Abstract
Objective:
To assess the natural history of prehospital blood pressure (BP) during emergency medical services (EMS) transport of suspected stroke and determine whether prehospital BP differs among types of patients with suspected stroke (ischemic stroke, TIA, intracerebral hemorrhage [ICH], or stroke mimic).
Methods:
A retrospective, cross-sectional, observational analysis of a centralized EMS database containing electronic records of patients transported by EMS to the emergency department (ED) with suspected stroke during an 18-month period was conducted. Hospital charts and neuroimaging were utilized to determine the final diagnosis (ischemic stroke, TIA, ICH, or stroke mimic).
Results:
A total of 960 patients were transported by EMS to ED with suspected stroke. Stroke was diagnosed in 544 patients (56.7%) (38.2% ischemic stroke, 12.2% TIA, 5.3% ICH) and 416 (43.2%) were considered mimics. Age-adjusted mean prehospital systolic BP (SBP) was higher in acute stroke patients (155.6 mm Hg; 95% confidence interval [CI]: 153.4–157.9 mm Hg) compared to mimics (146.1 mm Hg; 95% CI: 142.5–148.6 mm Hg; p < 0.001). Age-adjusted mean prehospital SBP was higher in ICH (172.3 mm Hg; 95% CI: 165.1–179.7 mm Hg) than in either ischemic stroke or TIA (154.7 mm Hg; 95% CI: 152.3–157.0 mm Hg; p < 0.001). Median (interquartile range) SBP drop from initial prehospital SBP to ED SBP was 4 mm Hg (−6 to 17 mm Hg). Mean prehospital SBP was strongly correlated with ED SBP (r = 0.82, p < 0.001).
Conclusions:
Prehospital SBP is higher in acute stroke relative to stroke mimics and highest in ICH. Given the stability of BP between initial EMS and ED measurements, it may be reasonable to test the feasibility and safety of prehospital antihypertensive therapy in patients with suspected acute stroke.
Elevated hospital admission blood pressure (BP) in patients with acute stroke is common1 and is associated with poor outcomes.2–5 There is limited randomized clinical trial evidence that acute BP reduction improves outcomes after intracerebral hemorrhage (ICH).6 One of the hypothesized reasons for the modest treatment effect is that hematoma enlargement and clinical deterioration have already begun by the time antihypertensive therapy is administered. It has been suggested that initiation of BP reduction in the prehospital setting may improve the efficacy of this approach.7
Recent pilot studies have assessed the feasibility of a prehospital BP-lowering strategy in patients with acute stroke symptoms using topical glyceryl trinitrate8 and sublingual lisinopril.9 At this point, however, the natural history of BP in the prehospital setting in patients with acute stroke symptoms is poorly understood. In contrast, the course of BP after hospital admission has been well described. Pressures are highest at admission, begin to spontaneously decline almost immediately,10 and this trend continues for several days.11–14 It is possible that admission pressures are similar to those taken closer to symptom onset. Alternatively, BP may acutely rise after arrival to the emergency department (ED), or the spontaneous decline seen after admission may actually begin shortly after symptom onset.
Admission BP and natural history over the first week after hospital admission have both been shown to differ with stroke type. Specifically, pressures are higher in patients with ICH, relative to those with ischemic stroke.11,15 It is unknown whether these associations are also present in the prehospital setting.
The objectives of this study were to (1) assess the natural history of prehospital BP during emergency medical services (EMS) transport of consecutive patients with suspected stroke, and (2) determine whether prehospital BP differed among types of patients with suspected stroke (ischemic stroke, TIA, ICH, or stroke mimic). We tested the hypotheses that (1) prehospital systolic BP (SBP) in patients with suspected stroke is not significantly different from that measured at admission to the ED, and (2) prehospital BP in ICH is higher than in patients with ischemic stroke.
METHODS
Study design.
We conducted a retrospective, cross-sectional, observational study of a prospectively maintained centralized EMS database of all consecutive patients with suspected stroke transported by EMS to the ED of the University of Alberta Hospital during an 18-month period (January 1, 2012, to July 31, 2013).
EMS service and suspected stroke.
The unified EMS service for the city of Edmonton attends to more than 157,000 events annually and transports patients to one of 13 provincially operated Edmonton Zone EDs. Since 2009, all land ambulance services have transitioned into a unified provincial system under Alberta Health Services.16 The University of Alberta Hospital is a tertiary health care center and one of 2 designated stroke centers within the Edmonton Zone, receiving the majority of patients transported by EMS with suspected stroke and admitting 1,000 to 1,200 stroke patients annually.
During a 9-1-1 call for suspected stroke, the emergency dispatcher used a stroke-specific interview algorithm to assign a Medical Priority Dispatch System event code for suspected stroke. This event code was subsequently communicated to EMS personnel who responded to the emergency call. The prehospital Cincinnati Stroke Screen17 in conjunction with an in-house stroke screen developed by provincial stroke neurologists was used by EMS personnel to determine a primary EMS impression of acute stroke.
