Abstract
Macrophages, via activation of the Toll-like receptors (TLR4), play an important role in the pathogenesis of hypertension and associated end-organ damage. There is accumulating evidence to suggest a protective role of the angiotensin AT2 receptor (AT2R) in pathological conditions involving inflammation and tissue injury. We have recently shown that AT2R stimulation is renoprotective, in part, via increased anti-inflammatory interleukin-10 (IL-10) production in renal epithelial cells, however the role of AT2R in macrophage inflammatory behavior is not known. The present study was designed to investigate whether AT2R activation exerts an anti-inflammatory response in TLR4-induced inflammation. The anti-inflammatory mechanisms of AT2R agonist C21 (1 µmol/ml) pre-treatment on the cytokine profile of THP-1 macrophages after activation by LPS (1 µg/ml) was studied. The AT2R agonist dose-dependently attenuated LPS-induced TNF-α and IL-6 production but increased IL-10 production. IL-10 was critical for the anti-inflammatory effect of AT2R stimulation, since IL-10 neutralizing antibody dose-dependently abolished the AT2R–mediated decrease in TNF-α level. Further, the enhanced IL-10 levels were associated with a sustained, selective increase in phosphorylation of extracellular signal-regulated kinase (ERK1/2), but not p38 MAPK. Blocking the activation of ERK1/2 prior to C21 pre-treatment completely abrogated this increased IL-10 production in response to AT2R agonist C21, while there was a partial reduction in IL-10 levels on inhibition of p38. We conclude that AT2R stimulation exerts a novel anti-inflammatory response in THP-1 macrophages via enhanced IL-10 production as a result of sustained, selective ERK1/2 phosphorylation, and thus may have protective role in hypertension and associated tissue injury.
Keywords: AT2 receptor, inflammation, macrophage, Interleukin-10
INTRODUCTION
Chronic inflammation, characterized by elevated cytokine and chemokine expression, has been shown to play a central role in the pathophysiology of hypertension and associated endorgan damage, including renal injury1–4. At the cellular level, chronic inflammation is mediated largely by macrophages. Also, macrophage infiltration invariably accompanies hypertensive organ damage, such as that to the blood vessels5, the heart6 and the kidneys7. Moreover, circulating monocytes are activated in hypertensive patients and produce increased amounts of pro-inflammatory cytokines, including tumor necrosis factor- α (TNF-α), interleukin-1 (IL-1) and transforming growth factor- β (TGF-β)8. In fact, recent evidence indicates that, rather than being a mere consequence of elevated blood pressure, macrophage activation actually serves as a causative factor in the development of hypertension9, 10.
The renin-angiotensin system (RAS) is a critical hormonal system that regulates blood pressure and is abnormally activated in hypertensive patients. Macrophages also express all major components of the RAS11. Angiotensin II (Ang II), via the AT1 receptor has been demonstrated to participate in the activation and pro-inflammatory polarization of leukocytes12–14. However, the precise cellular mechanisms are not well defined. One potential inflammatory pathway that has been implicated in hypertension is activation of innate immune receptors, specifically Toll-like receptor-4 (TLR4) signaling15, which leads to the production of an array of pro-inflammatory mediators. In fact, peripheral monocyte TLR4 expression is markedly increased in hypertensive patients compared to normotensive controls16. Further, Ang II via the AT1 receptor has been shown to up-regulate TLR4 expression17 and exacerbate pro-inflammatory cytokine production in response to TLR4 activation by lipopolysaccharide (LPS) in macrophages18. Accumulating evidence suggests that the AT2 receptor, which is generally considered to be a functional antagonist of the AT1 receptor, exerts an anti-inflammatory response19–21. We have previously shown that stimulation of AT2 receptor by the selective agonist Compound 21 (C21) attenuates pro-inflammatory signaling in response to LPS-activation of proximal tubule epithelial cells via increased IL-10 production22. However, whether AT2 receptor can induce IL-10 production and exert an anti-inflammatory response in LPS-activated macrophages has not been investigated.
In macrophages, multiple pathways exist to promote IL-10 production23. Activation of mitogen-activated protein kinases (MAPKs), specifically via p38 and extracellular signal-regulated kinase (ERK-1/2), has been shown to be required to increase IL-10 in macrophages and inhibition of either MAPK blunts IL-10 production24–28. Moreover, high ERK1/2 activation correlates well with the extent of IL-10 production in this cell-type23. Since the AT2 receptor has been linked to a sustained increase in ERK1/2 phosphorylation, it is possible that this might be a potential molecular mechanism by which the AT2 receptor agonist can enhance IL-10 production in macrophages.
The present study was designed to test the hypothesis that stimulation of the AT2 receptor attenuates TLR4-mediated pro-inflammatory cytokine production in macrophages via increased IL-10 production. We evaluated the effect of C21 on the production of TNF-α, IL-6 and IL-10 in LPS-activated THP-1 macrophages. We demonstrate that pre-treatment with C21 significantly attenuated the levels of pro-inflammatory cytokines. This was found to be dependent on increased IL-10 production in response to AT2 receptor activation. Moreover, this up-regulation of IL-10 is mediated via a sustained, selective increase in ERK1/2 phosphorylation.
MATERIALS AND METHODS
Cell culture and treatments
The human THP-1 monocytic cell-line (ATCC) was cultured in RPMI-1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS) and antibiotic/antimycotic cocktail (penicillin 100U/ml, streptomycin 100µg/ml and Amphotericin B 250 ng/ml) at 37°C in a humidified atmosphere with 5% CO2. Cell culture media and supplements were all purchased from (HyClone, ThermoFisher Scientific, Inc.). To differentiate monocytes to macrophages, 5×105 cells/well were treated with 40 nmol/L phorbol 12-myristate 13-acetate (PMA) (Sigma) for 48 hours in RPMI-1640 containing 5% FBS. At the end of the 48 hour incubation period, the medium was aspirated and cells were washed with RPMI-1640 without FBS and antibiotic/antimycotic and were incubated in this medium for 6–8 hours. To eliminate pre-existing cytokine production, the medium was replaced by fresh serum free medium before treatments were initiated. LPS (1µg/ml) (E.coli O55:B5, Sigma) was used to induce the production of pro-inflammatory cytokines. Cells were pre-treated with C21 (Custom synthesized) 60 minutes prior to the addition of LPS and the drug remained in the medium for the entire duration of treatment. Treatments with specific inhibitors including Candesartan (1µmol/l) (AstraZeneca) PD123319 (10µmol/l) (Pfizer), SB203580 (10µmol/l) (Cell Signaling Technology) and PD98059 (10µmol/l) (Cell Signaling Technology) were carried out as indicated in the text and figure legends.
Immunoblotting
Macrophages were washed twice with PBS and lysed on the plate using ice-cold cell lysis buffer (Cell Signaling Technology) containing protease (Roche) and phosphatase (Sigma) inhibitor cocktails. Equal amounts of protein in Laemmli buffer were loaded per well (15 µg/lane for AT1R, 45 µg/lane for AT2R and 30 µg/lane for ERK1/2 and p38 MAPK) and separated by SDSPAGE using a Tris-Glycine system. Proteins were then transferred to a PVDF membrane using the wet transfer protocol. The membrane was incubated in 5% non-fat dry milk in PBST for 1 hour, following overnight incubation with primary antibodies for AT1R (Biomolecular Integrations), AT2R (EZ Biolabs, Inc), p-ERK1/2 (Cell Signaling Technology) and p-p38 MAPK (Cell Signaling Technology) at a 1:1000 dilution. This was followed by washing with PBST and 1 hour incubation with appropriate HRP-conjugated secondary antibodies (Santa Cruz Biotechnology, Inc). Chemiluminesence was detected by the addition of Luminol HRP substrate (Santa Cruz Biotechnology, Inc.) and quantified by software-assisted densitometric analysis (Alpha Innotech Corp.). To ensure equal loading, blots were stripped and re-probed for β-Actin (BioVision) for AT1R and AT2R, total-p38 and total-ERK1/2 for p-p38 and p-ERK1/2, respectively (Cell Signaling Technology).
ELISA
Cytokines in the medium were assessed by kit-based ELISA using the manufacturers protocols (R&D Systems).
mRNA Expression by RT-PCR
Total RNA from the cells was extracted using the RNEasy kits (Qiagen) according to the manufacturer’s protocol. cDNA was synthesized by RT-PCR from 1 µg of total RNA using the SuperScript III First-Strand Synthesis System (Life Technologies). This cDNA was used as a template for the quantitative RT-PCR analysis of gene expressions of TNFA, IL6 and IL10 using TaqMan gene expression assays (Applied Biosystems). Relative quantification was determined using the delta-delta Ct method with GAPDH as a control.
