Abstract
Despite considerable effort over a century and the benefit of remarkable technical advances in the past few decades, we are still far from understanding mammalian cerebral cortex. With its six layers, modular architecture, canonical circuits, innumerable cell types, and computational complexity, isocortex remains a challenging mystery. In this review, we argue that identifying the structural and functional similarities between mammalian piriform cortex and reptilian dorsal cortex could help reveal common organizational and computational principles and by extension, some of the most primordial computations carried out in cortical networks.
Introduction
Despite considerable effort over a century and the benefit of remarkable technical advances in the past few decades, we are still far from understanding mammalian cerebral cortex. With its six layers, modular architecture, canonical circuits[1], innumerable cell types[2], and computational complexity[3], isocortex remains a challenging mystery. Isocortex most likely evolved from simpler layered circuits in the forebrain of ancestral amniotes, structures that we still find in mammals today, as paleo- and archi-cortices (piriform and hippocampal formations, respectively), together with a few “transitional” areas with n (3 ≤ n < 6) layers[4].
Among three-layered cortices in mammals, piriform cortex (PCx) is a good model system to investigate the function, dynamics and computational properties of cortical circuits. Understanding piriform cortex function, however, is made difficult by the complexity of the sensory space it subserves and the current lack of common metrics to describe the relevant psychophysical dimensions of olfactory perception[5].
Simple cortices are not limited to the olfactory system. In reptiles, the entire cerebral cortex is composed of only three layers and some of these cortices are primary sensory areas. The visual cortex of turtles (dorsal cortex, DCx) and the mammalian piriform cortex (PCx) hold very similar positions along their respective sensory pathways. They are just one processing station—the lateral geniculate nucleus (LGN), or the olfactory bulb (OB)—removed from their respective sense organ. Our current understanding of sensory processing in turtle visual cortex is still limited, but one notable advantage of this system is that its sensory input space is more easily defined.
Hodology and transcription factor expression during development suggest that the three layers of reptilian cortex may be homologous to layers 1,5, and 6 of the mammalian isocortex[6]. In this review, we argue that identifying the structural and functional similarities between PCx and DCx could help reveal common organizational and computational principles and by extension, some of the most primordial computations carried out in cortical networks.
Vertical Connectivity
The architecture of PCx and DCx is archetypal of a three-layered paleocortex. Layer 1 contains mainly dendrites of layer 2 principal cells, a few scattered interneurons and afferent and local axons. Layer 2 contains the densely packed somata of pyramidal cells, whose apical dendrites run radially towards the pial surface. Layer 3 contains basal dendrites of pyramidal cells, corticofugal and local axons, some interneurons and a few deep pyramidal neurons in PCx [7,8]. Incoming afferents to PCx run through the lateral olfactory tract (LOT)[9]; those to DCx through the lateral forebrain bundle (LFB)[10]. These input fibers fan out below the pial surface and make en-passant synapses on cortical neurons within the distal 50-100 µm of layer 1[11,12]. Afferent synapses impinge on both layer-1 interneurons and on distal dendrites of layer-2 pyramidal cells; interneurons provide both feed-forward and feedback inhibition to pyramidal cells which themselves provide recurrent excitation to other pyramidal neurons[12–16]. In both PCx and DCx, superficial layer-1 interneurons tend to receive a higher density of afferent input than pyramidal cells do[12,14,17] which, combined with a strong feed-back inhibition via layer-2/3 interneurons[14,15,17] may explain the observed strong inhibition evoked by sensory stimulation and the sparseness of pyramidal cell firing. To a first degree, PCx and DCx thus have a similar microcircuit layout: both exhibit distal dendritic excitation from sensory afferents, strong feed-forward inhibition, recurrent excitation through the so-called associational intracortical connections, and feed-back inhibition[18,19].
Different cell types have been identified in PCx. Most segregate into specific sub-layers of the piriform microcircuit. Excitatory neurons in layer 2 can be subdivided in semilunar (upper layer 2) and superficial pyramidal neurons (lower layer 2) while those in layer 3 comprise a few deep pyramidal cells and scattered multipolar spiny glutamatergic neurons[20–22]. Although they are embedded in the same basic connectivity scheme, semilunar and superficial pyramidal cells receive different ratios of afferent to associational inputs, and may thus belong to distinct functional sub-circuits[13] (but see[23]), consistent with morphological differences between their dendritic trees and their laminar position [24]. Although data on subpopulations of principal cells in DCx are few, analysis of Golgi-stained material also revealed different morphological classes of spiny neurons at different laminar and sublaminar positions in reptilian cortex[25,26]. PCx and DCx pyramidal neurons are also similar with respect to their dendritic electrophysiological properties, suggesting comparable integrative properties at the subcellular level[27,28]. Different subtypes of inhibitory interneurons have been identified in PCx, based on molecular markers, the morphology of their dendritic arbor and the distribution of their axonal projections (reviewed in [29]). These sub-classes seem to correlate with the type of inhibition they subserve, i.e., primarily feedback or feed-forward. Horizontal and neurogliaform interneurons in layer 1 receive afferent inputs from the LOT and mediate fast feed-forward inhibition targeting apical dendrites of layer-2 pyramidal cells. Bitufted, fast-spiking and regular spiking interneurons from layers 2 and 3 receive very little direct afferent input from the LOT but provide strong feed-back inhibition onto the somata and basal dendrites of pyramidal cells[14,17]. Similarly, different populations of inhibitory interneurons in turtle DCx subserve mainly feed-forward (subpial cells[16]) or feedback[16,30] inhibition. Axonal reconstructions of DCx interneurons[31] and immunocytochemical labeling[32,33] suggest the existence of morphologically and physiologically identifiable classes of inhibitory interneurons. It remains to be shown that those groupings also share functional similarities with those in PCx. Given the anatomical similarity of input projections to PCx and DCx, one may speculate that the inhibitory circuit topology of these two cortices could also be similar.
Horizontal Connectivity
In PCx, afferents from mitral/tufted (MT) cells appear to project throughout the cortex without any clear topographical relationship to their glomeruli of origin[9,34–37]. Although this does not rule out the possibility of some fine-scale topographical mapping of OB projections, (e.g., mitral vs. tufted cell projections[38]), it is now accepted that the glomerular clustering of olfactory receptor cells axons in OB is entirely discarded at the level of PCx[39]. In DCx, early tracing studies from Ulinski and colleagues suggested that the visual field is projected onto the rostro-caudal axis of DCx in the form of iso-azimuth lamellae covering the naso-temporal dimension of the visual field[10,40]. Such a mapping of projections still awaits physiological confirmation and fine thalamo-cortical projection tracing. If confirmed, this topographical mapping would differ from the topology of mammalian olfactory projections to PCx, at least along one cortical dimension.