Upon first EMS assessment and during transport to the ED, individual patient data (including serial vital sign measurements such as BP, heart rate, oxygen saturation, temperature, glucose, and Glasgow Coma scale score) were recorded on electronic patient health care reports. Prehospital BP recordings were measured according to the Standard Approach and Ongoing Assessment Medical Control Protocol, where at least 2 sets of BP readings are measured by EMS personnel every 15 to 30 minutes. The initial BP was measured by auscultation with subsequent measurements using a noninvasive BP cuff. Dispatch and individual electronic patient health care reports data were then prospectively uploaded to a centralized provincial EMS database at the Alberta Health Services.
Standard protocol approvals, registrations, and patient consents.
The research protocol of this study was approved for waiver of consent by the local institutional Human Research Ethics Review Board (University of Alberta).
Study population and data collection.
Eligible study patients were identified within the centralized Alberta Health Services database by dispatch event and/or EMS primary impression of acute stroke. Once identified, data from individual patient electronic patient health care reports were extracted and linked to ED records through the Emergency Department Information Systems to obtain vital sign measurements on ED arrival. Individual patient electronic patient health care reports data were also linked to in-hospital patient charts and neuroimaging to determine the final diagnosis of ischemic stroke, TIA, or ICH, as per the treating physician's discharge diagnosis. Ischemic stroke etiology was classified according to TOAST (Trial of Org 10172 in Acute Stroke Treatment) criteria.18 For the purpose of the study, stroke mimics were defined as any discharge diagnosis (other than acute stroke) for symptoms that prompted initial EMS activation for suspected stroke.
Statistical analyses.
All statistical analyses were performed using SPSS 22.0 (IBM SPSS Statistics 2014, Armonk, NY). The rate of missing data (notably in baseline characteristics) was low (<4.3%), and analyses were performed using pairwise deletion of missing data. Continuous variables are reported as means and SDs or medians and interquartile ranges, as appropriate. Dichotomous variables are reported as proportions. Differences in baseline characteristics in the 4 types of patients with suspected stroke were analyzed using χ2 test. Differences in groups of continuous variables (prehospital SBP) between acute stroke and stroke mimics and between the 4 groups were analyzed by 1-way analysis of variance followed by Tukey post hoc test for independent comparisons. Analysis of covariance was used to analyze the effects of age on the differences of mean prehospital SBP between groups. The degree of correlation between prehospital SBP and ED SBP was analyzed using Pearson correlation coefficient. Prehospital SBP variability was defined as the SD of mean prehospital SBP.
RESULTS
Study population.
A total of 1,060 eligible patients were identified within the study period. A total of 100 patients were excluded from analyses because of incomplete data (inability to link EMS data with in-hospital charts, n = 62; absence of EMS vital sign recordings during EMS transport, n = 13; and in-hospital chart unavailability for review, n = 25), leaving a total study population of 960 patients.
The mean ± SD age of the entire cohort was 70.4 ± 15.6 years, and 47.8% of patients were men. Acute stroke pathology was diagnosed in 544 patients (56.7%) and 416 (43.3%) were considered stroke mimics. Among acute stroke patients, 367 (38.2%) were diagnosed with acute ischemic stroke, 117 (12.2%) with TIA, and 51 (5.3%) with acute ICH. Nine patients were diagnosed with subarachnoid hemorrhage (0.9%) and were excluded a priori in subsequent analyses.