Statistical Analysis
Data are presented as means ±SEM. Student’s t-test was used to compare means of two groups. One-way ANOVA with post-hoc test (Tukey) for multiple comparisons was used to compare variations between more than 2 groups. A value of p<0.05 was considered statistically significant, with n=5–8 experiments per group.
RESULTS
Expression of angiotensin AT1 and AT2 receptors in THP-1 macrophages
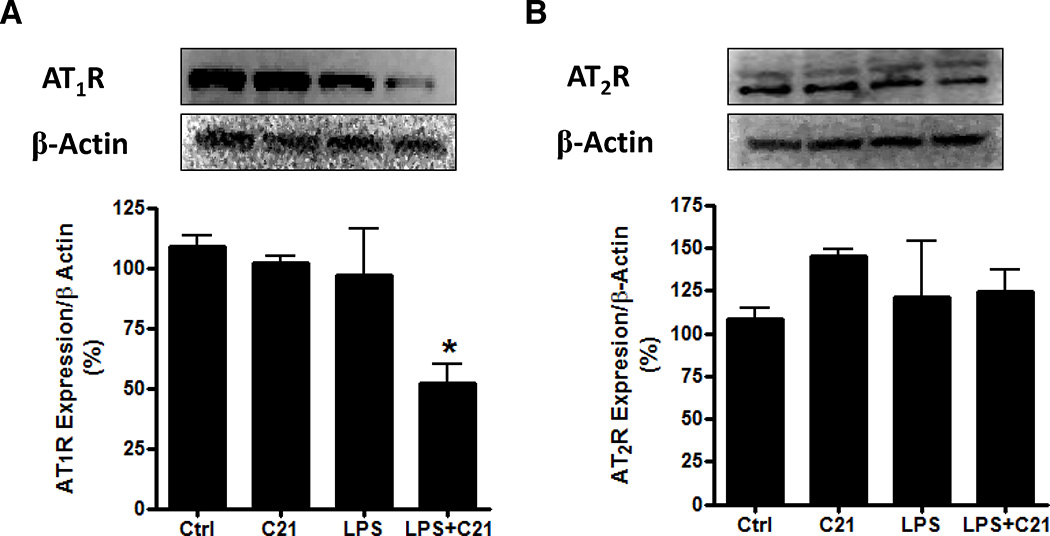
THP-1 macrophages express both, AT1R and AT2R. The expression of both receptor subtypes was not altered by C21 or LPS treatment (Figure 1A, B); however C21 pre-treatment lowered AT1R expression by ~50% in response to LPS (Figure 1A), which is in agreement with a number of reports demonstrating the AT2R-mediated down-regulation of AT1R in pathophysiological conditions29–31.
Figure 1. Expression of angiotensin II receptors in THP-1 macrophages.
Protein expression by immunoblotting of angiotensin AT1 (A) and AT2 (B) receptors in THP-1 macrophages after 24 hours treatment with vehicle (Ctrl), AT2R agonist C21 (C21; 1µmol/l), LPS (1µg/ml) or both LPS+C21. Cells were pre-treated with C21 (1µmol/l) for 60 minutes prior to LPS-activation. Data are represented as mean ± SEM. * indicates p<0.05 vs Ctrl THP-1 macrophages (n=5).
Effect of AT2R agonist (C21) and AT1R antagonist (candesartan) on cytokine production by LPS-activated THP-1 macrophages
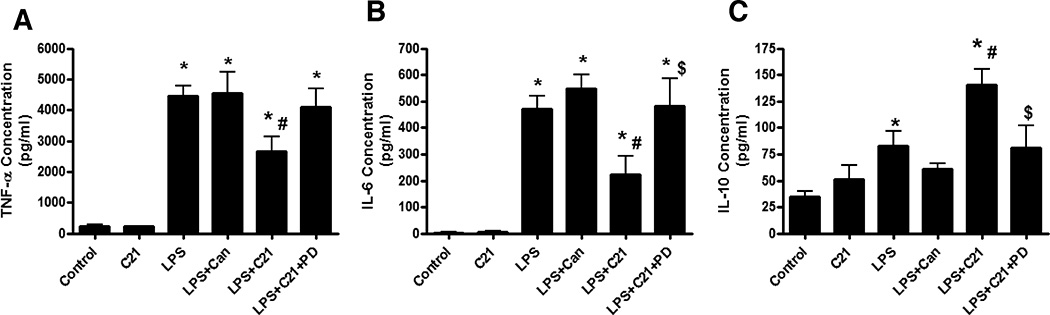
THP-1 Macrophages were treated with LPS (1 µg/ml) for 24 hours to induce the expression of pro-inflammatory cytokines (TNF-α and IL-6) and anti-inflammatory cytokine, IL-10. Pre-treatment with AT2R agonist C21 (1 µmol/L) attenuated the LPS-induced TNF-α and IL-6 production by ~33% and ~50%, respectively, with a concurrent 75% increase in IL-10 production. This anti-inflammatory response was blocked by AT2R antagonist PD123319 (10 µmol/L), suggesting an AT2 receptor-mediated effect. However, AT1R antagonist candesartan (1 µmol/L) pre-treatment did not alter the cytokine levels in response to LPS (Figure 2 A–C).
Figure 2. Effect of AT2R agonist (C21) and AT1R antagonist (Candesartan) on cytokine production by LPS-activated THP-1 macrophages.
THP-1 macrophages were incubated with either AT2R agonist (C21; 1µmol/l) or AT1R antagonist Candesartan (Can; 1µmol/l) for 60 minutes prior to activation with LPS (1µg/ml). To demonstrate the specificity of C21, an additional group of cells were incubated with AT2R antagonist PD123319 (PD; 10µmol/l) for 30 minutes prior to C21 pre-treatment. The cytokines TNF-α (A), IL-6 (B) and IL-10 (C) were assessed in the media 24 hours after LPS-activation by ELISA. Data are represented as mean ± SEM. * indicates p<0.05 vs Ctrl, # indicates p<0.05 vs LPS treated and $ indicates p<0.05 vs LPS+C21 treated THP-1 macrophages (n=6).
Anti-inflammatory effect of AT2R agonist (C21) on cytokine production by LPS-activated THP-1 macrophages is mediated via increased IL-10 production
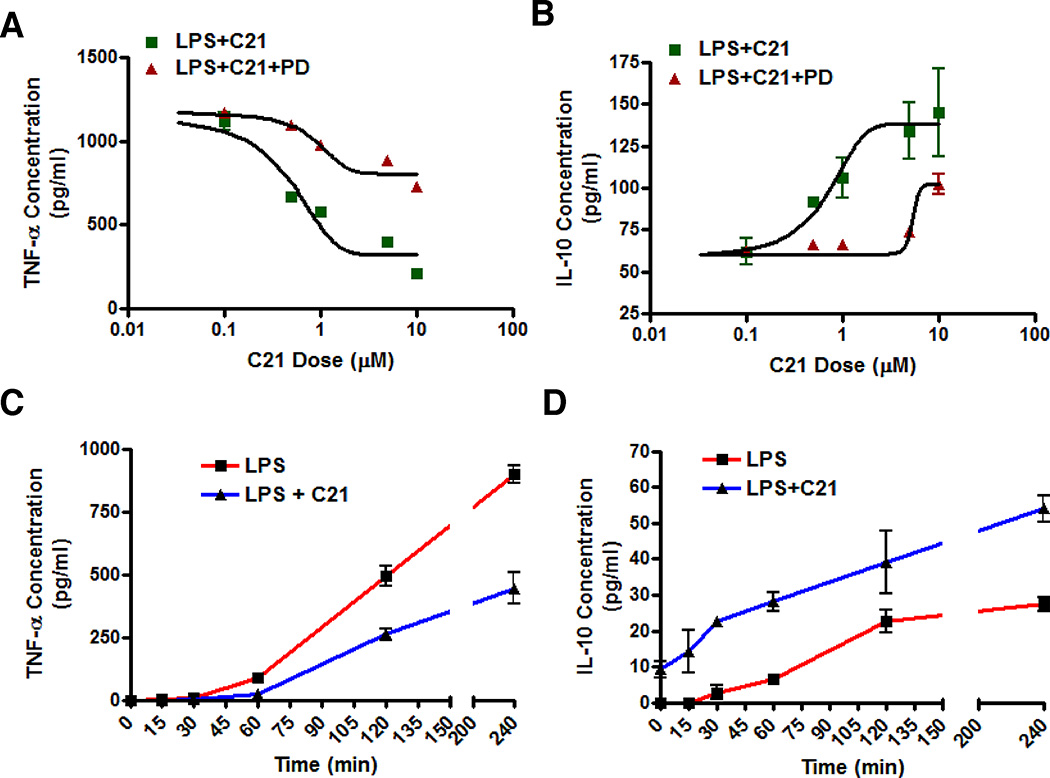
AT2R agonist C21 pre-treatment dose dependently (0.1–10 µmol/L) attenuated the production of TNF-α (Figure 3A) while IL-10 production was concurrently enhanced (Figure 3B) in LPS-activated THP-1 macrophages 24 hours post-LPS. Pre-treatment with C21 before LPS-activation resulted in lower TNF-α levels starting as early as 60 minutes post-LPS and this trend continued up to 4 hours (Figure 3C). Cells pre-treated with C21 for 1 hour produced measurable amounts of antiinflammatory IL-10 at the time of addition of LPS and the IL-10 levels continued to be higher up to 4 hours compared to LPS treated control cells (Figure 3D). Thus, AT2R agonist exerts an antiinflammatory effect on early cytokine production in THP-1 macrophages and this response is dose-dependent.