In both PCx and DCx, the density of sensory afferents varies over the cortical surface: high rostrally and laterally, it decreases progressively as one moves away from the entry point of the LOT (PCx) or the LFB (DCx). Hence, the balance between afferent and associational connectivity decreases along the rostro-caudal and latero-medial (or ventro-dorsal) axes[10,18,39,41,42]. PCx is subdivided into anterior and posterior regions, which differ not only in the density of afferent vs associational fibers[18] but also in the properties of odor-evoked responses[43,44]. PCx microcircuits may also contain fine-grain connectivity gradients: in vitro recordings from aPCx reveal that inhibition of pyramidal cells is asymmetric and stronger along the rostro-caudal axis of the anterior part of PCx, over distances as short as 200 µm[45]. In turtle, DCx has been classically divided into two different regions (D2 and D1) along the latero-medial axis[8,26]. This dichotomy rests mostly on cytoarchitectural features, related to the thickness of subcellular layer 3—thick in D2 laterally, thin in D1, with a significant transition zone between the two. Recent molecular data suggest that this separation may be correlated with higher expression level of layer-4 markers in D2 [46]. Confirmation of this division and of its potential functional significance needs additional work. Such gradients of connectivity across the cortical surface (in PCx and DCx) should be clearly described because any horizontal heterogeneity could influence the propagation and reverberation of activity across cortex, under the combined influences of spreading afferent input and widespread associational activity.
Given their reciprocal interconnections with high-order cortical areas and a lack of evident sensory topography, PCx and DCx are sometime described as associational rather than primary sensory cortices[19]. The major partners of PCx are the orbitofrontal cortex[47,48], the lateral entorhinal cortex[49,50] and the agranular insular cortex[50]. Connectivity to these downstream targets differs between aPCx and pPCx, supporting the notion that they play different functions. Similarly, DCx is reciprocally connected to dorso-medial (DMCx) and medial (MCx) cortices[25,26]. Those regions are, on the basis of hodology and position, often compared to parahippocampal and hippocampal cortices[26,51–53]. Both PCx and DCx are thus directly connected to associational networks, likely involved in controlling or modulating behavior.
PCx and DCx are further interconnected with other cortical-like areas, which also receive parallel sensory afferents from the OB or the LGN respectively. For PCx, these include the anterior olfactory nucleus (AON)[54,55], the olfactory tubercule (OT)[54], and the amygdala[50,56]. AON might be a first stage of odorant-feature processing, in turn used by PCx to detect complex odorant combinations[18,57,58]. DCx’s AON-equivalent could be the pallial thickening (PT), for it receives direct thalamic afferent input and projects to DCx[10,59]. If AON and PT also share functional characteristics, these similarities may point to common elementary processing streams of three-layered sensory cortices.
Coding and Sensory Representation
To a first degree, functional investigations of olfactory tuning on PCx neurons confirm anatomical results: the discretization of the olfactory bulb into glomerular domains disappears in PCx. Instead, odorants activate ensemble of PCx neurons, scattered over the cortical surface, with no apparent spatial clustering[35,60–62]. Both the dispersion of afferent bulbar inputs and a widespread network of associational connections likely contribute to the spatial spread and heterogeneity of PCx-neuron response selectivity[23,63,64]. This lack of visible organization of population responses is similar to that observed in the insect mushroom body, a structure directly postsynaptic to the antennal lobe, itself analogous to the olfactory bulb[65]. It may thus be a deep feature of this early encoding stage for odors[66].
A similar situation seems to hold true for DCx, although studies of RF mapping in turtle DCx are few[67]. In all such experiments, most cells were activated indiscriminately wherever a stimulus (typically a small dot) was flashed in the visual field, unlike thalamic neurons which exhibit spatially restricted RFs[68]. Voltage-sensitive-dye (VSD) recordings of DCx responses to stimulation of four visual quadrants yielded similar activity patterns across the cortical surface, consistent with the absence of clear retinotopic mapping of visual space along the surface of DCx[69]. Although VSD experiments reveal no functional evidence for the anatomical lamellae of thalamo-cortical projections[10], they do not necessarily disprove the older tracing studies. For example, widespread associational connections could easily mask the topography of thalamo-cortical projections.
If true, the absence of cortical retinotopy in DCx suggests a few remarks. (i) That three-layered reptilian visual cortex is not organized along the same principles as mammalian primary visual isocortex. (ii) That projections to a sensory three-layered cortex lack the functional, developmental or molecular substrates for spatial or functional segregation. Some have indeed argued that this diffuse organization represents the primordial structure of sensory cortex, prior to the evolution of isocortex in the synapsid and later, mammalian lineage[6]. (iii) That the computational properties of turtle primary visual cortex are more similar in essence to those of high-order cortices (e.g. parahippocampal, retrosplenial or infero-temporal), and that the true response properties of DCx neurons have yet to be discovered.
Until recently, functional experiments in PCx relied on sampling neuronal responses to limited sets of odors. Although these studies spanned stimulus sets large enough to identify the dispersion of RF selectivity across the cortical surface, they did not allow an evaluation of the actual “size” of these RFs along the many dimensions of odor space. Recent studies examined how PCx process patterns of activity in the bulb by direct stimulation of ensembles of glomeruli using photo-uncaging of glutamate[64] or optogenetic stimulation[70]. These studies indicate that individual PCx neurons respond selectively to distinct combinations of active glomeruli[64] and are sensitive to the temporal sequence of activation[70]. A more exhaustive exploration of this sensory space might allow one to better estimate the selectivity of PCx RFs, thereby facilitating comparisons with DCx. Although both PCx and DCx clearly exhibit no mapping of the first-order physical dimensions of their respective sensory space, they may both represent sensory features in some abstract and related feature spaces[39]. Mazurskaya[67] observed that, although DCx visual neurons respond unselectively to any flash of light, they may respond to pairs of flashes with sub- or supra-linear summation depending on the relative timing and spatial separation of the two stimuli, suggesting selectivity to high-order spatiotemporal correlations in the visual field. It could be that DCx neurons are selective to high-order correlations, and process spatio-temporal sequences of distributed visual cues in a manner similar to how PCx processes spatio-temporal activation of specific glomeruli.