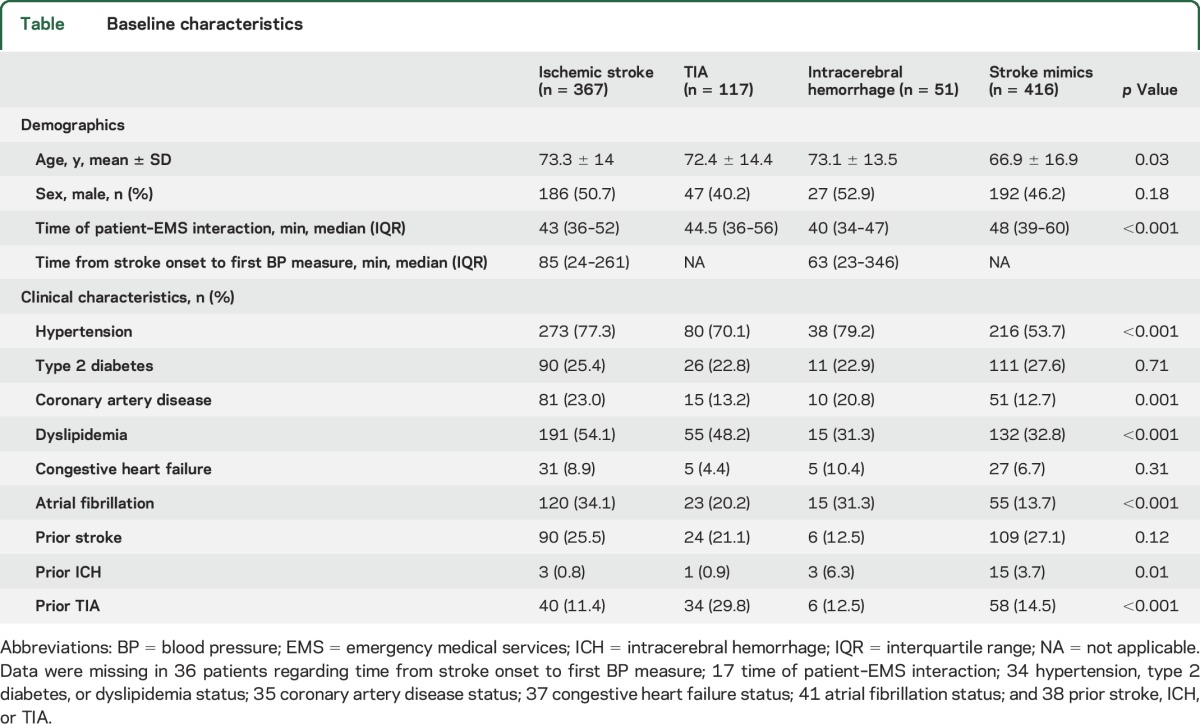
Patients diagnosed with stroke mimics were younger and had lower rates of hypertension and atrial fibrillation than patients with acute ischemic stroke/TIA/ICH (table). Conversely, patients with acute ischemic stroke had higher rates of coronary artery disease and dyslipidemia, while patients with TIA and ICH had higher rates of previous TIA and ICH, respectively.
Table.
Baseline characteristics
Prehospital BP in acute stroke and stroke mimic patients.
Initial prehospital SBP (mean ± SD) was higher in patients with acute stroke (159.7 ± 29.5 mm Hg) compared to those with stroke mimics (147.2 ± 31.2 mm Hg, p < 0.001). Similarly, mean prehospital SBP was higher in patients with acute stroke (156.4 ± 26.9 mm Hg) than in patients with stroke mimics (145.3 ± 25.4 mm Hg, p < 0.001). An age-adjusted analysis of covariance showed that mean prehospital SBP remained significantly higher in patients with acute stroke (adjusted means 155.6 mm Hg; 95% confidence interval [CI]: 153.4–157.9 mm Hg) than in patients with stroke mimics (adjusted means 146.1 mm Hg; 95% CI: 143.5–148.6 mm Hg; p < 0.001).
When divided into groups based on final diagnosis, initial prehospital SBP was higher in patients with ICH (175.5 ± 32.4 mm Hg, p ≤ 0.003) than in patients with ischemic stroke (157.8 ± 29.5 mm Hg), TIA (157.7 ± 25.7 mm Hg), and stroke mimics (147.2 ± 31.1 mm Hg). Patients with ICH also had higher mean prehospital SBP (172.3 ± 31.7 mm Hg, p < 0.001) when compared to ischemic stroke (154.9 ± 26.5 mm Hg), TIA (153.3 ± 23.1 mm Hg), and stroke mimics (145.4 ± 25.4 mm Hg) (figure 1A). Mean prehospital SBP remained highest in patients with ICH (adjusted means 172.3 mm Hg; 95% CI: 165.1–179.7 mm Hg) compared to patients with ischemic stroke or TIA (adjusted means 154.7 mm Hg; 95% CI: 152.3–157.0 mm Hg; p < 0.001) after adjusting for age. Similar results were observed for mean diastolic BP and mean arterial pressure during EMS transport (figure 1, B and C).
Figure 1. Relationship between mean prehospital blood pressure and final diagnosis.