Figure 3. Dose-dependent and time-dependent effects of AT2R agonist (C21) on cytokine production by LPS-activated THP-1 macrophages.
The dose-dependent effect of AT2R agonist C21 on TNF-α (A) and IL-10 (B) was assessed at 24 hours following LPS-activation (1µg/ml). C21 pre-treatment (0.1–10µmol/l) was given 60 minutes before LPS and cytokines in the media were assessed by ELISA. An additional set of cells was incubated with AT2R antagonist PD123319 (PD; 10µmol/l) for 30 minutes prior to C21 pre-treatment to demonstrate the receptor specificity of C21. The TNF-α (C) and IL-10 (D) production at earlier time points was also determined in the media at the indicated times after LPS-activation following C21 (1µmol/ml) pretreatment. Data are represented as mean ± SEM. * indicates p<0.05 vs respective control (n=6).
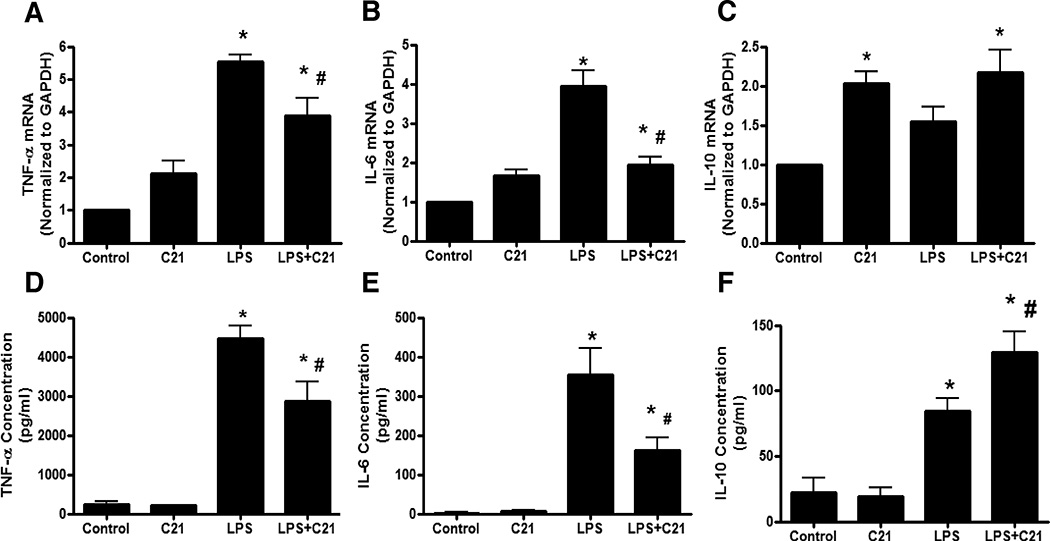
Cytokine production at the level of mRNA and protein released in the media was assessed at 24 hours post-LPS activation to determine the effect of chronic stimulation of AT2R. C21 pre-treatment significantly attenuated pro-inflammatory TNF-α and IL-6 mRNA as well as protein expression (Figure 4A, 4B, 4D, 4E). Conversely, IL-10 in the media was significantly higher in C21 pre-treated cells after LPS stimulation (Figure 4F). Interestingly, IL-10 mRNA expression was higher in C21 treated cells, even in the absence of LPS activation (Figure 4C), though this did not translate to higher IL-10 protein expression in this treatment group (Figure 4F). Further, a neutralizing antibody to IL-10 dose-dependently ameliorated the effect of C21 on LPS-induced TNF-α production (Figure 5). Thus, the increase in IL-10 production in response to C21 in the presence of LPS appears to be critical for attenuating the anti-inflammatory response to LPS.
Figure 4. Effect of AT2R agonist (C21) on mRNA and protein expression of cytokines by LPS-activated THP-1 macrophages.
Macrophages were treated with vehicle (Control), C21 (1µmol/ml), LPS (1µg/ml) or both, LPS and C21 (LPS+C21). C21 (1µmol/l) pre-treatment was started 60 minutes prior to LPS-activation. Expression of TNF-α, IL-6 and IL-10 at the level of mRNA (A, B, C) was determined by RT-PCR while TNF-α, IL-6 and IL-10 protein was quantified in the media (D, E, F) by ELISA 24 hours after LPS-activation. Data are represented as mean ± SEM. * indicates p<0.05 vs Control and # indicates p<0.05 vs LPS treated THP-1 macrophages (n=5–8).
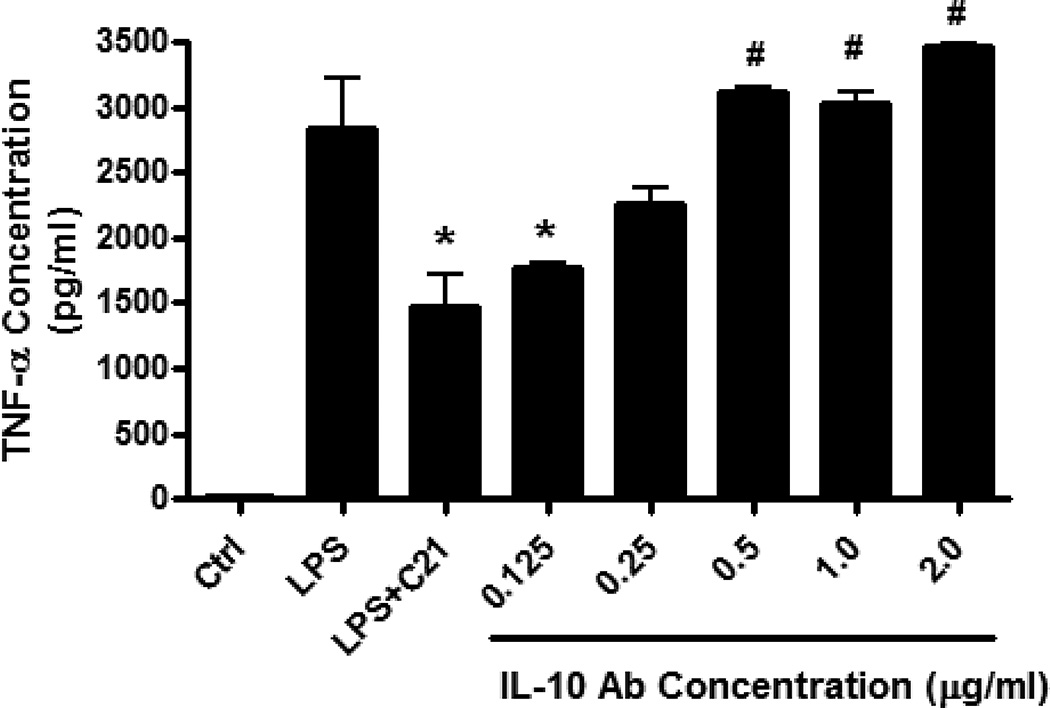
Figure 5. Dose-dependent inhibition of on the anti-inflammatory effect of AT2R agonist (C21) on TNF- α production by neutralizing interleukin-10 (IL-10) antibody.
Cells were incubated with increasing doses of neutralizing IL-10 antibody (0.125–2.5µg/ml) for 30 minutes prior to C21 pre-treatement (1µmol/l). Cells were activated with LPS (1µg/ml) 60 minutes after addition of C21. Cytokine concentrations in media were measured by ELISA. Data are represented as mean ± SEM. * indicates p<0.05 vs LPS, # indicates p<0.05 vs LPS+C21 treated THP-1 macrophages (n=5).