Cortical dynamics and oscillations
As observed in many sensory systems, PCx and DCx exhibit various types of oscillations. In PCx, these oscillations are usually split into 3 frequency bands: slow respiratory theta rhythm (1-15Hz); beta (15-35Hz); and gamma (40-100Hz)[71]. Although gamma has long been a focus of research in mammalian cortex, beta oscillations have, over recent years, grown in importance in olfactory studies. Interestingly, 20-Hz oscillations are a prominent feature of population activity also in some insect species[66]. Sensory evoked LFP responses in DCx and PCx both exhibit a noticeable increase in beta-frequency oscillations following sensory stimulation in both anaesthetized and awake cortical states[72–76]. It is currently difficult to assess whether beta oscillations in PCx and DCx share more than just a frequency and if they contribute to information processing in similar ways. The similarity, however, may be linked to common underlying mechanisms of generation. Except for the fact that beta oscillations in OB precede those in PCx and hippocampus [72,74,77] but require intact feedback between PCx and OB[78], we know little about the mechanistic origin and role of beta. Beta power in PCx appears correlated with behavioral context; it increases during learning of a discrimination task[73,79] and is correlated with pattern completion[73]. In DCx, visually evoked beta oscillations appear to be coherent across the surface of DCx, with a rostro-caudal phase-lag consistent with the propagation of waves[75,80]. It was suggested that some components of these waves may encode spatial information about the stimulus[69,81]. However, physiological data are still missing, and whether cortical waves in DCx are reliable enough to represent efficiently the spatiotemporal position of visual cues (or any other feature) remains conjectural.
Beta coherence has been investigated across different areas of PCx. Available data suggest rather short delays over long cortical distances between paired recording sites[62,72,77,82]. Nevertheless, the issue as to whether (and how) these oscillations propagate through the piriform network remains largely unexplored. Coherent beta oscillations between different olfactory areas have been observed, especially during odor learning and memory retrieval[79,83]. Theoretical work showed that beta frequencies are better suited than gamma oscillations to carry information over long distances[84]. This suggests that beta could contribute to synchronizing the activity of PCx with downstream targets. Assuming similarly distributed codes for PCx and DCx, beta oscillations might serve to support the formation of cell assemblies across their respective networks, synchronizing neurons by stimulus selectivity rather than position. Such role would probably require phase-locking of odor-evoked spiking to beta oscillations, to enable a concerted influence on downstream targets. Poo and Isaacson[62] showed that PCx neurons responses are phase-locked to beta oscillations as a result of a phase shift between excitatory and inhibitory synaptic drives. The preferred phase of firing was apparently cell- rather than stimulus-specific. More work is needed to elucidate whether or not cells with similar odor selectivity tend to have similar phase relationship to the beta oscillation cycle.
Conclusion
Piriform cortex and turtle dorsal cortex are good model systems to investigate sensory processing in cortical circuits; given their simple architecture, the mapping of elementary computations on specific circuit elements should be easier than with isocortex. Unfortunately, however, we have no clear understanding of the exact functional operations performed by these two cortices. PCx and DCx seem to process sensory inputs more like high-order cortical areas than primary sensory neocortex. According to this view, if we assume that the three-layered cortex of extant amniotes conserved functional features of the cortex of early amniotes (some 300 MYA), we would conclude that computations performed by high-order cortical areas are ancestral rather than evolved and that many operations found at initial stages of neocortical processing (first-order feature detection, local contrast enhancement…) appeared later in evolution, possibly linked to the additions of new layers (2/3,4), specific to mammalian neocortex. Despite obvious differences between visual and olfactory signals, sensory coding in PCx and DCx might follow a similar functional logic, focused on behaviorally relevant features. Haberly[18] postulated that PCx may function as a combinatorial/associative array, performing recognition of OB activity patterns encoded in specific cortical cell assemblies that may contribute, after reinforcement, to memory formation and recall of relevant sensory experiences. Experimental evidence shows that functional connectivity in PCx is modified during associative learning[44,73,78,79,85,86]. Similarly, lesion experiments in turtles suggest a role for dorsal and medial cortices in spatial learning and memory formation[52,87,88]. Visual processing in DCx might thus be closer to that in mammalian parahippocampal[89] or retrosplenial cortices[90]. Haberly[18] proposed that the topology and plasticity of PCx afferent and auto-associational connections are well suited to perform contextual learning of high-dimensional stimulus features. Plasticity has not yet been explored in DCx. But if DCx reveals experience-dependent changes in its functional connectivity, it would be an additional argument for considering PCx and DCx as equivalent networks, optimized for object recognition in a sensory landscape (made of odors or visual cues) whose relevant perceptual dimensions are dynamically shaped by sensory experience.
Highlights.
We review the recent and less recent literature on mammalian piriform cortex and on reptilian dorsal cortex.
We try to identify common organizational, dynamical and functional principles from those studies
We emphasize notable differences between these simpler cortices and known primary sensory neocortical areas
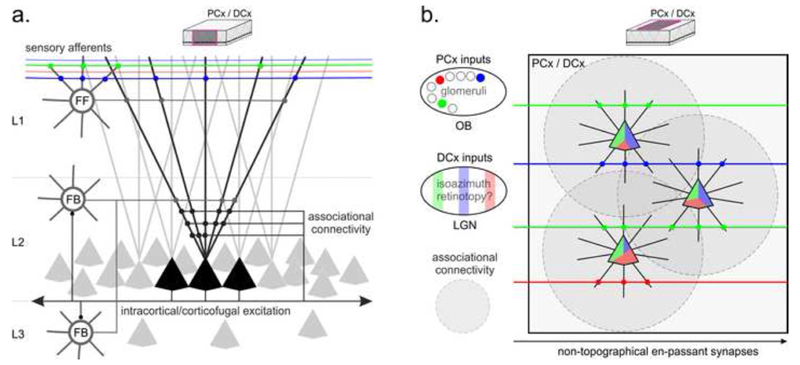
Figure. Connectivity in mammalian piriform cortex (PCx) and turtle dorsal cortex (DCx).