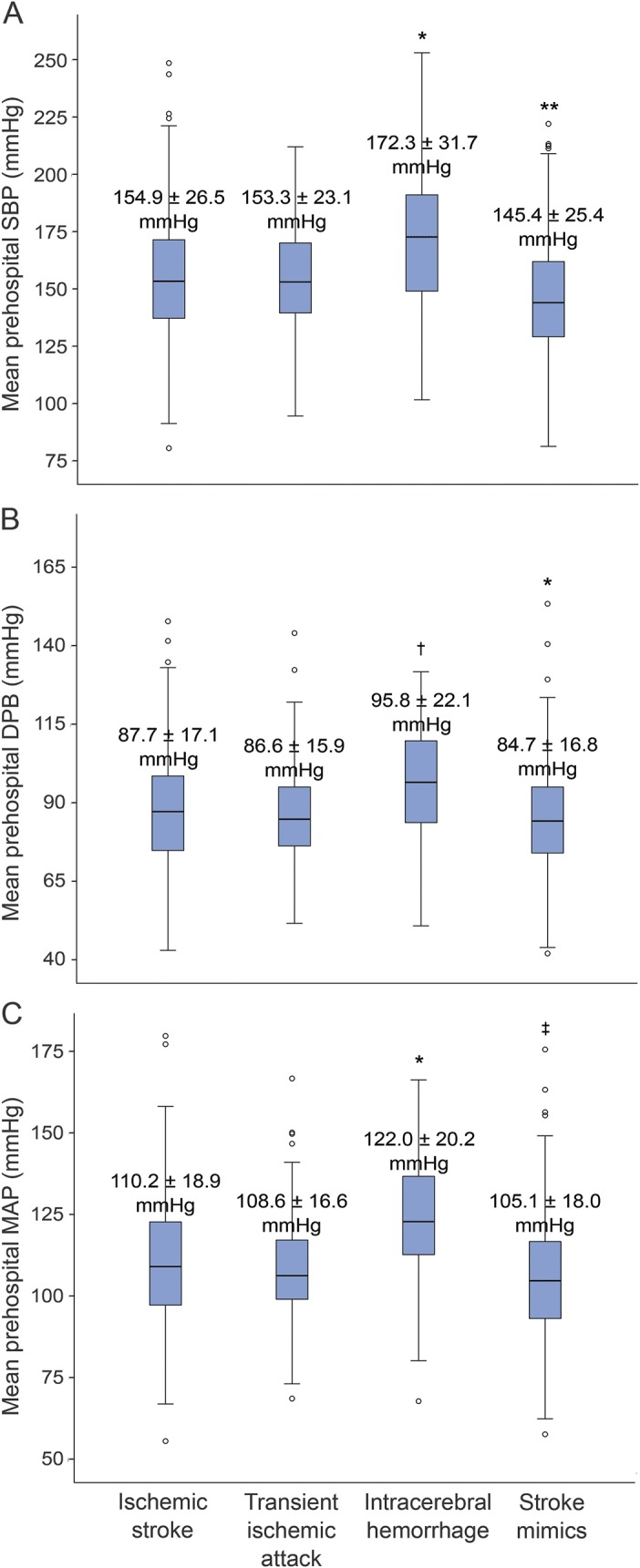
Box-and-whisker plots of (A) mean prehospital SBP by diagnosis classification, (B) mean prehospital DBP by diagnostic classification, and (C) mean prehospital MAP by diagnostic classification. The p values were obtained by analysis of variance followed by Tukey post hoc test for independent comparisons between categories. *p < 0.001, †p < 0.01 for ICH compared to ischemic stroke, TIA, and stroke mimics. **p < 0.02 for stroke mimics compared to ischemic stroke, TIA, and ICH. ‡p ≤ 0.001 for stroke mimics compared to ischemic stroke and ICH. DBP = diastolic blood pressure; ICH = intracerebral hemorrhage; MAP = mean arterial pressure; SBP = systolic blood pressure.
Prehospital BP during EMS transport.
The median (interquartile range) time of patient–EMS interaction (time of EMS arrival on-scene to ED arrival) was 45.0 (36–55) minutes. Patient–EMS interaction was slightly longer in patients with stroke mimics (48 [39–60] minutes) than in patients with acute stroke (43 [36–53] minutes, p < 0.001) (table). During EMS transport, prehospital BP was measured a mean 4 ± 1.9 times. In acute stroke patients, the median time from symptom onset to first prehospital BP reading was 75 (24–277) minutes.
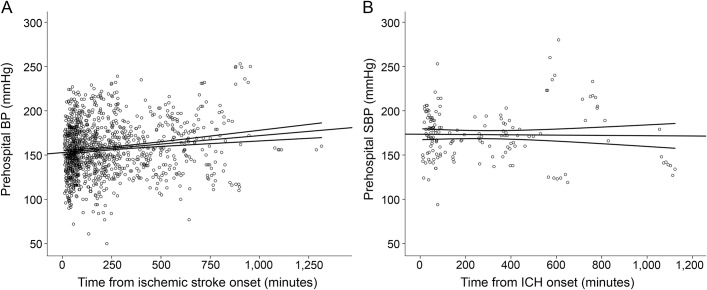
In relation to stroke onset, 74.8% of patients had their first BP measurement ≤4.5 hours from onset, 4.8% between 4.5 and 6 hours, 12.7% between 6 and 12 hours, and 7.7% >12 hours from onset. For acute stroke patients, prehospital SBP was very weakly correlated with the time from symptom onset to BP measurement (r = 0.14, p < 0.001). This did not differ in the subgroup of patients assessed within 4.5 hours of ischemic stroke onset (r = 0.1, p = 0.006) or in patients with ICH (r = −0.06, p = 0.39) (figure 2).
Figure 2. Relationship between time from onset and prehospital SBP in patients with acute stroke.