Involvement of p38 and ERK1/2 MAPKs in AT2R-mediated increase in IL-10 production
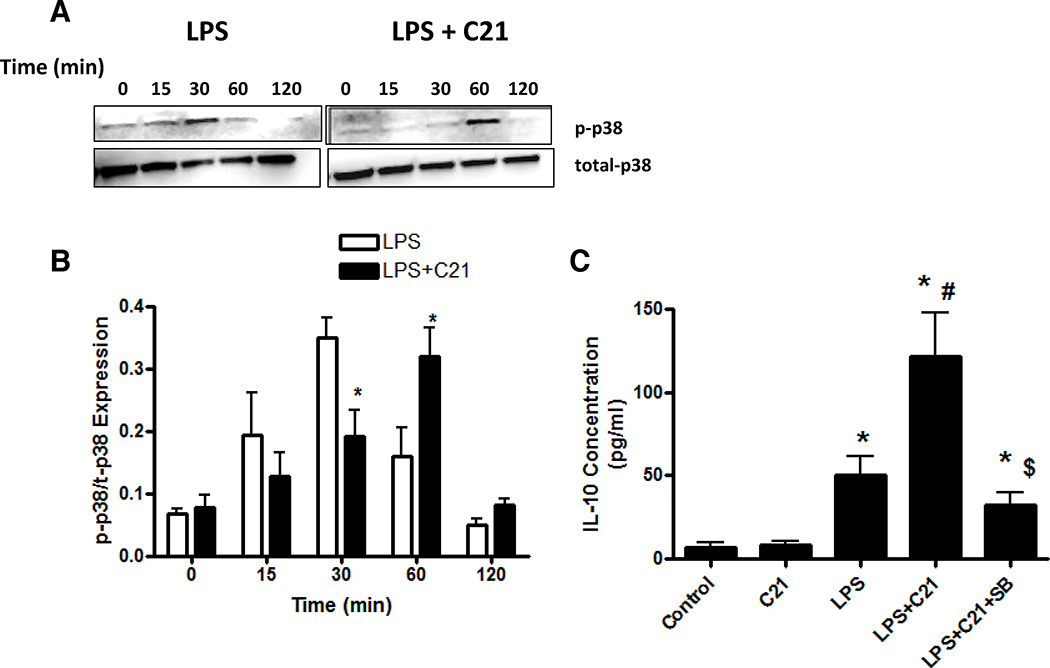
The activation of MAPKs, specifically p38 and ERK1/2, has been shown to be a key requirement for the regulation of IL-10 production in macrophages25, 28, 32, 33. LPS treatment led to a rapid phosphorylation of p38 and ERK1/2 which peaked at 30 minutes post-LPS and returned to basal levels by 2 hours post-LPS (Figure 6A,B 7A,B). C21 pre-treatment delayed the peak of p38 phosphorylation to 1 hour post-LPS (Figure 6A,B). Incubation with a p38 inhibitor (SB203580; 10µmol/L) prior to C21 pre-treatment partially abolished the C21-mediated increase in IL-10 at 24 hours post-LPS activation (Figure 6C).
Figure 6. Effect of pre-treatment with AT2R agonist (C21) on the phosphorylation of p38 MAPK.
The time course of p38 phosphorylation in response to LPS-activation with or without C21 was determined by immunoblotting (A) and the ratio of phophorylated to toal p38 was quantified (B). C21 pre-treatment (1µmol/l) was started 60 minutes prior to the addition of LPS (1µg/ml). Times indicated are after the addition of LPS. The effect of p38 inhibition on IL-10 production was also assessed (C). Macrophages were activated with LPS (1µg/ml) 60 minutes after addition of C21 (1µmol/l). An additional set of cells was pre-treated with p38 inhibitor SB203580 (10µmol/l) for 60 minutes before C21 was added. Cytokine concentrations in media were measured by ELISA 24 hours after LPS treatment. Data are represented as mean ± SEM. * indicates p<0.05 vs Ctrl, # indicates p<0.05 vs LPS treated and $ indicates p<0.05 vs LPS+C21 treated THP-1 macrophages (n=6).
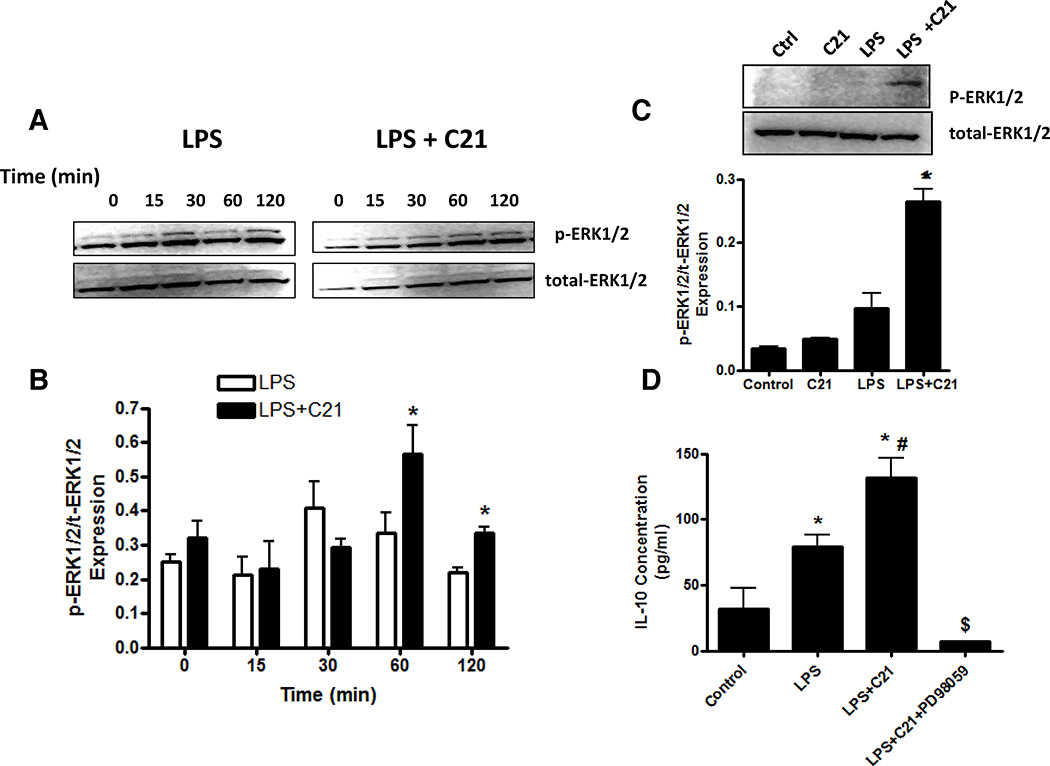
Figure 7. Effect of pre-treatment with AT2R agonist (C21) on the phosphorylation of ERK1/2.
The time course of ERK1/2 phosphorylation in response to LPS-activation with or without C21 was determined by immunoblotting (A) and the ratio of phophorylated to total ERK1/2 was quantified (B). C21 pre-treatment (1µmol/l) was started 60 minutes prior to the addition of LPS (1µg/ml). Times indicated are after the addition of LPS. To assess whether AT2R agonist led to a sustained increase in ERK1/2 phosphorylation, macrophages were activated with LPS (1µg/ml) 60 minutes after addition of C21 (1µmol/l) and cells were collected after 24 hours post-LPS treatment. Subsequently, the ratio of phophorylated to total ERK1/2 was quantified by immunoblotting (C). The effect of inhibition of ERK1/2 phosphorylation on IL-10 production was also assessed (D). Macrophages were activated with LPS (1µg/ml) 60 minutes after addition of C21 (1µmol/l). An additional set of cells was pre-treated with MEK inhibitor PD98059 (10µmol/l) for 60 minutes before C21 was added. Cytokine concentrations in media were measured by ELISA 24 hours after LPS treatment. Data are represented as mean ± SEM. * indicates p<0.05 vs Ctrl, # indicates p<0.05 vs LPS treated and $ indicates p<0.05 vs LPS+C21 treated THP-1 macrophages (n=6).
On the other hand, C21 pre-treatment resulted in a delayed, sustained increase in ERK1/2 phosphorylation (Figure 7A,B), which persisted up to 24 hours post-LPS treatment (Figure 7C). Moreover, pre-incubation of cells with a MEK inhibitor (PD98059; 10 µmol/L), which prevents ERK1/2 activation, prior to C21 pre-treatment completely prevented the C21-mediated increase in IL-10 at 24 hours post-LPS activation (Figure 7D), suggesting that the sustained, selective ERK1/2 phosphorylation associated with AT2R agonist is essential for the increase in IL-10 production. C21 treatment alone did not induce ERK1/2 phosphorylation at 24 hours (Figure 7C) or earlier time points (data not shown), suggesting that LPS-mediated signaling is required for C21 to exert its anti-inflammatory effect.
