatment of congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2003;88:2993–2998. doi: 10.1210/jc.2002-022026. [DOI] [PubMed] [Google Scholar]
- 30.Tiosano D, Vlodavsky E, Filmar S, et al. Ovarian adrenal rest tumor in a congenital adrenal hyperplasia patient with adrenocorticotropin hypersecretion following adrenalectomy. Horm Res Paediatr. 2010;74:223–228. doi: 10.1159/000295722. [DOI] [PubMed] [Google Scholar]
- 31.Crocker MK, Barak S, Millo CM, et al. Use of PET/CT with cosyntropin stimulation to identify and localize adrenal rest tissue following adrenalectomy in a woman with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2012;97:E2084–2089. doi: 10.1210/jc.2012-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charmandari E, Chrousos GP, Merke DP. Adrenocorticotropin hypersecretion and pituitary microadenoma following bilateral adrenalectomy in a patient with classic 21-hydroxylase deficiency. J Pediatr Endocrinol Metab. 2005;18:97–101. doi: 10.1515/jpem.2005.18.1.97. [DOI] [PubMed] [Google Scholar]
- 33.Bry-Gauillard H, Cartes A, Young J. Mitotane for 21-hydroxylase deficiency in an infertile man. N Engl J Med. 2014;371:2042–2044. doi: 10.1056/NEJMc1410041. [DOI] [PubMed] [Google Scholar]
- 34.Else T, Kim AC, Sabolch A, et al. Adrenocortical carcinoma. Endocr Rev. 2014;35:282–326. doi: 10.1210/er.2013-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chortis V, Taylor AE, Schneider P, et al. Mitotane therapy in adrenocortical cancer induces CYP3A4 and inhibits 5α-reductase, explaining the need for personalized glucocorticoid and androgen replacement. J Clin Endocrinol Metab. 2013;98:161–171. doi: 10.1210/jc.2012-2851. [DOI] [PubMed] [Google Scholar]
- 36.Sbiera S, Leich E, Liebisch G, et al. Mitotane Inhibits Sterol-O-Acyl Transferase 1 Triggering Lipid- Mediated Endoplasmic Reticulum Stress and Apoptosis in Adrenocortical Carcinoma Cells. Endocrinology. 2015;156:3895–3908. doi: 10.1210/en.2015-1367. [DOI] [PubMed] [Google Scholar]
- 37.Potter GA, Barrie SE, Jarman M, Rowlands MG. Novel steroidal inhibitors of human cytochrome P45017α (17α-hydroxylase-C17,20-lyase): potential agents for the treatment of prostatic cancer. J Med Chem. 1995;38:2463–2471. doi: 10.1021/jm00013a022. [DOI] [PubMed] [Google Scholar]
- 38.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–160. doi: 10.1016/S1470-2045(14)71205-7. [DOI] [PubMed] [Google Scholar]
- 41**.Auchus RJ, Buschur EO, Chang AY, et al. Abiraterone acetate to lower androgens in women with classic 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2014;99:2763–2770. doi: 10.1210/jc.2014-1258. This phase 1 nonrandomized, open-label, multiple-dose, clinical study used a CYP17A1 inhibitor, abiraterone acetate, to block androgen synthesis in adult women with 21-hydroxylase deficiency. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Attard G, Reid AH, Auchus RJ, et al. Clinical and biochemical consequences of CYP17A1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancer. J Clin Endocrinol Metab. 2012;97:507–516. doi: 10.1210/jc.2011-2189. [DOI] [PubMed] [Google Scholar]
- 43**.Turcu AF, Spencer-Segal JL, Farber RH, et al. Single-Dose Study of a Corticotropin-Releasing Factor Receptor-1 Antagonist in Women with 21-Hydroxylase Deficiency. J Clin Endocrinol Metab. 2016 doi: 10.1210/jc.2015-3574. jc20153574 (in press). In this proof of concept single-blind, placebo-controlled, fixed-sequence, single-dose trial of adult women with 21-hydroxylase deficiency, the CRH receptor type 1 antagonist NBI-77860 decreased ACTH and 17-hydroxyprogesterone in 6 of 8 participants. [DOI] [PMC free article] [PubMed] [Google Scholar]