Scatterplots of the relationship between time from ischemic stroke onset (A) and time from ICH onset (B) and prehospital SBP measurements. BP = blood pressure; ICH = intracerebral hemorrhage; SBP = systolic blood pressure.
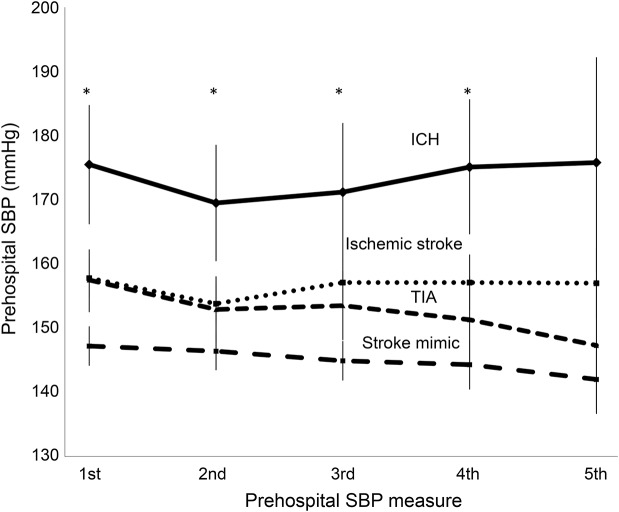
Mean prehospital SBP remained stable during EMS transport in all 4 patient types (figure 3). Patients with ICH maintained the highest mean prehospital SBP while patients with stroke mimics maintained the lowest prehospital SBP throughout EMS transport (p < 0.02). The overall mean variability of prehospital SBP was 11.1 ± 7.7 mm Hg, and this did not differ among groups (p = 0.46).
Figure 3. Natural history of prehospital SBP of patients with suspected stroke by diagnostic classification.
Linear plot of the natural history of prehospital SBP during emergency medical services transport by diagnostic classification. Error bars represent the 95% confidence interval of the mean. *p < 0.02 for ICH compared to ischemic stroke/TIA/mimics, except for the fifth prehospital measure between ICH and ischemic stroke (p = 0.06). ICH = intracerebral hemorrhage; SBP = systolic blood pressure.
Prehospital BP among ischemic stroke subtypes.
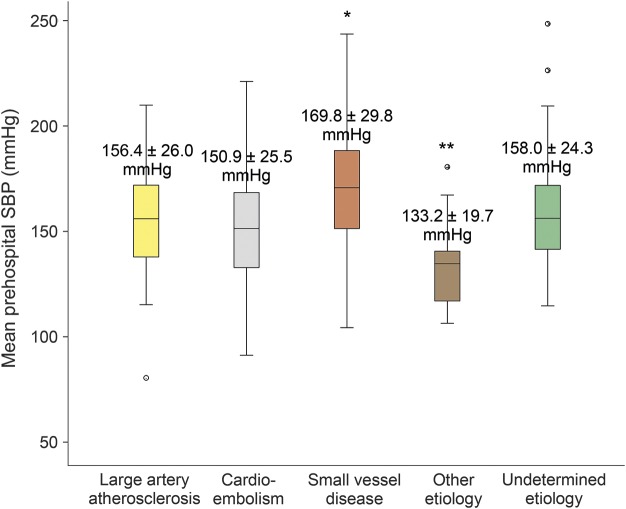
Among the 367 ischemic stroke patients, stroke etiology by TOAST classification was as follows: large artery atherosclerosis in 51 patients (13.9%), cardioembolic in 140 (38.1%), lacunar in 44 (12.0%), other determined etiology in 22 (6.0%), and cryptogenic in 110 (30.0%). Mean prehospital SBP in lacunar stroke was higher (169.8 ± 29.8 mm Hg, p < 0.001) than in cardioembolic stroke (150.9 ± 25.5 mm Hg) and stroke of other determined etiology (133.2 ± 19.7 mm Hg) (figure 4). There was a trend toward higher mean prehospital SBP in lacunar stroke when compared to large artery atherosclerosis (156.4 ± 26.0 mm Hg, p = 0.08) and cryptogenic stroke/stroke of undetermined etiology (158.0 ± 24.3 mm Hg, p = 0.06).
Figure 4. Relationship between mean prehospital SBP and ischemic stroke etiology.
Box-and-whisker plots of mean prehospital SBP by stroke etiology, defined according to TOAST classification. The p values were obtained by analysis of variance followed by Tukey post hoc test for independent comparisons between categories. *p < 0.001 for small vessel disease compared to cardioembolic and other determined etiology groups. **p < 0.02 for other determined etiology against all other groups. SBP = systolic blood pressure; TOAST = Trial of Org 10172 in Acute Stroke Treatment.
Relationship of prehospital BP and SBP on ED arrival.