DISCUSSION
Macrophages, via activation of the TLR4-mediated pro-inflammatory signaling, play an important role in the initiation and progression of hypertension and associated end-organ damage. Macrophages express AT2 receptors11, which have been demonstrated to attenuate TLR-mediated pro-inflammatory cytokine production in proximal tubule epithelial cells22. In the present study, we have demonstrated that pre-treatment of THP-1 macrophages with AT2R agonist C21 attenuates TNF-α and IL-6 production in response to activation of TLR4 by LPS. This effect is mainly a result of increased IL-10 production by macrophages via a sustained, selective increase in ERK1/2 phosphorylation.
Ang II via the AT1 receptor has been documented to promote pro-inflammatory cytokine and chemokine production in a manner similar to TLR4-mediated signaling in a number of tissues including endothelial cells34, 35, renal tubular epithelial cells36, dendritic cells37–39, and T lymphocytes40–42. However, its precise role in macrophages/monocytes is controversial. Incubation of monocytes with Ang II has been shown to induce the expression of the chemokine monocyte chemoattractant protein-1 (MCP-1)43, while no effect was observed on the production of cytokines44. The RAS is up-regulated during monocyte differentiation and macrophages express a relatively higher level of AT1 and AT2 receptors compared to monocytes11. Incubation of macrophages with Ang II via AT1R activation resulted in increased IL-6 production, without affecting TNF-α18, 45. Further, the interaction between Ang II and enhanced TLR4 signaling has been reported17, 46–48. However, interpretation of these findings has been made complicated by the fact that the commonly used AT1R blockers, including losartan and candesartan, have been found to exert anti-inflammatory actions independent of the AT1R18, 44.
Conversely, the anti-inflammatory role of the AT2R, which is generally considered to act as a functional antagonist of the AT1R, has been demonstrated in a number of in vitro and in vivo models19–22, 49. Here, we report that at higher concentration of LPS (1 µg/ml compared to 50 ng/ml used by Larrayoz et al.44), candesartan was ineffective in lowering pro-inflammatory cytokine production while AT2R agonist C21 significantly lowered both, TNF-α and IL-6 which was associated with an increase in the anti-inflammatory cytokine IL-10 production. Since this alteration in cytokine profile could be blocked by AT2R antagonist PD123319, we conclude this anti-inflammatory effect was a specific AT2 receptor mediated response. We found that pre-treatment with C21 in the presence of LPS also attenuated AT1R expression. The down-regulation of AT1R in response to AT2R stimulation under pathophysiological conditions has been reported in a number of experimental models29–31. In the present study, however, this observation may be unrelated to the anti-inflammatory response to AT2R agonist since the increase in pro-inflammatory cytokine levels did not appear to be mediated via AT1R activation.
We have previously shown that AT2R stimulation resulted in enhanced IL-10 secretion by proximal tubule epithelial cells22. A similar observation was reported in a specific subset of splenic CD8+AT2R+ T cells which produced uncharacteristically high amounts of IL-10 and AT2R stimulation by Ang II as well as by C21 further augmented the IL-10 production50. Here we report that C21 alone increased the IL-10 gene expression, however, this did not translate to increased IL-10 protein secretion, except in the presence of TLR4 activation by LPS. This could be a result of post-transcriptional modifications to IL-10 mRNA that have been shown to occur in immune cells as a means of regulation of IL-10 production in the absence of an inflammatory stimulus51.
Though there is considerable evidence to suggest an anti-inflammatory effect of AT2 receptor stimulation, the signaling pathways involved in mediating this response lack clear definition and are still a subject of debate. Moreover, the cell-types and experimental conditions greatly influence the downstream signaling cascades activated by the AT2R. Typically, AT2R stimulation results in the activation of phosphatases, including MAP kinase phosphatase-1 (MKP-1)52–54 and SH-2 domain containing phosphatase-1 (SHP-1)55–57, which ultimately leads to AT2R-mediated apoptosis. On the other hand, AT2R stimulation has also shown to promote cellular differentiation via a sustained increase in ERK1/2 phosphorylation58–61 which is independent of cAMP-mediated signaling62. In the present study, AT2R agonist pre-treatment resulted in a delayed increase in ERK1/2 phosphorylation which was sustained up to 24 hours post-LPS activation, however, AT2R agonist alone did not promote ERK1/2 phosphorylation at any of the time points studied, nor was IL-10 detectable in the medium. Thus, it appears that LPS-mediated signaling pathways are required for the augmented IL-10 production by AT2R agonist. It may be speculated that C21 pre-treatment ‘primes’ macrophages such that in the presence of an activating signal such as LPS, their polarization to the ‘alternatively activated’, anti-inflammatory M2 phenotype is favored over the pro-inflammatory, ‘classically activated’ M1 phenotype.
In macrophages, multiple pathways exist that can regulate the production of IL-10 depending upon the activating stimulus28, 63–66. Of these, activation of p38 and ERK1/2 MAPKs has been shown to be critical for induction of IL-10 synthesis23–28. We report that inhibition of p38 activation partially abrogated the AT2R-mediated increase in IL-10 while inhibition of ERK1/2 activation resulted in a complete lack of IL-10 production in response to AT2R stimulation, suggesting that p38 MAPK may contribute to, but is not essential for AT2R-mediated IL-10 expression. This observation could also be linked to the altered kinetics of p38 MAPK phosphorylation in response to LPS in the presence and absence of AT2R agonist pretreatment. However, the precise mechanisms that orchestrate these changes in time course of MAPK phosphorylation require further investigation.
Over the past decade, AT2R stimulation has emerged as a potential therapeutic target for the treatment of hypertension and end-organ damage21, 22, 29, 67, 68, particularly with concomitant AT1R blockade69, 70. Further, AT2R activation at the level of the kidney has been shown to promote vasodilation and natriuresis, thus affording renoprotection in the setting of hypertension29, 71, 72. Though administration of C21 has been shown to have a modest, if any, effect on lowering blood pressure21, 68, 73–75, its protective effects on inflammation, oxidative stress, fibrosis and vascular remodeling underscore the potential benefit of the addition of an AT2R agonist to the currently used classical anti-hypertensive drugs to retard the progression of hypertension and end-organ damage. Here, we identify macrophages, which play a central role in the initiation and progression of hypertension-associated target organ damage, as an additional target of AT2R stimulation.
In conclusion, the present study demonstrates a novel anti-inflammatory role for AT2R stimulation in macrophages involving the attenuation of TLR4-mediated pro-inflammatory cytokine production. We further demonstrate that ERK1/2-dependent increased IL-10 production is a key event involved in mediating this anti-inflammatory response. Thus, it may be speculated that stimulation of the AT2 receptor may be beneficial in hypertension owing to its protective effects not only on hemodynamic factors, such as vasodilation and Na+ excretion, but also as a consequence of attenuation of inflammation and associated end-organ injury.
Acknowledgments
This study was supported by grant R01 DK-61578 from the National Institutes of Health to TH. The authors also wish to thank Dr. Jianzhong Shen (Auburn University) for providing THP-1 cells. PD123319 was a generous gift from Pfizer, Inc. Candesartan was a generous gift from AstraZeneca, Inc.