(a). Transverse view (see inset) of the basic microcircuits. Sensory afferents from the Lateral Olfactory Tract (in PCx) or Lateral Forebrain Bundle (in DCx) make en-passant synapses in superficial layer 1 on distal segments of layer-2 pyramidal cell dendrites and on superficial inhibitory interneurons. Layer-2 pyramidal neurons receive recurrent excitation from other pyramidal cells (associational connectivity), feed-forward inhibition from superficial interneurons (FF), and feed-back inhibition from layer-2/3 interneurons (FB). (b) Top view (see inset) of PCx and DCx connectivity. Afferents from the olfactory bulb (OB) project to PCx without apparent topographical order. In DCx, there may be a coarse topography of lateral geniculate nucleus (LGN) projections that preserves visual isoazimuth neighborhoods [10,40]. In both cases, recurrent excitation through local (grey) and long-range (not shown) associational connections contributes to broadening the stimulus selectivity of pyramidal cells and may mask any local anisotropy in the spatial distribution of the primary sensory afferents (see color tiles).
Acknowledgements
This work was supported by the Max Planck Society, the European Research Council and the Human Frontier Science Program (JF). We thank Stephan Junek, Robert Naumann and Mike Hemberger for their comments on the manuscript.
Footnotes
Conflict Of Interest Form:
None
References
- 1.Douglas RJ, Martin R. Canonical Cortical Circuits. In: Shepherd GM, Grillner S, editors. Handbook of Brain Microcircuits. New York: Oxford University Press; 2010. pp. 15–21. [Google Scholar]
- 2.Markram H. Microcircuitry of the neocortex. In: Shepherd GM, Grillner S, editors. Handbook of Brain Microcircuits. Oxford university press; 2010. pp. 22–30. [Google Scholar]
- 3.Frégnac Y, Rudolph M, Davison AP, Destexhe A. Complexity in Neuronal Networks. In: Kepes F, editor. Biological Networks. World scientific; 2006. pp. 291–338. [Google Scholar]
- 4.Brodman K, Garey LJ. Brodmann’s Localization in the Cerebral Cortex. Springer-Verlag; 2006. [Google Scholar]
- 5.Arzi A, Sobel N. Olfactory perception as a compass for olfactory neural maps. Trends in Cognitive Sciences. 2011;15:537–545. doi: 10.1016/j.tics.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Aboitiz F, Zamorano F. Neural progenitors, patterning and ecology in neocortical origins. Frontiers in Neuroanatomy. 2013;7:1–15. doi: 10.3389/fnana.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neville KR, Haberly LB. Olfactory cortex. In: Shepherd GM, editor. The synaptic organization of the brain. Oxford, New York: UP; 2004. pp. 415–454. [Google Scholar]
- 8.Ulinski PS. The cerebral cortex of reptiles. Cerebral Cortex. 1990;8A:139–216. [Google Scholar]
- 9.Sosulski DL, Bloom ML, Cutforth T, Axel R, Datta SR. Distinct representations of olfactory information in different cortical centres. Nature. 2011;472:213–216. doi: 10.1038/nature09868. [Sosulski DL, Bloom ML, Cutforth T, Axel R, Datta SR: Distinct representations of olfactory information in different cortical centres. Nature 2011, 472:213–216] [Using dye electroporation of mitral and tufted cells in mice olfactory bulb, the authors traced axonal projections from individual glomeruli to PCx and cortical amygdala. They show that glomeruli project diffusely to PCx without spatial preference while in the cortical amygdala, glomeruli projections are patchy and reveal stereotyped spatial segregation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mulligan KA, Ulinski PS. Organization of Geniculocortical Projections in Turtles : Isoazimuth Lamellae in the Visual Cortex. The Journal of Comparative Neurology. 1990;296:531–547. doi: 10.1002/cne.902960403. [DOI] [PubMed] [Google Scholar]
- 11.Haberly L, Behan M. Structure of the piriform cortex of the opossum. III. Ultrastructural characterization of synaptic terminals of association and olfactory bulb afferent fibers. The Journal of Comparative Neurology. 1983;219:448–60. doi: 10.1002/cne.902190406. [DOI] [PubMed] [Google Scholar]
- 12.Smith LM, Ebner FF, Colonnier M. The Thalamocortical Projection in Pseudemys Turtles : A Quantitative Electron Microsco pic Study. The Journal of Comparative Neurology. 1980;461:445–461. doi: 10.1002/cne.901900304. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki N, Bekkers JM. Two Layers of Synaptic Processing by Principal Neurons in Piriform Cortex. The Journal of Neuroscience. 2011;31:2156–2166. doi: 10.1523/JNEUROSCI.5430-10.2011. [Suzuki N, Bekkers JM: Two Layers of Synaptic Processing by Principal Neurons in Piriform Cortex. The Journal of neuroscience 2011, 31:2156–2166] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki N, Bekkers JM. Microcircuits Mediating Feedforward and Feedback Synaptic Inhibition in the Piriform Cortex. The Journal of Neuroscience. 2012;32:919–931. doi: 10.1523/JNEUROSCI.4112-11.2012. [Suzuki N, Bekkers JM: Microcircuits Mediating Feedforward and Feedback Synaptic Inhibition in the Piriform Cortex. The Journal of neuroscience 2012, 32:919–931] [In this paper and the one above, the authors identified elementary circuit motifs in PCx using in vitro patch-clamp recordings from acute slices. In particular, they show that semilunar and superficial pyramidal cells receive distinct balances of afferent vs. associational synaptic inputs and that feedforward and feed-back inhibition in PCx originate from different inhibitory cell types segregating in distinct layers.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kriegstein R, Connors BW. Cellular Physiology of the Turtle Visual Cortex : Synaptic Properties and Intrinsic Circuitry. The Journal of Neuroscience. 1986;6:178–191. doi: 10.1523/JNEUROSCI.06-01-00178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mancilla JG, Fowler M, Ulinski PS. Responses of regular spiking and fast spiking cells in turtle visual cortex to light flashes. Visual Neuroscience. 1998;15:979–93. doi: 10.1017/s0952523898155190. [DOI] [PubMed] [Google Scholar]