Mean admission SBP on ED arrival was higher in patients with ICH (168.3 ± 34.0 mm Hg, p < 0.003) than in patients with ischemic stroke (153.2 ± 28.3 mm Hg), TIA (151.9 ± 25.2 mm Hg), and stroke mimics (142.2 ± 26.5 mm Hg). Initial prehospital SBP was strongly correlated to ED SBP (r = 0.74, p < 0.001), as was the mean prehospital SBP to ED SBP (r = 0.82, p < 0.001).
The median absolute difference in SBP from the initial prehospital BP measurement to admission BP measurement on ED arrival in all patients was 4 (−6 to 18) mm Hg. When broken down by type, the median absolute difference in initial prehospital SBP and ED SBP was 4 (−6 to 17) mm Hg in ischemic stroke, 6 (−7 to 18.5) mm Hg in TIA, 9 (−3.5 to 27.5) mm Hg in ICH, and 2 (−6 to 18) in stroke mimics, and this did not differ between groups (p = 0.36). The median %SBP reduction from initial prehospital measurement to ED was similar in all 4 groups (3% [−3.8% to 10%] in ischemic stroke, 4.4% [−4.7% to 11%] in TIA, 6.5% [−2.4% to 13.5%] in ICH, and 3% [−4% to 11.8%] in stroke mimics, p = 0.59).
DISCUSSION
Using robust prospectively collected regional EMS data and linkage to in-patient records, our findings suggest that prehospital SBP remains stable during EMS transport in patients with acute stroke symptoms. In addition, our study demonstrates that prehospital SBP is higher in acute stroke compared to stroke mimics, particularly in ICH.
There are very few published reports of prehospital BP in patients with suspected stroke. An observational analysis of 69 patients with acute stroke enrolled in a phase I recombinant tissue plasminogen activator study showed that elevated BP spontaneously declined up to a mean −29 ± 22 mm Hg within the first 90 minutes from stroke onset (first BP measurement taken at a mean 19 ± 13 minutes after symptom onset).10 In contrast, we found only a 4–mm Hg median SBP decrease from the first prehospital SBP to ED SBP, and elevated prehospital SBP appeared to be relatively independent of the time from stroke onset to assessment. Possible explanations for the discrepancy between studies may be attributable to sample size. Although BP does spontaneously decline shortly after hospital admission, prehospital BP measurements were relatively stable in our study. This may reflect the relatively brief transport time.
A 10-year retrospective population-based Japanese study of >106,000 patients with impaired consciousness showed that stroke diagnoses increased with the presence of elevated prehospital BP, particularly >160 mm Hg, relative to patients with other diagnoses.19 These data are consistent with our observation that elevated prehospital BP is higher in patients with acute stroke relative to other diagnoses.
To date, >10,000 patients have been enrolled in acute stroke BP treatment trials, yet optimal BP management in acute stroke remains controversial.6,20–24 Enrolling patients in the prehospital phase may help to elucidate whether early BP management is beneficial in acute stroke. This is of particular interest in acute ICH, where the phenomenon of hematoma expansion, a well-known predictor of poor prognosis and mortality,25 occurs most often within the first 3 hours from ICH onset.26 In BP-lowering trials conducted to date, very few patients with ICH have been enrolled this early after onset.6,27 Prehospital BP trials may therefore be the ideal setting to assess whether early BP treatment reduces hematoma expansion rates and improves clinical outcomes.
Nevertheless, there exists a theoretical concern regarding acute BP reduction in the EMS setting of undifferentiated stroke because of the fear of exacerbating hypoperfusion within the ischemic penumbra. Our findings suggest that higher prehospital SBP may be associated with lacunar infarcts due to small vessel disease, which, by definition, have smaller infarct volumes and thus may be at lower risk of exacerbated hypoperfusion.