Footnotes
CONFLICT OF INTREST/ DISCLOSURE STATEMENT
None
REFERENCES
- 1.Bautista LE. Inflammation, endothelial dysfunction, and the risk of high blood pressure: Epidemiologic and biological evidence. J Hum Hypertens. 2003;17:223–230. doi: 10.1038/sj.jhh.1001537. [DOI] [PubMed] [Google Scholar]
- 2.Chrysohoou C, Pitsavos C, Panagiotakos DB, Skoumas J, Stefanadis C. Association between prehypertension status and inflammatory markers related to atherosclerotic disease: The ATTICA Study. Am J Hypertens. 2004;17(7):568–573. doi: 10.1016/j.amjhyper.2004.03.675. [DOI] [PubMed] [Google Scholar]
- 3.Heijnen BFJ, Essen H, Schalkwijk CG, Janssen BJA, Struijker-Boudie HAJ. Renal inflammatory markers during the onset of hypertension in spontaneously hypertensive rats. Hypertens Res. 2014;37:100–109. doi: 10.1038/hr.2013.99. [DOI] [PubMed] [Google Scholar]
- 4.Stumpf C, John S, Jukic J, Yilmaz A, Raaz D, et al. Enhanced levels of platelet P-selectin and circulating cytokines in young patients with mild arterial hypertension. J Hypertens. 2005;23:995–1000. doi: 10.1097/01.hjh.0000166840.63312.12. [DOI] [PubMed] [Google Scholar]
- 5.Clozel M, Kuhn H, Hefti F, Baumgartner HR. Endothelial dysfunction and subendothelial monocyte macrophages in hypertension. Effect of angiotensin converting enzyme inhibition. Hypertension. 1991;18:132–141. doi: 10.1161/01.hyp.18.2.132. [DOI] [PubMed] [Google Scholar]
- 6.Haller H, Behrend M, Park JK, Schaberg T, Luft FC, Distler A. Monocyte infiltration and c-fms expression in hearts of spontaneously hypertensive rats. Hypertension. 1995;25:132–138. doi: 10.1161/01.hyp.25.1.132. [DOI] [PubMed] [Google Scholar]
- 7.Johnson RJ, Alpers CE, Yoshimura A, Lombardi D, Pritzl P, Floege J, et al. Renal injury from angiotensin II-mediated hypertension. Hypertension. 1992;19:464–474. doi: 10.1161/01.hyp.19.5.464. [DOI] [PubMed] [Google Scholar]
- 8.Chen NG, Abbasi F, Lamendola C, McLaughlin T, Cooke JP, Tsao PS, et al. Mononuclear cell adherence to cultured endothelium is enhanced by hypertension and insulin resistance in healthy nondiabetic volunteers. Circulation. 1999;100:940–943. doi: 10.1161/01.cir.100.9.940. [DOI] [PubMed] [Google Scholar]
- 9.Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Müller DN, Derer W, Goss J, Ziomber A, Dietsch P, Wagner H, van Rooijen N, Kurtz A, Hilgers KF, Alitalo K, Eckardt KU, Luft FC, Kerjaschki D, Titze J. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med. 2009;15(5):545–552. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 10.Kriska T, Cepura C, Gauthier KM, Campbell WB. Role of macrophage PPARγ in experimental hypertension. Am J Physiol –Heart C. 2014;306:H26–H32. doi: 10.1152/ajpheart.00287.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okamura A, Rakugi H, Ohishi M, Yanagitani Y, Takiuchi S, Moriguchi K, Fennessy PA, Higaki J, Ogihara T. Upregulation of renin-angiotensin system during differentiation of monocytes to macrophages. J Hypertens. 1999;17:537–545. doi: 10.1097/00004872-199917040-00012. [DOI] [PubMed] [Google Scholar]
- 12.Ma LJ, Corsa BA, Zhou J, Yang H, Li H, Tang YW, Babaev VR, Major AS, Linton MF, Fazio S, Hunley TE, Kon V, Fogo AB. Angiotensin type 1 receptor modulates macrophage polarization and renal injury in obesity. Am J Physiol Renal Physiol. 2011;300(5):F1203–F1213. doi: 10.1152/ajprenal.00468.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rafatian N, Milne RW, Leenen FH, Whitman SC. Role of renin-angiotensin system in activation of macrophages by modified lipoproteins. Am J Physiol Heart Circ Physiol. 2013;305(9):H1309–H1320. doi: 10.1152/ajpheart.00826.2012. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto S, Yancey PG, Zuo Y, Ma LJ, Kaseda R, Fogo AB, Ichikawa I, Linton MF, Fazio S, Kon V. Macrophage polarization by angiotensin II-type 1 receptor aggravates renal injury-acceleration of atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31(12):2856–2864. doi: 10.1161/ATVBAHA.111.237198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bomfim GF, Dos Santos RA, Oliveira MA, Giachini FR, Akamine EH, Tostes RC, Fortes ZB, Webb RC, Carvalho MHC. Toll-like receptor 4 contributes to blood pressure regulation and vascular contraction in spontaneously hypertensive rats. Clin Science. 2012;122:535–543. doi: 10.1042/CS20110523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marketou ME, Kontaraki JE, Zacharis EA, Kochiadakis GE, Giaouzaki A, Chlouverakis G, Vardas PE. TLR2 and TLR4 gene expression in peripheral monocytes in nondiabetic hypertensive patients: the effect of intensive blood pressure-lowering. J Clin Hypertens (Greenwich) 2012;14(5):330–335. doi: 10.1111/j.1751-7176.2012.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolf G, Bohlender J, Bondeva T, Roger T, Thaiss F, Wenzel UO. Angiotensin II upregulates toll-like receptor 4 on mesangial cells. J Am Soc Nephrol. 2006;17(6):1585–1593. doi: 10.1681/ASN.2005070699. [DOI] [PubMed] [Google Scholar]
- 18.An J, Nakajima T, Kuba K, Kimura A. Losartan inhibits LPS-induced inflammatory signaling through a PPARgamma-dependent mechanism in human THP-1 macrophages. Hypertens Res. 2010;33(8):831–835. doi: 10.1038/hr.2010.79. [DOI] [PubMed] [Google Scholar]
- 19.Rompe F, Artuc M, Hallberg A, Alterman M, Stroder K, Thone-Reineke C, Reichenback A, Schacherl J, Dahlof B, Bader M, Alenina N, Schwaninger M, Zuberbier T, Funke-Kaiser H, Schmidt C, Schunck W, Unger T, Steckelings UM. Direct angiotensin II type 2 receptor stimulation acts as anti-inflammatory through epoxyeicosatrienoic acid and inhibition of nuclear factor κB. Hypertension. 2010;55:924–931. doi: 10.1161/HYPERTENSIONAHA.109.147843. [DOI] [PubMed] [Google Scholar]
- 20.Abadir PM, Walton JD, Carey RM, Siragy HM. Angiotensin II type 2 receptors modulate inflammation through signal transducer and activator transcription proteins 3 phosphorylation and TNF-α production. J Interferon Cytokine Res. 2011;31:471–474. doi: 10.1089/jir.2010.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matavelli LC, Jiang H, Siragy HM. Angiotensin AT2 receptor stimulation inhibits early renal inflammation in renovascular hypertension. Hypertension. 2011;57:308–313. doi: 10.1161/HYPERTENSIONAHA.110.164202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhande IS, Ali Q, Hussain T. Proximal tubule Angiotensin AT2 receptors mediate an anti-inflammatory response via Interleukin-10: Role in renoprotection in obese rats. Hypertension. 2013;61:1218–1226. doi: 10.1161/HYPERTENSIONAHA.111.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saraiva M, O’Garra A. The regulation of Interleukin-10 production by immune cells. Nat Rev Immunol. 2010;10(3):170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 24.Zhang P, Martin M, Michalek SM, Katz J. Role of mitogen-activated protein kinases and NF-κB in the regulation of proinflammatory and anti-inflammatory cytokines by Porphyromonas gingivalis hemagglutinin B. Infect Immun. 2005;73(7):3990–3998. doi: 10.1128/IAI.73.7.3990-3998.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma W, Lim W, Gee K, Aucoin S, Nandan D, Kozlowski M, Diaz-Mitoma F, Kumar A. The p38 mitogen-activated kinase pathway regulates the human interleukin-10 promoter via the activation of Sp1 transcription factor in lipopolysaccharide-stimulated human macrophages. J Biol Chem. 2001;276(17):13664–13674. doi: 10.1074/jbc.M011157200. [DOI] [PubMed] [Google Scholar]