- 17.Stokes CCA, Isaacson JS. From Dendrite to Soma : Dynamic Routing of Inhibition by Complementary Interneuron Microcircuits in Olfactory Cortex. Neuron. 2010;67:452–465. doi: 10.1016/j.neuron.2010.06.029. [Stokes CCA, Isaacson JS: From Dendrite to Soma : Dynamic Routing of Inhibition by Complementary Interneuron Microcircuits in Olfactory Cortex. Neuron 2010, 67:452–465] [The authors investigated in vitro the connectivity and temporal dynamics of inhibition in PCx. They show that layer-1 interneurons receive stronger afferent input from the LOT than layer-2 pyramidal cell do and provide early-onset feed-forward inhibition to distal dendrites of pyramidal cells. On the other hand, layer-3 interneurons are activated exclusively by recurrent excitation from pyramidal cells and are responsible for a late onset feed-back inhibition onto a large fraction of local pyramidal cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haberly LB. Parallel-distributed Processing in Olfactory Cortex : New Insights from Morphological and Physiological Analysis of Neuronal Circuitry. Chemical Senses. 2001;26:551–576. doi: 10.1093/chemse/26.5.551. [DOI] [PubMed] [Google Scholar]
- 19.Shepherd GM. The microcircuit concept applied to cortical evolution : from three-layer to six-layer cortex. Frontiers in Neuroanatomy. 2011;5:1–15. doi: 10.3389/fnana.2011.00030. [Shepherd GM: The microcircuit concept applied to cortical evolution : from three-layer to six-layer cortex. frontiers in neuroanatomy 2011, 5:1–15] [The author reviews evidences for basic circuit motifs common to cortical circuits by comparing six-layer neocortex and three-layer olfactory, hippocampal and turtle cortices.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haberly LB. Structure of the Piriform Cortex of the Opossum. I. Description of Neuron Types With Golgi Methods. The Journal of Comparative Neurology. 1983;213:163–187. doi: 10.1002/cne.902130205. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki N, Bekkers JM. Neural Coding by Two Classes of Principal Cells in the Mouse Piriform Cortex. The Journal of Neuroscience. 2006;26:11938–11947. doi: 10.1523/JNEUROSCI.3473-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bekkers JM, Suzuki N. Neurons and circuits for odor processing in the piriform cortex. Trends in Neurosciences. 2013;36:429–438. doi: 10.1016/j.tins.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Poo C, Isaacson JS. A major role for Intracortical Circuits in the Strength and Tuning of Odor-Evoked Excitation in Olfactory Cortex. Neuron. 2011;72:41–48. doi: 10.1016/j.neuron.2011.08.015. [Poo C, Isaacson JS: A major role for Intracortical Circuits in the Strength and Tuning of Odor-Evoked Excitation in Olfactory Cortex. Neuron 2011, 72:41–48] [Using whole-cell voltage-clamp recording in vivo and local application of a GABAb receptor blocker, the authors investigated the effect of silencing intracortical recurrent excitatory inputs on the selectivity of PCx neurons to odors. They show that the tuning of PCx pyramidal cells to odor is primarily determined by the strength of intracortical associational inputs rather than by the degree of convergence of olfactory bulb inputs.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiegand HF, Beed P, Bendels MHK, Leibold C, Schmitz D, Johenning FW. Complementary Sensory and Associative Microcircuitry in Primary Olfactory Cortex. The Journal of Neuroscience. 2011;31:12149–12158. doi: 10.1523/JNEUROSCI.0285-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ulinski PS. Intrinsic organization of snake medial cortex: an electron microscopic and Golgi study. Journal of Morphology. 1977;152:247–79. doi: 10.1002/jmor.1051520208. [DOI] [PubMed] [Google Scholar]
- 26.Desan PH. The organization of the cerebral cortex of the pond turtle, Pseudemys scripta elegans. PhD thesis; Cambridge, MA: Harvard University; 1984. [Desan PH: The organization of the cerebral cortex of the pond turtle, Pseudemys scripta elegans. 1984, PhD Thesis, Harvard University] [In his doctoral thesis, Desan described the main morphological cell types present in turtle dorsal cortex and identified the principal afferent and efferent connections of the cerebral cortex of turtles using retrograde and anterograde tracers.] [Google Scholar]
- 27.Larkum ME, Watanabe S, Lasser-ross N, Rhodes P, Ross WN, Ledergerber D, Larkum ME. Dendritic Properties of Turtle Pyramidal Neurons. Journal of Neurophysiology. 2008;99:683–694. doi: 10.1152/jn.01076.2007. [Larkum ME, Watanabe S, Lasser-ross N, Rhodes P, Ross WN, Ledergerber D, Larkum ME: Dendritic Properties of Turtle Pyramidal Neurons. Journal of Neurophysiololy 2008, 99:683–694] [In this study, the authors performed whole-cell, dendritic and axonal patch-clamp recordings to characterize the electrophysiological properties of turtle cortex pyramidal neurons.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bathellier B, Margrie TW, Larkum ME. Properties of Piriform Cortex Pyramidal Cell Dendrites : Implications for Olfactory Circuit Design. The Journal of Neuroscience. 2009;29:12641–12652. doi: 10.1523/JNEUROSCI.1124-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki N, Bekkers JM. inhibitory interneurons in the piriform cortex. Clinical and Experimental Pharmacology and Physiology. 2007;34:1064–1069. doi: 10.1111/j.1440-1681.2007.04723.x. [DOI] [PubMed] [Google Scholar]
- 30.Connors BW, Kriegstein AFL. Cellular Physiology of the Turtle Visual Cortex : Distinctive Properties of Pyramidal and Stellate Neurons. The Journal of Neuroscience. 1986;6:164–177. doi: 10.1523/JNEUROSCI.06-01-00164.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colombe JB, Sylvester J, Block J. Subpial and Stellate Cells : Two Populations of Interneurons in Turtle Visual Cortex. The Journal of Comparative Neurology. 2004;471:333–351. doi: 10.1002/cne.20037. [DOI] [PubMed] [Google Scholar]
- 32.Reiner A. A comparison of the neurotransmitter-specific and neuropeptide-specific neuronal cell types present in turtle cortex to those present in mammalian isocortex: implications for the evolution of isocortex. Brain Behavioral Evolution. 1991;38:53–91. doi: 10.1159/000114379. [DOI] [PubMed] [Google Scholar]