Current guidelines for BP management in acute stroke suggest an SBP target of <180 mm Hg for all patients with ICH on ED arrival.28 Furthermore, the recommended target SBP in patients with ischemic stroke who are eligible for thrombolysis is <180 mm Hg.29 Given the stability of prehospital BP between EMS and ED measurement, it may be reasonable to target an SBP of <180 mm Hg in all patients presenting with suspected stroke and in a thrombolysis window while en route to hospital. The safety and feasibility of this approach needs to be assessed in a prospective trial. Although not target-based, studies of prehospital BP reduction are ongoing.30
Although this is a large observational study of consecutive patients with suspected stroke transported by EMS personnel, it is limited by its retrospective design and lack of standardized hypertension monitoring protocols. Conversely, individual EMS patient data were acquired in a prospective manner and subsequently uploaded to a centralized database and EMS staff members were unaware of the study, which may help to limit bias. Furthermore, we were able to achieve >90% linkage of individual EMS patient data to in-hospital patient charts and neuroimaging to appropriately classify patients by final diagnosis. Although all patients with suspected stroke identified by either dispatch event codes for suspected stroke or EMS primary impression of stroke within the study period were captured in our study, prehospital data of patients with an ultimate diagnosis of acute stroke who were not identified as such by EMS personnel (false-negative patients) were not accounted for. However, this is beyond the scope of the present analyses where the focus was to assess the natural history of prehospital BP in patients whom EMS personnel deem eligible for prehospital BP studies in acute stroke. Furthermore, patients with suspected stroke transported to other hospitals within the Edmonton Zone were not accounted for, which could potentially affect the generalizability of our results. However, as the primary designated stroke center within the Edmonton Zone with endovascular capabilities, we believe the bias is minimal, as persons with more severe strokes are transported to the University of Alberta Hospital. Nine patients (0.9%) with subarachnoid hemorrhage were excluded from analyses because the pathophysiology and management of these patients is so different from acute ischemic stroke and ICH patients. We had planned a priori to assess these patients separately, but the small number precluded this. Although it is difficult to differentiate a patient with subarachnoid hemorrhage from ischemic stroke or ICH in the prehospital setting, we believe the bias is minimal in our study. Finally, a higher than expected stroke mimic rate (43%) was observed in our study. This may be related to the previous observation that dispatch diagnosis of acute stroke has modest sensitivity and positive predictive value (approximately 45%).31
Prehospital BP is similar to that at hospital admission and remains stable during EMS transport in patients with acute stroke symptoms. Prehospital BP is higher in acute stroke patients compared to stroke mimic patients, particularly those with ICH. Although not reliably predictive of stroke or stroke type, elevated BP may be useful in patient selection for ongoing and future prehospital stroke treatment trials.
Supplementary Material
GLOSSARY
- BP
blood pressure
- ED
emergency department
- EMS
emergency medical services
- ICH
intracerebral hemorrhage
- SBP
systolic blood pressure
- TOAST
Trial of Org 10172 in Acute Stroke Treatment
AUTHOR CONTRIBUTIONS
L.C. Gioia: designed and conceptualized the study, collected data, performed analysis and interpretation of the data, and drafted the manuscript. R.T. Zewude: collected data, performed analysis, and revised the manuscript. M.P. Kate: designed and conceptualized the study and revised the manuscript. K. Liss: provided data and revised the manuscript. B. Rowe: designed and conceptualized the study and revised the manuscript. B. Buck: analysis and interpretation of data and revised the manuscript. T. Jeerakathil: helped with the statistical analyses and interpretation of the data and revised the manuscript. K. Butcher: designed and conceptualized the study, analyzed and interpreted the data, and revised the manuscript.
STUDY FUNDING
K.B. holds a Canada Research Chair in Cerebrovascular Disease, a Heart and Stroke Foundation of Alberta Professorship in Stroke Medicine, and a New Investigator Award from Alberta Innovates Health Solutions. L.C.G. and M.P.K. are supported by Clinical Research Fellowship bursaries from the Alberta Innovates Health Solutions. R.T.Z. received a Summer Studentship Award from Alberta Innovates Health Solutions. B.H.R. is supported by CIHR as a Tier I Canada Research Chair in Evidence-based Emergency Medicine through the Government of Canada (Ottawa, ON).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Qureshi AI, Ezzeddine MA, Nasar A, et al. . Prevalence of elevated blood pressure in 563,704 adult patients with stroke presenting to the ED in the United States. Am J Emerg Med 2007;25:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willmot M, Leonardi-Bee J, Bath PMW. High blood pressure in acute stroke and subsequent outcome: a systematic review. Hypertension 2004;43:18–24. [DOI] [PubMed] [Google Scholar]