- 26.Yi AK, Yoon JG, Yeo SJ, Hong SC, English BK, Krieg AM. Role of mitogen-activated protein kinases in CpG DNA-mediated IL-10 and IL-12 production: central role of extracellular signal-regulated kinase in the negative feedback loop of the CpG DNA-mediated Th1 response. J Immunol. 2002;168(9):4711–4720. doi: 10.4049/jimmunol.168.9.4711. [DOI] [PubMed] [Google Scholar]
- 27.Koscsó B, Csóka B, Selmeczy Z, Himer L, Pacher P, Virág L, Haskó G. Adenosine augments IL-10 production by microglial cells through an A2B adenosine receptor-mediated process. J Immunol. 2012;188(1):445–453. doi: 10.4049/jimmunol.1101224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chanteux H, Guisset AC, Pilette C, Sibille Y. LPS induces IL-10 production by human alveolar macrophages via MAPKinases- and Sp1-dependent mechanisms. Respir Res. 2007;8:71. doi: 10.1186/1465-9921-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ali Q, Wu Y, Hussain T. Chronic AT2 receptor activation increases renal ACE2 activity, attenuates AT1 receptor function and blood pressure in obese Zucker rats. Kidney Int. 2013;84(5):931–939. doi: 10.1038/ki.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shum M, Pinard S, Guimond MO, Labbé SM, Roberge C, Baillargeon JP, Langlois MF, Alterman M, Wallinder C, Hallberg A, Carpentier AC, Gallo-Payet N. Angiotensin II type 2 receptor promotes adipocyte differentiation and restores adipocyte size in high-fat/high-fructose diet-induced insulin resistance in rats. Am J Physiol Endocrinol Metab. 2013;304(2):E197–E210. doi: 10.1152/ajpendo.00149.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang J, Chen C, Ren H, Han Y, He D, Zhou L, Hopfer U, Jose PA, Zeng C. Angiotensin II AT(2) receptor decreases AT(1) receptor expression and function via nitric oxide/cGMP/Sp1 in renal proximal tubule cells from Wistar-Kyoto rats. J Hypertens. 2012;30(6):1176–1184. doi: 10.1097/HJH.0b013e3283532099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lucas M1, Zhang X, Prasanna V, Mosser DM. ERK activation following macrophage FcgammaR ligation leads to chromatin modifications at the IL-10 locus. J Immunol. 2005 Jul 1;175(1):469–477. doi: 10.4049/jimmunol.175.1.469. [DOI] [PubMed] [Google Scholar]
- 33.Kaiser F, Cook D, Papoutsopoulou S, Rajsbaum R, Wu X, Yang HT, Grant S, Ricciardi-Castagnoli P, Tsichlis PN, Ley SC, O'Garra A. TPL-2 negatively regulates interferon-beta production in macrophages and myeloid dendritic cells. J Exp Med. 2009;206(9):1863–1871. doi: 10.1084/jem.20091059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pastore L, Tessitore A, Martinotti S, Toniato E, Alesse E, Bravi MC, et al. Angiotensin II stimulates intercellular adhesion molecule-1 (ICAM-1) expression by human vascular endothelial cells and increases soluble ICAM-1 release in vivo. Circulation. 1999;100:1646–1652. doi: 10.1161/01.cir.100.15.1646. [DOI] [PubMed] [Google Scholar]
- 35.Pueyo ME, Gonzalez W, Nicoletti A, Savoie F, Arnal JF, Michel JB. Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-kappaB activation induced by intracellular oxidative stress. Arterioscler Thromb Vasc Biol. 2000;20:645–651. doi: 10.1161/01.atv.20.3.645. [DOI] [PubMed] [Google Scholar]
- 36.Rice EK, Tesch Gregory H, Cao Zemin, Cooper Mark E, Metz Christine N, Bucala Richard, Atkins Robert C, Nikolic-Paterson David J. Induction of MIF synthesis and secretion by tubular epithelial cells: A novel action of angiotensin II. Kidney Int. 2003;63:1265–1275. doi: 10.1046/j.1523-1755.2003.00875.x. [DOI] [PubMed] [Google Scholar]
- 37.Lapteva N, Ide K, Nieda M, Ando Y, Hatta-Ohashi Y, Minami M, Dymshits G, Egawa K, Juji T, Tokunaga K. Activation and suppression of renin-angiotensin system in human dendritic cells. Biochem Biophys Res Commun. 2002;296:194–200. doi: 10.1016/s0006-291x(02)00855-0. [DOI] [PubMed] [Google Scholar]
- 38.Muller DN, Shagdarsuren E, Park JK, Dechend R, Mervaala E, Hampich F, Fiebeler A, Ju X, Finckenberg P, Theuer J, et al. Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am J Pathol. 2002;161:1679–1693. doi: 10.1016/S0002-9440(10)64445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nahmod KA, Vermeulen ME, Raiden S, Salamone G, Gamberale R, Fernandez-Calotti P, Alvarez A, Nahmod V, Giordano M, Geffner JR. Control of dendritic cell differentiation by angiotensin II. FASEB J. 2003;17:491–493. doi: 10.1096/fj.02-0755fje. [DOI] [PubMed] [Google Scholar]
- 40.Crowley SD, Frey CW, Gould SK, Griffiths R, Ruiz P, Burchette JL, Howell DN, Makhanova N, Yan M, Kim HS, et al. Stimulation of lymphocyte responses by angiotensin II promotes kidney injury in hypertension. Am J Physiol Renal Physiol. 2008;295:F515–F524. doi: 10.1152/ajprenal.00527.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jurewicz M, McDermott DH, Sechler JM, Tinckam K, Takakura A, Carpenter CB, Milford E, Abdi R. Human T and natural killer cells possess a functional renin-angiotensin system: further mechanisms of angiotensin II-induced inflammation. J Am Soc Nephrol. 2007;18:1093–1102. doi: 10.1681/ASN.2006070707. [DOI] [PubMed] [Google Scholar]
- 42.Kvakan H, Kleinewietfeld M, Qadri F, Park JK, Fischer R, Schwarz I, Rahn HP, Plehm R, Wellner M, Elitok S, et al. Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation. 2009;119:2904–2912. doi: 10.1161/CIRCULATIONAHA.108.832782. [DOI] [PubMed] [Google Scholar]
- 43.Dai Q, Xu M, Yao M, Sun B. Angiotensin AT1 receptor antagonists exert anti-inflammatory effects in spontaneously hypertensive rats. Br J Pharmacol. 2007;152:1042–1048. doi: 10.1038/sj.bjp.0707454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Larrayoz IM1, Pang T, Benicky J, Pavel J, Sánchez-Lemus E, Saavedra JM. Candesartan reduces the innate immune response to lipopolysaccharide in human monocytes. J Hypertens. 2009;27(12):2365–2376. doi: 10.1097/HJH.0b013e3283314bc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakamura A1, Johns EJ, Imaizumi A, Yanagawa Y, Kohsaka T. Effect of beta(2)-adrenoceptor activation and angiotensin II on tumour necrosis factor and interleukin 6 gene transcription in the rat renal resident macrophage cells. Cytokine. 1999;11(10):759–765. doi: 10.1006/cyto.1999.0488. [DOI] [PubMed] [Google Scholar]
- 46.Ji Y, Liu J, Wang Z, Liu N. Angiotensin II Induces Inflammatory Response Partly Via Toll-Like Receptor 4-Dependent Signaling Pathway in Vascular Smooth Muscle Cells. Cell Physiol Biochem. 2009;23:265–276. doi: 10.1159/000218173. [DOI] [PubMed] [Google Scholar]
- 47.Bondeva T, Roger T, Wolf G. Differential Regulation of Toll-Like Receptor 4 Gene Expression in Renal Cells by Angiotensin II: Dependency on AP1 and PU.1 Transcriptional Sites. Am J Nephrol. 2007;27:308–314. doi: 10.1159/000102551. [DOI] [PubMed] [Google Scholar]
- 48.Wu J, Yang X, Zhang YF, Zhou SF, Zhang R, Dong XQ, Fan JJ, Liu M, Yu XQ. Angiotensin II up-regulated Toll-like receptor 4 and enhances lipopolysaccharide-induced CD40 expression in rat peritoneal mesothelial cells. Inflamm Res. 2009;58:473–482. doi: 10.1007/s00011-009-0012-z. [DOI] [PubMed] [Google Scholar]
- 49.Sabuhi R, Ali Q, Asghar M, Al-Zamily NRH, Hussain T. Role of angiotensin II AT2 receptor in inflammation and oxidative stress: Opposing effects in lean and obese rats. Am J Renal Physiol. 2011;300:F700–F706. doi: 10.1152/ajprenal.00616.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Curato C, Slavic S, Dong J, Skorska A, Altarche-Xifro W, Miteva K, Kaschina E, Thiel A, Imboden H, Wang I, Steckelings U, Steinhoff G, Unger T, Li J. Identification of non-cytotoxic and IL-10 producing CD8+AT2R+ T-cell population in response to ischemic heart injury. J Immunol. 2010;185:6286–6293. doi: 10.4049/jimmunol.0903681. [DOI] [PubMed] [Google Scholar]