- 33.Reiner A. Neurotransmitter organization and connections of turtle cortex: implications for the evolution of mammalian isocortex. Comparative Biochemical Physiology. 1993;104:735–748. doi: 10.1016/0300-9629(93)90149-x. [DOI] [PubMed] [Google Scholar]
- 34.Miyamichi K, Amat F, Moussavi F, Wang C, Wickersham I, Wall NR, Taniguchi H, Tasic B, Huang ZJ, He Z, et al. Cortical representations of olfactory input by trans-synaptic tracing. Nature. 2011;472:191–196. doi: 10.1038/nature09714. [Miyamichi K, Amat F, Moussavi F, Wang C, Wickersham I, Wall NR, Taniguchi H, Tasic B, Huang ZJ, He Z, et al.: Cortical representations of olfactory input by trans-synaptic tracing. Nature 2011, 472:191–196] [Using transynaptic-virus-dependent retrograde labelling, the authors traced the projections from mitral and tufted cells to the piriform cortex and cortical amygdala. They showed that afferent inputs to individual PCx neurons originate from multiple glomeruli that are distributed across the olfactory bulb and that mitral cells from the same glomerulus project independently to PCx neurons.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Illig KR, Haberly LB. Odor-Evoked Activity is Spatially Distributed in Piriform Cortex. The Journal of Comparative Neurology. 2003;457:361–373. doi: 10.1002/cne.10557. [DOI] [PubMed] [Google Scholar]
- 36.Apicella A, Yuan Q, Scanziani M, Isaacson JS. Pyramidal Cells in Piriform Cortex Receive Convergent Input from Distinct Olfactory Bulb Glomeruli. The Journal of Neuroscience. 2010;30:14255–14260. doi: 10.1523/JNEUROSCI.2747-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghosh S, Larson SD, Hefzi H, Marnoy Z, Cutforth T, Dokka K, Baldwin KK. Sensory msps in the olfactory cortex defined by long-range viral tracing of single neurons. Nature. 2011;472:217–220. doi: 10.1038/nature09945. [Ghosh S, Larson SD, Hefzi H, Marnoy Z, Cutforth T, Dokka K, Baldwin KK: Sensory msps in the olfactory cortex defined by long-range viral tracing of single neurons. Nature 2011, 472:217–220] [Using a viral-based anterograde tracing technique, these authors compared axonal projections of single mitral cells to different cortical regions. In particular, they demonstrate that the axonal arborizations of mitral cells originating from the same glomerulus have no more overlap in PCx than those originating from different glomeruli.] [DOI] [PubMed] [Google Scholar]
- 38.Igarashi KM, Ieki N, An M, Yamaguchi Y, Nagayama S, Kobayakawa K, Kobayakawa R, Tanifuji M, Sakano H, Chen WR, et al. Parallel Mitral and Tufted Cell Pathways Route Distinct Odor Information to Different Targets in the Olfactory Cortex. The Journal of Neuroscience. 2012;32:7970–7985. doi: 10.1523/JNEUROSCI.0154-12.2012. [Igarashi KM, Ieki N, An M, Yamaguchi Y, Nagayama S, Kobayakawa K, Kobayakawa R, Tanifuji M, Sakano H, Chen WR, et al.: Parallel Mitral and Tufted Cell Pathways Route Distinct Odor Information to Different Targets in the Olfactory Cortex. The Journal of Neuroscience 2012, 32:7970–7985] [This study compares mitral and tufted cells odor-evoked response profiles and show evidences that these two cell types target distinct, non-overlapping cortical subregions.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson DA, Sullivan RM. Cortical Processing of Odor Objects. Neuron. 2011;72:506–519. doi: 10.1016/j.neuron.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ulinski PS, Nautiyal J. Organization of Retinogeniculate Projections in Turtles of the Genera Pseudemys and Chrysemys. The Journal of Comparative Neurology. 1988;276:92–112. doi: 10.1002/cne.902760107. [DOI] [PubMed] [Google Scholar]
- 41.Hagiwara A, Pal SK, Sato TF, Wienisch M, Murthy VN, Shepherd GM. Optophysiological analysis of associational circuits in the olfactory cortex. Frontiers in Neural Circuits. 2012;6 doi: 10.3389/fncir.2012.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cosans CE, Ulinski PS. Spatial Organization of Axons in Turtle Visual Cortex : Intralamellar and Interlamellar Projections. The Journal of Comparative Neurology. 1990;296:548–558. doi: 10.1002/cne.902960404. [DOI] [PubMed] [Google Scholar]
- 43.Litaudon P, Amat C, Bertrand B, Vigouroux M, Buonviso N. Piriform cortex functional heterogeneity revealed by cellular responses to odours. European Journal of Neuroscience. 2003;17:2457–2461. doi: 10.1046/j.1460-9568.2003.02654.x. [DOI] [PubMed] [Google Scholar]
- 44.Kadohisa M, Wilson DA. Separate encoding of identity and similarity of complex familiar odors in piriform cortex. Proc Nat Acad Sci USA. 2006;103:15206–15211. doi: 10.1073/pnas.0604313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luna VM, Pettit DL. Asymetric rostro-caudal inhibition in the primary olfactory cortex. Nature Neuroscience. 2010;13:533–535. doi: 10.1038/nn.2524. [Luna VM, Pettit DL: Asymetric rostro-caudal inhibition in the primary olfactory cortex. Nature Neuroscience 2010, 13:533–535] [Using whole-cell patch-clamp and focal uncaging of glutamate on interneurons in PCx slices, the authors showed that inhibition onto layer-2 pyramidal neurons is asymetrical and stronger along the rostro-caudal axis of PCx.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dugas-ford J, Rowell JJ, Ragsdale CW. Cell-type homologies and the origins of the neocortex. Proc Nat Acad Sci USA. 2012;109:16974–16979. doi: 10.1073/pnas.1204773109. [Dugas-ford J, Rowell JJ, Ragsdale CW: Cell-type homologies and the origins of the neocortex. Proc. Nat. Acad. Sci. USA 2012, 109:16974–16979] [The authors investigated the distribution of molecular markers of layer-4 and layer-5 neocortical neurons in turtle and avian cortices. In particular, they found that in turtle DCx, layer-4 and layer-5 neocortical markers segregate horizontally along the rostro-caudal axis and are expressed by distinct cell ensembles.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ekstrand JJ, Domroese ME, Johnson DMG, Feig SL, Knodel SM, Behan M, Haberly LB. A New Subdivision of Anterior Piriform Cortex and Associated Deep Nucleus with Novel Features of Interest for Olfaction and Epilepsy. The Journal of Comparative Neurology. 2001;434:289–307. doi: 10.1002/cne.1178. [DOI] [PubMed] [Google Scholar]