- 3.Sare GM, Ali M, Shuaib A, Bath PMW. Relationship between hyperacute blood pressure and outcome after ischemic stroke: data from the VISTA collaboration. Stroke 2009;40:2098–2103. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed N, Wahlgren N, Brainin M, et al. . Relationship of blood pressure, antihypertensive therapy, and outcome in ischemic stroke treated with intravenous thrombolysis: retrospective analysis from Safe Implementation of Thrombolysis in Stroke–International Stroke Thrombolysis Register (SITS-ISTR). Stroke 2009;40:2442–2449. [DOI] [PubMed] [Google Scholar]
- 5.Dandapani BK, Suzuki S, Kelley RE, Reyes-Iglesias Y, Duncan RC. Relation between blood pressure and outcome in intracerebral hemorrhage. Stroke 1995;26:21–24. [DOI] [PubMed] [Google Scholar]
- 6.Anderson CS, Heeley E, Huang Y, et al. . Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med 2013;368:2355–2365. [DOI] [PubMed] [Google Scholar]
- 7.Saver JL, Starkman S, Eckstein M, et al. . Prehospital use of magnesium sulfate as neuroprotection in acute stroke. N Engl J Med 2015;372:528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ankolekar S, Fuller M, Cross I, et al. . Feasibility of an ambulance-based stroke trial, and safety of glyceryl trinitrate in ultra-acute stroke: the Rapid Intervention with Glyceryl Trinitrate in Hypertensive Stroke Trial (RIGHT). Stroke 2013;44:3120–3128. [DOI] [PubMed] [Google Scholar]
- 9.Shaw L, Price C, McLure S, et al. . Paramedic Initiated Lisinopril for Acute Stroke Treatment (PIL-FAST): results from the pilot randomised controlled trial. Emerg Med J 2014;31:994–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broderick J, Brott T, Barsan W, et al. . Blood pressure during the first minutes of focal cerebral ischemia. Ann Emerg Med 1993;22:1438–1443. [DOI] [PubMed] [Google Scholar]
- 11.Wallace JD. Blood pressure after stroke. JAMA 1981;246:2177. [PubMed] [Google Scholar]
- 12.Britton M, Carlsson A, de Faire U. Blood pressure course in patients with acute stroke and matched controls. Stroke 1986;17:861–864. [DOI] [PubMed] [Google Scholar]
- 13.Carlberg B, Asplund K, Hagg E. Factors influencing admission blood pressure levels in patients with acute stroke. Stroke 1991;22:527–530. [DOI] [PubMed] [Google Scholar]
- 14.Chamorro A, Vila N, Ascaso C, Elices E, Schonewille W, Blanc R. Blood pressure and functional recovery in acute ischemic stroke. Stroke 1998;29:1850–1853. [DOI] [PubMed] [Google Scholar]
- 15.Okumura K, Ohya Y, Maehara A, Wakugami K, Iseki K, Takishita S. Effects of blood pressure levels on case fatality after acute stroke. J Hypertens 2005;23:1217–1223. [DOI] [PubMed] [Google Scholar]
- 16.Chung T, Gaudet L, Vandenberghe C, et al. . Pre-hospital management of anaphylaxis in one Canadian Urban Centre. Resuscitation 2014;85:1077–1082. [DOI] [PubMed] [Google Scholar]
- 17.Kothari RU, Pancioli A, Liu T, Brott T, Broderick J. Cincinnati Prehospital Stroke Scale: reproducibility and validity. Ann Emerg Med 1999;33:373–378. [DOI] [PubMed] [Google Scholar]
- 18.Adams HP, Bendixen BH, Kappelle LJ, et al. . Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 19.Irisawa T, Iwami T, Kitamura T, et al. . An association between systolic blood pressure and stroke among patients with impaired consciousness in out-of-hospital emergency settings. BMC Emerg Med 2013;13:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandset EC, Bath PMW, Boysen G, et al. . The angiotensin-receptor blocker candesartan for treatment of acute stroke (SCAST): a randomised, placebo-controlled, double-blind trial. Lancet 2011;377:741–750. [DOI] [PubMed] [Google Scholar]
- 21.Potter JF, Robinson TG, Ford GA, et al. . Controlling hypertension and hypotension immediately post-stroke (CHHIPS): a randomised, placebo-controlled, double-blind pilot trial. Lancet Neurol 2009;8:48–56. [DOI] [PubMed] [Google Scholar]
- 22.Robinson TG, Potter JF, Ford GA, et al. . Effects of antihypertensive treatment after acute stroke in the Continue or Stop Post-Stroke Antihypertensives Collaborative Study (COSSACS): a prospective, randomised, open, blinded-endpoint trial. Lancet Neurol 2010;9:767–775. [DOI] [PubMed] [Google Scholar]
- 23.He J, Zhang Y, Xu T, et al. . Effects of immediate blood pressure reduction on death and major disability in patients with acute ischemic stroke: the CATIS randomized clinical trial. JAMA 2014;311:479–489. [DOI] [PubMed] [Google Scholar]
- 24.ENOS Trial Investigators, Bath PM, Woodhouse L, et al. . Efficacy of nitric oxide, with or without continuing antihypertensive treatment, for management of high blood pressure in acute stroke (ENOS): a partial-factorial randomised controlled trial. Lancet 2015;385:617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis SM, Broderick J, Hennerici M, et al. . Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006;66:1175–1181. [DOI] [PubMed] [Google Scholar]
- 26.Brott T, Broderick J, Kothari R, et al. . Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke 1997;28:1–5. [DOI] [PubMed] [Google Scholar]
- 27.Anderson CS, Huang Y, Wang JG, et al. . Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomised pilot trial. Lancet Neurol 2008;7:391–399. [DOI] [PubMed] [Google Scholar]
- 28.Morgenstern LB, Hemphill JC, Anderson C, et al. . Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2010;41:2108–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jauch EC, Saver JL, Adams HP, et al. . Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870–947. [DOI] [PubMed] [Google Scholar]
- 30.Field Administration of Stroke Therapy-Blood Pressure Lowering (FAST-BP). www.clinicaltrials.gov: NCT01811693. [Google Scholar]
- 31.Buck BH, Starkman S, Eckstein M, et al. . Dispatcher recognition of stroke using the National Academy Medical Priority Dispatch System. Stroke 2009;40:2027–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.