- 51.Powell MJ, Thompson SA, Tone Y, Waldmann H, Tone M. Posttranscriptional regulation of IL-10 gene expression through sequences in the 3'-untranslated region. J Immunol. 2000;165(1):292–296. doi: 10.4049/jimmunol.165.1.292. [DOI] [PubMed] [Google Scholar]
- 52.Hayashida W, Horiuchi M, Dzau VJ. Intracellular third loop domain of angiotensin II type-2 receptor. Role in mediating signal transduction and cellular function. J Biol Chem. 1996;271:21985–21992. doi: 10.1074/jbc.271.36.21985. [DOI] [PubMed] [Google Scholar]
- 53.Yamada T, Horiuchi M, Dzau VJ. Angiotensin II type 2 receptor mediates programmed cell death. Proc Natl Acad Sci. 1996;93:156–160. doi: 10.1073/pnas.93.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horiuchi M, Hayashida W, Kambe T, Yamada T, Dzau VJ. Angiotensin type 2 receptor dephosphorylates Bcl-2 by activating mitogen-activated protein kinase phosphatase-1 and induces apoptosis. J Biol Chem. 1997;272:19022–19026. doi: 10.1074/jbc.272.30.19022. [DOI] [PubMed] [Google Scholar]
- 55.Bedecs K, Elbaz N, Sutren M, Masson M, Susini C, Strosberg AD, Nahmias C. Angiotensin II type 2 receptors mediate inhibition of mitogen-activated protein kinase cascade and functional activation of SHP-1 tyrosine phosphatase. Biochem J. 1997;325:449–454. doi: 10.1042/bj3250449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elbaz N, Bedecs K, Masson M, Sutren M, Strosberg AD, Nahmias C. Functional trans-inactivation of insulin receptor kinase by growth-inhibitory angiotensin II AT2 receptor. Mol Endocrinol. 2000;14:795–804. doi: 10.1210/mend.14.6.0488. [DOI] [PubMed] [Google Scholar]
- 57.Shibasaki Y, Matsubara H, Nozawa Y, Mori Y, Masaki H, Kosaki A, Tsutsumi Y, Uchiyama Y, Fujiyama S, Nose A, et al. Angiotensin II type 2 receptor inhibits epidermal growth factor receptor transactivation by increasing association of SHP-1 tyrosine phosphatase. Hypertension. 2001;38:367–372. doi: 10.1161/01.hyp.38.3.367. [DOI] [PubMed] [Google Scholar]
- 58.Gendron L, Laflamme L, Rivard N, Asselin C, Payet MD, Gallo-Payet N. Signals from the AT2 (angiotensin type 2) receptor of angiotensin II inhibit p21ras and activate MAPK (mitogen-activated protein kinase) to induce morphological neuronal differentiation in NG108-15 cells. Mol Endocrinol. 1999;13(9):1615–1626. doi: 10.1210/mend.13.9.0344. [DOI] [PubMed] [Google Scholar]
- 59.Stroth U, Blume A, Mielke K, Unger T. Angiotensin AT(2) receptor stimulates ERK1 and ERK2 in quiescent but inhibits ERK in NGF-stimulated PC12W cells. Brain Res Mol Brain Res. 2000;78(1–2):175–180. doi: 10.1016/s0169-328x(00)00093-0. [DOI] [PubMed] [Google Scholar]
- 60.Hansen JL, Servant G, Baranski TJ, Fujita T, Iiri T, Sheikh SP. Functional reconstitution of the angiotensin II type 2 receptor and G(i) activation. Circ Res. 2000;87(9):753–759. doi: 10.1161/01.res.87.9.753. [DOI] [PubMed] [Google Scholar]
- 61.De Paolis P, Porcellini A, Savoia C, Lombardi A, Gigante B, Frati G, Rubattu S, Musumeci B, Volpe M. Functional cross-talk between angiotensin II and epidermal growth factor receptors in NIH3T3 fibroblasts. J Hypertens. 2002;20(4):693–699. doi: 10.1097/00004872-200204000-00027. [DOI] [PubMed] [Google Scholar]
- 62.Gendron L, Oligny JF, Payet MD, Gallo-Payet N. Cyclic AMP-independent involvement of Rap1/B-Raf in the angiotensin II AT2 receptor signaling pathway in NG108-15 cells. J Biol Chem. 2003;278(6):3606–3614. doi: 10.1074/jbc.M202446200. [DOI] [PubMed] [Google Scholar]
- 63.MacKenzie KF, Clark K, Naqvi S, McGuire VA, Nöehren G, Kristariyanto Y, van den Bosch M, Mudaliar M, McCarthy PC, Pattison MJ, Pedrioli PG, Barton GJ, Toth R, Prescott A, Arthur JS. PGE(2) induces macrophage IL-10 production and a regulatory-like phenotype via a protein kinase A-SIK-CRTC3 pathway. J Immunol. 2013;190(2):565–577. doi: 10.4049/jimmunol.1202462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okenwa C, Kumar A, Rego D, Konarski Y, Nilchi L, Wright K, Kozlowski M. SHP-1-Pyk2-Src protein complex and p38 MAPK pathways independently regulate IL-10 production in lipopolysaccharide-stimulated macrophages. J Immunol. 2013;191(5):2589–2603. doi: 10.4049/jimmunol.1300466. [DOI] [PubMed] [Google Scholar]
- 65.Elcombe SE, Naqvi S, Van Den Bosch MW, MacKenzie KF, Cianfanelli F, Brown GD, Arthur JS. Dectin-1 regulates IL-10 production via a MSK1/2 and CREB dependent pathway and promotes the induction of regulatory macrophage markers. PLoS One. 2013;8(3):e60086. doi: 10.1371/journal.pone.0060086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iyer SS, Ghaffari AA, Cheng G. Lipopolysaccharide-mediated IL-10 transcriptional regulation requires sequential induction of type I IFNs and IL-27 in macrophages. J Immunol. 2010;185(11):6599–6607. doi: 10.4049/jimmunol.1002041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carey RM, Padia SH. Role of angiotensin AT(2) receptors in natriuresis: Intrarenal mechanisms and therapeutic potential. Clin Exp Pharmacol Physiol. 2013;40(8):527–534. doi: 10.1111/1440-1681.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rehman A, Leibowitz A, Yamamoto N, Rautureau Y, Paradis P, Schiffrin EL. Angiotensin type 2 receptor agonist compound 21 reduces vascular injury and myocardial fibrosis in stroke-prone spontaneously hypertensive rats. Hypertension. 2012;59(2):291–299. doi: 10.1161/HYPERTENSIONAHA.111.180158. [DOI] [PubMed] [Google Scholar]
- 69.Carey RM, Howell NL, Jin XH, Siragy HM. Angiotensin type 2 receptor-mediated hypotension in angiotensin type-1 receptor-blocked rats. Hypertension. 2001;38(6):1272–1277. doi: 10.1161/hy1201.096576. [DOI] [PubMed] [Google Scholar]
- 70.Barber MN, Sampey DB, Widdop RE. AT(2) receptor stimulation enhances antihypertensive effect of AT(1) receptor antagonist in hypertensive rats. Hypertension. 1999;34(5):1112–1116. doi: 10.1161/01.hyp.34.5.1112. [DOI] [PubMed] [Google Scholar]
- 71.Ali Q, Hussain T. AT2 receptor non-peptide agonist C21 promotes natriuresis in obese Zucker rats. Hypertens Res. 2012;35(6):654–660. doi: 10.1038/hr.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hilliard LM, Jones ES, Steckelings UM, Unger T, Widdop RE, Denton KM. Sex-specific influence of angiotensin type 2 receptor stimulation on renal function: a novel therapeutic target for hypertension. Hypertension. 2012;59(2):409–414. doi: 10.1161/HYPERTENSIONAHA.111.184986. [DOI] [PubMed] [Google Scholar]
- 73.Bosnyak S, Welungoda IK, Hallberg A, Alterman M, Widdop RE, Jones ES. Stimulation of angiotensin AT2 receptors by the non-peptide agonist, Compound 21, evokes vasodepressor effects in conscious spontaneously hypertensive rats. Br J Pharmacol. 2010;159(3):709–716. doi: 10.1111/j.1476-5381.2009.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Paulis L, Becker ST, Lucht K, Schwengel K, Slavic S, Kaschina E, Thöne-Reineke C, Dahlof B, Baulmann J, Unger T, Steckelings UM. Direct angiotensin II type 2 receptor stimulation in Nω-nitro-L-arginine-methyl ester-induced hypertension: the effect on pulse wave velocity and aortic remodeling. Hypertension. 2012;59(2):485–492. doi: 10.1161/HYPERTENSIONAHA.111.185496. [DOI] [PubMed] [Google Scholar]
- 75.Gelosa P, Pignieri A, Fändriks L, de Gasparo M, Hallberg A, Banfi C, Castiglioni L, Turolo L, Guerrini U, Tremoli E, Sironi L. Stimulation of AT2 receptor exerts beneficial effects in stroke-prone rats: focus on renal damage. J Hypertens. 2009;27(12):2444–2451. doi: 10.1097/HJH.0b013e3283311ba1. [DOI] [PubMed] [Google Scholar]