- 48.Illig KR. Projections from Orbitofrontal Cortex to Anterior Piriform Cortex in the Rat Suggest a Role in Olfactory Information Processing. Journal of Comparative Neurology. 2006;488:224–231. doi: 10.1002/cne.20595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kerr KM, Agster KL, Furtak SC, Burwell RD. Functional Neuroanatomy of the Parahippocampal Region : The Lateral and Medial Entorhinal Areas. Hippocampus. 2007;17:697–708. doi: 10.1002/hipo.20315. [DOI] [PubMed] [Google Scholar]
- 50.Johnson DMG, Illig KR, Behan M, Haberly LB. New Features of Connectivity in Piriform Cortex Visualized by Intracellular Injection of Pyramidal Cells Suggest that “ Primary ” Olfactory Cortex Functions Like “ Association ” Cortex in Other Sensory Systems. The Journal of Neuroscience. 2000;20:6974–6982. doi: 10.1523/JNEUROSCI.20-18-06974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Northcutt RG. Evolution of the telencephalon in nonmammals. Annu Rev Neurosci. 1981;4:301–350. doi: 10.1146/annurev.ne.04.030181.001505. [DOI] [PubMed] [Google Scholar]
- 52.López JC, Vargas JP, Gómez Y, Salas C. Spatial and non-spatial learning in turtles : the role of medial cortex. Behavioural Brain Research. 2003;143:109–120. doi: 10.1016/s0166-4328(03)00030-5. [DOI] [PubMed] [Google Scholar]
- 53.Aboitiz F, Morales D, Montiel J. The evolutionary origin of the mammalian isocortex: towards an integrated developmental and functional approach. Behavioral and Brain sciences. 2003;26:535–52. doi: 10.1017/s0140525x03000128. [DOI] [PubMed] [Google Scholar]
- 54.Haberly LB, Price JL. Association and Commissural Fiber Systems of the Olfactory Cortex of the Rat. Journal of Comparative Neurology. 1978;178:711–740. doi: 10.1002/cne.901780408. [DOI] [PubMed] [Google Scholar]
- 55.Illig KR, Eudy JD. Contralateral Projections of the Rat Anterior Olfactory Nucleus. Journal of Comparative Neurology. 2009;512:115–123. doi: 10.1002/cne.21900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luna VM, Morozov A. Input-specific excitation of olfactory cortex microcircuits. Frontiers in Neural Circuits. 2012;6:1–7. doi: 10.3389/fncir.2012.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lei H, Mooney R, Katz LC. Synaptic Integration of Olfactory Information in Mouse Anterior Olfactory Nucleus. The Journal of Neuroscience. 2006;26:12023–12032. doi: 10.1523/JNEUROSCI.2598-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kay RB, Meyer EA, Illig KR, Brunjes PC. Spatial Distribution of Neural Activity in the Anterior Olfactory Nucleus Evoked by Odor and Electrical Stimulation. The Journal of Comparative Neurology. 2011;519:277–289. doi: 10.1002/cne.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heller SB, Uiinski PS. Morphology of geniculocortical axons in turtles of the genera Pseudemys and Chrysemys. Anatomy and Embryology. 1987;175:505–515. doi: 10.1007/BF00309685. [DOI] [PubMed] [Google Scholar]
- 60.Stettler DD, Axel R. Representations of Odor in the Piriform Cortex. Neuron. 2009;63:854864. doi: 10.1016/j.neuron.2009.09.005. [Stettler DD, Axel R: Representations of Odor in the Piriform Cortex. Neuron 2009, 63:854–864] [Using optical imaging of odor-evoked responses, this study demonstrates that different odorants activate distinct ensembles of cortical neurons that are distributed across PCx, without any apparent spatial segregation relative to the similarity between odorants.] [DOI] [PubMed] [Google Scholar]
- 61.Rennaker RL, Chen CF, Ruyle AM, Sloan AM, Wilson DA. Spatial and Temporal Distribution of Odorant-Evoked Activity in the Piriform Cortex. The Journal of Neuroscience. 2007;27:1534–1542. doi: 10.1523/JNEUROSCI.4072-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poo C, Isaacson JS. Odor Representations in Olfactory Cortex : “Sparse” Coding, Global Inhibition, and Oscillations. Neuron. 2009;62:850–861. doi: 10.1016/j.neuron.2009.05.022. [Poo C, Isaacson JS: Odor Representations in Olfactory Cortex : “Sparse” Coding, Global Inhibition, and Oscillations. Neuron 2009, 62:850–861] [These authors performed in vivo whole-cell recordings of PCx neurons. They show that inhibition to layer-2 cells is broadly tuned to odors while excitation is more selective and that the timing of action potentials is controlled by out-of-phase excitatory and inhibitory inputs oscillating in the beta frequency range.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Franks KM, Isaacson JS. Strong Single-Fiber Sensory Inputs to Olfactory Cortex : Implications for Olfactory Coding. Neuron. 2006;49:357–363. doi: 10.1016/j.neuron.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 64.Davison IG, Ehlers MD. Neural Circuit Mechanisms for Pattern Detection and Feature Combination in Olfactory Cortex. Neuron. 2011;70:82–94. doi: 10.1016/j.neuron.2011.02.047. [Davison IG, Ehlers MD: Neural Circuit Mechanisms for Pattern Detection and Feature Combination in Olfactory Cortex. Neuron 2011, 70:82–94] [The authors recorded extracellularly and intracellularly responses of PCx neurons to glomerular activation by photo-uncaging of glutamate in the olfactory bulb. They show that PCx neurons receive weak subthreshold inputs from individual glomeruli distributed across the olfactory bulb but respond above spike threshold to specfic combinations of glomeruli activation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perez-Orive J, Mazor O, Turner GC, Cassenaer S, Wilson RI, Laurent G. Oscillations and sparsening of odor representations in the mushroom body. Science. 2002;297:359–65. doi: 10.1126/science.1070502. [DOI] [PubMed] [Google Scholar]
- 66.Laurent G. Olfactory network dynamics and the coding of multidimensional signals. Nature Reviews Neuroscience. 2002;3:884–95. doi: 10.1038/nrn964. [DOI] [PubMed] [Google Scholar]
- 67.Mazurskaya PZ. Organization of receptive fields in the forebrain of Emys orbicularis. Neuroscience and Behavioral Physiology. 1973;6:311–8. doi: 10.1007/BF01182671. [DOI] [PubMed] [Google Scholar]
- 68.Boiko VP. Responses to visual stimuli in thalamic neurons of the turtle Emys orbicularis. Zhurnal Evolyutsionnoi Biokhimii i Fiziologii. 1980;14:57–63. doi: 10.1007/BF01148461. [DOI] [PubMed] [Google Scholar]
- 69.Senseman D, Robbins K. Modal behavior of cortical neural networks during visual processing. The Journal of Neuroscience. 1999;19:1–7. doi: 10.1523/JNEUROSCI.19-10-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haddad R, Lanjuin A, Madisen L, Zeng H, Murthy VN, Uchida N. Olfactory cortical neurons read out a relative time code in the olfactory bulb. Nature Neuroscience. 2013;16:949–957. doi: 10.1038/nn.3407. [Haddad R, Lanjuin A, Madisen L, Zeng H, Murthy VN, Uchida N: Olfactory cortical neurons read out a relative time code in the olfactory bulb. Nature Neuroscience 2013, 16:949–957] [This study investigates the response of PCx neurons to photo-stimulation of glomeruli and shows that PCx neurons are sensitive to the relative timing of activation of pairs of glomeruli, regardless of their spatial separation in the olfactory bulb.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kay LM, Beshel J, Brea J, Martin C, Kopell N, Rojas-li D. Olfactory oscillations : the what, how and what for. Trends in Neurosciences. 2009;32:207–214. doi: 10.1016/j.tins.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kay LM, Beshel J. A Beta Oscillation Network in the Rat Olfactory System During a 2-Alternative Choice Odor Discrimination Task. Journal of Neurophysiology. 2010;104:829–839. doi: 10.1152/jn.00166.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chapuis J, Wilson DA. Bidirectional plasticity of cortical pattern recognition and behavioral sensory acuity. Nature Neuroscience. 2012;15:155–161. doi: 10.1038/nn.2966. [Chapuis J, Wilson DA: Bidirectional plasticity of cortical pattern recognition and behavioral sensory acuity. Nature Neuroscience 2012, 15:155–161] [In this study, the authors show that the ability of adult rats to discriminate between odors depends on the past behavioral experience and correlates with functional changes in the encoding properties of PCx neuronal ensembles. It suggests that the capacity of the PCx network to perform pattern completion or pattern separation is adjustable to the animal’s experience.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Neville KR, Haberly LB. Beta and Gamma Oscillations in the Olfactory System of the Urethane-Anesthetized Rat. Journal of Neurophysiology. 2003;90:3921–3930. doi: 10.1152/jn.00475.2003. [DOI] [PubMed] [Google Scholar]
- 75.Prechtl JC, Cohen LB, Persaran B, Mitra PP, Kleinfeld D. Visual stimuli induce waves of electrical activity in turtle cortex. Proc Nat Acad Sci USA. 1997;94:7621–7626. doi: 10.1073/pnas.94.14.7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Flanigan WF. Sleep and wakefulness in chelonian reptiles. II. The red-footed tortoise, Geochelone carbonaria. Archives italiennes de biologie. 1974;112:253–77. [PubMed] [Google Scholar]
- 77.Gourévitch B, Kay LM, Martin C, Beshel J, Goure B. Directional Coupling From the Olfactory Bulb to the Hippocampus During a Go / No-Go Odor Discrimination Task. Journal of Neurophysiology. 2010;103:2633–2641. doi: 10.1152/jn.01075.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martin C, Gervais R, Messaoudi B, Ravel N. Learning-induced oscillatory activities correlated to odour recognition : a network activity. European Journal of Neuroscience. 2006;23:1801–1810. doi: 10.1111/j.1460-9568.2006.04711.x. [DOI] [PubMed] [Google Scholar]
- 79.Martin C, Gervais R, Chabaud P, Messaoudi B, Ravel N. Learning-induced modulation of oscillatory activities in the mammalian olfactory system : The role of the centrifugal fibres. Journal of physiology, Paris. 2004;98:467–478. doi: 10.1016/j.jphysparis.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 80.Prechtl JC, Bullock TH, Kleinfeld D. Direct evidence for local oscillatory current sources and intracortical phase gradients in turtle visual cortex. Proc Nat Acad Sci USA. 2000;97:877–882. doi: 10.1073/pnas.97.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nenadic Z, Ghosh BK, Ulinski PS. Modeling and Estimation Problems in the Turtle Visual Cortex. IEEE Trans Biomed Eng. 2002;49:753–762. doi: 10.1109/TBME.2002.800753. [DOI] [PubMed] [Google Scholar]
- 82.Freeman WJ. Distribution in time and space of prepyriform electrical activity. Journal of Neurophysiology. 1959;22:644–665. doi: 10.1152/jn.1959.22.6.644. [DOI] [PubMed] [Google Scholar]
- 83.Martin C, Beshel J, Kay LM. An Olfacto-Hippocampal Network Is Dynamically Involved in Odor-Discrimination Learning. Journal of Neurophysiology. 2007;98:2196–2205. doi: 10.1152/jn.00524.2007. [DOI] [PubMed] [Google Scholar]
- 84.Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Nat Acad Sci USA. 2000;97:1867–1872. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Choi GB, Stettler DD, Kallman BR, Bhaskar ST, Fleischmann A, Axel R. Driving Opposing Behaviors with Ensembles of Piriform Neurons. Cell. 2011;146:1004–1015. doi: 10.1016/j.cell.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cohen Y, Reuveni I, Barkai E, Maroun M. Olfactory learning-induced long-lasting enhancement of descending and ascending synaptic transmission to the piriform cortex. The Journal of Neuroscience. 2008;28:6664–9. doi: 10.1523/JNEUROSCI.0178-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grisham W, Powers AS. Function of the Dorsal and Medial Cortex of Turtles in Learning. 1989;103:991–997. doi: 10.1037//0735-7044.103.5.991. [DOI] [PubMed] [Google Scholar]
- 88.Blau A, Powers AS. Discrimination learning in turtles after lesions of the dorsal cortex or basal forebrain. Psychobiology. 1989;17:445–449. [Google Scholar]
- 89.Moser EI, Roudi Y, Witter MP, Kentros C, Bonhoeffer T, Moser M. Grid cells and cortical representation. Nature Reviews Neuroscience. 2014;15:466–481. doi: 10.1038/nrn3766. [DOI] [PubMed] [Google Scholar]
- 90.Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do ? Nature Reviews Neuroscience. 2009;10:792–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]